SUMMARY
Methicillin resistant Staphylococcus aureus (MRSA) is a global public-health threat. Methicillin resistance is carried on mobile genetic elements belonging to the Staphylococcal Cassette Chromosome (“SCC”) family. The molecular mechanisms that SCC elements exploit for stable maintenance and for horizontal transfer are poorly understood. Previously, we identified several conserved SCC genes with putative functions in DNA replication, including lp1413, which we found encodes a single-stranded (ss) DNA binding protein. We report here the 2.18 Å crystal structure of LP1413, which shows that it adopts a winged helix-turn-helix fold rather than the OB-fold normally seen in replication-related ssDNA binding proteins. However, conserved residues form a hydrophobic pocket not normally found in winged helix-turn-helix domains. LP1413 also has a conserved but disordered C-terminal tail. As deletion of the tail does not significantly affect cooperative binding to ssDNA, we propose that it mediates interactions with other proteins. LP1413 could play several different in vivo.
Graphical abstract
LP1413 is a conserved protein encoded by the SCC family of staphylococcal genomic islands. Mir-Sanchis et al report that it binds ssDNA, adopts a wHTH fold and contains an unusual hydrophobic pocket. That SCC elements encode an ssDNA-binding protein suggests a role for DNA replication in their poorly understood lifestyle.

INTRODUCTION
Although Staphylococcus aureus can cause life-threatening infections, it also appears in subclinical infections and as normal flora. Such variability is mainly due to the presence of mobile genetic elements such as plasmids, transposons, bacteriophages and genomic islands (Novick et al., 2001), including the islands known as Staphylococcal Cassette Chromosome, or SCC elements. SCCs carry a variety of passenger genes. Those carrying the mecA gene, which confers resistance to methicillin and other penicillin-type antibiotics, are known as SCCmec elements (Moellering, 2012). Despite the seriousness of MRSA as a public health problem, the mechanistic details of SCC element mobility remain surprisingly poorly understood at the molecular level (Chlebowicz et al., 2014; Haaber et al., 2017; Mašlaňová et al., 2013; Ray et al., 2016; Scharn et al., 2013; Smyth et al., 2011; Witte et al., 2008). To better define the possibilities, we are studying the set of core conserved genes carried by SCC elements.
Although highly mosaic, SCC elements share a common insertion site and a “recombinase locus” comprised of one or two site-specific recombinase genes surrounded by other conserved open reading frames (Ito et al., 1999; Mir-Sanchis et al., 2016; Misiura et al., 2013). The recombinases, which are the best understood of these proteins, catalyse excision from and insertion into the host chromosome (Ito et al., 1999; Misiura et al., 2013). The medical microbiology literature divides SCCmec elements into 13 different “types” according to DNA sequence variations in both their recombinase and mecA loci (Baig et al., 2018; International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC), 2009). However, because mecA is not found on all SCC elements, our previous bioinformatic analysis focused solely on the recombinase locus. We defined two different patterns of conserved genes in this locus, and found that they are likely to function in DNA replication (Mir-Sanchis et al., 2016)(Fig. S1). In both patterns, upstream of the recombinase operon is a single operon that encodes a large ATPase, Cch or Cch2, and one or two additional proteins. Cch and Cch2 belong to different families, but both have homologs among the replication initiator (“Rep”) proteins found in the otherwise-unrelated staphylococcal genomic islands called Staphylococcus aureus pathogenicity islands or SaPIs. We then focused on proteins from a type IV SCCmec element, which belongs to pattern 1 and is carried by an epidemic USA300 strain (Tenover and Goering, 2009). We showed that Cch is an active helicase and that its AAA+ ATPase domain is structurally related to the MCM replicative helicases from archaea and eukaryotes (Mir-Sanchis et al., 2016). In addition, we briefly characterized the other protein encoded by the Cch operon, which we termed LP1413, for Little Protein with domain of unknown function 1413. We found that it bound single stranded DNA (ssDNA) with high affinity but had only very weak affinity for dsDNA, and that it inhibited Cch’s helicase activity on short model substrates, presumably by blocking Cch from loading onto the substrate’s ssDNA tails, as we could not demonstrate direct Cch – LP1413 interactions.
ssDNA binding proteins play pivotal roles in processes where ssDNA intermediates are generated, such as replication, recombination and repair. Such proteins have been found in all domains of life and are mandatory for cell survival (Haseltine and Kowalczykowski, 2002; Shereda et al., 2008; Wold, 1997; Yadav et al., 2013). Many eukaryotic viruses and bacteriophages also harbor these genes (Kazlauskas and Venclovas, 2012, 2015; Kazlauskas et al., 2016; Nimonkar and Boehmer, 2002). Depending on the model system, ssDNA-binding proteins may be found in solution as monomers, dimers, heterotrimers (e.g. eukaryotic RPA) or tetramers (e.g. E. coli SSB) (Alberts and Frey, 1970; Bujalowski and Lohman, 1991; Ferrari et al., 1994; Kim and Richardson, 1993; Shereda et al., 2008; Zhang et al., 2014). Although not clearly related in sequence, ssDNA binding proteins that are involved in DNA replication usually use one or more copies of an oligonucleotide/oligosaccharide binding domain fold (OB-fold), to interact with the ssDNA (Theobald et al., 2003). This OB-motif consists of a highly twisted, five-stranded antiparallel β sheet, flanked by an α helix on one side (Theobald et al., 2003; Ginalski et al., 2004). Bacterial SSB proteins also have a conserved C-terminal tail that interacts with a variety of other proteins involved in replication, recombination and repair (Shereda et al., 2008).
Here we present the crystal structure and detailed biochemical characterization of LP1413, the small ssDNA-binding protein present at the 5′ end of the Cch-containing operon of pattern 1 SCC elements. Interestingly, LP1413 adopts a winged helix-turn-helix (wHTH) fold that is more common in ds-than ssDNA binding proteins. Unlike most wHTH domains, LP1413 has a conserved hydrophobic pocket that could mediate interactions with a single nucleotide or with other proteins. It also has a conserved but disordered C-terminal tail that could mediate interactions with other partners.
RESULTS
LP1413 forms a wHTH with a conserved hydrophobic pocket
We solved the LP1413 X-ray crystal structure by single-wavelength anomalous dispersion using SeMet-containing protein (Table 1). Although ssDNA (dT30) was present in the crystallization, it was not seen in the electron density. Moreover, crystals grown later in the absence of ssDNA had the same space group and unit cell dimensions as the one used for structure determination. There are two monomers per asymmetric unit (Fig. 1A), and crystal packing generates a loosely packed tetramer (Fig. 1B) that is discussed in more detail below. Unlike most ssDNA binding proteins, LP1413 adopts a wHTH fold composed of three α helices and three antiparallel β strands. wHTH domains are most commonly associated with dsDNA-binding.
Table 1.
Data collection, phasing and refinement statistics.
| Se-Met LP1413 (6BTC) | |
|---|---|
| Data Collection | |
|
| |
| Space group | P 61 2 2 |
| Cell dimensions | |
| a, b, c (Å) | 97 97 127.8 |
| α, β, γ (°) | 90 90 120 |
| Peak | |
| Wavelength | 0.9793 |
| Resolution (Å)a | 45.36 - 2.18 (2.255 - 2.18)a |
| R-meas | 0.108 (1.618) |
| I/σ (I) | 27.3 (2.16) |
| CC1/2 | (0.512) |
| Completeness (%) | 96.03 (71.03) |
| Anomalous Completeness (%) | 95.06 (65.5) |
| Redundancy | 27.9 (12) |
|
| |
| Refinement | |
|
| |
| Resolution (Å) | 50 – 2.18 |
| No. reflections | 18475 (1334) |
| R-work/Rfree | 0.234/0.258 |
| Reflections used for R-free | 1850 (136) |
| No. atoms | |
| Protein | 1343 |
| Ligand | 0 |
| Water | 117 |
| B factors | |
| Protein | 38.43 |
| Solvent | 39.82 |
| rms deviations from ideal | |
| Bond lengths (Å) | 0.006 |
| Bond angles (°) | 0.66 |
Statistics for the highest-resolution shell are shown in parentheses.
Figure 1.
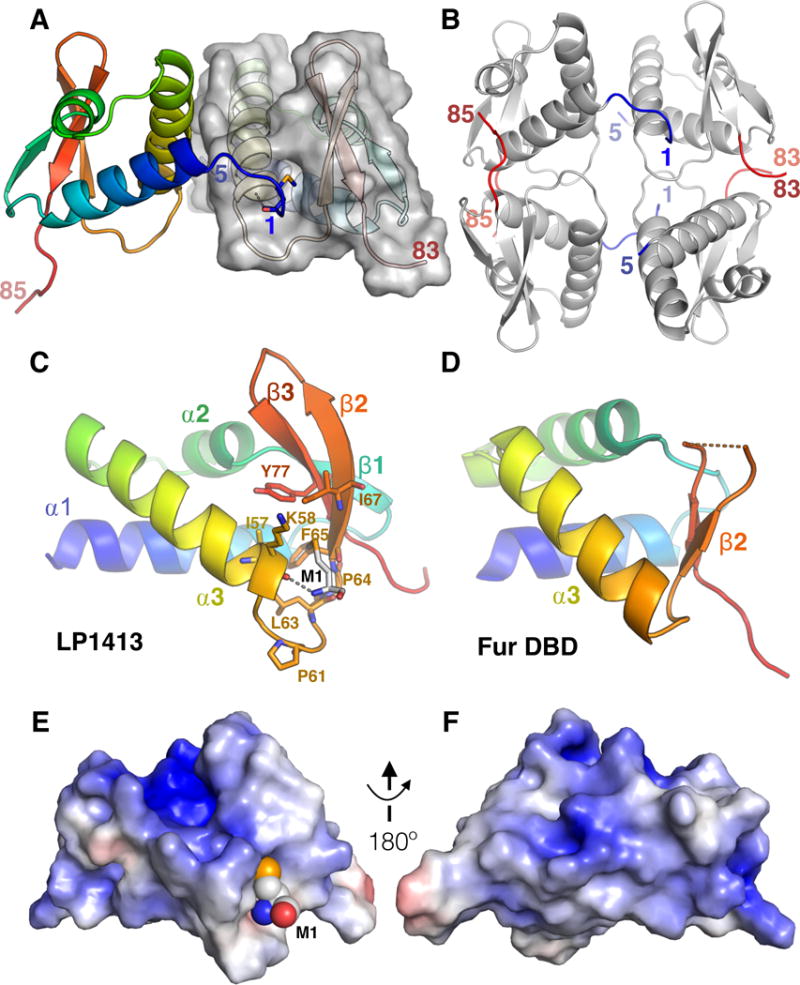
LP1413 structure. A. The two monomers in the asymmetric unit are shaded from blue (N) to red (C), with a partially transparent surface for monomer B. The model includes residues 1-85 of monomer A and 5-83 of B. M1 of monomer A (sticks) is inserted into a hydrophobic pocket on monomer B, while its NH3+ is hydrogen bonded to the carbonyl of K58 at the C-terminus of H3. B. Four copies of LP1413 form a loosely packed in the crystal. Residues M1-S5 and I79-C85 are colored blue and red respectively, and the first and last ordered residues in each chain are labeled. C, D: Comparison of LP1413 to a canonical wHTH domain. The DNA binding domain of Fur (Ferric uptake regulator; PDBid 4ETS (Butcher et al., 2012)), is shown as an example as it was the closest structural homolog to LP1413 found by the Dali server (Holm and Sander, 1995). They differ most in the region of the LP1413’s hydrophobic pocket: in LP1413, α3 and β2 are farther apart, with a longer turn connecting them that contains two conserved prolines (P61 and P64; shown as sticks, as are residues lining the pocket). E, F. Surface of LP1413 colored according to electrostatic potential (blue positive; red negative), as calculated by the APBS plugin to Pymol (Baker et al., 2001). M1 is shown as spheres. The view in E is the same as in part C, and that in F is rotated 180° about a vertical axis. See also Figure S1.
The LP1413 structure also reveals an unusual feature not found in most wHTH domains: a hydrophobic pocket between helix 3 and strand 2 (Fig. 1 C vs. D). To examine the conservation of this pocket, we retrieved 113 non-identical homologous sequences from the Uniref100 database (Suzek et al., 2015), checked that those most distant from our LP1413 were found in a similar genetic context to that of LP1413 (that is, adjacent to a DUF927 – containing protein (i.e. a Cch homolog) with serine-family site-specific recombinases nearby), and aligned them (Fig. S2), and mapped their conservation onto the structure (Fig. 2) (Ashkenazy et al., 2016). The residues lining the pocket are highly conserved, as are two prolines in the turn between helix 3 and strand 2. In the crystal, the hydrophobic pocket of monomer B is occupied by M1 of monomer A, and that of monomer A is partially occupied by M1 of monomer B (Fig S1). It is unclear whether or not M1 is the natural ligand for this pocket, as LP1413 is monomeric in the absence of DNA (Fig S3). We could also model a single nucleotide into the pocket with no steric clashes (Fig. 2B).
Figure 2.
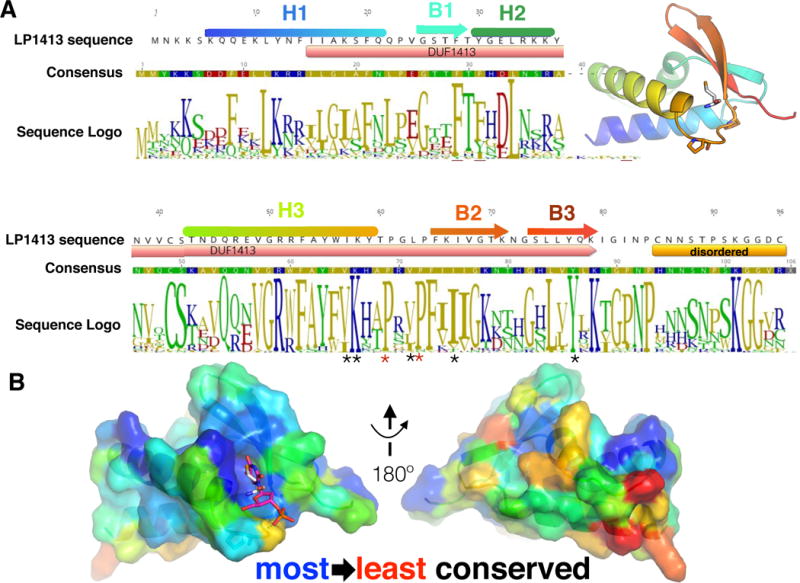
LP1413 sequence conservation. A. Sequence of LP1413 from SCCmec type IV, with secondary structure elements colored as in Fig 1. The region included in DUF 1413 and the disordered C-terminal tail are shown below the sequence. A consensus sequence and logo generated (using Geneious (Kearse et al., 2012)) from an alignment of 113 sequences retrieved from the Uniref100 database are shown, coloured according to polarity. * underneath the logo marks residues lining the hydrophobic pocket and red * the prolines in the turn. Inset: the same cartoon as in Fig. 1C, for reference. B. Views of LP1413 monomer B oriented as in Figure 1C (left) and rotated 180°, with the sequence conservation mapped onto the surface using Consurf (Ashkenazy et al., 2016). In the left panel, M1 of monomer A is shown as white sticks, and a modelled dT as magenta sticks. See also Figure S2.
LP1413 binds ssDNA tightly and cooperatively
Given that LP1413’s structure is quite unusual for an ssDNA binding protein, we decided to better characterize its ssDNA-binding activity. Previously we showed that it bound ssDNA much more tightly than dsDNA, and that it inhibited Cch’s helicase activity on short model substrates, presumably by blocking Cch from loading onto the ssDNA tails of those substrates, as the two proteins did not comigrate on a sizing column. First, we determined that LP1413 binds poly(dT)30 with nanomolar affinity and a Hill coefficient of 3.2 +/− 1. (Fig. 3, Fig. S4 and Table S1). Surprisingly for a small, monomeric protein, only one shifted band rather than a ladder appeared with this substrate and with dT25 and dT36. In addition, when comparing these 3 substrates, the mobility of the complex increased as the ssDNA length increased, suggesting that LP1413 wraps ssDNA around itself as do many other SSBs and consistent with regions of positive charge lying on both faces of the protein (Fig. 1E, F) (Shereda et al., 2008).
Figure 3.
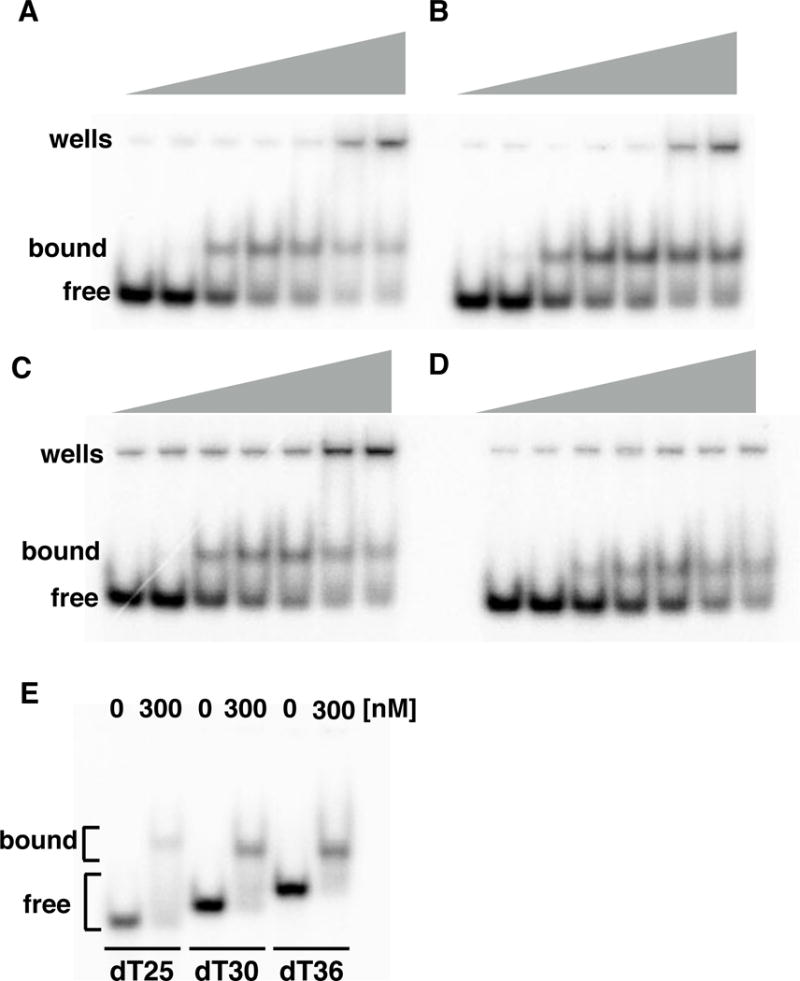
LP1413 binds ssDNA. A-D; EMSAs showing binding of WT and variant LP1413 to dT30. Protein variants used are noted over each gel (Δ5 and Δ18 are missing the last 5 and 18 C-terminal residues, respectively) and concentrations were 0,10, 30, 50, 100, 300 and 500 nM and DNA was 2nM. E. Comparison of the LP1413-ssDNA complexes formed with ssDNAs of different lengths. See also Figure S4.
As shown in Fig. 4, when longer ssDNA was used as substrate (60- and 88nt) several shifted bands appeared. In the case of the 88mer, one of the bands appearing at high concentrations of WT protein appears to run similarly to free DNA, but actually runs faster than free DNA when smaller, C-terminally truncated proteins (described below) are used. These results imply that compact protein-DNA complexes are formed, but further study is required to determine their stoichiometry.
Figure 4.
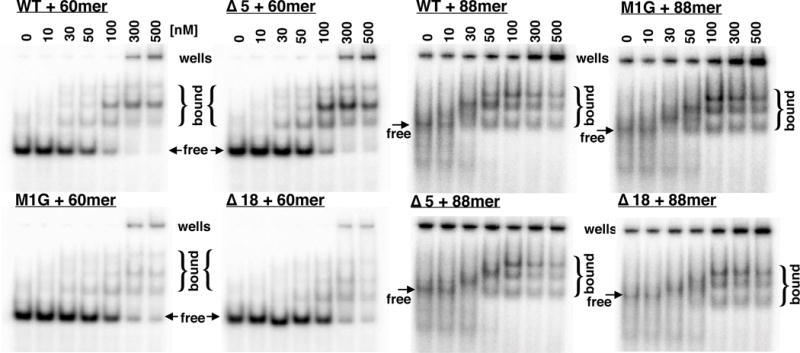
LP1413 forms multiple complexes with 60- and 88-nt ssDNAs. The protein variants, concentrations, and DNA substrates are denoted above each gel. DNA was 2nM in each assay. See Table S2 for oligonucleotide sequences.
Formation of an oligomer upon ssDNA binding could explain the Hill coefficient and the appearance of only a single shifted band with the 25-36nt substrates. A tetramer with approximate 222 symmetry (Fig. 1 B) can be found in the crystal, mediated by the interactions between M1 and the hydrophobic pocket and the between the ordered portion of the C-terminal tail and a neighboring protein. However, the significance of this tetramer is unclear because (1) these interactions do not bury a large amount of hydrophobic surface area and they differ in detail between the two halves of the tetramer and (2) as described below, removing these interactions has only a minor effect on ssDNA binding.
Deletions of the N- and C-termini have minor effects on ssDNA binding
Although the C-terminal tail of LP1413 was disordered in the crystal, its sequence is conserved, suggesting that it might mediate cooperative protein-protein interactions in the presence of ssDNA. To test that idea, we deleted the last five residues (K92 – C96; “Δ5”) and eighteen residues (K79-C96; “Δ18”) (Fig. 2A). Δ5 and Δ18 bound the dT30 substrate with 2.7- and 4.6-fold lower affinity, respectively, than WT, and with Hill coefficients that were smaller but within error of that for WT: 2.0 +/− 0.3 and 2.4 +/− 0.3, vs. 3.2 +/− 1 for WT (Table S1). Although the binding patterns with the longer substrates were too complicated to quantify, these truncations noticeably weakened binding to the 60mer but not to the 88mer (Fig 4). The sum of these results suggests that although the carboxy-terminal end of LP1413 is highly conserved, it is not critical for ssDNA binding.
In bacteriophage T4 SSB, removal of the amino terminal residues led to a loss of cooperativity (Shamoo et al., 1995). Although LP1413 is a monomer in solution (Fig. S3), in our structure M1 of one subunit docks into the hydrophobic pocket of another, indicating another possible mechanism for cooperative ssDNA binding (Fig. 1 and S1). To address this question, we generated an M1G variant (see material and methods and Fig. S3). M1G bound the dT30 substrate with 2.6-fold lower affinity than WT and with slightly lower cooperativity as well (Hill coefficient of 1.5 +/− 0.2) (Fig. 3, S4 and Table S1). However, as with the previous mutants, M1G binding to the 88-mer was indistinguishable from that of WT, indicating that although the M1 - hydrophobic pocket interactions may contribute in some way to ssDNA binding, they are not critical.
DISCUSSION
Although LP1413 has not been studied in vivo, our study suggests that it might play multiple roles (Fig. S5). On one hand, LP1413 shares multiple biochemical features with other, more canonical SSB proteins. It binds ssDNA cooperatively and with high affinity, it appears to wrap the ssDNA substrate around itself, and it has a conserved C-terminal tail. Furthermore, it is genetically linked to a helicase (Cch), suggesting a function in DNA replication. On the other hand, some features of LP1413 differ from those of canonical bacterial SSBs. First, although conserved, the sequence of its C-terminal tail differs from that of cannonical eubacterial and bacteriophage SSB proteins (Cernooka et al., 2017) (Shereda et al., 2008), indicating that it may interact with a different set of proteins. Second, it has a wHTH rather than an OB fold, suggesting that it might also bind dsDNA or dsRNA. We cannot rule out the possibility that in addition to binding ssDNA, LP1413 has high affinity for an as-yet-unidentified specific dsDNA or RNA sequence.
We hypothesize three non-exclusive scenarios for LP1413’s function in vivo: i) it acts as an SSB at the replication fork during SCCmec replication or transfer ii) it acts as a regulatory protein; iii) it aids in origin recognition and melting (Fig. S5). Although there is no definitive evidence for replication of SCC elements, it was recently reported that MRSA strains selected for enhanced resistance contained multiple copies of SCCmec (Gallagher et al., 2017).
The well-studied bacterial and bacteriophage SSBs play multiple roles at the replication fork (Shereda et al., 2008). As LP1413 is found in the same operon as a helicase, it seems likely that it aids in replication by preventing strand re-annealing (Fig. S5A). In addition to this simple role, SSBs generally interact with other replication proteins such as DNA polymerases, helicases, primases, and topoisomerase III (Shereda et al., 2008). Although we have not detected interactions between Cch (the helicase) and LP1413 in vitro, such interactions might still occur in vivo, perhaps stimulated by binding DNA or other proteins. LP1413’s conserved C-terminus and hydrophobic pocket are both good candidates for interactions with other proteins. Further experiments are needed to identify binding partners for LP1413, which could include proteins encoded by SSC or other mobile genetic elements as well as the host’s replication machinery. SSBs also protect ssDNA from endonucleases. This would be particularly important for SCC elements if they exploit conjugation machinery encoded by other genetic elements for horizontal transfer to new strains (Ramsay, 2016), as the transferred DNA would be single-stranded in that case. Cch might provide ssDNA to the unidentified transfer machinery, with LP1413 protecting that ssDNA during the process.
The closest structural homologs of LP1413 are dsDNA and dsRNA binding proteins (Fig. 1), suggesting that LP1413 might have a regulatory role in vivo (Fig. S5B). It might act through binding structured mRNAs, as some bacteriophage SSBs do (Borjac-Natour et al., 2004; von Hippel et al., 1982; Krisch and Allet, 1982; Shamoo et al., 1993) (Fulford et al., 1986; Model et al., 1982), or it might regulate transcription by binding to an as-yet-unidentified specific dsDNA sequence.
LP1413 might also play a role in origin recognition and/or melting. Although little is known about the SCCmec lifestyle, Cch, the helicase that shares an operon with LP1413, is homologous to Rep proteins from the Staphylococcus aureus pathogenicity islands (SaPIs). The SaPIs are known to replicate after they excise from the host chromosome, and their Rep proteins are self-loading helicases that initiate replication by recognizing multiple iterons surrounding an AT-rich region at the SaPI origin (Novick et al., 2010). In our previous work, Cch bound tightly to dsDNA, but we could not definitively identify a preferred binding site, nor have we yet identified an origin of replication for SCC elements (Mir-Sanchis et al., 2016). LP1413 may act as the specificity factor and work in coordination with Cch to initiate replication (Fig. S5C). This hypothesis would also explain our observation that the SaPIs, which use their Rep proteins to recognize the origin, do not encode any LP1413 homologs. Interestingly, some plasmid Rep proteins (which are unrelated to those of the SaPIs) act as both dsDNA and ssDNA binding proteins by means of two wHTH motifs (Wegrzyn et al., 2014).
Some mobile genetic elements are frugal in their genetic content primarily because physical space is a privilege during transfer. It is therefore common to find that one gene is involved in several functions: primase-helicase functions fusions, replication initiator proteins that also have helicase activity or ssDNA binding proteins that also have regulatory functions. LP1413 seems to be another of such examples. Whereas it is an ssDNA binding protein in vitro, its x-ray crystal structure suggests that might also be involved in dsDNA or RNA transactions.
We previously classified SCC conserved genes in two patterns that correlated with the type of recombinase (ccr) genes present (Mir-Sanchis et al., 2016) (Fig. S1). In group 1, the lp1413-cch operon lies upstream of the ccrAB recombinase genes, whereas in group 2, a different three-ORF operon lies upstream of the ccrC recombinase gene. This operon encodes a polymerase that might act as a primase (Iyer et al., 2008), a small hypothetical protein and cch2, a putative helicase with homology to a different family of SaPI Reps. Although lp1413 shows no homology with the small hypothetical protein, given its similar location they might share functions (Mir-Sanchis et al., 2016). There are no reported studies of the in vivo functions of the proteins encoded by either of these operons. Our work strongly suggests that they have role(s) in DNA replication, and suggests new directions for understanding the maintenance and horizontal transfer of SCC elements at the molecular level.
STAR METHODS
Contact for reagent and resource sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact Phoebe A. Rice (PRice@uchicago.edu)
Experimental model and subject details
The LP1413 protein described in this work is carried by SCCmec type IV and is identical to NCBI Reference Sequence WP_001036438.1. Although the “multispecies” record for this NCBI entry notes that identical proteins have been found in multiple strains of Staphylococcus aureus, staphylococcus argentus and staphylococcus epidermidis, we note that, as originally described in (Mir-Sanchis et al., 2016), we cloned this protein from genomic DNA from USA300 methicillin-resistant staphylococcus aureus strain 923, which was a gift of Robert Daum (Dept. of Pediatrics, University of Chicago).
Methods Details
LP1413 expression and purification
Untagged, WT LP1413 from SCCmec type IV was expressed and purified in E.coli Rosetta (DE3)pLysS (Novagen) from a pET-21a derivative, pET21aLP1413 (Novagen) as described previously (Mir-Sanchis et al., 2016). The primary purification procedures were (NH4)2SO4 fractionation and cation exchange chromatography, and while the procedure used here did not deviate from that described in (Mir-Sanchis et al., 2016) it is redescribed here for completeness. E.coli Rosetta (DE3)pLysS containing pET21aLP1413 were grown at 37 °C in LB (Luria Berta ni) broth supplemented with 100 μg ml−1 ampicillin. Once the culture reached an optical density of about 0.7 0.5 mM IPTG was added, followed by growth for another 3 hours at 37 °C. Cells were pelleted by c entrifugation in an F10S rotor for 10 min at 8,000 r.p.m, then resuspended 100 mM Tris, pH 8, 60% sucrose, 10% glycerol, 1 M NaCl, 1 mM EDTA and 1 mM DTT, then lysed in a microfluidizer (LV1-30K, Microfluidics). Debris was pelleted by centrifugation for 1 hour at 4 °C at 18,000 r.p.m. in an SS-34 rotor. LP1413 and other proteins were then precipitated by mixing the supernatant with 50% ammonium sulfate (weight/volume), followed by centrifugation at 18,000 r.p.m. for 1 h at 4 °C in an SS-34 rotor. For cation exchange chromatography, the pellet was redissolved in buffer A1 (25 mM HEPES, pH 7, 0.5 mM EDTA, 5% glycerol and 1 mM DTT). This sample was subjected to two rounds of chromatography on a HiPrep 16/10 SP FF column (GE Healthcare) followed by one round on a MonoS 5/50 GL (GE Healthcare) column. In all three cases, proteins were eluted by a gradient from buffer A1 to buffer B1 (buffer A1 plus 2M NaCl). After the first separation on the SP FF column, LP1413-containing fractions were pooled, diluted 1:2 with buffer A1 before reloading onto the same column. After the second separation on the SP FF column, LP1413-containing fractions were pooled and dialyzed overnight against 10% B1before loading onto the MonoS column. Fractions containing pure LP1413 were then concentrated and dialyzed at 4 °C overnig ht into storage buffer (20 mM Tris, pH 8, 0.5 mM EDTA, 200 mM NaCl, 20% glycerol and 1 mM DTT). Samples were rapidly frozen in small aliquots and stored at −80C.
Cloning, expression and purification of LP1413 Mutants
Mutants M1G, Δ5 and Δ18 were made with the quikchange kit (Agilent). See supplementary table 2 for primer sequences. For the M1G construct, an N-terminal His6 tag followed by a TEV cleavage site was added, such that cleavage leaves a single G in place of the WT M1 (Fig. S3). For Δ5 and Δ18 stop codons were inserted into the WT, untagged clone at the appropriate position (after S91 or Q78). Mutants Δ5 and Δ18 were expressed and purified as WT, although Δ18 was less soluble than WT LP1413. Mutant M1G was induced and lysed as WT but cell pellets were resuspended in buffer HA1 containing 50mM phosphate, 1M NaCl, 5% glycerol, 1mM DTT, pH7.5. Soluble proteins were passed through His Trap™ Column (GE Healthcare) twice and eluted with buffer HB1 containing 50mM phosphate, 1M NaCl, 5% glycerol, 1mM DTT, 2M NaCl, pH7.5. Positive fractions were treated with TEV protease overnight at 4° and passed through His Trap column again. The flow through was dialyzed overnight with HA1, loaded onto MonoS™ 5/50 GL (GE Healthcare), and eluted with buffer HB1. Positive fractions were mixed, concentrated and dialyzed at 4° overnight into storage buffer containing 20mM Tris pH8, 0.5mM EDTA, 200mM NaCl, 20% Glycerol and 1mM DTT. TEV cleavage was confirmed by PAGE (Fig. S3).
Selenomethionine (SeMet) - labeled LP1413 protein
Selenomethionine incorporation was achieved by suppression of methionine biosynthesis and the addition of selenomethionine to the media. E.coli Rosetta (DE3)pLysS containing pET21aLP1413 were grown at 37 °C in M9 medium plus additives (0.4% gl ucose, 10 mM NaCl, 0.1 mM CaCl2, 2 mM MgSO4, 1 μg ml−1 thiamine and 100 μg ml−1 ampicillin). A 20 ml initial culture was pelleted after 1 hour (8,000 r.p.m. in an F10S rotor at 4 °C), resuspended in 20ml of s ame media as above, then added to 1L of the same media as above. Once the culture reached an OD600 of 0.4–0.5, selenomethionine (to a final concentration of 60 mg/l) and a mix of other amino acids were added (l-isoleucine, l-leucine, l-lysine, l-phenylalanine, l-threonine and l-valine; final concentration 100mg/l total). After an additional 15 minutes of growth, 0.5 mM IPTG was added to induce LP1413 expression. The remainder of the purification procedure was exactly as for unlabeled LP1413, except that all buffers contained 10 mM DTT.
LP1413 Crystallization and Structure Determination
Native and SeMet-derivatized LP1413 protein were crystallized by the hanging drop vapor diffusion method. LP1413 (initially ~ 11 mg/ml) was mixed with poly(dT)30 to a 1.5:1 molar ratio (later we determined that the DNA was not in the crystals, perhaps due to the high salt). The complex was mixed in a 1:1 ratio with well solution containing 100mM NaCitrate, pH6, 10mM MgCl2, 1.8M Li2SO4 and 14% glycerol. Crystals grew to full size in 3 days and were flash-frozen in liquid N2 without additional cryoprotectant. Multiple data sets were collected at the GM/CA beamline 23ID-D at the Advanced Photon Source (Argonne, IL) and processed with HKL-2000 (Otwinowski, Z and Minor, W., 1997). Phases and an experimental map were generated from a single SeMet data set using the default parameters of Phenix Autosol (Adams et al., 2010). We used Coot for modeling (Emsley et al., 2010) and Phenix for refinement (Adams et al., 2010). Some early refinement runs used simulated annealing and some used the “real space” option, but the final refinement was carried out using energy minimization in reciprocal space, with individual B-factors but no TLS refinement. The asymmetric unit contains two copies of LP1413. Although M1 is the only methionine, an anomalous difference map (generated with CCP4 (Winn et al., 2011)) had 3 significant peaks (Fig. S1). The strongest peak was deep within the hydrophobic pocket of monomer B (see below) but corresponded to M1 of monomer A. The other two peaks were smaller, and most likely correspond to different positions for the largely-disordered N-terminus of monomer B, one within the hydrophobic pocket of monomer A and the other nearby. A representative region of the LP1413 model and experimental map are shown in Fig. S1.
Electrophoretic mobility shift assays. (EMSAs)
Substrate sequences are giving in Supplementary Table 2. Reactions were performed in 20 μl volume, where 2 μl of protein (10x) and 2 μl of DNA (20nM) were added to 14 μl of water and 2 μl of 10x reaction buffer. 1 μM oligonucleotide (sequences in Table S1) was 5′ end 32P-phosphorylated with T4 kinase, then a 10x excess of cold oligonucleotide was added and the mix was diluted to 20 nM. LP1413 protein was diluted in dilution buffer (20mM Tris pH 8, 100mM NaCl, 5% glycerol, 50ng μl−1 BSA, 5mM DTT), up to 10x the desired concentration. The final composition of reaction buffer contained 20mM Tris pH 8, 100mM NaCl, 5% glycerol, 50ng μl−1 BSA, 5mM DTT and 5mM MgSO4. After 30 minutes at room temperature, 5 μL of 80% glycerol was added and the entire volume was loaded on a 10% non-denaturing polyacrylamide gel. Gels were run in 0.5x TBE.
QUANTIFICATION AND STATISTICAL ANALYSIS
Gels were visualized and quantified by phosphor-imaging (BioRad Personal Molecular Imager). Background was subtracted and the area under the intensity profile curve for each band was measured. Material that was retained in the wells was included in the “bound” fraction. Dissociation constants and Hill coefficients were calculated by the equation θ = Bmax * ˆℎ/(dˆℎ + ˆℎ) where θ is the bound fraction/total, Bmax is the maximum binding, Kd is the protein concentration at which half DNA molecules are bound and h is the Hill slope. Experimental data were fitted with GraphPad Prism Software version 7, www.graphpad.com. The Hill plot shown in Figure S4 was generated by plotting the fitted curve as Log (Y/1 − Y) against Log[L] where Y=θ and [L] is protein concentration.
For each protein variant, EMSAs with 2 nM dT30 and protein concentrations of 0, 4, 5, 7.5, 10, 20, 30, 40, 50, 100, 200, 300, 400, 500 nM were run twice. To determine the Hill coefficients and apparent Kds, additional data from earlier gels were also used. In these earlier gels, the DNA concentration was also 2nM but fewer protein concentrations were used (0,10, 30, 50, 100, 300 and 500 nM). The earlier EMSA with WT LP1413 (Figure 3A) was run 3 times, those with M1G, Δ5 and Δ18 (Figures 3B,C, and D) were run twice. The mean and standard deviation of the fraction bound at each concentration, determined from both early and later experiments, are plotted in Figure S4.
The EMSA experiment in figure 3E (not subjected to further quantitation) was repeated 4 times. The EMSA experiments shown in figure 4 (also not subjected to further quantitation) were repeated as follows: for the 60-nt substrate, n=2; for the 88-nt substrate with WT and M1G protein, n=3; for the 88-nt substrate and Δ5 and Δ18, n=2.
Figures were made with Pymol (Baker et al., 2001) and GraphiteLifeExplorer (Hornus et al., 2013).
Data and Software Availability
Coordinates and structure factors have been deposited with the Protein Data Bank with PDBid 6BTC, and raw images with the SBGrid database as data set # 531, doi:10.15785/SBGRID/531. Raw gel images have been deposited with Mendeley Data: https://data.mendeley.com/datasets/vh896md947/draft?a=feba66cd-983d-4855-8e91-14e414a5e14e
Software used in this project was curated by SBGrid (Morin, et al., 2013).
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| E.coli Rosetta (DE3)pLysS (expression strain) | Novagen | N/A |
| Deposited Data | ||
| LP1413 coordinates and structure factors | This paper | PDB: 6BTC |
| Raw diffraction images | This paper | SBGrid data set#531 doi:10.15785/SBGRID/531 |
| Raw gel images | This paper | https://data.mendeley.com/datasets/vh896md947/draft?a=feba66cd-983d-4855-8e91-14e414a5e14e |
| Experimental Model | ||
| LP1413 protein (identical to the sequence used here) is found on SCC elements in many staphylococci. The NCBI Reference Sequence for this protein is WP_001036438.1. | N/A | https://www.ncbi.nlm.nih.gov/protein/WP_001036438.1 |
| Oligonucleotides | ||
| See table S2 for sequences of oligonucleotides used as primers and as EMSA substrates | This paper | N/A |
| Recombinant DNA | ||
| pET-21a expression vector containing LP1413 gene | Novagen; Mir-Sanchis et al., 2016 | pET21aLP1413 |
| Software and Algorithms | ||
| CCP4 | Winn et al., 2011 | www.ccp4.ac.uk |
| HKL-2000 | Otwinowski, Z and Minor, W., 1997 | http://www.hkl-xray.com/ |
| Phenix | Adams et al., 2010 | https://www.phenix-online.org/ |
| Coot | Emsley et al., 2010 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| GraphPad Prism Software version 7 | www.graphpad.com | |
| Pymol | Baker et al., 2001 | https://pymol.org/2/ |
| GraphiteLifeExplorer | Hornus et al., 2013 | http://www.lifeexplorer.info/ |
| SBgrid | Morin et al., 2013 | https://sbgrid.org/ |
Supplementary Material
Highlights.
LP1413 is an ssDNA-binding protein encoded by SCC-family genomic islands
It adopts a winged helix-turn-helix fold
it has an unusual, conserved hydrophobic pocket
it may function in SCC replication and/or horizontal transfer
Acknowledgments
We thank all members of GM/CA beam line 23ID at Advanced Photon Source (Argonne National Laboratory) for assistance in data collection. We thank Laura Swain assistance with model building and refinement, and the Crosson lab for the use of their sizing column. Funding provided by the National Institutes of Health, R01 GM121655 to PAR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
PAR designed the overall project. IMS and YZP purified and crystallized LP1413, and IMS carried out the subsequent crystallography and biochemistry. IMS and PAR wrote the paper.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts BM, Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970;227:1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Ashkenazy H, Abadi S, Martz E, Chay O, Mayrose I, Pupko T, Ben-Tal N. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44:W344–W350. doi: 10.1093/nar/gkw408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig S, Johannesen TB, Overballe-Petersen S, Larsen J, Larsen AR, Stegger M. Novel SCCmec type XIII (9A) identified in an ST152 methicillin-resistant Staphylococcus aureus. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2018;61:74–76. doi: 10.1016/j.meegid.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci U S A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjac-Natour JM, Petrov VM, Karam JD. Divergence of the mRNA targets for the Ssb proteins of bacteriophages T4 and RB69. Virol J. 2004;1:4. doi: 10.1186/1743-422X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujalowski W, Lohman TM. Monomers of the Escherichia coli SSB-1 mutant protein bind single-stranded DNA. J Mol Biol. 1991;217:63–74. doi: 10.1016/0022-2836(91)90611-9. [DOI] [PubMed] [Google Scholar]
- Butcher J, Sarvan S, Brunzelle JS, Couture JF, Stintzi A. Structure and regulon of Campylobacter jejuni ferric uptake regulator Fur define apo-Fur regulation. Proc Natl Acad Sci U S A. 2012;109:10047–10052. doi: 10.1073/pnas.1118321109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernooka E, Rumnieks J, Tars K, Kazaks A. Structural Basis for DNA Recognition of a Single-stranded DNA-binding Protein from Enterobacter Phage Enc34. Sci Rep. 2017;7:15529. doi: 10.1038/s41598-017-15774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowicz MA, Mašlaňová I, Kuntová L, Grundmann H, Pantůček R, Doškař J, van Dijl JM, Buist G. The Staphylococcal Cassette Chromosome mec type V from Staphylococcus aureus ST398 is packaged into bacteriophage capsids. Int J Med Microbiol IJMM. 2014;304:764–774. doi: 10.1016/j.ijmm.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari ME, Bujalowski W, Lohman TM. Co-operative binding of Escherichia coli SSB tetramers to single-stranded DNA in the (SSB)35 binding mode. J Mol Biol. 1994;236:106–123. doi: 10.1006/jmbi.1994.1122. [DOI] [PubMed] [Google Scholar]
- Fulford W, Russel M, Model P. Aspects of the growth and regulation of the filamentous phages. Prog Nucleic Acid Res Mol Biol. 1986;33:141–168. doi: 10.1016/s0079-6603(08)60022-7. [DOI] [PubMed] [Google Scholar]
- Gallagher LA, Coughlan S, Black NS, Lalor P, Waters EM, Wee B, Watson M, Downing T, Fitzgerald JR, Fleming GTA, et al. Tandem Amplification of the Staphylococcal Cassette ChromosomemecElement Can Drive High-Level Methicillin Resistance in Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00869-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginalski K, Kinch L, Rychlewski L, Grishin NV. BOF: a novel family of bacterial OB-fold proteins. FEBS Lett. 2004;567:297–301. doi: 10.1016/j.febslet.2004.04.086. [DOI] [PubMed] [Google Scholar]
- Haaber J, Penadés JR, Ingmer H. Transfer of Antibiotic Resistance in Staphylococcus aureus. Trends Microbiol. 2017 doi: 10.1016/j.tim.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Haseltine CA, Kowalczykowski SC. A distinctive single-strand DNA-binding protein from the Archaeon Sulfolobus solfataricus. Mol Microbiol. 2002;43:1505–1515. doi: 10.1046/j.1365-2958.2002.02807.x. [DOI] [PubMed] [Google Scholar]
- Holm L, Sander C. Dali: a network tool for protein structure comparison. Trends Biochem Sci. 1995;20:478–480. doi: 10.1016/s0968-0004(00)89105-7. [DOI] [PubMed] [Google Scholar]
- Hornus S, Lévy B, Larivière D, Fourmentin E. Easy DNA modeling and more with GraphiteLifeExplorer. PloS One. 2013;8:e53609. doi: 10.1371/journal.pone.0053609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC) Classification of Staphylococcal Cassette Chromosome mec (SCCmec): Guidelines for Reporting Novel SCCmec Elements. Antimicrob Agents Chemother. 2009;53:4961–4967. doi: 10.1128/AAC.00579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Katayama Y, Hiramatsu K. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob Agents Chemother. 1999;43:1449–1458. doi: 10.1128/aac.43.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer LM, Abhiman S, Aravind L. A new family of polymerases related to superfamily A DNA polymerases and T7-like DNA-dependent RNA polymerases. Biol Direct. 2008;3:39. doi: 10.1186/1745-6150-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas D, Venclovas C. Two distinct SSB protein families in nucleo-cytoplasmic large DNA viruses. Bioinforma Oxf Engl. 2012;28:3186–3190. doi: 10.1093/bioinformatics/bts626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas D, Venclovas Č. Viral DNA replication: new insights and discoveries from large scale computational analysis. BMC Bioinformatics. 2015;16:A2. [Google Scholar]
- Kazlauskas D, Krupovic M, Venclovas Č. The logic of DNA replication in double-stranded DNA viruses: insights from global analysis of viral genomes. Nucleic Acids Res. 2016;44:4551–4564. doi: 10.1093/nar/gkw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinforma Oxf Engl. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YT, Richardson CC. Bacteriophage T7 gene 2.5 protein: an essential protein for DNA replication. Proc Natl Acad Sci U S A. 1993;90:10173–10177. doi: 10.1073/pnas.90.21.10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisch HM, Allet B. Nucleotide sequences involved in bacteriophage T4 gene 32 translational self-regulation. Proc Natl Acad Sci U S A. 1982;79:4937–4941. doi: 10.1073/pnas.79.16.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mašlaňová I, Doškař J, Varga M, Kuntová L, Mužík J, Malúšková D, Růžičková V, Pantůček R. Bacteriophages of Staphylococcus aureus efficiently package various bacterial genes and mobile genetic elements including SCCmec with different frequencies. Environ Microbiol Rep. 2013;5:66–73. doi: 10.1111/j.1758-2229.2012.00378.x. [DOI] [PubMed] [Google Scholar]
- Mir-Sanchis I, Roman CA, Misiura A, Pigli YZ, Boyle-Vavra S, Rice PA. Staphylococcal SCCmec elements encode an active MCM-like helicase and thus may be replicative. Nat Struct Mol Biol. 2016;23:891–898. doi: 10.1038/nsmb.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiura A, Pigli YZ, Boyle-Vavra S, Daum RS, Boocock MR, Rice PA. Roles of two large serine recombinases in mobilizing the methicillin-resistance cassette SCCmec. Mol Microbiol. 2013;88:1218–1229. doi: 10.1111/mmi.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Model P, McGill C, Mazur B, Fulford WD. The replication of bacteriophage f1: gene V protein regulates the synthesis of gene II protein. Cell. 1982;29:329–335. doi: 10.1016/0092-8674(82)90149-0. [DOI] [PubMed] [Google Scholar]
- Moellering RC. MRSA: the first half century. J Antimicrob Chemother. 2012;67:4–11. doi: 10.1093/jac/dkr437. [DOI] [PubMed] [Google Scholar]
- Morin A, Eisenbraun BJ, Key J, Sanschagrin PC, Timony MA, Ottaviano M, Sliz P. Cutting edge: Collaboration gets the most out of software. eLife. 2013;2:e0145. doi: 10.7554/eLife.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimonkar AV, Boehmer PE. In vitro strand exchange promoted by the herpes simplex virus type-1 single strand DNA-binding protein (ICP8) and DNA helicase-primase. J Biol Chem. 2002;277:15182–15189. doi: 10.1074/jbc.M109988200. [DOI] [PubMed] [Google Scholar]
- Novick RP, Schlievert P, Ruzin A. Pathogenicity and resistance islands of staphylococci. Microbes Infect. 2001;3:585–594. doi: 10.1016/s1286-4579(01)01414-9. [DOI] [PubMed] [Google Scholar]
- Novick RP, Christie GE, Penadés JR. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol. 2010;8:541–551. doi: 10.1038/nrmicro2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Methods in Enzymology. Academic Press; New York: 1997. Processing of X-ray Diffraction Data Collected in Oscillation Mode. [DOI] [PubMed] [Google Scholar]
- Ramsay JP. Replicating methicillin resistance? Nat Struct Mol Biol. 2016;23:874. doi: 10.1038/nsmb.3303. [DOI] [PubMed] [Google Scholar]
- Ray MD, Boundy S, Archer GL. Transfer of the methicillin resistance genomic island among staphylococci by conjugation. Mol Microbiol. 2016 doi: 10.1111/mmi.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharn CR, Tenover FC, Goering RV. Transduction of staphylococcal cassette chromosome mec elements between strains of Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57:5233–5238. doi: 10.1128/AAC.01058-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamoo Y, Tam A, Konigsberg WH, Williams KR. Translational repression by the bacteriophage T4 gene 32 protein involves specific recognition of an RNA pseudoknot structure. J Mol Biol. 1993;232:89–104. doi: 10.1006/jmbi.1993.1372. [DOI] [PubMed] [Google Scholar]
- Shamoo Y, Friedman AM, Parsons MR, Konigsberg WH, Steitz TA. Crystal structure of a replication fork single-stranded DNA binding protein (T4 gp32) complexed to DNA. Nature. 1995;376:362–366. doi: 10.1038/376362a0. [DOI] [PubMed] [Google Scholar]
- Shereda RD, Kozlov AG, Lohman TM, Cox MM, Keck JL. SSB as an organizer/mobilizer of genome maintenance complexes. Crit Rev Biochem Mol Biol. 2008;43:289–318. doi: 10.1080/10409230802341296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth DS, Wong A, Robinson DA. Cross-species spread of SCCmec IV subtypes in staphylococci. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2011;11:446–453. doi: 10.1016/j.meegid.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzek BE, Wang Y, Huang H, McGarvey PB, Wu CH. UniRef clusters: a comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics. 2015;31:926–932. doi: 10.1093/bioinformatics/btu739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover FC, Goering RV. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J Antimicrob Chemother. 2009;64:441–446. doi: 10.1093/jac/dkp241. [DOI] [PubMed] [Google Scholar]
- Theobald DL, Mitton-Fry RM, Wuttke DS. Nucleic acid recognition by OB-fold proteins. Annu Rev Biophys Biomol Struct. 2003;32:115–133. doi: 10.1146/annurev.biophys.32.110601.142506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel PH, Kowalczykowski SC, Lonberg N, Newport JW, Paul LS, Stormo GD, Gold L. Autoregulation of gene expression. Quantitative evaluation of the expression and function of the bacteriophage T4 gene 32 (single-stranded DNA binding) protein system. J Mol Biol. 1982;162:795–818. doi: 10.1016/0022-2836(82)90548-4. [DOI] [PubMed] [Google Scholar]
- Wegrzyn K, Fuentes-Perez ME, Bury K, Rajewska M, Moreno-Herrero F, Konieczny I. Sequence-specific interactions of Rep proteins with ssDNA in the AT-rich region of the plasmid replication origin. Nucleic Acids Res. 2014;42:7807–7818. doi: 10.1093/nar/gku453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AGW, McCoy A, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W, Cuny C, Klare I, Nübel U, Strommenger B, Werner G. Emergence and spread of antibiotic-resistant Gram-positive bacterial pathogens. Int J Med Microbiol IJMM. 2008;298:365–377. doi: 10.1016/j.ijmm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- Yadav T, Carrasco B, Hejna J, Suzuki Y, Takeyasu K, Alonso JC. Bacillus subtilis DprA recruits RecA onto single-stranded DNA and mediates annealing of complementary strands coated by SsbB and SsbA. J Biol Chem. 2013;288:22437–22450. doi: 10.1074/jbc.M113.478347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhou R, Inoue J, Mikawa T, Ha T. Single molecule analysis of Thermus thermophilus SSB protein dynamics on single-stranded DNA. Nucleic Acids Res. 2014;42:3821–3832. doi: 10.1093/nar/gkt1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


