Abstract
Background
Characteristics and outcomes of tree nut (TN) oral food challenges (OFC) in patients with TN allergy or sensitization alone are poorly studied.
Objective
Determine the relationship between TN sensitization levels and OFC outcomes Methods: Open TN OFCs performed between 2007–2015 at a referral center were analyzed to compare outcome based on skin prick test (SPT) wheal size, food-specific IgE (sIgE), peanut co-allergy, and TN sensitization only versus TN allergy with sensitization to other TN’s. Delayed OFC was defined as >12 months from time of sIgE <2 kUA/L.
Results
Overall passage rate was 86% among 156 TN OFC in 109 patients (54 almond, 28 cashew, 27 walnut, 18 hazelnut, 14 pecan, 13 pistachio, and 2 Brazil nut). Passage rate was 76% (n=67) in patients with a history of TN allergy who were challenged to another TN to which they were sensitized, and 91% (n=65) among those with TN sensitization only (mean sIgE=1.53 kUA/L; range 0.35–9.14). Passage rate was 89% (n=110/124) if TN-sIgE <2 kUA/L, and 69% (11/16) if TN-sIgE ≥2 kUA/L. Among n=44 challenges in peanut allergic patients with TN co-sensitization, TN OFC passage rate was 96%. In 41 TN OFCs with TN SPT wheal size ≥3 mm, 61% passed, with mean wheal 4.8 mm (range 3–11) among those passing vs. 9 mm (range 3–20) in those failing.
Conclusion
TN challenges are frequently passed in TN-sensitized patients with or without a history of prior TN reactivity, despite TN SPT ≥3 mm or TN-sIgE ≥2 kUA/L. Nearly all peanut allergic but TN co-sensitization patients passed TN challenge, questioning how much clinically relevant “co-allergy” may exist.
Keywords: tree nut, peanut, oral food challenge, negative predictive value, tree nut allergy, tree nut sensitization, skin prick test, food-specific IgE, anaphylaxis
Introduction
Food allergy to tree nuts (TN) is estimated to affect approximately 1% of children in the United States based on self-report survey, and this rate has tripled over a 10-year period.1 Compared to other foods, TN and peanut IgE-mediated reactions are particularly associated with a higher degree of potential severity, and are attributed as leading causes of fatal food allergy induced anaphylaxis.1,2 Similar to peanut allergy (PA), TN allergy tends to persist into adulthood.3 Resolution rates for TN allergy may be less than those of peanut allergy, though few longitudinal TN allergy data exist. In one study of 101 children with history of clinical reactions to TN, resolution of TN allergy was reported in only 9% of patients,4 while recent data regarding peanut allergy report 22% of children regained tolerance based on oral food challenge (OFC) proven diagnosis.5 In another large, nationally representative study, 14.3% of those who at one time self-reported tree nut allergy later reported tolerance.1
In the setting of suspected TN reaction, skin prick testing (SPT) and serum-specific IgE (sIgE) help confirm an IgE-mediated food allergy and can potentially serve as a predictive measures to assess the necessity of OFC to confirm diagnosis. SPT and sIgE levels also have potential longitudinal utility to assess patient readiness to undergo OFC to determine if they are still allergic. In peanut, egg, and milk allergies sIgE <2.0 kUA/L is a proposed 50% negative predictive value (NPV) at which patients may pass OFC, and this should be offered.3,6 In TN allergy a higher threshold has been proposed based on a study that examined 39 TN OFCs performed in children 4–19 years of age at a referral center, noting a 58% NPV with TN sIgE <5.0 kUA/L, and 63% NPV with sIgE <2.0 kUA/L.4 Given the limited published data regarding TN allergy, additional studies are needed to help better guide clinical decision-making.
In clinical practice peanut allergic patients may often be screened for TN allergy. Tree nuts are drupaceous fruits and peanuts are legumes, but they share certain cross-reactive IgE-binding epitopes.7 It is common for PA patients to demonstrate some degree of TN co-sensitization, and vice versa. Patients allergic to one particular TN often demonstrate co-sensitization to other tree nuts as well.8,9 In PA patients, TN co-sensitization may occur in up to 86% of patients, though only 34% may display clinical reactivity TN (e.g. symptom development after ingestion).10 Furthermore, many providers instruct PA children to avoid TNs due to TN sensitization, despite no history of any TN reaction or low/absent sensitization. It is unclear if this cross-reactivity is clinically relevant, and OFC may be necessary to confirm a suspected IgE-mediated TN allergy.
There are no specific recommendations regarding the timing of when to perform OFC in relation to low-positive or negative TN test results.3,11 It has been postulated that OFC may be safely performed when sIgE test results are below the published 50% NPV cutoffs, though such values for TN are poorly established.12 It is unclear if declining or low sIgE, skin tests, or both are the superior predictor of being ready for OFC, or what to do when these values may be somewhat discrepant. Delaying an OFC may lead to additional, possibly unneeded, costs to both the families and the healthcare system.11 The objectives of this study were to provide additional data regarding the characteristics and outcomes of TN OFCs in patients with TN sensitization, both with and without a history of allergy to another TN, and to further examine TN OFC characteristics and outcomes in PA individuals with TN sensitization.
Methods
Study Design
A retrospective analysis was performed for all open TN OFCs conducted at the University of Michigan Division of Allergy and Clinical Immunology clinics between 2007 and 2015. Patients undergoing TN OFC were identified from the allergy division database using International Classification Diseases, Ninth Revision (ICD-9) coding, and Current Procedural Terminology (CPT) coding for OFC. Patients that had TN SPT and/or corresponding TN sIgE prior to challenge were included in the study. Patients with history of non-IgE mediated food allergy were excluded. TN SPT wheal size, TN-sIgE, coexisting food allergy, co-morbid allergic disease and features of the patient’s initial and any subsequent reactions were abstracted through chart review, and National Institutes of Allergy and Infectious Diseases/Food Allergy & Anaphylaxis Network anaphylaxis criteria were used to assess severity of documented reaction symptoms.13 Additionally, timing of OFC in relation to testing results was assessed, with delayed OFC defined as occurring >12 months from time of sIgE <2 kUA/L. Number of additional visits with testing from time of sIgE <2 kUA/L, age at time of OFC, and OFC outcome were also explored. Reasons for delaying OFC were not explored. Subjects were classified as TN allergic (chart documented clinical symptoms after TN ingestion), TN sensitized (positive tests alone without lifetime TN exposure), or avoiding TN despite no sensitization or reaction history. TN allergic individuals in the study were not challenged to any TN to which they had demonstrated previous symptomatic reactivity upon ingestion. These individuals were only challenged to TN to which they were sensitized and were being effectively managed as allergic to that item with avoidance recommended but had never actually ingested.
Statistical Analysis
The primary outcome was to investigate the passage rates of TN OFCs in relation to both SPT and sIgE results. Secondary outcomes included time to challenge (months), differences in OFC outcome based on sensitization vs. allergy, age at the time of OFC, number of additional visits, history of anaphylaxis, and allergen type between the two groups. Descriptive statistics were analyzed to characterize the population, and fisher exact tests and logistic regression used to assess bivariate relationships. Adjusted multiple regression models were used to determine predictive associations, and the stata margins command to determine predictive values for challenge outcomes. An a priori determined α of .05 was utilized for significance. All analyses were performed using Stata SE, version 13 (Stata Corp, College Station, Texas). The study was approved by the University of Michigan Institutional Review Board.
Results
There were 156 TN OFCs identified for analysis, performed in 109 patients between 2007 and 2015. Patient characteristics are detailed in Table 1. Co-morbid atopy was prominent. Half of the patients in the population were TN allergic (defined as reporting clinical symptoms with a TN ingestion and having positive skin or serum tests) (n=54), while 40% were only TN sensitized (positive TN allergy test only, without known symptoms attributable to ingestion) (n=43). The remaining (n=13) had been avoiding TN due to another food allergy, despite negative TN testing, for unclear reasons (e.g., presumed parent or provider preference that was poorly documented in the medical record). Most patients in the population (60%) had an additional food allergy, most commonly to peanut (42%). The most common presenting symptoms to any TN ingestion at initial diagnosis among TN allergic individuals were skin manifestations including hives, itching, flushing, and/or rash, and 28% had chart-documented symptoms consistent with anaphylaxis.
Table 1.
Study population characteristics (n=109)
| Characteristic (n=109) | |
|---|---|
| Male, no. (%) | 58 (53) |
| Mean age at tree nut allergy/sensitization diagnosis, years (median) | 4.5 (2.5) |
| Mean age at initial tree nut oral food challenge, years (median) | 11.2 (10.3) |
| a Tree nut allergic (any symptom at initial presentation), no. (%) | 54 (50) |
| Anaphylaxis, no. (%) | 15 (28) |
| Skin symptoms (hives, itching, flushing, rash), no. (%) | 36 (67) |
| Facial swelling, no. (%) | 16 (30) |
| Oropharyngeal symptoms (tongue or throat pruritus/swelling), no. (%) | 13 (24) |
| Respiratory symptoms (cough, wheezing, shortness of breath), no. (%) | 11 (20) |
| Vomiting, no. (%) | 13 (24) |
| Tree nut sensitized (no clinical symptoms), no. (%) | 43 (40) |
| Tree nut avoidance due to other food allergy (despite negative testing), no. (%) | 13 (12) |
| Concurrent food allergy, no. (%) | 65 (60) |
| Peanut allergy, no. (%) | 46 (42) |
| Egg allergy, no. (%) | 10 (9) |
| Milk allergy, no. (%) | 4 (4) |
| Other food allergy, no. (%) | 15 (14) |
| Atopic dermatitis, no. (%) | 42 (39) |
| Allergic rhinitis, no. (%) | 78 (72) |
| Asthma, no. (%) | 44 (40) |
These represent the most commonly reported symptoms and not necessarily an isolated single presenting symptom
Overall Characteristics of Tree Nut Challenges Within the Population
Challenge characteristics are outlined in Table 2. The overall OFC passage rate was 86%. Almond challenge was most commonly performed (n=54), with a 100% passage rate. Among n=67 TN challenges in patients with a prior reaction to TN (who were challenged to another TN to which they were sensitized) passage rate was 76%, while passage rate was 91% in n=65 TN sensitized challenges (e.g. no history of prior TN ingestion). The successful challenge rate (e.g., “pass”) was 71% in those with prior history of anaphylaxis (n=21) at initial diagnosis (Supplemental Table 1). Pass rate was the lowest (56%) among patients with facial swelling at initial presentation, and the highest pass rate was 83% in patients with skin symptoms or vomiting. Most challenges were performed in patients with TN sIgE <2 kUA/L (n=124), and these had an 89% passage rate.
Table 2.
Characteristics of tree nut oral food challenges
| No. | Pass rate | OFC with sIgE <2 kUA/L, no. (%) | OFC with sIgE ≥2 kUA/L, no. (%) | OFC with SPT <3 mm, no. (%) | OFC with SPT ≥3 mm, no. (%) | |
|---|---|---|---|---|---|---|
| All TN challenges | 156 | 86% | 124 (79) | 16 (10) | 101 (65) | 41 (26) |
| TN allergic | 67 | 76% | 60 (90) | 3 (4) | 39 (58) | 25 (37) |
| TN sensitized | 65 | 91% | 47 (72) | 13 (20) | 45 (69) | 16 (25) |
| Avoiding due to other FA | 24 | 100% | 17 (71) | - | 17 (71) | - |
| Almond | 54 | 100% | 42 (78) | 6 (11) | 37 (69) | 11 (20) |
| Cashew | 28 | 79% | 28 (100) | 0 (0) | 18 (64) | 9 (32) |
| Walnut | 27 | 82% | 19 (70) | 3 (11) | 15 (56) | 10 (37) |
| Hazelnut | 18 | 83% | 12 (67) | 4 (22) | 13 (72) | 2 (11) |
| Pecan | 14 | 79% | 10 (71) | 1 (7) | 11 (79) | 3 (21) |
| Pistachio | 13 | 69% | 11 (85) | 2 (15) | 8 (62) | 4 (31) |
| Brazil nut | 2 | 50% | 2 (100) | 0 (0) | 0 (0) | 2 (100) |
TN=tree nut; FA=food allergy; sIgE=specific IgE; SPT=skin prick test.
92% of TN challenges had both sIgE and SPT performed, 9% had SPT performed only, and 9% had sIgE performed only.
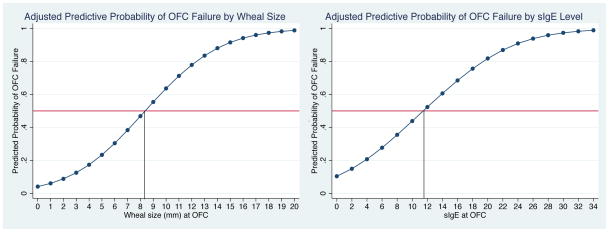
Among 101 challenges in patients with TN SPT wheal size <3 mm, the passage rate was 96%. Among OFCs in n=47 patients with either TN sIgE ≥2 kUA/L (mean 6.41 kUA/L; range 2.23–19.7 kUA/L) and/or TN SPT wheal size ≥3 mm (mean 6.5 mm; range 3–20 mm), the passage rate was 64% (partial data shown in Table 2). Passage rate was 61% among n=41 TN OFCs with wheal size ≥3 mm, with mean SPT of 4.8 mm (range 3–11) among those passing vs. 9 mm (range 3–20) in those failing challenge. Passage rate was 69% among n=16 OFCs with TN sIgE ≥2 kUA/L near or at the time of the OFC, with mean TN sIgE 5.12 kUA/L (range 2.23–9.14 kUA/L) among passed OFC vs. 9.27 kUA/L (range 2.38–19.7 kUA/L) among failed OFC. In n=65 TN OFCs in TN sensitized individuals, 59/65 challenges were successful, including n=10 in patients with sIgE ≥2 kUA/L (median sIgE 4.49 kUA/L; mean 5.24 kUA/L; range 2.23–9.14 kUA/L), n=11 in patients with SPT wheal size ≥3 mm (median wheal size 4 mm; mean 4.8 mm; range 3–11 mm). Figure 1 illustrates 50% NPV for both TN skin test wheal size and sIgE for all-comers within the entire sample (combined TN allergic and TN sensitized), adjusted for age, asthma, eczema, gender, and co-morbid peanut allergy. Of the n=156 TN OFCs, 94 were delayed (60%) with mean time to OFC among those that were delayed of 50 months (range 14–108), compared to 3.6 months among those who were not delayed. The mean number of additional visits with testing until OFC was performed in the delayed group was 2.1 (range 0–7).
Figure 1. 50% Negative Predictive Values for OFC to Tree Nut Among Tree Nut Allergic and Sensitized Individuals.
50% negative predictive values for the combined allergic and sensitized population, adjusted for patient age, eczema, asthma, gender, and peanut allergy. Left panel displays wheal size curve, and right panel tree nut sIgE curve.
Supplemental Table 2 presents data regarding the characteristics of failed challenges. Of the 22 failed OFCs, 14 had sIgE <2 kUA/L, and 4 with SPT wheal size <3 mm. The majority of failed challenges (73%) had recent SPT wheal size ≥3 mm, while only 23% had sIgE ≥2 kUA/L. Most patients failing OFC were TN allergic (73%). Cashew was the most frequently failed challenge (n=6), followed by walnut (n=5) and pistachio (n=4). No one failed almond challenge. Combining data, OFC pass rate was 96% in OFCs with SPT <3 mm, and was 89% if sIgE was <2 kUA/L.
Tree Nut Challenges Among Clinically Reactive vs. Sensitized Individuals
Among n=67 challenges in TN allergic patients, there were 25 OFCs undertaken in individuals with wheal size ≥3 mm, with a pass rate of 56%. There were only 3 OFCs with sIgE ≥2 kUA/L in this subgroup (all presented with anaphylaxis), one which passed (sIgE=3.92 kUA/L). In two of these failed challenges, sIgE was 12.8 kUA/L and 19.7 kUA/L.
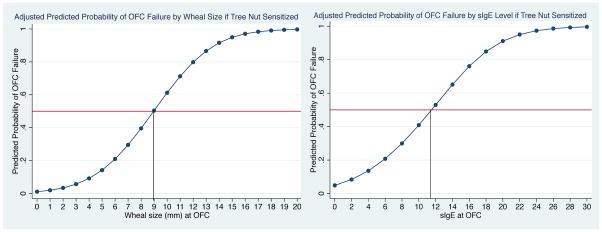
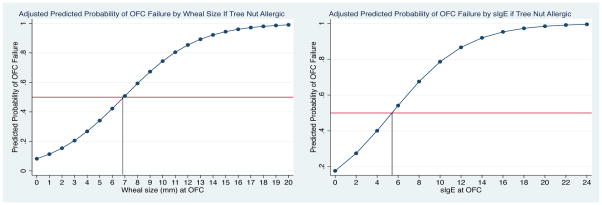
Table 3 outlines TN-sIgE and SPT results obtained in closest proximity to the time of OFC. For all TN challenges, mean sIgE was higher in failed OFC (2.88 kUA/L; median 0.35 kUA/L; range <0.35–19.7 kUA/L) vs. passed OFC (0.89 kUA/L; median 0.35 kUA/L; range <0.35–9.14 kUA/L), (p=0.01) This trend was also seen with SPT wheal size, with a mean of 7.4 mm (median 7.5 mm; range 0–20 mm) in failed OFC vs. 1.1 mm (median 0 mm; range 0–11 mm) in passed OFC (p<0.001). There was a higher mean sIgE (1.53 kUA/L; median 0.35 kUA/L; range 0.35–9.14 kUA/L) among challenges in the TN sensitized subset compared to the TN allergic individuals (0.99 kUA/L; median 0.35 kUA/L; range 0.35–19.7). However, mean SPT wheal size was slightly higher in TN allergic patients (2.4 mm; 95% CI, 1.5 mm to 3.3 mm) vs. TN sensitized patients (2.1 mm; 95% CI, 1.1 mm to 3.1 mm). In the 6 failed challenges in TN sensitized individuals, mean sIgE was 3.44 kUA/L (median 2.38 kUA/L; range 1.57–7.90 kUA/L), and mean SPT wheal size was 10.3 mm (median 10 mm; range 2–20 mm). Comparatively, in 16 failed challenges in TN allergic individuals, the mean TN sIgE was 2.68 kUA/L (median 0.35 kUA/L; range 0.35–19.7 kUA/L), and mean SPT wheal size was 6.1 mm (median 5 mm; range 0–16 mm). The 50% negative predictive values for TN challenge among TN sensitized individuals and TN allergic individuals are displayed in figures 2 and 3, respectively. The 50% NPV for cashew/pistachio and walnut/hazelnut/pecan challenges are displayed supplemental figure 1.
Table 3.
Tree nut-specific IgE and skin prick testing characteristics near time of oral food challenge
| Pass rate | Mean sIgE, all (median) | Mean sIgE, passed (median) | Mean sIgE, failed (median) | Mean wheal size, all (median) | Mean wheal size, passed (median) | Mean wheal size, failed (median) | |
|---|---|---|---|---|---|---|---|
| All TN challenges (n=156) | 86% | 1.14 (0.35) | 0.89 (0.35) | 2.88 (0.35) | 1.9 (0) | 1.1 (0) | 7.4 (7.5) |
| TN allergic (n=67) | 76% | 0.99 (0.35) | 0.51 (0.35) | 2.68 (0.35) | 2.4 (0) | 1.4 (0) | 6.1 (5.0) |
| TN sensitized (n=65) | 91% | 1.53 (0.35) | 1.36 (0.35) | 3.44 (2.38) | 2.1 (0) | 1.2 (0) | 10.3 (10.0) |
| Avoiding due to other FA (n=24) | 100% | 0.35 (0.35) | 0.35 (0.35) | - | 0 (0) | 0 (0) | - |
| Almond (n=54) | 100% | 1.18 (0.35) | 1.18 (0.35) | - | 1.27 (0) | 1.27 (0) | - |
| Cashew (n=28) | 79% | 0.49 (0.35) | 0.40 (0.35) | 0.80 (0.38) | 3.0 (0) | 0.7 (0) | 11.0 (10.5) |
| Walnut (n=27) | 82% | 1.36 (0.37) | 0.74 (0.35) | 4.16 (1.75) | 2.5 (0) | 1.4 (0) | 7.4 (7) |
| Hazelnut (n=18) | 83% | 1.31 (0.54) | 1.22 (0.54) | 1.96 (1.96) | 1.0 (0) | 0.9 (0) | 2.0 (2.0) |
| Pecan (n=14) | 79% | 2.15 (0.35) | 0.40 (0.35) | 10.03 (10.03) | 1.4 (0) | 0.5 (0) | 4.7 (5.0) |
| Pistachio (n=13) | 69% | 1.13 0.35) | 0.64 (0.35) | 2.24 (0.35) | 3.4 (0) | 1.9 (0) | 8 (4.0) |
| Brazil nut (n=2) | 50% | 0.35 (0.35) | 0.35 (0.35) | 0.35 (0.35) | 3.0 (3.0) | 3.0 (3.0) | 3.0 (3.0) |
TN=tree nut; sIgE=specific IgE; sIgE units= kUA/L; wheal size units=millimeters
Figure 2. 50% Negative Predictive Values for OFC to Tree Nut Among Tree Nut Sensitized Individuals.
50% negative predictive values for the tree nut sensitized population, adjusted for patient age, eczema, and peanut allergy. Left panel displays wheal size curve, and right panel tree nut sIgE curve. Reflects relationship with sensitized tree nuts in individuals with no history of tree nut allergy.
Figure 3. 50% Negative Predictive Values for OFC to Tree Nut Among Tree Nut Allergic Individuals.
50% negative predictive values for the tree nut sensitized population, adjusted for patient age, eczema, and peanut allergy. Left panel displays wheal size curve, and right panel tree nut sIgE curve. Reflects relationship with sensitized tree nuts other than the nut to which the patient has a primary allergy.
Tree Nut Challenges Among the Peanut/Tree Nut Co-Allergic Population
In this sample, 56/109 had a positive peanut SPT or sIgE (n=46 peanut allergic, n=10 peanut sensitized). Characteristics of the peanut allergic/sensitized patients are shown in Table 4. The initial peanut mean SPT wheal size was 9.7 mm and initial mean sIgE of 31.4 kUA/L, and 26% presented with symptoms constituting anaphylaxis. Sixty-five percent of the peanut allergic patients were TN sensitized, 20% were TN allergic, and 15% were avoiding TN despite negative TN testing and no exposure. There were n=68 TN OFCs performed in the 46 peanut allergic patients, with a passage rate of 96% (Supplemental table 3). There were only 3 failed challenges, occurring in 3 different patients (to walnut, hazelnut, and pistachio). Of the peanut allergic individuals, only 13% who were TN allergic were challenged, compared to 65% of TN sensitized individuals and 22% of individuals whom had been avoiding TN despite negative testing. Most TN challenges in these peanut allergic or sensitized individuals were performed in persons with negative TN testing at the time of challenge, including 51 patients with sIgE <2 kUA/L and 49 patients with SPT wheal size <3 mm. There were a total of 14 TN OFCs performed in the peanut allergic population with TN sIgE ≥2 kUA/L and/or SPT wheal size ≥3 mm.
Table 4.
Peanut allergic population characteristics challenged to tree nut (n=46)
| Characteristic (n=46) | |
|---|---|
| Male, no. (%) | 24 (52) |
| Mean age at peanut allergy diagnosis, years (median) | 2.5 (2.0) |
| Mean age at initial tree nut oral food challenge, years (median) | 10.2 (10.4) |
| Symptom at initial peanut allergy presentation | |
| Anaphylaxis, no. (%) | 12 (26) |
| Skin symptoms (hives, itching, flushing, rash), no. (%) | 43 (93) |
| Facial swelling, no. (%) | 13 (28) |
| Oropharyngeal symptoms (tongue or throat pruritus/swelling), no. (%) | 6 (13) |
| Respiratory symptoms (cough, wheezing, shortness of breath), no. (%) | 7 (15) |
| Vomiting, no. (%) | 14 (30) |
| Tree nut allergic, no. (%) | 9 (20) |
| Tree nut sensitized (no clinical symptoms), no. (%) | 30 (65) |
| Avoiding tree nut despite negative testing, no. (%) | 7 (15) |
| Atopic dermatitis, no. (%) | 21 (46) |
| Allergic rhinitis, no. (%) | 33 (72) |
| Asthma, no. (%) | 20 (43) |
| Initial mean peanut SPT wheal size, mm (median) | 9.7 (10.0) |
| Most recent mean peanut SPT wheal size, mm (median) | 9.0 (9.0) |
| Initial mean peanut sIgE, kUA/L (median) | 31.4 (3.0) |
| Most recent mean peanut sIgE, kUA/L (median) | 39.3 (8.0) |
Discussion
In one of the largest series of TN OFC to date, in this population we demonstrate a high TN challenge passage rate for both TN allergic patients (challenged to TN to which they are also sensitized) and TN sensitized-only patients, despite elevated TN sensitization levels as noted by the 50% NPV’s calculated for the population. These levels may provide reassurance that there is a larger margin of SPT or sIgE sensitization within which to strongly consider offering OFC, irrespective if the patient has reacted to another TN or are peanut allergic, as these challenges were very well tolerated. Most subjects were TN allergic to at least one nut (50%), though a large subgroup were only TN sensitized without any prior TN exposure (40%), and this sensitized group may have leveraged an overall high pass rate (86%). However, among the TN allergic individuals, OFC pass rates to other TN to which they are sensitized remained relatively high at 76%. It is notable that most challenges took place with recent SPT wheal size <3 mm, and/or sIgE <2 kUA/L, which may suggest that at this particular center, there may have been a bias towards challenging individuals with either absent or minimal sensitization, or a strong parental or provider preference for waiting until this scenario occurred. This is a limitation of the study. Because the study was retrospective, we were not able to assess these motivations. However, such a scenario may be relatively commonplace in clinical practice, given little guidance to influence decision-making regarding optimal timing and sensitization level for OFCs, and a general overall confusion of how to manage TN sensitization.
Fleischer et al. noted a 45% pass rate in 20 TN challenges (median sIgE 0.84 kUA/L).4 Our pass rate was higher, at 76% in 67 TN OFCs performed in TN allergic individuals (median sIgE 0.35 kUA/L, mean sIgE was 0.99 kUA/L), and at least half of our challenges took place with sIgE at <0.35 KU/L, which also may account for the higher pass rate, though our intent is not to directly compare these population. It is of note that in this study the median sIgE was <0.35 KU/L among 16 failed challenges (mean 2.68 kUA/L) demonstrating that non-detectable sIgE levels do not necessarily infer success. SPT wheal size in this subgroup was better associated with failure, though 56% passed OFCs with SPT wheal size ≥3 mm.
Discovery of TN sensitization in individuals who have never ingested any TN, or may be reactive to another TN but have never ingested the particular TN in question has become problematic in clinical practice. Sensitization in this context is difficult to interpret, poorly specific, and can lead to potentially unnecessary food avoidance through conservative management.14,15 We show that 91% percent of n=42 TN sensitized patients undergoing 65 TN OFCs were successful, and OFC in these scenarios may have high utility.
TN sensitization found while screening PN allergic individuals is equally problematic and also may lead to unnecessary TN avoidance. Peters et al recently showed that in an OFC proven peanut allergic population, TN sensitization rate was 61% for cashew, almond, or hazelnut.5 Another study using sIgE levels reported a higher rate of TN sensitization (88%) in peanut allergic patients, but clinical TN allergy was present in only 34% of patients.10 We note 42% of patients had peanut allergy, with 65% of these patients TN sensitized and 20% TN allergic. However, among the PN allergic/TN sensitized group, almost all TN OFCs were successful (96%), exceeding a prior study with a 69% passage rate in a similar clinical population.16 These data demonstrate the success rate of TN OFCs in peanut allergic individuals is potentially much higher than presumed, and may lend to consideration for a more pro-active management of such patients. These aggregate data would also question the utility of the common practice of screening for TN IgE sensitivity among individuals lacking any TN exposure, given most pass challenge. This is in particularly key with almond, where all 54 OFC were successful, 67% of which had peanut allergy. These findings may suggest that a more aggressive introduction approach may be possible with almond, though further study is necessary to validate this.
In exploring the timing of TN OFC, 60% of such OFCs were delayed >12 months. Fleischer et al. reported a 63% NPV with TN-sIgE <2 kUA/L, and recommended offering OFC given the relative likelihood of passing. 4 We show that 89% of 124 challenges with sIgE <2 kUA/L passed, further validating a high likelihood of passing OFC below this threshold, in a larger sample. Twelve months is a reasonable timeframe to perform OFC if there is mutual intent to perform this, and feel this is an appropriate if not conservative time marker to use. We have previously demonstrated that delaying OFC may lead to additional economic costs to families and the healthcare system.11 The high rate of success from these data provide further lack of justification that such delay is indicated or improves outcomes.
There were several limitations in this study. It was retrospective in nature, with all data obtained from electronic medical record review. All challenges were open and not double blinded, though open challenge is the standard for clinical practice but may be associated with subjective failure. Additionally, this study was performed in a single institution at a food allergy referral center, and the study population may include “higher risk” food allergic individuals, and thus be viewed as clustered data that may not generalize to other populations. While we highlight 50% NPV for where these OFCs were passed, these are applicable to our study population and may not necessarily generalize to other populations. Thus, caution should be used in interpreting those data. Most challenges were passed (86%), but the majority were performed with sIgE <2 kUA/L and/or SPT wheal size <3 mm, indicating a possible selection bias in the baseline sample available for analysis for performing OFC in patients that would likely pass OFC and provider and/or parent preference for OFC with minimal or absent sensitization. It is unclear if this occurred, as preferences for what patients were offered OFC (and why) were not explored. As stated earlier, we were not able to determine the motivations of the provider or child’s caregiver with respect to why challenge was not performed before sensitization levels declined to low or absent levels in some cases, or why avoidance was recommended with no evidence of TN sensitization. Furthermore, it is unclear if this pattern would actually be an outlier compared to other academic centers or community practices, given limited data regarding trends in tree nut challenge, including provider preferences for offering OFC. Nevertheless, there were still a number of challenges performed in sensitized patients, including those with a history of both peanut allergy and allergy to other tree nut, both well described risk factors for tree nut allergy.17 Despite these limitations the study provides useful TN data that may be of use to help guide clinical decisions regarding OFC and the need for TN avoidance in TN allergic or TN sensitized individuals, including the peanut allergic co-sensitized population.
In conclusion, patients with TN allergy (being challenged to a TN to which they are sensitized) or TN sensitization in this population frequently passed TN OFCs despite sIgE ≥2 kUA/L and/or SPT wheal size ≥3 mm. We propose that positive TN SPT (wheal size ≥3 mm) may be a better predictor of OFC outcome than sIgE in TN allergic individuals when both are available. Outcomes of TN OFCs in TN sensitized patients are difficult to predict using sIgE and/or SPT, and we would recommend that OFC should be performed despite sensitization to further clarify the clinical relevance of positive test results. Given the high success rate of TN OFCs in peanut allergic individuals, regardless of positive TN testing, we question the value of performing screening TN SPT or sIgE in patients without lifetime TN exposure. More specifically, we show that almond may be able to be introduced into the diet of peanut allergic patients without the need to perform SPT, sIgE, and/or OFC given 100% passed almond challenge in our sample. While this study contributes valuable data regarding TN allergy and TN sensitivity, additional studies are needed to help guide clinical decision-making in this area.
Supplementary Material
Acknowledgments
Funding source: This study was supported in part by a gift from an anonymous foundation, and by National Center for Advancing Translational Sciences Grant 2UL1TR000433. Dr. Greenhawt also received support from NCATS grant #2KL2TR000434. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Couch and Dr. Franxman report no funding sources.
Abbreviations
- sIgE
Food-specific IgE
- OFC
oral food challenge
- NPV
negative predictive value
- QoL
quality of life
- SPT
skin prick test
- ICD-9
International Classification Diseases, Ninth Revision
- CPT
coding, and Current Procedural Terminology
Footnotes
Clinical Trial Registration: not applicable
Conflicts of Interest: Dr. Greenhawt is an expert panel member of the NIAID-sponsored Guidelines for Peanut Allergy Prevention; has served as a consultant for the Canadian Transportation Agency and Aimmune Therapeutics; is a member of physician/medical advisory boards for Aimmune, Nutricia, Kaleo Pharmaceutical, Nestle, and Monsanto; is a member of the scientific advisory council for the National Peanut Board; has received honorarium for lectures from Thermo Fisher, ReachMD, and the Kentucky/Pennsylvania/Aspen/New York allergy societies, the ACAAI, the EAACI, and UCLA/Harbor Medical Center; and is a member of the Joint Taskforce on Allergy Practice Parameters. Dr. Couch and Dr. Franxman report no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 2.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 3.Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol. 2005;116:1087–93. doi: 10.1016/j.jaci.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Peters RL, Allen KJ, Dharmage SC, et al. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: A population-based assessment. J Allergy Clin Immunol. 2015;135:1257–66. e1–2. doi: 10.1016/j.jaci.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Perry TT, Matsui EC, Kay Conover-Walker M, Wood RA. The relationship of allergen-specific IgE levels and oral food challenge outcome. J Allergy Clin Immunol. 2004;114:144–9. doi: 10.1016/j.jaci.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 7.de Leon MP, Drew AC, Glaspole IN, Suphioglu C, O’Hehir RE, Rolland JM. IgE cross-reactivity between the major peanut allergen Ara h 2 and tree nut allergens. Molecular Immunology. 2007;44:463–71. doi: 10.1016/j.molimm.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol. 2001;108:881–90. doi: 10.1067/mai.2001.118515. [DOI] [PubMed] [Google Scholar]
- 9.Ewan PW. Clinical study of peanut and nut allergy in 62 consecutive patients: new features and associations. BMJ. 1996;312:1074–8. doi: 10.1136/bmj.312.7038.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maloney JM, Rudengren M, Ahlstedt S, Bock SA, Sampson HA. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J Allergy Clin Immunol. 2008;122:145–51. doi: 10.1016/j.jaci.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Couch C, Franxman T, Greenhawt M. The economic effect and outcome of delaying oral food challenge. Ann Allergy Asthma Immunol. 2016;116:420–4. doi: 10.1016/j.anai.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134:1016–25. e43. doi: 10.1016/j.jaci.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–7. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 14.Fleischer DM, Bock SA, Spears GC, et al. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011;158:578–83. e1. doi: 10.1016/j.jpeds.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Bird JA, Crain M, Varshney P. Food allergen panel testing often results in misdiagnosis of food allergy. J Pediatr. 2015;166:97–100. doi: 10.1016/j.jpeds.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 16.Ball H, Luyt D, Bravin K, Kirk K. Single nut or total nut avoidance in nut allergic children: outcome of nut challenges to guide exclusion diets. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2011;22:808. doi: 10.1111/j.1399-3038.2011.01191.x. [DOI] [PubMed] [Google Scholar]
- 17.Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.