Abstract
Study Objectives:
To assess effectiveness of a culturally and linguistically tailored telephone-delivered intervention to increase adherence to physician-recommended evaluation and treatment of obstructive sleep apnea (OSA) among blacks.
Methods:
In a two-arm randomized controlled trial, we evaluated effectiveness of the tailored intervention among blacks with metabolic syndrome, relative to those in an attention control arm (n = 380; mean age = 58 ± 13; female = 71%). The intervention was designed to enhance adherence using culturally and linguistically tailored OSA health messages delivered by a trained health educator based on patients’ readiness to change and unique barriers preventing desired behavior changes.
Results:
Analysis showed 69.4% of the patients in the intervention arm attended initial consultation with a sleep specialist, compared to 36.7% in the control arm; 74.7% of those in the intervention arm and 66.7% in the control arm completed diagnostic evaluation; and 86.4% in the intervention arm and 88.9% in the control arm adhered to PAP treatment based on subjective report. Logistic regression analyses adjusting for sociodemographic factors indicated patients in the intervention arm were 3.17 times more likely to attend initial consultation, compared to those in the control arm. Adjusted models revealed no significant differences between the two arms regarding adherence to OSA evaluation or treatment.
Conclusion:
The intervention was successful in promoting importance of sleep consultation and evaluation of OSA among blacks, while there was no significant group difference in laboratory-based evaluation and treatment adherence rates. It seems that the fundamental barrier to OSA care in that population may be the importance of seeking OSA care.
Keywords: race/ethnicity, blacks, adherence, obstructive sleep apnea, positive airway pressure, sleep consultation.
Statement of Significance
This study is the first to use a culturally and linguistically tailored intervention to address OSA evaluation and treatment among blacks by addressing specific challenges that black patients face and employed a systematic approach to deliver tailored messages according to each patient’s stage of readiness. While participants in the intervention arm were more likely to adhere to recommended OSA consultations, there was no significant difference between the two study arms regarding diagnostic OSA evaluations, although trends favored greater adherence among patients in the intervention arm. Our findings suggest that tailored behavioral approaches may be useful in ushering blacks into consultations with a sleep specialist for sleep disturbances.
INTRODUCTION
Obstructive sleep apnea (OSA) is a potentially life-threatening condition that is associated with several markers of cardiovascular disease (CVD) including obesity, hypertension, diabetes, and dyslipidemia.1 OSA is a prime example of a CVD-related disease that can be treated optimally, leading to improvement in hemodynamic functions2–4 and reduction in OSA morbidity.5 Unfortunately, the vast majority of OSA cases among blacks remain undiagnosed, despite alarming evidence that it is more prevalent in blacks compared to other groups.6 Underdiagnosis of OSA among blacks is an important public health problem; given the disproportionately higher burden of CVD burden in blacks compared to other racial/ethnic groups, OSA is a major target for intervention aimed at reduction in adverse OSA-related CVD outcomes.7–10
In the context of personalized medicine, blacks with metabolic syndrome and OSA may constitute an ideal group necessitating tailored behavioral interventions to address their sleep needs, thus preventing negative health outcomes. Data from a previous study by our group showed that few blacks adhered to physician-recommended OSA evaluations,11 leaving uncertain whether behavioral interventions targeted exclusively at improving adherence to OSA therapies8–11 is optimal for blacks. There is a critical gap in understanding modifiable factors preventing blacks from participating in available OSA clinical services. Several factors might underlie nonadherence to recommended OSA care. They include inadequate health-care access, language barriers, preference for care, lack of participation in autonomous decision-making, exposure to OSA health information without appropriate linguistic and cultural tailoring, poor health literacy, and mistrust of the health-care system.12 Whether underdiagnosis could be explained by lack of OSA awareness in that population12–16 or by refusal of recommended OSA care remains unclear. The foregoing evidence argues in favor of an evidence-based strategy to address specific barriers preventing or delaying access to sleep services among blacks with OSA.
In light of data from our previous study suggesting that few black patients with metabolic diseases adhered to physician-recommended OSA evaluations11 along with observations of their dysfunctional beliefs about sleep,12 we have engaged in a systematic effort to delineate factors hindering or enabling adoption of recommended OSA care in that population. These factors, emanating mostly from studies utilizing mixed-methods approaches, have informed the development and implementation of our National Institute of Health-funded randomized clinical trial ascertaining effectiveness of a behavioral intervention to promote adoption of health messages focusing principally on the importance of clinical consultations and laboratory-based OSA evaluations and treatment among blacks with metabolic syndrome.13–21 We employed an intervention strategy designed to increase adherence to recommended sleep consultations and evaluations and to increase adherence to treatment recommendations by addressing barriers that we identified during the formative phase (first 6 months) of the trial.12 The scope of the present paper is limited to the effectiveness of the behavioral intervention itself. Effectiveness of OSA treatment in improving clinical parameters in that population will be reported in a separate paper.
In this study, we evaluated effects of a tailored telephone- delivered intervention (TTI) to enhance adherence to recommended consultation, evaluation, and adherence to Positive Airway Pressure (PAP) treatment among blacks with metabolic syndrome and those at risk for OSA. The main objective of this study was to determine whether blacks exposed to the tailored intervention would exhibit greater likelihood of adhering to recommended OSA consultation, evaluation, and treatment, compared to those receiving only standard OSA literature. We also assessed whether baseline sociodemographics, health risks, medical conditions, and psychosocial factors would predict adherence status using multivariate-adjusted regression analyses.
METHODS
Participants
The primary aim of this randomized controlled trial (RCT) was to assess effectiveness of a culturally and linguistically telephone-delivered treatment intervention (TTI) to increase adherence to physician-recommended evaluation and treatment of OSA among blacks. The study sample was drawn from a well-characterized registry of black participants in the Metabolic Syndrome Outcome Cohort Study (MetSO).12,14 Patients were eligible for the RCT if they met the following criteria: (1) self-reported race/ethnicity as black/African American; (2) ≥18 years old; (3) documented metabolic syndrome from electronic medical records; and (4) have a positive OSA screening as measured by the Apnea Risk Evaluation System (ARES™) questionnaire.17 They were excluded if they met these criteria: (1) documented coexisting sleep problems; (2) presence of illness or disability in which death was expected within 2 years of enrollment; (3) intent to relocate from the New York city area within 1 year following enrollment; (4) non-English speaking; (5) not accessible via telephone; (6) impaired cognitive or functional ability; or (7) another family member participating in the study.
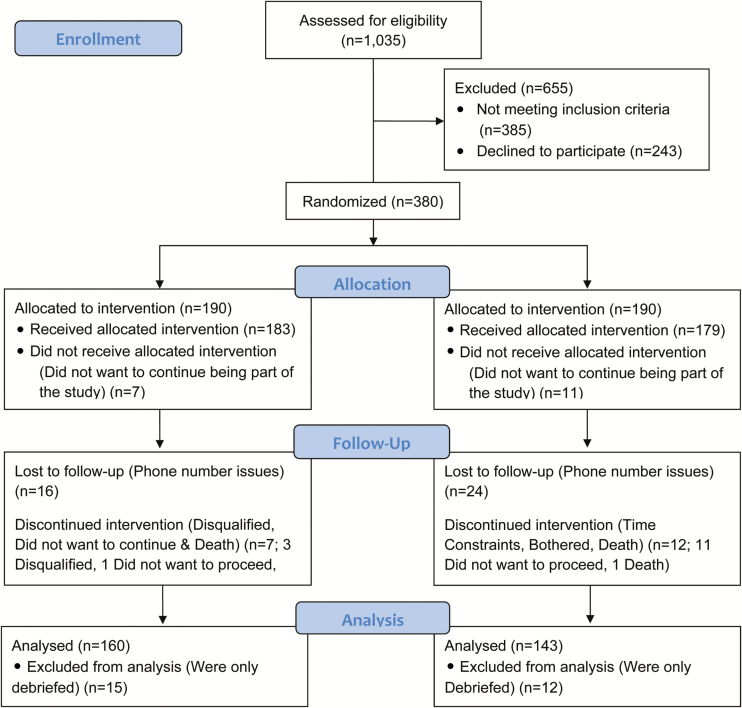
As illustrated in the CONSORT flowchart (Figure 1), a total of 1035 patients were screened for eligibility. Of those, 380 patients were equally randomized to either the intervention arm or the attention control arm. Seven (3.7%) patients from the intervention arm and 11 (5.8%) patients from the control arm withdrew from the trial. In the intervention arm, 23 (12.6%) patients were lost to follow-up; in the control arm, 36 (20.1%) patients were lost to follow-up. A total of 160 patients in the intervention arm and 143 patients in the control arm completed the trial yielding a follow-up rate of 80%. This was a clinic-based study enrolling patients from several local clinics. The institutional review boards at NYU Langone Medical Center (protocol #13-00182) and SUNY Downstate Medical Center (protocol #09-193) approved this study; and ethical standards were adhered to. All participants provided informed consent.
Figure 1—
Metabolic Syndrome Outcome Cohort Study (MetSO) Trial CONSORT flow diagram.
Procedures
From 2010 to 2014, black patients at four community-based clinics in Brooklyn, New York, were approached while attending routine clinical visits to describe the nature of the study and to solicit their participation. Trained study staff targeted only patients who were classified as having the metabolic syndrome using guidelines published by the National Heart, Lung and Blood Institute and the American Heart Association.22 Accordingly, patients were classified as having metabolic syndrome if they had at least three of the following five conditions: fasting glucose ≥100 mg/dL or receiving treatment for hyperglycemia, blood pressure ≥130/85 mm Hg or receiving drug therapy for hypertension, triglycerides ≥150 mg/dL or receiving drug treatment for hypertriglyceridemia, high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL among men or <50 mg/dL among women or receiving drug therapy for HDL-C, and a waist circumference ≥102 cm (40 in.) among men or ≥88 cm (35 in.) among women. Physician-diagnosed conditions were obtained by querying a hospital-based electronic medical record system (Allscripts, Sunrise Enterprise). If patients expressed interest, written consents were obtained and baseline data (sociodemographics, history of sleep problems, medical history, health risk behavior, and psychosocial measures) were gathered to develop the initial MetSO registry.12 Each participant received $10 for providing complete baseline data; those participating in the trial received $100. All patients in the registry received a packet including educational brochures about OSA, produced by the National Heart Lung and Blood Institute: “Sleep Apnea: Is Your Patient at Risk?” and the National Sleep Foundation “Sleep Apnea”.
Baseline Measurements
Patients were asked to complete several baseline questionnaires including the ARES Questionnaire17 to identify those at high OSA risk. The questionnaire solicits demographic and anthropometric data, diseases associated with risk of OSA (ie, hypertension, diabetes, heart disease, or stroke), the Epworth Sleepiness Scale,23 and frequency rating for snoring, waking up, choking, and having been told that patients stopped breathing during sleep. Validation studies showed that ARES has a sensitivity of 0.94, specificity of 0.79 (based on a clinical cutoff of apnea–hypopnea index >5), positive predictive value of 0.91, and negative predictive value of 0.86. Additionally, several other questionnaires were administered including the Medical Outcomes Study Short Form 36,18 the Center for Epidemiological Studies-Depression Scale,19,20 the Apnea Knowledge Test,16 the Self-Efficacy Scale,24 and the Change Assessment Scale.25
Randomization and Enrollment
Patients were eligible for randomization if they provided complete baseline questionnaire data and were at risk of OSA. Using contact information from the MetSO registry, the project coordinator contacted eligible patients for enrollment in the trial. The coordinator made up to five attempts on different days of the week and various times of the day to contact each patient. If initial contact could not be established due to no telephone response (eg, incorrect number, disconnected phone, phone in others’ home, blocked number, etc.) those patients were considered ineligible and, thus, not included into the trial. Randomization was carried out in pairs using a table of random permutations created by the statistician. One member of each pair was assigned to the intervention arm and the other to the attention control arm, using sealed envelopes. Both arms received standard care, which included a referral for a comprehensive OSA evaluation at a sleep clinic as well as standard literature about OSA evaluation and treatment procedures. However, compared to the attention control arm, patients in the intervention arm received tailored behavioral health interventions from a trained sleep health educator (See Table 1).
Table 1.
Description of the Processes of Patient Engagement in the MetSO Tailored Behavioral Intervention.
| MetSO Intervention Implementation (Phase I) |
| • The objective of the first contact was to assess knowledge, attitudes, and behaviors related to sleep and to improve patients’ understanding of OSA and the need for initial consultations and evaluations. Within 2 weeks of enrollment, the educator contacted each patient, providing a brief introduction and reiterating the purpose of the trial. The educator then proceeded to address barriers based on patients’ level of knowledge, perceived risk, and stage of readiness. During phone conversations, threatening, frightening, or judgmental statements were avoided. Rather, the educator emphasized positive reinforcement and enhancement of perceived self-efficacy to ensure desired behavior change. At the end of each call, the educator prompted patients for a verbal commitment to seek OSA care. Once a commitment was made, the educator contacted the sleep clinic to determine whether the patients had a consultation and evaluation and whether treatment has been prescribed if they were diagnosed. |
| • The decision not to adhere to recommended OSA care may be motivated by reasons that are not immediately apparent. Although a patient may have necessary knowledge for positive actions, negative emotions (eg, fear and denial) can influence their decision not to seek care. Thus, such emotions had to be acknowledged and addressed. Individual tailoring relied on an iterative process in which the educator considered patients’ incentives facilitating OSA evaluation and barriers limiting desired behaviors. Thus, the educator attempted to maximize the incentives and overcome identified barriers. Once the educator understood why a patient was not adhering, she endeavored to tailor the TTI to the patient’s stage of readiness, feelings, and ways of reasoning. For some patients, the educator tailored the TTI to increase their OSA knowledge. Others required assistance in overcoming deficits in self-efficacy through coaching and role-playing. Individual tailoring often required patience, particularly when patients had time constraints due to personal/familial challenges. For patients dealing with health problems, the educator was empathetic and understanding. For those beset by fear and denial, social and emotional support was necessary. |
| • For patients who did not keep their appointment, the educator would again attempt to elicit a verbal commitment to schedule one. The educator attempted to identify obstacles and worked jointly with the patients to overcome them. The cycle would be repeated until an initial consultation or evaluation was obtained. If appointments were kept, a positive reinforcing message was provided. For patients who reported that no diagnosis was made, they were congratulated and thanked and the intervention ended at that time; this was verified through chart reviews. |
| MetSO Intervention Implementation (Phase II) |
| • For patients who reported a diagnosis, the educator focused on three main questions: 1) Did the doctor say that you have OSA? 2) What therapy, if any, was suggested? and 3) Were you asked to go back for a follow-up visit? When? Those patients then moved to Phase II, where the focus was on treatment adherence. |
| • The TTI approach during Phase II was similar to Phase I. The main theme was to foster a certain level of comfort and confidence so that patients become accustomed to using positive airway pressure (PAP) therapy. The educator approached OSA as a chronic disease, requiring adequate self-management. Patients who indicated that they had received a diagnosis received a call to discuss the significance of the diagnosis and treatment recommendations. More importantly, the educator determined whether arrangements for such care had been made through the sleep clinic. If follow-up care was obtained, the educator identified obstacles and worked jointly with the patients to formulate strategies for overcoming such obstacles. The educator endeavored to enhance patients’ comfort and confidence using PAP until the end of the 6-month period when outcome ascertainment occurred. |
| • In cases where resistance to treatment was encountered, the educator provided further education on the benefits of PAP therapy including reduced daytime sleepiness, arterial blood desaturations, heart attack and pulmonary pressure, improved cognitive performance, increased quality of well-being, and longer survival. The educator addressed factors known to affect PAP adherence, including mask tolerance, nasal- related complaints, reduced motility as well as potential side effects such as facial skin discomfort, nasal stuffiness, rhinitis, and inability to tolerate the pressure. The educator encouraged patients to discuss the benefits of treating nasal congestion with heated humidification or nasal medications with their doctor, when necessary. The educator also highlighted the gains patients have experienced since initiating PAP. |
OSA, obstructive sleep apnea; TTI, telephone-delivered treatment intervention.
Intervention
Based on our own experience organizing health screening events, very few minority individuals participate in such events, some claiming distrust of the health-care systems, fear of finding that something might be wrong, or lack of awareness of the importance of medical screening examinations.15 The tailored behavioral intervention was designed to address perceived benefits of OSA evaluation and treatment, social factors that encourage or discourage desired behavior, and other facilitating and inhibiting factors derived from focus groups conducted with eligible patients from the MetSO registry. The intervention manual used to train the health educators was presented to our Community Steering Committee for final endorsement, which is essential in patient-engaged research.26–31 The manual included modules presenting rudiments of health intervention, appropriate use of stage-matched approaches, and messages regarding the importance of OSA care to prevent negative health outcomes. The formative phase was necessary to assess OSA barriers via focus groups, enabling tailoring of health messages; this was conducted using patients who were not enrolled in the trial. Final sleep messages were presented to a subset of patients and committee members to ensure that they were culturally sensitive, linguistically appropriate, practical, feasible, and acceptable to black patients. All sleep messages provided by the health educator were phrased in English at a fifth-grade reading level by a professional linguist who is familiar with the Flesch-Kincaid readability scoring system. This model has been utilized successfully in several studies involving minority groups.32–37
Conceptual Framework and Intervention Implementation
The TTI included components from PRECEDE38 and the Transtheoretical Model of Change.39 It was designed to correct misconceptions and address dysfunctional attitudes and beliefs about sleep; to facilitate access to sleep care (consultations, evaluations, and treatments); to enhance social support from existing networks to facilitate behavior change; to advocate the importance of OSA care for high-risk patients; and to provide sleep health messages from a trained sleep health educator of the same racial/ethnic background. The model conceptualizes readiness to change as a stage process,39 in which an individual moves from one stage to another, beginning with precontemplation, where individuals have no recognition for the need to change their behavior, to maintenance of change once they have successfully altered their behavior. By characterizing patients’ readiness to change, the educator was able to tailor the intervention based on identified factors preventing or facilitating adoption of desired behavior. In this TTI, the educator utilized the model to assist patients in working through barriers to behavioral change in ways that were responsive to different needs at various stages of readiness.
Group Tailoring
Tailoring of the intervention was anchored by the belief that behavioral intervention can largely achieve its goal if ethnic/cultural characteristics, experiences, norms, values, language, attitudes, beliefs, and social forces of the target population are incorporated in its design, delivery, and evaluation.40–44 Thus, consistent with recommended sleep health practices, we culturally and linguistically tailored the intervention for blacks in order to make it more effective in capturing attention, stimulating information processing, and motivating changes in the targeted health behavior. The TTI was tailored culturally and linguistically to conform to specific cultural characteristics of black patients identified via focus groups.
Individual Tailoring
Patients randomized to the TTI condition received up to 10 phone calls during the 6-month trial. Consistent with previous research, an average of 5 calls, lasting up to 25 min each was necessary to achieve desired outcomes.45 Individual tailoring of the TTI was based upon the principle that behavioral change is a process.39 For patients with low levels of motivation, the initial task of the educator was to increase motivation and commitment to receiving an OSA consultation and evaluation. For those who were already motivated, the educator facilitated purposive action toward desired goals. Motivation was enhanced by increasing knowledge, by modifying beliefs, and by increasing the value placed on OSA care. Willingness and ability to act on motivation was enhanced by providing cues to action, suggestions for overcoming barriers (eg, insufficient time to go to the clinic, unavailable means of transportation, fear of finding something wrong). A full description of the intervention components and procedures for training the health educator is provided in Table 1.
Attention Control Arm
Patients randomized to the attention control arm received comparable number of calls (up to 10, lasting an average of 10 minutes), providing general information about sleep and access to available sleep services as well as benefits of a healthy lifestyle. No tailored, stage-matched behavioral intervention was dispensed during those calls, which were made by a study staff uncertified as a sleep health educator. Standard care following positive screening for OSA consists of a referral for a comprehensive sleep evaluation and receipt of educational health literature, which patients would already have received at the time of enrollment. Instituting the procedure whereby patients in the attention control arm received periodic phone calls ensured that differences in adherence rates would be attributed solely to the delivery of stage-matched interventions.
Statistical Analysis
We performed analysis based on intent-to-treat assuming that all patients in the intervention arm were exposed to tailored OSA messages. Before assessing intervention effects, we verified comparability of patients in the control and intervention arms with respect to all available baseline sociodemographic and clinical data. We then compared patients who adhered to recommended OSA care and those who did not postenrollment using multivariate adjusted logistic modeling. This permitted estimation of the adherence rates for OSA consultation and laboratory-based evaluation as well as PAP treatment contrasting the two study arms; PAP use >4 hr a night for 70% of the nights was considered as the criterion for determining OSA treatment adherence satus.3,46,47 Although the study was designed to assess PAP subjectively and via telemetry, the present analysis relied on subjective data due to lack of adequate telemetric data. Adherence was a binary outcome (yes vs. no) for all three measured outcomes (initial consultation, evaluation, and treatment). While the primary objective was to ascertain the effectiveness of OSA messages, the secondary objective was to examine factors likely to facilitate adoption of OSA messages. Candidate predictors including sociodemographics, health risk, medical conditions, and psychosocial factors were examined.
For summary statistics, t-test and chi-square tests were performed to compare each outcome to the putative predictors depending on the type (continuous or categorical) and distribution (normal or not normal) of a predictor. Logistic regression modeling was employed to assess likelihood of adhering to recommended OSA care using a selected list of predictors for each dependent outcome (initial consultation, evaluation, and treatment). Three separate models were created depending on type of variable cluster: (1) sociodemographics, (2) medical, and (3) psychosocial. All variables in each cluster were included in each multivariate-adjusted logistic regression model. For each outcome, we provide point estimates along with 95% confidence interval for all predictors in the models. To account for missing values in the psychosocial questionnaires, the average of each participant’s completed questions was calculated. The average was then used or multiplied by the total number of questions (depending on the scoring method of the questionnaire). SAS 9.3 (Cary, North Carolina) was used for all descriptive and multivariate analyses.
RESULTS
Effect of Intervention on Adherence Status
As indicated in Tables 2–4, patients in both study arms were similar with respect to sociodemographic characteristics, health risks, medical comorbidity, and psychosocial measures. Patients in the control arm were more likely to report diabetes, relative to those in the intervention arm. Of note, this difference did not have any bearing on effects of the intervention on adherence status. Descriptive analysis showed that 69.4% of the patients in the intervention arm attended an initial consultation, compared to 36.7% in the control arm. Analysis also showed that 74.7% of those in the intervention arm and 66.7% of those in the control arm completed diagnostic OSA evaluations, and 86.4% in the intervention arm and 88.9% in the control arm adhered to OSA treatments. Since patients in the control arm exhibited a high level of adherence to recommended OSA evaluation and treatment, we contrasted adherers and nonadherers to determine whether they were different based on baseline measures, revealing no discernible differences with respect to demographic or clinical factors.
Table 2.
Demographic Characteristics of Patients in the MetSO Trial.
| Variables | Intervention (%) | Control (%) | p |
|---|---|---|---|
| Age | 60.2 ± 13.6 | 57.9 ± 13.0 | .113 |
| Gender | .986 | ||
| Male | 28.8 | 28.7 | |
| Female | 71.2 | 71.3 | |
| Income | .388 | ||
| <$10 000 | 43.0 | 48.0 | |
| ≥$10 000 | 57.0 | 52.0 | |
| Education | .719 | ||
| <High School | 30.4 | 32.3 | |
| >High School | 69.6 | 67.7 | |
| Birthplace | .837 | ||
| United States | 44.0 | 42.9 | |
| Other | 56.0 | 57.1 | |
| Smoking history | .939 | ||
| No | 67.3 | 67.7 | |
| Yes | 32.7 | 32.3 | |
| Alcohol history | .371 | ||
| No | 66.9 | 71.8 | |
| Yes | 33.1 | 28.2 | |
| BMI | .366 | ||
| <25 | 10.0 | 14.9 | |
| 25–30 | 21.2 | 21.3 | |
| >30 | 68.8 | 63.8 |
MetSO, Metabolic Syndrome Outcome Cohort Study; BMI, body mass index.
Values represent mean ± standard deviation for continuous variables or percentages for categorical variables
Table 4.
Psychosocial Characteristics of Patients in the MetSO Trial.
| Variables | Intervention (%) | Control (%) | p |
|---|---|---|---|
| Beck Anxiety Inventory Score | .649 | ||
| Minimal anxiety | 54.5 | 57.0 | |
| Mild anxiety | 45.5 | 43.0 | |
| Center for Epidemiologic Studies Depression Scale Score | .696 | ||
| No clinical significance | 69.5 | 67.5 | |
| Depressive symptoms | 30.5 | 32.5 | |
| Change Assessment Scale Score | .299 | ||
| Precontemplation | 36.7 | 32.6 | |
| Contemplation | 58.6 | 65.1 | |
| Preparation (action) | 4.7 | 2.3 |
MetSO, Metabolic Syndrome Outcome Cohort Study.
Values represent percentages for each measured baseline variable.
Table 3.
Medical Characteristics of Patients in the MetSO Trial.
| Variables | Intervention (%) | Control (%) | p |
|---|---|---|---|
| Hypertension | |||
| No | 7.7 | 7.1 | .835 |
| Yes | 92.3 | 92.9 | |
| Diabetes | .032 | ||
| No | 48.2 | 36.8 | |
| Yes | 51.7 | 63.2 | |
| Dyslipidemia | .849 | ||
| No | 31.1 | 30.2 | |
| Yes | 68.9 | 69.8 | |
| Heart problems | .654 | ||
| No | 70.4 | 72.6 | |
| Yes | 29.6 | 27.4 | |
| Arthritis | .828 | ||
| No | 50.9 | 49.7 | |
| Yes | 49.1 | 50.3 | |
| Respiratory problems | .738 | ||
| No | 76.9 | 78.4 | |
| Yes | 23.1 | 21.6 | |
| Cancer | .300 | ||
| No | 93.5 | 90.4 | |
| Yes | 6.5 | 9.6 |
MetSO, Metabolic Syndrome Outcome Cohort Study.
Values represent percentages for each measured baseline variable.
Multivariate Adjusted Logistic Regression Analysis
Adjusting for sociodemographic factors, analysis showed that patients in the intervention arm were 3.17 times more likely to have an initial consultation, relative to those in the control arm (odds ratio [OR] = 3.17, 95% confidence interval [CI]: 1.68–5.99, p = .001). As seen in Table 5, none of the sociodemographic factors showed significant associations with the likelihood of having an initial OSA consultation. In Table 6, we provide results of regression modeling considering medical factors as predictors of adherence to recommended OSA consultation. Of those factors, patients with a history of respiratory illness were more likely to adhere (OR = 2.27, 95% CI: 1.13–4.57, p = .021) and those with a history of dyslipidemia were less likely to adhere (OR = 0.43, 95% CI: 0.23–0.81, p = .009). In Table 7, we provide results of associations of the psychosocial measures with adherence. Analysis indicated that treatment self-efficacy was the only significant predictor of adherence status (OR = 1.11, 95% CI: 1.03–1.20, p = .007). Adjusted regression models revealed no significant differences between the two arms regarding adherence to OSA evaluations or treatments, although patients in the intervention showed a trend toward greater likelihood of adhering to recommended OSA evaluations (OR = 1.47, 95% CI: 0.52–4.19, NS). Patients in both the study arms were as likely to adhere to PAP treatment.
Table 5.
Demographic and Health Risk Predictors of Likelihood of Attending OSA Consultations.
| Variables | OR (95% CI) | p |
|---|---|---|
| Group | ||
| Control | 1 | |
| Intervention | 3.17 (1.68–5.99) | .001 |
| Age | 1.00 (0.97–1.02) | .681 |
| Gender | ||
| Male | 1 | |
| Female | 0.65 (0.33–1.30) | .227 |
| Income | ||
| <$10 000 | 1 | |
| ≥$10 000 | 0.68 (0.36–1.26) | .219 |
| Education | ||
| Less than HS | 1 | |
| HS or greater | 1.15 (0.57–2.33) | .706 |
| Birthplace | ||
| United States | 1 | |
| Other | 0.85 (0.44–1.65) | .637 |
| Smoking history | ||
| No | 1 | |
| Yes | 1.03 (0.51–2.08) | .925 |
| Alcohol history | ||
| No | 1 | |
| Yes | 0.75 (0.39–1.44) | .382 |
| BMI | ||
| <25 | 1 | |
| 25–30 | 0.74 (0.21–2.60) | .639 |
| >30 | 1.19 (0.41–3.48) | .749 |
| Epworth sleepiness | ||
| Normal | 1 | |
| Excessive | 1.14 (0.62–2.09) | .671 |
OSA, obstructive sleep apnea; BMI, body mass index; OR, odds ratio; CI, confidence interval.
Regression of baseline demographic and health risk factors on likelihood of attending initial OSA consultation.
Table 6.
Medical Predictors of Likelihood of Attending OSA Consultations.
| Variables | OR (95% CI) | p |
|---|---|---|
| Group | ||
| Control | 1 | |
| Intervention | 4.58 (2.57–8.16) | .001 |
| Hypertension | ||
| No | 1 | |
| Yes | 2.24 (0.80–6.30) | .125 |
| Diabetes | ||
| No | 1 | |
| Yes | 1.27 (0.72–2.23) | .407 |
| Dyslipidemia | ||
| No | 1 | |
| Yes | 0.43 (0.23- 0.81) | .009 |
| Heart problems | ||
| No | 1 | |
| Yes | 0.97 (0.52–1.79) | .918 |
| Arthritis | ||
| No | 1 | |
| Yes | 1.10 (0.63–1.91) | .741 |
| Respiratory problems | ||
| No | 1 | |
| Yes | 2.27 (1.13–4.57) | .021 |
| Cancer | ||
| No | 1 | |
| Yes | 1.67 (0.61–4.56) | .314 |
OSA, obstructive sleep apnea; OR, odds ratio; CI, confidence interval.
Regression of baseline medical variables on likelihood of attending initial OSA consultation.
Table 7.
Psychosocial Predictors of Likelihood of Attending OSA Consultation.
| Variables | OR (95% CI) | p |
|---|---|---|
| Group | ||
| Control | 1 | |
| Intervention | 4.89 (1.77–13.50) | .002 |
| BAI score | ||
| Minimal Anxiety | 1 | |
| Mild Anxiety | 2.77 (0.86–8.97) | .090 |
| CES-D score | ||
| No clinical significance | 1 | |
| Depressive symptoms | 0.37 (0.11–1.26) | .113 |
| CAS score | ||
| Precontemplation | 1 | |
| Contemplation | 0.49 (0.16–1.56) | .229 |
| Preparation (action) | 3.29 (0.21–5.75) | .395 |
| Self-efficacy score | ||
| Risk perception | 1.05 (0.94–1.18) | .389 |
| Outcome expectancies | 0.95 (0.88–1.02) | .157 |
| Treatment self-efficacy | 1.11 (1.03–1.20) | .007 |
| Apnea knowledge | 1.03 (0.82–1.29) | .834 |
OSA, objective sleep apnea; OR, odds ratio; CI, confidence interval; CES-D, Center for Epidemiological Studies-Depression.
Regression of psychosocial variables for likelihood of attending initial OSA consultation.
During the 6-month follow-up ascertainment, nearly all the patients in the intervention arm reported that the health educator helped them understand consequences of untreated OSA and to seek appropriate care (87%–95%). Likewise, they reported a high rate of positive rapport (95%–99%) and low rate of negative rapport (1%–3%) with the educator.
DISCUSSION
Prevalence studies have shown that individuals of the black race/ethnicity are 3 times as likely as those of the white race/ethnicity to have OSA,48 which is a significant public health concern, given the associated CVD burden.49–51 Yet, evidence has suggested that only 38% of blacks adhered to physician-recommended sleep evaluations.8 In this study, we evaluated effectiveness of a tailored TTI in increasing adherence to recommended consultation, evaluation and adherence to PAP treatment among blacks with metabolic syndrome. Our findings yielded two important observations. First, multivariate-adjusted logistic regressions indicated that blacks with metabolic syndrome and at risk of OSA, who were exposed to the intervention, were 3.17 times as likely as those in the attention control arm to adhere to recommended OSA consultations. Second, adjusted regression analyses showed no significant differences in the likelihood of adherence to recommended OSA diagnostic evaluations or PAP therapy, comparing patients in the two study arms.
This tailored behavioral intervention was designed principally to address dysfunctional attitudes and beliefs among blacks that were noted in previous mixed-methods studies we conducted in that community.6,9 Studies also suggested the need to use interventions that are culturally and linguistically tailored to ensure that the message is clear and acceptable to the intended patient population. Indeed, the sleep messages in this RCT were developed based on understanding the specific barriers and facilitators to receipt of OSA messages, which were identified through focus groups involving patients meeting criteria for the trial. Identified barriers were categorized as follows: problems sleeping in a strange and unfamiliar environment, unfamiliarity with the study protocol, and fear of being watched while sleeping. PAP barriers were related to the confining nature of the device, discomfort of wearing a mask while they slept, and concerns about their partner’s perceptions of treatment. By implementing an intervention tailored to address specific challenges black patients face as a group as well as delivering the messages according to patient’s stage of readiness, we were able to demonstrate a significant uptake of tailored OSA messages leading to desired behavior change. According to our adjusted models, black patients exposed to the intervention showed a nearly 4 times greater likelihood of adhering to recommended OSA consultation, which if replicated could have a significant public health impact in this vulnerable population that has heretofore remained underserved in regard to sleep services.
Contrary to our expectations, there was no significant difference between the two study arms regarding the likelihood of undergoing a diagnostic OSA evaluation, although trends favored greater adherence among patients in the intervention arm. Conceivably, the principal barrier to adequate OSA care among blacks may be the initial refusal to seek an initial consultation for evaluation of OSA, which may be hindered by various barriers as identified through focus groups. In another study with a population comprised of 54% blacks, consultation with a sleep specialist prior to the actual OSA evaluation was related to greater adherence to PAP treatment, with blacks exhibiting the lowest rates of PAP adherence,52 while in our study, the two study arms did not differ with regard to the likelihood of adhering to recommended PAP treatment. This led to the concern that patients in the control arm who adhered to both the OSA diagnostic studies and the PAP therapy might have been qualitatively different than those who did not, since they were not prompted in any way by the tailored intervention. Post hoc analysis contrasting adherers and nonadherers in the control arm revealed no significant group differences in sociodemographic, health risk, or psychosocial factors. Of note, no discernable factors emerged during final debriefing that would explain why individuals in the control arm showed a high likelihood of adhering to diagnostic OSA evaluations or PAP treatment. It is of interest to ascertain whether this observation could be replicated in other studies. One possible explanation is that patients with respiratory problems may have been more motivated to follow-up with recommended OSA care as regression analyses indicated that individuals with respiratory problems were twice as likely to adhere. However, no significant adherence bias can be inferred because patients in both intervention and control arms were as likely to report respiratory problems. Another possible explanation is that the education and/or support received from the study personnel could have contributed to their self-activation to follow through with recommended OSA care. Thus, such improvement in the attention control arm could have attenuated potential differences in the two study arms.
Contrary to results of our previous observational study, daytime sleepiness and obesity were not significant predictors of adherence status.8 Indeed, we found that none of the sociodemographic or health risk factors were predictive of adherence behavior. Of all the medical factors, respiratory illness seemed a strong motivator to seek OSA consultation, whereas individuals with dyslipidemia seemed less inclined to behave in a similar fashion. It is quite reasonable to expect patients with respiratory illnesses to show greater interest in seeking treatment for OSA, to which they might have been sensitized since OSA involves the respiratory system. It might have been expected that patients with dyslipidemia would be equally motivated to seek OSA care, but this was not evidenced in this trial. This finding should be explored in future studies, as OSA treatment has been shown to improve lipid profiles.53,54 Notably, baseline demographic or clinical measures could not account for this observation. It was also interesting to note that patients with other components of metabolic syndrome (obesity, diabetes, or hypertension) did not show differential level of motivation to seek OSA care, which is known to improve metabolic syndrome or its components.55–57
Consistent with previous research, self-efficacy proved a significant predictor of adherence behavior in this population. Specifically, patients scoring high on the treatment self-efficacy component exhibited a greater likelihood of seeking initial OSA consultations. It is worth noting that the other psychosocial factors did not have any significant bearing on adherence behavior. Furthermore, other factors such as history of screening or preventive behavior (eg, regular physical examinations, prostate screening, or mammography) were not associated with adherence status for any of the three OSA adherence measures. It is not clear why the other two self-efficacy components (outcome expectancy and risk perception) did not prove significant predictors as might have been suggested from published findings.52,58,59 Outcome expectancy has been noted as predictive of PAP adherence among whites. However, outcome expectancy is a future-oriented concept that may not be as salient for blacks who have a time orientation more often based in the “here and now”.60 With regard to risk perception, previous studies have indicated that nonwhites tend to have lower perceived risk of various health problems such as cancer, citing low awareness of family history or others in their social circle with the illness.61 A similar phenomenon may occur with regard to OSA among blacks, as this condition is underdiagnosed in this group, which may result in lower perceived risk. It would have been informative to assess whether such measures were in fact influenced by exposure to the intervention.
LIMITATIONS AND CONCLUSIONS
Many studies have shown low rates of OSA evaluation and PAP treatment among blacks. However, very few have investigated the underlying barriers and facilitators to OSA care in that group. A major strength of our study is that we engaged in a systematic effort to delineate factors hindering or enabling adoption of recommended OSA care among blacks using a tailored behavioral approach. To our knowledge, only one other study has attempted to employ a tailored approach to address low PAP adherence in a diverse sample, but we note that our study specifically addressed barriers observed among blacks. An important limitation of our study relates to the lack of adequate PAP telemetric data to explore group-based adherence differences. We note, however, that subjective data did not suggest significant differences between the two study arms. Another limitation is that it is not generalizable to other racial/ethnic groups, as the intervention was designed to address barriers elicited specifically from blacks during focus group discussions. Further, our findings are not generalizable to blacks as a whole as we note that there may have been some systematic differences in our study population, resulting in higher than average PAP adherence; of note, all of our participants had been previously diagnosed with metabolic syndrome, further limiting generalizability. Finally, we should note that the dropout rate of 20%, although relatively high, was comparable to those found in other studies.62 Furthermore, the use of intention-to-treat analysis obviates the need to assess systematic bias due to the dropout rate. Despite such limitations, results of our tailored intervention evidenced that sleep health information tailored to address specific barriers of racial/ethnic populations can lead to increased adoption of physician-recommended medical care, as demonstrated among blacks with metabolic syndrome and OSA. Success of the intervention may in part be attributable to the fact that it addressed sociocultural factors that might explain dissatisfaction with health-care services and mistrust of health-care providers common in that population.
FUNDING
This research was supported by funding from the National Institutes of Health: R01MD004113 and R01MD007716. NJW was supported by K23HL125939 from NHLBI. NJW is currently funded by a K grant: K23HL125939. The funding source had no role in the design, conduct, or analysis of the study, on in the decision to submit the manuscript for publication.
TRIAL REGISTRATION
Promoting Adherence to Sleep Apnea Treatment Among Blacks With Metabolic Syndrome (MetSO) https://clinicaltrials.gov/ct2/show/NCT01946659?term=sleep+apnea&rank= clinicaltrials.gov Identifier: NCT01946659
DISCLOSURE STATEMENT
None declared.
ACKNOWLEDGMENTS
The authors would like to thank Amy Linnea He for her assistance with statistical analyses and Anthony Collado for his assistance in the preparation of this manuscript.
REFERENCES
- 1. Chervin RD, Theut S, Bassetti C, Aldrich MS. Compliance with nasal CPAP can be improved by simple interventions. Sleep. 1997; 20(4): 284–289. [DOI] [PubMed] [Google Scholar]
- 2. Richards D, Bartlett DJ, Wong K, Malouff J, Grunstein RR. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep. 2007; 30(5): 635–640. [DOI] [PubMed] [Google Scholar]
- 3. Stepnowsky CJ, Palau JJ, Gifford AL, Ancoli-Israel S. A self- management approach to improving continuous positive airway pressure adherence and outcomes. Behav Sleep Med. 2007; 5(2): 131–146. [DOI] [PubMed] [Google Scholar]
- 4. Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2014; 1: CD007736. [DOI] [PubMed] [Google Scholar]
- 5. National Sleep Foundation. Sleep in America Poll, Washington, DC: National Sleep Foundation; 2005. [Google Scholar]
- 6. Williams NJ, Grandne MA, Snipes A, et al. Racial/ethnic disparities in sleep health and health care: importance of the sociocultural context. Sleep Health. 2015; 1(1): 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jean-Louis G, Youngstedt S, Grandner M, et al. Unequal burden of sleep-related obesity among black and white Americans. Sleep Health. 2015; 1(3): 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jean-Louis G, von Gizycki H, Zizi F, Dharawat A, Lazar JM, Brown CD. Evaluation of sleep apnea in a sample of black patients. J Clin Sleep Med. 2008; 4(5): 421–425. [PMC free article] [PubMed] [Google Scholar]
- 9. Jean-Louis G, Magai C, Cohen CI, et al. Ethnic differences in reported sleep problems in older adults. Sleep. 2001; 24(8): 926–933. [DOI] [PubMed] [Google Scholar]
- 10. Jean-Louis G, Grandner M. Importance of recognizing sleep health disparities and implementing innovative interventions to reduce these disparities. Sleep Med. 2016; 18: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams NJ, Grandner MA, Wallace DM, et al. Social and behavioral predictors of insufficient sleep among African Americans and Caucasians. Sleep Med. 2016; 18: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams NJ, Jean-Louis G, Ravenell J, et al. A community-oriented framework to increase screening and treatment of obstructive sleep apnea among blacks. Sleep Med. 2016; 18: 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams NJ, Nunes JV, Zizi F, et al. Factors associated with referrals for obstructive sleep apnea evaluation among community physicians. J Clin Sleep Med. 2015; 11(1): 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams NJ, Jean-Louis G, Brown CD, McFarlane SI, Boutin-Foster C, Ogedegbe G. Telephone-delivered behavioral intervention among blacks with sleep apnea and metabolic syndrome: study protocol for a randomized controlled trial. Trials. 2014; 15: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramos AR, Wallace DM, Pandi-Perumal SR, et al. Associations between sleep disturbances and diabetes mellitus among blacks with metabolic syndrome: Results from the Metabolic Syndrome Outcome Study (MetSO). Ann Med. 2015; 47: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith S, Lang C, Sullivan K, Warren J. Two new tools for assessing patients’ knowledge and beliefs about obstructive sleep apnea and continuous positive airway pressure therapy. Sleep Med. 2004; 5(4): 359–367. [DOI] [PubMed] [Google Scholar]
- 17. Levendowski DJ, Olmstead EM, Popovich D, Carper D, Berka C, Westbrook PR. Assessment of Obstructive Sleep Apnea Risk and Severity in Truck Drivers: Validation of a Screening Questionnaire. Sleep Diagnosis and Ther. 2007; 2(2): 20–26. [Google Scholar]
- 18. McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993; 31(3): 247–263. [DOI] [PubMed] [Google Scholar]
- 19. Williams JB. Standardizing the Hamilton Depression Rating Scale: past, present, and future. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 2):II6–12. [DOI] [PubMed] [Google Scholar]
- 20. Baca-Garcia E, Blanco C, Saiz-Ruiz J. et al. Assessment of reliability in the clinical evaluation of depressive symptoms among multiple investigators in a multicenter clinical trial. Psychiatry Res. 2001;102:163–173. [DOI] [PubMed] [Google Scholar]
- 21. Levendowski DJ, Morgan T, Montague J, Melzer V, Berka C, Westbrook PR. Prevalence of probable obstructive sleep apnea risk and severity in a population of dental patients. Sleep Breath. 2008; 12(4): 303–309. [DOI] [PubMed] [Google Scholar]
- 22. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112(17): 2735–2752. [DOI] [PubMed] [Google Scholar]
- 23. Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest. 1993,103:30–36. [DOI] [PubMed] [Google Scholar]
- 24. Stepnowsky CJ., Jr Improving measurement of CPAP self-efficacy. Sleep. 2004; 27(6): 1219–1220. [PubMed] [Google Scholar]
- 25. Rogers ES, Martin R, Anthony W. et al. Assessing readiness for change among persons with severe mental illness. Community Ment Health J. 2001;37:97–112. [DOI] [PubMed] [Google Scholar]
- 26. Grandner MA, Patel NP, Gehrman PR, Perlis ML, Jean-Louis G, Gooneratne N. Sleep-Related Attitudes, Beliefs and Practices in Black and White Adults. Sleep. 2010; 33: 375. [Google Scholar]
- 27. Bettman JR. An informed processing theory of consumer choice. Reading Massachussetts. Addison, TX: Wesley; 1979. [Google Scholar]
- 28. Basch CE. Focus group interview: an underutilized research technique for improving theory and practice in health education. Health Educ Q. 1987; 14(4): 411–448. [DOI] [PubMed] [Google Scholar]
- 29. Thackeray R, Neiger BL. Using social marketing to develop diabetes self-management education interventions. Diabetes Educ. 2002; 28(4): 536–534. [DOI] [PubMed] [Google Scholar]
- 30. Ling JC, Franklin BA, Lindsteadt JF, Gearon SA. Social marketing: its place in public health. Annu Rev Public Health. 1992; 13: 341–362. [DOI] [PubMed] [Google Scholar]
- 31. Pandi-Perumal SR, Akhter S, Zizi F, et al. Project Stakeholder Management in the Clinical Research Environment: How to Do it Right. Front Psychiatry. 2015; 6: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest. 1993; 103(1): 30–36. [DOI] [PubMed] [Google Scholar]
- 33. Basch C. Assessing health education needs: a multidimensional-multimethod approach. In: Lazes, Peal, ed. The Handbook of Health Education. 2 ed Rockville, MD: Aspen; 1987: 49–73. [Google Scholar]
- 34. Basch CE, Walker EA, Howard CJ, Shamoon H, Zybert P. The effect of health education on the rate of ophthalmic examinations among African Americans with diabetes mellitus. Am J Public Health. 1999; 89(12): 1878–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crane LA, Leakey TA, Ehrsam G, Rimer BK, Warnecke RB. Effectiveness and cost-effectiveness of multiple outcalls to promote mammography among low-income women. Cancer Epidemiol Biomarkers Prev. 2000; 9(9): 923–931. [PubMed] [Google Scholar]
- 36. Brouse CH, Basch C, Wolf RL, Shmukler C. Barriers to colorectal cancer screening with fecal occult blood testing in a predominantly minority urban population: A qualitative study. Am J Public Health. 2003; 93(8): 1268–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Myers RE, Ross EA, Wolf TA, Balshem A, Jepson C, Millner L. Behavioral interventions to increase adherence in colorectal cancer screening. Med Care. 1991; 29(10): 1039–1050. [DOI] [PubMed] [Google Scholar]
- 38. Green LW, Kreuter MW. Health Promotion Planning: An Educational and Environmental Approach. Mountain View, CA: Mayfield Publishing Company; 1991. [Google Scholar]
- 39. Prochaska J, Redding C, Evers KE. The transtheoretical model and stages of change. In: Glanz K, Lewis FM, Rimer BK, eds. Health Behavior and Health Education.San Francisco, CA: Jossey-Bass; 1997: 67–98. [Google Scholar]
- 40. Institute of Medicine . Focus on Health Communication: Placing Public Health in Perspective. 2008. [Google Scholar]
- 41. Institute of Medicine. Sepaking of Health: Assessing Health Communicaiton Strategies for Diverse Populations. Washington, DC: The National Academies Press; 2002. [PubMed] [Google Scholar]
- 42. Kreuter M. Integrating Culture into health information for African American women. American Behavioral Scientist. 2006; 49(6): 794–811. [Google Scholar]
- 43. Paul ED. Health Culture and Community. New York, NY: Russell Sage Foundation; 1955. [Google Scholar]
- 44. Green LW, Kreuter MW, Deeds SG, Partridge KB. Health Education Planning: A Diagnostic Approach. Mountain View, CA: Mayfield Publishing Company; 1980. [Google Scholar]
- 45. Walker EA, Schechter CB, Caban A, Basch CE. Telephone intervention to promote diabetic retinopathy screening among the urban poor. Am J Prev Med. 2008; 34(3): 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med. 1994; 149(1): 149–154. [DOI] [PubMed] [Google Scholar]
- 47. Lankford DA. Wireless CPAP patient monitoring: accuracy study. Telemed J E Health. 2004; 10(2): 162–169. [DOI] [PubMed] [Google Scholar]
- 48. Buxbaum SG, Elston RC, Tishler PV, Redline S. Genetics of the apnea hypopnea index in Caucasians and African Americans: I. Segregation analysis. Genet Epidemiol. 2002; 22(3): 243–253. [DOI] [PubMed] [Google Scholar]
- 49. Kendzerska T, Gershon AS, Hawker G, Leung RS, Tomlinson G. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med. 2014; 11(2): e1001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guven SF, Turkkani MH, Ciftci B, Ciftci TU, Erdogan Y. The relationship between high-sensitivity C-reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath. 2012; 16(1): 217–221. [DOI] [PubMed] [Google Scholar]
- 51. Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007; 176(12): 1274–1280. [DOI] [PubMed] [Google Scholar]
- 52. Smith SS, Lang CP, Sullivan KA, Warren J. A preliminary investigation of the effectiveness of a sleep apnea education program. J Psychosom Res. 2004; 56(2): 245–249. [DOI] [PubMed] [Google Scholar]
- 53. Börgel J, Sanner BM, Bittlinsky A, et al. Obstructive sleep apnoea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur Respir J. 2006; 27(1): 121–127. [DOI] [PubMed] [Google Scholar]
- 54. Nadeem R, Singh M, Nida M, et al. Effect of CPAP treatment for obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med. 2014; 10(12): 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011; 365(24): 2277–2286. [DOI] [PubMed] [Google Scholar]
- 56. Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013; 310(22): 2407–2415. [DOI] [PubMed] [Google Scholar]
- 57. Pamidi S, Wroblewski K, Stepien M, et al. Eight Hours of Nightly Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea Improves Glucose Metabolism in Patients with Prediabetes. A Randomized Controlled Trial. Am J Respir Crit Care Med. 2015; 192(1): 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Golay A, Girard A, Grandin S, et al. A new educational program for patients suffering from sleep apnea syndrome. Patient Educ Couns. 2006; 60(2): 220–227. [DOI] [PubMed] [Google Scholar]
- 59. Trupp RJ, Corwin EJ, Ahijevych KL, Nygren T. The impact of educational message framing on adherence to continuous positive airway pressure therapy. Behav Sleep Med. 2011; 9(1): 38–52. [DOI] [PubMed] [Google Scholar]
- 60. Kambin KK. African/Black Psychology in the American context: An African-Centered Approach. Tallahassee, FL: Nubian Nation Publications; 1998. [Google Scholar]
- 61. Jones RA, Steeves R, Williams I. How African American men decide whether or not to get prostate cancer screening. Cancer Nurs. 2009; 32(2): 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davis RM, Hitch AD, Nichols M, Rizvi A, Salaam M, Mayer-Davis EJ. A collaborative approach to the recruitment and retention of minority patients with diabetes in rural community health centers. Contemp Clin Trials. 2009; 30(1): 63–70. [DOI] [PubMed] [Google Scholar]