Abstract
Motivation
Identification of novel therapeutic effects for existing US Food and Drug Administration (FDA)-approved drugs, drug repurposing, is an approach aimed to dramatically shorten the drug discovery process, which is costly, slow and risky. Several computational approaches use transcriptional data to find potential repurposing candidates. The main hypothesis of such approaches is that if gene expression signature of a particular drug is opposite to the gene expression signature of a disease, that drug may have a potential therapeutic effect on the disease. However, this may not be optimal since it fails to consider the different roles of genes and their dependencies at the system level.
Results
We propose a systems biology approach to discover novel therapeutic roles for established drugs that addresses some of the issues in the current approaches. To do so, we use publicly available drug and disease data to build a drug-disease network by considering all interactions between drug targets and disease-related genes in the context of all known signaling pathways. This network is integrated with gene-expression measurements to identify drugs with new desired therapeutic effects based on a system-level analysis method. We compare the proposed approach with the drug repurposing approach proposed by Sirota et al. on four human diseases: idiopathic pulmonary fibrosis, non-small cell lung cancer, prostate cancer and breast cancer. We evaluate the proposed approach based on its ability to re-discover drugs that are already FDA-approved for a given disease.
Availability and implementation
The R package DrugDiseaseNet is under review for publication in Bioconductor and is available at https://github.com/azampvd/DrugDiseaseNet.
Supplementary information
Supplementary data are available at Bioinformatics online.
1 Introduction
Despite enormous investments in research and developments (R&D), it still takes approximately $800 million to $1 billion and 10–17 years to approve a new drug for clinical use (Adams and Brantner, 2006; Dickson and Gagnon, 2009; DiMasi et al., 2003). More than 90% of drugs fail to pass beyond the early stage of development and toxicity tests, and many of the drugs that go through early phases of the clinical trials fail because of adverse reactions, side effects, or lack of efficiency. Indeed, the rate of failure is still significantly higher than the rate of approval (Booth and Zemmel, 2004; Dickson and Gagnon, 2009; DiMasi et al., 2003). In order to overcome these challenges, drug repurposing, an approach aiming to find new indications for existing drugs (Chong and Sullivan, 2007), has emerged as an important strategy for drug discovery (Ashburn and Thor, 2004). This approach can also rescue drugs that are safe but fail to get to market due to the lack of efficacy against their initial clinical indication (Collins, 2011).
Repurposing approaches can be categorized as drug-based or disease-based. Disease-based approaches are developed to overcome the lack of knowledge about the pharmacology of a drug (Dudley et al., 2011). Drug-based approaches are preferred when drug data (e.g. transcriptomic data) are available. While each one of these approaches faces several challenges, successful repurposing approaches often take advantage of both drug and disease data. In this area, a number of approaches have been developed based on the analysis of transcriptomic data, such as gene expression signatures, defined as the changes in the expression of genes under a certain condition (e.g. administration of a drug, or a disease). Some of these approaches are based on the idea that if there is an anti-correlation between a drug-exposure gene expression signature and a disease gene expression signature, that drug may have a potential therapeutic effect on the disease (Lamb et al., 2006; Sirota et al., 2011). Drugs that are strongly anti-correlated with a disease are likely to be candidates for repurposing. Resources such as LINCS [new version of Connectivity Map (Lamb et al., 2006)] allow for systematic search of candidates for drug repurposing.
The Connectivity Map (CMap) project (Lamb et al., 2006) was the first systematic approach aimed at exploring functional connections between drugs, as well as between drugs and diseases. This project led to the first repository of genome-wide expression data from five human cancer cell lines exposed with 1309 compounds at different dosages, and integrated with other sources such as NCBI Gene Expression Omnibus (GEO). (Lamb et al., 2006) evaluate the similarity of a query signature, that can be a drug-exposure gene expression signature or a disease gene expression signature, to each drug signature in Connectivity Map database (reference data). In (Sirota et al., 2011) the authors developed a systematic approach based on the same idea originally proposed by (Lamb et al., 2006). In this work, they use drug-exposure gene expression signature from Connectivity Map as the reference data and query this reference data with every single disease gene expression signature by applying a pattern-matching method.
Systems biology can be used as an effective platform in drug discovery and development by leveraging the understanding of interactions between the different system components (Butcher et al., 2004; Kitano, 2002). In this paper, we propose a systems biology approach that takes advantage of prior knowledge of drug targets, disease-related genes and signaling pathways to construct a drug-disease network (DDN) composed of the genes that are most likely perturbed by a drug. By performing a system-level analysis on this network using disease gene expression signatures and drug-exposure gene expression signatures, our approach estimates the amount of perturbation caused by a drug on the genes that are associated to a disease of interest. Drugs are ranked based on the amount of perturbation they exercise on specific disease-related genes, and highest ranking drugs are proposed as candidates for repurposing.
We compare the results of our approach with the computational drug-repurposing approach proposed by (Sirota et al., 2011) using 19 datasets involving 4 diseases: idiopathic pulmonary fibrosis (IPF), non-small cell lung cancer (NSCLC), prostate cancer and breast cancer. We show that our approach provides a more accurate prediction based on its ability to identify drugs that are already approved for the disease of interest.
2 Materials and methods
2.1 Data sources
Disease and drug gene expression data. Large scale drug-exposure gene expression data are obtained from two databases: Connectivity Map and the Library of Integrated Network-Based Cellular Signatures (LINCS; Lamb et al., 2006; http://www.lincsproject.org/). Disease expression data are obtained from NCBI Gene Expression Omnibus (GEO; Edgar et al., 2002) and Lung Genomics Research Consortium (http://www.lung-genomics.org).
In Connectivity Map, drug expression data are measured from the exposure of five human cell lines to bioactive small molecules. Differentially expressed genes (DEGs) are identified using a moderated t-test (Smyth, 2005) by comparing treated samples and the corresponding control (untreated) samples. The resulting P-values are FDR adjusted (Benjamini and Yekutieli, 2001) to correct for multiple comparisons.
The LINCS program, the successor of Connectivity Map (Lamb et al., 2006), generated transcriptional gene expression data from cultured human cells exposed to small molecules and knock-down/overexpression of a single gene. The data is also available in GEO (GSE70138). This program provides DEGs in terms of z-score signatures by comparing two groups of samples (treatment versus control). In both Connectivity Map and LINCS, there are often more than one replicate for each drug. Replicates with at least (1%) DEGs (FDR-adjusted P-value < 0.025) are selected. Since measurements are carried out on different platforms, we standardize gene identifiers from chip specific probe identifiers to NCBI GeneID identifiers using the affy package (Gautier et al., 2004). We average across distinct probe expression values when multiple probes mapped to the same NCBI GeneID.
Drug-targets and disease-related genes. The proposed approach needs to construct a network that includes all the shortest paths between the drug targets and genes known to be associated to the disease of interest. Drug targets and disease-related genes (genes associated with the disease of interest) are retrieved from the Comparative Toxicogenomics Database (CTD; Mattingly et al., 2006) and Drugbank (Wishart et al., 2006). CTD is a database that provides curated data describing cross-species chemical-gene/protein interactions and gene-disease associations. Drugs with no known targets are removed from the study. Such drugs are mostly not FDA-approved.
Signaling pathways. We obtain signaling pathways from Kyoto Encyclopedia of Genes Genomics (KEGG; http://www.genome.ad.jp/kegg/). A signaling pathway in KEGG is modeled by a graph in which nodes represent genes or proteins, and directed edges between them represent signals between genes or proteins. The edges are weighted based on the various types of signals, such as activation, inhibition, etc.
2.2 Framework overview
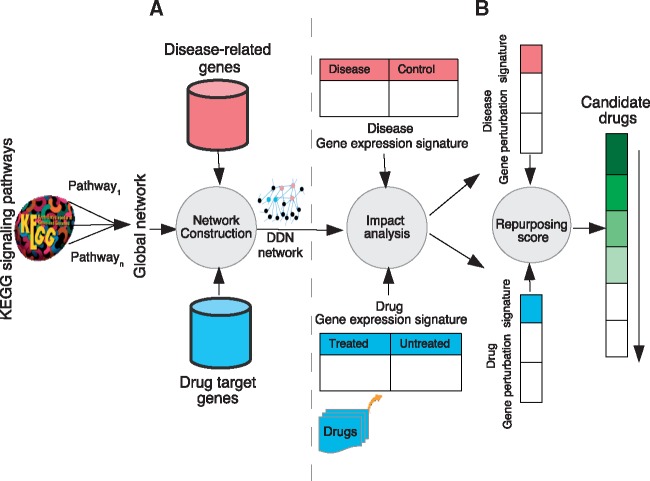
The first part of the framework consists in building the drug-disease network (DDN) by integrating knowledge about the disease-related genes, drug targets and gene-gene interaction knowledge. Then, a repurposing score is computed for each drug-disease pair by integrating expression data into this network. Figure 1 represents the proposed framework.
Fig. 1.
Framework overview. (A) We construct a global network (GN) that is the union of all KEGG human signaling pathways. For each drug-disease pair, we extract a subgraph of GN, namely DDN, consisting of all shortest paths between two sets of disease-related genes and drug targets. (B) We then generate gene perturbation signatures of drug-disease pairs by applying a system-level analysis on their gene expression signatures in the drug-disease network (DDN). A comparative analysis is applied on drug and disease gene perturbation signatures. A repurposing score is assigned to each drug-disease pair. Finally, a ranked list of drugs with potential therapeutic effects for the given disease is generated based on repurposing scores
Drug-disease network (DDN) construction. As shown in Figure 1A, first, we construct a global network (GN) by performing the union of all nodes and edges of KEGG human signaling pathways. In a number of KEGG pathways, a gene ‘a’ interacts with gene ‘b’, through an intermediate pathway ‘A’. This is represented by a link that starts from gene ‘a’ to gene ‘b’ through pathway ‘A’. For example, in the Adherence Junction pathway, TGFβR activates Smad3 through the TGF-beta signaling pathway. Interactions between genes belonging to the pathway ‘A’ and genes ‘a’ and ‘b’ are not included in our model. There are some interactions between genes/pathways through DNA or small molecules in KEGG. For instance, there is a link between MAPK signaling pathway and Phosphatidylinositol signaling system through a small molecule (compound) IP3 in KEGG. Such interactions are not part of the scope of this analysis and we do not include them in constructing the global network. We used ROntoTools package (Voichiţa and Drăghici, 2013; version 1.2.0) to calculate the union all KEGG signaling pathways that are represented by the adjacency matrices and obtain a unified adjacency matrix. In this step, we included some implicit interactions between the genes by performing the union of adjacency matrices representing KEGG signaling pathways. For example, suppose gene ‘a’ activate gene ‘b’ in pathway ‘A’ and gene ‘b’ activates gene ‘c’ in pathway ‘B’. A path between gene ‘a’ and gene ‘c’ may be constructed by our analysis, while there was no path between them before this analysis.
Next, given the two sets of disease-related genes as , and drug targets as , we extract a subgraph of GN that consists of all the shortest paths connecting genes belonging to these sets. It means that a gene from either Diseaset or Drugt can be a source or destination of the shortest path extracted from GN. This subgraph called Drug-disease network (DDN) represents all the interactions between drug targets and genes related to the given disease, through all the interactions described in KEGG signaling pathways.
Drug-disease repurposing score computation. In this stage, we capture the impact caused by a drug exposure or a disease on the genes that are specific to the condition of interest. In order to integrate the drug and disease gene expressions signatures, we generate gene perturbation signatures by computing the amount of perturbation upon the genes belonging to the drug-disease network (DDN) for all drug-disease pairs, as shown in Figure 1B. The gene perturbation signatures are calculated using the impact analysis method Drǎghici et al. (2007) on the subgraph of global network we constructed in previous step. The impact analysis (IA) takes into account the structure and dynamics of a signaling pathway by considering a number of important aspects, including the measured gene expression changes, the direction and type of every gene signal and the position and role of every gene in a pathway. A perturbation factor for each gene, PF(gi), is calculated using the impact analysis method Drǎghici et al. (2007), as follows:
A perturbation factor for each gene, PF(gi), is calculated using the impact analysis method (Drǎghici et al., 2007), as follows:
| (1) |
where the term denotes the signed normalized measured expression change of a gene gi, added to the sum of all perturbation factors of the genes gj that are direct upstream of the gene gi, normalized by the number of downstream genes of gj, . The coefficient βij represents the type of the interaction, for activation and induction and for inhibition and repression. The second term in Equation (1) involves the PF values of those genes that are upstream of the gene for which the perturbation factor is calculated. For a gene with no upstream genes, the PF will be the measured expression gene .
Next, we calculate the repurposing scores for drug-disease pairs by computing the Pearson correlation coefficient between their gene perturbation signatures. The result score is from –1 to 1, where a high positive score shows that the drug and the disease both cause similar perturbations in the system, and therefore, that drug may cause the same effect as the disease. Conversely, a high negative score shows that the drug and disease have opposite gene perturbation signatures. Our hypothesis is that if the perturbation caused by a particular drug in the system is the reverse of the perturbation caused by a disease, that drug may have the potential to treat the given disease. Thus, we rank drugs from the strongly anti-correlated to the strongly correlated, according to their repurposing pathway perturbation scores.
In order to estimate the statistical significance of drug candidate repurposing scores, we generate 1000 random drug gene expression signatures (by permuting gene labels) and then calculate random repurposing scores for all drug-disease pairs. We compute P-values as the percentage of the random scores higher than the observed score.
A systematic method to select repurposing candidates. We used a systematic method in order to rank repurposing candidates. To do this, given a ranked-list of drugs (drug instances) obtained by applying our approach on a disease dataset, we first compute a score for each drug that indicates how better or worse that drug is ranked in comparison to already FDA-approved drugs as follows:
| (2) |
where a denotes the number of already FDA-approved drugs (gold standards) that are ranked worse than Drugx, and b denotes the number of FDA-approved drugs that are ranked better than Drugx (Supplementary Fig. S3). For instance, if there were N FDA-approved drugs for a condition and an instance of a repurposing candidate were ranked higher than all N FDA approved drugs, the score of this candidate would be N. Conversely, if the candidate were ranked lower than all N FDA approved drugs, its score would be-N.
Using this objective measure, we then calculate an average score for each drug across different disease datasets (Supplementary Fig. S4B). Finally, we compute an average score for each distinct drug across different instances, if there are multiple instances for that drug (Supplementary Fig. S4C). We select the top 5% drug candidates from the ranked lists obtained by applying our approach on disease datasets and rank such drugs based on the scores computed by the this method, from highest to the lowest.
3 Results
To validate our approach, we analyzed 19 datasets from four different conditions: idiopathic pulmonary fibrosis (IPF; 6 datasets), non-small cell lung cancer (NSCLC; 4 datasets), prostate cancer (3 datasets) and breast cancer (6 datasets). The results of NSCLC, prostate cancer, and breast cancer are included in Supplementary Material. We compare the results of three computational drug repurposing approaches: our system-level approach, the most popular approach proposed by (Sirota et al., 2011; henceforth drug-disease) and a classical method based on disease and drug signature anti-correlation (henceforth anti-correlation).
Both the drug-disease and the anti-correlation approaches are based on the hypothesis that if gene expression signature is perturbed in one direction in a disease state, and in the opposite (reverse) direction upon a drug exposure, then that drug may have the potential therapeutic effect for the disease. The difference between the two approaches is related on the approach used to calculated the match between a disease and a drug. Given a disease gene expression signature (query signature) and a drug gene expression signatures (reference signature), the Sirota et al.’s drug-disease similarity approach calculate an enrichment score for the up-regulated and down-regulated disease genes [by applying a Kolmogorov–Smirnov (KS) test]. We use the R implementation of this approach available in the package DrugVsDisease (Pacini, 2013).
In contrast, the classical anti-correlation method calculates a similarity score for drug-disease pairs by computing the Pearson correlation coefficient between the drug gene expression signature and the given disease gene expression signature. Drugs are ranked from the highly anti-correlated to the highly correlated, according to their score.
In this study, we compare the various approaches based on their ability to identify drugs that have already been FDA-approved for that condition (gold standard), based exclusively on the molecular data. In essence, a good repurposing approach should place already approved drugs at the very top of the list of drugs proposed for that particular disease. We used the Wilcoxon rank sum test (Wilcoxon, 1945) to determine whether the proposed approach is significantly better than the existing approaches.
Supplementary Table S2 shows the proposed candidates and the preliminary evidence that support the usefulness of those candidates in treatment of four human diseases: IPF, NSCLC, prostate cancer and breast cancer.
Table 1.
Preliminary support by pre-clinical or clinical studies showing the therapeutic potential of the proposed candidates
Note: These candidates are currently FDA-approved but for other indications.
3.1 Drug repurposing using IPF data
The list of IPF datasets we used in our analysis is summarized in Supplementary Table S3. We compare the results of our approach with the existing approach proposed by (Sirota et al., 2011; drug-disease), as well as the classical method (anti-correlation). The lists of the top 10 drugs are summarized in Table 1.
Table 1.
A comparison between the results of three approaches: proposed, drug-disease, anti-correlation using IPF datasets (the top 10 drugs)
| Proposed | Drug-disease | Anti-correlation | Proposed | Drug-disease | Anti-correlation |
|---|---|---|---|---|---|
| GSE24206-early | GSE24206-advanced | ||||
| GSM1740570_saracatinib | GSM1746916_radicicol | GSM1746916_radicicol | GSM1740570_saracatinib | GSM1746916_radicicol | GSM1746916_radicicol |
| GSM1743214_nintedanib | GSM1746864_radicicol | GSM1746864_radicicol | GSM1743214_nintedanib | GSM1746864_radicicol | GSM1746864_radicicol |
| GSM1742836_celastrol | GSM1738326_mocetinostata | GSM1746893_radicicol | GSM1745714_buparlisib | GSM1738291_azacitidinea | GSM1746893_radicicol |
| GSM1745714_buparlisib | GSM1738291_azacitidinea | GSM1742836_celastrol | GSM1742836_celastrol | GSM1746893_radicicol | GSM1738290_azacitidinea |
| GSM1742552_linifanib | GSM1738794_garcinola | GSM1738290_azacitidinea | GSM1742552_linifanib | GSM1738772_ischemin | GSM1742836_celastrol |
| GSM1742850_nintedanib | GSM1745213_nilotiniba | GSM1738291_azacitidinea | GSM1740917_saracatinib | GSM1742836_celastrol | GSM1738291_azacitidinea |
| GSM1740917_saracatinib | GSM1737397_quizartiniba | GSM1745530_nilotiniba | GSM1739549_saracatinib | GSM1737397_quizartiniba | GSM1742552_linifanib |
| GSM1740731_CH5424802 | GSM1743996_sirolimusa | GSM1745213_nilotiniba | GSM1742850_nintedanib | GSM1738326_mocetinostata | GSM1743996_sirolimusa |
| GSM1743268_linifanib | GSM1742836_celastrol | GSM1742552_linifanib | GSM1740731_CH5424802 | GSM1742718_sorafeniba | GSM1742716_sorafeniba |
| GSM1739549_saracatinib | GSM1746893_radicicol | GSM1742716_sorafeniba | GSM1745213_nilotiniba | GSM1746811_ruxolitiniba | GSM1745530_nilotiniba |
| GSE44723 | GSE21369 | ||||
| GSM1741104_sunitiniba | GSM1737411_NVP-BGT226 | GSM1737409_NVP-BGT226 | GSM1737700_rucapariba | GSM1737352_everolimusa | GSM1737700_rucapariba |
| GSM1740080_sunitiniba | GSM1743823_fostamatiniba | GSM1737411_NVP-BGT226 | GSM1740570_saracatinib | GSM1737699_rucapariba | GSM1737699_rucapariba |
| GSM1742552_linifanib | GSM1745509_NVP-BEZ235 | GSM1740923_BI-2536a | GSM1745714_buparlisib | GSM1737700_rucapariba | GSM1738767_decitabinea |
| GSM1744393_gefitiniba | GSM1737409_NVP-BGT226 | GSM1740576_BI-2536a | GSM1740731_CH5424802 | GSM1738767_decitabinea | GSM1740731_CH5424802 |
| GSM1737353_everolimusa | GSM1738100_tranylcyprominea | GSM1745509_NVP-BEZ235 | GSM1745213_nilotiniba | GSM1746916_radicicol | GSM1737385_motesaniba |
| GSM1742436_GDC-0941 | GSM1741779_vorinostata | GSM1740570_saracatinib | GSM1742504_nintedanib | GSM1737385_motesaniba | GSM1737624_entinostat |
| GSM1743268_linifanib | GSM1742856_canertiniba | GSM1737412_NVP-BGT226 | GSM1737448_idelalisiba | GSM1742800_palbocicliba | GSM1744048_imatiniba |
| GSM1743214_nintedanib | GSM1742795_palbocicliba | GSM1737353_everolimusa | GSM1745530_nilotiniba | GSM1737990_mocetinostata | GSM1737698_rucapariba |
| GSM1741743_sirolimusa | GSM1739241_olapariba | GSM1745194_NVP-BEZ235 | GSM1742836_celastrol | GSM1737443_idelalisiba | GSM1738290_azacitidinea |
| GSM1740570_saracatinib | GSM1740387_CH5424802 | GSM1738308_entinostat | GSM1739679_dabrafeniba | GSM1742836_celastrol | GSM1741754_sirolimusa |
| LGRC-ILD | GSE1724 | ||||
| GSM1740570_saracatinib | GSM1738326_mocetinostata | GSM1738326_mocetinostata | GSM1741104_sunitiniba | GSM1746800_nilotiniba | GSM1746916_radicicol |
| GSM1739549_saracatinib | GSM1742795_palbocicliba | GSM1737624_entinostat | GSM1740570_saracatinib | GSM1746916_radicicol | GSM1742552_linifanib |
| GSM1740917_saracatinib | GSM1737410_NVP-BGT226 | GSM1739358_mocetinostata | GSM1739549_saracatinib | GSM1743953_linifanib | GSM1742836_celastrol |
| GSM1743214_nintedanib | GSM1741779_vorinostata | GSM1739435_belinostata | GSM1742552_linifanib | GSM1742836_celastrol | GSM1745213_nilotiniba |
| GSM1743268_linifanib | GSM1737624_entinostat | GSM1741779_vorinostata | GSM1745714_buparlisib | GSM1745958_dasatiniba | GSM1743268_linifanib |
| GSM1742552_linifanib | GSM1737411_NVP-BGT226 | GSM1737410_NVP-BGT226 | GSM1743268_linifanib | GSM1743268_linifanib | GSM1746864_radicicol |
| GSM1742504_nintedanib | GSM1739435_belinostata | GSM1746864_radicicol | GSM1740917_saracatinib | GSM1741215_velipariba | GSM1745626_vemurafenib |
| GSM1744170_GDC-0941 | GSM1737409_NVP-BGT226 | GSM1741767_vorinostata | GSM1740080_sunitiniba | GSM1741184_regorafeniba | GSM1743197_celastrol |
| GSM1745714_buparlisib | GSM1737455_vandetaniba | GSM1737409_NVP-BGT226 | GSM1742706_alvocidib | GSM1747067_mitoxantronea | GSM1744371_selumetinib |
| GSM1741265_saracatinib | GSM1738350_pracinostat | GSM1737642_mocetinostata | GSM1742850_nintedanib | GSM1741566_velipariba | GSM1746881_mitoxantronea |
Note: The P-values for Wilcoxon rank sum test comparing the results of the proposed approach and drug-disease approach using datasets GSE24206-early, GSE24206-advanced, GSE44723, GSE21369, LGRC- ILD and GSE1724 are 0.02, 0.02, 0.01, 0.02, 0.01 and 0.01, respectively. Highlighted drugs are FDA-approved for the treatment of IPF. The proposed approach was the only one that was able to rank the FDA-approved Nintedanib in the top 10. In contrast, none of the existing approaches was able to retrieve the FDA-approved drug in any of these six datasets.
Drugs that are currently FDA-approved but for other indications.
Gold standard: The gold standard for this disease is Nintedanib. This drug was approved for the treatment of IPF by FDA on October 2014. It is a small molecule inhibiting multiple tyrosine kinases (RTKs) and non-receptor tyrosine kinases (nRTKs). It is highlighted in Table 1.
As shown in Supplementary Table S4, We select the top 5% of drugs ranked lists obtained by applying our approach on 6 IPF datasets. We rank these drugs based on the scores computed by the systematic method from the highest to the lowest.
Proposed candidates: We propose Sunitinib (P = 0.0009), Dabrafenib (P = 0.0009) and Nilotinib (P = 0.0009) as repurposing candidates for treatment of IPF. Saracatinib, Linifanib, Buparlisib, GDC-0941 and Alvocidib are also highly ranked by our approach for treatment of IPF. Although these drugs are not approved by FDA yet, they can be considered for further experimental tests.
Sunitinib is a small molecule that inhibits multiple receptor tyrosine kinases (RTKs), including vascular endothelial growth factor receptors (VEGFR) and platelet-derived growth factor receptors (PDGFR). It is approved by FDA for the treatment of Gastrointestinal stromal tumor, advanced renal cell carcinoma and progressive well-differentiated pancreatic neuroendocrine tumors (Demetri et al., 2006; Motzer et al., 2007). It was investigated for its anti-fibrotic and anti-angiogenic properties. Its efficiency was experimentally proved in a bleomycin-induced mouse model and it has been proposed for the treatment of IPF (Knoerzer et al., 2013). Results of in vitro studies and animal models show that receptor tyrosine kinases, such as PDGFR, VEGFR and FGFR, and non-receptor tyrosine kinases, such as the Src family, play crucial roles in the pathogenesis of IPF (Grimminger et al., 2010; Richeldi et al., 2011).
Dabrafenib is approved by FDA for the treatment of patients with unresectable or metastatic melanoma. Recent clinical studies demonstrate that the extracellular signal regulated kinase (ERK) and mitogen-activated protein kinase (MAPK) are up-regulated in lung tissues of patients with IPF (Madala et al., 2012; Yoshida et al., 2002). In particular, results of studies on MAPK signaling pathways show that the level of serine/threonine-protein kinase B-Raf (BRAF) is increased in patients samples compared to the normal ones, suggesting the potential therapeutic effects of MEK/ERK inhibitors for pulmonary fibrosis (Madala et al., 2012; Olsen et al., 2014). This supports the idea that the BRAF inhibitor Dabrafenib may have atherapeutic effect on IPF.
Nilotinib is another FDA-approved drug we propose to be repurposed for the treatment of IPF. Nilotinib is a transduction inhibitor targeting BCR-ABL, c-kit and PDGF, that is approved by FDA for treatment of patients who are newly diagnosed with Philadelphia chromosome positive chronic myeloid leukemia (Ph + CML). It is also used for treatment of patients with Ph + CML in chronic phase and accelerated phase if they were resistant (or intolerant) to previous treatments. The potential roles of PDGFs in IPF have been shown by many studies (Antoniades et al., 1990; Allen and Spiteri, 2001; Cao et al., 2000; Homma et al., 1995; Wollin et al., 2015). The advantage of PDGF inhibition in IPF is well studied and supported by several studies (Abdollahi et al., 2005; Chaudhary et al., 2007; Wollin et al., 2015). Authors of (Grimminger et al., 2015; Rhee et al., 2011) confirmed the potential effect of Nilotinib in decreasing the extent of pulmonary fibrosis in a mouse model.
The phosphatidylinositol 3 kinase (PI3K) inhibitors Buparlisib and GDC-0941 are undergoing clinical trials for a number of diseases. Buparlisib is in Phase III of clinical trials for treatment of breast cancer and in and Phase II for several other solid tumors. GDC-0941(Pictilisib) has been used in clinical trials for the treatment of several cancers, including breast cancer. Preclinical studies proved that PI3K inhibitors have potential roles in treatment of IPF by interfering with the fibrogenic effects of signaling (Beyer and Distler, 2013; Conte et al., 2013; Hsu et al., 2017; Mercer et al., 2016). Based on this evidence, Buparlisib and GDC-0941 may have potential therapeutic effects on IPF.
The tyrosine kinase inhibitors Saracatinib and Linifanib are also highly ranked by our approach for treatment of IPF. Saracatinib (AZD0530) is an oral, tyrosine kinase inhibitor selective for Src. It underwent clinical tests at AstraZeneca for the treatment of cancer (Gucalp et al., 2011; Lara et al., 2009; Messersmith et al., 2010; Poole et al., 2010). However, it failed to show a sufficient efficacy in these studies. Subsequently, it was proposed for other usages such as Alzheimer’s disease (in Phase II; Nygaard et al., 2015). Linifanib (ABT-869) is also a multi-targeted receptor tyrosine kinase inhibitor that is intended to suppress tumor growth. It is investigated for treatment of leukemia (myeloid), myelodysplastic syndrome and solid tumors (Chen et al., 2016; Chiu et al., 2013; Wang et al., 2012). The efficiency and tolerability of Linifanib versus Sorafenib has been assessed in patients with advanced hepatocellular carcinoma (Cainap et al., 2013). The tyrosine kinase inhibitors are proven to be effective in treatment of IPF (Adamali and Maher, 2012; Beyer and Distler, 2013; Grimminger et al., 2015; Richeldi et al., 2011; Wollin et al., 2014). In particular, the Src kinase inhibitor Saracatinib is reported to be useful in treatment of IPF through targeting the signaling pathway (Hu et al., 2014). These represent additional and independent evidence supporting out findings that Linifanib and Saracatinib are expected to be useful in the treatment of IPF.
Alvocidib is a cyclin-dependent kinase (CDK) inhibitor that is undergoing clinical trials for a number of cancers: esophageal cancer, leukemia, lung cancer, liver cancer and lymphoma. Studies of murine models show that the CDK inhibitors block the epithelial apoptosis and decrease the tissue fibrosis in pulmonary fibrosis (Inoshima et al., 2004; Leitch et al., 2009). As a result, CDK inhibitors have been suggested as a novel therapeutic strategy against IPF (Zhou et al., 2014).
4 Conclusion
In this paper, we presented a systems biology approach to discover new uses of existing FDA-approved drugs. We take advantage of known knowledge of disease-related genes, drug targets information and signaling pathways to discover drugs with the potential desired effects on the given disease. We estimate a network of genes potentially perturbed by drugs and integrate this network with drug and disease gene expression signatures to conduct a more powerful analysis at system level. To evaluate the proposed approach for drug repurposing, we analyzed four different diseases (IPF, NSCLC, prostate cancer and breast cancer) using three approaches: proposed, drug-disease and anti-correlation. For each disease, there is at least one FDA-approved drug that is used to treat that disease in our input drug data. The already FDA-approved drugs for a given disease are considered as the gold standard because such drugs successfully passed all the pre-clinical and clinical trials for that disease and were demonstrated to be efficacious in each disease. The approach is validated by its ability to identify drugs that are already approved by FDA for these conditions. The proposed approach was able to find such drugs based on the molecular profile alone, while existing repurposing approaches failed to do so. Specific drugs have been identified as repurposing candidates for the four diseases studied here. Although the proposed approach is studied in the context of drug repurposing, it also can be used to identify novel targets for FDA-approved drugs and understanding their mechanism of action.
Supplementary Material
Acknowledgement
Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of any of the funding agencies.
Funding
This study was supported in part by the following grants: National Institutes of Health (R01 DK089167, R42 GM087013) and National Science Foundation (DBI-0965741). This study has also been supported by the Robert J. Sokol, MD, Endowed Chair in Systems Biology. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of any of the funding agencies.
Conflict of Interest: none declared.
References
- Abdollahi A. et al. (2005) Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J. Experimental Med., 201, 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul M., Hoosein N. (2002) Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett., 186, 99–105. [DOI] [PubMed] [Google Scholar]
- Adamali H.I., Maher T.M. (2012) Current and novel drug therapies for idiopathic pulmonary fibrosis. Drug Design Dev. Therapy, 6, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams C.P., Brantner V.V. (2006) Estimating the cost of new drug development: is it really $802 million? Health Affairs, 25, 420–428. [DOI] [PubMed] [Google Scholar]
- Alao J.P. et al. (2006) Histone deacetylase inhibitor, trichostatin a induces ubiquitin-dependent cyclin d1 degradation in mcf-7 breast cancer cells. Mol. Cancer, 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J.T., Spiteri M.A. (2001) Growth factors in mocetinostat pulmonary fibrosis: relative roles. Respiratory Res., 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato R.J. et al. (2008) Pilot study of rapamycin in patients with hormone-refractory prostate cancer. Clin. Genitourinary Cancer, 6, 97–102. [DOI] [PubMed] [Google Scholar]
- Antoniades H. et al. (1990) Platelet-derived growth factor in mocetinostat pulmonary fibrosis. J. Clin. Investig., 86, 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn T.T., Thor K.B. (2004) Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discovery, 3, 673–683. [DOI] [PubMed] [Google Scholar]
- Atienza D.M. et al. (1995) Phase II study of oral etoposide for patients with advanced breast cancer. Cancer, 76, 2485–2490. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Yekutieli D. (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat., 29, 1165–1188. [Google Scholar]
- Benzina S. et al. (2015) Deoxypodophyllotoxin isolated from juniperus communis induces apoptosis in breast cancer cells. Anti-Cancer Agents Med. Chem. (Formerly Current Medicinal Chemistry-anti-Cancer Agents), 15, 79–88. [DOI] [PubMed] [Google Scholar]
- Beyer C., Distler J.H. (2013) Tyrosine kinase signaling in fibrotic disorders: translation of basic research to human disease. Biochimica Et Biophysica Acta (BBA)-Mol. Basis Dis., 1832, 897–904. [DOI] [PubMed] [Google Scholar]
- Bhat-Nakshatri P. et al. (2013) Identification of FDA-approved drugs targeting breast cancer stem cells along with biomarkers of sensitivity. Sci. Rep., 3, 2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffa D.J. et al. (2004) Rapamycin inhibits the growth and metastatic progression of non-small cell lung cancer. Clin. Cancer Res., 10, 293–300. [DOI] [PubMed] [Google Scholar]
- Booth B., Zemmel R. (2004) Prospects for productivity. Nat. Rev. Drug Discovery, 3, 451–456. [DOI] [PubMed] [Google Scholar]
- Bradley D. et al. (2009) Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (national cancer institute trial 6862). Cancer, 115, 5541–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd G.T. et al. (1998) Phase I/II trial of all-trans retinoic acid and tamoxifen in patients with advanced breast cancer. Clin. Cancer Res., 4, 635–642. [PubMed] [Google Scholar]
- Butcher E.C. et al. (2004) Systems biology in drug discovery. Nat. Biotechnol., 22, 1253–1259. [DOI] [PubMed] [Google Scholar]
- Cainap C. et al. (2013) Phase III trial of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma (hcc). ASCO Annual Meeting Proc., 31, 249. [Google Scholar]
- Cao B. et al. (2000) The potential role of PDGF, IGF-1, TGF-beta expression in mocetinostat pulmonary fibrosis. Chin. Med. J., 113, 776–782. [PubMed] [Google Scholar]
- Carter S.L. et al. (2016) Iκbα mediates prostate cancer cell death induced by combinatorial targeting of the androgen receptor. BMC Cancer, 16, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamie K. et al. (2008) The effect of sirolimus on prostate-specific antigen (psa) levels in male renal transplant recipients without prostate cancer. Am. J. Transplantation, 8, 2668–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao B.H. et al. (2012) RET fusion genes in non–small-cell lung cancer. J. Clin. Oncol., 30, 4439–4441. [DOI] [PubMed] [Google Scholar]
- Chase A. et al. (2013) Ponatinib as targeted therapy for fgfr1 fusions associated with the 8p11 myeloproliferative syndrome. Haematologica, 98, 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N. et al. (2007) Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur. Respiratory J., 29, 976–985. [DOI] [PubMed] [Google Scholar]
- Chen J. et al. (2016) Linifanib (ABT-869) potentiates the efficacy of chemotherapeutic agents through the suppression of receptor tyrosine kinase- mediated akt/mtor signaling pathways in gastric cancer. Sci. Rep., 6, 29382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.-Y. et al. (2013) A synthetic podophyllotoxin derivative exerts anti-cancer effects by inducing mitotic arrest and pro-apoptotic ER stress in lung cancer preclinical models. PLoS One, 8, e62082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y.-L. et al. (2013) Exposure-response (safety) analysis to identify linifanib dose for a phase III study in patients with hepatocellular carcinoma. Clin. Therapeutics, 35, 1770–1777. [DOI] [PubMed] [Google Scholar]
- Choe K.S. et al. (2012) Aspirin use and the risk of prostate cancer mortality in men treated with prostatectomy or radiotherapy. J. Clin. Oncol., 30, 3540–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.Y. et al. (2015) Podophyllotoxin acetate triggers anticancer effects against non-small cell lung cancer cells by promoting cell death via cell cycle arrest, ER stress and autophagy. Int. J. Oncol., 47, 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C.R., Sullivan D.J. (2007) New uses for old drugs. Nature, 448, 645–646. [DOI] [PubMed] [Google Scholar]
- Collins F.S. (2011) Mining for therapeutic gold. Nat. Rev. Drug Discovery, 10, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte E. et al. (2013) Pi3k p110γ overexpression in mocetinostat pulmonary fibrosis lung tissue and fibroblast cells: in vitro effects of its inhibition. Laboratory Investig., 93, 566–576. [DOI] [PubMed] [Google Scholar]
- Demetri G.D. et al. (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet, 368, 1329–1338. [DOI] [PubMed] [Google Scholar]
- Detchokul S., Frauman A.G. (2011) Recent developments in prostate cancer biomarker research: therapeutic implications. Br. J. Clin. Pharmacol., 71, 157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson M., Gagnon J.P. (2009) The cost of new drug discovery and development. Discovery Med., 4, 172–179. [PubMed] [Google Scholar]
- DiMasi J. et al. (2003) The price of innovation: new estimates of drug development costs. J. Health Econ., 22, 151–186. [DOI] [PubMed] [Google Scholar]
- Drǎghici S. et al. (2007) A systems biology approach for pathway level analysis. Genome Res., 17, 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J.T. et al. (2011) Exploiting drug–disease relationships for computational drug repositioning. Brief. Bioinform., 12, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. et al. (2002) Gene Expression Omnibus: nCBI gene expression and hybridization array data repository. Nucleic Acids Res., 30, 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman S. et al. (2012) The mTOR pathway in lung cancer and implications for therapy and biomarker analysis. J.Thoracic Oncol., 7, 947–953. [DOI] [PubMed] [Google Scholar]
- Gainor J.F., Shaw A.T. (2013) Novel targets in non-small cell lung cancer: rOS1 and RET fusions. Oncologist, 18, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garattini E. et al. (2012) Retinoids and breast cancer: new clues to increase their activity and selectivity. Breast Cancer Res., 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier L. et al. (2004) affy–analysis of Affymetrix GeneChip data at the probe level. Bioinformatics, 20, 307–315. [DOI] [PubMed] [Google Scholar]
- Gautschi O. et al. (2017) Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J. Clin. Oncol., 35, 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y. et al. (2010) Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent. Neuro-Oncology, 12, 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhirsch A. et al. (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann. Oncol., 22, 1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordaliza M. et al. (2000) Antitumor properties of podophyllotoxin and related compounds. Curr. Pharmaceutical Design, 6, 1811–1839. [DOI] [PubMed] [Google Scholar]
- Goto T. et al. (1999) The effect of papaverine on morphologic differentiation, proliferation and invasive potential of human prostatic cancer lncap cells. Int. J. Urol., 6, 314–319. [DOI] [PubMed] [Google Scholar]
- Gridelli C. et al. (2008) The potential role of mTOR inhibitors in non-small cell lung cancer. Oncologist, 13, 139–147. [DOI] [PubMed] [Google Scholar]
- Grimminger F. et al. (2010) Targeting non-malignant disorders with tyrosine kinase inhibitors. Nat. Rev. Drug Discovery, 9, 956–970. [DOI] [PubMed] [Google Scholar]
- Grimminger F. et al. (2015) The role of tyrosine kinases in the pathogenesis of mocetinostat pulmonary fibrosis. Eur. Respir. J., 51, ERJ–01496. [DOI] [PubMed] [Google Scholar]
- Gucalp A. et al. (2011) Phase II trial of saracatinib (AZD0530), an oral SRC-inhibitor for the treatment of patients with hormone receptor-negative metastatic breast cancer. Clin. Breast Cancer, 11, 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel L.A. et al. (2002) Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the us. Cancer Causes Control, 13, 427–434. [DOI] [PubMed] [Google Scholar]
- Homma S. et al. (1995) Localization of platelet-derived growth factor and insulin-like growth factor I in the fibrotic lung. Am. J. Respiratory Crit. Care Med., 152, 2084–2089. [DOI] [PubMed] [Google Scholar]
- Hsu H.-S. et al. (2017) Involvement of ER stress, PI3K/AKT activation, and lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Sci. Rep., 7, 14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M. et al. (2014) Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J. Pharmacol. Experimental Therapeutics, 351, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. et al. (2016) Anticancer effect of deoxypodophyllotoxin induces apoptosis of human prostate cancer cells. Oncol. Lett., 12, 2918–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. et al. (2017) Papaverine selectively inhibits human prostate cancer cell (PC-3) growth by inducing mitochondrial mediated apoptosis, cell cycle arrest and downregulation of nf-κb/pi3k/akt signalling pathway. J. BU oN Off. J. Balkan Union Oncol., 22, 112. [PubMed] [Google Scholar]
- Imrali A. et al. (2016) Rapamycin inhibits prostate cancer cell growth through cyclin D1 and enhances the cytotoxic efficacy of cisplatin. Am. J. Cancer Res., 6, 1772. [PMC free article] [PubMed] [Google Scholar]
- Inoshima I. et al. (2004) Induction of CDK inhibitor p21 gene as a new therapeutic strategy against pulmonary fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol., 286, L727–L733. [DOI] [PubMed] [Google Scholar]
- Jacobs E.J. et al. (2005) A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J. Natl. Cancer Inst., 97, 975–980. [DOI] [PubMed] [Google Scholar]
- Jang E.R. et al. (2004) The histone deacetylase inhibitor trichostatin a sensitizes estrogen receptor α-negative breast cancer cells to tamoxifen. Oncogene, 23, 1724–1736. [DOI] [PubMed] [Google Scholar]
- Jin X. et al. (2017) Synergistic activity of the histone deacetylase inhibitor trichostatin a and the proteasome inhibitor ps-341 against taxane-resistant ovarian cancer cell lines. Oncol. Lett., 13, 4619–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. et al. (2004) Combination antiangiogenesis therapy with marimastat, captopril and fragmin in patients with advanced cancer. Br. J. Cancer, 91, 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar S. et al. (2013) Interaction of glucocorticoid receptor (GR) with estrogen receptor (er) α and activator protein 1 (AP1) in dexamethasone-mediated interference of ERα activity. J. Biol. Chem., 288, 24020–24034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast R.E., Halatsch M.-E. (2012) Matrix metalloproteinase-2 and-9 in glioblastoma: a trio of old drugs–captopril, disulfiram and nelfinavir–are inhibitors with potential as adjunctive treatments in glioblastoma. Arch. Med. Res., 43, 243–247. [DOI] [PubMed] [Google Scholar]
- Kaushik D. et al. (2015) Histone deacetylase inhibitors in castration-resistant prostate cancer: molecular mechanism of action and recent clinical trials. Therapeutic Adv. Urol., 7, 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B.D. (2008) Systematic review of the clinical effect of glucocorticoids on nonhematologic malignancy. BMC Cancer, 8, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-J., Bae S.-C. (2011) Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am. J. Trans. Res., 3, 166. [PMC free article] [PubMed] [Google Scholar]
- Kim K.-Y. et al. (2013) Interplay of reactive oxygen species, intracellular ca2+ and mitochondrial homeostasis in the apoptosis of prostate cancer cells by deoxypodophyllotoxin. J. Cell. Biochem., 114, 1124–1134. [DOI] [PubMed] [Google Scholar]
- Kitano H. (2002) Systems biology: a brief overview. Science, 295, 1662–1664. [DOI] [PubMed] [Google Scholar]
- Knoerzer D. et al. (2013) Therapeutic efficacy of Sunitinib and other broad spectrum receptor tyrosine kinase inhibitors (RTKI) in bleomycin-induced pulmonary fibrosis. J. Inflammation, 10, P38. [Google Scholar]
- Krusche B. et al. (2013) Synergistic inhibition of angiogenesis by artesunate and captopril in vitro and in vivo. Evidence-Based Complementary Alternative Med., 2013, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M.-C. et al. (2015) Colchicine significantly reduces incident cancer in gout male patients: a 12-year cohort study. Medicine, 94, e1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. et al. (2006) The connectivity map: using gene-expression signatures to connect small molecules, genes, and disease. Science, 313, 1929–1935. [DOI] [PubMed] [Google Scholar]
- Lane A.A., Chabner B.A. (2009) Histone deacetylase inhibitors in cancer therapy. J. Clin. Oncol., 27, 5459–5468. [DOI] [PubMed] [Google Scholar]
- Lara P.N., Jr et al. (2009) A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a california cancer consortium study. Anti-Cancer Drugs, 20, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch A. et al. (2009) Cyclin-dependent kinase inhibitor drugs as potential novel anti-inflammatory and pro-resolution agents. Br. J. Pharmacol., 158, 1004–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K.-T., Wang L.-H. (2016) New dimension of glucocorticoids in cancer treatment. Steroids, 111, 84–88. [DOI] [PubMed] [Google Scholar]
- Lin Z.-Y. et al. (2016) Anticancer effects of clinically acceptable colchicine concentrations on human gastric cancer cell lines. Kaohsiung J. Med. Sci., 32, 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M. et al. (2005) Expression of estrogen receptor α, retinoic acid receptor α and cellular retinoic acid binding protein II genes is coordinately regulated in human breast cancer cells. Oncogene, 24, 4362–4369. [DOI] [PubMed] [Google Scholar]
- Ma D. et al. (2016) Deoxypodophyllotoxin triggers parthanatos in glioma cells via induction of excessive ROS. Cancer Lett., 371, 194–204. [DOI] [PubMed] [Google Scholar]
- Ma Y. et al. (2017) Maintenance use of aspirin or other non-steroidal anti-inflammatory drugs (nsaids) and prostate cancer risk. Prostate Cancer Prostatic Dis., 1. [DOI] [PubMed] [Google Scholar]
- Madala S.K. et al. (2012) MEK-ERK pathway modulation ameliorates pulmonary fibrosis associated with epidermal growth factor receptor activation. Am. J. Respiratory Cell Mol. Biol., 46, 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire V. et al. (2013a) Polo-like kinase 1: a potential therapeutic option in combination with conventional chemotherapy for the management of patients with triple-negative breast cancer. Cancer Res., 73, 813–823. [DOI] [PubMed] [Google Scholar]
- Maire V. et al. (2013b) TTK/hMPS1 is an attractive therapeutic target for triple-negative breast cancer. PloS One, 8, e63712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly C. et al. (2006) The comparative toxicogenomics database (CTD): a resource for comparative toxicological studies. J. Experimental Zool. Part A: Comparative Experimental Biol., 305A, 689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maubant S. et al. (2015) Transcriptome analysis of Wnt3a-treated triple-negative breast cancer cells. PloS One, 10, e0122333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecham B.H. et al. (2004) Sequence-matched probes produce increased cross-platform consistency and more reproducible biological results in microarray-based gene expression measurements. Nucleic Acids Res., 32, e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer P.F. et al. (2016) Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax, thoraxjnl–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith W. et al. (2010) A phase II trial of saracatinib (AZD0530), an oral src inhibitor, in previously treated metastatic pancreatic cancer. ASCO Ann Meeting Proc., 28, e14515. [Google Scholar]
- Mottamal M. et al. (2015) Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules, 20, 3898–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R.J. et al. (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med., 356, 115–124. [DOI] [PubMed] [Google Scholar]
- Namazi S. et al. (2014) The role of captopril and losartan in prevention and regression of tamoxifen-induced resistance of breast cancer cell line MCF-7: an in vitro study. Biomed. Pharmacotherapy, 68, 565–571. [DOI] [PubMed] [Google Scholar]
- Nelson J., Harris R.E. (2000) Inverse association of prostate cancer and non-steroidal anti-inflammatory drugs (NSAIDs): results of a case-control study. Oncol. Rep., 7, 169–239. [DOI] [PubMed] [Google Scholar]
- Novello S. et al. (2011) Phase II study of sunitinib in patients with non-small cell lung cancer and irradiated brain metastases. J. Thoracic Oncol., 6, 1260–1266. [DOI] [PubMed] [Google Scholar]
- Núñez M. et al. (2013) Glibenclamide inhibits cell growth by inducing g0/g1 arrest in the human breast cancer cell line mda-mb-231. BMC Pharmacol. Toxicol., 14, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard H.B. et al. (2015) A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer’s disease. Alzheimer’s Res. Therapy, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen K.C. et al. (2014) Inhibition of transglutaminase 2, a novel target for pulmonary fibrosis, by two small electrophilic molecules. Am. J. Respiratory Cell Mol. Biol., 50, 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini C. (2013) DrugVsDisease: comparison of disease and drug profiles using Gene set Enrichment Analysis. Bioinformatics, R package version 2.4.0. [Google Scholar]
- Paroni G. et al. (2012) Synergistic antitumor activity of lapatinib and retinoids on a novel subtype of breast cancer with coamplification of ERBB2 and RARA. Oncogene, 31, 3431–3443. [DOI] [PubMed] [Google Scholar]
- Payen L. et al. (2001) The sulphonylurea glibenclamide inhibits multidrug resistance protein (mrp1) activity in human lung cancer cells. Br. J. Pharmacol., 132, 778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole C.J. et al. (2010) A randomized phase II clinical trial of the src inhibitor saracatinib (AZD0530) and carboplatin plus paclitaxel (C plus P) versus C plus p in patients (PTS) wiht advanced platinum-sensitive epithelial ovarian cancer (EOC). Ann. Oncol., 21 (Suppl. 8), 304–305. [Google Scholar]
- Price K.A. et al. (2010) Phase II trial of gefitinib and everolimus in advanced non-small cell lung cancer. J. Thoracic Oncol., 5, 1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X. et al. (2008) Glibenclamide exerts an antitumor activity through reactive oxygen species–c-jun nh (2)-terminal kinase pathway in human gastric cancer cell line mgc-803. Biochem. Pharmacol., 76, 1705–1715. [DOI] [PubMed] [Google Scholar]
- Ren M. et al. (2013) Novel fgfr inhibitor ponatinib suppresses the growth of non-small cell lung cancer cells overexpressing fgfr1. Oncol. Rep., 29, 2181–2190. [DOI] [PubMed] [Google Scholar]
- Rhee C.K. et al. (2011) Effect of nilotinib on bleomycin-induced acute lung injury and pulmonary fibrosis in mice. Respiration, 82, 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes L.V. et al. (2012) The histone deacetylase inhibitor trichostatin a alters microrna expression profiles in apoptosis-resistant breast cancer cells. Oncol. Rep., 27, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L. et al. (2011) Efficacy of a tyrosine kinase inhibitor in mocetinostat pulmonary fibrosis. N. Engl. J. Med., 365, 1079–1087. [DOI] [PubMed] [Google Scholar]
- Roh M. et al. (2004) Mechanism of histone deacetylase inhibitor trichostatin a induced apoptosis in human osteosarcoma cells. Apoptosis, 9, 583–589. [DOI] [PubMed] [Google Scholar]
- Rong Z. et al. (2013) Combined treatment of glibenclamide and cocl2 decreases mmp9 expression and inhibits growth in highly metastatic breast cancer. J. Experimental Clin. Cancer Res., 32, 32. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rooprai H.K. et al. (2001) Evaluation of the effects of swainsonine, captopril, tangeretin and nobiletin on the biological behaviour of brain tumour cells in vitro. Neuropathol. Appl. Neurobiol., 27, 29–39. [DOI] [PubMed] [Google Scholar]
- Rothwell P.M. et al. (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet, 377, 31–41. [DOI] [PubMed] [Google Scholar]
- Rundle-Thiele D. et al. (2016) Repurposing some older drugs that cross the blood–brain barrier and have potential anticancer activity to provide new treatment options for glioblastoma. Br. J. Clin. Pharmacol., 81, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkaria J.N. et al. (2007) Phase i trial of sirolimus combined with radiation and cisplatin in non-small cell lung cancer. J. Thoracic Oncol., 2, 751–757. [DOI] [PubMed] [Google Scholar]
- Savai R. et al. (2010) Targeting cancer with phosphodiesterase inhibitors. Expert Opin. Investigational Drugs, 19, 117–131. [DOI] [PubMed] [Google Scholar]
- Sharma N. et al. (2012) Inhibition of autophagy and induction of breast cancer cell death by mefloquine, an antimalarial agent. Cancer Lett., 326, 143–154. [DOI] [PubMed] [Google Scholar]
- Sirota M. et al. (2011) Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci. Trans. Med., 3, 96ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver C. et al. (2016) Low-dose aspirin or other nonsteroidal anti-inflammatory drug use and prostate cancer risk: a nationwide study. Cancer Causes Control, 27, 1067–1079. [DOI] [PubMed] [Google Scholar]
- Smyth G.K. (2005) Limma: linear models for microarray data. In: Bioinformatics and computational biology solutions using R and Bioconductor Springer, New York, NY, pp. 397–420.
- Socinski M. et al. (2006) Efficacy and safety of sunitinib in previously treated, advanced non-small cell lung cancer (nsclc): preliminary results of a multicenter phase ii trial. J. Clin. Oncol., 24 (Suppl 18), 7001–7001. [Google Scholar]
- Soria J.-C. et al. (2009) Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann. Oncol., 20, 1674–1681. [DOI] [PubMed] [Google Scholar]
- Sun Y. et al. (2016) Proliferation inhibition and apoptosis of breast cancer mcf-7 cells under the influence of colchicine. J. Buon, 3, 570–575. [PubMed] [Google Scholar]
- Sutton L.M. et al. (1997) Pharmacokinetics and clinical impact of all-trans retinoic acid in metastatic breast cancer: a phase ii trial. Cancer Chemotherapy Pharmacol., 40, 335–341. [DOI] [PubMed] [Google Scholar]
- Takai N., Narahara H. (2010) Preclinical studies of chemotherapy using histone deacetylase inhibitors in endometrial cancer. Obstetrics Gynecol. Int., 2010, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. et al. (2015) Refining the treatment of NSCLC according to histological and molecular subtypes. Nat. Rev. Clin. Oncol., 12, 511–526. [DOI] [PubMed] [Google Scholar]
- Vigushin D.M. et al. (2001) Trichostatin a is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin. Cancer Res., 7, 971–976. [PubMed] [Google Scholar]
- Voichiţa C., Drăghici S. (2013) ROntoTools: R Onto-Tools Suite. R package. [Google Scholar]
- Walsh C.A. et al. (2014) Global gene repression by the steroid receptor coactivator SRC-1 promotes oncogenesis. Cancer Res., 74, 2533–2544. [DOI] [PubMed] [Google Scholar]
- Wang E.S. et al. (2012) Phase 1 trial of linifanib (ABT-869) in patients with refractory or relapsed acute myeloid leukemia. Leukemia Lymphoma, 53, 1543–1551. [DOI] [PubMed] [Google Scholar]
- Wen C.-C. et al. (2011) Specific microtubule-depolymerizing agents augment efficacy of dendritic cell-based cancer vaccines. J. Biomed. Sci., 18, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon F. (1945) Individual comparisons by ranking methods. Biometrics, 1, 80–83. [Google Scholar]
- Wishart D.S. et al. (2006) DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res., 34, D668–D672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollin L. et al. (2014) Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Experimental Therapeutics, 349, 209–220. [DOI] [PubMed] [Google Scholar]
- Wollin L. et al. (2015) Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respiratory J., 51, ERJ–01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G. et al. (2013a) Curcumin enhances the anticancer effects of trichostatin a in breast cancer cells. Mol. Carcinogenesis, 52, 404–411. [DOI] [PubMed] [Google Scholar]
- Yan K.-H. et al. (2013b) Mefloquine induces cell death in prostate cancer cells and provides a potential novel treatment strategy in vivo. Oncol. Lett., 5, 1567–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. et al. (2000) Transcriptional activation of estrogen receptor α in human breast cancer cells by histone deacetylase inhibition. Cancer Res., 60, 6890–6894. [PubMed] [Google Scholar]
- Yasukagawa T. et al. (2012) Suppression of cellular invasion by glybenclamide through inhibited secretion of platelet-derived growth factor in ovarian clear cell carcinoma ES-2 cells. FEBS Lett., 586, 1504–1509. [DOI] [PubMed] [Google Scholar]
- Yoshida K. et al. (2002) MAP kinase activation and apoptosis in lung tissues from patients with idiopathic pulmonary fibrosis. J. Pathol., 198, 388–396. [DOI] [PubMed] [Google Scholar]
- Yuan P. et al. (2012) Oral etoposide monotherapy is effective for metastatic breast cancer with heavy prior therapy. Chin. Med. J., 125, 775–779. [PubMed] [Google Scholar]
- Yuan P. et al. (2015) Efficacy of oral etoposide in pretreated metastatic breast cancer: a multicenter phase 2 study. Medicine, 94, e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. et al. (2014) Chitinase 3–like 1 suppresses injury and promotes fibroproliferative responses in mammalian lung fibrosis. Sci. Trans. Med., 6, 240ra76–240ra76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.