Abstract
Objectives. To determine whether food bank provision of self-management support and diabetes-appropriate food improves glycemic control among clients with diabetes.
Methods. We screened 5329 adults for diabetes at food pantries (n = 27) affiliated with food banks in Oakland, California; Detroit, Michigan; and Houston, Texas, between October 2015 and September 2016. We individually randomized 568 participants with hemoglobin A1c (HbA1c) 7.5% or greater to waitlist control or 6-month intervention including food, diabetes education, health care referral, and glucose monitoring. The primary outcome was HbA1c at 6 months.
Results. Food security (relative risk [RR] = 0.85; 95% confidence interval [CI] = 0.73, 0.98), food stability (RR = 0.77; 95% CI = 0.64, 0.93), and fruit and vegetable intake (risk difference [RD] = 0.34; 95% CI = 0.34, 0.34) significantly improved among intervention participants. There were no differences in self-management (depressive symptoms, diabetes distress, self-care, hypoglycemia, self-efficacy) or HbA1c (RD = 0.24; 95% CI = −0.09, 0.58).
Conclusions. Food banks are ideally situated to provide diabetes-appropriate food to food-insecure households. Effective strategies for food banks to support improvements in diabetes clinical outcomes require additional study.
Public Health Implications. Moving chronic disease support from clinics into communities expands reach into vulnerable populations. However, it is unclear how community interventions should be integrated with clinical care to improve disease outcomes.
Trial Registration Number. NCT02569060
Income-related disparities in health persist despite sustained attention from the public health and health care sectors.1 Decades of research have shown that low socioeconomic status predisposes to poor health across multiple dimensions; more recent work focuses on reducing the negative health impacts of social determinants by implementing interventions tailored to high-risk populations, such as low-income adults with diabetes.
While diabetes prevalence has risen across the entire population, it is particularly high in the United States among the lowest-income adults, with 17.8% of the lowest-income tertile affected compared with 11.5% and 8.0% of the middle- and highest-income tertiles, respectively.2 These adults are also at highest risk for food insecurity or the lack of consistent access to enough food for an active, healthy life; approximately 37% of low-income (< 130% of the federal poverty level) households in the United States are food-insecure.3 Food insecurity may partially explain the observation that diabetes interventions are less effective in the lowest-income populations. Diet is a cornerstone of diabetes self-management, but diabetes-appropriate diets are more expensive and often financially out of reach for food-insecure households. In addition, adults with diabetes living in food-insecure households face other significant barriers to self-management, including cost-related medication nonadherence, poor clinical follow-up because of competing time demands, depression, and increased hypoglycemia risk.4–8 Such challenges likely contribute to the poor glycemic control observed among patients with diabetes living in food-insecure, compared with food-secure, households.9,10
Food banks have emerged as a potential partner in addressing challenges with traditional diabetes interventions in clinical settings for numerous reasons. First, food banks can support food-insecure households who have difficulty accessing diabetes-appropriate foods. While few diabetes interventions implemented in health care settings have the capacity to provide food, food distribution is the main expertise of food banks. Second, food banks reach highly vulnerable populations, many of which are also at highest risk of poor engagement in traditional clinical settings. The opportunity to reach patients who are not presenting regularly in clinical settings expands capacity for improving population health and reducing health disparities. Third, as funding for safety net clinical settings, such as federally qualified health centers, has fallen, access to diabetes self-management support of all kinds has decreased in low-income populations. Community-based organizations observe this to be an important unmet need for their clients. Fourth, patient-centered care efforts have encouraged models that provide self-management support in the places patients feel comfortable receiving it, which may mean expanding interventions outside of clinical settings and into the community.
With these trends in mind, we completed a pilot study in 2014 to test the feasibility of providing diabetes self-management support and diabetes-appropriate food to food bank clients with diabetes. This pre–post observational study demonstrated significant improvements in glycemic control and other diabetes self-management outcomes.11 To rigorously examine the model’s effectiveness, we implemented a randomized controlled trial of a food bank–based, multicomponent diabetes intervention with a primary outcome of hemoglobin A1c (HbA1c).
METHODS
We conducted this randomized controlled trial within Feeding America food banks. Feeding America is the largest US hunger relief charity and supports a nationwide network of 200 food banks. Food banks are organizations typically responsible for the sourcing, storage, and distribution of food to smaller agencies (such as food pantries) that are embedded in communities and provide free food directly to people in need. In most communities, this means that dozens or hundreds of food pantries have established formal relationships with the local or regional food bank to deliver large quantities of food to them. The food pantry then redistributes that food to clients. Feeding America food banks distribute food through approximately 60 000 food pantries and feeding programs.
Participating food banks were selected through an internal Feeding America competitive application process. Applications were submitted by 16 (8%) food banks. Those selected (Alameda County Community Food Bank, Oakland, CA; Gleaners Community Food Bank of Southeastern Michigan, Detroit, MI; and Houston Food Bank, Houston, TX) best demonstrated capacity to work with food pantry partners to reach the population of interest and implement study protocols with fidelity.
Study Design
Participants were recruited from food pantries affiliated with 1 of the 3 food banks via flyers, word of mouth, and in-person announcements. Interested adults were offered blood glucose testing with the GE100 Blood Glucose Monitoring System (General Electric Company, Taichung City, Taiwan); those with elevated glucose (nonfasting ≥ 160 mg/dL or fasting ≥ 140 mg/dL) or reporting a type 2 diabetes history were offered point-of-care HbA1c testing with the A1C Now+ system (PTS Diagnostics, Indianapolis, IN). Eligible participants had an on-site HbA1c of greater than or equal to 7.5%, identified as an existing or new pantry client, were aged 18 years or older, spoke English or Spanish, had a phone or mailing address, and intended to remain in the area for 12 months. We excluded clients who were cognitively impaired, pregnant or less than 6 weeks postpartum, or had a history of type 1 diabetes.
Randomization occurred at the level of the participant after written informed consent and a baseline survey. We used participant-level block randomization to ensure rolling enrollment across multiple pantries would distribute participants equally into study arms. Study staff generated the 1-to-1 randomization scheme for each food bank separately by using http://www.randomization.com. Food bank staff located at the food pantry where participants were recruited opened sequentially numbered, opaque envelopes to randomize. Participants were compensated with $15 gift cards after completing assessments. Food bank staff and participants were aware of study assignment; study staff were blinded.
Our original recruitment goal was 720 participants, which allowed for 20% loss to follow-up and 90% power to detect 0.4% reduction in HbA1c (9.5% to 9.1%). We closed recruitment after randomization of 568 participants, after extending recruitment as long as possible with available funding. With 20% loss to follow-up, we determined that 450 participants with follow-up data would provide 80% power to detect the same 0.4% HbA1c reduction. The 0.4% assumption was based on pilot study observations.
Intervention
The 6-month intervention included blood glucose and HbA1c testing at months 3 and 6, referral to primary care (for participants reporting no provider), formal diabetes self-management classes and 1-on-1 check-ins with educators, and twice-monthly food packages containing diabetes-appropriate foods. All intervention components were delivered by food bank staff and volunteers working at the food pantry.
Control participants continued to receive regular food pantry services for 6 months. After their wait period, they received a modified intervention. Future analyses will examine the impact of this modified intervention.
Diabetes self-management education.
The diabetes self-management education (DSME) program was tailored by study staff (nurse and diabetes educator, dietitian, and physician) to address participant challenges to self-management (literacy, numeracy, transportation barriers and costs, food-access barriers, and food insecurity). It was modeled on the American Association of Diabetes Educators AADE7 Self-Care Behaviors,12 adapted components from the Type 2 Diabetes BASICS curriculum,13 and drew from patient empowerment approaches.14,15 The curriculum included two 2-hour structured sessions (to be completed within the first 2 months of enrollment) and optional monthly 1-hour drop-in sessions covering a range of DSME topics, allowing class facilitators flexibility to address areas of participant interest. The choice to “require” attendance at only 2 classes was based on pilot study experience.11 Educators were food bank staff trained in curriculum delivery by a registered nurse and diabetes educator. They participated in monthly conference calls and additional in-service trainings with study staff to review curriculum implementation.
Participants also received written diabetes education materials at each food distribution, including simple, diabetes-appropriate recipes using foods included in the food packages. Educators conducted brief 1-on-1 check-ins with participants during food distributions for additional self-management support.
Food packages.
Food packages were designed to increase access to and consumption of foods appropriate for diabetes self-management. Participants were eligible to receive 11 food packages during the intervention, picked up twice monthly from the food pantry from which they were originally recruited. Packages were assembled by each of the 3 food banks independently to adhere to guidelines (e.g., dairy guidelines required 2 low-fat dairy items from the following list: half gallon fresh or shelf-stable milk, four 6-oz yogurts, 8-oz cottage cheese, or 8-oz cheese). Packages contained shelf-stable and perishable products, including lean proteins, eggs and low-fat dairy, legumes and nuts, fruits and vegetables, and whole grains. Canned products low in sodium and added sugars were included. Packages were offered in addition to other foods regularly available at the pantry during standard, ongoing food distributions.
In addition to meeting guidelines for diabetes management, food packages were designed to provide approximately 22 meals, or 20% to 25% of monthly food needs, scaled for household size in anticipation of sharing. Food quantity decisions were informed by the pilot, in which 20% of participants prescribed a diabetes diet at baseline reported that their diabetes-appropriate food lasted the entire month.11 Others reported their diabetes-appropriate food lasted 3 weeks (24%), 2 weeks (29%), 1 week (18%), or less than 1 week (10%). Although we provided more food in this intervention than in the pilot by changing from monthly to twice-monthly distributions, we were limited by food bank capacity, feasibility of scaling the intervention to other food banks, and participant challenges transporting food home. For example, participants noted challenges transporting food on the bus, by foot in icy conditions, and up stairs. Food banks used donated items and purchased in bulk whenever possible to lower costs, resulting in food package costs ranging from $12 to $18 for the smallest households.
Outcome Measures
Outcomes ascertainment was planned for 6 months after enrollment, allowing a window of 5 to 7 months to accommodate participants’ demanding schedules (many of whom were working, caring for children or dependents, and managing their own illness). Our primary outcome was on-site HbA1c. Secondary outcomes included food security (6 items)16; food stability (“Do you ever run out of the food you need to take care of your diabetes?”); fruit, vegetable, and sugar intake (11 dietary screening items)17,18; hypoglycemia (“In the past four weeks, how many times have you had a severe low blood sugar reaction such as passing out or needing help to treat the reaction?”)19; tradeoffs between food and medications or supplies (“In the last six months, how often did you put off buying diabetes supplies, like test strips or lancets, so that you would have money to buy food, or put off buying food so you would have money to buy diabetes supplies?”)11; cost-related medication nonadherence; diabetes distress (“feeling overwhelmed by the demands of living with diabetes” or “feeling that I am often failing with my diabetes routine”); depression (Patient Health Questionnaire–8)20; diabetes self-efficacy21–25; diabetes self-care26; and medication adherence (4-item scale).27 We also measured intervention satisfaction.
Statistical Analyses
We compared baseline characteristics of intervention and control groups by using the χ2 test for categorical variables and t test (normally distributed) or Wilcoxon rank sums test (not normally distributed) for interval variables.
We conducted intent-to-treat analyses of all outcomes. Because HbA1c testing was performed on most intervention participants with the pickup of their last (11th) food package (rather than timed for 6 months), there was a significant difference in average time between baseline and follow-up assessments for the intervention versus control groups (171.5 vs 174.9 days; P < .001). We therefore adjusted all analyses for baseline value and number of days between baseline and follow-up assessment by using generalized linear models with Huber–White robust standard errors (appropriate for individual-level randomization). We performed multivariable, modified least squares regression with an identity link to calculate risk differences in outcome means,28 and multivariable, modified Poisson regression with a logarithm link to obtain relative risks of binary outcomes.29 We treated hypoglycemic events as a dichotomous variable (0 vs 1+). Multiple sensitivity analyses with this variable, including treating it as a continuous variable and performing negative binomial regression with a logarithm link to account for overdispersion, did not substantively alter results.
We a priori defined a subgroup analysis examining glycemic control among intervention participants who fully engaged. We defined full engagement as attending both core DSME classes, picking up 9 or more of 11 food boxes, and reporting attending at least 1 primary care visit during the study period.
RESULTS
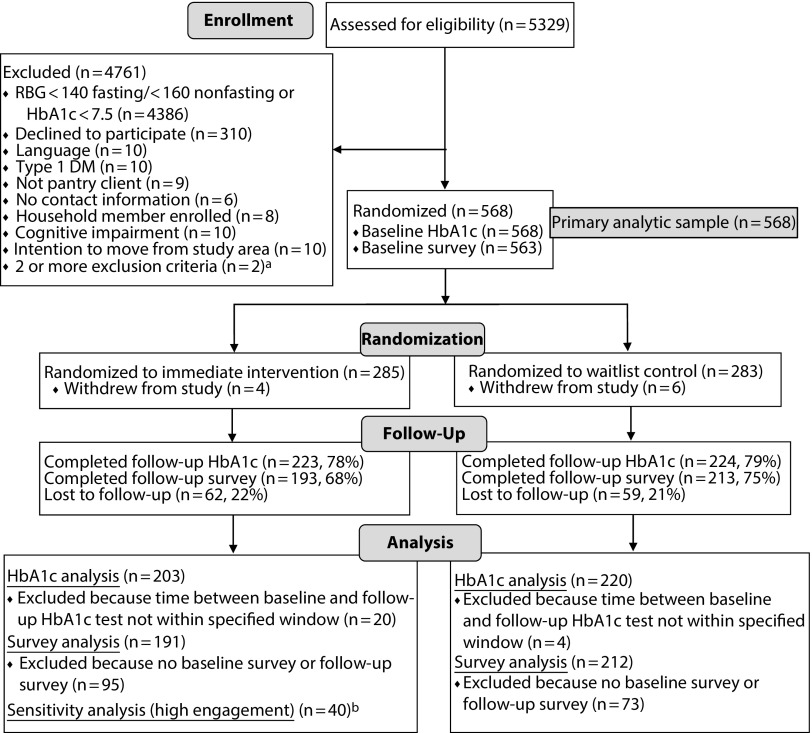
We screened 5329 pantry clients with either glucometer or HbA1c testing (Figure 1). We randomized 568 participants (285 intervention and 283 control), all of whom had a baseline HbA1c value. Participants were aged an average of 55 years and racially/ethnically diverse (52% Latino and 33% African American) with low educational attainment (48% without a high-school degree), as shown in Table 1. Participant characteristics were balanced across study arms.
FIGURE 1—
Flow of Participants Through Trial of Diabetes Self-Management Support in Food Banks: Detroit, MI; Houston, TX; and Oakland, CA, 2015–2016
Note. DM = diabetes mellitus; RBG = random blood glucose.
aSpecific exclusion criteria for participants with 2 or more exclusion criteria: no contact information and cognitive impairment (n = 1); no contact information and intention to move from study area (n = 1).
bHigh engagement = picked up ≥ 80% of diabetes-appropriate food boxes, attended ≥ 2 diabetes education classes, and had ≥ 1 primary care visit over the 6-mo follow-up period.
TABLE 1—
Baseline Characteristics of Participants in Trial of Diabetes Self-Management Support in Food Banks: Detroit, MI; Houston, TX; and Oakland, CA, 2015–2016
| Characteristic | All (n = 568), Mean ±SD or No. (%) | Control (n = 283), Mean ±SD or No. (%) | Intervention (n = 285), Mean ±SD or No. (%) |
| Age, y | 54.8 ±11.4 | 55.0 ±11.7 | 54.6 ±11.2 |
| Female | 384 (68.3) | 187 (66.8) | 197 (69.9) |
| Race/ethnicity | |||
| Latino or Hispanic | 293 (52.1) | 145 (51.8) | 148 (52.5) |
| White | 70 (12.5) | 36 (12.9) | 34 (12.1) |
| Black or African American | 183 (32.6) | 91 (32.5) | 92 (32.6) |
| Native American | 3 (0.5) | 0 (0.0) | 3 (1.1) |
| Asian or Pacific Islander | 8 (1.4) | 4 (1.4) | 4 (1.4) |
| Other | 5 (0.9) | 4 (1.4) | 1 (0.4) |
| Completed intervention in Spanish | 197 (34.7) | 100 (35.3) | 97 (34.0) |
| Has primary care provider | 523 (92.1) | 261 (92.2) | 262 (91.9) |
| Education | |||
| Some high school or less | 269 (48.0) | 131 (47.0) | 138 (48.9) |
| High-school graduate, GED, some college, AA, technical school | 251 (44.7) | 128 (45.9) | 123 (43.6) |
| College graduate or graduate degree | 41 (7.3) | 20 (7.2) | 21 (7.4) |
| Employment | |||
| Working full-time (≥ 35 h/week) | 75 (13.3) | 34 (12.1) | 41 (14.5) |
| Working part-time (< 35 h/week) | 68 (12.1) | 36 (12.9) | 32 (11.3) |
| Homemaker, unemployed, or retired | 307 (54.6) | 149 (53.2) | 158 (56.0) |
| Disabled | 102 (18.1) | 56 (20.0) | 46 (16.3) |
| Other | 10 (1.8) | 5 (1.8) | 5 (1.8) |
| New diagnosis of diabetes | 37 (6.6) | 20 (7.1) | 17 (6.1) |
| In general, self-reported health | |||
| Excellent or very good | 25 (4.4) | 14 (5.1) | 11 (3.9) |
| Good, fair, or poor | 534 (95.2) | 264 (94.6) | 270 (95.8) |
| Food insecure | 422 (75.5) | 204 (73.7) | 218 (77.3) |
| Study site | |||
| Detroit | 180 (31.7) | 90 (31.8) | 90 (31.6) |
| Houston | 268 (47.2) | 133 (47.0) | 135 (47.4) |
| Oakland | 120 (21.1) | 60 (21.2) | 60 (21.1) |
| Baseline measures of study outcomes | |||
| HbA1c | 9.75 ±1.77 | 9.74 ±1.76 | 9.75 ±1.79 |
| Fruits and vegetables, servings per day | 5.7 ±0.3 | 5.7 ±0.3 | 5.8 ±0.4 |
| Added sugar, teaspoons per day | 13.1 ±0.1 | 13.0 ±0.1 | 13.5 ±0.1 |
| Diabetes self-care score, range 1–100 | 71.2 ±18.4 | 72.4 ±18.2 | 69.9 ±18.6 |
| Diabetes self-efficacy score,a range 1–10 | 6.8 ±1.9 | 6.8 ±2.0 | 6.7 ±1.8 |
| Medication nonadherence score, range 1–4 | 1.2 ±1.2 | 1.2 ±1.2 | 1.2 ±1.1 |
| Food insecure, very low or low food security | 309 (74.5) | 154 (71.6) | 155 (77.5) |
| Food instability, responding affirmatively to running out of food to take care of diabetes | 226 (76.9) | 113 (74.3) | 113 (79.6) |
| Depressive symptoms, responding severe or moderately severe symptoms | 73 (18.0) | 29 (13.8) | 44 (22.6) |
| Diabetes distress, with clinically significant distress | 240 (62.8) | 124 (62.9) | 116 (62.7) |
| Cost-related medication nonadherence, responding affirmatively | 106 (25.7) | 52 (24.4) | 54 (27.1) |
| Food or medication trade-offs, responding affirmatively | 98 (23.8) | 55 (25.8) | 43 (21.6) |
| Food or supplies trade-offs, responding affirmatively | 97 (25.3) | 56 (28.3) | 41 (22.2) |
| Self-reported severe hypoglycemic episodes, reporting ≥ 1 event | 56 (14.8) | 29 (14.8) | 27 (14.8) |
Note. AA = associate’s degree; GED = general equivalency diploma.
Includes only participants with no missing values.
We collected 6-month HbA1c values within the prespecified window for 423 participants and follow-up surveys for 406 participants. Our primary analysis included 203 intervention and 220 control participants (72% retention). We observed statistically significant improvements in the intervention compared with the control group in outcomes related to food, including food security (P = .03), food stability (P = .01), and fruit and vegetable intake (P = .04). There was no significant difference in added sugar intake. No nonfood outcomes differed between the intervention and control groups (Table 2), except for tradeoffs between food and diabetes supplies (P = .03).
TABLE 2—
Primary and Secondary Outcomes in Trial of Diabetes Self-Management Support in Food Banks: Detroit, MI; Houston, TX; and Oakland, CA, 2015–2016
| Outcomes | Intervention | Control | RD (95% CI) or RR (95% CI) |
| HbA1c, mean | 9.12 | 8.88 | 0.24a (−0.09, 0.58) |
| Fruits and vegetables, mean servings per day | 4.2 | 3.9 | 0.34a,b |
| Added sugar, mean teaspoons per day | 10.9 | 11.0 | −0.03a,b |
| Diabetes self-care, mean score, range 1–100 | 76.3 | 75.5 | 0.77a (−2.68, 4.21) |
| Diabetes self-efficacy,c mean score, range 1–10 | 7.4 | 7.2 | 0.24a (−0.12, 0.61) |
| Medication nonadherence, mean score, range 1–4 | 0.8 | 1.0 | −0.17a (−0.39, 0.05) |
| Food insecure, % with very low or low food security | 60.0 | 69.4 | 0.85d (0.73, 0.98) |
| Food instability, % responding affirmatively to running out of food to take care of diabetes | 54.9 | 70.2 | 0.77d (0.64, 0.93) |
| Depressive symptoms, % responding severe or moderately severe symptoms | 9.4 | 6.7 | 1.18d (0.60, 2.32) |
| Diabetes distress, % with clinically significant distress | 48.4 | 49.7 | 0.98d (0.80,1.2) |
| Cost-related medication nonadherence, % responding affirmatively | 17.0 | 18.5 | 0.88d (0.56,1.37) |
| Food or medication trade-offs, % responding affirmatively | 15.7 | 24.1 | 0.70d (0.46,1.07) |
| Food or supplies trade-offs, % responding affirmatively | 15.3 | 26.8 | 0.62d (0.40, 0.95) |
| Self-reported severe hypoglycemic episodes, % reporting ≥ 1 event | 11.8 | 11.0 | 1.02d (0.56,1.86) |
Note. CI = confidence interval. All values are adjusted for baseline value and days between baseline and follow-up assessment.
Risk difference (RD).
Dietary intake variables were transformed, according to standard protocol, to generate estimates of daily intake. Back-transformation results in small standard errors with confidence intervals that do not accurately reflect statistical significance. The P value for fruit and vegetable intake was .04 and the P value for added sugar intake was .95.
Includes only participants with no missing values.
Relative risk (RR).
HbA1c at follow-up was not significantly different between the 2 groups (intervention 9.12% vs control 8.88%; P = .16). Percentage of participants with HbA1c less than 7.5% at follow-up also did not differ (intervention 24% vs control 29%; P = .19). Results did not change when we conducted a carry-forward analysis using baseline HbA1c values for participants without a follow-up HbA1c (n = 121) and including participants whose follow-up HbA1c was outside the prespecified window (n = 24).
Among intervention participants, 40 of 203 met criteria for full engagement (see Appendix available as a supplement to the online version of this article at http://www.ajph.org for characteristics). In subgroup analysis examining intervention participants who fully engaged compared with those who did not fully engage, HbA1c was significantly lower at follow-up among those who fully engaged (8.60% vs 9.24%; P = .02).
Intervention participants reported high satisfaction: 80% preferred the diabetes food package over standard pantry offerings; 78% were very or somewhat happy with the DSME; 99% found on-site glycemic monitoring very or somewhat helpful; 64% felt communication with their health care provider was very or somewhat helpful; and 98% found the diabetes food package very or somewhat helpful. Almost two thirds (64%) felt the program helped them to control their diabetes “a lot better” and 98% would recommend the program to friends or family. However, we also observed challenges with engagement: only 57% of participants felt that attending DSME was very or somewhat easy and 43% reported talking with their health care provider about the program.
DISCUSSION
In this randomized controlled trial of comprehensive diabetes self-management support and diabetes-appropriate foods for food pantry clients with poorly controlled diabetes, we observed significant improvements in outcomes related to food—the core operational expertise of food banks and food pantries. These outcomes included food security, food stability, and fruit and vegetable intake. We did not observe improvements in outcomes related to diabetes self-management or glycemic control.
There are multiple reasons the intervention may not have significantly affected glycemic control. First, it may not have been adequately comprehensive or lengthy. We carefully designed the intervention recognizing client barriers to frequent on-site presentation (e.g., transportation costs, dependent care, and job responsibilities). The decision to facilitate engagement by limiting intervention scope may have resulted in inadequate support.
A core intervention component was providing diabetes-appropriate food. Ethics required that participants retain the option for standard pantry food offerings also. Thus, although we may have increased access to diabetes-appropriate foods, we did not directly aim to reduce access to carbohydrates or added sugars. Providing diabetes-appropriate food without adequately facilitating clinical visits for medication titration or delivering a higher-intensity educational program focused on reducing carbohydrate intake may have blunted improvements in glycemic control that might have resulted directly from diabetes-appropriate food packages. Shifts within food banking to make all pantry food offerings more appropriate for clients with chronic disease may, over time, address this problem.
Although providing more food might amplify improvements we saw in food security, food stability, and dietary intake, it would require addressing operational challenges, participant barriers (transporting larger food packages), and additional costs. Although cost of the diabetes food package was high by food bank standards, it was relatively low by health care standards. Therefore, higher food “doses”—along with novel distribution strategies (e.g., home delivery) that reduce participant barriers—may be feasible if costs are shared among sectors (e.g., charitable feeding, insurance companies, health systems).
The DSME was designed to address substantial competing demands for time and resources faced by many people in food-insecure households. We attempted to reduce participation barriers (e.g., pairing classes with food distributions and reducing the number of sessions). Nonetheless, engagement was low; only 36% attended the 2 core classes, even with high satisfaction rates. The observation that these fully engaged participants improved their HbA1c significantly compared with less-engaged participants suggests there may be benefit to delivering a multicomponent intervention. Conversely, other factors specific to this subgroup may explain the improvements. Future work should explore factors allowing this subgroup to better engage and iteratively refine the intervention to enhance engagement.
Two core mechanisms by which we hoped to increase impact were linking to care participants without a health care provider and diagnosing with diabetes people who were unaware they had it. At enrollment, 8% of participants reported not having a health care provider (all of whom received a formal referral), and 6.6% were unaware that they had diabetes. Food bank staff briefly communicated with health care providers for these participants, as they did for other participants. However, because we were not able to tease out the individual impact of each study component, it is not clear how, if at all, communication between food bank staff and health care providers impacted engagement with clinical care. Future investigation of feedback loops between food banks and health care may prove valuable.
Health systems increasingly rely on community organizations as partners in health promotion and resources for improving clinical outcomes, particularly in populations with low socioeconomic status. For example, organizations that specialize in reducing household allergen exposure may become partners in reducing asthma exacerbations; organizations that provide home-delivered meals after a hospital admission may become partners in reducing readmission rates30; or, as this study examined, food banks may become partners in supporting access to diabetes-appropriate food to improve glycemic control. Our findings, in conjunction with the limited capacity of community-based organizations for implementing interventions, highlight the importance of rigorously testing these partnerships before broad-scale dissemination even in the presence of robust evidence of their impact in clinical settings.
Although community-based organizations such as food banks often have reach into communities that are the most vulnerable and hardest for clinical systems to reach, these communities also have additional barriers to clinical care and optimal self-management, which may make interventions less effective. The observation that fully engaged participants achieved greater improvement in glycemic control, although nonrandomized, and the relatively low percentage of participants who fully engaged suggest that engagement challenges observed in the clinical setting remain challenges in the community setting.
Although the core competency of food banks is food distribution, there have been few rigorous studies showing that food distribution improves outcomes. This study is the first randomized trial of which we are aware to demonstrate that targeted charitable feeding reduces food insecurity, stabilizes diet, and improves dietary intake. Although this may seem self-evident, access to free resources allows households under tight budget pressures to shift money toward other household necessities. Therefore, it is possible that charitable food access allows households to, for example, pay utility or rent bills but not improve food security. Our food-related findings highlight the importance of the large charitable feeding system31 and suggest increased attention to models bringing diabetes-appropriate food to the clinical setting, rather than bringing the patient with diabetes to the food bank.32
Limitations and Strengths
This study has limitations. Although point-of-care HbA1c testing in community settings was feasible, environmental conditions made testing challenging. However, basic reliability testing suggested acceptable device performance (data forthcoming). There was potential contamination between intervention and control groups because participants interacted at food pantries and within their communities. It is also possible that HbA1c improvements in both groups reflected an overall shift in participating food pantries toward more diabetic-friendly practices and procedures outside of the trial. We do not believe our study was substantially underpowered even though we did not reach recruitment goals. Lack of impact on self-management outcomes such as depressive symptoms, self-efficacy, and diabetes distress suggests we would not have observed significant changes in glycemic control even with a larger sample.
This study also has strengths. Community organizations rarely subject their work to the rigor of a randomized controlled trial. We increased generalizability by recruiting participants from many food pantries in 3 states. Finally, we maintained relatively high follow-up despite challenges conducting research within highly marginalized and very low-income populations.
Public Health Implications
Moving chronic disease support into communities can expand reach into highly vulnerable populations, but may be insufficient to achieve key disease management outcomes in the absence of closer integration with health care or more robust efforts to build patient self-management capacity.33 Both community-based organizations and health care systems are grappling with how to leverage their relative strengths to better meet the needs of low-income populations. Improving health outcomes and reducing health disparities requires continued exploration of how to scale effective interventions that build on the strengths of community organizations such as food banks, while also enhancing patient engagement with clinical care. This study reinforces the value of rigorously examining interventions designed for clinical implementation before broad dissemination through nonclinical systems.
ACKNOWLEDGMENTS
Funding for the Feeding America Intervention Trial for Health—Diabetes Mellitus (FAITH-DM) was provided by Feeding America, the Laura and John Arnold Foundation, the Urban Institute via a Robert Wood Johnson Foundation grant, National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award P30DK092924, and Centers for Disease Control and Prevention under award 3U48DP004998-01S1.
The authors wish to acknowledge and thank the staff, volunteers, and participants from each of the three food bank sites and Dean Schillinger for his helpful review of this article. We are grateful to Judy Quan for her statistical support.
Note. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
HUMAN PARTICIPANT PROTECTION
The research protocol was approved by the Western institutional review board. Additional review (for data analysis only) was provided by the University of California San Francisco institutional review board and the Urban Institute institutional review board. The manual of operations, including detailed protocol and study forms, can be accessed at Open Science Framework (https://osf.io). The authors can provide study data to qualified parties upon request.
REFERENCES
- 1.Chetty R, Stephner M, Abraham S et al. The association between income and life expectancy in the United States, 2001–2014. JAMA. 2016;315(16):1750–1766. doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 3.Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Economic Research Service, US Department of Agriculture. Statistical supplement to household food security in the United States in 2015. 2016. Administrative Publication Number 72. Available at: https://www.ers.usda.gov/webdocs/publications/79436/ap-072.pdf?v=42622. Accessed January 25, 2018. [DOI] [PubMed]
- 4.Ippolito MM, Lyles CR, Prendergast K, Marshall MB, Waxman E, Seligman HK. Food insecurity and diabetes self-management among food pantry clients. Public Health Nutr. 2017;20(1):183–189. doi: 10.1017/S1368980016001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014;127(4):303–310.e3. doi: 10.1016/j.amjmed.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Basu S, Berkowitz SA, Seligman H. The monthly cycle of hypoglycemia: an observational claims-based study of emergency room visits, hospital admissions, and costs in a commercially insured population. Med Care. 2017;55(7):639–645. doi: 10.1097/MLR.0000000000000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6–9. doi: 10.1056/NEJMp1000072. [DOI] [PubMed] [Google Scholar]
- 8.Seligman HK, Bolger AF, Guzman D, Lopez A, Bibbins-Domingo K. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia. Health Aff (Millwood) 2014;33(1):116–123. doi: 10.1377/hlthaff.2013.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkowitz SA, Meigs JB, DeWalt D et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the measuring economic insecurity in diabetes study. JAMA Intern Med. 2015;175(2):257–265. doi: 10.1001/jamainternmed.2014.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seligman HK, Jacobs EA, Lopez A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233–238. doi: 10.2337/dc11-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seligman HK, Lyles C, Marshall MB et al. A pilot food bank intervention featuring diabetes-appropriate food improved glycemic control among clients in three states. Health Aff (Millwood) 2015;34(11):1956–1963. doi: 10.1377/hlthaff.2015.0641. [DOI] [PubMed] [Google Scholar]
- 12.American Association of Diabetes Educators. AADE7 Self-Care Behaviors. 2018. Available at: https://www.diabeteseducator.org/living-with-diabetes/aade7-self-care-behaviors. Accessed January 25, 2018.
- 13.Type 2 Diabetes BASICS Curriculum Guide. 4th ed. St Louis Park, MN: Park Nicollet International Diabetes Center; 2014. [Google Scholar]
- 14.Peek ME, Wilkes AE, Roberson TS et al. Early lessons from an initiative on Chicago’s South Side to reduce disparities in diabetes care and outcomes. Health Aff (Millwood) 2012;31(1):177–186. doi: 10.1377/hlthaff.2011.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.University of Chicago. Improving diabetes care and outcomes on the South Side of Chicago. 2018. Available at: http://southsidediabetes.com. Accessed January 25, 2018.
- 16.US Department of Agriculture, Economic Research Service. US household food security survey module: six-item short form. 2012. Available at: https://www.ers.usda.gov/media/8282/short2012.pdf. Accessed August 18, 2015.
- 17. UCLA Center for Health Policy Research, California Department of Health Services, Public Health Institute. CHIS 2005 Adult Questionnaire. Version 6.4. Regents of the University of California. 2010. Available at: http://healthpolicy.ucla.edu/chis/design/Documents/CHIS2005_adult_q.pdf. Accessed January 25, 2018.
- 18.Epidemiology and Genomics Research Program, National Cancer Institute, Division of Cancer Control and Population Sciences. Diet screener in the 2005 CHIS: scoring procedures. 2015. Available at: https://epi.grants.cancer.gov/chis/dietscreener/scoring.html. Accessed January 25, 2018.
- 19.Seligman HK, Jacobs EA, Lopez A, Sarkar U, Tschann J, Fernandez A. Food insecurity and hypoglycemia among safety net patients with diabetes. Arch Intern Med. 2011;171(13):1204–1206. doi: 10.1001/archinternmed.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Lyles CR, Wolf MS, Schillinger D et al. Food insecurity in relation to changes in hemoglobin A1c, self-efficacy, and fruit/vegetable intake during a diabetes educational intervention. Diabetes Care. 2013;36(6):1448–1453. doi: 10.2337/dc12-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kavanagh DJ, Gooley S, Wilson PH. Prediction of adherence and control in diabetes. J Behav Med. 1993;16(5):509–522. doi: 10.1007/BF00844820. [DOI] [PubMed] [Google Scholar]
- 23.Johnston-Brooks CH, Lewis MA, Garg S. Self-efficacy impacts self-care and HbA1c in young adults with type I diabetes. Psychosom Med. 2002;64(1):43–51. doi: 10.1097/00006842-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar U, Fisher L, Schillinger D. Is self-efficacy associated with diabetes self-management across race/ethnicity and health literacy? Diabetes Care. 2006;29(4):823–829. doi: 10.2337/diacare.29.04.06.dc05-1615. [DOI] [PubMed] [Google Scholar]
- 25.Talbot F, Nouwen A, Gingras J, Gosselin M, Audet J. The assessment of diabetes-related cognitive and social factors: the multidimensional diabetes questionnaire. J Behav Med. 1997;20(3):291–312. doi: 10.1023/a:1025508928696. [DOI] [PubMed] [Google Scholar]
- 26.Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17(4):243–252. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Cheung YB. A modified least-squares regression approach to the estimation of risk difference. Am J Epidemiol. 2007;166(11):1337–1344. doi: 10.1093/aje/kwm223. [DOI] [PubMed] [Google Scholar]
- 29.Zou G. A. modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 30.Berkowitz SA, Terranova J, Hill C et al. Meal delivery programs reduce the use of costly health care in dual eligible Medicare and Medicaid beneficiaries. Health Aff (Millwood) 2018;37(4):535–542. doi: 10.1377/hlthaff.2017.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinfield NS, Mills G, Borger C for Feeding America. Hunger in America 2014. Chicago, IL: Feeding America; 2014. Available at: http://help.feedingamerica.org/HungerInAmerica/hunger-in-america-2014-full-report.pdf. Accessed January 25, 2018.
- 32.Smith S, Chang J, Brownell K. Food insecurity, its impact on health, and methods to address this modifiable SDH in primary care. Poster presented at: Society of Teachers of Family Medicine Annual Spring Conference; May 5–9, 2018; San Diego, CA.
- 33.Thom DH, Ghorob A, Hessler D, De Vore D, Chen E, Bodenheimer TA. Impact of peer health coaching on glycemic control in low-income patients with diabetes: a randomized controlled trial. Ann Fam Med. 2013;11(2):137–144. doi: 10.1370/afm.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]