Abstract
Inhalation therapy using small-interfering RNA (siRNA) is a potentially effective therapeutic strategy for lung cancer because of its high gene-silencing effects and sequence specificity. Previous studies reported that intratracheal administration of siRNA using pressurized metered dose inhalers or nebulizers could suppress tumor growth in murine lung metastatic models. Although dry powder inhalers are promising devices due to their low cost, good portability, and preservability, the anti-tumor effects of siRNA dry powder have not been elucidated. To evaluate the gene-silencing and anti-tumor effects of intratracheally delivered siRNA dry powder, vascular endothelial growth factor-specific siRNA (VEGF-siRNA) dry powder was administered intratracheally to mice with metastatic lung tumors consisting of B16F10 melanoma cells or Lewis lung carcinoma cells. A single intratracheal administration of VEGF-siRNA dry powder reduced VEGF levels in both bronchoalveolar lavage fluid and lung tumor tissue. Furthermore, repeated intratracheal administration of VEGF-siRNA dry powder suppressed the number of visible metastatic foci on the lung surface and tumor area in lung tissues. Taken together, intratracheal administration of siRNA dry powder could be a novel therapeutic strategy for lung cancer through the suppression of specific genes expressed in lung tumor tissue.
Keywords: siRNA, dry powder, intratracheal administration, lung cancer, vascular endothelial growth factor
Introduction
RNA interference using small interfering RNA (siRNA) is a mechanism of silencing gene expression that results in sequence-specific mRNA degradation.1 Because of its high gene-silencing effect, sequence specificity, and adjustability for targeting any disease-promoting gene, siRNA could apply to the treatment of intractable diseases, including cancer. For the clinical application of siRNA, an efficient delivery method to target cells is essential. With direct accessibility through the airways, the lung is an attractive target organ for siRNA delivery using inhalation systems.
Three types of inhalation systems are available: pressurized metered dose inhalers (MDIs), nebulizers, and dry powder inhalers (DPIs). Although MDIs are commonly used inhalers, the required propellants, such as chlorofluorocarbons or hydrofluoroalkanes, are unfavorable to the environment.2, 3 Nebulizers are also commonly used; however, they are not suitable for long-term storage, as nucleic acids are unstable during the process of liquid formation.4 On the contrary, DPIs present several advantages, as they do not require propellants and have good preservability, low cost, and good portability.5, 6 Thus, DPIs are the most suitable type of inhalation device for siRNA delivery. In our previous study, we demonstrated that intratracheal administration of siRNA dry powder with water-soluble chitosan as a non-viral vector produced by the supercritical carbon dioxide technique could suppress gene expression, not only in airways including the bronchus, bronchiole, and alveolus, but also inside lung tumors.7 This result indicated that intratracheal administration of siRNA could have therapeutic potential for lung cancer through the suppression of specific genes expressed in lung tumors. Several previous studies reported that intratracheal administration of RNA interference agents exerted an anti-tumor effect in murine lung metastasis models when administered using MDIs or nebulizers.8, 9, 10 However, the anti-tumor effect of intratracheal administration of siRNA dry powder on lung tumors is not known.
Spray-freeze-drying (SFD) is a novel powderization technique used to produce a highly porous low-density powder, with high dispersibility and reachability to the lungs.11 Mohri et al.12 reported that intratracheal administration of plasmid DNA dry powder produced by SFD could reach to intrapulmonary region and exhibit gene expression in the lungs. However, the gene-silencing effect of intratracheal administration of siRNA dry powder produced by SFD has not been elucidated.
Vascular endothelial growth factor (VEGF) stimulates neovascularization and strongly contributes to cancer progression. Inhibiting neovascularization by bevacizumab, a humanized monoclonal antibody against VEGF, has shown clinical effectiveness in various cancers, including lung cancer.13, 14, 15 Moreover, a previous study reported that local injection of VEGF-specific-siRNA (VEGF-siRNA) could inhibit tumor growth in a murine subcutaneous tumor model.16 Thus, pulmonary administration of VEGF-siRNA could be an effective therapeutic strategy to treat lung cancer when efficiently delivered to lung tumors.
The objective of this study was to investigate the gene-specific suppressive effects of the intratracheal administration of a novel VEGF-siRNA and chitosan dry powder produced by SFD on lung tumors and assess its inhibitory effect on tumor growth in a murine lung metastasis model.
Results
Effect of VEGF Gene-Specific Knockdown by VEGF-siRNA In Vitro
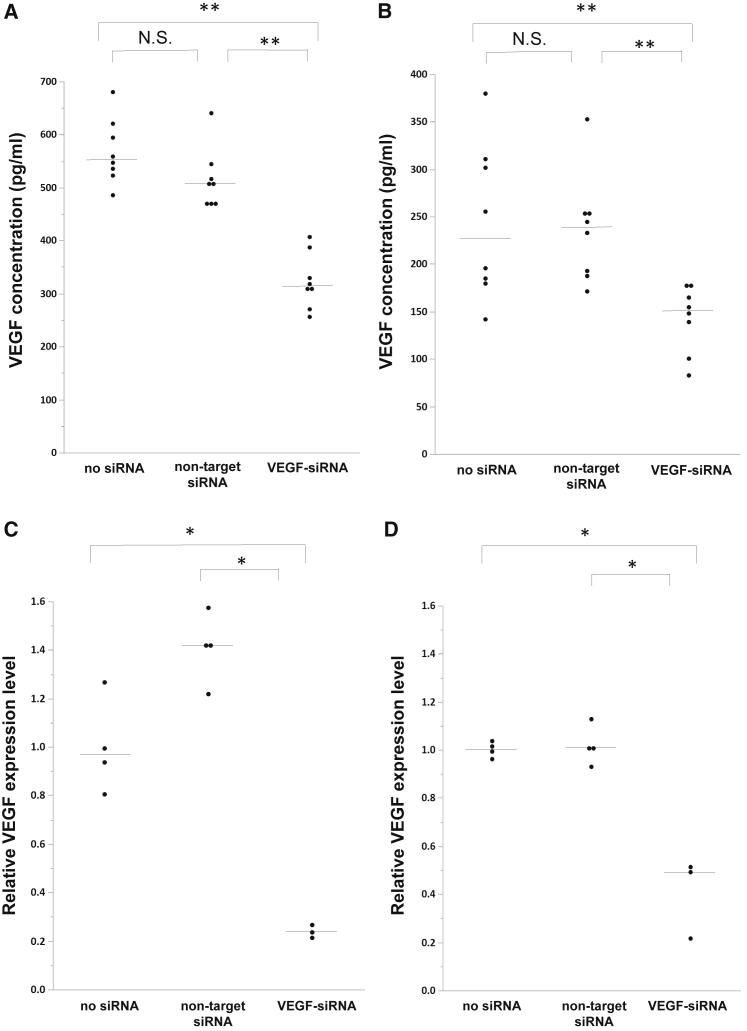
To confirm the gene-specific suppression of VEGF by VEGF-siRNA, VEGF-siRNA was transfected in two cancer cell lines (B16F10 and Lewis lung carcinoma [LLC]). In both cell lines, total VEGF protein in culture supernatant fluid and relative VEGF mRNA expression levels in the cells obtained from VEGF-siRNA-treated cells were significantly decreased compared with those obtained from cells treated with non-target siRNA or transfection reagent alone (Figures 1A–1D).
Figure 1.
Evaluation of Gene-Silencing Effect of VEGF-siRNA In Vitro
(A and C) B16F10 cells and (B and D) LLC cells were treated with VEGF-siRNA, non-target siRNA, or transfection reagent alone. (A and B) Concentrations of VEGF protein in culture supernatant fluid obtained from B16F10 (A) or LLC (B) cells were measured by enzyme-linked immunosorbent assay (n = 8/group). (C and D) VEGF-A mRNA levels in the cells obtained from B16F10 (C) or LLC (D) cells normalized to beta-actin levels were quantified by real-time RT-PCR (n = 3–4/group). p was determined using the Wilcoxon rank-sum test. *p < 0.05, **p < 0.01 between two groups. N.S., not significant. Bars denote the median values.
Suppression of VEGF Expression in Lung Tumor with a Single Intratracheal Administration of VEGF-siRNA and Chitosan Dry Powder
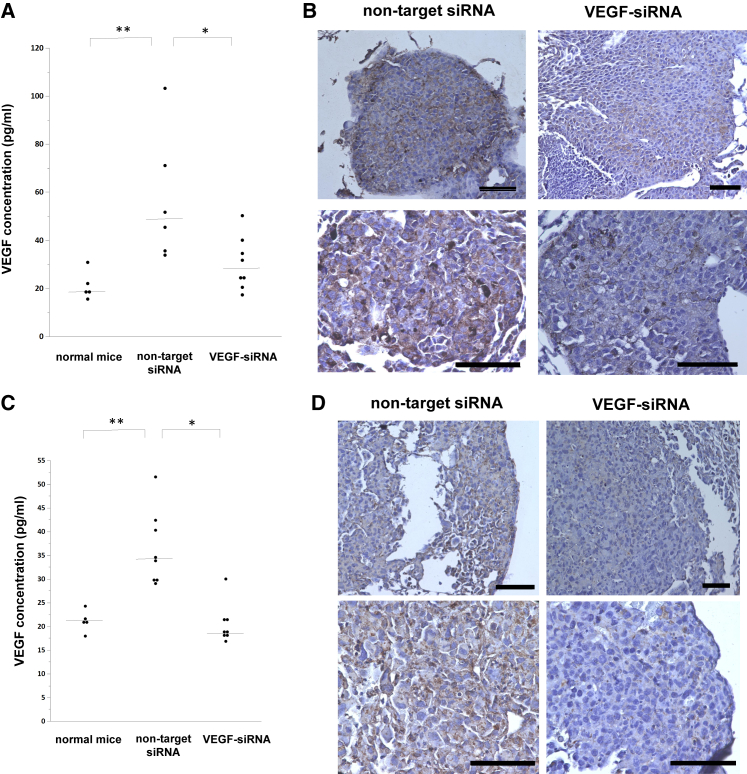
Next, we examined whether intratracheal administration of VEGF-siRNA dry powder could reduce VEGF expression in lung tumor tissue. We used water-soluble chitosan as a non-viral vector. As a preliminary experiment, we monitored VEGF protein levels in bronchoalveolar lavage fluid (BALF) obtained from tumor-bearing mice; the VEGF amount in BALF was significantly increased 18 days after B16F10 injection and 14 days after LLC injection. In tumor-bearing mice treated with VEGF-siRNA, the VEGF amount in BALF was significantly decreased compared with that of tumor-bearing mice treated with non-target siRNA and resembled that of non-tumor-bearing mice (Figures 2A and 2C). Furthermore, immunohistochemical staining of excised lung tumor sections using an anti-VEGF antibody confirmed the suppression of VEGF in lung tumor tissue obtained from tumor-bearing mice treated with VEGF-siRNA (Figures 2B and 2D). These results revealed that even a single intratracheal administration of VEGF-siRNA dry powder sufficiently suppressed VEGF expression in lung tumors.
Figure 2.
Evaluation of Gene-Silencing Effect of VEGF-siRNA Dry Power In Vivo
(A and B) B16F10 tumor-bearing mice (n = 6–8/group) or (C and D) LLC tumor-bearing mice (n = 8/group) were treated with a single administration of VEGF-siRNA dry powder or non-target siRNA dry powder. After 48 hr, bronchoalveolar lavage fluid samples were obtained from all subjects, and lungs were harvested. Same-aged, non-tumor-bearing mice served as controls (n = 5). (A and C) Concentrations of VEGF protein in bronchoalveolar lavage fluid obtained from B16F10 (A) or LLC (C) tumor-bearing mice were measured by ELISA. Bars denote the median values. (B and D) Representative images of immunohistochemical stainings of VEGF in lung tumors obtained from B16F10 (B) or LLC (D) tumor-bearing mice. Scale bar, 100 μm. p was determined using the Wilcoxon rank-sum test. *p < 0.05, **p < 0.01 between two groups.
Repeated Intratracheal Administration of VEGF-siRNA and Chitosan Dry Powder Inhibited Tumor Growth in the Lung
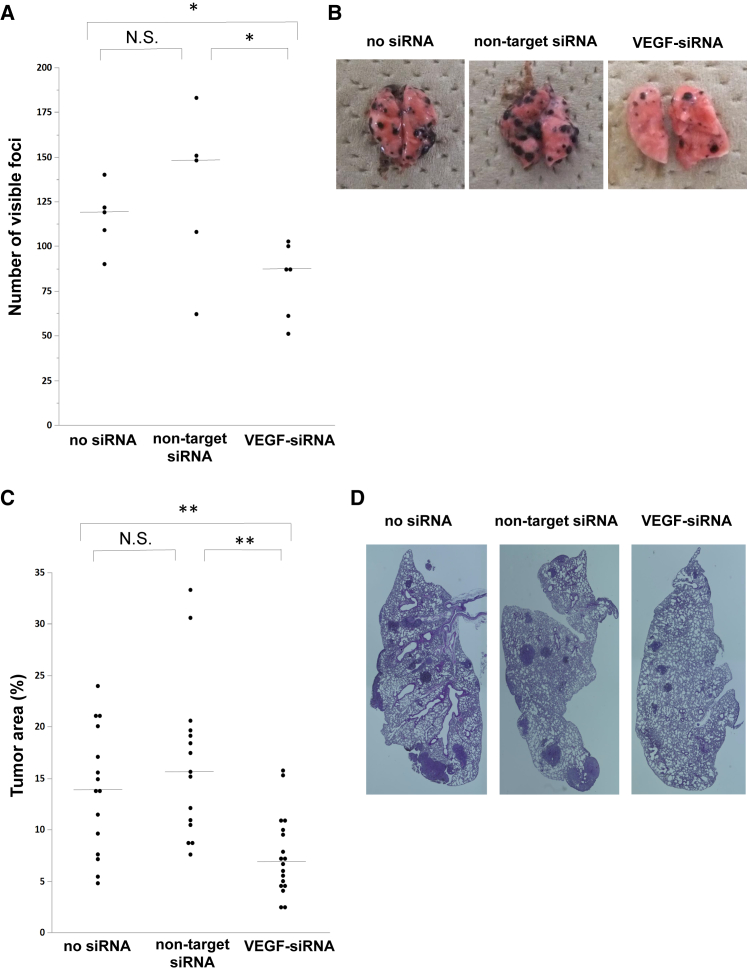
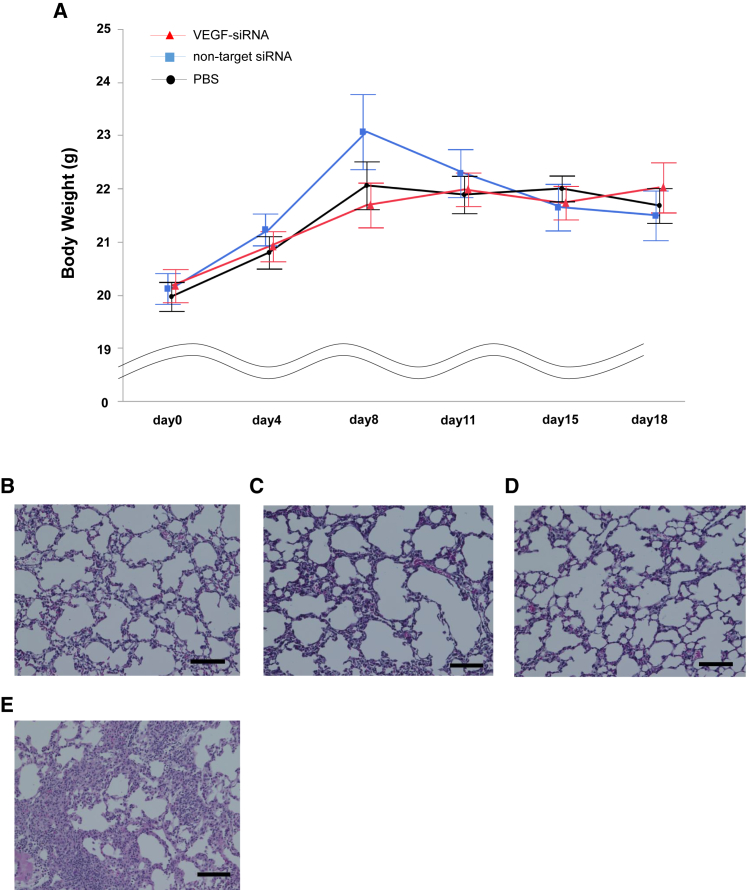
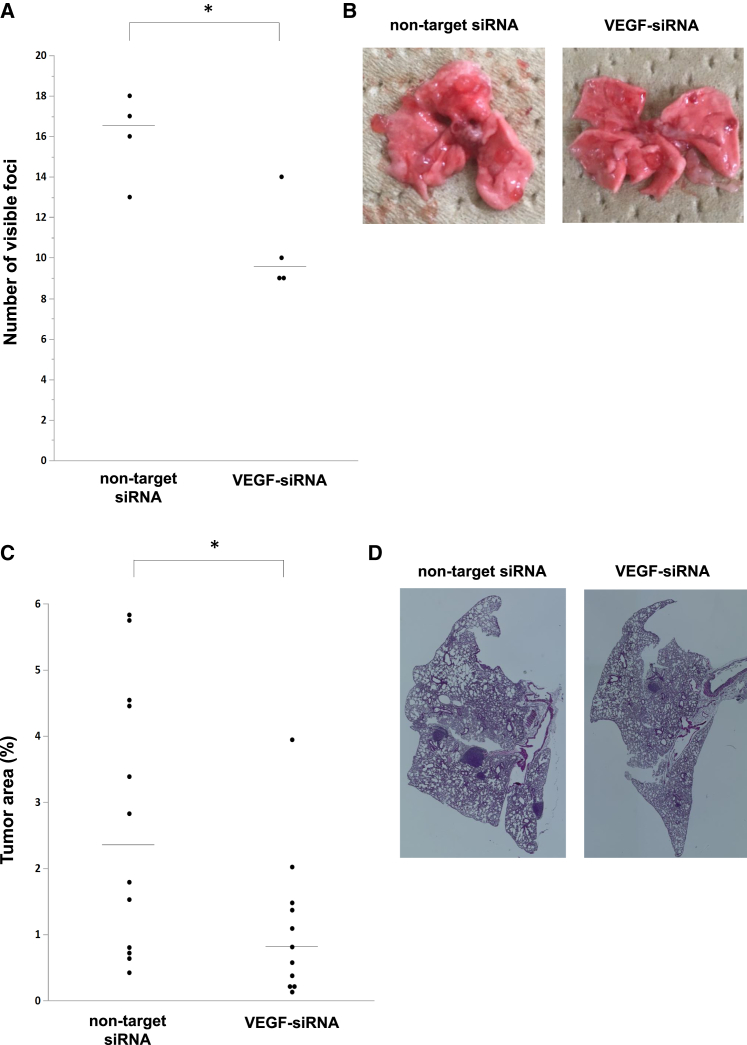
On the basis of the knockdown effect observed in lung tumor tissue with a single intratracheal administration of VEGF-siRNA, we examined whether repeated intratracheal administration of VEGF-siRNA dry powder could inhibit tumor growth in the lung. First, we examined the anti-tumor effect in mice carrying metastatic lung tumors consisting of B16F10 melanoma cells. As a preliminary experiment, we monitored visible tumors on the lung surface in tumor-bearing mice. Lung tumors were visible after 4 days and reached an evaluable tumor burden 18 days after B16F10 injection. In the VEGF-siRNA group, the number of visible foci was significantly reduced compared with that in the non-target-siRNA group or no siRNA group (Figures 3A and 3B). Moreover, the percentage of lung tumor area in the VEGF-siRNA group was also significantly reduced compared with that in the non-target-siRNA group or no siRNA group in histological examinations (Figures 3C and 3D). Additionally, there was no remarkable toxicity, such as body weight loss (Figure 4A) or inflammatory change in lung sections from mice treated with VEGF-siRNA (Figures 4B–4E).
Figure 3.
Anti-tumor Effect of VEGF-siRNA Dry Powder in B16F10 Lung Metastasis Model
Mice with lung metastases were treated with VEGF-siRNA dry powder (n = 6), non-target siRNA dry powder (n = 5), or PBS (n = 5) by intratracheal administration on days 4 and 11. (A and B) The number of tumor nodules on the lung surface was counted 18 days after injection of B16F10 cells. Bars denote the median values. (C and D) H&E-stained sections from each mouse treated with VEGF-siRNA, non-target siRNA, or PBS were randomly selected, and the ratio of tumor/whole-lung tissue area (%) was calculated using an image-analyzing system. p was determined using the Wilcoxon rank-sum test. *p < 0.05, **p < 0.01 between two groups. N.S., not significant.
Figure 4.
Toxicity of Intratracheal Administration of siRNA Dry Powder
(A) Body-weight fluctuation in mice with lung metastases treated with VEGF-siRNA dry powder, non-target siRNA dry powder, or PBS (mean ± SE). There was no significant difference in body weight between groups during the time course (n = 5–6/group). (B–E) Representative histological appearance of H&E-stained lung sections from mice treated with VEGF-siRNA (B), non-target siRNA (C), PBS (D), and bleomycin (E). The bleomycin group (intratracheal injection of 2.5 mg/kg body weight) served as a positive control. Scale bar, 100 μm.
Next, we examined the anti-tumor effect in mice carrying metastatic lung tumors consisting of LLC cells. According to a preliminary experiment, similar to B16F10 melanoma cells, tumors were visible on the lung surface 4 days after injection. However, some mice died on day 18 due to rapid tumor progression. Therefore, we optimized the time course as described in the Materials and Methods section. The number of visible foci and lung tumor area were significantly reduced compared with that in the non-target siRNA group (Figures 5A–5D).
Figure 5.
Anti-tumor Effect of VEGF-siRNA Dry Powder in LLC Lung Metastasis Model
Mice with lung metastases were treated with an intratracheal administration of VEGF-siRNA dry powder or non-target siRNA on days 4, 8, and 11 (n = 4/group). (A and B) The number of tumor nodules on the lung surface was counted 14 days after injection of LLC cells. Bars denote the median values. (C and D) H&E-stained sections from each mouse treated with VEGF-siRNA or non-target siRNA were randomly selected; the ratio of tumor/whole-lung tissue area (%) was calculated using an image analyzing system. p was determined using the Wilcoxon rank-sum test. *p < 0.05 between two groups.
In Vitro Inhalation Characteristics of VEGF-siRNA and Chitosan Dry Powders
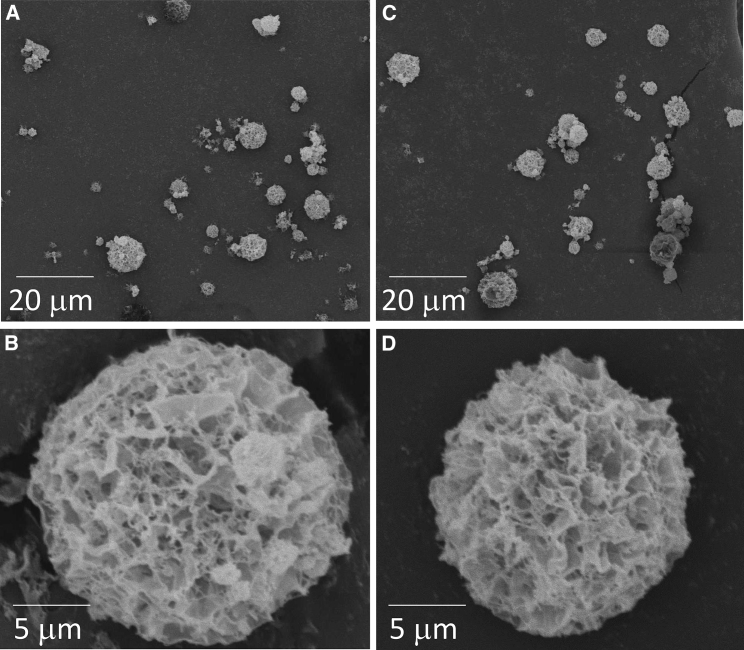
Figure 6 shows the morphology of particles prepared using the SFD technique. Both powder particles with VEGF-siRNA and non-target siRNA had a geometric diameter of around 10 μm. Because of their high porosity, these powders were expected to have low particle densities and small aerodynamic diameters suitable for inhalation. Indeed, the powders taken in a disposable tip were dispersed easily by releasing air compressed in a syringe, suggesting that they are suitable for use as inhalable formulations.
Figure 6.
Scanning Electron Micrographs of Spray-Freeze-Dried Powders
(A and B) Powders with VEGF-siRNA and (C and D) powders with non-target siRNA.
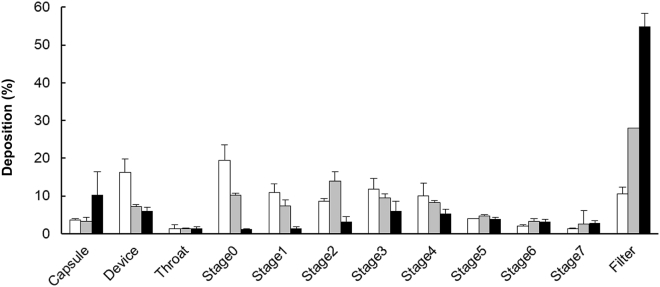
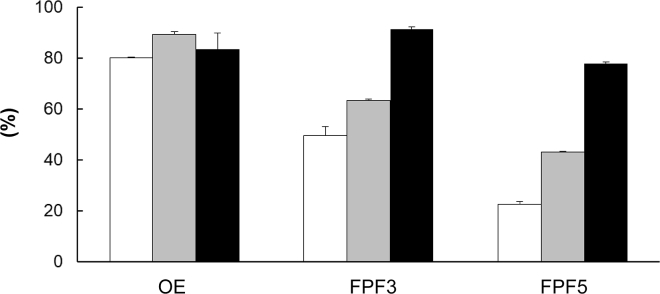
To evaluate the in vitro inhalation performance of the VEGF-siRNA and chitosan dry powder, the Anderson cascade impactor was used (Figure 7). The powder reached the lower stages depending on the pressure drop of the device, suggesting that the powder particles were small enough to reach the lower airway, including alveoli. Next, we calculated the output efficiency (OE) and fine particle fraction (FPF). As shown in Figure 8, OE exceeded 80% regardless of the device. FPF3 and FPF5 increased with increased pressure drop in the device. Even with Jethaler Single, FPF3 reached 50%, suggesting that the powder could be inhaled by patients with weak inhalation capacity.
Figure 7.
In Vitro Deposition Patterns of VEGF-siRNA and Chitosan Dry Powders
The powder was put in Jethaler single (white square), dual (gray square), and reverse (black square), and inhalation performance was evaluated as described in the Materials and Methods section. According to the cut-off diameters in each stage, stages 1 and 2 were classified as upper airway, stages 3 and 4 were classified as central airway, and stages 5–7 were classified as lower airway. Mean ± SD (n = 3).
Figure 8.
Output Efficiency and Fine Particle Fractions of VEGF-siRNA and Chitosan Dry Powders
The powder was placed in Jethaler single (white square), dual (gray square), and reverse (black square), and inhalation efficiency was determined as described in the Materials and Methods section. Mean ± SD (n = 3).
Discussion
In the present study, we demonstrated that a single intratracheal administration of a novel VEGF-siRNA and chitosan dry powder produced by SFD reduced VEGF in both BALF and lung tumor tissue in murine lung metastasis models. Moreover, repeated intratracheal administration of VEGF-siRNA dry powder reduced the number of visible foci on the lung surfaces and tumor area. Additionally, the powder particles were shown to have inhalable characteristics in vitro with regard to both their particle size and inhalation efficacy.
The most notable finding in this study was that even a single intratracheal administration of VEGF-siRNA and chitosan dry powder produced by SFD suppressed gene expression in lung tumor tissue. This effect was confirmed by the result that VEGF in BALF was reduced to a similar level as that of mice without lung tumors. SFD is a novel powderization technique used to produce a highly porous low-density powder, which is ideal for nucleic acids because of its good dispersibility.11, 17, 18, 19 The powders produced by SFD are considered more suitable for inhalation compared with those produced by conventional techniques. We previously demonstrated that intratracheal administration of EGFP-siRNA and chitosan dry powder produced by the supercritical CO2 technique reduced EGFP expression not only in the airways, but also inside metastatic lung tumor tissue.7 However, the effect of intratracheal administration of siRNA dry powders produced by SFD had not been elucidated. In the present study, we demonstrated that a single intratracheal administration of a novel siRNA dry powder produced by SFD could reduce gene expression in metastatic lung tumors.
We also clarified that repeated intratracheal administration of VEGF-siRNA and chitosan dry powder inhibited tumor growth in the lung. Several previous studies also showed an anti-tumor effect of intratracheal administration of RNA interference agents in murine lung metastasis models. However, these RNA interference agents were inhaled using MDIs or nebulizers.8, 9, 10, 20 While DPIs show several benefits compared with MDIs or nebulizers, little is known about intratracheal administration of nucleic acids inhaled using DPIs.12, 21, 22 The present study is the first that demonstrates a therapeutic effect of inhaled siRNA dry powder using disease models.
Because nucleic acids are easily degraded in vivo, an appropriate vector is required for their effective delivery to targeted cells. Viral vectors,23, 24 lipid vectors,25, 26 and cationic polymers27, 28 have been reported as appropriate vectors for inhalable siRNA. Among them, we focused on water-soluble chitosan because of its low toxicity. Indeed, no remarkable toxicity such as body weight loss or histological changes in the lungs were observed. Thus, we propose that chitosan is an ideal vector for siRNA delivery.
The VEGF-siRNA and chitosan dry powder is highly porous, a unique property among SFD powders. Its in vitro inhalation performance was very high even with Jethaler Single, which has the lowest pressure drop, suggesting that the powder is suitable for inhalation. We did not determine the mass median aerodynamic diameter (MMAD) of the powder because the log-probability plot, which is commonly applied to determine MMAD of inhalable powders, yielded a curve instead of a straight line (data not shown). We consider that some of the VEGF-siRNA and chitosan dry powder particles were broken into tiny fragments in the devices by the air turbulence, which would improve particle delivery deep into the lungs.
Our study has certain limitations. First, the detailed mechanism by which the siRNA dry powder reached the inside of lung tumors has not been determined. We speculate that there could be pathways operating between lung tumors and the airways. Another potential explanation is that intratracheal administration of siRNA dry powder could reduce gene expression not only in lung tumor, but also in other cell types, including bronchial and alveolar epithelial cells.7 Thus, VEGF-siRNA may indirectly suppress both intratumor VEGF expression and tumor growth by suppressing VEGF gene expression in other surrounding airway cells. Second, though we showed a therapeutic effect of intratracheal administration of siRNA dry powder in murine lung metastasis models, the effect of this system on primary lung cancer is unknown. Finally, because the drug deposition fractions in the upper airway are higher with DPIs than with MDIs in humans,29 higher doses of dry powders may be required to translate the present murine findings into human clinical practice. In addition, sufficient lung function will be necessary to enable inhalation of dry powder in humans; thus, patients with impaired lung function may not be able to use this device.
In summary, we demonstrated that a single intratracheal administration of a novel VEGF-siRNA and chitosan dry powder produced by SFD suppressed gene expression in lung tumors. Moreover, the repeated administration of the dry powder inhibited tumor growth in the lung. Intratracheal administration of siRNA and chitosan dry powder could be a novel therapeutic strategy for lung cancer due to its sequence specificity and high gene-silencing effect.
Materials and Methods
Each experiment was performed two or three times. Reproducible and representative data are shown as results.
Mice and Materials
Female C57BL/6 mice (8 to10 weeks old) were purchased from Japan SLC (Hamamatsu, Japan). Animals were maintained according to guidelines for the ethical use of animals in research at Hiroshima University. VEGF-siRNA (sense strand, 5′-CGAUGAAGCCCUGGAGUGCTT-3′; antisense strand, 5′-GCACUCCAGGGCUUCAUCGTT-3′) and non-target siRNA (sense strand, 5′-UACUAUUCGACACGCGAAGTT-3′; antisense strand, 5′-CUUCGCGUGUCGAAUAGUATT-3′) were purchased from Hokkaido System Science (Sapporo, Japan). Chitosan (molecular weight [MW] 2,000–5,000, water-soluble; Wako Pure Chemical Industries, Osaka, Japan), D-(-)-mannitol (Wako Pure Chemical Industries) and L-leucine (Sigma-Aldrich, St. Louis, MO, USA) were used as a non-viral vector and excipient, respectively.
Cell Line
B16F10 melanoma cells and LLC cells were purchased from ATCC (Manassas, VA, USA). These cells were cultured in DMEM (Gibco, Grand Island, NY, USA) containing 10% fetal calf serum and 1% penicillin and streptomycin. All cells were cultured at 37°C in a humidified atmosphere with 5% CO2.
Preparation of siRNA and Chitosan Dry Powder using SFD
A schematic diagram of the SFD technique is shown in Figure S1. A siRNA and chitosan dry powder was prepared as previously described.12 A total quantity of 66.5 mg of materials (composition ratio, siRNA 2%, chitosan 10%, mannitol 22%, L-leucine 66%) was dissolved in 5.0 mL of ultra-pure water. The solution was delivered at a rate of 5 mL/min and atomized at an air pressure of 150 kPa using a two-fluid nozzle for a spray dryer (SD-1000, Tokyo Rikakikai, Tokyo, Japan) placed approximately 10 cm above liquid nitrogen. Following atomization, the liquid nitrogen, including frozen droplets, was transferred to a freeze dryer (FDU-2100/DRC-1000; Tokyo Rikakikai) and pre-cooled at a shelf temperature of −40°C. After evaporation of the liquid nitrogen, frozen droplets were dried at a pressure of less than 2 Pa, while the shelf temperature was gradually increased from −40°C to 10°C over a period of 24 hr.
Intratracheal Administration of siRNA and Chitosan Dry Powder
Intratracheal administration of siRNA and chitosan dry powder was conducted as previously described.7 In brief, a 22G catheter was connected to a disposable yellow tip, and 0.5 mg siRNA and chitosan powder or 10 μL PBS was placed into the yellow tip (Figure S2A). Next, a three-way stopcock and 1 mL syringe containing 0.3 mL of air was connected to the yellow tip. Mice were anesthetized and orally intubated with the 22-gauge catheter connected to the yellow tip, three-way stopcock, and 1 mL syringe. By pushing the 1-mL syringe and opening the three-way stopcock, dry powder was dispersed into the trachea (Figures S2B and S2C). To confirm no visible powder remained in the tip, air was instilled into the connected yellow tip several times.
Verification of Knockdown Effect by VEGF-siRNA In Vitro
B16F10 cells or LLC cells (0.3 × 105/well) were plated on 24-well plates; 24 hr after plating, B16F10 cells or LLC cells were transfected with VEGF-siRNA (10 nM), non-target siRNA (10 nM), or transfection reagent alone. A lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA, USA) was used as a transfection reagent in each group. The cell culture supernatant fluid and the cells were collected 48 hr after transfection.
BALF Analysis
B16F10 cells (2.0 × 105) or LLC (3.0 × 105) cells were injected intravenously into mice through the tail vein. The siRNA dry powder was intratracheally administered 16 days (B16F10) or 12 days (LLC) after cell injection. Two days after administration, a bronchoalveolar lavage was performed using 1.5 mL PBS, and BALF was obtained from each mouse.
Quantification of VEGF Protein
Total VEGF protein in culture supernatant fluid or BALF was measured using a Mouse VEGF Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions.
Real-Time qPCR
Total RNA was isolated from cultured cells using an RNeasy Mini Kit (QIAGEN, Hilden, Germany). The reverse transcription reaction was performed using a High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions. Real-time qPCR was performed using a 7500 Fast Real-Time PCR system (Applied Biosystems) for evaluation of mouse VEGF-A expression (Applied Biosystems; assay ID, Mm00437306_m1), with beta-actin (Applied Biosystems; assay ID, Mm02619580_g1) used as a control housekeeping gene.
Evaluation of Anti-tumor Effect In Vivo
B16F10 cells (2.0 × 105) or LLC (3.0 × 105) cells were injected intravenously into mice through the tail vein. VEGF-siRNA or non-target-siRNA were intratracheally administered on days 4 and 11 (B16F10) or on days 4, 8, and 11 (LLC). Mice were sacrificed 18 days (B16F10) or 14 days (LLC) after injection, and the number of visible foci on the lung surface was manually counted.
Lung Histology
Mouse lung tissues were fixed in 10% formalin and embedded in paraffin. After sectioning 5-μm-thick slices, tissue was stained with H&E or used for immunohistochemical staining. To estimate the tumor burden, three randomly selected H&E sections were used to calculate tumor area using an image analyzing system (BZ Analyzer 2, Keyence, Osaka, Japan).
Immunohistochemistry of VEGF-A
Mouse lung sections were incubated with rabbit polyclonal antibody against mouse VEGF-A (Abcam, Cambridge, UK) at 4°C overnight. The immunoreaction was amplified using a VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA, USA) and visualized after incubation with a 3,3-diaminobenzidine solution acting as a chromogen. Sections were then counterstained with hematoxylin and dehydrated.
Morphological Analysis of the SFD Powders by Scanning Electron Microscopy
Small amounts of powder samples were placed in a disposable tip connected to a 1-mL disposable syringe with a three-way stopcock. The powders were manually dispersed on a specimen mount with double-sided tape by opening the three-way stopcock to release air compressed in the syringe and coated with platinum by a sputter coater (JFC-1600, JOEL, Tokyo, Japan). The morphology of the dry powders was examined by scanning electron microscopy (JSM-6060, JEOL).
In Vitro Inhalation Characteristics of VEGF-siRNA and Chitosan Dry Powders
The in vitro inhalation characteristics of the prepared dry powders were evaluated using an eight-stage Andersen cascade impactor (AN-200; SIBATA SCIENTIFIC TECHNOLOGY, Saitama, Japan). The cut-off diameters in stages 1 to 7 and filter were 11, 7.0, 4.7, 3.3, 2.1, 1.1, 0.65, and 0.43 μm, respectively. We prepared VEGF-siRNA and chitosan dry powder with 1% sodium fluorescein (FlNa) to quantify the amount of the powder recovered at each stage. The shape of the particles observed by scanning electron microscopy was not affected by the addition of FlNa. To prevent the dry powder from re-bounding off the plates and re-entering the air stream, the metal plates for the stages were coated with a thin layer of glycerin. After setting a no. 2 hydroxypropyl methylcellulose (HPMC) hard capsule (Shionogi Qualicaps Nara, Japan) containing 1 mg of the dry powder in an inhaler (Jethaler Single, Dual, and Reverse; Hitachi Automotive Systems, Isesaki, Japan), inspiration at a flow rate of 28.3 L/min and for a flow time of 5 s was carried out using a vacuum pump. The pressure drops at a constant flow rate (28.3 L/min) of the devices were set at 1.0, 5.1, and 8.7 kPa, and the peak flow rates that normal subjects could achieve with the devices were 34–115, 15–52, and 12–40 L/min, respectively.30 After inspiration, the dry powder deposited in each part (capsule, device, throat, stages, and filter) was washed out using 10 mL of PBS, and the concentration of FlNa was determined using a fluorescence microplate reader (EnSpire; PerkinElmer Japan, Yokohama, Japan).
OE, FPF3, and FPF5 were calculated by the following equations:
| (Equation 1) |
| (Equation 2) |
| (Equation 3) |
OE is the emission potential from the capsule and device, while FPF3 and FPF5 refer to the potential for delivery into the lung and deep lung, respectively.
Statistical Data Analysis
Statistical analyses were performed using the Wilcoxon rank-sum test. p values less than 0.05 were considered statistically significant. Analyses were performed using JMP pro 12 software (SAS Institute, Cary, NC, USA).
Author Contributions
K.M. participated in conception and design, development of methodology, collection of data, data analysis and interpretation, and manuscript writing. H.O. participated in conception and design, material support, development of methodology, collection of data, data analysis and interpretation, and manuscript writing. T.N. participated in conception and design, data analysis and interpretation, and revision of the manuscript. D.I. participated in conception and design and development of methodology. Y.H., T.M., S.M., H.I., K.F., and H.H. participated in data analysis and interpretation. A.S., T.I., and T.O. participated in collection of data and data analysis and interpretation. N.H. participated in conception and design, financial support, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Conflicts of Interest
There are no conflicts of interest to declare.
Acknowledgments
This work was supported by the Program of the Network-type Joint Usage/Research Center for Radiation Disaster Medical Science. This work was partially supported by JSPS KAKENHI (grant number JP 22390165) and by a grant from the Ryokufukai. We thank Yukari Iyanaga for excellent technical assistance.
Footnotes
Supplemental Information includes two figures and can be found with this article online at https://doi.org/10.1016/j.omtn.2018.07.009.
Supplemental Information
References
- 1.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Lam J.K.-W., Liang W., Chan H.-K. Pulmonary delivery of therapeutic siRNA. Adv. Drug Deliv. Rev. 2012;64:1–15. doi: 10.1016/j.addr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim K.H., Shon Z.H., Nguyen H.T., Jeon E.C. A review of major chlorofluorocarbons and their halocarbon alternatives in the air. Atmos. Environ. 2011;45:1369–1382. [Google Scholar]
- 4.Birchall J.C., Kellaway I.W., Gumbleton M. Physical stability and in-vitro gene expression efficiency of nebulised lipid-peptide-DNA complexes. Int. J. Pharm. 2000;197:221–231. doi: 10.1016/s0378-5173(00)00339-2. [DOI] [PubMed] [Google Scholar]
- 5.Claus S., Weiler C., Schiewe J., Friess W. How can we bring high drug doses to the lung? Eur. J. Pharm. Biopharm. 2014;86:1–6. doi: 10.1016/j.ejpb.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Rahimpour Y., Kouhsoltani M., Hamishehkar H. Alternative carriers in dry powder inhaler formulations. Drug Discov. Today. 2014;19:618–626. doi: 10.1016/j.drudis.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Ihara D., Hattori N., Horimasu Y., Masuda T., Nakashima T., Senoo T., Iwamoto H., Fujitaka K., Okamoto H., Kohno N. Histological quantification of gene silencing by intratracheal administration of dry powdered small-interfering RNA/chitosan complexes in the murine lung. Pharm. Res. 2015;32:3877–3885. doi: 10.1007/s11095-015-1747-6. [DOI] [PubMed] [Google Scholar]
- 8.Fujita Y., Takeshita F., Mizutani T., Ohgi T., Kuwano K., Ochiya T. A novel platform to enable inhaled naked RNAi medicine for lung cancer. Sci. Rep. 2013;3:3325. doi: 10.1038/srep03325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shim G., Choi H.-W., Lee S., Choi J., Yu Y.H., Park D.-E., Choi Y., Kim C.W., Oh Y.K. Enhanced intrapulmonary delivery of anticancer siRNA for lung cancer therapy using cationic ethylphosphocholine-based nanolipoplexes. Mol. Ther. 2013;21:816–824. doi: 10.1038/mt.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamora-Avila D.E., Zapata-Benavides P., Franco-Molina M.A., Saavedra-Alonso S., Trejo-Avila L.M., Reséndez-Pérez D., Méndez-Vázquez J.L., Isaias-Badillo J., Rodríguez-Padilla C. WT1 gene silencing by aerosol delivery of PEI-RNAi complexes inhibits B16-F10 lung metastases growth. Cancer Gene Ther. 2009;16:892–899. doi: 10.1038/cgt.2009.35. [DOI] [PubMed] [Google Scholar]
- 11.Maa Y.F., Nguyen P.A., Sweeney T., Shire S.J., Hsu C.C. Protein inhalation powders: spray drying vs spray freeze drying. Pharm. Res. 1999;16:249–254. doi: 10.1023/a:1018828425184. [DOI] [PubMed] [Google Scholar]
- 12.Mohri K., Okuda T., Mori A., Danjo K., Okamoto H. Optimized pulmonary gene transfection in mice by spray-freeze dried powder inhalation. J. Control. Release. 2010;144:221–226. doi: 10.1016/j.jconrel.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Sandler A., Gray R., Perry M.C., Brahmer J., Schiller J.H., Dowlati A., Lilenbaum R., Johnson D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 14.Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., Berlin J., Baron A., Griffing S., Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 15.Miller K., Wang M., Gralow J., Dickler M., Cobleigh M., Perez E.A., Shenkier T., Cella D., Davidson N.E. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 16.Takei Y., Kadomatsu K., Yuzawa Y., Matsuo S., Muramatsu T. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res. 2004;64:3365–3370. doi: 10.1158/0008-5472.CAN-03-2682. [DOI] [PubMed] [Google Scholar]
- 17.Kuo J.-H.S., Hwang R. Preparation of DNA dry powder for non-viral gene delivery by spray-freeze drying: effect of protective agents (polyethyleneimine and sugars) on the stability of DNA. J. Pharm. Pharmacol. 2004;56:27–33. doi: 10.1211/0022357022494. [DOI] [PubMed] [Google Scholar]
- 18.Okuda T., Suzuki Y., Kobayashi Y., Ishii T., Uchida S., Itaka K., Kataoka K., Okamoto H. Development of biodegradable polycation-based inhalable dry gene powders by spray freeze drying. Pharmaceutics. 2015;7:233–254. doi: 10.3390/pharmaceutics7030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otake H., Okuda T., Okamoto H. Development of spray-freeze-dried powders for inhalation with high inhalation performance and antihygroscopic property. Chem. Pharm. Bull. (Tokyo) 2016;64:239–245. doi: 10.1248/cpb.c15-00824. [DOI] [PubMed] [Google Scholar]
- 20.Garbuzenko O.B., Mainelis G., Taratula O., Minko T. Inhalation treatment of lung cancer: the influence of composition, size and shape of nanocarriers on their lung accumulation and retention. Cancer Biol. Med. 2014;11:44–55. doi: 10.7497/j.issn.2095-3941.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okuda T., Kito D., Oiwa A., Fukushima M., Hira D., Okamoto H. Gene silencing in a mouse lung metastasis model by an inhalable dry small interfering RNA powder prepared using the supercritical carbon dioxide technique. Biol. Pharm. Bull. 2013;36:1183–1191. doi: 10.1248/bpb.b13-00167. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno T., Mohri K., Nasu S., Danjo K., Okamoto H. Dual imaging of pulmonary delivery and gene expression of dry powder inhalant by fluorescence and bioluminescence. J. Control. Release. 2009;134:149–154. doi: 10.1016/j.jconrel.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Wu C.J., Huang W.C., Chen L.C., Shen C.R., Kuo M.L. Pseudotyped adeno-associated virus 2/9-delivered CCL11 shRNA alleviates lung inflammation in an allergen-sensitized mouse model. Hum. Gene Ther. 2012;23:1156–1165. doi: 10.1089/hum.2012.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong X.-S., Hu X.-B., Liu W., Sun Y.Q., Liu Z. Effects of RNA interference-induced Smad3 gene silencing on pulmonary fibrosis caused by paraquat in mice. Exp. Biol. Med. (Maywood) 2012;237:548–555. doi: 10.1258/ebm.2011.011280. [DOI] [PubMed] [Google Scholar]
- 25.Wu S.Y., McMillan N.A.J. Lipidic systems for in vivo siRNA delivery. AAPS J. 2009;11:639–652. doi: 10.1208/s12248-009-9140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moschos S.A., Jones S.W., Perry M.M., Williams A.E., Erjefalt J.S., Turner J.J., Barnes P.J., Sproat B.S., Gait M.J., Lindsay M.A. Lung delivery studies using siRNA conjugated to TAT(48-60) and penetratin reveal peptide induced reduction in gene expression and induction of innate immunity. Bioconjug. Chem. 2007;18:1450–1459. doi: 10.1021/bc070077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godbey W.T., Wu K.K., Mikos A.G. Poly(ethylenimine) and its role in gene delivery. J. Control. Release. 1999;60:149–160. doi: 10.1016/s0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 28.Beyerle A., Braun A., Merkel O., Koch F., Kissel T., Stoeger T. Comparative in vivo study of poly(ethylene imine)/siRNA complexes for pulmonary delivery in mice. J. Control. Release. 2011;151:51–56. doi: 10.1016/j.jconrel.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Iwanaga T., Kozuka T., Nakanishi J., Yamada K., Nishiyama O., Sano H., Murakami T., Tohda Y. Aerosol deposition of inhaled corticosteroids/long-acting β2-agonists in the peripheral airways of patients with asthma using functional respiratory imaging, a novel imaging technology. Pulm. Ther. 2017;3:219–231. [Google Scholar]
- 30.Hira D., Okuda T., Ichihashi M., Mizutani A., Ishizeki K., Okada T., Okamoto H. Influence of peak inspiratory flow rates and pressure drops on inhalation performance of dry powder inhalers. Chem. Pharm. Bull. (Tokyo) 2012;60:341–347. doi: 10.1248/cpb.60.341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.