Abstract
The role of the nfuA gene encoding an iron-sulfur ([Fe-S]) cluster-delivery protein in the pathogenic bacterium Pseudomonas aeruginosa was investigated. The analysis of nfuA expression under various stress conditions showed that superoxide generators, a thiol-depleting agent and CuCl2 highly induced nfuA expression. The expression of nfuA was regulated by a global [2Fe-2S] cluster containing the transcription regulator IscR. Increased expression of nfuA in the ΔiscR mutant under uninduced conditions suggests that IscR acts as a transcriptional repressor. In vitro experiments revealed that IscR directly bound to a sequence homologous to the Escherichia coli Type-I IscR-binding motifs on a putative nfuA promoter that overlapped the -35 element. Binding of IscR prevented RNA polymerase from binding to the nfuA promoter, leading to repression of the nfuA transcription. Physiologically, deletion of nfuA reduced the bacterial ability to cope with oxidative stress, iron deprivation conditions and attenuated virulence in the Caenorhabditis elegans infection model. Site-directed mutagenesis analysis revealed that the conserved CXXC motif of the Nfu-type scaffold protein domain at the N-terminus was required for the NfuA functions in conferring the stress resistance phenotype. Furthermore, anaerobic growth of the ΔnfuA mutant in the presence of nitrate was drastically retarded. This phenotype was associated with a reduction in the [Fe-S] cluster containing nitrate reductase enzyme activity. However, NfuA was not required for the maturation of [Fe-S]-containing proteins such as aconitase, succinate dehydrogenase, SoxR and IscR. Taken together, our results indicate that NfuA functions in [Fe-S] cluster delivery to selected target proteins that link to many physiological processes such as anaerobic growth, bacterial virulence and stress responses in P. aeruginosa.
Introduction
Pseudomonas aeruginosa is one of the most common gram-negative bacteria causing nosocomial infections with high mortality worldwide. During the host-microbe interaction, P. aeruginosa encounters oxidative stress generated either from phagocytic cells of the host innate immune response or from the killing mechanism of some bactericidal antibiotics [1]. Thus, the bacterial ability to endure oxidative stress is crucial for the survival of this pathogen during infection.
[Fe-S] clusters are ubiquitous prosthetic groups for numerous proteins that participate in various fundamental cellular activities, including gene regulation, iron/sulfur storage, aerobic and anaerobic respiration, and biosynthetic pathways [2]. To date, three types of [Fe-S] cluster biosynthetic machineries have been discovered in prokaryotes, i.e., ISC (iron-sulfur cluster), SUF (sulfur formation), and NIF (nitrogen fixation) (for a review see [2, 3]). The ISC and SUF systems contribute to the biogenesis of [Fe-S] clusters that are supplied for the maturation of most proteins in the cell; however, the NIF system is required for maturation of nitrogenase in nitrogen-fixing bacteria [4, 5]. P. aeruginosa possesses ISC machinery encoded by the iscRSUA-hscBA-fdx2-iscX gene cluster, which is responsible for [Fe-S] cluster biosynthesis [6]. IscR acts as a transcriptional repressor of the isc operon. Holo-IscR, a form containing a [2Fe-2S] cluster, directly binds to Type-I IscR-binding motifs located in the vicinity of the promoter and modulates isc gene expression [6].
NfuA was first reported in Escherichia coli as a [Fe-S] scaffolding protein required for maturation of a [Fe-S] cluster containing proteins under oxidative stress and iron starvation conditions [7] as well as in Azotobacter vinelandii as an intermediate [Fe-S] cluster carrier protein involved in [Fe-S] protein maturation [8]. A recent report in E. coli showed that NfuA is a non-SUF and non-ISC [4Fe-4S] carrier protein that is involved in maturation of [Fe-S] enzymes such as aconitase B and NADH dehydrogenase [9]. NfuA has two functionally related domains. The N-terminal domain is homologous to the ATC-type [Fe-S] carrier domain, which often has a variant of the CXXC domain that lacks the three C residues involved in the ligand for the cluster. The C-terminal domain is homologous to the Nfu domain of NifU, a U-type scaffold protein dehydrogenase involved in the assembly and transfer of [4Fe-4S] to target proteins [2, 9]. The physiological function of NfuA in P. aeruginosa is as yet unclear. Mutation of nfuA renders P. aeruginosa more susceptible to fluoroquinolone antibiotics than the wild type, under aerobic growth [10]. Here, we further investigated the gene regulation and the function of P. aeruginosa nfuA. The expression profile of nfuA, which could be induced in response to exposure to various oxidants together with the oxidant-vulnerable phenotypes of the nfuA mutant, suggests the contribution of NfuA to the protection of P. aeruginosa against oxidative stress. The induction of nfuA expression against the stress response was dependent on transcriptional control through [2Fe-2S] cluster-ligated IscR. Moreover, the nfuA mutant exhibited dramatically retarded growth under anaerobic conditions using nitrate as an electron due to the decrease in nitrate reductase activity level and showed attenuated virulence in a C. elegans host model system.
Results and discussion
The expression patterns of P. aeruginosa nfuA under stress conditions
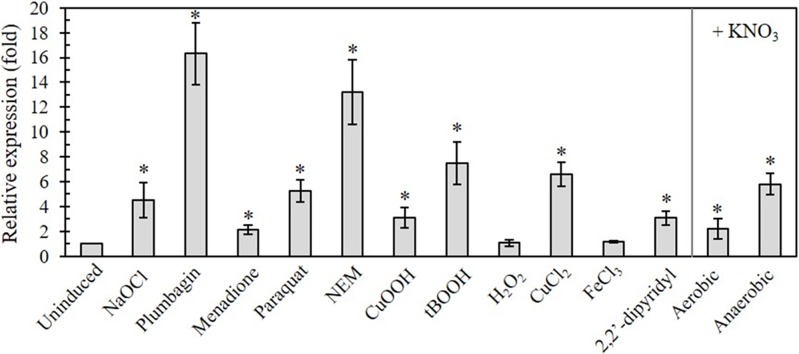
The expression of genes that contribute to protection against stress conditions is frequently induced in response to such stresses. We initially examined the effects of oxidative stress-generating agents including plumbagin (PB), menadione (MD) and paraquat (PQ) for the intracellular generation of superoxide anions, a thiol-depleting agent, N-ethylmaleimide (NEM), peroxides (cumene hydroperoxide [CuOOH], tert-butyl hydroperoxides [tBOOH], hydrogen peroxide [H2O2]) and sodium hypochlorite (NaOCl) on nfuA expression. Treatment of PAO1 with subgrowth inhibitory concentrations of oxidants induced the expression of nfuA (PB [16.3 ± 2.5-fold], MD [2.1 ± 0.4-fold], PQ [5.3 ± 0.9-fold], CuOOH [3.1 ± 0.8-fold], tBOOH [7.5 ± 1.7-fold] and NaOCl [5.1± 0.7-fold]) relative to the uninduced level (Fig 1). Treatment with NEM, a thiol-depleting agent, also induced the nfuA expression by 13.2 ± 2.6-fold. Nonsignificant induction of the gene expression was observed with H2O2-treated cells. The results are consistent with the observations from a transcriptome analysis, in which H2O2 did not induce nfuA expression in P. aeruginosa [11].
Fig 1. nfuA expression in response to stress conditions.
Analysis of the nfuA expression during normal growth and in response to oxidants. PAO1 and various mutant cultures were treated with 0.02% NaOCl, 0.5 mM Plumbagin, 0.5 mM Menadione, 0.5 mM Paraquat, 0.1 mM NEM, 1 mM CuOOH, 1 mM tBOOH, 1 mM H2O2, 0.5 mM CuCl2, 0.5 mM FeCl3, and 1 mM 2,2’-dipyridyl for 15 min under aerobic conditions. For anaerobic growth conditions, LB supplemented with nitrate (+ KNO3) was used. RNA extractions and qRT-PCR were performed as described in the Materials and Methods. The data are presented as the means ± SD from three independent experiments. The relative expression was analyzed using the 16S rRNA gene as the normalizing gene and is presented as the fold expression relative to the level of the uninduced condition.
Beside oxidant treatments, the level of intracellular iron is a major governing factor in bacterial oxidative stress. Experiments were extended to assess the effects of intracellular iron levels on nfuA expression. PAO1 cultures were treated with either 2,2’-dipyridyl, an intracellular iron chelator, or FeCl3 to create iron-deplete and iron-excess conditions, respectively. The results revealed no alteration in the nfuA expression level under the iron-excess condition, while the iron-deplete condition caused a 3.1 ± 0.6-fold increase in the expression level of nfuA compared with the uninduced control (Fig 1).
In summary, the expression of P. aeruginosa nfuA was highly induced (over 10-fold) in response to PB and NEM treatments and moderately induced (from 3 to 8-fold) by CuOOH, tBOOH, PQ, NaOCl and 2,2’-dipyridyl. The stress-inducible pattern of nfuA expression is comparable to that of genes in the iscR regulon [12, 13], where the expression is regulated by IscR, a [Fe-S] cluster containing a transcription regulator [6].
IscR derepresses nfuA expression under oxidant exposure
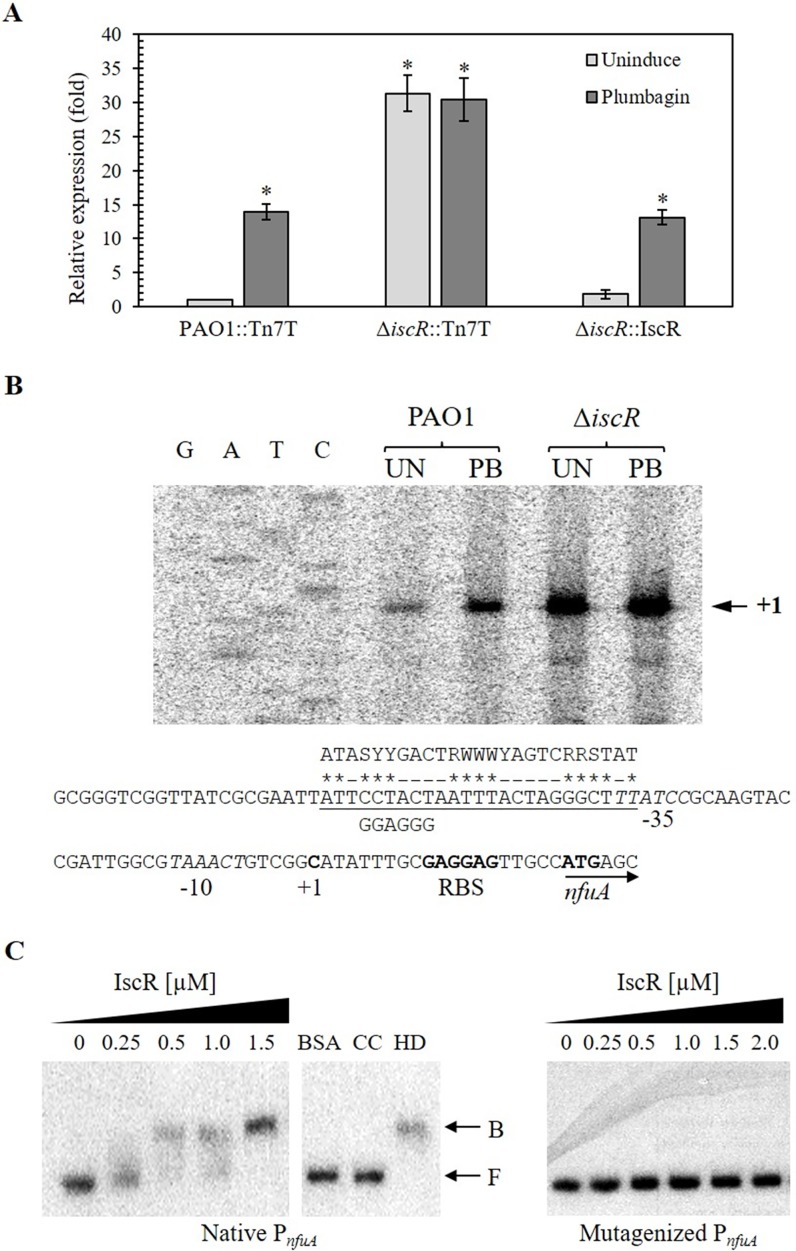
IscR is a transcriptional regulator regulating several genes implicated in [Fe-S] cluster biogenesis [6, 12, 13]. The pattern of nfuA expression in response to stress conditions showed similarity to the expression patterns of IscR-regulated genes [6]. The roles of IscR in the regulation of nfuA expression were examined. Treatment with PB, an inducer of IscR, in PAO1 and in an iscR mutant [6] showed that PB induced nfuA expression in PAO1 (13.9 ± 1.2-fold) but not in the iscR mutant, in which nfuA was constitutively highly expressed (Fig 2A). The PB induction was restored in the ΔiscR complemented strain (Fig 2A, ΔiscR::IscR). Moreover, a recent study indicates that copper ions exert their primary toxicity through degradation of [Fe-S] clusters [14]. As IscR contains an [2Fe-2S] cluster, we examined if treatment with copper ions would enhance the nfuA expression level. The results showed that treatment with CuCl2 at a sublethal concentration (5 mM) somewhat induced the nfuA expression (6.6 ± 1.0-fold), while the lower concentration ranging from 100 μM to 2.5 mM failed to induce the nfuA expression (data not shown).
Fig 2. IscR-regulated nfuA expression and nfuA promoter analysis.
(A) IscR-regulated nfuA expression. RNA samples were isolated from uninduced and 0.5 mM plumbagin (PB)-induced cultures of the indicated P. aeruginosa strains. qRT-PCR using primers BT2841 and BT2860 for monitoring nfuA expression and performed as described in the Materials and Methods. Relative expression (fold) is defined as the changes in the nfuA expression levels across multiple samples relative to the level of the uninduced culture of PAO1. The data are presented as the means ± SD from three independent experiments. (B) The primer extension assay was performed using 32P-labeled primer BT3577 and RNA extracted from P. aeruginosa PAO1 and ΔiscR grown under uninduced (UN) and 0.25 mM PB-induced conditions. G, A, T, and C represent the DNA ladder sequence prepared using 32P-labeled primer BT3577 and plasmid pPnfuA as the template. The arrowhead indicates the transcription start site (+1). The -10 and -35 elements are in italic type. The consensus sequence of the Type-I E. coli IscR-binding site is aligned above the corresponding underlined sequence, and the homologous bases are marked with asterisks. The mutated IscR-binding site on the nfuA promoter was aligned below the underlined sequence line. The putative ribosome-binding site (RBS) is indicated in bold type. (C) The electrophoretic mobility shift assay was performed using 32P-labeled native or mutagenized nfuA promoter fragments and increasing concentrations of purified IscR. CC and HD represent an addition of 1 μg unlabeled nfuA promoter and 2.5 μg of heterologous DNA (pUC18 plasmid), respectively, to the binding reaction mixtures containing 3.0 μM IscR. F and B indicate free and bound probes, respectively.
Inactivation of iscR leads to derepression of the nfuA promoter/operator and allows a high level of expression of nfuA. IscR acts as a transcriptional repressor of nfuA expression. Furthermore, oxidant induction of nfuA expression most likely results from oxidant reaction with the [Fe-S] cluster of IscR, leading to an alteration in the repressor-binding ability and derepression of the target promoter/operator. Thus, oxidant-induced expression of nfuA in PAO1 is dependent on iscR.
IscR specifically binds to the Type-I IscR-binding motif on the nfuA promoter
IscR binds specifically to the consensus sequence located adjacent to the target gene promoter [12, 13]. We experimentally identified the transcription start site (+1) to map the putative promoter region of nfuA using a primer extension experiment. Total RNA samples were purified from PAO1 cultures grown in uninduced and PB-induced conditions. Fig 2B demonstrates the primer extension products corresponding to the +1 site located at the C residue 20 nucleotides upstream of the nfuA translation initiation codon ATG. The putative nfuA promoter -35 and -10 elements were identified as TTATCC and TAAACT, respectively, and were separated by 17 nucleotides. The sequence ATTCCTACTAATTTACTAGGGCTTT, which shares a high score of identity (54%, 14 out of 25) with the consensus sequence for the Type-I E. coli IscR binding site ATASYYGACTRWWWYAGTCRRSTAT, was identified on the sequence overlapping the -35 motif (Fig 2B). The data suggest that IscR regulates the expression of nfuA through binding to its promoter region. The nfuA primer extension results from RNA prepared from uninduced and PB-induced samples showed similar size products and indicated that the transcription of nfuA under both conditions were driven by the same promoter. The higher amount of the primer extension products as judged from increased band intensity from the PB-induced sample was consistent with the real-time expression analysis in that PB is a potent inducer of nfuA expression (Fig 2A). The nfuA primer extension analysis of RNA samples prepared from uninduced and PB-induced ΔiscR mutants produced similar product sizes and quantities to as well as much higher product band intensities than the uninduced PAO1 sample, supporting constitutive high expression of nfuA in the mutant. The results are consistent with RT-PCR analysis of nfuA expression in ΔiscR (Fig 2A). Moreover, the results from Northern blot analysis hybridized with a nfuA-specific probe showed a positive band of 0.6 kb, indicating that nfuA is transcribed as a monocistronic mRNA (data not shown).
IscR regulation of its target gene expression has been partly elucidated in E. coli, Xanthomonas campestris and P. aeruginosa [6, 15–19]. IscR is known to act as either a repressor or an activator of gene expression, depending on the regulator binding to the promoters [6, 12, 13, 16]. IscR functions as a repressor of its own operon, iscRSUA, but as an activator of the sufABCDSE operon [15, 18]. The latter function requires coordinated regulators, i.e., OxyR, Fur and IHF [15, 18]. Our finding that the expression of nfuA was constitutively high in the iscR mutant suggests that IscR was a repressor for nfuA. This scenario is in line with that reported in E. coli, where IscR is a transcriptional repressor of nfuA [20]. Under physiological conditions, the [Fe-S] ligating IscR binds the nfuA promoter and represses its expression. Upon oxidation, disassembly of the [Fe-S] from the IscR leads to derepression of nfuA [20]. We showed here that oxidants and iron-depleting conditions are potent inducers of nfuA gene expression. Several oxidants are capable of causing oxidative damage, leading to disassembly of the [Fe-S] cluster, thereby deactivating the repressor IscR.
EMSA was conducted using purified P. aeruginosa IscR protein [6] and a nfuA promoter fragment containing the putative IscR binding box. The results showed that purified 6His-tagged IscR was specifically bound to the nfuA promoter fragment (Fig 2C). The binding specificity of purified IscR was demonstrated through the addition of excess unlabeled nfuA promoter fragment, which competed with the labeled promoter fragment in the IscR-binding complexes (Fig 2C). Furthermore, an unrelated protein (BSA) that was added did not bind to the nfuA promoter fragment. The results indicated that IscR binds specifically and directly to the nfuA promoter. Site-directed mutagenesis was performed to test if the proposed Type-I IscR-binding site (Fig 2B) functions as the binding site by mutating the putative Type-I IscR-binding site from part of the consensus sequence CCTACT to GGAGGG (Fig 2B). As shown in Fig 2C, increasing levels of purified IscR up to 2.0 mM (greater than the concentration at which purified IscR completely bound the wild-type nfuA promoter) was unable to bind the mutated nfuA promoter (Fig 2C). The results indicated that the Type-I IscR-binding site was responsible for the binding of IscR to the nfuA promoter. As the Type-I IscR-binding site overlapped the -35 promoter motif of the nfuA promoter (Fig 2B), binding of IscR would prevent RNA polymerase from binding to the promoter and thereby inhibiting the transcription of nfuA. These results support the role of IscR as a transcriptional repressor of nfuA gene expression. However, the requirement of [Fe-S] cluster for the binding of IscR to the nfuA promoter is under investigation.
The effects of nfuA inactivation on either [2Fe-2S] or [4Fe-4S] containing protein functions
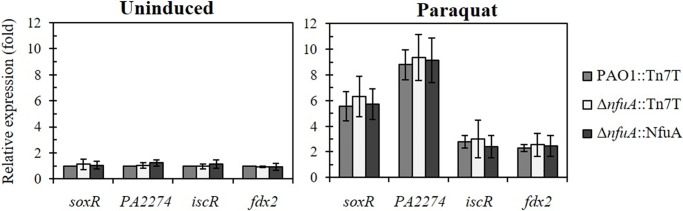
NfuA has been reported as an atypical [Fe-S] carrier contributing in part to the maturation of [Fe-S]-containing proteins such as aconitase (AcnB) and the complex I subunit NuoG dehydrogenase [9]. In P. aeruginosa, the role of NfuA is not known; thus, we investigated the role of NfuA in the functions of [2Fe-2S] and [4Fe-4S]-containing proteins in vivo. First, we tested whether inactivation of nfuA affects the regulatory function of the characterized [2Fe-2S]-containing transcriptional regulators, SoxR and IscR [16, 21]. Both regulators have a [2Fe-2S] cluster as a functional prosthetic group, and the holo-proteins ([2Fe-2S]-SoxR and [2Fe-2S]-IscR) function as the transcriptional repressor of their target genes [20]. Oxidation of [2Fe-2S] of SoxR and destabilization of [2Fe-2S] of IscR upon exposure to oxidants such as superoxide generators leads to activation or depression of the target genes [22]. If NfuA participates in maturation of these proteins, we would expect alterations in the expression patterns of SoxR- and IscR-regulated genes in response to oxidants. The results in Fig 3 show that deletion of nfuA did not affect the uninduced and the PQ-inducible expression profiles of a SoxR-regulated gene (PA2274 and soxR) and an IscR-regulated gene (fdx2 and iscR) compared to profiles obtained in the PAO1 wild type. The results suggest that nfuA is not involved in the maturation of the tested [2Fe-2S]-containing proteins.
Fig 3. Expression levels of soxR, iscR and their targeted genes in P. aeruginosa strains.
Analysis of the expression levels of genes encoding [2Fe-2S]-containing transcriptional regulators, namely, SoxR and IscR, and their targeted genes, soxR, PA2274, iscR and fdx2, in P. aeruginosa wild type (PAO1::Tn7T), ΔnfuA mutant (ΔnfuA::Tn7T) and the complemented strain (ΔnfuA::NfuA) grown in uninduced and 0.5 mM Paraquat-induced conditions. qRT-PCR was performed as described in the Materials and Methods, and the data are shown as the fold expression relative to the level of the uninduced PAO1 (PAO1::Tn7T).
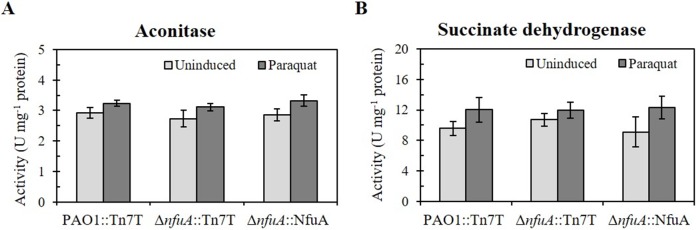
NfuA is known to function in [4Fe-4S] ligation and transfer to target proteins [2]. Next, the activities of two [4Fe-4S] clusters containing enzymes at their active sites, i.e., aconitase (Acn) and succinate dehydrogenase (Sdh), were monitored in the ΔnfuA mutant and in a PAO1 wild type. The Acn and Sdh activities in the ΔnfuA mutant were not significantly different from those in the PAO1 wild type under uninduced and paraquat-induced conditions (Fig 4A and 4B). By contrast, in E. coli, a decrease in aconitase activity has been observed in the ΔnfuA mutant [9]. P. aeruginosa NfuA plays no role in the maturation and transfer of [4Fe-4S] clusters to Acn and Sdh. This finding is in good agreement with the proposed role of NfuA, namely, that it is involved in the ligation and transfer of [Fe-S] clusters only to a subset of target proteins.
Fig 4. Determination of the activity of iron-sulfur cluster-containing enzymes in P. aeruginosa strains.
The enzyme activity of aconitase (A) and succinate dehydrogenase (B) was measured in the PAO1 and ΔnfuA mutant carrying the empty Tn7T vector (PAO1::Tn7T and ΔnfuA::Tn7T) as well as in the ΔnfuA mutant expressing wild-type NfuA or mutated NfuA (NfuAC152S, NfuAC155S, and NfuAC43,47S) under uninduced and Paraquat-induced conditions as described in the Materials and Methods. The data shown are the means ± SD from three independent experiments.
The role of nfuA in stress protection
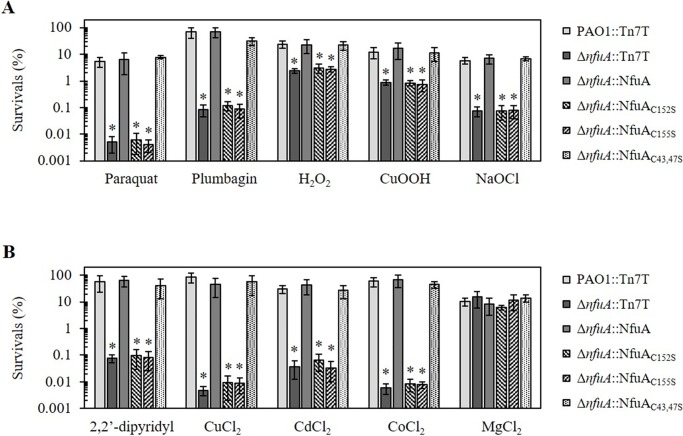
[Fe-S] clusters are known targets for reactions with oxidants. In several organisms, genes involved in [Fe-S] biogenesis are also involved in processes leading to oxidative stress resistance [6, 13, 19, 23–26]. We have reported that deletion of nfuA renders P. aeruginosa more susceptible to fluoroquinolone antibiotics [10]. To further evaluate the role of P. aeruginosa nfuA in processes of oxidative stress resistance, the level of resistance against various oxidants in the ΔnfuA mutant was determined and compared with that of the PAO1 wild type. The ΔnfuA mutant was extremely sensitive to superoxide generators, showing 104-fold and 103-fold increased sensitivity to PQ and PB, respectively, compared to the PAO1 wild type (Fig 5A). The sensitivities of the ΔnfuA mutant against peroxides, both H2O2 and CuOOH, and NaOCl were also increased 10-fold relative to the wild-type level. The altered phenotypes observed in the ΔnfuA mutant could be complemented by a single copy of nfuA that was transposed into the mutant chromosome using a mini-Tn7 vector (Fig 5A). The ΔnfuA mutant showed increased sensitivity to a variety of Reactive Oxygen Species, ROS and, particularly, to superoxide generators. [Fe-S] clusters in enzymes and proteins are proven targets for ROS [3, 14, 27].
Fig 5. Determination of the resistance levels against oxidative and metal stresses in P. aeruginosa strains.
The resistance levels of PAO1::Tn7T, ΔnfuA::Tn7T and the ΔnfuA::NfuA mutant strains expressing transposed wild-type NfuA or mutated NfuA (NfuAC152S, NfuAC155S, and NfuAC43,47S) against substances were determined using plate sensitivity assays on plates containing oxidants (A) i.e., 250 μM Paraquat, 1 mM Plumbagin, 0.5 mM H2O2, 1.6 mM CuOOH, and 0.045% NaOCl, and metals (B) i.e., 1.2 mM 2,2’-dipyridyl, 4.2 mM CuCl2, 0.8 mM CdCl2, 0.5 mM CoCl2, and 5 mM MgCl2. Survival (%) was defined as the percentage of colony-forming units (CFU) on plates containing oxidants over the number of CFU on plates without oxidants. The data shown are the means ± SD from three independent experiments. The asterisk indicates statistical significance (paired t-test, p < 0.05) compared with PAO1 treated with the same condition.
Next, we tested if nfuA has physiological roles in protecting bacteria from an iron-depleting condition. The ΔnfuA mutant showed 103-fold increased sensitivity to the intracellular iron chelator, 2,2’-dipyridyl, relative to that of the wild type. The phenotype suggests that there is less free intracellular Fe2+ available in the nfuA mutant. In addition, the level of intracellular free Fe2+ in the ΔnfuA mutant was indirectly determined using a streptonigrin sensitivity assay [28]. Streptonigrin is a redox cycling antibiotic that generates free radicals, and its bactericidal activity is enhanced by metal ions, notably Fe2+. The levels of free Fe2+ directly correlate with the bactericidal activity of the antibiotic [28]. Both the nfuA mutant and PAO1 showed the same survival rate of approximately 1% after treatment with 50 μg ml-1 streptonigrin for 2 h (data not shown), suggesting that the basal levels of free Fe2+ in the cytoplasm of the nfuA mutant and PAO1 wild type were similar. Thus, the defective [Fe-S] cluster transfer and assembly in the nfuA mutant does not lead to an increase and is more likely to cause a minor reduction in free intracellular ferrous iron. The increased susceptibility of the nfuA mutant to an iron chelator was probably due to defects in [Fe-S] assembly and transfer processes leading to a lowering of the total intracellular free iron pool. A similar phenotype has been observed in E. coli, where nfuA is shown to be important under iron-deprivation conditions [7].
Metal toxicities in bacteria have been linked to the ability of metals to either directly react with or replace various metal-containing proteins, resulting in induced oxidative stress [29–32]. Hence, the role of nfuA in conferring resistance against metal toxicities using a plate sensitivity assay was evaluated. The results in Fig 5B show that the nfuA mutant was more sensitive to copper (CuCl2, 104-fold), cadmium (CdCl2, 103-fold) and cobalt (CoCl2, 103-fold) than the PAO1 wild type. The ΔnfuA mutant metal-hypersensitive phenotypes were restored in the complemented strain (Fig 5B). Moreover, no alterations in the levels of resistance of the ΔnfuA mutant to the nonredox metal Mg were observed.
Redox metals, including Cu2+, Cd2+, and Co2+, and oxidants could exert their toxicities through the degradation of [4Fe-4S] clusters, resulting in a damaged cluster and release of Fe2+. The ferrous ion could participate in the Fenton reaction and generate highly reactive hydroxyl radicals [3, 14, 33]. NfuA has functions as a [Fe-S] cluster carrier and transfers the cluster to a subset of target proteins. In the nfuA mutant, this process would lead to a reduction in the number of functional [Fe-S] clusters transferred to target proteins and subsequent loss of enzymatic function, resulting in hypersensitive phenotypes to oxidants and redox metals. The data indicate that NfuA has important functions in [Fe-S] cluster ligation and transfer under stress conditions. Moreover, NfuA likely plays some roles in intracellular iron homeostasis that could be important under iron deprivation conditions.
Mutagenesis and functional analysis of conserved cysteine residues
P. aeruginosa NfuA contains two conserved motifs: C43XXXC47 of the degenerate ATC domain and C152XXC155 of the Nfu domain. Experiments were designed to examine the functionality of these two conserved domains in protecting PAO1 from stress. Site-directed mutagenesis changing C43S and C47S (C43,47S) of the ATC domain, C152S and C155S of the Nfu domains was performed to test the functionality of these motifs of the NfuA protein in protecting bacteria against stress. The mutated nfuA genes were cloned into a mini-Tn7 vector, and the recombinant clones were transposed into the P. aeruginosa ΔnfuA mutant. Expression of NfuAC43,47S complemented the phenotypes of increased sensitivity to oxidative stress (PQ, PB, H2O2, CuOOH and NaOCl), metal stress (CuCl2, CdCl2, CoCl3) and iron depletion (2,2’-dipyridyl) in the ΔnfuA mutant to levels similar to those attained by a parental strain (Fig 5A and 5B). By contrast, the expression of mutated nfuA that had either conserved cysteine residues C152 or C155 of the C152XXC155 motif of the Nfu domain converted to serine failed to complement the phenotypes of increased sensitivity to oxidative stress, metals and iron depletion in the ΔnfuA mutant (Fig 5A and 5B). Expression and Western blot analyses were performed to ensure that mutations of the nfuA gene (C152S, C155S, and C43,47S) did not affect expression of the genes at both transcriptional and translational levels (S1 Fig). The deletion analysis of NfuA indicates that NfuA domain is primarily essential for the protein functions. The ATC domain is thought to function in recognizing the target proteins and promote [Fe-S] cluster transferring processes [7, 9]. Our results showed that mutation at the C43S and C47S residues in the C43XXXC47 motif of the ATC type domain had no effects on the ability of mutated nfuA to complement the stress-hypersensitive phenotypes of the mutants, suggesting that the target protein recognition functions of the motif under stress conditions do not have primarily important roles for protecting the ΔnfuA mutant from lethal levels of oxidative stress, metals and iron-depletion conditions. The CXXC motif of Nfu domain is thought to be involved in the ligation and binding of [4Fe-4S] clusters and to facilitate transfer of the clusters to target proteins [9]. [Fe-S] clusters are targets involved in reactions with oxidants and reactive metals. The clusters are easily damaged by these stresses, which lead to decrease functionality or inactivation of these [Fe-S] containing proteins and result in increased sensitivity to lethal levels of these stresses. Thus, NfuA has ability to assist the bacteria in maintaining a balance between the transfer rates of newly formed clusters to replace stress-damaged clusters. This balance is likely important for the functionality of [Fe-S]-containing proteins involved in stress-protective mechanisms, which is reflected by the overall resistance levels to stress. As shown herein, mutations in the Nfu domain hinder the binding and transfer of [Fe-S] clusters to stress-damaged [Fe-S] clusters and prevent NfuA from complementing the stress hypersensitive phenotypes of the ΔnfuA mutant. The complementation of the stress hypersensitive phenotypes even suggest that NfuA could function as a chaperone and/or in the repair of stress damaged [Fe-S] clusters. Similar, results observed in E. coli have shown that mutations of a cysteine residue in the CXXC motif of the Nfu-type domain abolishes its ability to complement the stress hypersensitive phenotypes of nfuA mutants [7].
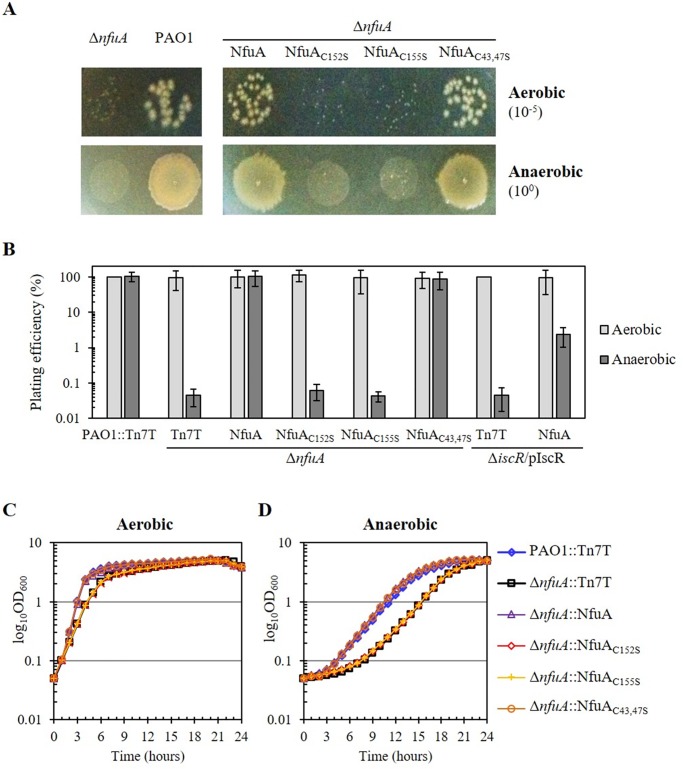
nfuA is required for proper growth under anaerobic condition
We observed that colonies on the LB agar plate of the ΔnfuA mutant grown under an aerobic atmosphere were smaller than those of the PAO1 wild type (Fig 6A). However, the plating efficiencies of the PAO1 wild type and ΔnfuA mutant on LB plates incubated under aerobic conditions did not differ (Fig 6A and 6B). Nonetheless, the aerobic growth of the ΔnfuA mutant in LB broth showed a small growth defect compared to the growth of the PAO1 wild type (Fig 6C), suggesting that nfuA is required for protecting bacteria against toxic metabolites generating oxidative stress from normal aerobic growth of P. aeruginosa. Moreover, the growth of the ΔnfuA mutant under an anaerobic atmosphere was monitored on both LB agar plates and broth. Under anaerobic condition, PAO1 was unable to grow on LB plated with or without glucose supplementation (data not shown). Thus, the medium was supplemented with nitrate to allow anaerobic growth of P. aeruginosa using nitrate respiration [34]. Unexpectedly, the ΔnfuA mutant exhibited a severe reduction of more than 103-fold in the plating efficiency relative to that of the PAO1 wild type (Fig 6A and 6B). Furthermore, the doubling time under anaerobic growth of the ΔnfuA mutant was considerably increased to 134 min compared to 119 min for PAO1 (Fig 6D). The reduced plating efficiency and growth retardation phenotypes of the ΔnfuA mutant was fully restored in the complemented strain (ΔnfuA::NfuA). However, the expression of the mutated nfuA genes in which conserved cysteine residues in the Nfu-type domain were mutated (either NfuAC152S or NfuAC155S) were unable to complement the growth defect phenotypes under both aerobic and anaerobic conditions. The expression of mutated nfuA in the ATC domain (NfuAC43,47S) complemented the growth retardation phenotypes to levels similar to those of wild-type NfuA (Fig 6A, 6B, 6C and 6D). The results illustrated the importance of nfuA to P. aeruginosa growth, especially under anaerobic conditions, and the involvement of the Nfu domain in binding and transferring [4Fe-4S] clusters to require enzymes for anaerobic growth. To our knowledge, this is the first report showing an essential role of nfuA for anaerobic growth in bacteria.
Fig 6. P. aeruginosa and ΔnfuA mutant plating efficiency and growth under aerobic and anaerobic conditions.
The plating efficiency and growth of PAO1 and various mutant strains were determined. Bacteria were grown in LB for aerobic conditions and in LB plus 1% KNO3 for anaerobic conditions. The plating efficiency was defined as the number of CFU under anaerobic conditions divided by the number of CFU under aerobic conditions. The asterisk indicates statistically significant differences (paired t-test, p < 0.05) compared with the aerobic condition. (A) The plating efficiency and colony morphology of PAO1 and ΔnfuA mutant strains under aerobic and anaerobic conditions was determined. The PAO1, ΔnfuA::NfuA strains expressing wild-type NfuA or mutated NfuA (NfuAC152S, NfuAC155S, and NfuAC43,47S) were spotted onto plates containing 1% KNO3 and incubated under aerobic and anaerobic conditions. (B) Plating efficiency of the PAO1 (PAO1::Tn7T), ΔnfuA mutant (ΔnfuA::Tn7T) and various complemented strains as in (A), with the addition of ΔiscR/pIscR and ΔiscR::NfuA/pIscR strains performed under aerobic and anaerobic conditions. Growth of P. aeruginosa strains under aerobic (C) and anaerobic (D) conditions in broth supplemented with 1% KNO3 incubated at 37°C with 180 rpm shaking was determined. The OD600nm was monitored at hourly intervals for 24 h. Typically representative results of five independent experiments are shown.
In PAO1, overexpression of iscR results in an anaerobic growth defect [6]. Here, we showed that IscR was a transcriptional repressor of nfuA. Hence, high levels of IscR lead to low nfuA expression in the IscR-overexpressing strain, which may be responsible for the observed anaerobic growth defect phenotype. An experiment was performed to test the assumption by expressing nfuA in an iscR-overexpressing strain and measuring the anaerobic growth. The results (Fig 6B) showed that increased expression of nfuA from the lacZ promoter (in strain ΔiscR::NfuA/pIscR) partially restored the anaerobic growth defect phenotype of the iscR-overexpressing strain to a level 102-fold higher than that of the iscR-overexpressing strain but just over 10-fold lower than the PAO1 level. These results confirm that low levels of nfuA expression in iscR-overexpressing strains contribute to the anaerobic-growth-defect phenotype of the strain.
Experiments were extended to determine the nfuA expression levels in anaerobic cultures in PAO1. One percent KNO3 was added into the culture medium to allow anaerobic growth of P. aeruginosa through nitrate respiration; we also tested the effect of nitrate supplementation on nfuA expression. The results showed that the presence of nitrate (LB + KNO3) induced a 2.2 ± 0.8-fold increase in nfuA expression under aerobic conditions compared to the expression in PAO1 grown aerobically in LB medium (Fig 1). The expression of nfuA in PAO1 grown in LB+KNO3 under anaerobic conditions was further induced (2.6-fold) compared to the expression in aerobically grown cells in a similar medium (Fig 1). Thus, the nfuA expression was also induced in response to the presence of 1% nitrate and in anaerobic conditions. This finding illustrates the important correlation between gene expression profiles and biochemical and physiological functions in the nitrate respiration under anaerobic conditions. Moreover, we found that addition of 1% nitrate could significantly increase nfuA expression in the ΔiscR mutant grown under aerobic condition suggesting that nitrate-induced the expression of nfuA is not mediated by IscR (data not shown). Thus, the mechanism by which nitrate induces nfuA expression is unknown.
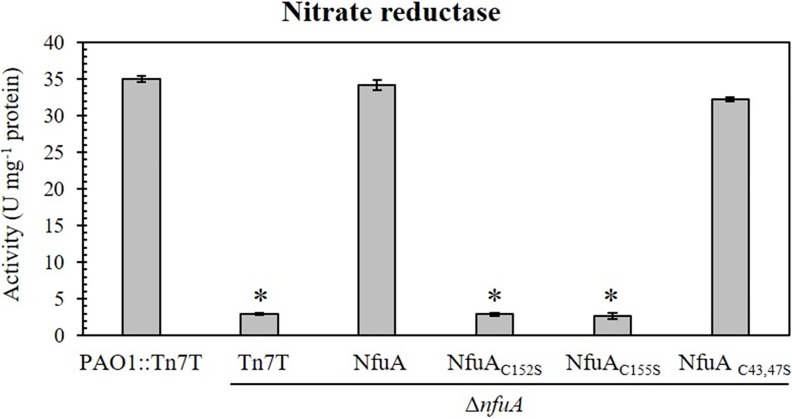
P. aeruginosa has a set of denitrification enzymes capable of reducing nitrate to molecular nitrogen, and these enzymes allow the bacterium to grow under anaerobic conditions in the presence of nitrate. As the ΔnfuA mutant exhibited a severe growth defect under anaerobic conditions, we postulated that NfuA may be needed for the denitrification process. Nitrate reductases contain [Fe-S] cluster prosthetic groups. The total nitrate reductase activity in the ΔnfuA mutant grown under anaerobic conditions was determined. The total nitrate reductase activity level in the ΔnfuA mutant (2.9 ± 0.2 U mg-1 protein) was significantly lower than that in the PAO1 wild type (35.0 ± 0.4 U mg-1 protein), and the activity could be restored to the wild-type level in the complemented strain ΔnfuA::NfuA (34.2 ± 0.7 U mg-1 protein) (Fig 7). The results suggest that NfuA is required for full activity of nitrate reductases, the enzymes that play a crucial role in maintaining proper growth under anaerobic conditions. However, direct involvement of NfuA in [Fe-S] delivery to nitrate reductase needs further investigation.
Fig 7. Determination of nitrate activity in P. aeruginosa strains.
Nitrate reductase activity was measured in the PAO1 and ΔnfuA mutant carrying Tn7T vector (PAO1::Tn7T and ΔnfuA::Tn7T), and the ΔnfuA mutant expressing wild-type NfuA or mutated NfuA (NfuAC152S, NfuAC155S, and NfuAC43,47S), as described in the Materials and Methods. The data shown are the means ± SD from three independent experiments.
Furthermore, the NfuA domain that is responsible for the NfuA effect on nitrate reductase enzyme activity was determined. The ΔnfuA mutant expressed mutant NfuA proteins (NfuAC152S, NfuAC155S and NfuAC43, 47S). As shown in Fig 7, the expression of NfuAC152S and NfuAC155S could not complement the reduced total nitrate reductase activity of the ΔnfuA mutant, indicating that the Nfu domain is critical for the NfuA effect on the activity of nitrate reductases. Mutation of C43 and C47 on the ATC domain (NfuAC43,47S) caused no adverse effect on the protein ability to complement the nitrate reductase activity of the ΔnfuA mutant (Fig 7).
More recently, it has been proposed in E. coli that NfuA assists ErpA in delivery of [Fe-S] cluster to the target proteins under unfavorable conditions such as oxidative stress [35]. High expression of erpA could complement the paraquat-sensitive phenotype of E. coli nfuA mutant [35]. Nevertheless, expression of a PAO1 erpA-homolog from pErpApa failed to restore the paraquat-sensitive phenotype of the ΔnfuA mutant (data not shown). Thus, P. aeruginosa NfuA may function differently from E. coli NfuA.
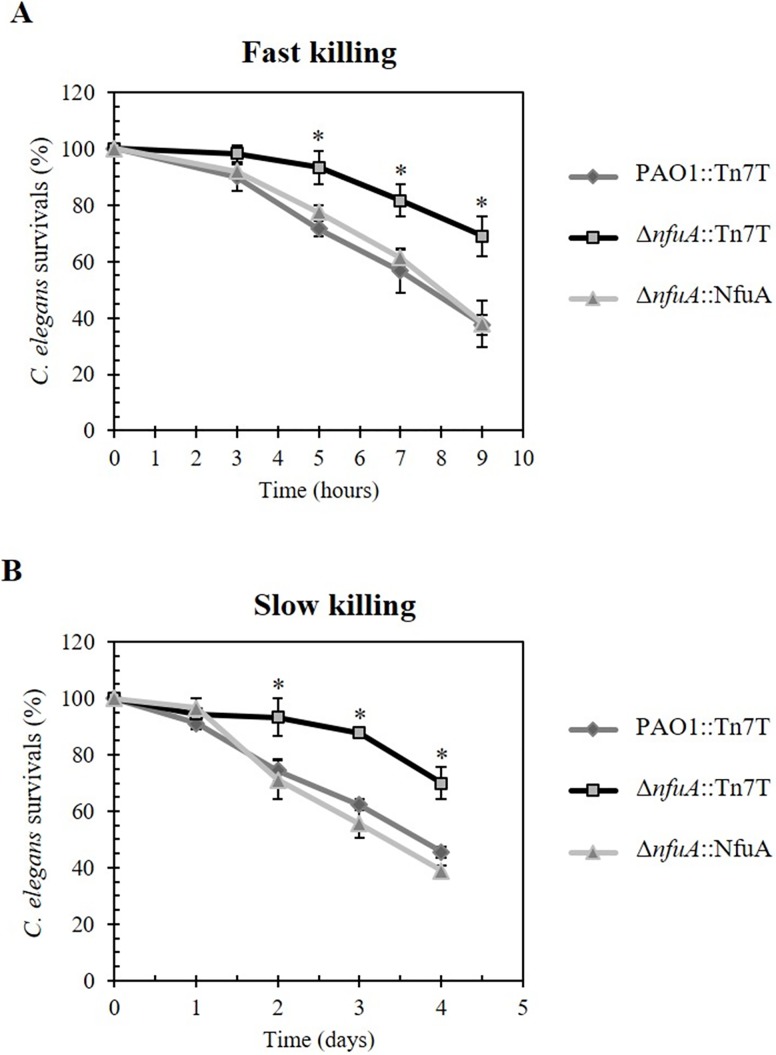
nfuA mutant has attenuated virulence in the Caenorhabditis elegans model host-pathogen system
The ΔnfuA mutant showed increased sensitivity to stresses and growth defects, and thus, we tested whether loss of nfuA function affects bacterial pathogenicity using a C. elegans model host system. C. elegans has been proposed as a model for studying host-pathogen interactions due to the susceptibility of C. elegans to different virulent phenotypes of P. aeruginosa [36, 37]. Fast and slow killing assays of P. aeruginosa towards C. elegans were performed. Fast killing is mediated by diffusible toxins released from P. aeruginosa and does not need live bacteria to kill the worms, whereas slow killing requires bacterial colonization in the worm gut to exhibit virulence [36]. The ΔnfuA mutant was attenuated for virulence by showing a roughly two-fold reduction in its ability to kill C. elegans compared to the PAO1 wild type in both fast and slow killing assays (Fig 8A and 8B). The phenotypes were restored in the complemented mutant (ΔnfuA::NfuA) strain.
Fig 8. P. aeruginosa virulence test using the Caenorhabditis elegans model host model.
Fast (A) and slow (B) killing assays were performed to determine the virulence of P. aeruginosa PAO1::Tn7T, ΔnfuA::Tn7T and the complemented strain (ΔnfuA::NfuA) as described in the Materials and Methods. Live worms were scored at 3, 5, 7 and 9 h for fast-killing experiments and at 1, 2, 3 and 4 days for slow-killing experiments. The data were analyzed from nine biological replicates, and the means ± SD are shown.
Thus, nfuA contributes to the virulence of P. aeruginosa. Defects in the nitrate dissimilation pathway have been shown to cause attenuation of the virulence phenotype and altered biofilm formation of P. aeruginosa [38]. We speculate, therefore, that the virulence attenuation in the ΔnfuA mutant is at least in part due to lowered nitrate reductase activity and anaerobic growth retardation. Physiologically, the ability of P. aeruginosa to grow in anaerobic conditions is also important for chronic infection in the cystic fibrosis lung, in which the bacteria must face an anaerobic microenvironment [39].
Materials and methods
Bacterial strains and growth conditions
All Pseudomonas aeruginosa strains were raised, maintained and experiments were conducted following procedures approved by the Committee of Biosafety, Faculty of Science, Mahidol University (MUSC2017-001).
All bacterial strains and plasmids used in this study are listed in Table 1. Pseudomonas aeruginosa (PAO1) strains were grown aerobically in the Luria-Bertani (LB) medium at 37°C with shaking at 180 rpm. As required, the medium were supplemented with 200 μg ml−1 carbenicillin (Cb), 25 μg ml−1 chloramphenicol (Cm) or 30 μg ml−1 gentamicin (Gm). To produce more synchronous growth, an overnight culture was inoculated into fresh LB medium to give an optical density at 600 nm (OD600nm) of 0.1. Exponential phase cells (OD600nm of about 0.6, after 3 h of growth) were used in all experiments.
Table 1. Bacterial strains and plasmids used in this study.
| Bacterial strain | Genotype or characteristic | Source |
|---|---|---|
| PAO1 | wild type | ATCC15692 |
| PAO1::Tn7T | PAO1 transposed with pUC18-Mini-Tn7T-Gm-LAC | In this study |
| ΔnfuA | PAO1 ΔnfuA mutant | [10] |
| ΔnfuA::Tn7T | ΔnfuA transposed with pUC18-Mini-Tn7T-Gm-LAC | In this study |
| ΔnfuA::NfuA | ΔnfuA transposed with pTn7T-NfuA | In this study |
| ΔnfuA::NfuAC152S | ΔnfuA transposed with pTn7T-NfuAC152S | In this study |
| ΔnfuA::NfuAC155S | ΔnfuA transposed with pTn7T-NfuAC155S | In this study |
| ΔnfuA::NfuAC43,47S | ΔnfuA transposed with pTn7T-NfuAC43,47S | In this study |
| ΔiscR | PAO1 ΔiscR mutant | [6] |
| ΔiscR/pBBR | ΔiscR harboring pBBR1MCS-4 | [6] |
| ΔiscR/pIscR | ΔiscR harboring pBBR1MCS-4 with full-length iscR | [6] |
| ΔiscR::NfuA/pIscR | ΔiscR transposed with pTn7T-NfuA harboring pBBR iscR | In this study |
Oxidant- and metal-induction experiments
PAO1 and mutant strains were subcultured from overnight cultures to yield an OD600nm of 0.1 and grown for 3 h. For induced cultures, either oxidants or metals were added to the cultures to the indicated concentrations, and the cultures were further grown for 15 min. Uninduced and induced cultures were harvested for RNA extractions and enzyme assays.
Site-directed mutagenesis of NfuA
PCR-based site-directed mutagenesis was performed as previously described [40] to replace the conserved cysteines (C43, C47, C152, and C155) of NfuA with serine residues. The mutagenic forward and reverse primers used to produce the mutant NfuA were BT3169 and BT3170 for NfuAC43,47S; BT2961 and BT2962 for NfuAC152S; and BT3238 and BT3239 for NfuAC155S. Two-step PCR amplification reactions were conducted using plasmid pNfuA [10] as the template and specific mutagenic primers. The PCR products were digested with Ecl136II and BamHI prior to cloning into pUC18-Mini-Tn7T-Gm-LAC [41] cut with the same enzymes. The resultant plasmids pTn7T-NfuAC43,47S, pTn7T-NfuAC152S and pTn7T-NfuAC155S expressing mutant NfuA proteins, NfuAC43,47S, NfuAC152S and NfuAC155S, respectively, were transposed into the ΔnfuA mutant. The accuracy of mutagenesis was verified by DNA sequencing.
Molecular techniques
General molecular techniques including DNA and RNA preparation, and bacterial transformation were performed using standard protocols [42]. The oligonucleotide primers used in this study are listed in Table 2.
Table 2. List of primers used in this study.
| Number | Sequence (5’→3’) | Purpose |
|---|---|---|
| BT3291 | CGAGACGAAGAGGTCGTT | Reverse primer for primer extension of nfuA |
| BT2879 | AACCGCTACGAGAACCTC | Forward primer for full length nfuA |
| BT3169 | CCTCCATCGCCTACTCCAAGCCGGGCGAGG | Forward primer for mutagenesis of NfuAC43,47S |
| BT3170 | TGGAGTAGGCGATGGAGGTTTCCGCGTAC | Reverse primer for mutagenesis of NfuAC43,47S |
| BT2961 | GCGGCGGTTCTCAGGGCTG | Forward primer for mutagenesis of NfuAC152S |
| BT2962 | CAGCCCTGAGAACCGCCGC | Reverse primer for mutagenesis of NfuAC152S |
| BT3238 | CAGGGCAGCGGGATGGTCGAC | Forward primer for mutagenesis of NfuAC155S |
| BT3239 | TCCCGCTGCCCTGACAACCG | Reverse primer for mutagenesis of NfuAC155S |
| BT4099 | ATTGGAGGGAATTTACTAGGGCTTTATCCG | Forward primer for mutagenesis of nfuA promoter |
| BT4100 | ATTCCCTCCAATAATTCGCGATAACCGACC | Reverse primer for mutagenesis of nfuA promoter |
| BT2781 | GCCCGCACAAGCGGTGGA | Forward primer for qRT-PCR of 16S rRNA |
| BT2782 | ACGTCATCCCCACCTTCC | Reverse primer for qRT-PCR of 16S rRNA |
| BT2841 | ACCATCCCGCAGCCCTG | Reverse primer for qRT-PCR of nfuA |
| BT2860 | ACCGCCATCGCCCTGAAG | Forward primer for qRT-PCR of nfuA |
| BT3186 | TATCTCCGAACGCCAAGG | Forward primer for qRT-PCR of iscR |
| BT3187 | GGTGGTGGGTCAGACAGG | Reverse primer for qRT-PCR of iscR |
| BT3555 | CGCAATGGCATCGAGATCGA | Forward primer for qRT-PCR of fdx2 |
| BT3556 | GATAGCCGCGAATCGGGCTC | Reverse primer for qRT-PCR of fdx2 |
| BT3046 | CCAGCGGGTCGGCATTCC | Forward primer for qRT-PCR of soxR |
| BT3047 | AGGCCTGGAGCGACAGGC | Reverse primer for qRT-PCR of soxR |
| BT3351 | ACCCGCCAGCCAGTTGTC | Forward primer for qRT-PCR of PA2274 |
| BT3352 | CACGCTTTTCGCCCCCAG | Reverse primer for qRT-PCR of PA2274 |
| Tn7S | GATGGGAACTGGGTGTAGCG | Reverse primer on pUC-Mini-Tn7T-Gm-LAC |
Construction of pTn7T-nfuA for a single copy complementation of the ΔnfuA mutant
A full-length nfuA excised from of pNfuA plasmid [10] digested with EcoRV and BamHI was cloned into the Ecl136II- and BamHI-cut pUC18-Mini-Tn7T-Gm-LAC [41] to generate pTn7T-NfuA. The recombinant plasmid was transposed into the ΔnfuA mutant, yielding a complemented mutant strain (ΔnfuA::NfuA), in which nfuA was expressed under lac promoter. Confirmation of transposition was performed as described [41].
Primer extension
Primer extension experiments were performed as previously described [6] using 10 μg total RNA, 32P- labeled primer BT3291, 200 U Superscript II RNaseH minus M-MLV reverse transcriptase (Life Technologies, USA) and incubated at 42°C for 60 min. The primer extension products were analyzed on a sequencing gel (8% polyacrylamide-7 M urea). A DNA ladder was generated using fmol DNA cycle sequencing system (Promega). The template for the sequencing ladder was an nfuA promoter fragment and the primer was 32P- labeled primer BT3291.
Site-directed mutagenesis on nfuA promoter
The putative IscR binding site 5’ATTCCTACTAATTTACTAGGGCTT3’ situated on the nfuA promoter was converted to 5’ATTGGAGGGAATTTACTAGGGCTT3’ using a mutagenic primer pair BT4099 and BT4100 and pPnfuA (pBBR1MCS-4 [43] containing putative nfuA promoter fragment amplified from PAO1 genomic DNA using primers BT2879 and BT3291) as DNA template. The mutagenized promoter was cloned into pBBR1MCS-4 to generated pP*nfuA and verified by DNA sequencing.
Gel mobility shift assay
6His-tagged IscR protein from P. aeruginosa was purified using the pET-Blue2 expression system as previously described [12]. The purity of IscR protein was more than 90% as judged by a major band corresponding to the 18 kDa protein observed in SDS-PAGE. Gel mobility shift assays were performed as previously described [44] using a 32P-labeled probe containing either wild type or mutagenized nfuA promoter in which putative IscR binding site was mutated. The probe was amplified from pPnfuA (for wild-type nfuA promoter) or pP*nfuA (for mutagenized nfuA promoter) with 32P-labeled BT2879 and BT3291 primers. Binding reactions consisting of 3 fmol of labeled probe in 25 μl of reaction buffer containing 20 mM Tris-HCl (pH 8.0), 50 mM KCl, 4 mM MgCl2, 0.5 mM EDTA, 0.02 mg ml-1 bovine serum albumin (BSA), 5 mM dithiothreitol (DTT), 10% (v/v) glycerol, 200 ng of poly(dI-dC) and varied concentrations of purified IscR were incubated at 25°C for 20 min. Protein-DNA complexes were separated by electrophoresis on a 7% nondenaturing PAGE in 0.5x Tris-borate-EDTA buffer at 4°C and visualized by exposure to X-ray film.
qRT-PCR
Reverse transcription and real-time PCR (qRT-PCR) was performed as previously described [6, 45]. Essentially, total RNA was extracted from uninduced and stress induced cultures. After DNase treatment, the RNA was reversed transcribed before 10 ng cDNA was added into a KAPA SYBR® FAST qPCR kit containing specific primer pair (BT2841 and BT2860 for nfuA, BT3186 and BT3187 for iscR, BT3555 and BT3556 for fdx2, BT3046 and BT3047 for soxR, BT3351 and BT3352 for PA2274 and BT2781 and BT2782 for 16S rRNA as an internal control). The reactions run on Applied Biosystems StepOnePlusTM under the following conditions: denaturation at 95°C for 10 s, annealing at 60°C for 30 s, and extension at 60°C for 30 s, for 40 cycles. Relative expression was calculated using STEPONE software v2.1 and expressed as folds of expression relative to the level of PAO1 wild type grown under uninduced condition. The experiments were independently repeated three times and the means ± standard deviations (SD) are shown.
Plate sensitivity assay
The oxidant resistance level was determined using a plate sensitivity assay as previously described [45]. Briefly, 10 μl of each dilution of 10-fold serially diluted exponential phase cells (adjusted to give OD600 of 0.1) was spotted onto LB agar plate containing appropriate concentrations of testing agents. The surviving colonies were scored after overnight incubation at 37°C. The resistance level for each agent was expressed as the % survival, defined as the percentage of the colony forming units (CFU) on plates containing testing agent over the CFU on plates without testing agent.
Enzymatic assays
Crude cell lysate preparation and total protein determination were performed as previously described [46]. Succinate dehydrogenase activity assay was carried out as previously described and enzyme activity was expressed as ΔA600 per min per milligram protein [47]. Aconitase activity was determined using Abcam’s Aconitase Assay Kit (ab83459). One unit of aconitase is defined as the amount of enzyme that isomerizes 1.0 μmol of citrate to isocitrate per min at pH 7.4 at 25°C. Nitrate reductase activity was monitored by measuring the reduction of nitrate to nitrite with methyl viologen as the electron donor as previously described [48]. One unit of activity is defined as the amount of enzyme capable of producing 1 nmol of NO2 per min at 25°C.
Nematode killing assays
All experiments using Caenorhabditis elegans were conducted following the protocol approved by the Institutional Animal Care and Use Committee of the Chulabhorn Research Institute (CRI-IACUC).
The virulence of P. aeruginosa strains was evaluated using a Caenorhabditis elegans model host system; both slow and fast killing experiments were conducted as previously described [12, 35, 49]. The fourth larval (L4) stage worms (approximately 30 animals per plate) were used to test the virulence of P. aeruginosa strains. Worm killing was scored as dead if touching-reflected movement was not detected under dissecting microscope. Scoring was done after 16, 24, 40, 48 and 64 h for fast killing and 1, 2, 3, 4, and 5 days for slow killing experiments. The experiments were carried out in blind fashion and data shown were analyzed from nine independently biological replicates.
Statistics
Group data are presented as means ± standard deviation (SD). The Student’s t-test was used to determine differences between means using the function of Excel (Microsoft, Washington) and the SPSS (version 17.0; SPSS Inc.) statistical package. Unless otherwise is stated, p values of < 0.05 were considered significant.
Supporting information
(A) Western analysis of mutated NfuA proteins. Crude protein extracts from exponential-phase cultures of the ΔnfuA mutant carrying miniTn7T containing 6His-tag NfuA-WT, C152S, C155S, C43,47S or a vector control (Tn7T) were partially purified using Ni-NTA column prior to loading (40 mg) onto SDS-PAGE. Western blot was performed using anti-6His antibody conjugated with HRP. (B) nfuA expression analysis. End-point reverse transcription PCR was performed using BT2841 and BT2842 primers and cDNAs was prepared from total RNA samples extracted from P. aeruginosa strain as templates.
(TIF)
Acknowledgments
The authors gratefully acknowledge Dr. Tatsanee Chuchue and Kumpanart Somsongkul for technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the Chulabhorn Research Institute (http://www.cri.or.th/en/index.php), Mahidol University (https://www.mahidol.ac.th) and Center of Excellence on Environmental Health and Toxicology, Ministry of Education, Thailand (http://www.eht.sc.mahidol.ac.th). A.R. was supported by the grants from the Center for Emerging Bacterial Infections (EBI) and the Central Instrument Facility (CIF grant) of Faculty of Science, the Mahidol University (http://science.mahidol.ac.th) and the joint funding of the Office of the Higher Education Commission and the Thailand Research Fund (MRG5980047), Thailand (https://www.trf.or.th). K.S. was supported by the Royal Golden Jubilee Ph.D. Scholarship (PHD/0047/2557) from Thailand Research Fund (http://rgj.trf.or.th/eng/rgje11.asp). P.V. was supported by the grant from Chulabhorn Graduate Institute (https://www.cgi.ac.th). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dwyer DJ, Kohanski MA, Collins JJ. Role of reactive oxygen species in antibiotic action and resistance. Curr Opin Microbiol. 2009;12(5):482–9. 10.1016/j.mib.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nat Rev Microbiol. 2010;8(6):436–46. 10.1038/nrmicro2356 [DOI] [PubMed] [Google Scholar]
- 3.Roche B, Aussel L, Ezraty B, Mandin P, Py B, Barras F. Iron/sulfur proteins biogenesis in prokaryotes: formation, regulation and diversity. Biochim Biophys Acta. 2013;1827(3):455–69. 10.1016/j.bbabio.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 4.Joerger RD, Jacobson MR, Premakumar R, Wolfinger ED, Bishop PE. Nucleotide sequence and mutational analysis of the structural genes (anfHDGK) for the second alternative nitrogenase from Azotobacter vinelandii. J Bacteriol. 1989;171(2):1075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi Y, Tokumoto U. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J Biol Chem. 2002;277(32):28380–3. 10.1074/jbc.C200365200 [DOI] [PubMed] [Google Scholar]
- 6.Romsang A, Duang-Nkern J, Leesukon P, Saninjuk K, Vattanaviboon P, Mongkolsuk S. The iron-sulphur cluster biosynthesis regulator IscR contributes to iron homeostasis and resistance to oxidants in Pseudomonas aeruginosa. PLoS One. 2014;9(1):e86763 Epub 2014/01/28. 10.1371/journal.pone.0086763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelini S, Gerez C, Ollagnier-de Choudens S, Sanakis Y, Fontecave M, Barras F, et al. NfuA, a new factor required for maturing Fe/S proteins in Escherichia coli under oxidative stress and iron starvation conditions. J Biol Chem. 2008;283(20):14084–91. 10.1074/jbc.M709405200 [DOI] [PubMed] [Google Scholar]
- 8.Bandyopadhyay S, Naik SG, O'Carroll IP, Huynh BH, Dean DR, Johnson MK, et al. A proposed role for the Azotobacter vinelandii NfuA protein as an intermediate iron-sulfur cluster carrier. J Biol Chem. 2008;283(20):14092–9. 10.1074/jbc.M709161200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Py B, Gerez C, Angelini S, Planel R, Vinella D, Loiseau L, et al. Molecular organization, biochemical function, cellular role and evolution of NfuA, an atypical Fe-S carrier. Mol Microbiol. 2012;86(1):155–71. 10.1111/j.1365-2958.2012.08181.x [DOI] [PubMed] [Google Scholar]
- 10.Daung-nkern J, Vattanaviboon P, Mongkolsuk S. Inactivation of nfuA enhances susceptibility of Pseudomonas aeruginosa to fluoroquinolone antibiotics. J Antimicrob Chemother. 2010;65(8):1831–2. 10.1093/jac/dkq194 [DOI] [PubMed] [Google Scholar]
- 11.Chang W, Small DA, Toghrol F, Bentley WE. Microarray analysis of Pseudomonas aeruginosa reveals induction of pyocin genes in response to hydrogen peroxide. BMC genomics. 2005;6:115 10.1186/1471-2164-6-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somprasong N, Jittawuttipoka T, Duang-Nkern J, Romsang A, Chaiyen P, Schweizer HP, et al. Pseudomonas aeruginosa thiol peroxidase protects against hydrogen peroxide toxicity and displays atypical patterns of gene regulation. J Bacteriol. 2012;194(15):3904–12. Epub 2012/05/23. 10.1128/JB.00347-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romsang A, Duang-Nkern J, Wirathorn W, Vattanaviboon P, Mongkolsuk S. Pseudomonas aeruginosa IscR-regulated ferredoxin NADP(+) reductase gene (fprB) functions in iron-sulfur cluster biogenesis and multiple Stress response. PloS One. 2015;10(7):e0134374 10.1371/journal.pone.0134374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A. 2009;106(20):8344–9. 10.1073/pnas.0812808106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo WS, Lee JH, Lee KC, Roe JH. IscR acts as an activator in response to oxidative stress for the suf operon encoding Fe-S assembly proteins. Mol Microbiol. 2006;61(1):206–18. 10.1111/j.1365-2958.2006.05220.x [DOI] [PubMed] [Google Scholar]
- 16.Schwartz CJ, Giel JL, Patschkowski T, Luther C, Ruzicka FJ, Beinert H, et al. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc Natl Acad Sci U S A. 2001;98(26):14895–900. 10.1073/pnas.251550898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Outten FW, Djaman O, Storz G. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol Microbiol. 2004;52(3):861–72. 10.1111/j.1365-2958.2004.04025.x [DOI] [PubMed] [Google Scholar]
- 18.Lee KC, Yeo WS, Roe JH. Oxidant-responsive induction of the suf operon, encoding a Fe-S assembly system, through Fur and IscR in Escherichia coli. J Bacteriol. 2008;190(24):8244–7. 10.1128/JB.01161-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuangthong M, Jittawuttipoka T, Wisitkamol R, Romsang A, Duang-nkern J, Vattanaviboon P, et al. IscR plays a role in oxidative stress resistance and pathogenicity of a plant pathogen, Xanthomonas campestris. Microbiol Res. 2015;170:139–46. Epub 2014/09/10. 10.1016/j.micres.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 20.Giel JL, Rodionov D, Liu M, Blattner FR, Kiley PJ. IscR-dependent gene expression links iron-sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol Microbiol. 2006;60(4):1058–75. 10.1111/j.1365-2958.2006.05160.x [DOI] [PubMed] [Google Scholar]
- 21.Gaudu P, Moon N, Weiss B. Regulation of the soxRS oxidative stress regulon. Reversible oxidation of the Fe-S centers of SoxR in vivo. J Biol Chem. 1997;272(8):5082–6. [DOI] [PubMed] [Google Scholar]
- 22.Crack JC, Green J, Hutchings MI, Thomson AJ, Le Brun NE. Bacterial iron-sulfur regulatory proteins as biological sensor-switches. Antioxid Redox Signal. 2012;17(9):1215–31. 10.1089/ars.2012.4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Zhuang W, Zhang S, Rensing C, Huang J, Li J, et al. Global regulator IscR positively contributes to antimonite resistance and oxidation in Comamonas testosteroni S44. Front Mol Biosci. 2015;2:70 10.3389/fmolb.2015.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sander K, Wilson CM, Rodriguez M Jr., Klingeman DM, Rydzak T, Davison BH, et al. Clostridium thermocellum DSM 1313 transcriptional responses to redox perturbation. Biotechnol Biofuels. 2015;8:211 10.1186/s13068-015-0394-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin TH, Tseng CY, Lai YC, Wu CC, Huang CF, Lin CT. IscR Regulation of Type 3 Fimbriae Expression in Klebsiella pneumoniae CG43. Front Microbiol. 2017;8:1984 10.3389/fmicb.2017.01984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergnes A, Viala JP, Ouadah-Tsabet R, Pocachard B, Loiseau L, Meresse S, et al. The iron-sulfur cluster sensor IscR is a negative regulator of Spi1 type III secretion system in Salmonella enterica. Cell Microbiol. 2017;19(4). 10.1111/cmi.12680 [DOI] [PubMed] [Google Scholar]
- 27.Jang S, Imlay JA. Hydrogen peroxide inactivates the Escherichia coli Isc iron-sulphur assembly system, and OxyR induces the Suf system to compensate. Mol Microbiol. 2010;78(6):1448–67. 10.1111/j.1365-2958.2010.07418.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitphati W1, Ngok-Ngam P, Suwanmaneerat S, Sukchawalit R, Mongkolsuk S. Agrobacterium tumefaciens fur has important physiological roles in iron and manganese homeostasis, the oxidative stress response, and full virulence. Appl Environ Microbiol. 2007;73(15):4760–8. 10.1128/AEM.00531-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green J, Rolfe MD, Smith LJ. Transcriptional regulation of bacterial virulence gene expression by molecular oxygen and nitric oxide. Virulence. 2014;5(8):794–809. 10.4161/viru.27794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasil ML. How we learnt about iron acquisition in Pseudomonas aeruginosa: a series of very fortunate events. Biometals. 2007;20(3–4):587–601. 10.1007/s10534-006-9067-2 [DOI] [PubMed] [Google Scholar]
- 31.Cassier-Chauvat C, Chauvat F. Responses to oxidative and heavy metal stresses in cyanobacteria: recent advances. Int J Mol Sci. 2014;16(1):871–86. 10.3390/ijms16010871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker KW, Skaar EP. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev. 2014;38(6):1235–49. 10.1111/1574-6976.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranquet C, Ollagnier-de-Choudens S, Loiseau L, Barras F, Fontecave M. Cobalt stress in Escherichia coli. The effect on the iron-sulfur proteins. J Biol Chem. 2007;282(42):30442–51. 10.1074/jbc.M702519200 [DOI] [PubMed] [Google Scholar]
- 34.Zennaro E, Ciabatti I, Cutruzzola F, D'Alessandro R, Silvestrini MC. The nitrite reductase gene of Pseudomonas aeruginosa: effect of growth conditions on the expression and construction of a mutant by gene disruption. FEMS Microbiol Lett. 1993;109(2–3):243–50. [DOI] [PubMed] [Google Scholar]
- 35.Py B, Gerez C, Huguenot A, Vidaud C, Fontecave M, Ollagnier de Choudens S, et al. The ErpA/NfuA complex builds an oxidation-resistant Fe-S cluster delivery pathway. J Biol Chem. 2018;293(20):7689–702. Epub 2018/04/08. 10.1074/jbc.RA118.002160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan MW, Mahajan-Miklos S, Ausubel FM. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci U S A. 1999;96(2):715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Utari PD, Quax WJ. Caenorhabditis elegans reveals novel Pseudomonas aeruginosa virulence mechanism. Trends Microbiol. 2013;21(7):315–6. 10.1016/j.tim.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 38.Yoon MY, Lee KM, Park Y, Yoon SS. Contribution of cell elongation to the biofilm formation of Pseudomonas aeruginosa during anaerobic respiration. PloS One. 2011;6(1):e16105 10.1371/journal.pone.0016105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer KL, Aye LM, Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol. 2007;189(22):8079–87. 10.1128/JB.01138-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romsang A, Leesukon P, Duangnkern J, Vattanaviboon P, Mongkolsuk S. Mutation of the gene encoding monothiol glutaredoxin (GrxD) in Pseudomonas aeruginosa increases its susceptibility to polymyxins. Int J Antimicrob Agents. 2015;45(3):314–8. 10.1016/j.ijantimicag.2014.10.024 [DOI] [PubMed] [Google Scholar]
- 41.Choi KH, Schweizer HP. mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc. 2006;1(1):153–61. 10.1038/nprot.2006.24 [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Russell DW, Russell DW. Molecular cloning: a laboratory manual: Cold spring harbor laboratory press Cold Spring Harbor, New York; 2001. [Google Scholar]
- 43.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166(1):175–6. [DOI] [PubMed] [Google Scholar]
- 44.Boonma S, Romsang A, Duang-Nkern J, Atichartpongkul S, Trinachartvanit W, Vattanaviboon P, et al. The FinR-regulated essential gene fprA, encoding ferredoxin NADP+ reductase: Roles in superoxide-mediated stress protection and virulence of Pseudomonas aeruginosa. PloS One. 2017;12(2):e0172071 10.1371/journal.pone.0172071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romsang A, Atichartpongkul S, Trinachartvanit W, Vattanaviboon P, Mongkolsuk S. Gene expression and physiological role of Pseudomonas aeruginosa methionine sulfoxide reductases during oxidative stress. J Bacteriol. 2013;195(15):3299–308. Epub 2013/05/21. 10.1128/JB.00167-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atichartpongkul S, Fuangthong M, Vattanaviboon P, Mongkolsuk S. Analyses of the regulatory mechanism and physiological roles of Pseudomonas aeruginosa OhrR, a transcription regulator and a sensor of organic hydroperoxides. J Bacteriol. 2010;192(8):2093–101. 10.1128/JB.01510-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enos-Berlage JL, Downs DM. Mutations in sdh (succinate dehydrogenase genes) alter the thiamine requirement of Salmonella Typhimurium. J Bacteriol. 1997;179(12):3989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacGregor CH, Schnaitman CA, Normansell DE. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974;249(16):5321–7. [PubMed] [Google Scholar]
- 49.Romsang A, Dubbs JM, Mongkolsuk S. The iron-sulfur cluster biosynthesis regulator IscR contributes to iron homeostasis and resistance to oxidants in Pseudomonas aeruginosa In: de Bruijn FJ, editor. Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria. John Wiley & Sons, USA: 2016. pp. 1090–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Western analysis of mutated NfuA proteins. Crude protein extracts from exponential-phase cultures of the ΔnfuA mutant carrying miniTn7T containing 6His-tag NfuA-WT, C152S, C155S, C43,47S or a vector control (Tn7T) were partially purified using Ni-NTA column prior to loading (40 mg) onto SDS-PAGE. Western blot was performed using anti-6His antibody conjugated with HRP. (B) nfuA expression analysis. End-point reverse transcription PCR was performed using BT2841 and BT2842 primers and cDNAs was prepared from total RNA samples extracted from P. aeruginosa strain as templates.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.