Abstract
Study Objectives:
We objectively measured body composition, energy expenditure, caloric intake, and sleep in a large, diverse sample of healthy men and women and determined how energy balance and diet associated with sleep physiology.
Methods:
Healthy adults (n = 50; 21–50 years) participated in an in-laboratory study involving two baseline sleep nights (BL1-2, 10 hours time-in-bed/night, 2200–0800 hours). Polysomnography was recorded on BL2. Demographic information, body composition, and energy expenditure measurements were collected at study admittance and on BL1. Daily food/drink intake was recorded both before (on BL1) and after (on BL2) the sleep measurement. Partial Pearson’s correlations assessed the relationship between energy balance and sleep physiology variables.
Results:
At baseline, greater fat-free mass associated with lower total sleep time (r = −0.52, p = .030), lower sleep efficiency (r = −0.53, p = .004), and greater wake after sleep onset (r = 0.55, p = .002). Higher body fat percentage (r = 0.39, p = .038) and being overweight (Body Mass Index [BMI] 25–30; p = .026) associated with more rapid eye movement (REM) sleep. Higher protein intake (r’s = 0.46–0.52; p’s < .001–.002) and lower carbohydrate intake (r’s = −0.31 to −0.34; p’s = .027–.046) on BL1 and BL2 associated with more REM sleep. Greater fiber consumption on BL1 and BL2 associated with more slow-wave sleep (SWS; r’s = 0.33–0.35; p’s = .02–.03). More SWS related to increased carbohydrate intake the following day (BL2, r = 0.32, p = .037).
Conclusions:
Body composition and diet were related to baseline sleep characteristics, including SWS and REM sleep duration and sleep maintenance. Future studies should further evaluate the influence of energy balance measures on sleep physiology, since dietary interventions may be useful in treating insufficient sleep, poor sleep quality, excessive sleepiness or other sleep disorders.
Keywords: Sleep architecture, polysomnography, energy balance, caloric intake, macronutrient intake.
Statement of Significance
Body composition (fat vs. fat-free mass) and macronutrient intake (calories from protein, carbohydrates, and fat) associate with rapid eye movement and slow-wave sleep duration as well as sleep efficiency. Future experimental studies are needed to manipulate macronutrient intake in order to demonstrate a cause and effect relationship between nutrition and sleep.
INTRODUCTION
Population studies consistently find that short sleep duration (habitual sleep ≤ 6 h/night) is a significant risk factor for weight gain and obesity.1–4 Laboratory studies demonstrate that sleep restriction leads to weight gain,5,6 increased caloric intake,5–10 delayed meal timing,5–7 and energy expenditure alterations.11–17 Although sleep duration affects energy balance, few studies have systematically examined the association between objective sleep characteristics (eg, sleep stage duration, sleep onset latency, and sleep efficiency) and objective energy balance measures.
Sleep is comprised of rapid eye movement (REM) sleep and non-REM sleep, with the latter comprised of stage 1, stage 2, and slow-wave sleep (SWS). REM sleep and SWS durations are highly variable between individuals but consistent within individuals across nights.18,19 Both SWS and REM sleep have been associated with energy balance measures in healthy children, adolescents, and adults. SWS duration has been negatively correlated with body mass index (BMI), waist circumference, ghrelin levels, intake during an ad libitum meal, saturated fat intake, and hunger ratings20–22 and has been positively related with fiber intake, lean body mass, and growth hormone release.23–26 REM sleep duration has been correlated with increased hunger ratings, higher BMI, and positive energy balance due to overeating22,27,28; however, other studies have shown that REM sleep duration is inversely correlated with waist circumference, BMI, and cardiovascular risk factors.20,21 In addition, a sleep fragmentation experiment showed that reduced REM sleep correlated with increased desire-to-eat ratings and decreased fullness ratings.29
Few studies have examined the relationship between objective measures of energy expenditure and sleep stages in healthy adults. When measuring energy expenditure during sleep, one study of 12 men found that metabolic rate and carbohydrate oxidation were higher during REM than during non-REM29 whereas two other studies (n = 7–13, men and women) found no differences in metabolic rate between sleep stages.30,31 To our knowledge, only one study (n = 27, men and women) has examined the relationship between sleep stage duration and resting metabolic rate (RMR, measured during wake): Schechter and colleagues32 found RMR and respiratory quotient (a measure of fat and carbohydrate oxidation) were not associated with the percentage of total sleep time spent in each sleep stage. More research is needed to determine if there is an association between RMR and sleep stage duration.
Although results are inconsistent, studies have shown that sleep latency, sleep efficiency, and wake after sleep onset are also related to energy balance measures in healthy individuals. Normal weight adults have exhibited higher sleep efficiency than overweight or obese individuals in some,2,27,33–35 but not all28,36 studies. In overweight adults, dieting related to increased sleep onset latency37 whereas in normal weight adults, controlled diet associated with decreased sleep onset latency (compared to ad libitum intake).26 Carbohydrate intake was related to shorter sleep onset latency in one study38 but with increased arousals during sleep in another study.26 In a population cohort of middle-age adults, sleep fragmentation was associated with lower carbohydrate intake and lower sleep efficiency was associated with higher caloric intake.39
We used objective measures to examine the relationships between demographic characteristics, body composition measures, and caloric intake and baseline sleep characteristics in a large, diverse sample of healthy men and women. We hypothesized that measures associated with positive energy balance (eg, higher BMI, greater body fat percentage, lower RMR, increased caloric intake) would associate with greater REM sleep and less SWS.
METHODS
Subjects
Healthy individuals, aged 21–50 years, were recruited in response to study advertisements. They reported habitual nightly sleep durations between 6.5 and 8.5 hours, habitual bedtimes between 2200 and 0000 hours, and habitual morning awakenings between 0600 and 0900 hours; these reports were confirmed objectively using actigraphy. They had no evidence of habitual napping, no sleep disturbances (ie, no complaints of insomnia, daytime sleepiness, or other sleep–wake disturbances), and did not show either extreme morningness or extreme eveningness, as assessed by questionnaire.40 Subjects were free of acute and chronic medical and psychological conditions, as established by interviews, clinical history, questionnaires, physical examinations, and blood (including a fasting blood glucose test) and urine tests. They were nonsmokers and did not participate in shift work, transmeridian travel, or irregular sleep/wake routines in the 60 days prior to the study. Enrolled subjects were monitored at home with actigraphy, sleep–wake diaries, and time-stamped call-ins to assess bedtime and waketime during the week before and after the in-laboratory phase. They were not permitted to use caffeine, alcohol, tobacco, and medications (except oral contraceptives) in the week before the experiment, as verified by urine screenings. Sleep disorders were excluded by a night of laboratory polysomnography and oximetry measurements.
The research protocols were approved by the Institutional Review Board of the University of Pennsylvania. All subjects provided written informed consent before enrollment and were compensated for participation.
Protocol
Subjects participated in a protocol in the Sleep and Chronobiology Laboratory at the Hospital of the University of Pennsylvania and were studied for 18 consecutive days continuously with daily clinical checks of vital signs and symptoms by nurses (with an independent physician on call). The protocol involved two baseline nights (BL1-2) of 10 hours time-in-bed (TIB; 2200–0800 hours). During the in-laboratory study phase, subjects could not leave the laboratory. Subjects were ambulatory and could watch television, read, play video or board games, and perform other sedentary activities between test bouts (completed while sitting at a computer) but were not allowed to exercise. Subjects wore a wrist actigraph continuously and on certain days wore ambulatory electroencephalography (EEG) and electrocardiography (ECG) recording equipment for 24-hours intervals. The light levels were held constant at <50 lux during scheduled wakefulness and <1 lux during scheduled sleep periods. Ambient temperature was maintained between 22°C and 24°C. Subjects were behaviorally monitored by trained staff continuously to ensure adherence.
Measures
A nurse recorded each subject’s height at study admittance. Body composition and metabolic measurements were collected after an overnight fast (beginning at 2200 hours) in the morning following BL1. Upon awakening, subjects were instructed to use the restroom. Each subject’s body composition was assessed and then he/she remained in bed in a supine position for the remainder of the metabolic testing period. Each subject’s body composition was measured using bioelectrical impedance analysis (Omron HBF-510W, 4-Limb device). Body weight, fat percentage, and FFM were collected. Metabolic rate (kcal/d) was measured using indirect calorimetry with a validated41,42 ventilated hood system (TrueOne 2400 Metabolic Cart, Parvo Medics, Sandy, UT). In the morning of the measurement, a flow meter calibration was conducted and before each use, the metabolic cart was calibrated with reference gas. After achieving a steady state (5 minutes), expired gases were collected for 10 minutes and used to calculate metabolic rate.15 For more detailed information about this procedure, please see.15 Trained technicians monitored subjects and instructed them to keep their eyes open to ensure they remained awake during the test.
Subjects selected their meals/snacks by choosing from various menu options, selecting additional food/drink available in the kitchen within the laboratory suite (which included a refrigerator, microwave, and toaster oven) and by making requests to the staff. To ensure subjects were provided sufficient time to eat each day, three eating opportunities were allotted during days with a 2200 hours bedtime (0900, 1235, and 1830 hours). In addition to these specified meal times, subjects could consume food/drink at any time other than when they were completing neurobehavioral tests or sleeping. Subjects could eat pre-ordered menu items or select from other foods available in the laboratory kitchen and could eat as much (or as little) as they wanted. Subjects retrieved their own food/drink from the kitchen and could eat at a table in the common area or privately in their bedrooms. All food was weighed and recorded prior to being provided to subjects. To improve the measurement accuracy of each food item’s weight, items were served in individual containers. Each day, a detailed description of the items and the amount consumed, and intake time were recorded by trained monitors. Additionally, any food/drink left over after each meal was weighed and recorded. The intake data were entered into The Food Processor SQL program (ESHA Research, Salem, OR), a validated43 professional nutrition analysis software and database program that provides components of food/drink intake including calories and macronutrients.
Polysomnography (PSG) recordings (EC3-A2, Fz-A1, O2-A1; two electrooculography's [EOG]—left outer canthus [LOC]/right outer canthus [ROC]; two submental electromyography [EMG]) were collected using digital ambulatory physiological recorders (Compumedics Profusion PSG3 recording system [128-Hz sampling]; Compumedics Limited, Abbotsford, Victoria, Australia) on BL2. All sleep stages were scored visually in continuous 30-second epochs according to Rechtschaffen and Kales44 by a trained scorer using commercial software (ProFusion PSG 3; Compumedics Limited). The EEGs and EOGs were referenced with A1 or A2 (Fz-A1, C3-A2, O2-A1, LOC-A2, and ROC-A1). A submental EMG was analyzed bipolarly. The sampling rate was 256 Hz. For sleep scoring, high-pass filters were set at 0.3 Hz for EEGs and EOGs and 10 Hz for EMG. Low-pass filters were set at 30 Hz for EEG and EOG and 100 Hz for EMG. Sleep onset latency was defined as three consecutive 30-second epochs of any sleep stage.
Statistical Analyses
Pearson’s r correlations and partial correlations analyzed the relationship between demographic, body composition, intake, and BL2 PSG sleep variables. Between-subjects ANOVAs examined differences in BL2 sleep and intake between BMI, gender and race groups. Statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 20.0 (Armonk, NY).
RESULTS
Baseline Sleep and Energy Balance Measurements
The sample (n = 50) used for correlations of demographic and energy balance measures with baseline sleep PSG measures consisted of 21 women (42.0%) and 29 men (58.0%), and 26 African Americans (52.0%) and 24 Caucasians (48.0%). Subjects were 21–50 years old (33.9 ± [SD] 9.1 years) and were either normal or overweight (normal: BMI < 25, overweight: BMI 25–30; 24.5 ± 3.6). Four subjects’ data were excluded from baseline sleep analyses due to a PSG recording issue (n = 1) or due to being outliers in total sleep time (n = 3, total sleep time was more than two SDs below the mean). See Table 1 for BL2 PSG sleep measurements. During BL1 (the day before the sleep recording), subjects (n = 50) consumed 2801.7 ± 726.8 kcal with 14.4 ± 2.9% kcal from protein, 58.3 ± 6.6% kcal from carbohydrates and 29.0 ± 5.5% kcal from fat, 211.1 ± 78.3 grams of sugar (29.9 ± 6.7% of total kcal), 24.5 ± 10.3 grams of fiber and 31.4 ± 15.0 grams of saturated fat (9.8 ± 3.4% of total kcal).
Table 1—
Baseline (10 hours TIB) Polysomnographic Sleep Measures (N = 46).
| Sleep variable | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|
| Total sleep time (TST, minutes) | 401.00 | 581.00 | 520.93 | 45.05 |
| Sleep efficiency (SE%, TST/TIB) | 66.80 | 96.80 | 86.83 | 7.51 |
| Sleep onset latency (SL, minutes) | 3.00 | 85.50 | 20.49 | 16.71 |
| Time spent in stage 1 sleep (S1, % TST) | 1.50 | 15.20 | 5.78 | 2.76 |
| Time spent in stage 2 sleep (S2, % TST) | 39.70 | 71.80 | 50.95 | 6.51 |
| Time spent in slow-wave sleep (SWS, % TST) | 4.20 | 33.00 | 16.96 | 6.84 |
| Time spent in rapid eye movement sleep (REM, % TST) | 14.80 | 34.10 | 26.31 | 4.06 |
| Wake after sleep onset (WASO, minutes) | 14.50 | 177.50 | 58.55 | 41.83 |
TIB = Time-in-bed.
In a subset of subjects (n = 36), body composition and RMR were assessed on BL1 morning following 10 hours of fasting. The mean RMR was 1591.5 ± 253.2 kcal/d with a respiratory quotient of 0.84 ± 0.05. Subjects weighed 72.2 ± 12.2 kg (body fat percentage: 28.8 ± 9.6%, FFM: 51.3 ± 10.6 kg).
Relationship Between Demographic Characteristics and Baseline Sleep
Age was significantly correlated with BL2 total sleep time (r = −0.43, p = .003), sleep efficiency (r = −0.43, p = .003), SWS (r = −0.35, p = .016) and wake after sleep onset (r = 0.45, p = .002). An ANOVA with gender and race as fixed factors and age as a covariate revealed significant race differences in stage 2 and SWS but no significant gender differences (all p > .16) or gender-by-race interactions (all p > .21). African Americans spent more time in stage 2 sleep (52.6 ± 5.2% vs. 49.0 ± 7.5%; F(1, 41) = 6.50, p = .015) and less time in SWS (15.2 ± 6.0% vs. 19.0 ± 7.3%; F(1, 41) = 5.61, p = .023) than Caucasians.
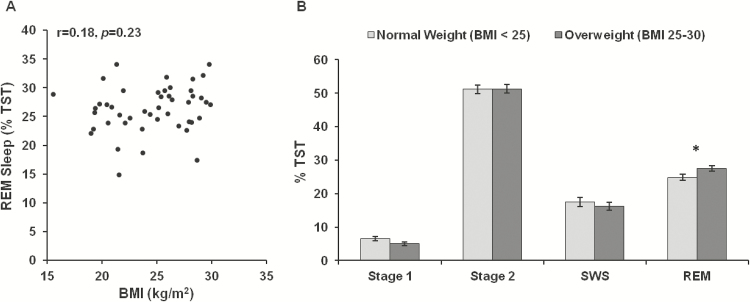
BMI was not significantly correlated with any BL2 sleep variable (all p > .16). When subjects were split into weight groups based on BMI, however, normal weight individuals (n = 22, 10 women and 12 men) exhibited less REM sleep than overweight individuals (n = 24, 10 women and 14 men) when covarying age and race (F(1, 42) = 5.35, p = .026; Figure 1). The two weight groups did not differ on any other sleep measures (stage 1 sleep: p = .084, all other p > .11).
Figure 1—
Relationship between body mass index (BMI) and baseline sleep measures. (A) BMI was not significantly correlated with any sleep variable (all p > .16), including rapid eye movement (REM) sleep. (B) When subjects were split into weight groups based on BMI, normal weight individuals (n = 22, 10 women and 12 men) exhibited significantly less REM sleep than overweight individuals (n = 24, 10 women and 14 men) when covarying age and race (*p = .026). Subjects in the two weight groups did not differ on any other sleep measure (all p > .08). Data are presented as mean ± SEM.
Relationship Between Demographic Characteristics and Baseline Intake
Age was not significantly correlated with any baseline intake measure (carbohydrate intake: r = −0.24, p = .089, sugar intake [grams]: r = −0.26, p = .068, all other p > .12). An ANOVA with gender and race as fixed factors revealed significant race differences in baseline protein and sugar intake, significant gender differences in caloric, sugar and saturated fat intake, but no significant gender-by-race interactions (all p > .60). African Americans consumed less protein (13.5 ± 1.9% vs. 15.4 ± 3.4%; F(1, 46) = 5.97, p = .018) and more sugar (32.0 ± 6.6% vs. 27.5 ± 6.1%; F(1, 46) = 6.20, p = .016) than Caucasians. Men consumed more calories (3094.5 ± 747.7 vs. 2397.4 ± 464.1 kcal; F(1, 46) = 14.66, p < .001) than women and although men consumed more grams of sugar (237.9 ± 83.5 vs. 174.3 ± 52.8; F(1, 46) = 10.77, p = .002) and saturated fat (35.8 ± 15.2 vs. 25.4 ± 12.7; F(1, 46) = 6.36, p = .015) than women, when sugar and saturated fat were calculated as a percentage of total intake there were no gender differences (all p > .30).
BMI was not significantly correlated with any baseline intake variables (fat: r = 0.24, p = .099, fiber: r = −0.27, p = .06, all other p > .13). When subjects were split into weight groups based on BMI, normal weight individuals consumed fewer calories from fat than overweight individuals (27.1 ± 5.1% vs. 30.8 ± 5.2%; F(1, 42) = 4.97, p = .031). Although not significant, normal weight subjects consumed more calories from carbohydrates than overweight subjects (60.1 ± 6.2% vs. 56.6 ± 6.5%; F(1, 49) = 3.76, p = .058); the two weight groups did not differ on any other baseline intake measure (all p > .11).
Greater consumption of sugar (grams: r = 0.81, p < .001) and saturated fat (grams: r = 0.76, p < .001; %kcal: r = 0.33, p = .02) associated with increased total caloric intake. Individuals who consumed more calories from protein consumed fewer total calories (r = −0.29, p = .043), fewer calories from carbohydrates (r = −0.48, p < .001) and less sugar (grams: r = −0.45, p = .001, %kcal: r = −0.45, p = 001). Individuals who consumed more calories from fat consumed fewer calories from carbohydrates (r = −0.85, p < .001) and fewer grams of fiber (r = −0.32, p = .024).
Relationship Between Body Composition and Baseline Sleep
A partial correlation analysis was conducted between BL2 sleep measures (listed in Table 1) and BL1 body composition and energy expenditure measures (RMR, respiratory quotient, weight, body fat percentage, and fat-free mass [FFM]) with age, gender and race as covariates (since previous studies have observed age, gender and race differences in sleep and/or body composition45–47). Higher RMR (r = 0.37, p = .047) and weight (r = 0.39, p = .035), specifically FFM (r = .55, p = .002), were significantly correlated with greater wake after sleep onset. FFM was also associated with less total sleep time (r = −0.52, p = .004) and a lower sleep efficiency (r = −0.53, p = .004). Body fat percentage was associated with less stage one sleep (r = −0.40, p = .033) and more REM sleep (r = 0.39, p = .038).
Relationship Between Baseline Intake and Baseline Sleep
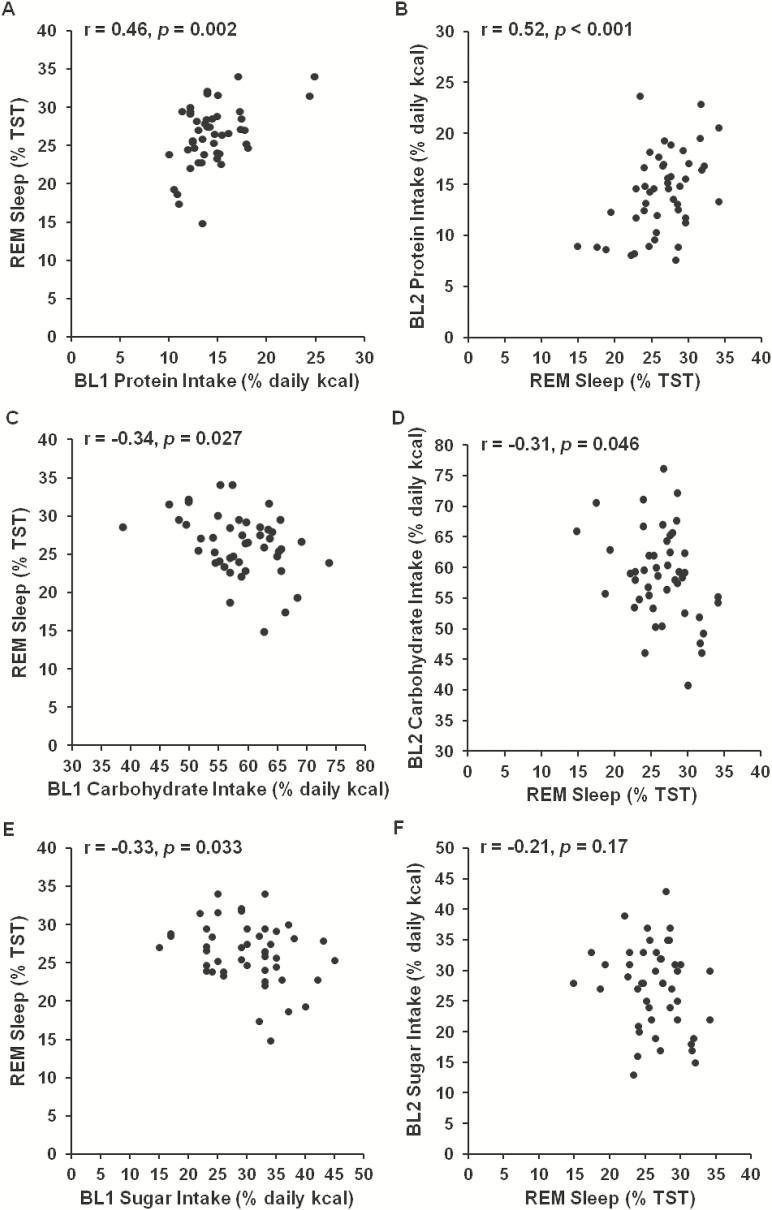
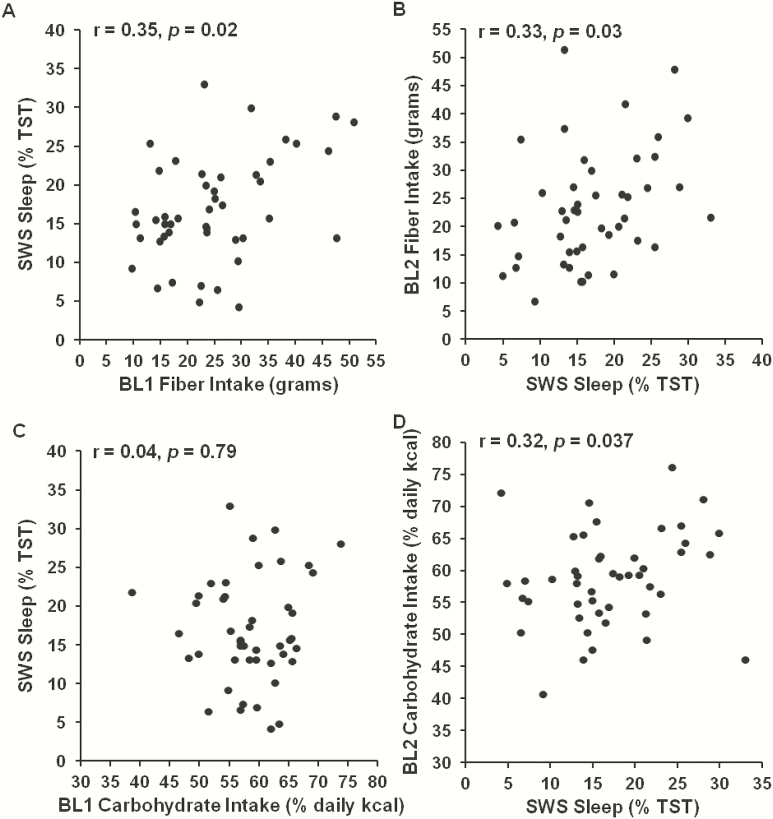
A partial correlation analysis was conducted between baseline (BL1) intake measures (total caloric intake [kcal], percentage of calories consumed from protein, carbohydrates and fat, and grams of sugar, fiber and saturated fat consumed) and sleep measures that night (BL2) (listed in Table 1) and with age, gender and race as covariates (since previous studies have observed age, gender and race differences in sleep and/or caloric intake).7,45,46 Greater protein intake was associated with less stage 2 sleep (r = −0.40, p = .007) and more REM sleep (r = 0.46, p = .002; Figure 2A). Greater carbohydrate intake (r = −0.34, p = .027; Figure 2C), and sugar consumption specifically (grams: r = −0.34, p = .026; %kcal: r = −0.33, p = .033; Figure 2E), were associated with less REM sleep. Sugar intake (%kcal) was associated with more stage 2 sleep (r = 0.44, p = .003).Carbohydrate intake did not associate with SWS duration (r = 0.04, p = .79, Figure 3C); however, whereas greater fiber intake was associated with more SWS (r = 0.35, p = .02; Figure 3A).
Figure 2—
Relationship between caloric intake variables and rapid eye movement (REM) sleep. Greater protein intake during the day preceding (A) and the day following (B) the baseline sleep measurement was significantly associated with more REM sleep. Greater carbohydrate intake during the day preceding (C) and the day following (D) the baseline sleep measurement was significantly associated with less REM sleep. Sugar intake during the day preceding (E), but not during the day following (F) the baseline sleep measurement was significantly associated with less REM sleep.
Figure 3—
Relationship between caloric intake variables and slow-wave sleep (SWS). Greater fiber intake during the day preceding (A) and the day following (B) the baseline sleep measurement was significantly associated with more SWS. Greater carbohydrate intake during the day following (D), but not the day preceding (C) the baseline sleep measurement was significantly associated with more SWS.
The relationship between BL2 sleep and intake during the following day (BL2, 08:00–22:00 hours) showed consistent results. Less stage 2 sleep (r = −0.34, p = .027) and more REM sleep (r = 0.52, p < .001; Figure 2B) were associated with greater protein intake, more REM sleep was associated with less carbohydrate intake (r = −0.31, p = .046; Figure 2D), but not less sugar intake (r = −0.21, p = .17; Figure 2F), and more SWS was associated with greater fiber intake (r = 0.33, p = .03; Figure 3B). In addition, more SWS was associated with greater carbohydrate intake (r = 0.32, p = .037; Figure 3D), shorter sleep onset latency was associated with both greater fat intake (r = 0.38, p = .027) and less carbohydrate intake (r = −0.39, p = .01), and less stage 1 sleep was associated with greater daily intake (r = −0.33, p = .028).
DISCUSSION
We examined the relationship between objective measurements of energy balance and objective baseline sleep characteristics and found the following: that greater FFM associated with less sleep; higher body fat percentage and being overweight associated with more REM sleep; and protein and carbohydrate intake bi-directionally associated with differences in SWS and REM sleep stage duration. These results highlight the importance of diet and body composition for sleep and are relevant to basic sleep research focused on understanding the basis of individual differences in sleep physiology and to clinical sleep research focused on improving sleep quality in healthy sleepers and patients with sleep disorders. The association between sleep physiology and diet also suggests improved sleep hygiene may be beneficial for weight management.
In our study, when all subjects were allowed the same amount of TIB for sleep, subjects with a lower RMR and less FFM showed decreased wake after sleep onset, and individuals with lower FFM also showed increased total sleep time and higher sleep efficiency. Individuals with shorter sleep onset latencies consumed more calories from fat and carbohydrates the following day and those with a higher body fat percentage or were overweight exhibited increased REM sleep. Consistent with these findings, previous studies have observed that higher BMIs associate with longer sleep duration among adults48 and that obese individuals experience greater excessive daytime sleepiness.48 Moreover, men with increased REM sleep (and less SWS) exhibited greater positive energy balance due to overeating in a controlled laboratory study.22 Similarly, rats who become obese due to a high-fat diet exhibited more sleep, specifically greater REM sleep.49 These findings suggest that overweight individuals may experience more sleepiness at night and have less trouble initiating and maintaining sleep. In addition, although findings are inconsistent, some evidence suggests energy expenditure is higher during REM sleep than during other stages.50 One possible homeostatic response to excess fat is an increase in REM sleep to increase energy expenditure during sleep. Studies comparing sleep architecture between healthy adults with similar weights but different body compositions (ie, amount of lean mass) would clarify this association. Our finding also highlights the importance of using body composition measures rather than relying only on BMI (weight divided by height), since BMI does not take into account differences in lean mass or body fat percentage.
Few studies have assessed the effect of diet on nighttime sleep. We found that increased protein intake and decreased carbohydrate intake associated with more REM sleep and that increased fiber intake associated with more SWS when intake was assessed the day before and after the baseline night sleep measurement. Although previous studies have observed differences in sleep onset latency, total sleep time or sleep efficiency when varying the carbohydrate content of meals,26,37,38 to our knowledge this is the first time a link between protein and carbohydrate intake with REM sleep stage duration has been demonstrated. In overweight and obese adults, increased protein intake improved self-reported sleep quality51; future studies are needed to assess if changes in REM sleep duration underlie subjective ratings of sleep quality. The relationship between fiber and SWS was also demonstrated in a recent paper26 measuring sleep and intake in healthy men and women who were similar in age to our sample. Dietary fiber is found in fruits, vegetables, grains, and legumes, promotes healthy digestion, and has been linked to the maintenance of a healthy diet and decreased risk for heart disease.52,53 Hypothesized functions of SWS include recuperation, tissue damage repair, and restoration of brain energy sources. A healthy diet may promote these processes and thus extend time spent in SWS. Future experiments, in animals and humans, are needed to establish a causal link between fiber and SWS duration and determine if increased fiber consumption specifically, or a healthy diet more generally, increases SWS duration and subsequent recuperation processes in brain and body.
Our study has the following limitations. Although caloric intake was ad libitum, subjects were only allowed to consume food and drink provided by hospital and laboratory staff; foods that contained caffeine (including chocolate) were prohibited. Therefore, subjects may have desired to eat certain foods that were unavailable to them. Our subjects were healthy, between the ages of 21–50 years, and had BMIs in the normal to overweight range. The results may therefore not generalize to other age groups (eg, adolescents or the elderly), or to obese individuals.
In conclusion, we observed several reliable relationships between measures of diet and energy balance with sleep characteristics. Higher FFM associated with less sleep, lower sleep efficiency, and greater wake after sleep onset, and having more body fat or being overweight associated with increased REM sleep duration. A diet higher in protein and lower in carbohydrate related with more REM sleep and increased fiber consumption related with more SWS. Future research is needed to further assess the impact of diet and body composition on sleep, since this may have clinical applications for patients with insomnia, insufficient sleep, poor sleep quality, or excessive sleepiness. The association between sleep physiology and diet suggests improved sleep hygiene may be beneficial for weight management.
FUNDING
This research was supported by NIH grants R01 NR004281 (DFD) and F31 AG044102 (AMS); the Department of the Navy, Office of Naval Research Award No. N00014-11-1-0361 (NG); National Aeronautics and Space Administration NNX14AN49G (NG) and Clinical and Translational Research Center (CTRC) grant UL1TR000003.
DISCLOSURE STATEMENT
Dr. Spaeth and Dr. Goel have no conflicts of interest. Dr. Dinges is compensated for serving on a scientific advisory council for Mars, Inc. Clinical Trial Registry: ClinicalTrials.gov NCT02128737 and ClinicalTrials.gov NCT02130791.
ACKNOWLEDGMENTS
The authors thank the subjects who participated in the experiments and the faculty and staff of the Division of Sleep and Chronobiology who helped acquire the data, specifically Claire Fox for analyzing the polysomnography data.
REFERENCES
- 1. Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among U.S. adults. Obesity. 2014; 22(2): 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moraes W, Poyares D, Zalcman I, et al. Association between body mass index and sleep duration assessed by objective methods in a representative sample of the adult population. Sleep Med. 2013; 14(4): 312–318. [DOI] [PubMed] [Google Scholar]
- 3. Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004; 27(4): 661–666. [DOI] [PubMed] [Google Scholar]
- 4. Chaput JP, Després JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec family study. Sleep. 2008; 31(4): 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 2013; 36(7): 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013; 110(14): 5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spaeth AM, Dinges DF, Goel N. Gender and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr. 2014; 100(2): 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011; 94(2): 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calvin AD, Carter RE, Adachi T, et al. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest. 2013; 144(1): 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008; 1(5): 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shechter A, Rising R, Albu JB, St-Onge MP. Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am J Clin Nutr. 2013; 98(6): 1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hursel R, Rutters F, Gonnissen HK, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation in healthy men on energy expenditure, substrate oxidation, physical activity, and exhaustion measured over 48 h in a respiratory chamber. Am J Clin Nutr. 2011; 94(3): 804–808. [DOI] [PubMed] [Google Scholar]
- 13. Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012; 4(29): 129ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benedict C, Hallschmid M, Lassen A, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011; 93(6): 1229–1236. [DOI] [PubMed] [Google Scholar]
- 15. Spaeth AM, Dinges DF, Goel N. Resting metabolic rate varies by race and by sleep duration. Obesity (Silver Spring). 2015; 23(12): 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short- term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009; 90(6): 1476–1482. [DOI] [PubMed] [Google Scholar]
- 17. Bromley LE, Booth JN, III, Kilkus JM, Imperial JG, Penev PD. Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep. 2012; 35(7): 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tucker AM, Dinges DF, Van Dongen HP. Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res. 2007; 16(2): 170–180. [DOI] [PubMed] [Google Scholar]
- 19. Buckelmüller J, Landolt HP, Stassen HH, Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006; 138(1): 351–356. [DOI] [PubMed] [Google Scholar]
- 20. Rao MN, Blackwell T, Redline S, et al. Association between sleep architecture and measures of body composition. Sleep. 2009; 32(4): 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Theorell-Haglöw J, Berne C, Janson C, Sahlin C, Lindberg E. Associations between short sleep duration and central obesity in women. Sleep. 2010; 33(5): 593–598. [PMC free article] [PubMed] [Google Scholar]
- 22. Rutters F, Gonnissen HK, Hursel R, Lemmens SG, Martens EA, Westerterp-Plantenga MS. Distinct associations between energy balance and the sleep characteristics slow wave sleep and rapid eye movement sleep. Int J Obes (Lond). 2012; 36(10): 1346–1352. [DOI] [PubMed] [Google Scholar]
- 23. Shapiro CM, Catterall J, Warren P, et al. Lean body mass and non-rapid eye movement sleep. Br Med J (Clin Res Ed). 1987; 294(6563): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gronfier C, Luthringer R, Follenius M, et al. A quantitative evaluation of the relationships between growth hormone secretion and delta wave electroencephalographic activity during normal sleep and after enrichment in delta waves. Sleep. 1996; 19(10): 817–824. [DOI] [PubMed] [Google Scholar]
- 25. Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000; 284(7): 861–868. [DOI] [PubMed] [Google Scholar]
- 26. St-Onge MP, Roberts A, Shechter A, Choudhury AR. Fiber and saturated fat are associated with sleep arousals and slow wave sleep. J Clin Sleep Med. 2016; 12(1): 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wojnar J, Brower KJ, Dopp R, et al. Sleep and body mass index in depressed children and healthy controls. Sleep Med. 2010; 11(3): 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arun R, Pina P, Rubin D, Erichsen D. Association between sleep stages and hunger scores in 36 children. Pediatr Obes. 2015; 11(5): e9–e11. [DOI] [PubMed] [Google Scholar]
- 29. Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr. 2013; 109(4): 748–756. [DOI] [PubMed] [Google Scholar]
- 30. Gonnissen HK, Drummen M, Rosique Esteban N, Schoffelen PF, Westerterp-Plantenga MS. Overnight energy expenditure determined by whole-body indirect calorimetry does not differ during different sleep stages. Am J Clin Nutr. 2013; 98(4): 867–871. [DOI] [PubMed] [Google Scholar]
- 31. Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011; 589(Pt 1): 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shechter A, O’Keeffe M, Roberts AL, Zammit GK, RoyChoudhury A, St-Onge MP. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am J Physiol Regul Integr Comp Physiol. 2012; 303(9):R883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kahlhöfer J, Karschin J, Breusing N, Bosy-Westphal A. Relationship between actigraphy-assessed sleep quality and fat mass in college students. Obesity (Silver Spring). 2016; 24(2): 335–341. [DOI] [PubMed] [Google Scholar]
- 34. Wirth MD, Hébert JR, Hand GA, et al. Association between actigraphic sleep metrics and body composition. Ann Epidemiol. 2015; 25(10): 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bailey BW, Allen MD, LeCheminant JD, et al. Objectively measured sleep patterns in young adult women and the relationship to adiposity. Am J Health Promot. 2014; 29(1): 46–54. [DOI] [PubMed] [Google Scholar]
- 36. Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int J Obes (Lond). 2015; 39(1): 39–44. [DOI] [PubMed] [Google Scholar]
- 37. Karklin A, Driver HS, Buffenstein R. Restricted energy intake affects nocturnal body temperature and sleep patterns. Am J Clin Nutr. 1994; 59(2): 346–349. [DOI] [PubMed] [Google Scholar]
- 38. Afaghi A, O’Connor H, Chow CM. High-glycemic-index carbohydrate meals shorten sleep onset. Am J Clin Nutr. 2007; 85(2): 426–430. [DOI] [PubMed] [Google Scholar]
- 39. Dashti HS, Zuurbier LA, de Jonge E, et al. Actigraphic sleep fragmentation, efficiency and duration associate with dietary intake in the Rotterdam study. J Sleep Res. 2016; 25(4): 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989; 74(5): 728–738. [DOI] [PubMed] [Google Scholar]
- 41. Bassett DR, Howley ET, Thompson DL, et al. Validity of inspiratory and expiratory methods of measuring gas exchange with a computerized system. J Appl Physiol. 2001; 91(1): 218–224. [DOI] [PubMed] [Google Scholar]
- 42. Cooper JA, Watras AC, O’Brien MJ, et al. Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J Am Diet Assoc. 2009; 109(1): 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hise ME, Sullivan DK, Jacobsen DJ, Johnson SL, Donnelly JE. Validation of energy intake measurements determined from observer-recorded food records and recall methods compared with the doubly labeled water method in overweight and obese individuals. Am J Clin Nutr. 2002; 75(2): 263–267. [DOI] [PubMed] [Google Scholar]
- 44. Rechtschaffen A, Kales A, eds. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: UCLA Brain Information Service; 1968. [Google Scholar]
- 45. Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: implications for cardiometabolic disease risk. Soc Sci Med. 2013; 79: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004; 27(7): 1255–1273. [DOI] [PubMed] [Google Scholar]
- 47. Gallagher D, Song MY. Evaluation of body composition: practical guidelines. Prim Care. 2003; 30(2): 249–265. [DOI] [PubMed] [Google Scholar]
- 48. Panossian LA, Veasey SC. Daytime sleepiness in obesity: mechanisms beyond obstructive sleep apnea—a review. Sleep. 2012; 35(5): 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luppi M, Cerri M, Martelli D, et al. Waking and sleeping in the rat made obese through a high-fat hypercaloric diet. Behav Brain Res. 2014; 258: 145–152. [DOI] [PubMed] [Google Scholar]
- 50. Katayose Y, Tasaki M, Ogata H, Nakata Y, Tokuyama K, Satoh M. Metabolic rate and fuel utilization during sleep assessed by whole-body indirect calorimetry. Metabolism. 2009; 58(7): 920–926. [DOI] [PubMed] [Google Scholar]
- 51. Zhou J, Kim JE, Armstrong CL, Chen N, Campbell WW. Higher-protein diets improve indexes of sleep in energy-restricted overweight and obese adults: results from 2 randomized controlled trials. Am J Clin Nutr. 2016; 103(3): 766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burton-Freeman B. Dietary fiber and energy regulation. J Nutr. 2000; 130(2S Suppl): 272–275S. [DOI] [PubMed] [Google Scholar]
- 53. Eilat-Adar S, Sinai T, Yosefy C, Henkin Y. Nutritional recommendations for cardiovascular disease prevention. Nutrients. 2013; 5(9): 3646–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]