Abstract
Spinal cord injury (SCI) leads to rapid destruction of neuronal tissue, resulting in devastating motor and sensory deficits. This is exacerbated by damage to spinal cord blood vessels and loss of vascular integrity. Thus, approaches that protect existing blood vessels or stimulate the growth of new blood vessels might present a novel approach to minimize loss or promote regeneration of spinal cord tissue following SCI. In light of the remarkable power of chronic mild hypoxia (CMH) to stimulate vascular remodeling in the brain, the goal of this study was to examine how CMH (8% O2 for up to 7 days) affects blood vessel remodeling in the spinal cord. We found that CMH promoted the following: (i) endothelial proliferation and increased vascularity as a result of angiogenesis and arteriogenesis, (ii) increased vascular expression of the angiogenic extracellular matrix protein fibronectin as well as concomitant increases in endothelial expression of the fibronectin receptor α5β1 integrin, (iii) strongly upregulated endothelial expression of the tight junction proteins claudin-5, ZO-1 and occludin, and (iv) astrocyte activation. Of note, the vascular remodeling changes induced by CMH were more extensive in white matter. Interestingly, hypoxic-induced vascular remodeling in spinal cord blood vessels was markedly attenuated in mice lacking endothelial α5 integrin expression (α5-EC-KO mice). Taken together, these studies demonstrate the considerable remodeling potential of spinal cord blood vessels and highlight an important angiogenic role for the α5β1 integrin in promoting endothelial proliferation. They also imply that stimulation of the α5β1 integrin or controlled use of mild hypoxia might provide new approaches for promoting angiogenesis and improving vascular integrity in spinal cord blood vessels.
Keywords: Hypoxia, spinal cord, vascular remodeling, endothelial proliferation, fibronectin, integrin
INTRODUCTION
Blood vessels of the spinal cord play an essential role in supplying the oxygen and metabolic demands of spinal cord neurons and glial cells. Abrupt loss of vascularization during spinal cord injury (SCI) quickly leads to cell death and destruction of spinal cord tissue, resulting in profound motor and sensory deficits [1,2]. Mechanical injury tears blood vessels in the immediate vicinity of damage and also causes blood vessels in adjacent areas to become leaky and dysfunctional [3]. This in turn, leads to activation of several pathological mechanisms including tissue edema, excitotoxicity, free radical damage and neuroinflammation, all of which amplify the secondary expansion of tissue destruction [4,5]. Interestingly, an endogenous angiogenic response occurs immediately after tissue damage, but this ultimately fails to produce functionally stable new blood vessels [1,2]. This lack of functional blood supply impedes endogenous tissue repair and also limits potential repair strategies. With this in mind, it becomes apparent that approaches that either protect existing blood vessels or stimulate the growth of new blood vessels might present a way to minimize loss of spinal cord tissue following SCI [6].
Surprisingly, little is known about the vascular plasticity of spinal cord blood vessels or the types of stimuli or factors that promote this plasticity. Within the brain, a number of studies have demonstrated the remarkable power of chronic mild hypoxia (CMH) to stimulate vascular remodeling [7–9]. Over a series of studies, it has been shown that exposure of mice or rats to mild hypoxia (8% O2) for 2–3 weeks promotes a marked angiogenic response, resulting in greater than 50% increases in vessel density over a 2 week period. These brain studies also revealed that hypoxic induction of vascular remodeling is not just limited to capillaries, as first described, but also includes significant arteriogenic remodeling, demonstrating that CMH promotes vascular remodeling at all stages of the vascular tree [10]. Significantly, in the brain, CMH also promotes marked upregulation of the tight junction proteins claudin-5 and ZO-1 at the blood-brain barrier (BBB), suggestive of increased vascular integrity [11,12].
The molecular mechanisms that mediate hypoxic-induced vascular remodeling in the brain have been well defined and include hypoxic inducible factor-1-alpha (HIF-1α), vascular endothelial growth factor (VEGF) and angiopoietin 2 (Ang2) [13–15]. In addition we have shown that the extracellular matrix (ECM) protein fibronectin is strongly upregulated on remodeling cerebral blood vessels in mice exposed to CMH [12,9], as well as in animal models of ischemic stroke and multiple sclerosis [16,17], clinical situations where angiogenic remodeling is thought to play a significant role. These studies also indicate that angiogenic endothelial cells strongly upregulate expression of the fibronectin receptor α5β1 integrin. The functional significance of the fibronectin-α5β1 integrin signaling pathway in promoting vascular remodeling in the brain was demonstrated by the observation that transgenic mice lacking α5 integrin expression specifically in endothelial cells (α5-EC-KO transgenic mice) display attenuated endothelial cell proliferation and a delayed hypoxic-induced angiogenic response [18].
As the plasticity of spinal cord blood vessels has yet to be fully explored, the main goal of this study was to investigate how CMH regulates vascular remodeling in the spinal cord. In particular, we wanted to examine how CMH influences angiogenesis, arteriogenesis, endothelial expression of the fibronectin-α5β1 integrin signaling axis, and endothelial expression of tight junction proteins, and then determine if α5β1 integrin mediated-signaling drives the vascular remodeling process.
MATERIALS AND METHODS
Animals
The studies described have been reviewed and approved by The Scripps Research Institute Institutional Animal Care and Use Committee. Wild-type female SJL/J mice were purchased from JAX labs and maintained under pathogen-free conditions at The Scripps Research Institute (TSRI). The generation of the Tie2-Cre/α5 integrin+/− strain, and α5 integrinflox/flox strains of mice have been described previously [19–21]. Both strains were backcrossed >6 times onto the C57BL/6 background and maintained under specific pathogen-free conditions in the closed breeding colony of The Scripps Research Institute (TSRI). Breeding of these two strains generated mice that lacked expression of the α5integrin specifically in endothelial cells (referred to here as α5 endothelial cell knockout mice (α5-EC-KO) mice; i.e. α5f/−; Tie2-Cre), accounting for 25% of the offspring, as well as mice that were Tie2-Cre negative, having two copies of the α5 integrin gene (α5+/f), which were used as controls. Genotyping was performed using previously described protocols [19–21].
Chronic Hypoxia Model
8–10 week old wild-type female SJL/J mice or α5-EC-KO and their WT littermate controls, were housed 4 to a cage, and placed into a hypoxic chamber (Biospherix, Redfield, NY) maintained at 8% O2 for periods up to 7 days. Littermate control mice were kept in the same room under similar conditions except that they were kept at ambient sea-level oxygen levels (normoxia, approximately 21% O2 at sea-level) for the duration of the experiment. Every few days, the chamber was briefly opened for cage cleaning and food and water replacement as needed.
Immunohistochemistry and antibodies
Immunohistochemistry was performed as described previously [22] on 10 µm frozen sections of cold phosphate buffered saline (PBS) perfused spinal cords taken from mice subject to either normoxia (control) or hypoxic conditions. The following antibodies were used in this study: rat monoclonal antibodies reactive for CD31 (clone MEC13.3) and the integrin subunit α5 (clone 5H10-27 (MFR5)) (BD Pharmingen (La Jolla, CA), hamster anti-CD31 (clone 2H8, Abcam, Cambridge, MA), rabbit anti-fibronectin and anti-laminin (Sigma, St. Louis, MO), mouse anti-α-SMA-Cy3 conjugate (Sigma, clone 1A4), rabbit anti-claudin-5, rabbit anti-occludin and rabbit anti-ZO-1) (all from Invitrogen, Carlsbad, CA), and mouse anti-Ki67 (Vector laboratories, Burlingame, CA). Secondary antibodies used included goat anti-rabbit Cy3, goat anti-rat Cy3, goat anti-mouse Cy3, and goat anti-rabbit Cy5 (far red) (all from Jackson Immunoresearch, Baltimore, PA) and anti-rat Alexa Fluor 488 and anti-hamster Alexa 488 (both from Invitrogen).
Image analysis
Images were taken using a 10X or 20X objective on a Zeiss Imager M1.m microscope. All analysis was performed in the spinal cord. Within these regions, and for each antigen, images of three randomly selected areas were taken at 10X or 20X magnification in both the white and gray matter, and three sections per spinal cord analyzed to calculate the mean for each subject. For each antigen in each experiment, exposure time was set to convey the maximum amount of information without saturating the image. Exposure time was maintained constant for each antigen across the time-course of hypoxic exposure. All data analysis was performed using NIH Image J software. This includes quantification of total vascular (CD31-positive) area, and the number of CD31/Ki67 dual-positive cells and α-SMA-positive vessels. To quantify the expression level of vascular fibronectin, α5 integrin, and the tight junction proteins claudin-5, occludin and ZO-1, NIH Image J software was used to measure the total fluorescent signal per field of view. Each experiment was performed with three different animals per condition, and the results expressed as the mean ± SEM. Statistical significance was assessed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison post-hoc test, in which p < 0.05 was defined as statistically significant.
RESULTS
Chronic mild hypoxia (CMH) promotes a strong vascular remodeling response in spinal cord blood vessels
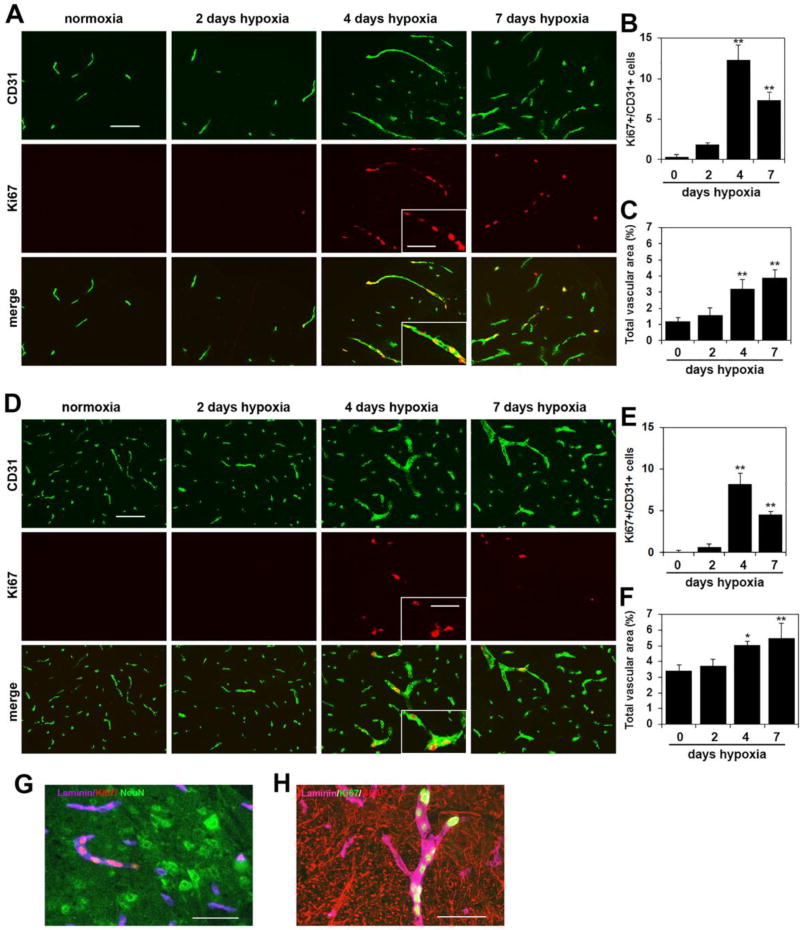
To examine how chronic mild hypoxia (CMH) influences vascular remodeling in blood vessels of the spinal cord, 10 week old mice were exposed to normoxia (control) or hypoxia (8% O2) for 2, 4 or 7 days. Spinal cords were then analyzed by CD31/Ki67 dual-immunofluorescence (dual-IF) for the endothelial cell marker CD31 and the cell proliferation marker Ki67, to quantify total vascular area and endothelial proliferation. Consistent with previous reports [23,24], under control (normoxic) conditions, CD31 staining revealed that blood vessel density in spinal cord gray matter was much higher (approximately 4–5 fold) than white matter (compare top left panel in Figure 1D with that in Figure 1A). When we examined endothelial proliferation, CD31/Ki67 dual-IF showed that proliferating endothelial cells were rarely seen in the spinal cords of control (normoxic) mice but that CMH triggered a marked endothelial proliferation response. After 2 days CMH, very few Ki67-positive cells were observed, but after 4 days CMH, many CD31/Ki67 dual-positive cells were present throughout the spinal cord, both in gray and white matter. Interestingly, many of the proliferating Ki67-positive endothelial cells displayed a linear arrangement (Figure 1A), indicating localized growth and expansion of pre-existing blood vessels. Strikingly, despite vascular density being highest in the gray matter, the greatest number of proliferating endothelial cells was found in white matter. In both regions, quantification revealed that the number of proliferating endothelial cells reached a maximum after 4 days CMH and declined thereafter. Compared with normoxic conditions, 4 days CMH increased the number of CD31/Ki67 dual-positive cells per field of view (FOV) from 0.33 ± 0.33 under normoxic conditions to 12.33 ± 1.85 in the white matter (p < 0.01) (Figure 1B) and from 0.11 ± 0.2 under normoxic conditions to 8.22 ± 1.26 in the gray matter (p < 0.01) (Figure 1E). Furthermore, this endothelial proliferation response correlated with an obvious expansion of the area covered by blood vessels (total vascular area) such that after 7 days hypoxia, the area covered by blood vessels expressed as a % of the total FOV had increased from 1.18 ± 0.24% under normoxic conditions to 3.90 ± 0.50% in the white matter (p < 0.01) (Figure 1C) and from 3.43 ± 0.34% under normoxic conditions to 5.50 ± 0.93% in the gray matter (p < 0.01) (Figure 1F). This demonstrates that endothelial proliferation and vascular expansion occur both in white and gray matter, but makes the point that vascular remodeling is more extensive in white matter, where the higher rate of endothelial proliferation translates into a greater expansion of total vascular area. This is also evident in the IF pictures shown in Figures 1A and 1D. To investigate the relationship between new vessels and astrocytes or neurons, we performed triple-staining. This showed that newly generated vessels (dual-positive for laminin and Ki67) were often seen growing towards neuron-rich (NeuN-positive) areas and were also closely associated with GFAP-positive astrocyte endfeet (Figures 1G and H respectively).
Figure 1.
Chronic mild hypoxia (CMH) stimulates vascular remodeling in spinal cord blood vessels. Dual-IF was performed on frozen sections of spinal cord white matter (A) or gray matter (D) taken from mice exposed to normoxia or 2, 4 or 7 days hypoxia using antibodies specific for CD31 (AlexaFluor-488) and Ki67 (Cy-3). Scale bar = 100 µm, except for the inset where scale bar = 50 µm. Note that little endothelial proliferation is seen after 2 days hypoxia, but after 4 days hypoxia a strong proliferative response is observed, before declining at later time-points. Proliferating endothelial cells are seen grouped together in a linear arrangement within a single vessel, in which an angiogenic sprout is observed (see inset). B and E. Quantification of endothelial proliferation at different time-points of CMH in white matter (B) and gray matter (E). Results are expressed as the mean ± SEM (n = 3 mice/group). C and F. Quantification of the influence of CMH on total vascular area in white matter (C) and gray matter (F). Results are expressed as the mean ± SEM in which vascular area is represented as a % of the FOV (n = 3 mice/group). Note that endothelial proliferation and vascular expansion occur both in white matter and gray matter, but vascular remodeling is more extensive in white matter, where the higher rate of endothelial proliferation translates into a greater expansion of total vascular area. * p < 0.05, ** p < 0.01 vs. normoxia (0 days hypoxia). G Triple-IF for laminin (Cy-5), Ki67 (Cy-3) and NeuN (AlexaFluor-488). H. Triple-IF for laminin (Cy-5), Ki67 (AlexaFluor-488) and GFAP (Cy-3).
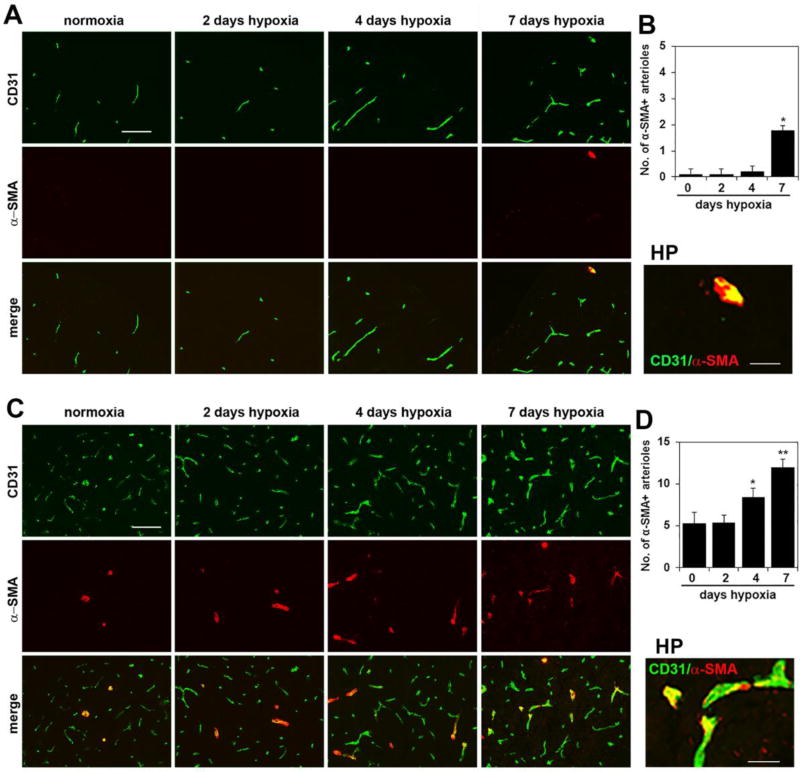
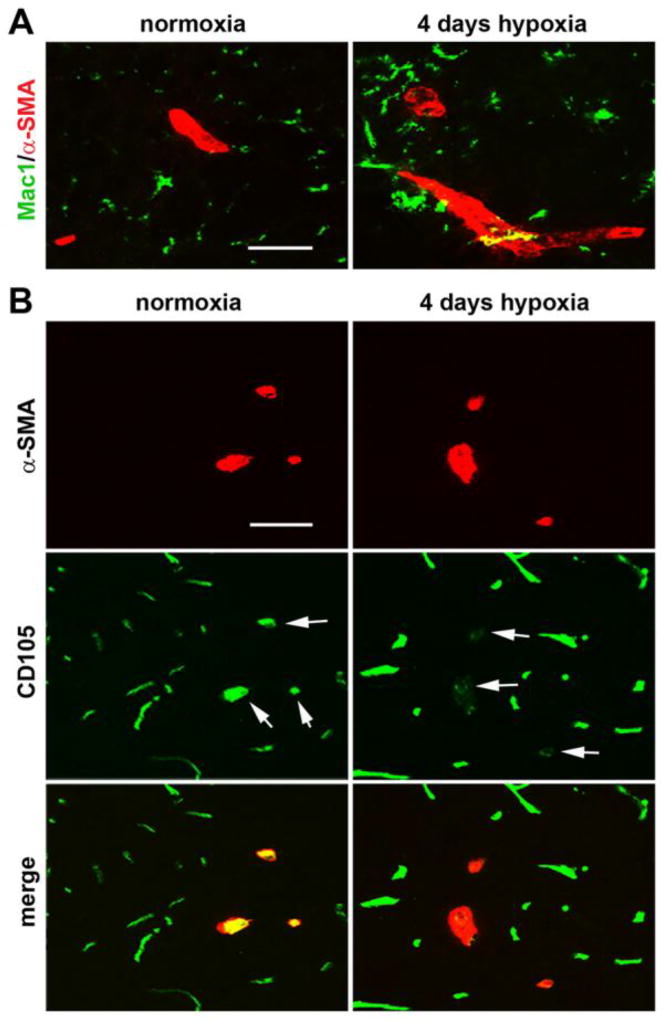
In a recent study in brain tissue, we showed that in addition to promoting angiogenic remodeling, CMH also stimulates an active arteriogenic remodeling response, culminating in an increased density of arterioles [10]. To examine if a similar mechanism also exists in the spinal cord, we performed dual-IF on spinal cord tissue with the endothelial marker CD31 and the smooth muscle cell marker α-smooth muscle actin (α-SMA), which by virtue of the higher level of smooth muscle cells within arterial vessels, preferentially labels arterial vessels. As shown in Figure 2, under normoxic conditions, most of the α-SMA-positive vessels were located in gray matter (panel C), with barely any observed in white matter (panel A). Interestingly, 7 days CMH significantly increased the density of α-SMA-positive vessels both in spinal cord white matter (increased from 0.11 ± 0.19 SMA-positive vessels/FOV under normoxic conditions to 1.78 ± 0.19 α-SMA-positive vessels/FOV, p < 0.05) and in gray matter (increased from 5.33 ± 1.33 SMA-positive vessels/FOV to 12.0 ± 1.0 α-SMA-positive vessels/FOV, p < 0.01). Thus CMH also stimulates an arteriogenic response in spinal cord blood vessels. In a previous study we showed that arteriogenic vessels could be identified by downregulated expression of CD105 (endoglin), an anciliary TGF-β receptor [10]. Using this as a tool to identify arteriogenic vessels in the hypoxic spinal cord, we confirmed that as in the brain, while α-SMA-positive arterial vessels in the normoxic spinal cord express high levels of CD105, remodeling arterioles in the hypoxic spinal cord strongly downregulate this receptor (see arrows in Figure 3B). As the process of arteriogenesis has been shown to be more dependent on signals derived from monocytic cells than on angiogenic factors such as VEGF [25,26], we next examined the distribution of Mac-1-positive cells in hypoxic spinal cord. This revealed that in contrast to arterial vessels under normoxic conditions, arteriogenic vessels in the hypoxic spinal cord were surrounded by aggregates of Mac-1-positive cells (Figure 3A).
Figure 2.
Chronic mild hypoxia (CMH) stimulates arteriogenic remodeling in spinal cord blood vessels. Dual-IF was performed on frozen sections of spinal cord white matter (A) or gray matter (C) taken from mice exposed to normoxia or 2, 4 or 7 days hypoxia using antibodies specific for CD31 (AlexaFluor-488) and alpha-smooth muscle actin (α-SMA, Cy-3). Scale bar = 100 µm. High power (HP) images, scale bar = 25 µm. B and D. Quantification of the number of arterial vessels at different time-points of CMH in white matter (B) and gray matter (D). Results are expressed as the mean ± SEM (n = 3 mice/group). Note that under normoxic conditions, most of the α-SMA-positive vessels were located in spinal cord gray matter, with barely any observed in white matter, but 7 days CMH significantly increased the density of α-SMA-positive vessels both in white and gray matter. * p < 0.05, ** p < 0.01 vs. normoxia (0 days hypoxia).
Figure 3.
Characterization of arteriogenic events in the hypoxic spinal cord. A. Dual-IF was performed on frozen sections of spinal cord gray matter taken from mice exposed to normoxia or 4 days hypoxia using antibodies specific for Mac-1 (AlexaFluor-488) and α-SMA (Cy-3). Scale bar = 25 µm. Note that in contrast to arterial vessels in the normoxic spinal cord, arteriogenic vessels in the hypoxic spinal cord were surrounded by aggregates of Mac-1-positive cells. B. Dual-IF was performed on frozen sections of spinal cord gray matter taken from mice exposed to normoxia or 4 days hypoxia using antibodies specific for CD105 (AlexaFluor-488) and α-SMA (Cy-3). Scale bar = 25 µm. Note that while α-SMA-positive arterial vessels in the normoxic spinal cord express high levels of CD105, remodeling arterioles in the hypoxic spinal cord strongly downregulate this receptor (see arrows).
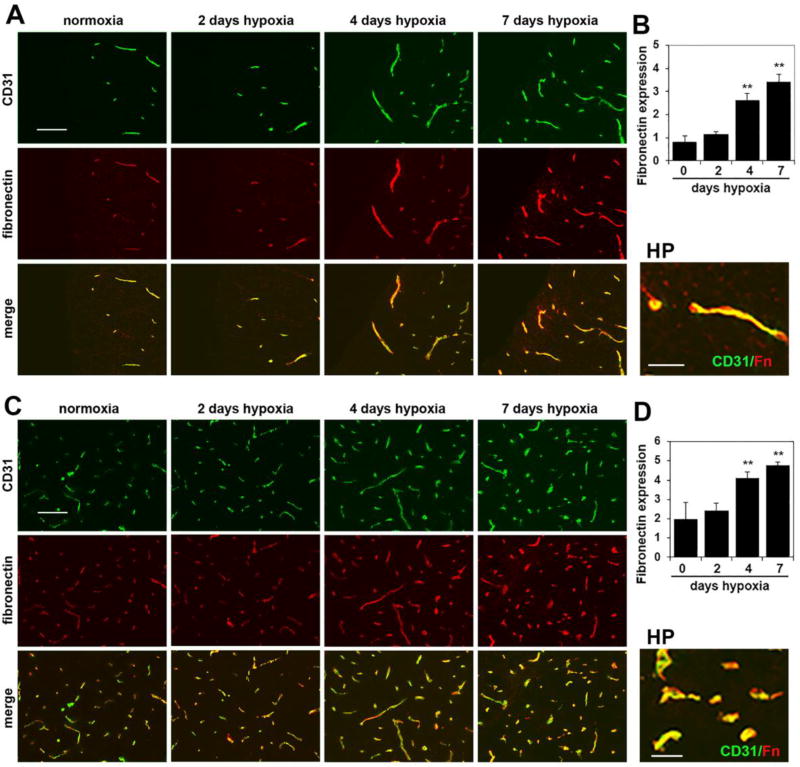
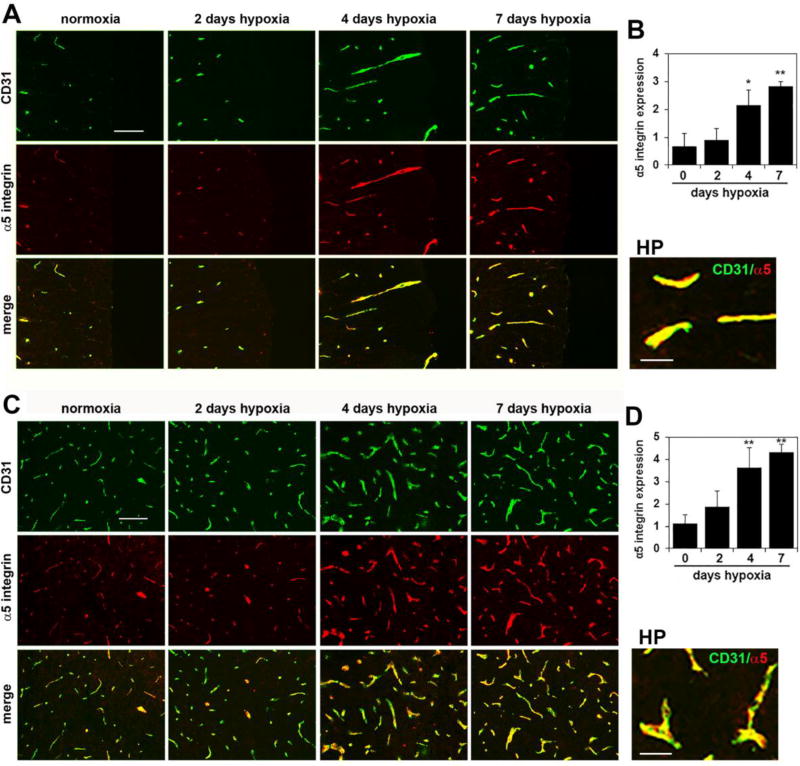
Chronic mild hypoxia strongly upregulates the angiogenic proteins fibronectin and α5β1 integrin on spinal cord blood vessels
In previous work in brain tissue, we demonstrated that fibronectin and the fibronectin receptor α5β1 integrin are strongly upregulated on angiogenic blood vessels and that these proteins play an important role in driving endothelial proliferation and angiogenic remodeling in response to CMH [12,18,9]. As a first step in determining whether the same mechanism regulates vascular remodeling in spinal cord blood vessels, we examined whether CMH triggered changes in fibronectin and α5β1 integrin expression in spinal cord blood vessels. As shown in Figure 4, CD31/fibronectin dual-IF revealed that after 4 and 7 days CMH, vascular expression levels of fibronectin were strongly upregulated on spinal cord blood vessels. Quantification of expression levels by fluorescent densitometry using NIH Image J software showed that following 7 days CMH, vascular fibronectin expression levels were increased from 0.82 ± 0.25 fluorescent units under normoxic conditions to 3.44 ± 0.31 fluorescent units in the white matter (p < 0.01) (Figure 4B) and from 2.0 ± 0.83 fluorescent units under normoxic conditions to 4.78 ± 0.17 fluorescent units in the gray matter (p < 0.01 (Figure 4D). In parallel, CMH also strongly increased endothelial expression of the fibronectin receptor α5 integrin within spinal cord blood vessels (Figure 5). Quantification revealed that 7 days CMH increased expression of α5 integrin within spinal cord blood vessels from 0.68 ± 0.45 fluorescent units under normoxic conditions to 2.83 ± 0.16 fluorescent units in the white matter (p < 0.01) (Figure 5B) and from 1.14 ± 0.37 fluorescent units under normoxic conditions to 4.32 ± 0.35 fluorescent units in the gray matter (p < 0.01) (Figure 4D). Thus, CMH promotes a striking upregulation of fibronectin and the endothelial α5β1 integrin within spinal cord blood vessels.
Figure 4.
Chronic mild hypoxia (CMH) upregulates fibronectin expression in spinal cord blood vessels. Dual-IF was performed on frozen sections of spinal cord white matter (A) or gray matter (C) taken from mice exposed to normoxia or 2, 4 or 7 days hypoxia using antibodies specific for CD31 (AlexaFluor-488) and fibronectin (Cy-3). Scale bar = 100 µm. High power (HP) images, scale bar = 25 µm. B and D. Quantification of fibronectin fluorescent signal at different time-points of CMH in white matter (B) and gray matter (D). Results are expressed as the mean ± SEM (n = 3 mice/group). Note that under normoxic conditions, vascular fibronectin expression is low, but after 4 or 7 days CMH, fibronectin expression is strongly increased, both in white and gray matter. ** p < 0.01 vs. normoxia (0 days hypoxia).
Figure 5.
Chronic mild hypoxia (CMH) upregulates endothelial α5 integrin expression in spinal cord blood vessels. Dual-IF was performed on frozen sections of spinal cord white matter (A) or gray matter (C) taken from mice exposed to normoxia or 2, 4 or 7 days hypoxia using antibodies specific for CD31 (AlexaFluor-488) and α5 integrin (Cy-3). Scale bar = 100 µm. High power (HP) images, scale bar = 25 µm. B and D. Quantification of endothelial α5 integrin signal at different time-points of CMH in white matter (B) and gray matter (D). Results are expressed as the mean ± SEM (n = 3 mice/group). Note that under normoxic conditions, endothelial expression of α5 integrin is low, but after 4 or 7 days CMH, α5 integrin expression is strongly increased, both in white and gray matter. * p < 0.05, ** p < 0.01 vs. normoxia (0 days hypoxia).
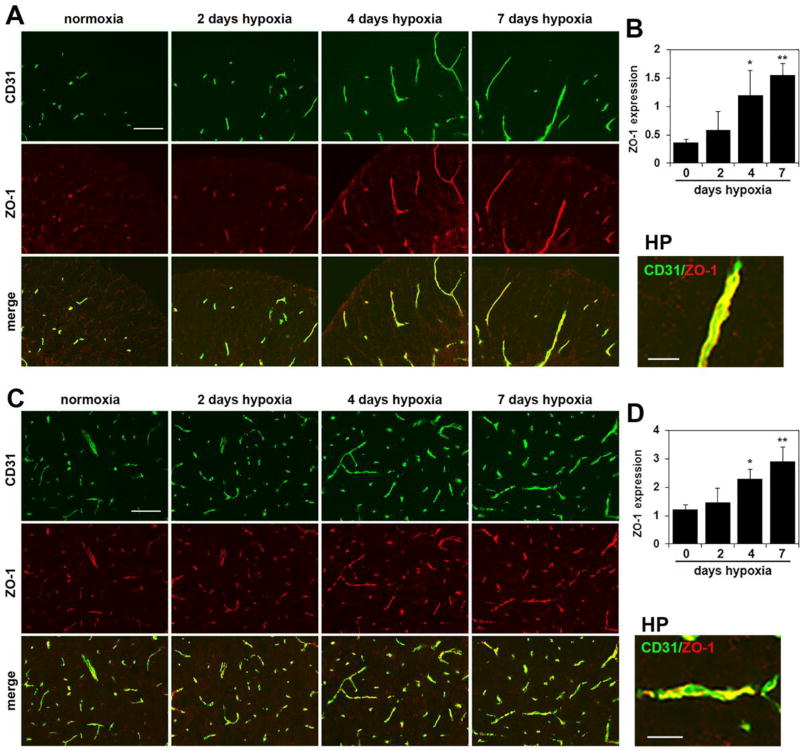
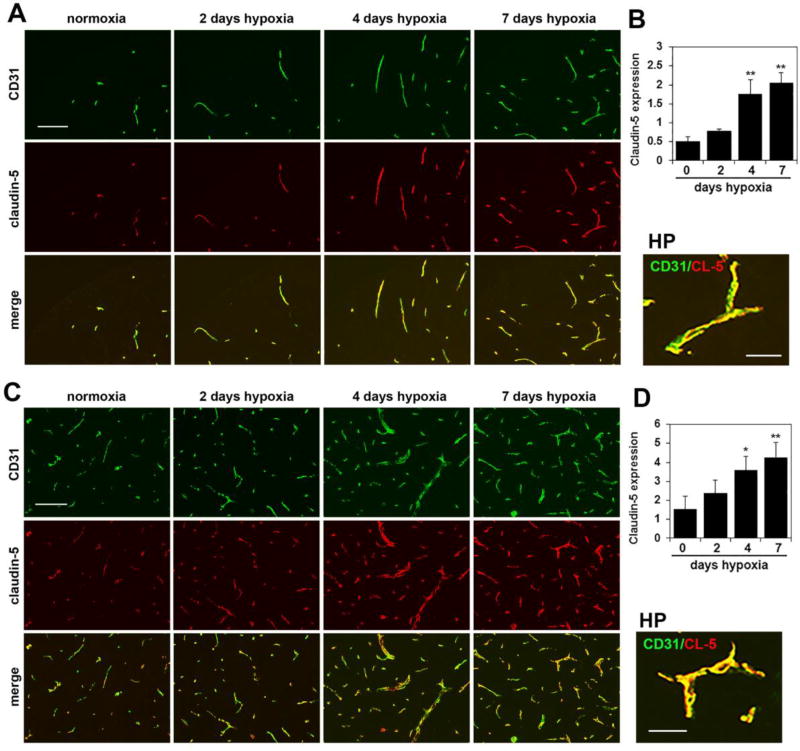
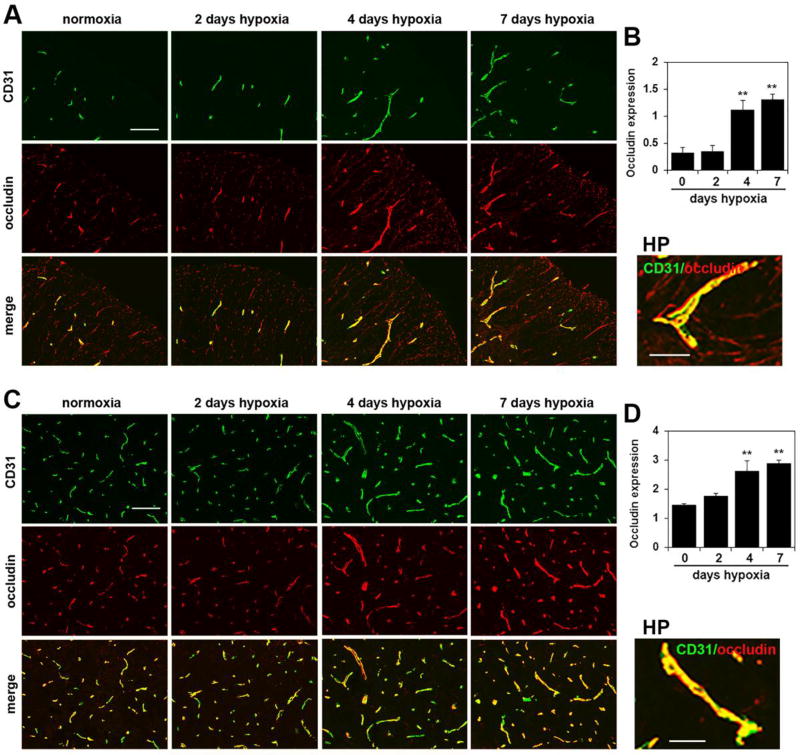
Chronic mild hypoxia also upregulates endothelial expression of tight junction proteins on spinal cord blood vessels
Endothelial tight junction proteins are one of the major molecular mechanisms responsible for maintaining the integrity of the blood-brain barrier (BBB) [27–29]. Several years ago, we made the observation that CMH stimulates a marked upregulation of the tight junction proteins claudin-5 and ZO-1 in cerebral blood vessels [12]. To examine whether a similar regulation occurs in the spinal cord, we performed CD31/claudin-5, CD31/ZO-1 and CD31/occludin dual-IF on frozen sections of spinal cord. As shown in Figures 5–7, CMH exposure for 4 or 7 days triggered strong upregulation of all three tight junction proteins in spinal cord blood vessels. Fluorescent densitometry showed that 7 days CMH increased endothelial expression of claudin-5 from 0.50 ± 0.12 fluorescent units under normoxic conditions to 2.06 ± 0.26 fluorescent units in the white matter (p < 0.01) and from 1.53 ± 0.67 fluorescent units under normoxic conditions to 4.27 ± 0.77 fluorescent units in the gray matter (p < 0.01) (Figure 6). In parallel, 7 days CMH increased endothelial ZO-1 expression from 0.37 ± 0.06 fluorescent units under normoxic conditions to 1.56 ± 0.19 fluorescent units in the white matter (p < 0.01) and from 1.21 ± 0.16 fluorescent units under normoxic conditions to 2.92 ± 0.50 fluorescent units in the gray matter (p < 0.01) (Figure 7) and also increased endothelial occludin expression from 0.33 ± 0.09 fluorescent units under normoxic conditions to 1.32 ± 0.09 fluorescent units in the white matter (p < 0.01) and from 1.46 ± 0.04 fluorescent units under normoxic conditions to 2.89 ± 0.11 fluorescent units in the gray matter (p < 0.01) (Figure 8). This shows that CMH triggers strong endothelial upregulation of the tight junction proteins claudin-5, ZO-1 and occludin.
Figure 7.
Chronic mild hypoxia (CMH) upregulates endothelial ZO-1 expression in spinal cord blood vessels. Dual-IF was performed on frozen sections of spinal cord white matter (A) or gray matter (C) taken from mice exposed to normoxia or 2, 4 or 7 days hypoxia using antibodies specific for CD31 (AlexaFluor-488) and ZO-1 (Cy-3). Scale bar = 100 µm. High power (HP) images, scale bar = 25 µm. B and D. Quantification of endothelial ZO-1 signal at different time-points of CMH in white matter (B) and gray matter (D). Results are expressed as the mean ± SEM (n = 3 mice/group). Note that after 4 or 7 days CMH, ZO-1 expression is strongly increased, both in white and gray matter. * p < 0.05, ** p < 0.01 vs. normoxia (0 days hypoxia).
Figure 6.
Chronic mild hypoxia (CMH) upregulates endothelial claudin-5 expression in spinal cord blood vessels. Dual-IF was performed on frozen sections of spinal cord white matter (A) or gray matter (C) taken from mice exposed to normoxia or 2, 4 or 7 days hypoxia using antibodies specific for CD31 (AlexaFluor-488) and claudin-5 (Cy-3). Scale bar = 100 µm. High power (HP) images, scale bar = 25 µm. B and D. Quantification of endothelial claudin-5 signal at different time-points of CMH in white matter (B) and gray matter (D). Results are expressed as the mean ± SEM (n = 3 mice/group). Note that after 4 or 7 days CMH, claudin-5 expression is strongly increased, both in white and gray matter. * p < 0.05, ** p < 0.01 vs. normoxia (0 days hypoxia).
Figure 8.
Chronic mild hypoxia (CMH) upregulates endothelial occludin expression in spinal cord blood vessels. Dual-IF was performed on frozen sections of spinal cord white matter (A) or gray matter (C) taken from mice exposed to normoxia or 2, 4 or 7 days hypoxia using antibodies specific for CD31 (AlexaFluor-488) and occludin (Cy-3). Scale bar = 100 µm. High power (HP) images, scale bar = 25 µm. B and D. Quantification of endothelial occludin signal at different time-points of CMH in white matter (B) and gray matter (D). Results are expressed as the mean ± SEM (n = 3 mice/group). Note that after 4 or 7 days CMH, occludin expression is strongly increased, both in white and gray matter. * p < 0.05, ** p < 0.01 vs. normoxia (0 days hypoxia).
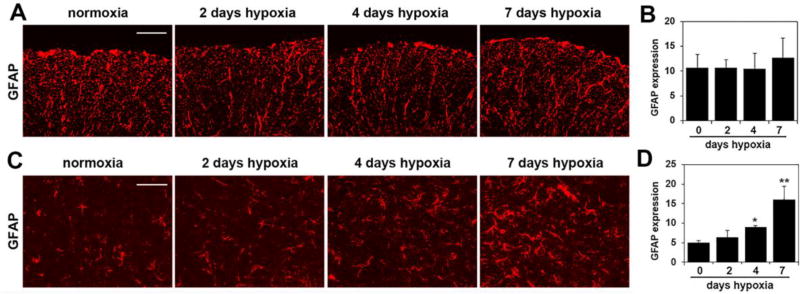
Chronic mild hypoxia also triggers marked astrocyte activation, specifically in the gray matter
In light of our previous finding that CMH stimulates astrocyte activation in the brain [12], we also examined whether a similar regulation occurs in the spinal cord. Under control (normoxic) conditions GFAP staining revealed a strong and robust expression within the fibrous astrocytes of white matter, but in contrast, within the gray matter, only a relatively low density of GFAP-positive cells were detected. Interestingly, the influence of CMH on GFAP expression within the white and gray matter was quite different. In contrast to the white matter, where CMH had no obvious effect on GFAP expression, in the gray matter, CMH triggered a gradual increase both in the number of GFAP-positive cells and also in the size and staining intensity of GFAP-positive cells. Quantification showed that CMH had no noticeable effect on the total amount of GFAP fluorescent signal in the white matter, but in the gray matter, 7 days CMH increased the GFAP signal from 5.04 ± 0.56 fluorescent units under normoxic conditions to 16.04 ± 3.51 fluorescent units (p < 0.01) (Figure 9).
Figure 9.
The influence of chronic mild hypoxia (CMH) on astrocyte activation in the spinal cord. IF was performed on frozen sections of spinal cord white matter (A) or gray matter (C) taken from mice exposed to normoxia or 2, 4 or 7 days hypoxia using GFAP antibodies (Cy-3). Scale bar = 100 µm. B and D. Quantification of GFAP fluorescent signal at different time-points of CMH in white matter (B) and gray matter (D). Results are expressed as the mean ± SEM (n = 3 mice/group). Note that under normoxic conditions, robust GFAP expression was observed within the fibrous astrocytes of white matter, but in contrast, within the gray matter, very few GFAP-positive cells were detected. Quantification showed that CMH had no noticeable effect on the total amount of GFAP fluorescent signal in the white matter, but in the gray matter, 7 days CMH strongly increased the GFAP signal. * p < 0.05, ** p < 0.01 vs. normoxia (0 days hypoxia).
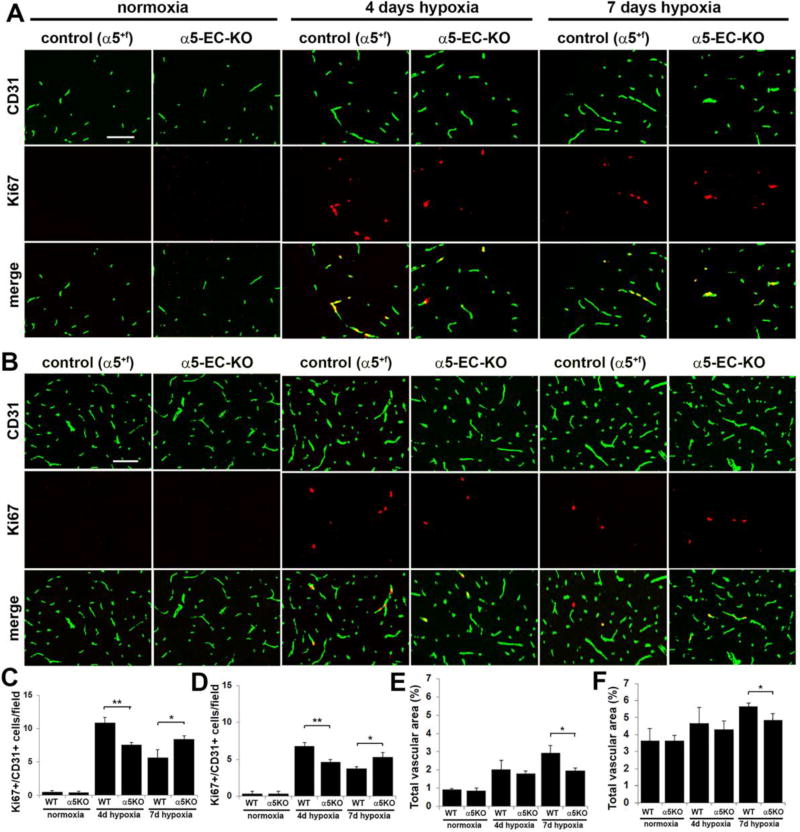
Mice deficient in α5 integrin show reduced spinal cord vascular remodeling in response to cerebral hypoxia
As our lab previously demonstrated that transgenic mice lacking α5 integrin expression in endothelial cells (α5-EC-KO transgenic mice) showed attenuated cerebrovascular remodeling in response to mild hypoxia [18], we wanted to examine if the same mechanism is also relevant in the spinal cord. Mice were subject to mild hypoxia (8% O2) for 0, 4, or 7 days and their spinal cords were analysed for vessel density and endothelial proliferation by CD31/Ki67 dual-IF. As shown in Figure 10C and D, after 4 days hypoxia, the number of dual-positive Ki67+/CD31+ cells per field of view in the spinal cord of α5-EC-KO mice was significantly lower than in WT littermates, both in the white matter (7.56 ± 0.35 compared with 10.89 ± 0.76, p < 0.01) and in the gray matter (4.67 ± 0.33 compared with 6.78 ± 0.51, p < 0.01). Interestingly however, after 7 days hypoxia, this ranking reversed, such that the number of dual-positive Ki67+/CD31+ cells in the spinal cord of α5-EC-KO mice became significantly higher than in controls, both in the white matter (8.44 ± 0.51 compared with 5.67 ± 1.2, p < 0.05) and in the gray matter (5.33 ± 0.58 compared with 3.78 ± 0.19, p < 0.05). Quantification of total vascular area in the spinal cord revealed that under normoxic conditions, there was no difference between α5-EC-KO and control mice, either in the white matter (0.85 ± 0.14 compared with 0.91 ± 0.04) or the gray matter (3.66 ± 0.30 compared with 3.63 ± 0.73) (Figure 10E and F). However, in keeping with the reduced endothelial proliferation response, the hypoxic-associated expansion in total vascular area observed after 7 days CMH was markedly reduced in α5-EC-KO mice both in the white matter (1.97 ± 0.13 compared with 2.92 ± 0.41, p < 0.05) and in the gray matter (4.86 ± 0.36 compared with 5.65 ± 0.19, p < 0.05). Taken together, these studies demonstrate that mice lacking endothelial cell α5 integrin expression showed a significantly attenuated and delayed spinal cord vascular remodeling response to cerebral hypoxia.
Figure 10.
Hypoxic-induced spinal cord vascular remodeling is delayed in α5-EC-KO mice. Frozen sections of spinal cord white matter (A) or gray matter (B) taken from control or α5-EC-KO mice exposed to normoxia or 4 or 7 days hypoxia (8% O2) were examined for BEC proliferation and total vascular area, as assessed by CD31/Ki67 dual-IF. Scale bar = 100µm. C and D. Quantification of CD31+/Ki67+ proliferating BEC in the white matter (C) and gray matter (D), in which each experiment was performed with three different animals per condition, and the results expressed as the mean ± SEM of the number of CD31+/Ki67+ cells per field of view. Note that hypoxia promoted an increase in the number of proliferating BEC, though this response was delayed in α5-EC-KO mice in both white and gray matter. * p < 0.05, ** p < 0.01. E and F. Quantification of the influence of CMH on total vascular area in white matter (E) and gray matter (F). Results are expressed as the mean ± SEM in which vascular area is represented as a % of the FOV (n = 3 mice/group). Note that 7 days hypoxia promoted an increase in the total vascular area, though this response was delayed in α5-EC-KO mice both in white and gray matter. * p < 0.05.
DISCUSSION
Spinal cord injury (SCI) leads to rapid cell death and destruction of tissue, resulting in profound motor and sensory deficits [1,2]. This injury tears blood vessels in the area of damage and also causes neighboring blood vessels to become leaky and dysfunctional, thus compounding the problem [3]. Lack of functional vasculature impedes endogenous tissue repair as well as limiting repair strategies. With this in mind, it becomes apparent that approaches that can protect existing blood vessels or stimulate the growth of new blood vessels might present a way to minimize loss or promote regeneration of spinal cord tissue following SCI. In light of the remarkable power of chronic mild hypoxia (CMH) to stimulate vascular remodeling in the brain [7–9], the goal of this study was to examine the influence of CMH (8% O2 for up to 7 days) on blood vessel remodeling in the spinal cord. We found that over a period of 7 days, CMH promoted the following events within spinal cord blood vessels: (i) endothelial proliferation and increased vascularity as a result of angiogenic and arteriogenic responses, (ii) increased vascular expression of the pro-angiogenic ECM protein fibronectin as well as concomitant increases in endothelial expression of the fibronectin receptor α5β1 integrin, (iii) upregulated endothelial expression of the tight junction proteins claudin-5, ZO-1 and occludin, and (iv) astrocyte activation. Of note, the vascular remodeling changes induced by CMH were generally more extensive in white matter, while the astrocyte activation response was most evident in gray matter. Interestingly, hypoxic-induced vascular remodeling in spinal cord blood vessels was markedly attenuated in mice lacking endothelial α5 integrin expression (α5-EC-KO mice). Taken together, these studies demonstrate the considerable remodeling potential of spinal cord blood vessels and highlight an important angiogenic role for the α5β1 integrin in promoting endothelial proliferation. They also suggest that stimulation of the α5β1 integrin or controlled use of mild hypoxia might provide new therapeutic approaches to promote angiogenesis and improve vascular integrity in spinal cord blood vessels.
Vascular remodeling induced by CMH is more extensive in white matter
It is well-established that blood vessel density in the gray matter is much higher (approximately 4–5 fold) than in white matter, presumably because of the higher metabolic requirements of the neuron-rich tissue in gray matter [23,24]. Interestingly though, in our current study, we found that the vascular remodeling changes induced by CMH were more extensive in the white matter, which is a rather surprising result. CMH triggered endothelial proliferation, enhanced expression of the pro-angiogeneic fibronectin-α5β1 integrin signaling axis and increased vascularity in the gray matter, but these changes were noticeably stronger in the white matter. It’s currently unknown what accounts for this difference. One possibility is that the blood supply to the neuron-rich gray matter is already optimized and has a higher degree of functional reserve, and is thus able to handle small changes in delivery of oxygen, glucose and other nutrients, whereas blood vessels in the white matter are already working at 100% of capacity and are forced to enhance vessel density if they are to continue to provide sufficient oxygen in the face of hypoxic challenge. An alternative possibility is that other compensatory mechanisms exist in the gray matter to maintain energy delivery to the sensitive neuronal population, and one such defined mechanism is the astrocyte-neuron lactate shuttle, whereby astrocytes provide neighboring neurons with the energy substrate lactate, from which neurons then derive ATP [30]. Yet another reason could be that oligodendrocytes, the most abundant cell type within white matter, are highly sensitive to the effects of hypoxia and thus trigger a stronger adaptive vascular remodeling response to cope with the hypoxic challenge. While it is currently unclear which of these explanations, if any, accounts for the differential effect we see in vascular remodeling between white and gray matter, future studies should clarify our understanding of this process.
Does mild hypoxia provide therapeutic benefit in spinal cord injury (SCI) by optimising vascularity and vascular integrity?
Over the last fifteen years a number of studies have shown that mild hypoxia, when delivered intermittently, can promote neuroplasticity in the injured spinal cord, leading to functional recovery of breathing capacity and limb motor function [31,32]. While most of the research into the underlying mechanisms have centered on growth factor release, neurotransmitters and electrophysiology [33], to date no-one has examined the role of blood vessels in this process. The work we present here shows that mild hypoxia when administered chronically, exerts profound changes in blood vessels of the spinal cord, leading to enhanced vascular density and increased expression of endothelial tight junction proteins, suggestive of tightened vascular integrity. Most of the hypoxic work in SCI recovery has involved the use of intermittent hypoxic training (IHT) protocols, whereby a series of short relatively mild hypoxic exposures leads to demonstrable clinical benefit [31,32]. The current challenge in the field is to titrate back the dosing of hypoxia so as to trigger beneficial effects without inducing any pathological events.
The angiogenic role of α5β1 integrin
In addition to using mild hypoxia as a means of stimulating beneficial changes prior to insult (pre-conditioning) or after insult (post-conditioning), our study also makes the important point that pharmacological manipulation of the fibronectin-α5β1 integrin axis might offer an alternative approach to stimulate vascular growth and integrity. Accumulating data taken from different systems suggests that the fibronectin-α5β1 integrin axis plays an important role in angiogenesis. Mice with global KO of the fibronectin or α5 integrin genes show an embryonic lethal phenotype and aberrant blood vessel formation in the embryo [34,21]. Similar vascular defects are also apparent in α5 integrin-null embryoid bodies and teratoma cells [35]. Importantly, while differentiated endothelial cells express low levels of α5β1 integrin, angiogenic endothelial cells strongly upregulate this integrin, and functional blockade of the α5β1 integrin in tumor-induced neovasculature inhibits angiogenesis and tumor growth in vivo [36]. We previously demonstrated that angiogenic endothelial cells in the CNS upregulate two different fibronectin receptors, the α5β1 and αvβ3 integrins [37]. Interestingly however, while hypoxic-induced CNS angiogenesis is unaffected in β3 integrin KO mice [37], transgenic mice deficient in endothelial α5 integrin (α5-EC-KO mice) showed delayed and attenuated CNS vascular remodeling in response to hypoxic challenge [18], suggesting that while αvβ3 integrin is dispensable for the angiogenic response, α5β1 integrin plays an important role in driving endothelial proliferation and CNS angiogenesis [18]. The current study confirms a similar role for α5β1 integrin in promoting vascular remodeling in the spinal cord. Having shown an important role for this integrin, in future studies our goal is to identify reagents (e.g.; peptide agonists) that stimulate α5β1 integrin or its downstream signaling pathways so as to enhance the growth of spinal cord blood vessels.
Acknowledgments
This work was supported by the NIH R56 grant NS095753 (RM). This is manuscript number 29533 from The Scripps Research Institute.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- 1.Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006;23:264–280. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- 2.Oudega M. Spinal cord injury and repair: role of blood vessel loss and endogenous angiogenesis. Adv Wound Care. 2010;1:335–340. [Google Scholar]
- 3.Echeverry S, Shi XQ, Rivest S, Zhang J. Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J Neurosci. 2011;31:10819–10828. doi: 10.1523/JNEUROSCI.1642-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramlett HM, Dietrich WD. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- 5.Ek CJ, Habgood MD, Callaway JK, Dennis R, Dziegielewska KM, Johansson PA, Potter A, Wheaton B, Saunders NR. Spatio-temporal progression of grey and white matter damage following contusion injury in rat spinal cord. PLoS One. 2010;5:e12021. doi: 10.1371/journal.pone.0012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fassbender JM, Whittemore SR, Hagg T. Targeting microvasculature for neuroprotection after SCI. Neurotherapeutics. 2011;8:240–251. doi: 10.1007/s13311-011-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207:3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- 8.LaManna JC, Vendel LM, Farrell RM. Brain adaptation to chronic hypobaric hypoxia in rats. J Appl Physiol. 1992;72:2238–2243. doi: 10.1152/jappl.1992.72.6.2238. [DOI] [PubMed] [Google Scholar]
- 9.Milner R, Hung S, Erokwu B, Dore-Duffy P, LaManna JC, del Zoppo GJ. Increased expression of fibronectin and the α5β1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol Cell Neurosci. 2008;38:43–52. doi: 10.1016/j.mcn.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boroujerdi A, Welser-Alves J, Tigges U, Milner R. Chronic cerebral hypoxia promotes arteriogenic remodeling events that can be identified by reduced endoglin (CD105) expression and a switch in β1 integrins. J Cereb Blood Flow Metab. 2012;32:1820–1830. doi: 10.1038/jcbfm.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boroujerdi A, Milner R. Defining the critical hypoxic threshold that promotes vascular remodeling in the brain. Exp Neurol. 2015;263:132–140. doi: 10.1016/j.expneurol.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Welser JV, Dore-Duffy P, Del Zoppo GJ, LaManna JC, Milner R. In the hypoxic central nervous system, endothelial cell proliferation is followed by astrocyte activation, proliferation, and increased expression of the α6β4 integrin and dystroglycan. Glia. 2010;58:1157–1167. doi: 10.1002/glia.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxic inducible factor 1α in the brain of rats during chronic hypoxia. J Appl Physiol. 2000;89:1937–1942. doi: 10.1152/jappl.2000.89.5.1937. [DOI] [PubMed] [Google Scholar]
- 14.Kuo N-T, Benhayon D, Przybylski RJ, Martin RJ, LaManna JC. Prolonged hypoxia increases vascular endothelial growth factor mRNA and protein in adult mouse brain. J Appl Physiol. 1999;86:260–264. doi: 10.1152/jappl.1999.86.1.260. [DOI] [PubMed] [Google Scholar]
- 15.Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and de-adaptation to prolonged mild hypoxia. J Appl Physiol. 2002;93:1131–1139. doi: 10.1152/japplphysiol.00318.2002. [DOI] [PubMed] [Google Scholar]
- 16.Boroujerdi A, Welser-Alves J, Milner R. Extensive vascular remodeling in the spinal cord of pre-symptomatic experimental autoimmune encephalomyelitis mice; increased vessel expression of fibronectin and the α5β1 integrin. Exp Neurol. 2013;250:43–51. doi: 10.1016/j.expneurol.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Liu F, Welser-Alves JV, McCullough LD, Milner R. Upregulation of fibronectin and the α5β1 and αvβ3 integrins on blood vessels within the cerebral ischemic penumbra. Exp Neurol. 2012;233:283–291. doi: 10.1016/j.expneurol.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Welser-Alves JV, van der Flier A, Boroujerdi A, Hynes RO, Milner R. An angiogenic role for the α5β1 integrin in promoting endothelial cell proliferation during cerebral hypoxia. Exp Neurol. 2012;237:46–54. doi: 10.1016/j.expneurol.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 20.van der Flier A, Badu-Nkansah K, Whittaker CA, Crowley D, Roderick T, Bronson DT, Lacy-Hulbert A, Hynes RO. Endothelial α5 and αv Integrins Cooperate in Remodeling of the Vasculature During Development. Development. 2010;137:2439–2449. doi: 10.1242/dev.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in α5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 22.Milner R, Campbell IL. Developmental regulation of β1 integrins during angiogenesis in the central nervous system. Mol Cell Neurosci. 2002;20:616–626. doi: 10.1006/mcne.2002.1151. [DOI] [PubMed] [Google Scholar]
- 23.Hassler O. Blood supply to human spinal cord. A microangiographic study. Arch Neurol. 1966;15:302–307. doi: 10.1001/archneur.1966.00470150080013. [DOI] [PubMed] [Google Scholar]
- 24.Turnbull IM, Brieg A, Hassler O. Blood supply of cervical spinal cord in man. A microangiographic cadaver study. J Neurosurg. 1966;24:951–965. doi: 10.3171/jns.1966.24.6.0951. [DOI] [PubMed] [Google Scholar]
- 25.Buschmann IR, Busch H-J, Mies G, Hossmann K-A. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation. 2003;108:610–615. doi: 10.1161/01.CIR.0000074209.17561.99. [DOI] [PubMed] [Google Scholar]
- 26.Todo K, Kitagawa K, Sasaki T, Omura-Matsuoka E, Terasaki Y, Oyama N, Yagita Y, Hori M. Granulocyte-macrophage colony-stimulating factor enhances leptomeningeal collateral growth induced by common carotid artery occlusion. Stroke. 2008;39:1875–1882. doi: 10.1161/STROKEAHA.107.503433. [DOI] [PubMed] [Google Scholar]
- 27.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview. Structure, regulation and clinical implications. Neurobiology of Disease. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neourosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 29.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier; development, composition and regulation. Vascular Pharmacology. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 30.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis- a mechanism coupling neuronal activity to glucose utilization. Pro Natl Acad Sci. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller DD, Johnson SM, Olson EBJ, Mitchell GS. Synaptic pathways to phrenic motorneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23:2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia imrpoves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Rothi EJ, Lee K-Z, Dale EA, Reier PJ, Mitchell GS, Fuller DD. Intermittent hypoxia and neurorehabilitation. J Appl Physiol. 2015;119:1455–1465. doi: 10.1152/japplphysiol.00235.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 35.Taverna D, Hynes RO. Reduced blood vessel formation and tumor growth in alpha5-integrin-negative teratocarcinomas and embryoid bodies. Cancer Res. 2001;61:5255–5261. [PubMed] [Google Scholar]
- 36.Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Welser JV, Milner R. Absence of the αvβ3 integrin dictates the time-course of angiogenesis in the hypoxic central nervous system: accelerated endothelial proliferation correlates with compensatory increases in α5β1 integrin expression. J Cereb Blood Flow Metab. 2010;30:1031–1043. doi: 10.1038/jcbfm.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]