Abstract
Metformin is everywhere. Originally introduced in clinical practice as an anti-diabetic agent, its role as a therapeutic agent is expanding to include treatment of pre-diabetes, gestational diabetes, polycystic ovarian disease, and more recently, experimental studies, as well as observations in randomized clinical trials, suggest that metformin could have a place in the treatment or prevention of preeclampsia. This article provides a brief overview of the history of metformin in the treatment of diabetes, reviews the results of meta-analyses of metformin in gestational diabetes, and the treatment of obese non-diabetic pregnant women to prevent macrosomia. We highlight the results of a randomized clinical trial in which metformin administration in early pregnancy did not reduce the frequency of large-for-gestational-age infants (primary endpoint), but did decrease the frequency of preeclampsia (a secondary endpoint). The mechanisms by which metformin may prevent preeclampsia include a reduction in the production of anti-angiogenic factors (soluble vascular endothelial growth factor receptor-1 and soluble endoglin), and improving endothelial dysfunction, probably through an effect on the mitochondria. Another potential mechanism whereby metformin may play a role in the prevention of preeclampsia is its ability to modify cellular homeostasis and energy disposition, mediated by mTOR. Metformin has a molecular weight of 129 Dalton, and therefore, readily crosses the placenta. There is considerable evidence suggesting that this agent is safe during pregnancy. New literature on the role of metformin in the prevention of cancer, a chemotherapeutic adjuvant, and in prolonging life and protecting against aging, is briefly reviewed. Herein we discuss the mechanisms of action and potential benefits of metformin.
Suddenly, it’s metformin time. Want to live longer and be healthier(1–6)? Take metformin(3, 4). Don’t want to get cancer? Take metformin(7–10). Do you have polycystic ovary syndrome(11–14), congestive heart failure(15), chronic liver disease(15), chronic kidney disease(15), multiple sclerosis(16, 17), renal tubulointerstitial fibrosis(18), or nonalcoholic fatty liver disease(19)— metformin (Figure 1).
Figure 1. Different effects of metformin and its signaling pathways.
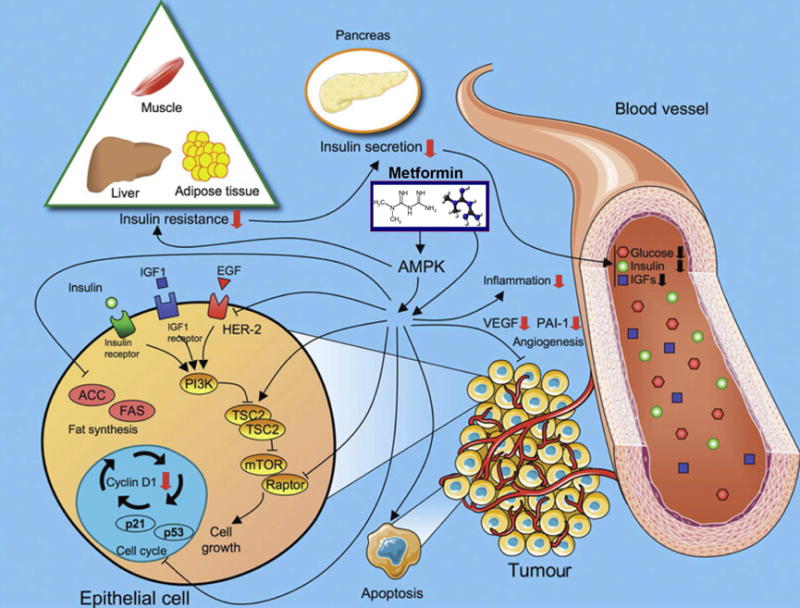
Metformin reduces insulin resistance, secretion, glucose blood levels, inflammation, and angiogenesis as well as reduction in cell growth and metabolism that mediates its anti-tumor activity. These effects are regulated by both AMPK-dependent or -independent mechanisms that lead to the inhibition of mTOR signaling.
(Abbreviations: ACC, acetyl-CoA carboxylase; AMPK, 5′ adenosine monophosphate-activated protein kinase; IGF, Insulin-like growth factor; EGF, Epidermal growth factor; FAS, fatty acid synthase; PAI-1, plasminogen-activator inhibitor-1; PI3K, Phosphatidylinositol-4,5-bisphosphate 3-kinase; TSC2, tuberous sclerosis 2; mTOR, mechanistic target of rapamycin; VEGF, vascular endothelial growth factor)
Reproduced with permission from Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012; 122:253-70 and http://diabetesmanager.pbworks.com/f/1255202482/metformin.JPG
The good news for obstetricians is that preeclampsia has the potential to be added to this list (20). This could be quite important as preeclampsia affects about 5-7% of pregnancies worldwide, is a leading cause of maternal and perinatal morbidity and mortality, and imposes substantial costs on the healthcare system(20). We felt it was time to review how metformin works and what it can offer obstetricians for treating pregnancy disorders, other than diabetes and obesity, now and into the future.
Metformin: from the Pharaohs to the Present
Metformin (dimethylbiguanide) is a constituent of many herbal remedies, and the Ebers Papyrus, written in 1500 B.C.E.(21), (Figure 2) records its use in Egypt since the time of the Pharaohs. In Europe, herbal remedies derived from the plant Galega officinalis (Figure 3) that contained metformin have been prescribed to treat polyuria and other symptoms of diabetes since the Middle Ages(21–24), but it was not until the early 1900s that guanidine was identified as responsible for the hypoglycemic effects of G. officinalis extracts(25, 26).
Figure 2. Ebers papyrus.

This document, 20 meters long, contains a collection of medical texts considered to be the most comprehensive account of the practice in Egyptian medicine. Its encyclopedic content addresses not only multiple illnesses, e.g., treatment for diabetes, but also crocodile bites, mental illness, and treatment for death (half an onion and froth of a beer…). The papyrus was purchased by the Chief of Egyptology (Georg Ebers) at the University of Leipzig in Germany where it currently resides. The story goes that the papyrus was discovered between the legs of a mummy.
Adapted from http://spheresoflight.com.au/axismundi/content/images/ebers-papyrus-colonnes1-2.jpg.
Figure 3. Galega officinalis.

This plant, also known as goat’s rue, French lilac, or Italian fitch, was used for many years to treat the symptoms of diabetes. In 1920, the anti-diabetic class of drugs called biguanides, originating from this plant, was introduced for the treatment of diabetes.
Adapted from http://www.naturalmedicinefacts.info/plant/galega-officinalis.html.
Guanidine was too toxic for clinical use, and isoamylene guanidine (Galegine) was used as an anti-diabetic agent in the 1920s until the development of metformin and phenformin(27, 28). Phenformin was withdrawn from clinical use because it caused lactic acidosis(29), and although metformin did not have this side-effect, its use, as well as the use of other biguanide derivatives to treat diabetes, was displaced by insulin, which was purified and synthesized in 1921 and used clinically to treat diabetes in humans the following year(30, 31).
Nevertheless, research with biguanides continued, as they were effective in treating malaria, and the hypoglycemic effects of the anti-malarial agent chloroguanidine hydrochloride eventually paved the way for the development of metformin for the treatment of diabetes by Professor Jean Sterne at the Hopital Laennec in Paris, who coined the name “glucophage” (“glucose eater”) for metformin (Figure 4)(32). Two unexpected side-effects of some biguanides—lactic acidosis and increased cardiac mortality—then caused the biguanides to be withdrawn from clinical use in the United States(22, 33–38); however, metformin was relatively safe and, after 20 years of clinical use in Europe, metformin was approved by the FDA in 1995 for the treatment of diabetes in theUnited States. A joint consensus statement by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) now recommends metformin as initial oral therapy for patients with type 2 diabetes(39, 40). Recently, the Professional Practice Committee of the American Diabetes Association has recommended the use of metformin for patients with prediabetes (fasting glucose 100-125 mg/dl, 2-hr post-load glucose 140-199 mg/dl, or A1C 5.7-6.4%), especially in those who are younger than 60 years old, have a BMI >35 kg/m2, or have a history of gestational diabetes(41–43).
Figure 4. Professor Jean Sterne at the Hospital Laennec, Paris, France.

Introduction of metformin (“glucophage”) into clinical medicine.
Reproduced with permission from Bailey CJ, Day C. Metformin: its botanical background. Practical Diabetes International. 2004;21(3):115-117.
Several mechanisms of action are considered responsible for this effect, including: 1) a decreased in hepatic glucose production by suppression of gluconeogenesis(44, 45); 2) an increased insulin suppression of endogenous glucose production by the liver(44, 45); 3) reduction of glucose absorbtion by the gastro-intestinal tract(44, 45); By far the most important mechanism is the reduction in hepatic glucose production that is considered mediated by the activation of the global energy sensor in cells, adenosine monophosphate-activated protein kinase (AMPK) (Figure 1).(46)
Metformin was mostly used in non-pregnant diabetic patients until Coetzee and Jackson reported its use in the late 1970s with pregnant diabetics from South Africa(47–50), after which metformin became the treatment of choice for gestational diabetes(51–68) and, in the 1990s, for type 2 diabetes (69, 70) because of its ease of administration and high compliance rate.
Important results from the meta-analyses: metformin reduces the frequency of gestational hypertension in gestational diabetes
In 2013, the efficacy and safety of metformin in the management of gestational diabetes had been compared to that of insulin in five randomized clinical trials(56, 71–74). Gui et al.(75) published a systematic review and meta-analysis in which metformin was shown to be superior to insulin in reducing maternal weight gain during pregnancy as well as in the frequency of gestational hypertension (Figure 5a,); however, metformin did not change the frequency of large-for-gestational-age (LGA) or small-for-gestational-age (SGA) fetuses or of hypoglycemia and preeclampsia(75). Two subsequent meta-analyses confirmed metformin’s effect on maternal weight gain during pregnancy and on gestational hypertension(76, 77); furthermore, Butalia et al.(77) reported that, compared to insulin, metformin significantly decreased the frequency of neonatal hypoglycemia, LGA neonates, and admissions to a neonatal intensive care unit (Figure 5b,)(56, 72–74, 77–83). Gui et al.(75) suggested that metformin reduced the rate of gestational hypertension because of its effects on endothelial function and its decrease in the production of reactive oxygen species, the two mechanisms implicated in the pathophysiology of preeclampsia(84–94).
Figure 5. Results of the meta-analyses comparing the efficacy of treatment with metformin vs. insulin in women with gestational diabetes.
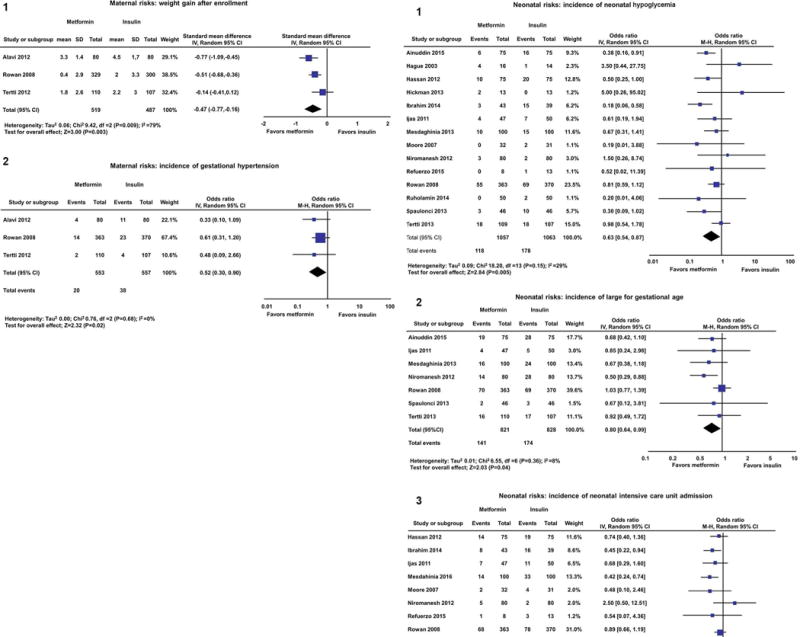
a. The panels present the beneficial effects of metformin vs. insulin in women with gestational diabetes that indicate a reduction in: 1) maternal weight gain during pregnancy; 2) gestational hypertension. Reproduced with permission from Gui J, Liu Q, Feng L. Metformin vs insulin in the management of gestational diabetes: a meta-analysis. PLoS One. 2013 May 27; 8:e64585.
b. The panels present the beneficial effects of metformin vs. insulin in women with gestational diabetes that indicate a reduction in: 1) neonatal hypoglycemia; 2) large-for-gestational-age neonates; and 3) admissions to the neonatal intensive care unit.Reproduced with permission from Butalia S, Gutierrez L, Lodha A, Aitken E, Zakariasen A, Donovan L. Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta-analysis. Diabet Med. 2017; 34:27-36.
(Abbreviations: CI, confidence interval; IV, inverse variance; M-H, Mantel-Haenszel).
Two trials from the UK report conflicting results of treating obese, non-diabetic, pregnant women with metformin
In a randomized controlled clinical trial called “Efficacy of Metformin in Pregnant Obese Women” (EMPOWaR), the effect of metformin was compared to placebo in non-diabetic, obese (defined here as a BMI>30kg/m2), pregnant women(95). Women randomized to receiving metformin began treatment between 12-16 weeks of gestation with a starting dose of 500 mg that was increased, as necessary, to a maximum tolerable dose not exceeding 2,500 mg. In this trial, metformin had no significant effect on birth weight, maternal weight gain during gestation, preeclampsia, or combined adverse pregnancy outcomes, including miscarriage, termination of pregnancy, or fetal or neonatal death(95).
The treatment of obese (defined here as a BMI>35 kg/m2), non-diabetic, pregnant women with metformin was compared to placebo in another randomized clinical trial in the UK — the Fetal Medicine Foundation trial(96). In this trial, women randomized to receiving metformin started treatment between 12-18 weeks of gestation at a dose of 1g/day that was increased by 0.5g/week to a maximum dose of 3g/day. The goal was to reduce the rate of LGA infants(96): the primary outcome was a reduction of median neonatal birthweight by 0.3 standard deviations, which would represent a 50% reduction in the incidence of LGA neonates. Although metformin did not reduce the frequency of LGA neonates, it significantly reduced the frequency of preeclampsia and maternal weight gain, although not the rate of gestational diabetes(96). The finding that metformin decreased the frequency of preeclampsia was consistent with the findings reported in a meta-analysis by Gui et al.(75) and Feng and Yang(76).
Trial design and execution may explain the contradictory results of the two UK trials?
Differences in trial design, execution and compliance are the most likely explanations for the contradictory results obtained in these two randomized clinical trials from the United Kingdom. In the Fetal Medicine Foundation trial, women had a higher BMI (>35 kg/m2 vs. >30 kg/m2), were treated with a higher starting and maximum dose of metformin, and were more compliant than women in the EMPOWaR trial(95, 96). In the Fetal Medicine Foundation trial, almost 80% of women took at least 50% of the total number of tablets prescribed, and 91% of those prescribed more than 2.5 g/day did so(96). By contrast, in the EMPOWaR trial, compliance was defined as ingestion of at least one tablet for at least 29% of the days between randomization and delivery, and only 67% of women fulfilled this criterion(95). Suboptimal compliance in randomized trials is well known to cause negative results(97–101). It is also noteworthy that only 13% (443/3329) of eligible patients consented to participate in the EMPOWaR study, whereas 47% (400/844) of eligible women were recruited to the Fetal Medicine Foundation study, making the latter group more representative of women who meet the study’s eligibility criteria and to whom the study’s results apply.
Mechanisms by which metformin may prevent preeclampsia
The role of angiogenic and antiangiogenic factors in the genesis of preeclampsia
For more than 100 years, preeclampsia was thought to be caused by the release of “toxic factors” from an ischemic placenta, hence, the name “toxemia” (see references(102–117) for a review). The most widely known ‘toxins’ at this time are sFlt-1 (or sVEGFR-1) and soluble endoglin(118–142).
It has been hypothesized that sVEGFR-1 is produced in preeclampsia because the placenta is ischemic or hypoxic, and sVEGFR-1 antagonizes angiogenic molecules, such as VEGF and placental growth factor (PlGF)(119, 143–150). Soluble endoglin is a cell surface co-receptor for transforming growth factor (TGF)-β1, which blocks TGF-β1–mediated activation of endothelial nitric oxide synthase (eNOS) and promotes vasorelaxation(124). There is excessive production of sVEGFR-1 and soluble endoglin in the uterus of preeclamptic patients(121) proportional to the severity of the disease (151–153) that causes maternal plasma concentrations of sVEGFR-1 and soluble endoglin to increase before preeclampsia is diagnosed, making them potential biomarkers for the disease(111, 120, 128, 129, 132, 133, 137, 138, 154–179). The increase of sVEGFR-1 causes a parallel decrease of maternal plasma concentrations of PIGF in preeclampsia(119, 143–150).
The administration of sFlt-1 (or sVEGFR-1) and/or soluble endoglin to animals produces changes characteristic of preeclampsia. For example, if sVEGFR-1 is administered to rats using an adenovirus vector, the animals develop hypertension, proteinuria, and glomerular endotheliosis(180). Additionally, if sVEGFR1 and soluble endoglin are given to pregnant rats, the animals not only develop preeclampsia but also liver dysfunction, thrombocytopenia, and intrauterine growth restriction(124); similar results have been obtained in mice(141). This condition is indistinguishable from the HELLP syndrome observed in humans(181–184).
Although the emphasis on the toxic factors produced by an ischemic placenta has been on the balance between angiogenic and anti-angiogenic factors, evidence now suggests that cytokines, such as TNF-α and IL-10, are altered in early and late preeclampsia, and the changes correlate with the type of histopathological changes in the placenta(185). The importance of ischemic placental disease, not limited to preeclampsia, has been the subject of several recent studies, i.e., preterm labor, preterm PROM, fetal growth restriction, fetal death, and other complications of pregnancy(186–189).
Brownfoot et al.(20) from the Translational Obstetrics Group of the Department of Obstetrics and Gynecology, Mercy Hospital for Women, at the University of Melbourne, Heidelberg, Victoria, Australia, reported that metformin reduced the production of soluble fms-like tyrosine kinase-1 (sFlt-1, also known as soluble vascular endothelial growth factor receptor 1 or sVEGFR-1) and soluble endoglin (sENG) in a dose-dependent manner by endothelial cells, villous trophoblast, and villous explants (Figure 6); the report also suggested that metformin regulates these anti-angiogenic factors at the level of the mitochondria. Metformin also decreased the expression of vascular cell adhesion molecule 1 (VCAM1), expressed by endothelial cells that are dysfunctional or have been stimulated by incubation with tumor necrosis factor alpha (TNF-α), a cytokine increased in the circulation of patients with preeclampsia(190–195).
Figure 6. Effect of metformin on soluble fms-like tyrosine kinase-1 (sFlt1)/soluble vascular endothelial growth factor receptor-1 (sVEGFR-1) secretion, soluble endoglin, and isoforms e15a and i13 expression in endothelial cells and placental tissue.

Metformin reduced in a dose-dependent manner sFlt1 from (A) endothelial cells, (B) villous cytotrophoblast cells, and (C) preterm preeclamptic placental villous explants. Metformin also reduced endothelial cell expression of (D) the sFlt-1 i13 isoform, (E) villous cytotrophoblast cells, and (F) preterm preeclamptic placental villous explant messenger RNA expression of sFlt-1 e15a. Metformin reduced soluble endoglin secretion from (G) endothelial cells and (H) villous cytotrophoblast cells, but it did not change soluble endoglin secretion from (I) preterm preeclamptic placental villous explants. (The single asterisk indicates P <0.05; the double asterisk indicates P <0.01; the triple asterisk indicates P <0.0001; and the quadruple asterisk indicates P <0.00001).
(Abbreviations: mM, millimolar; sENG, soluble endoglin; sFlt-1, Soluble fms-like tyrosine kinase-1).
Modified with permission from Brownfoot FC, Hastie R, Hannan NJ, Cannon P, Tuohey L, Parry LJ, et al. Metformin as a prevention and treatment for preeclampsia: effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am J Obstet Gynecol. 2016; 214:356.e1-356.e15.
However, the most persuasive evidence that metformin has a vascular effect is the finding that it reverses the impairment of vascular relaxation induced by incubating the maternal blood vessels obtained from the omentum at the time of cesarean delivery with placental conditioned media of patients with preeclampsia(20). Metformin also abrogated the reduction of angiogenic sprouting induced in human omental vessel explants by incubation with VEGFR-1 (Figure 7)(20). Overall, Brownfoot et al.’s findings suggest that metformin may have a role in the prevention of preeclampsia through its effect on cell metabolism, on the anti-angiogenic state, and, most likely, on other processes associated with this obstetrical syndrome(20).
Figure 7. Effect of soluble fms-like tyrosine kinase-1 (sFlt1)/soluble vascular endothelial growth factor receptor-1 (sVEGFR-1) and metformin on angiogenesis.

Omental vessel rings cultured with sFlt1/sVEGFR-1 reduced the vessel outgrowth (white arrow, middle panel). This effect was resolved when metformin (1 mmol/L) was added to the culture media (right panel). (Abbreviations: sFlt-1, Soluble fms-like tyrosine kinase-1)
Reproduced with permission from Brownfoot FC, Hastie R, Hannan NJ, Cannon P, Tuohey L, Parry LJ, et al. Metformin as a prevention and treatment for preeclampsia: effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am J Obstet Gynecol. 2016; 214:356.e1-356.e15.
Preeclampsia as a mitochondrial disorder and the effect of metformin on mitochondrial function
Torbergsen et al.(196) first suggested that mitochondria might be involved in the pathogenesis of preeclampsia based upon the high frequency of preeclampsia in a family suffering from mitochondrial dysfunction. Six of 10 women with a mitochondrial disease had at least one pregnancy complicated by preeclampsia or eclampsia, whereas none had been hypertensive in the non-pregnant state. Torbergsen et al. suggested that these women developed preeclampsia because their dysfunctional mitochondria could not meet the increased energy demands of pregnancy, and the failure of adequate cellular energy production led to an accumulation of ADP, which is able to mediate most of the changes seen in preeclampsia(89, 197) ADP is a vasoconstrictor, causes platelet aggregation, and breaks down to uric acid.
In 1990, Shanklin and Sibai(198) reported that mitochondria from small vessels, myometrial smooth muscle, myometrial interstitial cells, circulating leukocytes, epidermal and dermal cells, and hepatocytes in women with preeclampsia all had morphologic changes that were quite different from those seen in normal pregnancies(198). The tissues of women with preeclampsia showed central disruption in mitochondrial morphology and changes in the Golgi apparatus, endoplasmic reticulum, and small, unidentified microvesicles, and these mitochondrial changes were not limited to uterine tissues, thus implying that mitochondrial dysfunction was a systemic disorder. That same year, Berkowitz et al.(199) reported a case of abnormal mitochondrial morphology observed in an endomyocardial biopsy obtained from a 20-year-old nulliparous woman, known to have mitochondrial myopathy, who developed severe preeclampsia(199).
Since that time, the following findings have further implicated mitochondria in the pathogenesis of preeclampsia:
Comparative proteomics analysis of placental mitochondria in normal patients and those with preeclampsia has shown up-regulation of four proteins and down-regulation of 22 proteins. Using bioinformatic tools, differentially expressed proteins in this study were identified as participating in many critical processes of preeclampsia, such as reactive oxygen species generation, apoptosis, fatty acid oxidation, respiratory chain function, and the tricarboxylic acid cycle(200);
the median maternal whole blood mitochondrial DNA copy number was higher in women with preeclampsia than in those who experienced a normal pregnancy (p<0.001)(201), suggesting that an influx of mitochondrial DNA into the maternal circulation may act as a danger signal/alarmin responsible for the sterile (absence of infection) intravascular inflammatory processes of this condition(202, 203);
the placentas of patients with preeclampsia(204), as well as those from pregnancies with preeclampsia and SGA(205), overexpress the microRNA (miR)-210, which has, as potential targets, regulation of transcription of the innate immune response. Additionally, it was demonstrated that miR-210 was induced by hypoxia, and RNA interference knockdown resulted in autophagosomal and siderosomal iron accumulation, implicating siderosis or interstitial trophoblasts as mechanisms in preeclampsia and SGA(206); and
aside from its effects on sterile inflammation and iron metabolism, miR-210 also modulates mitochondrial function. The laboratory of Professor Leslie Myatt provided evidence that placental mitochondrial dysfunction is mediated, at least in part, by miR-210 by demonstrating that mitochondrial complexes I, III, and IV decreased in preeclampsia, along with a decrease in the iron-sulfur cluster scaffold homologue (ISCU)(207). Importantly, transfection of cells with miR-210 resulted in a significant reduction in oxygen consumption by mitochondria, mitochondrial respiratory deficiency, and production of ROS(207).
Recently, Professor Gennady Sukhikh’s Research Institute in Moscow has reported the following findings, indicating that mitochondrial functional changes in the placenta occur in both early- and late-onset preeclampsia when compared to normal pregnancy(208):
Women with early-onset preeclampsia had 1) a two-fold increase in the mRNA expression of OPA (optic atrophy)-1 and a three-fold increase in OPA-1 protein expression (cleaved and uncleaved forms) in patients with early-onset preeclampsia. This gene is involved in mitochondrial fusion and in the cristae structure of the inner mitochondrial membrane, a fine-tuned process crucial for mitochondrial quality control; 2) a five-fold decrease in the mitochondrial transcription factor (TFAM)-A; 3) a 1.5-fold increase of the relative placental mitochondrial DNA copy number; and 4) increased mitochondrial respiration in the presence of Complex I substrates(208).
An increase in the P/O ratio (a measure for how much ATP is synthesized per 2 electrons transferred to oxygen) was observed in both early and late preeclampsia. Thus, early-onset preeclampsia is associated with mitochondrial activation, up-regulation of OPA-1, active DNA replication (resulting from a high respiration rate), and TFAM down-regulation, while both early and late preeclampsia are associated with an elevated P/O ratio(208).
Collectively, this evidence, coupled with the conduction of workshop recently held by the International Federation of Placental Associations on the role of mitochondria in placental function(209), suggests that mitochondrial dysfunction plays a major role in the pathogenesis of preeclampsia.
Brownfoot et al.(20) found that metformin can improve endothelial dysfunction, reduce the expression of vascular adhesion molecule 1 mRNA induced by TNFα, and improve whole-blood vessel angiogenesis impaired by sFlt-1 or sVEGFR-1. Given the evidence that metformin acts through the mitochondrial electron transport chain (ETC) by inhibiting complex I(46, 210–212) (Figure 8), Brownfoot et al.(20) investigated whether the effects on sFlt-1 and soluble endoglin production are regulated through the mitochondrial electron transport chain.
Figure 8. Effect of metformin on the mitochondrial respiratory transport chain complex 1.
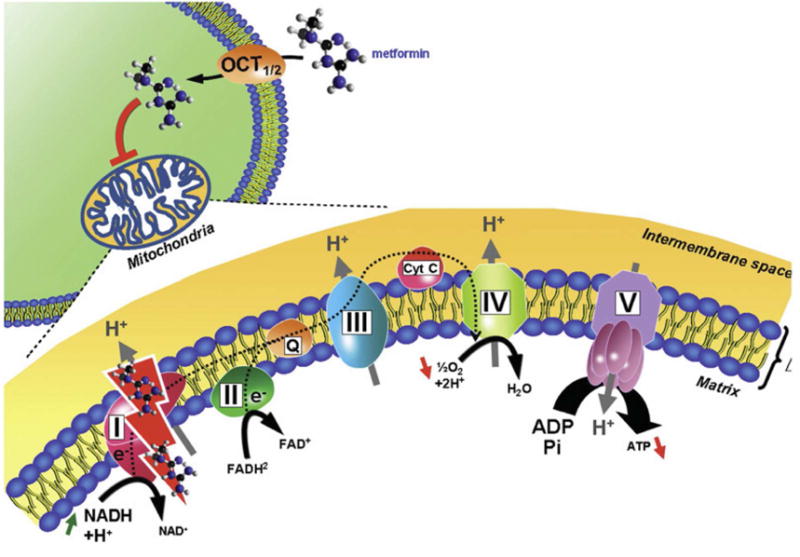
Metformin crosses the plasma membrane of the cell by passive diffusion, and the mitochondria is its main intracellular target. Metformin inhibits mitochondrial respiratory transport chain complex 1 and induces a decrease in the reduced form of nicotinamide adenosine dinucleotide (NADH) oxidation, proton pumping across the inner mitochondrial membrane, and the oxygen consumption rate, leading to a reduction of adenosine triphosphate (ATP) synthesis. (Abbreviations: ADP, Adenosine pyrophosphate; ATP, Adenosine triphosphate; Cyt c, cytochrome complex; FAD, flavin adenine dinucleotide; H+, hydrogen ion; H2O, water; NAD, Nicotinamide Adenine Dinucleotide; OCT, organic cation transporter)
Reproduced with permission from Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012; 122:253-70.
The reduction in the secretion of anti-angiogenic factors by metformin was reversed when succinate (whose metabolism via complex II bypasses complex I) was used as an electron source(20). However, succinate is also an inhibitor of HIF-1α(213), which has been implicated in the exaggerated production of sFlt-1 and in the pathophysiology of preeclampsia(214, 215); therefore, succinate could also be acting by inhibiting HIF-1α, rather than the mitochondrial electron transport chain. However, Brownfoot et al.(20) demonstrated that other electron transport chain inhibitors (antimycin and rotenone) blocked secretion of sFlt-1 and soluble endoglin, suggesting that the electron transport chain is the major effector of the benefits of metformin. Indeed, succinate in the absence of metformin appears to have no effect(20). The role that ROS and the electron transport chain play in the genesis of preeclampsia could be parsed both pharmacologically and genetically(216–219). The changes in the mitochondrial function in preeclampsia suggest there are perturbations in the cellular energy balance of patients with this syndrome that affect cell growth and division (especially in the placenta and the fetus); all are modified by metformin.
The biological basis for the effects of metformin on fetal growth
Nutrient sensing – key for the survival of all living forms
“Cell growth and division are the two most fundamental features of life”(220). All organisms must be able to detect nutrient levels in their environment to coordinate growth and development. This is true of bacteria that must choose whether to grow, remain stationary, and, in the case of motile bacteria, determine in which direction to move depending upon the availability of nutrients. Bacteria evolved specific chemoreceptors for this purpose, which coordinate information received from the environment with specialized structures, such as flagella, to move toward nutrients. The evolutionary history of nutrient-sensing pathways from bacteria to humans is displayed in Figure 9(221).
Figure 9. Nutrient-sensing pathways in the evolution of species from unicellular to multicellular organisms.
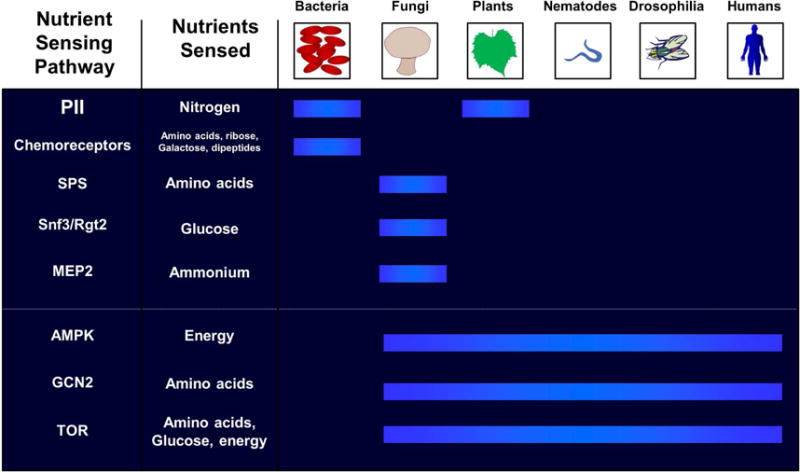
Unicellular organisms have two distinct nutrient pathways (PII and chemoreceptors), while fungi evolved three distinct nutrient-sensing pathways (SPS, Snf3/Rgt2, and MEP2). are denoted, followed by the sensing pathways that are conserved from yeast to man. Blue bars indicate the presence of the nutrient-sensing pathways used by different organisms. (Abbreviations: AMPK, 5′ adenosine monophosphate-activated protein kinase; GCN2, general control nonderepressible 2; MEP, 2-C-methyl-D-erythritol 4-phosphate; SPS, Ssy1-Ptr3-Ssy5; TOR, target of rapamycin)
Reproduced with permission from Chantranupong L, Wolfson RL, Sabatini DM. Nutrient-sensing mechanisms across evolution. Cell. 2015; 161:67-83.
The evolution from prokaryotes (a two-compartment system) to eukaryotes (Figure 10) created unique opportunities for storage: eukaryotes have a third compartment that allows intracellular storage – here, nutrient sensing can also occur (see Figure 10)(221). Nutrient sensing became more complex in metazoans, as they were required to maintain homeostasis of different tissues and organs. Indeed, over the course of millions of years, specific pathways have evolved for glucose, amino-acids, and energy, and metazoans eventually evolved the endocrine and paracrine systems to meet their requirements for nutrient sensing. The coordinated actions of hormones such as insulin, leptin, and ghrelin regulate the organism’s response to the presence or absence of nutrients, modulate anabolic and catabolic processes, and control feeding behavior by signaling the brain(222–224).
Figure 10. Mechanisms of nutrient-sensing within unicellular and multicellular organisms.
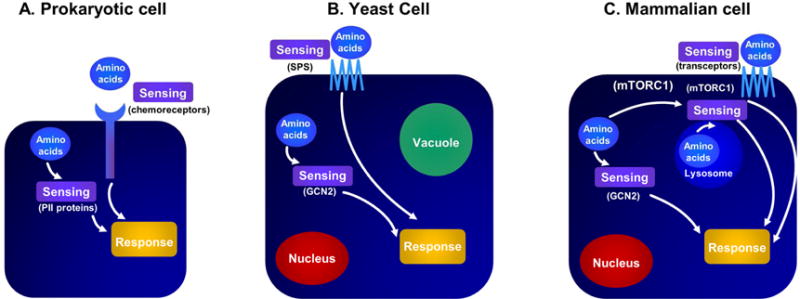
Panel A: Prokaryotes can sense amino acids through a variety of sensors present in the cytosol and extracellular compartments. Panel B: Similarly, yeast cells sense extracellular amino acids via plasma membrane transporters and cytosolic sensors. Eukaryote cells have another potential compartment, such as a vacuole, where sensing may occur. Panel C: In mammalian cells, sensing may occur via cell membrane transporters in the cytosol and within the lysosome. (Abbreviations: mTORC1, mechanistic target of rapamycin complex 1;)
Reproduced with permission from Chantranupong L, Wolfson RL, Sabatini DM. Nutrient-sensing mechanisms across evolution. Cell. 2015; 161:67-83.
Fetal-placental nutritient sensing
Nutrient sensing is a crucial requirement for fetal development. The key structure in the placenta responsible for nutritient sensing is the syncytiotrophoblast, which covers the villous tree and is in direct contact with the maternal blood in the intervillous space. In this strategic location, the nutritient-sensing mechanisms of the syncytiotrophoblast can detect changes in maternal blood composition, enabling the placenta to constantly monitor it, not only regarding the nutritional status of maternal circulation but also for any threat it contains to the fetus (i.e. microorganisms). Thus, it is easy to envision that the syncytiotrophoblast and other components of the placenta contain the nutritient-sensing systems that allow the fetus to regulate its own growth by extracting nutrition from the maternal blood.
Disorders in nutrient-sensing pathways may lead to fetal growth disorders, and studies by Professors Powell and Jansson have provided unique insights into nutrient sensing by the human placenta(225–227). For example, down-regulation of mechanistic target of rapamycin (mTOR) in the placenta has been reported in SGA/growth-restricted fetuses (see Figure 11)(226). Whether this represents a primary defect in the sensing mechanisms or an adaptive response by the feto-placental unit remains to be determined. Similarly, when the placenta senses an excess of nutrients (as in obesity, diabetes, or other metabolic disorders) mTOR activation and fetal growth acceleration may be expected to occur.
Figure 11. Effect of maternal nutrition on the placental nutritional sensing system and fetal growth.
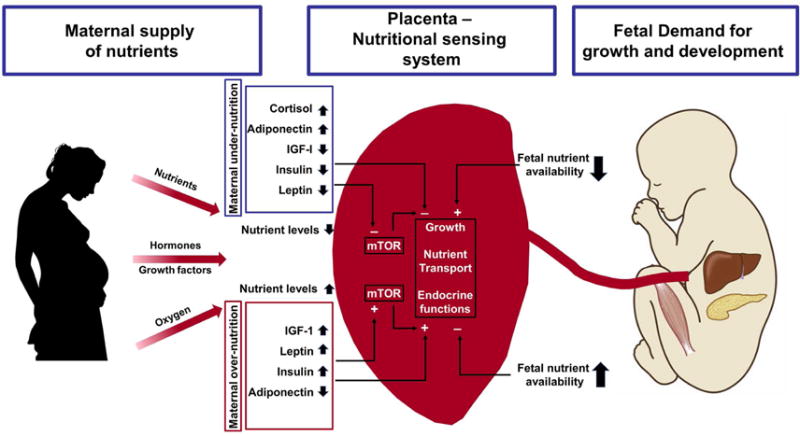
The placenta plays a critical role in modulating maternal-fetal resource allocation, thereby affecting fetal growth and the long-term health of the offspring. Maternal under-nutrition decreases circulating levels of IGF-I, leptin, and insulin and increases maternal serum adiponectin concentrations, leading to low fetal nutrient availability. Maternal over-nutrition is associated with increased insulin, Insulin-like growth factor-1 (IGF-1), and leptin concentrations in the maternal circulation and decreased levels of circulating levels of adiponectin, leading to fetal overgrowth. The placenta integrates maternal and fetal nutritional signals with information from intrinsic nutrient sensors such as mammalian target of rapamycin (mTOR) signaling.
(Abbreviations: IGF-1, Insulin-like growth factor-1; mTOR, mechanistic target of rapamycin) Modified with permission from Jansson T, Powell TL. Role of placental nutrient sensing in developmental programming. Clin Obstet Gynecol. 2013; 56:591-601; and Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta. 2012; 33 Suppl 2: e23-e29 and https://clipartfest.com/download/2ccb316331956e87398d72be2d6d14e49c1a9be8.html
Nutrient sensing occurs through several pathways(225), the most important of which are:
the AMPK pathway, which is a global energy sensor in cells;
glycogen synthase 3 (GSK-3), which acts as a glucose sensor;
the hexose-amino signaling pathway, which depends on the availability of nutrients such as glucose, glutamine, and acetyl-CoA;
the amino-acid response signal transduction pathway, which is activated under conditions essential amino-acids deficiency or imbalance; and
the mechanistic target of rapamycin complex 1 (mTORC1) pathway, which integrates nutrient and growth factor signaling.
Each of these nutritient-sensing pathways is present in the human placenta(225–227). There are also changes in these pathways in cases of fetal growth restriction caused by maternal starvation or impaired placentation where there is down-regulation of placental nutrient transport(225, 226).
The mechanistic target of rapamycin (mTOR) translates maternal nutritional status via the placenta to the fetus
Rapamycin is an anti-fungal agent produced by Streptomyces higroscopicus(228) that inhibits the growth of Candida albicans and that was first discovered in soil on Easter Island (Rapa Nui). The story of its discovery is similar to that of penicillin: its isolation from Aspergillus penicillinum and its capacity to inhibit bacterial growth. On the other hand, rapamycin’s popularity is due to its powerful immunosuppressive properties, which made it a drug of choice for patients with renal transplants(229), and not its antimicrobial properties.
Rapamycin can also prolong the life cycle of many species as diverse as worms and mice, and its signaling system has been implicated as the master regulator of cellular growth and metabolism. A family of molecules that are the mechanistic target of the rapamycin (mTOR) complex has been attributed a major role in the biology of cell metabolism, growth, longevity, and even preterm birth(230–236).
Rapamycin acts by interacting with a family of TOR molecules that is highly-conserved functionally and evolutionarily from yeast to humans, and it contains two multiprotein complexes that are functionally and structurally different: the first, mTORC1, is rapamycin-sensitive; and the second, mTORC2, is rapamycin-resistant(237). Both complexes control cell growth and metabolism in response to the availability of nutrients, energy, and growth factors. Upon activation, the serine/threonine kinases activate a cascade of intracellular processes involving the mitochondria and the nucleus of the cell to promote cellular growth and, to a certain extent, aging. Rapamycin prolongs lifespan by inhibiting the effects of mTOR. (See references(238, 239) for an in-depth discussion of this topic.)
mTOR is a major human nutrient-sensing receptor in the placenta(225). This signaling system is highly expressed in the syncytiotrophoblast, and its activity is regulated by glucose and amino-acid concentrations(226, 227, 240).
mTOR has key properties in the placenta(225, 226) that include its activation by insulin, IGF-1, and leptin, and its inhibition by cortisol. mTOR regulates two key amino-acid transport systems (A and L). The activation of mTOR is positively correlated with the first-trimester maternal body mass index, linking maternal over-nutrition and nutritient-sensing by the placenta; while its expression is down-regulated when fetal growth is restricted (in both animal models and humans). The mTOR Akt-mTOR-HIF-1α signaling pathway also affects placental angiogenesis by its ability to increase VEGF and endoglin expression in response to hypoxia in a trophoblast cell line(241).
Safety of metformin during pregnancy
Metformin has a molecular weight of 129 dalton, and it crosses the placenta in direct diffusion without affecting the facilitated transfer of glucose(242). Indeed, an ex vivo dually perfused human placental lobule demonstrated the rapid transfer of metformin from maternal to fetal circulation with a lag time of 1.7±0.28 minutes similarly observed in women with normal pregnancies and those diagnosed with gestational diabetes mellitus(242). In vivo studies reported the detection of metformin in the umbilical cord blood of neonates from women with polycystic ovarian syndrome who were treated with metformin throughout gestation(243, 244). Similar concentrations of metformin in the umbilical artery and umbilical vein suggest negligible metformin metabolism by the fetus (244). Several meta-analyses that studied the teratogenic effect of metformin on embryonic development found that this drug carries no increased risk for congenital malformations(245, 246) and is currently classified as category B in the United States and as category C in Australia (247, 248). Similarly, no excess of fetal or neonatal complications could be demonstrated when the administration of metformin was compared to glyburide and insulin(249). In addition, a study that investigated the neurodevelopmental effect at two years of age could not identify a significant difference between children exposed in utero to metformin and those exposed to insulin(250). Moreover, there were no differences in fat measurements, total fat mass, and percentage of body fat(251). Those who were exposed to metformin during fetal life had a larger upper arm circumference and bigger subscapular skin folds and biceps, suggesting a better fat distribution than children exposed to insulin(251).
Maternal side-effects reported with the use of metformin are mainly gastro-intestinal, i.e., nausea and diarrhea(248). The rate of hypoglycemia is lower than that reported with insulin(79). In addition, rare side-effects such as mild erythema and decreased vitamin B12 absorption have been associated with long-term administration(252).
A role for metformin in cancer and aging
Originally introduced for the treatment of diabetes, metformin is now gaining attention as a potential anti-cancer agent (7–10) (Figure 1). The first observation that metformin might reduce the risk of cancer was made in a population-based case-control study of type 2 diabetic patients treated with metformin(253). A cohort study of type 2 diabetic patients newly treated with metformin later followed in which the frequency of cancer was significantly lower in patients receiving metformin than in the controls who had never received metformin, after adjusting for body mass index, hemoglobin A1C, smoking and the use of other drugs (254): this finding has subsequently been confirmed in several other studies(7–10). In a meta-analysis(255), metformin-treated diabetic patients had a 31% reduction in the incidence of cancer and a 34% reduction in cancer mortality after adjusting for body mass index(255). This epidemiologic evidence has coalesced with experimental work in animals(256–263). Animal experiments have elucidated the mechanisms underlying these epidemiological findings, i.e., suppression of cancer stem cells(264); inhibition of epithelial-to-mesenchymal transition(265), (which is implicated in metastasis); and interference with glucose(266), protein(267, 268), and lipid(269) metabolism of the neoplastic cells. There is further evidence that metformin may also have an adjunctive effect in patients receiving chemotherapy(270), and there are now more than 100 ongoing trials registered in clinicaltrials.gov studying the role of metformin in cancer treatment (see references(45, 271, 272) for a review of this topic and the mechanisms of action underlying these effects of metformin).
Finally, an unbiased search for genes that extend lifespan has identified a disproportionate number of genes that function in mitochondrial metabolism(273), similar observatons werealso found by targeting mitochondrial genes(274). Not surprisingly, drugs that inhibit mitochondrial function were examined in this context, and metformin was shown to extend longevity in worms(2) and mice(3) (although not in Drosophila(275)). Metformin has now been found to target several age-related pathways(3, 276, 277), but the mechanisms by which metformin extends lifespan are far from clear. A randomized clinical trial, TAME (“Targeting aging with metformin”)(6), has been planned to test the effect of metformin on the time to the new occurrence of a composite outcome that includes cardiovascular events, cancer, dementia, and mortality as an endpoint in 3,000 subjects who are 65-79 years of age(6).
Metformin exerts many of its growth inhibitory and anti-neoplastic effects through the nuclear pore complex and acyl-CoA dehydrogensase family member-10 (ACAD10)(278). Nucleocytoplasmic shuttling regulates mTORC activity, an effect that can be reversed by RNA silencing. Biguanides inhibit growth by suppressing mitochondrial respiration, which limits the transit of the complex RagA-RagC GTPase heterodimer through the nuclear pore complex(278). By preventing access to the nucleus, RagC becomes incapable of stimulating mTORC1 and, therefore, stimulates cellular growth (Figure 12)(278). Wu et al. proposed that the nuclear pore complex and ACAD10 are involved in insulin action and regulation of blood glucose concentration(278). Whether these mechanisms are also responsible for the anti-diabetic actions of metformin and its ability to improve insulin sensitivity is unknown at this time.
Figure 12. Metformin suppresses cell growth and promotes longevity.
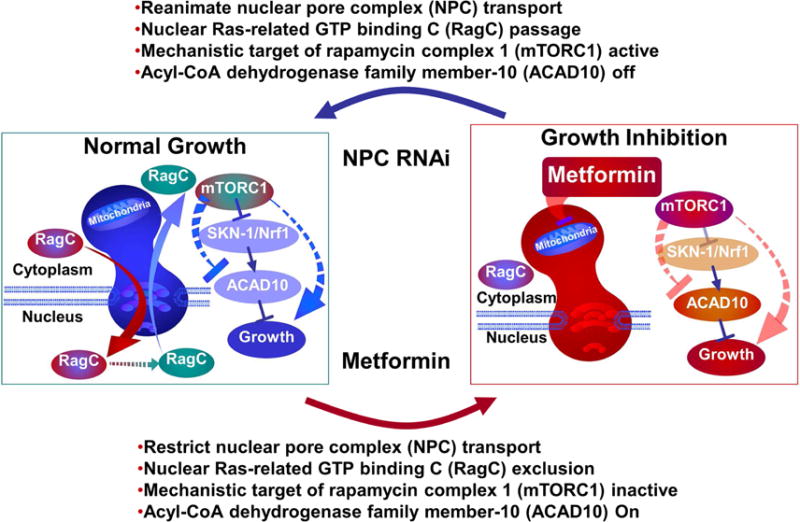
Metformin slows Caenorhabditis elegans (roundworm) growth by inhibiting the mitochondrial electron transport chain, which limits the transit of the RagC protein through the nuclear pore complex resulting in a reduced activity of the mechanistic target of rapamycin complex 1 (mTORC1). The metformin-induced inhibition of mTORC1 leads to the upregulation of the transcription factor Skn-1/Nrf-2 (a regulator of antioxidant genes) and the expression of acyl-CoA dehydrogenase family member-10 (ACAD10) gene.
(Abbreviations: ACAD10, acyl-CoA dehydrogenase family member-10; mTORC1, mechanistic target of rapamycin complex 1; NPC, nuclear pore complex; RagC, Ras-related GTP binding C; RNAi, RNA interference; Skn-1/Nrf-2, protein skinhead-1/nuclear-factor-erythroid-related factor-2).
Reproduced with permission from Wu L, Zhou B, Oshiro-Rapley N, Li M, Paulo JA, Webster CM, Mou F, Kacergis MC, Talkowski ME, Carr CE, Gygi SP, Zheng B, Soukas AA. An ancient, unified mechanism for metformin growth inhibition in C. elegans and cancer. Cell. 2016; 167:1705-1718.e13.
Conclusion
Metformin, long known to be an herbal medicine, has evolved from its use as a popular treatment for diabetes into a drug with a significantly wider array of beneficial effects ranging from cancer treatment to extending longevity, as well as, in our field, gestational hypertension and preeclampsia in obese women. Current evidence suggests that metformin’s wide-ranging beneficial effects are mediated by at least two primary mechanisms: suppression of intracellular metabolic activity of mitochondria and the cellular nutritient-sensing system mediated by mTOR.
Table 1.
Association between prepregnancy body mass index and the risk of mild and severe preeclampsia and gestational hypertension according to ethnicity in a multinomial regression model (n= 35,422 for preeclampsia Caucasian women and n=36,936 for gestational hypertension).
| Ethnicity | Body mass index (kg/me | Preeclampsia | Preeclampsia | Gestational hypertension | Gestational hypertension |
|---|---|---|---|---|---|
| Mild | Severe | Mild | Severe | ||
| Caucasian | 17 | 0.7 (0.6 - 0.8) | 0.8 (0.5 - 1.1) | 0.7 (0.6 - 0.8) | 0.4 (0.2 - 0.7) |
| 20 | Reference | Reference | Reference | Reference | |
| 25 | 1.8 (1.5-2.1) | 1.7 (1.1-2.5) | 1.8 (1.6-2.0) | 3.6 (2.0-6.5) | |
| 30 | 3.0 (2.3-3.8) | 3.4 (2.1-5.6) | 3.0 (2.6–3.5) | 8.8 (4.4-17.6) | |
| 35 | 4.9 (3.5- | 7.6 (4.2-13.7) | 4.9 (4.0-6.1) | 17.4 (8.5–35.9) | |
| African American | 17 | 1.0 (0.7–1.3) | 1.4 (0.8–2.3) | 1.0 (0.8–1.2) | 1.4 (0.6–3.6) |
| 20 | 1.4 (1.1–1.6) | 1.6 (1.1-2.3) | 1.2 (1.0-1.3) | 1.9 (1.0- 3.7) | |
| 25 | 2.2 (1.8-2.7) | 2.1 (1.4-3.2) | 1.5 (1.4–1.7) | 3.0 (1.6- 5.8) | |
| 30 | 3.1 (2.5–3.9) | 3.2 (2.1-5.0) | 2.2 (1.9–2.5) | 4.9 (2.5–9.6) | |
| 35 | 4.2 (3.1–5.7) | 5.3 (2.9–9.7) | 3.3 (2.7 -4.0) | 8.0 (3.7–17.5) |
Values displayed are adjusted Odds ratio and (95% Confidence interval). Odds ratio was adjusted for maternal age, smoking status, marital status, socio-economic status, parity and maternal height.
Table modified from Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM. Epidemiology 2007;18(2):234-239.
Table 2.
Summary of Metabolic changes in Pregnancy and Preeclampsia
| Normal Pregnancy | Preeclampsia | |||
|---|---|---|---|---|
| Early | Late | Early | Late | |
| Maternal metabolism | Anabolic | Catabolic | Anabolic | Catabolic |
| Glucose intolerance | ~ | ↓ | ~↓ | ↓↓ |
| Insulin sensitivity | ~ | ↓ | ~ ↓ | ↓↓ |
| Free fatty acids | ↑ | ↑↑ | ↑↑ | ↑↑↑ |
| Triglycerides | ↑ | ↑↑ | ↑↑↑ | ↑↑↑ |
| Cholesterol | ~ | ↑ | ~↑ | ↑ |
~ Similar compared to non-pregnant controls.
↑, ↑↑, ↑↑↑: elevated (relative degree increasing by number of arrows) compared to non-pregnancy controls.
↓, ↓↓: lower compared to non-pregnant controls.
Adapted from von Versen-Hoeynck FM and Powers RW. Frontiers in Bioscience 2007;12:2457-2470 and Jeyabalan, A., C. A. Hubel, et al. (2014). Metabolic syndrome and preeclampsia. Chesley’s hypertensive disorders in pregnancy. R. N. Taylor, J. M. Roberts, F. G. Cunningham and M. D. Lindheimer, Elsevier: 133-160.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PloS one. 2010;5(1):e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228–39. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Haes W, Frooninckx L, Van Assche R, Smolders A, Depuydt G, Billen J, et al. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci U S A. 2014;111(24):E2501–9. doi: 10.1073/pnas.1321776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crandall Jill. Metformin in Longevity Study (MILES) 2015 [Google Scholar]
- 6.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. Cell metabolism. 2016;23(6):1060–5. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang ZJ, Bi Y, Li S, Zhang Q, Zhao G, Guo Y, et al. Reduced risk of lung cancer with metformin therapy in diabetic patients: a systematic review and meta-analysis. American journal of epidemiology. 2014;180(1):11–4. doi: 10.1093/aje/kwu124. [DOI] [PubMed] [Google Scholar]
- 8.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PloS one. 2013;8(8):e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Lai ST, Xie L, Zhao JD, Ma NY, Zhu J, et al. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes research and clinical practice. 2014;106(1):19–26. doi: 10.1016/j.diabres.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Wu L, Zhu J, Prokop LJ, Murad MH. Pharmacologic Therapy of Diabetes and Overall Cancer Risk and Mortality: A Meta-Analysis of 265 Studies. Scientific reports. 2015;5:10147. doi: 10.1038/srep10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victor VM, Rovira-Llopis S, Banuls C, Diaz-Morales N, Lopez-Domenech S, Escribano-Lopez I, et al. Metformin modulates human leukocyte/endothelial cell interactions and proinflammatory cytokines in polycystic ovary syndrome patients. Atherosclerosis. 2015;242:167–73. doi: 10.1016/j.atherosclerosis.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Tan X, Li S, Chang Y, Fang C, Liu H, Zhang X, et al. Effect of metformin treatment during pregnancy on women with PCOS: a systematic review and meta-analysis. Clinical and investigative medicine Medecine clinique et experimentale. 2016;39(4):E120–31. doi: 10.25011/cim.v39i4.27091. [DOI] [PubMed] [Google Scholar]
- 13.Feng L, Lin XF, Wan ZH, Hu D, Du YK. Efficacy of metformin on pregnancy complications in women with polycystic ovary syndrome: a meta-analysis. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2015;31(11):833–9. doi: 10.3109/09513590.2015.1041906. [DOI] [PubMed] [Google Scholar]
- 14.Brock B, Smidt K, Ovesen P, Schmitz O, Rungby J. Is metformin therapy for polycystic ovary syndrome safe during pregnancy? Basic & clinical pharmacology & toxicology. 2005;96(6):410–2. doi: 10.1111/j.1742-7843.2005.pto_02.x. [DOI] [PubMed] [Google Scholar]
- 15.Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, et al. Clinical Outcomes of Metformin Use in Populations With Chronic Kidney Disease, Congestive Heart Failure, or Chronic Liver Disease: A Systematic Review. Annals of internal medicine. 2017;166(3):191–200. doi: 10.7326/M16-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. Journal of immunology (Baltimore, Md: 1950) 2009;182(12):8005–14. doi: 10.4049/jimmunol.0803563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negrotto L, Farez MF, Correale J. Immunologic Effects of Metformin and Pioglitazone Treatment on Metabolic Syndrome and Multiple Sclerosis. JAMA neurology. 2016;73(5):520–8. doi: 10.1001/jamaneurol.2015.4807. [DOI] [PubMed] [Google Scholar]
- 18.Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, et al. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1alpha expression and oxygen metabolism. Diabetes. 2011;60(3):981–92. doi: 10.2337/db10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat A, Sebastiani G, Bhat M. Systematic review: Preventive and therapeutic applications of metformin in liver disease. World J Hepatol. 2015;7(12):1652–9. doi: 10.4254/wjh.v7.i12.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brownfoot FC, Hastie R, Hannan NJ, Cannon P, Tuohey L, Parry LJ, et al. Metformin as a prevention and treatment for preeclampsia: effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. American Journal of Obstetrics & Gynecology. 2016;214:356.e1–15. doi: 10.1016/j.ajog.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Witters LA. The blooming of the French lilac. J Clin Invest. 2001;108(8):1105–7. doi: 10.1172/JCI14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12(8):553–64. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 23.Bailey CJ, Day C. Metformin: its botanical background. Practical Diabetes International. 2004;21(3):115–7. [Google Scholar]
- 24.Thomas I, Gregg B. Metformin; a review of its history and future: from lilac to longevity. Pediatric diabetes. 2017;18(1):10–6. doi: 10.1111/pedi.12473. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe C. Studies in the metabolic changes induced by administration of guanidine bases. J Biol Chem. 1918;33:253–65. [Google Scholar]
- 26.Sterne J. Pharmacology and mode of action of the hypoglycaemic guanidine derivatives. In: Campbell GD, editor. Oral hypoglycaemic agents: pharmacology and therapeutics. Vol. 9. Academic Press; 1969. pp. 193–245. [Google Scholar]
- 27.Muller H, Rheinwein H. Pharmacology of galegin. Arch Expll Path Pharm. 1927;125:212–28. [Google Scholar]
- 28.Simonnet H, Tanret G. Sur les propietes hypoglycemiantes du sulfate de galegine. Bull Soc Chim Biol Paris. 1927;8 [Google Scholar]
- 29.Nattrass M, Alberti KG. Biguanides. Diabetologia. 1978;14(2):71–4. doi: 10.1007/BF01263443. [DOI] [PubMed] [Google Scholar]
- 30.Paulesco N. Recherches sur le role du pancreas dans l’assimilation nutritive. Arch Int Physiol. 1921;17:85–109. [Google Scholar]
- 31.Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Canadian Medical Association journal. 1922;12(3):141–6. [PMC free article] [PubMed] [Google Scholar]
- 32.Sterne J. Treatment of diabetes mellitus with N,N-dimethylguanylguanidine (LA. 6023, glucophage) Therapie. 1959;14:625–30. [PubMed] [Google Scholar]
- 33.Bernier GM, Miller M, Springate CS. Lactic acidosis and phenformin hydrochloride. Jama. 1963;184:43–6. doi: 10.1001/jama.1963.03700140099014. [DOI] [PubMed] [Google Scholar]
- 34.Ewy GA, Pabico RC, Maher JF, Mintz DH. Lactate acidosis associated with phenformin therapy and localized tissue hypoxia. Report of a case treated by hemodialysis. Annals of internal medicine. 1963;59:878–83. doi: 10.7326/0003-4819-59-6-878. [DOI] [PubMed] [Google Scholar]
- 35.Tranquada RE, Bernstein S, Martin HE. Irreversible lactic acidosis associated with phenformine therapy. Report of three cases. Jama. 1963;184:37–42. doi: 10.1001/jama.1963.03700140093013. [DOI] [PubMed] [Google Scholar]
- 36.Davidson MB, Bozarth WR, Challoner DR, Goodner CJ. Phenformin, hypoglycemia and lactic acidosis. Report of attempted suicide. The New England journal of medicine. 1966;275(16):886–8. doi: 10.1056/NEJM196610202751606. [DOI] [PubMed] [Google Scholar]
- 37.Proctor DW, Stowers JM. Fatal lactic acidosis after an overdose of phenformin. British medical journal. 1967;4(5573):216. doi: 10.1136/bmj.4.5573.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shirriffs GG, Bewsher PD. Hypothermia, abdominal pain, and lactic acidosis in phenformin-treated diabetic. British medical journal. 1970;3(5721):506. doi: 10.1136/bmj.3.5721.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 41.American Diabetes A. 4. Prevention or Delay of Type 2 Diabetes. Diabetes Care. 2016;39(Suppl 1):S36–8. doi: 10.2337/dc16-S007. [DOI] [PubMed] [Google Scholar]
- 42.Diabetes Prevention Program Research G. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. The lancet Diabetes & endocrinology. 2015;3(11):866–75. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 2006;49(3):434–41. doi: 10.1007/s00125-006-0141-7. [DOI] [PubMed] [Google Scholar]
- 45.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell metabolism. 2014;20(6):953–66. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clinical science (London, England: 1979) 2012;122(6):253–70. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coetzee EJ, Jackson WP. Metformin in management of pregnant insulin-independent diabetics. Diabetologia. 1979;16(4):241–5. doi: 10.1007/BF01221950. [DOI] [PubMed] [Google Scholar]
- 48.Coetzee EJ, Jackson WP. Diabetes newly diagnosed during pregnancy: A 4-year study at Groote Schuur Hospital. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1979;56(12):467–75. [PubMed] [Google Scholar]
- 49.Jackson WP, Coetzee EJ. Side-effects of metformin. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1979;56(26):1113–4. [PubMed] [Google Scholar]
- 50.Coetzee EJ, Jackson WP. Pregnancy in established non-insulin-dependent diabetics. A five-and-a-half year study at Groote Schuur Hospital. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1980;58(20):795–802. [PubMed] [Google Scholar]
- 51.Elliott BD, Langer O, Schuessling F. Human placental glucose uptake and transport are not altered by the oral antihyperglycemic agent metformin. Am J Obstet Gynecol. 1997;176(3):527–30. doi: 10.1016/s0002-9378(97)70541-6. [DOI] [PubMed] [Google Scholar]
- 52.Simmons D, Walters BN, Rowan JA, McIntyre HD. Metformin therapy and diabetes in pregnancy. The Medical journal of Australia. 2004;180(9):462–4. [PubMed] [Google Scholar]
- 53.Vanky E, Salvesen KA, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Human reproduction (Oxford, England) 2004;19(8):1734–40. doi: 10.1093/humrep/deh347. [DOI] [PubMed] [Google Scholar]
- 54.Coustan DR. Pharmacological management of gestational diabetes: an overview. Diabetes Care. 2007;30(Suppl 2):S206–8. doi: 10.2337/dc07-s217. [DOI] [PubMed] [Google Scholar]
- 55.Ecker JL, Greene MF. Gestational diabetes–setting limits, exploring treatments. The New England journal of medicine. 2008;358(19):2061–3. doi: 10.1056/NEJMe0802623. [DOI] [PubMed] [Google Scholar]
- 56.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin versus insulin for the treatment of gestational diabetes. The New England journal of medicine. 2008;358(19):2003–15. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 57.Paglia MJ, Coustan DR. The use of oral antidiabetic medications in gestational diabetes mellitus. Current diabetes reports. 2009;9(4):287–90. doi: 10.1007/s11892-009-0044-3. [DOI] [PubMed] [Google Scholar]
- 58.Glueck CJ, Goldenberg N, Streicher P, Wang P. The contentious nature of gestational diabetes: diet, insulin, glyburide and metformin. Expert opinion on pharmacotherapy. 2002;3(11):1557–68. doi: 10.1517/14656566.3.11.1557. [DOI] [PubMed] [Google Scholar]
- 59.Glueck CJ, Wang P, Kobayashi S, Phillips H, Sieve-Smith L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertility and sterility. 2002;77(3):520–5. doi: 10.1016/s0015-0282(01)03202-2. [DOI] [PubMed] [Google Scholar]
- 60.Glueck CJ, Goldenberg N, Streicher P, Wang P. Metformin and gestational diabetes. Current diabetes reports. 2003;3(4):303–12. doi: 10.1007/s11892-003-0022-0. [DOI] [PubMed] [Google Scholar]
- 61.Homko CJ, Reece EA. Insulins and oral hypoglycemic agents in pregnancy. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2006;19(11):679–86. doi: 10.1080/14767050600863376. [DOI] [PubMed] [Google Scholar]
- 62.Metzger BE. Diet and medical therapy in the optimal management of gestational diabetes mellitus. Nestle Nutrition workshop series Clinical & performance programme. 2006;11:155–65. doi: 10.1159/000094449. discussion 65-9. [DOI] [PubMed] [Google Scholar]
- 63.Eyal S, Easterling TR, Carr D, Umans JG, Miodovnik M, Hankins GD, et al. Pharmacokinetics of metformin during pregnancy. Drug metabolism and disposition: the biological fate of chemicals. 2010;38(5):833–40. doi: 10.1124/dmd.109.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Current diabetes reports. 2012;12(1):33–42. doi: 10.1007/s11892-011-0249-0. [DOI] [PubMed] [Google Scholar]
- 65.Langer O. Oral hypoglycemic agents: do the ends justify the means? Maternal health, neonatology and perinatology. 2015;1:19. doi: 10.1186/s40748-015-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma RC, Schmidt MI, Tam WH, McIntyre HD, Catalano PM. Clinical management of pregnancy in the obese mother: before conception, during pregnancy, and post partum. The lancet Diabetes & endocrinology. 2016;4(12):1037–49. doi: 10.1016/S2213-8587(16)30278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magon N, Seshiah V. Gestational diabetes mellitus: Non-insulin management. Indian J Endocrinol Metab. 2011;15(4):284–93. doi: 10.4103/2230-8210.85580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kampmann U, Madsen LR, Skajaa GO, Iversen DS, Moeller N, Ovesen P. Gestational diabetes: A clinical update. World journal of diabetes. 2015;6(8):1065–72. doi: 10.4239/wjd.v6.i8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rasouli N, Kern PA, Reece EA, Elbein SC. Effects of pioglitazone and metformin on beta-cell function in nondiabetic subjects at high risk for type 2 diabetes. American journal of physiology Endocrinology and metabolism. 2007;292(1):E359–65. doi: 10.1152/ajpendo.00221.2006. [DOI] [PubMed] [Google Scholar]
- 70.Feig DS, Murphy K, Asztalos E, Tomlinson G, Sanchez J, Zinman B, et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): a multi-center randomized controlled trial. BMC pregnancy and childbirth. 2016;16(1):173. doi: 10.1186/s12884-016-0954-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ijas H, Vaarasmaki M, Morin-Papunen L, Keravuo R, Ebeling T, Saarela T, et al. Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG: an international journal of obstetrics and gynaecology. 2011;118(7):880–5. doi: 10.1111/j.1471-0528.2010.02763.x. [DOI] [PubMed] [Google Scholar]
- 72.Moore LE, Briery CM, Clokey D, Martin RW, Williford NJ, Bofill JA, et al. Metformin and insulin in the management of gestational diabetes mellitus: preliminary results of a comparison. The Journal of reproductive medicine. 2007;52(11):1011–5. [PubMed] [Google Scholar]
- 73.Niromanesh S, Alavi A, Sharbaf FR, Amjadi N, Moosavi S, Akbari S. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes research and clinical practice. 2012;98(3):422–9. doi: 10.1016/j.diabres.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 74.Tertti K, Ekblad U, Koskinen P, Vahlberg T, Ronnemaa T. Metformin vs. insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes, obesity & metabolism. 2013;15(3):246–51. doi: 10.1111/dom.12017. [DOI] [PubMed] [Google Scholar]
- 75.Gui J, Liu Q, Feng L. Metformin vs insulin in the management of gestational diabetes: a meta-analysis. PloS one. 2013;8(5):e64585. doi: 10.1371/journal.pone.0064585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feng Y, Yang H. Metformin - a potentially effective drug for gestational diabetes mellitus: a systematic review and meta-analysis. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2016:1–8. doi: 10.1080/14767058.2016.1228061. [DOI] [PubMed] [Google Scholar]
- 77.Butalia S, Gutierrez L, Lodha A, Aitken E, Zakariasen A, Donovan L. Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta-analysis. Diabetic medicine: a journal of the British Diabetic Association. 2017;34(1):27–36. doi: 10.1111/dme.13150. [DOI] [PubMed] [Google Scholar]
- 78.Hickman MA, McBride R, Boggess KA, Strauss R. Metformin compared with insulin in the treatment of pregnant women with overt diabetes: a randomized controlled trial. American journal of perinatology. 2013;30(6):483–90. doi: 10.1055/s-0032-1326994. [DOI] [PubMed] [Google Scholar]
- 79.Spaulonci CP, Bernardes LS, Trindade TC, Zugaib M, Francisco RP. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol. 2013;209(1):34.e1–7. doi: 10.1016/j.ajog.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 80.Ibrahim MI, Hamdy A, Shafik A, Taha S, Anwar M, Faris M. The role of adding metformin in insulin-resistant diabetic pregnant women: a randomized controlled trial. Archives of gynecology and obstetrics. 2014;289(5):959–65. doi: 10.1007/s00404-013-3090-7. [DOI] [PubMed] [Google Scholar]
- 81.Ruholamin S, Eshaghian S, Allame Z. Neonatal outcomes in women with gestational diabetes mellitus treated with metformin in compare with insulin: A randomized clinical trial. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences. 2014;19(10):970–5. [PMC free article] [PubMed] [Google Scholar]
- 82.Ainuddin J, Karim N, Hasan AA, Naqvi SA. Metformin versus insulin treatment in gestational diabetes in pregnancy in a developing country: a randomized control trial. Diabetes research and clinical practice. 2015;107(2):290–9. doi: 10.1016/j.diabres.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Refuerzo JS, Gowen R, Pedroza C, Hutchinson M, Blackwell SC, Ramin S. A pilot randomized, controlled trial of metformin versus insulin in women with type 2 diabetes mellitus during pregnancy. American journal of perinatology. 2015;30(2):163–70. doi: 10.1055/s-0034-1378144. [DOI] [PubMed] [Google Scholar]
- 84.Rodgers GM, Taylor RN, Roberts JM. Preeclampsia is associated with a serum factor cytotoxic to human endothelial cells. Am J Obstet Gynecol. 1988;159(4):908–14. doi: 10.1016/s0002-9378(88)80169-8. [DOI] [PubMed] [Google Scholar]
- 85.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161(5):1200–4. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 86.Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7(3):375–84. doi: 10.1111/j.1538-7836.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- 87.Myatt L, Kossenjans W, Sahay R, Eis A, Brockman D. Oxidative stress causes vascular dysfunction in the placenta. J Matern Fetal Med. 2000;9(1):79–82. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<79::AID-MFM16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 88.Hubel CA, Kozlov AV, Kagan VE, Evans RW, Davidge ST, McLaughlin MK, et al. Decreased transferrin and increased transferrin saturation in sera of women with preeclampsia: implications for oxidative stress. Am J Obstet Gynecol. 1996;175(3 Pt 1):692–700. doi: 10.1053/ob.1996.v175.a74252. [DOI] [PubMed] [Google Scholar]
- 89.Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol. 1996;174(1 Pt 1):288–91. doi: 10.1016/s0002-9378(96)70410-6. [DOI] [PubMed] [Google Scholar]
- 90.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11(6):342–52. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 91.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(Suppl A):S43–8. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31(Suppl):S66–9. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):287–99. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215(1 Suppl):S1–S46. doi: 10.1016/j.ajog.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chiswick C, Reynolds RM, Denison F, Drake AJ, Forbes S, Newby DE, et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. The lancet Diabetes & endocrinology. 2015;3(10):778–86. doi: 10.1016/S2213-8587(15)00219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Syngelaki A, Nicolaides KH, Balani J, Hyer S, Akolekar R, Kotecha R, et al. Metformin versus Placebo in Obese Pregnant Women without Diabetes Mellitus. New England Journal of Medicine. 2016;374(5):434–43. doi: 10.1056/NEJMoa1509819. [DOI] [PubMed] [Google Scholar]
- 97.Cramer JA, Spilker B. Patient compliance in medical practice and clinical trials. Raven Press; 1991. pp. 387–92. [Google Scholar]
- 98.Cramer JA. Effect of partial compliance on cardiovascular medication effectiveness. Heart. 2002;88(2):203–6. doi: 10.1136/heart.88.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cramer J, Rosenheck R, Kirk G, Krol W, Krystal J, Group VANS Medication compliance feedback and monitoring in a clinical trial: predictors and outcomes. Value Health. 2003;6(5):566–73. doi: 10.1046/j.1524-4733.2003.65269.x. [DOI] [PubMed] [Google Scholar]
- 100.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–7. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 101.Shingler SL, Bennett BM, Cramer JA, Towse A, Twelves C, Lloyd AJ. Treatment preference, adherence and outcomes in patients with cancer: literature review and development of a theoretical model. Curr Med Res Opin. 2014;30(11):2329–41. doi: 10.1185/03007995.2014.952715. [DOI] [PubMed] [Google Scholar]
- 102.Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Seminars in perinatology. 1988;12(4):302–23. [PubMed] [Google Scholar]
- 103.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet (London, England) 2005;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 104.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annual review of pathology. 2010;5:173–92. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 105.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Seminars in nephrology. 2011;31(1):33–46. doi: 10.1016/j.semnephrol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Redman CW. Preeclampsia: a multi-stress disorder. La Revue de medecine interne. 2011;32(Suppl 1):S41–4. doi: 10.1016/j.revmed.2011.03.331. [DOI] [PubMed] [Google Scholar]
- 108.Tranquilli AL, Landi B, Giannubilo SR, Sibai BM. Preeclampsia: No longer solely a pregnancy disease. Pregnancy hypertension. 2012;2(4):350–7. doi: 10.1016/j.preghy.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 109.Staff AC, Benton SJ, von Dadelszen P, Roberts JM, Taylor RN, Powers RW, et al. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013;61(5):932–42. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 110.Staff AC, Dechend R, Redman CW. Review: Preeclampsia, acute atherosis of the spiral arteries and future cardiovascular disease: two new hypotheses. Placenta. 2013;34(Suppl):S73–8. doi: 10.1016/j.placenta.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 111.Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol. 2014;10(9):531–40. doi: 10.1038/nrneph.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roberts JM. Pathophysiology of ischemic placental disease. Seminars in perinatology. 2014;38(3):139–45. doi: 10.1053/j.semperi.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Redman CW, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol. 2015;213(4 Suppl):S9 e1–S9-11. doi: 10.1016/j.ajog.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 114.Hod T, Cerdeira AS, Karumanchi SA. Molecular Mechanisms of Preeclampsia. Cold Spring Harbor perspectives in medicine. 2015;5(10) doi: 10.1101/cshperspect.a023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Myatt L, Roberts JM. Preeclampsia: Syndrome or Disease? Current hypertension reports. 2015;17(11):83. doi: 10.1007/s11906-015-0595-4. [DOI] [PubMed] [Google Scholar]
- 116.Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. 2015;213(4 Suppl):S115–22. doi: 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sircar M, Thadhani R, Karumanchi SA. Pathogenesis of preeclampsia. Current opinion in nephrology and hypertension. 2015;24(2):131–8. doi: 10.1097/MNH.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 118.Korzeniewski SJ, Romero R, Chaiworapongsa T, Chaemsaithong P, Kim CJ, Kim YM, et al. Maternal plasma angiogenic index-1 (placental growth factor/soluble vascular endothelial growth factor receptor-1) is a biomarker for the burden of placental lesions consistent with uteroplacental underperfusion: a longitudinal case-cohort study. Am J Obstet Gynecol. 2016;214(5):629.e1–e17. doi: 10.1016/j.ajog.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Goncalves LF, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190(6):1541–7. doi: 10.1016/j.ajog.2004.03.043. discussion 7-50. [DOI] [PubMed] [Google Scholar]
- 120.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2005;17(1):3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 121.Bujold E, Romero R, Chaiworapongsa T, Kim YM, Kim GJ, Kim MR, et al. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2005;18(1):9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 122.Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol. 2005;193(3 Pt 2):984–9. doi: 10.1016/j.ajog.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 123.Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, et al. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne’s syndrome) The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2006;19(10):607–13. doi: 10.1080/14767050600922677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 125.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. The New England journal of medicine. 2006;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 126.Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196(4):326.e1–13. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21(1):25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21(5):279–87. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21(1):9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Than NG, Romero R, Hillermann R, Cozzi V, Nie G, Huppertz B. Prediction of preeclampsia - a workshop report. Placenta. 2008;29(Suppl A):S83–5. doi: 10.1016/j.placenta.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Toft JH, Lian IA, Tarca AL, Erez O, Espinoza J, Eide IP, et al. Whole-genome microarray and targeted analysis of angiogenesis-regulating gene expression (ENG, FLT1, VEGF, PlGF) in placentas from pre-eclamptic and small-for-gestational-age pregnancies. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21(4):267–73. doi: 10.1080/14767050801924118. [DOI] [PubMed] [Google Scholar]
- 132.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2009;22(11):1021–38. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chaiworapongsa T, Romero R, Savasan ZA, Kusanovic JP, Ogge G, Soto E, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2011;24(10):1187–207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Romero R, Chaiworapongsa T, Erez O, Tarca AL, Gervasi MT, Kusanovic JP, et al. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23(12):1384–99. doi: 10.3109/14767051003681121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vaisbuch E, Whitty JE, Hassan SS, Romero R, Kusanovic JP, Cotton DB, et al. Circulating angiogenic and antiangiogenic factors in women with eclampsia. Am J Obstet Gynecol. 2011;204(2):152.e1–9. doi: 10.1016/j.ajog.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Soto E, Romero R, Kusanovic JP, Ogge G, Hussein Y, Yeo L, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(5):498–507. doi: 10.3109/14767058.2011.591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chaiworapongsa T, Romero R, Korzeniewski SJ, Kusanovic JP, Soto E, Lam J, et al. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol. 2013;208(4):287.e1–e15. doi: 10.1016/j.ajog.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chaiworapongsa T, Romero R, Korzeniewski SJ, Cortez JM, Pappas A, Tarca AL, et al. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014;27(2):132–44. doi: 10.3109/14767058.2013.806905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Adekola H, Romero R, Chaemsaithong P, Korzeniewski SJ, Dong Z, Yeo L, et al. Endocan, a putative endothelial cell marker, is elevated in preeclampsia, decreased in acute pyelonephritis, and unchanged in other obstetrical syndromes. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015;28(14):1621–32. doi: 10.3109/14767058.2014.964676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chaiworapongsa T, Romero R, Whitten AE, Korzeniewski SJ, Chaemsaithong P, Hernandez-Andrade E, et al. The use of angiogenic biomarkers in maternal blood to identify which SGA fetuses will require a preterm delivery and mothers who will develop pre-eclampsia. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015:1–15. doi: 10.3109/14767058.2015.1048431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Szalai G, Romero R, Chaiworapongsa T, Xu Y, Wang B, Ahn H, et al. Full-length human placental sFlt-1-e15a isoform induces distinct maternal phenotypes of preeclampsia in mice. PloS one. 2015;10(4):e0119547. doi: 10.1371/journal.pone.0119547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chaiworapongsa T, Romero R, Whitten AE, Korzeniewski SJ, Chaemsaithong P, Hernandez-Andrade E, et al. The use of angiogenic biomarkers in maternal blood to identify which SGA fetuses will require a preterm delivery and mothers who will develop pre-eclampsia. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2016;29(8):1214–28. doi: 10.3109/14767058.2015.1048431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chaiworapongsa T, Romero R, Gotsch F, Kusanovic JP, Mittal P, Kim SK, et al. Acute pyelonephritis during pregnancy changes the balance of angiogenic and anti-angiogenic factors in maternal plasma. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23(2):167–78. doi: 10.3109/14767050903067378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ogge G, Romero R, Kusanovic JP, Chaiworapongsa T, Dong Z, Mittal P, et al. Serum and plasma determination of angiogenic and anti-angiogenic factors yield different results: the need for standardization in clinical practice. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23(8):820–7. doi: 10.3109/14767050903366119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Powers RW, Jeyabalan A, Clifton RG, Van Dorsten P, Hauth JC, Klebanoff MA, et al. Soluble fms-Like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in preeclampsia among high risk pregnancies. PloS one. 2010;5(10):e13263. doi: 10.1371/journal.pone.0013263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125(7):911–9. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baltajian K, Bajracharya S, Salahuddin S, Berg AH, Geahchan C, Wenger JB, et al. Sequential plasma angiogenic factors levels in women with suspected preeclampsia. Am J Obstet Gynecol. 2016;215(1):89.e1–e10. doi: 10.1016/j.ajog.2016.01.168. [DOI] [PubMed] [Google Scholar]
- 148.Karumanchi SA. Angiogenic Factors in Preeclampsia: From Diagnosis to Therapy. Hypertension. 2016;67(6):1072–9. doi: 10.1161/HYPERTENSIONAHA.116.06421. [DOI] [PubMed] [Google Scholar]
- 149.Kim MY, Buyon JP, Guerra MM, Rana S, Zhang D, Laskin CA, et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. Am J Obstet Gynecol. 2016;214(1):108.e1–e14. doi: 10.1016/j.ajog.2015.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Malshe AK, Sibai BM. Angiogenic and Antiangiogenic Markers for Prediction and Risk Classification of Preeclampsia. Clinical obstetrics and gynecology. 2017:134–40. doi: 10.1097/GRF.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 151.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. The New England journal of medicine. 2004;350(7):672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 152.Lai J, Garcia-Tizon Larroca S, Peeva G, Poon LC, Wright D, Nicolaides KH. Competing risks model in screening for preeclampsia by serum placental growth factor and soluble fms-like tyrosine kinase-1 at 30-33 weeks’ gestation. Fetal diagnosis and therapy. 2014;35(4):240–8. doi: 10.1159/000359968. [DOI] [PubMed] [Google Scholar]
- 153.Tsiakkas A, Saiid Y, Wright A, Wright D, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 30-34 weeks’ gestation. Am J Obstet Gynecol. 2016;215(1):87.e1–e17. doi: 10.1016/j.ajog.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 154.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46(5):1077–85. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 155.Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196(4):326.e1–13. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol. 2007;197(3):244.e1–8. doi: 10.1016/j.ajog.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 157.Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension. 2007;49(4):818–24. doi: 10.1161/01.HYP.0000258404.21552.a3. [DOI] [PubMed] [Google Scholar]
- 158.Diab AE, El-Behery MM, Ebrahiem MA, Shehata AE. Angiogenic factors for the prediction of pre-eclampsia in women with abnormal midtrimester uterine artery Doppler velocimetry. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2008;102(2):146–51. doi: 10.1016/j.ijgo.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 159.Stepan H, Geipel A, Schwarz F, Kramer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol. 2008;198(2):175.e1–6. doi: 10.1016/j.ajog.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 160.Garovic VD. The role of angiogenic factors in the prediction and diagnosis of preeclampsia superimposed on chronic hypertension. Hypertension. 2012;59(3):555–7. doi: 10.1161/HYPERTENSIONAHA.111.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hagmann H, Thadhani R, Benzing T, Karumanchi SA, Stepan H. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clinical chemistry. 2012;58(5):837–45. doi: 10.1373/clinchem.2011.169094. [DOI] [PubMed] [Google Scholar]
- 162.Holmes VA, Young IS, Patterson CC, Maresh MJ, Pearson DW, Walker JD, et al. The role of angiogenic and antiangiogenic factors in the second trimester in the prediction of preeclampsia in pregnant women with type 1 diabetes. Diabetes Care. 2013;36(11):3671–7. doi: 10.2337/dc13-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, Troisi R, et al. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol. 2012;207(5):407.e1–7. doi: 10.1016/j.ajog.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 164.Chaiworapongsa T, Romero R, Korzeniewski SJ, Kusanovic JP, Soto E, Lam J, et al. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol. 2013;208(4):287.e1–e15. doi: 10.1016/j.ajog.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Myers JE, Kenny LC, McCowan LM, Chan EH, Dekker GA, Poston L, et al. Angiogenic factors combined with clinical risk factors to predict preterm pre-eclampsia in nulliparous women: a predictive test accuracy study. BJOG: an international journal of obstetrics and gynaecology. 2013;120(10):1215–23. doi: 10.1111/1471-0528.12195. [DOI] [PubMed] [Google Scholar]
- 166.Poston L. Prediction and diagnosis of pre-eclampsia; the scientific basis. Pregnancy hypertension. 2013;3(2):57. doi: 10.1016/j.preghy.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 167.Verlohren S, Stepan H, Dechend R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clinical science (London, England: 1979) 2012;122(2):43–52. doi: 10.1042/CS20110097. [DOI] [PubMed] [Google Scholar]
- 168.Akolekar R, Zaragoza E, Poon LC, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2008;32(6):732–9. doi: 10.1002/uog.6244. [DOI] [PubMed] [Google Scholar]
- 169.Foidart JM, Munaut C, Chantraine F, Akolekar R, Nicolaides KH. Maternal plasma soluble endoglin at 11-13 weeks’ gestation in pre-eclampsia. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2010;35(6):680–7. doi: 10.1002/uog.7621. [DOI] [PubMed] [Google Scholar]
- 170.Crovetto F, Figueras F, Triunfo S, Crispi F, Rodriguez-Sureda V, Peguero A, et al. Added value of angiogenic factors for the prediction of early and late preeclampsia in the first trimester of pregnancy. Fetal diagnosis and therapy. 2014;35(4):258–66. doi: 10.1159/000358302. [DOI] [PubMed] [Google Scholar]
- 171.Garcia-Tizon Larroca S, Tayyar A, Poon LC, Wright D, Nicolaides KH. Competing risks model in screening for preeclampsia by biophysical and biochemical markers at 30-33 weeks’ gestation. Fetal diagnosis and therapy. 2014;36(1):9–17. doi: 10.1159/000362518. [DOI] [PubMed] [Google Scholar]
- 172.Moore Simas TA, Crawford SL, Bathgate S, Yan J, Robidoux L, Moore M, et al. Angiogenic biomarkers for prediction of early preeclampsia onset in high-risk women. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014;27(10):1038–48. doi: 10.3109/14767058.2013.847415. [DOI] [PubMed] [Google Scholar]
- 173.Crovetto F, Figueras F, Triunfo S, Crispi F, Rodriguez-Sureda V, Dominguez C, et al. First trimester screening for early and late preeclampsia based on maternal characteristics, biophysical parameters, and angiogenic factors. Prenatal diagnosis. 2015;35(2):183–91. doi: 10.1002/pd.4519. [DOI] [PubMed] [Google Scholar]
- 174.Widmer M, Cuesta C, Khan KS, Conde-Agudelo A, Carroli G, Fusey S, et al. Accuracy of angiogenic biomarkers at 20weeks’ gestation in predicting the risk of pre-eclampsia: A WHO multicentre study. Pregnancy hypertension. 2015;5(4):330–8. doi: 10.1016/j.preghy.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 175.Di Martino D, Cetin I, Frusca T, Ferrazzi E, Fuse F, Gervasi MT, et al. Italian Advisory Board: sFlt-1/PlGF ratio and preeclampsia, state of the art and developments in diagnostic, therapeutic and clinical management. European journal of obstetrics, gynecology, and reproductive biology. 2016;206:70–3. doi: 10.1016/j.ejogrb.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 176.Kienast C, Moya W, Rodriguez O, Jijon A, Geipel A. Predictive value of angiogenic factors, clinical risk factors and uterine artery Doppler for pre-eclampsia and fetal growth restriction in second and third trimester pregnancies in an Ecuadorian population. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2016;29(4):537–43. doi: 10.3109/14767058.2015.1012063. [DOI] [PubMed] [Google Scholar]
- 177.Khalil A, Maiz N, Garcia-Mandujano R, Penco JM, Nicolaides KH. Longitudinal changes in maternal serum placental growth factor and soluble fms-like tyrosine kinase-1 in women at increased risk of pre-eclampsia. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2016;47(3):324–31. doi: 10.1002/uog.15750. [DOI] [PubMed] [Google Scholar]
- 178.Lai J, Pinas A, Poon LC, Agathokleous M, Nicolaides KH. Maternal serum placental growth factor, pregnancy-associated plasma protein-a and free beta-human chorionic gonadotrophin at 30-33 weeks in the prediction of pre-eclampsia. Fetal diagnosis and therapy. 2013;33(3):164–72. doi: 10.1159/000345090. [DOI] [PubMed] [Google Scholar]
- 179.Nucci M, Poon LC, Demirdjian G, Darbouret B, Nicolaides KH. Maternal serum placental growth factor (PlGF) isoforms 1 and 2 at 11-13 weeks’ gestation in normal and pathological pregnancies. Fetal diagnosis and therapy. 2014;36(2):106–16. doi: 10.1159/000357842. [DOI] [PubMed] [Google Scholar]
- 180.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142(2):159–67. doi: 10.1016/s0002-9378(16)32330-4. [DOI] [PubMed] [Google Scholar]
- 182.Romero R, Vizoso J, Emamian M, Duffy T, Riely C, Halford T, et al. Clinical significance of liver dysfunction in pregnancy-induced hypertension. American journal of perinatology. 1988;5(2):146–51. doi: 10.1055/s-2007-999675. [DOI] [PubMed] [Google Scholar]
- 183.Abildgaard U, Heimdal K. Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): a review. European journal of obstetrics, gynecology, and reproductive biology. 2013;166(2):117–23. doi: 10.1016/j.ejogrb.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 184.Szabo S, Mody M, Romero R, Xu Y, Karaszi K, Mihalik N, et al. Activation of villous trophoblastic p38 and ERK1/2 signaling pathways in preterm preeclampsia and HELLP syndrome. Pathology oncology research: POR. 2015;21(3):659–68. doi: 10.1007/s12253-014-9872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Weel IC, Baergen RN, Romao-Veiga M, Borges VT, Ribeiro VR, Witkin SS, et al. Association between Placental Lesions, Cytokines and Angiogenic Factors in Pregnant Women with Preeclampsia. PloS one. 2016;11(6):e0157584. doi: 10.1371/journal.pone.0157584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ananth CV, Peltier MR, Chavez MR, Kirby RS, Getahun D, Vintzileos AM. Recurrence of ischemic placental disease. Obstetrics and gynecology. 2007;110(1):128–33. doi: 10.1097/01.AOG.0000266983.77458.71. [DOI] [PubMed] [Google Scholar]
- 187.Ananth CV, Peltier MR, Kinzler WL, Smulian JC, Vintzileos AM. Chronic hypertension and risk of placental abruption: is the association modified by ischemic placental disease? Am J Obstet Gynecol. 2007;197(3):273.e1–7. doi: 10.1016/j.ajog.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 188.Ananth CV, Smulian JC, Vintzileos AM. Ischemic placental disease: maternal versus fetal clinical presentations by gestational age. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23(8):887–93. doi: 10.3109/14767050903334885. [DOI] [PubMed] [Google Scholar]
- 189.Ananth CV, Vintzileos AM. Ischemic placental disease: epidemiology and risk factors. European journal of obstetrics, gynecology, and reproductive biology. 2011;159(1):77–82. doi: 10.1016/j.ejogrb.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 190.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. British journal of obstetrics and gynaecology. 1995;102(1):20–5. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 191.Bartha JL, Romero-Carmona R, Escobar-Llompart M, Comino-Delgado R. The relationships between leptin and inflammatory cytokines in women with pre-eclampsia. BJOG: an international journal of obstetrics and gynaecology. 2001;108(12):1272–6. doi: 10.1111/j.1471-0528.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 192.Velzing-Aarts FV, Muskiet FA, van der Dijs FP, Duits AJ. High serum interleukin-8 levels in afro-caribbean women with pre-eclampsia. Relations with tumor necrosis factor-alpha, duffy negative phenotype and von Willebrand factor. American journal of reproductive immunology (New York, NY: 1989) 2002;48(5):319–22. doi: 10.1034/j.1600-0897.2002.01087.x. [DOI] [PubMed] [Google Scholar]
- 193.Visser W, Beckmann I, Knook MA, Wallenburg HC. Soluble tumor necrosis factor receptor II and soluble cell adhesion molecule 1 as markers of tumor necrosis factor-alpha release in preeclampsia. Acta Obstet Gynecol Scand. 2002;81(8):713–9. [PubMed] [Google Scholar]
- 194.Tosun M, Celik H, Avci B, Yavuz E, Alper T, Malatyalioglu E. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-alpha in normal pregnancies and in pregnancies complicated by preeclampsia. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23(8):880–6. doi: 10.3109/14767051003774942. [DOI] [PubMed] [Google Scholar]
- 195.Szarka A, Rigo J, Jr, Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC immunology. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Torbergsen T, Oian P, Mathiesen E, Borud O. Pre-eclampsia–a mitochondrial disease? Acta Obstet Gynecol Scand. 1989;68(2):145–8. doi: 10.3109/00016348909009902. [DOI] [PubMed] [Google Scholar]
- 197.Masoura S, Makedou K, Theodoridis T, Kourtis A, Zepiridis L, Athanasiadis A. The involvement of uric acid in the pathogenesis of preeclampsia. Current hypertension reviews. 2015;11(2):110–5. doi: 10.2174/1573402111666150529130703. [DOI] [PubMed] [Google Scholar]
- 198.Shanklin DR, Sibai BM. Ultrastructural aspects of preeclampsia. II. Mitochondrial changes. Am J Obstet Gynecol. 1990;163(3):943–53. doi: 10.1016/0002-9378(90)91102-i. [DOI] [PubMed] [Google Scholar]
- 199.Berkowitz K, Monteagudo A, Marks F, Jackson U, Baxi L. Mitochondrial myopathy and preeclampsia associated with pregnancy. Am J Obstet Gynecol. 1990;162(1):146–7. doi: 10.1016/0002-9378(90)90837-w. [DOI] [PubMed] [Google Scholar]
- 200.Shi Z, Long W, Zhao C, Guo X, Shen R, Ding H. Comparative proteomics analysis suggests that placental mitochondria are involved in the development of pre-eclampsia. PloS one. 2013;8(5):e64351. doi: 10.1371/journal.pone.0064351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Qiu C, Hevner K, Enquobahrie DA, Williams MA. A case-control study of maternal blood mitochondrial DNA copy number and preeclampsia risk. International journal of molecular epidemiology and genetics. 2012;3(3):237–44. [PMC free article] [PubMed] [Google Scholar]
- 202.Bonney EA. Preeclampsia: a view through the danger model. Journal of reproductive immunology. 2007;76(1–2):68–74. doi: 10.1016/j.jri.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mando C, De Palma C, Stampalija T, Anelli GM, Figus M, Novielli C, et al. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. American journal of physiology Endocrinology and metabolism. 2014;306(4):E404–13. doi: 10.1152/ajpendo.00426.2013. [DOI] [PubMed] [Google Scholar]
- 204.Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol. 2009;200(6):661.e1–7. doi: 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 205.Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196(3):261.e1–6. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 206.Lee DC, Romero R, Kim JS, Tarca AL, Montenegro D, Pineles BL, et al. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. The American journal of pathology. 2011;179(2):590–602. doi: 10.1016/j.ajpath.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33(10):816–23. doi: 10.1016/j.placenta.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vishnyakova PA, Volodina MA, Tarasova NV, Marey MV, Tsvirkun DV, Vavina OV, et al. Mitochondrial role in adaptive response to stress conditions in preeclampsia. Scientific reports. 2016;6:32410. doi: 10.1038/srep32410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bianco-Miotto T, Blundell C, Buckberry S, Chamley L, Chong S, Cottrell E, et al. IFPA meeting 2015 workshop report I: placental mitochondrial function, transport systems and epigenetics. Placenta. 2016;48(Suppl 1):S3–S6. doi: 10.1016/j.placenta.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 210.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–14. [PMC free article] [PubMed] [Google Scholar]
- 211.Hur KY, Lee MS. New mechanisms of metformin action: Focusing on mitochondria and the gut. Journal of diabetes investigation. 2015;6(6):600–9. doi: 10.1111/jdi.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–6. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 214.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453(7198):1117–21. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 215.Tal R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol Reprod. 2012;87(6):1–8. doi: 10.1095/biolreprod.112.102723. [DOI] [PubMed] [Google Scholar]
- 216.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494(7436):256–60. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, et al. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38(1):33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- 219.Portela LV, Gnoatto J, Brochier AW, Haas CB, de Assis AM, de Carvalho AK, et al. Intracerebroventricular metformin decreases body weight but has pro-oxidant effects and decreases survival. Neurochem Res. 2015;40(3):514–23. doi: 10.1007/s11064-014-1496-7. [DOI] [PubMed] [Google Scholar]
- 220.Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging. 2009;1(4):357–62. doi: 10.18632/aging.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chantranupong L, Wolfson RL, Sabatini DM. Nutrient-sensing mechanisms across evolution. Cell. 2015;161(1):67–83. doi: 10.1016/j.cell.2015.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clinical chemistry. 2004;50(9):1511–25. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 223.Hopkins M, Blundell JE. Energy balance, body composition, sedentariness and appetite regulation: pathways to obesity. Clinical science (London, England: 1979) 2016;130(18):1615–28. doi: 10.1042/CS20160006. [DOI] [PubMed] [Google Scholar]
- 224.Hu F, Xu Y, Liu F. Hypothalamic roles of mTOR complex I: integration of nutrient and hormone signals to regulate energy homeostasis. American journal of physiology Endocrinology and metabolism. 2016;310(11):E994–e1002. doi: 10.1152/ajpendo.00121.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jansson T, Powell TL. Role of placental nutrient sensing in developmental programming. Clinical obstetrics and gynecology. 2013;56(3):591–601. doi: 10.1097/GRF.0b013e3182993a2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Roos S, Powell TL, Jansson T. Placental mTOR links maternal nutrient availability to fetal growth. Biochemical Society transactions. 2009;37(Pt 1):295–8. doi: 10.1042/BST0370295. [DOI] [PubMed] [Google Scholar]
- 227.Rosario FJ, Kanai Y, Powell TL, Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. The Journal of physiology. 2013;591(3):609–25. doi: 10.1113/jphysiol.2012.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. The Journal of antibiotics. 1975;28(10):721–6. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 229.Lamming DW. Inhibition of the Mechanistic Target of Rapamycin (mTOR)-Rapamycin and Beyond. Cold Spring Harbor perspectives in medicine. 2016;6(5):a025924. doi: 10.1101/cshperspect.a025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature reviews Molecular cell biology. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hirota Y, Cha J, Yoshie M, Daikoku T, Dey SK. Heightened uterine mammalian target of rapamycin complex 1 (mTORC1) signaling provokes preterm birth in mice. Proc Natl Acad Sci U S A. 2011;108(44):18073–8. doi: 10.1073/pnas.1108180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nature reviews Molecular cell biology. 2014;15(3):155–62. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 233.Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125(1):25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Deng W, Cha J, Yuan J, Haraguchi H, Bartos A, Leishman E, et al. p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J Clin Invest. 2016;126(8):2941–54. doi: 10.1172/JCI87715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kennedy BK, Lamming DW. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell metabolism. 2016;23(6):990–1003. doi: 10.1016/j.cmet.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Xie J, Wang X, Proud CG. mTOR inhibitors in cancer therapy. F1000Research. 2016;5:2078. doi: 10.12688/f1000research.9207.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Aylett CH, Sauer E, Imseng S, Boehringer D, Hall MN, Ban N, et al. Architecture of human mTOR complex 1. Science (New York, NY) 2016;351(6268):48–52. doi: 10.1126/science.aaa3870. [DOI] [PubMed] [Google Scholar]
- 238.Xu S, Cai Y, Wei Y. mTOR Signaling from Cellular Senescence to Organismal Aging. Aging and disease. 2014;5(4):263–73. doi: 10.14336/AD.2014.0500263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wei Y, Zhang YJ, Cai Y, Xu MH. The role of mitochondria in mTOR-regulated longevity. Biological reviews of the Cambridge Philosophical Society. 2015;90(1):167–81. doi: 10.1111/brv.12103. [DOI] [PubMed] [Google Scholar]
- 240.Lager S, Powell TL. Regulation of nutrient transport across the placenta. Journal of pregnancy. 2012;2012:179827. doi: 10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fujita D, Tanabe A, Sekijima T, Soen H, Narahara K, Yamashita Y, et al. Role of extracellular signal-regulated kinase and AKT cascades in regulating hypoxia-induced angiogenic factors produced by a trophoblast-derived cell line. The Journal of endocrinology. 2010;206(1):131–40. doi: 10.1677/JOE-10-0027. [DOI] [PubMed] [Google Scholar]
- 242.Nanovskaya TN, Nekhayeva IA, Patrikeeva SL, Hankins GD, Ahmed MS. Transfer of metformin across the dually perfused human placental lobule. Am J Obstet Gynecol. 2006;195(4):1081–5. doi: 10.1016/j.ajog.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 243.Charles B, Davoren P, Hague W, McIntyre D, Norris R, Xiaonian X. Metformin crosses the placenta: a modulator for fetal insulin resistance? British medical journal. 2004;327(7420):880–1. [Google Scholar]
- 244.Vanky E, Zahlsen K, Spigset O, Carlsen SM. Placental passage of metformin in women with polycystic ovary syndrome. Fertility and sterility. 2005;83(5):1575–8. doi: 10.1016/j.fertnstert.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 245.Cassina M, Dona M, Di Gianantonio E, Litta P, Clementi M. First-trimester exposure to metformin and risk of birth defects: a systematic review and meta-analysis. Human reproduction update. 2014;20(5):656–69. doi: 10.1093/humupd/dmu022. [DOI] [PubMed] [Google Scholar]
- 246.Gilbert C, Valois M, Koren G. Pregnancy outcome after first-trimester exposure to metformin: a meta-analysis. Fertility and sterility. 2006;86(3):658–63. doi: 10.1016/j.fertnstert.2006.02.098. [DOI] [PubMed] [Google Scholar]
- 247.Gray SG, McGuire TM, Cohen N, Little PJ. The emerging role of metformin in gestational diabetes mellitus. Diabetes, obesity & metabolism. 2017 doi: 10.1111/dom.12893. [DOI] [PubMed] [Google Scholar]
- 248.Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. The New England journal of medicine. 2008;358(1):47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 249.Nicholson W, Baptiste-Roberts K. Oral hypoglycaemic agents during pregnancy: The evidence for effectiveness and safety. Best Pract Res Clin Obstet Gynaecol. 2011;25(1):51–63. doi: 10.1016/j.bpobgyn.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 250.Wouldes TA, Battin M, Coat S, Rush EC, Hague WM, Rowan JA. Neurodevelopmental outcome at 2 years in offspring of women randomised to metformin or insulin treatment for gestational diabetes. Arch Dis Child Fetal Neonatal Ed. 2016;101:F488–F93. doi: 10.1136/archdischild-2015-309602. [DOI] [PubMed] [Google Scholar]
- 251.Rowan JA, Rush EC, Obolonkin V, Battin M, Wouldes T, Hague WM. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition at 2 years of age. Diabetes Care. 2011;34(10):2279–84. doi: 10.2337/dc11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lautatzis ME, Goulis DG, Vrontakis M. Efficacy and safety of metformin during pregnancy in women with gestational diabetes mellitus or polycystic ovary syndrome: a systematic review. Metabolism: clinical and experimental. 2013;62(11):1522–34. doi: 10.1016/j.metabol.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 253.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ (Clinical research ed) 2005;330(7503):1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32(9):1620–5. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer prevention research (Philadelphia, Pa) 2014;7(9):867–85. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mohammed A, Janakiram NB, Brewer M, Ritchie RL, Marya A, Lightfoot S, et al. Antidiabetic Drug Metformin Prevents Progression of Pancreatic Cancer by Targeting in Part Cancer Stem Cells and mTOR Signaling. Translational oncology. 2013;6(6):649–59. doi: 10.1593/tlo.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Experimental gerontology. 2005;40(8–9):685–93. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 258.Anisimov VN. Do metformin a real anticarcinogen? A critical reappraisal of experimental data. Annals of translational medicine. 2014;2(6):60. doi: 10.3978/j.issn.2305-5839.2014.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Afzal M, Kazmi I, Gupta G, Rahman M, Kimothi V, Anwar F. Preventive effect of Metformin against N-nitrosodiethylamine-initiated hepatocellular carcinoma in rats. Saudi Pharm J. 2012;20(4):365–70. doi: 10.1016/j.jsps.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging. 2011;3(2):148–57. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412(2):211–21. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 262.Tajima K, Nakamura A, Shirakawa J, Togashi Y, Orime K, Sato K, et al. Metformin prevents liver tumorigenesis induced by high-fat diet in C57Bl/6 mice. American journal of physiology Endocrinology and metabolism. 2013;305(8):E987–E98. doi: 10.1152/ajpendo.00133.2013. [DOI] [PubMed] [Google Scholar]
- 263.Tomimoto A, Endo H, Sugiyama M, Fujisawa T, Hosono K, Takahashi H, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008;99(11):2136–41. doi: 10.1111/j.1349-7006.2008.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Vazquez-Martin A, Lopez-Bonetc E, Cufi S, Oliveras-Ferraros C, Del Barco S, Martin-Castillo B, et al. Repositioning chloroquine and metformin to eliminate cancer stem cell traits in pre-malignant lesions. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2011;14(4–5):212–23. doi: 10.1016/j.drup.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Menendez JA. Metformin against TGFbeta-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell cycle (Georgetown, Tex) 2010;9(22):4461–8. doi: 10.4161/cc.9.22.14048. [DOI] [PubMed] [Google Scholar]
- 266.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nature reviews Cancer. 2009;9(8):563–75. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Green AS, Chapuis N, Maciel TT, Willems L, Lambert M, Arnoult C, et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood. 2010;116(20):4262–73. doi: 10.1182/blood-2010-02-269837. [DOI] [PubMed] [Google Scholar]
- 268.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer research. 2006;66(21):10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 269.Lettieri Barbato D, Vegliante R, Desideri E, Ciriolo MR. Managing lipid metabolism in proliferating cells: new perspective for metformin usage in cancer therapy. Biochimica et biophysica acta. 2014;1845(2):317–24. doi: 10.1016/j.bbcan.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 270.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(20):3297–302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Daugan M, Dufay Wojcicki A, d’Hayer B, Boudy V. Metformin: An anti-diabetic drug to fight cancer. Pharmacological research. 2016;113(Pt A):675–85. doi: 10.1016/j.phrs.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 272.Sosnicki S, Kapral M, Weglarz L. Molecular targets of metformin antitumor action. Pharmacological reports: PR. 2016;68(5):918–25. doi: 10.1016/j.pharep.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 273.Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nature genetics. 2003;33(1):40–8. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- 274.Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, et al. Rates of behavior and aging specified by mitochondrial function during development. Science (New York, NY) 2002;298(5602):2398–401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 275.Slack C, Foley A, Partridge L. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PloS one. 2012;7(10):e47699. doi: 10.1371/journal.pone.0047699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lopez-Otin C, Galluzzi L, Freije JM, Madeo F, Kroemer G. Metabolic Control of Longevity. Cell. 2016;166(4):802–21. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 277.Novelle MG, Ali A, Dieguez C, Bernier M, de Cabo R. Metformin: A Hopeful Promise in Aging Research. Cold Spring Harbor perspectives in medicine. 2016;6(3):a025932. doi: 10.1101/cshperspect.a025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wu L, Zhou B, Oshiro-Rapley N, Li M, Paulo JA, Webster CM, et al. An Ancient, Unified Mechanism for Metformin Growth Inhibition in C. elegans and Cancer. Cell. 2016;167(7):1705–18 e13. doi: 10.1016/j.cell.2016.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]


