Abstract
The post-translational glycosylation of select proteins by O-linked mannose (O-mannose or O-man) is a conserved modification from yeast to humans and has been shown to be necessary for proper development and growth. The most well studied O-mannosylated mammalian protein is α-dystroglycan (α-DG). Hypoglycosylation of α-DG results in varying severities of congenital muscular dystrophies, cancer progression and metastasis, and inhibited entry and infection of certain arenaviruses. Defects in the gene products responsible for post-translational modification of α-DG, primarily glycosyltransferases, are the basis for these diseases. The multitude of clinical phenotypes resulting from defective O-mannosylation highlights the biomedical significance of this unique modification. Elucidation of the various O-mannose biosynthetic pathways is imperative to understanding a broad range of human diseases and for the development of novel therapeutics. In this review, we will focus on recent discoveries delineating the various enzymes, structures and functions associated with O-mannose-initiated glycoproteins. Additionally, we discuss current gaps in our knowledge of mammalian O-mannosylation, discuss the evolution of this pathway, and illustrate the utility and limitations of model systems to study functions of O-mannosylation.
Keywords: O-mannosylation, congenital muscular dystrophy, dystroglycan, glycosylation
Introduction
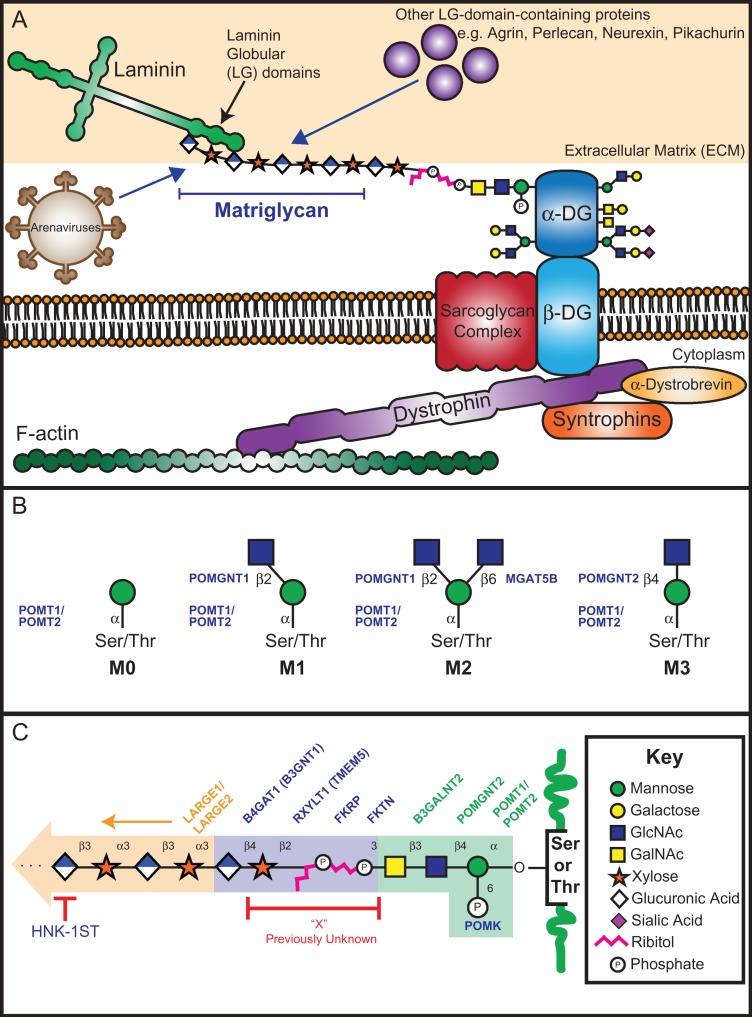
Oxygen-linked alpha-mannose (hereinafter referred to as either O-mannose or O-man) covalently attached to serine or threonine residues was discovered nearly 50 years ago in yeast (Sentandreu and Northcote 1968) and nearly 40 years ago in rat brain (Finne et al. 1979). This post-translational modification is conserved from fungi to humans and plays a role in a wide variety of human diseases, such as congenital muscular dystrophy (CMD), cancer metastasis and viral entry (Dobson et al. 2013; Panin and Wells 2014). Almost 20 years ago, it was discovered that the causative genes for CMD were primarily glycosyltransferase (GT) genes that specifically modify the most well studied O-mannosylated protein, α-dystroglycan (α-DG) (Michele et al. 2002; Moore et al. 2002; Muntoni et al. 2002; Yoshida et al. 2001). The human DAG1 gene (dystrophin-associated glycoprotein 1) encodes the 895-residue dystroglycan precursor protein, which is post-translationally cleaved into the peripheral membrane subunit (α-DG, residues 1–653) and the transmembrane subunit (β-DG, residues 654–895) (Holt et al. 2000). α-DG is post-translationally processed in the secretory pathway by furin to generate the mature α-DG (313–653). In addition to being modified by N-linked glycans (Ervasti and Campbell 1991), α-DG is highly O-glycosylated (serine- or threonine-linked) within its mucin-like domain (residues 316–485) which includes mucin-type, O-GalNAc (N-acetylgalactosamine)-initiated structures as well as O-mannose-initiated structures (Barresi and Campbell 2006; Chiba et al. 1997; Praissman and Wells 2014; Sasaki et al. 1998; Smalheiser et al. 1998; Wells 2013). The best-characterized function of α-DG is its role in the dystrophin-glycoprotein complex (DGC), in which α-DG contributes a glycan-dependent link between the actin cytoskeleton and extracellular matrix (ECM) (Ervasti and Campbell 1993) (Figure 1A).
Fig. 1.
The DGC and core O-mannose structures. (A) The DGC consists of the cytosolic dystrophin protein which connects the actin cytoskeleton to other intracellular and extracellular proteins. The heavily glycosylated peripheral membrane protein α-DG participates in the DGC and mediates interactions with ECM proteins through the GAG-like repeating disaccharide, matriglycan. In addition to binding to LG-domain-containing proteins, matriglycan has been implicated in interactions with certain arenaviruses which utilize α-DG as their primary cell-surface receptor. (B) Four classifications of core O-mannose structures are presented here. Enzymes that catalyze the sugar transfer are indicated. (C) Summary of the fully elaborated core M3 functional glycan. Previously unknown region of this structure (“X”) is indicated in red. The phospho-trisaccharide, as well as the enzymes that catalyze the indicated sugar or phosphate transfer for this region, are highlighted in green. The linker and priming region as well as the linker and priming enzymes are indicated in blue, while matriglycan is highlighted in orange. Glycan symbol representation is adapted from Varki et al. (2015).
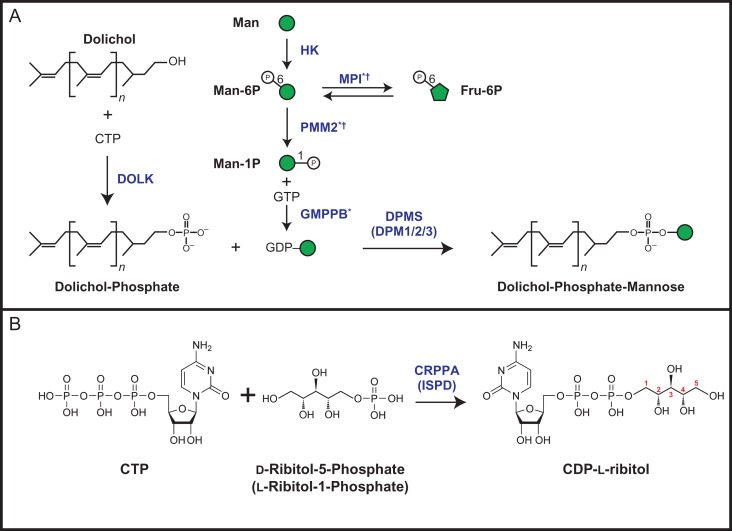
The biosynthesis of O-mannose-initiated glycans begins on the cytosolic face of the endoplasmic reticulum (ER) with the generation of the lipid-linked mannosyl donor molecule dolichol-phosphate mannose (Dol-P-Man or DPM). The enzymatic activity of the DPM synthase complex (DPMS, comprised of DPM1, DPM2 and DPM3) catalyzes the transfer of mannose from guanidine diphosphate mannose (GDP-Man) to dolichol-phosphate (Maeda et al. 2000). In addition to O-mannosylation, various mannosyltransferases involved in asparagine-linked (N-linked) glycosylation (Aebi 2013), tryptophan-linked C-mannosylation (Doucey et al. 1998) and glycosylphosphatidylinositol-anchor biosynthesis (Kang et al. 2005; Orlean 1990) are dependent on Dol-P-Man as a donor substrate.
Four major classifications of core O-mannose-initiated glycans are proposed in Figure 1B, where the last three have been extensively reviewed by Praissman and Wells (2014) and Yoshida-Moriguchi et al. (2013). Hereinafter, we will refer to core O-mannose substructures consistent with the nomenclature proposed by Praissman and Wells (2014). O-mannose glycan biosynthesis is initiated in the ER by the Protein O-mannosyltransferase 1 (POMT1) and Protein O-mannosyltransferase 2 (POMT2) enzyme complex which catalyzes the transfer of mannose from Dol-P-Man to the hydroxyl oxygen of serine or threonine side chains (Manya et al. 2004). We refer to a serine or threonine residue linked with a single α-mannose as core M0. Core M0 can be modified with β1,2-linked N-Acetylglucosamine (GlcNAc) by Protein O-Linked Mannose N-Acetyl-glucosaminyltransferase 1 (POMGNT1), resulting in the core M1 structure. The core M1 structure can be modified with β1,6-linked GlcNAc by the activity of Mannosyl (α1,6)-Glycoprotein β1,6-N-Acetyl-Glucosaminyltransferase (MGAT5B), yielding the core M2 structure. Core M3 glycans are generated by the addition of β1,4-linked GlcNAc to core M0 that is catalyzed by Protein O-Linked Mannose N-Acetyl-glucosaminyltransferase 2 (POMGNT2).
While M1 and M2 cores can be further elaborated, most of the recent research focus has been on the M3 core that can become functionally modified to bind laminin globular (LG)-domain-containing proteins. The core M3 structure is extended with β1,3-linked GalNAc by β-1,3-N-Acetylgalactosaminyltransferase 2 (B3GALNT2). After assembly of this trisaccharide, the six-position of mannose is subject to phosphorylation by Protein O-Mannose Kinase (POMK, formerly SGK196) in the ER, in which this reaction product will be referred to as the “phospho-trisaccharide” throughout this review (Yoshida-Moriguchi et al. 2013). Once formed, the phospho-trisaccharide is further elaborated by the activities of the recently discovered phospho-ribitol transferases and the priming enzymes, which will be discussed later. Finally, the synthesis of matriglycan, a glycosaminoglycan (GAG)-like repeating disaccharide of xylose (Xyl) and glucuronic acid (GlcA) ([-Xyl-α3-GlcA-β3-]n), is polymerized on the primed structure by the activities of the bifunctional GTs LARGE1 (formerly LARGE) and/or its paralog LARGE2 (formerly GYLTL1B) in the Golgi apparatus (Ashikov et al. 2013; Inamori et al. 2012, 2013) (Figure 1C). Matriglycan is the functional epitope that binds the LG-domain-containing proteins in the ECM. This linkage appears to be critical for the structural integrity of skeletal muscle and proper brain development (Yoshida-Moriguchi and Campbell 2015).
A disruption of this link between α-DG and the ECM results in a subset of CMDs. When mutations in specific genes result in hypoglycosylation or loss of α-DG, these diseases are referred to as dystroglycanopathies. The evolving nomenclature of dystroglycanopathies is chiefly classified by the origin of the genetic defect, where primary dystroglycanopathies result from mutation in the DAG1 gene (Geis et al. 2013; Hara, Balci-Hayta et al. 2011), while secondary dystroglycanopathies result from defects in any of the genes encoding enzymes directly modifying of α-DG (Beedle et al. 2012; Brockington et al. 2010) (Table I). Secondary dystroglycanopathies include a wide range of muscular dystrophic phenotypes including the most severe case of Walker–Warburg syndrome (WWS) to the less severe limb-girdle muscular dystrophies (LGMDs). Recently, tertiary dystroglycanopathies have been described as originating from defects in genes required for the proper biosynthesis of donor substrate molecules, such as Dol-P-Man or CDP-ribitol, used by the α-DG modifying enzymes and will be discussed further in this review (Figure 2) (Barone et al. 2012; Lefeber et al. 2009, 2011; Riemersma, Froese et al. 2015; Yang et al. 2013).
Table I.
Proteins involved with functional glycosylation of α-DG
| Gene product name (former name) | UniProt ID | Function | Core glycan | Subcellular localization | Dystroglycanopathy classification based on mutation |
|---|---|---|---|---|---|
| DAG1 | Q14118 | Extracellular glycoprotein that acts as a receptor for LG-domain-containing ECM proteins |  |
Plasma membrane/extracellular space | Primary |
| POMT1 | Q9Y6A1 | Transfers α-mannose from DPM to serine or threonine residues in a complex with POMT2 | M0, M1, M2, M3 | ER | Secondary |
| POMT2 | Q9UKY4 | Transfers α-mannose from DPM to serine or threonine residues in a complex with POMT1 | M0, M1, M2, M3 | ER | Secondary |
| POMGNT1 | Q8WZA1 | Transfers β1,2-GlcNAc to O-mannose on serine or threonine residues | M1, M2 | Golgi | Secondary |
| POMGNT2 (AGO61, GTDC2) | Q8NAT1 | Transfers β1,4-GlcNAc to O-mannose on serine or threonine residues | M3 | ER | Secondary |
| MGAT5B (GNT-VB, GNT-IX) | Q3V5L5 | Transfers β1,6-GlcNAc to O-mannose on serine or threonine residues | M2 | Golgi | Not Observed |
| B3GALNT2 | Q8NCR0 | Transfers β1,3-GalNAc to core M3 | M3 | ER | Secondary |
| POMK (SGK196) | Q9H5K3 | Carbohydrate kinase that phosphorylates 6 position of O-mannose | M3 | ER | Secondary |
| FKTN (FCMD) | O75072 | Transfers l-ribitol-1-phosphate to the core M3 trisaccharide at the 3 position of GalNAc in a phosphodiester linkage | M3 | Golgi | Secondary |
| FKRP | Q9H9S5 | Transfers l-ribitol-1-phosphate to the RboP product of FKTN in a phosphodiester linkage | M3 | Golgi | Secondary |
| RXYLT1 (TMEM5) | Q9Y2B1 | Transfers β1,2-Xyl to ribitol | M3 | Golgi | Secondary |
| B4GAT1 (B3GNT1) | O43505 | Transfers a β1,4-GlcA to an underlying Xyl | M3 | Golgi | Secondary |
| LARGE1 (LARGE) | O95461 | Polymerizes an α1,3-Xyl-β1,3-GlcA repeat | M3 | Golgi | Secondary |
| LARGE2 (GYLTL1B) | Q8N3Y3 | Polymerizes an α1,3-Xyl-β1,3-GlcA repeat | M3 | Golgi | Not observed |
| HNK-1ST (CHST10) | O43529 | Presumably sulfates terminal GlcA of matriglycan | M3 | Golgi | Not observed |
| DPM1 | O60762 | Transfers mannose from GDP-mannose to dolichol monophosphate to form dolichol-phosphate mannose (Dol-P-Man) | M1, M2, M3 | ER | Tertiary |
| DPM2 | O94777 | Regulatory subunit of the DPM synthase complex | M1, M2, M3 | ER | Tertiary |
| DPM3 | Q9P2X0 | Tethers catalytic subunit DPM1 to the ER | M1, M2, M3 | ER | Tertiary |
| DOLK | Q9UPQ8 | Phosphorylates dolichol to produce dolichol-phosphate | M1, M2, M3 | ER | Tertiary |
| CRPPA (ISPD) | A4D126 | Catalyzes the transfer of a pyrophosphate group from CTP to synthesize CDP-l-ribitol | M3 | Cytosolic | Tertiary |
| HK | P19367 | Phosphorylates mannose to Produce mannose-6-phosphate | M1, M2, M3 | Mitochondrial/Cytosolic | Not observed |
| P52790 | |||||
| P52789 | |||||
| MPI | P34949 | Catalyzes the isomerization of mannose-6-phosphate and fructose-6-phosphate | M1, M2, M3 | Cytosolic | Tertiaryab |
| PMM2 | O15305 | Catalyzes the isomerization of mannose-6-phosphate to mannose-1-phosphate | M1, M2, M3 | Cytosolic | Tertiaryab |
| GMPPB | Q9Y5P6 | Synthesizes GDP-mannose from GTP and mannose-1-phosphate | M1, M2, M3 | Cytosolic | Tertiarya |
aIndicates CDG.
bIndicates Putative Dystroglycanopathy.
Fig. 2.
Donor synthesis of Dol-P-Man and CDP-ribitol. (A) Reaction scheme for the synthesis of Dol-P-Man. Dolichol is phosphorylated by the CTP-mediated kinase activity of Dolichol kinase (DOLK) to form Dolichol-Phosphate (Dol-P). To generate GDP-Man, Mannose (Man) is phosphorylated by Hexokinase (HK) to yield Mannose-6-Phosphate (Man-6P) and can undergo isomerization by the activity of Mannose-6-Phosphate Isomerase (MPI) for conversion to Fructose-6-Phosphate (Fru-6P). Phosphomannomutase 2 (PMM2) converts Man-6P to Man-1P. Man-1P and GTP is converted to GDP-Man by the activity of GDP-Mannose Pyrophosphorylase B (GMPPB). Dolichol-Phosphate-Mannose (Dol-P-Man) is synthesized from Dol-P and GDP-Man by the activities of the DPM synthase (DPMS or DPM1/2/3) complex. A superscript *indicates that the gene has been implicated in CDG (congenital disorders of glycosylation) and †indicates a putative dystroglycanopathy [(Belaya et al. 2015; Luo et al. 2017; Schollen et al. 2000; Sparks et al. 1993), see Table I]. (B) Reaction scheme for the synthesis of CDP-l-ribitol from CTP and ribitol-5-phosphate by the activity of CRPPA (ISPD). Carbon numbering assignments for ribitol in CDP-l-ribitol, based on IUPAC rules, are shown in red. This figure is available in black and white in print and in color at Glycobiologyonline.
An astonishing amount of progress has been made by multiple laboratories over the past 4 years in order to elucidate the central link between the core M3 phospho-trisaccharide and matriglycan. Until 2016; the linker region (which has been frequently named “X” in the literature) was unknown (Figure 1C). The identification of previously unknown activities of multiple causal gene products including B4GAT1 (formerly B3GNT1), TMEM5, ISPD, FKTN, and FKRP further assisted in the complete assignment of the unknown linker region. Structural determination of both POMGNT1 and POMK and mechanistic studies on POMGNT1 and POMGNT2 provided insight into substrate specificity and regulation. This review focuses on recently published work, expands on the current gaps in the field, and highlights future directions.
Phosphodiester linkages connecting matriglycan to α-DG
In 2010, it was inferred that the functional glycan, matriglycan was connected to core M3 substructures by an unknown moiety known as “X” (Figure 1C), presumably via a phosphate group linked to position 6 of α-mannose of the core M3 phospho-trisaccharide [GalNAc-β1,3-GlcNAc-β1,4(6-phospho)-Man] (Yoshida-Moriguchi et al. 2010). These conclusions were made from experiments demonstrating that reactivity with an α-DG glyco-specific antibody, IIH6, was abolished upon treatment of α-DG with aqueous hydrofluoric acid (HFaq) which chemically cleaves phosphodiester bonds (Ilg et al. 1996). Interestingly, phosphate analysis of native α-DG purified from rabbit skeletal muscle revealed 4–5 moles of phosphate per mole of protein (Yoshida-Moriguchi et al. 2010), which could either be attributed to multiple sites of core M3-type modification or additional phosphates within the functional glycan. In 2016, our group (Praissman et al. 2016) and others (Kanagawa et al. 2016) identified ribitol-phosphate (RboP)-containing species within the functional glycan on α-DG by mass spectrometry. Kanagawa et al. demonstrated that the RboP incorporation occurred in tandem and was linked to carbon-3 of GalNAc, instead of the previously hypothesized mannose-6-phosphate of the core M3 glycan (Kanagawa et al. 2016) (Figure 1C). Interestingly, the Kato and Khoo laboratories detected a novel, lower abundance modification of a single glycerol-phosphate (GroP) moiety linked to the core M3 phospho-trisaccharide on truncated, recombinant α-DG (Yagi et al. 2016). In this study, no tandem GroP or GroP-RboP modifications were detected, nor was the GroP modification extended with Xyl-GlcA repeats, which suggests that GroP might serve as a molecular brake for core M3 functional glycan synthesis under certain physiological conditions. The discovery of tandem ribitol-phosphate moieties within the α-DG functional glycan identifies a gap in the symbol representation (Varki et al. 2015). Therefore, we suggest to represent the linear ribitol (reduced ribose) as a pink zigzag line containing five points, as shown in Figure 1.
CDP-l-ribitol—A Novel “Nucleotide-Sugar Alcohol” In Mammals
Having determined that RboP is a part of the functional M3 glycan brought up the question of the donor for the FKTN and FKRP enzymes. One hint came from an effort to identify host factors required for Lassa virus (LASV) cellular entry, which is known to utilize α-DG as its primary cellular receptor (Cao et al. 1998; Oldstone and Campbell 2011). Using a gene trap–insertion screen in the near-haploid HAP1 human cell line (Carette et al. 2011) the dystroglycan gene DAG1, in addition to other genes known to be causal for CMD and involved with α-DG modification were identified (Table I) (Jae et al. 2013). Among these genes, ISPD (isoprenoid synthase domain-containing) was of unknown function and not predicted to have any GT domains. ISPD was of particular interest due to reports of WWS patients harboring mutations in this gene (Roscioli et al. 2012; Willer et al. 2012). Near the end of 2015, four independent research groups demonstrated that recombinant ISPD was able to utilize CTP and d-ribitol-5-phosphate (as well as ribose-5-phosphate or ribulose-5-phosphate) to generate CDP-l-ribitol (or CDP-ribose or CDP-ribulose, respectively, Figure 2B) (Gerin et al. 2016; Kanagawa et al. 2016; Praissman et al. 2016; Riemersma, Froese et al. 2015). Note that by IUPAC convention as well as stereospecific numbering nomenclature d-ribitol-5-phosphate is the preferred name but identical to l-ribitol-1-phosphate and only CDP-l-ribitol (alternatively referred to as CDP 5-ester of D-ribitol) exists for this polyol nucleotide where the carbon in ribitol nearest the phosphate is the 1 carbon (Korte et al. 1976). While all groups converged on demonstrating ISPD's enzymatic activity and requirement for α-DG glycosylation, three of these studies provided unique contributions to understanding the function of ISPD. Riemersma et al. solved the 2.4 Å crystal structure of human ISPD (residues 43–451) and mapped disease causing mutations to the N-terminal cytidyltransferase domain (Riemersma, Froese et al. 2015), while the Bommer laboratory successfully detected a CDP-pentitol, likely CDP-l-ribitol (CDP-Rbo), in rat muscle and mouse myotubes (Gerin et al. 2016). Furthermore, Kanagawa et al. demonstrated that supplementation of CDP-Rbo to cells deficient in ISPD can restore functional glycosylation of α-DG, and that further investigation of CDP-Rbo supplementation therapy using animal models should be considered as a potential therapeutic (Kanagawa et al. 2016).
Interestingly, all groups have defined the enzymatic activity of ISPD in similar, yet alternative ways. For instance, Kanagawa et al. use the broadest classification of ISPD as a CDP-ribitol synthase (i.e. an enzyme that catalyzes the linking together of two molecules). Riemersma et al. classify ISPD as a cytidyltransferase, where a transferase is an enzyme that catalyzes the transfer of a particular moiety from one molecule to another, consistent with the classification used in defining homologous enzymes, such as TarI, in bacterial systems (Baur et al. 2009). However, following mammalian enzyme nomenclature, ISPD is most appropriately defined as a pyrophosphorylase (i.e. an enzyme that catalyzes the transfer of a pyrophosphate group from one molecule to another). Praissman et al. and Gerin et al. define ISPD as a CDP-ribitol pyrophosphorylase (Gerin et al. 2016; Praissman et al. 2016). This terminology is more specific than synthase and is most consistent with the naming schemes of other mammalian sugar-nucleotide biosynthetic enzymes, like GDP-Mannose Pyrophosphorylase B (GMPPB). Accordingly, we propose to rename ISPD to CDP-l-ribitol (ribose, ribulose) pyrophosphorylase A, or CRPPA.
It is puzzling why this cytidine-containing nucleotide-sugar was not previously identified in mammals. However, it may be due to the coelution of CDP-glucose during sugar-nucleotide analysis by HPLC (Gerin et al. 2016). Outstanding areas of research include identification of the pentose reductase and the location of this activity in the CDP-Rbo biosynthetic pathway in mammals (i.e. does the reductase act on ribose-5-phosphate and then CRPPA acts or does CRPPA convert ribose-5-phosphate to CDP-ribose and then is it reduced to CDP-ribitol?). Upon identification of a pentose reductase involved in this pathway, it would be intriguing to determine if any mutations in the reductase are causal for aberrant α-DG glycosylation as multiple dystroglycanopathies are still of unknown genetic etiology.
Another gene identified in the gene trap–insertion screen (Carette et al. 2011) was SLC35A1 (Solute Carrier Family 35 Member A1), a CMP-sialic acid transporter (Patnaik and Stanley 2006). SLC35A1 mutations are causal for CMD and result in defective α-DG glycosylation (Riemersma, Sandrock et al. 2015). While it has yet to be demonstrated, it is enticing to hypothesize that SLC35A1 is also a CDP-Rbo transporter as SLC35A1-deficient HAP1 cells lack the functional glycan as detected by IIH6 staining, independent of sialic acid (Riemersma, Sandrock et al. 2015).
Fukutin and FKRP—enzymes that utilize CDP-l-ribitol
LARGE1/2-mediated matriglycan synthesis on core M3 glycans requires extension of the phospho-trisaccharide by several recently characterized phosphoglycosyltransferases (Figure 1C). The genes FKTN (Fukutin) and FKRP (Fukutin related protein) were initially predicted to encode phosphoryl-ligand transferases based on sequence analysis (Aravind and Koonin 1999) and were established to be medial-Golgi-resident proteins (Esapa et al. 2002; Lynch et al. 2012). Mutations in FKTN and FKRP result in α-DG hypoglycosylation (Brockington et al. 2001; Kobayashi et al. 1998) and are causative for Fukuyama-type CMD (FCMD), LGMD and WWS (Taniguchi-Ikeda et al. 2016). Recently, recombinantly expressed FKTN, lacking the transmembrane domain, was shown to transfer RboP from CDP-Rbo to a phospho-trisaccharide peptide or purified fragments of rabbit α-DG, and once this transfer has occurred, FKRP can then transfer the second RboP group (Gerin et al. 2016; Kanagawa et al. 2016). Nuclear magnetic resonance (NMR) analyses of the reaction products revealed that FKTN transfers alditol-1-P to the C3 position of GalNAc through a phosphodiester linkage and FKRP transfers the second RboP to the C5 (note: we refer to this as C5 using IUPAC nomenclature) position of the underlying RboP by a phosphodiester linkage (Kanagawa et al. 2016) (Figure 1C). Thus, this tandem RboP addition mediated by the sequential phosphoglycosyltransferase activities of FKTN and FKRP, respectively, occurs in the Golgi after addition of the phosphate to the 6-position of the M3 trisaccharide by POMK in the ER (Yoshida-Moriguchi et al. 2013).
Despite understanding of the enzymatic activities of FKTN and FKRP, further studies are warranted in order to establish their unique donor and acceptor substrate specificities. Comparative structural analyses of FKTN and FKRP will aid in understanding the acceptor substrate requirements and processive nature of this enzyme pair. It is unclear if GTs that extend the phospho-trisaccharide can recognize the distal 6-phosphate on the core M3 mannose (transferred by POMK) as a part of their functional specificity. In regard to the GroP modification mentioned above (Yagi et al. 2016), it is unknown if human ISPD can synthesize CDP-glycerol using CTP and glycerol-3-phosphate, however, the homologous bacterial IspD enzyme has been shown to catalyze such a reaction (Majumdar et al. 2009). Regardless of the source of CDP-glycerol, it has yet to be determined if FKTN or FKRP can transfer GroP from CDP-glycerol. Given that only GroP modifications on the phospho-trisaccharide were identified and that no tandem GroP or GroP-RboP modifications were detected, one hypothesis is that FKTN (and not FKRP) might be able to transfer the initial GroP from CDP-glycerol and that the stringency of FKRP's acceptor substrate specificity prohibits priming.
The priming enzymes
Collectively, we refer to B4GAT1 and TMEM5 as the priming enzymes. The β1,4-glucuronyltransferase B4GAT1 (formerly B3GNT1) was identified in 2014 as the enzyme responsible for adding β1,4-linked GlcA to an underlying β-linked Xyl which serves as a primer for extension by LARGE1 (Praissman et al. 2014; Willer et al. 2014). Among other α-DG-related gene products of unknown function, the Wells and Campbell groups showed that recombinant TMEM5 can hydrolyze UDP-Xyl in the absence of any acceptor substrate and could transfer 14C-labeled Xyl to a truncated α-DG construct (α-DG-Fc340) that was expressed in a TMEM5-deficient patient cell line, demonstrating that TMEM5 is a xylosyltransferase (Praissman et al. 2016). Subsequently, Manya et al. established that TMEM5 transfers Xyl in a β-linkage to the ribitol moiety of the FKRP reaction product based on NMR studies, and this TMEM5 reaction product is an acceptor substrate for B4GAT1 (Manya et al. 2016). The anomeric configuration of Xyl, as predicted by Praissman et al. (2014) and Willer et al. (2014), is in complete agreement with the substrate specificity of B4GAT1, which can complete priming of the β-linked Xyl via transfer of β1,4-linked GlcA and allow for LARGE1/2 to synthesize matriglycan (Figure 1C).
Manya et al. identified the Xyl-linkage position of the TMEM5 reaction product using NMR spectroscopy and refer to TMEM5 as a ribitol β-1,4 Xylosyltransferase (Manya et al. 2016). This carbon numbering is based on the fact that d-ribitol-5-phosphate is derived from d-ribose-5-phosphate and CDP-ribitol is synthesized from CTP and d-ribitol-5-phosphate. The IUPAC name, however, for CDP-ribitol is {[[(2 R,3 S,4 R,5 R)-5-(4-amino-2-oxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2 R,3 S,4 S)-2,3,4,5-tetrahyroxypentyl] hydrogen phosphate} that is more commonly referred to as CDP-l-ribitol or the CDP 5-ester of d-ribitol. Manya et al. maintained the carbohydrate nomenclature and thus referred to the carbon on ribitol as the 4-carbon that TMEM5 acts on. However, in CDP-l-ribitol, carbon-1 of ribitol is directly linked to the β-phosphate of the nucleotide phosphate (Figure 2B). FKTN and FKRP thus transfer alditol-1-phosphate moieties, in nomenclature agreement with the teichoic acid synthesis enzymes in bacteria (Figure 1C) (Korte et al. 1976). Therefore, based on the NMR analyses by Manya et al. and following IUPAC nomenclature, we refer to TMEM5 as a ribitol β-1,2 Xylosyltransferase (Figure 1C). We propose renaming TMEM5 to RXYLT1 consistent with its defined activity and the naming convention used for the CAZy (Carbohydrate-Active enZYmes Database) GT8 family.
Functional relevance of core M1 and M2 structures on α-DG and other proteins
While progress has been made in elucidating the link between the core M3 O-man glycan structure and matriglycan (Gerin et al. 2016; Kanagawa et al. 2016; Praissman et al. 2014, 2016; Willer et al. 2014), little is known about the functional relevance of core M1 and M2 structures. Core M1 structures are formed by the POMGNT1-dependent extension of the initial mannose residue with β1,2-linked GlcNAc (Figure 1B) and can be further extended to form the classical tetrasaccharide (Neu5Ac-α2,3-Gal-β1,4-GlcNAc-β1,2-Man), a Lewis X epitope (Gal-β1,4-(Fuc-α1,3)-GlcNAc-β1,2-Man) or a Human Natural Killer-1 epitope (HNK-1; 3S-GlcA-β1,3-Gal-β1, 4-GlcNAc-β1,2-Man), among others (Praissman and Wells 2014) using GTs and modifying enzymes that are involved in multiple glycan pathways. Core M1 structures account for over 15% of brain O-glycans (Stalnaker et al. 2011) and are far more abundant in the mucin-like domain of α-DG than core M3 structures (Harrison et al. 2012; Nilsson et al. 2010; Stalnaker et al. 2010). Mutations in POMGNT1 leading to loss of core M1 glycans are causal for various forms of CMDs (Falsaperla et al. 2016), and core M1 structures are necessary for functional glycosylation of α-DG (Liu et al. 2006). One hypothesis is that these structures serve as a scaf-fold for the core M3 GTs (Kuwabara et al. 2016; Xiong et al. 2006). Evidence that overexpressed POMGNT1 co-precipitates with overexpressed FKTN and forms a complex (Xiong et al. 2006) suggests one potential model to be tested for the role of POMGNT1 in the generation of functional M3 glycan structures. Further support for this model comes from a POMGNT1 crystal structure showing a lectin-like stem domain capable of binding the enzyme's reaction product (Figure 1B) (Kuwabara et al. 2016). Thus, POMGNT1’s binding to M1 sites may assist in the recruitment of core M3 GTs through protein–protein associations to facilitate elaboration of nearby core M3 sites.
Core M1 structures also serve as a precursor for core M2 structures (Figure 1B). Multiple core M2 structures exist, including HNK-1 and Lewis X epitope-containing structures (Praissman and Wells 2014; Stalnaker et al. 2010). Core M2 structures account for ~5% of brain O-glycans (Stalnaker et al. 2011). Altering core M2 levels changes integrin-dependent cell adhesion and migration in vitro (Abbott et al. 2006, 2008), however, a mouse model lacking core M2 shows no neuronal development problems and does not impede functional glycosylation of α-DG (Kanekiyo et al. 2013; Lee et al. 2012). While clear biological roles of the core M1 and M2 glycan structures are not fully understood, their functional relevance may become more apparent as research into α-DG and other O-mannosylated proteins continues.
Expanding the O-mannosylated proteome beyond α-DG
Hypoglycosylation of α-DG that results from mutations in GTs explains the CMD phenotypes observed in skeletal muscle, but it does not fully explain the associated spectrum of neurological phenotypes seen in patients. While hypoglycosylation of α-DG and loss-of-function mutations in dystrophin (see Figure 1) both disrupt the DGC and lead to muscle disease (CMDs and Duchenne's muscular dystrophy, respectively), critical neurological complications are only observed in the severe forms of CMD (Falsaperla et al. 2016; Yiu and Kornberg 2015). In line with this observation, similar levels of O-man initiated structures were found to be present in the brain of DAG1 knockout mice compared to WT mice, suggesting that there must be O-mannosylated proteins other than α-DG (Stalnaker et al. 2011). This begged the question: Other than α-DG, are there additional proteins that are functionally modified by O-mannose-initiated glycans?
Recently, two groups (Lommel et al. 2013; Vester-Christensen et al. 2013) identified E-cadherin as an O-mannosylated protein in mammals (Baenziger 2013). Cadherins are a class of cell-surface membrane glycoproteins that have multiple extracellular cadherin (ECs) domains. Clausen et al. identified O-mannose sites in EC2-5 of classical Types 1 and 2 cadherins, EC2-3 of the clustered protocadherins, and plexins, as well as all known O-mannose sites on α-DG (Vester-Christensen et al. 2013). Additionally, Strahl et al. demonstrated that O-mannosylation is essential for E-cadherin mediated cell adhesion in mouse embryos (Lommel et al. 2013). In order to simplify the O-glycoproteome, Clausen's group used their “SimpleCell” breast cancer line that contains a genetic inactivation of POMGNT1. Lectin-weak affinity chromatography (LWAC) followed by identification using mass spectrometry was used to elucidate the O-mannose glycoproteome. While the data strongly suggest that cadherin/plexin-derived peptides are O-glycosylated, the lectin (Concanavalin A; ConA) used for LWAC enrichment of α-mannose also has known affinity for other hexose sugars, including α-glucose (Goldstein and Poretz 2012; Goldstein et al. 1973). Hexoses are indistinguishable in mass spectrometric analysis of glycopeptides, therefore, experiments to eliminate alternative possibilities, such as O-glucosyl modification of cadherins/plexins, should be performed. For example, it is imperative to determine if these presumed O-mannosyl modified proteins are sensitive to α-mannosidase treatment. Further efforts should be put forth to understand how those putative mannose residues on cadherins/plexins either remain unextended as core M0, or are extended into core M1, M2 or M3 structures in biologically pertinent cell lines and tissues, such as muscle and brain.
Two other groups (Dwyer et al. 2012; Yaji et al. 2015) found that receptor protein tyrosine phosphatase ζ (RPTPζ)/phosphacan is also O-mannosylated in mouse brain. More specifically, Dwyer et al. found that RPTPζ/phosphacan is O-mannosylated and hypoglycosylated in brains of POMGNT1-knockout mice, a model of Muscle-Eye-Brain disease (Dwyer et al. 2012). In this study, they also demonstrate that RPTPζ/phosphacan is not modified by LARGE1 in the mouse brain, suggesting that there are only core M1 and core M2 structures present on this protein. In fact, Morise et al. found that RPTPζ/phosphacan in mouse brain has the O-mannose-linked HNK-1 glycan epitope that is an elaboration of the core M1/M2 structure (Morise et al. 2014). In a similar vein, the Lewis X epitope was found to be mainly expressed on RPTPζ/phosphacan in the developing mouse brain (Yaji et al. 2015). Since the Lewis X epitope almost disappeared in POMGNT1-knockout mouse brains, it was suggested that the O-man glycan is responsible for presenting the Lewis X epitope as well. Taken together, the abnormal glycosylation of RPTPζ/phosphacan in POMGNT1-knockout mice brains may contribute to the spectrum of neurological phenotypes seen in mutant-POMGNT1, POMT1 and POMT2 CMDs (Dwyer et al. 2012). A summary of currently known and putative O-mannosylated proteins is presented in Supplemental Table I (Abbott et al. 2008; Bartels et al. 2016; Bleckmann et al. 2009; Pacharra et al. 2012, 2013; Vester-Christensen et al. 2013; Winterhalter et al. 2013).
Evolutionary perspectives of O-mannosylation and model organisms
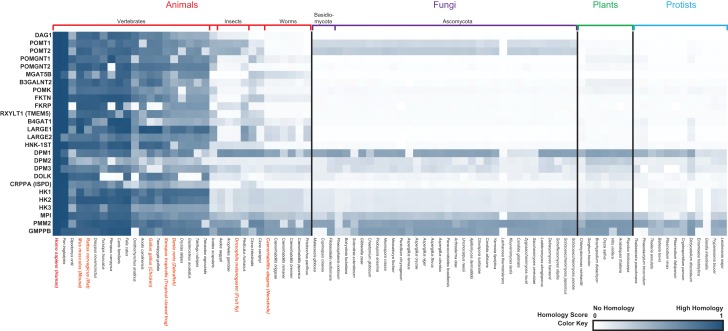
O-mannosylation of α-DG is evolutionarily conserved, and has been extensively studied in mammals (Yoshida-Moriguchi and Campbell 2015). α-DG has also been studied in the powerful model organism Drosophila melanogaster (Nakamura et al. 2010). All subunits of the DGC are present in Drosophila, however, only one isoform from DAG1 splicing has a glycosylated mucin-like domain similar to human α-DG. Unlike human α-DG, all DAG1 isoforms in flies are a single polypeptide and are not cleaved into α and β subunits (Sciandra et al. 2015). It is unclear if α-DG in flies plays a similar biological role to α-DG in humans. Interestingly, in Drosophila there are no identified elaborated O-Man structures (Aoki et al. 2008) nor obvious homologs for most of the enzymes needed to make and elaborate the core M3 glycan [(Grewal et al. 2005), Figure 3]. However, there are homologs for POMT1/2, rotated (rt) and twisted (tw) (Ichimiya et al. 2004; Nakamura et al. 2010), indicating there are O-mannosylated proteins present in this organism that is relatively distant from mammals on the evolutionary tree. Phylogenetic analysis of human LARGE1, in addition to other genes involved with functional glycosylation of α-DG, indicates orthologues are present in most metazoans, especially vertebrates. Divergence begins in insects followed by complete absence of most of the genes in fungi and lower organisms. This suggests higher organisms have evolved a uniquely multifaceted functional glycosylation, presumably to accommodate increasing complexity in tissue structure and function (Figure 3) (Sadreyev et al. 2015). However, due to the evolutionary conservation of O-mannosyl modification of proteins, reduced heterogeneity of structures outside of the mammalian clade, and the lower costs associated with research, using model organisms, like Drosophila, presents unique advantages for studying the first step of protein O-mannosylation.
Fig. 3.
Phylogenetic profile of genes involved with functional glycosylation of α-DG. A phylogenetic profile heatmap of human genes (left) involved with functional glycosylation of α-DG (from Table I) was generated using PhyloGene (Sadreyev et al. 2015). Species analyzed are indicated at the bottom, and the heatmap is categorized according to indicated taxa (top and vertical black lines) and subtaxa (additional colored vertical line separators). Model organisms that have been described in this review or used in dystroglycan studies (not mentioned in this review) are labeled in orange, and Homo sapiens is labeled in red. Protein sequence similarity values range from 0 (white, no homology) to 1 (blue, high homology relative to H. sapiens).
The zebrafish (Danio rerio) has also emerged as a powerful vertebrate model organism to study dystroglycanopathies and other neuromuscular disorders (Pappalardo et al. 2013; Steffen et al. 2007). Genetic manipulation of zebrafish embryos is fast, effective and inexpensive, and all of the human dystroglycanopathy-related genes have been identified in zebrafish (Moore et al. 2008; Wood and Currie 2014). While homozygous dystroglycan mutations in mice are embryonic lethal (Williamson et al. 1997), morpholino-mediated knockdown of dystroglycan results in viable zebrafish, however, with disorganized muscle and disruption of the DGC (Parsons et al. 2002).
Recent zebrafish studies knocking down genes implicated in secondary dystroglycanopathies, such as those described in Table I, have recapitulated the muscle, eye and brain phenotypes typically observed in CMD patients, such as hydrocephaly, reduced eye size, impaired muscle development and reduced α-DG glycosylation (Avsar-Ban et al. 2010; Buysse et al. 2013; Di Costanzo et al. 2014; Manzini et al. 2012; Praissman et al. 2016; Stevens et al. 2013). Interestingly, while FKTN and FKRP knockdown in zebrafish results in hypoglycosylated α-DG and reduced laminin binding (Kawahara et al. 2010; Thornhill et al. 2008), other studies suggest that FKTN and FKRP may also play roles in protein secretion, the unfolded protein response, and angiogenesis (Lin et al. 2011; Wood et al. 2011). For tertiary dystroglycanopathies (Table I and Figure 2), morpholino-mediated knockdown of the zebrafish DPMS complex and ISPD resulted in anticipated hypoglycosylation of α-DG and a dystrophic muscle phenotype (Marchese et al. 2016; Roscioli et al. 2012).
Other aspects of O-mannosylation
The loss of α-DG expression and hypoglycosylation has also been documented in many types of epithelial and neuronal cancers, with aberrant glycosylation of α-DG being implicated in cancer progression and metastasis (de Bernabe et al. 2009; Martin et al. 2007; Muschler et al. 2002; Sgambato et al. 2003; Shen et al. 2012). It has been shown that maturation of Core M3-type glycans on α-DG is critical for binding of lymphocytic choriomeningitis virus (LCMV) and LASV, in addition to other arenaviruses, Mobala and Oliveros (Kunz, Rojek, Kanagawa et al. 2005). Furthermore, LCMV and LASV compete with laminin for binding α-DG glycans (Kunz, Rojek, Perez et al. 2005; Kunz et al. 2001). Ubiquitous expression of α-DG in various tissues and the competitive binding nature of LCMV and LASV with LG-domain-containing proteins indicate these viruses have evolved to infect a broad range of cell types and the potential disruption of cell-ECM homeostasis likely contributes to pathogenesis.
Further insights into the mechanism of ECM receptor binding to the functional glycan on α-DG have come out of the Hohenester and Campbell laboratories with a high-resolution (1.4 Å) crystal structure of matriglycan bound to the LG4 and LG5 domains of laminin-α2 (Briggs et al. 2016). Structural analysis of this complex show the coordination of a single Ca2 + ion by a single [-GlcA-β3-Xyl-α3-] repeat, providing a snapshot of this high affinity Ca2+-dependent protein-carbohydrate interaction. Precise regulation of the control of matriglycan chain length is currently unknown, however expression levels of human natural killer-1 sulfotransferase (HNK-1ST) and LARGE1 have been implicated in the regulatory mechanism (Nakagawa et al. 2012, 2013). Overexpression of LARGE1 results in increased levels of α-DG glycosylation (Barresi et al. 2004; Patnaik and Stanley 2005), whereas sulfation of the functional glycan, likely on a non-reducing end GlcA residue, by HNK-1ST reduces levels of LARGE-mediated α-DG glycosylation (Nakagawa et al. 2012, 2013) (Figure 1C). While the sulfation has been determined to be within the functional moiety on core M3 glycans (Nakagawa et al. 2013), the exact site of HNK-1ST-mediated sulfation has yet to be formally demonstrated. Although the precise range of matriglycan GlcA-Xyl repeats is unknown, the large shifts in electrophoretic mobility of α-DG in SDS-PAGE (>60 kDa which is approximately >200 repeats) suggests that the LARGE1-dependent modification might serve as a long scaf-fold potentially allowing multiple LG-domain-containing ECM proteins to bind and tether to the cell-surface. However, LG-domain-containing ligand proteins have been shown to competitively bind to α-DG (Kanagawa et al. 2004). Thus, it remains to be determined whether a single matriglycan chain can bind multiple LG-domain-containing proteins concurrently. Complete structural determination of the fully elaborated core M3 glycan built in vitro by chemoenzymatic synthesis using recombinant enzymes is necessary to define the comprehensive three dimensional structure and will allow for direct testing of the impact of repeat length and binding to other proteins of interest, in addition to being used as a tool to identify other proteins that may participate in the DGC.
Using Chinese Hamster ovary cell mutants that lacks both O-mannose and complex N-glycans, work from the Stanley laboratory suggests that when overexpressed in these systems, LARGE1 can modify substrates other than O-mannose, such as N-glycans and mucin-type O-glycans on α-DG (Aguilan et al. 2009; Patnaik and Stanley 2005). Additionally, LARGE1 overexpression in neural stems cells deficient in POMT2 or both POMT2 and α-DG resulted in the reporting of IIH6-reactive proteins and laminin-binding epitopes that were PNGaseF-sensitive (which removes N-glycans), suggesting that LARGE1 could potentially modify N-glycans on proteins other than α-DG (Zhang and Hu 2012; Zhang et al. 2011). However, it was recently shown that LARGE2, and not LARGE1, is capable of modifying proteoglycans, such as Glypican-4, presumably by extending the non-reducing end GlcA on a common core GAG tetrasaccharide linker, GlcAβ1-3-Galβ1-3-Galβ1-4Xylβ-, with matriglycan when overexpressed in dystroglycan-deficient mouse embryonic stem cells (Inamori et al. 2016). While LARGE1 and LARGE2 catalyze the same bifunctional GT reaction (Inamori et al. 2012, 2013), LARGE1 is highly expressed in skeletal muscle, heart and brain, whereas LARGE2 is highly expressed in kidneys and testis (Grewal et al. 2005; Peyrard et al. 1999). This differential tissue expression, different pH optima for GT activities (Inamori et al. 2013), and different substrate specificities (Inamori et al. 2016) suggests that LARGE1 and LARGE2 may not have overlapping functions. Thus, in a tissue-specific manner, LARGE1- and/or LARGE2-mediated hyperglycosylation of non-native structures (other than O-mannose, such as proteoglycans) may serve as a compensatory mechanism, and potential therapeutic approach, for dystroglycanopathies where there are no O-mannose structures present.
Recent progress has been made in understanding the structural aspects of enzymes in the O-mannosylation pathway. Specifically, elucidation of the POMGNT1 structure revealed a carbohydrate-binding stem domain and further support for the promiscuity of this enzyme towards various glycopeptides substrates (Kuwabara et al. 2016). Additionally, POMK is an unusual pseudokinase that lacks certain primary sequence elements thought to be required for kinase activity (Yoshida-Moriguchi and Campbell 2015), however, a crystal structure of zebrafish POMK reveals the mechanism in which the enzyme recognizes the GalNAc-β3-GlcNAc-β4-Man trisaccharide acceptor substrate and catalyzes the phosphate transfer from ATP (Zhu et al. 2016).
Given that the POMGNTs dictate the core structure to be synthesized, an understanding of substrate specificity for these enzymes needs to be elucidated. Our group recently investigated the substrate specificities of POMGNT1 and POMGNT2 and found POMGNT1 to be promiscuous while an identified amino acid motif, R-X-R-X-X-I-X-X-T(O-Man)-P-T, that appears to only be present and conserved in vertebrate α-DGs is the preferred substrate for POMGNT2 (Halmo et al. 2017). Thus, POMGNT2 appears to be the “gatekeeper” enzyme for the generation of functional M3 glycans. Elucidation of POMGNT2 structure, to complement POMGNT1’s, will greatly facilitate our understanding of what sites of O-man can be elaborated into full-length M3 glycans capable of binding LG-domain-containing ECM proteins.
Concluding remarks
Although major accomplishments have been made in understanding the uniquely complex functional modification of α-DG, there are still many unknowns in the field. These questions include: 1. What are the mechanisms behind the unique specificities of the pathway enzymes?, 2. What are the origins and significance of the GroP modification?, 3. What is the functional relevance of core M1 and M2 glycans?, 4. What is the role of the furin-cleaved N-terminus of α-DG? and 5. Are newly identified proteins that contain O-mannose further extended and functional?. Many of these studies could take advantage of the aforementioned model organisms established for the study of CMDs. Now that the vast majority of enzyme functions involved with core M3 elaboration have been identified, structural analyses can be undertaken to establish catalytic mechanisms, identify how CMD patient mutations affect enzymatic functions, and propose testable mechanisms to ameliorate these defects. It should also be noted that there are still CMD and cobblestone lissencephaly patients with unknown genetic etiology or known etiology where the functional link to O-mannosylation has not been established such as mutations in TMTC3 (Jerber et al. 2016). In conclusion, while it would seem inconceivable, from an evolutionary point of view, that such an elaborate modification that requires multiple gene products and at least one novel donor substrate is targeted to only a single cell-surface protein, α-DG, on two sites (T317 and T379), no other proteins have yet to be identified with M3 glycans (Hara, Kanagawa et al. 2011; Yagi et al. 2013). With the aid of the research findings described here in the last few years and the advancements in the tools used to study these biological molecules and processes (Porterfield et al. 2014; Steentoft et al. 2011; Sun et al. 2016; Zhao et al. 2013), continued research will help us understand the evolutionary perspective of O-mannosylation and achieve the ultimate goal of discovering novel therapeutics relevant to the associated human diseases.
Supplementary Material
Acknowledgements
We thank all members of the Wells Laboratory for helpful discussions.
Supplementary data
Funding
The National Institutes of Health [R01GM111939 to L.W., P41GM103490 to L.W. (Senior Investigator), P01GM107012 to L.W. (Senior Investigator), T32GM107004 to S.M.H.].
Conflict of interest statement
None declared.
Abbreviations
α-DG, α-Dystroglycan; B3GALNT2, β1,3-N-Acetylgalactosaminyltransferase 2; B4GAT1, β1,4-Glucuronyltransferase 1; β-DG, β-Dystroglycan; CAZy, Carbohydrate-Active enZYmes Database; CDP-Rbo, CDP-ribitol; CRPPA, CDP-ribitol pyrophosphorylase A; ConA, Concanavalin A; CDG, congenital disorders of glycosylation; CMD, congenital muscular dystrophy; CDP, cytidine diphosphate; CMP, cytidine monophosphate; Dol-P-Man or DPM, dolichol-phosphate mannose; DPM synthase complex comprised of DPM1; DAG1, dystrophin-associated glycoprotein 1; DGC, dystrophin-glycoprotein complex; ER, endoplasmic reticulum; EC, extracellular cadherin; ECM, extracellular matrix; Fru-6P, Fructose-6-Phosphate; FKTN, Fukutin; FKRP, Fukutin related protein; FCMD, Fukuyama-type congenital muscular dystrophy; Gal, galactose; GalNAc, N-Acetylgalactosamine; GlcA, glucuronic acid; GlcNAc, NAcetylglucosamine; GroP, glycerol-phosphate; GAG, glycosaminoglycan; GT, glycosyltransferase; GDP-Man, guanidine diphosphate mannose; GTP, guanidine triphosphate; GMPPB, GDP-Mannose Pyrophosphorylase B; Hfaq, aqueous hydrofluoric acid; HK, Hexokinase; HPLC, high pressure liquid chromatography; HNK-1, Human Natural Killer-1; HNK-1ST, Human Natural Killer-1 sulfotransferase; IUPAC, International Union of Pure and Applied Chemistry; ISPD, isoprenoid synthase domain-containing; LG, laminin globular; LASV, Lassa virus; LWAC, Lectin-Weak Affinity Chromatography; LARGE1, Like-acetyl-glucosaminyltransferase 1; LARGE2, Like-acetyl-glucosaminyltransferase 2; LGMD, limb-girdle muscular dystrophy; LCMV, Lymphocytic choriomeningitis virus; Man, mannose; Man-1P, Mannose-1-Phosphate; Man-6P, Mannose-6-Phosphate; MPI, Mannose-6-Phosphate Isomerase; MGAT5B, Mannosyl (α1,6)-Glycoprotein β1,6-N-Acetyl-Glucosaminyltransferase; N-linked, asparagine-linked; Neu5Ac, N-Acetylneuraminic acid; NMR, nuclear magnetic resonance; oxygen-linked mannose; O-linked, serine- or threonine-linked; PNGaseF, Peptide:N-Glycosidase F; PMM2, Phosphomannomutase 2; POMGNT1, Protein O-Linked Mannose N-Acetyl-glucosaminyltransferase 1; POMGNT2, Protein O-Linked Mannose N-Acetyl-glucosaminyltransferase 2; POMK, Protein O-Mannose Kinase; POMT1, Protein O-Mannosyltransferase 1; POMT2, Protein O-Mannosyltransferase 2; RPTPζ, receptor protein tyrosine phosphatase ζ; RboP, ribitol-phosphate; SLC35A1, Solute Carrier Family 35 Member A1; TMEM5, Transmembrane Protein 5; WWS, Walker–Warburg syndrome; Xyl, xylose
References
- Abbott KL, Matthews RT, Pierce M. 2008. Receptor tyrosine phosphatase beta (RPTPbeta) activity and signaling are attenuated by glycosylation and subsequent cell surface galectin-1 binding. J Biol Chem. 283:33026–33035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott KL, Troupe K, Lee I, Pierce M. 2006. Integrin-dependent neuroblastoma cell adhesion and migration on laminin is regulated by expression levels of two enzymes in the O-mannosyl-linked glycosylation pathway, PomGnT1 and GnT-Vb. Exp Cell Res. 312:2837–2850. [DOI] [PubMed] [Google Scholar]
- Aebi M. 2013. N-linked protein glycosylation in the ER. Biochim Biophys Acta. 1833:2430–2437. [DOI] [PubMed] [Google Scholar]
- Aguilan JT, Sundaram S, Nieves E, Stanley P. 2009. Mutational and functional analysis of Large in a novel CHO glycosylation mutant. Glycobiology. 19:971–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Porterfield M, Lee SS, Dong B, Nguyen K, McGlamry KH, Tiemeyer M. 2008. The diversity of O-linked glycans expressed during Drosophila melanogaster development reflects stage- and tissue-specific requirements for cell signaling. J Biol Chem. 283:30385–30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L, Koonin EV. 1999. The fukutin protein family - predicted enzymes modifying cell-surface molecules. Cur Biol. 9:R836–R837. [DOI] [PubMed] [Google Scholar]
- Ashikov A, Buettner FF, Tiemann B, Gerardy-Schahn R, Bakker H. 2013. LARGE2 generates the same xylose- and glucuronic acid-containing glycan structures as LARGE. Glycobiology. 23:303–309. [DOI] [PubMed] [Google Scholar]
- Avsar-Ban E, Ishikawa H, Manya H, Watanabe M, Akiyama S, Miyake H, Endo T, Tamaru Y. 2010. Protein O-mannosylation is necessary for normal embryonic development in zebrafish. Glycobiology. 20:1089–1102. [DOI] [PubMed] [Google Scholar]
- Baenziger JU. 2013. O-mannosylation of cadherins. Proc Natl Acad Sci USA. 110:20858–20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone R, Aiello C, Race V, Morava E, Foulquier F, Riemersma M, Passarelli C, Concolino D, Carella M, Santorelli F et al. 2012. DPM2-CDG: A muscular dystrophy-dystroglycanopathy syndrome with severe epilepsy. Ann Neurol. 72:550–558. [DOI] [PubMed] [Google Scholar]
- Barresi R, Campbell KP. 2006. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 119:199–207. [DOI] [PubMed] [Google Scholar]
- Barresi R, Michele DE, Kanagawa M, Harper HA, Dovico SA, Satz JS, Moore SA, Zhang WL, Schachter H, Dumanski JP et al. 2004. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med. 10:696–703. [DOI] [PubMed] [Google Scholar]
- Bartels MF, Winterhalter PR, Yu J, Liu Y, Lommel M, Mohrlen F, Hu H, Feizi T, Westerlind U, Ruppert T et al. 2016. Protein O-Mannosylation in the Murine Brain: Occurrence of Mono-O-Mannosyl Glycans and Identification of New Substrates. PLoS One. 11:e0166119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur S, Marles-Wright J, Buckenmaier S, Lewis RJ, Vollmer W. 2009. Synthesis of CDP-activated ribitol for teichoic acid precursors in Streptococcus pneumoniae. J Bacteriol. 191:1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beedle AM, Turner AJ, Saito Y, Lueck JD, Foltz SJ, Fortunato MJ, Nienaber PM, Campbell KP. 2012. Mouse fukutin deletion impairs dystroglycan processing and recapitulates muscular dystrophy. J Clin Invest. 122:3330–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaya K, Rodriguez Cruz PM, Liu WW, Maxwell S, McGowan S, Farrugia ME, Petty R, Walls TJ, Sedghi M, Basiri K et al. 2015. Mutations in GMPPB cause congenital myasthenic syndrome and bridge myasthenic disorders with dystroglycanopathies. Brain. 138:2493–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckmann C, Geyer H, Lieberoth A, Splittstoesser F, Liu Y, Feizi T, Schachner M, Kleene R, Reinhold V, Geyer R. 2009. O-glycosylation pattern of CD24 from mouse brain. Biol Chem. 390:627–645. [DOI] [PubMed] [Google Scholar]
- Briggs DC, Yoshida-Moriguchi T, Zheng T, Venzke D, Anderson ME, Strazzulli A, Moracci M, Yu L, Hohenester E, Campbell KP. 2016. Structural basis of laminin binding to the LARGE glycans on dystroglycan. Nat Chem Biol. 12:810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington M, Blake DJ, Prandini P, Brown SC, Torelli S, Benson MA, Ponting CP, Estournet B, Romero NB, Mercuri E et al. 2001. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha 2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am J Hum Genet. 69:1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington M, Torelli S, Sharp PS, Liu K, Cirak S, Brown SC, Wells DJ, Muntoni F. 2010. Transgenic overexpression of LARGE induces alpha-dystroglycan hyperglycosylation in skeletal and cardiac muscle. PLoS One. 5:e14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse K, Riemersma M, Powell G, van Reeuwijk J, Chitayat D, Roscioli T, Kamsteeg EJ, van den Elzen C, van Beusekom E, Blaser S et al. 2013. Missense mutations in beta-1,3-N-acetylglucosaminyltransferase 1 (B3GNT1) cause Walker-Warburg syndrome. Hum Mol Genet. 22:1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. 1998. Identification of α-Dystroglycan as a Receptor for Lymphocytic Choriomeningitis Virus and Lassa Fever Virus. Science. 282:2079–2081. [DOI] [PubMed] [Google Scholar]
- Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G et al. 2011. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 477:340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T. 1997. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J Bio Chem. 272:2156–2162. [DOI] [PubMed] [Google Scholar]
- de Bernabe DB, Inamori K, Yoshida-Moriguchi T, Weydert CJ, Harper HA, Willer T, Henry MD, Campbell KP. 2009. Loss of alpha-dystroglycan laminin binding in epithelium-derived cancers is caused by silencing of LARGE. J Biol Chem. 284:11279–11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Costanzo S, Balasubramanian A, Pond HL, Rozkalne A, Pantaleoni C, Saredi S, Gupta VA, Sunu CM, Yu TW, Kang PB et al. 2014. POMK mutations disrupt muscle development leading to a spectrum of neuromuscular presentations. Hum Mol Genet. 23:5781–5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CM, Hempel SJ, Stalnaker SH, Stuart R, Wells L. 2013. O-Mannosylation and human disease. Cell Mol Life Sci. 70:2849–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucey MA, Hess D, Cacan R, Hofsteenge J. 1998. Protein C-mannosylation is enzyme-catalysed and uses dolichyl-phosphate-mannose as a precursor. Mol Biol Cell. 9:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer CA, Baker E, Hu H, Matthews RT. 2012. RPTPzeta/phosphacan is abnormally glycosylated in a model of muscle-eye-brain disease lacking functional POMGnT1. Neuroscience. 220:47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. 1991. Membrane organization of the dystrophin-glycoprotein complex. Cell. 66:1121–1131. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. 1993. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 122:809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esapa CT, Benson MA, Schroder JE, Martin-Rendon E, Brockington M, Brown SC, Muntoni F, Kroger S, Blake DJ. 2002. Functional requirements for fukutin-related protein in the Golgi apparatus. Hum Mol Genet. 11:3319–3331. [DOI] [PubMed] [Google Scholar]
- Falsaperla R, Pratico AD, Ruggieri M, Parano E, Rizzo R, Corsello G, Vitaliti G, Pavone P. 2016. Congenital muscular dystrophy: from muscle to brain. Ital J Pediatr. 42:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J, Krusius T, Margolis RK, Margolis RU. 1979. Novel mannitol-containing oligosaccharides obtained by mild alkaline borohydride treatment of a chondroitin sulfate proteoglycan from brain. J Biol Chem. 254:10295–10300. [PubMed] [Google Scholar]
- Geis T, Marquard K, Rodl T, Reihle C, Schirmer S, von Kalle T, Bornemann A, Hehr U, Blankenburg M. 2013. Homozygous dystroglycan mutation associated with a novel muscle-eye-brain disease-like phenotype with multicystic leucodystrophy. Neurogenetics. 14:205–213. [DOI] [PubMed] [Google Scholar]
- Gerin I, Ury B, Breloy I, Bouchet-Seraphin C, Bolsee J, Halbout M, Graff J, Vertommen D, Muccioli GG, Seta N et al. 2016. ISPD produces CDP-ribitol used by FKTN and FKRP to transfer ribitol phosphate onto alpha-dystroglycan. Nat Commun. 7:11534. 10.1038/ncomms11534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein IJ, Poretz RD. 2012. Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins In: Liener IE, Sharon N, Goldstein IJ, editors. The Lectins Properties, Functions and Applications in Biology and Medicine. Cambridge, MA, USA: Academic Press. p. 33–247. [Google Scholar]
- Goldstein IJ, Reichert CM, Misaki A, Gorin PA. 1973. An “extension” of the carbohydrate binding specificity of concanavalin A. Biochim Biophys Acta. 317:500–504. [DOI] [PubMed] [Google Scholar]
- Grewal PK, McLaughlan JM, Moore CJ, Browning CA, Hewitt JE. 2005. Characterization of the LARGE family of putative glycosyltransferases associated with dystroglycanopathies. Glycobiology. 15:912–923. [DOI] [PubMed] [Google Scholar]
- Halmo SM, Singh D, Patel S, Wang S, Edlin M, Boons GJ, Moremen KW, Live D, Wells L. 2017. Protein O-linked mannose beta-1,4-N-acetylglucosaminyltransferase 2 (POMGNT2) is a gatekeeper enzyme for functional glycosylation of alpha-dystroglycan. J Biol Chem. 292:2101–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Balci-Hayta B, Yoshida-Moriguchi T, Kanagawa M, Beltran-Valero de Bernabe D, Gundesli H, Willer T, Satz JS, Crawford RW, Burden SJ et al. 2011. A dystroglycan mutation associated with limb-girdle muscular dystrophy. N Engl J Med. 364:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Kanagawa M, Kunz S, Yoshida-Moriguchi T, Satz JS, Kobayashi YM, Zhu Z, Burden SJ, Oldstone MB, Campbell KP. 2011. Like-acetylglucosaminyltransferase (LARGE)-dependent modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc Natl Acad Sci USA. 108:17426–17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R, Hitchen PG, Panico M, Morris HR, Mekhaiel D, Pleass RJ, Dell A, Hewitt JE, Haslam SM. 2012. Glycoproteomic characterization of recombinant mouse alpha-dystroglycan. Glycobiology. 22:662–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt KH, Crosbie RH, Venzke DP, Campbell KP. 2000. Biosynthesis of dystroglycan: processing of a precursor propeptide. FEBS Lett. 468:79–83. [DOI] [PubMed] [Google Scholar]
- Ichimiya T, Manya H, Ohmae Y, Yoshida H, Takahashi K, Ueda R, Endo T, Nishihara S. 2004. The twisted abdomen phenotype of Drosophila POMT1 and POMT2 mutants coincides with their heterophilic protein O-mannosyltransferase activity. J Biol Chem. 279:42638–42647. [DOI] [PubMed] [Google Scholar]
- Ilg T, Stierhof YD, Craik D, Simpson R, Handman E, Bacic A. 1996. Purification and structural characterization of a filamentous, mucin-like proteophosphoglycan secreted by Leishmania parasites. J Bio Chem. 271:21583–21596. [DOI] [PubMed] [Google Scholar]
- Inamori K, Hara Y, Willer T, Anderson ME, Zhu Z, Yoshida-Moriguchi T, Campbell KP. 2013. Xylosyl- and glucuronyltransferase functions of LARGE in alpha-dystroglycan modification are conserved in LARGE2. Glycobiology. 23:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamori K, Yoshida-Moriguchi T, Hara Y, Anderson ME, Yu L, Campbell KP. 2012. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science. 335:93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamori KI, Beedle AM, de Bernabe DB, Wright ME, Campbell KP. 2016. LARGE2-dependent glycosylation confers laminin-binding ability on proteoglycans. Glycobiology. 26:1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jae LT, Raaben M, Riemersma M, van Beusekom E, Blomen VA, Velds A, Kerkhoven RM, Carette JE, Topaloglu H, Meinecke P et al. 2013. Deciphering the Glycosylome of Dystroglycanopathies Using Haploid Screens for Lassa Virus Entry. Science. 340:479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerber J, Zaki MS, Al-Aama JY, Rosti RO, Ben-Omran T, Dikoglu E, Silhavy JL, Caglar C, Musaev D, Albrecht B et al. 2016. Biallelic Mutations in TMTC3, Encoding a Transmembrane and TPR-Containing Protein, Lead to Cobblestone Lissencephaly. Am J Hum Genet. 99:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa M, Kobayashi K, Tajiri M, Manya H, Kuga A, Yamaguchi Y, Akasaka-Manya K, Furukawa J, Mizuno M, Kawakami H et al. 2016. Identification of a Post-translational Modification with Ribitol-Phosphate and Its Defect in Muscular Dystrophy. Cell Rep. 14:2209–2223. [DOI] [PubMed] [Google Scholar]
- Kanagawa M, Saito F, Kunz S, Yoshida-Moriguchi T, Barresi R, Kobayashi YM, Muschler J, Dumanski JP, Michele DE, Oldstone MB et al. 2004. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell. 117:953–964. [DOI] [PubMed] [Google Scholar]
- Kanekiyo K, Inamori K, Kitazume S, Sato K, Maeda J, Higuchi M, Kizuka Y, Korekane H, Matsuo I, Honke K et al. 2013. Loss of branched O-mannosyl glycans in astrocytes accelerates remyelination. J Neurosci. 33:10037–10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JY, Hong Y, Ashida H, Shishioh N, Murakami Y, Morita YS, Maeda Y, Kinoshita T. 2005. PIG-V involved in transferring the second mannose in glycosylphosphatidylinositol. J Biol Chem. 280:9489–9497. [DOI] [PubMed] [Google Scholar]
- Kawahara G, Guyon JR, Nakamura Y, Kunkel LM. 2010. Zebrafish models for human FKRP muscular dystrophies. Hum Mol Genet. 19:623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Nakahori Y, Miyake M, Matsumura K, Kondo-Iida E, Nomura Y, Segawa M, Yoshioka M, Saito K, Osawa K et al. 1998. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 394:388–392. [DOI] [PubMed] [Google Scholar]
- Korte F, Goto M, Anno K. 1976. Nucleic acids, proteins, and carbohydrates. 3.4:2.2 Carbohydrates; Cell Wall Substances: Teichoic Acids. New York: Academic Press; 231, p. viii. [Google Scholar]
- Kunz S, Rojek JM, Kanagawa M, Spiropoulou CF, Barresi R, Campbell KP, Oldstone MB. 2005. Posttranslational modification of α-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J Virol. 79:14282–14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S, Rojek JM, Perez M, Spiropoulou CF, Oldstone MB. 2005. Characterization of the interaction of lassa fever virus with its cellular receptor α-dystroglycan. J Virol. 79:5979–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S, Sevilla N, McGavern DB, Campbell KP, Oldstone MB. 2001. Molecular analysis of the interaction of LCMV with its cellular receptor α-dystroglycan. J Cell Biol. 155:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara N, Manya H, Yamada T, Tateno H, Kanagawa M, Kobayashi K, Akasaka-Manya K, Hirose Y, Mizuno M, Ikeguchi M et al. 2016. Carbohydrate-binding domain of the POMGnT1 stem region modulates O-mannosylation sites of alpha-dystroglycan. Proc Natl Acad Sci USA. 113:9280–9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Matthews RT, Lim JM, Swanier K, Wells L, Pierce JM. 2012. Developmental expression of the neuron-specific N-acetylglucosaminyltransferase Vb (GnT-Vb/IX) and identification of its in vivo glycan products in comparison with those of its paralog, GnT-V. J Biol Chem. 287:28526–28536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefeber DJ, de Brouwer APM, Morava E, Riemersma M, JHM Schuurs-Hoeijmakers, Absmanner B, Verrijp K, van den Akker WMR, Huijben K, Steenbergen G et al. 2011. Autosomal recessive dilated cardiomyopathy due to DOLK mutations results from abnormal dystroglycan O-Mannosylation. Plos Genet. 7:e1002427. 10.1371/journal.pgen.1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefeber DJ, Schonberger J, Morava E, Guillard M, Huyben KM, Verriip K, Grafakou O, Evangelioi A, Preijers FW, Manta P et al. 2009. Deficiency of Dol-P-Man Synthase Subunit DPM3 Bridges the Congenital Disorders of Glycosylation with the Dystroglycanopathies. Am J Hum Genet. 85:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YY, White RJ, Torelli S, Cirak S, Muntoni F, Stemple DL. 2011. Zebrafish Fukutin family proteins link the unfolded protein response with dystroglycanopathies. Hum Mol Genet. 20:1763–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ball SL, Yang Y, Mei P, Zhang L, Shi H, Kaminski HJ, Lemmon VP, Hu H. 2006. A genetic model for muscle-eye-brain disease in mice lacking protein O-mannose 1,2-N-acetylglucosaminyltransferase (POMGnT1). Mech Dev. 123:228–240. [DOI] [PubMed] [Google Scholar]
- Lommel M, Winterhalter PR, Willer T, Dahlhoff M, Schneider MR, Bartels MF, Renner-Muller I, Ruppert T, Wolf E, Strahl S. 2013. Protein O-mannosylation is crucial for E-cadherin-mediated cell adhesion. Proc Natl Acad Sci USA. 110:21024–21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Cai S, Maxwell S, Yue D, Zhu W, Qiao K, Zhu Z, Zhou L, Xi J, Lu J et al. 2017. Novel mutations in the C-terminal region of GMPPB causing limb-girdle muscular dystrophy overlapping with congenital myasthenic syndrome. Neuromuscul Disord. 27:557–564. [DOI] [PubMed] [Google Scholar]
- Lynch TA, Lam LT, Man NT, Kobayashi K, Toda T, Morris GE. 2012. Detection of the dystroglycanopathy protein, fukutin, using a new panel of site-specific monoclonal antibodies. Biochem Bioph Res Co. 424:354–357. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Tanaka S, Hino J, Kangawa K, Kinoshita T. 2000. Human dolichol-phosphate-mannose synthase consists of three subunits, DPM1, DPM2 and DPM3. Embo J. 19:2475–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Shah MH, Bitok JK, Hassis-LeBeau ME, Meyers CLF. 2009. Probing phosphorylation by non-mammalian isoprenoid biosynthetic enzymes using H-1-P-31-P-31 correlation NMR spectroscopy. Mol Biosyst. 5:935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manya H, Chiba A, Yoshida A, Wang X, Chiba Y, Jigami Y, Margolis RU, Endo T. 2004. Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc Natl Acad Sci USA. 101:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manya H, Yamaguchi Y, Kanagawa M, Kobayashi K, Tajiri M, Akasaka-Manya K, Kawakami H, Mizuno M, Wada Y, Toda T et al. 2016. The Muscular Dystrophy Gene TMEM5 Encodes a Ribitol beta1,4-Xylosyltransferase Required for the Functional Glycosylation of Dystroglycan. J Biol Chem. 291:24618–24627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini MC, Tambunan DE, Hill RS, Yu TW, Maynard TM, Heinzen EL, Shianna KV, Stevens CR, Partlow JN, Barry BJ et al. 2012. Exome Sequencing and Functional Validation in Zebrafish Identify GTDC2 Mutations as a Cause of Walker-Warburg Syndrome. Am J Hum Genet. 91:541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese M, Pappalardo A, Baldacci J, Verri T, Doccini S, Cassandrini D, Bruno C, Fiorillo C, Garcia-Gil M, Bertini E et al. 2016. Dolichol-phosphate mannose synthase depletion in zebrafish leads to dystrophic muscle with hypoglycosylated alpha-dystroglycan. Biochem Biophys Res Commun. 477:137–143. [DOI] [PubMed] [Google Scholar]
- Martin LT, Glass M, Dosunmu E, Martin PT. 2007. Altered expression of natively glycosylated alpha dystroglycan in pediatric solid tumors. Hum Pathol. 38:1657–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H et al. 2002. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 418:417–422. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Goh HT, Hewitt JE. 2008. Genes required for functional glycosylation of dystroglycan are conserved in zebrafish. Genomics. 92:159–167. [DOI] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA et al. 2002. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 418:422–425. [DOI] [PubMed] [Google Scholar]
- Morise J, Kizuka Y, Yabuno K, Tonoyama Y, Hashii N, Kawasaki N, Manya H, Miyagoe-Suzuki Y, Takeda S, Endo T et al. 2014. Structural and biochemical characterization of O-mannose-linked human natural killer-1 glycan expressed on phosphacan in developing mouse brains. Glycobiology. 24:314–324. [DOI] [PubMed] [Google Scholar]
- Muntoni F, Brockington M, Blake DJ, Torelli S, Brown SC. 2002. Defective glycosylation in muscular dystrophy. Lancet. 360:1419–1421. [DOI] [PubMed] [Google Scholar]
- Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. 2002. A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 62:7102–7109. [PubMed] [Google Scholar]
- Nakagawa N, Manya H, Toda T, Endo T, Oka S. 2012. Human natural killer-1 sulfotransferase (HNK-1ST)-induced sulfate transfer regulates laminin-binding glycans on alpha-dystroglycan. J Biol Chem. 287:30823–30832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa N, Takematsu H, Oka S. 2013. HNK-1 sulfotransferase-dependent sulfation regulating laminin-binding glycans occurs in the post-phosphoryl moiety on alpha-dystroglycan. Glycobiology. 23:1066–1074. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Stalnaker SH, Lyalin D, Lavrova O, Wells L, Panin VM. 2010. Drosophila Dystroglycan is a target of O-mannosyltransferase activity of two protein O-mannosyltransferases, Rotated Abdomen and Twisted. Glycobiology. 20:381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Nilsson J, Larson G, Grahn A. 2010. Characterization of site-specific O-glycan structures within the mucin-like domain of α-dystroglycan from human skeletal muscle. Glycobiology. 20:1160–1169. [DOI] [PubMed] [Google Scholar]
- Oldstone MB, Campbell KP. 2011. Decoding arenavirus pathogenesis: essential roles for alpha-dystroglycan-virus interactions and the immune response. Virology. 411:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlean P. 1990. Dolichol phosphate mannose synthase is required in vivo for glycosyl phosphatidylinositol membrane anchoring, O mannosylation, and N glycosylation of protein in Saccharomyces cerevisiae. Mol Cell Biol. 10:5796–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacharra S, Hanisch FG, Breloy I. 2012. Neurofascin 186 is O-mannosylated within and outside of the mucin domain. J Proteome Res. 11:3955–3964. [DOI] [PubMed] [Google Scholar]
- Pacharra S, Hanisch FG, Muhlenhoff M, Faissner A, Rauch U, Breloy I. 2013. The lecticans of mammalian brain perineural net are O-mannosylated. J Proteome Res. 12:1764–1771. [DOI] [PubMed] [Google Scholar]
- Panin VM, Wells L. 2014. Protein O-mannosylation in metazoan organisms. Curr Protoc Protein Sci. 75:12, Unit 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo A, Pitto L, Fiorillo C, Alice Donati M, Bruno C, Santorelli FM. 2013. Neuromuscular disorders in zebrafish: state of the art and future perspectives. Neuromolecular Med. 15:405–419. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Campos I, Hirst EM, Stemple DL. 2002. Removal of dystroglycan causes severe muscular dystrophy in zebrafish embryos. Development. 129:3505–3512. [DOI] [PubMed] [Google Scholar]
- Patnaik SK, Stanley P. 2005. Mouse large can modify complex N- and mucin O-glycans on alpha-dystroglycan to induce laminin binding. J Biol Chem. 280:20851–20859. [DOI] [PubMed] [Google Scholar]
- Patnaik SK, Stanley P. 2006. Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 416:159–182. [DOI] [PubMed] [Google Scholar]
- Peyrard M, Seroussi E, Sandberg-Nordqvist AC, Xie YG, Han FY, Fransson I, Collins J, Dunham I, Kost-Alimova M, Imreh S et al. 1999. The human LARGE gene from 22q12.3-q13.1 is a new, distinct member of the glycosyltransferase gene family. Proc Natl Acad Sci USA. 96:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield M, Zhao P, Han HY, Cunningham J, Aoki K, Von Hoff DD, Demeure MJ, Pierce JM, Tiemeyer M, Wells L. 2014. Discrimination between Adenocarcinoma and Normal Pancreatic Ductal Fluid by Proteomic and Glycomic Analysis. J Proteome Res. 13:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praissman JL, Live DH, Wang S, Ramiah A, Chinoy ZS, Boons GJ, Moremen KW, Wells L. 2014. B4GAT1 is the priming enzyme for the LARGE-dependent functional glycosylation of alpha-dystroglycan. eLife. 3:e03943. 10.7554/eLife.03943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praissman JL, Wells L. 2014. Mammalian O-mannosylation pathway: glycan structures, enzymes, and protein substrates. Biochemistry-Us. 53:3066–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praissman JL, Willer T, Sheikh MO, Toi A, Chitayat D, Lin YY, Lee H, Stalnaker SH, Wang S, Prabhakar PK et al. 2016. The functional O-mannose glycan on α-dystroglycan contains a phospho-ribitol primed for matriglycan addition. eLife. 5:e14473. 10.7554/eLife.14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma M, Froese DS, van Tol W, Engelke Udo F, Kopec J, van Scherpenzeel M, Ashikov A, Krojer T, von Delft F, Tessari M et al. 2015. Human ISPD Is a Cytidyltransferase Required for Dystroglycan O-Mannosylation. Chem Biol. 22:1643–1652. [DOI] [PubMed] [Google Scholar]
- Riemersma M, Sandrock J, Boltje TJ, Bull C, Heise T, Ashikov A, Adema GJ, van Bokhoven H, Lefeber DJ. 2015. Disease mutations in CMP-sialic acid transporter SLC35A1 result in abnormal alpha-dystroglycan O-mannosylation, independent from sialic acid. Hum Mol Genet. 24:2241–2246. [DOI] [PubMed] [Google Scholar]
- Roscioli T, Kamsteeg EJ, Buysse K, Maystadt I, van Reeuwijk J, van den Elzen C, van Beusekom E, Riemersma M, Pfundt R, Vissers LE et al. 2012. Mutations in ISPD cause Walker-Warburg syndrome and defective glycosylation of alpha-dystroglycan. Nat Genet. 44:581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadreyev IR, Ji F, Cohen E, Ruvkun G, Tabach Y. 2015. PhyloGene server for identification and visualization of co-evolving proteins using normalized phylogenetic profiles. Nucleic Acids Res. 43:W154–W159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Yamada H, Matsumura K, Shimizu T, Kobata A, Endo T. 1998. Detection of O-mannosyl glycans in rabbit skeletal muscle alpha-dystroglycan. Bba-Gen Subjects. 1425:599–606. [DOI] [PubMed] [Google Scholar]
- Schollen E, Dorland L, de Koning TJ, Van Diggelen OP, Huijmans JG, Marquardt T, Babovic-Vuksanovic D, Patterson M, Imtiaz F, Winchester B et al. 2000. Genomic organization of the human phosphomannose isomerase (MPI) gene and mutation analysis in patients with congenital disorders of glycosylation type Ib (CDG-Ib). Hum Mutat. 16:247–252. [DOI] [PubMed] [Google Scholar]
- Sciandra F, Bigotti MG, Giardina B, Bozzi M, Brancaccio A. 2015. Genetic Engineering of Dystroglycan in Animal Models of Muscular Dystrophy. Bio Med Res Int. 2015:635792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentandreu R, Northcote DH. 1968. The structure of a glycopeptide isolated from the yeast cell wall. Biochem J. 109:419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambato A, Migaldi M, Montanari M, Camerini A, Brancaccio A, Rossi G, Cangiano R, Losasso C, Capelli G, Trentini GP et al. 2003. Dystroglycan expression is frequently reduced in human breast and colon cancers and is associated with tumor progression. Am J Pathol. 162:849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JG, Xu CY, Li X, Dong MJ, Jiang ZN, Wang J, Wang LB. 2012. Dystroglycan is associated with tumor progression and patient survival in gastric cancer. Pathol Oncol Res. 18:79–84. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Haslam SM, Sutton-Smith M, Morris HR, Dell A. 1998. Structural analysis of sequences O-linked to mannose reveals a novel Lewis X structure in cranin (Dystroglycan) purified from sheep brain. J Bio Chem. 273:23698–23703. [DOI] [PubMed] [Google Scholar]
- Sparks SE, Krasnewich DM. 1993. PMM2-CDG (CDG-Ia) In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH et al. editors. GeneReviews(R). Seattle (WA): University of Washington, Seattle. [PubMed] [Google Scholar]
- Stalnaker SH, Aoki K, Lim JM, Porterfield M, Liu M, Satz JS, Buskirk S, Xiong Y, Zhang P, Campbell KP et al. 2011. Glycomic analyses of mouse models of congenital muscular dystrophy. J Biol Chem. 286:21180–21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker SH, Hashmi S, Lim J-M, Aoki K, Porterfield M, Gutierrez-Sanchez G, Wheeler J, Ervasti JM, Bergmann C, Tiemeyer M et al. 2010. Site Mapping and Characterization of O-Glycan Structures on α-Dystroglycan Isolated from Rabbit Skeletal Muscle. J Biol Chem. 285:24882–24891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steentoft C, Vakhrushev SY, Vester-Christensen MB, Schjoldager KTBG, Kong Y, Bennett EP, Mandel U, Wandall H, Levery SB, Clausen H. 2011. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat Methods. 8:977–982. [DOI] [PubMed] [Google Scholar]
- Steffen LS, Guyon JR, Vogel ED, Beltre R, Pusack TJ, Zhou Y, Zon LI, Kunkel LM. 2007. Zebrafish orthologs of human muscular dystrophy genes. BMC genomics. 8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens E, Carss KJ, Cirak S, Foley AR, Torelli S, Willer T, Tambunan DE, Yau S, Brodd L, Sewry CA et al. 2013. Mutations in B3GALNT2 cause congenital muscular dystrophy and hypoglycosylation of alpha-dystroglycan. Am J Hum Genet. 92:354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Yu SH, Zhao P, Meng L, Moremen KW, Wells L, Steet R, Boons GJ. 2016. One-Step Selective Exoenzymatic Labeling (SEEL) Strategy for the Biotinylation and Identification of Glycoproteins of Living Cells. J Am Chem Soc. 138:11575–11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi-Ikeda M, Morioka I, Iijima K, Toda T. 2016. Mechanistic aspects of the formation of alpha-dystroglycan and therapeutic research for the treatment of alpha-dystroglycanopathy: A review. Mol Aspects Med. 51:115–124. [DOI] [PubMed] [Google Scholar]
- Thornhill P, Bassett D, Lochmuller H, Bushby K, Straub V. 2008. Developmental defects in a zebrafish model for muscular dystrophies associated with the loss of fukutin-related protein (FKRP). Brain. 131:1551–1561. [DOI] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, Stanley P, Hart G, Darvill A, Kinoshita T et al. 2015. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology. 25:1323–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester-Christensen MB, Halim A, Joshi HJ, Steentoft C, Bennett EP, Levery SB, Vakhrushev SY, Clausen H. 2013. Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc Natl Acad Sci. 110:21018–21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L. 2013. The o-mannosylation pathway: glycosyltransferases and proteins implicated in congenital muscular dystrophy. J Biol Chem. 288:6930–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer T, Inamori K, Venzke D, Harvey C, Morgensen G, Hara Y, Beltran Valero de Bernabe D, Yu L, Wright KM, Campbell KP. 2014. The glucuronyltransferase B4GAT1 is required for initiation of LARGE-mediated alpha-dystroglycan functional glycosylation. eLife. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer T, Lee H, Lommel M, Yoshida-Moriguchi T, de Bernabe DB, Venzke D, Cirak S, Schachter H, Vajsar J, Voit T et al. 2012. ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker-Warburg syndrome. Nat Genet. 44:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, IbraghimovBeskrovnaya O, Campbell KP. 1997. Dystroglycan is essential for early embryonic development: Disruption of Reichert's membrane in Dag1-null mice. Hum Mol Genet. 6:831–841. [DOI] [PubMed] [Google Scholar]
- Winterhalter PR, Lommel M, Ruppert T, Strahl S. 2013. O-glycosylation of the non-canonical T-cadherin from rabbit skeletal muscle by single mannose residues. FEBS Lett. 587:3715–3721. [DOI] [PubMed] [Google Scholar]
- Wood AJ, Currie PD. 2014. Analysing regenerative potential in zebrafish models of congenital muscular dystrophy. Int J Biochem Cell Biol. 56:30–37. [DOI] [PubMed] [Google Scholar]
- Wood AJ, Muller JS, Jepson CD, Laval SH, Lochmuller H, Bushby K, Barresi R, Straub V. 2011. Abnormal vascular development in zebrafish models for fukutin and FKRP deficiency. Hum Mol Genet. 20:4879–4890. [DOI] [PubMed] [Google Scholar]
- Xiong H, Kobayashi K, Tachikawa M, Manya H, Takeda S, Chiyonobu T, Fujikake N, Wang F, Nishimoto A, Morris GE et al. 2006. Molecular interaction between fukutin and POMGnT1 in the glycosylation pathway of alpha-dystroglycan. Biochem Biophys Res Commun. 350:935–941. [DOI] [PubMed] [Google Scholar]
- Yagi H, Kuo CW, Obayashi T, Ninagawa S, Khoo KH, Kato K. 2016. Direct Mapping of Additional Modifications on Phosphorylated O-glycans of alpha-Dystroglycan by Mass Spectrometry Analysis in Conjunction with Knocking Out of Causative Genes for Dystroglycanopathy. Mol Cell Proteomics. 15:3424–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Nakagawa N, Saito T, Kiyonari H, Abe T, Toda T, Wu SW, Khoo KH, Oka S, Kato K. 2013. AGO61-dependent GlcNAc modification primes the formation of functional glycans on alpha-dystroglycan. Sci Rep. 3:3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaji S, Manya H, Nakagawa N, Takematsu H, Endo T, Kannagi R, Yoshihara T, Asano M, Oka S. 2015. Major glycan structure underlying expression of the Lewis X epitope in the developing brain is O-mannose-linked glycans on phosphacan/RPTPbeta. Glycobiology. 25:376–385. [DOI] [PubMed] [Google Scholar]
- Yang AC, Ng BG, Moore SA, Rush J, Waechter CJ, Raymond KM, Willer T, Campbell KP, Freeze HH, Mehta L. 2013. Congenital disorder of glycosylation due to DPM1 mutations presenting with dystroglycanopathy-type congenital muscular dystrophy. Mol Genet Metab. 110:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu EM, Kornberg AJ. 2015. Duchenne muscular dystrophy. J Paediatr Child Health. 51:759–764. [DOI] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Campbell KP. 2015. Matriglycan: a novel polysaccharide that links dystroglycan to the basement membrane. Glycobiology. 25:702–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Willer T, Anderson ME, Venzke D, Whyte T, Muntoni F, Lee H, Nelson SF, Yu LP, Campbell KP. 2013. SGK196 Is a Glycosylation-Specific O-Mannose Kinase Required for Dystroglycan Function. Science. 341:896–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Yu LP, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MBA, Schachter H, Wells L, Campbell KP. 2010. O-Mannosyl Phosphorylation of Alpha-Dystroglycan Is Required for Laminin Binding. Science. 327:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M et al. 2001. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell. 1:717–724. [DOI] [PubMed] [Google Scholar]
- Zhang P, Hu H. 2012. Differential glycosylation of alpha-dystroglycan and proteins other than alpha-dystroglycan by like-glycosyltransferase. Glycobiology. 22:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang P, Hu H. 2011. LARGE expression augments the glycosylation of glycoproteins in addition to alpha-dystroglycan conferring laminin binding. PLoS One. 6:e19080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Stalnaker SH, Wells L. 2013. Approaches for site mapping and quantification of O-linked glycopeptides. Methods Mol Biol. 951:229–244. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Venzke D, Walimbe AS, Anderson ME, Fu Q, Kinch LN, Wang W, Chen X, Grishin NV, Huang N et al. 2016. Structure of protein O-mannose kinase reveals a unique active site architecture. Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.