Abstract
Purpose
We investigated serum cytokine and T-cell responses directed against tumour-associated antigens (TAAs) in association with survival of patients with glioblastoma multiforme (GBM).
Patients and Methods
Peripheral blood from 205 treatment-naïve patients with glioma (GBM = 145; non-GBM = 60) was obtained on the day of surgery to measure (i) circulating T-cells reacting to viral antigens and TAAs, in the presence or absence of cytokine conditioning with IL-2/IL-15/IL-21 or IL-2/IL-7, and (ii) serum cytokine levels (IL-4, IL-5, IL-6, TNF-α, IFN-γ and IL-17A). Patients were followed-up for at least 1000 days post-surgery. Survivin protein and gene expression in resected GBM tumour tissue were confirmed by immunohistochemistry and real-time polymerase chain reaction, respectively. Antigen-specific T-cell responses were gauged by ICS (intracellular cytokine production). Associations between patient survival and immunological reactivity patterns were analysed using univariate and multivariate statistics.
Results
Approximately 2% of patients with GBM and 18% of patients with non-GBM glioma, were alive beyond 1000 days of surgery. Univariate analysis indicated that the combination of three cytokines (IL-4/IL-5/IL-6, p = .0022; IFN-γ/TNF-α/IL-17A, p = .0083) but not a ‘partial’ combination of these cytokines, the IFN-γ immune response to EBV-EBNA-1 (p < .0001) as well as T-cell responses to the survivin97–111 peptide (p = .0152) correlated with longer survival among patients with GBM. Multivariate analysis identified survivin97–111-directed IFN-γ production with IL-2/IL-15/IL-21 conditioning (p = .024), and the combined presence of serum IFN-γ/TNF-α/IL-17a (p = .003) as independent predictors of survival.
Conclusion
Serum cytokine patterns and lymphocyte reactivity to survivin97–111, particularly with IL-2, IL-15 and IL-21 conditioning may be instrumental in predicting survival among patients with GBM. This has implications for clinical follow-up of patients with GBM and the targeted development of immunotherapy for patients with CNS tumours.
Keywords: Glioblastoma multiforme, Cytokine networks, Survivin, Immune response, Survival benefit, Immunotherapy
Highlights
-
•
Cytokine combinations of IL-4/5/6 or IFN-γ/TNF-α/IL-17A may predict survival in patients with GBM.
-
•
IL-2/15/21-amplified survivin peptide-specific T-cell responses may independently predict survival among patients with GBM.
-
•
Large-scale clinical studies to validate these readouts as biomarkers of survival in GBM are warranted.
1. Introduction
The World Health Organization (WHO) estimated that circa 2% of all human cancers occur in the central nervous system (CNS) [1]. The glioma WHO classification system includes (i) diffuse or anaplastic astrocytoma (isocitrate dehydrogenase (IDH)-wildtype/-mutant/not otherwise specified (NOS)); (ii) oligodendroglioma or anaplastic oligodendroglioma (IDH-mutant and 1p/19q-co-deletion/NOS); (iii) oligoastrocytoma or anaplastic oligoastrocytoma (NOS) and (iv) glioblastoma multiforme (GBM, IDH-wildtype/-mutant/NOS). GBM is the most common and aggressive clinical manifestation of glioma, with a 5-year survival rate of <3% compared to other primary gliomas, which have a 5-year survival rate of at least 50% [1, 2]. Patients diagnosed with primary GBM (IDH-wildtype) represent approximately 90% of cases, while those with secondary GBM (IDH-mutant) represent the remaining 10% [2]. The clinical outcome in patients with GBM remains poor despite advanced surgery, first-line temozolomide therapy and radiotherapy [3, 4], sometimes administered with adjunctive anti-vascular endothelial growth factor (VEGF) antibody (bevacizumab) immunotherapy [5].
Sporadic CNS inflammation has been attributed to GBM oncogenesis, with the involvement of pro-inflammatory and pleiotropic cytokines, e.g. interferon gamma (IFN-γ), tumour necrosis factor alpha (TNF-α), interleukin (IL) 6, IL-8, and IL-17A [[6], [7], [8]]. Non-specific inflammation may facilitate mutations in genes encoding proteins involved in the receptor tyrosine kinase pathway, including epidermal growth factor receptor (EGFR), as well as enzymes required for DNA repair and genetic stability [7]. The most frequent EGFR mutation associated with GBM is variant III of the receptor (EGFRvIII) [9, 10].
A key factor that contributes to the reduced survival of patients with GBM is the local immunosuppressive tumour microenvironment, including regulatory T-cell (Treg) induction and programmed cell death 1 (PD-1) expression [[11], [12], [13]]. Productive immune responses directed against tumour-associated antigens (TAA) i.e. EGFRvIII, survivin (encoded by baculoviral inhibitor of apoptosis repeat-containing 5, BIRC5 gene) or other antigenic targets are likely to be subdued. Production of immune-tolerising cytokines such as transforming growth factor beta (TGF-β), IL-10, IL-4 and IL-13, as well as the expression of the IL-13 receptor, which itself is a target for GBM immunotherapy [14] play a critical role in the balance of protective and pathological immune responses [[15], [16], [17]]. Temozolomide itself contributes to intratumoural immune suppression, affecting tumour-infiltrating lymphocyte (TIL) numbers as well as anti-tumour immune responses [18]. Adjunct immunotherapies e.g. tumour vaccination of patients with GBM [19] or the application of T cells expressing chimeric antigen receptors (CARs) targeting EGFRvIII (clinical trials identifier: NCT02209376), IL-13 Rα2 [14], or Her2 (clinical trials identifier: NCT02442297) are currenty tested in patients for safety and to improve treatment outcomes.
We studied immune responses in peripheral blood of patients using the determination of cytokines (by ELISA) and immune reactivity to specific target antigens defined by cytokine production to gain a better understanding of the global immune reactivity pattern in association with survival in patients with GBM.
2. Material and Methods
2.1. Patient Characteristics
The study was approved by the Regional Ethics Review Board (Regionala etikprövningsnämnden) at Karolinska Institutet, Stockholm (ethical permit number: 2013/576-31). 205 patients with glioma were selected to participate in the study, following written informed consent. The largest group comprised patients with GBM (WHO grade IV CNS tumour, n = 145), while patients with non-GBM glioma (n = 60) comprised individuals diagnosed with astrocytoma, oligodendroglioma/oligoastrocytoma or anaplastic oligoastrocytoma (WHO grades II-III CNS tumours) [2]. Venous blood for laboratory studies was drawn on the day of surgery and prior to initiation of cancer therapy. A description of the patient cohort is provided in Table 1.
Table 1.
Summary of the clinical characteristics of patients with glioma included in this study. GBM: Glioblastoma multiforme.
| Patient characteristics | Glioma |
||||
|---|---|---|---|---|---|
| Histology |
Grade |
||||
| GBM | Non-GBM | IV | III | II | |
| Sample size(N) | 145 | 60 | 145 | 18 | 42 |
| Age median(years) | 63 | 40 | 63 | 38 | 42 |
| Age range(years) | 16–80 | 20–75 | 16–80 | 22–62 | 20–75 |
| Sex(male/female) | 99/46 | 38/22 | 99/46 | 10/8 | 28/14 |
2.2. Whole Blood Assay (WBA)
Venous blood from the patients with glioma was first diluted at a ratio of 1:1.5 with RPMI 1640 Glutamax medium (ThermoFisher Scientific, Carlsbad, CA) and supplemented with antibiotics (penicillin, 100 IU/ml and streptomycin, 100 μg/ml) (ThermoFisher Scientific, Carlsbad, CA). The diluted blood was then conditioned in following manner: i) without cytokines (RPMI only); ii) human IL-7 (10 ng/ml) and IL-2 (500 IU/ml) or iii) human IL-2 (1000 IU/ml), IL-15 (10 ng/ml) and IL-21 (10 ng/ml) (Prospec, Ness-Ziona, Israel) and added to 96-well microtiter plates containing a panel of tumour-associated antigens (TAA) and viral antigens (Supplementary Table S1). The survivin97–111 peptide (TLGEFLKLDRERAKN) was tested separately since it induced superior immune reactivity in circulating lymphocytes and TIL in an initial screening test. The plates were incubated at 37 °C with 5% CO2 for seven days. Incubation with medium alone was used as negative control while 5 μg/ml phytohaemagglutinin (PHA, Sigma-Aldrich, St. Louis, MO), 30 ng/ml OKT3 (anti-human CD3 monoclonal antibody, Biolegend, CA) and 10 ng/ml SEA+SEB (Staphylococcal Enterotoxin A and B, Sigma-Aldrich, St. Louis, MO) were used separately as positive controls.
2.3. Blood Serum Preparation and Cytokine ELISA
For plasma preparation, a fraction of whole blood was layered onto Ficoll-Paque Plus solution (GE Healthcare, Uppsala, Sweden) and centrifuged at 1260 ×g for 10 min. The resulting layer of serum was removed and stored at −80 °C. Cytokines (IFN-γ, TNF-α, IL-17A, IL-4, IL-5 and IL-6) as well as WBA supernatants (IFN-γ production by circulating lymphocytes) were quantified with commercially available enzyme-linked immunosorbent assay (ELISA) kits (MABTECH, Stockholm, Sweden) according to the manufacturer's instructions.
2.4. Immunohistochemistry
Immunostaining for survivin was performed on 4 μm sections of formalin-fixed paraffin-embedded tissue using the Leica Bond-Max automated immunostaining system (Leica Biosystems AB, Kista, Sweden). For antigen retrieval, samples were incubated for 20 min at 100 °C with Bond Epitope Retrieval Solution 1 (Leica Biosystems AB, Kista, Sweden). Slides were stained for 30 min at room temperature with the survivin polyclonal antibody RB-9245 (Thermo scientific, Carlsbad, CA), diluted at 1:200. The percentage of positive cells was evaluated using a semi-quantitative score: 1+ <10%, 2+ = 10–20%, 3+ = 20–50% and 4+ >50% (the high-expression group refers to scores 4 and 3, while the ‘low-expression’ group includes scores 2 and 1).
2.5. Real-Time Polymerase Chain Reaction (RT-PCR)
Qualitative BIRC5 PCR: Total RNA was extracted from flash-frozen tumour specimens obtained during surgery. The tumour tissue was lysed using Qiazol tissue lysis reagent (Qiagen inc. Hilden, Germany) and total RNA was obtained by ethanol precipitation according to supplier's instructions. 5 μg total RNA was subjected to reverse transcription using an Oligo DT protocol of a RevertAid first strand cDNA synthesis kit (Thermo Fisher Scientific inc. Waltham, MA, USA) according to the manufacturer's instructions. 10 μl cDNA from each cDNA reaction was used to amplify the BIRC5 variant specific product in a 20 μl PCR reaction using BIRC5 commercially available transcript specific primers published previously [20] and according to the manufacturer's instructions (ThermoFisher Scientific, Carlsbad, CA). β-actin was used as positive control for housekeeping gene transcription. The PCR reaction was initiated at 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s. Difference in cycle threshold (delta cycle threshold, ΔCt) for each sample refers to the difference between Ct values of BIRC5 and β-actin. Cut-off for values of Ct and ΔCt is lower than 35 and 10 respectively.
2.6. Statistical Methods, Survival Curves, Patient Stratification
The Kaplan-Meier (K-M) survival analysis was used in order to estimate the number of patients who survived over the follow-up period of 1200 days. Univariate analysis was performed by comparing single parameters (clinical and immunological parameters as well as antigen-specific immune responses) with the overall survival of patients with GBM. Cox Proportional hazards model (forward and backward stepwise analysis) for multivariate analysis was applied to account for individual parameters/factors that could predict improved survival of patients when analysed in combination with all the parameters considered in the study. For the relationship between PBMC IFN-γ production and patient survival, cut-offs for K-M analysis were based on ‘detectable’ or ‘non-detectable’ levels (assay detection limit provided by the manufacturer) or the median concentration values of cytokines in samples which could generate the greatest hazard ratios between two groups.
3. Results
3.1. Clinical Characteristics and Survival Pattern of Patients With Malignant Glioma
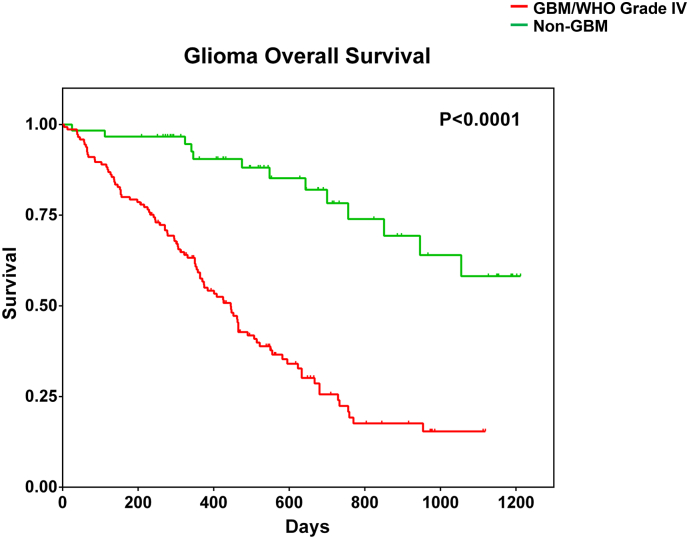
Patients with glioma (n = 205) enrolled in this study were categorised into two distinct groups: (i) patients with GBM (n = 145) and (ii) patients with non-GBM malignant glioma (n = 60; astrocytoma, oligodendroglioma or oligoastrocytoma with different subtypes). Demographic information and clinical characteristics of the patient cohort is provided in Table 1. In agreement with previous reports, patients with GBM displayed a significantly poorer survival profile compared to patients with non-GBM gliomas (18% vs 2%, p < .0001) (Fig. 1).
Fig. 1.
Overall survival pattern of patients with malignant glioma in the study cohort. Patients with different diagnoses of malignant glioma were clinically followed up for at least 1200 days (40 months) post-surgery. Kaplan-Meier survival analysis was performed after segregating patients into two groups: patients with GBM/WHO CNS tumour grade IV malignant glioma or patients with non-GBM malignant glioma (WHO CNS tumour grades 1-III).
3.2. Detectability of Serum Cytokine Levels in Patients With Malignant Glioma
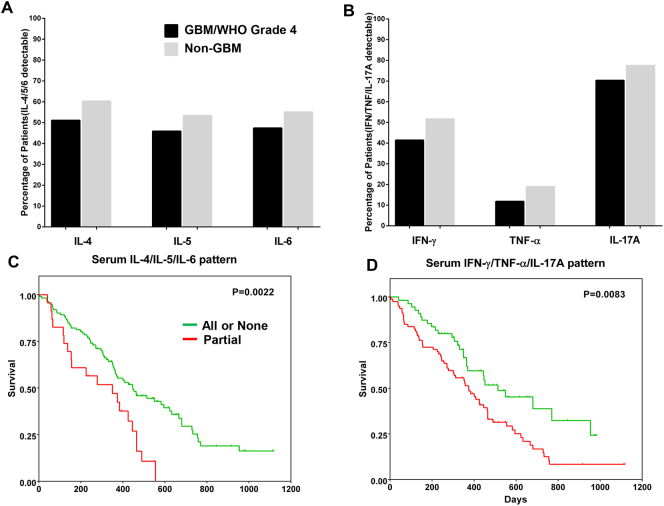
Cytokines were separated into two functional groups: (i) Th2/anti-inflammatory, comprising IL-4, IL-5 and IL-6 and (ii) Th1/inflammatory, consisting of IFN-γ, TNF-α and IL-17A. IL-6 was included in the first group due to its pleiotropic properties [21]. Between 45% and 50% of patients with GBM (WHO grade IV CNS tumour) exhibited detectable levels of IL-4, IL-5 and IL-6 in serum, while 55–60% of patients with non-GBM malignant glioma had detectable levels of each cytokine tested (Fig. 2A). Compared to patients with non-GBM malignant gliomas, a smaller fraction of patients with GBM had detectable levels of serum IFN-γ (41% vs 52%), TNF-α (12% vs 19%) and IL-17A (70% vs 78%) (Fig. 2B). We also observed that the median values of actual cytokine concentrations in the serum samples did not differ between the two patient groups (Supplementary Fig. S1).
Fig. 2.
Serum cytokine levels and their combinatorial effect on the survival pattern of patients with GBM based on univariate analysis. A sandwich ELISA was used for measuring levels of cytokines designated as anti-inflammatory or pleiotropic (IL-4, IL-5 and IL-6) (A) as well as cytokines with pro-inflammatory or Th1 attributes (IFN-γ, TNF-α and IL-17A) (B) in the sera of patients with glioma (GBM and non-GBM) and expressed in pg/ml. Shown are fractions (in percentages) of proportion of individuals in the respective patient groups (GBM vs non-GBM) who had detectable/non-detectable amounts of cytokine in serum compared to the entire sub-cohort (GBM = 145 patients; non-GBM patients = 60), based on the detection limit of the commercial ELISA kits. The GBM and non-GBM group of patients exhibited a similar pattern in terms of cytokine levels in serum, with minor differences. Kaplan-Meier survival analyses showing the combined effect of the two functional networks of serum cytokines: IL-4/IL-5/IL-6 (C) and IFN-γ/TNF-α/IL-17A (D) on the survival pattern of patients with GBM up to 1200 days post-surgery. Detection of ‘all’ or ‘none’ of the cytokines as opposed to only partial combinations (one or two cytokines) in each functional network was correlated with improved survival. The interaction between individual cytokines in the two functional networks was also ascertained using Spearman's correlation.
3.3. Combinatorial Effect of Serum Cytokines on Patient Survival Patterns
Univariate analysis was then used for visualising if serum cytokine combinations affected patient survival. We found that the combination of circulating IL-4/IL-5/IL-6 – either all present or all absent (‘all’ or ‘none’) – correlated strongly with a better survival profile (p = .0022) among the patients with GBM compared to only a ‘partial’ combination i.e. one or two cytokines instead of all three (Fig. 2C). The scenario was similar for serum IFN-γ/TNF-α/IL-17A; patients with all three cytokines or none showed a tendency for an improved survival pattern (p = .0083) (Fig. 2D).
3.4. Antigen-Specific IFN-γ Production and Association With Survival Pattern of Patients with GBM
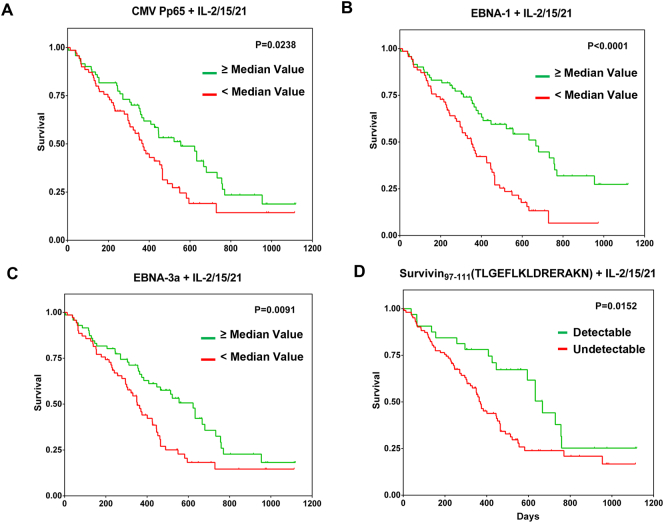
IFN-γ responses of peripheral blood mononuclear cells (PBMCs) from patients with GBM were measured by ELISA after stimulation with the antigens listed in Supplementary Table S1, with or without cytokine conditioning with IL-2/IL-15/IL-21 or IL-2/IL-7 (Methods section). CMV pp65-specific IFN-γ production by PBMCs correlated closely with a better survival profile of the patients in the presence of IL-2/IL-15/IL-21 conditioning (Fig. 3A). This was also found to be true for IFN-γ production in response to EBNA-1 (Fig. 3B), EBNA-3a (Fig. 3C) and the survivin97–111 peptide TLGEFLKLDRERAKN (Fig. 3D). Conversely, IFN-γ production in response to the NY-ESO180–94 peptide ARGPESRLLEFYLAM or EGFRvIII1–13 peptide LEEKKGNYVVTDH did not correlate with improved survival of patients with GBM (data not shown). The survivin97–111 peptide was tested separately, in addition to the survivin peptide mix due to its strong induction of IFN-γ production in TIL and PBMCs in an initial screen performed in our laboratory (data not shown).
Fig. 3.
Antigen-specific IFN-γ responses and survival of patients with GBM based on univariate analysis. Diluted peripheral blood of patients with GBM was exposed to viral targets (CMV pp65 (A), EBNA-1 (B) and EBNA-3a (C)) as well as the surviving97–111 peptide (D) over a 7-day period. Supernatants were then harvested for measuring antigen-specific IFN-γ production. Kaplan-Meier survival curves show the relationship between IFN-γ concentrations and patient survival based on median concentration values for the viral targets (since the net IFN-γ was very high after medium control subtraction) or detectable and non-detectable IFN-γ levels for the survivin peptide (since the net IFN-γ production was generally low after medium control subtraction) following antigen stimulation. Blood samples from 145 patients with GBM were used in all whole blood assays, using the entire set of candidate targets, except for the testing of survivin97–111 peptide, for which blood samples from 134 patients with GBM were used. Only antigen-specific IFN-γ responses with a statistically significant positive correlation with patient survival are shown. IFN-γ production in response to other TAAs tested i.e. NY-ESO-1, survivin (combination of peptides spanning the entire surviving protein) and EGFRvIII peptide pools, respectively did not exhibit a statistically significant correlation with improved survival of patients with GBM (data not shown).
3.5. Targeted IFN-γ Responses to Survivin97–111 Is Associated With Improved Survival of Patients With GBM
Clinical and immunological parameters, as well as the antigen-specific immune responses were submitted to univariate analysis to test their relationship to patient survival (Table 2). The more general immunological factors are distinguished from the antigen-specific immune responses: the former designate (non-antigen-specific) serum cytokines measurements, while the latter represent target-specific IFN-γ production in peripheral blood with or without cytokine conditioning. The following clinical and immunological parameters were found to be strongly associated with improved survival of patients with GBM: age (p = .0439), tumour recurrence (p = .0397), Karnofsky Performance Status (KPS) of patients (p = .0258), radiotherapy (p < .0001), chemotherapy (p < .0001), serum levels of IL-4/IL-5/IL-6 (p = .0022) as well as IFN-γ/TNF-α/IL-17A (p = .0083). Antigen-specific immune responses, defined by IFN-γ production of PBMCs to EBNA-1 (p < .0001), EBNA-3a (p = .0091), CMV pp65 (p = .0238) as well as the survivin97–111 peptide TLGEFLKLDRERAKN (p = .0152) also correlated with improved survival of patients with GBM, yet only in the presence of IL-2/IL-15/IL-21 (Table 2). None of the antigen-specific immune response parameters in the unconditioned and in IL-2/IL-7-conditioned groups were statistically significant (data not shown).
Table 2.
Univariate analysis of clinical and immunological parameters in relation to survival of patients with GBM.
| Factor | P value | |
|---|---|---|
| Clinical | ||
| Age | 0.0439a | |
| Median | 63 | |
| Range | 16–80 | |
| Gender | 0.5543 | |
| M | 99 | |
| F | 46 | |
| Recurrence | 0.0397a | |
| 2 | 15 | |
| 1 | 35 | |
| 0 | 95 | |
| Tumour diameter(cm) | 0.7450 | |
| Median | 4.5 | |
| Range | 1–8 | |
| Radiology Oedema | 0.1079 | |
| No | 9 | |
| Moderate | 79 | |
| Severe | 57 | |
| Tumour Localisation | 0.4205 | |
| Frontal | 40 | |
| Parietal | 30 | |
| Temporal | 59 | |
| Rest | 16 | |
| Mental status | 0.0594 | |
| Normal | 111 | |
| Minor confusion | 25 | |
| Major/Gross confusion or Unconscious | 9 | |
| KPS score | 0.0258a | |
| >80 | 104 | |
| ≤80 | 41 | |
| RPA classification before surgery | 0.0435a | |
| 5 + 6 | 121 | |
| 3/4 | 22 | |
| ND | 2 | |
| Extent of resection | 0.2174 | |
| Complete | 65 | |
| Partial | 77 | |
| ND | 3 | |
| Radiotherapy | <0.0001a | |
| Yes | 107 | |
| No | 37 | |
| ND | 1 | |
| Chemotherapy | <0.0001a | |
| ≥ 2 | 70 | |
| 1 | 67 | |
| 0 | 8 | |
| Infection | 0.1191 | |
| Yes | 127 | |
| No | 18 | |
| Immunological | ||
| Serum IL-4/IL-5/IL6 | 0.0022a | |
| All or none | 112 | |
| Partial | 23 | |
| ND | 10 | |
| Serum IFN-γ/TNF-α/IL-17A | 0.0083a | |
| All or none | 55 | |
| Partial | 80 | |
| ND | 10 | |
| Antigen-specific immune response | ||
| EBNA-1(IL-2/15/21) | <0.0001a | |
| IFN < 460.5 pg/ml | 70 | |
| IFN ≥460.5 pg/ml | 71 | |
| ND | 4 | |
| EBNA-3a(IL-2/15/21) | 0.0091a | |
| IFN < 86.1 pg/ml | 70 | |
| IFN ≥86.1 pg/ml | 71 | |
| ND | 4 | |
| CMV Pp65(IL-2/15/21) | 0.0238a | |
| IFN < 224.5/ml | 70 | |
| IFN ≥224.5/ml | 71 | |
| ND | 4 | |
| Survivin97–111(IL-2/15/21) | 0.0152a | |
| Undetectable | 102 | |
| Detectable | 32 | |
| ND | 11 | |
KPS: Karnofsky Performance Status; RPA: Recursive Partitioning Analysis; ND: Not defined.
Included in multivariate analysis.
3.6. Multivariate Analysis of Parameters to Visualise Independent Predictors of Survival in GBM
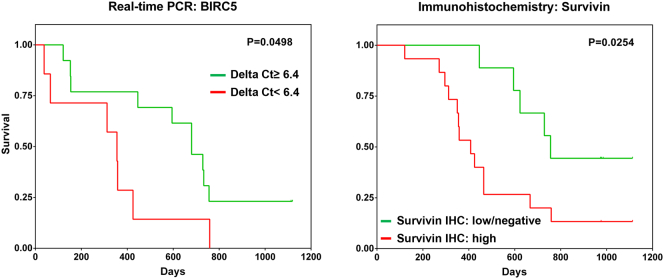
Next, we determined whether the above-mentioned parameters/factors could independently affect the survival of patients with GBM using multivariant analysis. All parameters and factors that gave a p value of <0.05 in the univariate analysis, including data from the three separate culture conditions (non-cytokine-conditioned blood, IL-2/IL-7-conditioned and IL-2/IL-15/IL-21-conditioned), that were fitted into the forward-backward stepwise Cox regression model (proportional hazards). Parameters that resulted in a significant p value (<0.05) pertaining to the survival pattern of patients with GBM are listed in Table 3. Radiotherapy (p < .0001; HR = 0.3368), chemotherapy (p = .028; HR = 0.7143) and the combination of detectable levels of serum IFN-γ/TNF-α/IL-17A correlated positively with an improved survival profile (p = .003; HR = 1.7851), with borderline significance for serum IL-4/IL-5/IL-6 (p = .052; HR = 2.2645). PBMC produced IFN-γ in response to survivin97–111 peptide only with IL-2/IL-15/IL-21 conditioning (and not in the presence of IL-2/IL-7 or ‘unconditioned’), and was identified as the sole factor reflecting an antigen-specific immune response associated with improved survival of the patients (p = .024; HR = 2.0756) (Supplementary Fig. S2). Approximately 24% of patients with GBM exhibited IFN-γ responses to the survivin97–111 peptide in peripheral blood conditioned with IL-2/IL-15/IL-21 (Supplementary Fig. S2). The effect of EBNA-1-specific IFN-γ production (with IL-2/IL-15) on patient survival followed a similar trend to the survivin peptide, albeit with borderline statistical significance (p = .051; HR = 1.6397). Survivin protein expression and gene (BIRC5) transcription in GBM lesions were also confirmed by immunohistochemistry and polymerase chain reaction, respectively (Fig. 4), in agreement with previous reports [22, 23]. 15/25 patients with GBM (60%) exhibited high expression (≥20%) of survivin protein in the tumour tissue based on immunohistochemistry data (data not shown).
Table 3.
Forward and backward stepwise multivariate analysis model of confirmed prognostic variables (Cox proportional hazards model) from univariate analyses to determine single factors to predict survival of patients with GBM.
| Stepwise(COX) | HR | P | 95% CI | |
|---|---|---|---|---|
| Radiotherapy | 0.3368 | <0.0001 | 0.20435 | 0.55502 |
| Chemotherapy | 0.7143 | 0.028 | 0.52857 | 0.96521 |
| EBNA1 | 1,0.6397 | 0.051 | 0.99820 | 2.69339 |
| Survivin97–111 | 2.0756 | 0.024 | 1.09916 | 3.91960 |
| IL4/5/6 | 1.7851 | 0.052 | 0.99582 | 3.19990 |
| IFN-γ/TNF-α/IL-17A | 2.2645 | 0.003 | 1.33067 | 3.85354 |
Fig. 4.
Intratumoural survivin/BIRC5 expression in relation to survival of patients with GBM. Kaplan-Meier survival analyses showing BIRC5 gene expression (A) as well as survivin protein expression (B) in GBM tissue samples in relation to the survival pattern of patients with GBM. Molecular analysis of BIRC5 gene transcription levels in GBM tissue was measured by real-time polymerase chain reaction, while survivin protein expression in paraffin-embedded GBM tissue sections was detected using immunohistochemistry. For BIRC5 gene transcription, low expression indicates a delta cycle threshold (CT) value of >6.4, while high expression indicates a delta CT value of <6.4. Twenty samples from patients with GBM were tested. ΔCT range was 3.56–10.00 and the median ΔCT value was 6.4. Thirteen samples exhibited ΔCT ≥ 6.4 and the remaining seven samples ΔCT <6.4.
4. Discussion
Cytokine networks appear to be crucial in cancer initiation and disease establishment [24, 25] orchestrating immune responses that sustain health or promote disease. This report provides the first evidence that a combination of serum IL-4/IL-5/IL-6 or IFN-γ/TNF-α/IL-17A, detectable prior to surgery and cancer therapy, can predict the survival profile of patients with GBM in the follow-up phase (see Supplementary Fig. S5 for the consort diagram). We also show that circulating T cells specific for EBV, CMV, or the survivin97–111 peptide target can be conditioned with a cocktail of IL-2/IL-15/IL-21 to produce IFN-γ which correlates with patient survival. To the best of our knowledge, this is also the first study describing PBMC reactivity gauged by IFN-γ production to a single survivin peptide, as an independent predictor of improved survival among patients with GBM.
Chiorean and colleagues reported previously an association between the upregulation of serum IL-8, endothelial hyperplasia as well as coagulation necrosis, while VEGF upregulation was found to be linked with ischemic necrosis in patients with GBM [8]. The authors, however, did not find any correlation with patient survival. Previous studies have assessed individual serum cytokines in relation to the survival of patients with cancer [26, 27], although the effect of cytokine networks remained underexplored. Cytokine networks, rather than individual cytokines, provide a ‘high-resolution’ insight into disease mechanisms involved in pathogenesis and progression i.e. the IL-15/IL-32 network in pulmonary tuberculosis (a pertinent example of chronic inflammation) [28], the IL-17/IL-6/TNF-α/TGF-β axis in colorectal cancer [24] and IFN-γ/IL-6/TNF-α/IL-1β cross-talk in atherosclerosis [29]. In line with this, the pleiotropic cytokine IL-6 can support plasma cell differentiation and maintenance in the host synergistically with IL-5, in addition to promoting inflammation on its own [21, 30]. Together with IL-4, IL-6 can trigger the reduction of circulating Treg numbers by promoting FoxP3 downregulation, which is highly favourable for successful cell-based therapies in cancer [31]. Thus, the combinatorial effect of IL-4, IL-5 and IL-6 may help to control the tumour burden in patients by sustaining the production of TAA-specific antibodies as well as antigen-specific T-cell reactivity without Treg-associated disruption of productive immune responses [32]. IL-4 and IL-5 can nevertheless be involved in inflammatory processes; IL-5 is associated with eosinophil induction in airway hyperreactivity and asthma [33], while IL-4 upregulation is linked to IL-12p40 production by dendritic cells, Th1 cell induction and cytotoxic lymphocyte activation [25, 34]. Since a survival benefit was also observed when no serum cytokines were detected, the total absence of systemic hyper-immune activation may be as likely as the presence of a balanced cytokine network, in this case IL-4/5/6 and/or IFN-γ/TNF-α/IL-17A, to contribute to clinically relevant and durable anti-tumour immune responses that may be considered biologically relevant in clinical immune-monitoring (Fig. 5). Furthermore, no statistical significance was seen when the individual cytokines were tested for an effect on survival benefit (Supplementary Fig. S6).
Fig. 5.
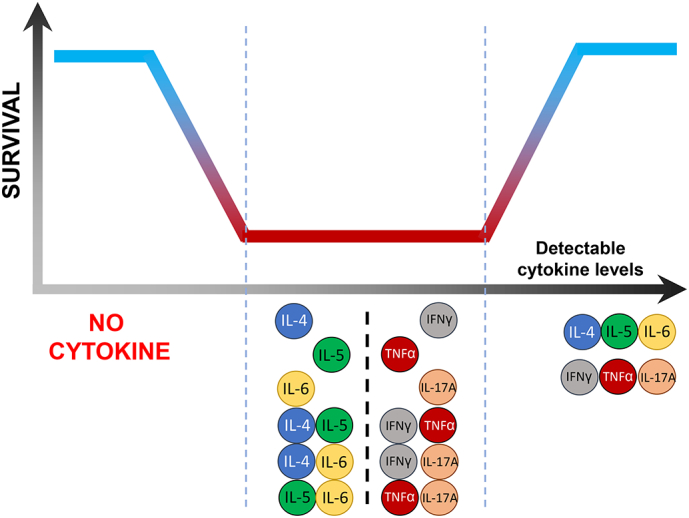
Schematic representation of the contribution of cytokine networks to the survival dynamics of patients of GBM. As presented in this study, the absence (far left of the diagram) or presence (far right of the diagram) of the combination of circulating IL-4/IL-5/IL-6 or IFN-γ/TNF-α/IL-17A measurable in blood reflects a favourable prognosis for improved survival of patients with GBM (grade IV brain tumour). Conversely, the presence of only one or two of the cytokines, shown in the middle section of the diagram, does not appear to favour the survival of these patients based on our data. Thus, the role of cytokine networks in GBM summarised in this figure could serve as a starting point for biological and clinical validation as a reliable biomarker in larger cohorts of patients with glioma.
We observed that exclusively in the presence of IL-2/IL-15/IL-21 conditioning, detectable anti-survivin97–111 IFN-γ production by PBMCs correlated with 70% of survival at 600 days post-surgery (also reflected in the multivariate analysis) as opposed to under 25% among non-responding patients with GBM. Encoded by the BIRC5 gene, survivin is an anti-apoptotic protein which associates with caspase-9 to inhibit the intrinsic apoptotic pathway, while promoting mitosis in cells; its overexpression in cancer cells perpetrates uncontrolled proliferation leading to disease progression [35, 36]. Downregulation of survivin expression in glioblastoma cell lines (G55T2 and U-87 MG) via siRNA-based knockdown of the Special AT-rich Sequence-Binding Protein 1 (SATB1) regulator has been shown to induce apoptosis and cell growth arrest [37]. Furthermore, the survivin-based conjugate vaccine SurVaxM was recently tested in a phase I clinical trial involving patients with GBM (NCT02455557), while IMA950, a multi-peptide conjugate vaccine candidate also incorporating the survivin peptide TLGEFLKLDRERAKN, showed promising immunogenicity in patients with GBM, which is currently in phase II clinical trials (NCT01920191) for HLA-A*02+ individuals [38]. The survivin97–111 TLGEFLKLDRERAKN peptide is also presented by frequent HLA-DR alleles (which trigger CD4+ T-cell responses) [39, 40]. We could also detect survivin-directed immune reactivity among CD4+ and CD8+ TIL in patients with GBM (Supplementary Fig. S4). We have previously shown that TIL from GBM and pancreatic adenocarcinoma, after cultivation with IL-2/IL-15/IL-21, display properties that are highly desirable for cellular therapy i.e. autologous tumour killing, TAA-specific IFN-γ production, and accumulation within the central memory phenotype [41, 42]. Taken together, our finding strongly suggests that survivin97–111-specific cellular immune-reactivity, enhanced by IL-2/IL-15/IL-21 is integral to the host-protective anti-tumour response in GBM.
EBV-directed IFN-γ production (anti-EBNA-1 reactivity) was also found to be associated with patient survival after multivariate analysis, albeit only with borderline statistical significance. Akin to survivin97–111-specific immune-reactivity, this was observed exclusively with IL-2/IL-15/IL-21 conditioning and not with IL-2/IL-7 conditioning or without cytokine conditioning. Anti-CMV/EBV immune responses may not only indicate (viral) target-specific cellular immune responses; impaired immune responses to both or either pathogen often reflects immune dysfunction [[43], [44], [45], [46]]. In contrast, strong “intact” immune responses indicate ‘immune-fitness', as demonstrated in patients with pulmonary tuberculosis: well-preserved anti-CMV/EBV IFN-γ responses of PBMCs correlate with better survival following standard drug therapy [47]. Thus, it is plausible to conclude that amplification of clinically relevant T-cell responses to TLGEFLKLDRERAKN with IL-2/IL-15/IL-21, makes this cytokine combination a strong candidate to expand antigen-specific T-cells for adoptive immunotherapy.
Patients diagnosed with GBM are given an intravenous dose of corticosteroids i.e. dexamethasone at admission to reduce brain oedema and facilitate clinical improvement. However, the systemic effects of corticosteroids may abrogate a sizeable proportion of pro-inflammatory responses, including antigen-specific T-cell activity [48]. Our observation that IL-2/IL-15/IL-21 conditioning of whole blood can exclusively evoke survivin97–111-directed as well as anti-EBV cellular immune responses suggests that immunosuppressed patients can also benefit from this strategy.
This study has several benefits for strengthening future personalised immunotherapy as well as immune-monitoring protocols. The WBA (whole-blood-assay) can be easily used to screen patients with GBM or advanced cancers of different histologies for the presence of survivin97–111-specific T cells, which may be isolated and expanded in vitro with IL-2/IL-15/IL-21 for reinfusion. Additionally, peptide-specific T-cell receptors (TCRs) recognising survivin97–111 may be cloned and transferred for transgenic expression in effector T cells, as was recently reported for the KRAS G12D mutation in patients with colorectal cancer [49]. The significance of cytokine networks i.e. IL-4/IL-5/IL-6 and IFN-γ/TNF-α/IL-17A in predicting patient survival also broadens the examination of other biological mediators that can be easily measured in blood samples using established and qualified diagnostic methods.
Acknowledgments
Acknowledgement
We are thankful for Dr. Lalit Rane's assistance (PCR) and Dr. Christoph Illies for the survivin staining in GBM tissue (immunohistochemistry).
Funding Sources
This study was supported by the Söderberg Foundation to MM. The funding agency was not involved in study design, data collection, data analysis, interpretation, writing of the report.
Conflicts of Interest
The cytokine cocktail IL-2, IL-15 and IL-21 has been filed for IP with regard to TIL expansion and antigen-specific T-cells (E.D., M.M.).
Author Contributions
LZ performed the experiments, analysed and interpreted the data, prepared the figures and wrote the manuscript; MR performed the literature search, wrote the manuscript and provided scientific input; XL provided assistance with statistical analyses and scientific input; DV provided assistance with statistical analyses, AvL, QM and NH provided technical assistance with experiments; GS provided patient material and relevant clinical information; JK contributed with technical input; H-MA contributed with scientific input; EJ contributed to the study design and provided scientific input; I-HP contributed to the study design; ED contributed to the study design and provided patient material as well as relevant clinical information; MM designed and initiated the study, interpreted the data, provided scientific input and wrote the manuscript.
Disclaimer
The authors declare that the cytokine cocktail IL-2, IL-15 has been filed for IP (ED, MM).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.06.014.
Appendix A. Supplementary data
Supplementary tables
Supplementary Table S1: List of antigens used in the whole blood assays (WBA).
Supplementary Table S2: Multivariate analysis of WBA in the presence of IL-2/IL-7 conditioning.
Supplementary figures
Supplementary Fig. S1: Anti-inflammatory/pleiotropic as well as pro-inflammatory/Th1 cytokine levels in sera of patients with malignant glioma. Serum levels of IL-4, IL-5, IL-6, IFN-γ, TNF-α and IL-17A were measured by sandwich ELISA, and individual concentrations of cytokine levels (pg/ml) among patients with GBM vs non-GBM are shown. As depicted in the figure, the medians between the two patient groups across all the cytokines tested did not differ significantly, although there appears to be a trend toward meagerly higher levels among patients with non-GBM malignant glioma (except for TNF-α).
Supplementary Fig. S2: Serum cytokine profiles in patients with GBM. Based on the actual concentration of cytokines measured by ELISA, patients were categorized for the survival analysis based on the detection of none, one, two or three cytokines (belonging to the ‘anti-inflammatory/pleiotropic’ or ‘pro-inflammatory/Th1’ grouping system) in serum. The number of patients ascribed to each category as well as the relative proportion to the total number of patients with GBM (%) is shown. Left panel, IL-4/IL-5IL-6; right panel, IFN-γ/TNF-α/IL-17A.
Supplementary Fig. S3: Immune-reactivity in blood of patients with GBM to survivin97–111 peptide (TLGEFLKLDRERAKN). Specific IFN-γ responses to survivin97–111 peptide was tested with blood samples from 134 patients with GBM using the same WBA method described in this study. 23.9% of patients with GBM (n = 32) showed positive reactivity to the survivin97–111 peptide, defined by detectable IFN-γ production in WBA supernatants, while the remaining 76.1% of patients (n = 102) did not have a measurable IFN-γ response.
Supplementary Fig. S4: Immune-reactivity of glioma TIL (tumor-infiltrating lymphocytes) to survivin. TIL were expanded as reported earlier (ref. 40) using IL-2, IL-15 and IL-21 and tested directed against the survivin peptide mix in a standard 6 h intracellular cytokine release assay (ICS). Addition of medium or PMA, served as the respective negative or positive control. TIL were first gated on CD3+ T-cells, then sequentially on CD3+CD4+, CD3+CD8+ and on CD3+CD4-CD8- (double, negative, DN) T-cells. The latter cell population represents strong activated T-cells that downregulated CD4+ or CD8+. Percentages are shown for IFN-γ and TNF-α producing cells in the parental T-cell population. Survivin-specific IFN-γ and TNF-α production in CD4+, CD8+, as well as in DN (double negative, i.e. CD3-CD4-CD8-) TIL.
Supplementary Fig. S5: Statistical workflow of the univariate and multivariate analyses steps. Flow diagram showing how the parameters for the univariate and multivariate (Cox proportional hazards model) analyses were selected and included.
Supplementary Fig. S6: Serum levels of individual cytokines and their effect on the survival pattern of patients with GBM based on univariate analysis. Cytokine levels (IL-4, IL-5, IL-6, IFN-γ, TNF-α and IL-17A) were measured using a sandwich ELISA in the sera of patients with glioma (GBM and non-GBM) and expressed in pg/ml. Kaplan-Meier survival analyses show the individual effect of serum cytokines on the survival pattern of patients with GBM up to 1200 days post-surgery, based on whether the cytokines were detected (identical approach to Fig. 2 in the main manuscript). The interaction between individual cytokines was also ascertained using Spearman's correlation. None of the individual cytokines tested affected patient survival.
References
- 1.WHO . International Agency for Research on Cancer, World Health Organisation; Lyon: 2014. World Cancer Report 2014. [Google Scholar]
- 2.Louis D.N., Perry A., Reifenberger G. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Vaios E.J., Nahed B.V., Muzikansky A., Fathi A.T., Dietrich J. Bone marrow response as a potential biomarker of outcomes in glioblastoma patients. J Neurosurg. 2016:1–7. doi: 10.3171/2016.7.JNS16609. [DOI] [PubMed] [Google Scholar]
- 4.Barani I.J., Larson D.A. Radiation therapy of glioblastoma. Cancer Treat Res. 2015;163:49–73. doi: 10.1007/978-3-319-12048-5_4. [DOI] [PubMed] [Google Scholar]
- 5.Larson E.W., Peterson H.E., Lamoreaux W.T. Clinical outcomes following salvage Gamma Knife radiosurgery for recurrent glioblastoma. World J Clin Oncol. 2014;5(2):142–148. doi: 10.5306/wjco.v5.i2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wainwright D.A., Sengupta S., Han Y., Ulasov I.V., Lesniak M.S. The presence of IL-17A and T helper 17 cells in experimental mouse brain tumors and human glioma. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sowers J.L., Johnson K.M., Conrad C., Patterson J.T., Sowers L.C. The role of inflammation in brain cancer. Adv Exp Med Biol. 2014;816:75–105. doi: 10.1007/978-3-0348-0837-8_4. [DOI] [PubMed] [Google Scholar]
- 8.Chiorean R., Berindan-Neagoe I., Braicu C. Quantitative expression of serum biomarkers involved in angiogenesis and inflammation, in patients with glioblastoma multiforme: correlations with clinical data. Cancer Biomarkers. 2014;14(2–3):185–194. doi: 10.3233/CBM-130310. [DOI] [PubMed] [Google Scholar]
- 9.Gan H.K., Cvrljevic A.N., Johns T.G. The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J. 2013;280(21):5350–5370. doi: 10.1111/febs.12393. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen M.W., Meltorn M., Damstrup L., Poulsen H.S. The type III epidermal growth factor receptor mutation. Biological significance and potential target for anti-cancer therapy. Ann Oncol. 2001;12(6):745–760. doi: 10.1023/a:1011177318162. [DOI] [PubMed] [Google Scholar]
- 11.Waziri A. Glioblastoma-derived mechanisms of systemic immunosuppression. Neurosurg Clin N Am. 2010;21(1):31–42. doi: 10.1016/j.nec.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Antonios J.P., Soto H., Everson R.G. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight. 2016;1(10) doi: 10.1172/jci.insight.87059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowther D.E., Goods B.A., Lucca L.E. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight. 2016;1(5) doi: 10.1172/jci.insight.85935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown C.E., Alizadeh D., Starr R. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debinski W., Gibo D.M., Slagle B., Powers S.K., Gillespie G.Y. Receptor for interleukin 13 is abundantly and specifically over-expressed in patients with glioblastoma multiforme. Int J Oncol. 1999;15(3):481–486. doi: 10.3892/ijo.15.3.481. [DOI] [PubMed] [Google Scholar]
- 16.Perng P., Lim M. Immunosuppressive mechanisms of malignant gliomas: parallels at non-CNS sites. Front Oncol. 2015;5:153. doi: 10.3389/fonc.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harshyne L.A., Nasca B.J., Kenyon L.C., Andrews D.W., Hooper D.C. Serum exosomes and cytokines promote a T-helper cell type 2 environment in the peripheral blood of glioblastoma patients. Neuro Oncol. 2016;18(2):206–215. doi: 10.1093/neuonc/nov107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sengupta S., Marrinan J., Frishman C., Sampath P. Impact of temozolomide on immune response during malignant glioma chemotherapy. Clin Dev Immunol. 2012;2012:831090. doi: 10.1155/2012/831090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rampling R., Peoples S., Mulholland P.J. A cancer research UK first time in human phase I trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clin Cancer Res. 2016;22(19):4776–4785. doi: 10.1158/1078-0432.CCR-16-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J.H., Zhang Y.C., Qian H.Q. Survivin antisense oligodeoxynucleotide inhibits growth of gastric cancer cells. World J Gastroenterol. 2004;10(8):1121–1124. doi: 10.3748/wjg.v10.i8.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirai K., Suzuki Y., Oka K. Nuclear survivin expression predicts poorer prognosis in glioblastoma. J Neurooncol. 2009;91(3):353–358. doi: 10.1007/s11060-008-9720-4. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarti A., Noll E., Black P.M. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20(4):1063–1068. doi: 10.1200/JCO.2002.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 24.West N.R., Mccuaig S., Franchini F., Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15(10):615–629. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- 25.Iwami K., Natsume A., Wakabayashi T. Cytokine networks in glioma. Neurosurg Rev. 2011;34(3):253–263. doi: 10.1007/s10143-011-0320-y. [discussion 63-4] [DOI] [PubMed] [Google Scholar]
- 26.Enewold L., Mechanic L.E., Bowman E.D. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 2009;18(1):215–222. doi: 10.1158/1055-9965.EPI-08-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres C., Linares A., Alejandre M.J. Prognosis relevance of serum cytokines in pancreatic cancer. Biomed Res Int. 2015;2015:518284. doi: 10.1155/2015/518284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montoya D., Inkeles M.S., Liu P.T. IL-32 is a molecular marker of a host defense network in human tuberculosis. Sci Transl Med. 2014;6(250):250ra114. doi: 10.1126/scitranslmed.3009546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Autieri M. Pro- and anti-inflammatory cytokine networks in atherosclerosis. Int Scholar Res Notices. 2012;2012 [Google Scholar]
- 30.Cassese G., Arce S., Hauser A.E. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171(4):1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 31.Kastner L., Dwyer D., Qin F.X. Synergistic effect of IL-6 and IL-4 in driving fate revision of natural Foxp3+ regulatory T cells. J Immunol. 2010;185(10):5778–5786. doi: 10.4049/jimmunol.0901948. [DOI] [PubMed] [Google Scholar]
- 32.Fujio K., Okamura T., Sumitomo S., Yamamoto K. Regulatory T cell-mediated control of autoantibody-induced inflammation. Front Immunol. 2012;3:28. doi: 10.3389/fimmu.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee M., Sehmi R., Nair P. Anti-IL5 therapy for asthma and beyond. World Allerg Org J. 2014;7(1):32. doi: 10.1186/1939-4551-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okada H., Kuwashima N. Gene therapy and biologic therapy with interleukin-4. Curr Gene Ther. 2002;2(4):437–450. doi: 10.2174/1566523023347625. [DOI] [PubMed] [Google Scholar]
- 35.Sah N.K., Khan Z., Khan G.J., Bisen P.S. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006;244(2):164–171. doi: 10.1016/j.canlet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Mellai M., Caldera V., Patrucco A., Annovazzi L., Schiffer D. Survivin expression in glioblastomas correlates with proliferation, but not with apoptosis. Anticancer Res. 2008;28(1A):109–118. [PubMed] [Google Scholar]
- 37.Fromberg A., Rabe M., Oppermann H., Gaunitz F., Aigner A. Analysis of cellular and molecular antitumor effects upon inhibition of SATB1 in glioblastoma cells. BMC Cancer. 2017;17(1):3. doi: 10.1186/s12885-016-3006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winograd E.K., Ciesielski M.J., Fenstermaker R.A. Novel vaccines for glioblastoma: clinical update and perspective. Immunotherapy. 2016;8(11):1293–1308. doi: 10.2217/imt-2016-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widenmeyer M., Griesemann H., Stevanovic S. Promiscuous survivin peptide induces robust CD4+ T-cell responses in the majority of vaccinated cancer patients. Int J Cancer. 2012;131(1):140–149. doi: 10.1002/ijc.26365. [DOI] [PubMed] [Google Scholar]
- 40.Dutoit V., Herold-Mende C., Hilf N. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain. 2012;135(Pt 4):1042–1054. doi: 10.1093/brain/aws042. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z., Meng Q., Bartek J., Jr. Tumor-infiltrating lymphocytes (TILs) from patients with glioma. Oncoimmunology. 2017;6(2) doi: 10.1080/2162402X.2016.1252894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng Q., Liu Z., Rangelova E. Expansion of tumor-reactive T cells from patients with pancreatic cancer. J Immunother. 2016;39(2):81–89. doi: 10.1097/CJI.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 43.Riddell S.R., Watanabe K.S., Goodrich J.M., Li C.R., Agha M.E., Greenberg P.D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 44.Icheva V., Kayser S., Wolff D. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. 2013;31(1):39–48. doi: 10.1200/JCO.2011.39.8495. [DOI] [PubMed] [Google Scholar]
- 45.La Rosa C., Diamond D.J. The immune response to human CMV. Future Virol. 2012;7(3):279–293. doi: 10.2217/fvl.12.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merlo A., Turrini R., Dolcetti R. The interplay between Epstein-Barr virus and the immune system: a rationale for adoptive cell therapy of EBV-related disorders. Haematologica. 2010;95(10):1769–1777. doi: 10.3324/haematol.2010.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagu T., Aboud S., Rao M. Strong anti-Epstein Barr virus (EBV) or cytomegalovirus (CMV) cellular immune responses predict survival and a favourable response to anti-tuberculosis therapy. Int J Infect Dis. 2017;56:136–139. doi: 10.1016/j.ijid.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 48.Dietrich J., Rao K., Pastorino S., Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4(2):233–242. doi: 10.1586/ecp.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran E., Robbins P.F., Lu Y.C. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375(23):2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables
Supplementary Table S1: List of antigens used in the whole blood assays (WBA).
Supplementary Table S2: Multivariate analysis of WBA in the presence of IL-2/IL-7 conditioning.
Supplementary figures
Supplementary Fig. S1: Anti-inflammatory/pleiotropic as well as pro-inflammatory/Th1 cytokine levels in sera of patients with malignant glioma. Serum levels of IL-4, IL-5, IL-6, IFN-γ, TNF-α and IL-17A were measured by sandwich ELISA, and individual concentrations of cytokine levels (pg/ml) among patients with GBM vs non-GBM are shown. As depicted in the figure, the medians between the two patient groups across all the cytokines tested did not differ significantly, although there appears to be a trend toward meagerly higher levels among patients with non-GBM malignant glioma (except for TNF-α).
Supplementary Fig. S2: Serum cytokine profiles in patients with GBM. Based on the actual concentration of cytokines measured by ELISA, patients were categorized for the survival analysis based on the detection of none, one, two or three cytokines (belonging to the ‘anti-inflammatory/pleiotropic’ or ‘pro-inflammatory/Th1’ grouping system) in serum. The number of patients ascribed to each category as well as the relative proportion to the total number of patients with GBM (%) is shown. Left panel, IL-4/IL-5IL-6; right panel, IFN-γ/TNF-α/IL-17A.
Supplementary Fig. S3: Immune-reactivity in blood of patients with GBM to survivin97–111 peptide (TLGEFLKLDRERAKN). Specific IFN-γ responses to survivin97–111 peptide was tested with blood samples from 134 patients with GBM using the same WBA method described in this study. 23.9% of patients with GBM (n = 32) showed positive reactivity to the survivin97–111 peptide, defined by detectable IFN-γ production in WBA supernatants, while the remaining 76.1% of patients (n = 102) did not have a measurable IFN-γ response.
Supplementary Fig. S4: Immune-reactivity of glioma TIL (tumor-infiltrating lymphocytes) to survivin. TIL were expanded as reported earlier (ref. 40) using IL-2, IL-15 and IL-21 and tested directed against the survivin peptide mix in a standard 6 h intracellular cytokine release assay (ICS). Addition of medium or PMA, served as the respective negative or positive control. TIL were first gated on CD3+ T-cells, then sequentially on CD3+CD4+, CD3+CD8+ and on CD3+CD4-CD8- (double, negative, DN) T-cells. The latter cell population represents strong activated T-cells that downregulated CD4+ or CD8+. Percentages are shown for IFN-γ and TNF-α producing cells in the parental T-cell population. Survivin-specific IFN-γ and TNF-α production in CD4+, CD8+, as well as in DN (double negative, i.e. CD3-CD4-CD8-) TIL.
Supplementary Fig. S5: Statistical workflow of the univariate and multivariate analyses steps. Flow diagram showing how the parameters for the univariate and multivariate (Cox proportional hazards model) analyses were selected and included.
Supplementary Fig. S6: Serum levels of individual cytokines and their effect on the survival pattern of patients with GBM based on univariate analysis. Cytokine levels (IL-4, IL-5, IL-6, IFN-γ, TNF-α and IL-17A) were measured using a sandwich ELISA in the sera of patients with glioma (GBM and non-GBM) and expressed in pg/ml. Kaplan-Meier survival analyses show the individual effect of serum cytokines on the survival pattern of patients with GBM up to 1200 days post-surgery, based on whether the cytokines were detected (identical approach to Fig. 2 in the main manuscript). The interaction between individual cytokines was also ascertained using Spearman's correlation. None of the individual cytokines tested affected patient survival.