Here we demonstrate the use of an RNA-based analysis of specific taxa of interest, including bacteria and fungi, within microbial communities. This multiplex method may be useful as a means to identify samples with specific combinations of taxa and to gain information on how specific populations vary over time and space or in response to perturbation. A rapid means to measure bacterial and fungal populations may aid in the study of host response to changes in microbial communities.
KEYWORDS: NanoString, bacteria, clinical microbiology, cystic fibrosis, fungi, microbial communities
ABSTRACT
Here, we report an approach to detect diverse bacterial and fungal taxa in complex samples by direct analysis of community RNA in one step using NanoString probe sets. We designed rRNA-targeting probe sets to detect 42 bacterial and fungal genera or species common in cystic fibrosis (CF) sputum and demonstrated the taxon specificity of these probes, as well as a linear response over more than 3 logs of input RNA. Culture-based analyses correlated qualitatively with relative abundance data on bacterial and fungal taxa obtained by NanoString, and the analysis of serial samples demonstrated the use of this method to simultaneously detect bacteria and fungi and to detect microbes at low abundance without an amplification step. Compared at the genus level, the relative abundances of bacterial taxa detected by analysis of RNA correlated with the relative abundances of the same taxa as measured by sequencing of the V4V5 region of the 16S rRNA gene amplified from community DNA from the same sample. We propose that this method may complement other methods designed to understand dynamic microbial communities, may provide information on bacteria and fungi in the same sample with a single assay, and with further development, may provide quick and easily interpreted diagnostic information on diverse bacteria and fungi at the genus or species level.
IMPORTANCE Here we demonstrate the use of an RNA-based analysis of specific taxa of interest, including bacteria and fungi, within microbial communities. This multiplex method may be useful as a means to identify samples with specific combinations of taxa and to gain information on how specific populations vary over time and space or in response to perturbation. A rapid means to measure bacterial and fungal populations may aid in the study of host response to changes in microbial communities.
INTRODUCTION
Cystic fibrosis (CF) is a life-limiting genetic disease associated with chronic lung infection that often leads to progressive lung function decline interspersed with pulmonary exacerbations (1–4). CF lung infections are often polymicrobial, heterogeneous between subjects, and variable within individuals over time and can contain bacteria, fungi, and viruses (5–14). The characterization of microbes within CF sputum samples containing diverse bacteria and fungi has been performed using various methods, including culturing on different media, sequencing of PCR amplicons for rRNA genes for bacteria (or ribosomal DNA [rDNA] for fungi in this study) or internal transcribed spacer (ITS) sequences from bacteria and fungi, respectively, and metagenomic sequencing of community DNA. In this work, we assess the use of the NanoString nCounter technology (15) to detect specific bacterial and fungal taxa in sputum samples from individuals with CF-related respiratory infections.
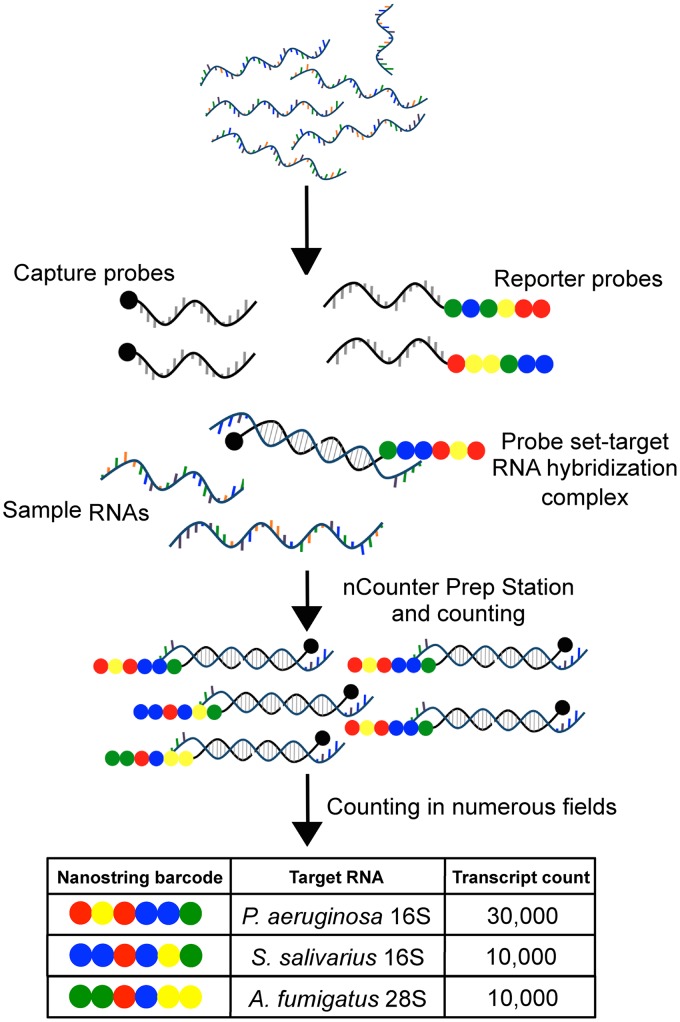
The NanoString methodology detects RNAs using color-coded probe pairs that are optically detected. Together, a fluorescent reporter probe and a capture probe recognize an approximately 100-bp sequence within the target RNA. The target RNA-probe complexes are then captured and counted based on their unique fluorescent probe signatures (Fig. 1). Barczak et al. (16) previously demonstrated the use of this technology to detect species-specific mRNAs of bacteria, parasites, and fungal species and viruses in samples of blood and urine containing one pathogen, but not in clinical samples with complex communities such as CF sputum. In addition, NanoString-based methods were used to monitor 75 Pseudomonas aeruginosa virulence-associated mRNAs in RNA from CF sputum (17) and to detect pathogen mRNAs in samples from single-species mammalian infection models (18–21).
FIG 1 .
NanoString detection method schematic. Frozen samples are lyophilized, and total RNA is extracted. Total RNA is mixed with the probe sets, with each containing a reporter and capture probe for each target RNA. After hybridization, the excess probes are removed and the probe-target complexes are aligned and immobilized in the nCounter cartridge. Fluorescent color sequences are counted and tabulated, yielding counts that correspond to the number of target molecules.
We designed and tested taxon-specific NanoString probe sets for the simultaneous detection of 42 CF-associated bacterial and fungal genera or species by monitoring levels of rRNAs. Probe fidelity was determined using control RNA mixtures as well as by comparison to results obtained by culture and previously established DNA-based community profiling methods. This technology can be used to monitor diverse taxa of interest, including but not limited to, bacterial and fungal targets, in complex microbe-containing samples. We demonstrate its use in monitoring changes in populations over time. Future applications of this approach include the development of improved diagnostics and strategies to monitor changes in microbial communities in response to perturbation.
RESULTS
Probe selection and design.
The bacteria and fungi commonly found in CF respiratory sputum have been well described by culture and deep sequencing methods (5, 6, 14, 22–26). With this knowledge of species likely to be present, we designed a set of NanoString probes to detect taxon-specific rRNA sequences within total RNA isolated from sputum. In addition to the 42 bacterial and fungal genera or species that are frequently detected in CF sputum, we included a few other taxa that commonly occur in non-CF respiratory samples (Table 1). In some cases, we designed genus-level probes (e.g., Streptococcus and Staphylococcus) in addition to probes that could detect specific species or complexes within these genera. For other taxa, such as Burkholderia, only a genus-level probe was included to streamline analysis and limit costs for this proof of concept study. For each taxon that we chose to monitor, a NanoString probe set was designed to target rRNA sequences from bacterial (16S or 23S rRNA) or fungal (18S or 28S rRNA) taxa. Two probes that recognized adjacent sequences within a consecutive 100-nucleotide (nt) region were designed: one was modified to serve as the capture probe, and the other was modified to serve as the reporter probe (Fig. 1). The sequences for the two probes were designed to have >95% identity to the comparable region in all publically available genomes for the target taxon (inclusivity criteria) and less than 90% sequence identity to any non-rRNA gene sequence in the genomes of bacteria, fungi, or humans available in GenBank at the time of probe design (exclusivity criteria) as described in Materials and Methods. The sequences and inclusivity and exclusivity assessments are presented in Table S1 in the supplemental material.
TABLE 1 .
Taxa detected with the NanoString probe sets
| Bacterium detected | Fungus detected |
|---|---|
| Achromobacter xylosoxidans | Aspergillus fumigatus |
| Actinomyces odontolyticus | Candida albicans |
| Anaerococcus prevotii | Candida dubliniensis |
| Burkholderia spp. | Candida glabrata |
| Corynebacterium pseudodiptheriticum | Candida lusitaniae |
| Fusobacterium nucleatum | Candida tropicalis |
| Gemella haemolysans | Candida parapsilosis |
| Gemella morbillorum | Exophiala dermatitidis |
| Haemophilus influenzae | Malassezia globosa |
| Haemophilus parainfluenzae | Malassezia restricta |
| Mycobacterium spp. | Rhodotorula glutinis |
| Mycobacterium abscessus | Rhodotorula mucilaginosa |
| Neisseria spp. | Scedosporium apiospermum |
| Porphyromonas catoniae | Trichosporon mycotoxinivorans |
| Prevotella melaninogenica | |
| Prevotella oris | |
| Pseudomonas aeruginosa | |
| Ralstonia pickettii | |
| Rothia dentocariosa/mucilaginosa | |
| Staphylococcus spp. | |
| Staphylococcus aureus | |
| Stenotrophomonas maltophilia | |
| Streptococcus spp. | |
| Streptococcus oralis/mitis/pneumonia | |
| Streptococcus anginosus | |
| Streptococcus intermedius/constellatus | |
| Streptococcus salivarius | |
| Veillonella atypica and Veillonella dispar |
NanoString probe sets designed for this study. The target RNA, abbreviations used in Fig. 2, probe designations, and target sequence for each probe set are presented. The inclusivity screen reflects probe recognition of strains among all strain sequences within a species; minimum percentage of identity between sequences and the number of sequences analyzed is shown. In cases where the RefSeq database did not contain multiple sequences, the inclusivity screen was performed using the GenBank database (db), as indicated. The exclusivity screen was used to confirm that the probes would not cross-react with other microbial or human sequences. The exclusivity screen for bacteria was performed using the RefSeq database, and fungi were analyzed against the GenBank database. Cross-reactivity indicates other species that may cross-react with the probe sets. Most probe sets were used in every experiment, though some were only included in the analysis of samples in the exacerbation series. The ITS1 fungus-specific probes were not used in these studies because ITS1 RNA was more than 100-fold less abundant than 18S and 28S rRNAs from the same RNA preparation. Download TABLE S1, XLSX file, 0.1 MB (19.7KB, xlsx) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Analysis of specificity of probes using mixes of RNA from single-species cultures.
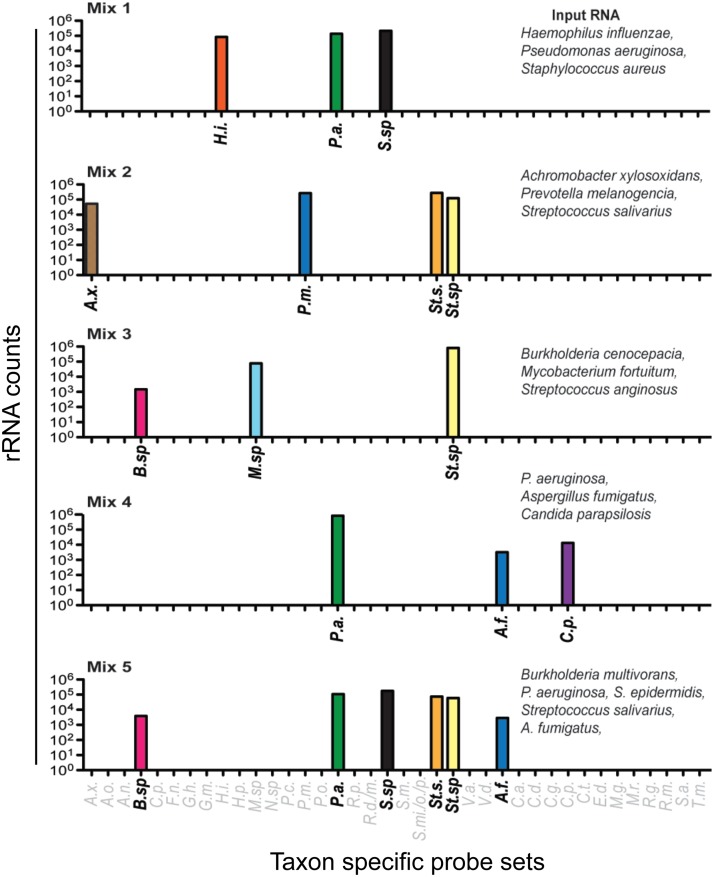
To accompany the computational analysis, which suggested that taxon-targeting rRNA probes would be specific based on exclusivity and inclusivity criteria, we performed control experiments using mixes of total RNA from single-species cultures of 12 bacteria and two different fungi to assess potential cross-reactivity. We prepared mixes of RNA extracted from single-species cultures. Each mix, containing equivalent amounts of RNA from three to five species, was analyzed using the panel of NanoString probe sets. The species from which the RNA samples included in each mix were derived are indicated in Fig. 2. (For strain information, see Table S2 in the supplemental material.)
FIG 2 .
Specificity of select bacterial rRNA targeting probe sets. Control mixes were prepared with total RNA from three to five species per mix and analyzed using all of the probe sets listed on the x axis. Strain details are shown in Table S2. The probe sets for taxa not present in any of the RNA mixes (in gray font) yielded no counts above background, indicating no cross-reaction with RNAs in the mixes. All data are presented as counts, and all taxon abbreviations are defined in Table S1. The species detected by each probe set is defined by the first letters of the genus and species name, except for Streptococcus-targeting probe sets, which are abbreviated “St.” to differentiate them from Staphylococcus-targeting probe sets, which are abbreviated “S.” Probe sets that detect multiple species within a genus are indicated by “sp” as for the probe sets that detect Staphyloccocus species (S.sp), Streptococcus species (St.sp), and Burkholderia spp. (B.sp).
Strains used in this study. The strains used in control mixes and their lab collection identifiers are listed. For each of control mixes (shown in Fig. 2), the specific strain used is indicated. Download TABLE S2, XLSX file, 0.1 MB (35.3KB, xlsx) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Taxon-specific probe sets gave strong positive signals, measured as counts, when cognate rRNAs were present in the mix (Fig. 2). The average positive signal was 1,000-fold higher than the signals from other probe sets. There were no cases of false positives due to cross-reaction with other probes. (Counts for all probes are shown in Table S3A in the supplemental material.) As shown for mixes 2 and 5, the Streptococcus salivarius RNA reacted with the species-specific Streptococcus salivarius probe and the Streptococcus probe, while Streptococcus anginosus only reacted with the genus-level Streptococcus probe as no species-specific probe was present. Both Staphylococcus aureus and Staphylococcus epidermidis reacted with the Staphylococcus probe. Two different Burkholderia species, B. multivorans and B. cenocepacia, were detected by the Burkholderia species probe. Each mixture that included Pseudomonas aeruginosa included RNA from a distinct clinical isolate (strains detailed in Table S2), and each reacted equally well with the species-specific P. aeruginosa probe. Candida parapsilosis and Aspergillus fumigatus RNAs reacted specifically with their cognate probes and did not cross-react with probes from other Candida or Aspergillus species, respectively. Some species-specific probes were omitted from this analysis but were assessed by comparison to culture and MiSeq data as discussed below.
NanoString data. (A) Raw transcript counts detected by NanoString analysis of control mixes for Fig. 2. Background was set at 3 times the number of counts for a highest negative-control probe (NEG). Negative-control probes were included by the manufacturer. The geometric mean of positive-control signals can be used to normalize across samples. A dash indicates “not analyzed” in that sample. This occurs when a particular probe set was not included in the analysis. Only the values in boldface are above background. (B) NanoString counts for samples in Fig. S1. Samples A, C, and D were derived from aliquots of sputum from subjects 1, 3, and 4, respectively, in Table S3D. (C) Counts detected by NanoString analysis for samples from exacerbation series shown in Fig. 3. The data normalized to the positive-control transcript levels are shown. Background was set at 3 times the number of counts for a negative-control probe (NEG). Negative- and positive-control probes were included by the manufacturer. (D) Transcript counts detected by NanoString analysis for patient samples shown in Fig. 4. The table cells with orange shading indicate the highest background value for each sample. Background was set at 3 times the number of counts for a negative-control probe (NEG). Positive- and negative-control probes were included by the manufacturer. The geometric mean of positive-control signals can be used to normalize across samples, but normalization does not affect relative abundance calculations. A dash indicates “not analyzed” in that sample. Download TABLE S3, XLSX file, 0.1 MB (56.6KB, xlsx) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To assess the range of counts for which probe set signals were linear, we analyzed RNA mix 1, which contained RNA from Pseudomonas aeruginosa, Haemophilus influenzae, and Staphylococcus aureus, with total RNA amounts from 0.0005 to 5 ng. We found that the probe sets for P. aeruginosa, H. influenzae and S. aureus all yielded the expected linear increase in counts that correlated with sample input with the linear range between 100 and >70,000 counts (R2 > 0.999 for all three taxa) (Table S3A).
RNA-derived NanoString profiles were consistent across replicates.
As a first step toward evaluating the NanoString panel described above for the analysis of microbial communities within sputum, we obtained deidentified sputa that were expected to contain many of the taxa targeted by the probes that we had designed. We extracted total RNA from five sputum samples (sputum samples A to E) and analyzed the RNA in two independent runs. In every case, we found that rRNA counts for each bacterial and fungal taxon strongly correlated between replicates indicating the high reproducibility of the method (see Fig. S1A in the supplemental material) (R2 > 0.999 each sample). The counts from bacterium- and fungus-targeting probes are plotted separately (Fig. S1A) as the counts for bacterial RNAs were 100- to 1,000-fold higher than those for the fungi, suggesting that the bacteria were more abundant. In addition, we separately recovered RNA from homogenized sputum that had been divided into three aliquots (samples F, G, and H). Again, we found that the relative abundances of different taxa present in the three replicates from each sample were highly similar (Fig. S1B) (R2 > 0.9 in each pairwise comparison per patient).
Reproducibility of NanoString analysis of sputum. (A) Microbial profiles in CF sputum RNA (sputum samples A to E) analyzed in duplicate by NanoString analysis are shown. Bacteria and fungi are presented separately. Shown is correlation of count numbers by NanoString between duplicates for samples A to E. (B) Microbial community profiles presented as percentage of abundance in three replicate RNAs extracted in parallel from three separate sputum samples (F, G, and H). The legend is the same as in panel A. Download FIG S1, PDF file, 0.6 MB (604.8KB, pdf) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In the NanoString method, probes bind directly to their RNA targets without a cDNA synthesis step or any other enzymatic manipulation. Double-stranded DNA is not available for hybridization (Fig. 1). To confirm that the NanoString analysis of rRNA was detecting RNA and not DNA, three sputum-derived RNA samples were analyzed before and after RNase treatment. The total number of counts for all probe sets above background was reduced by >99% for all samples after RNase treatment, and the community patterns determined by NanoString were markedly altered after RNase treatment, indicating that RNA, and not DNA, was giving rise to the detected counts (see Fig. S2B and Table S3B in the supplemental material).
RNase treatment of extracted RNA depletes detection of patient sample microbial communities by NanoString. Microbial communities were detected from total RNA of three sputum samples (F, G, and H) pre- and post-RNase treatment with NanoString probes. (A) Total detection (sum of counts from all probe sets) from pre- and post-RNase-treated samples. (B) Probe-specific transcript detection pre- and post-RNase-treatment. The different species/genera detected are indicated by the different colors and symbols. The data values are presented in Table S3B. Download FIG S2, PDF file, 0.1 MB (78.9KB, pdf) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Simultaneous analysis of bacteria and fungi using NanoString in sputum sample series from six patients hospitalized for disease exacerbations.
One of the impetuses for designing this RNA-based method for complex culture analysis of CF sputum is to overcome several challenges associated with current culture based protocols, including (i) the need to analyze CF samples using at least six different culture media (blood agar, chocolate agar, MacConkey agar, Sabouraud dextrose agar [SDA], mannitol salt agar, and OFPBL [oxidation/fermentation-polymyxin-bacitracin-lactose] agar selective for Burkholderia) to detect potential pathogens of interest, (ii) the inability to readily identify some pathogens, such as Achromobacter xylosoxidans, by growth characteristics alone, (iii) the wide variation in growth kinetics and colony morphology types within a species, and (iv) the use of a small, fixed volume of sputum, which may limit the detection of less abundant or heterogeneously distributed species. To assess the accuracy of NanoString 16S rRNA analysis as a technique both for detecting species of interest within a mixed community and for assessing changes in sputum microbiota across samples from the same subject, we obtained and analyzed sample series from six hospitalized individuals undergoing treatment for disease exacerbation (designated E1 to E6). In each series, a sample was obtained on the first day of treatment and then at least one more and as many as six more samples were obtained on subsequent days. A total of 25 sputa were analyzed. In addition to the clinical culture analysis of the day 0 sample (Table 2), all specimens were plated on sheep blood agar (SBA), Pseudomonas isolation agar (PIA), and SDA with gentamicin in our research lab (culture plates shown in Fig. S3 in the supplemental material). In addition, total RNA was extracted from 100 µl of each sputum sample, and 18 ng of total extracted sputum RNA was analyzed by NanoString. All probe sets were included in the analysis of each sample. Figure 3A presents data for all positive signals.
TABLE 2 .
Clinical culture report for day 0 samples from the E series
| Day 0 sample | Clinical culture reporta |
|---|---|
| E1 | Moderate Exophiala dermatitidis and a few MSSA strains |
| E2 | Achromobacter xylosoxidans |
| E3 | Pseudomonas aeruginosa |
| E4 | Burkholderia cenocepacia and MSSA |
| E5 | MRSA and mucoid Pseudomonas aeruginosa |
| E6 | MRSA, MSSA, and mucoid and nonmucoid Pseudomonas aeruginosa |
MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant S. aureus.
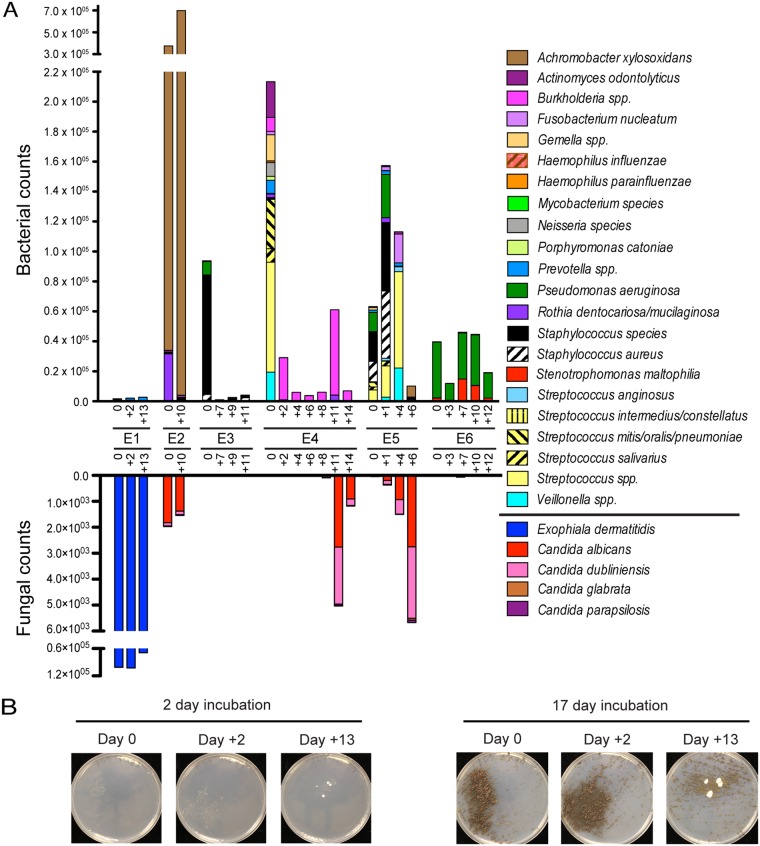
FIG 3 .
Bacterial and fungal community composition in exacerbation series from six subjects. (A) Serial sputum samples from six individuals with CF were obtained during treatment for pulmonary exacerbation and analyzed by NanoString (data in Table S3C). Microbes present in multiple subjects or abundantly in at least one subject are graphed as total counts normalized to positive controls as a stacked plot in which each segment indicates the number of counts for the indicated taxon. Bacteria and fungi are plotted on the upper and lower y axes, respectively, though the data for bacterial and fungal probe sets were obtained in the same reaction. (B) Exacerbation series E1 showed high levels of Exophiala dermatitidis by plate culture on SDA only after extended incubation.
Culture analysis and NanoString analysis of sputum series from six hospitalized patients. Shown are cultures of the E1 to E6 sputum series on sheep blood agar (SBA), Pseudomonas isolation agar (PIA), and Sabouraud dextrose agar (SDA) plates. Plates were incubated for at least 48 h before imaging. Samples were collected on day 0 (date of admission for treatment of exacerbation), and the number of days posttreatment is shown for subsequent samples. For each series, RNA was analyzed by NanoString, and data above background are shown for the bacterium- and fungus-targeting probes included in the code set. Exacerbation series E1 showed high levels of Exophiala dermatitidis in plate cultures only after extended incubation for all days, and the plate from day 0 is shown after a 2-week incubation as an example. The clinical culture data for the day 0 samples are shown in Table 2. The NanoString data, shown as stacked plots, for each sample are the same data shown in Fig. 3 and presented in Table S3C and are represented here for ease of comparison. A different scale is used on the y axis to better enable examination of lower-abundance taxa. Download FIG S3, PDF file, 1.4 MB (1.4MB, pdf) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The analysis of sputum RNA from the E1 series showed high counts for the fungus Exophilia dermatitidis, with no counts above background for any other fungi; the counts for E. dermatitidis were ~100-fold higher than those for any bacterial species, suggesting that E. dermatitidis was a dominant microbe in these samples (Fig. 3A). Among the bacteria that were positively detected, none of the CF pathogens commonly reported were observed (Fig. S3; Table S3C). While E. dermatitidis was not evident on culture plates after 48 h of incubation at 37°C, the characteristic brown colonies of E. dermatitidis were clearly abundant on both SBA and SDA after more than a week (Fig. 3B). E. dermatitidis was in the final clinical culture report of the day 0 sample (Table 2).
In the two samples from the E2 series, NanoString counts for Achromobacter xylosoxidans were 10-fold higher than any other bacterial or fungal species at both time points (Fig. 3A), and Achromobacter xylosoxidans was the only species detected in the clinical microbiology analysis of the day 0 sample. Small, nondescript white colonies consistent with those formed by Achromobacter xylosoxidans were also detected on blood agar, PIA, and SBA plates in our culture-based studies (Fig. S3, E2). Low counts for Candida albicans were also detected and yeast colonies were evident on the SDA medium plates (Fig. S3, E2).
In series E3, the most abundant NanoString signal from CF-associated pathogens that are routinely reported clinically was P. aeruginosa (Fig. 3A, green bars); this species was the only species reported in the clinical microbiology analysis of the day 0 sample and was clearly evident on the day 0 sample on PIA (Table 2; Fig. S3, E3). P. aeruginosa was not detected by either NanoString or by culture at the later time points. The genus-level Staphylococcus probe set (black bars) gave very high counts, and the Staphylococcus aureus probe set (black-hatched bars) yielded 10-fold-fewer counts in the day 0 sample (Fig. S3; Table S3C). The presence of non-aureus Staphylococcus species, which are not reported in the clinical microbiological analysis, was confirmed by the presence of large numbers of nonhemolytic staphylococci on the blood agar plate at the day 0 time point, and the number of staphylococcal colonies on the blood agar plate decreased on samples from subsequent days concomitant with the reduction in Staphylococcus NanoString signal (Fig. S3, E3). Interestingly, the S. aureus NanoString counts remained low but constant over the samples examined (Table S3C), suggesting that this signal was not due to cross-reaction with other RNA from other Staphylococcus spp. that grew on the blood agar plates.
In samples from subject E4, counts for Burkholderia (pink bars) were high in the RNA from the sputum samples (Fig. 3); Burkholderia colonies that were slow-growing, characteristic of small colony variants, began to emerge on SBA after 48 h (Fig. S3, E4). The weak growth observed on plates from E4 was confirmed to be Burkholderia by 16S rRNA gene sequencing of amplified DNA from single isolates, and the clinical microbiology reported Burkholderia cenocepacia as the dominant pathogen with “few” S. aureus counts. Staphylococcus aureus counts were elevated in the day 0 sample, but not above the conservative threshold set in this study (see Materials and Methods) (Table S3C). Candida colonies on SDA were more abundant at later time points, and the signals from Candida albicans and Candida dubliniensis increased concomitantly in samples from later days. We confirmed that both C. albicans and C. dubliniensis were present by sequencing of the ITS1 region amplified from genomic DNA from individual colonies.
In samples from series E5, Pseudomonas aeruginosa (green bars), Staphylococcus spp. (black bars), and Staphylococcus aureus (black-hatched bars) represented the strongest signals in the early time points (day 0 and day 1) (Fig. 3, E5). Both P. aeruginosa (mucoid) and S. aureus (methicillin-resistant S. aureus [MRSA]) were detected in the clinical microbiology analysis of the day 0 sample (Table 2). P. aeurginosa was detected on PIA medium and staphylococcal colonies were evident on the SBA plate in our laboratory analyses (Fig. S3, E5). E5 sample from day 6 showed P. aeruginosa and S. aureus signals to be 100-fold lower than on days 0 and 1, and the counts for Achromobacter (brown bars) were the highest among all taxa at this time point (>7,000 counts) (Fig. 3; Table S3C). Small gray colonies that are typical for Achromobacter appeared on the PIA and SBA plates at these late time points (Fig. S3). C. albicans and C. dubliniensis were detected by NanoString with increasing levels at later days, and this was concordant with the culture analysis (Fig. S3, E5). We confirmed that both of these species were present by sequencing of the ITS1 region amplified from DNA from individual colonies. Candida glabrata and Candida parapsilosis were detected, though at low levels, in E5 by NanoString, and their presence among the colonies on the day 6 SDA plate was confirmed by sequencing of the ITS1 sequence amplified from the DNA of single isolates. The presence of multiple Candida spp. within single CF sputum samples is consistent with previous reports (14).
In series E6, the strongest signals were for P. aeruginosa (green bars) and Stenotrophomonas maltophilia (red bars), with much lower but positive signals from Achromobacter xylosoxidans and Burkholderia species (Fig. 3; Table S3C). Of these taxa, only P. aeruginosa was reported in the clinical microbiology report for day 0 (Table 2). The clinical lab reported isolation of Staphylococcus aureus from the E6 day 0 culture (Table 2), and while the NanoString counts were above the highest background count, they were much lower than those for the other bacterial pathogens (Table S3C). The levels of S. maltophilia rRNA increased on days 7 and 10. Bacterial growth consistent with P. aeruginosa was observed on blood agar plates on day 0; Candida colonies were only observed on days 3, 7, and 12, and an elevated NanoString signal for Candida albicans was observed on days 3 and 7 (Fig. S3).
Together, these data indicate that all of the pathogens reported upon clinical microbiology analysis of the day 0 sputum samples were clearly present in the NanoString data, with the exception of reports of Staphylococcus aureus in the clinical cultures from E1 and E6 (Table 2; Table S3C). Staphylococcus aureus was not evident in our laboratory culture of a separate aliquot of the same sample. The NanoString counts for the predominant bacterial pathogens were high (>3,800 counts), regardless of whether the strains grew robustly on medium, such as P. aeruginosa in E3, or slowly, such as Burkholderia cenocepacia in E4 and Exophiala in E1. Together, these data indicate that the NanoString methodology can specifically detect bacteria (E2 to E6) and fungi (E1, E2, E4, and E5) within samples using a single assay.
In addition, the NanoString approach can provide insights into the dynamics of bacterial and fungal species abundance over time or in response to treatment, especially when one taxon is of lower abundance. As one example, in subjects E4 and E5, the number of Candida colonies and Candida NanoString counts increased over the course of treatment, especially when sputum was analyzed on SDA plus gentamicin, a medium that suppresses the growth of bacteria (Fig. S3), consistent with previous reports of bacterial suppression of fungal growth in clinical cultures (27). The only fungi detected among these samples were Exophiala and Candida spp., and no samples had counts above background from probe sets for other fungi encountered in CF samples (Aspergillus fumigatus, Scedosporium, and Trichosporon). Except for samples from the E1 series, the total probe counts from all fungal rRNA-specific probe sets were more than 1,000× lower than the total counts from probe sets designed to target bacterial rRNAs.
Relative abundance of CF pathogens determined by NanoString correlates with Illumina sequencing of community DNA and culture-based analysis of sputum.
Eighteen sputum samples collected during outpatient visits were divided for separate RNA and DNA extractions. We compared data obtained by NanoString analysis of RNA to data obtained by well-validated deep sequencing analysis of 16S rRNA sequences amplified from DNA. Comparison to clinical microbiological culture data was also performed. We only focused on the bacteria in these analyses as the fungi were predicted to be of low abundance in most samples based on the clinical culture analysis. Total RNA was analyzed using the NanoString probes described above, and the total DNA was subjected to amplification and sequencing of V4-V5 16S rRNA-encoding genes by Illumina MiSeq (Fig. 4A). Clinical culture analysis of the 18 samples found that most of the bacterial pathogens frequently reported from CF sputum cultures were detected in at least one sample, including P. aeruginosa, Achromobacter xylosoxidans, Burkholderia spp., Ralstonia spp., Stenotrophomonas maltophilia, and Staphylococcus aureus; two other commonly reported CF pathogens (Haemophilus influenzae and Mycobacterium spp.) were not detected in the clinical microbiology analyses, though low levels of Haemophilus influenzae may not have been detected by the culture methods used.
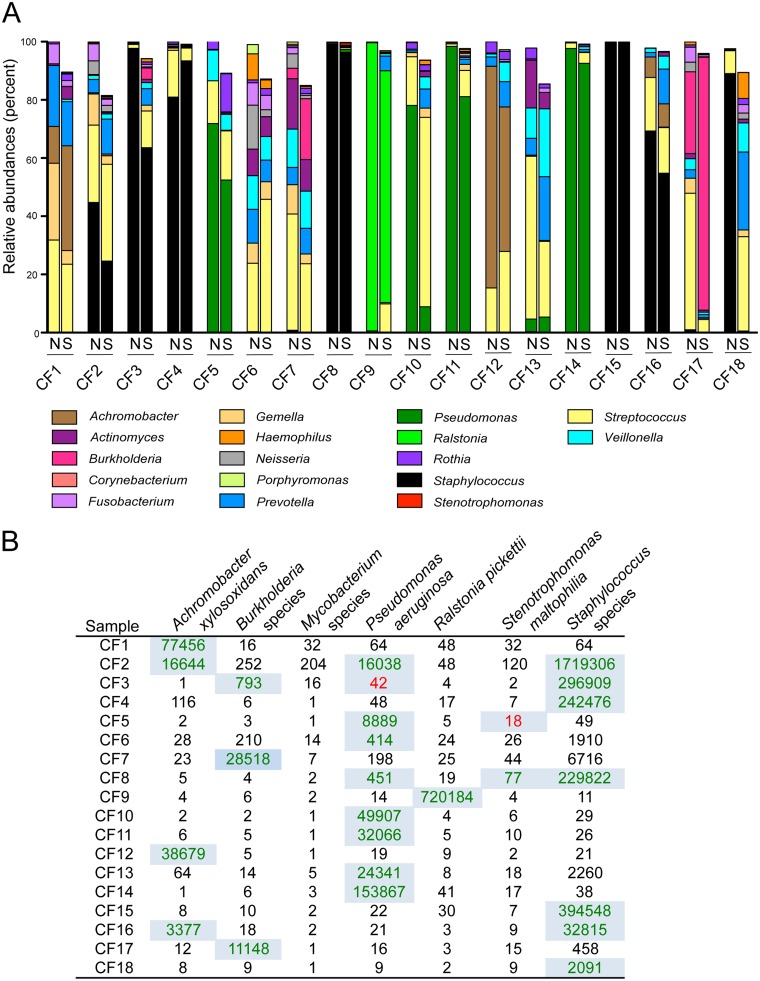
FIG 4 .
Bacterial community profiles detected by NanoString and 16S rRNA gene deep sequencing (MiSeq) methods correlate and are confirmed by culture. (A) Eighteen CF sputum samples, labeled with the subject number (CF1 to -18), were analyzed by both NanoString (N) and 16S rRNA gene deep sequencing (MiSeq [S]). The percentages of relative abundance of taxa detected by both methods are shown. Only the taxa measured in our NanoString code set are included in the deep sequencing profiles, but relative abundance was determined as a percentage of total reads, not only reads represented in the NanoString code set (data in Table S4). In cases where NanoString probes detected different species within the same genus, counts from individual species-specific probes were summed to correspond with the genus-level assignments for the MiSeq data. (B) The counts for NanoString probe sets correlating with CF pathogens detected by culture are shown. See Table S3D for data for all genera analyzed. Highlighted numbers indicate that the corresponding pathogen was also detected by clinical culture analysis. The numbers in red in CF3 and CF5 were reported as being “rare” in the clinical microbiology analysis and were at very low levels (<0.5%) in the MiSeq-based community profiles for those samples. For CF sputum specimens, the clinical lab reports staphylococci only if they are identified as S. aureus.
Illumina MiSeq analysis amplified 16S rRNA sequences from 18 sputum samples. (A) Relative abundance of the 19 genera detected by NanoString and MiSeq. Raw counts were divided by the total counts for all taxa in each sample. (B) 16S rRNA gene deep sequencing reads for genera analyzed by one of the NanoString probe sets. In cases where the reads for a taxon within a sample were less than >0.1% of total reads, the value was set to zero. Download TABLE S4, XLSX file, 0.1 MB (72.5KB, xlsx) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
All of the genera at greater than 5% abundance in the Illumina-based community profiles of CF sputum were the taxa also detected by NanoString probes (Table S4). This result was not surprising since the taxa included in the NanoString code set were selected based on their representation in prior Illumina-based community profiling of CF sputum. On average, 92% ± 6% of the deep sequencing reads corresponded to taxa that were represented among the NanoString probes (Table S4A). For both data sets, the relative abundances were determined by division of the counts, for NanoString, or reads, for MiSeq, for the taxon of interest by the total number of counts or reads. For the MiSeq relative abundances, total reads, including those for taxa that were not assessed by NanoString, were used in the calculation of the percentage of abundance. Overall, there was high concordance between the data obtained by the two methods, with an average Pearson correlation coefficient of 0.803 across all 18 samples with all taxa, including both CF-related pathogens and species that are also frequent members of the oral microbiome. Together, these data indicate that there is minimal cross-reaction between different NanoString probes. Furthermore, these data show that for CF sputum collected during outpatient visits, NanoString analysis of RNA, and Illumina analysis of sequences amplified from DNA yielded similar results. Future studies will determine if data obtained from community RNA versus DNA differs in other contexts, such as over the course of intensive antibiotic treatment that might increase the fraction of nonviable or dead cells.
Clinical culture results from analysis of these 18 samples described 26 instances in which one of the above bacterial pathogens was detected by culture and several samples contained two or three pathogens. For 24 of the 26 bacterial pathogen detection events, there was a clear positive signal in the NanoString data that indicated the presence of the cultured pathogen (highlighted in green font with blue background in Fig. 4B); there was also a signal for the pathogen in the MiSeq data in each case (Fig. 4A; Table S4). In two instances, culture analysis detected only “rare” colonies of P. aeruginosa (CF3) or Stenotrophomonas maltophilia (CF5), which can mean as few as one colony, and the NanoString signal was not clearly positive (red text with blue background in Fig. 4B). MiSeq analysis found Pseudomonas or Stenotrophomonas to be at very low relative abundance (0.01 and 0.2%) in samples CF3 and CF5, respectively (Table S4). In one case (CF18), the pathogen in the clinical culture report was Staphylococcus aureus, and this species was robustly detected by NanoString but at very low levels in the Illumina sequencing data (Fig. 4A; Table S4A). The reason for this discrepancy is not known, but it is worth noting that the sequencing depth was not aberrantly low for this sample. These data suggest that nucleic acid based-methods, which involve the analysis of larger sample aliquots, may be capable of providing quantitative or semiquantitative information on the levels or activity of a taxon and may reinforce clinical microbiology data when few colonies of a species are observed in culture. One benefit of the NanoString method is that no amplification is performed prior to analysis aiding in quantitation. The high concordance between the NanoString, MiSeq, and culture data further supports the validity of the probes and their use in describing microbial communities.
DISCUSSION
Here, we report an RNA-based method for detecting target species within complex microbial communities. We describe this approach for the analysis of sample types that have been well characterized by nontargeted approaches, such as the sequencing of the metagenome or of amplified rRNA and ITS regions from community DNA in order to identify the taxa present. While the NanoString technology has been applied to the detection of mRNAs from microbial species within clinical samples or in tissues from animal experiments, we show the application of this technology to the analysis of rRNAs, which are highly abundant molecules with sequences that are highly conserved across the genus and species levels. In contrast to amplification-based methods in which conserved primer-binding sequences are required, the regions detected within the rRNAs can be different for different taxa, thus allowing for different taxonomic levels to be detected in the same multiplex reaction. Because of the use of a large (~100-nucleotide [nt]) region for hybridization to the two probes, computational analysis of the probes suggests that this method can accommodate some sequence divergence within species or genus, but this was not explicitly tested in these experiments.
Because fungi can grow slowly in laboratory cultures, as shown for Exophiala in samples from subject E1, and may be unsuspected in some chronic contexts, a method that detects both bacteria and fungi has significant clinical value. We found that taxon-specific NanoString probe sets successfully detected fungi, including C. albicans, C. dubliniensis, C. glabrata, A. fumigatus, and Exophiala. In our initial analyses using the NanoString method, we included probes that were complementary to ITS1 RNA sequences, in addition to probes that bound fungal 28S rRNA (see Table S1 for sequences). The ITS1 RNA counts were over 100-fold less abundant, suggesting that ITS1 RNA, which is processed from rRNA-containing RNAs, is less stable and thus less abundant than rRNAs.
We demonstrate significant concordance between this method and Illumina-based sequencing of amplified 16S rRNA sequences from community DNA for many of the organisms examined (Fig. 4A), including Pseudomonas, Achromobacter, Burkholderia, Ralstonia, Stenotrophomonas, Staphylococcus, Actinomyces, Fusobacterium, Gemella, Neisseria, Rothia, Porphyromonas, and Veillonella. Consistent with a recent study that showed a relationship between deep sequencing and detection by culture when extended culture techniques were used (12), we found correlations between NanoString data and clinical microbiology culture assessment as well.
This method is most useful in the analysis of sample sets that have already been well characterized by nontargeted approaches, such as the sequencing of the metagenome or of amplified rRNA and ITS regions from community DNA. Once microbial community members of interest have been identified within a particular habitat, the number of tools available for gaining additional data on specific taxa within the community increases to include quantitative PCR, fluorescent in situ hybridization methods, and plate-based studies, among other methods. We propose that the NanoString method be added to the list of techniques for the analysis of specific taxa within a community and that it may be particularly useful for analyzing the relationships between microbes from different domains, which cannot be profiled using single primer sets, and between species within a genus, which may lack resolution by other methods. Multiple probes for each taxon that target both small and large subunit rRNAs may enhance confidence in the detection of low-abundance species.
In addition to the rRNA analyses described here, we have published a NanoString analysis of various P. aeruginosa mRNAs in clinical samples, and the inclusion of species- or strain-specific mRNAs could be useful in analyzing phylogenetically close taxa (17). The combination of probes for rRNAs and mRNAs, however, could be challenged by the difficulties in detecting lower-abundance mRNA signals when used with rRNAs, which are orders of magnitude more abundant. Interfering complementary RNAs may be useful in reducing signals from highly abundant RNAs in order to enable the simultaneous analysis of probes for RNAs that are present in vastly different amounts, but we have not explored this strategy as part of this work. With further development, it is also possible to monitor host and microbial transcripts at the same time to better understand the interplay between host and microbe, particularly during changes in health.
The agreement between NanoString and 16S rRNA gene and rDNA deep sequencing data for most taxa was striking considering the differences in target molecule (rRNA versus rRNA-encoding DNA) and the technological differences between the methodologies. A number of environmental community profiling studies have compared results from the analysis of extracted rRNA gene and rDNA sequences, often with the goal of determining if RNA can be used to identify the more metabolically active members of the community (28–31). rRNA, while more stable than mRNA, is degraded by stable, active RNases, and thus it is tempting to speculate that the presence of RNA may serve as a more sensitive indicator of an active cell than the presence of DNA (32). If this is the case, the high concordance between NanoString and MiSeq data would suggest that the majority of microbes in the CF sputum samples from these stable patients are alive. While our data do not address whether RNA and DNA equivalently reflect the presence of live and dead cells, with taxon-specific probes in hand, studies that test this idea can now be performed.
This detection method, like any nucleic acid analysis method, has the potential for methodological bias. Complicating factors for the NanoString technology could include variable lysis, differences in transcription frequency of rRNA genes in different taxa, and differences in probe hybridization efficiency: thus, validation strategies must be employed. While the bead beating-based methods used here have been shown to be efficient at lysing fungi and other difficult-to-lyse taxa, the nucleic acid extraction methods used need to be assessed for other taxa that were not present in our sample sets, such as mycobacteria.
The studies described here represent the first step in employing the NanoString technology to the characterization of complex microbial communities using rRNA. In addition to analyzing and monitoring bacteria and fungi, host markers could also be added to the code sets to gain knowledge about the host state and response (33–36). Furthermore, the NanoString technology has already been introduced into clinical practice for the purpose of cancer cell profiling. While many additional validation experiments would need to be performed before a clinical diagnostic tool could be developed, the quick sample turnaround time and semiquantitative outputs may allow for more rapid assessment of samples (compared to traditional culture techniques, which may take days to weeks), as well as protocols for the assessment of the efficacy of treatments. This method provides direct quantitation of rRNAs present in a sample and thus can give insight into the number of rRNA molecules by sample weight, volume, or total RNA. Future studies are necessary to determine if rRNA levels correlate with meaningful metrics such as cell number or population activity.
One application of this methodology is the identification of community RNAs that contain significant levels of specific mixed-species microbial populations for use in more extensive analyses, such as total RNA sequencing. In addition, the development of a sensitive methodology for the detection of fungi in human-derived samples can be readily adapted to address other research questions, such as those regarding the roles of fungi in ulcerative colitis or asthma and reactive airway disease (37–41), as well as in environmental settings. Finally, the development of taxon-specific probes that detect microbial rRNAs sets the stage for future studies aimed at understanding whether an analysis of microbial rRNA can provide an indication of the metabolic activity or pathogenic potential of specific microbial populations within complex communities.
MATERIALS AND METHODS
Probe design.
We detected microbial rRNAs using custom-designed species- and genus-specific probe sets. The probe sequences were designed by the NanoString probe design team to recognize all available sequences in GenBank for the taxon of interest and to not cross-hybridize to off-target sequences in other bacteria, other fungi, or human genomes. All probe set sequences are listed in Table S1. Measurements were made using the NanoString nCounter system (NanoString Technologies, Seattle, WA) as previously described (15). As described in Table S1, the probes were purchased in batches, referred to as code sets and the code set composition was slightly modified over time to include additional taxa or to achieve different levels of specificity (for example, replacing a genus-level probe with a species-level probe). Each probe contained either capture or detection elements. Each code set also contained six synthetic positive-control probes that detect spiked transcripts added at a range of different concentrations and eight negative-control probes that are not predicted to hybridize to any transcripts in the bacterial, fungal, or human transcriptomes analyzed (NanoString Technologies).
Analysis of RNA from in vitro-grown cultures.
To assess the specificity of the RNA probes for specific species, equal amounts of purified total RNA from each microorganism were mixed, and 5 ng of the RNA mixture was used. The strains used in this study are listed in Table S2. RNA from laboratory cultures was prepared from cells harvested from 1 to 3 ml of liquid culture for all strains. Pseudomonas aeruginosa was grown in lysogeny broth (LB) with aeration on a roller drum. Achromobacter xylosoxidans, Staphylococcus aureus, Burkholderia cenocepacia, and Mycobacterium fortuitum were grown in tryptic soy broth (TSB) with aeration on a roller drum. Prevotella melaninogenica was grown under anoxic conditions using a GasPak EZ anaerobic container system, and Streptococcus salivarius and Streptococcus anginosus were grown statically in an atmosphere with 5% CO2 in Todd-Hewitt broth amended with 0.5% yeast extract. Haemophilus influenzae was grown in brain heart infusion (BHI) broth supplemented with 10 µg/ml NADH and 10 µg/ml hemin with static incubation in a 5% CO2 atmosphere. All cultures were inoculated from a single colony into 5 ml of the specified medium. Liquid cultures were grown at 37°C for 16 to 18 h, except for M. fortuitum, which was grown for 48 h. Cells were frozen and lyophilized prior to RNA extraction using the methods described in the following section.
RNA and DNA isolation from sputum.
Frozen aliquots (100 µl) were lyophilized for 5 to 16 h, as necessary. Lyophilized cells were disrupted using a mixture of 0.1-, 0.5-, and 1-mm zirconia-silicon beads (70 µl each) that was added to the dry pellet prior to agitation. Samples were lysed using 5 cycles of 30 s, with 20-s pauses in between, on an Omni Bead Ruptor homogenizer (Omni International, Inc.) set at 5.65 m/s. The disrupted sample was resuspended in lysis buffer (200 µl of 0.25 µg/µl lysostaphin and 3 µg/µl lysozyme in 10 mM Tris and 1 mM EDTA [TE] buffer, pH 8.0), and incubated at room temperature for 5 to 10 min. RNA isolation was then performed using a Direct-zol kit (Zymo Research), with TRIzol, according to the manufacturer’s suggested protocol. RNA was eluted in two 20-µl volumes of elution buffer before being frozen at −80°C until use. No DNase treatment was performed. For RNA isolation reproducibility experiments, sputa were split into triplicate samples after homogenization and then processed in parallel following the RNA isolation protocol described above. For DNA isolation from sputum, lyophilized and disrupted sputum aliquots were resuspended in lysis buffer (described above) and incubated at room temperature for 5 to 10 min. Total DNA was isolated using Qiagen genomic DNA purification reagents, following the manufacturer’s protocol for Gram-negative bacterial DNA isolation. DNA pellets were resuspended in 40 µl TE buffer and frozen at −80°C until use.
rRNA analysis using NanoString probe sets.
We empirically determined that 15 to 20 ng total sputum RNA was best for balancing the ability to detect less abundant species without overloading the optical detection method. For control mixes of RNA isolated from pure culture, 5 ng total RNA was analyzed, unless otherwise specified. Sample RNA was hybridized to the probe sets for 12 to 16 h at 65°C using the NanoString nCounter Prep Station instrument according to the manufacturer’s instructions. Targets were counted on the nCounter using 255 fields of view per sample, and output results were extracted using nSolver Analysis software (NanoString Technologies).
Background determination and detection of positive samples.
A series of internal negative-control probes that do not target sequences in any known organisms are included in every sample, and the signals associated with these probes inform the relative quality and background hybridization in each analysis. Following the manufacturer’s recommendation of using limits of detection based on the maximum signal for internal negative-control probes, we chose a background subtraction method in which 3 times the maximum detection by internal negative controls (3 × Max) was subtracted from all probe counts. Prior to analysis, a background subtraction of the 3 × Max value was performed for all control mixes and patient samples in the graphs. In the tables, counts without background subtraction and counts for the negative controls that were used to calculate the background are shown. For comparisons of levels across samples, we normalized samples based on the mean of the positive controls.
Patient cohort for CF sputum collection.
The samples were collected in accordance with protocols approved by Center for the Protection of Human Subjects at Dartmouth. Expectorated sputum samples used in the comparison of NanoString and Illumina sequencing methods were collected from adult subjects with CF during a routine office visit. Enrolled subjects provided two separate sputum samples during a single visit, one of which was analyzed by standard culture methods for this specimen type at the Dartmouth Hitchcock Medical Center clinical microbiology laboratory. The second sputum sample was divided into aliquots (one for DNA extraction and one for RNA extraction) and then frozen on-site using a portable −80°C freezer shuttle (Sterling Ultracold) prior to storage in an upright −80°C freezer. Sputum samples were stored at −80°C for at least 1 h and often for multiple days or weeks before analysis.
In serial samples collected from the same patient, samples were collected upon admission for treatment of a disease exacerbation as well as over the course of treatment. Sputum samples were frozen upon collection and stored at −80°C until samples were processed for RNA isolation as described below.
DNA deep sequence analysis of bacterial 16S rRNA genes.
Total genomic DNA (gDNA) isolated from 18 sputum samples was used as the template for the amplification and analysis of the V4-V5 regions of bacterial 16S rRNA genes by deep sequencing at the Marine Biological Laboratory (Woods Hole, MA) using primers and adaptors as described previously (42) (https://vamps.mbl.edu/resources/primers.php). The amplification reaction mixtures contained 1 × Platinum Hi-Fi Taq buffer, 2 mM MgSO4, 0.2 mM deoxynucleoside triphosphates (dNTPs), 0.2 to 0.4 µM each primer, 12.5 U of platinum Hi-Fi Taq, up to 25 ng of template, and water to a final volume of 100 µl. This volume was divided into replicate 33-µl reaction mixtures to reduce amplification bias, and we included a no-template negative-control reaction mixture for each primer set. Cycling conditions were an initial denaturation for 3 min at 94°C, followed by 30 cycles of 30 s at 94°C, 45 s at 57°C, and 1 min at 72°C, with a final extension for 2 min at 72°C. The replicates were combined, and the positive and negative reactions were visualized on a PerkinElmer Caliper LabChipGX. We cleaned and size selected the products with Agencourt Ampure XP beads (0.75× sample volume) and quantitated the recovered product using a PicoGreen assay (Life Technologies/Invitrogen). The products were pooled, and the pool was size selected with a 1× volume of Ampure XP.
The library pool was sequenced on an Illumina MiSeq to produce 250-nt paired-end reads. We used the “merge-illumina-pairs” scripts distributed in the Illumina Utilities library (available from https://github.com/meren/illumina-utils) (43) to analyze the reads. This script removed any read pair with more than three mismatches in the ~80-nt-long overlapping region and also required at least 66% of the nucleotides in the nonoverlapping region to have greater than a Q30 score (44). The program vsearch (45) removes chimeras both de novo and in comparison to the RDP classifier training reference database (46). These data sets serve as input to GAST (47) for assigning and posting taxonomic compositions of each analyzed microbial community on VAMPS (48). For the comparative analyses with the NanoString methods, a threshold of 0.01% was set for positive signals.
Analysis of RNase-treated RNA.
To determine the specificity of NanoString detection for RNA versus DNA, RNase treatment was performed on 3 samples after RNA isolation (F, G, and H). For these three samples, 1 µl RNaseA was added to 5 µl of isolated RNA, and the mixture was incubated at 37°C for 1 h. The samples were then diluted 1:10 in 45 µl of 10 mM Tris-HCl (pH 7.5), 300 mM NaCl, 5 mM EDTA (pH 7.5), and 2 µl of RNase A-T1 mix (Thermo Fisher catalog no. EN0551). This mixture was incubated for 1 h at 37°C. Nontreated RNA samples were then diluted 1:10, and equal volumes of RNase-treated and untreated matched samples were used for the NanoString code set.
Culture analysis of serial sputum samples.
In addition to the clinical laboratory analysis of sputum, we also analyzed the sputum by culture in our laboratory. Approximately 10 µl of sputum was removed with a metal Scienceware Microspoon and plated on tryptic soy agar (TSA) with 5% sheep blood (P1100; Northeast Laboratory Service), Pseudomonas isolation agar, or Sabouraud dextrose agar plus 100 µg/ml gentamicin made according to the manufacturer’s directions. Plates were incubated for 24 to 48 h at 37°C and then imaged.
In order to identify isolates that grew on plates, clinical isolates were struck to single colonies and grown as single-colony cultures in either liquid LB medium (bacteria), liquid YPD (yeast extract-peptone-dextrose) medium (Candida species), or liquid glucose minimal medium (Aspergillus fumigatus). Bacterial 16S rRNA genes (forward primer, GTGSTGCAYGGYTGTCGTCA; reverse primer, ACGTCRTCCMCACCTTCCTC) and the fungal ITS1 region (forward primer, GTAAAAGTCGTAACAAGGTTTC; reverse primer, GTTCAAAGAYTCGATGATTCAC) were PCR amplified, and the identity of each clinical isolate was determined after sequencing each amplicon using the Applied Biosystems 3730 DNA analyzer (Thermo Fisher Scientific).
Statistics.
Significance of correlation to determine reproducibility and linearity of microbial detection by NanoString was calculated using linear regression in Graphpad Prism 5. Correlations between NanoString and MiSeq sequencing were performed using R to determine the Pearson r correlation coefficient and its P value for each subject across selected taxa and for the selected taxa across all subjects using the “cor.test” function in the “stats” package (v3.3.0) with default settings. Significance of correlation between Candida colony numbers and NanoString counts and the significance of differences were calculated using linear regression or the Mann-Whitney test in GraphPad Prism 5.
Data availability.
The Illumina sequencing data have been deposited under SRA accession no. SRP153115 (https://trace.ncbi.nlm.nih.gov/Traces/sra/sra.cgi), with links to BioProject accession no. PRJNA480715 in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/).
ACKNOWLEDGMENTS
Research reported in this publication was supported by grants from the National Institutes of Health to D.A.H. (R01 GM108492 to D.A.H.). Support from the Cystic Fibrosis Foundation Research Development Program (STANTO011R0) was provided as pilot grants to J.D.S., A.H.G., and D.A.H. The Dartmouth Lung Biology Center and CF Translational Research Core were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant no. P30GM106394 and by the CFF RDP (CFRDP STANTO11R0). A.H.G. was supported by the CF Foundation Clinical Research Scholars Program under award no. GIFFOR17Y5 and the Dartmouth Clinical and Translational Science Institute (SYNERGY) under award no. KL2TR001088 (Green, P.I.) from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Farrell PM. 2000. Improving the health of patients with cystic fibrosis through newborn screening. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Adv Pediatr 47:79–115. [PubMed] [Google Scholar]
- 2.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, Moninger TO, Michalski AS, Hoffman EA, Zabner J, Stoltz DA, Welsh MJ. 2014. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB Jr, Zabner J, Welsh MJ. 2010. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, Zabner J, Welsh MJ. 2014. pH modulates the activity and synergism of the airway surface liquid antimicrobials beta-defensin-3 and LL-37. Proc Natl Acad Sci U S A 111:18703–18708. doi: 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, Young VB, LiPuma JJ. 2012. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A 109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price KE, Hampton TH, Gifford AH, Dolben EL, Hogan DA, Morrison HG, Sogin ML, O’Toole GA. 2013. Unique microbial communities persist in individual cystic fibrosis patients throughout a clinical exacerbation. Microbiome 1:27. doi: 10.1186/2049-2618-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmody LA, Zhao J, Schloss PD, Petrosino JF, Murray S, Young VB, Li JZ, LiPuma JJ. 2013. Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. Ann Am Thorac Soc 10:179–187. doi: 10.1513/AnnalsATS.201211-107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salipante SJ, Sengupta DJ, Rosenthal C, Costa G, Spangler J, Sims EH, Jacobs MA, Miller SI, Hoogestraat DR, Cookson BT, McCoy C, Matsen FA, Shendure J, Lee CC, Harkins TT, Hoffman NG. 2013. Rapid 16S rRNA next-generation sequencing of polymicrobial clinical samples for diagnosis of complex bacterial infections. PLoS One 8:e65226. doi: 10.1371/journal.pone.0065226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SH, Clark ST, Surendra A, Copeland JK, Wang PW, Ammar R, Collins C, Tullis DE, Nislow C, Hwang DM, Guttman DS, Cowen LE. 2015. Global analysis of the fungal microbiome in cystic fibrosis patients reveals loss of function of the transcriptional repressor Nrg1 as a mechanism of pathogen adaptation. PLoS Pathog 11:e1005308. doi: 10.1371/journal.ppat.1005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmody LA, Zhao J, Kalikin LM, LeBar W, Simon RH, Venkataraman A, Schmidt TM, Abdo Z, Schloss PD, LiPuma JJ. 2015. The daily dynamics of cystic fibrosis airway microbiota during clinical stability and at exacerbation. Microbiome 3:12. doi: 10.1186/s40168-015-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boutin S, Graeber SY, Weitnauer M, Panitz J, Stahl M, Clausznitzer D, Kaderali L, Einarsson G, Tunney MM, Elborn JS, Mall MA, Dalpke AH. 2015. Comparison of microbiomes from different niches of upper and lower airways in children and adolescents with cystic fibrosis. PLoS One 10:e0116029. doi: 10.1371/journal.pone.0116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings LA, Kurosawa K, Hoogestraat DR, SenGupta DJ, Candra F, Doyle M, Thielges S, Land TA, Rosenthal CA, Hoffman NG, Salipante SJ, Cookson BT. 2016. Clinical next generation sequencing outperforms standard microbiological culture for characterizing polymicrobial samples. Clin Chem 62:1465–1473. doi: 10.1373/clinchem.2016.258806. [DOI] [PubMed] [Google Scholar]
- 13.Hahn A, Sanyal A, Perez GF, Colberg-Poley AM, Campos J, Rose MC, Pérez-Losada M. 2016. Different next generation sequencing platforms produce different microbial profiles and diversity in cystic fibrosis sputum. J Microbiol Methods 130:95–99. doi: 10.1016/j.mimet.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willger SD, Grim SL, Dolben EL, Shipunova A, Hampton TH, Morrison HG, Filkins LM, O’Toole GA, Moulton LA, Ashare A, Sogin ML, Hogan DA. 2014. Characterization and quantification of the fungal microbiome in serial samples from individuals with cystic fibrosis. Microbiome 2:40. doi: 10.1186/2049-2618-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. 2008. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 16.Barczak AK, Gomez JE, Kaufmann BB, Hinson ER, Cosimi L, Borowsky ML, Onderdonk AB, Stanley SA, Kaur D, Bryant KF, Knipe DM, Sloutsky A, Hung DT. 2012. RNA signatures allow rapid identification of pathogens and antibiotic susceptibilities. Proc Natl Acad Sci U S A 109:6217–6222. doi: 10.1073/pnas.1119540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gifford AH, Willger SD, Dolben EL, Moulton LA, Dorman DB, Bean H, Hill JE, Hampton TH, Ashare A, Hogan DA. 2016. Use of a multiplex transcript method for analysis of Pseudomonas aeruginosa gene expression profiles in the cystic fibrosis lung. Infect Immun 84:2995–3006. doi: 10.1128/IAI.00437-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanning S, Xu W, Solis N, Woolford CA, Filler SG, Mitchell AP. 2012. Divergent targets of Candida albicans biofilm regulator Bcr1 in vitro and in vivo. Eukaryot Cell 11:896–904. doi: 10.1128/EC.00103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu W, Solis NV, Filler SG, Mitchell AP. 2016. Pathogen gene expression profiling during infection using a NanoString nCounter platform. Methods Mol Biol 1361:57–65. doi: 10.1007/978-1-4939-3079-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung D, Barker BM, Carey CC, Merriman B, Werner ER, Lechner BE, Dhingra S, Cheng C, Xu W, Blosser SJ, Morohashi K, Mazurie A, Mitchell TK, Haas H, Mitchell AP, Cramer RA. 2014. ChIP-seq and in vivo transcriptome analyses of the Aspergillus fumigatus SREBP SrbA reveals a new regulator of the fungal hypoxia response and virulence. PLoS Pathog 10:e1004487. doi: 10.1371/journal.ppat.1004487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng S, Clancy CJ, Xu W, Schneider F, Hao B, Mitchell AP, Nguyen MH. 2013. Profiling of Candida albicans gene expression during intra-abdominal candidiasis identifies biologic processes involved in pathogenesis. J Infect Dis 208:1529–1537. doi: 10.1093/infdis/jit335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fodor AA, Klem ER, Gilpin DF, Elborn JS, Boucher RC, Tunney MM, Wolfgang MC. 2012. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS One 7:e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cystic Fibrosis Foundation 2011. Cystic Fibrosis Foundation patient registry 2010 annual data report. Cystic Fibrosis Foundation, Bethesda, MD. [Google Scholar]
- 24.Gilligan PH. 2014. Infections in patients with cystic fibrosis: diagnostic microbiology update. Clin Lab Med 34:197–217. doi: 10.1016/j.cll.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filkins LM, Hampton TH, Gifford AH, Gross MJ, Hogan DA, Sogin ML, Morrison HG, Paster BJ, O’Toole GA. 2012. Prevalence of streptococci and increased polymicrobial diversity associated with cystic fibrosis patient stability. J Bacteriol 194:4709–4717. doi: 10.1128/JB.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madan JC, Koestler DC, Stanton BA, Davidson L, Moulton LA, Housman ML, Moore JH, Guill MF, Morrison HG, Sogin ML, Hampton TH, Karagas MR, Palumbo PE, Foster JA, Hibberd PL, O’Toole GA. 2012. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio 3:e00251. doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hockey LJ, Fujita NK, Gibson TR, Rotrosen D, Montgomerie JZ, Edwards JE Jr.. 1982. Detection of fungemia obscured by concomitant bacteremia: in vitro and in vivo studies. J Clin Microbiol 16:1080–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamke J, Taylor MW, Schmitt S. 2010. Activity profiles for marine sponge-associated bacteria obtained by 16S rRNA vs 16S rRNA gene comparisons. ISME J 4:498–508. doi: 10.1038/ismej.2009.143. [DOI] [PubMed] [Google Scholar]
- 29.West NJ, Obernosterer I, Zemb O, Lebaron P. 2008. Major differences of bacterial diversity and activity inside and outside of a natural iron-fertilized phytoplankton bloom in the Southern Ocean. Environ Microbiol 10:738–756. doi: 10.1111/j.1462-2920.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 30.Brinkmann N, Martens R, Tebbe CC. 2008. Origin and diversity of metabolically active gut bacteria from laboratory-bred larvae of Manduca sexta (Sphingidae, Lepidoptera, Insecta). Appl Environ Microbiol 74:7189–7196. doi: 10.1128/AEM.01464-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inkinen J, Jayaprakash B, Santo Domingo JW, Keinänen-Toivola MM, Ryu H, Pitkänen T. 2016. Diversity of ribosomal 16S DNA- and RNA-based bacterial community in an office building drinking water system. J Appl Microbiol 120:1723–1738. doi: 10.1111/jam.13144. [DOI] [PubMed] [Google Scholar]
- 32.McKillip JL, Jaykus LA, Drake M. 1998. rRNA stability in heat-killed and UV-irradiated enterotoxigenic Staphylococcus aureus and Escherichia coli O157:H7. Appl Environ Microbiol 64:4264–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweeney TE, Wong HR, Khatri P. 2016. Robust classification of bacterial and viral infections via integrated host gene expression diagnostics. Sci Transl Med 8:346ra91. doi: 10.1126/scitranslmed.aaf7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang RY, Weng KF, Huang YC, Chen CJ. 2016. Elevated expression of circulating miR876-5p is a specific response to severe EV71 infections. Sci Rep 6:24149. doi: 10.1038/srep24149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pallin DJ, Bry L, Dwyer RC, Lipworth AD, Leung DY, Camargo CA Jr, Kupper TS, Filbin MR, Murphy GF. 2016. Toward an objective diagnostic test for bacterial cellulitis. PLoS One 11:e0162947. doi: 10.1371/journal.pone.0162947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, Cook S, Tankou S, Stuart F, Melo K, Nejad P, Smith K, Topçuolu BD, Holden J, Kivisäkk P, Chitnis T, De Jager PL, Quintana FJ, Gerber GK, Bry L, Weiner HL. 2016. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 7:12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, Brown J, Funari VA, Wang HL, Crother TR, Arditi M, Underhill DM, Iliev ID. 2016. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe 19:865–873. doi: 10.1016/j.chom.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Underhill DM, Pearlman E. 2015. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity 43:845–858. doi: 10.1016/j.immuni.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, Rotter JI, Wang HL, McGovern DP, Brown GD, Underhill DM. 2012. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodridge HS, Simmons RM, Underhill DM. 2007. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol 178:3107–3115. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 41.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. 2005. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun 73:30–38. doi: 10.1128/IAI.73.1.30-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huse SM, Young VB, Morrison HG, Antonopoulos DA, Kwon J, Dalal S, Arrieta R, Hubert NA, Shen L, Vineis JH, Koval JC, Sogin ML, Chang EB, Raffals LE. 2014. Comparison of brush and biopsy sampling methods of the ileal pouch for assessment of mucosa-associated microbiota of human subjects. Microbiome 2:5. doi: 10.1186/2049-2618-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eren AM, Vineis JH, Morrison HG, Sogin ML. 2013. A filtering method to generate high quality short reads using Illumina paired-end technology. PLoS One 8:e66643. doi: 10.1371/journal.pone.0066643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minoche AE, Dohm JC, Himmelbauer H. 2011. Evaluation of genomic high-throughput sequencing data generated on Illumina HiSeq and genome analyzer systems. Genome Biol 12:R112. doi: 10.1186/gb-2011-12-11-r112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. 2016. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM. 2014. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huse SM, Dethlefsen L, Huber JA, Welch DM, Relman DA, Sogin ML. 2008. Exploring microbial diversity and taxonomy using SSU rRNA hypervariable tag sequencing. PLoS Genet 4:e1000255. doi: 10.1371/journal.pgen.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huse SM, Welch DM, Voorhis A, Shipunova A, Morrison HG, Eren AM, Sogin ML. 2014. VAMPS: a website for visualization and analysis of microbial population structures. BMC Bioinformatics 15:41. doi: 10.1186/1471-2105-15-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NanoString probe sets designed for this study. The target RNA, abbreviations used in Fig. 2, probe designations, and target sequence for each probe set are presented. The inclusivity screen reflects probe recognition of strains among all strain sequences within a species; minimum percentage of identity between sequences and the number of sequences analyzed is shown. In cases where the RefSeq database did not contain multiple sequences, the inclusivity screen was performed using the GenBank database (db), as indicated. The exclusivity screen was used to confirm that the probes would not cross-react with other microbial or human sequences. The exclusivity screen for bacteria was performed using the RefSeq database, and fungi were analyzed against the GenBank database. Cross-reactivity indicates other species that may cross-react with the probe sets. Most probe sets were used in every experiment, though some were only included in the analysis of samples in the exacerbation series. The ITS1 fungus-specific probes were not used in these studies because ITS1 RNA was more than 100-fold less abundant than 18S and 28S rRNAs from the same RNA preparation. Download TABLE S1, XLSX file, 0.1 MB (19.7KB, xlsx) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains used in this study. The strains used in control mixes and their lab collection identifiers are listed. For each of control mixes (shown in Fig. 2), the specific strain used is indicated. Download TABLE S2, XLSX file, 0.1 MB (35.3KB, xlsx) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
NanoString data. (A) Raw transcript counts detected by NanoString analysis of control mixes for Fig. 2. Background was set at 3 times the number of counts for a highest negative-control probe (NEG). Negative-control probes were included by the manufacturer. The geometric mean of positive-control signals can be used to normalize across samples. A dash indicates “not analyzed” in that sample. This occurs when a particular probe set was not included in the analysis. Only the values in boldface are above background. (B) NanoString counts for samples in Fig. S1. Samples A, C, and D were derived from aliquots of sputum from subjects 1, 3, and 4, respectively, in Table S3D. (C) Counts detected by NanoString analysis for samples from exacerbation series shown in Fig. 3. The data normalized to the positive-control transcript levels are shown. Background was set at 3 times the number of counts for a negative-control probe (NEG). Negative- and positive-control probes were included by the manufacturer. (D) Transcript counts detected by NanoString analysis for patient samples shown in Fig. 4. The table cells with orange shading indicate the highest background value for each sample. Background was set at 3 times the number of counts for a negative-control probe (NEG). Positive- and negative-control probes were included by the manufacturer. The geometric mean of positive-control signals can be used to normalize across samples, but normalization does not affect relative abundance calculations. A dash indicates “not analyzed” in that sample. Download TABLE S3, XLSX file, 0.1 MB (56.6KB, xlsx) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Reproducibility of NanoString analysis of sputum. (A) Microbial profiles in CF sputum RNA (sputum samples A to E) analyzed in duplicate by NanoString analysis are shown. Bacteria and fungi are presented separately. Shown is correlation of count numbers by NanoString between duplicates for samples A to E. (B) Microbial community profiles presented as percentage of abundance in three replicate RNAs extracted in parallel from three separate sputum samples (F, G, and H). The legend is the same as in panel A. Download FIG S1, PDF file, 0.6 MB (604.8KB, pdf) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
RNase treatment of extracted RNA depletes detection of patient sample microbial communities by NanoString. Microbial communities were detected from total RNA of three sputum samples (F, G, and H) pre- and post-RNase treatment with NanoString probes. (A) Total detection (sum of counts from all probe sets) from pre- and post-RNase-treated samples. (B) Probe-specific transcript detection pre- and post-RNase-treatment. The different species/genera detected are indicated by the different colors and symbols. The data values are presented in Table S3B. Download FIG S2, PDF file, 0.1 MB (78.9KB, pdf) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Culture analysis and NanoString analysis of sputum series from six hospitalized patients. Shown are cultures of the E1 to E6 sputum series on sheep blood agar (SBA), Pseudomonas isolation agar (PIA), and Sabouraud dextrose agar (SDA) plates. Plates were incubated for at least 48 h before imaging. Samples were collected on day 0 (date of admission for treatment of exacerbation), and the number of days posttreatment is shown for subsequent samples. For each series, RNA was analyzed by NanoString, and data above background are shown for the bacterium- and fungus-targeting probes included in the code set. Exacerbation series E1 showed high levels of Exophiala dermatitidis in plate cultures only after extended incubation for all days, and the plate from day 0 is shown after a 2-week incubation as an example. The clinical culture data for the day 0 samples are shown in Table 2. The NanoString data, shown as stacked plots, for each sample are the same data shown in Fig. 3 and presented in Table S3C and are represented here for ease of comparison. A different scale is used on the y axis to better enable examination of lower-abundance taxa. Download FIG S3, PDF file, 1.4 MB (1.4MB, pdf) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Illumina MiSeq analysis amplified 16S rRNA sequences from 18 sputum samples. (A) Relative abundance of the 19 genera detected by NanoString and MiSeq. Raw counts were divided by the total counts for all taxa in each sample. (B) 16S rRNA gene deep sequencing reads for genera analyzed by one of the NanoString probe sets. In cases where the reads for a taxon within a sample were less than >0.1% of total reads, the value was set to zero. Download TABLE S4, XLSX file, 0.1 MB (72.5KB, xlsx) .
Copyright © 2018 Grahl et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
The Illumina sequencing data have been deposited under SRA accession no. SRP153115 (https://trace.ncbi.nlm.nih.gov/Traces/sra/sra.cgi), with links to BioProject accession no. PRJNA480715 in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/).