Abstract
Male germ-line stem cells are the only cell type in postnatal mammals that have the capability to self-renew and to contribute genes to the next generation. Genetic modification of these cells would provide an opportunity to study the biology of their complex self-renewal and differentiation processes, as well as enable the generation of transgenic animals in a wide range of species. Although retroviral vectors have been used as an efficient method to introduce genes into a variety of cell types, postnatal male germ-line stem cells have seemed refractory to direct infection by these viruses. In addition, expression of genes transduced into several types of stem cells, such as embryonic or hematopoietic, is often attenuated or silenced. We demonstrate here that in vitro retroviral-mediated gene delivery into spermatogonial stem cells of both adult and immature mice results in stable integration and expression of a transgene in 2–20% of stem cells. After transplantation of the transduced stem cells into the testes of infertile recipient mice, approximately 4.5% of progeny from these males are transgenic, and the transgene is transmitted to and expressed in subsequent generations. Therefore, there is no intrinsic barrier to retroviral transduction in this stem cell, and transgene expression is not extinguished after transmission to progeny.
Spermatogonial stem cells, the postnatal male germ-line stem cells of animals, proliferate throughout life and, after puberty, provide daughter cells that differentiate into spermatozoa (1, 2). These stem cells are the only cells in the postnatal animal that undergo self-renewal and contribute genes to subsequent generations; therefore, among stem cells, they are unique and essential for continuity of a species. Consequently, spermatogonial stem cells represent a valuable resource for experimental modification of the mammalian genome, but current techniques to generate transgenic animals are based primarily on cells found in the female: oocytes, fertilized eggs, and blastocysts (3–5). Emphasis on the use of female-derived cells for transgenesis results in part from their availability. In contrast, it is difficult to identify postnatal male germ-line stem cells and they are less accessible. Moreover, attempts to infect these stem cells with viral vectors or transfect them by other techniques have met with little success (6–10).
Underlying the difficulty in working with male germ-line stem cells is the complexity of spermatogenesis and the absence of any distinguishing morphological or biochemical markers for the stem cell. Only a functional assay can identify the stem cell. Nonetheless, the male germ line has several possible positive attributes for use in generating transgenic animals. Spermatogenesis is a very productive and highly organized process (11). In the rat, 107 spermatozoa are generated per gram of testicular tissue each day, and similar rates of production are characteristic of all mammals (12). Furthermore, the process of spermatogenesis continues throughout life of the adult male; thus, the number of offspring that can be derived from a single male far exceeds that from a female. At the foundation of this process are spermatogonial stem cells that can self-renew as well as generate daughter cells that undergo mitotic proliferation to produce spermatocytes, which enter meiosis, and ultimately generate haploid spermatozoa (1, 2). An additional advantage of the system is that spermatogonial stem cell populations can be obtained and readily cryopreserved from many mammalian species (13, 14). Therefore, genetic modification of the spermatogonial stem cell has the potential to produce a large number of transgenic offspring in diverse species.
Development of the spermatogonial stem cell transplantation technique provided access to this cell and an opportunity to manipulate the male germ line, because during the transfer of donor stem cells to recipient testes they can be subjected to genetic modification (15, 16). In previous studies we found that these cells can be transduced, at a low efficiency (1 in 280), by a retroviral vector either in vitro or in vivo during the transplantation process, and subsequently reestablish spermatogenesis that was maintained for 6 months in recipient seminiferous tubules (17). Because retroviral integration requires cell replication, the results indicate that stem cells are capable of proliferation under in vitro culture conditions, suggesting the potential for various approaches to genetic modification through the male germ line. However, despite a long history of retrovirus studies over the last 4 decades, it has seemed extremely difficult or impossible to demonstrate vertical transmission of integrated provirus to the next generation through the male germ line when postnatal males were exposed to the retrovirus (6, 9, 18). Thus, it is not clear that a reporter transgene introduced and expressed in germ cells of recipient males would be transmitted to progeny by mature spermatozoa. Furthermore, expression of genes in retroviral vectors often is silenced or undergoes diminution in target cells such as embryonic stem (ES) cells or hematopoietic stem cells (6, 19, 20). Consequently, if a retrovirally transduced gene present in male germ cells was transmitted to progeny it might be nonfunctional. We show here that a reporter transgene can be introduced by a retroviral vector into 2–20% of cultured spermatogonial stem cells and that after transplantation of these cells into recipient males the transgene is transmitted and expressed in 4.5% of progeny, and transmission to subsequent generations with expression also occurs. Success required efficient donor stem cell transduction, a high level of fertility in recipient males, and stable transgene integration with expression in progeny.
Materials and Methods
Animals.
Donor immature mouse (pup) testis cells were obtained from C57BL/6 × 129/SvCP (B6/129) F2 hybrid mice or C57BL/6 (B6) mice at 5–7 days of age. Donor adult cells were obtained from B6 mouse testes of males made cryptorchid at 6–8 weeks of age and used 2 months later (21). Two types of mice were used as recipients. One type was compound mutants of the W locus (Wv/W54, Wv/W, Wv/Wv; The Jackson Laboratory) that have mutations in the c-kit receptor tyrosine kinase; their testes contain no differentiating germ cells (22). Maintenance and breeding of W mutant mice were described (15, 22, 23). The second type of recipient was B6/129 F1 hybrid mice. All recipient mice were pups (5–14 days old) at the time of transplantation.
In Vitro Manipulation of Spermatogonial Stem Cells and Transplantation.
In vitro culture and infection procedures were based on our previous studies with slight modifications (15–17, 24). After a two-step digestion of donor testes, 6 × 106 cells were placed on mitomycin C-treated SIM mouse embryo-derived Thioguanine- and Ouabain-resistant fibroblast cell line (STO) feeder cells (1.2 × 106 cells in a 25-cm2 tissue culture flask). The next day, donor cells were treated for 2 h with retrovirus-containing medium (2 ml, 4 μg/ml polybrene) obtained from a 24-h culture of retrovirus-producer cells (17, 19). Virus-producing cells were eliminated from the virus-containing medium by using a 0.22-μm filter. After the 2-h exposure to retrovirus, donor cells were incubated with fresh culture medium for 1 h. This infection cycle was repeated 4 times each day for 2 days. On the third day, cells were washed 3 times with PBS and digested with trypsin. A single cell suspension of cultured donor cells was transplanted into testes of recipient pups in which anesthesia was induced by hypothermia (25). Each recipient testis received 2 μl of cell suspension (Table 1). The retrovirus vector used was based on the Moloney murine leukemia virus that carries the lacZ structural gene with the Pgk-1 promoter (Gen− PGKβ-gal; refs. 17 and 19). The virus titer was 3.0 ± 0.5 × 105 (mean ± SEM, n = 13) colony-forming units/ml on NIH/3T3 cells.
Table 1.
Transgenic mice from retroviral vector transduction of mouse spermatogonial stem cells
| Exp. | Donor (age)* | Recipient mouse number† | Recipient (age)‡ | Testis weight, mg§
|
Colonies/testis¶
|
Area, mm2/testis‖
|
Transplant to first transgenic** | Transgenic/total progeny (%)‡‡ | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | L | R | L | R | L | ||||||
| 1 | B6/129 F2 (5 day) | 5483 | Wv/W54 (12 day) | 87.4 | 29.5 | 12 | 1 | 27.5 | 12.1 | 114 | 10/174 (5.7) |
| 5484 | Wv/Wv (5 day) | 25.9 | 47.3 | 3 | 9 | 4.7 | 21.3 | — | — | ||
| 5485 | Wv/W54 (12 day) | 22.6 | 23.1 | 4 | 0 | 4.3 | 0 | — | — | ||
| 5486 | Wv/W (5 day) | 74.4 | 48.0 | 12 | 1 | 12.0 | 0.3 | 114 | 8/111 (7.2) | ||
| 2 | B6 (5–7 day) | 5650 | B6/129 (7 day) | 144.7 | 130.7 | 0 | 0 | 0 | 0 | — | 0/103 (0) |
| 5651 | Wv/W54 (14 day) | 49.5 | 43.0 | 4 | 4 | 9.6 | 2.7 | 168 | 1/101 (1.0) | ||
| 5654 | Wv/W54 (8 day) | 35.8 | 50.0 | 1 | 1 | 2.6 | 3.3 | — | 0/95 (0) | ||
| 5655 | Wv/W54 (8 day) | 50.0 | 36.8 | 4 | 2 | 5.7 | 5.2 | 238 | 1/13 (7.7) | ||
| 3 | B6 (adult) | 5763 | B6/129 (8 day) | 227.6 | 92.4 | 0 | 0 | 0 | 0 | — | 0/100 (0) |
| 5764 | Wv/W54 (12 day) | 49.9 | 44.5 | 3 | 4 | 5.7 | 7.5 | 172 | 6/80 (7.5) | ||
| 5767 | Wv/W54 (10 day) | ND | 45.5 | — | 3 | — | 4.1 | — | 0/3 (0) | ||
| 5768 | B6/129 (7 day) | 143.7 | 135.5 | 0 | 0 | 0 | 0 | — | 0/103 (0) | ||
| 5769 | B6/129 (7 day) | 145.2 | 152.2 | 0 | 0 | 0 | 0 | — | 0/102 (0) | ||
Donor cell strain; adult, cryptorchid.
The number of cultured cells injected per recipient testis (1.2, 2.0, and 4.0 × 105 in Exps. 1, 2, and 3, respectively) was the adjusted number of the original cultured testis cell population introduced. Cultured cells (originally 6 × 106 testis cells) were recovered after 3 days and suspended in 100, 60, and 30 μl for Exps. 1, 2, and 3, respectively. Feeder cells cannot make colonies and were not included in calculations. Of each cell suspension, 2 μl were injected into a testis. Therefore, in Exp. 1, 6 × 106 cells per 100 μl × 2 μl injected per testis = 1.2 × 105 cultured testis cells were injected per recipient testis. All cells recovered from culture cannot be injected into a recipient testis because the seminiferous tubule volume and number of recipients are limited.
Strain of recipient mouse and approximate age in days at time of donor cell transplantation.
R, right; L, left; ND, not determined.
Number of individual blue colonies in each testis. Each colony generally represents the progeny of one donor stem cell. Totals for W recipients: Exp. 1 = 42; Exp. 2 = 16; Exp. 3 = 10.
Total blue surface area of all colonies in each testis that represents area of spermatogenesis expressing the transgene. Totals for W recipients: Exp. 1 = 82.2 mm2; Exp. 2 = 29.1 mm2; Exp. 3 = 17.3 mm2.
Time in days from transplantation of transduced donor cells to birth of first transgenic pup sired by the recipient.
Numerator is the number of transgenic progeny; denominator is the total number of progeny from each recipient. Totals for W recipients: Exp. 1 = 18/285 (6.3%); Exp. 2 = 2/209 (1.0%); Exp. 3 = 6/83 (7.2%).
Analyses.
Recipient males were analyzed first by mating with wild-type C57BL/6 × SJL F1 hybrid females, and progeny were examined for expression of the lacZ gene by staining ear or tail samples, and in some instances embryos (11–18 days of gestation), with 5-bromo-4-chloro-3-indolyl β-d-galactosidase (X-gal; refs. 26 and 27). Mating continued for 250–390 days after cell transplantation. After completion of progeny examination, testes of recipient males were removed and stained with X-gal, the number of blue stretches of seminiferous tubule was counted (colonies), and total blue area (mm2) occupied by colonies was measured as described (28). A donor spermatogonial stem cell is defined by its ability to produce a blue-staining colony in a recipient testis, and each colony is thought to result from the spread of a single stem cell (27, 28). In progeny of some recipients, the integration site of the viral transgene was examined by Southern blot analysis. Genomic DNA (8 μg) extracted from tail samples of transgenic and control offspring was digested with NcoI, separated on a 0.8% agarose gel, and transferred to a nylon membrane (Hybond-N4, Amersham Pharmacia). The membrane was hybridized for 16 h at 45°C with a 32P-labeled PCR-amplified fragment of lacZ cDNA (1,991 bp; Random Primed DNA labeling kit, Roche Diagnostics) in the presence of formamide. To examine the morphology of spermatogenesis derived from modified stem cells, testes of recipients were stained with X-gal and processed for paraffin sections and counterstained with nuclear fast red.
Results
Pup and Adult Male Germ-Line Stem Cells Are Efficiently Transduced.
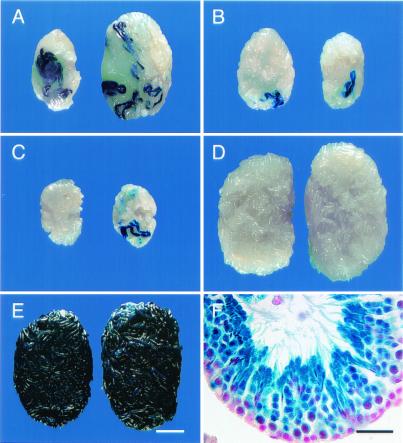
Although cryptorchid adult donor testes contain a higher concentration of stem cells than pup testes (29), the latter seem to be more efficiently infected by retroviral vectors during a 7-day culture period (17). Because the exposure to virus was only 2 days of a 3-day culture period in the present experiments and the relative effect of stem cell concentration and transduction efficiency was unknown for this shorter period, both pup and adult testis cells were studied (Table 1). A similar number (6 × 106) of testis cells of each type was cultured with virus, and the number of donor cells injected into each recipient testis was the equivalent of 1.2 to 4 × 105 of the testis cells originally placed in culture (Table 1, Legend). Both pup and cryptorchid adult stem cells were transduced by the vector, and the transgene was expressed in differentiating germ cells as evidenced by the formation of blue colonies of spermatogenesis (Fig. 1 A–C; Table 1, columns 7 and 8).
Figure 1.
Macroscopic and histological appearance of W and wild-type B6/129-recipient testes that were injected with spermatogonial stem cells transduced by a retroviral vector containing a lacZ reporter gene. (A) Testes of W male 5483 that was fertile and produced transgenic progeny (killed at 379 days). Note size of testes, number of blue colonies, and total blue area. (B) Testes of W male 5654 that was fertile but produced no transgenic progeny (killed at 319 days). Note size of testes and small number of blue colonies. (C) Testes of W male 5485 that was not fertile (killed at 307 days). Testes are small; total weight is ≈40% of A and 53% of B. (D) Testes of wild-type B6/129 male 5650 (killed at 273 days). Note normal size and absence of blue colonies. (E) Testes of 5486-4 progeny of W male 5486 (killed at 320 days). Testes are normal size and all tubules stain blue. (F) Histological section of seminiferous tubule from testes of W male 5486. Note normal organization of spermatogenesis and production of spermatozoa from stem cell transduced by vector. All germ cells carry the transgene and stain blue. Right testes appear on Right in A–E. Stain: A–F, 5-bromo-4-chloro-3-indolyl β-d-galactosidase (X-gal); F, nuclear fast red counterstain. [Bars = 2 mm (A–E) and 30 μm (F).]
None of the B6/129 F1 hybrid pup recipient testes displayed blue colonies, perhaps indicating that endogenous spermatogenesis prevented sufficient seeding of transplanted cells to produce spermatogenesis from a transduced donor stem cell (Table 1). Therefore, only W recipients were used for further analysis. A total of 42 and 16 colonies of donor cell-derived spermatogenesis expressing the transgene were found in recipient W testes in Exps. 1 and 2, respectively (Table 1). On the basis of the number of testis cells placed in culture and injected per testis, 43.8 [42 colonies/(0.12 × 106 cells injected per testis × 8 testes)] and 13.3 colonies of spermatogenesis expressing the transgene were produced per 106 testis cells exposed to viral vector. Cryptorchid adult testis cells produced 8.3 colonies of spermatogenesis expressing the transgene per 106 testis cells cultured with viral vector.
Combining the data from the two pup experiments, 28.6 [(43.8 + 13.3)/2] colonies per 106 cultured donor testis cells were produced. When pup and cryptorchid adult testis cells carrying a reporter transgene were transplanted without culture, they generated 140 ± 19 and 358 ± 37 (mean ± SEM, n = 15 and 18, respectively) colonies per 106 cells. Therefore, in pup testis cell suspensions exposed to a retroviral vector, about 1 in 4.9 (28.6/140) or 20% of originally cultured stem cells survived in vitro and were transduced by the retroviral vector as evidenced by blue colony formation. Similar calculations indicate that only about 1 in 43 (8.3/358) or 2.3% of cryptorchid adult stem cells form colonies of spermatogenesis expressing the transgene after transplantation. Although the relationship of transduction efficiency of pup and adult stem cells (28.6/8.3 = 3.4) in the present study was similar to our previous findings (2.47/0.69 = 3.6; 17), the absolute level of transduction in this study was 11.6-fold (28.6/2.47) and 12-fold (8.3/0.69) greater for pup and adult stem cells, respectively.
Transplantation of Transduced Spermatogonial Stem Cells Restores Fertility to Infertile Males, and Expression of the Transgene Occurs in Recipient Testes.
Previous studies have demonstrated that donor cell colonization is superior in pup compared with adult W recipients (29). Therefore, donor testis cells cultured with the retroviral vector were transplanted to recipient pup testes of W or wild-type (B6/129) F1 hybrid mice (Table 1). Donor cells were derived from both pup and cryptorchid adult testes because each provides stem cells that may exhibit distinct beneficial potential in susceptibility to transduction, stem cell concentration, or ability to colonize recipients (refs. 17 and 29 and above). Four wild-type recipients produced progeny within 52–80 days of donor cell transplantation. When these animals were killed, however, the testes were of normal size, and no areas of spermatogenesis expressing the transgene were found (Table 1; Fig. 1D). These findings strongly suggested that spermatogenesis and progeny resulted from endogenous stem cell activity.
Seven of nine W recipients produced progeny within 76–222 days of donor cell transplantation. Five of seven W recipients that received pup donor cells became fertile, and both W recipients of adult cells became fertile. Thus, donor testis cells from both pup and cryptorchid adult produced similar levels of fertility. Testis weight of fertile W males (49.6 ± 4.3 mg; mean ± SEM) was significantly greater (P = 0.03) than for infertile W males (29.7 ± 5.9; Table 1; Fig. 1 A and C). Previous studies indicate that in W recipients testis weight is proportional to the number of seminiferous tubule cross-sections with complete spermatogenesis (29). Colonies of spermatogenesis with germ cells expressing the transgene were found in 16 of 17 W testes from fertile and infertile W recipients (Table 1). Complete spermatogenesis with normal organizational structure was present in areas of the seminiferous tubules expressing the transgene (Fig. 1F). The number of blue colonies in testes of fertile males producing transgenic progeny was 4.7 ± 1.3 (mean ± SEM) compared with 1.7 ± 0.7 for fertile males with no transgenic progeny (P < 0.06). A similar comparison of total blue area per testis, indicating the extent of transgene-expressing germ cells, was 8.8 ± 2.4 mm2 and 3.3 ± 0.4 mm2 (P < 0.05) for fertile W males with and without transgenic progeny, respectively (Table 1).
The Transgene Is Integrated in Chromosomes of Stem Cells and Transmitted to Progeny.
Consistent with the absence of blue-stained areas of colonization in B6/129 F1 hybrid recipients, none of these males produced transgenic progeny. However, 5 of 7 fertile W recipients (out of 9 W recipients total) produced transgenic progeny. In Exps. 1 and 2, using pup donor cells, 6.3% (18/285) and 1.0% (2/209) of progeny were transgenic, respectively. Cryptorchid adult cells in Exp. 3 produced 7.2% (6/83) transgenic progeny. Overall, 4.5% (26/577) of progeny carried the transgene. At this level of transduction, it is likely that most transduced stem cells contained only one viral integration, in which case one-half of the resulting spermatozoa would carry the transgene. Therefore, about 9.0% of spermatozoa were derived from transduced stem cells. Interestingly, recipient males 5484 and 5486 were not immunologically compatible with donor cells (B6/129), because both WV and W alleles of these recipients are maintained on a B6 genetic background (The Jackson Laboratory; ref. 22). However, colonies of spermatogenesis were present in both 5484 and 5486 when killed at 307 and 358 days after transplanting, respectively. In addition, progeny were produced by 5486 for more than 7 months.
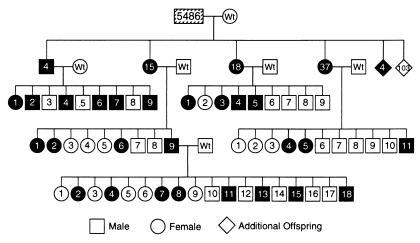
Transmission of the transgene was studied in 2 recipient male lines (5483 and 5486), and expression of the transgene was demonstrated for 3 generations. The pedigree for one of these lines (5486) demonstrates that both male and female progeny transmit the transgene to both sexes, and inheritance of the transgene is Mendelian (Fig. 2). Testes of transgenic progeny (e.g., 5486-4♂) from recipient males stain completely blue, indicating the presence and expression of the transgene in all cells (Fig. 1E); testes of transgenic males in subsequent generations demonstrate similar staining (data not shown).
Figure 2.
Pedigree of recipient W male 5486 demonstrating transmission of transgene for 3 generations. Diagonal lines in 5486 indicate only some seminiferous tubules stain blue and express the transgene. Solid symbols indicate the transgene is expressed in these progeny. Numbers in boxes and circles are identification numbers of individual animals. In the first generation, the numbers in diamonds are population size; 4 animals contained the transgene and 103 were negative. None of these animals were assigned identification numbers or mated. At weaning, females and males were caged separately and then analyzed for the transgene; therefore, identification numbers for each sex are consecutive.
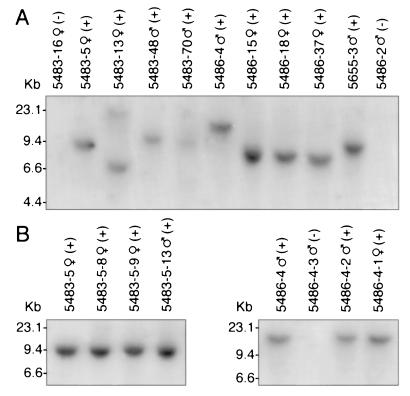
Because retroviral vectors integrate at diverse locations in the chromosomes, progeny generated by spermatozoa arising from different colonies in recipient testes are likely to have distinct viral integration sites. Southern blot analyses of progeny from three recipient males (5483, 5486, and 5655) confirmed that viral integration had occurred at different chromosomal locations in individual donor stem cells (Fig. 3A). Thus, analysis of chromosomal DNA from four progeny of recipient 5483 (5, 13, 48, and 70) was able to distinguish at least three different patterns of viral DNA migration on the gel, and one animal (5483-13) appears to have two integration sites (Fig. 3A). Analysis of DNA from four progeny of recipient 5486 (4, 15, 18, and 37) distinguished at least two different viral integration sites, and DNA from 5655-3 produced a band of a size different from most others on the gel (Fig. 3A). However, when chromosomal DNA of progeny in each of two lines (5483-5 and 5486-4) was examined, the integration site was identical in the mother (5483-5♀) and progeny, or father (5486-4♂) and progeny (Figs. 2 and 3B), indicating stable chromosomal integration of the viral vector and transgene.
Figure 3.
Southern blot showing integration pattern of retroviral vector in tail DNA from progeny of recipient W males 5483 and 5486. (A) DNA from three progeny of 5483 (5, 13, and 48) generate bands that migrate to different levels in the gel, indicating three distinct integration sites. A difference in sizes of bands for animals 5483-48 and 5483-70 cannot be distinguished on this gel separation. DNA from animal 5483-13 generates two bands and may have two sites of vector integration. DNA from two progeny of 5486 (4 and 15) generate bands of different sizes. Bands for progeny 15, 18, and 37 of W male 5486 on this gel appear similar. DNA from only one offspring of 5655 (5655-3) was examined. Nontransgenic mice display no band. (B) Progeny 8, 9, and 13 from 5483-5♀ (Left) have the same band migration and thus vector integration site as the parent, indicating stable integration of the transgene. Progeny 1 and 2 from 5486-4♂ (Right) have the same integration site as the parent, again indicating stable integration of the transgene. Results of staining for lacZ are indicated by + or − .
Discussion
Exposure of testis cell populations of both pup and adult mice to a retroviral vector followed by transplantation of these cells to recipient testes resulted in production of progeny that express the transgene. We previously used a similar approach to modify the spermatogonial stem cell genome by retrovirus transduction but were unable to determine whether the transgene was stably integrated and transmitted to progeny or whether expression was extinguished (17). In the present study, to address these questions three experimental modifications were developed. First, donor testis cells containing stem cells for viral infection were cultured for 3 days, instead of 7 days as in the previous study. Under present culture conditions, stem cell number decreases ≈50% and 90% after 2 and 7 days, respectively (data not shown). Thus, using a 3-day rather than a 7-day culture period would be expected to increase the number of stem cells transplanted ≈4-fold. Transduction efficiency was increased about 12-fold in the experiments reported here compared with previous studies (17); therefore, other factors such as cell cycle kinetics of the stem cell in vitro must also contribute to enhanced retroviral infection of stem cells during the shorter culture period. Second, recipient mice were W pups rather than busulfan-treated wild-type adults as used previously, which increases donor-cell colonization of injected testes. Transplanted donor cells generate a greater number (9.4-fold) of colonies that are larger (4-fold) in pup than in adult W recipients, which results in a 35- to 40-fold increase in area of spermatogenesis (29). In part, the increase in colonization of pup compared with adult testes results from the absence of tight junctions between Sertoli cells before about 12 days postpartum (2). The barrier generated by formation of the Sertoli cell tight junctions likely inhibits stem cell migration from the lumen of the tubule to their niche on the basement membrane. Other factors, including testis growth activity, hormonal milieu, and growth factors, may also be superior in pup testes when stem cells are normally undergoing mitotic expansion (30). Third, the recipient W mice received no busulfan treatment, which was used to prepare wild-type recipients in previous experiments. Busulfan destroys endogenous germ cells but also may have deleterious effects on somatic supporting cells (31, 32). A particular advantage of W recipients is the absence of endogenous germ cells that can compete with transplanted cells (22, 23, 29). Neither pup nor adult retrovirus-infected donor stem cells colonized wild-type pup recipients. This result probably reflects both competition from endogenous germ cells in wild-type recipients as well as the low number of transduced donor stem cells that could generate a blue colony.
Retroviral vectors have been used often to transduce multiple cell types in vitro and many cells of the mouse in vivo (33). Indeed, they are the most common agent used for human somatic cell gene-therapy trials (34). However, in ES cells, expression of Moloney murine leukemia viral vectors is severely restricted. Moreover, although these cells may carry the vector, the block to expression is maintained in later stages of development (19, 20). Similarly, silencing of retrovirally transduced genes in long-term self-renewing hematopoietic stem cells is often seen (20, 35, 36). In addition, infection of postimplantation and postnatal germ cells has been extremely difficult. Germ-line transmission was not seen when retrovirus was injected into sheep embryos in utero (18), and was very low when postimplantation fetal stages of the mouse were exposed to retrovirus (37). Despite many studies with retroviruses, there are no reports that show direct infection or transduction of postnatal male germ-line stem cells (reviewed in ref. 6). Intraperitoneal injection of retrovirus in postnatal mice infects Leydig cells but not germ cells (9). Therefore, an explanation for the low rate of stem cell transduction (1 in 280) in our previous studies (17) was the existence of a block to expression in male germ-line stem cells as has been observed in ES cells and hematopoietic stem cells. However, the results of the present study indicate that ≈2.3% and 20% of adult cryptorchid and pup spermatogonial stem cells, respectively, can be transduced, which is more than 10-fold greater than reported (17). Despite a lower transduction efficiency for cryptorchid adult compared with pup cultured spermatogonial stem cells found in this and a previous study (17), the percent of transgenic pups was similar for adult (7.2%, Exp. 3) and pup (4.0%, Exps. 1 and 2) transplanted cells (Table 1). Further study will be needed to explain this difference, which may be related to the small number of observations for transgenic pup production from cryptorchid adult stem cells. Nonetheless, high transduction efficiencies can be obtained (20% for pup cells) and a high percent (4.5%) of transgenics obtained with retroviral vectors. These findings indicate that postnatal male germ-line stem cells can be transduced readily, and the vector can be integrated stably. Furthermore, expression of the retroviral-delivered transgene is not silenced as often occurs in ES cells and hematopoietic stem cells.
The development and maintenance of spermatogenesis from B6/129 F2 donor testis cells in two W recipients (5484 and 5486) with B6 genetic background indicate that spermatogonial stem cell transplantation should be feasible in other donor and recipient combinations with less than complete immunological compatibility. In previous studies, spermatogonial transplantation between random-bred Sprague–Dawley rats was successful, and donor cell-derived colonies persisted in recipient testes for at least 6 months (38). In addition, mouse donor cell-derived spermatogenesis persisted in Sprague–Dawley rat recipients with little immunosuppressive treatment for at least 3 months (38). Therefore, several experiments now indicate that some immunological incompatibility between donor and recipient can be overcome in spermatogonial stem cell transplantation. It has been suggested that the testis enjoys a level of immunological privilege because of Fas ligand expression and perhaps other unknown factors (39, 40). One might anticipate that allogeneic spermatogonial stem cell transplantation within a species will be successful between unrelated males with little or no immunosuppressive treatment, thus facilitating use of the technique for transgenesis.
Current methods to generate transgenic animals are based on female-derived cells, e.g., oocytes, fertilized eggs, and blastocysts (3, 41). Indeed, most transgenic mice result from injection of DNA into the pronucleus of fertilized eggs or the transfection of ES cells, which are subsequently transplanted to blastocysts. The efficiency of these two techniques in generating transgenic offspring and germ-line transmission of the foreign DNA is variable, but frequently in the range of 5–10% (41, 42). Moreover, the time required from the initial experiment to produce a transgenic animal for breeding and production of progeny is typically a minimum of 6 months. In the experiments reported here, 4.5% of the progeny from W recipient males produced progeny expressing the transgene in an average of 5.4 months after donor cell transplantation, thus providing a potentially competitive alternative to female-based transgenic methods.
Several approaches using male germ cells to produce transgenic mice have been reported. Perhaps the most well known and controversial is mixing a foreign gene with spermatozoa (43). Although success with this system has been claimed, results are controversial (44). However, when sperm heads are pretreated and then exposed to DNA followed by microinjection into the egg, transgenic mice result at levels slightly lower than direct injection of a gene into the pronucleus (45). In another approach, using techniques similar to stem cell transplantation, the foreign gene is injected into the seminiferous tubules, and the testis is electroporated (8). Sertoli cells are readily transfected, and a few small areas of germ cells expressing the gene can be identified. However, no transgenic progeny were produced. In a modification of this approach, using the green fluorescent protein (GFP) as a marker, the procedure is performed on young males when the tubules are lined with early germ-cell differentiation stages. Many cells become transfected, and a few spermatozoa that are fluorescent can be identified and injected into oocytes to produce young (46). By using this system, a total of 5 transgenic offspring resulted from ≈20 injected oocytes. Because spermatozoa were selected and injected into oocytes 1–2 weeks after electroporation, it is possible that the transgene was either integrated into the chromosomes of germ cells at late stages of differentiation or carried into the oocyte and subsequently integrated in the male pronucleus of the egg, as in the sperm head injection technique (described previously). Production of spermatozoa requires 35 days from differentiating type A spermatogonia in mice; therefore, it is unlikely that transgenic spermatozoa were derived from transfected stem cells.
Clearly, the male germ line offers several approaches for the introduction of foreign genes into a species, and transduction or transfection of spermatogonial stem cells is perhaps the most promising. The efficiency of the technique and time necessary to produce progeny are comparable to female-based systems, and improvements in the technique can be anticipated. First, higher viral titer preparations with enhanced infection rates or improved expression characteristics would be immediately beneficial. Second, procedures to enrich the stem cells in testis cell populations will increase colonization by transfected cells and will decrease the period from cell transplantation to production of offspring (29). Third, although stem cells can survive and proliferate in vitro (17, 47), significant expansion of stem cell number in culture would greatly improve the efficiency of transgenesis. Because male germ-line stem cells are present in the testes throughout postnatal life and methods are available to manipulate, cryopreserve, and transplant these cells in many mammalian species, the development of culture techniques will make the use of these stem cells a powerful approach to germ-line modification and improvement in a broad range of species.
Acknowledgments
We thank Drs. R. Behringer, H. Kubota, and E. Sandgren for critical evaluation of the manuscript and helpful comments; and P. Soriano for the retroviral producer cell line. We also thank O. Jacenko and M. Campbell for advice and help with DNA analysis. We appreciate the assistance of C. Freeman and R. Naroznowski with animal maintenance and experimentation; and J. Hayden for help with photography. Microscopic sections were produced in the Institute for Human Gene Therapy, Cellular Morphology Core, University of Pennsylvania (5-P30-DK-47747-07). Financial support for the research was from the National Institutes of Health National Institute of Child Health and Human Development Grant 36504; the Commonwealth and General Assembly of Pennsylvania; and by the Robert J. Kleberg, Jr., and Helen C. Kleberg Foundation.
Abbreviation
- ES
embryonic stem
References
- 1.Russell L D, Ettlin R A, SinhaHikim A P, Clegg E D. In: Histological and Histopathological Evaluation of the Testis. Russell L D, Ettlin R A, SinhaHikim A P, Clegg E D, editors. Clearwater, FL: Coche River Press; 1990. pp. 1–40. [Google Scholar]
- 2.de Kretser D M, Kerr J B. In: The Physiology of Reproduction. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 1177–1290. [Google Scholar]
- 3.Palmiter R D, Brinster R L. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capecchi M R. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- 5.Smithies O, Koller B H. In: Biology of Mammalian Germ Cell Mutagenesis. Allen J W, Bridges B A, Lyon M F, Moses M J, Russell L B, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. pp. 321–331. [Google Scholar]
- 6.Jaenisch R. In: Molecular Biology of RNA Tumor Viruses. Stephenson J R, editor. New York: Academic; 1980. pp. 131–162. [Google Scholar]
- 7.Kim J H, Jung-Ha H S, Lee H T, Chung K S. Mol Reprod Dev. 1997;46:515–526. doi: 10.1002/(SICI)1098-2795(199704)46:4<515::AID-MRD10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki Y, Fujimoto H, Ando H, Ohyama T, Hirota Y, Noce T. Biol Reprod. 1998;59:1439–1444. doi: 10.1095/biolreprod59.6.1439. [DOI] [PubMed] [Google Scholar]
- 9.Panthier J J, Gounon P, Condamine H, Jacob F. J Virol. 1989;63:2134–2142. doi: 10.1128/jvi.63.5.2134-2142.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muramatsu T, Shibata O, Ryoki S, Ohmori Y, Okumura J. Biochem Biophys Res Commun. 1997;233:45–49. doi: 10.1006/bbrc.1997.6361. [DOI] [PubMed] [Google Scholar]
- 11.Russell L D, Ren H P, Sinha Hikim I, Schulze W, Sinha Hikim A P. Am J Anat. 1990;188:21–30. doi: 10.1002/aja.1001880104. [DOI] [PubMed] [Google Scholar]
- 12.Wing T Y, Christensen A K. Am J Anat. 1982;165:13–25. doi: 10.1002/aja.1001650103. [DOI] [PubMed] [Google Scholar]
- 13.Avarbock M R, Brinster C J, Brinster R L. Nat Med. 1996;2:693–696. doi: 10.1038/nm0696-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobrinski I, Avarbock M R, Brinster R L. Mol Reprod Dev. 2000;57:270–279. doi: 10.1002/1098-2795(200011)57:3<270::AID-MRD9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 15.Brinster R L, Avarbock M R. Proc Natl Acad Sci USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinster R L, Zimmermann J W. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagano M, Shinohara T, Avarbock M R, Brinster R L. FEBS Lett. 2000;475:7–10. doi: 10.1016/s0014-5793(00)01606-9. [DOI] [PubMed] [Google Scholar]
- 18.Porada C D, Tran N, Eglitis M, Moen R C, Troutman L, Flake A W, Zhao Y, Anderson W F, Zanjani E D. Hum Gene Ther. 1998;9:1571–1585. doi: 10.1089/hum.1998.9.11-1571. [DOI] [PubMed] [Google Scholar]
- 19.Soriano P, Friedrich G, Lawinger P. J Virol. 1991;65:2314–2319. doi: 10.1128/jvi.65.5.2314-2319.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherry S R, Biniszkiewicz D, van Parijs L, Baltimore D, Jaenisch R. Mol Cell Biol. 2000;20:7419–7426. doi: 10.1128/mcb.20.20.7419-7426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinohara T, Avarbock M R, Brinster R L. Dev Biol. 2000;220:401–411. doi: 10.1006/dbio.2000.9655. [DOI] [PubMed] [Google Scholar]
- 22.Silvers W K. The Coat Colors of Mice. New York: Springer; 1979. pp. 206–241. [Google Scholar]
- 23.Ogawa T, Dobrinski I, Avarbock M R, Brinster R L. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa T, Aréghaga J M, Avarbock M R, Brinster R L. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 25.Suckow M A, Danneman P, Brayton C. The Laboratory Mouse. Boca Raton, FL: CRC; 2001. [Google Scholar]
- 26.Zambrowicz B P, Imamoto A, Fiering S, Herzenberg L A, Kerr W G, Soriano P. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagano M, Avarbock M R, Brinster R L. Biol Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobrinski I, Ogawa T, Avarbock M R, Brinster R L. Mol Reprod Dev. 1999;53:142–148. doi: 10.1002/(SICI)1098-2795(199906)53:2<142::AID-MRD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.Shinohara T, Orwig K E, Avarbock M R, Brinster R L. Proc Natl Acad Sci USA. 2001;98:6186–6191. doi: 10.1073/pnas.111158198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orth J M. In: Cell and Molecular Biology of the Testis. Desjardins C, Ewing L L, editors. New York: Oxford Univ. Press; 1993. pp. 3–42. [Google Scholar]
- 31.Boujrad N, Hochereau-de Reviers M T, Carreau S. Biol Reprod. 1995;53:1345–1352. doi: 10.1095/biolreprod53.6.1345. [DOI] [PubMed] [Google Scholar]
- 32.Meistrich M L. APMIS. 1998;106:37–45. doi: 10.1111/j.1699-0463.1998.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 33.Miller A D. Curr Top Microbiol Immunol. 1992;158:1–24. doi: 10.1007/978-3-642-75608-5_1. [DOI] [PubMed] [Google Scholar]
- 34.Raponi M, Szpirer C. In: Ex Vivo Cell Therapy. Schindhelm K, Nordon R, editors. London: Academic; 1999. pp. 293–322. [Google Scholar]
- 35.Kalberer C P, Pawliuk R, Imren S, Bachelot T, Takekoshi K J, Fabry M, Eaves C J, London I M, Humphries R K, Leboulch P. Proc Natl Acad Sci USA. 2000;97:5411–5415. doi: 10.1073/pnas.100082597. . (First Published May 2, 2000; 10.1073/pnas.100082597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klug C A, Cheshier S, Weissman I L. Blood. 2000;96:894–901. [PubMed] [Google Scholar]
- 37.Soriano P, Gridley T, Jaenisch R. Genes Dev. 1987;1:366–375. doi: 10.1101/gad.1.4.366. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa T, Dobrinski I, Brinster R L. Tissue Cell. 1999;31:461–472. doi: 10.1054/tice.1999.0060. [DOI] [PubMed] [Google Scholar]
- 39.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke R C. Nature (London) 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 40.D'Alessio A, Riccioli A, Lauretti P, Padula F, Muciaccia B, De Cesaris P, Filippini A, Nagata S, Ziparo E. Proc Natl Acad Sci USA. 2001;98:3316–3321. doi: 10.1073/pnas.051566098. . (First Published February 27, 2001; 10.1073/pnas.051566098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood S A, Allen N D, Rossant J, Auerbach A, Nagy A. Nature (London) 1993;365:87–89. doi: 10.1038/365087a0. [DOI] [PubMed] [Google Scholar]
- 42.Brinster R L, Chen H Y, Trumbauer M E, Yagle M K, Palmiter R D. Proc Natl Acad Sci USA. 1985;82:4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavitrano M, Camaioni A, Fazio V M, Dolci S, Farace M G, Spadafora C. Cell. 1989;57:717–723. doi: 10.1016/0092-8674(89)90787-3. [DOI] [PubMed] [Google Scholar]
- 44.Brinster R L, Sandgren E P, Behringer R R, Palmiter R D. Cell. 1989;59:239–241. doi: 10.1016/0092-8674(89)90282-1. [DOI] [PubMed] [Google Scholar]
- 45.Perry A C, Wakayama T, Kishikawa H, Kasai T, Okabe M, Toyoda Y, Yanagimachi R. Science. 1999;284:1180–1183. doi: 10.1126/science.284.5417.1180. [DOI] [PubMed] [Google Scholar]
- 46.Huang Z, Tamura M, Sakurai T, Chuma S, Saito T, Nakatsuji N. FEBS Lett. 2000;487:248–251. doi: 10.1016/s0014-5793(00)02271-7. [DOI] [PubMed] [Google Scholar]
- 47.Nagano M, Avarbock M R, Leonida E B, Brinster C J, Brinster R L. Tissue Cell. 1998;30:389–397. doi: 10.1016/s0040-8166(98)80053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]