Abstract
Study Objectives:
Investigate whether a realistic “dose” of shortened sleep, relative to a well-rested state, causes a decline in adolescents’ learning and an increase in inattentive and sleepy behaviors in a simulated classroom setting.
Methods:
Eighty-seven healthy 14.0- to 16.9-year olds underwent a 3-week sleep manipulation protocol, including two 5-night sleep manipulation conditions presented in a randomly counterbalanced within-subjects cross-over design. Wake time was held constant. Bedtimes were set to induce Short Sleep (SS; 6.5 hours in bed) versus Healthy Sleep (HS; 10 hours in bed). During the morning at the end of each condition, participants underwent a simulated classroom procedure in which they viewed lecture-based educational videotapes and completed relevant quizzes. Their behaviors in the simulated classroom were later coded by condition-blind raters for evidence of inattention and sleepiness.
Results:
Adolescents had a longer average sleep period during HS (9.1 hours) than SS (6.5 hours). Compared to scores during HS, adolescents scored significantly lower on the quiz, showed more behaviors suggestive of inattention and sleepiness in the simulated classroom, and were reported by adolescents themselves and by their parents to be more inattentive and sleepy during SS. However, the impact of the manipulation on quiz scores was not mediated by changes in attention or sleepiness.
Conclusions:
Although effect sizes were modest, these findings suggest that previously-reported correlations between sleep duration and academic performance reflect true cause–effect relationships. Findings add to the growing evidence that the chronically shortened sleep experienced by many adolescents on school nights adversely impacts their functioning and health.
Keywords: Sleep deprivation, adolescence, learning.
Statement of significance
For over a century, advocates for adolescent sleep guidelines have pointed, in part, to poor school performance as a consequence of short sleep. However, prior research has been largely correlational, which has precluded confident cause–effect conclusions. This study is the first to show that an experimentally manipulated, realistic “dose” of shortened sleep (five nights at 6.5 hours of sleep; similar to a school week) causes a decline in learning, as well as behaviors suggestive of sleepiness and inattention, in a classroom-like setting. Findings lend confidence that the short sleep experienced by millions of adolescents on school nights can negatively affect school functioning.
INTRODUCTION
Concerns that children and adolescents get too little sleep date back at least a century, with a common theme being that school performance is hampered when students are poorly rested.1 These concerns have been echoed in recent policy papers by the American Academy of Pediatrics2 and the National Sleep Foundation.3 Adolescents are often cited as being at particular risk, as sleep duration dips across adolescence on school nights,4 and adolescents in developed countries have shown a greater reduction in sleep over the past century than have younger children5 or adults.6
However, findings on adolescent sleep and school performance have been surprisingly mixed. A 2010 meta-analysis found that the association between sleep duration and school performance was small, inconsistent, less prominent in adolescents than in younger children, and weaker than the link between school performance and other aspects of sleep (eg, sleep quality).7 Several recent studies have confirmed those findings or even reported that the short sleep that many adolescents get is “optimal” for maximizing academic test scores.8–10 Even when evidence suggests that sleep duration is related to school performance, the mechanism remains unclear. It is commonly thought that inadequate sleep induces inattention and/or daytime sleepiness in the classroom, but there have been few true tests of mediation, and those that have studied mediation have tended to rely on self-report.11 Critics have pointed out that the literature is prone to potential reporter biases, relies heavily on correlational designs, and lacks realistic experimental work that uses school-relevant outcome measures.1,12 As a result, some have questioned the scientific foundation for current guidelines related to adolescent sleep.1,10,13
The primary goal of this study was to test whether, compared to a healthy sleep (HS) condition, experimental short sleep (SS) causes adolescents to have objectively verifiable learning deficits, inattention, and daytime sleepiness in a simulated classroom. To maximize generalizability to real-world settings, the SS condition was of realistic intensity and duration (around 6.5 hours for five nights, as in a school week), assessments occurred during what would normally be school hours, and the simulated classroom involved a developmentally-relevant task: an educational lecture. Secondarily, this study tested whether SS induced inattention and sleepiness in daily life, as reported by adolescent participants and their parents. Finally, we explored whether any effect of the sleep manipulation on learning was statistically mediated by changes in attention or sleepiness.
METHODS
The study was approved and overseen by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center. Adolescent participants provided informed assent and their parents provided informed consent. Data collection occurred exclusively during summer breaks from school to eliminate the potential for adverse effects on actual scholastic performance. The procedures and sample overlap with those described in our previous papers that focused on dietary outcomes and emotion regulation.14–17 The current paper reflects a larger sample and a unique focus on learning and learning- relevant behaviors.
Participants
Healthy adolescents aged 14.0–16.9 years and their parents were recruited through flyers posted throughout a regional pediatric medical center, supplemented by a research coordinator nested within a clinic that serves low-income adolescents to ensure diverse representation. Eligibility was established by telephone screening and verified in person during the study’s initial visit. Exclusions included a previously diagnosed psychiatric disorder, a history of neurological illness or injury, a body mass index > 30, an IQ < 70, daily consumption of > 1 coffee or energy drink or > 2 caffeinated soft drinks, parent-suspected illegal substance use, use of medication that can affect sleep or daytime alertness, pregnancy, or obligations that would require a bedtime later than 10 pm or a wake time prior to 6 am.
Procedures
The sleep manipulation protocol has been detailed previously.14–17 Briefly, each adolescent participant underwent a 3-week protocol. Wake time was held constant, set at the time the participant would need to awaken to arrive for an office visit at 8:30 am. The initial week (sleep stabilization) was intended to ensure that participants were on a steady morning-rising schedule. This was deemed particularly important because adolescents can shift to a later sleep phase when not in school; the sleep stabilization nights allowed all teens to adjust to a similar sleep phase prior to the experimental conditions. During that initial week, participants were asked to awaken at the predetermined time, but were permitted to self-select their bedtimes. Those who were unable to rise on time were considered ineligible for randomization.
Eligible participants then entered a within-subjects, randomized cross-over experiment comparing five night spans (Monday–Friday nights) of SS versus HS, with the order of conditions randomly counterbalanced across participants. Adolescents adjusted their bedtime to allow for 6.5 hours in bed during the SS condition and 10 hours in bed during the HS condition. The 6.5 hour requirement for SS is similar to what 20%–25% of adolescents experience regularly on school nights,4,10 and has been shown to alter mood, mood regulation, and dietary behaviors.14,18,17 Conversely, a nightly sleep opportunity of 10 hours has been shown to induce sleep satiety in adolescents and eliminated carry-over effects in our prior work.14,18–20
Participants were asked to be fully ready for bed at the prescribed bedtime, with lights out and electronics off. They were instructed not to nap and to consume no more than one coffee or energy drink, or no more than two caffeinated sodas per day. Parents were asked to support their adolescents in achieving these goals. Participants slept at home, monitored via sleep diaries and actigraphy.
Participants and a parent came in for office visits on the Saturday morning after the stabilization, SS, and HS conditions, at which time we collected our primary outcome measures. We endeavoured to maintain consistent timing of activities for each participant, with all data collected between 8:30 and 11:30 am. Between conditions, participants had a two-night washout period on Saturday and Sunday nights, identical to the initial sleep stabilization week.
Measures
Background Information
During the initial visit, parents reported family income and participant age, sex, ethnicity, and race via questionnaires. Parents and participants independently reported on the participant’s typical school grades using the 8-point scale developed by Wolfson and Carskadon21; the two reports correlated strongly (rho = 0.85, p < .001). Participants also were tested individually using a validated intelligence screener (Kaufman Brief Intelligence Test-222).
Sleep Monitoring (Adherence)
Participants wore a Micro Motionlogger SleepWatch (Ambulatory Monitoring, Inc.; AMI) on their non-dominant wrist and reported their bedtime and wake time in a daily sleep diary. The SleepWatch contains a sensitive 3-plane accelerometer and memory to archive data on movement patterns. Default settings were used to collect data in “zero crossing” mode. Participants were asked to wear the units at all times except during participation in contact sports, bathing/showering, and swimming. Trained coders uploaded the actigraph data weekly and, with the support of the sleep diaries and follow-up queries to participants and parents, identified artifact-free periods for processing via AMI software using the validated Sadeh algorithm.23 Sleep onset and offset, sleep period (time between onset and offset, ignoring nocturnal awakenings), time asleep (sleep period minus nocturnal awakening time) and sleep efficiency (time asleep divided by sleep period) were the primary sleep monitoring outcomes. All sleep indexes reflected the nightly average across each condition. Participants who did not average at least one hour longer nightly sleep period during HS than SS were considered non-adherent to the sleep regimen and subsequently were not included in analyses.
Simulated Classroom (Primary Outcomes)
Videotaped sessions of primary-school children in simulated classrooms have been used for years to assess attention24 and are sensitive to sleepiness.25 We developed a protocol that captures key elements of those primary-school simulated classrooms—which require self-regulation of attention and arousal, have increased ecological validity over office tests, and which archive behavioral performance to allow objective scoring—while engaging adolescents in a developmentally-relevant task: viewing an educational lecture. Three lecture-based videos were licensed from “The Western Tradition” series by the Annenberg Foundation (St. Louis, MO), based on the series’ professional production quality, thematically-coherent half-hour segments, and content that is of potential relevance to high school students.
To introduce adolescents to the task, they viewed the first video in the series in a group setting during the initial visit. On experimental week visits, they viewed one of two other videos (order randomly counterbalanced) while seated individually behind a table in a room about 6 feet away from a 19-inch screen, with subdued indoor lighting. No paper, cell phones, or other distracting materials were allowed. Their behaviors were recorded using a high-definition camera mounted next to the screen and focused on the adolescent’s torso and head. Data captured at 256 Hz via electrooculogram (EOG) further documented eye saccades. Following each video during the experimental conditions, participants completed a brief quiz over its content.
Adolescents’ behaviors were later coded by two trained, condition-blinded raters using concurrent camera and EOG data. Each rater marked spans of time when specific, potentially-overlapping events occurred, including: (1) “Inattention,” defined as looking away from the screen for ≥1 second, (2) “Yawning,” with or without vocalization or stretching, and (3) “Eyes Closed” for ≥1 second. The first was used as an objective index of attention, while the latter two captured outward manifestations of sleepiness. Raters worked independently, reconciling their coding every few sessions to resolve discrepancies and create final ratings for analyses. Data files were converted to a series of 1-second epochs, which were summed for each rated behavior across the 24 minutes of each recording after the initial, series-wide video introduction. Interrater reliability was strong across all three behaviors (intraclass correlation coefficients > 0.83). Final (consensus) behavior rating scores also correlated moderately across the experimental weeks (rho = 0.51–0.70, p < .001), consistent with the measurement of a similar construct under different conditions.
Quizzes were comprised of multiple-choice and true–false questions covering video content, developed specifically for this study. The 22 preliminary items on each quiz were administered to all participants, but trimmed to final scales for analysis based upon data from the first 20 subjects, ignoring condition. Item trimming balanced several factors: (1) difficulty, reflecting a moderate mix of correct and incorrect responses, (2) equal coverage of material across the full span of each video, and (3) consistency in item format. The final 16-item scales queried evenly across content from each quarter of each video, using two multiple-choice and two true–false questions per quarter. In the final sample, internal consistency was acceptable (alphas = 0.7). Quiz scores correlated significantly across the experimental weeks (rho = 0.52, p < .001) and with overall intelligence (rho = 0.46–0.52, p ≤ .001) and school grades per parent-report (rho = 0.33–0.35, p < .005) and self-report (rho = 0.37–0.42, p ≤ .001), suggesting the measurement of a consistent construct that is relevant to real-world scholastic functioning.
Attention and Sleepiness in Daily Life (Secondary Outcomes)
At each Saturday morning assessment, parents and participants independently completed previously-validated questionnaires querying about attention (nine items) and daytime sleepiness (five items) “over the past 5 days, including today.”14,18 Questionnaire items are provided in Supplementary Appendix. In the current sample, each form showed strong internal consistency (alphas = 0.8–0.9) and moderate stability across sessions (rho = 0.24–0.67, p ≤ .04).
Analytic Approach
Preliminary Analyses
We explored whether there were differences in characteristics of participants included in the final analyses versus those who either were randomized but subsequently dropped out or were non-adherent to sleep instructions using t tests (normally- distributed variables), Wilcoxon Signed Rank Tests (non- normal continuous variables), or chi-square (categorical data). The sleep variables, which were normally-distributed, were compared across the two experimental conditions using paired- sample t tests.
Primary and Secondary Analyses
Although quiz scores were roughly normal in distribution, the other video and questionnaire outcomes were highly skewed and did not consistently follow an alternative distribution (eg, Poisson). Accordingly, Wilcoxon Tests compared the SS and HS conditions on all primary and secondary outcomes. In all cases, paired-sample t tests yielded similar findings, but only Wilcoxon findings are presented for parsimony. An alpha threshold of 0.05 was adopted for these hypothesis-driven analyses.
Exploratory Analyses
Potential moderators/modifiers of cross-condition effects were tested in two steps. First, we computed difference scores for each sleep, primary outcome, and secondary outcome variable across the sleep manipulation (eg, quiz scores during SS minus those during HS). We then assessed whether these difference scores systematically varied across the order of sleep conditions, order in which the videos were presented, sex, or race (Caucasian vs. African American) using independent-sample Wilcoxon tests, and across participant age, IQ, parent- and teen-reported academic grades, and family income using Spearman correlations. We also explored whether participants who showed the greatest change in sleep period or in sleep quality (as indexed by sleep efficiency) across conditions also experienced differential changes on the primary and secondary outcome variables via Spearman correlations.
Finally, we explored whether cross-condition effects on quiz scores were statistically mediated by changes in attention or sleepiness using a repeated-measures General Linear Model (GLM) with quiz scores as the within-subjects variable. Effect sizes were examined with and without covariates, which included: cross-condition change in coded inattention, eyes closed, and yawning; parent-reported inattention and sleepiness; and participant-reported inattention and sleepiness.
To partially control for the large number of exploratory analyses while still allowing the potential for hypothesis-generating findings, the alpha threshold for exploratory analyses was set at 0.01.
RESULTS
Sample Composition
Of 116 adolescents who were randomized, five dropped out mid-study, 10 were non-adherent to the sleep regimen, and we were unable to gather simulated classroom data during at least one sleep condition for 14 due to scheduling problems or equipment malfunction. Characteristics of the final sample of 87 adolescents are provided in Table 1. The final sample mirrored the demographics of the community in which the study took place, with a similar number of Caucasian and African American adolescents, average overall intelligence, and a median family income similar to the local population. More females than males volunteered. Per actigraphy, the participants averaged a sleep period just short of 7 hours during the initial week (roughly midnight to 7 am). Randomization was successful; the order of conditions was not associated with any variable listed in Table 1 (p > .10).
Table 1.
Sample Features at the Time of Randomization. Categorical Variables Are Listed in Percentages, Normally-Distributed Variables Are Summarized as Mean ± Standard Deviation, and Non-normal Continuous Variables Are Summarized as Median (25th, 75th Percentile).
| Percent, mean ± SD, or median (25th, 75th percentile) | |
|---|---|
| Female (%) | 66% |
| Race/Ethnicitya (%): | |
| White | 45% |
| Blacka | 46% |
| Multiracial | 6% |
| Other/not reported | 3% |
| Age (y) | 15.46 ± 0.77 |
| Intelligence (norm average = 90–110) | 97.52 ± 12.16 |
| Parent-report GPA (US 4-point scale) | 3.5 (3, 4) |
| Participant-report GPA (US 4-point scale) | 3.5 (2.5, 4) |
| Family income (in $1000s of US Dollars) | 40–50 (20–30, 80–90) |
| Sleep onset (time) | 00:14 ± 1:13 |
| Sleep offset (time) | 07:10 ± 0:38 |
| Sleep period (hours between onset and offset) | 6.94 ± 0.99 |
| Time asleep (sleep period minus nocturnal awakening time) | 6.50 ± 0.72 |
| Sleep efficiency (time asleep divided by sleep period) | 0.92 ± 0.05 |
| Parent-report inattention (raw) | 1.56 (0.00, 5.00) |
| Parent-report sleepiness (raw) | 2.00 (0.00, 4.00) |
| Participant-report inattention (raw) | 6.00 (1.00, 7.00) |
| Participant-report sleepiness (raw) | 4.00 (2.00, 6.00) |
One participant self-identified as Hispanic black; otherwise none self-identified as Hispanic.
Compared to the final sample, the 29 who were lost or dropped were slightly older (Mean = 16.2 vs. 15.5 years). However, retained participants did not differ from those who were lost/dropped in their assigned order of sleep conditions; sex, race or intelligence; family income; sleep during the stabilization week; or parent- or self-reported inattention or sleepiness during that initial week (p > .10).
Sleep During the Experimental Conditions
As shown in Table 2, adolescents’ sleep period averaged 2.53 (SD = 0.67) hours longer during HS than SS, p < .001. As planned, this was due to earlier bedtimes during HS; rise times were nearly identical across conditions.
Table 2.
Actigraph-Estimated Sleep Features Across Experimental Conditions.
| Short Sleep (SS) | Healthy Sleep (HS) | p | |
|---|---|---|---|
| Sleep onset (time) | 00:42 ± 0:43 | 22:11 ± 0:52 | <.001 |
| Sleep offset (time) | 07:12 ± 0:35 | 07:13 ± 0:34 | .784 |
| Sleep period (h) | 6.53 ± 0.99 | 9.06 ± 0.81 | <.001 |
| Time asleep (h) | 6.05 ± 0.72 | 8.08 ± 1.07 | <.001 |
| Sleep efficiency | 0.93 ± 0.05 | 0.89 ± 0.07 | <.001 |
Simulated Classroom Performance (Primary Outcomes)
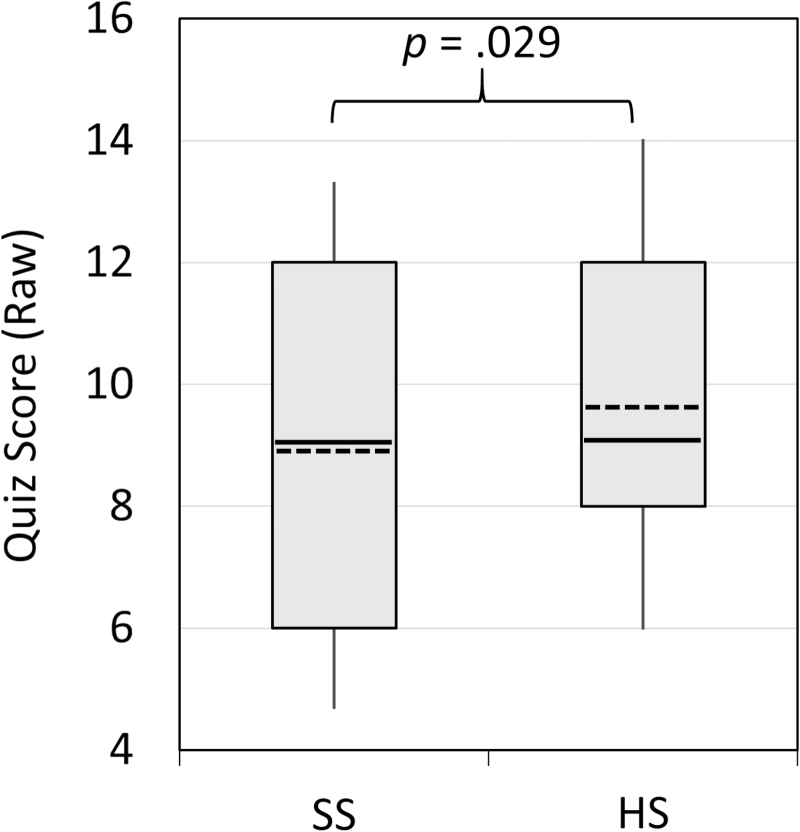
As shown in Figure 1, adolescents scored modestly but significantly higher on video quizzes following HS compared to SS, z = 2.18, p = .029. Closer inspection of the data indicated that this was primarily because teens who scored above the median following HS averaged substantially lower scores during SS (mean decline = 1.8 points), whereas those who scored at or below the median following HS averaged little change in score (mean rise of 0.2 points).
Figure 1.
Quiz scores across the Short Sleep (SS) and Healthy Sleep (HS) conditions. The bottom and top boundaries of each box reflect the 25th and 75th percentiles, respectively, and the tips of each “whisker” line reflect the 10th and 90th percentiles. Within each box the median is a solid line, while the mean is a dashed line.
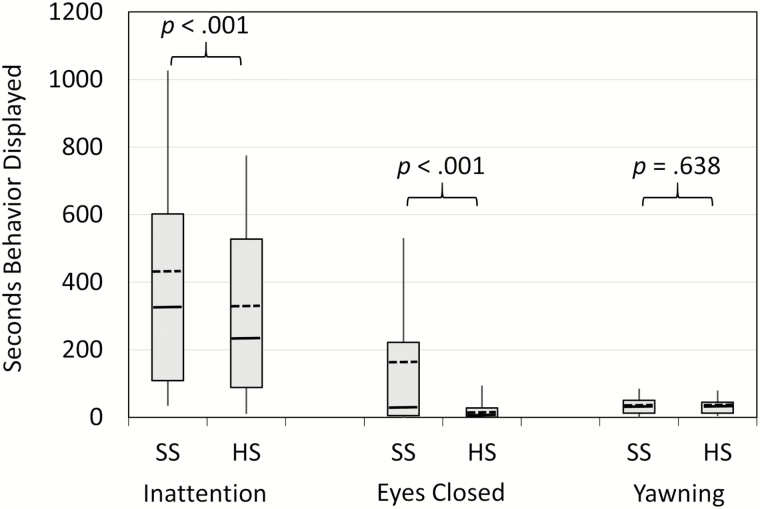
Figure 2 illustrates the changes in behaviors during our simulated classroom procedure. Adolescents showed significantly greater inattention and protracted eye closure during SS than HS, z > 3.67, p < .001. They did not differ in yawning across conditions, z = 0.47, p = .638.
Figure 2.
Behaviors during the simulated classroom procedure across the Short Sleep (SS) and Healthy Sleep (HS) conditions. The bottom and top boundaries of each box reflect the 25th and 75th percentiles, respectively, and the tips of each “whisker” line reflect the 10th and 90th percentiles. Within each box the median is a solid line, while the mean is a dashed line.
Behaviors in Daily Life (Secondary Outcomes)
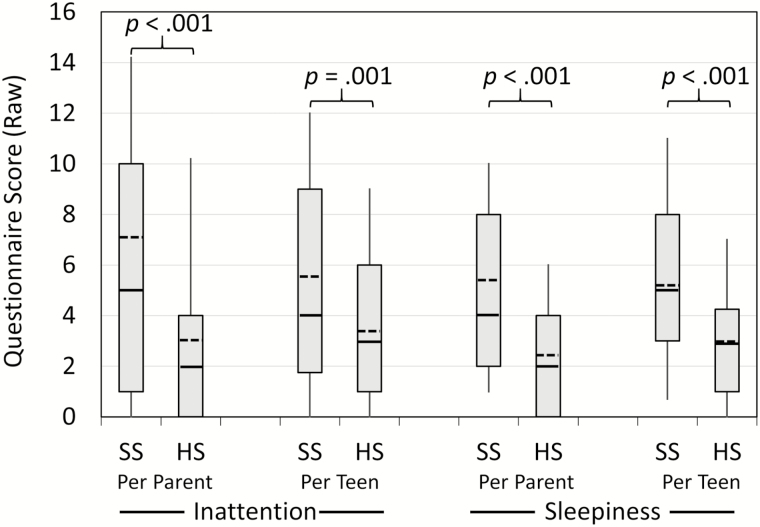
As shown in Figure 3, parents and adolescents alike reported that the participant showed significantly greater inattention and daytime sleepiness during SS than during HS, z > 3.37, p ≤ .001.
Figure 3.
Parent- and adolescent-reported inattentive and sleepy behaviors across the Short Sleep (SS) and Healthy Sleep (HS) conditions. The bottom and top boundaries of each box reflect the 25th and 75th percentiles, respectively, and the tips of each “whisker” line reflect the 10th and 90th percentiles. Within each box the median is a solid line, while the mean is a dashed line.
Moderator Effects (Exploratory Analyses)
There was no significant difference due to age, sex, parent- or adolescent-reported academic grades, or the order in which the videos were presented on the strength of the cross-condition effect on any sleep, simulated classroom, or daily behavior variable. The sleep manipulation affected parent- and participant-reported sleepiness more strongly amongst higher-income families than lower-income families (rho = 0.31–0.36, p ≤ .004), and amongst Caucasian adolescents than amongst African American adolescents (z = 2.73–3.76, p ≤ .006). Higher IQ was also associated with a greater effect of the manipulation on participant-reported sleepiness (rho = 0.29, p = .006), but not on parent-reported sleepiness (rho = 0.15, p > .10), on any simulated classroom outcome, nor on any sleep variable. Neither family income nor ethnicity significantly moderated the effect of the sleep manipulation on the simulated classroom quiz or behavior ratings; adolescent sleep onset, offset, or period; or parent- or participant-reported attention. The effect of the sleep manipulation on coded inattention and eyes closed appeared greater in those who underwent HS first (followed by SS) than amongst those who had the opposite sequence (z = 2.81–4.30, p ≤ .005). However, the functional significance of this is not clear; the order of conditions a participant underwent had no significant effect on any of the sleep variables, parent- or participant-reported attention or sleepiness, quiz scores, or yawning behaviors.
Greater declines in sleep efficiency from SS to HS were associated with greater improvements in coded attention in the simulated classroom (rho = 0.33, p = .005), but not with cross-condition change in other coded behaviors, quiz scores, or parent- or participant-reported inattention or sleepiness. There was no significant association between cross-condition change in sleep period and cross-condition change in any primary or secondary outcome variable. While these exploratory moderator analyses offer points of potential exploration in future research, we caution that some might be spurious, as 120 such analyses were run in all.
Mediator Effects (Exploratory Analyses)
GLM confirmed the effect of sleep condition on quiz scores without any covariates entered, η2partial = 0.059, p = .027. This effect persisted after controlling for cross-condition changes in coded behaviors in the simulated classroom and parent- and adolescent-reported attention and sleepiness, η2partial = 0.073, p = .030. We found no evidence that the impact of the sleep manipulation on quiz scores could be attributed to observed changes in attention or daytime sleepiness.
DISCUSSION
To our knowledge, this is the first study to objectively document that experimentally induced SS affects adolescents’ learning and behaviors in a classroom-like setting. Compared to when well-rested, after five nights of SS adolescents showed diminished learning from lecture-format educational videos, and also displayed evidence of increased sleepiness and difficulty paying attention while watching the lectures. Further, the impact of the sleep manipulation on attention and sleepiness extended into real-world settings, based upon parent- and adolescent-report. However, we did not find evidence that the decrement in learning induced by SS was mediated by inattention or daytime sleepiness; that learning effect remained even after controlling for cross-condition changes in observed behaviors and in parent- and adolescent-report of daytime sleepiness and inattention.
This study used a randomly counterbalanced within-subjects cross-over design that maximized statistical power and allowed for true cause–effect inference, overcoming a common critique of the otherwise largely correlational research literature.12 Balancing this rigor, the sleep manipulation mirrored what many adolescents experience on a regular basis during the school year: five consecutive nights of less than 7 hours of sleep.4 That condition was compared against a sleep period similar to what adolescents obtain regularly on non-school nights (eg, weekends).4 This leads to two alternative perspectives; the longer sleep condition could be viewed as sleep extension (compared to sleep on school nights) or the shorter condition could be viewed as shortened sleep (compared to sleep on non-school nights). While acknowledging both perspectives, we favor the latter. Sleep during this study’s longer experimental condition fits squarely within all three recently-released guidelines for healthy adolescent sleep,3,26,27 supporting the our choice of the term “HS.” While it is common on school nights for adolescents to sleep as little as in our SS condition, that amount of sleep falls far below guidelines and, to our knowledge, below the optimal sleep level reported in any empirical study in any age range.
The outcome assessment was also designed to balance rigor with real-world relevance (also called ecological validity). Though the study maintained tight control over the measurement context, the learning task—an educational lecture—was selected based on its relevance to high school students. The task occurred on the morning after five nights of shortened sleep, a temporal analogue to Friday morning class. Further, the simulated classroom allowed for objective, condition-blind coding of adolescents’ behaviors, overcoming concerns about reporter biases.12 Of note, the sleep manipulation had a similar effect on both objective behavioral ratings and on parent- and adolescent-report of inattention and sleepiness, suggesting that effects are robust and not a measurement artifact.
Findings provide a key bridge across a previously vexing research gap. Experimental sleep manipulation studies demonstrate cause–effect relationships, but the few that have been published with adolescents have tended to use unrealistically extreme or short-term manipulations27–32 and outcome measures that are dissimilar to classroom functioning.19,25,28–37 On the other hand, correlational studies have focused on real-world circumstances, but have not allowed for confident cause–effect inferences.12 While the current study did not perfectly mimic real-world circumstances, findings converge with prior correlational work to suggest that real-world associations between sleep and academic performance reflect true cause–effect relationships.
The effect on classroom learning was relatively small, consistent with the small pooled effect sizes reported across correlational studies.7 Small effects can still be important on the population level, but it is worth acknowledging that sleep duration is only one of many contributors to academic performance. Indeed, the greatest impact of inadequate sleep may be on other metrics. For example, both correlational and experimental data show an impact of shortened sleep on adolescent mood,2,14,31,35,38 obesity/dietary choices,15,17,21,38 and risky behaviors.38–41 Further, the impact of inadequate sleep may extend to academically- relevant constructs that were not the focus of this study, such as executive functioning (eg, planning or persistence on getting homework done or studying effectively),11,18,37 overnight memory consolidation,42 or school attendance.39
Limitations and Next Research Steps
A key limitation of the study was that there were only two sleep conditions; the experimental contrast between SS and well-rested conditions offers clear evidence of causation, but does not pinpoint the “optimal” sleep duration for adolescents.12 The manipulation also focused on sleep duration, though sleep timing (relative to the circadian clock) and sleep consistency also may be important.23,38,43 Because the sleep manipulation focused on shifting bedtimes, not rise times, it does not speak directly to the impact of school start time on sleep and academic performance. Future experimental research should parametrically vary sleep duration, timing and regularity; these are challenging studies to conduct, but would inform critical debates with public health relevance.43 Similarly, it may be fruitful to systematically vary participant sleep during the initial adaptation period and washout periods, thereby manipulating the opportunity for recovery sleep and assessing the potential for sleep “banking,” as has been described in adults.44
Inferences were also limited by our outcome measures. Ethical principles ruled out a shortened sleep protocol during the school year, so we could not directly measure changes in actual academic functioning. Preliminary findings provided reassurance that our learning measures (multiple-choice quizzes), which were designed to be difficult to minimize ceiling effects, correlated with measures of intelligence and actual school grades. However, the quizzes were not independently validated, and the simulated classroom was an approximation of one type of classroom setting (lectures on the history of Western civilization), was administered only once per condition, was video-recorded and clearly part of a research study (which could alter adolescent behaviors), and was not administered in a way that allowed for examination of time of day (eg, first class of the day vs. later in the day). Future research could address ethics concerns by extending the sleep of short-sleeping adolescents during the school year (eg, using a sleep manipulation yoked to each teen’s habitual sleep), and outcome measures could be sampled more frequently, less conspicuously, across multiple days, and across varying times of day and types of classes (eg, lecture vs. interactive/lab).
Future research should also examine potential mediators and moderators of effect. Unexpectedly, we found that the impact of shortened sleep on learning was independent of the impact on attention or daytime sleepiness. This may have been due to measurement factors. For example, our video coding scheme may have failed to capture important information (eg, participants daydreaming but still appearing to watch the video). However, parent- and adolescent-reported inattention and daytime sleepiness in daily life also failed to statistically mediate the impact of the sleep manipulation on quiz scores. Inattention and daytime sleepiness can be measured in multiple ways, but to remain plausible mediators they would have to be conceptualized in a way that is neither introspectively accessible nor behaviorally manifest. Alternatively, the effect of the sleep manipulation on quiz scores may have related to changes in motivation, interest, memory encoding, or another unmeasured factor, awaiting future research. We encourage further work in this area, including more detailed analyses of how changes in sleep (eg, sleep architecture or quality) might relate to changes in learning.
Our data began to examine effect moderators. The sleep manipulation had similar effects on sleep patterns, and on learning and behaviors in the simulated classroom, across age, sex, race, and family income levels. Interestingly, lower-income and African American adolescents and their parents reported fewer effects of the manipulation on daytime sleepiness, for reasons that remain unclear. It is important to continue to investigate potential demographic moderators, especially when refining population-based guidelines. Exploratory inspection of raw data also suggested that teens who scored above the median on the quiz following HS averaged substantially lower scores during SS, whereas those who scored poorly after HS scored just as badly during SS. The reason for this dissociation warrants further investigation, as it cannot be accounted for by statistical regression to the mean, and the effect of the sleep manipulation on quiz scores did not systematically vary by teens’ IQ or typical academic grades. At least in theory, there may be individual differences in adolescents’ sleep need and vulnerability to sleep restriction, though to date no reliable markers for such individual differences have been validated in youth.
CONCLUSIONS
Despite study limitations, present findings confirm that 6.5 hours of nightly sleep—equivalent to what millions of high school students experience regularly on school nights—can adversely affect learning, attention, and arousal in a classroom-like situation. This adds to a growing literature showing that, even as “optimal” sleep is debated (a debate that might come to different conclusions depending on the chosen outcome43), such chronically SS can have substantial effects on adolescents’ mental and physical health.2,38 The overall body of evidence supports public health efforts to remove barriers to adolescent sleep, to prioritize the need for sleep, and to routinely screen for and address inadequate sleep and sleep disorders in clinical practice.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at SLEEP online.
FUNDING
Financial support was provided by the National Institutes of Health (R01 HL092149; UL1 RR026314).
DISCLOSURE STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGMENTS
Particular thanks to Tori Van Dyk for feedback on an earlier draft of this paper, and to the many research associates who helped make this work possible, including Ellen Manegold, Teresa Smith, Heather Strong, Dan Strotman, Sindhia Swaminathan, and Drs. Katie Baum, Roshni Rao, and Melissa Stern.
REFERENCES
- 1. Matricciani LA, Olds TS, Blunden S, Rigney G, Williams MT. Never enough sleep: a brief history of sleep recommendations for children. Pediatrics. 2012; 129(3): 548–556. [DOI] [PubMed] [Google Scholar]
- 2. Owens J. Adolescent Sleep Working Group; Committee on Adolescence Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014; 134(3): e921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015; 1: 233–243. [DOI] [PubMed] [Google Scholar]
- 4. National Sleep Foundation. Summary of Findings: 2006 Sleep In America Poll. Washington, DC: National Sleep Foundation; 2006. [Google Scholar]
- 5. Matricciani L, Olds T, Petkov J. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev. 2012; 16(3): 203–211. [DOI] [PubMed] [Google Scholar]
- 6. Bin YS, Marshall NS, Glozier N. Secular trends in adult sleep duration: a systematic review. Sleep Med Rev. 2012; 16(3): 223–230. [DOI] [PubMed] [Google Scholar]
- 7. Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bögels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med Rev. 2010; 14(3): 179–189. [DOI] [PubMed] [Google Scholar]
- 8. Tonetti L, Fabbri M, Filardi M, Martoni M, Natale V. Effects of sleep timing, sleep quality and sleep duration on school achievement in adolescents. Sleep Med. 2015; 16(8): 936–940. [DOI] [PubMed] [Google Scholar]
- 9. Short MA, Gradisar M, Lack LC, Wright HR. The impact of sleep on adolescent depressed mood, alertness and academic performance. J Adolesc. 2013; 36(6): 1025–1033. [DOI] [PubMed] [Google Scholar]
- 10. Eide E, Showalter M. Sleep and student achievement. East Econ J. 2012: 1–13. [Google Scholar]
- 11. Perkinson-Gloor N, Lemola S, Grob A. Sleep duration, positive attitude toward life, and academic achievement: the role of daytime tiredness, behavioral persistence, and school start times. J Adolesc. 2013; 36(2): 311–318. [DOI] [PubMed] [Google Scholar]
- 12. Matricciani L, Blunden S, Rigney G, Williams MT, Olds TS. Children’s sleep needs: is there sufficient evidence to recommend optimal sleep for children? Sleep. 2013; 36(4): 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell IG, Feinberg I. Later school start times? Not so fast. The Davis Enterprise. March 8, 2015. [Google Scholar]
- 14. Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry. 2014; 55(2): 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beebe DW, Zhou A, Rausch J, Noe O, Simon SL. The impact of early bedtimes on adolescent caloric intake varies by chronotype. J Adolesc Health. 2015; 57(1): 120–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon SL, Field J, Miller LE, DiFrancesco M, Beebe DW. Sweet/dessert foods are more appealing to adolescents after sleep restriction. PLoS One. 2015; 10(2): e0115434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beebe DW, Simon S, Summer S, Hemmer S, Strotman D, Dolan LM. Dietary intake following experimentally restricted sleep in adolescents. Sleep. 2013; 36(6): 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beebe DW, Fallone G, Godiwala N, et al. Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. J Child Psychol Psychiatry. 2008; 49(9): 915–923. [DOI] [PubMed] [Google Scholar]
- 19. Beebe DW, Difrancesco MW, Tlustos SJ, McNally KA, Holland SK. Preliminary fMRI findings in experimentally sleep-restricted adolescents engaged in a working memory task. Behav Brain Funct. 2009; 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beebe DW, Rose D, Amin R. Attention, learning, and arousal of experimentally sleep-restricted adolescents in a simulated classroom. J Adolesc Health. 2010; 47(5): 523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998; 69(4): 875–887. [PubMed] [Google Scholar]
- 22. Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Scale-Second Edition Manual. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- 23. Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994; 17(3): 201–207. [DOI] [PubMed] [Google Scholar]
- 24. Barkley RA. Attention-Deficit Hyperactivity Disorder: A handbook for diagnosis and treatment. 2nd ed. New York, NY: Guilford; 1998. [Google Scholar]
- 25. Fallone G, Acebo C, Arnedt JT, Seifer R, Carskadon MA. Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Perceptual and Motor Skills. 2001; 93(1): 213–229. [DOI] [PubMed] [Google Scholar]
- 26. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a statement of the American academy of sleep medicine. J Clin Sleep Med. 2016; 12(6): 785–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tremblay MS, Carson V, Chaput JP, et al. Canadian 24-hour movement guidelines for children and youth: an integration of physical activity, sedentary behaviour, and Sleep. Appl Physiol Nutr Metab. 2016; 41(6): S311–S327. [DOI] [PubMed] [Google Scholar]
- 28. Carskadon MA, Harvey K, Dement WC. Acute restriction of nocturnal sleep in children. Percept Motor Skills. 1981; 53: 103–112. [Google Scholar]
- 29. Carskadon MA, Harvey K, Dement WC. Sleep loss in young adolescents. Sleep. 1981; 4(3): 299–312. [DOI] [PubMed] [Google Scholar]
- 30. Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10-14. Sleep. 1998; 21(8): 861–868. [PubMed] [Google Scholar]
- 31. Lo JC, Ong JL, Leong RL, Gooley JJ, Chee MW. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep. 2016; 39(3): 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kopasz M, Loessl B, Valerius G, et al. No persisting effect of partial sleep curtailment on cognitive performance and declarative memory recall in adolescents. J Sleep Res. 2010; 19(1, pt 1): 71–79. [DOI] [PubMed] [Google Scholar]
- 33. Louca M, Short MA. The effect of one night’s sleep deprivation on adolescent neurobehavioral performance. Sleep. 2014; 37(11): 1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiang F, VanDyke RD, Zhang J, Li F, Gozal D, Shen X. Effect of chronic sleep restriction on sleepiness and working memory in adolescents and young adults. J Clin Exp Neuropsychol. 2011; 33(8): 892–900. [DOI] [PubMed] [Google Scholar]
- 35. Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, Harvey AG. Sleep deprivation in adolescents and adults: changes in affect. Emotion. 2010; 10(6): 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Voderholzer U, Piosczyk H, Holz J, et al. Sleep restriction over several days does not affect long-term recall of declarative and procedural memories in adolescents. Sleep Med. 2011; 12(2): 170–178. [DOI] [PubMed] [Google Scholar]
- 37. Cohen-Zion M, Shabi A, Levy S, Glasner L, Wiener A. Effects of partial sleep deprivation on information processing speed in adolescence. J Int Neuropsychol Soc. 2016; 22(4): 388–398. [DOI] [PubMed] [Google Scholar]
- 38. Shochat T, Cohen-Zion M, Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Med Rev. 2014; 18(1): 75–87. [DOI] [PubMed] [Google Scholar]
- 39. Hysing M, Harvey AG, Linton SJ, Askeland KG, Sivertsen B. Sleep and academic performance in later adolescence: results from a large population-based study. J Sleep Res. 2016; 25(3): 318–324. [DOI] [PubMed] [Google Scholar]
- 40. Davis AL, Avis KT, Schwebel DC. The effects of acute sleep restriction on adolescents’ pedestrian safety in a virtual environment. J Adolesc Health. 2013; 53(6): 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garner AA, Miller MM, Field J, Noe O, Smith Z, Beebe DW. Impact of experimentally manipulated sleep on adolescent simulated driving. Sleep Med. 2015; 16(6): 796–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Bruin EJ, van Run C, Staaks J, Meijer AM. Effects of sleep manipulation on cognitive functioning of adolescents: a systematic review. Sleep Med Rev. 2016. [DOI] [PubMed] [Google Scholar]
- 43. Beebe DW. The cumulative impact of adolescent sleep loss: next steps. Sleep. 2016; 39(3): 497–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking sleep: realization of benefits during subsequent sleep restriction and recovery. Sleep. 2009; 32(3): 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.