ACYL-COA:DIACYLGLYCEROL ACYLTRANSFERASE1 confers freezing tolerance, and its expression and activity could underlie adaptive variation in freezing tolerance in Boechera stricta, a wild relative of Arabidopsis thaliana.
Abstract
Freezing limits plant growth and crop productivity, and plant species in temperate zones have the capacity to develop freezing tolerance through complex modulation of gene expression affecting various aspects of metabolism and physiology. While many components of freezing tolerance have been identified in model species under controlled laboratory conditions, little is known about the mechanisms that impart freezing tolerance in natural populations of wild species. Here, we performed a quantitative trait locus (QTL) study of acclimated freezing tolerance in seedlings of Boechera stricta, a highly adapted relative of Arabidopsis (Arabidopsis thaliana) native to the Rocky Mountains. A single QTL was identified that contained the gene encoding ACYL-COENZYME A:DIACYLGLYCEROL ACYLTRANSFERASE1 (BstDGAT1), whose expression is highly cold responsive. The primary metabolic enzyme DGAT1 catalyzes the final step in assembly of triacylglycerol (TAG) by acyl transfer from acyl-CoA to diacylglycerol. Freezing tolerant plants showed higher DGAT1 expression during cold acclimation than more sensitive plants, and this resulted in increased accumulation of TAG in response to subsequent freezing. Levels of oligogalactolipids that are produced by SFR2 (SENSITIVE TO FREEZING2), an indispensable element of freezing tolerance in Arabidopsis, were also higher in freezing-tolerant plants. Furthermore, overexpression of AtDGAT1 led to increased freezing tolerance. We propose that DGAT1 confers freezing tolerance in plants by supporting SFR2-mediated remodeling of chloroplast membranes.
Low temperature is a major environmental factor influencing the geographical distribution of plant species and affecting crop yield (Hannah et al., 2006; Zheng et al., 2015). Freezing injury can result from a combination of mechanical stress and extreme dehydrative forces exerted by extracellular ice formation (Steponkus, 1984; Thomashow, 1999). Plants have evolved a wide range of adaptations to cope with freezing conditions. In temperate zones they generally have the capacity to acquire freezing tolerance in a process of acclimation that is set in motion after a period of low, “chilling” temperature (i.e. between 0°C and 8°C). In overwintering species, diminishing temperature and light in autumn act as cues for plants to become cold acclimated and prepare for winter frost. Cold acclimation involves the synthesis of osmotically active and cryoprotective molecules, which partly relies on the orchestrated response of a high number of cold regulated genes and transcription factors (Zhao et al., 2015). In addition, chilling temperature potentially affects a broad spectrum of metabolic and physiological processes, including photosynthesis and growth, triggering adjustments to optimize chances of survival and reproduction during periods of freezing (Huner et al., 1998; Demmig-Adams et al., 2017).
Membranes are particularly sensitive cellular structures because low temperature decreases their fluidity, a requirement for proper membrane function and integrity, and cellular water loss due to ice formation results in deformations and close apposition of membranes, causing leakiness and loss of bilayer structure (Steponkus et al., 1998). To prevent these adverse effects, membrane lipid compositions are modulated during cold acclimation so as to increase the level of fatty acid unsaturation and the amount of bilayer-stabilizing lipids. This can be achieved by comprehensive regulation of genes and enzymes acting in lipid metabolic pathways. Part of the changes in lipid and acyl chain compositions under chilling conditions are thought to be due to rebalancing of the main pathways of glycerolipid assembly at the chloroplast and the endoplasmic reticulum (ER) membranes, the prokaryotic and eukaryotic pathways, respectively (Johnson and Williams, 1989; Welti et al., 2002; Li et al., 2015, 2016). The complex mechanisms modulating membrane lipid properties are thought to be fundamental to low temperature adaptation, but their relevance to freezing tolerance is less well understood (Chen and Thelen, 2013; Ruelland, 2017).
In contrast to lipid modifying activities transcriptionally induced during cold acclimation, SENSITIVE TO FREEZING2 (SFR2) is absolutely required for freezing survival in Arabidopsis (Arabidopsis thaliana) after acclimation, but its enzyme activity is posttranslationally stimulated at freezing temperatures (Thorlby et al., 2004). SFR2 transfers galactosyl groups from monogalactosyldiacylglycerol (MGDG) to other galactolipids to generate oligogalactolipids, including tri- and tetragalactosyldiacylglycerol (TGDG and TeGDG, respectively), which are thought to stabilize chloroplast membranes, and diacylglycerol (DAG; Fourrier et al., 2008; Moellering et al., 2010). The latter has been suggested to be acylated through an unknown activity to triacylglycerol (TAG), which is sequestered in cytoplasmic oil droplets (Moellering and Benning, 2011; Barnes et al., 2016). The accumulation of TAGs in vegetative plant tissues has been observed in response to various abiotic stress conditions, including temperature stresses and drought (Degenkolbe et al., 2012; Gasulla et al., 2013; Higashi et al., 2015), but the functional significance and underlying metabolic pathways are still largely unknown.
Despite major advances in understanding cold signaling, cold acclimation, and freezing protection in model and crop species, and extensive studies of natural variation in freezing tolerance in Arabidopsis accessions (Oakley et al., 2014; Gehan et al., 2015; Zuther et al., 2015; Horton et al., 2016), the question of which genes and mechanisms underlie freezing tolerance of wild species has remained largely unanswered. As freezing tolerance generally exhibits continuous variation (Collins et al., 2008), it is amenable to quantitative trait locus (QTL) mapping to identify relevant chromosomal regions. For example, studies in Arabidopsis and Medicago truncatula have identified QTL regions containing C-repeat binding factor (CBF) genes (Alonso-Blanco et al., 2005; Tayeh et al., 2013). Complementary to the commonly used annual model and crop species, the close Arabidopsis relative Boechera stricta has emerged as a perennial ecological genomics plant model (Schranz et al., 2007; Rushworth et al., 2011). This Brassicaceae species native to the Rocky Mountains occurs along a broad elevational and latitudinal gradient and inhabits highly undisturbed sites with diverse abiotic and biotic conditions often characterized by low winter temperatures, and B. stricta populations are locally adapted to these ecological variables (Anderson et al., 2012). Several genomic tools and resources have been developed for comparative genomic studies of B. stricta, including a recombinant inbred line (RIL) population for QTL studies (Schranz et al., 2007; Lee and Mitchell-Olds, 2013) from parental lines SAD12 from Colorado and LTM from Idaho, which are members of the “East” and “West” subspecies, respectively, and have been sequenced to high coverage (Lee et al., 2017). These resources have allowed identification of molecular determinants of various ecologically important traits in B. stricta (Schranz et al., 2009; Anderson et al., 2011; Prasad et al., 2012). In a recent study, freezing tolerance in adult B. stricta plants was shown to be higher in the northern LTM ecotype than in the southern SAD12 ecotype, and the phenotypic variation was associated with a QTL locus containing several CBF paralogs (Heo et al., 2014, 2018). However, the expression of genes implicated in the complex trait of freezing tolerance could differ between developmental stages (Novillo et al., 2007). Tissues of young plants are often more prone to freezing-induced injury, which calls for effective cryoprotective mechanisms, especially in overwintering species such as B. stricta. In order to identify such mechanisms, we devised this study.
We found that seedlings of the LTM ecotype displayed higher freezing tolerance than SAD12, and the trait correlated with a single QTL locus, which we named SKI (Seedling Koude Intolerance). BstDGAT1, encoding acyl-CoA:diacylglycerol O-acyltransferase1, was identified as the likely candidate gene within the QTL interval. We therefore further characterized BstDGAT1 and found that its expression was highly cold responsive and correlated with freezing tolerance in the two B. stricta lines. Upstream sequence analysis showed several known cold stress-related motifs to be exclusively present in the more freezing tolerant ecotype. This was associated with higher accumulation of major molecular species of the acylation product TAG during acclimation and subsequent freezing. Lipidomics analysis indicated higher conversions of phosphatidylcholine (PC) to TAG during cold acclimation and from MGDG to TAG during freezing, which could support adaptive changes in membrane and nonmembrane lipid compositions. Specifically, higher TAG levels upon freezing were associated with higher accumulation of TGDG and TeGDG, which implicated a link with SFR2, a major determinant of freezing tolerance. Overexpression of AtDGAT1 in Arabidopsis also resulted in a substantial increase in freezing tolerance. Together, the data suggest a mechanism by which DGAT1-mediated TAG assembly confers tolerance to freezing stress.
RESULTS
Identification of the SKI Locus in B. stricta
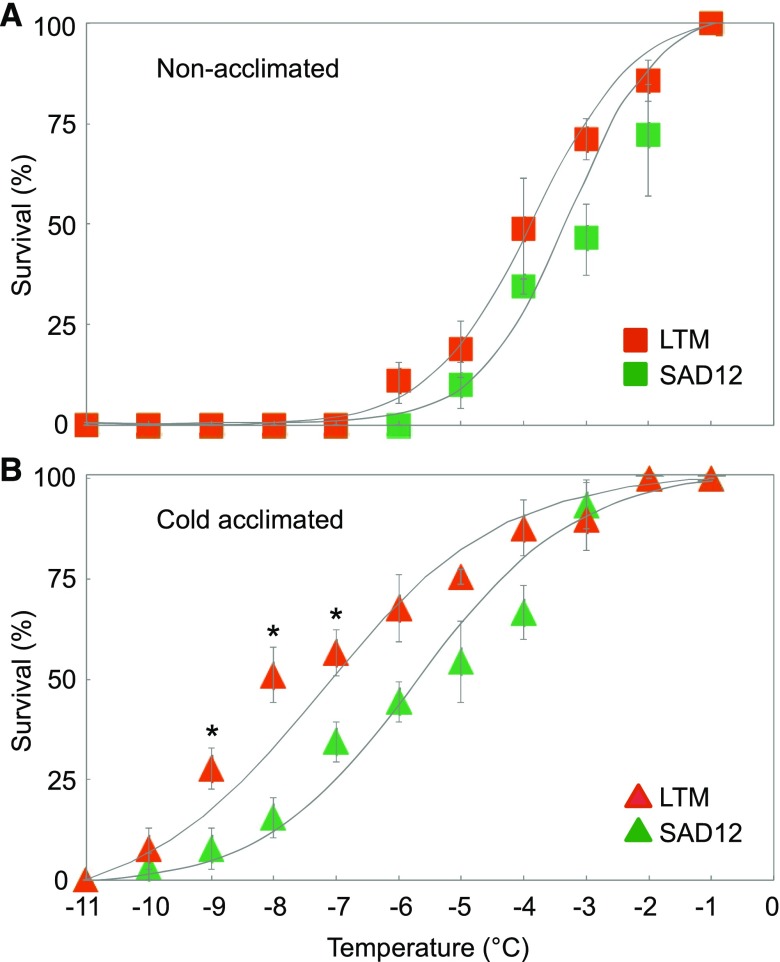
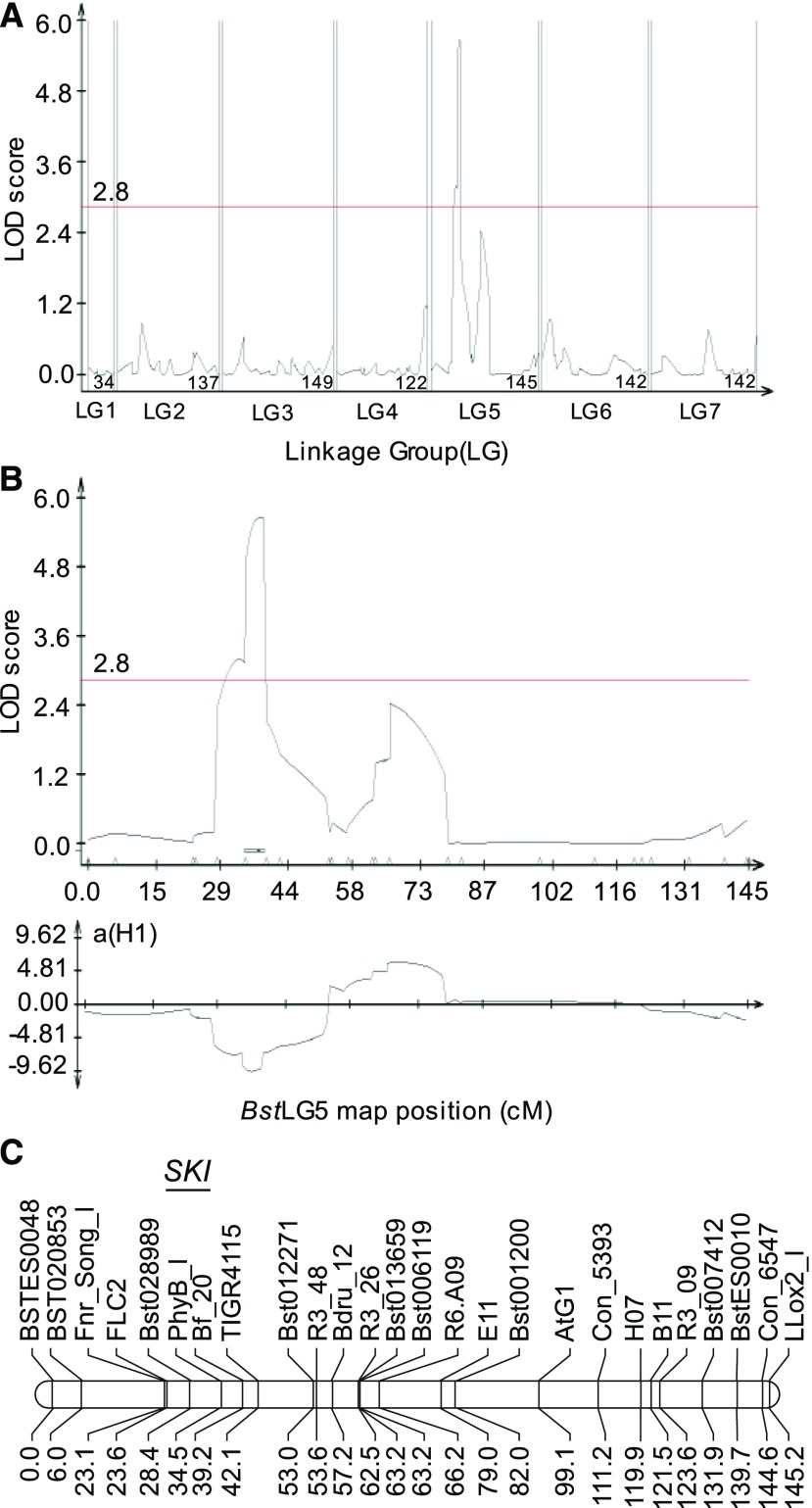
Freezing survival of B. stricta seedlings was studied following an agar plate-based assay that has been used in the past for the discovery of multiple essential components of cold acclimation and freezing tolerance mechanisms in Arabidopsis (Xin and Browse, 1998; Lee et al., 2002). While basal freezing tolerance of 20-d-old seedlings of the RIL parental lines SAD12 and LTM was similar, significant differences in survival were found when seedlings were cold-acclimated for 48 h at 4°C before freezing treatment, and LTM was more tolerant of freezing than SAD12 (Fig. 1). Median lethal temperatures (LT50s) of cold-acclimated SAD12 and LTM seedlings were −5.7°C and −7.2°C, respectively, and the greatest difference was found at −8°C, at which only 16% of SAD12 seedlings survived compared to 52% of LTM. Subsequently, cold-acclimated RIL seedlings were tested at −8°C, resulting in a normal trait frequency distribution (Supplemental Table S1). Based on these data, a single major QTL was detected on the short arm of linkage group 5 (LG5) at 37.5 centimorgans (cM) from the first marker and centered on the markers PhyB.I and Bf_20 (Fig. 2). We named this QTL Seedling Koude Intolerance (SKI; “koude” being the Dutch word for cold). With a maximum likelihood of odds (LOD) score of 5.67, SKI explained 22.4% of the total phenotypic variance and had 9.52 of the additive effect of the LTM allele.
Figure 1.
Freezing survival of seedlings of B. stricta SAD12 (from Colorado) and LTM (from Idaho) ecotypes. Non-acclimated (A) and cold acclimated plants (B) were subjected to a range of subzero temperatures, and survival was scored after thawing. Fitted response curves were used for calculation of LT50, the temperature at which 50% of the plants died. Significant differences (P < 0.01, Student’s t test) are indicated by asterisks. Data represent means (±sd) of five agar plates containing 30 seedlings each.
Figure 2.
Mapping of the SKI QTL for freezing tolerance of B. stricta seedlings. A, QTL analysis results of freezing tolerance across the seven LGs using the SAD12 × LTM RIL population. The red line shows the LOD significance threshold of 2.8. B, A single significant QTL above the LOD threshold for seedling survival assay was identified on LG5 (top) and its estimated additive effect (bottom). The negative value of the additive effect indicates that the LTM ecotype allele confers enhanced freezing tolerance. C, The precise two-LOD interval and marker names of the SKI major QTL on B. stricta LG5.
DGAT1 Is a Candidate Gene Underlying SKI
The comparative genetic Brassicaceae framework between the species B. stricta, Arabidopsis, Arabidopsis lyrata, and Capsella rubella (Schranz et al., 2007) allowed identification of potential candidate genes underlying the QTL. The one-LOD confidence interval for the SKI QTL was 6 cM and was contained within genomic block H corresponding to Arabidopsis chromosome 2, approximately from At2g18790 to At2g20050. Using functional annotations and Arabidopsis expression databases, a total of 19 candidate genes were found (Supplemental Table S2). Only At2g19450 (DGAT1) was highly and specifically induced at 4°C (Kilian et al., 2007). DGAT1 activity has previously been associated not only with seed oil accumulation but also, in vegetative tissues, with normal growth and abiotic stress responses (Lu and Hills, 2002; Yang et al., 2011; Kelly et al., 2013; Kong et al., 2013; Tjellström et al., 2015; Fan et al., 2017). Based on its transcriptional induction at chilling temperature in Arabidopsis, its potential role in TAG biosynthesis at low temperature, and our interest in lipid functions in abiotic stress responses, we decided to study DGAT1 in B. stricta as a candidate gene underlying SKI.
BstDGAT1 Sequence Polymorphisms and Induced Expression in Seedlings at Low Temperature
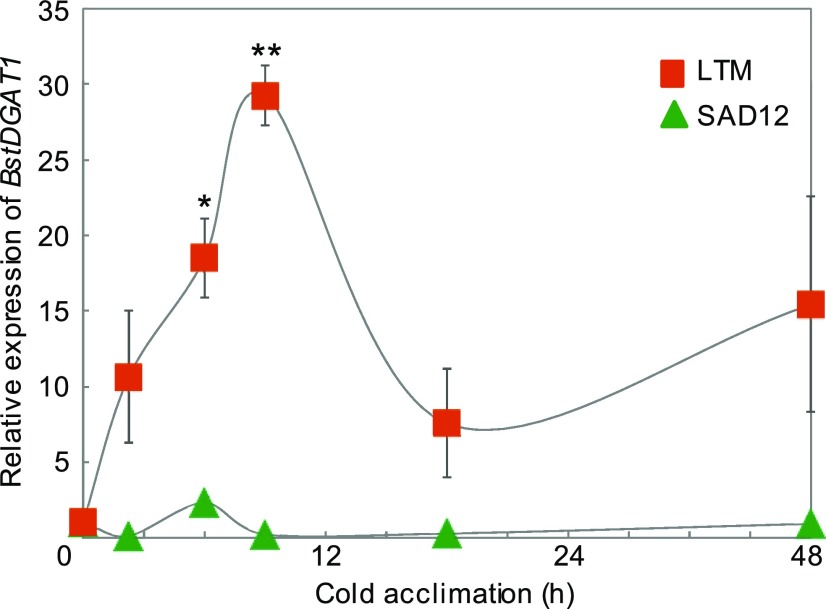
The BstDGAT1 coding sequences of LTM and SAD12 (Supplemental Fig. S1) showed conserved regions including those encoding multiple predicted transmembrane domains, which in Brassica napus sequester the enzyme to the ER (Weselake et al., 2006). Comparison of DGAT1 coding sequences from LTM and SAD12 (Supplemental Fig. S1) identified five single nucleotide polymorphisms (SNPs), one of which was nonsynonymous, resulting in an amino acid change in the variable N-terminal predicted cytosolic region of the protein (Lung and Weselake, 2006; Supplemental Fig. S2). For BstDGAT1 expression analysis, 18-d-old seedlings of B. stricta were cold-acclimated for up to 2 d at 4°C (Fig. 3). Whereas in LTM a 30-fold, transient increase in DGAT1 transcripts was found with a peak at 12 h, SAD12 displayed only a modest increment at 8 h. Thus, the greater acquisition of freezing tolerance in LTM seedlings was associated with high BstDGAT1 expression, whereas SAD12, with inferior hardiness, showed lower expression of this gene.
Figure 3.
Time course of relative BstDGAT1 expression in B. stricta SAD12 and LTM seedlings during cold acclimation at 4°C. Values are relative to the control (no cold treatment). Significant ecotypic differences are indicated by asterisks (*P < 0.05 and **P < 0.01; Student’s t test ); data represent means (±se) of three biological replicates.
To study whether the different expression profiles could be related to variation in upstream cis-regulatory elements, the 4-kb upstream genomic sequences of both ecotypes were used for motif analysis (Supplemental Table S3). Interestingly, LTM, but not SAD12, had several potential cis-element motifs commonly associated with stress responses, including MYB- and MYC-type transcription factor recognition sites associated with cold stress signaling. These sequence polymorphisms could therefore underlie the higher induction of BstDGAT1 at low temperature in LTM.
Making use of a collection of genomic sequence data of 79 B. stricta individuals from across the species range (Lee et al., 2017), the geographical distribution patterns of the discovered SNPs in the exon (Supplemental Fig. S1) and two of the identified upstream motifs, MYB2AT and ARR1AT (Supplemental Table S3), were analyzed and visualized in maps (Supplemental Fig. S3). Interestingly, while the exonic SNP showed a rare and scattered distribution of the LTM allele, the two upstream motifs present in the LTM genotype showed high prevalence at higher latitude.
To test the hypothesis that variation in BstDGAT1 expression in B. stricta ecotypes could contribute to the observed differences in freezing tolerance through modulation of TAG biosynthesis, we set out to quantitatively investigate glycerolipid and TAG molecular species compositions of SAD12 and LTM seedlings after cold acclimation and freezing treatment.
Remodeling of Polar Glycerolipids of B. stricta Seedlings during Cold Acclimation
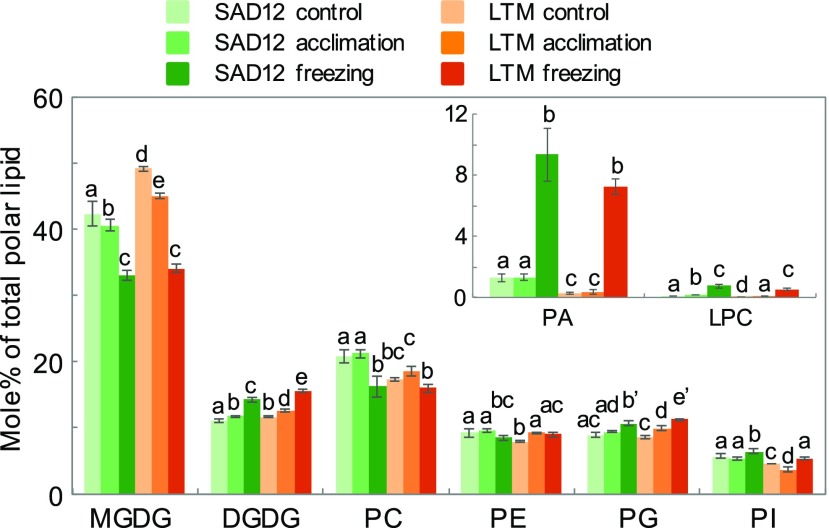
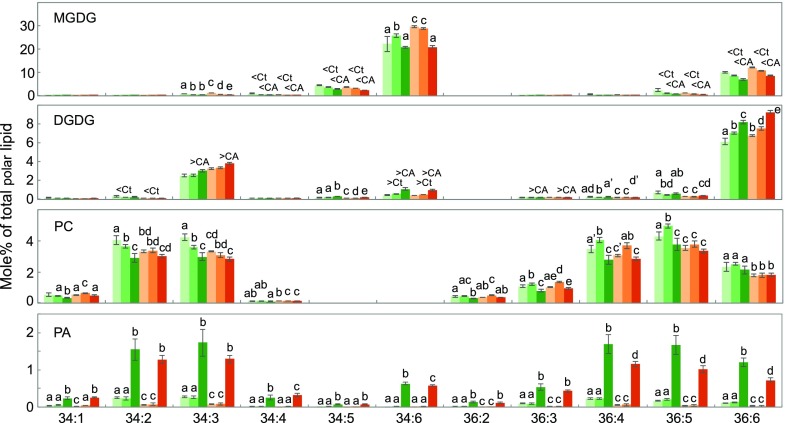
In lipidomics analysis of control, cold-acclimated, and cold-acclimated freezing-treated plants, 156 molecular species belonging to different classes of polar glycerolipids were detected, including the galactolipids MGDG and digalactosyldiacylglycerol (DGDG), the phospholipids phosphatidylcholine (PC), phosphatidylglycerol, phosphatidylethanolamine (PE), phosphatidylinositol, phosphatidyl-Ser, phosphatidic acid (PA), and two lyso-phospholipids (LPC and LPE, respectively; Fig. 4; Supplemental Table S3). Basal levels of MGDG and DGDG, the main constituents of chloroplast membranes, were higher in LTM than in SAD12 seedlings (Fig. 4). Similar to Arabidopsis, MGDG consisted mainly of the eukaryotic species 36:6 (18:3-18:3; x:y meaning x acyl carbon atoms containing y double bonds), and a prokaryotic species 34:6 (18:3-16:3), the presence of sn2-16:3 being indicative of prokaryotic, chloroplastic precursors (Fig. 5). SAD12 was enriched in phospholipids, particularly PC, the dominant glycerolipid in extraplastidial membranes, including the plastid outer envelope membrane (Fig. 4). While LTM had a higher basal MGDG level, it also showed a more pronounced cold-induced decrease, including in 36:6-MGDG (Figs. 4 and 5). Simultaneously, 36:6-DGDG increased in both ecotypes, reaching a higher level in LTM. In contrast, SAD12 showed increased 34:6-MGDG (Fig. 5). During cold acclimation in SAD12 but not LTM, several 18:2-containing PC species tended to increase, particularly the most abundant 36:5-PC, while the monounsaturated PC species 34:2 and 34:3 declined (Fig. 5). The most desaturated, plastidial phosphatidylglycerol species 34:4 increased in both ecotypes upon acclimation but reached higher levels in LTM under all experimental conditions (Supplemental Table S3). Conversely, the most abundant phosphatidylinositol species, 34:3 and 34:2, were higher in SAD12 than LTM and decreased upon acclimation (Supplemental Table S4). While basal PA levels showed a considerable difference with SAD12 containing 4.4 times as much PA as LTM, there was no increase in either upon cold acclimation (Fig. 4). In conclusion, cold acclimation induced mostly similar adjustments in LTM and SAD12 membrane lipids, but LTM showed a more pronounced plastidial lipid rebalancing, while SAD12 showed increased prokaryotic MGDG and remodeling in PC.
Figure 4.
Polar glycerolipids of B. stricta SAD12 and LTM seedlings subjected to control conditions, cold acclimation, or cold acclimation followed by freezing. Immediately after treatment, total lipids were extracted from the aerial parts of plants for quantitative lipidomics analysis. The minor phospholipids PA and LPC are shown in the inset. Bars marked with different letters indicate statistical differences within a lipid class (P < 0.05, ANOVA); differences between genotypes under the same conditions are at P < 0.01 unless marked with a prime (P < 0.05); data represent means (±sd, n = 5–6).
Figure 5.
Molecular species of selected polar glycerolipid classes of B. stricta SAD12 and LTM seedlings subjected to control conditions, cold acclimation, or cold acclimation followed by freezing. Bar colors indicate ecotype and treatment, and letters indicate statistical differences as in Figure 4. For molecular species that do not display genotype-by-treatment interactions, significant differences from control (Ct) or cold-acclimated (CA) values within the same genotype are indicated by >/<Ct or >/<CA, respectively. Data represent means (±sd, n = 5–6).
Freezing-Induced Turnover of MGDG and PC in SAD12 and LTM Seedlings
Freezing treatment triggered much more drastic changes in the lipidome, featuring major reductions in MGDG and PC (Fig. 4). The decline of total MGDG and 34:6-MGDG was greater in LTM than in SAD12 (Figs. 4 and 5). As in acclimation, this was accompanied in both ecotypes by increases in DGDG that were comparable in size, but reached higher levels in LTM (Fig. 4). DGDG was enriched in 34:6, making up only 3.4 to 3.8 mol % of control DGDG, but 6.1 to 7.7 mol % of DGDG after freezing.
The induced turnover of PC was greater in SAD12 than in LTM (Fig. 4), and several major species that were reduced in SAD12, e.g. 34:2-, 34:3-, 36:5-, and 36:6-PC, were not significantly affected in LTM (Fig. 5). Also, the decrease in PE in SAD12 was not matched in LTM (Supplemental Table S4). The simultaneous accumulations in the according molecular species of PA indicated PC and PE hydrolysis, with higher amounts of several PA species in SAD12 (Fig. 5). The emergence of 34:6-PA, a normally very minor molecular species with the hallmark fatty acid composition of MGDG, suggested another conditional metabolic pathway. Freezing also triggered increments in all species of LPC and LPE (Fig. 4; Supplemental Table S4). Accumulation of 34:6-PA, LPC, and LPE during freezing have previously been reported in Arabidopsis, suggesting conserved elements in the response (Welti et al., 2002).
Freezing-Induced TAG Accumulation in B. stricta Seedlings Was Higher in LTM Than in SAD12 Ecotypes
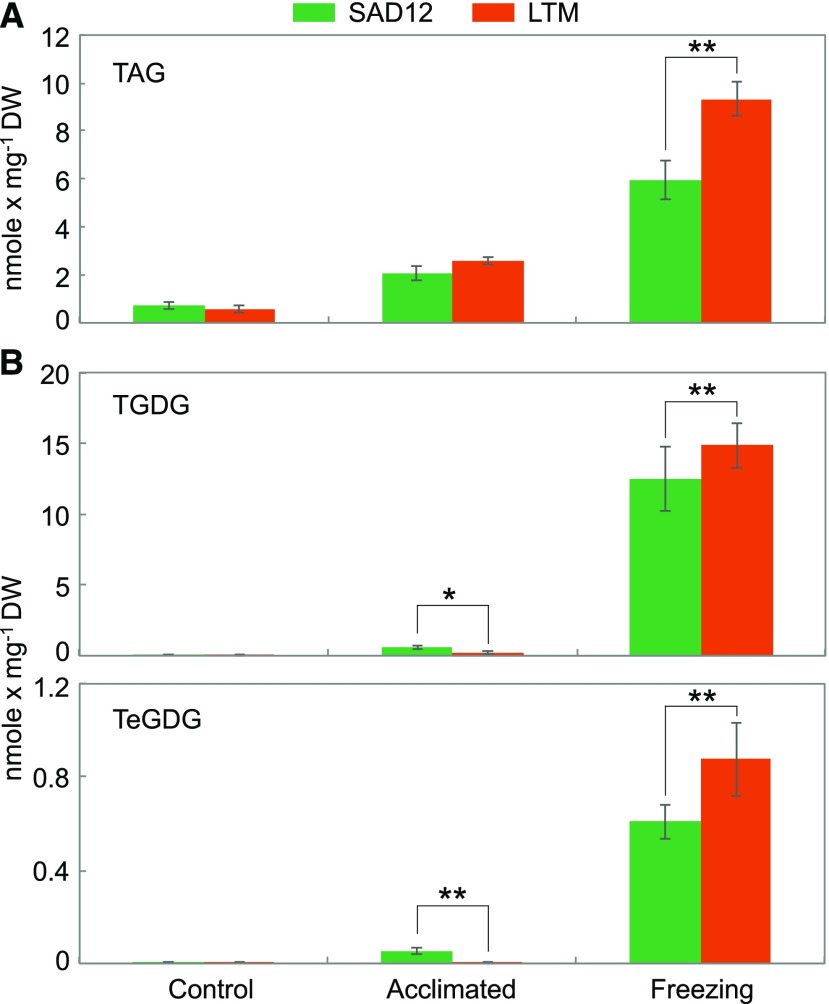
For TAG analysis, a recently developed electrospray ionization mass spectrometry (ESI-MS)-based quantitative TAG profiling approach was deployed (Li et al., 2014). As expected in vegetative plant tissues, steady state TAG quantities of SAD12 and LTM were low (0.6–0.7 nmol/mg dry weight [DW]), which was comparable to concentrations of minor phospholipids like PA and phosphatidyl-Ser (Fig. 6; Supplemental Table S5). Cold acclimation induced increases to 2.0 to 2.3 nmol/mg DW in both ecotypes. However, freezing treatment showed a quantitative difference: While both ecotypes displayed substantial increases, LTM accumulated more TAG than SAD12 (Fig. 6). Total TAG of SAD12 and LTM amounted to 5.9 and 9.3 nmol/mg DW, corresponding to 8.4 and 16.7 times steady state levels, respectively. A similar difference between the ecotypes in TAG responses was found when expressed relative to the total amount of polar membrane lipids (P < 0.001; Supplemental Table S5).
Figure 6.
Accumulation of TAG, TGDG, and TeGDG in B. stricta SAD12 and LTM seedlings subjected to control conditions, cold acclimation, or cold acclimation followed by freezing. Significant ecotypic differences are indicated by asterisks (*P < 0.05 and **P < 0.01; ANOVA); data represent means (±sd, n = 5–6).
Molecular Species Composition of Acclimation- and Freezing-Induced TAG in LTM and SAD12 Ecotypes
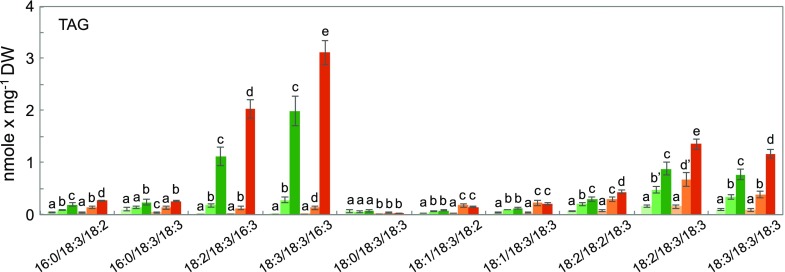
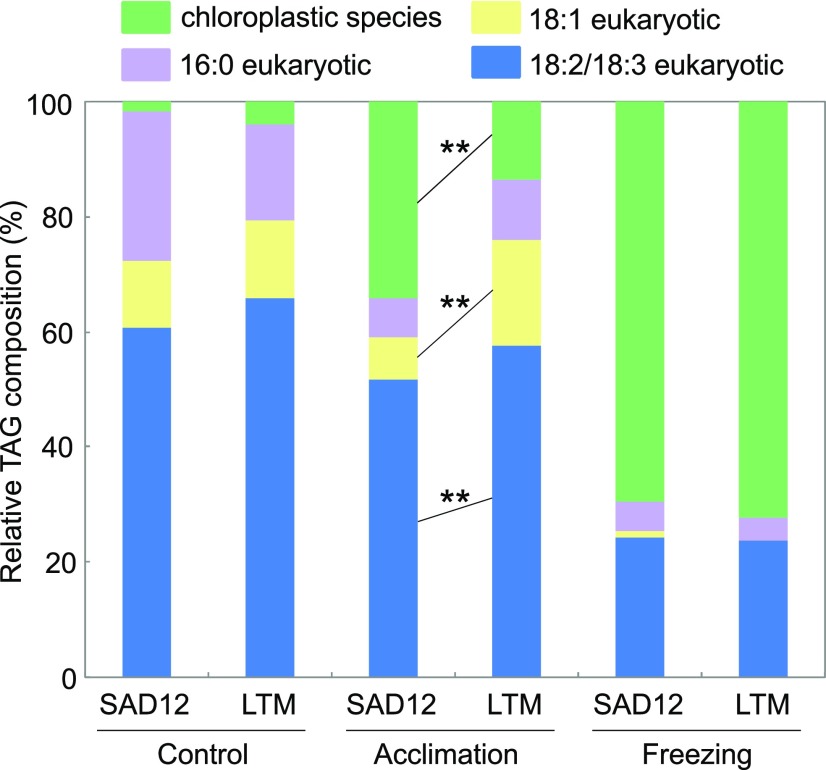
Detailed analysis of TAG formed during freezing showed the prevalence of several highly unsaturated molecular species, featuring two 16:3-species with apparent chloroplast lipid precursors, 18:3/18:3/16:3 and 18:2/18:3/16:3, and two eukaryotic species, 18:3/18:3/18:3 and 18:2/18:3/18:3 (Fig. 7; Supplemental Table S5). Although cold acclimation resulted in similar total TAG levels in both ecotypes, there were differences in molecular species (Fig. 7). LTM accumulated higher amounts of eukaryotic TAGs, including 18:1-containing molecular species. In contrast, TAG from SAD12 was enriched in 16:3-TAGs, making up 24 mol % and 11 mol % of total TAG in SAD12 and LTM, respectively. If only the lipid formed during cold acclimation is considered, the enrichment is even more conspicuous, with 16:3-containing molecular species making up 34.3 mol % and 13.6 mol % of total TAG increments in SAD12 and LTM, respectively (Fig. 8).
Figure 7.
Molecular species of TAG accumulated in B. stricta SAD12 and LTM seedlings subjected to control conditions, cold acclimation, or cold acclimation followed by freezing. Bar colors indicate ecotype and treatment, and letters indicate statistical differences as in Figure 4; data represent means (±sd, n = 5–6).
Figure 8.
Relative molecular species compositions of TAG in B. stricta SAD12 and LTM seedlings subjected to control conditions, cold acclimation, or cold acclimation followed by freezing. TAG classes indicated by different colors were characterized by distinctive acyl chain compositions. Chloroplastic species contained 16:3, indicative of a galactolipid precursor. Based on a χ2 test, overall relative TAG molecular species compositions in control conditions and in response to freezing were similar, but TAGs formed during cold acclimation differed (P < 0.01). Three groups of TAG molecular species formed during acclimation showed ecotypic differences, as indicated by asterisks (**P < 0.01, n = 5–6; ANOVA).
The amounts of major freezing-induced TAG species were 1.5 to 1.8 times higher in LTM than in SAD12 (Fig. 7; Supplemental Table S5), and in contrast to cold acclimation, they displayed a highly similar distribution of molecular species (Fig. 8). In both ecotypes, they featured a marked increase in 16:3-containing molecular species, which accounted for only 2 to 4 mol % of total TAG in controls, but ∼70 mol% of freezing-induced TAG (Fig. 8).
Accumulation of TGDG and TeGDG Suggested Differential SFR2-Like Activity in Response to Cold Acclimation and Freezing
Previous studies have shown the freezing-induced formation of 16:3-containing TAG species to be contingent on the activity of SFR2 in Arabidopsis, which is essential for freezing tolerance (Moellering et al., 2010; Barnes et al., 2016). This enzyme has the capacity to transgalactosylate MGDG in the chloroplast to generate not only DGDG but also, in a processive fashion, TGDG and TeGDG. To investigate SFR2-like activity in B. stricta, TGDG and TeGDG were analyzed. Interestingly, under conditions of cold acclimation, SAD12, but not LTM, showed accumulations of these lipids (Fig. 6). In contrast, freezing induced marked increases of TGDG and TeGDG in both ecotypes, mainly consisting of 34:6 and 36:6 molecular species (data not shown), but the tolerant ecotype LTM displayed higher accumulations (Fig. 6).
Overexpression of AtDGAT1 in Arabidopsis Enhances Freezing Tolerance of Seedlings
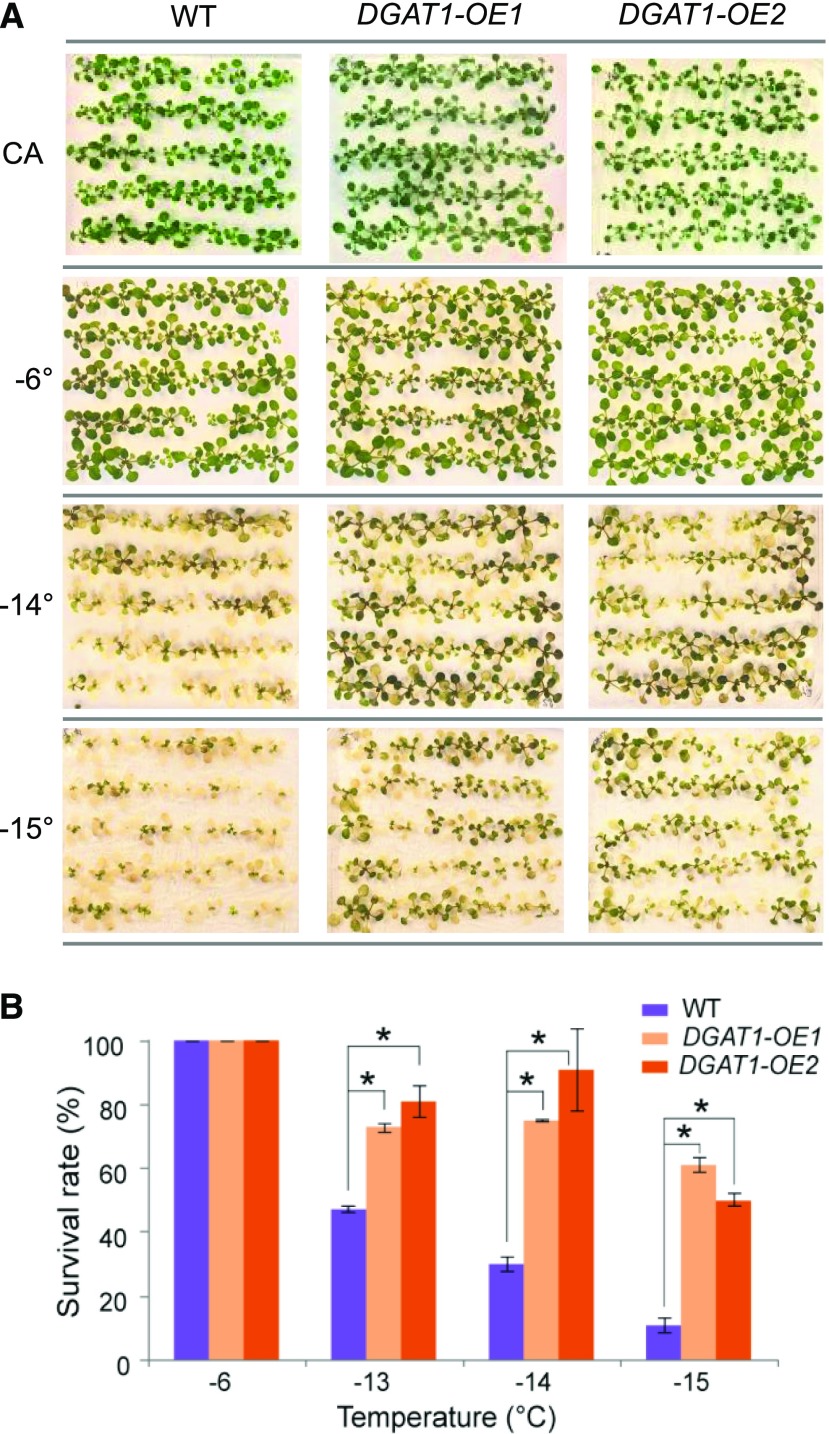
To further assess the role of DGAT1 in freezing stress, Arabidopsis was transformed with a 35S-AtDGAT1 construct. The resulting DGAT1 overexpressing plants showed enhanced freezing tolerance compared to the wild type (Fig. 9; Supplemental Fig. S4). After treatment at −15°C, the majority (75–89%) of wild-type plants were killed but 50 to 75% of DGAT1-OE transgenic plants survived, suggesting that DGAT1 positively regulates freezing tolerance.
Figure 9.
Freezing tolerance of Arabidopsis AtDGAT1 overexpressor plants. A, Two-week-old seedlings of Arabidopsis wild type (WT) and 35S-DGAT1 lines DGAT1-OE1 and DGAT1-OE2 were cold-acclimated for four 4 d (CA) and subsequently subjected to freezing temperatures as indicated. Pictures were taken 3 d after thawing of plates. B, Survival rates were scored, and significant differences compared to the wild type are indicated by asterisks (* P < 0.01, Student’s t test); data represent means (±sd) of three to four agar plates containing 40 to 50 seedlings each.
DISCUSSION
This study identified SKI as single major QTL underlying natural variation in freezing tolerance of B. stricta seedlings, and within this genomic region, we selected BstDGAT1 as a candidate gene based on the strong cold-induced transcriptional up-regulation of the Arabidopsis ortholog and the accumulation of TAG at chilling and freezing temperatures (Moellering et al., 2010; Degenkolbe et al., 2012; Barnes et al., 2016). The presented results suggest a function for DGAT1 to enhance freezing tolerance by allowing coherent glycerolipid changes pertaining to cold acclimation and freezing responses and highlight a pivotal role of plasticity in chloroplast lipid metabolism.
Cold-Induced Expression of BstDGAT1 Is Associated with the Presence of Conserved Upstream Elements and Freezing Tolerance in B. stricta Ecotypes LTM and SAD12
The BstDGAT1 alleles of the LTM and SAD12 lines (Supplemental Fig. S1) contained a single nonsynonymous sequence polymorphism encoding an amino acid in a segment of the hydrophilic N-terminal region, which in B. napus promotes self-association into dimers (Weselake et al., 2006; Caldo et al., 2017; Supplemental Fig. S2). Variation within this domain is thought to account for differences in enzymatic properties of DGAT1 isozymes from different sources (Liu et al., 2012). Analysis of the geographic distribution of the nonsynonymous LTM SNP in 79 genomes across the species range of B. stricta showed that variant to be rare and scattered (Supplemental Fig. S3).
BstDGAT1 transcription was differentially induced during cold acclimation in both ecotypes, in association with freezing tolerance (Fig. 3), and this prompted us to search for polymorphisms in the upstream regulatory regions. Notably, the LTM allele contained several potential cis-regulatory elements involved in abiotic stress responses that were missing from SAD12 due to SNPs (Supplemental Table S3). These elements included motifs that recognize transcription factors such as INDUCER OF CBF EXPRESSION1, CALMODULIN-BINDING TRANSCRIPTION ACTIVATOR3, and MYB2, which are also present in the Arabidopsis CBF promoter regions. Conversely, the SAD12 allele contained an extra potential binding motif for MYB15, a negative regulator of CBF expression and freezing tolerance in Arabidopsis (Agarwal et al., 2006). Hence, the presence of extra upstream cis-regulatory motifs could underlie the differences in transcriptional responses and freezing tolerance. Interestingly, in contrast to the exon SNP, analysis of the geographic distribution of the upstream LTM MYB2AT and ARR1AT sequence variants showed these motifs to be prevalent in B. stricta populations at higher latitude (Supplemental Fig. S3). In spite of the notable differences in distribution patterns, it can at present not be concluded whether the observed divergence in promoter sequences is due to local adaptive selection and/or reflects population structure.
Highly Freezing-Tolerant B. stricta LTM Seedlings Accumulate More TAG and Oligogalactolipids in Response to Freezing
Relating with BstDGAT1 transcription, the amount of TAGs produced during freezing was higher in LTM than in SAD12 (Fig. 6). The reported freezing-induced TAG production in Arabidopsis has been linked to the activity of SFR2 on plastidial galactolipids, which is a major established determinant of freezing tolerance in Arabidopsis (Warren et al., 1996; Thorlby et al., 2004; Moellering et al., 2010; Barnes et al., 2016). This raised the question whether TAG accumulation in B. stricta seedlings could be similarly coupled to galactolipid metabolism. To address this, lipid profiling was performed, including not only TAG but a comprehensive membrane glycerolipidome. Freezing-induced TAG species were suggested to be predominantly derived from MGDG-derived lipid precursors (Fig. 8). The simultaneous decreases in MGDG and increases in DGDG, as well as TGDG and TeGDG, implicated SFR2-like galactosyltransferase activity potentially supplying DAG substrate to DGAT1 (Figs. 4 and 6). Quantitative analysis showed that the magnitude of these changes was greater in LTM than in SAD12, consistent with a potential link to the generation of freezing tolerance. Also, eukaryotic TAG molecular species accumulated under freezing stress, reaching higher levels in LTM than in SAD12 (Fig. 7; Supplemental Table S5). In Arabidopsis, freezing-induced TAGs appear to be derived from mixed metabolic origin, partly from SFR2-generated plastidial DAGs and partly, independent of SFR2, from eukaryotic lipid precursors, likely PC (Moellering et al., 2010; Barnes et al., 2016). These results suggest that DAG acylation could be independently upregulated at freezing temperature. While the higher levels of oligogalactolipids and TAGs in the LTM freezing response could confer ecotype-dependent differences in freezing tolerance, investigation of other lipids not only after freezing but also in response to cold acclimation provided evidence for additional variation in regulation of lipid fluxes potentially associated with acquisition of freezing tolerance.
During Cold Acclimation, B. stricta LTM Accumulates More TAG from PC and SAD12 Accumulates More TAG from MGDG
Whereas during cold acclimation total TAG accumulated to similar levels in both ecotypes, there were differences in several distinct groups of molecular species. The predominant eukaryotic TAGs accumulated to higher levels in LTM than in SAD12, similar to freezing (Figs. 7 and 8; Supplemental Table S5), suggesting conversion from PC. PC is a pivotal intermediate in glycerolipid biosynthesis as it provides precursors for eukaryotic MGDG synthesis in the plastid as well as desaturated fatty acids for de novo synthesis at the ER (Li-Beisson et al., 2010). As low temperature inhibits growth, the demand for new membrane lipid is reduced, and TAGs may be synthesized from excess lipid precursors normally utilized for biosynthesis of MGDG and other glycerolipids. The higher amounts of several of these TAG species in LTM suggested a more efficient conversion compared to SAD12. Consistently, only SAD12 displayed a concomitant accumulation of the major 18:3/18:2 PC molecular species, which in Arabidopsis is considered precursor in eukaryotic MGDG biosynthesis and tends to increase at low temperature (Fig. 5; Somerville and Browse, 1991; Wang et al., 2014; Li et al., 2015). Notably, LPC accumulated during acclimation, which is metabolically linked to PC via an acyl editing cycle (Fig. 4; Bates et al., 2012).
Whereas LTM showed higher eukaryotic TAGs during cold acclimation, TAGs in SAD12 were enriched in 16:3 (Fig. 8), and concomitant accumulation of TGDG and TeGDG indicated SFR2 activity (Fig. 6). The unexpected cold-induced activation of SFR2 and associated acyltransferase activity in B. stricta SAD12 seedlings could therefore be a sign of more sensitive membranes as compared to LTM. This concurs with recent suggestions that SFR2 activity under nonfreezing conditions does not always promote stress tolerance (Wang et al., 2016; Mueller et al., 2017) and could reflect accidental activation by membrane leakiness (Barnes et al., 2016).
During Cold Acclimation, B. stricta LTM Seedlings Accumulate More TAG from Nascent Fatty Acyl Chains
In addition to more highly unsaturated molecular species, 18:1-containing TAGs accumulated during cold acclimation (Fig. 7; Supplemental Table S5), which likely resulted from acyltransferase activity using nascent fatty acyl-CoA emerging from the chloroplast with 18:1-CoA being the major exported molecular species (Ohlrogge and Browse, 1995; Bates, 2016). This subpool also increased more in LTM than in SAD12 (Figs. 7 and 8), another indication of differentially organized TAG metabolism at low temperature, possibly reflecting higher activity of DGAT1 in LTM. Consistently, whereas in different plant species the alternative major acyltransferase PHOSPHOLIPID:DIACYLGLYCEROL ACYLTRANSFERASE1 (PDAT1) uses more unsaturated acyl chains from PC for DAG acylation at the ER (Ståhl et al., 2004; Fan et al., 2013), type I DGAT readily uses 18:1-CoA as a fatty acyl substrate (Zhou et al., 2013; Aznar-Moreno et al., 2015) and has been localized to the chloroplast envelope (Kaup et al., 2002) where newly formed fatty acyl chains exit. A putative function of DGAT1 in channeling of nascent fatty acyl chains to TAG bypasses PC metabolism and could aid in preventing lipid overload of the PC editing cycle. Notably, unlike all other TAG molecular species, 18:1-TAGs only increased upon acclimation but not during the freezing phase (Figs. 7 and 8). This is consistent with their formation from newly synthesized acyl-CoA since de novo fatty acid synthesis is known to be dependent on photosynthesis and thus arrested at dark during freezing conditions (Ohlrogge and Jaworski, 1997; Rawsthorne, 2002). Interestingly, a recent detailed study of B. napus DGAT1 suggests a mechanism for direct modulation of activity by acyl-CoA levels (Caldo et al., 2017).
Direct channeling of nascent acyl chains to TAG may have additional benefits in photosynthetic acclimation. Low temperature can cause formation of reactive oxygen species and photoinhibition as the source-sink balance between absorbed light energy and carbon metabolism is disturbed (Huner et al., 1998). Plants have evolved adaptive strategies to prevent this, e.g. by restoring sink capacity through up-regulation of the Calvin cycle (Stitt and Hurry, 2002; Hüner et al., 2014; Demmig-Adams et al., 2017). In this light, TAG biosynthesis could represent an alternative sink pathway for excess energy and reduced carbon, as was suggested for nutrient-deprived algae (Li et al., 2012; Goold et al., 2015; Zienkiewicz et al., 2016). Notably, the regulation of TAG metabolism at low temperature in a perennial species such as B. stricta is likely to differ from annual plant species, since it is governed by different trade-offs between allocation of resources, particularly carbon, into growth and acclimation pathways, and vegetative versus reproductive development (Anderson et al., 2014; Wingler, 2015).
Based on the obtained results from this study, a working model was made highlighting ecotypic lipid differences that are suggested to link DGAT1 function with freezing tolerance (Fig. 10). Freezing tolerance of the two B. stricta ecotypes was positively associated with transcriptional induction of BstDGAT1 during cold acclimation, and several aspects of TAG accumulation and freezing-induced biosynthesis of oligogalactolipids. Notably, in unbiased comprehensive lipidomics analysis, TAG molecular species stood out among all lipids in genotype-by-treatment interactions of the highest-ranking significance, complementing the identification of BstDGAT1 by QTL analysis, and in keeping with DGAT1’s proposed adaptive function (Supplemental Table S6). In SAD12 seedlings, TAG acylation may be limiting, and several unique observations could be diagnostic of their associated higher sensitivity: Cold treatment was sufficient to induce accumulation of 16:3-TAG, TGDG, and TeGDG, and freezing appeared to induce more PC hydrolysis to PA, likely reflecting phospholipase D (PLD) activity. A similar inverse correlation of freezing tolerance and apparent PLD activity was found in Arabidopsis leaves, where freezing-induced PLDα1 activity accounted for half of the PA rise, which negatively associated with freezing tolerance (Welti et al., 2002).
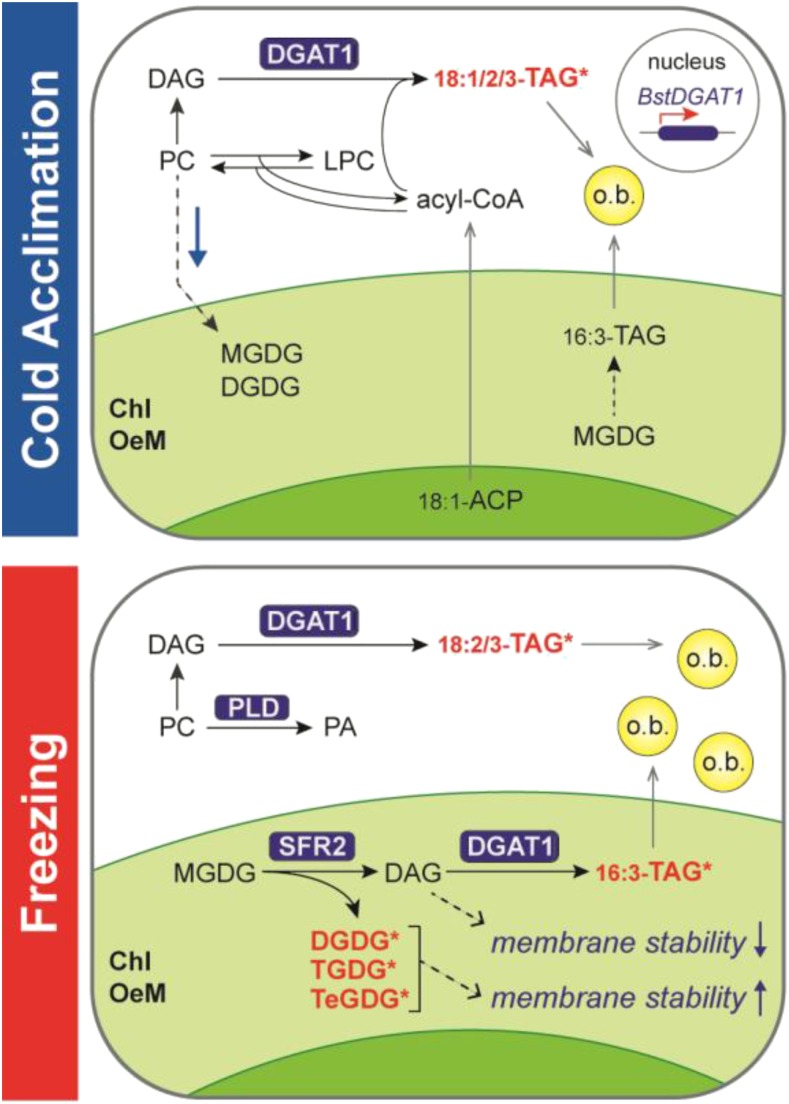
Figure 10.
Model of DGAT1 function in B. stricta seedlings in response to cold acclimation and subsequent freezing. During cold acclimation (top panel), BstDGAT1 is transcriptionally upregulated. As low temperature reduces the requirement for eukaryotic galactolipid biosynthesis (blue arrow), excess PC is converted to DAG and subsequently acylated to 18:1-, 18:2-, and 18:3-rich molecular species of TAG. Simultaneously, turnover of MGDG in the chloroplast results in accumulation of low amounts of 16:3-containing, chloroplastic TAG. In contrast, in response to freezing (bottom panel), high amounts of 16:3-TAG accumulate, together with oligogalactolipids DGDG, TGDG, and TeGDG, suggesting concomitant activation of SFR2. DGAT1, which is most likely coresident with SFR2 in the chloroplast outer envelope membrane (Chl OeM), is suggested to use SFR2-generated DAG as acylation substrate and promote chloroplast membrane resilience during freezing by removing membrane destabilizing DAG molecules, while allowing SFR2-mediated synthesis of membrane-stabilizing DGDG, TGDG, and TeGDG. Our study of natural variation in B. stricta ecotypes has identified BstDGAT1 as a candidate QTL locus for freezing survival and found firm positive associations of freezing tolerance with acclimation-induced BstDGAT1 expression and accumulation of TAGs and oligogalactolipids during freezing. The associated differential activities of DGAT1 and SFR2 support their proposed functional link underlying an adaptive mechanism of freezing tolerance. Freezing also induces PC hydrolysis to PA, presumably by PLD activity, which correlates negatively with freezing tolerance in B. stricta ecotypes. DAG acylation reactions indicated outside the chloroplast are localized to the ER or to the chloroplast outer envelope membrane. TAGs are contained in cytoplasmic oil bodies (o.b.). Lipids with significant genotype-by-treatment interactions that positively associate with freezing tolerance are indicated in red and by asterisks. (L)PC, (lyso)phosphatidylcholine; ACP, acyl carrier protein.
Potential Function of DGAT1 and TAG Accumulation in SFR2-Mediated Freezing Tolerance of B. stricta Seedlings
SFR2 was discovered in Arabidopsis as an essential component of acclimated freezing tolerance in photosynthetic tissues converting the main lipid constituent of the chloroplast, MGDG, into oligogalactolipids (Warren et al., 1996; Thorlby et al., 2004; Moellering et al., 2010). The latter are thought to stabilize the membrane bilayer structure by increasing membrane surface hydration and replacing the non-bilayer-prone lipid MGDG (Moellering et al., 2010; Moellering and Benning, 2011). However, SFR2 also produces DAG, which has the potential to counteract these effects, since it favors nonbilayer structures and decreases surface hydration (Goñi and Alonso, 1999). Thus, for SFR2 to exert its membrane protective effects, its activity must be coupled to an enzyme activity that removes DAG. We propose such a function for DAG acylation by DGAT1, which is expected to be coresident with SFR2 at the chloroplast envelope (Kaup et al., 2002; Fourrier et al., 2008). The correlated differential transcriptional induction of BstDGAT1 and subsequent generation of plastidial type TAGs and oligogalactolipids under freezing stress in the two B. stricta ecotypes suggested that DGAT1 activity could be required for and limiting to the SFR2 pathway and thus underlie the corresponding ecotypic freezing tolerance levels. The link of DGAT1 with this pivotal determinant of freezing tolerance would fit with the large effect expected from the QTL-underlying gene. Interestingly, a recent study suggested a similar function for PDAT1-mediated TAG biosynthesis in heat-stressed Arabidopsis (Mueller et al., 2017).
Based on DGAT1 transcriptional induction during cold acclimation, the model could explain why SFR2 imparts freezing tolerance only in cold acclimated plants even though SFR2 itself is not transcriptionally induced or activated at chilling temperature in Arabidopsis (Thorlby et al., 2004; Moellering et al., 2010). Also, the previous finding of functional SFR2 alleles in chill- and freezing-sensitive plant species suggests the requirement of an additional component for freezing tolerance (Fourrier et al., 2008). While SFR2 functions in other stresses in other species remain to be elucidated (Gasulla et al., 2013; Wang et al., 2016; Mueller et al., 2017), we suggest that in freezing-tolerant plant species, SFR2 could collaborate with DGAT1 to protect chloroplasts under freezing stress. In this light, the potential exploitation of SFR2-mediated freezing tolerance for generating resistant crops has to take into account that merely engineering high SFR2 activity is expected to be detrimental rather than beneficial, but an approach based on genetic enhancement of DAG acylation through DGAT1 could be fruitful. This is supported by our finding that overexpression of AtDGAT1 in Arabidopsis increased seedling freezing tolerance (Fig. 9).
CONCLUSION
Two adaptive responses in B. stricta, namely, redirection of PC and fatty acyl chains to TAG biosynthesis during cold acclimation and SFR2-dependent chloroplast membrane remodeling during freezing, may be supported by the DAG acyltransferase activity of DGAT1. It cannot be concluded whether the TAG accumulations and other lipid changes seen during the acclimation phase merely reflected aspects of metabolic adjustments to low temperature or represented mechanisms that influence freezing survival. However, lipidome analysis after freezing provided evidence for a function for DGAT1 in SFR2-dependent freezing tolerance. The suggested positive contribution was further supported by DGAT1 overexpression in Arabidopsis (Fig. 9; Supplemental Fig. S4). Besides functions for TAG biosynthesis in decreasing membrane lipid and preventing adverse lipid accumulations, it could act in metabolic acclimation to enhance sink capacity under cold stress and give rise to lipid droplets as potential sources of energy and lipid precursors. Lipid droplets with associated proteins are emerging as organelles that are required for fatty acid respiration and could have a myriad of specialized adaptive functions under environmental stress (Aubert et al., 2010; Gidda et al., 2016; Kim et al., 2016; Pyc et al., 2017).
While this manuscript was under review, the article by Tan et al. (2018) was published, providing complementary evidence for a function of DGAT1 in the freezing response of Arabidopsis. They found that dgat1 mutants accumulate less TAG in response to freezing and are less tolerant, complementary to our results showing increased tolerance of DGAT1 overexpressor plants and a similar association in B. stricta ecotypes between BstDGAT1 expression, TAG accumulation, and freezing tolerance. Interestingly, the activity of several diacylglycerol kinase isozymes was implicated in the mutants to convert excess DAG into PA, which induced reactive oxygen species formation (Tan et al., 2018). Also, B. stricta SAD12 seedlings showed not only lower TAG but higher levels of PA molecular species, suggesting that a similar mechanism could contribute to their lower freezing tolerance. The combined data strongly support a function of DGAT1 to impart freezing tolerance through regulation of DAG and PA turnover as well as chloroplastic galactolipid remodeling, while our study adds the important notion that this most likely contributes to freezing tolerance in natural plant populations.
The previously found tight association, based on the same B. stricta RIL population, of freezing tolerance in adult plants with photosynthetic performance parameters suggests that freezing tolerance could be genetically or mechanically linked to prevention of photoinhibition (Heo et al., 2014). Interestingly, although in this study we identified a different QTL, SKI, exclusive for freezing tolerance of seedlings, our data also point at a key role for chloroplast resilience in freezing tolerance, and we suggest an underlying mechanistic model. Importantly, the SKI locus has also been identified as a QTL for juvenile winter survival in B. stricta under field conditions, underscoring its potential adaptive significance (Anderson et al., 2014). Conclusive evidence of BstDGAT1 genetic variation underlying the trait variation will require reciprocal allele transformation and fine mapping. The presented data introduce DGAT1 as an element of acclimated freezing tolerance in plants. Further elucidation of the mechanism by which DGAT1 confers tolerance will be interesting and may provide useful knowledge in a climate of frequent temperature extremes.
MATERIALS AND METHODS
Plant Material
For the QTL experiment, 108 F6 RILs of Boechera stricta were used. The parental lines used throughout this study were collected in Gunninson County, Colorado (ecotype SAD12), and Lemhi County, Idaho (ecotype LTM, ‘Lost Trail Meadow’; Schranz et al., 2005, 2007). In Arabidopsis (Arabidopsis thaliana) experiments, Col-0 was the wild type.
Transformation of Arabidopsis
For the AtDGAT1 construct used for transformation, the coding sequence of AtDGAT1 fused to the 35S promoter was cloned into a DsRed vector with a BAR-selectable marker. The construct was transformed into Arabidopsis Col-0 plants by Agrobacterium tumefaciens-mediated transformation. For tolerance assays, homozygous plants from four transgenic lines, C7-8, E5-13, C7-2, and E5-4, renamed AtDGAT1-OE1, -OE2, -OE3, and -OE4, respectively, were selected by DsRed2 visual marker.
RNA Extraction and RT-qPCR of Arabidopsis
To confirm transgene expression, RT-qPCR was performed using tissue samples from rosette leaves taken from 3-week-old Arabidopsis plants grown in soil (Supplemental Table S7). Total RNA was extracted using a RNeasy Plant Mini Kit (Qiagen) and used for cDNA synthesis with the QuantiTect Reverse Transcription Kit (Qiagen). Briefly, ∼300 to 400 ng RNA was first treated to remove genomic DNA contamination for 5 min at 42°C, followed by reverse transcription at 42°C for 30 min and 3 min deactivation at 95°C. The cDNA was then diluted 10-fold and used as a PCR template. Transcript levels of DGAT1 were measured using the LightCycler480 (Roche) and the SYBR Green I Master Kit (Roche) following the manufacturer’s protocols. The analysis was performed twice for each sample with biological replicates of three independent plant samples. The PROTEIN PHOSPHATASE 2A SUBUNIT A3 gene (AT1G13320, GenBank accession NM_101203) was used as an endogenous control to normalize the RNA level for each sample. PCR primers for DGAT1 (amplicon size 86 bp) are as follows: forward, CATGAGCTATGCATCGCAGT; reverse, ATGAAGACCAAAGGCACCTG. Primers for PP2AA3 (amplicon size 61 bp) are as follows: forward, TAACGTGGCCAAAATGATGC; reverse, GTTCTCCACAACCGCTTGGT.
Growth Conditions
Surface-sterilized B. stricta seeds were sown on petri dishes (30 seeds per plate), containing 0.8% (w/v) agar (Daishin agar; Duchefa Biochemie)-solidified 0.5× Murashige and Skoog medium. To ensure uniform germination, plates were kept in the dark at 4°C for 14 d before transfer to a growth chamber (Microclima 1000E; Snijders Labs) programmed with a 14-h-light/10-h-dark cycle with irradiant photon flux 100 µmol m−2 s−1, and a 20°C/16°C temperature regime. For cold acclimation of B. stricta, 18-d-old seedlings were subjected to 4°C at 14 h light/10 h dark for 48 h, while control plants were kept at standard growth conditions. Freezing treatment was imposed by lowering the temperature from 4°C to −1°C at 1.25°C h−1. After 1 h at −1°C, ice crystals were sprinkled on the plates to induce freezing and prevent supercooling. Subsequently, temperature was decreased over a 4-h interval to the final temperature, which was maintained for 6 h. For survival assays of parental and RIL lines, seedlings were thawed for 12 h in the dark at 4°C and then placed back in the standard growth regime. Survival was visually scored 10 d after freezing. Median lethal temperature (LT50) was estimated from fitted response curves and calculated in R (procedure: http://lukemiller.org/index.php/2010/02/calculating-lt50-median-lethal-temperature-aka-ld50-quickly-in-r/). The survival response curve was used to select the target temperature of −8°C for QTL and biochemical assays. For freezing survival assays of Arabidopsis lines, surface-sterilized seeds were sown on agar plates containing 1% (w/v) agar, 0.5× Murashige and Skoog, 0.5% Suc, and 0.1% MES (pH 5.8) and stratified for 3 d at 4°C, and then grown for 10 to 14 d at 20°C in continuous light at 40 µmol m−2 s−1; plants were acclimated for 4 d at 4°C. After freezing to the indicated temperatures, plates were thawed at 4°C in the dark for 14 h and then returned to the original growth conditions for recovery. After 3 d, survival rate was calculated per plate as percentage of well-developed seedlings scored before acclimation (40–50 seedlings per plate) showing green leaves after freezing treatment. Each genotype was tested with three to four replicates in two to three independent experiments.
QTL Analysis
Seedling freezing tolerance at −8°C was assessed for 108 randomly selected RILs. A total of 150 seeds for each RIL was put on five agar plates, and survival rates were measured from germinated seedlings (Supplemental Table S1). Average seedling survival for RILs was used to map QTLs using a set of 196 mapped markers on the B. stricta genetic map (Schranz et al., 2007). QTLs were detected by composite interval mapping with a 1-cM step size using a 10-cM window and five background cofactors selected via a forward and backward stepwise regression method using WinQTL Cartographer v2.5. A significance threshold value (LOD score) corresponding to a genome-wide significance of α = 0.05 was used for QTL detection and was determined using permutation tests with 1000 replicates. A single QTL was found on LG5. To exclude chromosomal rearrangements that could interfere with the procedure, the Arabidopsis and B. stricta corresponding regions were investigated, showing a long contig (scaffold 18351) being collinear between the two species (Supplemental Table S8). Synteny was assessed by alignment of the regions by a comparative genomics web tool (genomevolution.org; Supplemental Fig. S5). Using the established comparative genomic framework between Arabidopsis and B. stricta (Schranz et al., 2007), 19 out of a total of 126 genes in the region were selected as candidates (Supplemental Table S2). Selection criteria were functional annotations and Arabidopsis gene expression patterns during cold treatment using eFP browser databases.
Isolation of the DGAT1 Candidate Gene and Expression Pattern Analysis in B. stricta
Genomic DNA was isolated from LTM and SAD12 using the DNeasy plant mini kit (Qiagen), and PCR products were purified using the GeneJET PCR purification kit according to the manufacturer’s instructions (Thermo Scientific). PCR primer pairs for DGAT1 were designed using the Primer3 software using data from B. stricta genomic scaffold sequences obtained from the Mitchell-Olds laboratory at Duke University. PCR conditions were optimized for each template-primer system. DNA sequencing was conducted by GATC Biotech in Germany. Based on obtained nucleotide sequences in two B. stricta genotypes, coding and peptide sequences of the LTM and SAD12 DGAT1 alleles were predicted by GeneMark (opal.biology.gatech.edu). Predicted coding sequences were utilized to design primers for gene expression. Analysis of RNA samples from leaves was done for LTM and SAD12 parental genotypes exposed to 3, 8, 12, 24, and 48 h of cold treatment (n = 3). Total RNA was isolated with the RNeasy plant mini kit (Qiagen) according to the manufacturer’s instructions. First, DNA was digested during the preparation by DNase I and the RNA was eluted in RNase-free water. The quality and concentration were measured with a Nano-drop, and then cDNA was synthesized with oligo d(T)18 primer and SuperScript III Reverse Transcriptase (Life Technologies) from 5 μg of total RNA. One microliter of cDNA template was amplified using the Platinum SYBR Green qPCR supermix-UDG (Invitrogen) in a 20-μL qPCR reaction according to the manufacturer’s protocol with gene-specific primers for DGAT1 (forward, AGGTTTGGCTCAACGGTATG; reverse, GAAGATCATGTTGCCCACCT). Relative expression values were calculated by the ΔΔCt method using ACTIN2 as a control.
Analysis of BstDGAT1 Potential cis-Regulatory Elements
To analyze the 4-kb regions upstream of SAD12 and LTM BstDGAT1, a motif search was performed using two programs: PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/; Lescot et al., 2002) and NewPLACE (https://sogo.dna.affrc.go.jp/cgi-bin/sogo.cgi?lang=en&pj=640&action=page&page=newplace). NewPLACE was rewritten by Akio Miyao based on the original SIGNAL SCAN (Prestridge, 1991).
Lipid Analysis
For each lipid extraction 8 to 12 seedlings were used, and five to six replicates were conducted per condition and genotype. The aboveground parts of control, cold-acclimated, and cold-acclimated-freezing-treated seedlings were cut off and immediately immersed in 3 mL isopropanol with 0.01% butylated hydroxytoluene at 75°C. After 15 min, 1.5 mL chloroform and 0.6 mL water were added, followed by agitation at 19°C for 1 h. Subsequently, the remaining plant material was extracted three more times with 4 mL chloroform/methanol (2:1, by volume), and the combined extracts were evaporated in a heated (45°C) vacuum concentrator. Dried lipids were redissolved in chloroform and transferred to 1.5 mL vials for MS analysis of lipid molecular species. The nonlipid residues were dried at 110°C overnight and weighed to obtain a DW proxy of extracted plant material.
Quantitative profiling of polar lipids and triacylglycerols TAG by ESI-MS was conducted at the KS Lipidomics Research Center (Manhattan, KS) as described elsewhere (Welti et al., 2002). Triacylglycerol structures were identified by ESI-MS/MS with a triple quadrupole MS in multiple neutral loss scan modes, and quantification was based on standard curves for response adjustments of individual TAG molecular species (Li et al., 2014).
Statistical Procedures
Lipidome data analysis was performed in R. A linear model was fit to the raw data of each lipid molecular species. Next, the residuals were checked for normality by performing a Shapiro-Wilk test, and traits that did not follow a normal distribution were transformed using a Box-Cox transformation (Supplemental Table S9). A new linear model was then fit to the transformed data. All traits, after transformation if applicable, were also visually checked for normality with a QQ plot on the residuals. Next, an ANOVA was performed to determine whether a significant interaction of genotype and treatment was present (P < 0.05). If not, the interaction was removed from the model and simple genotype and treatment effects were determined. Either significant interaction or simple effects were then analyzed with a Tukey posthoc multiple comparison test, provided by the multcomp package. For comparison of the distribution of different types of TAG lipids (Fig. 8), a χ2 test was used to determine significant differences in distribution between treatment and genotypes.
Accession Numbers
BstDGAT1 sequence data can be retrieved under Bostr.18351s0404 at the Phytozome 12 plant genomics resource (https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Bstricta). Arabidopsis sequence data can be found in the Arabidopsis Genome Initiative database under the following accession numbers: DGAT1 (AT2G19450), PDAT1 (AT5G13640), and SFR2 (AT3G06510).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. BstDGAT1 alleles of B. stricta SAD12 and LTM ecotypes.
Supplemental Figure S2. DGAT1 amino acid sequences of B. stricta SAD12 and LTM ecotypes.
Supplemental Figure S3. Geographical distribution of DGAT1 nonsynonymous coding SNP and polymorphic upstream sequence motifs in populations of B. stricta.
Supplemental Figure S4. Freezing tolerance of Arabidopsis AtDGAT1 overexpressor lines.
Supplemental Figure S5. Genomic synteny alignment of the Arabidopsis and Boechera stricta DGAT1-containing regions.
Supplemental Table S1. RIL freezing survival data for QTL analysis.
Supplemental Table S2. Candidate gene selection for the SKI seedling freezing tolerance QTL.
Supplemental Table S3. Potential BstDGAT1 cis-regulatory elements specific for B. stricta LTM or SAD12 ecotypes.
Supplemental Table S4. Glycerolipid molecular species compositions of B. stricta seedlings in response to cold acclimation and freezing treatment.
Supplemental Table S5. TAG molecular species compositions of control, cold acclimation, and acclimation-freezing-treated B. stricta SAD12 and LTM seedlings.
Supplemental Table S6. B. stricta glycerolipid species and classes ranked by significance of genotype-by-treatment interaction.
Supplemental Table S7. Average relative expression of AtDGAT1 in Arabidopsis 35S-AtDGAT1 lines.
Supplemental Table S8. Syntenic DGAT1-containing regions of Arabidopsis and B. stricta.
Supplemental Table S9. Lipids transformed by Box-Cox transformation.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Dongsheng Feng and Dwight Tomes of DuPont Pioneer for their valuable input on the project. We also thank Xavi Zarza and Chris van Schie for critical reading and helpful suggestions. The lipid profile data were acquired at Kansas Lipidomics Research Center.
Footnotes
Research by J.-Y.H. was supported by DuPont Pioneer. I.K. and C.T. are supported by the Netherlands Organization for Scientific Research ALW Graduate Program Grant 83115004. B.W. was supported by the Swedish Research Council. T.M.-O. was supported by the National Institutes of Health (NIH; Grant R01 GM086496). Instrument acquisition and method development at the Kansas Lipidomics Research Center was supported by National Science Foundation (NSF) grants MCB 0455318, MCB 0920663, DBI 0521587, and DBI 1228622, Kansas INBRE (NIH Grant P20 RR16475 from the INBRE program of the National Center for Research Resources), NSF EPSCoR Grant EPS-0236913, Kansas Technology Enterprise Corporation, and Kansas State University.
Articles can be viewed without a subscription.
References
- Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK (2006) A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem 281: 37636–37645 [DOI] [PubMed] [Google Scholar]
- Alonso-Blanco C, Gomez-Mena C, Llorente F, Koornneef M, Salinas J, Martínez-Zapater JM (2005) Genetic and molecular analyses of natural variation indicate CBF2 as a candidate gene for underlying a freezing tolerance quantitative trait locus in Arabidopsis. Plant Physiol 139: 1304–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT, Lee CR, Mitchell-Olds T (2011) Life-history QTLS and natural selection on flowering time in Boechera stricta, a perennial relative of Arabidopsis. Evolution 65: 771–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT, Inouye DW, McKinney AM, Colautti RI, Mitchell-Olds T (2012) Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc Biol Sci 279: 3843–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT, Lee CR, Mitchell-Olds T (2014) Strong selection genome-wide enhances fitness trade-offs across environments and episodes of selection. Evolution 68: 16–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert Y, Vile D, Pervent M, Aldon D, Ranty B, Simonneau T, Vavasseur A, Galaud JP (2010) RD20, a stress-inducible caleosin, participates in stomatal control, transpiration and drought tolerance in Arabidopsis thaliana. Plant Cell Physiol 51: 1975–1987 [DOI] [PubMed] [Google Scholar]
- Aznar-Moreno J, Denolf P, Van Audenhove K, De Bodt S, Engelen S, Fahy D, Wallis JG, Browse J (2015) Type 1 diacylglycerol acyltransferases of Brassica napus preferentially incorporate oleic acid into triacylglycerol. J Exp Bot 66: 6497–6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AC, Benning C, Roston R (2016) Chloroplast membrane remodeling during freezing stress is accompanied by cytoplasmic acidification activating SENSITIVE TO FREEZING 2. Plant Physiol 171: 2140–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD. (2016) Understanding the control of acyl flux through the lipid metabolic network of plant oil biosynthesis. Biochim Biophys Acta 1861: 1214–1225 [DOI] [PubMed] [Google Scholar]
- Bates PD, Fatihi A, Snapp AR, Carlsson AS, Browse J, Lu C (2012) Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol 160: 1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldo KMP, Acedo JZ, Panigrahi R, Vederas JC, Weselake RJ, Lemieux MJ (2017) Diacylglycerol acyltransferase 1 is regulated by its N-terminal domain in response to allosteric effectors. Plant Physiol 175: 667–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Thelen JJ (2013) ACYL-LIPID DESATURASE2 is required for chilling and freezing tolerance in Arabidopsis. Plant Cell 25: 1430–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NC, Tardieu F, Tuberosa R (2008) Quantitative trait loci and crop performance under abiotic stress: where do we stand? Plant Physiol 147: 469–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenkolbe T, Giavalisco P, Zuther E, Seiwert B, Hincha DK, Willmitzer L (2012) Differential remodeling of the lipidome during cold acclimation in natural accessions of Arabidopsis thaliana. Plant J 72: 972–982 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Stewart JJ, Adams WW III (2017) Environmental regulation of intrinsic photosynthetic capacity: an integrated view. Curr Opin Plant Biol 37: 34–41 [DOI] [PubMed] [Google Scholar]
- Fan J, Yan C, Zhang X, Xu C (2013) Dual role for phospholipid:diacylglycerol acyltransferase: enhancing fatty acid synthesis and diverting fatty acids from membrane lipids to triacylglycerol in Arabidopsis leaves. Plant Cell 25: 3506–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Yu L, Xu C (2017) A central role for triacylglycerol in membrane lipid breakdown, fatty acid β-oxidation, and plant survival under extended darkness. Plant Physiol 174: 1517–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourrier N, Bédard J, Lopez-Juez E, Barbrook A, Bowyer J, Jarvis P, Warren G, Thorlby G (2008) A role for SENSITIVE TO FREEZING2 in protecting chloroplasts against freeze-induced damage in Arabidopsis. Plant J 55: 734–745 [DOI] [PubMed] [Google Scholar]
- Gasulla F, Vom Dorp K, Dombrink I, Zähringer U, Gisch N, Dörmann P, Bartels D (2013) The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: a comparative approach. Plant J 75: 726–741 [DOI] [PubMed] [Google Scholar]
- Gehan MA, Park S, Gilmour SJ, An C, Lee CM, Thomashow MF (2015) Natural variation in the C-repeat binding factor cold response pathway correlates with local adaptation of Arabidopsis ecotypes. Plant J 84: 682–693 [DOI] [PubMed] [Google Scholar]
- Gidda SK, Park S, Pyc M, Yurchenko O, Cai Y, Wu P, Andrews DW, Chapman KD, Dyer JM, Mullen RT (2016) Lipid droplet-associated proteins (LDAPs) are required for the dynamic regulation of neutral lipid compartmentation in plant cells. Plant Physiol 170: 2052–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goñi FM, Alonso A (1999) Structure and functional properties of diacylglycerols in membranes. Prog Lipid Res 38: 1–48 [DOI] [PubMed] [Google Scholar]
- Goold H, Beisson F, Peltier G, Li-Beisson Y (2015) Microalgal lipid droplets: composition, diversity, biogenesis and functions. Plant Cell Rep 34: 545–555 [DOI] [PubMed] [Google Scholar]
- Hannah MA, Wiese D, Freund S, Fiehn O, Heyer AG, Hincha DK (2006) Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol 142: 98–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JY, Feng D, Niu X, Mitchell-Olds T, Van Tienderen PH, Tomes D, Schranz ME (2014) Identification of quantitative trait loci and a candidate locus for freezing tolerance in controlled and outdoor environments in the overwintering crucifer Boechera stricta. Plant Cell Environ 37: 2459–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JY, Van Tienderen PH, Schranz ME (2018) Cloning and functional analysis of three cold regulated CBF genes in the overwintering crucifer Boechera stricta. Int J Agric Biol 20: 594–600 [Google Scholar]
- Higashi Y, Okazaki Y, Myouga F, Shinozaki K, Saito K (2015) Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci Rep 5: 10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MW, Willems G, Sasaki E, Koornneef M, Nordborg M (2016) The genetic architecture of freezing tolerance varies across the range of Arabidopsis thaliana. Plant Cell Environ 39: 2570–2579 [DOI] [PubMed] [Google Scholar]
- Huner NPA, Öquist G, Sarhan F (1998) Energy balance and acclimation to light and cold. Trends Plant Sci 3: 224–230 [Google Scholar]
- Hüner NPA, Dahal K, Kurepin LV, Savitch L, Singh J, Ivanov AG, Kane K, Sarhan F (2014) Potential for increased photosynthetic performance and crop productivity in response to climate change: role of CBFs and gibberellic acid. Front Chem 2: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G, Williams JP (1989) Effect of growth temperature on the biosynthesis of chloroplastic galactosyldiacylglycerol molecular species in Brassica napus leaves. Plant Physiol 91: 924–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup MT, Froese CD, Thompson JE (2002) A role for diacylglycerol acyltransferase during leaf senescence. Plant Physiol 129: 1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AA, van Erp H, Quettier AL, Shaw E, Menard G, Kurup S, Eastmond PJ (2013) The sugar-dependent1 lipase limits triacylglycerol accumulation in vegetative tissues of Arabidopsis. Plant Physiol 162: 1282–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D’Angelo C, Bornberg-Bauer E, Kudla J, Harter K (2007) The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50: 347–363 [DOI] [PubMed] [Google Scholar]
- Kim EY, Park KY, Seo YS, Kim WT (2016) Arabidopsis small rubber particle protein homolog SRPs play dual roles as positive factors for tissue growth and development and in drought stress responses. Plant Physiol 170: 2494–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Chen S, Yang Y, An C (2013) ABA-insensitive (ABI) 4 and ABI5 synergistically regulate DGAT1 expression in Arabidopsis seedlings under stress. FEBS Lett 587: 3076–3082 [DOI] [PubMed] [Google Scholar]
- Lee C-R, Mitchell-Olds T (2013) Complex trait divergence contributes to environmental niche differentiation in ecological speciation of Boechera stricta. Mol Ecol 22: 2204–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-R, Wang B, Mojica JP, Mandáková T, Prasad KVSK, Goicoechea JL, Perera N, Hellsten U, Hundley HN, Johnson J, et al. (2017) Young inversion with multiple linked QTLs under selection in a hybrid zone. Nat Ecol Evol 1: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Guo Y, Ohta M, Xiong L, Stevenson B, Zhu JK (2002) LOS2, a genetic locus required for cold-responsive gene transcription encodes a bi-functional enolase. EMBO J 21: 2692–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30: 325–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Baughman E, Roth MR, Han X, Welti R, Wang X (2014) Quantitative profiling and pattern analysis of triacylglycerol species in Arabidopsis seeds by electrospray ionization mass spectrometry. Plant J 77: 160–172 [DOI] [PubMed] [Google Scholar]
- Li Q, Zheng Q, Shen W, Cram D, Fowler DB, Wei Y, Zou J (2015) Understanding the biochemical basis of temperature-induced lipid pathway adjustments in plants. Plant Cell 27: 86–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Shen W, Zheng Q, Fowler DB, Zou J (2016) Adjustments of lipid pathways in plant adaptation to temperature stress. Plant Signal Behav 11: e1058461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Moellering ER, Liu B, Johnny C, Fedewa M, Sears BB, Kuo M-H, Benning C (2012) A galactoglycerolipid lipase is required for triacylglycerol accumulation and survival following nitrogen deprivation in Chlamydomonas reinhardtii. Plant Cell 24: 4670–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, et al. (2010) Acyl-lipid metabolism. Arabidopsis Book 8: e0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Siloto RMP, Lehner R, Stone SJ, Weselake RJ (2012) Acyl-CoA:diacylglycerol acyltransferase: molecular biology, biochemistry and biotechnology. Prog Lipid Res 51: 350–377 [DOI] [PubMed] [Google Scholar]
- Lu C, Hills MJ (2002) Arabidopsis mutants deficient in diacylglycerol acyltransferase display increased sensitivity to abscisic acid, sugars, and osmotic stress during germination and seedling development. Plant Physiol 129: 1352–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung SC, Weselake RJ (2006) Diacylglycerol acyltransferase: a key mediator of plant triacylglycerol synthesis. Lipids 41: 1073–1088 [DOI] [PubMed] [Google Scholar]
- Moellering ER, Benning C (2011) Galactoglycerolipid metabolism under stress: a time for remodeling. Trends Plant Sci 16: 98–107 [DOI] [PubMed] [Google Scholar]
- Moellering ER, Muthan B, Benning C (2010) Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330: 226–228 [DOI] [PubMed] [Google Scholar]
- Mueller SP, Unger M, Guender L, Fekete A, Mueller MJ (2017) Diacylglycerol acyltransferase-mediated triacylglyerol synthesis augments basal thermotolerance. Plant Physiol 175: 486–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novillo F, Medina J, Salinas J (2007) Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc Natl Acad Sci USA 104: 21002–21007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley CG, Ågren J, Atchison RA, Schemske DW (2014) QTL mapping of freezing tolerance: links to fitness and adaptive trade-offs. Mol Ecol 23: 4304–4315 [DOI] [PubMed] [Google Scholar]
- Ohlrogge J, Browse J (1995) Lipid biosynthesis. Plant Cell 7: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Physiol Plant Mol Biol 48: 109–136 [DOI] [PubMed] [Google Scholar]
- Prasad KVSK, Song B-H, Olson-Manning C, Anderson JT, Lee C-R, Schranz ME, Windsor AJ, Clauss MJ, Manzaneda AJ, Naqvi I, et al. (2012) A gain-of-function polymorphism controlling complex traits and fitness in nature. Science 337: 1081–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestridge DS. (1991) SIGNAL SCAN: a computer program that scans DNA sequences for eukaryotic transcriptional elements. Comput Appl Biosci 7: 203–206 [DOI] [PubMed] [Google Scholar]
- Pyc M, Cai Y, Greer MS, Yurchenko O, Chapman KD, Dyer JM, Mullen RT (2017) Turning over a new leaf in lipid droplet biology. Trends Plant Sci 22: 596–609 [DOI] [PubMed] [Google Scholar]
- Rawsthorne S. (2002) Carbon flux and fatty acid synthesis in plants. Prog Lipid Res 41: 182–196 [DOI] [PubMed] [Google Scholar]
- Ruelland E. (2017) Plant responses to chilling temperatures. In Shabala S, ed, Plant Stress Physiology. CABI, Wallingford, Oxfordshire, UK, pp 97–137 [Google Scholar]
- Rushworth CA, Song BH, Lee CR, Mitchell-Olds T (2011) Boechera, a model system for ecological genomics. Mol Ecol 20: 4843–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schranz ME, Dobes C, Koch MA, Mitchell-Olds T (2005) Sexual reproduction, hybridization, apomixis, and polyploidization in the genus Boechera (Brassicaceae). Am J Bot 92: 1797–1810 [DOI] [PubMed] [Google Scholar]
- Schranz ME, Windsor AJ, Song BH, Lawton-Rauh A, Mitchell-Olds T (2007) Comparative genetic mapping in Boechera stricta, a close relative of Arabidopsis. Plant Physiol 144: 286–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schranz ME, Manzaneda AJ, Windsor AJ, Clauss MJ, Mitchell-Olds T (2009) Ecological genomics of Boechera stricta: identification of a QTL controlling the allocation of methionine- vs branched-chain amino acid-derived glucosinolates and levels of insect herbivory. Heredity (Edinb) 102: 465–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C, Browse J (1991) Plant lipids: metabolism, mutants, and membranes. Science 252: 80–87 [DOI] [PubMed] [Google Scholar]
- Ståhl U, Carlsson AS, Lenman M, Dahlqvist A, Huang B, Banaś W, Banaś A, Stymne S (2004) Cloning and functional characterization of a phospholipid:diacylglycerol acyltransferase from Arabidopsis. Plant Physiol 135: 1324–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus PL. (1984) Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol 35: 543–584 [Google Scholar]
- Steponkus PL, Uemura M, Joseph RA, Gilmour SJ, Thomashow MF (1998) Mode of action of the COR15a gene on the freezing tolerance of Arabidopsis thaliana. Proc Natl Acad Sci USA 95: 14570–14575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Hurry V (2002) A plant for all seasons: alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Curr Opin Plant Biol 5: 199–206 [DOI] [PubMed] [Google Scholar]
- Tan W-J, Yang Y-C, Zhou Y, Huang L-P, Xu L, Chen Q-F, Yu L-J, Xiao S (2018) DIACYLGLYCEROL ACYLTRANSFERASE and DIACYLGLYCEROL KINASE modulate triacylglycerol and phosphatidic acid production in the plant response to freezing stress. Plant Physiol 177: 1185–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayeh N, Bahrman N, Devaux R, Bluteau A, Prosperi J-M, Delbreil B, Lejeune-Hénaut I (2013) A high-density genetic map of the Medicago truncatula major freezing tolerance QTL on chromosome 6 reveals colinearity with a QTL related to freezing damage on Pisum sativum linkage group VI. Mol Breed 32: 279–289 [Google Scholar]
- Thomashow MF. (1999) Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50: 571–599 [DOI] [PubMed] [Google Scholar]
- Thorlby G, Fourrier N, Warren G (2004) The SENSITIVE TO FREEZING2 gene, required for freezing tolerance in Arabidopsis thaliana, encodes a β-glucosidase. Plant Cell 16: 2192–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjellström H, Strawsine M, Ohlrogge JB (2015) Tracking synthesis and turnover of triacylglycerol in leaves. J Exp Bot 66: 1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Hersh HL, Benning C (2016) SENSITIVE TO FREEZING2 aides in resilience to salt and drought in freezing-sensitive tomato. Plant Physiol 172: 1432–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kazachkov M, Shen W, Bai M, Wu H, Zou J (2014) Deciphering the roles of Arabidopsis LPCAT and PAH in phosphatidylcholine homeostasis and pathway coordination for chloroplast lipid synthesis. Plant J 80: 965–976 [DOI] [PubMed] [Google Scholar]
- Warren G, McKown R, Marin AL, Teutonico R (1996) Isolation of mutations affecting the development of freezing tolerance in Arabidopsis thaliana (L.) Heynh. Plant Physiol 111: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X (2002) Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J Biol Chem 277: 31994–32002 [DOI] [PubMed] [Google Scholar]
- Weselake RJ, Madhavji M, Szarka SJ, Patterson NA, Wiehler WB, Nykiforuk CL, Burton TL, Boora PS, Mosimann SC, Foroud NA, et al. (2006) Acyl-CoA-binding and self-associating properties of a recombinant 13.3 kDa N-terminal fragment of diacylglycerol acyltransferase-1 from oilseed rape. BMC Biochem 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A. (2015) Comparison of signaling interactions determining annual and perennial plant growth in response to low temperature. Front Plant Sci 5: 794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Browse J (1998) Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc Natl Acad Sci USA 95: 7799–7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yu X, Song L, An C (2011) ABI4 activates DGAT1 expression in Arabidopsis seedlings during nitrogen deficiency. Plant Physiol 156: 873–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Lang Z, Zhu J-K (2015) Cold responsive gene transcription becomes more complex. Trends Plant Sci 20: 466–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Chapman SC, Christopher JT, Frederiks TM, Chenu K (2015) Frost trends and their estimated impact on yield in the Australian wheatbelt. J Exp Bot 66: 3611–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X-R, Shrestha P, Yin F, Petrie JR, Singh SP (2013) AtDGAT2 is a functional acyl-CoA:diacylglycerol acyltransferase and displays different acyl-CoA substrate preferences than AtDGAT1. FEBS Lett 587: 2371–2376 [DOI] [PubMed] [Google Scholar]
- Zienkiewicz K, Du Z-Y, Ma W, Vollheyde K, Benning C (2016) Stress-induced neutral lipid biosynthesis in microalgae – Molecular, cellular and physiological insights. Biochim Biophys Acta 1861: 1269-1281 [DOI] [PubMed] [Google Scholar]
- Zuther E, Juszczak I, Lee YP, Baier M, Hincha DK (2015) Time-dependent deacclimation after cold acclimation in Arabidopsis thaliana accessions. Sci Rep 5: 12199. [DOI] [PMC free article] [PubMed] [Google Scholar]