Abstract
Background:
Cigarette smoking has been linked with several factors associated with cardiac dysfunction. We hypothesized that cigarette smoking is associated with left ventricular (LV) structure, function and incident heart failure (HF) hospitalization.
Methods:
We investigated 4129 (never smoker n=2884, current smoker n=503, and former smoker n=742) African American participants (mean age 54 years, 63% women) without a history of HF or coronary heart disease (CHD) at baseline in the Jackson Heart Study. We examined the relationship between cigarette smoking and LV structure and function using cardiac magnetic resonance imaging (CMR) among 1092 participants, cigarette smoking and brain natriuretic peptide (BNP) levels among 3325 participants and incident HF hospitalization among 3633 participants with complete data.
Results:
After adjustment for confounding factors, current smoking was associated with higher mean LV mass index and lower mean LV circumferential strain (P <0.05, for all) compared with never smoking. Smoking status, intensity and burden were associated with higher mean BNP levels (all P <0.05). Over 8.0 years (7.7–8.0) median follow-up, there were 147 incident HF hospitalizations. After adjustment for traditional risk factors and incident CHD, current smoking (HR 2.82, 95%CI 1.71~4.64), smoking intensity among current smokers (≥20 cigarettes/day: HR 3.48, 95%CI 1.65~7.32) and smoking burden among ever smokers (≥15 pack-years: HR 2.06 95%CI 1.29~3.3) were significantly associated with incident HF hospitalization compared with never smoking.
Conclusions:
In African Americans, cigarette smoking is an important risk factor for LV hypertrophy, systolic dysfunction and incident HF hospitalization even after adjusting for effects on CHD.
Keywords: smoking, heart failure, cardiac MRI, Jackson Heart Study (JHS), blacks
Introduction
Cigarette smoking is a risk factor for heart failure (HF) independent of traditional risk factors.1–5 Whereas cigarette smoking increases the risk of coronary artery disease (CHD), a major cause of HF, there may be other effects of smoking that result in cardiac dysfunction and HF.6 For example, smoking acutely increases systolic and diastolic blood pressure, total systemic vascular resistance, pulmonary artery pressure and pulmonary vascular resistance, all known risk factors for HF.7 Furthermore, smoking is associated with carbon monoxide exposure which has been reported to increase oxidative stress and lead to impaired mitochondrial function, inflammation, impaired endothelial function and worsening renal function, all of which have been implicated in the pathophysiology of HF. 8–13
African Americans have a doubling in the incidence of HF compared to other races.14 The prevalence of current cigarette smoking among African Americans has declined in recent years, but remains approximately 18% among adults.15 Although some epidemiologic studies have demonstrated a significant association of current cigarette smoking to risk of developing HF, there are limited data specific to African Americans who are substantially affected by cardiovascular diseases.
We hypothesized that cigarette smoking is associated with cardiac remodeling, left ventricular dysfunction and incident heart failure (HF) hospitalization in African Americans. To test this hypothesis, we examined the association of cigarette smoking status, intensity and burden with cardiac structure and function and incident HF hospitalization in the Jackson Heart Study (JHS).
Methods
Study participants
The JHS is a large prospective community-based observational study designed to investigate risk factors for cardiovascular diseases in African Americans. Details of the JHS study design, recruitment and data collection have been described previously.16 Briefly, 5301 African American participants residing in the Jackson, Mississippi tri-county area (Hinds, Rankin and Madison) were recruited for the baseline exam between 2000 and 2004 and completed 3 subsequent study follow-up visits (Visit 1: 2000–2004, Visit 2: 2005–2008, Visit 3: 2009–2012). The JHS was approved by the Institutional Review Boards of Jackson State University, Tougaloo College and the University of Mississippi Medical Center in Jackson, Mississippi. All study participants provided written informed consent. The data, analytic methods and study materials can be made available to other researchers for purposes of reproducing the results or replicating the procedure by following the Jackson Heart Study publications procedures and data use agreements.
For the present analysis, we excluded all individuals with history of CHD or HF (n = 717), missing CHD/HF data (n=5), missing information on smoking status (n = 33) or missing information on study covariates (n = 422) at Visit 1 (Figure 1).
Figure 1.
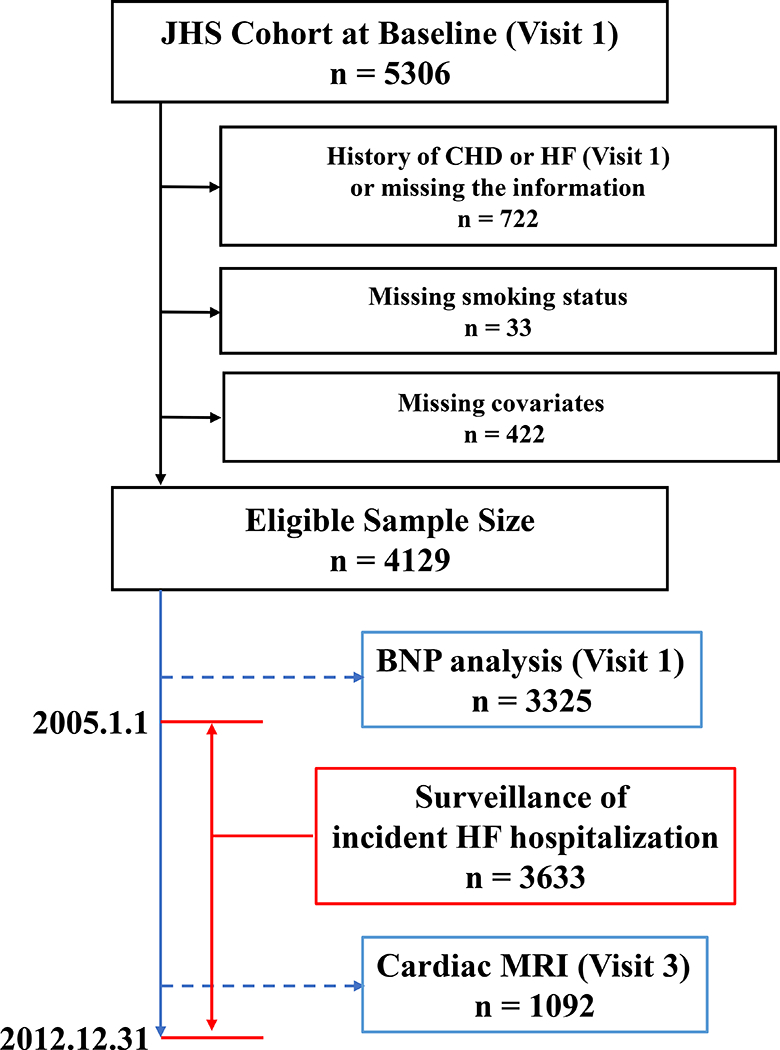
Exclusion criteria and the numbers of participants for each study.
Smoking information
Smoking information was obtained via questionnaire at both Visits 1 and 3. Participants who smoked >400 cigarettes in their lifetime were defined as ever smokers. Participants who gave a positive response to the question, “Do you now smoke cigarettes?” were classified as current smokers. Those who responded negatively to both of these questions were classified as never smokers.12 Participants who smoked >400 cigarettes but no longer smoked at the time of the examination were classified as former smokers. Cigarettes per day (smoking intensity) and pack-years (smoking burden) were also collected. Smoking burden and data related to time since quitting in former smokers is available in Supplemental Table 1.
Clinical covariates
At each examination, systolic and diastolic blood pressures were measured in the right arm of participants twice using the random-0 blood pressure sphygmomanometer (Hawksley and Sons Limited, Sussex, United Kingdom). The first blood pressure was obtained after allowing the participant to rest for 5 minutes in a seated position and the second blood pressure was obtained after waiting 1 additional minute. The average of the two measurements was used. Body mass index (BMI) was calculated as body weight (kg) / (height (m))2. Self-reported anti-hypertensive medication use was collected at the time of each examination. Venous blood samples were drawn from each participant after more than twelve hours of fasting. Fasting plasma glucose, hemoglobin A1c, and serum creatinine levels were assessed using standard laboratory techniques. Diabetes mellitus was defined as the use of diabetes medications, a hemoglobin A1c ≥ 6.5%, or a fasting blood glucose ≥ 126 mg/dL at baseline. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease Study equation.17
Cardiac and aortic MRI
Cardiac magnetic resonance (CMR) images were obtained with 1.5T MR Siemens Espree scanner (Siemens Medical Solutions, Erlangen, Germany) at Visit 3 (Figure 1) in a randomly selected subset of 1092 participants without incident CHD between Visits 1 and 3. Cine and tagged imaging were performed to assess LV mass, volumes and deformation parameters. LV mass and volumes were indexed to body surface area measured at Visit 3. LV peak mid-wall circumferential strain was assessed at the apex, middle and base of the LV, and these three variables were averaged to determine total LV circumferential strain. All strain variables are negative values; more negative values indicate greater circumferential shortening. The coefficients of variation of each LV strain variable are: base strain 19.0%, mid strain 20.2%, apex strain 18.3% and total strain 15.5%. CMR aortic pulse wave velocity (PWV) was calculated as follows: PWV (m/s) = distance (mm) / transit time between ascending to the diaphragm level of descending aorta (ms). Transit time was calculated as the average time difference using the least squares estimate between all data points on the systolic upslope of the ascending and descending aortic flow curves after peak flow normalization, and distance from ascending to descending aorta was measured using the oblique sagittal image through the thoracic aorta.
Brain natriuretic peptide (BNP) measurements
Plasma BNP levels were measured at Visit 1 using a chemiluminescent immunoassay performed on the Siemens Advia Centaur (Siemens Medical Solutions, Erlangen, Germany) (Figure 1).18 The coefficient of variation of the assay was previously discribed.18 We included the histogram of raw BNP data in Supplemental Data (Supplemental figure).
Outcomes of the longitudinal study
The primary outcome was time to HF hospitalization. In the JHS cohort, HF hospitalization surveillance began January 1, 2005. Among participants who survived to January 1, 2005, we assessed the cumulative incidence of HF hospitalization from January 1, 2005 through December 31, 2012 (Figure 1). Potential HF hospitalizations were identified and adjudicated as previously described.19 In brief, hospitalization data were obtained from the hospital discharge index from all catchment area hospitals and annual follow-up information. Hospitalization data from noncatchment area hospitals were obtained after participant consent. The self-reported data from annual follow-up were confirmed with the hospital discharge index data. The primary diagnoses based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were reviewed by trained medical personnel and adjudicated by trained adjudicators based on signs and symptoms, clinical documentation, labs, chest x rays and other imaging modalities including echocardiography, multiple gated acquisition scans and MRIs.20 Incident CHD was ascertained through directed patient queries during annual telephone follow-up and ongoing surveillance of hospitalizations, and subsequently confirmed through the review of hospital records.
Statistical Analysis
Data are presented as mean with standard deviations for normally distributed continuous variables, median with interquartile ranges for non-normally distributed continuous variables and frequencies and proportions for categorical variables. Analysis of variance with post-hoc Bonferroni test, Mann–Whitney U test and Chi-squared test were used for comparison of variables between smoking status groups if applicable. Relationships between smoking variables and BNP levels and cardiac structure and function were examined as cross-sectional analyses at Visit 1 and Visit 3 respectively. Relationships between smoking variables and incident heart failure hospitalization were assessed as prospective longitudinal analyses.
Cross-sectional study
Relationships between smoking status (current, former, never), intensity among current smokers (cigarettes / day) and burden among ever smokers (pack-years) and cardiac structure and function measured using CMR (LV volume variables, LV EF, LV mass index, LV mass / volume, and LV systolic strain variables) and BNP levels were assessed using linear regression analysis. Two models, minimally and further adjusted models were constructed to evaluate associations of smoking and cardiac structure and function. Model 1 included adjustment for age and sex, whereas Model 2 additionally included BMI, systolic blood pressure, use of anti-hypertension medications, history of diabetes mellitus and eGFR based on a previous meta-analysis which examined several risk factors and incident HF and we additionally included eGFR to determine if the effect of smoking is independent of its effect on renal function.12, 21 Medication use may affect the relationship between smoking status and LV structure and function. Thus, we created another model to examine the relationship between smoking status and LV structure and function assessed by cardiac MRI with additional adjustment for classes of medications (calcium channel blocker use, beta blocker use, angiotensin converting enzyme inhibitor or angiotensin receptor blocker use, and diuretics use; Supplemental Table 2) instead of anti-hypertensive medication use. To examine the possibility of unmeasured differences in blood pressure that may affect the associations, we additionally included average ambulatory systolic blood pressure (ABPM) instead of office systolic blood pressure (Model 3). ABPM was performed as previously described.22 ABPM was evaluated only at Visit 1 and the number of included participants who underwent ABPM was small (n=711). Therefore, we were only able to examine the relationship between smoking status and BNP levels (Model 3). Aortic valve stenosis (AS) and mitral valve regurgitation (MR) can increase LV load and promote LV hypertrophy. Thus, we additionally adjusted for AS and MR severity evaluated by echocardiography at Visit 1. Analyses of smoking status and BNP levels (Supplemental Table 3, Model 4), and smoking status and incident HF hospitalization (Supplemental Table 4, Model 5) were additionally performed with adjustment for the grade of AS and MR. Detailed information on the echocardiographic methods is described in the supplemental material. BNP levels were natural log transformed because they were not normally distributed. To visualize the relationship between smoking intensity and burden and BNP levels, restricted cubic spline curves were used. The analysis was adjusted using multiple covariates (model 2) and we used 3 knots. Knots were located at 10 (half-pack), 20 (pack) and 40 cigarettes (2 packs) / day for intensity, and 7.5, 15 and 30 pack-years for burden. For this analysis, the y axes were expressed as adjusted geometric mean ratios with 95% CI.
Longitudinal study
We constructed Kaplan-Meier curves for cumulative survival free from incident HF for smoking status (current, former, never), intensity among current smokers (cigarettes / day) and burden among ever smokers (pack-years), and compared using log-rank tests. Cox proportional hazards models were used to estimate the hazard ratios (HR) of incident HF using smoking status, intensity and burden category groups. Censoring was applied to both loss to follow-up and deaths. The assumption of proportionality was tested using Schoenfeld residuals. No significant deviations from proportionality were observed. Several models were constructed to evaluate associations of smoking information with outcomes. The same models that were used in the cross-sectional analyses were used, except in Model 2 incident CHD was additionally included as a time-dependent variable to evaluate the influence of incident CHD on incident HF. All statistical analyses were performed with STATA version 14 (STATA Corp, College Station, TX). A 2-sided P value <0.05 was considered significant.
Results
Baseline characteristics
Among the study participants (n=4129), 503 (12%) were current smokers, 742 (18%) were former smokers and 2884 (70%) were never smokers. Never smokers were more likely to be women than the other smoking status groups. Former smokers were older, had higher prevalence of hypertension and diabetes than the other smoking status groups. Current smokers had higher prevalence of current drinking, lower prevalence of achieving the recommended physical activity level and had higher mean eGFR than the other smoking status groups. The prevalence of taking calcium channel blockers, beta-blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers and diuretics were higher in former smokers than the other groups, and the prevalence of taking these anti-hypertensive medications in current smokers were lower than in never smokers (Table 1).
Table 1.
Baseline characteristics
| Variable | Overall (n=4129) |
Never Smoker (n=2884) |
Former Smoker (n=742) |
Current Smoker (n=503) |
|---|---|---|---|---|
| Age, years | 54 ± 13 | 53 ± 13 | 60 ± 11 | 51 ± 11 |
| Female Sex, n (%) | 2607 (63) | 1983 (69) | 381 (51) | 243 (48) |
| BMI, kg/m2 | 31 ± 7 | 32 ± 7 | 31 ± 6 | 29 ± 7 |
| SBP, mmHg | 127 ± 17 | 126 ± 16 | 128 ± 17 | 128 ± 18 |
| DBP, mmHg | 76 ± 9 | 76 ± 9 | 75 ± 9 | 77 ± 9 |
| SBP (ABPM), mmHg | 135 ± 28 | 135 ± 32 | 136 ± 15 | 137 ± 16 |
| DBP (ABPM), mmHg | 79 ± 27 | 79 ± 32 | 78 ± 10 | 82 ± 10 |
| Hypertension, n (%) | 2084 (50) | 1398 (49) | 453 (61) | 233 (46) |
| Diabetes, n (%) | 691 (17) | 458 (16) | 168 (23) | 65 (13) |
| Current Alcohol Use, n (%) | 1964 (48) | 1223 (42) | 367 (50) | 374 (74) |
| Physical Activity, n (%) | ||||
| Poor | 1949 (47) | 1321 (46) | 340 (46) | 288 (57) |
| Intermediate | 1341 (32) | 955 (33) | 253 (34) | 133 (26) |
| Recommended | 839 (20) | 608 (21) | 149 (20) | 82 (16) |
| Tc / HDL ratio | 4.1 ± 1.3 | 4.1 ± 1.3 | 4.2 ± 1.4 | 4.2 ± 1.5 |
| eGFR, ml/min/1.73m2 | 87 ± 17 | 87 ± 17 | 85 ± 17 | 93 ± 18 |
| Calcium channel blocker use | 650 (16) | 440 (16) | 154 (21) | 56 (11) |
| Beta blocker use | 301 (7) | 201 (7) | 68 (9) | 32 (7) |
| ACEI or ARB use | 506 (12) | 345 (12) | 126 (17) | 35 (7) |
| Diuretic use | 1072 (27) | 739 (26) | 249 (34) | 84 (17) |
| Average number of cigarettes / day | 10 (6, 20) | 10 (8, 20) | ||
| Pack-years of cigarettes | 21 (11, 39) | 17 (9, 31) |
ABPM: ambulatory blood pressure, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, BMI: body mass index, DBP: diastolic blood pressure, eGFR: estimated glomerular filtration rate, SBP: systolic blood pressure, Tc / HDL ratio: Total cholesterol / high density lipoprotein cholesterol ratio.
Smoking status and cardiac structure and function assessed by CMR at visit 3
After adjustment for confounding factors, current smoking was associated with higher mean LV mass index (beta coefficient 5.24, 95% CI 2.71~7.77) and LV mass / volume ratio (beta coefficient 0.12, 95% CI 0.06~0.18), whereas smoking status was not associated with mean LV volume measurements or LVEF (Table 2). However, current smoking was significantly associated with lower mean LV systolic function assessed with LV circumferential strain (total peak systolic circumferential strain: beta coefficient 0.74, 95% CI 0.26~1.22). Current smoking also was associated with higher mean pulse wave velocity in Model 2 (beta coefficient 1.25, 95% CI 0.09~2.41) (Table 2). After additional adjustment for medication class (Model 3), the relationship between smoking status and cardiac structure and function was not remarkably changed (Supplemental Table 2). However, in this model, former smoking was associated with lower LV mass index.
Table 2.
Smoking status and cardiac structure and function assessed by CMR at Visit 3
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Never Smoker (n=791) |
Former Smoker (n=198) |
Current Smoker (N=103) |
Never Smoker (n=791) |
Former Smoker (n=198) |
Current Smoker (n=103) |
|
| Variable | β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | ||
| LVEDVI, ml/m2 | Ref. | −22.27 (−4.55, 0.14) | −1.86 (−4.81, 1.08) | Ref. | −2.09 (−4.36, 0.18) | −2.35 (−5.29, 0.60) |
| LVESVI, ml/2 | Ref. | −1.06 (−2.55, 0.43) | 0.12 (−1.80, 2.05) | Ref. | −1.02 (−1.92, 1.94) | 0.01 (−1.92, 1.94) |
| SVI, ml/2 | Ref. | −1.21 (−2.68, 0.25) | −1.99 (−3.88, 0.10) | Ref. | −1.07 (−2.53, 0.39) | −2.35 (−4.24, −0.47) |
| LVEF, % | Ref. | 0.01 (−1.41, 1.43) | −0.75 (−2.58, 1.08) | Ref. | 0.09 (−1.33, 1.51) | −0.82 (−2.66, 1.02) |
| LVMI, g/m2 | Ref. | −2.09 (−4.21, 0.02) | 4.97 (2.24, 7.69) † | Ref. | −1.73 (−3.69, 0.22) | 5.24 (2.71, 7.77) † |
| LV mass / volume | Ref. | 0.01 (−0.04, 0.06) | 0.10 (0.04, 0.17) † | Ref. | 0.01 (−0.03, 0.06) | 0.12 (0.06, 0.18) † |
| LV Strain# | ||||||
| Total Strain, % | Ref. | 0.02 (−0.36, 0.41) | 0.56 (0.07, 1.06) * | Ref. | 0.01 (−0.37, 0.38) | 0.74 (0.26, 1.22) † |
| Base Strain, % | Ref. | 0.08 (−0.39, 0.55) | 0.60 (−0.01, 1.20) | Ref. | 0.08 (−0.38, 0.55) | 0.71 (0.12, 1.31) * |
| Mid Strain, % | Ref. | −0.13 (−0.63, 0.38) | 0.50 (−0.15, 1.15) | Ref. | −0.16 (−0.66, 0.33) | 0.71 (0.07, 1.34) * |
| Apex Strain, % | Ref. | 0.16 (−0.31, 0.63) | 0.61 (0.01, 1.21) * | Ref. | 0.14 (−0.32, 0.59) | 0.82 (0.23, 1.41) † |
| PWV, m/sec | Ref. | 0.23 (−0.65, 1.12) | 1.42 (0.27, 2.57) * | Ref. | 0.24 (−0.64, 1.12) | 1.25 (0.09, 2.41) * |
LVEDV: left ventricular end-diastolic volume, LVEF: left ventricular ejection fraction, LVESV: left ventricular end-systolic volume, LVMI: left ventricular mass index, PWV: pulse wave velocity from ascending aorta to descending aorta. #: Strain indicators represent mean peak systolic circumferential strain. Visit 3 smoking status information was used for the analysis. Model 1: adjusted for age and sex. Model 2: further adjusted for systolic blood pressure, anti-hypertensive medication use, body mass index, diabetes, and estimated glomerular filtration rate.
p<0.05
p<0.01.
Smoking status, intensity, and burden and BNP levels at visit 1
After adjustment for confounding factors, current smoking (Model 2: beta coefficient 0.182, 95% CI 0.074~0.290), smoking intensity among current smokers (Model 2, ≥20 cigarettes/day vs. never smokers: beta coefficient 0.298, 95%CI 0.122~0.474) and smoking burden among ever smokers (Model 2, ≥30 pack-years vs. never smoker: beta coefficient 0.139, 95%CI 0.018~0.260) were significantly associated with higher mean BNP levels (Table 3). Even after adjustment for mean systolic ambulatory blood pressure instead of office systolic blood pressure, the association was not remarkably changed (Table 3, Model 3). After adjustment for grade of AS and MR severity, the association was not remarkably changed (Supplemental Table 3). Figure 2 shows the restricted cubic spline between smoking intensity (average cigarettes / day), smoking burden (pack-years) and log transformed BNP levels. Log-transformed BNP levels were associated were positively associated with increased smoking intensity and burden.
Table 3.
Smoking status, intensity, burden and BNP levels (log transformed) at Visit 1
| Smoking Status | Never Smoker | Former Smoker | Current Smoker |
|---|---|---|---|
| Model | β (95%CI) | β (95%CI) | |
| Model 1 | Ref. | 0.000 (−0.094, 0.095) | 0.218 (0.111, 0.325) † |
| Model 2 | Ref. | 0.042 (−0.053, 0.137) | 0.182 (0.074, 0.290) † |
| Model 3 | Ref. | 0.165 (−0.026, 0.357) | 0.329 (0.061, 0.597) * |
| Smoking Intensity‡ | Never Smoker | <10 Cigarettes/day | 10–19 Cigarettes/day | ≥ 20 Cigarettes/day |
|---|---|---|---|---|
| Model | β (95%CI) | β (95%CI) | β (95%CI) | |
| Model 1 | Ref. | 0.101 (−0.086, 0.288) | 0.203 (0.038, 0.367) * | 0.341 (0.165, 0.516) † |
| Model 2 | Ref. | 0.065 (−0.123, 0.252) | 0.151 (−0.015, 0.317) | 0.298 (0.122, 0.474) † |
| Model 3 | Ref. | 0.135 (−0.297, 0.568) | 0.321 (−0.098, 0.741) | 0.711 (0.206, 1.215) † |
| Smoking Burden‡ | Never Smoker | < 7.5 Pack-years | 7.5-15 Pack-years | 15-30 Pack-years | ≥ 30 Pack-years |
|---|---|---|---|---|---|
| Model | β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | |
| Model 1 | Ref. | 0.036 (−0.132, 0.204) | 0.083 (−0.061, 0.228) | 0.075 (−0.050, 0.200) | 0.119 (−0.002, 0.240) |
| Model 2 | Ref. | 0.030 (−0.139, 0.199) | 0.104 (−0.040, 0.248) | 0.069 (−0.055, 0.193) | 0.139 (0.018, 0.260) * |
| Model 3 | Ref. | −0.001 (−0.385, 0.384) | 0.112 (−0.216, 0.441) | 0.213 (−0.056, 0.482) | 0.375 (0.124, 0.626) † |
body mass index, systolic blood pressure, anti-hypertensive medication use, diabetes and estimated glomerular filtration rate. Model 3: adjusted for Model 2, but including mean systolic ambulatory blood pressure instead of office systolic blood pressure
p<0.05,
p<0.01.
Smoking intensity analyses include only never smoker and current smoker. Smoking burden analyses include never smoker and ever smoker.
Figure 2. Smoking Intensity, Burden, and Brain Natriuretic Peptide Levels.
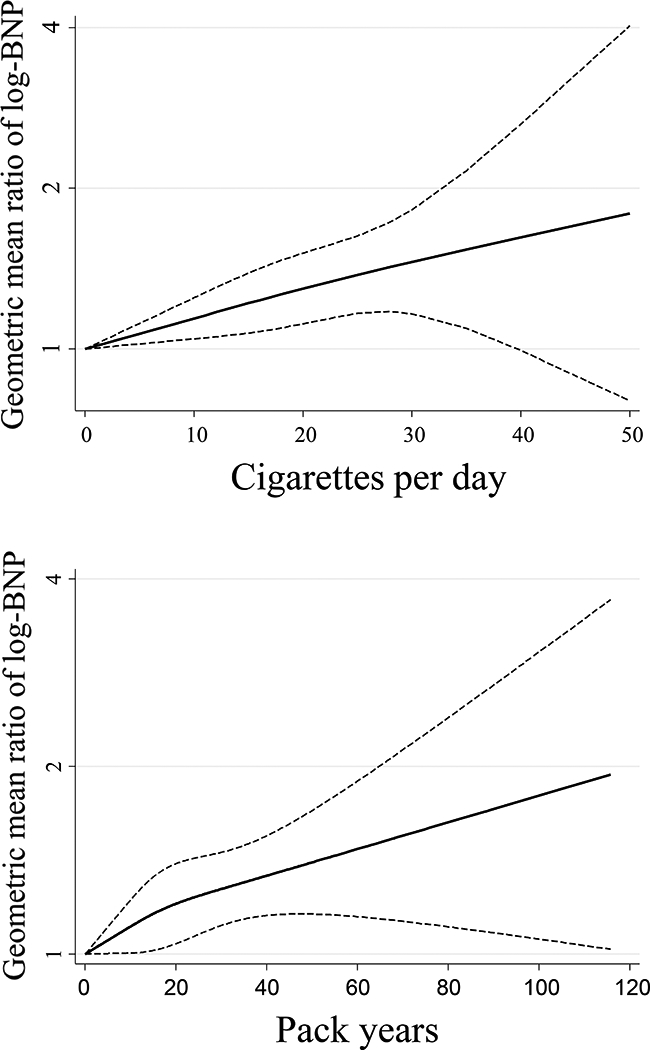
Restricted cubic spline analyses demonstrate increased log-transformed BNP levels with increased smoking intensity (top panel) and burden (bottom panel).
Smoking status, intensity and burden and incident HF hospitalizations
At the time of January 1st 2005, 3633 participants out of 4129 were alive and eligible for the longitudinal analysis. Over a median follow-up of 8.0 years (interquartile range, 7.7–8.0 years), there were 147 incident HF hospitalizations (incidence rate: 5.46 per 1,000 person-years). Both former and current smokers had a higher incidence of HF than never smokers (log-rank P <0.01) (Figure 3). Current smoking was associated with increased incident HF hospitalizations after adjustment for conventional risk factors and incident CHD as a time dependent variable (HR 2.82, 95% CI, 1.71~4.64) (Table 4). Furthermore, smoking intensity among current smokers (model 2, ≥20 cigarettes/day vs never smoker: HR 3.48, 95% CI 1.65~7.32) was associated with incident HF hospitalization in multivariable analyses (Table 4). Smoking burden among ever smokers was associated with incident HF, albeit not linearly (model 2, ≥15 pack-years vs never smoker: HR 2.06, 95% CI 1.29~3.33, P<0.01 and ≥30 pack-years vs never smoker: HR 1.60, 95% CI 1.00~2.56) (Table 4). After additional adjustment for valvular heart disease, the association was not remarkably changed (Supplemental Table 4, Model 5).
Figure 3. Kaplan Meier Survival Curves of the Study Participants.
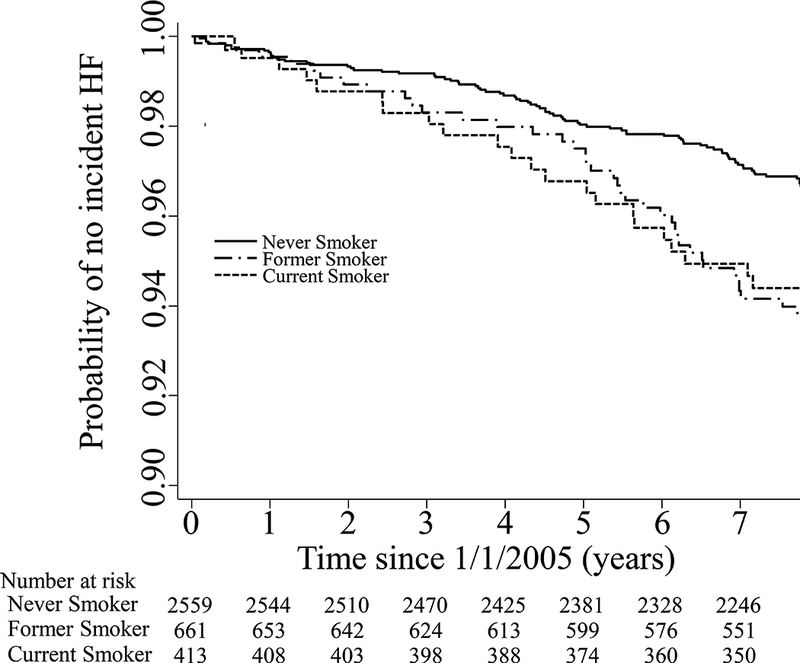
Kaplan Meier Curves separated by smoking status.
Table 4.
Smoking status, intensity, burden and incident HF hospitalization
| Smoking Status | Never Smoker | Former Smoker | Current Smoker |
|---|---|---|---|
| Model | H.R. (95%CI) | H.R. (95%CI) | |
| Model 1 | Ref (1) | 1.46 (0.99, 2.13) | 2.25 (1.39, 3.65) † |
| Model 2 | Ref (1) | 1.44 (0.98, 2.12) | 2.82 (1.71, 4.64) † |
| Smoking Intensity‡ | Never Smoker | <10 Cigarettes/day | 10–19 Cigarettes/day | ≥ 20 Cigarettes/day |
|---|---|---|---|---|
| Model | H.R. (95%CI) | H.R. (95%CI) | H.R. (95%CI) | |
| Model 1 | Ref (1) | 1.42 (0.52, 3.89) | 2.08 (0.99, 4.35) | 2.67 (1.30, 5.50) † |
| Model 2 | Ref (1) | 1.57 (0.57, 4.35) | 2.64 (1.24, 5.62) * | 3.48 (1.65, 7.32) † |
| Smoking Burden‡ | Never Smoker | < 7.5 Pack-years | 7.5-15 Pack-years | 15-30 Pack-years | ≥ 30 Pack-years |
|---|---|---|---|---|---|
| Model | β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | |
| Model 1 | Ref. | 0.304 (0.042, 2.192) | 1.349 (0.701, 2.598) | 2.194 (1.385, 3.475) * | 1.479 (0.928, 2.356) |
| Model 2 | Ref. | 0.310 (0.043, 2.240) | 1.425 (0.735, 2.765) | 2.056 (1.290, 3.275) * | 1.601 (1.002, 2.557) * |
Model 1: adjusted for age and sex. Model 2: further adjusted for systolic blood pressure, anti-hypertensive medication use, body mass index, diabetes, estimated glomerular filtration rate and incident coronary heart disease as a time dependent variable.
p<0.05,
p<0.01.
Smoking intensity and burden analyses include only never smoker and current smoker.
The number of each smoking status is as follows; Never smoker n=2884, Former smoker n=742, Current smoker n=503. The number of each smoking intensity is as follows; <10 Cigarettes/day n=143, 10–19 Cigarettes/day n=187, ≥ 20 Cigarettes/day n=162. The number of each smoking burden is as follows; <7.5 pack years n=234, 7.5–15 pack years n=256, 15–30 pack years n=362, ≥30 n=393.
Discussion
In our community-based cohort of African Americans, current smoking was associated with a higher LV mass and lower LV circumferential strain assessed by CMR. Current smoking status, higher levels of smoking intensity and burden were associated with higher mean BNP levels at baseline. Furthermore, current smoking, higher levels of smoking intensity and burden also were associated with increased risk of incident HF hospitalization after adjusting for possible confounding factors including incident CHD.
Limited evidence on the relationship between smoking and HF currently exists.1–5, 23, 24 In the Health, Aging, and Body Composition Study, both current smoking and past smoking were associated with incident HF independently of incident CHD, and smoking burden was associated with incident HF among former smokers, but not among current smokers.1 The impact of current smoking on incident HF was higher in our study (HRs 1.93 vs 2.82 in ours) than in their study. To our knowledge, their study was the first which examined the relationship between smoking status and incident HF with adjustment for incident CHD, as well as examination of the relationships between smoking burden and incident HF. Our study results are consistent with the Health Aging, and Body Composition Study, and extend the findings to a large cohort of African Americans. However, in contrast to the Health, Aging, and Body Composition Study, our study showed dose-dependent associations of smoking intensity among current smokers on incident HF accounting for incident CHD. This difference may be attributed to the difference in the numbers of current smokers in their study (n=221) and ours (n=503). In their study, past smoking was associated with incident HF, and there was dose dependency between smoking burden and incident HF among former smokers. Thus, the relationship between smoking behavior and incident HF could be different due to other factors including different ethnicities (40% African Americans in the ABC study and 100% African Americans in our study). Recently published papers reported the relationships between smoking and different phenotypes of incident HF. In the PREVEND study, current smoking was associated with incident HF with reduced ejection fraction (HFrEF).4 On the other hand, in the Framingham Heart Study, current smoking was associated with incident HF with preserved ejection fraction (HFpEF).3 In the JHS, the information of phenotypes of incident HF is currently not available, and further investigation is warranted to determine whether smoking status is associated with specific phenotypes of incident HF in African Americans.
There are several previous studies which examined the relationship between smoking and LV mass.25–30 Many of them showed a positive association; however, there are some studies which showed neutral or negative associations.27, 28 We showed a positive association between current smoking and LV mass among those without CHD. These observations are supported by several previous studies which demonstrated a positive relationship between current smoking and LV hypertrophy25, 26. Our study confirmed this finding in a large community based cohort of African Americans. Importantly, our study used CMR data. CMR is a more accurate technique for assessment of LV wall thickness, volumes and ejection fraction compared to echocardiography; therefore, CMR offers diagnostic advantages over echocardiography.31–33 Furthermore, our results are consistent with other studies evaluating risk factors such as smoking and CMR-derived measures of cardiac structure.29 One prior study which showed a negative association between smoking and LV mass or LV mass index used a unique cohort of Army Training Regiment recruits with an average age of 20 years.28 Thus, the results of that study may not be generalizable to the general community or older smokers. In our analysis, after additionally adjusted for class of medication use, former smoking was associated with lower LV mass index. The mechanism of this association is unclear at this point, and further investigation on this issue is warranted.
A recently published study showed a significant correlation between current smoking and LV diastolic dysfunction assessed by tissue Doppler echo imaging.26 This study also found a non-significant relationship between smoking and global longitudinal strain. Another study showed an adverse association between smoking status and burden on LV circumferential strain assessed by CMR among those without any symptoms or history of cardiovascular disease.34 These study findings are somewhat limited by their cross-sectional design and lack of HF outcomes. Our study builds upon this work by utilizing a large cohort of African Americans and demonstrating and linking the associations of adverse structural and functional cardiac effects of cigarette smoking with incident HF hospitalizations.
In our study, smoking status, intensity and burden were associated with higher BNP levels. Nadruz and colleagues showed that among those free of overt CHD and HF, cumulative cigarette exposure assessed by pack-years was associated with higher N terminal pro-BNP (NT pro-BNP) levels, and active smokers had a higher incidence of elevated NT pro-BNP levels after 15 years of follow up.35 Otsuka and colleagues also showed smoking status was positively associated with higher NT pro-BNP levels.36 Our study results showed that all measures of smoking (status, intensity and burden) were associated with higher BNP levels, and extend these findings to large cohort of African Americans. Both larger LV mass index and higher BNP levels reflect higher LV wall stress. Based on our findings, it is possible that current smoking, smoking intensity, and smoking burden are associated with higher LV wall stress, increasing the risk of HF.
Cigarette smoking has been associated with higher levels of inflammatory cytokines and, dysfunction and death of endothelial cells through increased oxidative stress.9, 10, 37, 38 Endothelial dysfunction and inflammation may affect cardiac structure and function either through direct influences on the myocardium or indirectly by accelerating arterial atherosclerosis and augmented LV afterload. In turn, carbon monoxide exposure may cause LV hypertrophy and systolic dysfunction independently of its effect on endothelial function or blood pressure.11 These collective effects of smoking may result in the larger LV mass and systolic dysfunction seen in our study. Furthermore, cigarette smoking is independently associated with worsening of kidney function.12 Thus, cigarette smoking-related alterations of cardiac structure and function, combined with impairment of renal function, may lead to incident HF independently of CHD. In this study, smoking burden among ever smokers was associated with incident HF, albeit nonlinearly. This may be related to a longer time since quitting in the group with the highest smoking burden (≥ 30 pack-years). Regardless, higher smoking burdens (both ≥ 15 pack-years and ≥ 30 pack-years) were significantly associated with more incident HF hospitalization.
In the current study, former smoking was not associated with adverse cardiac remodeling, impaired cardiac function, BNP levels or incident HF hospitalization after adjusting for possible confounding factors. These findings suggest that smoking cessation may be an important strategy to reduce the risk of impaired cardiac function and HF in current smokers.
Our study has a few limitations including lack of information about the type of cigarettes (including tar concentration or menthol) that the participants smoked. Self-reported smoking status was not confirmed with cotinine levels, which are currently unavailable in JHS. Our data were obtained from an all-African American cohort in Jackson, MS and may not be generalizable to other ethnic/racial groups or other regions. Unmeasured confounding may have influenced the results. It is also possible that HF cases may have been missed (or misclassified); however the definition for HF that was utilized has been previously used and validated in other JHS analyses as well as Atherosclerosis Risk in Communities Study (ARIC). In our study, the relationships between smoking status and BNP levels and cardiac structure and function were assessed at Visits 1 and 3, respectively. The longitudinal relationship between smoking status and HF hospitalization was evaluated beginning 5 years after Visit 1 (2005). Therefore, time differences of performed examinations may limit the causal inference of the effect of smoking on cardiac structure and function and BNP with the relationship between smoking and HF hospitalization. Finally, due to the lack of follow-up echocardiograms and appropriate clinical data, we were unable to assess the type of HF (ie HF with preserved versus reduced EF).
Our study also has several strengths. To our knowledge, this study is the first prospective study to show a dose relationship between cigarette smoking and incident HF in a large cohort of African Americans. African Americans have a higher incidence of HF than whites, Hispanics and Asians.14, 39 Thus, smoking cessation may be a potential strategy to attenuate the higher rate of HF in African Americans. Due to superior reliability, we used CMR instead of echocardiography to assess cardiac structure and function.40
Cigarette smoking is a well-known risk factor for cardiovascular disease. However, the influences on cardiac structure and function may not be fully recognized due to the strong association with CHD. In our study, cigarette smoking was associated with higher mean LV mass index and LV mass / volume assessed with CMR among those without known CHD. Smoking intensity and burden also are associated with higher mean BNP levels and incident HF in a dose-dependent manner. Therefore, in African Americans, cigarette smoking is a strong risk factor for higher LV mass and systolic dysfunction, and incident HF hospitalization independent of its effects on incident CHD.
Supplementary Material
Clinical Perspective.
What is new?
Cigarette smoking is a well-known risk factor for atherosclerotic cardiovascular disease; however, less is known about the risk for heart failure (particularly in African Americans).
We found that current cigarette smoking status, smoking intensity (cigarettes per day) and smoking burden (pack-years) were independently associated with higher left ventricular mass, lower left ventricular strain, higher brain natriuretic peptide levels and higher risk of incident heart failure hospitalization in African Americans.
These relationships were significant after adjustment for coronary heart disease suggesting mechanisms beyond atherosclerosis probably contribute to myocardial dysfunction and increased risk of heart failure in smokers.
What are the clinical implications?
African Americans are disproportionately affected by cardiovascular diseases including heart failure and they are more likely to die from smoking-related diseases compared with whites.
Our findings suggest that smoking is associated with structural and functional left ventricular abnormalities that lead to heart failure in African Americans and that smoking cessation should be encouraged in those with risk factors for heart failure.
Acknowledgements
The authors also wish to thank the staff and participants of the JHS. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Sources of Funding
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201300049C and HHSN268201300050C), Tougaloo College (HHSN268201300048C), and the University of Mississippi Medical Center (HHSN268201300046C and HHSN268201300047C) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority
Health and Health Disparities (NIMHD). Michael Hall has also received support from NIH/NIDDK 1K08DK099415–01A1 and NIH/NIGMS P20GM104357. Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (P50HL120163).
Footnotes
Disclosures
All authors have no significant disclosures to declare.
University of Mississippi Medical Center, Jackson, MS (DK, LRC, ERF, MDW, JB, AC, MEH); Jackson Heart Study, Jackson, MS (DK, LRC, RJM, WBW, ERF, AC, MEH); Duke University School of Medicine, Durham, NC (RJM); Tougaloo College, Tougaloo, MS (WBW); Johns Hopkins University, Baltimore, MD (MJB); University of Louisville, Louisville, KY (APD, RJK, AB); Wake Forest University School of Medicine, Winston-Salem, NC (CJR); Boston University School of Public Health, Boston, MA (EJB); Vanderbilt University Medical Center, Nashville, TN (RMR); American Heart Association Tobacco Regulation and Addiction Center (A-TRAC) (WBW, MJB, APD, CJR, RJK, EJB, JB, AB, RMR, MDW, MEH).
References
- 1.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Smith AL, Bauer DC, Newman AB, Kim L, Bibbins-Domingo K, Tindle H, Harris TB, Tang WW, Kritchevsky SB and Butler J. Cigarette smoking exposure and heart failure risk in older adults: the Health, Aging, and Body Composition Study. Am Heart J. 2012;164:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed AA, Patel K, Nyaku MA, Kheirbek RE, Bittner V, Fonarow GC, Filippatos GS, Morgan CJ, Aban IB, Mujib M, Desai RV, Allman RM, White M, Deedwania P, Howard G, Bonow RO, Fletcher RD, Aronow WS and Ahmed A. Risk of Heart Failure and Death After Prolonged Smoking Cessation: Role of Amount and Duration of Prior Smoking. Circ Heart Fail. 2015;8:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG and Levy D. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ and van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–1431. [DOI] [PubMed] [Google Scholar]
- 5.Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu WC, Manson JE, Margolis K, Johnson KC, Allison M, Corbie-Smith G, Rosamond W, Breathett K and Klein L. Risk Factors for Incident Hospitalized Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Postmenopausal Women. Circ Heart Fail. 2016;9 pii: e002883. doi.org/ 10.1161/CIRCHEARTFAILURE.115.002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox KF, Cowie MR, Wood DA, Coats AJ, Gibbs JS, Underwood SR, Turner RM, Poole-Wilson PA, Davies SW and Sutton GC. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J. 2001;22:228–236. [DOI] [PubMed] [Google Scholar]
- 7.Nicolozakes AW, Binkley PF and Leier CV. Hemodynamic effects of smoking in congestive heart failure. Am J Med Sci. 1988;296:377–380. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J and Piantadosi CA. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J Clin Invest. 1992;90:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, El-Chami MF, Bhakta S, Winchester DE, Al-Mallah MH, Sanchez Shields M, Deedwania P, Mehta LS, Phan BA and Benowitz NL. Cardiovascular Effects of Exposure to Cigarette Smoke and Electronic Cigarettes: Clinical Perspectives From the Prevention of Cardiovascular Disease Section Leadership Council and Early Career Councils of the American College of Cardiology. J Am Coll Cardiol. 2015;66:1378–1391. [DOI] [PubMed] [Google Scholar]
- 10.Levitzky YS, Guo CY, Rong J, Larson MG, Walter RE, Keaney JF Jr., Sutherland PA, Vasan A, Lipinska I, Evans JC and Benjamin EJ. Relation of smoking status to a panel of inflammatory markers: the framingham offspring. Atherosclerosis. 2008;201:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bye A, Sorhaug S, Ceci M, Hoydal MA, Stolen T, Heinrich G, Tjonna AE, Najjar SM, Nilsen OG, Catalucci D, Grimaldi S, Contu R, Steinshamn S, Condorelli G, Smith GL, Ellingsen O, Waldum H and Wisloff U. Carbon monoxide levels experienced by heavy smokers impair aerobic capacity and cardiac contractility and induce pathological hypertrophy. Inhal Toxicol. 2008;20:635–646. [DOI] [PubMed] [Google Scholar]
- 12.Hall ME, Wang W, Okhomina V, Agarwal M, Hall JE, Dreisbach AW, Juncos LA, Winniford MD, Payne TJ, Robertson RM, Bhatnagar A and Young BA. Cigarette Smoking and Chronic Kidney Disease in African Americans in the Jackson Heart Study. J Am Heart Assoc. 2016;5 pii: e003280. doi: 10.1161/JAHA.116.003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reboul C, Boissiere J, Andre L, Meyer G, Bideaux P, Fouret G, Feillet-Coudray C, Obert P, Lacampagne A, Thireau J, Cazorla O and Richard S. Carbon monoxide pollution aggravates ischemic heart failure through oxidative stress pathway. Sci Rep. 2017;7:39715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL and Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamal A, Homa DM, O’Connor E, Babb SD, Caraballo RS, Singh T, Hu SS and King BA. Current cigarette smoking among adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64:1233–1240. [DOI] [PubMed] [Google Scholar]
- 16.Taylor HA Jr. The Jackson Heart Study: an overview. Ethn Dis. 2005;15:S6–1–3. [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N and Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 18.Fox ER, Musani SK, Bidulescu A, Nagarajarao HS, Samdarshi TE, Gebreab SY, Sung JH, Steffes MW, Wang TJ, Taylor HA and Vasan RS. Relation of obesity to circulating B-type natriuretic peptide concentrations in blacks: the Jackson Heart Study. Circulation. 2011;124:1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mentz RJ, Greiner MA, DeVore AD, Dunlay SM, Choudhary G, Ahmad T, Khazanie P, Randolph TC, Griswold ME, Eapen ZJ, O’Brien EC, Thomas KL, Curtis LH and Hernandez AF. Ventricular conduction and long-term heart failure outcomes and mortality in African Americans: insights from the Jackson Heart Study. Circ Heart Fail. 2015;8:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keku E, Rosamond W, Taylor HA Jr., Garrison R, Wyatt SB, Richard M, Jenkins B, Reeves L and Sarpong D Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis. 2005;15:S6-62–70. [PubMed] [Google Scholar]
- 21.Yang H, Negishi K, Otahal P and Marwick TH. Clinical prediction of incident heart failure risk: a systematic review and meta-analysis. Open Heart. 2015;2:e000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravenell J, Shimbo D, Booth JN 3rd, Sarpong DF, Agyemang C, Beatty Moody DL, Abdalla M, Spruill TM, Shallcross AJ, Bress AP, Muntner P and Ogedegbe G Thresholds for Ambulatory Blood Pressure Among African Americans in the Jackson Heart Study. Circulation. 2017;135:2470–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avery CL, Loehr LR, Baggett C, Chang PP, Kucharska-Newton AM, Matsushita K, Rosamond WD and Heiss G. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2012;60:1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB and Health ABCS Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markus MR, Stritzke J, Baumeister SE, Siewert U, Baulmann J, Hannemann A, Schipf S, Meisinger C, Dorr M, Felix SB, Keil U, Volzke H, Hense HW, Schunkert H and Study MKAC. Effects of smoking on arterial distensibility, central aortic pressures and left ventricular mass. Int J Cardiol. 2013;168:2593–2601. [DOI] [PubMed] [Google Scholar]
- 26.Nadruz W Jr., Claggett B, Goncalves A, Querejeta-Roca G, Fernandes-Silva MM, Shah AM, Cheng S, Tanaka H, Heiss, Kitzman DW and Solomon SD. Smoking and Cardiac Structure and Function in the Elderly: The ARIC Study (Atherosclerosis Risk in Communities). Circ Cardiovasc Imaging. 2016;9:e004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasegawa T, Boden-Albala B, Eguchi K, Jin Z, Sacco RL, Homma S and Di Tullio MR. Impaired flow-mediated vasodilatation is associated with increased left ventricular mass in a multiethnic population. The Northern Manhattan Study. Am J Hypertens. 2010;23:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne JR, James LE, Eleftheriou KI, Hawe E, Mann J, Stronge A, Banham K, World M, Humphries SE, Pennell DJ and Montgomery HE. The association of left ventricular mass with blood pressure, cigarette smoking and alcohol consumption; data from the LARGE Heart study. Int J Cardiol. 2007;120:52–58. [DOI] [PubMed] [Google Scholar]
- 29.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA and Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leigh A, Balfour P, Kaplan R, Swett K, Kansal MM, Talavera GA, Perreira K, Blaha M, Benjamin E, Robertson R, Bhartnagar A, Rodriguez CJ. Association of the Intensity and Duration of Cigarette Smoking Exposure with Cardiac Structure and Function in Current Daily Smokers: The Echocardiographic Study of Hispanic/Latinos. OpenHeart. 2017;4:e000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buddhe S, Lewin M, Olson A, Ferguson M and Soriano BD. Comparison of left ventricular function assessment between echocardiography and MRI in Duchenne muscular dystrophy. Pediatr Radiol. 2016;46:1399–1408. [DOI] [PubMed] [Google Scholar]
- 32.Corona-Villalobos CP, Sorensen LL, Pozios I, Chu L, Eng J, Abraham MR, Abraham TP, Kamel IR and Zimmerman SL. Left ventricular wall thickness in patients with hypertrophic cardiomyopathy: a comparison between cardiac magnetic resonance imaging and echocardiography. Int J Cardiovasc Imaging. 2016;32:945–954. [DOI] [PubMed] [Google Scholar]
- 33.Brunklaus A, Parish E, Muntoni F, Scuplak S, Tucker SK, Fenton M, Hughes ML and Manzur AY. The value of cardiac MRI versus echocardiography in the pre-operative assessment of patients with Duchenne muscular dystrophy. Eur J Paediatr Neurol. 2015;19:395–401. [DOI] [PubMed] [Google Scholar]
- 34.Rosen BD, Saad MF, Shea S, Nasir K, Edvardsen T, Burke G, Jerosch-Herold M, Arnett DK, Lai S, Bluemke DA and Lima JA. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic: individuals the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;47:1150–1158. [DOI] [PubMed] [Google Scholar]
- 35.Nadruz W Jr., Goncalves A, Claggett B, Querejeta Roca G Shah AM, Cheng S, Heiss G, Ballantyne CM and Solomon SD. Influence of cigarette smoking on cardiac biomarkers: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Heart Fail. 2016;18:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otsuka T, Kawada T, Seino Y, Ibuki C, Katsumata M and Kodani E. Relation of smoking status to serum levels of N-terminal pro-brain natriuretic peptide in middle-aged men without overt cardiovascular disease. Am J Cardiol. 2010;106:1456–1460. [DOI] [PubMed] [Google Scholar]
- 37.Messner B and Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34:509–515. [DOI] [PubMed] [Google Scholar]
- 38.McEvoy JW, Nasir K, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, Barr RG, Budoff MJ, Szklo M, Navas-Acien A, Polak JF, Blumenthal RS, Post WS and Blaha MJ. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics−-2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 40.Breitenbach I, Harringer W, Tsui S, Amorim MJ, Herregods MC, Bogaert J, Goiti JJ and Gerosa G. Magnetic resonance imaging versus echocardiography to ascertain the regression of left ventricular hypertrophy after bioprosthetic aortic valve replacement: results of the REST study. J Thorac Cardiovasc Surg. 2012;144:640–645 e641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


