Abstract
Introduction
Phosphodiesterase type 5 inhibitors (PDE5-Is) are first-line drugs for erectile dysfunction. Non-arteritic anterior ischemic optic neuropathy (NAION) has been linked with PDE5-I use. However, no meta-analysis or conclusive review has explored the association between NAION and PDE5-I use.
Aim
To investigate the association between PDE5-I use and risk of NAION.
Methods
A comprehensive literature search was conducted using online databases in October 2017 to obtain studies researching the association between PDE5-I application and occurrence of NAION. Summarized unadjusted risk ratios (RRs) with 95% CIs were calculated for the strength of this association. This study was conducted in accordance to Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines and registered in PROSPERO under number CRD42017080865.
Main Outcome Measures
The strength of association between PDE5-I use and risk of NAION was assessed through pooled unadjusted RRs and 95% CIs.
Results
5 original articles with 6 clinical observations were included in the meta-analysis. No significant higher risk of NAION was observed after the use of PDE5-Is within a 1-month period (RR = 1.16, 95% CI = 0.98-1.39, P = .09). Subgroup analyses indicated 2 PDE5-Is were significantly related to NAION (tadalafil: RR = 2.14, 95% CI = 1.20–3.84, P = .01; sildenafil: RR = 2.25, 95% CI = 1.29–3.94, P = .004).
Conclusions
Although we found no association between NAION and PDE5-I use, our results should be interpreted cautiously because we included only observational studies and could not control for potential confounders. Because NAION is a rare ocular disease and difficult to diagnose, this association should be confirmed in prospective comparative studies with larger samples and more rigorous designs.
Liu B, Zhu L, Zhong J, et al. The Association Between Phosphodiesterase Type 5 Inhibitor Use and Risk of Non-Arteritic Anterior Ischemic Optic Neuropathy: A Systematic Review and Meta-Analysis. Sex Med 2018;6:185−192.
Key Words: Association, Meta-Analysis, Non-Arteritic Anterior Ischemic Optic Neuropathy, Phosphodiesterase Type 5 Inhibitors, Systematic Review
Introduction
Erectile dysfunction (ED) is a disorder affecting approximately 1% to 10% of men younger than 40 years old. The prevalence rate increases to 20% to 100% in men older than 60 years.1 Older age plays a vital role in the existence of ED, although other lifestyle factors are related to the onset of ED, such as tobacco or alcohol abuse, lack of exercise, obesity, sleeping disorders, and so on.2 The interaction of ED with different systematic diseases such as diabetes also has been researched.3
Phosphodiesterase type 5 inhibitors (PDE5-Is) are first-line drugs for ED and have proved effective and relatively safe. Common mild adverse events include transient headache, dizziness, and blurred vision; however, some rare ocular complications can occur, leading to serious harm to patients’ visual acuity.4 Non-arteritic anterior ischemic optic neuropathy (NAION) has been linked to PDE5-I use. These patients often complain of unilateral, sudden, and painless loss of vision when awakening. Although the detailed pathogenesis is unclear, NAION is considered to be caused by hypoperfusion of ciliary arteries owing to systematic hypotension or some vascular diseases, without evidence of arteritis.5 PDE5-Is block PDE5 to degrade cyclic guanosine monophosphates, which maintain relaxation of vascular smooth muscle. Cyclic guanosine monophosphates are activated and upregulated by the release of nitric oxide, so PDE5-Is could amplify the pharmacologic effect of nitric oxide donors.6 PDE5 mainly exists in the corpus cavernosum, but some PDE5-Is such as sildenafil and vardenafil also act on the PDE6 subtype appearing in ocular blood vessels.7 Although hypoperfusion caused by PDE5-Is might play a role in NAION, ED and NAION supposedly share some risk factors such as smoking, diabetes, and obstructive sleep apnea.8
Since 2005, after extensive reports about NAION and its potential association with PDE5-I use, the Food and Drug Administration (FDA) mandated pharmaceutical companies place warnings on their drug inserts and perform prospective studies to determine whether there is an association between PDE5-I use and NAION. In a case-crossover study sponsored by Pfizer and performed by Campbell et al,9 a doubly increased risk of NAION was found in patients who recently used a PDE5-I compared with those who used a PDE5-I in a more remote period. A similar study sponsored by Eli Lilly was completed in February 2016 but has not been published (NCT01131104)10 and a study sponsored by Bayer is ongoing (NCT00867815).11 In an analysis by Pomeranz12 of data on adverse events from the FDA, reports of ischemic optic neuropathy associated with PDE5-I use were more numerous than the number of cases from the published literature. However, some previous articles also reported no significant association between NAION and PDE5-I use13, 14 and the causality between them could not be elucidated clearly in current publications. Therefore, we conducted a systematic review and meta-analysis to investigate their association through combined statistics to provide some further evidence for the occurrence of NAION and rational use of PDE5-Is.
Methods
This systematic review and meta-analysis was conducted in accordance to Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. The protocol of this review was registered in PROSPERO (registration number CRD42017080865).
Search Strategy
A comprehensive literature search was conducted using the PubMed, Embase, Cochrane Library, CBM, CNKI, and VIP databases and the last search was in October 2017. No data range criteria were applied when searching the original studies. Unpublished data were screened in databases such as OpenGrey and Web of Science. Search terms were phosphodiesterase type 5 inhibitors or PDE5-Is or PDE5A inhibitors or PDE5 inhibitors or PDEIs and non-arteritic anterior ischemic optic neuropathy or NAION. No language restriction was applied and references of related studies were scanned.
Inclusion and Exclusion Criteria
We defined study eligibility according to PICOS (patient population, intervention or exposure, comparator, outcome, and study design; Supplementary Figure 1). Studies focusing on the association between PDE5-I use and risk of NAION were included. Unadjusted relative ratios (RRs) or odds ratios (ORs) in these studies were extracted directly or were calculated. Editorials, reviews, conference proceedings and abstracts, case reports, animal experiments, and repeated publications were excluded. 2 reviewers (B.L. and L.Z.) independently screened related records meeting the inclusion criteria, and disagreement was resolved by a 3rd reviewer (T.D.).
Data Extraction and Quality Assessment
Data from included studies were checked carefully and extracted by 2 independent reviewers. The following information was collected on a prepared standard form: first author, year of publication, country, ethnicity, study period, type and timing of PDE5-I use, case and control sample sizes, and number of patients with NAION. RRs or ORs for the risk of NAION from PDE5-I use also were extracted, if possible.
The level of evidence of each study was accessed by the GRADE approach by 2 reviewers independently.15 The quality of non-randomized controlled trials also was assessed by the Newcastle-Ottawa Scale.16 Literature with an assessment score of at least 7 stars was considered high quality.
Statistics Analysis
Unadjusted RRs or ORs of PDE5-I use for the risk of NAION were extracted or calculated from all studies with their 95% CIs. Pooled unadjusted RRs and 95% CIs were calculated to assess the strength of association between PDE5-I use and NAION. Subgroup analyses were conducted based on study region, study design, and type of PDE5-I. Apparent heterogeneity existed if the P value was less than 0.10 by χ2 test17 and the random-effect model was applied to achieve the pooled RR. Lack of heterogeneity was considered when the P value was higher than .10 and the fixed-effect model was used. Results in this study were regarded as significant only with a 2-sided P value less than .05. Publication bias was evaluated by inverted funnel plot visual inspection.18 All statistical analyses were conducted by RevMan 5.3 (Cochrane Collaboration, Oxford, UK).
Results
Characteristics and Quality Assessment of Included Studies
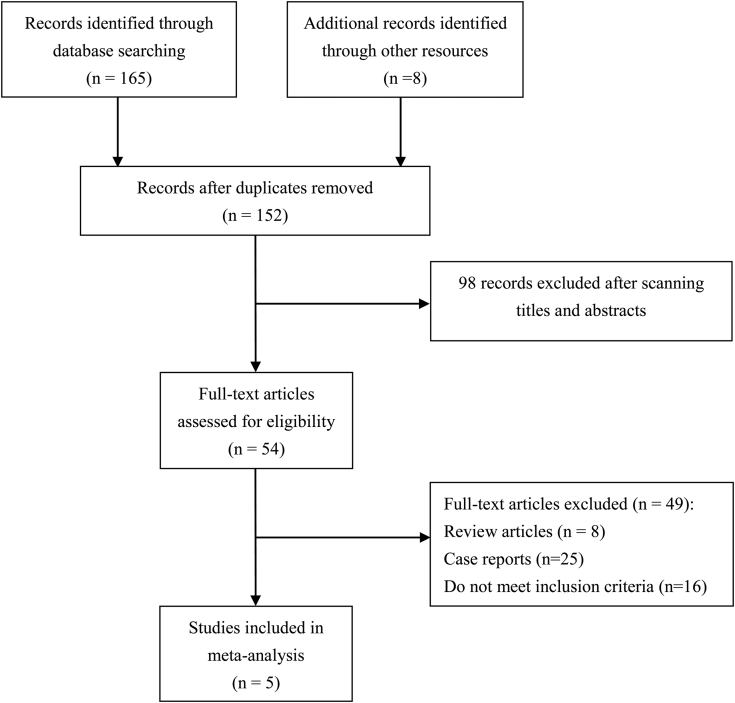
6 clinical trials from 5 studies were included in this study. More than 5 million patients were observed and researched for the association between PDE5-I use and risk of NAION. A flowchart of the screening process of the meta-analysis is presented in Figure 1. Of the 5 studies, 1 was a prospective case-crossover study,19 1 was a retrospective case-crossover study,12 1 was a retrospective case-control study,13 and 2 were retrospective cohort studies.14, 20 The study by Flahavan et al19 was conducted mostly in Caucasians in the United Kingdom, and the others were conducted in mixed populations. The study period of these trials lasted from 1 to 5 years and the PDE5-Is in all studies consisted of tadalafil, sildenafil, and vardenafil. Other basic information is listed in Table 1. For level-of-evidence assessment, the study by Flahavan el al19 was rated level 2b and the others were rated level 3.9, 13, 14, 20 All 5 studies scored at least 7 stars and were regarded as high quality.
Figure 1.
Flow diagram of meta-analysis.
Table 1.
Baseline characteristics of included studies
| Study | Country | Main ethnicity | Study period | Study design | LOE | Total participants, N | Mean or median age (y) | PDE5-I period of use | PDE5-I users/controls (within 1 mo) | PDE5-I users/controls (within 1 y) | Incidence of NAION in PDE5-I users (%) | Quality score∗ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flahavan et al, 2017 | UK | Caucasian | 2010–2015 | Prospective case-crossover study | 2b | 26 | 61.3 | Within 1 mo and 1 y of index date | 24/24 | 26/26 | NA | ★★★★★ |
| Nathoo et al, 2015 | Canada | Mixed | 2000–2011 | Retrospective case-control study | 3 | 1,238,399 | 69.8 | Within 1 mo and 1 y of index date | 67/85,373 | 75/91,559 | NA | ★★★★★★★★ |
| Campbell et al, 2015 | mixed | Mixed | 2008–2012 | Retrospective case-crossover study | 3 | 43 | 61.4 | Within 1 mo and 2 mo of index date | 43/43 | NA | NA | ★★★★★★★ |
| French et al, 2008 | USA | Mixed | 2004–2005 | Retrospective cohort study | 3 | 3,686,212 | 67 | Within 1 mo of index date | 8,344/3,677,868 | NA | 15/8,344 (0.18%) | ★★★★★★★ |
| 3,762,037 | 68 | 84,169/3,677,868 | 129/84,169 (0.15%) | |||||||||
| Margo and French, 2007 | USA | Mixed | 2004–2005 | Retrospective cohort study | 3 | 4,157,357 | 64 | Within 1 mo of index date | 479,489/3,677,868 | NA | 442/479,489 (0.09%) | ★★★★★★★ |
LOE = level of evidence; NA = not applicable; NAION = non-arteritic anterior ischemic optic neuropathy; PDE5-I = phosphodiesterase type 5 inhibitor.
Points of Newcastle-Ottawa Scale score, where 1 star equals 1 point.
Association Between PDE5-I Use and Risk of NAION
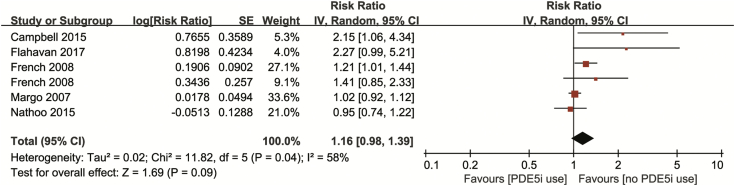
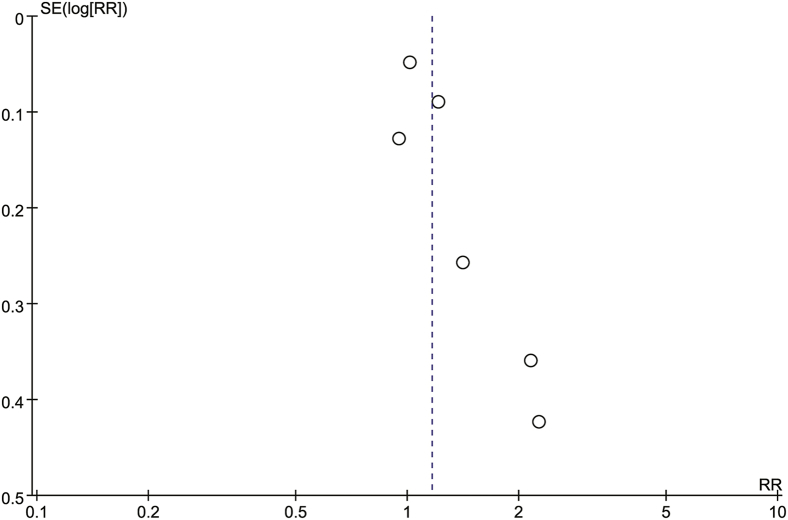
All 6 trials from 5 studies included RRs or ORs with 95% CIs of the risk of NAION after PDE5-I use during a 1-month period. Our combined result indicated no significant increase of NAION (RR = 1.16, 95% CI = 0.98–1.39, P = .09), with obvious heterogeneity (I2 = 58%, P = .04; Figure 2). No obvious publication biases were detected among the included 5 studies through the inverted funnel plot visual inspection (Figure 3).
Figure 2.
Forest plot for risk of non-arteritic anterior ischemic optic neuropathy after PDE5-I use within 1-month period. PDE5-I = phosphodiesterase type 5 inhibitor; SE = standard error.
Figure 3.
Funnel plot of pooled result of risk of non-arteritic anterior ischemic optic neuropathy after phosphodiesterase type 5 inhibitor use within 1-month period. RR = risk ratio; SE = standard error.
2 studies also researched the occurrence of NAION after PDE5-I use within 1 year. We found no significant increase of NAION within 1 year after using PDE5-Is (RR = 1.77, 95% CI = 0.52–6.04, P = .36). Heterogeneity appeared to be significant (I2 = 89%, P = .003; Figure 4).
Figure 4.
Forest plot for risk of non-arteritic anterior ischemic optic neuropathy after PDE5-I use within 1-year period. PDE5-I = phosphodiesterase type 5 inhibitor.
Subgroup Analyses
Results of subgroup analyses are listed in Table 2. For region of study, 2 were conducted in the United States and the results showed no significant differences (RR = 1.70, 95% CI = 0.98–1.16, P = .12); 1 was conducted in Canada, 1 was conducted in the United Kingdom, and 1 was conducted in different countries (Table 2). In 2 case-crossover studies, the pooled RR showed a significant increased risk of NAION from PDE5-Is (RR = 2.20, 95% CI = 1.29–3.76, P = .004) and no heterogeneity existed (I2 = 0%, P = .92). Result of 3 cohort studies showed no significant association (RR = 1.07, 95% CI = 0.98–1.16, P = .12). Only 1 study was designed as a case-control study, with no significant association between PDE5-I application and NAION (RR = 0.95, 95% CI = 0.74–1.22, P = .69). 2 studies included RRs or ORs of the risk of NAION from different PDE5-Is, including tadalafil, sildenafil, and vardenafil (the study by Nathoo et al13 was not included because it was underpowered). After combining the related statistics, the RRs for the significant increased risk of NAION were 2.14 for tadalafil (95% CI = 1.20–3.84, P = .01) and 2.25 for sildenafil (95% CI = 1.29–3.94, P = .004). No significant influence on the occurrence of NAION was detected for vardenafil (RR = 0.71, 95% CI = 0.10–5.02, P = .73).
Table 2.
Subgroup analyses of association between risk of non-arteritic anterior ischemic optic neuropathy and PDE5-I use (within 1-month period)
| Subgroup | Studies, n | Heterogeneity |
RR (95%CI) | |
|---|---|---|---|---|
| I2 | P value | |||
| Region of study | ||||
| USA | 2 | 50% | .13 | 1.07 (0.98–1.16) |
| UK | 1 | NA | 2.27 (0.99–5.21) | |
| Mixed countries∗ | 1 | NA | 2.15 (1.06–4.34)† | |
| Study design | ||||
| Case-crossover study | 2 | 0% | .92 | 2.20 (1.29–3.76)† |
| Cohort study | 2 | 50% | .13 | 1.07 (0.98–1.16) |
| Type of PDE5-I | ||||
| Tadalafil | 2 | 0% | .54 | 2.14 (1.20–3.84)† |
| Sildenafil | 2 | 0% | .63 | 2.25 (1.29–3.94)† |
| Vardenafil | 1 | NA | 0.71 (0.10–5.02) | |
NA = not applicable; PDE5-I = phosphodiesterase type 5 inhibitor; RR = relative ratio.
United States, United Kingdom, France, Germany, Italy, and Spain.
P < .05.
Discussion
The association between PDE5-I use and NAION was uncertain according to previous studies. In our analysis, no significant higher risk of NAION was observed after the use of PDE5-Is within 1 month or 1 year. Subgroup analysis indicated tadalafil and sildenafil might increase the risk of NAION, whereas vardenafil did not; however, the validity of this conclusion was limited to the inadequate number of original studies.
PDE5-Is increase the concentration of cyclic guanosine monophosphates in cavernosal tissue and penile arteries by blocking PDE5, thus enhancing the role of nitric oxide.21 Probably related to systematic hypotension caused by vasodilation, the potential biological mechanisms of PDE5-Is on NAION are worth discussion. PDE6, also combined with PDE5-Is, exists in retinal blood vessels but not in ciliary arteries. The latter plays a role in NAION and is mainly responsible for transient changes in color perception, a common adverse event of PDE5-I use.6 The local autoregulation of the optic nerve head also is disturbed by PDE5-Is; however, none of these explanations have been confirmed by experimental researches or clinical trials.22 Furthermore, the monitoring of blood flow from short posterior ciliary arteries adjacent to the optic disc head showed no changes with the use of PDE5-Is in patients.23
The study by Nathoo et al13 supported no apparent association between PDE5-I use and NAION in a case-control clinical observation. In a large cohort study conducted by Margo and French20 composed of more than 4 million male participants, only a marginal significance was reported. In addition, in a review including more than 100 clinical trials of sildenafil in patients with ED from different countries, no NAION cases were reported.24 In contrast, 2 case-crossover studies performed by Flahavan et al19 and Campbell et al,9 respectively, indicated a significant increased risk of NAION after patients took PDE5-Is. A case-crossover study is appropriate for some acute and rare diseases with transient exposure to the risk factor. All included cases also served as controls during the period without the use of PDE5-Is, thus avoiding some biases in traditional case-control studies. Nevertheless, some shortcomings were obvious during the design and conduction process. Because the prevalence of NAION has been evaluated at 10.3 cases per 100,000 in the US population older than 50 years,25 it is difficult to collect sufficient samples with such a low incidence and the study period often lasted for several years, during which treatment strategies varied greatly. An exposure window of PDE5-I also needed to be accessed for the effect time and the aftereffect should not be included. In consequence, investigators reported the relative risk within a specific period rather than a cumulative effect. The study by Campbell et al also omitted the use of PDE5-Is on the same day as the appearance of NAION and the interaction between them was confusing, bringing about more inaccuracies for the present small sample.
Apart from problems in study design, NAION presented some other difficulties for researchers. Different confounding factors contribute to NAION and ED, such as smoking, older age, diabetes, hypertension, and hyperlipidemia.19 The exact diagnosis of NAION also is a challenge for ophthalmologists. Although all studies confirmed NAION according to the International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) nomenclature, NAION could not be distinguished from other ischemic optic neuropathies and no code for non-arteritic diseases exists. 2 studies made the diagnosis of NAION based on clinical evidence of ischemic optic neuropathy with the exclusion of temporal arteritis, giant cell arteritis, or polymyalgia rheumatica and divided their patients into “NAION” and “possible NAION” groups.19, 20 Inaccurate diagnosis might lead to an overestimate or underestimate of sample size, thus influencing the calculated RR. Because of the inadequate number of NAION cases, some studies used databases as the source of samples, in which the information recorded might not have met the inclusion criteria strictly and some recalls were incorrect during a long-term period.
In subgroup analysis according to type of PDE5-I, we found that tadalafil and sildenafil were significantly associated with NAION, whereas vardenafil was not. This result should be considered cautiously, because only 1 or 2 studies were included in this subgroup analysis. The number of studies and sample sizes were insufficient to reach a valid conclusion. Based on our primary outcome showing no obvious influence of PDE5-I use on the occurrence of NAION, this subgroup analysis was only exploratory and needs to be confirmed by more rigorous comparative studies.
Our meta-analysis had several limitations. (i) Only 5 published articles with 6 trials were included in our study and NAION samples were not large enough to achieve a persuasive conclusion. No randomized controlled trials were included to confirm the causal relation. A retrospective cohort design also can show the “depletion of susceptibilities” phenomenon and inconsistent data collection. Although the use of databases decreases the selection bias from non-response, some important information could not be obtained, such as diagnostic code, confounders, exposure information, complementary therapy, and so on. Apart from this, all studies focused on 3 PDE5-Is, namely, tadalafil, sildenafil, and vardenafil, which were used most widely. The present results should be applied cautiously to other PDE5-Is. (ii) The diagnosis of NAION remained difficult, because there is no ICD-9 code specific for NAION. Clinicians must exclude those patients with evidence of arteritis, and some patients were regarded as “possible NAION” cases because of insufficient findings. This might cause inaccuracy when including case samples. (iii) Most RRs or ORs from original studies were unadjusted and it was impossible for us to acquire the adjusted pooled RRs. Because NAION had many confounding factors and shared some similar risks with ED, it was difficult for us to determine a strong relation between PDE5-I use and NAION. Furthermore, limited subgroup analyses were conducted because occurrence of NAION was investigated only after 1-month or 1-year PDE5-I use in the included studies. 4 studies were carried out in mixed populations and no information of ethnicity or race could be collected. We could not perform subgroup analysis based on race or ethnicity.
Conclusion
We concluded from this meta-analysis that no valid causal association exists between PDE5-I use and the occurrence of NAION. Although tadalafil and sildenafil were found to significantly increase the risk of NAION and vardenafil did not, this result should be regarded as exploratory because few original articles focused on this point. In light of these limitations, our results should be interpreted cautiously. Because NAION is a rare and unique ocular disease with many confounding risk factors, this association should be verified in more prospective comparative studies such as those sponsored by Eli Lilly and Bayer. Clinicians should prescribe PDE5-Is carefully after weighing their benefits against probable severe adverse events.
Statement of authorship
Category 1
-
(a)Conception and Design
- Bing Liu; Tuo Deng
-
(b)Acquisition of Data
- Bing Liu; Linxin Zhu; Tuo Deng
-
(c)Analysis and Interpretation of Data
- Bing Liu; Linxin Zhu; Tuo Deng
Category 2
-
(a)Drafting the Article
- Bing Liu; Tuo Deng
-
(b)Revising It for Intellectual Content
- Bing Liu; Linxin Zhu; Jingxiang Zhong; Guohua Zeng; Tuo Deng
Category 3
-
(a)Final Approval of the Completed Article
- Bing Liu; Linxin Zhu; Jingxiang Zhong; Guohua Zeng; Tuo Deng
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Funding: Natural Science Foundation of Guangdong Province (grant 2017A030310547) and China Postdoctoral Science Foundation (grants 2017M612636 and 2017M622912).
Supplementary data related to this article can be found at https://doi.org/10.1016/j.esxm.2018.03.001.
Supplementary data
Supplementary Figure 1.
PICOS (patient population, intervention or exposure, comparator, outcome, and study design) worksheet for this study.
References
- 1.Shamloul R., Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–165. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 2.Yafi F.A., Jenkins L., Albersen M. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLay K.J., Haney N., Hellstrom W.J. Modifying risk factors in the management of erectile dysfunction: a review. World J Mens Health. 2016;34:89–100. doi: 10.5534/wjmh.2016.34.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yafi F.A., Sharlip I.D., Becher E.F. Update on the safety of phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction. Sex Med Rev. 2018;6:242–252. doi: 10.1016/j.sxmr.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Kerr N.M., Chew S.S., Danesh-Meyer H.V. Non-arteritic anterior ischaemic optic neuropathy: a review and update. J Clin Neurosci. 2009;16:994–1000. doi: 10.1016/j.jocn.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Glossmann H., Petrischor G., Bartsch G. Molecular mechanisms of the effects of the sildenafil (VIAGRA) Exp Gerontol. 1999;34:305–318. doi: 10.1016/s0531-5565(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 7.Pomeranz H.D. Erectile dysfunction agents and nonarteritic anterior ischemic optic neuropathy. Neurol Clin. 2017;35:17–27. doi: 10.1016/j.ncl.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y., Zhou L.M., Lou H. The association between obstructive sleep apnea and nonarteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. Curr Eye Res. 2016;41:987–992. [Google Scholar]
- 9.Campbell U.B., Walker A.M., Gaffney M. Acute nonarteritic anterior ischemic optic neuropathy and exposure to phosphodiesterase type 5 inhibitors. J Sex Med. 2015;12:139–151. doi: 10.1111/jsm.12726. [DOI] [PubMed] [Google Scholar]
- 10.A prospective case-crossover study to evaluate the possible association between the use of PDE5 inhibitors and the risk of acute nonarteritic anterior ischemic optic neuropathy (NAION) http://ichgcp.net/clinical-trials-registry/NCT01131104 Available at: Accessed December 20, 2017.
- 11.Prospective case crossover study to assess whether PDE5 inhibitor exposure in men with erectile dysfunction increases the risk for the development of non-arteritic anterior ischemic optic neuropathy (NAION) http://ichgcp.net/clinical-trials-registry/NCT00867815 Available at: Accessed December 20, 2017.
- 12.Pomeranz H.D. Cases of ischemic optic neuropathy associated with phosphodiesterase-5 inhibitor use reported to the Food and Drug Administration Adverse Event Reporting System. J Neuroophthalmol. 2016;36:221–222. doi: 10.1097/WNO.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 13.Nathoo N.A., Etminan M., Mikelberg F.S. Association between phosphodiesterase-5 inhibitors and nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2015;35:12–15. doi: 10.1097/WNO.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 14.French D.D., Margo C.E. Post-marketing surveillance of ischaemic optic neuropathy in male veterans co-prescribed phosphodiesterase-5 inhibitors with organic nitrates or alpha-blockers. Drug Saf. 2008;31:241–247. doi: 10.2165/00002018-200831030-00005. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M., Davey Smith G., Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flahavan E.M., Li H., Gupte-Singh K. Prospective case-crossover study investigating the possible association between nonarteritic anterior ischemic optic neuropathy and phosphodiesterase type 5 inhibitor exposure. Urology. 2017;105:76–84. doi: 10.1016/j.urology.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 20.Margo C.E., French D.D. Ischemic optic neuropathy in male veterans prescribed phosphodiesterase-5 inhibitors. Am J Ophthalmol. 2007;143:538–539. doi: 10.1016/j.ajo.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res. 2004;16:S11–S14. doi: 10.1038/sj.ijir.3901208. [DOI] [PubMed] [Google Scholar]
- 22.Grunwald J.E., Siu K.K., Jacob S.S. Effect of sildenafil citrate (Viagra) on the ocular circulation. Am J Ophthalmol. 2001;131:751–755. doi: 10.1016/s0002-9394(00)00944-2. [DOI] [PubMed] [Google Scholar]
- 23.Harris A., Kagemann L., Ehrlich R. The effect of sildenafil on ocular blood flow. Br J Ophthalmol. 2008;92:469–473. doi: 10.1136/bjo.2007.131789. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics National Health and Nutritional Examination Survey (NHANES) http://www.cdc.gov/ nchs/nhanes.htm Available at:
- 25.Hattenhauer M.G., Leavitt J.A., Hodge D.O. Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997;123:103–107. doi: 10.1016/s0002-9394(14)70999-7. [DOI] [PubMed] [Google Scholar]