Abstract
Social isolation presents a risk factor and worsens outcome to cerebrovascular diseases; however, the underlying mechanisms remain underspecified. This study examines the effect of social environment on microglial reactivity after global cerebral ischemia, to test the hypothesis that social isolation leads to greater microglial responses. Adult female and male mice were pair-housed or socially isolated for one week prior to cardiac arrest/cardiopulmonary resuscitation (CA/CPR) or the sham procedure, and following either 2 or 24 hours of reperfusion, microglia samples were enriched and analyzed for gene expression. At the 2-hour time point, microglia from both females and males exhibited ischemia-induced inflammation, characterized by the gene expression increase of tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β) and interleukin 6 (IL-6), regardless of the housing conditions. However, at 24 hours post-ischemia, social housing attenuated microglial pro-inflammatory gene expression in a sex-specific manner. At this time point, the ischemia-induced increased expression of IL-1β and IL-6 was attenuated by social interaction in microglia from male mice, while among female mice social attenuation of the inflammatory response was observed in the microglial expression of cell surface protein major histocompatibility complex II (MHC II). A second study examined behavioral and physiological measures 96 hours after ischemic injury. At this time point, female and male mice displayed increased locomotion and exploratory behavior following CA/CPR relative to controls. Regardless of sex, ischemia also elicited neuroinflammation and neurodegeneration, both of which were modulated by the social environment. Hippocampal nitric oxide (iNOS), cortical TNF-α, and counts of Fluoro-Jade C positive stained cells in the CA1 region of the hippocampus, were increased in the isolated CA/CPR group relative to sham controls and the pair-housed CA/CPR groups. Together, these data indicate that female and male mice exhibit similar outcome measures and social modulation at 96 hours post-ischemic injury, nonetheless, that social environment influences microglial reactivity to global cerebral ischemia in a sex-specific manner.
Keywords: Social isolation, Global cerebral ischemia, Cardiac arrest/cardiopulmonary resuscitation, Neuroinflammation, Microglia
1. Introduction
Social factors are important determinants of human health and well-being. Indeed, social isolation is a major risk factor for disease onset and progression, and is associated with increased all-cause mortality [1–4]. A wide array of human and animal studies recapitulate the negative consequences social isolation has on cerebral ischemia outcome [5–6]. Nevertheless, the physiological mechanisms that mediate the detrimental effects of social isolation on cerebral ischemia survival and recovery remain poorly understood [7–8].
Inflammation is part of the physiological cascade that leads to neuronal death following cerebral ischemia [9]. The first phase of ischemia-induced inflammation is mediated by microglia and begins immediately upon cessation of blood flow; it includes the activation and proliferation of these cells [10]. As damaged neurons begin to degenerate, they release danger signals that further promote microglial activation, giving rise to the second phase of ischemia-induced inflammation [11]. Both phases occur within the first 24 hours post-ischemia and are primarily mediated by microglia, the innate immune cells of the brain [10, 12–14]. When investigating the early inflammatory response to cerebral ischemia, the present work focuses on microglia due to their immediate role in the ischemic cascade [15], their responsiveness to environmental stressors [16–18], and prior evidence of their involvement in social neuroprotection [19–24].
Sex-differences in microglial biology remain understudied. This is remarkable, as neurodegenerative diseases characterized by neuroinflammation, such as Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis and multiple sclerosis, are sexually dimorphic [25–28] and sex-differences in microglia potentially underlie the observed sex-differences in incidence and severity. Cerebral ischemia is also a sexually dimorphic disease [29–35] and differences in immune responses between females and males have been suggested as a potential mediator of sex-differences in cerebral ischemia outcome [36–38]. An increase in the number of microglia/macrophages following focal cerebral ischemia in male mice, relative to their female counterparts [39], provides evidence for sexual dimorphisms in the inflammatory response to ischemia. The presence of microglia following ischemic injury serves as an indicator of inflammation and subsequent damage [40], suggesting that differences in microglia numbers contribute to increased damage among males. Moreover, elevated CD11b in sham females relative to sham males and a significant increase in the CD11b receptor following cerebral ischemia only among male mice [41], provide additional evidence in support of sexual dimorphisms and their influence on ischemic injury.
The role of microglia in global cerebral ischemia outcome became evident in a study where administration of minocycline, a tetracycline antibiotic that attenuates microglial activation, reduced ischemia-induced inflammation, neurodegeneration, and behavioral deficits [42]. Due to the potential of these innate immune cells to become sensitized by environmental stressors [16–18] and the detrimental consequences of social isolation on cerebral ischemia outcome [6, 19, 24, 43], the present work investigates social influences on ischemia-induced microglial reactivity profile at 2 and 24 hours following cardiac arrest/cardiopulmonary resuscitation (CA/CPR), and evaluates behavioral and physiological outcome at 96 hours post-ischemic injury, in both female and male mice. These studies aim to test the hypothesis that social isolation leads to a prolonged and maladaptive microglial response, which can result in greater ischemia-induced neuroinflammation and neurodegeneration. Thus, the findings will contribute to the body of knowledge concerning social modulation of the inflammatory response to cerebral ischemia and further examine sexual dimorphisms in this heterogeneous disease.
2. Materials and Methods
These studies were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and under protocols approved by the Ohio State Institutional Animal Care and Use Committee. All data were collected by individuals who were uninformed of experimental assignment.
2.1 Experimental design
2.1.1 Experiment 1
Female (n=48) and male (n=48) c57/bl6 mice remained socially isolated (n=24/per sex) or were pair-housed (n=24/per sex) with an ovariectomized female. One week later mice received a cardiac arrest/cardiopulmonary resuscitation (CA/CPR; n=12/per group) or sham control (n=12/per group) procedure, and 2 hours later brain tissue was collected for microglia enrichment using a Percoll gradient. Immediately after the enrichment, mRNA was extracted using the Qiagen microkit and stored in −80°C. Once all samples were collected, cDNA was synthesized and gene expression was used to determine microglial reactivity profile. The same experimental design was completed on a second cohort, for which tissue was collected 24 hours after the ischemic or control procedure.
2.1.2 Experiment 2
Female (n=60) and male (n=60) c57/bl6 mice remained socially isolated (n=30/per sex) or were paired (n=30/per sex) with an ovariectomized female. One week later mice received a CA/CPR (n=15/per group) or sham control (n=15/per group) procedure. At 96 hours post-ischemia, during the light-phase, mice were tested in the open field, elevated plus maze and forced swim test, and then brain tissue was collected for gene expression and histological analysis.
2.2 Animals
Young adult c57bl/6 female and male mice (49–56 days of age, Charles River, Wilmington, MA) were housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved vivarium with a 14:10 light/dark cycle. Upon arriving to our facility, all animals were provided ad libitum access to food and filtered tap water, as well as cotton nesting material. Animals were singly housed in cages that were situated in ventilated racks with controlled temperature and humidity, and allowed one week to habituate prior to experimental assignment.
2.3 Ovariectomy
The stimulus females used for social enrichment arrived our facility prior to the beginning of the studies. Following a 1 week acclimation period, they were anesthetized with isoflurane (induced at ~3% and maintained at ~1.5%). Both ovaries were removed under sterile surgery and buprenorphine (0.05 mg/kg; SC) was administered. To minimize animal usage, the same stimulus females were used in all experiments described in this paper.
2.4 Housing manipulation
At day 0, the experimental mice were either paired with an ovariectomized female or kept in social isolation. Ovariectomized females were used as the pairing stimulus to minimize the aggressive and territorial behavior often exhibited between male mice [44] and because the ovariectomy procedure eliminates the influence of the female estrous cycle over male physiology and behavior [45].
2.5 Cardiac arrest/Cardiopulmonary resuscitation
Cardiac arrest/cardiopulmonary resuscitation (CA/CPR) was used to induce ~8 minutes of global cerebral ischemia in vivo. Mice were anesthetized with 3% isoflurane in air and then maintained on 1.5% isoflurane during the procedure. CA/CPR was induced as previously described [46], but with minor modifications. A temperature probe was inserted in the temporalis muscle, and this temperature served as an index of brain temperature. A rectal temperature probe provided feedback to a heating blanket (Harvard Apparatus, Holliston, MA) to maintain body temperature within the appropriate range. A cannula (Fine Science, Foster City, CA) was inserted into the right femoral artery to measure blood pressure (Columbus Instruments, Columbus, OH). A PE 10 catheter was placed into the right jugular vein for drug administration. An intubation tube was connected to a ventilator (Columbus Instruments, Columbus, OH) and mice were ventilated with a tidal volume of 120 uL and a respiratory rate of 160 breaths per minutes. Following all the preparatory steps, mice were allowed to stabilize for 10 minutes, during which blood pressure and temperature were recorded. At the end of the acclimation period, body temperature was decreased to 27°C by circulating cold water through a coil system underneath the animal and placement of an alcohol patch over the ventrum in order to prevent damage to peripheral organs [47]. To induce cardiac arrest, potassium chloride (KCl, 50 μl, 0.5 M, 4°C) was injected via the jugular catheter and the mice were detached from the ventilator. Slow re-warming of the body via a thermal blanket began when the body temperature reached 27°C after approximately 4 minutes of the procedure. At 7.5 minutes into the arrest period, the mouse was reattached to the ventilator and ventilated with 100% oxygen with a tidal volume of 150 uL and a respiratory rate of 190 breaths per minutes. At 8 minutes following the KCL injection, cardiopulmonary resuscitation was initiated via an injection of epinephrine (EPI, 16 μg in 0.6 cc saline, 37°C) into the jugular vein catheter followed by chest compressions. Mice were maintained on 100% oxygen for 25 minutes following return of spontaneous circulation and then extubated, followed by the removal of catheters, skin suturing, and buprenorphine (0.05 mg/kg; SC) administration. Once the mice had recovered, they were returned to their normal housing conditions until tissue collection. The procedure described above was similar for CA/CPR and sham animals, except that the control animals received isotonic saline instead of KCL or epinephrine, and did not experience chest compressions.
2.6 Behavioral testing
Locomotor activity was assessed for 30 minutes using the open field, a clear acrylic 40 × 40 × 37.5 cm chamber inside a ventilated cabinet (Med Associates, St Albans, VT, USA). A frame located at the base of the chamber with 32 photobeams in a 16 × 16 arrangement, detected horizontal movements. Similarly, a row of beams above detected rearing activity (Open Field Photobeam Activity System, San Diego Instruments, San Diego, CA, USA). Prior to beginning the test, the chamber was cleaned and half a cup of corn bedding was spread inside. Each mouse was placed in the center of the open field and following 2 minutes of acclimation, the testing time began. Prior to each test, the open field chambers were cleaned with a mild soapy water solution. Data were analyzed to determine total locomotor activity, rearing (i.e. exploratory drive), and central tendency during the first 5 minutes of testing time (i.e. reduced anxiety-like behavior in a novel environment).
Anxiety-like behavior was further assessed using the elevated plus maze, a plus-shaped apparatus elevated one meter above the floor with two enclosed arms (50 × 10 × 40 cm) and two open arms (50 × 10 cm; San Diego Instruments, San Diego, CA, USA). Following cleaning of the equipment with 70% ethanol, a mouse was placed in the center of the test apparatus and behavior was recorded for 5 minutes. Later, an uninformed observer scored the videos using Observer software (Exeter Software, Setauket, NY) to record the number of entries into each arm and time spent on each arm type.
Depressive-like behavior was assessed using the forced swim test. Mice were placed in a 4 L Plexiglas beaker filled to a depth of 15 cm with water maintained at 27±1°C. Prior to each test, the beaker was cleaned with 70% ethanol and water was replaced. Behavior was recorded for 5 minutes and later an uninformed observer scored the videos to determine the amount of time spent swimming versus floating using Observer software (Version 5, Exeter Software, Setauket, NY, USA).
2.7 Tissue collection
All mice were euthanized via cervical dislocation. Anesthetics were not provided to avoid altering the natural immune system response. For experiment 1, whole brains were dissected for microglial enrichment. For experiment 2, whole brains were dissected and hemispheres were separated; alternating hemispheres, one was stored in RNA Later for gene expression analysis while the other was post-fixed for histological analysis.
2.8 Microglia enrichment
A Percoll (GE Healthcare, Sweden) gradient was used to enrich microglia, as previously described [48], but with minor modifications. Following euthanasia, the brains were dissected and homogenized by mashing through a 70 μm nylon mesh cell strainer and flushing homogenate with 1× PBS onto a 50 mL conical tube. Once clearing the strainer and reaching a volume of 20 mL, the homogenate was centrifuged at 500 g for 6 minutes at 10°C. The supernatant was discarded and the cell pellet was resuspended in 3 mL of the 70% Percoll solution, transferred to a 15 mL conical tube, and overlaid with the discontinuous Percoll density gradient (3 mL of 50% Percoll, 3 mL of 35% Percoll and 2 mL of 1× PBS, respectively). Following a 20 minute spin at 2000 g, microglia were collected from the interfase between the 70% and 50% Percoll layers. Enriched cells were washed with 1× PBS, and used to assess gene expression.
2.9 Gene expression
mRNA from enriched microglia samples was extracted using the RNeasy microkit while mRNA from the hippocampus and prefrontal cortex samples were extracted using the Rneasy minikit (both from Qiagen, Germany). Extractions were performed according to the manufacturer’s instructions. cDNA was synthesized using M-MLV reverse transcription and diluted 1:10 for subsequent qPCR. The qPCR reaction was run using endogenous control eukaryotic 18S, probes from Applied Biosystems (Myh2, Mm01332564_m1; Tnfα, Mm00443260_g1; Il1β, Mm00434228_m1; Il6, Mm00446190_m1; Nos2, Mm00440502_m1), and Taqman Universal Master Mix (all from Life Technologies), on an ABI 7000 Sequencing Detection System. Relative gene expression was calculated using a relative standard curve, and normalized to the 18S signal. For experiment 1, fold change relative to sham (isolated and paired combined) was determined.
2.10 Post-fixation and cryoprotection
A brain hemisphere was post-fixed overnight in 4% paraformaldehyde (PFA) in 0.1M PBS. Following fixation of the tissue with 4% PFA, brains were incubated overnight for cyroprotection with 30% sucrose in 0.1M PBS. Then, the tissue was frozen and stored in −80°C until sectioning.
2.11 Immunohistochemistry
Coronal brain slices were sectioned at 20 μm using a cryostat and mounted on Superfrost Plus Slides (Fisher Scientific, USA). For neuronal degeneration, Fluoro-Jade C (catalogue # AG325, EMD Millipore, USA) staining was performed. Using coplin jars, slides were submerged in 100% EtOH for 3 minutes and in 70% EtOH for 1 minute. With in-between rinses with distilled water, slides were incubated in 0.06% potassium permanganate for 15 minutes and then in 0.001% Fluoro-Jade staining solution for 30 minutes, while gently shaking. Slides dried overnight and then were coverslippped with Vectashield Antifade Mounting Medium with DAPI (catalogue # H-1200, Vector Laboratories, USA).
2.12 Imaging
Images were captured using a Nikon Eclipse E800 fluorescence microscope and analyzed using Image J software (Java, USA). An individual blinded to experimental assignment completed imaging and histological analysis. To examine neuronal degeneration, three sets of serial images were collected per mouse, all at 20× magnification. Each set focused on a specific region of the hippocampus, the cornu ammonis region 1 (CA1), CA3, or dentate gyrus (DG), and included a total of three images. Using Image J, a region of interest (ROI) box of 500 μm × 287 μm was overlaid on each image and positive stained cells were counted. For each mouse, image counts of a hippocampus region were then averaged and graphed.
2.13 Statistical analysis and data visualization
For all data sets, the Grubbs test (α=0.05) was used once to detect outliers within groups. Samples with a Z-score>2 were removed prior to completing statistical analysis and graphing. Consistently, a p<0.05 was used to determine significance, and female and male data were analyzed separately.
In experiment 1, throughout the different parameters analyzed, the paired and single sham groups did not differ statistically, and so the data were combined into one sham group. Normality was violated and non-parametric Analysis of Variance (ANOVA) on ranks were completed to determine overall significant differences and Dunn’s pairwise multiple comparisons were used to identify specific group differences, using SigmaPlot 13.0. For experiment 2, two-way ANOVAs were performed using GraphPad Prism 6; when significant Sidak multiple comparisons were applied. GraphPad Prism 6 was used for graph visualization. In all figures, data are presented as mean + SEM; group differences were considered significant at p<0.05 and are identified by different letters.
3. Results
3.1 Experiment 1
3.1.1 Microglial reactivity profile following cardiac arrest/cardiopulmonary resuscitation
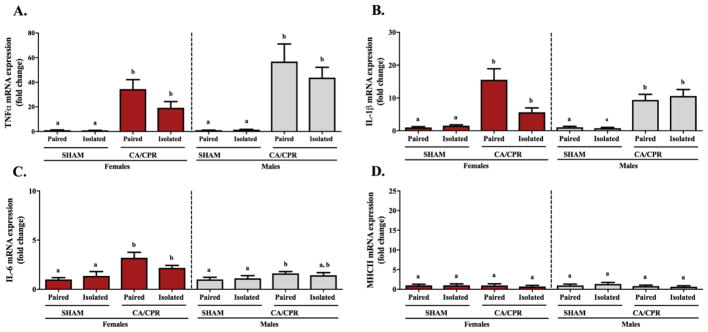
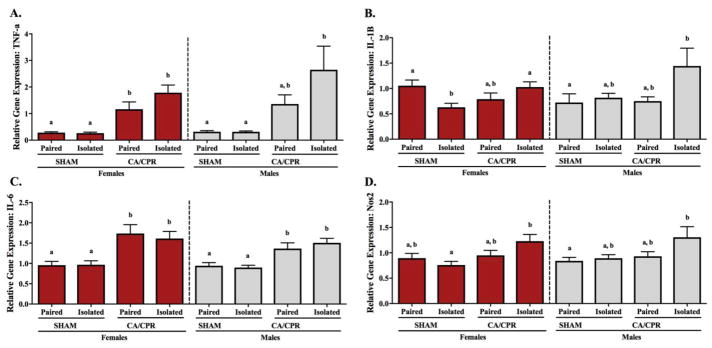
At 2 hours following CA/CPR, whole brain microglia from female and male mice exhibited an ischemia-induce increase in the expression of pro-inflammatory cytokines, with no effect related to housing conditions (Figure 1). Microglial TNF-α gene expression in females varied significantly among the groups (Figure 1A; X2(2)=32.078, p<0.05), exhibiting increased expression in the paired and isolated CA/CPR groups relative to the sham controls (p<0.05 for both). Similarly, in the males there were significant differences among the groups (X2(2)=32.289, p<0.05), with the paired and isolated CA/CPR males expressing greater TNF-α than the sham controls (p<0.05 for both). Microglial IL-1β varied significantly among the treatment groups in both female (Figure 1B; X2(2)=26.468, p<0.05) and male (X2(2)=30.436, p<0.05) mice; in both cases, paired and isolated CA/CPR groups exhibited increased IL-1β gene expression relative to the sham controls (p<0.05 for all). The microglial gene expression of IL-6 in females and males varied significantly among the groups (Figure 1C; X2(2)=17.382, p<0.05 and X2(2)=7.321, p<0.05, respectively). Among the female mice, IL-6 gene expression was elevated in the paired and isolated CA/CPR groups relative to sham controls (p<0.05 for both). However, among males, IL-6 expression was elevated relative to the controls only in the paired CA/CPR group (p<0.05). At this time point, there were no significant differences among treatment groups in the gene expression of MHC II (Figure 1D; females: X2(2)=0.674, p>0.05 and males: X2(2)=1.228, p>0.05).
Figure 1.
Two hours after CA/CPR microglia enriched from the whole brain of female and male mice exhibit an ischemia-induced inflammatory response, regardless of their social experience. Gene expression of pro-inflammatory cytokines TNF-α (A), IL-1β (B) and IL-6 (C), are significantly upregulated in the CA/CPR groups relative to the sham controls. At this time point expression of MHC II (D) remained comparable across all groups.
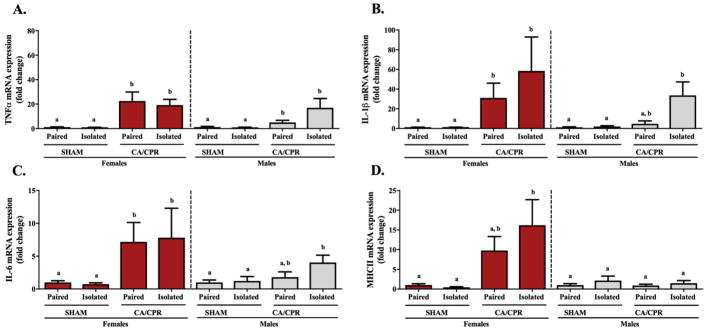
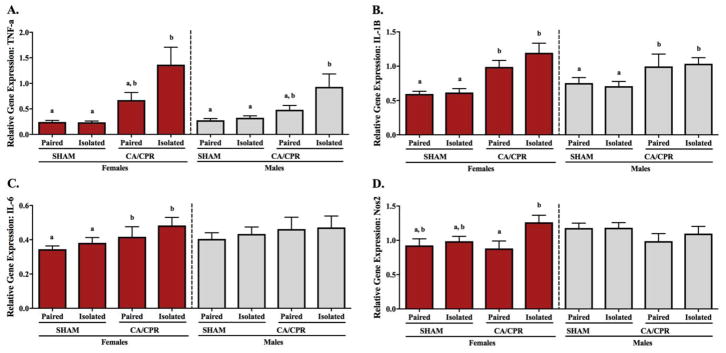
Twenty-four hours following CA/CPR, the ischemia-induced inflammatory response is exacerbated in microglia from socially isolated mice. Microglial TNF-α gene expression in the female and male mice displayed variation among the groups (Figure 2A; X2(2)=29.342, p<0.05 and X2(2)=11.439, p<0.05, respectively). In both cases, paired and isolated CA/CPR groups exhibited increased TNF-α gene expression relative to the sham controls (p<0.05 for all). The microglial IL-1β gene expression in females varied significantly among the groups (Figure 2B; X2(2)=20.484, p<0.05), exhibiting increased expression in the paired and isolated CA/CPR groups relative to the sham controls (p<0.05 for both). Similarly, for the males there were significant differences among the groups (X2(2)=16.729, p<0.05). Multiple comparisons revealed that the isolated CA/CPR group expressed greater IL-1β gene expression levels than the sham controls (p<0.05), whereas the paired CA/CPR group exhibited an intermediate phenotype comparable to both the sham controls and isolated CA/CPR group (p>0.05 for both). Microglial IL-6 gene expression in females and males displayed significant variation among the groups (Figure 2C; X2(2)=16.192, p<0.05 and X2(2)=7.940, p<0.05, respectively). In the female mice, the paired and isolated CA/CPR groups exhibited increased expression relative to the sham controls (p<0.05 for both). In the male mice, only the isolated CA/CPR group had significantly elevated IL-6 relative to the sham group (p<0.05). The paired CA/CPR displayed an intermediate phenotype that did not differ from either the sham controls or the isolated CA/CPR group (p>0.05 for both). At this time point, microglial MHC II gene expression in the female mice varied significantly among the groups (Figure 2D; X2(2)=9.200, p<0.05). Specifically, the isolated CA/CPR females exhibited increased MHC II gene expression relative to the sham controls (p<0.05), while the paired CA/CPR females exhibited an intermediate phenotype that was comparable to both the sham and isolated CA/CPR groups (p>0.05 for both). Similar to the MHC II expression at 2 hours, the male mice microglial MHC II gene expression at 24 hours did not display any group differences (X2(2)=0.139, p>0.05).
Figure 2.
Twenty-four hours after CA/CPR microglia from female mice display an ischemia-induced inflammatory response regardless of their housing condition, whereas in the male mice this response is exaggerated by social isolation (A–C). Among the males, microglial gene expression of IL-1β and IL-6 were increased following CA/CPR only in those that were socially isolated, while the pair-housed exhibited an intermediate phenotype comparable to both the isolated CA/CPR and sham groups. The gene expression of MHC II (D) was increased following ischemia only among female mice, a response that was exacerbated by social isolation.
3.2 Experiment 2
3.2.1 Behavioral outcome following cardiac arrest/cardiopulmonary resuscitation
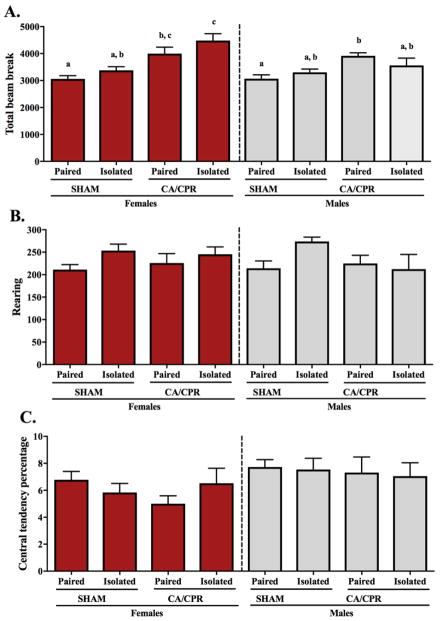
There was variation in total locomotor activity among female and male mice 96 hours after the ischemic insult (Figure 3A). Females exhibited a social environment (F(1,54)=4.244, p<0.05) and an ischemia main effect (F(1,54)=27.54, p<0.05), with both ischemia and isolation increasing total number of beam breaks in the open field. However, there was no interaction between the variables (F(1,54)=0.1821, p>0.05). The pair-housed sham females displayed significantly less locomotor activity than both CA/CPR groups (p<0.05 for both). The isolated sham females total beam break was comparable to the paired sham and paired CA/CPR groups (p>0.05 for both), but significantly lower than the isolated CA/CPR females (p<0.05). Among the CA/CPR females, locomotor activity was highest in the isolated female mice; which did not differ statistically from the pair-housed females (p>0.05). Among the males, ischemic conditions induced hyperlocomotion (F(1,45)=11.37, p<0.05), however, no interaction (F(1,45)=3.147, p>0.05) or social environment main effect (F(1,45)=0.1410, p>0.05) was exhibited in total number of beam breaks. Multiple comparisons reveal that paired CA/CPR males exhibit increased locomotion relative to the paired sham controls (p<0.05), but no additional significant differences between the other groups (p>0.05 for all). Neither rearing activity (Figure 3B) during the 30 minutes in the open field, nor central tendency (Figure 3C) over the first 5 minutes in the open field, varied among the female or male groups. Rearing activity in the females exhibited no main effect by the social environment (F(1,53)=3.753, p>0.05) or the ischemic conditions (F(1,53)=0.03994, p>0.05), and no interaction (F(1,53)=0.5110, p>0.05). In the males, there was also no housing (F(1,46)=1.427, p>0.05) or ischemia (F(1,46)=1.657, p>0.05) main effects, and no interaction (F(1,46)=3.337, p>0.05) over rearing. Central tendency percentage did not vary among females; no ischemic (F(1,53)=0.4758, p>0.05) or housing (F(1,53)=0.1301, p>0.05) main effect, and no interaction (F(1,53)=2.411, p>0.05). Similarly, in the males, central tendency percentage remained comparable within the experimental groups; no main effect by the social environment (F(1,44)=0.06449, p>0.05) or ischemic condition (F(1,44)=0.2626, p>0.05), and no interaction (F(1,44)=0.0016, p>0.05).
Figure 3.
Ninety-six hours after receiving the CA/CPR procedure mice exhibit ischemia-induced hyperlocomotion in the open field test (A). Ischemia increased total locomotion among the females, with the unusual behavior being exacerbated by isolation. Among the males, hyperlocomotion was exhibited only among the pair-housed mice. Exploratory drive and anxiety-like behavior as interpreted by rearing activity (B) and central tendency (C), respectively did not vary among neither the female nor male groups.
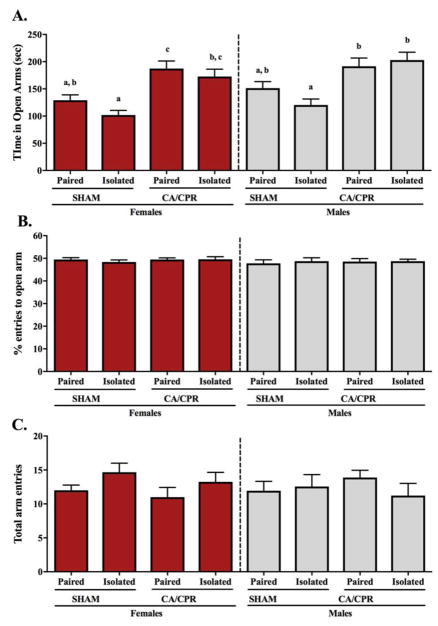
In the elevated plus maze (EPM), females and males exhibit an ischemia-induced increase in exploratory behavior, as indicated by increased time spent in the open arms of the maze. Specifically, female and male mice exhibited an ischemia main effect (F(1,52)=31.69, p<0.05 and F(1,45)=21.62, p<0.05 respectively), with CA/CPR increasing the time spent in the open arms of the maze (Figure 4A). However, there was no main effect of social environment (F(1,52)=3.344, p>0.05 for females and F(1,45)=0.5321, p>0.05 for males) or interaction between the experimental variables (F(1,52)=0.2982, p>0.05 for females and F(1,45)=2.556, p>0.05 for males). Among the females, sham controls spent an equivalent amount of time in the open arms of the EPM regardless of their social environment (p>0.05). Between the CA/CPR females, there was also no difference in time spent in the open arms in response to the housing conditions (p>0.05). The isolated sham females exhibited anxiety-like behavior (i.e. reduced time in the open arms) relative to the CA/CPR groups (p<0.05 for both), whereas the pair-housed sham females displayed increased anxiety-like behavior relative to the paired CA/CPR (p<0.05) but not the isolated CA/CPR females (p>0.05). Among the males, paired and isolated sham mice spent an equivalent amount of time in the open arms of the EPM (p>0.05), and so did the paired and isolated CA/CPR mice (p<0.05). The paired sham males spent similar time in the open arms of the EPM than the CA/CPR groups (p>0.05 for both), however, the socially isolated sham males displayed anxiety-like behavior (i.e. reduced time in the open arms of the EPM) relative to the CA/CPR groups (p<0.05 for both). No differences were displayed in percent entries into the open arm (Figure 4B) or the total arm entries (Figure 4C) of the EPM among the female or male mice. Percent entries into the open arms of the EPM was comparable among the females, displaying no main effect of housing (F(1,50)=0.3092, p>0.05) or ischemic conditions (F(1, 50)=0.3948, p>0.05), and no interaction (F(1,50)=0.4239, p>0.05). Similarly, males did not exhibit main effects (F(1,44)=0.1431, p>0.05 for housing and F(1,44)=0.06038, p>0.05 for ischemic condition) or interaction between the variables (F(1,44)=0.06955, p>0.05) in the percent entries to the open arms of the EPM. Among the females, total arm entries did not display a social environment (F(1,51)=3.774, p>0.05) or ischemic (F(1,51)=0.9117, p>0.05) main effect, or interaction between the two experimental variables (F(1,51)=0.02710, p>0.05). In the males, there were also no main effects (F(1,43)=0.3871, p>0.05 for housing and F(1,43)=0.03458, p>0.05 for ischemic condition) or interaction (F(1,43)=1.027, p>0.05) over the total arm entries.
Figure 4.
Ninety-six hours after receiving the CA/CPR procedure, female and male mice do not exhibit anxiety-like behavior, as interpreted by an increase in time spent in the open arms of the elevated plus maze test (A). Among the sham controls, paired mice exhibit an intermediate phenotype between the less anxious CA/CPR groups and the more anxious isolated sham mice. There was no variation on the percent entries to open arm (B) or total arm entries (C), suggesting no differences in exploratory behavior.
In the forced swim test (FST), all animals displayed comparable behavior; within the female and male groups, floating time on the FST was unaffected by housing or ischemic injury (data not shown). Females displayed no social environment (F(1,45)=0.04176, p>0.05) or ischemic (F(1,45)=2.532, p>0.05) main effect, and no interaction between these variables (F(1,45)=2.522, p>0.05) on floating time. Males also exhibited comparable floating time; no main effect by housing (F(1,41)=1.668, p>0.05) or ischemic conditions (F(1,41)=0.6390, p>0.05), and no interaction (F(1,41)=0.06395, p>0.05). Latency to float was also consistent throughout female and male experimental groups, regardless of social environment or ischemic conditions (data not shown). Females displayed no housing (F(1,47)=1.007, p=0.3208) or ischemic main effect (F(1,47)=0.1912, p>0.05), nor an interaction between these variables (F(1,47)=0.7988, p>0.05) on latency to float. Similarly, males displayed comparable latency to float; no social environment (F(1,41)=3.438, p>0.05) or ischemic (F(1,41)=0.01943, p>0.05) main effect, and no interaction (F(1,41)=1.042, p>0.05). All together, these data suggest that neither isolation nor ischemia affected depressive-like behavior in this study.
3.2.2 Gene expression of inflammatory markers following global cerebral ischemia
Hippocampal gene expression of pro-inflammatory cytokines and iNOS varied in response to social environment and ischemic conditions. Hippocampal TNF-α gene expression displayed no interaction between the variables (F(1,47)=2.405, p>0.05 for females and F(1,35)=3.247, p>0.05 for males) and no social environment main effect (F(1,47)=2.141, p>0.05 for females and F(1,35)=3.195, p>0.05 for males). However, in both females and males, TNF-α gene expression exhibited an ischemia main effect (F(1,47)=34.02, p<0.05 and F(1,35)=22.13, p<0.05 respectively), where ischemia resulted in increased expression of the pro-inflammatory cytokine (Figure 5A). Among the females, TNF-α expression was elevated following CA/CPR regardless of housing conditions (p<0.05 for all CA/CPR versus sham comparisons), but paired and isolated mice within the sham or CA/CPR group displayed comparable TNF-α levels (p>0.05 for both). Among the males, TNF-α gene expression did not vary between the pair-housed and isolated sham groups (p>0.05). Relative to the sham controls, socially isolated CA/CPR male mice exhibited increased TNF-α expression (p<0.05 for both), whereas the paired CA/CPR group displayed an intermediate phenotype that was comparable to the shams and the isolated CA/CPR group (p>0.05 for all comparisons). In the hippocampus, IL-1β gene expression varied in both female and male mice (Figue 5B). Among the females, there was an interaction between the social environment and ischemic procedure (F(1,45)=10.03, p<0.05), but no separate main effects (F(1,48)=0.7955, p>0.05 for housing and F(1,48)=0.3952, p>0.05 for ischemic conditions). Multiple comparisons revealed that the isolated sham group expressed lower IL-1β levels than the paired sham and isolated CA/CPR groups (p<0.05 for both), with no further statistical differences within the female groups. Among the males, there was no interaction (F(1,35)=2.923, p>0.05) and no ischemia main effect (F(1,35)=3.511, p>0.05) on IL-1β expression in the hippocampus. However, hippocampal IL-1β exhibited a housing main effect (F(1,35)=5.085, p<0.05), with the pro-inflammatory cytokine being increased by isolation. Specifically, isolated CA/CPR males displayed elevated IL-1β gene expression relative to the paired sham group (p<0.05), while the isolated sham and paired CA/CPR groups exhibited an intermediate phenotype comparable to both, the low expression of the paired shams and the high expression of the isolated CA/CPR mice (p>0.05 for all comparisons). Hippocampal IL-6 gene expression displayed no interaction (F(1,49)=0.1926, p>0.05 for females and F(1,34)=0.8998, p>0.05 for males), and no housing main effect (F(1,49)=0.1384, p>0.05 for females and F(1,34)=0.2389, p>0.05 for males). Nonetheless, there was an ischemia main effect in both the females and males (Figure 5C; F(1,49)=20.92, p<0.05 and F(1,34)=27.51, p<0.05 respectively). Among the females, IL-6 expression was significantly elevated following CA/CPR regardless of housing conditions (p<0.05 for all CA/CPR versus sham comparisons), but paired and isolated mice within the sham or CA/CPR groups had comparable IL-6 expression (p>0.05 for both). Similarly, in the males, IL-6 expression in the hippocampus was significantly increased among the CA/CPR groups (p<0.05 for all CA/CPR versus sham comparisons) and no differences were detected between the paired and isolated mice within the sham or CA/CPR groups (p>0.05 for both). In the hippocampus, nitric oxide synthase 2 (NOS2; i.e. iNOS) expression varied in both female and male mice (Figure 5D). iNOS expression exhibited an ischemia main effect in females (F(1,48)=6.429, p<0.05) with increased expression following CA/CPR, but no social environment main effect (F(1,48)=0.4774, p>0.05) or interaction between the variables (F(1,48)=4.022, p>0.05). There was an ischemia-induce increase in hippocampal iNOS mRNA expression among the isolated females (p<0.05). However, the iNOS expression levels in the pair-housed sham and CA/CPR groups remained comparable to the lower expression of the isolated sham females and the higher expression of the isolated CA/CPR ones (p>0.05 for all comparisons). The males exhibited a social environment (F(1,36)=5.883, p<0.05) and an ischemia (F(1,36)=4.235, p<0.05) main effect, with both ischemia and isolation increasing hippocampal iNOS expression; but there was no interaction between the experimental variables (F(1,36)=2.434, p>0.05). Multiple comparisons revealed that isolated CA/CPR males displayed elevated iNOS gene expression relative to the paired sham group (p<0.05), while the isolated sham and paired CA/CPR groups exhibited an intermediate phenotype not significantly different from either the paired shams or the isolated CA/CPR mice (p>0.05 for all comparisons).
Figure 5.
Ninety-six hours after receiving the CA/CPR procedure, hippocampal gene expression of pro-inflammatory cytokines and Nos2 (i.e. iNOS) were increased, a response that was exacerbated among animals that were socially isolated. Ischemia resulted in increased hippocampal expression of TNF-α, which among males was exacerbated by isolation (A). Socially isolated sham females expressed lower IL-1β expression than paired sham and isolated CA/CPR females (B), suggesting that only in isolation CA/CPR can affect the levels of this pro-inflammatory cytokine. Among the males, IL-1β was significantly elevated in the isolated CA/CPR group relative to the pair-housed sham group. In the hippocampus of female and male mice, IL-6 gene expression was increased by ischemic conditions regardless of social environment (C). iNOS expression on females was increased following CA/CPR only among isolated mice (D). In the males, iNOS was significantly elevated in the isolated CA/CPR group relative to the pair-housed sham group.
Cortical gene expression of pro-inflammatory cytokines and iNOS varied in response to social environment and ischemic conditions. Gene expression of TNF-α among female mice, exhibited an ischemia main effect (Figure 6A; F(1,47)=17.19, p<0.05), with CA/CPR increasing its expression. However, it did not display a social environment main effect (F(1,47)=3.349, p>0.05) or an interaction (F(1,47)=3.476, p>0.05). TNF-α gene expression did not vary significantly between the pair-housed and isolated sham females (p>0.05). Relative to the sham controls, the socially isolated CA/CPR female mice exhibited increased TNF-α expression (p<0.05 for both), whereas the paired CA/CPR group displayed an intermediate phenotype that was comparable to the shams and the isolated CA/CPR groups (p>0.05 for all comparisons). Among male mice, there was a social environment (F(1,44)=4.563, p<0.05) and an ischemia (F(1,44)=12.11, p<0.05) main effect, with both isolation and ischemia increasing TNF-α expression in the cortex; but no interaction between the variables (F(1,44)=2.955, p>0.05). For females and males, IL-1β expression in the cortex exhibited an ischemia main effect (Figure 6B; F(1,47)=28.58, p<0.05 and F(1,45)=7.454, p<0.05 respectively), where ischemic conditions resulted in increased expression of the pro-inflammatory cytokine. However, there was no social environment main effect (F(1,47)=1.561, p>0.05 for females and F(1,45)=0.0009221, p>0.05 for males) or interaction (F(1,47)=1.040, p>0.05 for females and F(1,45)=0.1660, p>0.05 for males). Among the females, IL-1β expression was elevated following CA/CPR regardless of housing conditions (p<0.05 for all CA/CPR versus sham comparisons), but the paired and isolated CA/CPR groups had comparable IL-1β gene expression and so did the paired and isolated SHAM groups (p>0.05 for both). In the males, no multiple comparison differences were identified for IL-1β gene expression in the cortex (p>0.05). Cortical IL-6 gene expression among the female mice displayed an ischemia main effect (Figure 6C; F(1,47)=4.651, p<0.05) with CA/CPR increasing the expression of IL-6; but no social environment main effect (F(1,47)=1.617, p>0.05) or interaction (F(1,47)=0.1292, p>0.05). Multiple comparisons detected no further differences. Among the male mice, there was no variation in IL-6 gene expression in the cortex; no interaction (F(1,43)=0.03494, p>0.05) and no housing (F(1,43)=0.1378, p>0.05) or ischemia (F(1,43)=0.8534, p>0.05) main effect. Gene expression of iNOS in the cortex of females exhibited a social environment main effect (Figure 6D; F(1,46)=5.464, p<0.05) with isolation increasing its expression, but no ischemia-induced main effect (F(1,46)=1.502, p>0.05) or interaction between experimental variables (F(1,46)=2.830, p>0.05). Within the sham controls there was no variation in response to housing conditions (p>0.05). However, among the CA/CPR groups, isolated females exhibited increased iNOS expression relative to their pair-housed counterparts (p<0.05). The sham females displayed an intermediate phenotype that was comparable to the lower IL-6 expression of the paired CA/CPR mice and the higher IL-6 expression of the isolated CA/CPR mice (p>0.05 for all comparisons). Among the males, cortical expression of iNOS did not vary between the experimental groups; they displayed no interaction between the social and ischemic conditions (F(1,46)=0.3551, p>0.05), and no main effects (F(1,46)=0.4074, p>0.05 for social and F(1,46)=2.423, p>0.05 for ischemic conditions).
Figure 6.
Ninety-six hours after receiving the CA/CPR procedure, gene expression of pro-inflammatory cytokines and Nos2 (i.e. iNOS) in the cortex were increased, a response that was exacerbated among animals that were socially isolated. Cortical expression of TNF-α in females and males was elevated 96 hours after CA/CPR, an effect that was exaggerated by isolation (A). IL-1β expression was also increased by the ischemic procedure, regardless of housing conditions or sex of the mice (B). In the cortex, IL-6 levels were upregulated following ischemia in the females, but remained unaffected in the males (C). iNOS expression on female mice was elevated following CA/CPR only in isolated mice, and did not vary among the males (D).
3.2.3 Neurodegeneration following global cerebral ischemia
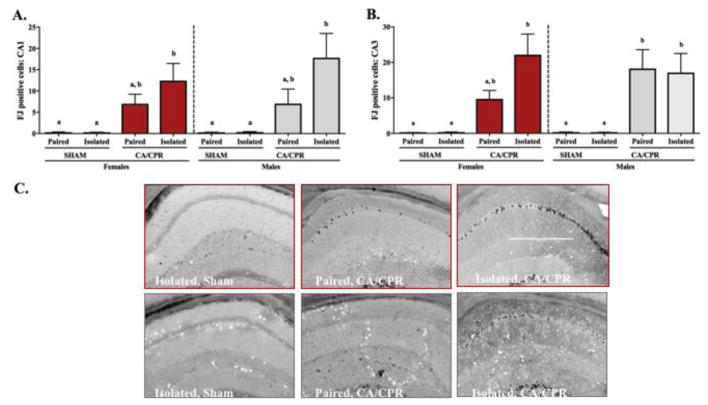
Neuronal degeneration, as identified by positive Fluoro-Jade C staining in the CA1, CA3 and DG regions of the hippocampus, increased in both the female and male hippocampus following global cerebral ischemia. Social interaction attenuated ischemia-induced neurodegeneration; among females this was evident in the CA1 and CA3 regions of the hippocampus, while among the males it was only observed in the CA1. Females exhibited an ischemia main effect (Figure 7A; F(1,54)=17.63, p<0.05) on the Fluoro-Jade C positive cells in the CA1 region of the hippocampus, but no interaction (F(1,54)=0.8352, p>0.05) or housing effect (F(1,54)=0.8352, p>0.05). Multiple comparisons revealed that the socially isolated CA/CPR group had increased neurodegeneration relative to the sham groups (p<0.05 for both), whereas the paired CA/CPR group was comparable to sham controls and the isolated CA/CPR group (p>0.05 for all). The males also exhibited an ischemia main effect in CA1 neurodegeneration (F(1,47)=18.21, p<0.05), and no interaction or housing main effect (F(1,47)=1.339, p>0.05 and F(1,47)=1.186, p>0.05 respectively). Significant differences were identified between the isolated CA/CPR group and the sham controls by multiple comparisons (p<0.05 for both). In both females and males, Fluoro-Jade C positive cells within the CA3 region of the hippocampus, exhibited an ischemia main effect (Figure 7B; F(1,54)=24.87, p<0.05 and F(1,47)=26.90, p<0.05 respectively), where ischemic conditions resulted in increased Fluoro-Jade C staining. However, there was no social environment main effect (F(1,54)=2.447, p>0.05 for females and F(1,47)=0.2885, p<0.05 for males) or interaction between the variables (F(1,54)=2.388, p>0.05 for females and F(1,47)=0.2876, p>0.05 for males). Among the females, CA3 neurodegeneration did not vary significantly between the pair-housed and isolated sham groups (p>0.05). Relative to the sham controls, socially isolated CA/CPR female mice exhibited increased CA3 neurodegeneration (p<0.05 for both), while the paired CA/CPR mice displayed an intermediate phenotype that was comparable to both the sham controls and the isolated CA/CPR group (p>0.05 for all comparisons). Among the males, CA3 neurodegeneration was elevated following CA/CPR regardless of housing conditions (p<0.05 for all CA/CPR versus sham comparisons), but paired and isolated mice within the sham or CA/CPR groups displayed comparable levels of Fluoro-Jade C positive staining (p>0.05 for both). Neurogegeneration was also evident in the DG region of the hippocampus. Both males and females displayed a significant ischemia main effect (Data not shown; F(1,54)=24.49, p<0.05 for females and F(1,47)=18.4, p<0.05 for males), but no interaction (F(1,54)=0.14, p>0.05 for females and F(1,47)=0.10, p>0.05 for males) or housing effect (F(1,54)=0.15, p>0.05 for females and F(1,47)=0.10, p>0.05 for males). Multiple comparisons revealed that regardless of housing, for both females and males, the CA/CPR groups had increased neurodegeneration relative to the sham groups (p<0.05 for both).
Figure 7.
Neurodegeneration, as indicated by fluoro-jade c positive staining, was increased in both female and male mice at 96 hours post-ischemia. Among females and males, cell death in the CA1 region of the hippocampus was increased in the isolated CA/CPR group relative to sham controls, while the paired CA/CPR group exhibited an intermediate phenotype comparable to both the shams and isolated CA/CPR group (A). This pattern was also present for the CA3 region among females, however, among the males, amelioration of ischemia-induced neurodegeneration from the social environment was not detected (B). A representative image of fluoro-jade c positive staining is presented for each group (C). Scale bar in panel 3C 500 μm.
4. Discussion
Microglial activation is identified by the production and secretion of inflammatory markers [49–51]. Thus, increased expression of pro-inflammatory cytokines within these cells following an ischemic insult is indicative of the neuroinflammatory response. Two hours after CA/CPR, gene expression of pro-inflammatory cytokines TNF-α, IL-1β and IL-6, increased following global cerebral ischemia in microglia enriched from the whole brains of both female and male mice, regardless of their housing conditions (Figure 1A–C). However, 24 hours after resuscitation, microglial gene expression of these pro-inflammatory cytokines exhibited sexual dimorphisms and social modulation (Figure 2A–C). The 2 hour time point evaluates immediate neuroinflammation, as pro-inflammatory cytokine secretion from the innate immune cells of the central nervous system occurs within minutes of ischemic onset [15, 52–53]. When investigating the neuroinflammatory response to intracerebroventricular lipopolysaccharide stimulation in young and aged mice, hippocampal and cortical gene expression of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 were increased at 2 hours regardless of the animals age, whereas at the 8 hour time point, lipopolysaccharide-induce inflammation was exacerbated in the older mice relative to the young adults [54]. Together with the numerous experimental evidence supporting microglial sensitization with aging [18, 55–58], these findings support that primed microglia modulate the inflammatory response and that a time course may reveal differences in microglial priming that may not be apparent immediately after a neuroinflammatory stimulus.
At 24 hours after resuscitation, social attenuation of the ischemia-induced increase of pro-inflammatory cytokines was only evident in microglia from male mice (Figure 2). Gene expression of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 increased in the microglia from female mice that had CA/CPR relative to SHAM controls, with no effect based on their social environment. On the other hand, among the males, social interaction attenuated the ischemia-induced increase in microglial expression of IL-1β and IL-6. Reduced inflammation among socially integrated male mice relative to their isolated counterparts in the CA/CPR model of global cerebral ischemia had been previously reported [19, 22, 24], but had not been investigated among female mice. The estrous cycle of experimental females was not monitored, and because estrogen promote microglial resting state by restoring their ramified morphology and decreasing their MHC II expression [59], this must be noted as a confounding factor. Nonetheless, the present findings suggest sex-differences in the social modulation of inflammatory responses to CA/CPR. Furthermore, it provides evidence in support of sexual dimorphisms in the social modulation of inflammatory responses; as previously suggested by a study in which one week following peripheral lipopolysaccharide administration to rats, group housing attenuated the inflammatory response in males but exacerbated it in females [60].
Pro-inflammatory conditions and neurodegeneration can upregulate microglial expression of cell-surface antigen presenting molecule major histocompatibility complex II (MHC II) [60–61]. At 2 hours post-ischemia, MHC II expression in microglia remained comparable across all groups regardless of sex or housing conditions; nonetheless, by 24 hours, sex-differences and modulation by the social environment were apparent (Figures 1D and 2D). In the males, MHC II expression did not vary in response to either housing or ischemic conditions at the later time point. In contrast, among the females, isolation exacerbated the ischemia-induced increase of MHC II 24 hours after resuscitation. Sex-differences in the expression of MHC II following cerebral ischemia have been previously reported. In a model of hypoxic encephalopathy, 1 day after injury, neonatal brains from male and female mice exhibit a comparable inflammatory response; however, at 3 days post-injury, microglial activation, characterized by increased expression of MHC II, was greater among males than females [36]. The injury-induced increase of MHC II was greater among the neonatal males, while in the young adults, MHC II induction was only detected among females. The different trends might result from variation in the experimental designs, such as age of the animals, ischemia models, or the techniques used to measure MHC II expression. Nonetheless, both results provide additional support to the hypothesis that the microglial response to cerebral injury is sex-specific.
A prolonged and maladaptive microglial response to CA/CPR in isolated animals, characterized in males by increased expression of pro-inflammatory cytokines and in females by increased expression of MHC II, supports the hypothesis that isolation can prime or sensitize microglia. The increase in the expression of microglial priming marker MHC II following one week of isolation served as the first indication of isolation-induced microglial sensitization [24]. Moreover, social attenuation of the neuroinflammatory response to cerebral ischemia, following oxygen-glucose depravation ex vivo [24], provides additional evidence in support of microglia priming as the mechanism underlying the detrimental effects of isolation on ischemic outcome.
Pre-clinical studies have examined the behavioral consequences of cerebral ischemia, with the great majority identifying deficits following injury [19, 22, 42, 46–47]. Post-ischemia, locomotor and affective behaviors are the most commonly tested [63]. At 96 hours post-ischemia, mice in the present study exhibited increased locomotor activity relative to sham controls (Figure 3A). The ischemic effect was evident among females and males, suggesting that ischemia-induced hyperlocomotion occurs regardless of sex. These data are consistent with previous findings reporting increased locomotor activity at 2, 3 and 5 days post-injury [22, 46, 47], a behavior that resolves between 6 and 21 days post-ischemia [42, 46, 64]. Among the males, housing condition had no effect on total locomotion; however, the socially isolated females displayed increased locomotor activity relative to their pair-housed counterparts. The ischemic effect in the males appears to be determined by the pair-housed animals; the paired sham group having significantly fewer beam breaks than the paired CA/CPR group, while among the isolated sham and CA/CPR groups, total beam breaks were comparable. In a previous study with a similar experimental design, at 5 days post-ischemic injury, hyperlocomotion was evident among isolated and pair-housed male mice [22]. Variations between the studies that might account for the different observations include the length of housing manipulation and duration of open field test. In the females, modulation of locomotor behavior by housing followed a different pattern, with the ischemia-induced locomotion increase in both the pair-housed and socially isolated female mice. The total beam break mean was greatest among the isolated females that experienced ischemia suggesting that social interaction assists in the resolution of the hyperlocomotion. Altogether, the locomotion data obtained from the open field test indicate that at 96 hours post-CA/CPR both female and male mice exhibit hyperlocomotion, a behavior that is ameliorated by social interaction among the females.
Affective dysfunction, such as the development of anxiety-like and depressive-like behavior, has been identified in animal models of global cerebral ischemia [22, 42, 46, 63]. Reduced central tendency in the open field test or decreased time spent in the open arms of the elevated plus maze are indicative of anxiety-like behavior [64–65]. At 96 hours post-ischemia, neither female nor male mice exhibited a change in time spent in the center of the open field during the first 5 minutes of the testing period (Figure 3C). This observation was comparable to past work reporting no difference in open field central tendency at 3 or 6 days post-ischemic injury [46]. Nonetheless, other studies have reported a reduction in time spent in the center of the open field at 4, 5 and 21 days post-ischemia [22, 42, 63]. The one prior study examining social influences on CA/CPR outcome did not report a housing effect on anxiety-like behavior in the open field [22]. In the elevated plus maze, another behavioral test used to measure anxiety-like behavior, the mice in the present study exhibited an ischemia-induced increase in exploratory behavior and reduced anxiety-like behavior as interpreted from time spent in the open arms of the maze (Figure 4A). Contrary to the current study, a reduction in time spent in the open arms of the elevated plus maze was reported 7 days post-ischemia [46], and this behavior persisted at 21 days [64]. A possible explanation for the differences between the past and present findings could be the timing of the behavioral testing; at 96 hours post-ischemic injury, hyperactivity is a potential confounding factor in the elevated plus maze; increased locomotor activity may be interpreted as increased exploratory behavior, and it might not be until resolution of the locomotor dysfunction that the anxiety-like behavior phenotype is apparent [63]. Depressive-like behavior, another affect disorder commonly reported in ischemia survivors, has also been measured in experimental models of this heterogeneous disease. At 96 hours post-ischemia, female and male mice regardless of their housing or procedure assignment, displayed comparable latency to float and floating time (data not shown). Contrary to our findings, previous work identified that following 5 days of the CA/CPR procedure animals increase the time spent floating in the forced swim test; furthermore, they report that the depressive-like behavior phenotype is ameliorated in animals that were pair-housed relative to those that were socially isolated [22]. Besides the inclusion of animals from both sexes, the main difference between the past work and the present study is the length of social interaction prior to ischemic onset; one versus two weeks. Previous work reporting that 7 days of social manipulation is sufficient to alter physiology and detect the housing influences on ischemic outcome [24, 66], supports the current experimental design. Furthermore, clinically reported sexual dimorphisms in cerebral ischemia and multiple failed clinical trials, highlight that incorporating animals from both sexes on experimental models of this disease is necessary [32].
The hippocampus is the brain region most vulnerable to cerebral ischemia, due to its high energy demands [68]. When examining hippocampal gene expression of pro-inflammatory markers 96 hours post-ischemic injury, ischemia elicits an inflammatory response which can be attenuated by social interaction in both female and male mice (Figure 5). Expression of TNF-α and IL-6 mRNA significantly increased among the ischemic groups relative to shams. Among the paired CA/CPR males, TNF-α hippocampal gene expression exhibited an intermediate phenotype that was comparable to both the high expression of the isolated CA/CPR group and the low expression of the sham controls. IL-1β gene expression in the hippocampus of females remained comparable between the paired sham and CA/CPR animals; however, among the isolated female mice, IL-1β gene expression increased following ischemic injury. In the males, hippocampal IL-1β increased in the socially isolated mice relative to their pair-housed counterparts, as determined by increased IL-1β expression in the isolated CA/CPR males. These findings are similar to earlier work in which following 1 and 3 days of CA/CPR, male mice experience increased expression of pro-inflammatory cytokines [69], a response that is attenuated by social interaction [19, 22, 24]. In addition to TNF-α, IL-1β and IL-6, inducible nitric oxide (iNOS) gene expression was examined in the present study. Upregulation of iNOS following cerebral ischemia results in increased nitric oxide [70], an inflammatory mechanism that contributes to neuronal cell death [71]. For both females and males, there was an ischemia-induce increase in the hippocampal expression of iNOS, determined by increased iNOS in the isolated CA/CPR group. Altogether, the present findings support the previously reported attenuation of the ischemia-induced neuroinflammatory response within the hippocampus of male mice following global cerebral ischemia induced by CA/CPR, and evidence that the same is true among female mice. When interpreting these findings, it is important to note that the inflammation assessment at 96 hours post-ischemia is influenced by resident glia and peripheral immune cells which either had infiltrated the brain following injury or were present in the blood vessels at the time of tissue collection.
Together with the hippocampus, the striatum and the cortex are selectively vulnerable to cerebral ischemia damage [72]. With social attenuation of the inflammatory profile of microglia enriched from the whole brain following ischemic injury, we examined the beneficial effects of social interaction on the inflammatory response in another relevant brain region, the cortex (Figure 6). Gene expression of pro-inflammatory cytokines TNF-α, IL-1β and IL-6, increased in the cortex of females at 96 hours post-ischemia. The same was true for TNF-α and IL-1β cortical gene expression among males. For both males and females, the ischemia-induce increase in TNF-α was primarily due to the isolated CA/CPR group. iNOS gene expression in the cortex of males remained comparable across all groups; however, among the females, it displayed an increase among the isolated sham and CA/CPR groups relative to the pair-housed counterparts. Together, the gene expression suggests that both the hippocampus and cortex display social modulation of the ischemia-induced inflammatory response; furthermore, it provides evidence to support that this occurs regardless of sex. Differences such as social attenuation of hippocampal TNF-α expression in males but not females, and the ischemia-induced increase in cortical iNOS among isolated CA/CPR females but not isolated CA/CPR males, suggests differential mechanisms of action between how females and males respond to ischemia and/or how the social environment modulates the ischemia-induced inflammatory response; an area that warrants further investigation.
In the brain, the ischemic cascade ultimately leads to neuronal cell death [73]. Following 96 hours of CA/CPR, hippocampal neurodegeneration in females and males increased relative to sham controls (Figure 7); furthermore, the counts of Fluoro-Jade C positive stained cells were greater among socially isolated animals. In the CA1 region of the hippocampus, social amelioration of neurodegeneration was evident regardless of sex. Nonetheless, in the CA3 region of the hippocampus, the reduction in Fluoro-Jade C positive stained cell counts among pair-housed mice was present among females but not males. Previous studies examining male mice have reported social attenuation of cell death in the CA1, CA2, CA3 and DG regions of the hippocampus, at 7 days post-ischemic injury [19, 22]. These studies also examined microglia activity using histological analysis, and identified that at 1 and 7 days following CA/CPR proportional area of the microglia positive staining was significantly greater than sham controls in only socially isolated animals [19, 24]. Despite an overall agreement of social modulation on ischemia-induced hippocampal cell death, our inability to fully reproduce these findings in the CA3 region of the hippocampus is likely the result of differences in the timing of tissue collection after CA/CPR. Reduced survival rates among male mice relative to female mice, also might have compromised the present findings. In the outcome study, female mice had a 97% chance of survival to the CA/CPR procedure, whereas the males had a 73% chance. It is probable that mice with greater damage did not survive, which may have resulted in a reduced mean for Fluoro-Jade C positive cell counts or neurodegeneration among the CA/CPR male groups. Overall, data collected from these experiments identified social influences on cerebral ischemia outcome in both female and male mice, with minor variations suggesting sex-specific mechanisms. However, additional work must be completed to establish the causal mechanisms by which sex and social influences modulate the inflammatory response, neuronal damage, and behavioral outcomes.
4. Conclusions
Extensive evidence supports the conclusion that social isolation is detrimental to cerebral ischemia outcome, whereas social integration protects against ischemic damage [73–74]. Cerebral ischemia, induced by cardiac arrest or stroke, is a heterogeneous disease; it triggers a multitude of events, including inflammation, excitotoxicity, oxidative stress and neurodegeneration [15], and it can be influenced by factors including sex and aging [76]. The present study suggests that social environment can modulate microglial reactivity to cerebral ischemia in both female and male mice, and that this might occur through sex-specific mechanisms. Observed differences in the microglial reactivity profile at 24 hours post-ischemia may contribute to the sex-differences and social influences that have been reported in long-term ischemic outcome. The current experiments also identified social influences on ischemia-induced neuroinflammation and neurodegeneration, regardless of sex. However, contrary to previous studies, housing effects were not detected in behavioral outcome measures at the 96 hour time point [22]. Together, the present findings suggest that social interaction ameliorates ischemic outcome by modulating neuroinflammation and neurodegeneration. Furthermore, differential gene expression of inflammatory markers together with the microglial data implies that the social influences on the ischemia-induced inflammatory response might occur in a sex-specific manner.
Highlights.
Social modulation of microglial reactivity to CA/CPR is sex-specific.
Microglia from socially isolated females display increased MHC II after CA/CPR.
Isolation exacerbates ischemia-induced IL-1β and IL-6 expression in male microglia.
Social modulation of ischemia-induced inflammation is evident regardless of sex.
Ischemia-induced neurodegeneration is greater in isolated animals.
Acknowledgments
We thank Randy J. Nelson and Kate Karelina for suggestions and/or assistance throughout the completion of this work.
Funding Sources
This research was in part supported by grants from the National Institute of Mental Health [MH107002-01A1 to ACD] and the National Institute of Neurological Disorders and Stroke [NS092388-01A1 to RJN and ACD].
Abbreviations
- CA/CPR
cardiac arrest/cardiopulmonary resuscitation
- MHC II
major histocompatibility complex II
- NF-κB
nuclear factor kappa
- TNF-α
tumor necrosis factor alpha
- IL-1β
interleukin 1 beta
- IL-6
interleukin 6
- iNOS
inducible nitric oxide synthase
- OPF
open field
- EPM
elevated plus maze
- FST
forced swim test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241(4865):540–5. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell MJ, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet. 2010;376(9735):112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 3.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7(7) doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karelina K, DeVries AC. Modeling Social Influences on Human Health. Psychosom Med. 2011;73(1):67–74. doi: 10.1097/PSY.0b013e3182002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boden-Albala B, Litwak E, Elkind MSV, Rundek T, Sacco RL. Social isolation and outcomes post stroke. Neurology. 2005;64(11):1888–1892. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- 6.Craft TKS, et al. Social interaction improves experimental stroke outcome. Stroke. 2005;36(9):2006–2011. doi: 10.1161/01.STR.0000177538.17687.54. [DOI] [PubMed] [Google Scholar]
- 7.Cacioppo JT, Hawkley LC. Social Isolation and Health, with an Emphasis on Underlying Mechanisms. Perspect Biol Med. 2003;46(3):S39–S52. [PubMed] [Google Scholar]
- 8.Yang YC, McClintock MK, Kozloski M, Li T. Social isolation and adult mortality: the role of chronic inflammation and sex differences. J Health Soc Behav. 2013;54(2):183–203. doi: 10.1177/0022146513485244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstein JR, Koerner IP, Möller T. Microglia in ischemic brain injury. Future Neurol. 2010;5(2):227–246. doi: 10.2217/fnl.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8(4):279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeter M, Jander S, Huitinga I, Witte OW, Stoll G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke. 1997;28(2):382–6. doi: 10.1161/01.str.28.2.382. [DOI] [PubMed] [Google Scholar]
- 13.Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: A study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003;183(1):25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 14.Schilling M, Besselmann M, Müller M, Strecker JK, Ringelstein EB, Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: An investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005;196(2):290–297. doi: 10.1016/j.expneurol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Experimental Neurology. 2012;233(1):40–48. doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norden DM, Muccigrosso MM, Godbout JP. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology. 2015;96(PA):29–41. doi: 10.1016/j.neuropharm.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niraula A, Sheridan JF, Godbout JP. Microglia Priming with Aging and Stress. Neuropsychopharmacology. 2017;42(1):318–333. doi: 10.1038/npp.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weil ADZM, Norman GJ, Barker JM, Su AJ, Nelson RJ. Social isolation potentiates cell death and inflammatory responses after global ischemia. Mol Psychitry. 2008;13(10):913–915. doi: 10.1038/mp.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci U S A. 2009;106(14):5895–5900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karelina K, Norman GJ, Zhang N, DeVries AC. Social contact influences histological and behavioral outcomes following cerebral ischemia. Exp Neurol. 2009;220(2):276–282. doi: 10.1016/j.expneurol.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Norman GJ, Zhang N, Morris JS, Karelina K, Berntson GG, DeVries AC. Social interaction modulates autonomic, inflammatory, and depressive-like responses to cardiac arrest and cardiopulmonary resuscitation. Proc Natl Acad Sci U S A. 2010;107(37):16342–7. doi: 10.1073/pnas.1007583107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venna VR, et al. NF-κB contributes to the detrimental effects of social isolation after experimental stroke. Acta Neuropathol. 2012;124(3):425–438. doi: 10.1007/s00401-012-0990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudier-Diaz MM, Zhang N, Haines AH, Surbhi, Zhou M, DeVries AC. Social interaction modulates the neuroinflammatory response to global cerebral ischemia in male mice. Brain Res. 2017;1673 doi: 10.1016/j.brainres.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payami H, et al. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet. 1996;58(4):803–811. [PMC free article] [PubMed] [Google Scholar]
- 26.Logroscino G, et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010;81(4):385–390. doi: 10.1136/jnnp.2009.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirdefeldt K, Adami H-O, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26(S1):1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 28.Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol. 2012;8(5):255–263. doi: 10.1038/nrneurol.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alkayed NJ, Harukuni I, Kimes aS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29(1):159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- 30.Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. ILAR J. 2004;45(2):147–159. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- 31.Rosamond W, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 32.Reeves MJ, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. The Lancet Neurology. 2008;7(10):915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vagnerova K, Koerner IP, Hurn PD. Gender and the Injured Brain. Anesth Analg. 2008;107(1):201–214. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turtzo LC, McCullough LD. Sex differences in stroke. Cerebrovascular Diseases. 2008;26(5):462–474. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Go AS, et al. Heart disease and stroke statistics-2013 update: A Report from the American Heart Association. Circulation. 2013;127(1) doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirza Ma, Ritzel R, Xu Y, McCullough LD, Liu F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J Neuroinflammation. 2015;12(1):32. doi: 10.1186/s12974-015-0251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 38.Spychala MS, Honarpisheh P, McCullough LD. Sex differences in neuroinflammation and neuroprotection in ischemic stroke. Journal of Neuroscience Research. 2017;95(1–2):462–471. doi: 10.1002/jnr.23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy S, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res. 2013;4(5):1–19. doi: 10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α PU.1 pathway. Nat Med. 2011;17(1):64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison HW, Filosa JA. Sex differences in astrocyte and microglia responses immediately following middle cerebral artery occlusion in adult mice. Neuroscience. 2016;339:85–99. doi: 10.1016/j.neuroscience.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neigh GN, et al. Anxiety after cardiac arrest/cardiopulmonary resuscitation: Exacerbated by stress and prevented by minocycline. Stroke. 2009;40(11):3601–3607. doi: 10.1161/STROKEAHA.109.564146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venna VR, Xu Y, Doran SJ, Patrizz A, McCullough LD. Social interaction plays a critical role in neurogenesis and recovery after stroke. Transl Psychiatry. 2014;4(1):e351. doi: 10.1038/tp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Loo PLP, Van Zutphen LFM, Baumans V. Male management: coping with aggression problems in male laboratory mice. Lab Anim. 2003;37(4):300–313. doi: 10.1258/002367703322389870. [DOI] [PubMed] [Google Scholar]
- 45.Ingersoll DW, Weinhold LL. Modulation of male mouse sniff, attack, and mount behaviors by estrous cycle-dependent urinary cues. Behav Neural Biol. 1987;48(1):24–42. doi: 10.1016/s0163-1047(87)90544-9. [DOI] [PubMed] [Google Scholar]
- 46.Neigh GN, et al. Cardiac arrest/cardiopulmonary resuscitation increases anxiety-like behavior and decreases social interaction. J Cereb Blood Flow Metab. 2004;24:372–82. doi: 10.1097/01.WCB.0000112323.75217.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kofler J, et al. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136(1):33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23(3):309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanisch UK. Microglia as a source and target of cytokines. GLIA. 2002;40(2):140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 50.Kawanokuchi J, et al. Production of interferon-γ by microglia. Mult Scler J. 2006;12(5):558–564. doi: 10.1177/1352458506070763. [DOI] [PubMed] [Google Scholar]
- 51.Lynch MA. The multifaceted profile of activated microglia. Molecular Neurobiology. 2009;40(2):139–156. doi: 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- 52.Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7(1):111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- 53.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87(5):779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29(11):1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godbout JP, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19(10):1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 56.Sierra A, Gottfried-Blackmore AC, Mcewen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55(4):412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 57.Wynne AM, Henry CJ, Godbout JP. Immune and behavioral consequences of microglial reactivity in the aged brain. Integr Comp Biol. 2009;49(3):254–266. doi: 10.1093/icb/icp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol. 2010;226(1–2):181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: Role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci. 2007;25(10):3039–3046. doi: 10.1111/j.1460-9568.2007.05563.x. [DOI] [PubMed] [Google Scholar]
- 60.Yee JR, Prendergast BJ. Sex-specific social regulation of inflammatory responses and sickness behaviors. Brain Behav Immun. 2010;24(6):942–951. doi: 10.1016/j.bbi.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng H, Han HS, Cheng D, Sun GH, Yenari MA. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke. 2003;34(10):2495–2501. doi: 10.1161/01.STR.0000091269.67384.E7. [DOI] [PubMed] [Google Scholar]
- 62.Wojtera M, Sobow T, Kloszewska I, Liberski PP, Brown DR, Sikorska B. Expression of immunohistochemical markers on microglia in Creutzfeldt-Jakob disease and Alzheimer’s disease: morphometric study and review of the literature. Folia Neuropathol. 2012;50(1):74–84. [PubMed] [Google Scholar]
- 63.Herson PS, Palmateer J, Hurn PD, DeVries AC. Evaluating Behavioral Outcomes from Ischemic Brain Injury. ANIMAL MODELS OF BEHAVIORAL ANALYSIS. 2011;50:307–327. [Google Scholar]
- 64.Neigh GN, et al. Cardiac arrest and cardiopulmonary resuscitation dysregulates the hypothalamic-pituitary-adrenal axis. J Cereb Blood Flow Metab. 2009;29(10):1673–82. doi: 10.1038/jcbfm.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. European Journal of Pharmacology. 2003;463(1–3):3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 66.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karelina K, et al. Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke. 2011;42(12):3606–3611. doi: 10.1161/STROKEAHA.111.628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nikonenko AG, Radenovic L, Andjus PR, Skibo GG. Structural features of ischemic damage in the hippocampus. Anatomical Record. 2009;292(12):1914–1921. doi: 10.1002/ar.20969. [DOI] [PubMed] [Google Scholar]
- 69.Norman GJ, et al. Cardiopulmonary arrest and resuscitation disrupts cholinergic anti-inflammatory processes: a role for cholinergic α7 nicotinic receptors. J Neurosci. 2011;31(9):3446–52. doi: 10.1523/JNEUROSCI.4558-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yenari MA, Kauppinen TM, Swanson RA. Microglial Activation in Stroke: Therapeutic Targets. Neurotherapeutics. 2010;7(4):378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15(6):252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 72.Block F. Global ischemia and behavioural deficits. Prog Neurobiol. 1999;58(3):279–295. doi: 10.1016/s0301-0082(98)00085-9. [DOI] [PubMed] [Google Scholar]
- 73.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14(11):1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friedler B, Crapser J, McCullough L. One is the deadliest number: the detrimental effects of social isolation on cerebrovascular diseases and cognition. Acta Neuropathologica. 2015;129(4):493–509. doi: 10.1007/s00401-014-1377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Venna VR, McCullough LD. Role of social factors on cell death, cerebral plasticity and recovery after stroke. Metab Brain Dis. 2015;30(2):497–506. doi: 10.1007/s11011-014-9544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]