Abstract
Alzheimer’s disease (AD) is the most common form of dementia that is often accompanied by mood and emotional disturbances and seizures. There is growing body of evidence that neurons expressing γ-aminobutyric acid (GABA) play an important role in regulation of cognition, mood, and emotion as well as seizure susceptibility, but participation of GABAergic neuronal pathology in Alzheimer’s disease (AD) is not understood well at present. Here, we report that transgenic mice expressing human amyloid precursor protein Swedish-Deutch-Iowa mutant (APPSweDI) exhibit early loss of neurons expressing GAD67, a GABA synthesizing enzyme, in advance to the loss of pyramidal neurons in hippocampal CA1 region. The loss of GAD67+ neurons in APPSweDI mice accompanied with decreased spatial cognition as well as increased anxiety-like behaviors and kainic acid-induced seizure susceptibility at early phase. In the hippocampal CA1 region, GAD67+ neurons expressed high basal levels of neuronal nitric oxide synthase (nNOS) and nitrosative stress (nitrotyrosine). Similarly, GAD67+ neurons in primary cortical and hippocampal neuron cultures also expressed high basal levels of nNOS and degenerated in response to lower Aβ concentrations due to their high basal levels of nitrosative stress. Given the role of GABAergic neurons in cognitive and neuropsychiatric functions, this study reports the role of nNOS mediated nitrosative stress in dysfunction of GABAergic neurons and its potential participation in early development of cognitive and neuropsychiatric symptoms in AD.
Keywords: GABAergic neurons, hippocampus, nNOS, nitrosative stress, amyloid-β (Aβ)
Introduction
The area of brain hippocampus plays the major role in the process of learning and memory and is the most affected area in Alzheimer’s disease (AD). Neural network of hippocampus is composed of different types of neurons (e.g. pyramidal, granular, and GABAergic neurons). Among them, GABAergic neurons are the local neurons which comprise a relatively small population but distributed throughout the hippocampus to provide inhibitory inputs to virtually all excitatory units (Chamberland S and Topolnik L, 2012). AD brains are associated with GABAergic neuronal degeneration and thus disruptions in the GABAergic neurotransmission [see review (Lanctot KL et al., 2004)]. In addition, transgenic mice expressing human Aβ precursor protein (APP), tau, or apolipoprotein E4 allele (AopE4) also develop age-dependent loss of GABAergic neurons in the hippocampus with decreased learning/memory functions (Andrews-Zwilling Y et al., 2010; Levenga J et al., 2013; Verret L et al., 2012). In hippocampus, GABAergic neurons play a crucial role in long-term potentiation (LTP) (Meredith RM et al., 2003), a form of neuroplasticity playing a critical role in memory formation. These observations, therefore, suggest a potential involvement of GABAergic neuronal pathology in development of cognitive impairment in AD.
GABAergic neurons also play an important role in regulation of mood and emotion (Shiah IS and Yatham LN, 1998) and defects in their functions have been implicated in schizophrenia, depression, and anxiety (Luscher B et al., 2011; Nuss P, 2015; Taylor SF and Tso IF, 2015). In parallel with the disruption of GABAergic system in AD brains (Lanctot KL, Herrmann N, Mazzotta P, Khan LR and Ingber N, 2004), depression is also reported as the most frequent comorbidity of AD (Modrego PJ, 2010). Moreover, similar depression-like behaviors were also observed in mouse models of AD (Romano A et al., 2015). Anxiety is another significant complication in AD (Kaiser NC et al., 2014) and anxiety-like behaviors were reported in transgenic mice expressing human mutant APP and ApoE4 (Espana J et al., 2010; Tong LM et al., 2014). Loss of GABAergic neurons also shifts the neuronal excitation and inhibition balance towards excitation (Ben-Ari Y and Represa A, 1990) and thus increases the probability of seizure onset (Knopp A et al., 2005; Wozny C et al., 2003). In AD, seizures are highly prevalent (Born HA, 2015; Vossel KA et al., 2013), and, accordingly, animal models of AD also show increased spontaneous seizure activities as well as increased seizure susceptibility to proconvulsant agents (Born HA, 2015). These studies document the role of GABAergic degeneration in development of cognitive, neurological, and psychiatric symptoms in AD.
Nitric oxide (NO) is a key signaling molecule throughout the body. NO is produced by a family of enzymes called nitric oxide synthases (NOS). There are three NOS isoforms, neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS), all of which differ in physiological role and expression profile. nNOS is an enzyme responsible for the synthesis of NO in neurons but not all neurons are known to express nNOS. In hippocampus and cortex, nNOS is primarily expressed in specific subpopulations of GABAergic neurons (Tricoire L and Vitalis T, 2012), where it regulates GABA release (Gasulla J and Calvo DJ, 2015; Getting SJ et al., 1996; Lee JJ, 2009; Maggesissi RS et al., 2009; Wall MJ, 2003; Yang Q et al., 2007), local blood flow (Cauli B and Hamel E, 2010; Cauli B et al., 2004; Perrenoud Q et al., 2012), and seizure and epileptiform activities (Demchenko IT and Piantadosi CA, 2006; Gholipour T et al., 2010; Rajasekaran K et al., 2003). nNOS is activated by calcium (Ca2+)-dependent calmodulin and secondary modification of nNOS (e.g. denitrosylation and phosphorylation) (Gingerich S and Krukoff TL, 2008; Hayashi Y et al., 1999; Komeima K et al., 2000; Qu ZW et al., 2012). Under physiological conditions, the activated nNOS produces NO from L-arginine (coupled nNOS reaction). However, under oxidative stress conditions where its cofactor tetrahydro-biopterin is decreased, nNOS also produces superoxide anion (O2•−), in addition to NO, and thus leading to generation of peroxynitrite (ONOO−), the most reactive NO-derived oxidant (Pall ML, 2007; Xia Y et al., 1996). AD brains are reported to have decreased level of tetrahydrobiopterin (Barford PA et al., 1984) and increased level of nitrotyrosine (N-Tyr) (Sultana R et al., 2009). In addition, nNOS expressing neurons in different areas of AD brains were reported to be highly susceptible to Aβ induced neurodegeneration (Thorns V et al., 1998). These reports indicate a potential role of nNOS dysregulation in neuronal degeneration in AD, but the involvement of nNOS dysfunction in GABAergic neuronal degeneration and associated cognitive and neuropsychiatric disorders in AD is not well understood at present.
In this study, we report that transgenic mice expressing human APP Swedish-Dutch-Iowa mutant (APPSweDI) had loss of GAD67 immunoreactivity in stratum pyramidale of hippocampal CA1 region in advance to the loss of pyramidal neurons. The GAD67+ neurons expressed high basal levels of nNOS and nitrosative stress and exhibited higher vulnerability to Aβ induced cytotoxicity than other types of neurons. The loss of GAD67 expressing cells accompanied with increased cognitive deficits and neuropsychiatric-like symptoms as well as increased seizure susceptibility in APPSweDI mice. Although pathological relationship between the observed loss of GAD67+ neurons and increased cognitive/neuropsychiatric deficits in APPSweDI is not completely understood, these data suggest the potential role of nNOS dysregulation in cell death or hypo-function of GABAergic neurons and thus loss of GABAergic function for cognitive and neuropsychiatric functions in AD mouse model.
Experimental Procedure
Animals
All animal procedures were in accordance with the animal experiment guidelines of the Medical University of South Carolina and National Institute of Health. Wild-type (C57Bl/6J; The Jackson Laboratories, Bar Harbor, ME; https://www.jax.org/strain/000664) and APPSweDI mice [C57BL/6-Tg(Thy1-APPSweDutIowa)BWevn/Mmjax; The Jackson Laboratories; https://www.jax.org/strain/007027] were housed in cages under controlled temperature (21 ± 1°C) and humidity (55 ± 10%), with a 12-hr light/12-hr dark cycle. Equal number of male and female WT and APPSweDI mice (6 and 14 months old) were used.
Histology and immuno-fluorescent staining
Cryosections (40 μm thick) obtained from 4% paraformaldehyde-fixed brain tissues or paraffin-embedded sections obtained from formalin-fixed brain tissues were used for Nissl staining (Nissl stain kit; IHCWORLD, Woodstock, MD) or immunofluorescent staining for Aβ (BC05; Wako, Osaka, Japan, Cat# 010-26903), GAD67 (Millipore, Billerica, MA, Cat# MAB5406, RRID: AB_2278725), Neurogranin (Millipore, Cat# AB5620, RRID: AB_91937), nNOS (Santa Cruz, Dallas, TX, Cat# sc-5302, RRID: AB_626757) and N-Tyr (Millipore, Cat# 487923, RRID: AB_212231). For immunofluorescent staining of neuron culture, the neurons cultured on poly-D-lysine (Sigma-Aldrich, Saint Louis, MO) coated chamber slides (Nunc Lab-Tek, Rochester, NY) were fixed in formaldehyde and incubated with MAP2 (Santa Cruz, Cat# sc-20172, RRID: AB_2250101), GAD67, nNOS, or N-Tyr. DAPI (4′,6-diamidino-2-phenylindole; Thermo-Fisher Scientific, Houston, TX) was used for staining of nuclei. BX60 Olympus fluorescent/light microscope equipped with DP-70 camera (Olympus, Tokyo, Japan) was used for histological and immunofluorescent imaging. The number of stained cells were manually counted in three randomly selected microscope fields.
Western immunoblot analysis
For Western immunoblot of hippocampal tissue, hippocampi of mice were dissected and homogenized in SDS sample loading buffer (50 mM Tris-HCl, pH 6.8, 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, and10% glycerol) by sonication and 20μg of protein/well was resolved in SDS-PAGE gel (4–20%, Life Science Research, Hercules, CA, USA) and then transferred to polyvinylidene fluoride membranes. The membrane was blocked with 5% non-fat dry milk in TBST (10 mM Tris-HCl, pH 7.4, 0.15 M NaCl, 0.05% Tween-20) and incubated with antibodies against GAD67, EMX1 (Santa Cruz, Cat# sc-28220, RRID: AB_2262220), Aβ/6E10, nNOS, p35/p25 (Cell Signaling Technology, Danvers, MA, Cat# 2680S, RRID: AB_1078214), phospho-Ser9-GSK-3β (p-GSK-3β S9; Cell Signaling Technology, Cat# 9336, RRID: AB_331405), Cleaved Caspase 3 (Cell Signaling Technology, Cat# 9664, RRID: AB_2070042), or β-actin (Santa Cruz, Cat# sc-47778, RRID: AB_626632), then with appropriate secondary antibody-conjugated with horseradish peroxidase (HRP). The signal of HRP was detected by ECL-plus Western blotting detection system (Amersham Pharmacia Biotech, Piscataway, NJ, USA)
Morris water maze test
Morris water maze was employed to assess spatial learning and memory according to previously published methods with modification (Won JS et al., 2013). The test was performed in a circular pool (124 cm in diameter/60 cm in depth) filled with water clouded by nontoxic white paint. The circular pool consisted of four equal virtual quadrants. A circular area (radius 20 cm from the center of the platform) was defined as the target zone, equivalent to 4.9% of the total water maze area. All other experimental conditions are identical with our previous report (Won JS, Kim J, Annamalai B, Shunmugavel A, Singh I and Singh AK, 2013).
Open field test for anxiety-like and exploratory behaviors
Open field test was performed to assess anxiety-like and exploratory behaviors as described previously with modification (Miyakawa T et al., 2001). Briefly, the mice were placed in the center of an open arena (length 1 m; width 1 m; side walls 20 cm height) for 5 min and explorative behavior was recorded by a video-tracking system (Accuscan, Ohio). Total distance traveled and the relative time spent in the center of the arena were recorded and quantified. In addition, number and time spent in freezing (immobility longer than 3 seconds), grooming, rearing, and defecation were measured.
Kainic Acid (KA) treatment
The mice were treated with repeated low dose (RLD) of KA (5mg/kg/i.p. per every 30 min, up to a maximum of 6 times) based on previous report (Tse K et al., 2014). Following the first KA injection, the time to reach status epilepticus (stage 5 in Racine scale; behaviors such as generalized tonic-clonic convulsions with lateral recumbence or jumping and wild running followed by generalized convulsions (Racine RJ, 1972)) were analyzed. Following the last KA injection, the number of mice that reached stage 5 seizure and survival rate were analyzed. At 3 days after KA injection, the survived mice were sacrificed and the brains were extracted for histological analysis.
Primary neuronal culture
Primary neuron cultures were prepared from the cerebral cortex and hippocampus of embryos of Sprague Dawley rats at embryonic day 17 (E17) as described in our previous report (Won JS, Kim J, Annamalai B, Shunmugavel A, Singh I and Singh AK, 2013). The cultured neurons were maintained in Neurobasal media (Invitrogen, Carlsbad, CA) supplemented with 2% B27 supplement (Invitrogen), 0.5 mM glutamine, 25 μM glutamate, 50 units/ml penicillin, 50 μg/ml streptomycin under humidified atmosphere of 5% CO2 and 95% O2, at 37 °C. All experiments presented in this work were performed on neuronal cells at 7 days in vitro.
Preparation of Aβ
Synthetic human Aβ25-35 and Aβ1-42 peptides were purchased from Anaspec (Fremont, CA). Soluble, oligomeric, and fibrillary forms of Aβ1-42 peptides was generated as previously described (Stine WB et al., 2011). Briefly, Aβ1-42 peptide was solved in hexafluoro-2-propanol (HFIP), lyophilized in vacuum centrifugation, and reconstituted in dimethylsulfoxide (monomeric Aβ1-42). Then the monomeric Aβ1-42 peptide was further incubated in phenol-free F-12 cell culture media for 24h at 4°C for preparation of oligomeric Aβ1-42 or resuspended in 10 mM HCl for 24h at 37°C for preparation of fibrillary Aβ1-42. Aggregation of Aβ1-42 was confirmed by Western blot analysis.
Neuronal death/viability assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay was performed for analysis of apoptotic neuronal death by using commercial kit (Roche, Indianapolis, IN). For analysis of cell specific apoptosis, the TUNEL assay was combined with immunofluorescent staining using antibody specific to MAP2 (pan neuronal marker; Santa Cruz) or GAD67 (GABAergic neuronal marker; Millipore). For neuronal viability analysis, cell counting kit-8 (CCK-8, Dojindo Laboratories, Tokyo, Japan) was used. Briefly, primary cultured neurons (4×103 cells/well in 96-well plates) were incubated with Aβ peptides in the presence or absence of Nω-Propyl-L-arginine hydrochloride (NPLA, Tocris, Bristol, UK) or 3-morpholinosydnonimine (SIN-1) (Tocris) for 24 hours, then CCK-8 was added to each well and further incubated for 3 hours at 3° C. The neuronal viability was detected by spectrometrically at 450 nm using SpectraMax 190 microplate reader (Molecular devices, Sunnyvale, CA).
Assay of nitro tyrosine
Brain levels of nitro tyrosine (N-Tyr) was performed using OxiSelect Nitrotyrosine ELISA Kit (Cell Biolabs, Inc., San Diego, CA). Briefly, brain tissues (n=3) were homogenized (dounce homogenizer) in assay diluent provided with the kit and equal amounts (500 μg) of brain lysates and nitrated BSA standards were added to an N-Tyr coated enzyme Immunoassay (EIA) plate and followed by incubation with anti-nitro tyrosine antibody. Following washing, the pates were incubated HRP conjugated secondary antibody and the levels of N-Tyr were measured by incubation with 3,3′,5,5′-tetramethylbenzidine (TMB) solution and colorimetric analysis at 450 nm.
Statistical analysis
Statistical analysis was performed using Graphpad prism. An unpaired student t test was used for comparison between two groups. One-way ANOVA with Tukey’s multiple comparison test were used for comparison of multiple groups. Criterion for significance was p < 0.05.
Results
Loss of GABAergic neurons in stratum pyramidale of hippocampal CA1 of APPSweDI mice
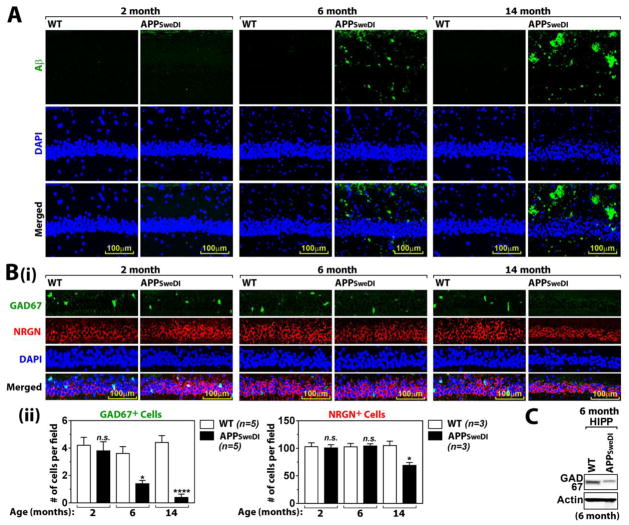
Pyramidal neurons in CA1 region of hippocampus (stratum pyramidale) play critical roles in learning and memory (Kesner RP et al., 2011) as well as mood and emotions (Freeman-Daniels E et al., 2011; Modol L et al., 2011). In this study, we investigated Aβ42 deposition and associated neuronal loss in CA1 stratum pyramidale of APPSweDI mice. For detection of human Aβ deposition, the brain sections from 2, 6, and 14 months old APPSweDI mice and their age-matched wild type (WT) controls (C57BL/6 mice) were immunostained for Aβ42 using the antibody specific to Aβ42 peptide (BC05). Figure 1A shows no significant deposition of human Aβ42 in stratum pyramidale of hippocampal CA1 of WT mice at all ages as well as APPSweDI mice at 2 months of age, increased deposition of Aβ42 in the same brain region of 6 months old APPSweDI mice, and the even greater deposition of Aβ42 in 14 months old APPSweDI mice.
Fig. 1. Age dependent Aβ accumulation and degeneration of GABAergic neurons in hippocampus.
A. Aβ42 accumulation in CA1 of Hippocampus of WT and APPSwDI mice (2, 6, and 14 months old) were analyzed by immunofluorescent staining with BC05 antibody. DAPI was used for staining for nucleus. B. Age dependent loss of GABAergic neurons (GAD67+ cells) or pyramidal neurons (neurogranin/NRGN) were investigated (i). The graph shows the # of GABAergic or pyramidal neurons (ii). C. The expression of GAD67 in hippocampus (HIPP) was analyzed by Western blot. All columns are means of individual data and T-bars are standard error mean: n.s. P > 0.05. *. P ≤ 0.05. ****. P ≤ 0.0001 as compared to WT mice.
Next, sections from the mice were immunostained with antibody specific to GAD67 for detection of GABAergic neurons or neurogranin (NRGN) for detection of pyramidal neurons (Xiong K et al., 2008). We observed no loss of GABAergic and pyramidal neurons at 2 months of age (Fig. 1B). However, increased Aβ deposition paralleled with reduced number of GAD6 + neurons in stratum pyramidale of hippocampal CA1 of APPSweDI mice as compared to that of WT at 6 months of age (Fig. 1B). Moreover, almost complete loss of GAD67+ neurons was observed in 14 months old APPSweDI mice (Fig. 1B). Accordingly, Western analysis of hippocampal lysates shows decreased levels of GAD67 protein in APPSweDI mice as compared to WT mice at 6 months of age (Fig. 1C). On the other hand, APPSweDI mice did not show any obvious changes in the number of NRGN+ neurons (pyramidal neurons) at 6 months of age as compared to WT but these neurons were lost in advanced disease at 14 months of age. Studies have shown that GABAergic neurons have capacity to downregulate GAD67 expression yielding GABAergic hypo-function without neuronal loss (Zamberletti E et al., 2014). Therefore, the decreased GAD67 immunoreactivity and thus GABAergic hypo-function in stratum pyramidale of hippocampal CA1 of APPSweDI mice may not be only due to the loss of GABAergic neurons, but also due to the decreased gene expression of GAD67. Interestingly, we observed predominant deposition of human Aβ in stratum oriens and stratum radiatum in APPSweDI mice (Fig. 1A). However, we observed relatively less deposition of Aβ in stratum pyramidale, where nNOS+ GAD67+ and NRGN+ neurons were undergoing degeneration (Fig. 1B). Overall, these data indicate occurrence of GABAergic hypo-function (decreased GAD67 expression or degeneration of GABAergic neurons) at early stage of disease in advance to the loss of pyramidal neurons in APPSweDI mice.
Alterations of cognitive function and neuropsychiatric behaviors of APPSweDI mice
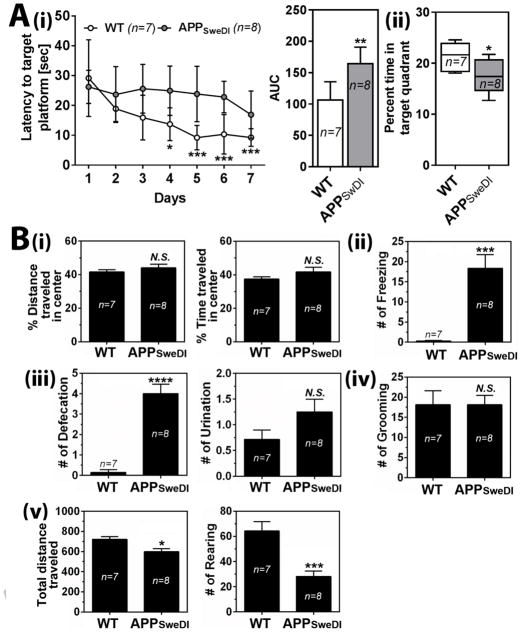
Since GABAergic neurons play important role in memory and learning processes (Andrews-Zwilling Y et al., 2012; Meredith RM, Floyer-Lea AM and Paulsen O, 2003; Shinoda Y et al., 2011) as well as regulation of mood and emotion (Luscher B, Shen Q and Sahir N, 2011; Nuss P, 2015; Shiah IS and Yatham LN, 1998; Taylor SF and Tso IF, 2015), we next investigated relationship between the GABAergic hypo-function and developments of cognitive deficits and neuropsychiatric-like symptoms. For analysis of cognitive function, 6 months old APPSweDI mice and WT mice were subjected to Morris water maze test. Fig. 2A shows that APPSweDI mice had longer escape latency with shorter time spent in target quadrant than WT mice, indicating that the APPSweDI mice develop defective spatial learning and memory dysfunction at 6 months of age.
Fig. 2. Cognitive deficits and neuropsychiatric-like symptoms in APPSwDI mice (6 months old).
A. Spatial learning (latency to invisible target platform) and memory (% time to spent in target quadrant without platform) functions were analyzed by Morris water maze test. In left panel of A-i, open (WT mice) and solid (APPSwDI mice) circles represent mean and T-bars represent standard error means. In right panel of A-i, the area under the curve (AUC) of the overall learning was calculated and represented by bar graph. In panel A-ii, columns show 75% of distribution; horizontal bar in each column is median; and vertical T-bars are minimum and maximum values of the data. B. Open field test was test for anxiety-like behaviors, such as distance/time travelled in the center area (i), number of freezing (ii), number of defecation and urination (iii), number of grooming (iv), and total travelled distance and number of rearing (v). All columns are means of individual data and T-bars are standard error mean: n.s. P > 0.05. *. P ≤ 0.05. **. P ≤ 0.01. ***. P ≤0.001. ****. P ≤ 0.0001 as compared to WT mice.
Next, to analyze neuropsychiatric-like symptoms in APPSweDI mice, 6 months old APPSweDI mice and WT mice were subjected to open field test. Fig. 2B-i shows that APPSweDI mice did not show any decreases in distance and time traveled in center, a typical behavioral measure for anxiety-like behavior (Walsh RN and Cummins RA, 1976). However, the mice developed increased sympathetic reactivity (defecation and urination) (Fig. 2B-ii) with increased frequency of freezing (Fig. 2B-iii), another typical indicators of high anxiety-like behavior (Gray JA, 1971). APPSweDI mice did not show any differences in grooming behavior (Fig. 2B-iv), a displacement behavior (Espejo EF, 1997), but developed decreased locomotor and exploratory activities as observed by decreased total distance traveled and number of rearing compared to WT (Fig. 2B-v). These data suggest possible involvement of early loss of GABAergic neurons in increased anxiety-like behaviors and decreased locomotor/exploration activities in APPSweDI mice.
Altered seizure susceptibility of APPSweDI mice
GABAergic neurons in hippocampus are known to play a critical role in modulation of seizure activity (Esclapez M et al., 1997). Therefore, we next assessed the susceptibility of APPSweDI mice to kainic acid (KA)-induced seizure activity. KA is an epileptogenic glutamate analogue and a reliable tool to study temporal lobe epilepsy, the most common type of epilepsy. Since treatment with repeated low dose (RLD) of KA treatment develops a robust and reliable mouse model of status epilepticus (Tse K, Puttachary S, Beamer E, Sills GJ and Thippeswamy T, 2014), 6 months old APPSweDI and WT mice were treated with RLD of KA and seizure activities, during and after treatments, were analyzed based on Racine scale (see Materials and Method section) (Racine RJ, 1972).
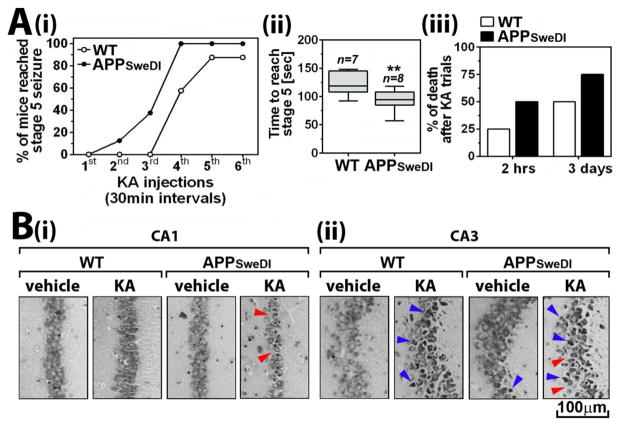
Figs. 3A-i and ii show the percent of mice that reached stage 5 and the time to reach stage 5 seizure in Racine scale (most severe behaviors including generalized tonic-clonic seizure with lateral recumbence or jumping and wild running). APPSweDI mice reached stage 5 with lesser doses of KA and in shorter time than WT mice (Figs. 3A-i and ii). APPSweDI mice also exhibited higher lethality than WT (Fig. 3A-iii). Three days after the trials, the brain sections from surviving mice were subjected to Nissl staining for assay of neuronal morbidity. Figure 3B shows that the number of pyramidal cells in CA1 and CA3 of KA-treated APPSweDI mice were reduced to greater degree than KA-treated WT mice. Moreover, greater empty spaces in pyramidal layer (blue arrow head) and higher number of dark Nissl bodies (blue arrow head) in KA-treated APPSweDI mice indicate higher degree of active neurodegeneration in these mice. Previous studies have reported that CA3 damage occurs more rapidly than CA1 (within 48hr) (Ben-Ari Y et al., 1979; Charriaut-Marlangue C et al., 1996; Friedman LK, 1998; Hartley Z and Dubinsky JM, 1993). Accordingly, we observed more post-epileptic neurodegeneration in CA3 compared to CA1 (Fig. 3B). In this study, we observed that some of pyramidal neurons in CA3 hippocampus of APPSweDI mice also underwent neurodegeneration without KA treatment (Fig. 3B-ii), suggesting that pyramidal neurons in CA3 may be more susceptible to Aβ toxicity than those in CA1. Overall, these data suggest the possible involvement of early loss of GABAergic neurons in increased vulnerability of APPSweDI mice to KA induced seizure and post-seizure neurodegeneration.
Fig. 3. Altered seizure susceptibility of APPSweDI mice (6 months old).
A. Seizure responses of mice to KA was assessed for analysis of seizure vulnerability of the mice. The mice were treated with repeated low dose of KA (5mg/kg; 30 min intervals) till each mouse reached stage 5 Racine scale. The number of KA treatment (i) and time to reach stage 5 seizure (ii) and death of mice following the KA treatment (iii) were analyzed. The columns in panel A-ii show 75% of distribution; horizontal bar in each column is median; and vertical T-bars are minimum and maximum values of the data: **. P ≤ 0.01 as compared to WT mice. B. Three days of KA treatment, hippocampal morphology and pyramidal neuronal status in CA1 (i) and CA3 (ii) regions were assessed by Nissle staining. Red and blue arrow heads indicate neuronal loss (empty areas) and degenerating neurons (dark Nissl bodies), respectively.
GABAergic neurons have high vulnerability to Aβ-induced cytotoxicity
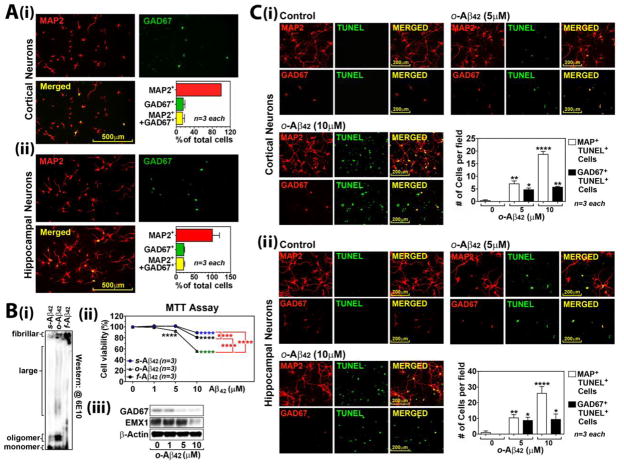
Since APPSweDI mice had loss of GAD67+ immunoreactivity in CA1 hippocampus prior to the loss of pyramidal neurons at early stages (6 months old) of disease (Fig. 1), we next investigated the vulnerability of GABAergic neurons to Aβ-induced cytotoxicity using primary rat neuron culture. For identification of GABAergic neurons in culture, the primary cultured cortical and hippocampal neurons were immunostained for MAP2 (pan neuronal marker) and GAD67 (GABAergic neuronal marker). Fig. 4A shows that both cortical and hippocampal neuron cultures contained 15~20% of GAD67+/MAP+ GABAergic neurons (Fig. 4A). Next, the cultured cortical and hippocampal neurons were treated with soluble (s-), oligomeric (o-), and fibrillary (f-) forms of Aβ42. The different Aβ42 assemblies of Aβ42 (s-, o-, and f- forms) were generated as described previously (Stine WB, Jungbauer L, Yu C and LaDu MJ, 2011) and characterized by Western analysis (Fig. 4B-i). As reported previously (Tomic JL et al., 2009), o-Aβ42 was most toxic to the cultured neurons than s-Aβ42 and f-Aβ42 as shown by MTT cell viability assay (Fig. 4B-ii). Next, we examined the effect of o-Aβ42 treatment on the expression of GAD67 (marker for GABAergic neurons) and EMX-1 (marker for glutamatergic neurons) (Chan CH et al., 2001) in the cortical neuron culture. Fig. 4B-iii shows that treatment of the cultured neurons with lower concentration of o-Aβ42 (5 μM) selectively decreased the expression of GAD67 without affecting EMX-1 expression, while higher concentration of o-Aβ42 (10 μM) reduced expressions of both GAD67 and EMX-1. o-Aβ42 at lower concentration (5 μM) selectively induced apoptosis of GAD67+ neurons while higher concentration of o-Aβ42 (10 μM) also induced apoptosis of other types of neurons (MAP2+/GAD67−) in both cortical and hippocampal neuron cultures (Fig. 4C). Similar to the effects of o-Aβ42, Aβ23-35 peptide, a main toxic part of the full-length Aβ peptides (Mattson MP et al., 1992), also decreased the viability of cultured cortical neurons in a dose dependent manner (data not shown). Aβ23-35 peptide at low concentration (10 μM) selectively decreased GAD67 expression, but without affecting EMX-1 expression, while Aβ23-35 peptide at higher concentration (50–100 μM) reduced expressions of both GAD67 and EMX-1. Accordingly, lower concentration of Aβ23-35 (10 μM) selectively induced apoptosis of GAD6 + neurons, while higher concentration of Aβ23-35 (50 μM) increased apoptosis of other MAP2+ neurons in addition to GAD67+ neurons. These data indicate that GABAergic neurons are relatively more vulnerable to lower concentrations of Aβ than other types of neurons.
Fig. 4. Higher vulnerability of GABAergic neurons than glutamatergic neurons to Aβ-induced cytotoxicity.
A. Primary rat cortical and hippocampal neurons were stained for MAP2 (pan neuronal marker) and GAD67 (GABAergic neuronal marker) (i) for quantification of GABAergic neurons in culture (ii). B. Soluble (s-), oligomeric (o-), and fibrillary (f-) forms of Aβ42 were generated in vitro and characterized by Western blot (i) and their neurotoxicity was assessed in primary cultured cortical neurons by MTT assay (ii). The cortical neuron cultures were treated with o-Aβ42 peptide and expressions of GAD67 vs. EMX1 were analyzed by Western analysis (iii). C. Apoptotic death of GABAergic neurons (TUNEL assay) was also analyzed by double immunostaining for MAP2 (pan neuronal marker) and TUNEL or GAD67 (GABAergic neuronal marker) and TUNEL in primary cultured cortical (i) and hippocampal neurons (ii). All columns and lines are means of individual data and T-bars are standard error mean: *. P ≤ 0.05. **. P ≤ 0.01. ***. P ≤ 0.001. ****. P ≤ 0.0001.
GABAergic neurons in CA1 area of hippocampus express high basal levels of nNOS and nitrosative stress
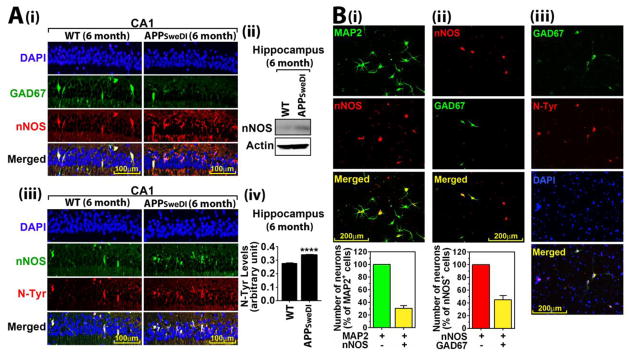
In AD brains, neurons expressing nNOS are highly susceptible to NFTS and Aβ induced neurodegeneration (Thorns V, Hansen L and Masliah E, 1998). Since nNOS is known to be expressed primarily in specific subtypes of GABAergic neurons (Tricoire L and Vitalis T, 2012), we examined whether GAD67+ neurons in hippocampal CA1 express nNOS. Figure 5A-i shows that GAD67+ neurons in stratum pyramidale of hippocampal CA1 region in WT mice (6 months old) express high levels of nNOS. In APPSweDI mice of the same age (6 months), GAD67+ neurons also expressed nNOS but the number of GAD67 and nNOS double positive neurons was decreased as the number of GAD67+ neurons was decreased (Fig. 1B-i). However, APPSweDI mice showed increased nNOS expression as shown by Western analysis of hippocampal tissue lysates (Fig. 5A-ii), specifically in pyramidal neurons in hippocampal CA1 (Fig. 5A-i). Similar aberrant expression of nNOS in pyramidal neurons in hippocampus was also reported previously in AD brains (Luth HJ et al., 2000; Tang Z et al., 2013) but underlying mechanism is not understood at present. Interestingly, the nNOS and GAD67 double positive neurons in WT mice and nNOS positive pyramidal neurons in APPSweDI mice expressed high N-Tyr immuno-reactivity (Fig, 5A-iii and iv), indicating the basal (GABAergic neurons) or induced (pyramidal neurons) nitrosative stress in those neurons.
Fig. 5. Expression of nNOS and nitrotyrosine in GABAergic neurons.
A. Expression of nNOS in GABAergic and pyramidal neurons (i and ii) and its co-localization with nitrotyrosine (N-Tyr) were analyzed by immuno-fluorescent staining of CA1 regions of hippocampus in 6 months old WT and APPSwDI mice (iii). The levels of N-Tyr were also measured in hippocampus by ELISA (iv). B. Presence of nNOS+ GABAergic (GAD67+) neurons and their co-localization with N-Tyr were analyzed in primary cultured rat cortical neurons. All columns are means of individual data (n=3) and T-bars are standard error mean: ****. P ≤ 0.0001.
Next, we examined whether nNOS+ GABAergic neurons in culture also express high basal levels of nitrosative stress. Figure 5B-i shows that ~30% of MAP2+ neurons in culture-expressed nNOS. In addition, figure 5B-ii shows that ~40% of nNOS+ neurons expressed GAD67+. These observations indicate that 12% of MAP2+ neurons express both nNOS and GAD67 (Fig. 5B-ii). Similar to the observation in stratum pyramidale of hippocampal CA1 of WT mice, the nNOS expressing GABAergic neurons in culture also expressed high basal levels of N-Tyr immunoreactivity (Fig. 5B-iii), thus suggesting that nNOS expressing GABAergic neurons express higher basal levels of nitrosative stress compared to other neurons even under normal conditions.
Involvement of nitrosative stress in high vulnerability of nNOS expressing GABAergic neurons to Aβ cytotoxicity
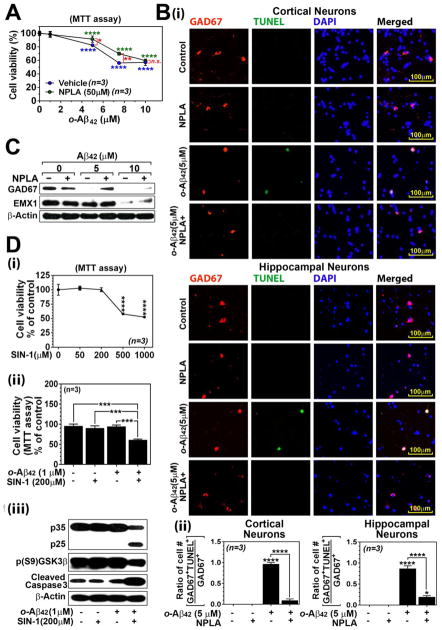
We next investigated the involvement of nitrosative stress in Aβ-induced neurotoxicity of nNOS and GAD67 double positive neurons. Fig. 6A-i show that treatment of cultured neurons with selective nNOS inhibitor Nω-propyl-L-arginine (NPLA; 50 μM) significantly protected against neuronal death induced by lower concentrations (5 – 7.5 μM) of o-Aβ42. However, NPLA was unable to block loss of neuronal viability induced by higher concentration of o-Aβ42 (10 μM) (Fig. 6A-i). These data indicate that nNOS activity participates in neuronal degeneration under conditions of lower load of Aβ. However, inability of nNOS inhibitor in the protection of neurons at higher concentration of o-Aβ42 (10 μM) suggests the participation of additional cell death mechanisms at higher Aβ load. Next, the effect of NPLA (50 μM) on Aβ-induced GABAergic neuronal death was examined by double-immunofluorescent staining of neuron culture for GAD67 and TUNEL. Figure. 6B-i and ii show that NPLA treatment almost completely inhibited o-Aβ42 (5 μM) induced apoptosis of GAD67+ neurons. As observed by Western analysis (Fig. 6C), NPLA treatment also restored the GAD67 expression decreased by low concentration of o-Aβ42 (5 μM). However, NPLA was not able to restore the expressions of GAD67 and EMX1 decreased by higher concentration of o-Aβ42 (10 μM) (Fig. 6C). These data document that o-Aβ42 induces apoptosis of GABAergic neurons at lower load of Aβ is mediated via nNOS-dependent pathway. However, additional mechanisms of cell death pathways may participate at high load of Aβ during the late stages of AD.
Fig. 6. Role of nitrosative stress in Aβ-induced GABAergic neuronal loss.
Effect of nNOS inhibitor, Nω-propyl-L-arginine (NPLA; 20μM), on o-Aβ42 induced loss of neuronal viability (MTT assay) (A), apoptosis of GABAergic neurons (TUNEL assay) (B), and decreased protein levels of GAD67 and EMX-1 (C) were analyzed in primary rat cortical and hippocampal neuron cultures. Loss of cell viability (MTT assay) by serial concentrations of 3-morpholinosydnonimine (SIN-1, a donor of ONOO−) (D-i), effect of subtoxic concentrations of SIN-1 and o-Aβ42 on cell viability (D-ii), p35 cleavage to p25, GSK3β phosphorylation (Ser9), active (cleaved) caspase 3 were analyzed (D-iii). All columns or lines are means of individual data and T-bars are standard error mean: n.s. P > 0.05. *. P ≤ 0.05. **. P ≤ 0.01. ***. P ≤ 0.001. ****. P ≤ 0.0001 as compared to WT mice.
Since nNOS expressing GABAergic neurons also express high levels of cellular N-Tyr (Fig. 5B-iii) and are highly susceptible to Aβ-induced neurotoxicity (Fig. 4C), we next assessed whether ONOO− and Aβ cooperatively induce neuronal death. For it, the cultured rat neurons were treated with subtoxic concentrations of o-Aβ42 and 3-morpholinosydnonimine (SIN-1). SIN-1 is known to generate stoichiometric amounts of NO and O2− and thus ONOO− (Hogg N et al., 1992). Figure 6D-ii shows that combination of subtoxic concentrations of SIN-1 (200 μM; Fig. 6D-i) and o-Aβ42 (1 μM; Fig. 4B-ii), which did not induce cell death individually, synergistically reduced cell viability (Fig. 6B-ii). Consistent with this data, combination of subtoxic concentrations of SIN-1 and o-Aβ42 also induced p35 cleavage to p25, a critical event in aberrant Cdk5 activation, and activations of GSK3β (dephosphorylation of Ser9) and caspase 3 (cleaved caspase 3) which are involved in neuronal degeneration under AD conditions (Fig. 5D-iii) (Camins A et al., 2006; Pei JJ et al., 2008; Snigdha S et al., 2012). These data document that nNOS expressing GABAergic neurons are highly vulnerable to Aβ-induced toxicity due to nitrosative stress induced mechanisms.
Discussion
GABAergic neurons play critical roles in learning and memory (Andrews-Zwilling Y, Gillespie AK, Kravitz AV, Nelson AB, Devidze N, Lo I, Yoon SY, Bien-Ly N, Ring K, Zwilling D, Potter GB, Rubenstein JL, Kreitzer AC and Huang Y, 2012; Meredith RM, Floyer-Lea AM and Paulsen O, 2003; Shinoda Y, Sadakata T, Nakao K, Katoh-Semba R, Kinameri E, Furuya A, Yanagawa Y, Hirase H and Furuichi T, 2011), neuropsychiatric behaviors (mood and anxiety states) (Luscher B, Shen Q and Sahir N, 2011; Nuss P, 2015; Shiah IS and Yatham LN, 1998; Taylor SF and Tso IF, 2015), and suppression of seizure activity (Cohen I et al., 2002; Knopp A, Kivi A, Wozny C, Heinemann U and Behr J, 2005; Wozny C, Kivi A, Lehmann TN, Dehnicke C, Heinemann U and Behr J, 2003). Patients with AD frequently exhibit GABAergic dysfunction (Lanctot KL, Herrmann N, Mazzotta P, Khan LR and Ingber N, 2004) along with neuropsychiatric symptoms (Egashira N et al., 2005; Kaiser NC, Liang LJ, Melrose RJ, Wilkins SS, Sultzer DL and Mendez MF, 2014; Modrego PJ, 2010; Romano A, Pace L, Tempesta B, Lavecchia AM, Macheda T, Bedse G, Petrella A, Cifani C, Serviddio G, Vendemiale G, Gaetani S and Cassano T, 2015) as well as late onset seizures before and after the onset of cognitive deficits (Born HA, 2015; Mendez M and Lim G, 2003; Romanelli MF et al., 1990; Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, Seeley WW, Geschwind MD, Gorno-Tempini ML, Shih T, Kirsch HE, Garcia PA, Miller BL and Mucke L, 2013). However, answers to when, where, and how GABAergic neurons degenerate under AD conditions and what is the impact of GABAergic neuronal loss on cognitive deficits and neuropsychiatric symptoms in AD are still elusive.
In this study, we report that number of neurons expressing GAD67 in CA1 hippocampus of APPSweDI mice decreases at early stages of disease with relatively lower Aβ load in advance to the loss of pyramidal neurons (Fig. 1). In the CNS, GAD67 is known to provide more than 90% of GABA (Asada H et al., 1997), thus suggesting that the loss of GAD67 immunoreactivity and expression may directly induce GABAergic hypo-function in the CA1 of hippocampus. GAD67+ neuronal loss accompanies with the loss of spatial learning and memory as well as increased anxiety-like behaviors (Fig. 2) and increased vulnerability to KA-induced seizures in APPSweDI mice (Fig. 3). In primary cultured neurons, GAD67+ neurons are highly vulnerable to Aβ-induced cytotoxicity than other types of neurons (Fig. 4). GAD67+ neurons in primary neuron cultures as well as in hippocampal CA1 region express high basal levels of nNOS and nitrosative stress (Fig. 5) and treatment of those neurons with pharmacological nNOS inhibitor protects against Aβ-induced apoptosis of GAD67+ neurons, while ONOO− donor treatment enhances Aβ-induced apoptosis of other types of neurons (Fig. 6). These observations suggest the potential role of nNOS-mediated nitrosative stress in early development of GABAergic hypo-function (e.g. decrease in GAD67 expression and/or degeneration of GAD67+ neurons) under early AD condition.
In this study, we observed early loss of GAD67+ and nNOS+ neurons, in advance to the loss of pyramidal neurons, in stratum pyramidale of CA1 hippocampus of 6 months old APPSweDI mice (Fig. 1B). In addition, the loss of GAD67+ and nNOS+ neurons accompanies with the increased cognitive deficits and neuropsychiatric-like symptoms (Figs. 2 and 3). The role of GAD67+ and nNOS+ neurons in regulation of cognitive function and neuropsychiatric behaviors under conditions of AD is not well understood at present; therefore, it is not clear whether the degeneration of these neurons causes the observed cognitive deficits and neuropsychiatric-like deficits APPSweDI mice. However, the reported efficacies of GABAergic agonists, such as lorazepam and carbamazepine, against behavioral and psychiatric symptoms of dementia (Anton RF et al., 1986; Gleason RP and Schneider LS, 1990; Lanctot KL, Herrmann N, Mazzotta P, Khan LR and Ingber N, 2004; Lemke MR, 1995; Olin JT et al., 2001; Tariot PN et al., 1994; Tariot PN et al., 1999) support the importance of GABAergic neuronal protection for controlling cognitive, neurological, and psychiatric symptoms in early AD conditions.
Hippocampal GABAergic neurons play important roles for modulation of seizure activities (Cohen I, Navarro V, Clemenceau S, Baulac M and Miles R, 2002; Knopp A, Kivi A, Wozny C, Heinemann U and Behr J, 2005; Orban-Kis K et al., 2015; Wozny C, Kivi A, Lehmann TN, Dehnicke C, Heinemann U and Behr J, 2003). Therefore, the loss of hippocampal GABAergic transmission by decreased GAD67 expression or GABAergic neuronal degeneration may increase incidence of seizures in AD patients. AD patients are reported to have increased risk for late-onset seizures and neuronal network abnormalities (Born HA, 2015; Mendez M and Lim G, 2003; Romanelli MF, Morris JC, Ashkin K and Coben LA, 1990; Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, Seeley WW, Geschwind MD, Gorno-Tempini ML, Shih T, Kirsch HE, Garcia PA, Miller BL and Mucke L, 2013). Accordingly, this study also documents that increased seizure susceptibility of APPSweDI mice to KA treatment at 6-month age accompanies with decreased number of GAD67+ neurons in hippocampal CA1 region as well as decreased expression of GAD67 protein in CA1 hippocampus (Fig. 3A). In addition to the loss of GAD67+ cells, we also observed increased expression of nNOS and nitrosative stress in pyramidal neurons in 6-month-old APPSweDI mice (Fig. 5A). At present, the role of increased nNOS expression and nitrosative stress in pyramidal neurons in KA-induced seizure activity is not understood. Previous studies reported that NO produced by nNOS promotes initiation of seizure-like events in hippocampus and entorhinal cortex slice cultures (Kovacs R et al., 2009). Therefore, loss of GABAergic neurons as well as increased nNOS expression and nitrosative stress in pyramidal neurons may participate in increased seizure susceptibility of APPSweDI mice. Interestingly, APPSweDI mice exhibited more post-seizure damage in the hippocampus than WT mice (Fig. 3B). Recent studies reported that an estimated 10 to 22 percent of AD patients develop late-onset seizures and those who develop seizures typically have more severe symptoms and rapid disease progression (Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, Seeley WW, Geschwind MD, Gorno-Tempini ML, Shih T, Kirsch HE, Garcia PA, Miller BL and Mucke L, 2013). Taken together, these studies document that the loss of hippocampal GABAergic neurons and thus increased seizure susceptibility may influence disease course and thus severity of AD. Therefore, protection of hippocampal GABAergic neurons may be important in modulation of seizure-associated worse outcome in AD.
Under physiological conditions, nNOS catalyzes the oxidation of L-arginine resulting in the formation of L-citrulline and NO (coupled nNOS reaction). However, when the levels of its cofactor (tetrahydrobiopterin) and substrate (L-arginine) are low, nNOS produces relatively higher amounts of O2− (uncoupled nNOS reaction) (Pall ML, 2007; Xia Y, Dawson VL, Dawson TM, Snyder SH and Zweier JL, 1996). Under such conditions, the nNOS produced O2− readily reacts with NO to form ONOO−, which in turn causes cellular and tissue nitrosative damage (Koppenol WH, 2001; Pacher P et al., 2007). In this study, we observed that nNOS expressing GAD67+ neurons have higher intrinsic nitrosative stress compared to other types of cells in CA1 of hippocampus as well as in neuron culture (Fig. 5) and the number of these neurons was greatly reduced in APPSweDI mice as well as in neuron culture treated with Aβ. In addition, the observed inhibitory effect of nNOS inhibitor on Aβ-induced GABAergic neuronal death as compared to synergistic effect of ONOO− donor on Aβ-induced death of other types of neurons suggests that nitrosative stress, especially in nNOS expressing GABAergic neurons, may participate in increased susceptibility to Aβ-induced neuronal death. These data further postulate that nNOS expressing GABAergic neurons in the hippocampus degenerate at earlier stages of AD with relatively lower Aβ load due to their high intrinsic nitrosative stress.
In summary, the present study documents that APPSweDI mice exhibit selective loss of nNOS expressing GABAergic neurons in CA1 stratum pyramidale in advance to the loss of pyramidal neurons during early disease development. The nNOS expressing GABAergic neurons were highly susceptible to Aβ probably due to their intrinsic high basal levels of nitrosative stress. The loss of GABAergic neurons is accompanied with development of cognitive deficits and neuropsychiatric-like symptoms as well as increased seizure susceptibility in APPSweDI mice. GABAergic neurons are known to play critical roles in learning and memory, neuropsychiatric behaviors (mood and anxiety states), and suppression of seizure activity. Therefore, protection of nNOS expressing GABAergic neurons by drugs targeting these early stages of the disease may potentially alleviate cognitive, neurological, and psychiatric symptoms at the early stages of disease.
Highlights.
APPSwDI transgenic mice develop early loss of GAD67 expression in CA1 of hippocampus.
GAD67+ neurons exhibit high basal expression of nNOS and nitrosative stress.
These neurons are highly vulnerable to Aβ due to the high intrinsic nitrosative stress.
The loss of GAD67+ neurons correlates with cognitive and neuropsychiatric deficits.
These suggest a role of nitrosative stress in of Alzheimer’s GABAergic pathology.
Acknowledgments
This work was supported in part by grants from VA and NIH (BX001062, BX001072, NS072511, and NS037766). We also acknowledge Ms. Joyce Bryan for their help in procurement of animals and supplies.
Abbreviations
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- APPSweDI
human amyloid precursor protein Swedish-Deutch-Iowa mutant
- GABA
γ-aminobutyric acid
- KA
kainic acid
- N-Tyr
nitrotyrosine
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NPLA
Nω-Propyl-L-arginine hydrochloride
- NRGN
neurogranin
- O2•−
superoxide anion
- ONOO−
peroxynitrite
- RLD
repeated low dose
- SIN-1
3-morpholinosydnonimine
- WT
wild type
Footnotes
Conflicts of interest
The authors declare no actual or potential conflicts of interest for this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, et al. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Zwilling Y, Gillespie AK, Kravitz AV, Nelson AB, Devidze N, Lo I, Yoon SY, Bien-Ly N, et al. Hilar GABAergic interneuron activity controls spatial learning and memory retrieval. PloS one. 2012;7:e40555. doi: 10.1371/journal.pone.0040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Waid LR, Fossey M, AuBuchon P. Case report of carbamazepine treatment of organic brain syndrome with psychotic features. J Clin Psychopharmacol. 1986;6:232–234. [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, et al. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barford PA, Blair JA, Eggar C, Hamon C, Morar C, Whitburn SB. Tetrahydrobiopterin metabolism in the temporal lobe of patients dying with senile dementia of Alzheimer type. Journal of neurology, neurosurgery, and psychiatry. 1984;47:736–738. doi: 10.1136/jnnp.47.7.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Represa A. Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends in neurosciences. 1990;13:312–318. doi: 10.1016/0166-2236(90)90135-w. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Ottersen OP, Naquet R. Evidence suggesting secondary epileptogenic lesion after kainic acid: pre treatment with diazepam reduces distant but not local brain damage. Brain research. 1979;165:362–365. doi: 10.1016/0006-8993(79)90571-7. [DOI] [PubMed] [Google Scholar]
- Born HA. Seizures in Alzheimer’s disease. Neuroscience. 2015;286:251–263. doi: 10.1016/j.neuroscience.2014.11.051. [DOI] [PubMed] [Google Scholar]
- Camins A, Verdaguer E, Folch J, Canudas AM, Pallas M. The role of CDK5/P25 formation/inhibition in neurodegeneration. Drug news & perspectives. 2006;19:453–460. doi: 10.1358/dnp.2006.19.8.1043961. [DOI] [PubMed] [Google Scholar]
- Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Frontiers in neuroenergetics. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S, Topolnik L. Inhibitory control of hippocampal inhibitory neurons. Front Neurosci. 2012;6:165. doi: 10.3389/fnins.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CH, Godinho LN, Thomaidou D, Tan SS, Gulisano M, Parnavelas JG. Emx1 is a marker for pyramidal neurons of the cerebral cortex. Cerebral cortex. 2001;11:1191–1198. doi: 10.1093/cercor/11.12.1191. [DOI] [PubMed] [Google Scholar]
- Charriaut-Marlangue C, Aggoun-Zouaoui D, Represa A, Ben-Ari Y. Apoptotic features of selective neuronal death in ischemia, epilepsy and gp 120 toxicity. Trends in neurosciences. 1996;19:109–114. doi: 10.1016/s0166-2236(96)80039-7. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Demchenko IT, Piantadosi CA. Nitric oxide amplifies the excitatory to inhibitory neurotransmitter imbalance accelerating oxygen seizures. Undersea & hyperbaric medicine : journal of the Undersea and Hyperbaric Medical Society, Inc. 2006;33:169–174. [PubMed] [Google Scholar]
- Egashira N, Iwasaki K, Takashima A, Watanabe T, Kawabe H, Matsuda T, Mishima K, Chidori S, et al. Altered depression-related behavior and neurochemical changes in serotonergic neurons in mutant R406W human tau transgenic mice. Brain research. 2005;1059:7–12. doi: 10.1016/j.brainres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Hirsch JC, Khazipov R, Ben-Ari Y, Bernard C. Operative GABAergic inhibition in hippocampal CA1 pyramidal neurons in experimental epilepsy. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12151–12156. doi: 10.1073/pnas.94.22.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana J, Gimenez-Llort L, Valero J, Minano A, Rabano A, Rodriguez-Alvarez J, LaFerla FM, Saura CA. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer’s disease transgenic mice. Biological psychiatry. 2010;67:513–521. doi: 10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Espejo EF. Effects of weekly or daily exposure to the elevated plus-maze in male mice. Behavioural brain research. 1997;87:233–238. doi: 10.1016/s0166-4328(97)02286-9. [DOI] [PubMed] [Google Scholar]
- Freeman-Daniels E, Beck SG, Kirby LG. Cellular correlates of anxiety in CA1 hippocampal pyramidal cells of 5-HT1A receptor knockout mice. Psychopharmacology (Berl) 2011;213:453–463. doi: 10.1007/s00213-010-2030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LK. Selective reduction of GluR2 protein in adult hippocampal CA3 neurons following status epilepticus but prior to cell loss. Hippocampus. 1998;8:511–525. doi: 10.1002/(SICI)1098-1063(1998)8:5<511::AID-HIPO9>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Gasulla J, Calvo DJ. Enhancement of tonic and phasic GABAergic currents following nitric oxide synthase inhibition in hippocampal CA1 pyramidal neurons. Neuroscience letters. 2015;590:29–34. doi: 10.1016/j.neulet.2015.01.058. [DOI] [PubMed] [Google Scholar]
- Getting SJ, Segieth J, Ahmad S, Biggs CS, Whitton PS. Biphasic modulation of GABA release by nitric oxide in the hippocampus of freely moving rats in vivo. Brain research. 1996;717:196–199. doi: 10.1016/0006-8993(96)00127-8. [DOI] [PubMed] [Google Scholar]
- Gholipour T, Ghasemi M, Riazi K, Ghaffarpour M, Dehpour AR. Seizure susceptibility alteration through 5-HT(3) receptor: modulation by nitric oxide. Seizure. 2010;19:17–22. doi: 10.1016/j.seizure.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Gingerich S, Krukoff TL. Activation of ERbeta increases levels of phosphorylated nNOS and NO production through a Src/PI3K/Akt-dependent pathway in hypothalamic neurons. Neuropharmacology. 2008;55:878–885. doi: 10.1016/j.neuropharm.2008.06.058. [DOI] [PubMed] [Google Scholar]
- Gleason RP, Schneider LS. Carbamazepine treatment of agitation in Alzheimer’s outpatients refractory to neuroleptics. J Clin Psychiatry. 1990;51:115–118. [PubMed] [Google Scholar]
- Gray JA. The Psychology of Fear and Stress. New York: McGraw-Hill Companies; 1971. [Google Scholar]
- Hartley Z, Dubinsky JM. Changes in intracellular pH associated with glutamate excitotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:4690–4699. doi: 10.1523/JNEUROSCI.13-11-04690.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Nishio M, Naito Y, Yokokura H, Nimura Y, Hidaka H, Watanabe Y. Regulation of neuronal nitric-oxide synthase by calmodulin kinases. The Journal of biological chemistry. 1999;274:20597–20602. doi: 10.1074/jbc.274.29.20597. [DOI] [PubMed] [Google Scholar]
- Hogg N, Darley-Usmar VM, Wilson MT, Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992;281(Pt 2):419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser NC, Liang LJ, Melrose RJ, Wilkins SS, Sultzer DL, Mendez MF. Differences in anxiety among patients with early- versus late-onset Alzheimer’s disease. The Journal of neuropsychiatry and clinical neurosciences. 2014;26:73–80. doi: 10.1176/appi.neuropsych.12100240. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Morris AM, Weeden CSS. Spatial, temporal, and associative behavioral functions associated with different subregions of the hippocampus. In: Zentall TR, Wasserman EA, editors. The oxford handbook of comparative cognition. New York: Oxfrd University Press; 2011. pp. 322–344. [Google Scholar]
- Knopp A, Kivi A, Wozny C, Heinemann U, Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. The Journal of comparative neurology. 2005;483:476–488. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]
- Komeima K, Hayashi Y, Naito Y, Watanabe Y. Inhibition of neuronal nitric-oxide synthase by calcium/ calmodulin-dependent protein kinase IIalpha through Ser847 phosphorylation in NG108-15 neuronal cells. The Journal of biological chemistry. 2000;275:28139–28143. doi: 10.1074/jbc.M003198200. [DOI] [PubMed] [Google Scholar]
- Koppenol WH. 100 years of peroxynitrite chemistry and 11 years of peroxynitrite biochemistry. Redox report : communications in free radical research. 2001;6:339–341. doi: 10.1179/135100001101536517. [DOI] [PubMed] [Google Scholar]
- Kovacs R, Rabanus A, Otahal J, Patzak A, Kardos J, Albus K, Heinemann U, Kann O. Endogenous nitric oxide is a key promoting factor for initiation of seizure-like events in hippocampal and entorhinal cortex slices. J Neurosci. 2009;29:8565–8577. doi: 10.1523/JNEUROSCI.5698-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot KL, Herrmann N, Mazzotta P, Khan LR, Ingber N. GABAergic function in Alzheimer’s disease: evidence for dysfunction and potential as a therapeutic target for the treatment of behavioural and psychological symptoms of dementia. Can J Psychiatry. 2004;49:439–453. doi: 10.1177/070674370404900705. [DOI] [PubMed] [Google Scholar]
- Lee JJ. Nitric oxide modulation of GABAergic synaptic transmission in mechanically isolated rat auditory cortical neurons. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2009;13:461–467. doi: 10.4196/kjpp.2009.13.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke MR. Effect of carbamazepine on agitation in Alzheimer’s inpatients refractory to neuroleptics. J Clin Psychiatry. 1995;56:354–357. [PubMed] [Google Scholar]
- Levenga J, Krishnamurthy P, Rajamohamedsait H, Wong H, Franke TF, Cain P, Sigurdsson EM, Hoeffer CA. Tau pathology induces loss of GABAergic interneurons leading to altered synaptic plasticity and behavioral impairments. Acta neuropathologica communications. 2013;1:34. doi: 10.1186/2051-5960-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Molecular psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luth HJ, Holzer M, Gertz HJ, Arendt T. Aberrant expression of nNOS in pyramidal neurons in Alzheimer’s disease is highly co-localized with p21ras and p16INK4a. Brain Res. 2000;852:45–55. doi: 10.1016/s0006-8993(99)02178-2. [DOI] [PubMed] [Google Scholar]
- Maggesissi RS, Gardino PF, Guimaraes-Souza EM, Paes-de-Carvalho R, Silva RB, Calaza KC. Modulation of GABA release by nitric oxide in the chick retina: different effects of nitric oxide depending on the cell population. Vision research. 2009;49:2494–2502. doi: 10.1016/j.visres.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M, Lim G. Seizures in elderly patients with dementia: epidemiology and management. Drugs & aging. 2003;20:791–803. doi: 10.2165/00002512-200320110-00001. [DOI] [PubMed] [Google Scholar]
- Meredith RM, Floyer-Lea AM, Paulsen O. Maturation of long-term potentiation induction rules in rodent hippocampus: role of GABAergic inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:11142–11146. doi: 10.1523/JNEUROSCI.23-35-11142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modol L, Darbra S, Pallares M. Neurosteroids infusion into the CA1 hippocampal region on exploration, anxiety-like behaviour and aversive learning. Behav Brain Res. 2011;222:223–229. doi: 10.1016/j.bbr.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Modrego PJ. Depression in Alzheimer’s disease. Pathophysiology, diagnosis, and treatment. Journal of Alzheimer’s disease : JAD. 2010;21:1077–1087. doi: 10.3233/jad-2010-100153. [DOI] [PubMed] [Google Scholar]
- Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatric disease and treatment. 2015;11:165–175. doi: 10.2147/NDT.S58841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olin JT, Fox LS, Pawluczyk S, Taggart NA, Schneider LS. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9:400–405. [PubMed] [Google Scholar]
- Orban-Kis K, Szabadi T, Szilagyi T. The loss of Ivy cells and the hippocampal input modulatory O-LM cells contribute to the emergence of hyperexcitability in the hippocampus. Rom J Morphol Embryol. 2015;56:155–161. [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological reviews. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall ML. Nitric oxide synthase partial uncoupling as a key switching mechanism for the NO/ONOO- cycle. Med Hypotheses. 2007;69:821–825. doi: 10.1016/j.mehy.2007.01.070. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Sjogren M, Winblad B. Neurofibrillary degeneration in Alzheimer’s disease: from molecular mechanisms to identification of drug targets. Current opinion in psychiatry. 2008;21:555–561. doi: 10.1097/YCO.0b013e328314b78b. [DOI] [PubMed] [Google Scholar]
- Perrenoud Q, Rossier J, Ferezou I, Geoffroy H, Gallopin T, Vitalis T, Rancillac A. Activation of cortical 5-HT(3) receptor-expressing interneurons induces NO mediated vasodilatations and NPY mediated vasoconstrictions. Frontiers in neural circuits. 2012;6:50. doi: 10.3389/fncir.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu ZW, Miao WY, Hu SQ, Li C, Zhuo XL, Zong YY, Wu YP, Zhang GY. N-methyl-D-aspartate receptor-dependent denitrosylation of neuronal nitric oxide synthase increase the enzyme activity. PLoS One. 2012;7:e52788. doi: 10.1371/journal.pone.0052788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalography and clinical neurophysiology. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rajasekaran K, Jayakumar R, Venkatachalam K. Increased neuronal nitric oxide synthase (nNOS) activity triggers picrotoxin-induced seizures in rats and evidence for participation of nNOS mechanism in the action of antiepileptic drugs. Brain research. 2003;979:85–97. doi: 10.1016/s0006-8993(03)02878-6. [DOI] [PubMed] [Google Scholar]
- Romanelli MF, Morris JC, Ashkin K, Coben LA. Advanced Alzheimer’s disease is a risk factor for late-onset seizures. Archives of neurology. 1990;47:847–850. doi: 10.1001/archneur.1990.00530080029006. [DOI] [PubMed] [Google Scholar]
- Romano A, Pace L, Tempesta B, Lavecchia AM, Macheda T, Bedse G, Petrella A, Cifani C, et al. Depressive-like behavior is paired to monoaminergic alteration in a murine model of Alzheimer’s disease. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2015:18. doi: 10.1093/ijnp/pyu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiah IS, Yatham LN. GABA function in mood disorders: an update and critical review. Life sciences. 1998;63:1289–1303. doi: 10.1016/s0024-3205(98)00241-0. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Sadakata T, Nakao K, Katoh-Semba R, Kinameri E, Furuya A, Yanagawa Y, Hirase H, et al. Calcium-dependent activator protein for secretion 2 (CAPS2) promotes BDNF secretion and is critical for the development of GABAergic interneuron network. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:373–378. doi: 10.1073/pnas.1012220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha S, Smith ED, Prieto GA, Cotman CW. Caspase-3 activation as a bifurcation point between plasticity and cell death. Neuroscience bulletin. 2012;28:14–24. doi: 10.1007/s12264-012-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine WB, Jungbauer L, Yu C, LaDu MJ. Preparing synthetic Abeta in different aggregation states. Methods Mol Biol. 2011;670:13–32. doi: 10.1007/978-1-60761-744-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Sowell R, Butterfield DA. Nitrated Proteins in the Progression of Alzheimer’s Disease: A Proteomics Comparison of Mild Cognitive Impairment and Alzheimer’s Disease Brain. In: Veasey SC, editor. Oxidative Neural Injury. Humana Press; 2009. pp. 137–157. [Google Scholar]
- Tang Z, Bereczki E, Zhang H, Wang S, Li C, Ji X, Branca RM, Lehtio J, et al. Mammalian target of rapamycin (mTor) mediates tau protein dyshomeostasis: implication for Alzheimer disease. J Biol Chem. 2013;288:15556–15570. doi: 10.1074/jbc.M112.435123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariot PN, Erb R, Leibovici A, Podgorski CA, Cox C, Asnis J, Kolassa J, Irvine C. Carbamazepine treatment of agitation in nursing home patients with dementia: a preliminary study. J Am Geriatr Soc. 1994;42:1160–1166. doi: 10.1111/j.1532-5415.1994.tb06982.x. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Jakimovich LJ, Erb R, Cox C, Lanning B, Irvine C, Podgorski CA. Withdrawal from controlled carbamazepine therapy followed by further carbamazepine treatment in patients with dementia. J Clin Psychiatry. 1999;60:684–689. doi: 10.4088/jcp.v60n1007. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Tso IF. GABA abnormalities in schizophrenia: a methodological review of in vivo studies. Schizophrenia research. 2015;167:84–90. doi: 10.1016/j.schres.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorns V, Hansen L, Masliah E. nNOS expressing neurons in the entorhinal cortex and hippocampus are affected in patients with Alzheimer’s disease. Experimental neurology. 1998;150:14–20. doi: 10.1006/exnr.1997.6751. [DOI] [PubMed] [Google Scholar]
- Tomic JL, Pensalfini A, Head E, Glabe CG. Soluble fibrillar oligomer levels are elevated in Alzheimer’s disease brain and correlate with cognitive dysfunction. Neurobiology of disease. 2009;35:352–358. doi: 10.1016/j.nbd.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong LM, Djukic B, Arnold C, Gillespie AK, Yoon SY, Wang MM, Zhang O, Knoferle J, et al. Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Abeta accumulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:9506–9515. doi: 10.1523/JNEUROSCI.0693-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire L, Vitalis T. Neuronal nitric oxide synthase expressing neurons: a journey from birth to neuronal circuits. Frontiers in neural circuits. 2012;6:82. doi: 10.3389/fncir.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse K, Puttachary S, Beamer E, Sills GJ, Thippeswamy T. Advantages of repeated low dose against single high dose of kainate in C57BL/6J mouse model of status epilepticus: behavioral and electroencephalographic studies. PloS one. 2014;9:e96622. doi: 10.1371/journal.pone.0096622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA neurology. 2013;70:1158–1166. doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ. Endogenous nitric oxide modulates GABAergic transmission to granule cells in adult rat cerebellum. The European journal of neuroscience. 2003;18:869–878. doi: 10.1046/j.1460-9568.2003.02822.x. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychological bulletin. 1976;83:482–504. [PubMed] [Google Scholar]
- Won JS, Kim J, Annamalai B, Shunmugavel A, Singh I, Singh AK. Protective role of S-nitrosoglutathione (GSNO) against cognitive impairment in rat model of chronic cerebral hypoperfusion. J Alzheimers Dis. 2013;34:621–635. doi: 10.3233/JAD-121786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozny C, Kivi A, Lehmann TN, Dehnicke C, Heinemann U, Behr J. Comment on “On the origin of interictal activity in human temporal lobe epilepsy in vitro”. Science. 2003;301:463. doi: 10.1126/science.1084237. author reply 463. [DOI] [PubMed] [Google Scholar]
- Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Luo DW, Patrylo PR, Luo XG, Struble RG, Clough RW, Yan XX. Doublecortin-expressing cells are present in layer II across the adult guinea pig cerebral cortex: partial colocalization with mature interneuron markers. Experimental neurology. 2008;211:271–282. doi: 10.1016/j.expneurol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Chen SR, Li DP, Pan HL. Kv1.1/1.2 channels are downstream effectors of nitric oxide on synaptic GABA release to preautonomic neurons in the paraventricular nucleus. Neuroscience. 2007;149:315–327. doi: 10.1016/j.neuroscience.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zamberletti E, Beggiato S, Steardo L, Jr, Prini P, Antonelli T, Ferraro L, Rubino T, Parolaro D. Alterations of prefrontal cortex GABAergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiology of disease. 2014;63:35–47. doi: 10.1016/j.nbd.2013.10.028. [DOI] [PubMed] [Google Scholar]