Abstract
Functional neuroimaging research has recently revealed brain network interactions during performance on creative thinking tasks—particularly among regions of the default and executive control networks—but the cognitive mechanisms related to these interactions remain poorly understood. Here we test the hypothesis that the executive control network can interact with the default network to inhibit salient conceptual knowledge (i.e., pre-potent responses) elicited from memory during creative idea production. Participants studied common noun-verb pairs and were given a cued-recall test with corrective feedback to strengthen the paired association in memory. They then completed a verb generation task that presented either a previously studied noun (high-constraint) or an unstudied noun (low-constraint), and were asked to “think creatively” while searching for a novel verb to relate to the presented noun. Latent Semantic Analysis of verbal responses showed decreased semantic distance values in the high-constraint (i.e., interference) condition, which corresponded to increased neural activity within regions of the default (posterior cingulate cortex and bilateral angular gyri), salience (right anterior insula), and executive control (left dorsolateral prefrontal cortex) networks. Independent component analysis of intrinsic functional connectivity networks extended this finding by revealing differential interactions among these large-scale networks across the task conditions. The results suggest that interactions between the default and executive control networks underlie response inhibition during constrained idea production, providing insight into specific neurocognitive mechanisms supporting creative cognition.
Keywords: creativity, divergent thinking, cognitive control, functional connectivity, default network, executive control network
Creative cognition involves flexibly combining concepts stored in memory to form novel and useful associations. Previous theories have emphasized the contribution of associative mechanisms that passively unfold within semantic memory networks (i.e., spreading activation), with closely associated concepts generally considered less creative than remote associations (Mednick, 1962). Although memory provides a foundation for creative thought, evidence suggests that it can also act as a source of interference by biasing search processes toward salient conceptual knowledge (e.g., recalling known uses for an object during the alternate uses task; Gilhooly et al., 2007), thus requiring cognitive control to inhibit pre-potent response tendencies (Benedek et al., 2012). Neuroimaging research has demonstrated that memory and cognitive control are supported by the brain’s default and executive control networks (Zabelina & Andrews-Hanna, 2016), and that the interaction of these systems supports performance on creative thinking tasks (Beaty, Benedek, Silvia, & Schacter, 2016). Nevertheless, the cognitive mechanisms associated with such interactions remain poorly understood. In the present research, we developed an experimental paradigm to examine brain activity and network connectivity underlying conceptual interference during creative idea production.
Neurocognitive Mechanisms Supporting Creative Idea Production
One of the most influential models of the creative thought process is the associative theory described by Mednick (1962). According to this framework, individual differences in creative thinking ability can be explained by variation in the structural organization of concepts within semantic networks. Memory’s contribution to creative thought is apparent across both domain-general (e.g., divergent thinking) and domain-specific (e.g., creative writing) contexts, both of which require drawing upon acquired knowledge to construct novel and useful solutions to open-ended problems. Despite the importance of memory to creativity, substantial evidence suggests that it can also function to constrain idea production, such as in the phenomenon of functional fixedness in convergent problem solving (Duncker, 1945; Osman, 2008) and divergent thinking (Chrysikou & Weisberg, 2005; Ward, Patterson, & Sifonis, 2004). In the alternate uses task (AUT), for example, people are typically asked to produce creative uses for common objects. Behavioral research has linked performance deficits on the AUT and other divergent thinking tasks to an inability to transcend salient conceptual knowledge (i.e., recalling the known uses of an object; Beaty & Silvia, 2012; Chrysikou et al., 2016; Gilhooly et al., 2007). Moreover, when presented with pictorial examples of possible responses to open-ended problems prior to idea generation, people often produce responses that conform to the examples (Jansson & Smith, 1991; Smith et al., 1993), even when instructed to avoid doing so (Chrysikou & Weisberg, 2005; Ward et al., 2004).
Behavioral evidence suggests that the constraining effects of memory can be mitigated by cognitive control. Several control processes have been found to support creative task performance, including pre-potent response inhibition (Benedek et al., 2012), broad retrieval ability (Benedek et al., 2012b; Silvia, Beaty, & Nusbaum, 2013), and conceptual category switching (Finke et al., 1992; Nusbaum & Silvia, 2011). Benedek et al. (2012) reported a correlation between ideational fluency in the alternate uses task and pre-potent response inhibition—the ability to overcome interference by suppressing dominant responses (i.e., an overlearned or salient response tendency with a strong association to a given concept or behavior; Friedman & Miyake, in press). Other work has examined the contribution of broad retrieval ability—an executive function associated with controlled memory retrieval—and shown positive correlations with various tasks involving divergent thinking (Beaty & Silvia, 2013; Benedek et al., 2012; Silvia et al., 2013). Such findings suggest that idea production is supported in part by the interaction of memory systems and cognitive control.
The involvement of memory in creativity is further supported by neuroimaging evidence. Several fMRI studies have reported activation of regions within the brain’s default network, a set of cortical midline, medial temporal, and posterior inferior parietal regions associated with self-generated cognition (e.g., mind-wandering and memory retrieval; Andrews-Hanna et al., 2014; Buckner et al., 2008; Schacter et al., 2012). Benedek et al. (2014a) found that generating creative metaphors elicits activation of the posterior cingulate and left angular gyrus, core default network regions associated with memory retrieval and semantic integration (Badre & Wagner, 2007; McAvoy et al., 2016). Further evidence comes from large-scale individual differences studies reporting correlations among divergent thinking ability and default network structure and function (Chen et al., 2015; Jauk et al., 2015; Jung et al., 2016).
Neuroimaging research has also implicated brain regions within the executive control network—a system comprised of lateral prefrontal and anterior inferior parietal cortices implicated in the executive control of attention and cognition (Seeley et al., 2007; Spreng et al., 2010; Vincent et al., 2008). The dorsolateral prefrontal cortex (DLPFC), for example, has been implicated in studies of general creative cognitive processing (Chen et al., in press; Gonen-Yaacovi et al., 2013; Wu et al., 2015) and artistic performance (Beaty, 2015; Boccia et al., 2015; Pinho et al., 2014, 2016). DLPFC activation during creative task performance is thought to reflect the involvement of executive control processes (Beaty et al., 2016; Benedek et al., 2014a; Chen et al., 2015, in press). Another prefrontal region commonly implicated in neuroimaging studies of creative cognition is the left inferior frontal gyrus (IFG; Gonen-Yaacovi et al., 2013; Vartanian et al., 2014). The IFG shows robust activation during cognitive tasks that require controlled memory retrieval, particularly those requiring the selection of a target concept among a set of competing alternatives (Grindrod et al., 2008; Zhang et al., 2004).
Although specific regions within the default and executive control networks have shown consistent activation in fMRI studies, the extent to which these regions cooperate during creative task performance has only recently been explored. This emerging literature has reported default and control network interaction across several creative tasks and domains (Beaty et al., 2016; Christoff et al., 2016; Jung, Mead, Carrasco, & Flores, 2013; Zabelina & Andrews-Hanna, 2016). In an fMRI study of divergent thinking, Beaty et al. (2015) found increased functional connectivity between regions of the default (PCC), control (DLPFC), and salience (insula) networks across the task duration. Other research using verbal creativity tasks has further demonstrated interactions among regions within these networks (e.g., Green et al., 2015; Mayseless et al., 2015). Studies of artistic performance have also reported co-activation of regions with the default and control networks, including musical improvisation (Pinho et al., 2016), visual art (Ellamil et al., 2012), and poetry production (Liu et al., 2015). Mounting evidence thus suggests that creative cognition involves functional connectivity of the default and executive control networks.
The Present Research
Although neuroimaging studies have reported interactions among large-scale brain systems during creative task performance, the cognitive mechanisms associated with these interactions remain poorly understood. One way in which the control network may interact with the default network is by managing conceptual interference that can arise during memory retrieval. During divergent thinking tasks, for example, people often begin by recalling known uses for objects before eventually generating novel uses (Benedek et al., 2014b; Gilhooly et al., 2007). Importantly, however, people with higher cognitive abilities tend not to show this effect (Beaty & Silvia, 2012). These findings suggest that concepts strongly associated to a given cue can act as a source of interference, and that cognitive control may function to suppress such salient conceptual knowledge, which is reflected in functional interactions among regions within the default and executive control networks.
Here we sought to test this hypothesis by developing a paradigm to experimentally induce conceptual interference during idea production. We used a modified version of the verb generation task which has recently been used to assess creative cognition in several behavioral and neuroimaging studies (Green et al., 2015, 2016; Prabhakaran et al., 2014). In these studies, participants were presented with a series of nouns and asked to “think creatively” while searching for novel verbs to relate to the nouns; verbal responses were recorded and analyzed using Latent Semantic Analysis (LSA), a computational method for quantifying semantic similarity between words in a given semantic space (cf. Green, 2016). Green and colleagues have demonstrated that compared to a condition where participants are not instructed to think creatively, the instruction to think creatively yields significantly greater semantic distance values between the nouns and participant generated verbs. Prabhakaran et al. (2013) provided evidence for the validity of the verb generation task as an assessment of creative thought, reporting strong correlations between semantic distance values in the creativity instructions condition and a range of established markers of creative cognition and behavior, including performance on the alternate uses task and self-reported creative achievement
In the present research, we asked participants to study a list of noun and verb pairs (e.g., shoe-walk), and then use either studied (‘High-Constraint’) or non-studied nouns (‘Low-Constraint’) to generate novel verb associations during functional magnetic resonance imaging (fMRI); a cued-recall control condition (‘Recall’) presented the studied nouns and asked participants to recall the studied noun. Consistent with past work, participant’s verbal responses were analyzed using LSA. We hypothesized that verb responses generated with studied nouns would be less semantically distant than those generated with unstudied nouns because the previously encoded verbs act as a source of semantic interference (i.e., a pre-potent response). We hypothesized that compared to the Recall condition, the High- and Low-Constraint conditions would similarly engage the left IFG, consistent with past research past research using the verb generation task (e.g., Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997) but that the High-Constraint condition would be associated with greater activity of regions within the executive and salience networks, in light of their role in cognitive control and network switching, respectively (Uddin, 2015). Critically, we further hypothesized that compared to the Low-Constraint condition, the High-Constraint condition would show increased activity within regions of the executive, salience, and default networks, because this condition places greater demands on brain network dynamics related to the activation of a pre-potent response in memory (default), suppression of this response via cognitive control (executive), and dynamic switching mechanisms that facilitate default-control network interactions (salience).
The involvement of executive processes as a function of ideational constraints would also be consistent with past work on the role of cognitive control (Chrysikou, Weber, & Thompson-Schill, 2014) and “hypofrontal” states (Mayseless & Shamay-Tsoory, 2015). On the one hand, the added task demand of overcoming conceptual interference should require greater cognitive control, reflected in increased activation of frontal brain regions (cf. Chrysikou et al., 2014). On the other hand, generating verbs in response to unstudied nouns should be less constraining and thus require relatively fewer executive resources (cf. Mayseless et al., 2015). We further predicted that the executive and default networks would show greater functional connectivity during the interference condition compared to the control condition, reflecting the inhibition of pre-potent responses stemming from their activation in the default network (Beaty et al., 2016).
Method
Participants
The total sample comprised 26 healthy adults from the University of North Carolina at Greensboro (UNCG). Two participants were excluded from the analyses due to excessive head movement, resulting in a final sample of 24 (15 females, mean age: 24.19, age range: 18–47). Participants were paid in cash for their involvement in the study. All participants were right-handed with normal or corrected-to-normal vision, and reported no history of neurological disorder or psychotropic medication.
Experimental Task and Procedure
The experimental procedure consisted of three phases. In a study phase, a list of 36 noun-verb pairs (e.g., shoe-walk) was presented for two iterations (3.5 minutes each), and participants were asked to remember the word pairs for a later memory task. The study phase was completed during an initial anatomical MRI scan because functional imaging data from this phase were not of interest. Noun-verb pairs presented during the study phase were derived from a recent study examining the validity of the verb generation task as a measure of creative cognitive ability (Prabhakaran et al., 2013). Verbs associated with nouns during the study phase reflected the most common responses generated by participants in this study (e.g., phone-call). Semantically similar noun-verb pairs were used to maximize conceptual interference during the subsequent verb generation phase: the aim was to strengthen preexisting semantic associations in memory and thus promote pre-potent response tendencies when attempting to generate a new verb associate.
In a cued-recall phase, participants’ memory for the studied noun-verb pairs was assessed. The purpose of this recall test was to further strengthen the encoded noun-verb associations, thus MRI data were not obtained during this phase. Participants were presented with the 36 nouns from the study phase and asked to recall the associated verb. A trial displayed a previously studied noun with a question mark (e.g., shoe-?). Participants were instructed to speak the associated verb into an MRI compatible microphone (Optoacoustics; Mazor, Israel). Verbal responses were recorded online by an experimenter to ensure compliance and to assess recall performance. Following each response period, the correct noun-verb association was displayed for participants to restudy, with the goal of further strengthening these associations in memory.
A final generation phase consisted of the main fMRI tasks of interest: two verb generation tasks and one control task (i.e., cued recall). Each condition included 18 trials presented in a pseudo-random order that was fixed across participants. Stimuli comprised the 36 previously studied nouns and 18 novel nouns (also used in Prabhakaran et al., 2013). All trials presented a noun with the condition name listed above: ‘Create’ or ‘Recall.’ For ‘Create’ trials, participants were asked to “think creatively” and come up with a verb to associate with the presented noun (cf. Green, 2016; Green et al., 2015, in press; Prabhakaran et al. 2013; Weinberger et al., 2016).
The ‘Create’ condition was divided into two sub-conditions that differed in terms of ideational constraints. The ‘Low-Constraint’ condition presented unstudied nouns for verb generation; the ‘High-Constraint’ condition presented previously studied nouns. Participants were told during initial instruction that some trials would include nouns from the study phase, but that they should always try to generate a new verb, not the one they had studied. The ‘High-Constraint’ condition was hypothesized to induce greater conceptual interference by activating the previously studied verb association, requiring inhibitory mechanisms to overcome pre-potent response tendencies. The ‘Low-Constraint’ condition was expected to induce relatively less conceptual interference because the noun cues were not previously associated with a semantically related verb during the study phase. The cued-recall task was similar to the recall task used in the second phase: a previously studied noun was displayed with the word ‘Recall’ above, and participants were asked to recall the associated verb. This recall condition served as a baseline control that permitted a direct contrast of brain activity related to memory retrieval and idea generation within high- and low-constraints.
The experimental design followed the same procedure across all conditions. Visual stimuli were presented using E-Prime and viewed through a mirror attached to the head coil. Trials consisted of a jittered fixation cross (4–6 s), a thinking period displaying the condition (‘Create’ or ‘Recall’) above a noun paired with a question mark (e.g., shoe-?; 7 s), and a response period requiring participants to speak their responses into the MRI microphone (3 s); the duration of the thinking period was based on reaction time data provided in Prabhakaran et al. (2013). Verbal responses were recorded online during functional imaging by an experimenter. Recall responses were subsequently coded for accuracy, and Create responses were coded for semantic distance via Latent Semantic Analysis (Green, 2016); behavioral data from three subjects were not included due to technical issues with the MRI microphone. Only valid responses (i.e., verbs) were included in the LSA. Semantic distance was derived by computing the inverse of the semantic similarity values (Prabhakaran et al. 2013).
MRI Data Acquisition and Preprocessing
Participants completed the fMRI task in a single run. Whole-brain imaging was performed on a 3T Siemens Magnetom MRI system (Siemens Medical Systems, Erlangen, Germany) using a 16-channel head coil. BOLD-sensitive T2*-weighted functional images were acquired using a single shot gradient-echo EPI pulse sequence (TR = 2000 ms, TE = 30 ms, flip angle = 78°, 32 axial slices, 3.5 × 3.5 × 4.0 mm, distance factor 0%, FoV = 192×192 mm, interleaved slice ordering) and corrected online for head motion. The first two volumes were discarded to allow for T1 equilibration effects. A high resolution T1 scan was acquired for anatomic normalization. Imaging data were slice-time corrected and realigned using the Statistical Parametric Mapping (SPM) 12 package (Wellcome Institute of Cognitive Neurology, London). Functional volumes were co-registered and resliced to a voxel size of 3mm3, normalized to the MNI template brain (Montreal Neurological Institute), and smoothed with an 8 mm3 isotropic Gaussian kernel.
Functional Network Connectivity
To explore differences in functional network connectivity across conditions, we used independent component analysis (ICA) implemented in the CONN Toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012). ICA is a data-driven method for identifying spatiotemporal voxel clusters, also known as intrinsic connectivity networks (Calhoun, Adali, Pearlson, & Pekar, 2001), and has been widely used to identify brain networks in resting-state and task-based fMRI data. For our purposes, ICA was employed to extract brain networks associated with the three task conditions and to explore correlational patterns among these networks. We identified 20 independent components within the fMRI task data, using a dimensionality reduction of 64 (Calhoun et al., 2001). We then extracted networks of interest (i.e., group-level spatial maps) to explore functional interactions during the task conditions: anterior default (medial prefrontoal cortex), posterior default (posterior cingulate/precuneus and bilateral angular gyri), left executive (left dorsolateral prefrontal cortex and left anterior inferior parietal lobe), right executive (right dorsolateral prefrontal cortex and right anterior inferior parietal lobe), and salience (bilateral insulae and anterior cingulate cortex). Network maps were specified as regions of interest (ROI) to examine between-network connectivity differences associated with the three task conditions.
fMRI Univariate Analysis
Functional imaging analysis was conducted using SPM12. We specified fixed-effects models for each participant in a first-level analysis. All events within an experimental trial were modeled in the design matrix. Subject-specific movement parameters were modeled as regressors of no interest. Contrasts were computed for the three conditions of interest—verb generation for studied nouns (High-Constraint), verb generation for unstudied nouns (Low-Constraint), and cued recall for studied nouns (Recall)—and entered into a second-level random-effects model. Unless otherwise noted, results are reported when significant at a voxel-level threshold of p < .05 familywise error (FWE) corrected and cluster size k ≥ 10.
Functional connectivity analysis was conducted using the CONN Toolbox in Matlab (Whitfield-Gabrieli & Nieto-Castanon, 2012). For each participant, CONN implemented CompCor, a method for identifying principal components associated with segmented white matter (WM) and cerebrospinal fluid (CSF; Behzadi et al., 2007). These components were entered as confounds along with realignment parameters in a first-level analysis (Whitfield-Gabrieli & Nieto-Castanon, 2012). For second-level analyses, t-tests on Fisher’s Z-transformed correlations were computed to test for differences between task conditions. Functional connectivity results are reported when significant at threshold of p < .05 false discovery rate (FDR) corrected.
Results
Behavioral Results
Participants accurately recalled a majority of the noun-verb pairs during the first (M = 76%, SD = .16) and second (M = 94%, SD = .06) cued-recall tests. Recall performance improved significantly from the first to the second memory test (t = 6.28, p < .001), suggesting that the restudy period effectively boosted memory for the noun-verb pairs1. Regarding semantic distance, results showed that the semantic distance between noun cues and verb responses was significantly greater in the Low-Constraint (M = .76, SD = .03) compared to the High-Constraint condition (M = .74, SD = .05; t(21) = 2.12, p = .04), suggesting that the interference manipulation affected idea production, reflected in moderately decreased semantic distance in the High-Constraint condition.
Verb Generation and Memory Retrieval
The fMRI analysis began by contrasting the two verb generation conditions separately with the memory retrieval condition. Generating verb associations for new nouns compared to recalling studied associations (Low-Constraint > Recall) revealed significant activation of four clusters: left IFG (BA 45), left superior frontal gyrus (BA 8), caudate, and cerebellum (see Table 1). The reverse contrast (Recall > Low-Constraint) showed activation of several clusters corresponding to the default network, including the precuneus (PCC; 31), medial prefrontal cortex (mPFC; BA 10), bilateral angular gyri (AG; BA 40), and right middle temporal gyrus (MTG; BA 21).
Table 1.
Univariate analysis contrasting verb generation (‘High-Constraint’ and ‘Low-Constraint’) and memory retrieval (‘Recall’)
| Peak (MNI) | |||||||
|---|---|---|---|---|---|---|---|
| Region | Lat. | BA | x | y | z | k | T |
| Low-Constraint > Recall | |||||||
| Inferior Frontal G. | L | 45 | −51 | 35 | 14 | 641 | 9.75 |
| Superior Frontal G. | L | 8 | −3 | 17 | 56 | 361 | 10.64 |
| Caudate | L | – | −15 | 5 | 8 | 85 | 8.09 |
| Cerebellum | R | – | 18 | −70 | −31 | 114 | 7.29 |
| Recall > Low-Constraint | |||||||
| Medial Prefrontal C. | R | 10 | 3 | 53 | −4 | 30 | 7.00 |
| Middle Temporal G. | R | 21 | 60 | −13 | −19 | 39 | 9.11 |
| Angular G. | L | 40 | −57 | −61 | 38 | 48 | 9.06 |
| R | 40 | 60 | −52 | 38 | 565 | 13.19 | |
| Precuneus | R | 31 | 12 | −55 | 35 | 562 | 11.12 |
| Middle Frontal G. | R | 8 | 30 | 35 | 47 | 25 | 6.51 |
| High-Constraint > Recall | |||||||
| Inferior Frontal G. | L | 45 | −51 | 17 | 23 | 1035 | 10.74 |
| Superior Frontal G. | – | 6 | 0 | 26 | 44 | 569 | 12.24 |
| Anterior Insula | R | 13 | 30 | 26 | −7 | 63 | 9.42 |
| Thalamus | L | – | −6 | −4 | −1 | 235 | 9.13 |
| Cerebellum | L | – | −33 | −58 | −37 | 21 | 7.36 |
| R | – | 18 | −73 | −28 | 398 | 9.59 | |
| Recall > High-Constraint | |||||||
| Precuneus | R | 31 | 12 | −55 | 29 | 29 | 7.18 |
| Inferior Parietal L. | R | 40 | 60 | −40 | 47 | 95 | 7.49 |
Notes. Lat. = Laterality, BA = Brodmann area, k = cluster size, L/R = Left/right; C = Cortex; G = Gyrus; L = Lobule. Results are corrected for multiple comparisons (p < .05, FWE-corrected; k > 10).
We then assessed activity related to verb generation for studied nouns (i.e., the interference condition) compared to memory retrieval (High-Constraint > Recall). Results showed significant activation within six voxel clusters (see Table 1). Two of these clusters overlapped with the previous contrast—left IFG (BA 45) and cerebellum—with additional clusters found within the right anterior insula (BA 13), MFG (BA 6), and thalamus. The reverse contrast (Recall > High-Constraint) yielded activity of two clusters within the precuneus (BA 31) and right inferior parietal lobe (IPL; BA 40).
Semantic Interference During Verb Generation
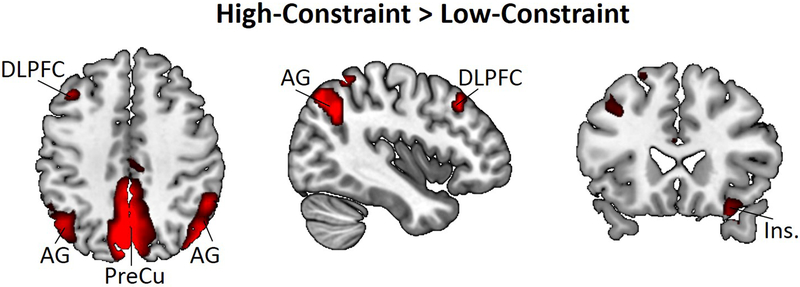
Our next analysis examined brain activity during semantic interference by contrasting the High-Constraint and Low-Constraint conditions (High-Constraint > Low-Constraint). Initial results showed activation of a small cluster within the precuneus (p < .05, FWE corrected). At a reduced threshold (p < .001, uncorrected), additional clusters were found in the left DLPFC (BA 9), PCC (BA 23), bilateral AG (BA 40), and right anterior insula (BA 13; see Table 2 and Figure 1). The reverse contrast (Low-Constraint > High-Constraint) did not reveal significant activation related to the Low-Constraint condition at both the corrected and uncorrected thresholds. These findings provide preliminary evidence that constrained idea generation involves relatively greater activation of regions of the default, executive, and salience networks.
Table 2.
Univariate analysis contrasting ‘High-Constraint’ and ‘Low-Constraint’ verb generation
| Peak (MNI) | |||||||
|---|---|---|---|---|---|---|---|
| Region | Lat. | BA | x | y | z | k | T |
| High-Constraint > Low-Constraint | |||||||
| Precuneus | L | 7 | −3 | −55 | 38 | 1141 | 6.03 |
| R | 7 | 9 | −55 | 71 | 13 | 4.18 | |
| Angular G. | L | 40 | −45 | −61 | 41 | 1622 | 4.34 |
| R | 40 | 48 | −49 | 32 | 269 | 5.23 | |
| Posterior Cingulate C. | – | 23 | 0 | −25 | 26 | 24 | 3.98 |
| Middle Frontal G. (DLPFC) | L | 9 | −42 | 26 | 44 | 16 | 4.25 |
| Anterior Insula | R | 13 | 30 | 20 | −28 | 27 | 4.10 |
| Lingual G. | – | 18 | 0 | −76 | −10 | 15 | 3.78 |
| Cerebellum | – | – | 0 | −61 | −1 | 43 | 4.41 |
| Low-Constraint > High-Constraint | |||||||
| – | – | – | – | – | – | – | |
Notes. Lat. = Laterality, BA = Brodmann area, k = cluster size, L/R = Left/right; C = Cortex; DLPFC = dorsolateral prefrontal cortex; G = Gyrus; L = Lobule. Results are uncorrected for multiple comparisons (p < .001, uncorrected; k > 10).
Figure 1. Univariate results contrasting ‘High-Constraint’ and ‘Low-Constraint’ verb generation.
Notes. AG = angular gyrus; DLPFC = dorsolateral prefrontal cortex; INS = insula; PreCu = precuneus.
Functional Network Connectivity
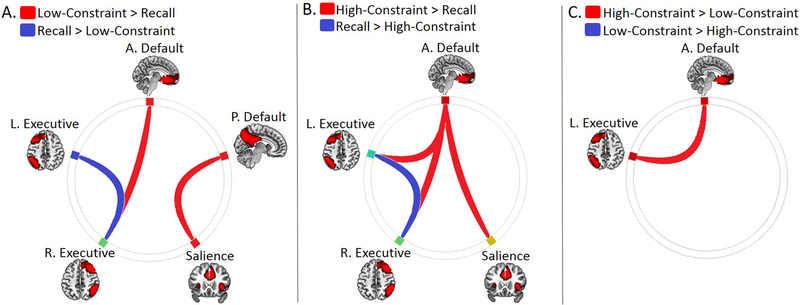
Our next set of analyses examined interactions among the five networks of interest identified using ICA—anterior default, posterior default, left executive, right executive, and salience—during verb generation and memory retrieval. We first contrasted network connections associated with the Low-Constraint condition compared to memory retrieval (Low-Constraint > Recall). Results showed increased functional coupling of the posterior default and salience networks, as well as the anterior default and right executive networks (see Figure 2a). The reverse contrast (Recall > Low-Constraint) revealed greater coupling of the right and left executive networks.
Figure 2. Between-network functional connectivity contrasting verb generation (‘High-Constraint’ and ‘Low-Constraint’) and memory retrieval (‘Recall’).
Notes. A = anterior; P = posterior; R = Right; L = Left.
We then assessed network connections associated with the High-Constraint condition compared to memory retrieval (High-Constraint > Recall). Similar to the results of the Low-Constraint condition, we found increased coupling of the anterior default network with the right executive network (see Figure 2b). In addition, the anterior default network showed further coupling with two other networks: left executive and salience. The reverse contrast (Recall > High-Constraint) revealed similar connectivity as shown in the other reverse contrast (Recall > Low-Constraint) between the left and right executive networks.
Finally, we contrasted the High-Constraint condition with the Low-Constraint condition (High-Constraint > Low-Constraint). This analysis revealed greater connectivity between the anterior default and left executive network (see Figure 2c). The reverse contrast (Low-Constraint > High-Constraint) did not show significant connectivity differences. In sum, the verb generation conditions were associated with increased functional coupling among the default, salience, and executive control networks.
Discussion
Creative cognition has previously been associated with functional interactions among large-scale brain networks, but the mechanisms underlying these dynamics remain largely unknown. The present research isolated one core component of creative cognition—overcoming conceptual interference during idea production—and identified specific patterns of functional connectivity among these systems. Univariate analysis contrasting High-Constraint vs. Low-Constraint verb generation revealed greater activity within core regions of the default (bilateral AG and precuneus), salience (right anterior insula), and control (DLPFC) networks (p < .001, uncorrected). We extended these findings to the level of large-scale brain networks, and demonstrated that semantic interference induces differential interactions among these systems. The findings provide initial insight into the neurocognitive mechanisms underlying brain network interactions during constrained idea production.
As expected, we found greater activation of the left IFG during both generative conditions when compared to memory retrieval. The left IFG has previously been implicated in fMRI studies using the verb generation task (Crescentini et al., 2010; Thompson-Schill, 2003), and it has shown consistent activation in neuroimaging studies of creative cognition (Gonen-Yaacovi et al., 2013; Wu et al., 2015). A substantial body of evidence has shown that the left IFG supports semantic retrieval processes, particularly during cognitive tasks involving the search and selection of target concepts among a large set of competing alternatives (Crescentini et al., 2010; Grindrod et al., 2008). We also found common activation of the cerebellum and subcortical structures (e.g., caudate) in the generative conditions compared to the control condition. Although we did not make predictions about the involvement of these regions, their activation is consistent with recent neuroimaging research on creative cognition, such as studies implicating the cerebellum (Saggar et al., 2015, in press) and caudate (Jauk et al., 2015). Notably, the only difference that emerged across generative conditions when contrasted with the control task was unique activation of the right anterior insula during constrained generation. As a key node of the salience network, the right anterior insula plays a central role in detecting behaviorally relevant stimuli (Uddin, 2015) and has also been shown to function as a switching mechanism between the default and executive control networks (Menon & Uddin, 2010; Sridharan, Levitin, & Menon, 2008). The right anterior insula thus may support constrained generation by both detecting previously encoded information (i.e., the studied noun-verb association) and facilitating interactions between the default and control networks.
Using ICA, we extended the univariate findings by extracting networks associated with the task conditions. Between-network connectivity analyses revealed differential network configurations underlying verb generation with high- and low-constraints compared to memory retrieval. Although both generative conditions elicited increased connectivity among the default, salience, and control networks, the networks showed greater convergence during high-constraint generation compared to low-constraint generation. Specifically, the anterior default network cluster acted as a hub during high-constraint generation, showing connections to the salience and bilateral executive networks. During low-constraint generation, in contrast, results revealed a relatively sparse connectivity profile, with the three networks diverging into discrete, pairwise connections. This pattern suggests that constrained idea production elicits greater network convergence compared to low-constraint production, a pattern that may reflect greater coordination among brain systems supporting memory retrieval and cognitive control.
This interpretation was further reinforced by the contrast of the two generative conditions. Compared to low-constraint generation, the high-constraint condition was associated with greater functional coupling between the anterior default and left executive networks. We suspect that the high-constraint condition caused semantic interference by activating a pre-potent response (i.e., the encoded verb association), which in turn recruited cognitive control circuits to inhibit its activation and redirect search processes. This explanation is consistent with studies demonstrating the involvement of left lateral prefrontal cortex in the selection of non-dominant semantic associates (Jefferies, 2013; Whitney, Kirk, O’Sullivan, Ralph, & Jefferies, 2011). Moreover, the observed functional coupling of the left executive network with default regions during constrained generation is in line with a recent fMRI study reporting increased functional connectivity between the PCC and left lateral prefrontal regions as a function of increasing demands on semantic retrieval (Krieger-Redwood et al., 2016).
The constraining effect of semantic interference on idea production was supported by the behavioral results showing decreased semantic distance in this condition relative to the low-constraint condition. This observation is consistent with research described on the serial order effect in divergent thinking tasks—the tendency to produce stereotypical responses (or known uses for objects) at the early stages of idea generation (Beaty & Silvia, 2012)—although the current paradigm and behavioral studies vary considerably in terms of time allotted for idea production. Nevertheless, because the response period for verb generation was limited to a short duration (7 s; cf. Green et al., 2015), we suspect that the probability of activating salient semantic associates at the onset of the generation period was maximized by the interference manipulation.
Our findings extend previous research reporting default and control network coupling by clarifying the interaction of cognitive control and memory systems during creative idea production. Recent work has identified other task contexts associated with cooperation of these brain systems (for reviews, see Beaty et al., 2016; Zabelina & Andrews-Hanna, 2016). In a study of visual art production, Ellamil et al. (2012) found greater functional connectivity among core regions of the default and control networks during the evaluation of previously generated ideas. Although we suspect that default-control network coupling reflected the involvement of inhibitory mechanisms, in the context of Ellamil et al. (2012), an alternate interpretation of such coupling is that it reflects increased demands on idea evaluation. Default-control network interactions were reported in a study of poetry generation (Liu et al., 2015). In this study, professional poets were asked to freely generate novel poetry in one condition, and then revise their ideas in a separate condition. Compared to generation, the revision condition was associated with increased coupling among default and control network regions. Similar to the present study, the generation tasks used in these studies were relatively less constraining compared to the evaluation and revision tasks. Likewise, the generation of novel verb associations in the semantic interference condition reported here required increased cognitive control demands because it was more constraining than generating verbs with less interference, reflected in greater coupling of the control network with default regions.
The verb generation task employed in this study was motivated by a similar task described in recent behavioral and neuroimaging studies (for a review, see Green, 2016). Green and colleagues modified the classic verb generation task by cuing participants to “think creatively” as they searched for verbs to relate to presented noun; they were also the first research group to apply Latent Semantic Analysis to quantify semantic distance of participant responses (Prabhakaran et al. 2013). In a series of neuroimaging studies, Green et al. provide evidence that compared to uncued conditions with no creativity instructions, cuing participants to think creatively elicits robust activation within frontopolar cortex (Green et al., 2015), a region involved in relational integration and flexible thinking (Bendetowicz et al., in press; Boorman et al., 2009; Green et al., 2006). It is important to point out that our task similarly instructed people to think creatively during the verb generation task, but unlike Green and colleagues, we did not observe activation within frontopolar cortex. We suspect that this discrepancy is critically related to differences in task contexts and corresponding fMRI contrasts. Specifically, Green et al. (2015) assessed brain activity related to cued (creativity instructions) vs. uncued (no creativity instructions). In contrast, our study did not include an uncued condition but rather cued participants to think creatively in both generative conditions (i.e., ‘High-Constraint’ and ‘Low-Constraint’ verb generation); our paradigm also included an experimental manipulation designed to induce semantic interference during creative verb generation. Taken together, these key differences in task demands and fMRI contrasts between our study and those of Green and colleagues raise challenges for comparing results across studies. We nevertheless think that both approaches can shed light on the neurocognitive mechanisms associated with creative idea production.
Costs and Benefits of Memory to Creative Thought
The present study illustrates how memory can constrain idea production, pointing to a potential cost of memory to creativity. On the other hand, an emerging literature has documented a central role of semantic (Beaty et al., 2014; Benedek et al., 2012b; Kenett et al., 2014, in press) and episodic (Addis et al., 2016; Madore et al., 2015, 2016) memory systems to creative cognition. The present work illustrates how semantic memory can constrain creative thought by activating salient conceptual knowledge during idea generation, but it is clear that memory also benefits creativity by providing acquired knowledge and retrieval mechanisms (Abraham, in press). We think that it is useful to briefly consider our study in the context of this broader literature in order to highlight ways in which memory and creativity may interact.
Contemporary models of memory posit at least two systems. A semantic system stores information about the meaning of concepts and their interrelations; such concepts are thought to be represented in a network that is relatively fixed in its structural organization, although networks can be updated and reorganized through learning and experience. An episodic system, in contrast, stores information about personal past experiences (i.e., episodes; Tulving, 2002); this system is thought to represent past events in a constructive manner, extracting and combining relevant constituent components (i.e., people, places, objects, and actions) into a coherent mental representation. Episodic memory has been shown to support both recalling past experiences and imagining future experiences that have not yet occurred (Schacter et al., 2012), providing evidence for the involvement of constructive episodic retrieval processes (Schacter & Addis, 2007).
Recent behavioral experiments (Madore et al., 2015, 2016) have highlighted the contribution of episodic memory to divergent thinking by using an episodic specificity induction, where 1) participants are trained to recall as much detail as possible about a recently experienced event (e.g., a short video clip), and 2) the effect of this brief training on performance of subsequent tasks is examined. Several studies have shown that specificity inductions selectively enhance performance on subsequent tasks that draw on episodic memory—including imagining future experiences (Madore, Gaesser, & Schacter, 2014; Madore et al., in press) and solving means-end problems (Madore & Schacter, 2014)—by increasing access to episodic content via facilitation of constructive processes (Schacter & Madore, 2016). More directly relevant to the present research, Madore et al. (2015, 2016) demonstrated selective benefits of the specificity induction to tasks involving divergent thinking (i.e., the alternate uses task and the consequences task) but not convergent thinking (i.e., the remote associates task). These findings suggest that divergent thinking relies in part on episodic memory and related retrieval mechanisms involved in the extraction and combination of stored information.
Limitations and Future Directions
The present research used a novel semantic interference manipulation to examine brain mechanisms underlying constrained idea production. Our results extend recent work by clarifying the contribution of the default and executive control networks to creative cognition, and suggest that the interaction of these networks may reflect the interplay of memory systems and cognitive control. Future research should continue to explore brain network interactions supporting creative task performance as the verb generation task employed in this study likely reflects only one aspect of creative cognition. Moreover, the recall condition employed as a control task was notably different from the two idea generation conditions (i.e., High- and Low-Constraint), so comparisons of these generative conditions with the recall condition should be made with caution. Future studies could employ more comparable control tasks such as the uncued verb generation task used in previous studies that asks participants to generate a verb associate without creativity instructions (e.g., Green et al., 2015). Moreover, the difference between the High- and Low-Constraint conditions in terms of semantic distance was marginally significant, so future research should attempt to replicate and extend this finding in a laboratory or neuroimaging context. Our study focused on the impact of semantic memory, but recent evidence suggests that episodic memory also contributes to performance on creative thinking tasks (Madore et al., 2015; Madore et al., 2016). Understanding the specific contributions of these memory systems may clarify the default network’s role in creative cognition, and provide critical insight into how and when memory systems interact with cognitive control to support the production of novel and useful ideas.
Acknowledgments
R.E.B, P.J.S., and M.B. were supported by grant RFP-15–12 from the Imagination Institute (www.imagination-institute.org), funded by the John Templeton Foundation. The opinions expressed in this publication are those of the authors and do not necessarily reflect the view of the Imagination Institute or the John Templeton Foundation. D.L.S was supported by National Institute of Mental Health R01 MH060941.
Footnotes
Note that the second cued-recall test included half the number of trials (n = 18) as the first recall period (n = 36) because half of the studied cues were used for the ‘High-Constraint’ generation condition to induce interference.
References
- Abraham A (in press). The imaginative mind Human Brain Mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, & Wagner AD (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia, 45, 2883–2901. [DOI] [PubMed] [Google Scholar]
- Beaty RE (2015). The neuroscience of musical improvisation. Neuroscience & Biobehavioral Reviews, 51, 108–117. [DOI] [PubMed] [Google Scholar]
- Beaty R, Benedek M, Kaufman S, & Silvia P (2015). Default and executive network coupling supports creative idea production. Scientific Reports, 5, 10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Silvia PJ, & Schacter DL (2016). Creative cognition and brain network dynamics. Trends in Cognitive Sciences, 20, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, & Silvia PJ (2012). Why do ideas get more creative across time? An executive interpretation of the serial order effect in divergent thinking tasks. Psychology of Aesthetics, Creativity, and the Arts, 6, 309–319. [Google Scholar]
- Beaty RE, & Silvia PJ (2013). Metaphorically speaking: Cognitive abilities and the production of figurative language. Memory & Cognition, 41, 255–267. [DOI] [PubMed] [Google Scholar]
- Beaty RE, Silvia PJ, Nusbaum EC, Jauk E, & Benedek M (2014). The roles of associative and executive processes in creative cognition. Memory & Cognition, 42, 1186–1197. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendetowicz D, Urbanski M, Aichelburg C, Levy R, & Volle E (in press). Brain morphometry predicts individual creative potential and the ability to combine remote ideas. Cortex. [DOI] [PubMed] [Google Scholar]
- Benedek M, Beaty R, Jauk E, Koschutnig K, Fink A, Silvia PJ, … & Neubauer AC (2014a). Creating metaphors: The neural basis of figurative language production. NeuroImage, 90, 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Franz F, Heene M, & Neubauer AC (2012). Differential effects of cognitive inhibition and intelligence on creativity. Personality and Individ Differences, 53, 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Jauk E, Beaty RE, Fink A, Koschutnig K, & Neubauer AC (2016). Brain mechanisms associated with internally directed attention and self-generated thought. Scientific Reports, 6, 22959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Jauk E, Fink A, Koschutnig K, Reishofer G, Ebner F, & Neubauer AC (2014b). To create or to recall? Neural mechanisms underlying the generation of creative new ideas. NeuroImage, 88, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Jauk E, Sommer M, Arendasy M, & Neubauer AC (2014c). Intelligence, creativity, and cognitive control: The common and differential involvement of executive functions in intelligence and creativity. Intelligence, 46, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia M, Piccardi L, Palermo L, Nori R, & Palmiero M (2015). Where do bright ideas occur in our brain? Meta-analytic evidence from neuroimaging studies of domain-specific creativity. Frontiers in Psychology, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman ED, Behrens TE, Woolrich MW, & Rushworth MF (2009). How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron, 62, 733–743. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, & Pekar JJ (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Beaty RE, Wei D, Yang J, Sun J, Liu W, … & Qiu J (in press). Longitudinal alterations of frontoparietal and frontotemporal networks predict future creative cognitive ability. Cerebral Cortex. [DOI] [PubMed] [Google Scholar]
- Chen QL, Xu T, Yang WJ, Li YD, Sun JZ, Wang KC, … Qiu J (2015). Individual differences in verbal creative thinking are reflected in the precuneus. Neuropsychologia, 75, 441–449. [DOI] [PubMed] [Google Scholar]
- Christoff K, Irving ZC, Fox KC, Spreng RN, & Andrews-Hanna JR (2016). Mind-wandering as spontaneous thought: A dynamic framework. Nature Reviews Neuroscience, 17, 718–731. [DOI] [PubMed] [Google Scholar]
- Chrysikou EG, Motyka K, Nigro C, Yang S, & Thompson-Schill SL (2016). Functional fixedness in creative thinking tasks depends on stimulus modality. Psychology of Aesthetics, Creativity, and the Arts, 10, 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG, Weber M, & Thompson-Schill SL (2014). A matched filter hypothesis for cognitive control. Neuropsychologia, 62, 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG, & Weisberg RW (2005). Following the wrong footsteps: Fixation effects of pictorial examples in a design problem-solving task. Journal of Experimental Psychology: Learning, Memory, and Cognition, 31, 1134. [DOI] [PubMed] [Google Scholar]
- Crescentini C, Shallice T, & Macaluso E (2010). Item retrieval and competition in noun and verb generation: An fMRI study. Journal of Cognitive Neuroscience, 22, 1140–1157. [DOI] [PubMed] [Google Scholar]
- Duncker K (1945). On problem-solving. Psychological Monographs, 58, i–113. [Google Scholar]
- Ellamil M, Dobson C, Beeman M, & Christoff K (2012). Evaluative and generative modes of thought during the creative process. NeuroImage, 59, 1783–1794. [DOI] [PubMed] [Google Scholar]
- Finke RA, Ward TB, & Smith SM (1992). Creative cognition: Theory, research, and applications. Cambridge: MIT Press. [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, & Christoff K (2015). The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage, 111, 611–621. [DOI] [PubMed] [Google Scholar]
- Friedman NP, & Miyake A (in press). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhooly KJ, Fioratou E, Anthony SH, & Wynn V (2007). Divergent thinking: strategies and executive involvement in generating novel uses for familiar objects. Br J Psychol, 98, 611–625. [DOI] [PubMed] [Google Scholar]
- Gonen-Yaacovi G, de Souza LC, Levy R, Urbanski M, Josse G, & Volle E (2013). Rostral and caudal prefrontal contribution to creativity: A meta-analysis of functional imaging data. Frontiers in Human Neuroscience, 7, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AE (2016). Creativity, within reason: Semantic distance and dynamic state creativity in relational thinking and reasoning. Current Directions in Psychological Science, 25, 28–35. [Google Scholar]
- Green AE, Cohen MS, Raab HA, Yedibalian CG, & Gray JR (2015). Frontopolar activity and connectivity support dynamic conscious augmentation of creative state. Human Brain Mapping, 36, 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJ, Shamosh NA, & Dunbar KN (2006). Frontopolar cortex mediates abstract integration in analogy. Brain Research, 1096, 125–137. [DOI] [PubMed] [Google Scholar]
- Green AE, Spiegel KA, Giangrande EJ, Weinberger AB, Gallagher NM, & Turkeltaub PE (in press). Thinking cap plus thinking zap: tDCS of frontopolar cortex improves creative analogical reasoning and facilitates conscious augmentation of state creativity in verb generation. Cerebral Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindrod CM, Bilenko NY, Myers EB, & Blumstein SE (2008). The role of the left inferior frontal gyrus in implicit semantic competition and selection: An event-related fMRI study. Brain Research, 1229, 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauk E, Neubauer AC, Dunst B, Fink A, & Benedek M (2015). Gray matter correlates of creative potential: A latent variable voxel-based morphometry study. NeuroImage, 111, 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E (2013). The neural basis of semantic cognition: Converging evidence from neuropsychology, neuroimaging and TMS. Cortex, 49, 611–625. [DOI] [PubMed] [Google Scholar]
- Jung RE, Flores RA, & Hunter D (2016). A new measure of imagination ability: Anatomical brain imaging correlates. Frontiers in Psychology, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Mead BS, Carrasco J, & Flores RA (2013). The structure of creative cognition in the human brain. Frontiers in Human Neuroscience, 7, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Redwood K, Jefferies E, Karapanagiotidis T, Seymour R, Nunes A, Ang JWA, … & Smallwood J (2016). Down but not out in posterior cingulate cortex: Deactivation yet functional coupling with prefrontal cortex during demanding semantic cognition. NeuroImage, 141, 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Erkkinen MG, Healey ML, Xu Y, Swett KE, Chow HM, & Braun AR (2015). Brain activity and connectivity during poetry composition: Toward a multidimensional model of the creative process. Human Brain Mapping, 36, 3351–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Addis DR, & Schacter DL (2015). Creativity and memory: Effects of an episodic-specificity induction on divergent thinking. Psychological Science, 26, 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Gaesser B, & Schacter DL (2014). Constructive episodic simulation: Dissociable effects of a specificity induction on remembering, imagining, and describing in young and older adults. Journal of Experimental Psychology: Learning, Memory, and Cognition,40, 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Jing HG, & Schacter DL (2016). Divergent creative thinking in young and older adults: Extending the effects of an episodic specificity induction. Memory & Cognition, 44, 974–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, & Schacter DL (2014). An episodic specificity induction enhances means-end problem solving in young and older adults. Psychology and Aging, 29, 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP Szpunar KK, Addis DR, & Schacter DL (in press). Episodic specificity induction impacts activity in a core brain network during construction of imagined future experiences. Proceedings of the NationalAcademy of Sciences USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayseless N, Eran A, & Shamay-Tsoory SG (2015). Generating original ideas: The neural underpinning of originality. NeuroImage, 116, 232–239. [DOI] [PubMed] [Google Scholar]
- Mayseless N, & Shamay-Tsoory SG (2015). Enhancing verbal creativity: Modulating creativity by altering the balance between right and left inferior frontal gyrus with tDCS. Neuroscience, 291,167–176. [DOI] [PubMed] [Google Scholar]
- McAvoy M, Mitra A, Coalson RS, d’Avossa G, Keidel JL, Petersen SE, & Raichle ME (2016). Unmasking language lateralization in human brain intrinsic activity. Cerebral Cortex, 26, 1733–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S (1962). The associative basis of the creative process. Psychological Review, 69, 220. [DOI] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function,214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum EC, & Silvia PJ (2011). Are intelligence and creativity really so different? Fluid intelligence, executive processes, and strategy use in divergent thinking. Intelligence, 39, 36–45. [Google Scholar]
- Osman M (2008). Positive transfer and negative transfer/antilearning of problem-solving skills. Journal of Experimental Psychology: General, 137, 97. [DOI] [PubMed] [Google Scholar]
- Pinho AL, de Manzano Ö, Fransson P, Eriksson H, & Ullén F (2014). Connecting to create: Expertise in musical improvisation is associated with increased functional connectivity between premotor and prefrontal areas. The Journal of Neuroscience, 34, 6156–6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho AL, Ullén F, Castelo-Branco M, Fransson P, & de Manzano Ö (2016). Addressing a paradox: Dual strategies for creative performance in introspective and extrospective networks. Cerebral Cortex, 26, 3052–3063. [DOI] [PubMed] [Google Scholar]
- Prabhakaran R, Green AE, & Gray JR (2013). Thin slices of creativity: Using single-word utterances to assess creative cognition. Behavioral Research Methods, 46, 641–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineberg AE, Andrews-Hanna JR, Depue BE, Friedman NP, & Banich MT (2015). Resting-state networks predict individual differences in common and specific aspects of executive function. NeuroImage, 104, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggar M, Quintin EM, Kienitz E, Bott NT, Sun Z, Hong WC, … & Hawthorne G (2015). Pictionary-based fMRI paradigm to study the neural correlates of spontaneous improvisation and figural creativity. Scientific reports, 5, 10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggar M, Quintin EM, Bott NT, Kienitz E, Chien YH, Hong DW, … & Reiss AL (in press). Changes in brain activation associated with spontaneous improvization and figural creativity after design-thinking-based training: A Longitudinal fMRI Study. Cerebral Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, & Szpunar KK (2012). The future of memory: Remembering, imagining, and the brain. Neuron, 76, 677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL & Addis DR (2007). The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society (B), 362, 773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, & Madore KP (2016). Remembering the past and imagining the future: Identifying and enhancing the contribution of episodic memory. Memory Studies, 9, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Beaty RE, & Nusbaum EC (2013). Verbal fluency and creativity: General and specific contributions of broad retrieval ability (Gr) factors to divergent thinking. Intelligence, 41, 328–340. [Google Scholar]
- Spreng RN, Gerlach KD, Turner GR, & Schacter DL (2015). Autobiographical planning and the brain: Activation and its modulation by qualitative features. Journal of Cognitive Neuroscience, 27, 2147–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, & Schacter DL (2013). Intrinsic architecture underlying the relations among the default, dorsal attention, and executive control networks of the human brain. Journal of Cognitive Neuroscience, 25, 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, & Schacter DL (2010). Default network activity, coupled with the executive control network, supports goal-directed cognition. NeuroImage, 53, 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, & Menon V (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences, 105, 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL (2003). Neuroimaging studies of semantic memory: inferring “how” from “where”. Neuropsychologia, 41, 280–292. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, & Farah MJ (1997). Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences, 94, 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16, 55–61. [DOI] [PubMed] [Google Scholar]
- Vartanian O, Bouak F, Caldwell JL, Cheung B, Cupchik G, Jobidon M-E, … Smith I (2014). The effects of a single night of sleep deprivation on fluency and prefrontal cortex function during divergent thinking. Frontiers in Human Neuroscience, 8, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, & Buckner RL (2008). Evidence for a executive control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100, 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, & Poldrack RA (2001). Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron, 31, 329–338. [DOI] [PubMed] [Google Scholar]
- Ward TB, Patterson MJ, & Sifonis CM (2004). The role of specificity and abstraction in creative idea generation. Creativity Research Journal, 16, 1–9. [Google Scholar]
- Weinberger AB, Iyer H, & Green AE (2016). Conscious augmentation of creative state enhances “real” creativity in open-ended analogical reasoning. PloS one, 11, e0150773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Ralph MAL, & Jefferies E (2011). The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex, 21, 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yang W, Tong D, Sun J, Chen Q, Wei D, … Qiu J (2015). A meta-analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Human Brain Mapping, 36, 2703–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabelina DL, & Andrews-Hanna JR (2016). Dynamic network interactions supporting internally-oriented cognition. Current Opinion in Neurobiology, 40, 86–93. [DOI] [PubMed] [Google Scholar]
- Zabelina DL, & Robinson MD (2010). Creativity as flexible cognitive control. Psychology of Aesthetics, Creativity, and the Arts, 4, 136. [Google Scholar]
- Zhang JX, Feng C-M, Fox PT, Gao J-H, & Tan LH (2004). Is left inferior frontal gyrus a general mechanism for selection? NeuroImage, 23, 596–603. [DOI] [PubMed] [Google Scholar]