SUMMARY
The enhancer regions of the myogenic master regulator MyoD give rise to at least two enhancer RNAs. CEeRNA regulates transcription of the adjacent MyoD gene while DRReRNA affects expression of Myogenin in trans. We found that DRReRNA is recruited at the Myogenin locus where it colocalizes with Myogenin nascent transcripts. DRReRNA associates with the Cohesin complex and such association correlates with its transactivating properties. Despite being expressed in undifferentiated cells, Cohesin is not loaded on Myogenin until the cells start expressing DRReRNA, which is then required for Cohesin chromatin recruitment and maintenance. Functionally, depletion of either Cohesin or DRReRNA reduces chromatin accessibility, prevents Myogenin activation, and hinders muscle cell differentiation. Thus, DRReRNA ensures spatially appropriate Cohesin loading in trans to regulate gene expression.
Keywords: Non-Coding RNA, Enhancer RNA, Muscle Gene Transcription, Myogenin, Cohesin
eTOC Blurb
A non-coding RNA transcribed from an enhancer region on mouse chromosome 7 (eRNA) recruits Cohesin to regulate transcription of the Myogenin gene located on chromosome 1.

INTRODUCTION
Enhancers activate or increase the rate of transcription independently of their position and orientation respect to target genes (Maniatis et al., 1987) (Levine, 2010) (Bulger and Groudine, 2011) (Spitz and Furlong, 2012). Tridimensional enhancer-promoter interactions are mediated by DNA, RNA, and protein components, including transcription factors and chromatin-binding CTCF and Cohesin complexes (Bonev and Cavalli, 2016). In addition to serving as platforms for transcription factors recruitment, enhancers are themselves transcribed into noncoding RNA (enhancer RNAs, eRNAs) (De Santa et al., 2010) (Kim et al., 2010) (Orom et al., 2010) (Natoli and Andrau, 2012). Even though it remains to be conclusively demonstrate that they exert a general regulatory role on transcription (Struhl, 2007) (Natoli and Andrau, 2012) (Kaikkonen et al., 2013) (Espinosa, 2016), several studies indicate that eRNAs contribute to gene activation (Orom et al., 2010) (Melo et al., 2013) (Li et al., 2013) (Mousavi et al., 2013) (Lai et al., 2013) (Schaukowitch et al., 2014) (Hsieh et al., 2014) (Maruyama et al., 2014) (Ilott et al., 2015) (Pnueli et al., 2015) (Yang et al., 2016) (Bose et al., 2017) (Scionti et al., 2017) (Alvarez-Dominguez et al., 2017) (Yang et al., 2017). The majority of eRNAs act locally, regulating transcription of neighboring genes through the involvement of transcriptional activators and coactivators, such as Mediator (Lai et al., 2013), the CBP acetyltransferase (Bose et al., 2017), the negative elongation factor NELF (Schaukowitch et al., 2014), or transcription factors (Sigova et al., 2015) (Beagan et al., 2017) (Weintraub et al., 2017). Long-range enhancer-promoter interactions are frequently observed. For instance, expression of the Sonic hedgehog (SHH) gene is regulated by an enhancer located 1 Mb away and point mutations of this enhancer segregate with polydactyly in both humans and mice (Lettice et al., 2003) (Amano et al., 2009) (Li et al., 2012). The IRS gene, involved in the pathogenesis of type-2 diabetes mellitus, is physically connected to two sites positioned ~ 0.5 and 1Mb away from its promoter (Li et al., 2012). Enhancers frequently bypass nearby genes (de Laat and Duboule, 2013) so that only ~ 7% of looping interactions are with the nearest gene (Sanyal et al., 2012). In mouse embryonic stem cells, 76% of enhancer nodes interact beyond their closest active gene with more than 40% of the enhancer-promoter interactions occurring between different chromosomes (interchromosomal interactions) (Zhang et al., 2013). Similar results were observed in mouse neural stem cells and neural progenitors (Zhang et al., 2013). Interchromosomal interactions have also been documented by live-cell imaging in mouse embryonic stem cells as well as in human retinal cells (Maass et al., 2018). An understanding of the role played by eRNAs in long-range transcriptional regulation remains at this moment incomplete.
Cohesin complexes have an architectural role in establishing chromosomal organization (Kagey et al., 2010) (Phillips-Cremins et al., 2013) (Merkenschlager and Nora, 2016) (Vian et al., 2018). However, gene transcription was not significantly affected by Cohesin acute depletion (Rao et al., 2017). Chromatin loop domains were eliminated but histone modifications were not altered and widespread ectopic gene activation not observed in the absence of Cohesin. Rather, Cohesin seemed to regulate appropriate super-enhancers (SEs) topology and function as in its absence SEs collide to form de novo higher-order intra- and inter-chromosomal hubs affecting a very small set of genes (Rao et al., 2017).
Here, we report that DRReRNA, a noncoding RNA transcribed from the enhancer regions of the master regulator MyoD (located on mouse chromosome 7), acts in trans at the Myogenin locus (located on mouse chromosome 1). Despite being transcribed in close vicinity of MyoD, DRReRNA is not recruited at and does not directly affect transcription of its neighboring gene. Rather it localizes at and influences transcription of Myogenin. By ChIRP-seq and single-molecule RNA FISH, we document recruitment and colocalization of DRReRNA with nascent Myogenin transcripts. Mass spectrometry identified several subunits of the Cohesin complex as DRReRNA interactors. Reducing DRReRNA prevented recruitment of the Cohesin complex at Myogenin, and decreasing either DRReRNA or Cohesin hindered chromatin remodeling, Myogenin expression and cell differentiation. These data indicate that DRReRNA directs specific and appropriate Cohesin loading in trans to regulate muscle gene expression.
RESULTS
DRReRNA Acts in Trans to Regulate Myogenin
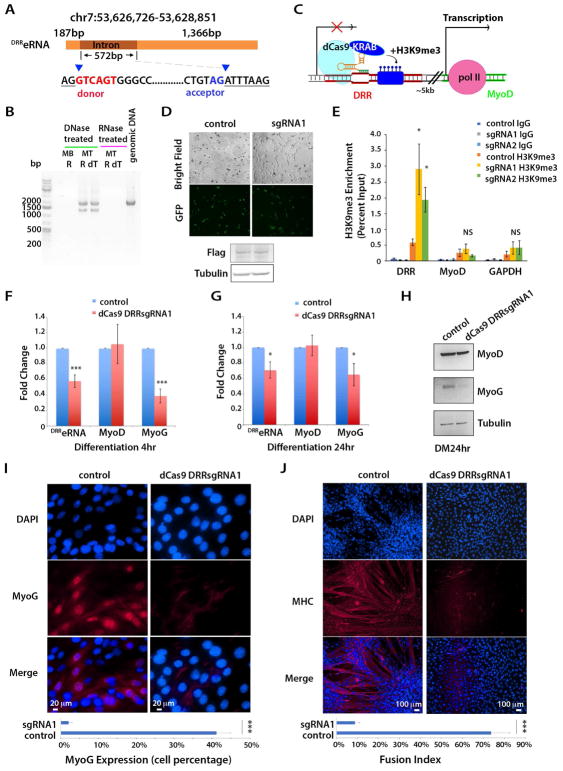
An evolutionarily conserved enhancer element, the distal regulatory region (DRR), located 5kb from the transcriptional start site (TSS) of the MyoD gene (Tapscott et al., 1992) (Asakura et al., 1995) is transcribed into a noncoding RNA (Mousavi et al., 2013) (Mueller et al., 2015). Because of the overlap with the DRR enhancer, we designated the corresponding RNA transcript DRR eRNA (DRReRNA) (Mousavi et al., 2013). Employing RNA derived from either undifferentiated skeletal muscle C2C12 cells (myoblasts, MB) or differentiated myotubes (MT) and DRR-specific primers, the DRReRNA appeared as a C2C12 MT transcript of ~2Kb. Treatment with DNase or RNase indicated that the amplified bands result from RNA reverse transcription, not amplification of contaminating DNA (Figure 1A,B). In agreement with data indicating DRReRNA polyadenylation (Mousavi et al., 2013), DRReRNA could be amplified using oligodT primers (Figure 1B) and displayed transcriptional unidirectionality (Figure S1A) (Mousavi et al., 2013), thus conforming to 1d-eRNAs classification (Natoli and Andrau, 2012). In addition to a ~2Kb transcript, we noted the presence of a ~1.5Kb transcript, suggesting potential splicing (Figure 1B). Sequencing of the DRR bands revealed that unspliced transcripts encompassed a 572 bp region flanked by intron donor and acceptor sites (Figure 1A).
Figure 1. DRReRNA is Required for Myogenin Expression.
(A) Schematic representation of DRReRNA transcripts and donor and acceptor sites. (B) Reverse-transcription PCR analysis of DRReRNA. C2C12 MB (MB); C2C12 MT (MT); R, random hexamers; dT, oligo dT primers used in RT. PCR products were run on a 1.0% agarose gel. (C) Schematic representation of DRRsgRNAs-directed dCAS9-KRAB at the DRR locus. (D) Detection of dCAS9-KRAB-GFP-FLAG fusion protein expressed in C2C12 cells transduced with control or dCAS9-KRAB-DRRsgRNA1 lentivirus (top panel). Cell extracts were immunoblotted with Flag and tubulin antibodies (bottom panel). (E) H3K9me3 ChIP-qPCR at DRR, and MyoD and GAPDH promoters in C2C12 cells transduced with control dCAS9-KRAB, dCAS9-KRAB-DRRsgRNA1 and dCAS9-KRAB-DRRsgRNA2 viruses, respectively. Data are presented as mean ± SD (n=5), (*) P < 0.05. NS, not significant. (F,G) Relative expression of DRReRNA, MyoD, and Myogenin transcripts measured by RT-qPCR in C2C12 cells transduced with either control dCAS9-KRAB or dCAS9-KRAB-DRRsgRNA1 and induced to differentiate for either 4 hr or 24 hr. Data are presented as mean ± SD (n=3), (*) P < 0.05, (***) P < 0.005. (H) Immunoblotting with MyoD, Myogenin, and tubulin antibodies of cell extracts derived from C2C12 cells transduced with either dCAS9-KRAB or dCAS9-KRAB-DRRsgRNA1 and differentiated (differentiation medium, DM) for 24 hr. (I) Immunofluorescence detection of Myogenin (red) in C2C12 cells transduced with either dCAS9-KRAB or dCAS9-KRAB-DRRsgRNA1 and differentiated for 24 hr. Quantification of Myogenin-positive control and dCAS9-KRAB-DRRsgRNA1 cells. Data are presented as mean ± SD (10 randomly chosen microscopic fields), (***) P < 0.005. (J) Immunofluorescence detection of Myosin Heavy Chain (MHC) (red) in C2C12 cells transduced with either dCAS9-KRAB or dCAS9-KRAB-DRRsgRNA1 and differentiated for 48 hr. Fusion index (percentage of myotubes containing > 3 nuclei) in control and dCAS9-KRAB-DRRsgRNA1 cells. Data are presented as mean ± SD (10 randomly chosen microscopic fields), (***) P < 0.005.
To directly evaluate whether the DRReRNA 2Kb transcript could undergo splicing, we cloned it into the splicing reporter plasmid RHCglo (Singh and Cooper, 2006). The resulting plasmid (RHCglo-DRR) or the parental vector were transiently transfected in human embryonic kidney 293 cells, in C2C12 MB or C2C12 cells induced to differentiate. RNA recovered from RHCglo-DRR-transfected cells and amplified with vector-specific primers gave rise to a single band of ~1.5Kb (Figure S1B,C), the same length observed for endogenous DRReRNA. Approximately 95% of spliced DRReRNA was localized to the nucleus (Figure S1D). Transfection of either unspliced or spliced forms of DRReRNA activated Myogenin expression to a comparable extent, without affecting that of MyoD (Figure S1E–G). Approximately 80% of overexpressed DRReRNA localized to the nucleus (Figure S1H). Since majority of endogenous or overexpressed spliced DRReRNA localized to the nucleus it’s likely that nuclear spliced DRReRNA is the active isoform. However, we cannot conclusively rule out that nuclear unspliced or small quantity of overexpressed cytoplasmic DRReRNA may also be functional. Non-polyadenylated eRNAs are unstable, rapidly degraded with a half-life of approximately 7 minutes (De Santa et al., 2010; Schaukowitch et al., 2014). Unexpectedly, DRReRNA decay time evaluated by 5-ethynyl uridine (EU) pulse-labeling was found to be ~ 30 minutes (Figure S1I). These results indicate that mouse DRReRNA is a muscle-specific, spliced, unidirectional, and polyadenylated transcript with a longer half-life than previously characterized non-polyadenylated eRNAs.
siRNA-mediated DRReRNA depletion impairs Myogenin expression and hinders the myogenic differentiation program without directly affecting MyoD transcription (Mousavi et al., 2013). Since eRNAs regulate transcription prevalently in cis (Orom et al., 2010) (Lai et al., 2013), it was important to rigorously document DRReRNA’s effect in trans using several and independent experimental approaches. Therefore, we transduced C2C12 cells with lentiviruses expressing the nuclease-defective dCas9-KRAB repressor and DRR-specific single guide RNAs (sgRNAs) (Figure 1C,D). dCas9-KRAB can be targeted at putative distal enhancer regions where it deposits H3K9me3 resulting in transcriptional repression (Gilbert et al., 2013) (Thakore et al., 2015). ChIP-qPCR showed that dCas9-KRAB in combination with two DRRsgRNAs, DRR-sgRNA1 or DRR-sgRNA2, induced H3K9me3 at the DRR without significantly affecting MyoD or GAPDH promoter regions (Figure 1E). DRReRNA and Myogenin transcripts and protein were reduced in cells transduced with dCas9-KRAB-DRR-sgRNA1 lentivirus compared to cells receiving control vector (dCas9-KRAB only). MyoD transcripts were slightly diminished, but not significantly affected and MyoD protein wasn’t reduced by dCas9-KRAB-DRR-sgRNA1 (Figure 1F–H). Immunostaining with antibodies against Myogenin and Myosin Heavy Chain, markers of early and late differentiation, respectively, documented their impaired expression and inability of dCAs9-KRAB-DRR-sgRNA1-transduced cells to differentiate and to form multinucleated myotubes (Figure 1I,J). Similar results were obtained with dCas9-KRAB-DRR-sgRNA2 lentivirus (Figure S2). To further confirm the effects of reducing DRReRNA, we employed RNase H-activating antisense oligonucleotides (ASO). Transfection of DRReRNA ASO in C2C12 cells reduced DRReRNA and Myogenin transcripts without affecting MyoD (Figure S3A). Thus, either preventing DRReRNA transcription by dCas9-KRAB-sgRNA directed targeting or depleting its transcripts by ASO or siRNA (Mousavi et al., 2013) resulted in decreased Myogenin transcription without apparently affecting MyoD.
We next investigated whether DRReRNA exerted a similar role in primary mouse myocytes. Isolated primary mouse myoblasts were transfected with DRReRNA-siRNA and their RNA collected after 24 hours of culture in differentiation medium. Real-time quantitative PCR revealed that the DRReRNA and Myogenin transcripts were decreased whereas those of MyoD levels were unchanged (Figure S3B). In complementary experiments, freshly FACS-isolated satellite cells were transduced with either control or DRReRNA-expressing retrovirus. DRReRNA overexpression resulted in premature Myogenin expression without significantly affecting MyoD (Figure S3C). Due to cross-regulation (Weintraub et al., 1991), the slight MyoD increase observed upon DRReRNA expression may be consequence of increased Myogenin expression. Overall, these results confirm and reinforce earlier findings that DRReRNA influences transcription of Myogenin in trans without significantly affecting that of MyoD in cis.
DRReRNA is Recruited to the Myogenin Locus and Colocalizes with Myogenin Nascent Transcripts
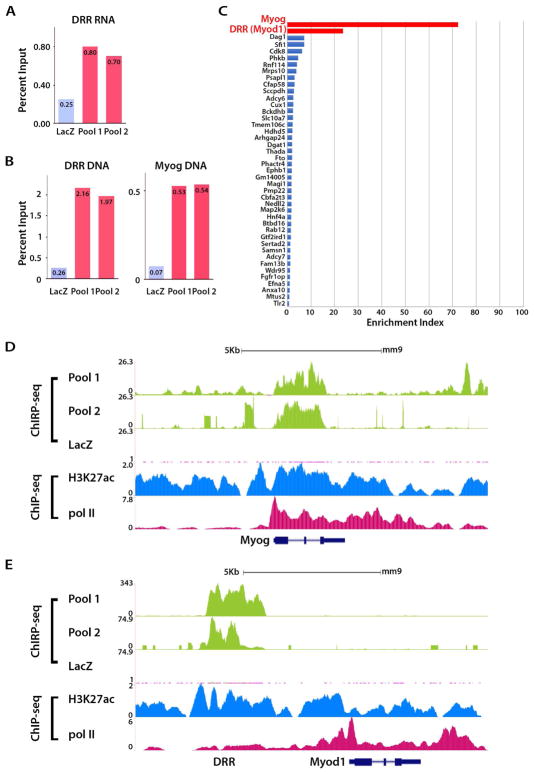
To assess whether DRReRNA associates with chromatin, we performed Chromatin Isolation by RNA Purification (ChIRP) (Chu et al., 2011). Twenty-two biotinylated non-overlapping and complementary DNA oligonucleotides tiling 1.2 Kb of DRReRNA were split in two pools (11 “even-Pool2” and 11 “odd-Pool1” oligonucleotides). As a negative control, we employed biotinylated DNA oligonucleotides targeting the lacZ mRNA. Given the DRReRNA expression profile (Figure S1), ChIRP-seq was performed on chromatin derived from differentiated C2C12 cells. Both DRReRNA Pool1 and Pool2 ChIRP samples were enriched for DRReRNA, and DRR and Myogenin genomic DNA over the ChIRP LacZ sample (Figure 2A,B). Several DRReRNA ChIRP-seq peaks appeared to be enriched over lacZ control (Table S1). Only enrichments present in both Pool1 and Pool2 DRReRNA DNA probes in two biological replicates were further considered. We ranked the DRReRNA ChIRP-seq peaks according to the ChIRP Enrichment Index (CEI) (Experimental Procedures) and, to evaluate specificity of ChIRP peaks, calculated the normalized distribution of the reads in specific peak areas as described in Experimental Procedures. This analysis revealed that the top two DRReRNA-enriched ChIRP regions were observed at Myogenin and DRR while the MyoD gene located 5kb downstream to DRR was devoid of DRReRNA binding (Figure 2C–E). Except for Myogenin, none of the genes assigned to the DRReRNA ChIRP-seq peaks was affected by DRReRNA siRNA (Table S1), indicating that DRReRNA recruitment is not functionally relevant for these genes.
Figure 2. DRReRNA is Recruited to the Myogenin Locus.
(A,B) PCR quantification, expressed as percentage of input, of RNA (DRR) and DNA (DRR and Myogenin) recovered after lacZ ChIRP or DRReRNA ChIRP with two different biotinylated probe sets (Pool1 and Pool2) in C2C12 MT. (C) Enrichment index of DRReRNA ChIRP-seq peaks at assigned genes obtained by calculating the area and width of low-error peaks of two independent replicates as described in Experimental Procedures. (D) DRReRNA ChIRP-seq profiles at the Myogenin locus in C2C12 MT. The signals in Pool 1 track were obtained with “odd” DRReRNA and those in Pool 2 with “even” DRReRNA oligonucleotide probes. ChIP-seq tracks for H3K27ac and polII (Dell’Orso et al., 2016). (E) DRReRNA ChIRP profiles at the DRR and MyoD locus in C2C12 MT.
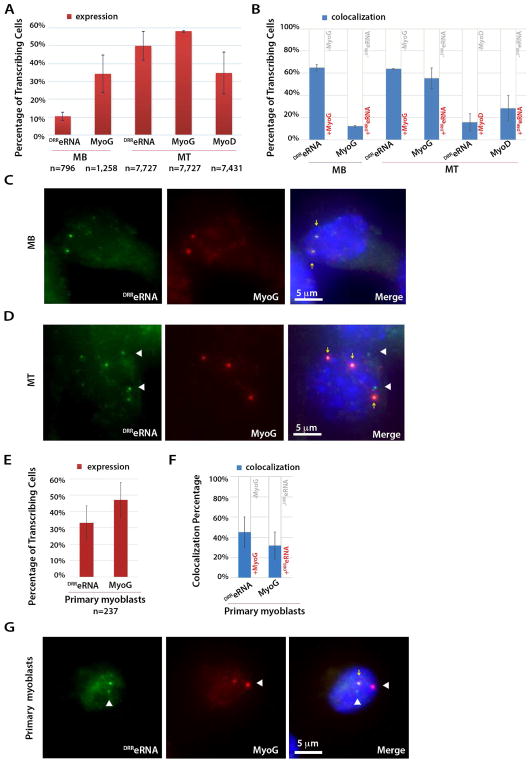
To visualize DRReRNA, Myogenin and MyoD nascent transcription we performed single-molecule RNA-fluorescence in situ hybridization (smFISH). smFISH enables detection of RNA at the site of transcription in individual cells (Femino et al., 1998). Cy3-labeled DRReRNA exonic and Cy5-labeled Myogenin intronic probes were employed to identify the corresponding transcripts. The percentage of DRReRNA-positive cells increased from 10% to 50% and for Myogenin from 34% to 58% during the transition from C2C12 MB to MT, respectively (Figure 3A,C,D). Robust co-localization of DRReRNA and pre-mRNA Myogenin transcripts was observed in both MB (65%) and MT (64%) (Figure 3B–D, Figure S4A,B, Table S2). The inverse pre-mRNA Myogenin to DRReRNA colocalization increased from 12% in MB to 55% in MT (Figure 3B). In MT, Cy5-labeled MyoD intronic probes identified MyoD transcripts in 35% of the cells (Figure 3A). Colocalization of DRReRNA and pre-mRNA MyoD transcripts occurred in 15% of the cells and, conversely, pre-mRNA MyoD to DRReRNA was observed in 28% of MT (Figure 3B, Table S2). Thus, in MT, colocalization of DRReRNA and pre-mRNA Myogenin transcripts occurred 4.2-fold (64%) more frequently than with pre-mRNA MyoD transcripts (15%). We note that, due to the close proximity (5kb) of their sites of transcription, the percentage of DRReRNA and MyoD colocalization might have been overestimated, not reflecting an actual colocalization of the two transcripts but rather their juxtaposition. Neither DRReRNA nor pre-mRNA Myogenin transcripts were detected in mouse embryonic fibroblasts (Figure S4C,D). Next, we investigated whether DRReRNA- pre-mRNA Myogenin colocalization was accompanied by colocalization of the corresponding MyoD and Myogenin loci. 3D DNA FISH experiments revealed that during cell differentiation, from myoblasts to myotubes, the distance separating MyoD and Myogenin loci was reduced and their colocalization occurred in approximately 1% of C2C12 MB and 5.5% of C2C12 MT (Figure S4E–H), indicating low frequency contacts of the two loci (see Discussion). Finally, we conducted smFISH in primary muscle cells (Figure 3E–G). DRReRNA transcripts were present in 33% and Myogenin pre-mRNA in 47% of primary myoblasts, respectively (Figure 3E). DRReRNA-pre-mRNA Myogenin colocalization events were detected in 45% and the converse pre-mRNA Myogenin to DRReRNA colocalization in 32% of primary myoblasts (Figure 3F,G, Table S2). Thus, using two independent experimental approaches, ChIRP-seq and smFISH, DRReRNA was found to preferentially localize at sites of active Myogenin transcription.
Figure 3. DRReRNA Colocalizes with Pre-mRNA Myogenin.
(A) Single molecule RNA FISH (smFISH) quantification of signals corresponding to DRReRNA and pre-mRNA Myogenin transcripts in undifferentiated C2C12 myoblasts (MB) and C2C12 cells differentiated for 24 hr (myotubes, MT); and of pre-mRNA MyoD transcripts in C2C12 MT. Cells containing at least one spot in the nucleus were classified as “transcribing”. Data are presented as mean± SD. Total number of signal-positive cells is indicated. (B) Percentage of observed DRReRNA-pre-mRNA Myogenin and pre-mRNA Myogenin-DRReRNA colocalization signals in C2C12 MB and MT; and of DRReRNA-pre-mRNA MyoD and pre-mRNA-MyoD-DRReRNA colocalization signals in C2C12 MT. (C) Representative images of smFISH in C2C12 MB cells using DRReRNA (Cy3, green) and Myogenin intronic probes (Cy5, red). Colocalizated fluorescent spots are identified by yellow arrows. (D) Representative images of smFISH in C2C12 MT cells using DRReRNA probes and Myogenin intronic probes. Colocalizated fluorescent spots are identified by yellow arrows. DRReRNA non-colocalizated fluorescent spots are identified by white arrowheads. (E) smFISH quantification for DRReRNA and pre-mRNA Myogenin transcripts in primary myoblasts. Cells containing at least one spot in the nucleus were classified as “transcribing”. Data are presented as mean± SD. The total number of signal-positive cells is indicated. (F) Percentage of observed DRReRNA-pre-mRNA Myogenin and pre-mRNA Myogenin-DRReRNA in primary myoblasts. (G) Representative images of smFISH in primary myoblasts using DRReRNA and Myogenin intronic probes. Colocalizated fluorescent spots are identified by yellow arrows. DRReRNA and pre-mRNA Myogenin non-colocalizated fluorescent spots are identified by white arrowheads.
DRReRNA Functionally Associates with the Cohesin Complex
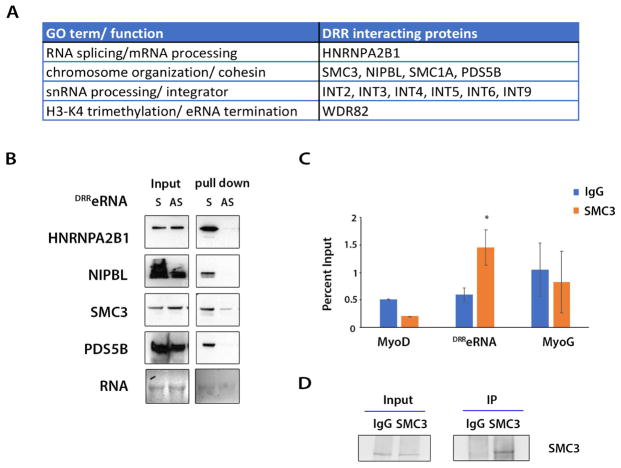
RNA emerging from sites of transcription is stabilized and processed by interacting proteins (Moore and Proudfoot, 2009). We determined whether DRReRNA can associate with protein partners by employing streptavidin bead-mediated capture of biotinylated DRReRNA and C2C12 nuclear extracts. The purified polypeptides were identified by quantitative liquid chromatography tandem-mass spectrometry (LM-MS/MS). Only polypeptides purified by DRReRNA in two biological replicates were further considered as potential interactors (Table S3). In addition to members of Integrator (INT) and WDR82, both of which are involved in eRNA biogenesis (Lai et al., 2015) (Austenaa et al., 2015), we identified the heterogenous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1), a nuclear RNA-binding protein involved in RNA splicing which has been purified with long non-coding RNAs and pri-miRNA (Ng et al., 2013) (Carpenter et al., 2013) (Liu et al., 2016). Several members of the Cohesin complex were also purified by sense DRReRNA (Figure 4A). To validate the RNA-protein interactions identified by mass spectrometry, sense and anti-sense biotinylated DRReRNA were incubated with C2C12 nuclear extracts and captured by streptavidin-coated magnetic beads. The beads were extensively washed and purified RNA-protein complexes subjected to immunoblot analysis with specific antibodies. HNRNPA2B1, the cohesin SMC3, the cohesin loading factor NIPBL, and cohesin associated factor PDS5B could be detected after purification with sense and not anti-sense biotinylated DRReRNA (Figure 4B). RNA-immunoprecipitation (RIP)-qPCR performed with control IgG or SMC3 antibody revealed an interaction between SMC3 and DRReRNA but not MyoD nor Myogenin transcripts (Figure 4C,D).
Figure 4. DRReRNA Interacts with Components of the Cohesin Complex.
(A) DRReRNA-interacting proteins identified by mass spectrometry. (B) Biotinylated sense (S) and antisense (AS) DRReRNAs were incubated with C2C12 MT nuclear extracts and RNA-protein complexes were immunoblotted with HNRNPA2B1, NIPBL, SMC3, or PDS5B antibodies. (C) C2C12 nuclear extracts were immunoprecipitated with either IgG or SMC3 antibody, the associated RNAs were reversed transcribed and amplified using specific primers for MyoD, DRReRNA, or Myogenin transcripts. Relative enrichment was measured by RT-qPCR. Data are presented as mean ± SD (n=3), (*) P < 0.05. (D) Input and immunoprecipitated SMC3 protein were documented by immunoblotting.
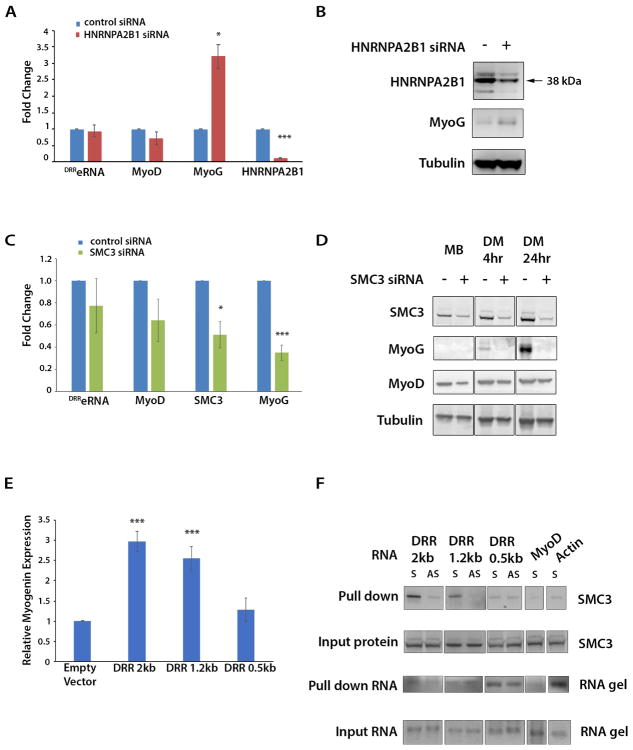
To evaluate their functional role, we reduced either HNRNPA2B1 or SMC3. While HNRNPA2B1siRNA increased Myogenin transcripts and protein (Figure 5A,B), decreasing SMC3 levels hindered Myogenin expression. In contrast, MyoD expression was reduced only in undifferentiated myoblasts but recovered during cell differentiation (Figure 5C,D). Next, we tested whether the transactivating properties of DRReRNA are related to specific sequences or secondary structures and if they correlate with its ability to interact with SMC3. RNA secondary structure prediction was investigated by folding DRReRNA in silico using RNAfold (Hofacker, 2003). Calculation of base-pair probabilities in the thermodynamic ensemble identified several DRReRNA domains. Domains A and C have a higher probability of base-pairing than domains B and D (Figure S5A–C). Full-length DRReRNA (2kb) activated Myogenin expression and interacted with SMC3 whereas a construct expressing the DRReRNA domain D alone (0.5kb) neither activated Myogenin not interacted with SMC3. A construct containing DRReRNA domains A,B, and D interacted with SMC3 and activated Myogenin, suggesting that domains A and B are involved in conferring both transactivating and SMC3 binding properties (Figure 5E,F, Figure S5D). While more detailed studies will be necessary to precisely identify the DRReRNA sequences linking structure-function, these findings indicate that discrete regions of DRReRNA are required to interact with SMC3 and activate Myogenin expression.
Figure 5. Depletion of DRReRNA-Interacting Proteins HNRNPA2B1 and SMC3.
(A) Relative expression measured by RT-qPCR of DRReRNA, MyoD, Myogenin, and HNRNPA2B1 transcripts in C2C12 cells transfected with either control or HNRNPA2B1-siRNA. Data are presented as mean ± SD (n=3), (*) P < 0.05, (***) P < 0.005. (B) C2C12 cells were transfected with either control or HNRNPA2B1siRNA and cell extracts immunoblotted with HNRNPA2B1, Myogenin, or tubulin antibodies. (C) Relative expression measured by RT-qPCR of DRReRNA, MyoD, SMC3, and Myogenin transcripts from 48 hours differentiated C2C12 transfected with either control or SMC3siRNA. Data are presented as mean ± SD (n=3), (*) P < 0.05, (***) P < 0.005. (D) C2C12 cells were transfected with either control or SMC3siRNA and either collected as undifferentiated myoblasts (MB) or induced to differentiate for 4 hr or 24 hr (DM, differentiation medium). Cell extracts were immunoblotted with SMC3, Myogenin, MyoD, or tubulin antibodies. (E) C2C12 cells were transfected with empty vector, DRR 2kb, 1.2kb, or 0.5kb expression vectors (Mousavi et al., 2013) and Myogenin mRNA levels measured by RT-qPCR. Data are presented as mean ± SD (n=3), (***) P < 0.005. (F) Biotinylated sense (S) and antisense (AS) DRReRNA 2kb, 1.2kb or 0.5kb transcripts were incubated with C2C12 MT nuclear extracts and the eluted RNA-protein complexes were subjected to immunoblotting with SMC3 antibodies. Sense MyoD and actin transcripts were employed as control.
DRReRNA Promotes Cohesin Recruitment and Maintenance at the Myogenin Locus
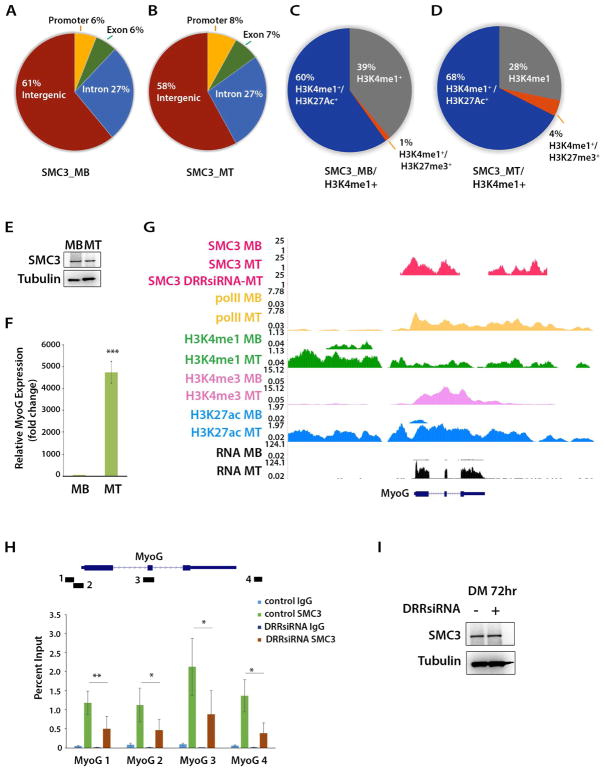
The association of DRReRNA transcripts with several members of the Cohesin complex justified an investigation of the genome-wide SMC3 distribution in C2C12 MB and MT. The SMC3 antibody we employed has been validated in previous ChIP-seq experiments (Kagey et al., 2010) (Busslinger et al., 2017). In both C2C12 MB and MT, SMC3 peaks were mainly detected at intergenic (61%) and intronic (27%) regions. A smaller percentage of peaks was present at promoter (6%–8%) and exons (6%–7%) (Figure 6A,B). Approximately one-third of the SMC3 peaks occurred at H3K4me1 regions and, within these regions, majority of SMC3 peaks were found at active enhancers (H3K4me1+/H3K27ac+) and only at 1%–4% of poised enhancers (H3K4me1+/H3K27me3+) (Figure 6C,D and Table S4). SMC3+/H3K4me1+/H3K27ac+ increased from 60% in MB to 68% in MT and the newly acquired MT regions preferentially occurred at MT super-enhancers (27%) versus typical enhancers (8%) (Table S4).
Figure 6. DRReRNA-Dependent Recruitment of SMC3 at the Myogenin Locus.
(A) Distribution of SMC3 ChIP-seq signals in C2C12 MB and (B) C2C12 MT. (C) Distribution of SMC3 at H3K4me1+, H3K4me1+/H3K27Ac+ or H3K4me1+/H3K27me3+ regions in C2C12 MB. (D) Distribution of SMC3 at H3K4me1+, H3K4me1+/H3K27Ac+ or H3K4me1+/H3K27me3+ regions in C2C12 MT. (E) Cell extracts derived from C2C12 MB and MT were immunoblotted with SMC3 and tubulin antibodies. (F) Relative levels of Myogenin transcripts in C2C12 MB and MT. Data are presented as mean ± SD (n=3), (***) P < 0.005. (G) SMC3 ChIP-seq profiles at the Myogenin locus in C2C12 MB, control C2C12 MT and DRReRNA siRNA-transfected C2C12 MT. PolII, H3K4me1, H3K4me1, H3K4me3, and H3K27ac tracks are from (Dell’Orso et al., 2016). (H) SMC3 ChIP-qPCR at Myogenin in C2C12 cells transfected after 8 hr of differentiation with either control or DRReRNAsiRNA and further differentiated to 72 hr. Data are presented as mean ± SD (n=3), (*) P < 0.05, (**) P < 0.01. (I) Extracts from control and DRReRNAsiRNA transfected C2C12 cells employed in (H) were subjected to immunoblotting with SMC3 or tubulin antibodies.
Despite being comparably expressed in C2C12 MB and MT (Figure 6E), SMC3 was recruited at Myogenin only in C2C12 MT when Myogenin is activated (Figure 6F,G). Since DRReRNA and Myogenin transcripts concordantly increase during muscle cell differentiation (Mousavi et al., 2013) (Figure S1), we asked whether DRReRNA may regulate SMC3 recruitment. To this end, we performed SMC3 ChIP-seq in cells transfected with control or DRReRNA-siRNAs. In DRReRNA siRNA-transfected cells, SMC3 recruitment was impaired at Myogenin (Figure 6G, SMC3 DRRsiRNA-MT track). This was not a general phenomenon as at other regions SMC3 loading was not affected by DRReRNA-siRNA (Figure S5A–D). NIPBL loading at Myogenin was also regulated and prevented in DRReRNA-siRNA transfected cells (Figure S6E). Reducing the DRReRNA levels hampers muscle cell differentiation raising concerns that DRReRNA-siRNA may indirectly affect SMC3 recruitment by maintaining the cells in an undifferentiated state. To clarify this point, we first determined the timing of SMC3 loading during C2C12 cell differentiation and reduced DRReRNA after SMC3 was engaged at Myogenin. A ChIP-qPCR time-course revealed increased SMC3 loading starting 8 hr and remaining until the end of the time-course 72 hr after the cells were induced to differentiate (Figure S6F). Accordingly, control or DRReRNA-siRNA were transfected after 8 hr and cells collected for ChIP-qPCR after 72 hr. The results of these experiments indicated that decreasing DRReRNA after SMC3 was fully recruited resulted in reduced SMC3 loading at the Myogenin locus (Figure 6H). SMC3 expression was not affected by DRReRNA-siRNA (Figure 6I). Overall, these findings document that DRReRNA is required for Cohesin recruitment and maintenance at Myogenin.
DRReRNA and Cohesin Regulate Chromatin Accessibility
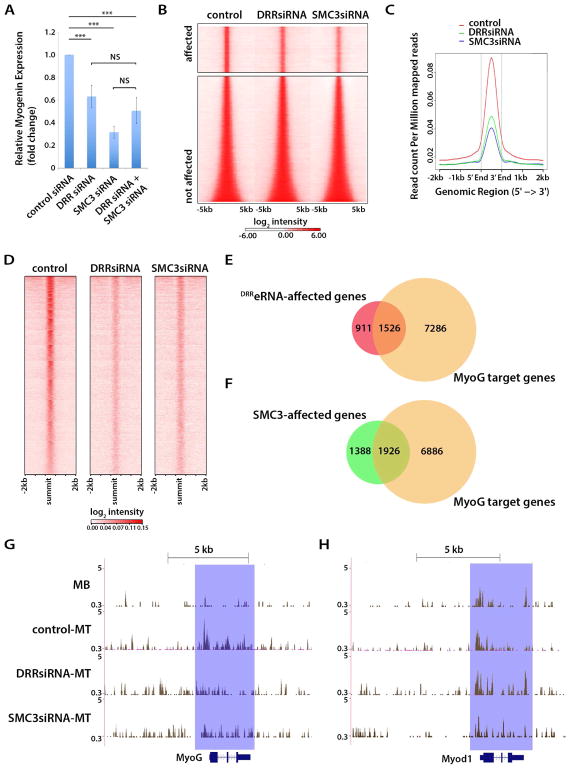
We reasoned that should DRReRNA and SMC3 control the same regulatory step, their combined reduction wouldn’t be expected to have an additive negative effect on Myogenin expression. To test this supposition C2C12 cells were transfected with either SMC3, DRReRNA, or a combination of the two siRNAs and Myogenin expression monitored by qPCR. Reducing either DRReRNA or SMC3 levels decreased Myogenin expression which wasn’t further decreased by concomitant knock-down of DRReRNA and SMC3 (Figure 7A).
Figure 7. DRReRNA and Cohesin Regulate Chromatin Accessibility.
(A) Relative expression of Myogenin transcripts measured by RT-qPCR in C2C12 cells transfected with control, DRReRNA, SMC3, or a combination of DRReRNA and SMC3 siRNAs and induced to differentiate for 16 hr. Data are presented as mean ± SD (n=5), (***) P < 0.005, NS, not significant. (B) Heatmaps representing ATAC-seq signal intensity for regions affected or not affected by DRReRNA (DRRsiRNA) or SMC3siRNA in C2C12 cells differentiated for 16 hr. (C) Averaged ATAC-seq signal densities for DRRsiRNA affected peaks in control, DRReRNA (DRRsiRNA), or SMC3siRNA-transfected C2C12 cells differentiated for 16 hr. Signals were centered at the ATAC-seq peaks summit. (D) Heatmaps representing ATAC-seq signal intensity for regions affected by DRReRNA (DRRsiRNA) or SMC3siRNA in C2C12 cells differentiated for 16 hr. (E) Venn diagrams illustrating overlap of Myogenin-occupied and DRReRNA-affected genes (P = 1.442E-27, against the average overlap of 1000 randomly sampled mm9 gene sets of the same sizes). (F) Venn diagrams illustrating overlap of Myogenin-occupied and SMC3-affected genes (P = 1.086E-27). (G) ATAC-seq tracks at the Myogenin locus in C2C12 myoblasts (MB), C2C12 cell transfected with control, DRReRNA (DRRsiRNA), or SMC3siRNA and differentiated for 16 hr (MT). (H) ATAC-seq tracks at the MyoD locus in C2C12 myoblasts (MB), C2C12 cell transfected with control, DRReRNA (DRRsiRNA), or SMC3siRNA and differentiated for 16hr (MT).
Since DRReRNA regulates chromatin remodeling at the Myogenin locus (Mousavi et al., 2013), we investigated whether SMC3 may participate to this process. Chromatin accessibility of control, DRReRNA, or SMC3-siRNA transfected cells was probed by Assay for Transposase Accessible Chromatin with high-throughput sequencing (ATAC-seq). Out of total 39,330 ATAC peaks, 11,283 (28.7%) were reduced in DRReRNA and 13,802 (35%) in SMC3 knock-down cells, respectively (Figure 7B–D, Table S4). Regions with reduced ATAC-seq peaks in DRReRNA knock-down cells were assigned by proximity (−10/+10Kb from the peak summit) to 2,437 genes and to 3,314 genes in SMC3 knock-down cells, respectively (Table S5). Sixty-two percent (1,526/2,437) of the assigned genes within reduced ATAC peaks in DRReRNA and fifty-eight percent (1,926/3,314) in SMC3 knock-down cells are direct Myogenin targets (Figure 7E,F) (Table S5) and include genes which are upregulated during differentiation (Dell’Orso et al., 2016) and correspond to gene ontology biological terms muscle system process, muscle structure development, muscle contraction, regulation of cell differentiation, and muscle organ development (Table S6). ATAC peaks at the Myogenin locus increased, both in frequency and magnitude, in cells undergoing differentiation (Figure 7G, MB and control-MT tracks), concomitant with Myogenin activation. Reducing either DRReRNA (DRRsiRNA-MT) or SMC3 (SMC3-siRNA-MT) decreased ATAC peaks, indicating impaired Myogenin chromatin accessibility (Figure 7G). In addition to reducing chromatin accessibility, DRReRNA depletion impaired RNA polymerase II recruitment at Myogenin (Figure 7SA). A distinct ATAC-seq pattern was observed at the MyoD locus. Consistent with MyoD being expressed in both C2C12 myoblasts and myotubes (Davis et al., 1987), chromatin at its locus was already accessible in myoblasts. While SMC3 siRNA reduced ATAC peaks, DRReRNA siRNA was without consequence on chromatin accessibility at MyoD (Figure 7H). Thus, while both DRReRNA and SMC3 concordantly regulate chromatin accessibility of Myogenin, SMC3 affects MyoD accessibility independently of DRReRNA.
DISCUSSION
MyoD enhancer regions exert regulatory functions, in part, by giving rise to at least two eRNAs coherently regulating myogenesis but acting with distinct mechanisms. The CEeRNA regulates transcription of the adjacent MyoD gene in cis (Mousavi et al., 2013) (Scionti et al., 2017) whereas the DRReRNA promotes Myogenin transcription in trans (Mousavi et al., 2013) (Mueller et al., 2015). While eRNAs generally regulate expression of neighboring genes in cis, some eRNAs, such as ncRNA-a7, DRReRNA, KLK3-eRNA, and Bloodlinc affect transcription also in trans (Orom et al., 2010) (Mousavi et al., 2013) (Hsieh et al., 2014) (Alvarez-Dominguez et al., 2017). Blocking transcription of DRReRNA by RNA-guided epigenetic repression or promoting its degradation was without immediate consequences on transcription of the adjacent MyoD gene located only 5kb from the DRR. DRReRNA was enriched at the DRR, the site of its own transcription, and at Myogenin. Consistent with its function, and despite the vicinity, DRReRNA was not recruited at MyoD. Moreover, DRReRNA colocalized with Myogenin nascent transcripts, confirming physical presence of DRReRNA at sites of Myogenin transcription. These observations indicate that the ability of DRReRNA to selectively regulate PolII occupancy and chromatin accessibility at Myogenin (Mousavi et al., 2013) correlates with its physical presence at Myogenin. DRReRNA and Myogenin nascent transcripts identified in myoblast cultures are likely to be present in cells that have stochastically initiate differentiation. While the distance separating the two loci decreased during differentiation, the low frequency (1%–5%) of MyoD and Myogenin loci colocalization and lack of Hi-C contacts between MyoD and Myogenin (Doynova et al., 2017) do not immediately support a model where DRReRNA is transferred from its site of transcription to Myogenin via physical interaction of the two chromosomes on which the loci reside. However, we cannot exclude that dynamic and transient chromosomes association, not captured by Hi-C and 3D DNA FISH, may bring in close proximity DRR and Myogenin.
DRReRNA was found to associate with Integrator and WDR82, protein complexes involved in eRNA biogenesis (Lai et al., 2015) (Austenaa et al., 2015). Several polypetides of the Cohesin complex were also identified. The levels of SMC3, the core subunit of the Cohesin complex, were comparable in undifferentiated and differentiated muscle cells. However, SMC3 recruitment at Myogenin coincided with DRReRNA and Myogenin transcriptional activation and was dependent on DRReRNA. Reducing DRReRNA, even after SMC3 was initially recruited, impaired SMC3 recruitment at Myogenin. While DRReRNA and SMC3 concordantly regulated Myogenin chromatin accessibility during cell differentiation, reducing DRReRNA did not affect MyoD accessibility or transcription. Altogether, these findings indicate that DRReRNA-mediated Cohesin recruitment mechanistically differs from estrogen receptor-regulated transcription where the Cohesin complex is present on many estrogen receptors-regulated enhancers even before ligand treatment and contributes to gene activation at least in part by stabilizing E2/ER-alpha/eRNA-induced enhancer-promoter looping (Li et al., 2013) (Li et al., 2016). Structure-function experiments suggested that two DRReRNA domains, A and B, are involved in SMC3 binding and transactivation. Domain A is predicted to fold with high base-pair probabilities. Cohesin complexes mediate DNA-DNA-interactions by topological embrace and loop distant DNA sequences (Uhlmann et al., 2000) (Hadjur et al., 2009) (Phillips-Cremins et al., 2013) (Ji et al., 2016). It is therefore tempting to speculate that self-paired DRReRNA regions may serve as platform for Cohesin recruitment.
A remaining and important question relates to the mechanism/s by which eRNAs acting in trans identify their cognate targets. Lack of polyadenylation on a substantial percentage of eRNAs may explain their instability (De Santa et al., 2010) (Schaukowitch et al., 2014), limiting their range of action to neighboring genes (Kim and Shiekhattar, 2016). Interestingly, the trans-acting DRReRNA, ncRNA-a7, KLK3eRNA, and Bloodlinc are polyadenylated. We found that DRReRNA has a half-life of ~30 min, above the ~7 min observed for other eRNAs (De Santa et al., 2010) (Schaukowitch et al., 2014). Polyadenylation may afford selected eRNAs the stability necessary to explore the nuclear space and identify target sequences on which to act upon. Our findings that DRReRNA is recruited at Myogenin and colocalizes with Myogenin nascent transcripts suggest that target recognition could be mediated by either formation of RNA:RNA interaction of DRReRNA with intronic regions of the Myogenin nascent transcripts or RNA/DNA triple helix at the Myogenin locus. Although their formation may be deleterious (Sollier and Cimprich, 2015), RNA loops are often observed near transcriptional star sites of transcribed genes where they can induce nucleosome depletion and chromatin decondensation (Powell et al., 2013) (Sanz et al., 2016) and positively correlate with H3S10P, a histone mark induced by ncRNA-a (Ginno et al., 2013) (Castellano-Pozo et al., 2013) (Lai et al., 2013). Aligning DRR sequences to regions enriched for ChIRP-seq peaks at Myogenin has not detected obvious overlaps. It remains formally possible that non-contiguous, short stretches of DRReRNA nucleotides complementarily recognize sequences present at the Myogenin locus. Other polyadenylated eRNAs may employ alternative targeting strategies as they interact with and affect transcription of hundreds of loci (Orom et al., 2010) (Alvarez-Dominguez et al., 2017), arguing against their recognition mode be based on base-pair complementarity. Another possibility is that high-valency disordered regions of eRNAs and their associated proteins participate in the formation of biomolecular condensates or phase-separated domains to compartmentalize transcription (Hyman et al., 2014) (Banani et al., 2017) (Hnisz et al., 2017). By regulating Myogenin, the master regulator of muscle cell differentiation (Hasty et al., 1993) (Nabeshima et al., 1993), DRReRNA indirectly controls expression of the entire muscle differentiation program. This is consistent with the observation that DRReRNA knock-down impaired chromatin remodeling of genes whose transcription is induced during cell differentiation and that are direct Myogenin targets, ultimately preventing muscle cell differentiation. Irrespective of the relevant and presently unaddressed target recognition modalities, our findings describe the dynamics and effectors through which an eRNA generated by a muscle-specific enhancer orchestrates muscle gene expression in trans and regulates cell differentiation.
EXPERIMENTAL PROCEDURES
Cell lines and mouse embryonic fibroblasts
Phoenix, HEK293 and HEK293T cells (ATCC) were cultured in DMEM supplemented with 10% FBS. C2C12 cells (ATCC) were grown in 1× Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco), 20% HyClone FBS [GE Healthcare]). For C2C12 cell differentiation, FBS was replaced with 2% horse serum (Gibco) supplemented with 1× insulin-transferrin-selenium (Gibco). All cells were cultured at 37°C with 5% CO2. Mouse embryonic fibroblasts were cultured in DMEM supplemented with 10% FBS.
Bacterial strains
DH5α and TOP10 cells were purchased from Thermo Fisher Scientific, NEB® 5-alpha Competent E. coli (High Efficiency) were purchased from New England BioLabs. Competent E. coli were stored at −80°C and used to propagate plasmids growing in LB medium at 37°C.
Satellite cells isolation by FACS (fluorescence-activated cell sorting)
Satellite cells (SCs) were isolated from 3 months old adult wild type mice following the method described by (Liu et al., 2013). Hindlimb muscle were minced then digested with collagenase for 1.5 hr into muscle slurry. SCs were then released from the digested muscle by collagenase/dispase treatment for additional 30 min. After removing the debris, total cells were incubated with the following primary antibodies: biotin conjugated VCAM1, Pacific Blue-labeled Sca1, APC-labeled CD31/CD45, and SYTOX Green. Satellite cells were sorted by gating on positive VCAM1- and negative on Pacific Blue-labeled Sca1, APC-labeled CD31/CD45, and SYTOX Green staining using BD Influx or FACSAria Cell Sorters.
Plasmids
RHCglo vector was kindly provided by Dr. Thomas A. Cooper (Baylor College of Medicine). DRR was subcloned into RHCglo vector using BspEI and PpuMI restriction enzyme sites. dCAS9-KRAB plasmids were purchased from Addgene (#71236). The DRRsgRNAs were subcloned into dCAS9-KRAB vector using BsmBI restriction enzyme. For in vitro transcription, pCRII TOPO vector was purchased from ThermoFisher (#K4600-01). All plasmids and corresponding sequence information are available upon request.
Antibodies
Antibodies used were anti-H3K9me3 (ab8898, abcam), anti-Myh (MF20, DSHB), anti-tubulin (E7, DSHB), anti-MyoD (c-20, Santa Cruz), anti-Myogenin (F5D, Santa Cruz), anti-HNRNPA2B1 (sc-374052, Santa Cruz), anti-PDS5B (A300-537A, Bethyl), anti-SMC3 (ab9263, Abcam) and anti-NIPBL (A301-779A, Bethyl), anti-RNA polymerase II (Abcam), and anti-FLAG (Sigma). Alexa Fluor® 658 goat anti-mouse (H+L) was used as secondary antibodies for immunofluorescence.
Transfections and RNA Interference
Plasmid transient transfections were performed using Lipofectamine 2000 (Invitrogen). Cells were transfected with control, SMC3, HNRNPA2B1, or DRReRNA siRNAs with RNAiMAX (Invitrogen), 10nM final per each siRNA, according to manufacturer’s protocol.
Lentiviral production
dCAS9-KRAB lentiviruses were generated using HEK293T cells (Life Technologies) following the method described by (Thakore et al., 2015). 1 day before transfection, cells were seeded at 60% confluency in 100mm cell culture dish (Corning). Cells were transfected the next day at 80–90% confluency. For each plate, 10μg of plasmid containing the vector of interest, 10 μg of pMD2.G and 15μg of psPAX2 (Addgene) vectors were transfected using 100 μl of Lipofectamine 2000. 24 hr after transfection the media was changed. Virus supernatant was harvested 48hr post-transfection, filtered with a 0.45-mm PVDF filter (Millipore). C2C12 cells were transduced (Sigma) in 100mm cell culture dish.
Retroviral production
DRReRNA retroviruses were generated using Phoenix cells following the method described by (Caretti et al., 2004). DRReRNA was cloned into pHAN-puro retroviral vector. 10μg of vector was transfected into Phoenix cells using 20 μl of Lipofectamine 2000. 24hr after transfection the media was changed. Virus supernatant was harvested twice every 24hr post-transfection, filtered with a 0.45-mm PVDF filter (Millipore) and concentrated using Retro-X™ Concentrator (Clontech). Satellite cells were transduced in 24-well collagen-coated cell culture plates (Corning).
RNA subcellular localization
C2C12 cells were differentiated for 24hr. Cells were harvested and nuclear and cytoplasmic RNAs were extracted from them using PARIS kit (Life Technologies). Purified RNAs were immediately processed for reverse transcription and quantitative PCR.
RNA half-life measurement
C2C12 cells were incubated with 0.2 mM 5-ethynyl Uridine in differentiation medium (DMEM, 2% Horse serum, 1X Insulin/Transferrin/Selenium) for 24 hours. After pulsing, cells were washed 3 times with PBS and collected for RNA extraction after 0, 15, 30 min, 1 hr and 2 hr. Total RNA was extracted by Trizol (Invitrogen) according to manufacturer’s instructions and 5 μg of total RNA were incubated for 30 min with 0.5mM of Biotin Azide for the Click-it reaction. (Click-it nascent RNA capture kit, Invitrogen, C10365). After RNA precipitation, biotinylated RNA was collected by using 50μl of Dynabeads® MyOne™ Streptavidin T1 magnetic beads (Invitrogen, 656-01) per 500ng of biotinylated RNA and washed according with manufacturer’s instructions. After the final wash, samples were immediately used for cDNA synthesis (High capacity cDNA Reverse Transcription Kit, Applied Biosystem, 4368813).
Single molecule RNA fluorescent in situ hybridization (smFISH)
DRReRNA probe sets were synthesized and labeled with cyanine dye Cy3 and Myogenin and MyoD probe sets were labeled with Cy5, respectively. CTCF probes were synthesized and labeled with Cy5. The probe sets were purchased from Biosearch Technologies (Petaluma, CA). C2C12 myoblasts, 24 hr-differentiated C2C12 myotubes, and mouse embryonic fibroblasts were grown on coverslips. smFISH was performed according to the Stellaris RNA FISH protocol with minor changes (Biosearch Technologies, Petaluma, CA). Coverslips were pre-hybridized 2–5 min in wash buffer (2× SSC, 10% deionized formamide). 50μl of probe hybridization solution (10% dextran sulfate, 10% deionized formamide and 2× SSC) containing 100nM of probe was used for each coverslip hybridization within the humidified chamber for 4 hours at 37°C. Coverslips were washed in 37°C wash buffer for 2×30 min at 37°C, rinsed quickly with 2× SSC, and washed in PBS for 5 min. Coverslips were briefly dried and mounted on microscope slides using ProLong Gold antifade reagent with DAPI overnight (Life Technologies). Slides were imaged on a custom-built microscope. This microscope consisted of an ASI Rapid Automated Modular Microscope System (RAMM) base, Hamamatsu ORCA-Flash4 V2 CMOS camera (C11440), Lumencor SpectraX excitation light source, ASI High Speed Filter Wheel, and ASI MS-2000 Small XY stage. Excitation of the DAPI, Quasar 570, Quasar 670 was performed using the SpectraX violet, red, and green light sources respectively. Specific emission filters were used to select for the associated emission spectra. Microscope hardware was controlled by the Micro-Manager software (https://micro-manager.org/wiki/Citing_Micro-Manager). A grid of 10 by 10 field of views resulting in 100 tiles per sample was imaged. We obtained 25 Z-stacks at 0.5 micron intervals for each channel. The maximum intensity projections were computed in Image J/FIJI and corrected for field non-uniformity of the excitation light source.
3D DNA FISH
Probes for MyoD and MyoG DNA fluorescence in situ hybridization (FISH) were purchased from Empire Genomics. Three-dimensional FISH (3D FISH) experiments were performed on C2C12 myoblasts treated with a 500 μg/ml thymidine double blocking to arrest cells in G1/S phase. 3D FISH on synchronized myoblasts and C2C12 myotubes were conducted per manufacturer protocol (http://www.empiregenomics.com/files/store/products/FISH_probes/FISH_Protocol.pdf). Cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100. Fixed cells were then treated with liquid nitrogen freeze-thaw cycles, glycerol, 0.1N HCl, 200 μg/mL RNase A, and equilibrated in 50% formamide/2X SSC. Cells and probe were co-denatured on a heat block at 73°C for 5 min, hybridized for 48 hours at 37°C, and washed. Cells were counterstained with 1 μg/mL 4′,6′-diamidino-2-phenylindole (DAPI) and mounted with Vectashield mounting medium. FISH slides were imaged with Leica DM6000 wide-field fluorescent microscope while 3D FISH slides were imaged with Leica SP5 NLO confocal microscope using 63X or 100X objective lenses. Three-dimensional reconstruction was conducted using Imaris v7.70. A combination of MatLab and Imaris softwares was used to analyze the spatial distribution of MyoD and MyoG. Imaris software generated the xyz coordinates and volume of each nuclei and DNA probe. In MatLab, each probe was assigned to the closest nuclear center and given a cellular ID. The relative 3D distances between MyoD and MyoG were then determined within each cell how far these probes lied from their corresponding nuclear center.
RNA pull-down
The DRR was inserted into the pCRII plasmid vector using TA cloning. The linearized template was then used for in vitro transcription to incorporate biotinylated UTP with MAXIscript Transcription Kit (Life Technologies). In vitro transcription reactions were first treated with TURBO DNase (Life Technologies). The transcripts were analyzed by MOPS-agarose gel electrophoresis. 12×106 C2C12 cells differentiated for 48hr were pelleted to obtain nuclear extract using nuclear complex Co-IP kit (Active Motif) with 20 U/ml RNaseOut (Invitrogen). The protein supernatant was pre-cleared by applying 40 μl of equilibrated streptavidin-coupled Dynabeads (Invitrogen) for 20 min at 4°C. To block binding of unspecific RNA binding proteins, 20 mg/ml yeast tRNA was added and incubated for 20 min at 4°C. 50pmol of biotinylated RNA was added to pre-cleared nuclear extract and incubated for 2hr at room temperature, followed by addition of 60μl of equilibrated streptavidin-coupled Dynabeads (Invitrogen) and incubation for 12hr at 4°C. Beads were washed with high salt IP buffer for 5 min and 3×5 min in IP buffer. To release proteins from the beads / RNA, proteins were denaturated in 65μl of water with 25μl of 4× LDS sample buffer and 10μl of reducing reagents at 95°C for 5 min, and subjected to and subjected to Western analysis with 4%–12% NuPAGE Bis-Tris gels. RNA was released from the beads by incubation in 95% formamide, 10mM EDTA, pH 8.2 for 5 min at 65°C according to Dynabeads product’s suggestion.
Mass spectrometry
Biotinylated DRReRNA-protein complexes captured on streptavidin-conjugated beads were processed at the Laboratory of Proteomics and Analytical Technologies of the National Cancer Institute at Frederick, MD. Samples were resuspended in 25 mM NH4HCO3, pH 8.4 and heated at 95°C for 5 min before overnight digestion with 2 ug of trypsin at 37°C. The beads were washed twice with 25 mM NH4HCO3, pH 8.4 for maximum yield and the supernatant containing the tryptic digest was obtained after beads centrifugation. The tryptic digest was lyophilized and then reconstituted in 25% ACN/0.1% FA for fractionation using strong cation exchange (SCX) liquid chromatography (LC). The SCX fractionation was performed as described (Das et al., 2010). All LC-MS/MS analysis was performed on an LTQ Velos Pro ion trap mass spectrometer (Thermo Scientific, San Jose, CA). Dried tryptic digests were resuspended in 12 ul of 0.1% TFA and 6 ul were loaded onto Acclaim™ PepMap™ 100 C18 LC column (Thermo Scientific, CA) using a Thermo Easy nLC 1000 liquid chromatograpy system (Thermo Scientific, CA) connected online with the mass spectrometer. After sample injection, the column was washed and peptides eluted using a linear gradient. The mass spectrometer was operated in a data dependent mode in which each full MS scan was followed by 15 data-dependent MS/MS scans in the LTQ using the 15 most abundant ions and collision-induced dissociation for fragmentation.
RNA Immunoprecipitation
6×106 C2C12 cells differentiated for 48hr were prepared following the method described by (Klattenhoff et al., 2013). Nuclear pellets were resuspended in 2ml PBS, 2ml nuclear isolation buffer (1.28 M sucrose; 40 mM Tris-HCl pH 7.5; 20 mM MgCl2; 4% Triton X-100) and 6ml dH2O and the suspension incubated on ice for 20 min. After pelleting by centrifugation at 2,500 G for 15 min, nuclear pellets were resuspended in 1ml RIP buffer (150 mM KCl, 25 mM Tris pH 7.4, 0.5mMDTT, 0.5% NP40, 1mM PMSF, protease inhibitor and 20U/ml RNaseout (Invitrogen)). Nuclei were homogenized by 20 strokes using a dounce homogenizer, followed by centrifuging for 10 min at 13,000 rpm. 5μg antibody against SMC3 (Abcam, ab9263), or IgG (Abcam, ab171870 were added and incubated for 4–5 hr at 4°C. Immuno-complexes were captured by addition of 40μl protein G Dynabeads (Invitrogen). Beads were washed 3 times with RIP buffer for 5 min, followed by resuspending in 1ml Trizol (Thermofisher). Isolated RNA was reverse-transcribed and subjected to qPCR-analysis.
Quantitative RT-PCR
Total RNA was extracted using TRIzol Reagent (Invitrogen) according to manufacturer’s protocol. cDNA was synthesized with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) containing random primers and using 500 ng of extracted RNA per sample. Reverse transcribed cDNA was diluted 1:10 and SYBR green real-time PCR was performed on the Applied Biosystems StepOne Plus Real-Time PCR system. mRNA levels were normalized to 18S.
Chromatin isolation by RNA Purification (ChIRP)
C2C12 cells were differentiated for 48h, trypsinized for 5 min at 37°C; trypsin was quenched with >2× volume of media, cells were re-suspended into single cell suspension and collected in a 50mL Falcon tube by centrifugation (800RCF for 5 min). The cell pellet was washed once with PBS and re-suspended in freshly made 1% glutaraldehyde for crosslinking. Cells were crosslinked for 10 min at room temperature on a shaker. The cross-linking reaction was quenched with 1/10th volume of 1.25 M glycine at room temperature for 5 min. Cells were spun at 2000RCF for 5 min and the pellet was washed with 20 mL of chilled PBS once, followed by a second wash with 1 ml chilled PBS per 20 million cells. Each ml was transferred to an Eppendorf tube and spun at 2000RCF for 4 min at 4 C. Pellets were individually weighed and lysed with Lysis Buffer in a volume equal to10X of the pellet’s mass. Nuclei were sonicated using Bioruptor in 15 ml Falcon tubes at highest setting with 30 seconds ON, 45 seconds OFF pulse intervals. Lysates were cleared at 16100RCF for 10 min at 4°C; 1ml of chromatin was transferred to 15 mL Falcon tube and 10 μL were collected for DNA INPUT. 2 mL of Hybridization Buffer and a mix of specific probes (100 pmol probe per 1 mL chromatin, 1μL of 100 pmol/μL probe per 1 mL chromatin), were added to each tube, mixed and Incubated at 37°C for 4 hr with shaking. C-1 magnetic beads were washed with 1mL of Lysis Buffer three times and resuspended in the original volume of Lysis Buffer, supplemented with fresh PMSF, P.I and Superase-in. After 4 hr of hybridization, 100 μL of beads were added to each tube and incubated at 37°C for 30 min with shaking. Beads were collected and washed with 1 mL of pre-warmed wash buffer for 5 min at 37C, five times. DNA, including input, was eluted in 150uL of Elution Buffer supplemented with 10 μL RNase A (10 mg/mL) and RNaseH (10 U/μL), and treated with proteinase K for 50 min. DNA was extracted with 300uL of phenol:chloroform:isoamyl per sample, precipitated O/N at −20°C in ethanol and finally resuspended in 30uL of EB.
ChIP-qPCR and ChIP followed by sequencing (ChIP-Seq)
Chromatin immunoprecipitation was performed as previously described (Mousavi et al., 2012) using antibodies against SMC3 (Abcam, Ab9236), NIPBL (Bethyl, A301-779A), RNA polymerase II (Abcam) or IgG (Abcam). Real-time PCR was performed with a SyberGreen MasterMix (Applied Biosystems) on a StepOnePlus real-time PCR system (Applied Biosystems). Oligonucleotides employed in ChIP-qPCR for the are reported in Supplemental Information. For sequencing, the DNA fragments were blunt-end ligated to the Illumina adaptors and amplified via Mondrian SP Workstation system (NuGEN). Libraries were sequenced for 50 cycles on Illumina HiSeq3000. Mock DNA (input DNA) was used against the matched sample data to call enriched regions and control for the false-positive detection rate (FDR).
ATAC-seq
ATAC-seq was performed according to a published protocol (Buenrostro et al., 2013) with minor modification. Briefly, 5×104 C2C12 cells were pelleted, washed with 50μl of 1xPBS and lysed in 50μl of Lysis Buffer (10mM Tris-HCl, pH7.4, 10mM NaCl, 3mM MgCl2, 0.1% of IGEPAL CA-630). To tag and fragment accessible chromatin, nuclei were centrifuged at 500× g for 10 min and re-suspended in 40μl of transposition reaction mix with 2μl Tn5 transposase (Illumina Cat# FC-121-1030). The reaction was incubated at 37°C with shaking at 300rpm for 30 min. DNA-fragments were then purified and amplified by PCR (12–15 cycles based on the amplification curve). C2C12 MB and MT samples were multiplexed using primers Ad2.1–4 paired with Ad1 for final library amplification as described previously (Buenrostro et al., 2013). Purified libraries were then sequenced on HiSeq2500 Illumina instrument.
QUANTIFICATION AND STATISTICAL ANALYSIS
smFISH image analysis
smFISH analysis was performed using custom IDL software as previously described (Coulon et al., 2014). smFISH spots in the maximum projections were analyzed with the IDL software Localize using the manual bandpass threshold. Cells were then segmented into nucleus and cytoplasm using CellProfiler (Broad Institute, http://www.cellprofiler.org. Masks generated by CellProfiler were then used to identify nuclear spots with the IDL software FishAuxiliary. This software also outputs the image coordinates of the nuclear spots. Colocalization between spots in different channels was determining using their Euclidean distance. Spots less than or equal to 5 pixels from each other were considered localized. Cells containing at least one spot in the nucleus were classified as “transcribing”. Custom software is available at larsonlab.net. Confocal images were obtained on a Zeiss LSM780 laser scanning confocal microscope. The imaging was performed using a 63X objective, 405nM, 561nM, and 633nM laser excitation, pinhole sizes between 1 and 3, zoom of 0.6 to 0.8 at 2048×2048. Several Z planes were imaged per field of view, spanning 8–11 microns. These sets of Z planes were then processed by the Zen software to extract the maximum intensity projections. These images were then 2×2 pixel binned with ImageJ.
Mass spectrometry data analysis
Acquired MS/MS spectra were searched against a mouse uniprot protein database, 2014 using a SEQUEST and Fixed Value PSM validator algorithm in the Proteome Discoverer 1.4 software (Thermo Scientific, CA). The precursor ion tolerance was set at 1.5 Da and the fragment ions tolerance was set at 0.6 Da along with methionine oxidation included as dynamic modification. Only fully tryptic peptides with up to two miscleavages with charge state dependent cross correlation Xcorr ≥ 2.1 for [M+H]1+, ≥ 2.5 for [M+2H]2+ and ≥ 3.2 for [M+3H]3+ and delta correlation (ΔCn) ≥ 0.08 were considered as positive identification.
ChIRP-seq Profiling and Data Analysis
ChIRP-seq and reads were generated on Illumina HiSeq 2000/2500 machines, demultiplexed by CASAVA from Illumina, and aligned to the mouse reference genome (mm9) using Bowtie2 with the parameters for local alignments (Langmead et al., 2009). Peaks were generated using MACS2 (Zhang et al., 2008) with default parameters of narrow peak calling. After selecting common peaks among replicates using BEDOPS (Neph et al., 2012), the peaks are annotated by ChIPseeker package in R (Yu et al., 2015) and the characteristics of peaks were profiled using rtracklayer package in R (Lawrence et al., 2009). To evaluate ChIRP enrichment, we implemented the ChIRP Enrichment Index (CEI). Based on the observed ChIRP peak pattern, the specificity of ChIRP enrichment is proportional to peak widths. After calculating the error rate (ER) using eq.(1), we selected peaks with low error rates. Then, we calculated the CEI using eq.(2) and ranked the peaks by CEI to measure the ChIRP enrichment.
| (1) |
where
P1 is the num. of reads in peak area in the first replicates
P2 is the num. of reads in peak area in the second replicates
| (2) |
where
P1 is the num. of reads in peak area in the first replicates
P2 is the num. of reads in peak area in the second replicates
Peak width represents the number of reads located within the start and the end of the ChIRP-enriched peak regions determined by ChIPseeker (Bioconductor R package). The peak area was calculated using rtracklayer (Bioconductor R package) as log2-calculated area-under-the curve (AUC) and represents the sum of the total number of mapped reads belonging to the individual peaks obtained from a BigWig file. The number of reads in P1 and P2 were corrected for the sequencing depth.
ChIP-Seq Analysis
Sequencing data were generated with an Illumina’s HiSeq 3000 system. Raw sequencing data were processed with bcl2fastq/2.17.1 to generate Fastq files. Adapter sequences were removed using trimgalore/0.4.2. Reads of 50 bases were aligned to the mouse genome build mm9 with Bowtie/1.1.1 (Langmead et al., 2009), allowing two mismatches. Uniquely mapped and non-redundant reads were used for peak calling using MACS 1.4.2 (Zhang et al., 2008) with a p-value cutoff of 1.0E-05 (Transcription factor model was applied). Bigwig files were generated with BedGraphToBigWig (Kent et al., 2010) and Bedtools/2.25.0 (Quinlan and Hall, 2010). Peaks are assigned to gene-centric genomic regions with PAPST (Bible et al., 2015) (www.ncbi.nlm.nih.gov/geo/). Fastq files of H3K27ac, H3K27me3, and H3K4me1 in C2C12 and MB (myoblasts) MT (myotubes) were from published datasets (Dell’Orso et al., 2016) (accession number GSE76010). The distribution of SMC3 in intergenic, intragenic and promoter (±1kb) regions from the summits of the regions among the different stages of the cells were identified by PAVIS (Huang et al., 2013). Distribution of SMC3 at super- and typical enhancers was determined by Bedtools with default parameters (Quinlan and Hall, 2010).
ATAC-seq Analysis
Sequencing data were generated with an Illumina’s HiSeq 2500 system via pair-end sequencing. Raw sequencing data were processed with bcl2fastq/2.17.1 to generate fastq files. Adapter sequences were removed using cutadapt/1.12 (https://cutadapt.readthedocs.io/en/stable). Reads of 50 bases were aligned to the mouse genome build mm9 with Bowtie/1.1.1 (Langmead et al., 2009), allowing two mismatches. Uniquely mapped and non-redundant reads were used for peak calling using MACS 1.4.2 (Zhang et al., 2008) with a p-value cutoff of 1.0E-03. Peaks were assigned to the closest TSS with PAPST (Bible et al., 2015).
Gene Ontology Analysis
GO analysis of genes whose chromatin accessibility is affected by either DRReRNA or SMC3 siRNA was performed with ToppGene Suite (http://toppgene.cchmc.org) (Chen et al., 2009).
DATA AND SOFTWARE AVAILABILITY
The accession number for ChIP-seq, RNA-seq, and ChIRP-seq data reported in this paper is GEO: GSE113248.
Peak locations derived from ChIRP-seq are listed in Table S1.
Mass spectrometry data are reported in Table S3.
Peak numbers relative to SMC3/H3K4me1/H3K27ac/H3K27me3 ChIP-seq are reported in Table S4.
ATAC-Seq positive regions are listed in Table S5
Supplementary Material
Highlights.
The enhancer RNA DRReRNA is recruited at and regulates expression of Myogenin in trans
DRReRNA Interacts with the Cohesin Complex
DRReRNA is Required for Cohesin Loading and Maintenance at the Myogenin Locus
DRReRNA and Cohesin Remodel Chromatin
Acknowledgments
We thank the NIAMS Flow Cytometry Section, the NIAMS Laboratory Animal Care and Use Section, the NIAMS High-Throughput Sequencing Unit, and the NIAMS Biodata Mining and Discovery Section. This study utilized the high-performance computational capabilities of the Helix Systems at the National Institutes of Health, Bethesda, MD (http://helix.nih.gov). This work was supported in part by the Intramural Research Program of the NIAMS at the NIH and the Center for Cancer Research of the NCI.
Footnotes
Supplemental information includes seven figures and seven tables.
AUTHOR CONTRIBUTIONS
P-F.T. performed most of the experiments. S.D.O. performed ChIRP-seq and ATAC-seq experiments. J.R. and D.R.L. assisted with smFISH experiments. K.O.V. and A.H.J. isolated and FACS purified satellite cells. A.A.S. and M.H. assisted with RNA immunoprecipitation. K.D.K., K.J., H.W.S., J.P., and D.A. analyzed RNA-seq, ChIP-seq, ChIRP-seq, and ATAC-seq data. A.H.W. assisted with protein purification. M.T., D.W., L.V., E.R., and T.R. assisted with 3D DNA FISH experiments. P-F.T., S.D.O. and V.S. conceived the study. V.S. supervised the project and wrote the manuscript with input from the authors.
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Dominguez JR, Knoll M, Gromatzky AA, Lodish HF. The Super-Enhancer-Derived alncRNA-EC7/Bloodlinc Potentiates Red Blood Cell Development in trans. Cell reports. 2017;19:2503–2514. doi: 10.1016/j.celrep.2017.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Sagai T, Tanabe H, Mizushina Y, Nakazawa H, Shiroishi T. Chromosomal dynamics at the Shh locus: limb bud-specific differential regulation of competence and active transcription. Developmental cell. 2009;16:47–57. doi: 10.1016/j.devcel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Asakura A, Lyons GE, Tapscott SJ. The regulation of MyoD gene expression: conserved elements mediate expression in embryonic axial muscle. Dev Biol. 1995;171:386–398. doi: 10.1006/dbio.1995.1290. [DOI] [PubMed] [Google Scholar]
- Austenaa LM, Barozzi I, Simonatto M, Masella S, Della Chiara G, Ghisletti S, Curina A, de Wit E, Bouwman BA, de Pretis S, et al. Transcription of Mammalian cis-Regulatory Elements Is Restrained by Actively Enforced Early Termination. Molecular cell. 2015;60:460–474. doi: 10.1016/j.molcel.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beagan JA, Duong MT, Titus KR, Zhou L, Cao Z, Ma J, Lachanski CV, Gillis DR, Phillips-Cremins JE. YY1 and CTCF orchestrate a 3D chromatin looping switch during early neural lineage commitment. Genome research. 2017;27:1139–1152. doi: 10.1101/gr.215160.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bible PW, Kanno Y, Wei L, Brooks SR, O’Shea JJ, Morasso MI, Loganantharaj R, Sun HW. PAPST, a User Friendly and Powerful Java Platform for ChIP-Seq Peak Co-Localization Analysis and Beyond. PLoS One. 2015;10:e0127285. doi: 10.1371/journal.pone.0127285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonev B, Cavalli G. Organization and function of the 3D genome. Nature reviews Genetics. 2016;17:661–678. doi: 10.1038/nrg.2016.112. [DOI] [PubMed] [Google Scholar]
- Bose DA, Donahue G, Reinberg D, Shiekhattar R, Bonasio R, Berger SL. RNA Binding to CBP Stimulates Histone Acetylation and Transcription. Cell. 2017;168:135–149. e122. doi: 10.1016/j.cell.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nature methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger M, Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011;144:327–339. doi: 10.1016/j.cell.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger GA, Stocsits RR, van der Lelij P, Axelsson E, Tedeschi A, Galjart N, Peters JM. Cohesin is positioned in mammalian genomes by transcription, CTCF and Wapl. Nature. 2017;544:503–507. doi: 10.1038/nature22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes & development. 2004;18:2627–2638. doi: 10.1101/gad.1241904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S, Aiello D, Atianand MK, Ricci EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M, Lawrence JB, et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science. 2013;341:789–792. doi: 10.1126/science.1240925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano-Pozo M, Santos-Pereira JM, Rondon AG, Barroso S, Andujar E, Perez-Alegre M, Garcia-Muse T, Aguilera A. R loops are linked to histone H3 S10 phosphorylation and chromatin condensation. Molecular cell. 2013;52:583–590. doi: 10.1016/j.molcel.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37:W305–311. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Molecular cell. 2011;44:667–678. doi: 10.1016/j.molcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon A, Ferguson ML, de Turris V, Palangat M, Chow CC, Larson DR. Kinetic competition during the transcription cycle results in stochastic RNA processing. eLife. 2014:3. doi: 10.7554/eLife.03939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Bosley AD, Ye X, Chan KC, Chu I, Green JE, Issaq HJ, Veenstra TD, Andresson T. Comparison of strong cation exchange and SDS-PAGE fractionation for analysis of multiprotein complexes. J Proteome Res. 2010;9:6696–6704. doi: 10.1021/pr100843x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- de Laat W, Duboule D. Topology of mammalian developmental enhancers and their regulatory landscapes. Nature. 2013;502:499–506. doi: 10.1038/nature12753. [DOI] [PubMed] [Google Scholar]
- De Santa F, Barozzi I, Mietton F, Ghisletti S, Polletti S, Tusi BK, Muller H, Ragoussis J, Wei CL, Natoli G. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010;8:e1000384. doi: 10.1371/journal.pbio.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Orso S, Wang AH, Shih HY, Saso K, Berghella L, Gutierrez-Cruz G, Ladurner AG, O’Shea JJ, Sartorelli V, Zare H. The Histone Variant MacroH2A1.2 Is Necessary for the Activation of Muscle Enhancers and Recruitment of the Transcription Factor Pbx1. Cell reports. 2016;14:1156–1168. doi: 10.1016/j.celrep.2015.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doynova MD, Markworth JF, Cameron-Smith D, Vickers MH, O’Sullivan JM. Linkages between changes in the 3D organization of the genome and transcription during myotube differentiation in vitro. Skelet Muscle. 2017;7:5. doi: 10.1186/s13395-017-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JM. Revisiting lncRNAs: How Do You Know Yours Is Not an eRNA? Molecular cell. 2016;62:1–2. doi: 10.1016/j.molcel.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Femino AM, Fay FS, Fogarty K, Singer RH. Visualization of single RNA transcripts in situ. Science. 1998;280:585–590. doi: 10.1126/science.280.5363.585. [DOI] [PubMed] [Google Scholar]
- Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginno PA, Lim YW, Lott PL, Korf I, Chedin F. GC skew at the 5′ and 3′ ends of human genes links R-loop formation to epigenetic regulation and transcription termination. Genome research. 2013;23:1590–1600. doi: 10.1101/gr.158436.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjur S, Williams LM, Ryan NK, Cobb BS, Sexton T, Fraser P, Fisher AG, Merkenschlager M. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460:410–413. doi: 10.1038/nature08079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Hnisz D, Shrinivas K, Young RA, Chakraborty AK, Sharp PA. A Phase Separation Model for Transcriptional Control. Cell. 2017;169:13–23. doi: 10.1016/j.cell.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofacker IL. Vienna RNA secondary structure server. Nucleic Acids Res. 2003;31:3429–3431. doi: 10.1093/nar/gkg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Fei T, Chen Y, Li T, Gao Y, Wang X, Sun T, Sweeney CJ, Lee GS, Chen S, et al. Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7319–7324. doi: 10.1073/pnas.1324151111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Loganantharaj R, Schroeder B, Fargo D, Li L. PAVIS: a tool for Peak Annotation and Visualization. Bioinformatics. 2013;29:3097–3099. doi: 10.1093/bioinformatics/btt520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Weber CA, Julicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol. 2014;30:39–58. doi: 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- Ilott NE, Heward JA, Roux B, Tsitsiou E, Fenwick PS, Lenzi L, Goodhead I, Hertz-Fowler C, Heger A, Hall N, et al. Corrigendum: Long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nature communications. 2015;6:6814. doi: 10.1038/ncomms7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Dadon DB, Powell BE, Fan ZP, Borges-Rivera D, Shachar S, Weintraub AS, Hnisz D, Pegoraro G, Lee TI, et al. 3D Chromosome Regulatory Landscape of Human Pluripotent Cells. Cell stem cell. 2016;18:262–275. doi: 10.1016/j.stem.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010 doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikkonen MU, Spann NJ, Heinz S, Romanoski CE, Allison KA, Stender JD, Chun HB, Tough DF, Prinjha RK, Benner C, et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Molecular cell. 2013;51:310–325. doi: 10.1016/j.molcel.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Zweig AS, Barber G, Hinrichs AS, Karolchik D. BigWig and BigBed: enabling browsing of large distributed datasets. Bioinformatics. 2010;26:2204–2207. doi: 10.1093/bioinformatics/btq351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Shiekhattar R. Diverse regulatory interactions of long noncoding RNAs. Curr Opin Genet Dev. 2016;36:73–82. doi: 10.1016/j.gde.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–583. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Gardini A, Zhang A, Shiekhattar R. Integrator mediates the biogenesis of enhancer RNAs. Nature. 2015;525:399–403. doi: 10.1038/nature14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Orom UA, Cesaroni M, Beringer M, Taatjes DJ, Blobel GA, Shiekhattar R. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501. doi: 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M, Gentleman R, Carey V. rtracklayer: an R package for interfacing with genome browsers. Bioinformatics. 2009;25:1841–1842. doi: 10.1093/bioinformatics/btp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, de Graaff E. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12:1725–1735. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- Levine M. Transcriptional enhancers in animal development and evolution. Curr Biol. 2010;20:R754–763. doi: 10.1016/j.cub.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Ruan X, Auerbach RK, Sandhu KS, Zheng M, Wang P, Poh HM, Goh Y, Lim J, Zhang J, et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell. 2012;148:84–98. doi: 10.1016/j.cell.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X, et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498:516–520. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Notani D, Rosenfeld MG. Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nature reviews Genetics. 2016;17:207–223. doi: 10.1038/nrg.2016.4. [DOI] [PubMed] [Google Scholar]
- Liu H, Liang C, Kollipara RK, Matsui M, Ke X, Jeong BC, Wang Z, Yoo KS, Yadav GP, Kinch LN, et al. HP1BP3, a Chromatin Retention Factor for Co-transcriptional MicroRNA Processing. Molecular cell. 2016;63:420–432. doi: 10.1016/j.molcel.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cheung TH, Charville GW, Hurgo BM, Leavitt T, Shih J, Brunet A, Rando TA. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell reports. 2013;4:189–204. doi: 10.1016/j.celrep.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass PG, Barutcu AR, Weiner CL, Rinn JL. Inter-chromosomal Contact Properties in Live-Cell Imaging and in Hi-C. Molecular cell. 2018;69:1039–1045. e1033. doi: 10.1016/j.molcel.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Goodbourn S, Fischer JA. Regulation of inducible and tissue-specific gene expression. Science. 1987;236:1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Maruyama A, Mimura J, Itoh K. Non-coding RNA derived from the region adjacent to the human HO-1 E2 enhancer selectively regulates HO-1 gene induction by modulating Pol II binding. Nucleic Acids Res. 2014;42:13599–13614. doi: 10.1093/nar/gku1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo CA, Drost J, Wijchers PJ, van de Werken H, de Wit E, Oude Vrielink JA, Elkon R, Melo SA, Leveille N, Kalluri R, et al. eRNAs are required for p53-dependent enhancer activity and gene transcription. Molecular cell. 2013;49:524–535. doi: 10.1016/j.molcel.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M, Nora EP. CTCF and Cohesin in Genome Folding and Transcriptional Gene Regulation. Annu Rev Genomics Hum Genet. 2016;17:17–43. doi: 10.1146/annurev-genom-083115-022339. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell. 2009;136:688–700. doi: 10.1016/j.cell.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Mousavi K, Zare H, Dell’orso S, Grontved L, Gutierrez-Cruz G, Derfoul A, Hager GL, Sartorelli V. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Molecular cell. 2013;51:606–617. doi: 10.1016/j.molcel.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi K, Zare H, Wang AH, Sartorelli V. Polycomb protein Ezh1 promotes RNA polymerase II elongation. Molecular cell. 2012;45:255–262. doi: 10.1016/j.molcel.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller AC, Cichewicz MA, Dey BK, Layer R, Reon BJ, Gagan JR, Dutta A. MUNC, a long noncoding RNA that facilitates the function of MyoD in skeletal myogenesis. Mol Cell Biol. 2015;35:498–513. doi: 10.1128/MCB.01079-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- Natoli G, Andrau JC. Noncoding transcription at enhancers: general principles and functional models. Annu Rev Genet. 2012;46:1–19. doi: 10.1146/annurev-genet-110711-155459. [DOI] [PubMed] [Google Scholar]
- Neph S, Kuehn MS, Reynolds AP, Haugen E, Thurman RE, Johnson AK, Rynes E, Maurano MT, Vierstra J, Thomas S, et al. BEDOPS: high-performance genomic feature operations. Bioinformatics. 2012;28:1919–1920. doi: 10.1093/bioinformatics/bts277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SY, Bogu GK, Soh BS, Stanton LW. The long noncoding RNA RMST interacts with SOX2 to regulate neurogenesis. Molecular cell. 2013;51:349–359. doi: 10.1016/j.molcel.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Orom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Cremins JE, Sauria ME, Sanyal A, Gerasimova TI, Lajoie BR, Bell JS, Ong CT, Hookway TA, Guo C, Sun Y, et al. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell. 2013;153:1281–1295. doi: 10.1016/j.cell.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L, Rudnizky S, Yosefzon Y, Melamed P. RNA transcribed from a distal enhancer is required for activating the chromatin at the promoter of the gonadotropin alpha-subunit gene. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:4369–4374. doi: 10.1073/pnas.1414841112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell WT, Coulson RL, Gonzales ML, Crary FK, Wong SS, Adams S, Ach RA, Tsang P, Yamada NA, Yasui DH, et al. R-loop formation at Snord116 mediates topotecan inhibition of Ube3a-antisense and allele-specific chromatin decondensation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13938–13943. doi: 10.1073/pnas.1305426110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SSP, Huang SC, Glenn St Hilaire B, Engreitz JM, Perez EM, Kieffer-Kwon KR, Sanborn AL, Johnstone SE, Bascom GD, Bochkov ID, et al. Cohesin Loss Eliminates All Loop Domains. Cell. 2017;171:305–320. e324. doi: 10.1016/j.cell.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489:109–113. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz LA, Hartono SR, Lim YW, Steyaert S, Rajpurkar A, Ginno PA, Xu X, Chedin F. Prevalent, Dynamic, and Conserved R-Loop Structures Associate with Specific Epigenomic Signatures in Mammals. Molecular cell. 2016;63:167–178. doi: 10.1016/j.molcel.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaukowitch K, Joo JY, Liu X, Watts JK, Martinez C, Kim TK. Enhancer RNA facilitates NELF release from immediate early genes. Molecular cell. 2014;56:29–42. doi: 10.1016/j.molcel.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scionti I, Hayashi S, Mouradian S, Girard E, Esteves de Lima J, Morel V, Simonet T, Wurmser M, Maire P, Ancelin K, et al. LSD1 Controls Timely MyoD Expression via MyoD Core Enhancer Transcription. Cell reports. 2017;18:1996–2006. doi: 10.1016/j.celrep.2017.01.078. [DOI] [PubMed] [Google Scholar]
- Sigova AA, Abraham BJ, Ji X, Molinie B, Hannett NM, Guo YE, Jangi M, Giallourakis CC, Sharp PA, Young RA. Transcription factor trapping by RNA in gene regulatory elements. Science. 2015;350:978–981. doi: 10.1126/science.aad3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Cooper TA. Minigene reporter for identification and analysis of cis elements and trans factors affecting pre-mRNA splicing. Biotechniques. 2006;41:177–181. doi: 10.2144/000112208. [DOI] [PubMed] [Google Scholar]
- Sollier J, Cimprich KA. Breaking bad: R-loops and genome integrity. Trends Cell Biol. 2015;25:514–522. doi: 10.1016/j.tcb.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz F, Furlong EE. Transcription factors: from enhancer binding to developmental control. Nature reviews Genetics. 2012;13:613–626. doi: 10.1038/nrg3207. [DOI] [PubMed] [Google Scholar]
- Struhl K. Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nature structural & molecular biology. 2007;14:103–105. doi: 10.1038/nsmb0207-103. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ, Lassar AB, Weintraub H. A novel myoblast enhancer element mediates MyoD transcription. Mol Cell Biol. 1992;12:4994–5003. doi: 10.1128/mcb.12.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakore PI, D’Ippolito AM, Song L, Safi A, Shivakumar NK, Kabadi AM, Reddy TE, Crawford GE, Gersbach CA. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nature methods. 2015;12:1143–1149. doi: 10.1038/nmeth.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann F, Wernic D, Poupart MA, Koonin EV, Nasmyth K. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell. 2000;103:375–386. doi: 10.1016/s0092-8674(00)00130-6. [DOI] [PubMed] [Google Scholar]
- Vian L, Pekowska A, Rao SSP, Kieffer-Kwon KR, Jung S, Baranello L, Huang SC, El Khattabi L, Dose M, Pruett N, et al. The Energetics and Physiological Impact of Cohesin Extrusion. Cell. 2018 doi: 10.1016/j.cell.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub AS, Li CH, Zamudio AV, Sigova AA, Hannett NM, Day DS, Abraham BJ, Cohen MA, Nabet B, Buckley DL, et al. YY1 Is a Structural Regulator of Enhancer-Promoter Loops. Cell. 2017;171:1573–1588. e1528. doi: 10.1016/j.cell.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S, et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Yang XH, Nadadur RD, Hilvering CR, Bianchi V, Werner M, Mazurek SR, Gadek M, Shen KM, Goldman JA, Tyan L, et al. Transcription-factor-dependent enhancer transcription defines a gene regulatory network for cardiac rhythm. eLife. 2017:6. doi: 10.7554/eLife.31683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Su Z, Song X, Liang B, Zeng F, Chang X, Huang D. Enhancer RNA-driven looping enhances the transcription of the long noncoding RNA DHRS4-AS1, a controller of the DHRS4 gene cluster. Sci Rep. 2016;6:20961. doi: 10.1038/srep20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang LG, He QY. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31:2382–2383. doi: 10.1093/bioinformatics/btv145. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nussbaum C, Myers RM, Brown M, Li W, et al. Model-based analysis of ChIP-Seq (MACS) Genome biology. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wong CH, Birnbaum RY, Li G, Favaro R, Ngan CY, Lim J, Tai E, Poh HM, Wong E, et al. Chromatin connectivity maps reveal dynamic promoter-enhancer long-range associations. Nature. 2013;504:306–310. doi: 10.1038/nature12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.