Abstract
Study Objectives:
The association between restless legs syndrome (RLS) and Parkinson’s disease (PD) has been extensively studied with inconclusive results; therefore, we prospectively examined the associations of the presence of RLS with development of incident PD.
Methods:
From a nationally representative prospective cohort of almost 3.5 million US veterans (age: 60 ± 14 years, 93% male, median follow-up time of 7.8 years [interquartile range: 6.4–8.4 years]), we created a propensity-matched cohort of 100882 PD-free patients and examined the association between prevalent RLS and incident PD. This association was also assessed in the entire cohort. Associations were examined using Cox models.
Results:
There were 68 incident PD events (0.13%, incidence rate 1.87 [1.48–2.37]/10000 patient-years) in the RLS-negative group, and 185 incident PD events (0.37%, incidence rate 4.72 [4.09–5.45]/10000 patient-years) in the RLS-positive group in the propensity-matched cohort. Prevalent RLS was associated with more than twofold higher risk of incident PD (hazard ratio [HR]: 2.57, 95% confidence interval [CI]: 1.95–3.39) compared to RLS-negative patients. Qualitatively similar results were found when we examined the entire 3.5 million cohort: Prevalent RLS was associated with more than twofold higher risk of incident PD (multivariable adjusted HR: 2.81, 95%CI: 2.41–3.27).
Conclusion:
RLS and PD share common risk factors. In this large cohort of US veterans, we found that prevalent RLS is associated with higher risk of incident PD during 8 years of follow-up, suggesting that RLS could be an early clinical feature of incident PD.
Keywords: restless legs syndrome, Parkinson’s disease.
Statement of Significance
The association of restless legs syndrome (RLS) and Parkinson’s disease (PD) is a less studied topic with conflicting and debatable results. The findings of our study show that RLS is associated with a higher risk of PD, suggesting that RLS could be an early clinical feature of PD. These results could be highly significant and beneficial to neurologists or other physicians who play an important role in the management of RLS patients. Also, for researchers it justifies the need for more effective investigation approach on pathological basis of RLS and RLS development into PD. In addition, future researchers can set this study as a point reference.
INTRODUCTION
Restless legs syndrome (RLS) is a common disorder characterized by a convincing urge to move the lower limbs, accompanied by unpleasant sensations, symptoms that are aggravated during rest and alleviated by activity.1,2 RLS may be idiopathic or secondary, with several cross-sectional studies indicating an association between RLS and various chronic diseases including hypertension, cardiovascular diseases, obesity, diabetes, rheumatoid arthritis, osteoarthritis, chronic obstructive pulmonary disease, depression, cancer, and hyperthyroidism.3 RLS is also known to develop secondary to other medical conditions such as iron deficiency and chronic kidney disease or can be of a familial origin.1,4 There are factors that may exacerbate RLS symptoms such as excessive alcohol, caffeine, and nicotine use, drugs (tricyclic antidepressants, dopamine antagonists, and serotonin reuptake inhibitors) as well as older age.5
The association between RLS and Parkinson’s disease (PD) has been extensively studied6 on the basis that dopaminergic hypofunction in the central nervous system is present in both diseases,7 and it has been suggested that RLS is a possible preclinical marker of PD.8 However, there is still insufficient evidence to support an identical pathophysiologic mechanism for both the diseases.9 Previous cross-sectional, prospective, and retrospective studies showed that RLS symptoms appeared before the onset of PD,10–12 but the majority of them were limited by low event numbers and gave inconclusive results. Two recent, cross-sectional studies concluded that men with RLS are more likely to have concurrent PD, suggesting that RLS could be an early clinical feature of PD.13,14 However, a recent study showed that prevalence of RLS in PD patients was not significantly different from the general population, which suggests that these two diseases may not share the same mechanism.15 Until now, epidemiologic studies assessing the association between RLS and PD generated conflicting results.11–13
Given that previous studies reported contradictory results, and the fact that majority of them had cross-sectional designs with low event numbers, we examined in a historic prospective cohort study the association of RLS with the development of incident PD in a large, nationally representative contemporary group of US veterans. Based on previous findings, we hypothesized that RLS is associated with higher risk of development of incident PD.
METHODS
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the institutional review committees of the Memphis and Long Beach Veterans Affairs (VA) Medical Centers. Given the large sample size, anonymity of the patients studied, and nonintrusive nature of the research, the requirement for written consent was waived.
Study Setting and Cohort Definition
We used data from the Racial and Cardiovascular Risk Anomalies in chronic kidney disease (CKD) (RCAV) study. This study examines risk factors and outcomes of incident CKD, as detailed previously.16,17 RCAV cohort selected patients with estimated glomerular filtration rate (eGFR) ≥60 ml/min/1.73m2 from among all US veterans who had serum creatinine measurements performed between October 1, 2004, and September 30, 2006 (n = 4447691), which comprised approximately 94% of the entire VA patient population receiving medical care in a VA health-care facility during this time period.18 We identified RLS from the VA Inpatient and Outpatient Medical SAS Datasets using The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes (Table e-1). Figure e-1 shows the algorithm used for cohort definition. We included patients with baseline eGFR ≥60 ml/min/1.73m2 and without a diagnosis of PD prior to the diagnosis of RLS. The final cohort included 3481506 patients, of which 3423031 patients were without RLS and 58475 patients had prevalent RLS. Using this cohort, we created a propensity score-matched cohort of 50441 patients in each group using 1:1 matching, as shown in Figure e-1.
Exposure Outcomes and Covariates
We defined incident PD as our outcome of interest. RLS was defined by the presence of a relevant ICD-9-CM code at baseline, and incident PD was defined as a new ICD-9-CM code for PD (Table e-1) during the follow-up period. Patients with an existing diagnosis of PD before or within 60 days of the diagnosis of RLS were excluded. Sensitivity analyses were also performed in which patients with an existing diagnosis of PD before or within 365 days of the diagnosis of RLS were excluded.
Sociodemographic characteristics and comorbid conditions were obtained, as described previously.17 We obtained data on patients’ age, gender, and race from the VA Corporate Data Warehouse and from Medicare and on comorbidities from the VA Inpatient and Outpatient Medical SAS Datasets using ICD-9-CM diagnostic and procedure codes and Current Procedural Terminology codes, as shown in Table e-2. All comorbidities were detected at baseline. Anemia was defined as follow: baseline hemoglobin <13 g/dL for men and <12 g/dL for women.
Statistical Analysis
We generated summary statistics using proportions, means ± SD, or medians (interquartile ranges [IQR]). We compared continuous variables with the Student’s t test or the Mann–Whitney U test, as appropriate. The associations between prevalent RLS and incident PD were assessed using the Kaplan–Meier method, and Cox proportional hazard models (for time to event analyses).
The start of the follow-up period was the date when patients were diagnosed with RLS. Patients were followed until the diagnosis of PD or were censored at the date of last health care or administrative visit or on July 26, 2013.
We applied a propensity score matching method to account for baseline differences in clinical and demographic characteristics between those with and without RLS. We used logistic regression (see Table 1) to determine characteristics associated with RLS, which we then used to calculate propensity scores with STATA’s “psmatch2” command suite. We used a one-to-one nearest neighbor matching without replacement. The propensity score was calculated from the following variables: gender, age, race/ethnicity, marital status, income, baseline eGFR, comorbidities at baseline (hypertension, diabetes, congestive heart failure, cardiovascular disease, peripheral vascular disease, rheumatic heart disease, cerebrovascular disease, dementia, lung disease, liver disease, rheumatic disease, HIV/AIDS, malignancy, depression, thyroid disease, iron deficiency, insomnia, anemia, and gout), baseline neuroleptic usage, and body mass index (BMI). After propensity score matching the standardized differences were minimal (Table 2), and the distribution of the propensity score was very similar in RLS-positive and RLS-negative patients (Figure e-2).
Table 1.
Predictors of Presence of Restless Legs Syndrome Using Logistic Regression Analysis in the Entire Cohort.
| Odds Ratio (OR) | 95% confidence interval of OR | |
|---|---|---|
| Age (+10 years) | 0.95 | 0.94–0.95 |
| Gender: female vs. male (ref.) | 1.40 | 1.36–1.45 |
| Race: | ||
| White (ref.) | 1.00 | 1.00–1.00 |
| African American | 0.36 | 0.34–0.37 |
| Hispanic | 0.40 | 0.37–0.44 |
| Other race | 0.71 | 0.66–0.76 |
| Income (+1 log) | 1.01 | 0.99–1.02 |
| Marital status: Unmarried vs married (ref.) | 0.68 | 0.66–0.69 |
| Baseline eGFR (+10 ml/min./1.73m2) | 0.97 | 0.96–0.98 |
| Presence of diabetes vs absence of diabetes (ref.) | 1.05 | 1.02–1.07 |
| Presence of hypertension vs absence of hypertension (ref.) | 1.07 | 1.05–1.09 |
| Presence of cardiovascular diseasea vs. absence of cardiovascular diseasea (ref.) | 1.07 | 1.05–1.10 |
| Presence of congestive heart failure vs. absence of congestive heart failure (ref.) | 0.87 | 0.83–0.91 |
| Presence of cerebrovascular disease vs. absence of cerebrovascular disease (ref.) | 1.02 | 0.99–1.06 |
| Presence of peripheral arterial disease vs. absence of peripheral arterial disease (ref.) | 1.13 | 1.09–1.17 |
| Presence of chronic lung disease vs. absence of chronic lung disease (ref.) | 1.31 | 1.28–1.34 |
| Presence of dementia vs. absence of dementia (ref.) | 0.63 | 0.55–0.72 |
| Presence of rheumatologic disease vs. absence of rheumatologic disease (ref.) | 1.13 | 1.06–1.22 |
| Presence of liver disease vs. absence of liver disease (ref.) | 0.86 | 0.79–0.93 |
| Presence of malignancy vs. absence of malignancy (ref.) | 0.85 | 0.83–0.88 |
| Presence of AIDS/HIV vs. absence of AIDS/HIV (ref.) | 1.01 | 0.88–1.16 |
| Presence of depression vs. absence of depression (ref.) | 1.51 | 1.47–1.55 |
| Presence of thyroid disease vs. absence of thyroid disease (ref.) | 1.27 | 1.24–1.30 |
| Presence of rheumatic heart disease vs. absence of rheumatic heart disease (ref.) | 1.42 | 1.35–1.49 |
| Presence of iron deficiency vs. absence of iron deficiency (ref.) | 1.74 | 1.69–1.79 |
| Presence of insomnia vs. absence of insomnia (ref.) | 2.89 | 2.84–2.95 |
| Presence of anemia vs. absence of anemia (ref.) | 0.87 | 0.84–0.90 |
| Presence of gout vs. absence of gout (ref.) | 1.10 | 1.06–1.13 |
| Presence of neuroleptic use vs. absence of neuroleptic use (ref.) | 1.01 | 0.97–1.05 |
| Body mass index (+5 kg/m2) | 1.10 | 1.09–1.11 |
Abbreviations: AIDS, Acquired immundeficiency syndrome; eGFR, estimated glomerular filtration rate; HIV, human immundeficiency virus; OR, odds ratio.
a Cardiovascular disease was defined as acute myocardial infraction, angina, coronary artery disease, previous coronary artery bypass grafting, or percutaneous coronary intervention.
Table 2.
Baseline Characteristics of the Study Population.
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| RLS negative (n = 3423031) | RLS positive (n = 58475) | Std. Diff. | RLS negative (n = 50441) | RLS positive (n = 50441) | Std. Diff. | |
| Age, years | 60 ± 14 | 59 ± 12 | −0.039 | 59 ± 14 | 59 ± 12 | −0.001 |
| Gender (male) | 3190956 (93) | 53390 (91) | 0.099 | 46313 (92) | 46276 (92) | 0.003 |
| Outcome | ||||||
| Incident Parkinson disease | 3175 (0.09) | 192 (0.3) | N/A | 68 (0.1) | 185 (0.3) | N/A |
| Race | −0.260 | −0.006 | ||||
| White | 2417842 (78) | 49861 (90) | 44280 (87) | 45463 (90) | ||
| African American | 537759 (18) | 3798 (7) | 5306 (11) | 3551 (7) | ||
| Hispanic | 70608 (2) | 565 (1) | 440 (1) | 532 (1) | ||
| Other race | 66472 (2) | 989 (2) | 415 (1) | 895 (2) | ||
| Marital status | ||||||
| Married | 1821040 (56) | 36108 (64) | −0.187 | 31611 (63) | 31442 (62) | 0.007 |
| Single | 367114 (11) | 3523 (6) | 4428 (9) | 3359 (7) | ||
| Divorced | 845666 (26) | 13225 (24) | 11584 (23) | 12611 (25) | ||
| Widow | 240770 (7) | 3427 (6) | 2818 (6) | 3029 (6) | ||
| Other sociodemographic | ||||||
| Mean per capita income, USD | 22824 (11647–35989) | 24241 (13041–33982) | 0.058 | 24046 (12454–35485) | 23846 (12937–33522) | −0.001 |
| Other | ||||||
| Baseline eGFR, ml/min./1.73m2 | 84 ± 16 | 83 ± 15 | −0.076 | 83 ± 15 | 83 ± 15 | 0.006 |
| BMI, kg/m2 | 29.2 ± 5.7 | 30.1 ± 5.9 | 0.169 | 30.2 ± 6.2 | 30.2 ± 5.9 | 0.001 |
| Comorbidities | ||||||
| Hypertension | 2023591 (59) | 36778 (63) | 0.050 | 31812 (63) | 31816 (63) | <0.001 |
| Diabetes mellitus | 808359 (24) | 15448 (26) | 0.053 | 13510 (27) | 13627 (27) | 0.005 |
| Cardiovascular diseasea | 387355 (11) | 7906 (14) | 0.063 | 7066 (14) | 7070 (14) | <0.001 |
| Congestive heart failure | 149982 (4) | 2779 (5) | 0.014 | 2441 (5) | 2501 (5) | 0.006 |
| Cerebrovascular disease | 204286 (6) | 3819 (7) | 0.016 | 3481 (7) | 3377 (7) | −0.008 |
| Peripheral arterial disease | 185129 (5) | 3871 (7) | 0.046 | 3455 (7) | 3464 (7) | 0.001 |
| Chronic lung disease | 621355 (18) | 14239 (24) | 0.142 | 12715 (25) | 12632 (25) | −0.004 |
| Dementia | 26619 (0.8) | 262 (0.4) | −0.041 | 213 (0.4) | 236 (0.4) | 0.007 |
| Rheumatologic disease | 47515 (1) | 1055 (2) | 0.031 | 909 (2) | 915 (2) | 0.001 |
| Liver disease | 41265 (1) | 649 (1) | −0.011 | 571 (1) | 601 (1) | 0.006 |
| All malignancies | 352076 (10) | 5222 (9) | −0.053 | 4606 (9) | 4522 (9) | −0.006 |
| AIDS/HIV | 21348 (1) | 235 (0.4) | −0.035 | 218 (0.4) | 217 (0.4) | <−0.001 |
| Depression | 309509 (9) | 9290 (16) | 0.195 | 8382 (17) | 8422 (17) | 0.002 |
| Thyroid disease | 403609 (12) | 10010 (17) | 0.140 | 8738 (17) | 8635 (17) | −0.005 |
| Rheumatic heart disease | 68647 (2) | 2031 (3) | 0.085 | 1801 (4) | 1829 (4) | 0.003 |
| Iron deficiency | 205857 (6) | 6417 (11) | 0.166 | 5620 (11) | 5731 (11) | 0.007 |
| Insomnia | 371468 (11) | 16903 (29) | 0.445 | 14853 (29) | 14814 (29) | −0.002 |
| Anemia | 276095 (8) | 4176 (7) | −0.040 | 3667 (7) | 3629 (7) | −0.003 |
| Gout | 241801 (7) | 4970 (9) | 0.040 | 4351 (9) | 4340 (9) | −0.001 |
| Medication | ||||||
| Neuroleptic use at baseline | 155346 (5) | 3297 (6) | 0.042 | 3001 (6) | 3006 (6) | <0.001 |
Dichotomous/dummy variables are presented as number of patients and (percentage); continous variables are presented as mean ± SD or median (interquartile range, IQR).
Abbreviations: AIDS, Acquired immuno deficiency syndrome; BMI, body mass index; eGFR, estimated glomerular filtration rate; HIV, human immundeficiency virus; N/A, not applicable; IQR, interquartile range; RLS, restless legs syndrome; Std.Diff, standardized difference; USD, US dollars.
a Cardiovascular disease was defined as acute myocardial infraction, angina, coronary artery disease, previous coronary artery bypass grafting or percutaneous coronary intervention.
All associations were examined in unadjusted models using our propensity-matched cohort of 100882 patients. We performed subgroup analyses, and effect modification was detected based on interaction terms. We also analyzed the entire cohort in a sensitivity analysis. For each of these analyses, four models were examined based on the level of multivariable adjustment: 1, crude model; 2, Model 1: adjusted for age and sex; 3, Model 2: variables from Model 1 and race, income, marital status, and baseline eGFR; 4, Model 3: variables from Model 2 and comorbidities (diabetes, hypertension, cardiovascular disease, congestive heart failure, cerebrovascular disease, peripheral artery disease, lung disease, dementia, rheumatic disease, liver disease, malignancy, AIDS/HIV, depression, thyroid disease, rheumatic heart disease, iron deficiency, insomnia, anemia, and gout), BMI, and baseline neuroleptic use. Statistical analyses were performed using Stata MP version 12 (Stata Corporation, College Station, Texas).
RESULTS
Baseline Characteristics
The mean ± SD age of the cohort at baseline was 60 ± 14 years, 93% were male, 78% and 17% of patients were white and black, respectively, 44% were unmarried, 24% of the patients were diabetic, and the mean baseline eGFR was 84 ± 16 ml/min/1.73m2. Baseline characteristics of patients categorized by RLS status are shown in Table 2. In the original cohort (n = 3481506), patients with RLS were more likely to be white, female, and married; had higher prevalence of hypertension, CVD, depression, diabetes mellitus, thyroid disease, chronic lung disease, insomnia, gout, and iron deficiency; and were slightly younger. These differences disappeared after propensity score matching and the baseline characteristics became balanced (Table 2).
Predictors of RLS
In our multivariable logistic regression model, younger age, female gender, white race, being married, lower eGFR, higher BMI, and most of the comorbidities (such as diabetes, hypertension, peripheral artery disease, chronic lung disease, rheumatologic disease, depression, thyroid disease, rheumatic heart disease, iron deficiency, insomnia and gout) were associated with a higher risk of prevalent RLS (Table 1).
Incident PD in the Propensity-Matched Cohort
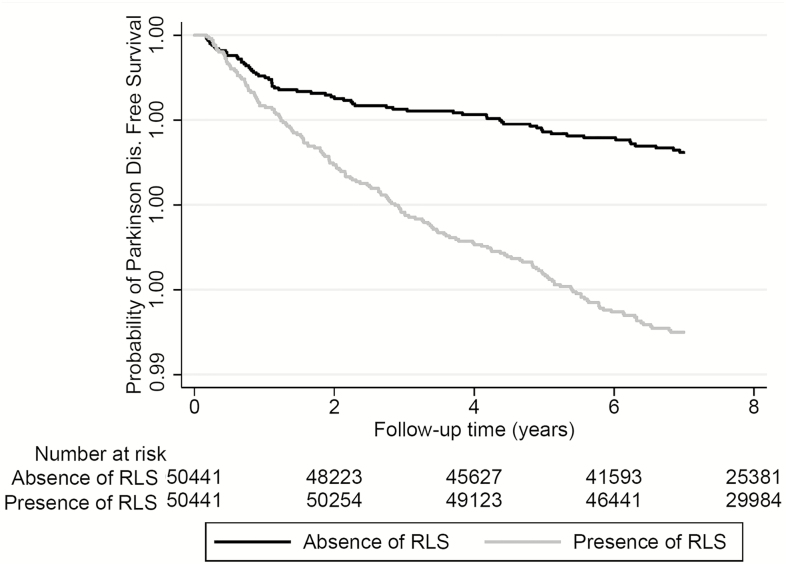
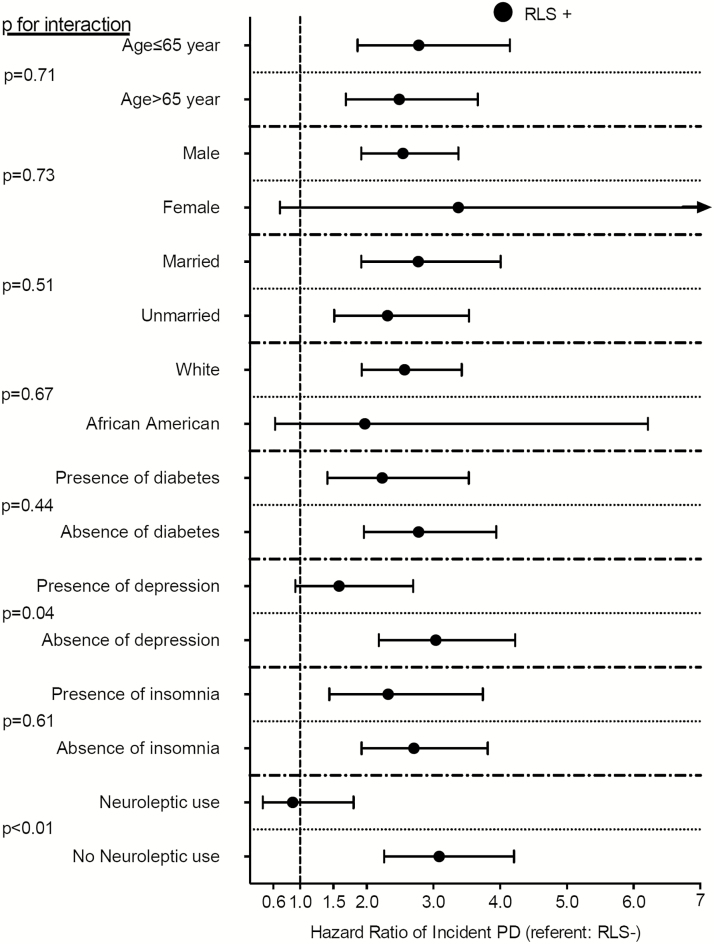
The median follow-up time was 8.1 years (IQR: 7.2–8.5 years) in the propensity-matched cohort. There were 253 incident PD events (0.25%, incidence rate 3.35 [2.96–3.79]/10000 patient-years) in the propensity-matched cohort. There were 68 incident PD events (0.13%, incidence rate 1.87 [1.48–2.37]/10000 patient-years) in the RLS-negative group, and 185 incident PD events (0.37%, incidence rate 4.72 [4.09–5.45]/10000 patient-years) in the RLS-positive group. Figure 1 shows the associations between prevalent RLS and probability of the incident PD event in the propensity-matched cohort. Prevalent RLS was associated with a more than twofold higher risk of incident PD (hazard ratio [HR]: 2.57, 95% confidence interval [CI]: 1.95–3.39) compared to RLS-negative patients (Table 3). Similar results were found in almost all subgroups (Figure 2). Results were qualitatively similar when we included antidepressant medication use in our propensity score (HR: 2.77, 95%CI: 1.94–3.97) and also when we excluded PD cases diagnosed during the first year after the RLS diagnosis (HR: 3.41, 95%CI: 2.39–4.86) in the sensitivity analyses.
Figure 1.
Kaplan–Meier curves of incident Parkinson Disease in patients with and without restless legs syndrome in the propensity-matched cohort. Abbreviations: RLS, restless legs syndrome.
Table 3.
Association Between Presence of Restless Legs Syndrome and Incident Parkinson Disease Using Cox Proportional Regression Models in the Propensity-Matched Cohort (n = 100882) and the Entire Cohort (n = 3481506) With Different Levels of Adjustment.
| Presence of RLS vs absence of RLS (ref.) | Hazard ratios (HR) | 95% Confidence interval of HR |
|---|---|---|
| PS matched cohort | 2.57 | 1.95–3.39 |
| Entire cohort crude model | 3.20 | 2.77–3.71 |
| Entire cohort Model 1a | 3.36 | 2.90–3.88 |
| Entire cohort Model 2b | 3.55 | 3.05–4.12 |
| Entire cohort Model 3c | 2.81 | 2.41–3.27 |
Abbreviations: AIDS, acquired immundeficiency syndrome; BMI, body mass index; eGFR, estimated glomerular filtration rate; HIV, human immundeficiency virus; HR, hazard ratios; PS, propensity score; RLS, restless legs syndrome.
Model 1a: Adjusted for age, sex.
Model 2b: Adjusted for age, sex, race, income, marital status, and baseline eGFR.
Model 3c: Adjusted for age, sex, race, income, marital status, baseline eGFR, comorbidities (diabetes, hypertension, cardiovascular disease, congestive heart failure, cerebrovascular disease, peripheral artery disease, lung disease, dementia, rheumatic disease, liver disease, malignancy, AIDS/HIV, depression, thyroid disease, rheumatic heart disease, iron deficiency, insomnia, anemia, and gout), BMI, and neuroleptic use at baseline.
Figure 2.
Association between restless legs syndrome and incident Parkinson Disease in different subgroups of patients in the propensity-matched cohort. Abbreviations: PD, Parkinson Disease; RLS, restless legs syndrome.
Incident PD in the Entire Cohort
The median follow-up time was 7.8 years (IQR: 6.4–8.4 years) in the entire cohort. There were 3175 incident PD events (0.09%, incidence rate 1.35 [1.31–1.40]/10000 patient-years) in the RLS-negative group and 192 incident PD events (0.33%, incidence rate 4.24 [3.68–4.88]/10000 patient-years) in the RLS-positive group. Figure e-3 shows the associations between prevalent RLS and the probability of incident PD in the overall cohort. Prevalent RLS was associated with a more than threefold higher risk of incident PD (HR: 3.20, 95%CI: 2.77–3.71) compared to RLS-negative patients in our crude model (Table 3). This association remained qualitatively similar (HR: 2.81, 95%CI: 2.41–3.27) after adjustment for important confounders in our final Cox regression model (Table 3). Similar results were found in almost all subgroups (Figure e-4).
DISCUSSION
In this large cohort of US veterans, we examined the association between RLS and the development of incident PD. Our findings confirm that prevalent RLS is associated with higher risk of incident PD during 8 years of follow-up. In our study, the explanation for the observed association between RLS and PD is unclear. Since the cause of RLS remains unknown and the absolute incidence of PD in patients with RLS is very low, it is difficult to postulate a common pathophysiological background for the two diseases, although the response to dopaminergic agents in both the conditions may suggest this.
Several pathophysiologic mechanisms have been described which indicate a potential pathophysiological connection between RLS and PD. The nigrostriatal dopaminergic system is primarily affected in PD, and RLS is also believed to have an underlying dopaminergic pathophysiology.19 In addition, there is pathological evidence of dopamine dysfunction in both the diseases,20 and previous data support the hypothesis that primary iron deficiency produces a dopaminergic abnormality characterized by an overly activated dopaminergic system, rather than dopamine cell depletion, as part of the RLS pathology. The findings of functional imaging studies in RLS have been inconclusive, showing mild reduction in postsynaptic dopaminergic status.21 Another study suggested presynaptic dopaminergic dysfunction in the striatum,22 in contrast to PD. However, a fourth study showed normal values for presynaptic dopaminergic function.23 It is possible that the extrastriatal dopaminergic system may be variably involved in RLS,9 but the association of RLS severity with the severity of nondopaminergic symptoms in PD, such as cognitive, autonomic, mood, or psychotic disorders, raises the possibility of a nondopaminergic mechanism in RLS as well.24 Nondopaminergic systems, such as the noradrenergic system, might play a role in the possible link between RLS and PD.24 As far as we know, there are no studies that used animal models specifically examining the link between RLS and PD. Two sonographic studies suggest different pathological processes underlying idiopathic and PD-associated RLS,25,26 revealing a significant decrease in substantia nigra region echogenicity in idiopathic RLS compared to other groups (RLS–PD, idiopathic PD, and healthy control group). In contrast, the RLS–PD group had increased echogenicity of the substantia nigra area compared to the control group. As for genetic studies, the literature appears to support a link between the parkin mutation and idiopathic RLS.27 A recent genetic study identified the MEIS1 locus for RLS in the basal ganglia,28 suggesting that RLS has components of a neurodevelopmental disorder. In addition, pathways within the basal ganglia circuit are affected in PD as well; hence, the pathophysiological relation of RLS and PD is even more likely. A recent study of patient with idiopathic PD found the prevalence of RLS to be 45%,29 providing stronger evidence of this association. Other genetic studies have yielded mostly negative or inconclusive results.30 However, earlier studies using olfactory tests suggested that the pathophysiology of RLS differs from PD.31 PD and RLS may share similar pathophyisological mechanisms as mentioned earlier, but smoking plays an uncertain role in these diseases. Several studies have shown that smoking relieves Parkinson symptoms, and it is associated with a lower risk of developing PD.32,33 However, the relationship between smoking and RLS is controversial. Some studies show that smoking alleviates RLS symptoms,34,35 others concluded that there is no influence,36–38 yet others found a higher incidence of smokers among RLS patients.39 Sleep disorders are a common feature in PD.40 Insomnia is thought to be a nonspecific, prodromal symptom in PD, whereas sleep disturbances in RLS are a common consequence due to the nocturnal occurrence of symptoms.41 Accordingly, the approach to insomnia should be cautious because it may be difficult to differentiate RLS sleep-onset insomnia from prodromal insomnia in PD. The association between RLS and PD remained present even after matching and adjusting for insomnia in our models.
Most of the predictors of RLS, such as female gender, white race, lower eGFR, higher BMI, diabetes, hypertension, chronic lung disease, rheumatologic disease, depression, thyroid disease, rheumatic heart disease, iron deficiency, gout, and insomnia were similar in our study as in previous studies.1–3,37,42,43 In contrast to previous epidemiological studies,44 we found younger age (≤65 years) to be a predictor of RLS.1,45 The explanation for this discrepancy could be due to differences in how RLS patients were identified (ICD-9 codes vs. standardized or self-reported questionnaires) or to differences in the source populations (male, elderly, and chronic kidney disease US veterans vs. other populations). We identified some predictors that were never examined in previous studies such as peripheral artery disease. This association can be explained by the fact that the symptoms in these cases did not meet RLS diagnostic criteria, and the results were actually due to misclassification in RLS assessment.
It must be acknowledged that the pathophysiologic basis of RLS remains poorly understood, and RLS may be an especially complex disease,46 however the symptoms of RLS respond to drugs increasing dopamine, dopaminergic hypofunction in the central nervous system.7 Therefore, neuroleptics, drugs that block dopamine receptors may cause or exacerbate symptoms of RLS. Based on a few case reports of neuroleptic-induced RLS,47–54 the interaction between neuroleptic use and RLS should be recognized and taken into account, although the quality of some of this data has been questioned55 and has provided conflicting results.56–58 In our study, neuroleptic use at baseline was not associated with higher risk of prevalent RLS.
There are few studies that examined the development of PD after the onset of RLS,59,60 and these yielded inconclusive results. Major limitations of these studies were their cross-sectional and/or retrospective design or having had very low numbers of events. In a study examining the prevalence of PD among RLS patients, 4 out of 85 patients referred for RLS developed PD, which was higher than the ~1% prevalence of PD in the general population older than 60 years.61 In a recent study examining a family with high prevalence of RLS, 2 of the 30 members with RLS also suffered from PD.62 However, none of these studies included control groups. To the best of our knowledge, there were only two recent studies (a cross-sectional and a prospective longitudinal study) including mostly men, which concluded that RLS was associated with a higher prevalence of PD across all age-groups and that RLS was an early clinical feature of PD, not a risk factor.10,14 In these studies, RLS was assessed based on self-report by standardized questionnaires corresponding to the International RLS Study Group (IRLSSG) criteria, without verification by physical examination, and PD was identified by biannual self-reported questionnaires and by reviewing the medical records and death certificates of deceased study participants. The findings of these cross-sectional studies support our results, namely, that there is an association between RLS and PD. To the best of our knowledge, our study has the largest sample size, and it is one of the few studies that followed patients who were PD-free at baseline in a historical prospective manner. It remains unclear if the observed associations are explained by a pathological link leading from RLS to PD, versus both diseases having a common root.
It is important to note that in our study, the estimated risk of incident PD events in the RLS group is relatively small, suggesting that the connection between the two diseases may not be very strong and/or specific. In contrast, other diseases such as idiopathic rapid eye movement (REM) sleep behavior disorder have stronger effects.63 Iranzo et al. indicated that the majority of patients with idiopathic REM sleep behavior disorder develop incident PD or dementia with Lewy bodies within a median of 7.5 years.63
Strengths of our study are the large sample size and event numbers and the fact that it is representative of veterans in the entire United States. Second, to our knowledge, this is one of the first large studies using a propensity score-matched approach to balance measured confounders. This epidemiological approach serves to balance the measured confounders between the subcohort with exposures and the subcohort without it. Third, the historical prospective design allowed us to conclude that RLS might be an early indicator of PD, whereas previous cross-sectional studies could only conclude associations. Finally, the long follow-up time and relatively high event numbers provided enough power to conduct adjusted analyses.
Our study also has several limitations that need to be acknowledged. The method of propensity score matching only accounts for confounding by variables that are available for analysis. Therefore, we cannot rule out residual confounding. We used diagnostic codes to define all conditions in lieu of standardized or self-reported questionnaires or clinical diagnosis to diagnose RLS and PD. The diagnostic performance of these codes is not well documented, and they may not correlate well with clinical diagnoses. A report from 2010 indicated that the ICD-9 code for Parkinsonism has good sensitivity (75%) and excellent specificity (99.1%).64 Additionally, we were not able to differentiate the source of the RLS ICD-9 codes, which could have been assigned by neurologists or other physicians. A potential restriction to a diagnosis assigned by a neurologist might be more valid for RLS than one assigned by nonneurologists. The limitation of using an ICD-9 code for RLS is important, as there is a possibility of inexperienced physicians coding leg motor restlessness as RLS, which was previously shown to be a predictor of PD.65 In addition, the significant predictors of RLS in our study were similar to those found in previous studies.1–3,37,42,43 We were unable to assess the associations between the severity of RLS and PD. We also have no data about the treatment of RLS. The clinical severity of RLS can vary, and its natural course can involve transient episodes of remissions and relapses, especially in milder forms seen in young to middle-aged adults. It is therefore possible that patients suffering from milder forms of RLS or those in remission during the evaluation period may have been misclassified in our study. Although excessive alcohol, caffeine intake, and nicotine use may aggravate RLS symptoms, we do not have reliable information about these in our database; therefore, we could not match and adjust for these confounders in our models. In addition, we did not have data regarding usage of dopamine agonists or levodopa. Patients with more comorbidities could have more frequent follow-up visits with their physicians, which may result in higher recognition rate of PD in these patients. However, we adjusted for these comorbidities in our multivariable models. In addition, the study population consisted mostly of male and elderly US veterans. Hence, the results may not be accurately representative of other populations such as women, younger patients, non-US patients, or patients with chronic kidney disease. Finally, we did not have information about several RLS-like syndromes (e.g., akathisia, nocturnal leg cramps, peripheral neuropathy, painful legs and moving toes, positional discomfort, lumbosacral radiculopathy, and attention-deficit/ hyperactivity disorder), which could have resulted in a possible overestimation or misclassification in RLS assessment. However, results were similar when we adjusted for all the comorbidities that may cause the enumerated disorders.
In this large cohort of US veterans, we found that prevalent RLS is associated with higher risk of incident PD during 8 years of follow-up. Our results suggest that RLS could be an early clinical feature of incident PD. However, there are several remaining open questions. First, the pathological basis of RLS development into PD remains unanswered. Second, based on the iron–dopamine theory, it would be interesting to examine the relationship between RLS and schizophrenia (hyperdopaminergic activity). Finally, it would be interesting to know whether treatment of RLS could help prevent or delay the development of PD. Further studies are needed to confirm our results and answer these proposed questions.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
FUNDING
This study is supported by grant 1R01DK096920 from the NIH to CPK and KKZ, and by resources from the US Department of Veterans Affairs. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). Grant support for DB: KTIA-NAP-13-1-2013-0001 from the Hungarian National Brain Research Program.
AUTHORS’ NOTE
This paper is dedicated for the memory of Dr Ferenc Fornadi.
This is not a clinical trial. The institution at which the work was performed: VA Medical Center, Memphis, TN, USA.
Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs. The results of this paper have not been published previously in whole or part. Molnar and Kovesdy had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
AUTHORS’ ROLE
Study concept and design. MZM, CPK, SS and DB.
Acquisition of data. CPK, MZM.
Analysis and interpretation of data. MZM, CPK, SS, DB, and KKZ.
Drafting of the manuscript and approval of the final version. SS and MZM.
Critical revision of the manuscript for important intellectual content and approval of the final version. MZM, CPK, SS, DB, KF and KKZ.
DISCLOSURE STATEMENT
SS reports no disclosures. DB reports no disclosures. KF reports no disclosures. KKZ is an employee of the Department of Veterans affairs. He reports no other disclosures. CPK is an employee of the Department of Veterans affairs. He reports no other relevant disclosures. MZM reports no disclosures.
Supplementary Material
REFERENCES
- 1. Garcia-Borreguero D, Egatz R, Winkelmann J, Berger K. Epidemiology of restless legs syndrome: the current status. Sleep Med Rev. 2006; 10(3): 153–167. [DOI] [PubMed] [Google Scholar]
- 2. Szentkirályi A, Madarász CZ, Novák M. Sleep disorders: impact on daytime functioning and quality of life. Expert Rev Pharmacoecon Outcomes Res. 2009; 9(1): 49–64. [DOI] [PubMed] [Google Scholar]
- 3. Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009; 32(5): 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allen RP, Earley CJ. Defining the phenotype of the restless legs syndrome (RLS) using age-of-symptom-onset. Sleep Med. 2000; 1(1): 11–19. [DOI] [PubMed] [Google Scholar]
- 5. Kushida CA. Clinical presentation, diagnosis, and quality of life issues in restless legs syndrome. Am J Med. 2007; 120(1 Suppl 1): S4–S12. [DOI] [PubMed] [Google Scholar]
- 6. Garcia-Borreguero D, Odin P, Serrano C. Restless legs syndrome and PD: a review of the evidence for a possible association. Neurology. 2003; 61(6 Suppl 3): S49–S55. [DOI] [PubMed] [Google Scholar]
- 7. Allen RP. Controversies and challenges in defining the etiology and pathophysiology of restless legs syndrome. Am J Med. 2007; 120(1 Suppl 1): S13–S21. [DOI] [PubMed] [Google Scholar]
- 8. Chaudhuri KR, Healy DG, Schapira AH; National Institute for Clinical Excellence Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006; 5(3): 235–245. [DOI] [PubMed] [Google Scholar]
- 9. Tan EK. Restless legs syndrome and Parkinson’s disease: is there an etiologic link? Journal of neurology 2006; 253Suppl 7: VII33–VII37. [DOI] [PubMed] [Google Scholar]
- 10. Wong JC, Li Y, Schwarzschild MA, Ascherio A, Gao X. Restless legs syndrome: an early clinical feature of Parkinson disease in men. Sleep. 2014; 37(2): 369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nomura T, Inoue Y, Miyake M, Yasui K, Nakashima K. Prevalence and clinical characteristics of restless legs syndrome in Japanese patients with Parkinson’s disease. Mov Disord. 2006; 21(3): 380–384. [DOI] [PubMed] [Google Scholar]
- 12. Krishnan PR, Bhatia M, Behari M. Restless legs syndrome in Parkinson’s disease: a case-controlled study. Mov Disord. 2003; 18(2): 181–185. [DOI] [PubMed] [Google Scholar]
- 13. Tan EK, Lum SY, Wong MC. Restless legs syndrome in Parkinson’s disease. J Neurol Sci. 2002; 196(1-2): 33–36. [DOI] [PubMed] [Google Scholar]
- 14. Gao X, Schwarzschild MA, O’Reilly EJ, Wang H, Ascherio A. Restless legs syndrome and Parkinson’s disease in men. Mov Disord. 2010; 25(15): 2654–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rijsman RM, Schoolderman LF, Rundervoort RS, Louter M. Restless legs syndrome in Parkinson’s disease. Parkinsonism Relat Disord. 2014; 20Suppl 1: S5–S9. [DOI] [PubMed] [Google Scholar]
- 16. Molnar MZ, Lu JL, Kalantar-Zadeh K, Kovesdy CP. Association of incident restless legs syndrome with outcomes in a large cohort of US veterans. J Sleep Res. 2016; 25(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molnar MZ, Alhourani HM, Wall BM, et al. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015; 61(5): 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kovesdy CP, Norris KC, Boulware LE, et al. Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation. 2015; 132(16): 1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ondo WG, Lai D. Association between restless legs syndrome and essential tremor. Mov Disord. 2006; 21(4): 515–518. [DOI] [PubMed] [Google Scholar]
- 20. Connor JR, Wang XS, Allen RP, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009; 132(Pt 9): 2403–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turjanski N, Lees AJ, Brooks DJ. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology. 1999; 52(5): 932–937. [DOI] [PubMed] [Google Scholar]
- 22. Ruottinen HM, Partinen M, Hublin C, et al. An FDOPA PET study in patients with periodic limb movement disorder and restless legs syndrome. Neurology. 2000; 54(2): 502–504. [DOI] [PubMed] [Google Scholar]
- 23. Trenkwalder C, Walters AS, Hening WA, et al. Positron emission tomographic studies in restless legs syndrome. Mov Disord. 1999; 14(1): 141–145. [DOI] [PubMed] [Google Scholar]
- 24. Verbaan D, van Rooden SM, van Hilten JJ, Rijsman RM. Prevalence and clinical profile of restless legs syndrome in Parkinson’s disease. Mov Disord. 2010; 25(13): 2142–2147. [DOI] [PubMed] [Google Scholar]
- 25. Kwon DY, Seo WK, Yoon HK, Park MH, Koh SB, Park KW. Transcranial brain sonography in Parkinson’s disease with restless legs syndrome. Mov Disord. 2010; 25(10): 1373–1378. [DOI] [PubMed] [Google Scholar]
- 26. Ryu JH, Lee MS, Baik JS. Sonographic abnormalities in idiopathic restless legs syndrome (RLS) and RLS in Parkinson’s disease. Parkinsonism Relat Disord. 2011; 17(3): 201–203. [DOI] [PubMed] [Google Scholar]
- 27. Adel S, Djarmati A, Kabakci K, et al. Co-occurrence of restless legs syndrome and Parkin mutations in two families. Mov Disord. 2006; 21(2): 258–263. [DOI] [PubMed] [Google Scholar]
- 28. Spieler D, Kaffe M, Knauf F, et al. Restless legs syndrome-associated intronic common variant in Meis1 alters enhancer function in the developing telencephalon. Genome Res. 2014; 24(4): 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Limousin N, Konofal E, Karroum E, et al. Restless legs syndrome, rapid eye movement sleep behavior disorder, and hypersomnia in patients with two parkin mutations. Mov Disord. 2009; 24(13): 1970–1976. [DOI] [PubMed] [Google Scholar]
- 30. Mylius V, Möller JC, Strauch K, Oertel WH, Stiasny-Kolster K. No significance of the COMT val158met polymorphism in restless legs syndrome. Neurosci Lett. 2010; 473(2): 151–154. [DOI] [PubMed] [Google Scholar]
- 31. Adler CH, Gwinn KA, Newman S. Olfactory function in restless legs syndrome. Mov Disord. 1998; 13(3): 563–565. [DOI] [PubMed] [Google Scholar]
- 32. Hernán MA, Zhang SM, Rueda-deCastro AM, Colditz GA, Speizer FE, Ascherio A. Cigarette smoking and the incidence of Parkinson’s disease in two prospective studies. Ann Neurol. 2001; 50(6): 780–786. [DOI] [PubMed] [Google Scholar]
- 33. Ishikawa A, Miyatake T. Effects of smoking in patients with early-onset Parkinson’s disease. J Neurol Sci. 1993; 117(1-2): 28–32. [DOI] [PubMed] [Google Scholar]
- 34. Mountifield JA. Restless leg syndrome relieved by cessation of smoking. CMAJ. 1985; 133(5): 426–427. [PMC free article] [PubMed] [Google Scholar]
- 35. Oksenberg A. Alleviation of severe restless legs syndrome (RLS) symptoms by cigarette smoking. J Clin Sleep Med. 2010; 6(5): 489–490. [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang J, Lam SP, Li SX, Li AM, Kong AP, Wing YK. Restless legs symptoms in adolescents: epidemiology, heritability, and pubertal effects. J Psychosom Res. 2014; 76(2): 158–164. [DOI] [PubMed] [Google Scholar]
- 37. Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008; 70(1): 35–42. [DOI] [PubMed] [Google Scholar]
- 38. Lavigne GL, Lobbezoo F, Rompré PH, Nielsen TA, Montplaisir J. Cigarette smoking as a risk factor or an exacerbating factor for restless legs syndrome and sleep bruxism. Sleep. 1997; 20(4): 290–293. [PubMed] [Google Scholar]
- 39. Schlesinger I, Erikh I, Avizohar O, Sprecher E, Yarnitsky D. Cardiovascular risk factors in restless legs syndrome. Mov Disord. 2009; 24(11): 1587–1592. [DOI] [PubMed] [Google Scholar]
- 40. Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol. 2015; 14(1): 57–64. [DOI] [PubMed] [Google Scholar]
- 41. Allen RP, Picchietti DL, Garcia-Borreguero D, et al. ; International Restless Legs Syndrome Study Group. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014; 15(8): 860–873. [DOI] [PubMed] [Google Scholar]
- 42. Lee HB, Hening WA, Allen RP, et al. Restless legs syndrome is associated with DSM-IV major depressive disorder and panic disorder in the community. J Neuropsychiatry Clin Neurosci. 2008; 20(1): 101–105. [DOI] [PubMed] [Google Scholar]
- 43. Pereira JC Jr, Pradella-Hallinan M Lins Pessoa Hd. Imbalance between thyroid hormones and the dopaminergic system might be central to the pathophysiology of restless legs syndrome: a hypothesis. Clinics (Sao Paulo). 2010; 65(5): 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gao X, Schwarzschild MA, Wang H, Ascherio A. Obesity and restless legs syndrome in men and women. Neurology. 2009; 72(14): 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ghorayeb I, Tison F. [Epidemiology of restless legs syndrome]. Rev Neurol (Paris). 2009; 165(8-9): 641–649. [DOI] [PubMed] [Google Scholar]
- 46. Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011; 12(7): 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duggal HS, Mendhekar DN. Clozapine-associated restless legs syndrome. J Clin Psychopharmacol. 2007; 27(1): 89–90. [DOI] [PubMed] [Google Scholar]
- 48. Horiguchi J, Yamashita H, Mizuno S, et al. Nocturnal eating/drinking syndrome and neuroleptic-induced restless legs syndrome. Int Clin Psychopharmacol. 1999; 14(1): 33–36. [PubMed] [Google Scholar]
- 49. Kang SG, Lee HJ, Kim L. Restless legs syndrome and periodic limb movements during sleep probably associated with olanzapine. J Psychopharmacol. 2009; 23(5): 597–601. [DOI] [PubMed] [Google Scholar]
- 50. Khalid I, Rana L, Khalid TJ, Roehrs T. Refractory restless legs syndrome likely caused by olanzapine. J Clin Sleep Med. 2009; 5(1): 68–69. [PMC free article] [PubMed] [Google Scholar]
- 51. Kraus T, Schuld A, Pollmächer T. Periodic leg movements in sleep and restless legs syndrome probably caused by olanzapine. J Clin Psychopharmacol. 1999; 19(5): 478–479. [DOI] [PubMed] [Google Scholar]
- 52. Pinninti NR, Mago R, Townsend J, Doghramji K. Periodic restless legs syndrome associated with quetiapine use: a case report. J Clin Psychopharmacol. 2005; 25(6): 617–618. [DOI] [PubMed] [Google Scholar]
- 53. Urbano MR, Ware JC. Restless legs syndrome caused by quetiapine successfully treated with ropinirole in 2 patients with bipolar disorder. J Clin Psychopharmacol. 2008; 28(6): 704–705. [DOI] [PubMed] [Google Scholar]
- 54. Wetter TC, Brunner J, Bronisch T. Restless legs syndrome probably induced by risperidone treatment. Pharmacopsychiatry. 2002; 35(3): 109–111. [DOI] [PubMed] [Google Scholar]
- 55. Hoque R, Chesson AL.Jr. Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010; 6(1): 79–83. [PMC free article] [PubMed] [Google Scholar]
- 56. Brown LK, Dedrick DL, Doggett JW, Guido PS. Antidepressant medication use and restless legs syndrome in patients presenting with insomnia. Sleep Med. 2005; 6(5): 443–450. [DOI] [PubMed] [Google Scholar]
- 57. Dimmitt SB, Riley GJ. Selective serotonin receptor uptake inhibitors can reduce restless legs symptoms. Arch Intern Med. 2000; 160(5): 712. [DOI] [PubMed] [Google Scholar]
- 58. Jagota P, Asawavichienjinda T, Bhidayasiri R. Prevalence of neuroleptic-induced restless legs syndrome in patients taking neuroleptic drugs. J Neurol Sci. 2012; 314(1-2): 158–160. [DOI] [PubMed] [Google Scholar]
- 59. Peralta CM, Frauscher B, Seppi K, et al. Restless legs syndrome in Parkinson’s disease. Mov Disord. 2009; 24(14): 2076–2080. [DOI] [PubMed] [Google Scholar]
- 60. Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol. 2002; 59(3): 421–424. [DOI] [PubMed] [Google Scholar]
- 61. Walters AS, LeBrocq C, Passi V, et al. A preliminary look at the percentage of patients with Restless Legs Syndrome who also have Parkinson Disease, Essential Tremor or Tourette Syndrome in a single practice. J Sleep Res. 2003; 12(4): 343–345. [DOI] [PubMed] [Google Scholar]
- 62. Young JE, Vilarino-Guell C, Lin SC, Wszolek ZK, Farrer MJ. Clinical and genetic description of a family with a high prevalence of autosomal dominant restless legs syndrome. Mayo Clin Proc. 2009; 84: 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Iranzo A, Fernández-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014; 9(2): e89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. St. Sauver J, Buntrock J, Rademacher D, et al. Comparison of Mayo Clinic Coding Systems. Technical Report #83. Department of Health Sciences Research Mayo Clinic, Rochester, Minnesota; 2010. [Google Scholar]
- 65. Gjerstad MD, Tysnes OB, Larsen JP. Increased risk of leg motor restlessness but not RLS in early Parkinson disease. Neurology. 2011; 77(22): 1941–1946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.