Abstract
Increasing predictability of animal models of posttraumatic stress disorder (PTSD) has required active collaboration between clinical and preclinical scientists. Modeling PTSD is challenging as it is heterogeneous disorder with 20+ symptoms. Clinical research is increasingly utilizing objective biological measures (e.g. imaging, peripheral biomarkers) or non-verbal behaviors/physiological responses to complement verbally reported symptoms. This shift toward more objectively measurable phenotypes enables refinement of current animal models of PTSD, and supports incorporation of homologous measures across species. We reviewed >600 articles to examine the ability of current rodent models to probe biological phenotypes of PTSD (e.g. sleep disturbances, hippocampal and fear-circuit dysfunction, inflammation, glucocorticoid receptor hypersensitivity) in addition to behavioral phenotypes. Most models reliably produced enduring generalized anxiety- and/or depression-like behaviors, as well as hyperactive fear circuits, glucocorticoid receptor hypersensitivity, and response to chronic SSRIs. Although a few paradigms probed fear conditioning/extinction and/or utilized peripheral immune, sleep, and non-invasive imaging measures, which we argue should be incorporated more to enhance translation. There was little data in females, at different ages across the lifespan, or on temporal trajectories of phenotypes post-stress, which would inform model utility and experimental design for treatment studies. Overall, preclinical (and clinical) PTSD researchers are increasingly incorporating homologous biological measures to assess markers of risk, response and treatment outcome. This shift is exciting, as we and many others hope it will support translation of drug efficacy not only from animal models to clinical trials, but potentially improve predictability of stageII for stageIII clinical trials.
Keywords: PTSD, animal model, social defeat, single prolonged stress, shock, predator stress, immobilization, unpredictable variable stress
Introduction
Globally the prevalence rate for posttraumatic stress disorder (PTSD) is 4-6% with the disorder being described by Koenen et al. (2017, p. 2) as a “life sentence due to its association with increased risk of chronic disease, accelerated aging, and premature mortality” (1). Efficacious prophylactic and therapeutic agents are urgently needed (2), and animal models are critical to establish causality of putative mechanisms and verify potential treatment efficacy. Unfortunately, PTSD continues to be diagnosed via a menu of 20+ separate self-reported symptoms, with little input from neuroscience-based research. Animal models of PTSD rely primarily on face validity for complex human symptoms or predictive validity for current treatments (selective serotonin reuptake inhibitors, SSRIs), validity types which offer relatively poor translation or bias towards detection of “me too” drugs.
Recently, neuroscience- and molecular-based clinical research has identified PTSD-related phenotypes operationalized not by symptoms but by biological markers (e.g. circuit changes, peripheral biomarkers) or non-verbal dimensional behaviors/physiological responses (e.g. sleep, fear response physiology). This shift enables refinement of current animal models of PTSD to probe novel and hopefully more translatable mechanisms of PTSD. Given these recent advances it is good time to examine current rodent models of PTSD and their efficacy in recapitulating these potentially more translatable phenotypes. This review aims to guide readers in identifying paradigms that probe particular PTSD-relevant behavioral and biological constructs of interest for their drug/molecular target (e.g. treat extinction deficits, sleep disturbances, circuit abnormalities). We will in particular highlight biological measures incorporated by these models that can support cross-species translation.
PTSD is triggered by multiple trauma types (physical vs. emotional) and given the heterogeneity in biological and environmental factors that likely mediate PTSD in humans, expecting a “one size fits all” PTSD model in rodents is a fool’s errand. Instead, rodent tests should be utilized interpreted within the PTSD-related phenotypes they do and do not produce. This approach will allow for drug targeting at specific mechanisms and constructs, and hopefully result in enhanced translation to the clinic. Within this context, our guidelines in choosing which paradigms to evaluate were: the paradigm must (1) focus on outcome variables that endure long after the trauma/stress has ended (e.g. >1 week after the stressor is terminated), (2) measure more than 1 behavioral outcome variable for reliability/robustness, (3) have replicable effects across more than one laboratory, and (4) present an unpredictable, inescapable severe stressor (e.g. vary stressor intensity, duration) to avoid habituation and mimic “life threatening” aspects of trauma associated with PTSD (3). We also did not review animal models of fear conditioning and extinction per se, as although this construct is highly relevant to PTSD, excellent reviews as how these models pertain to PTSD can be found elsewhere (within 24-48 hrs; (4; 5)). Overall, paradigms that fit these criteria included those that used foot shocks, predator stress, single prolonged stress, immobilization stress, unpredictable variable stress, and social defeat.
We reviewed these paradigms both for efficacy in evoking PTSD-like constructs (learned fear and extinction, avoidance, reduced motivation/reward, arousal and cognitive deficits) in addition to biological and physiological phenotypes associated with PTSD (Table 1). One of the most consistent is increased glucocorticoid receptor (GR) sensitivity and enhanced negative feedback of the hypothalamo-pituitary-adrenal (HPA) axis (6). Other established biological phenotypes include increased activity/function of amygdala, reduced function and structural abnormalities in prefrontal cortex (PFC) and hippocampus (6–9). PTSD is consistently associated with increased inflammation both as a risk factor and in relation to symptom state (10). Finally, sleep disturbances, including reduced sleep duration or fragmented rapid eye movement (REM), are commonly described in PTSD (6). These phenotypes can be assessed across species as outcome measures of risk or enduring stress response. When conducting our review we were most interested in what PTSD-related behavioral and biological phenotypes were and were not reliably produced in each paradigm (Table 1) (e.g. consistent over cohorts and laboratories). We also examined approaches to categorize “resilient” vs. ”susceptible” animals, and identify biological/behavioral risk factors (e.g. immune response, early-life stress) that predict individual variance in susceptibility. This is not meant to be an extensive review of each model, but instead to highlight robust and replicable findings for model across phenotypes.
Table 1.
Operational measures of PTSD-related constructs and phenotypes
| Behavioral phenotypes | Operational measures |
|---|---|
| Pain perception Chronic pain, ↑ thermal pain perception |
Thermal: acetone, Hargreaves and hot-plate tests Mechanical: von Frey test Inflammatory: formalin test |
| Generalized avoidance/anxiety | EPM, LD, MB, novelty-suppressed feeding test, OF, SAAT, SIT |
| Avoidance of trauma-related cues | Freezing or avoidance behavior when in presence of stress-related cue (e.g. odor, stress context) |
| Fear learning and extinction ↑ conditioned fear and ↓ extinction of conditioned fear | Pavlovian fear conditioning (contextual and cued auditory fear responses) as assessed by freezing or startle reactivity with and without the presence of conditioned cues |
| Arousal ↑ startle, ↑ somatic signs of arousal (HR, GSR) | ASR and startle habituation; PPI |
| Depression ↑ depression symptoms (BDI, HAM-D) |
Despair/helplessness: FST, TST Anhedonia: ICSS, PRT, SP |
| Memory deficits (hippocampal function) ↓ processing speed, working memory, attention, response inhibition | EPM, MWM, NORT, RAWM |
| Biological phenotypes | Assessments |
| Fear circuit dysfunctions (PFC-Amy) ↑ amygdala activity, ↓ PFC activity during emotional tasks in fMRI, ↓ functional connectivity between PFC and ACC or Amy |
Functional: cFos-positive cells, LTP/LTD Structure/Morphology: dendritic spines length/number, number of cells and apoptosis rate, functional connectivity (imaging) |
| Changes in HPA axis functioning Altered GR sensitivity, hypersensitive negative feedback, genomic changes in HPA gene pathways |
Plasma: CORT/ACTH (baseline or in response to a subsequent stress, or DST) Central: CRF, CRFR1/2, GR, MR expression or protein levels |
| Inflammation Altered inflammatory state measured by changes in serum levels of inflammatory markers |
Plasma: levels of anti-inflammatory cytokine (IL-10) pro-inflammatory cytokines (IL-1β, TNF-α, IL-6, IL-12, IL-18), TLR2 and TLR4, NLRP3) Central: microglial activation; levels of anti-inflammatory and pro-inflammatory cytokines (described above) |
| Hippocampal dysfunction Functional: altered activity, altered hippocampal-dependent memory Structural: ↓ volume and/or connectivity |
Functional: cFos-positive cells, LTP/LTD Structure/Morphology: number of cells and apoptosis rate, HP volume, dendritic spines length/number, functional connectivity (imaging) |
| Sleep disturbances ↑ awakenings from REM, ↑ number and density/↓ duration of REM, recurrent nightmares | NREM, REM, EEG bands |
ACC, anterior cingulate cortices; ACTH, adrenocorticotropic hormone; Amy, amygdala; ASR, acoustic startle response; BDI, Beck Depression Inventory; CORT, corticosterone; CRF, corticotropin-releasing factor; CRFR1/2, corticotropin-releasing factor receptor type 1 or 2; DST, dexamethasone suppression test; EEG, electroencephalography; EPM, elevated plus maze; FST, forced swim test; GR, glucocorticoid receptors; GSR, galvanic skin response; HAM-D, Hamilton Rating Scale for Depression; HP, hippocampus; HR, heart rate; ICSS, intracranial self-stimulation; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; IL-12, interleukin-12; IL-18, interleukin-18; LD, light-dark box; LTP/LTD, long-term potentiation/depression; MB, marble burying; MR, mineralocorticoid receptors; MWM, Morris water maze; NLRP3, NACTH, LRR and PYD domains-containing proteins 3; NORT, novel object recognition task; NREM, non-rapid eye movement; OF, open field; PFC, prefrontal cortex; PPI, prepulse inhibition of acoustic startle; PRT, probabilistic reward task; RAWM, radial arm water maze; REM, rapid eye movement; SAAT, social approach-avoidance test; SIT, social interaction test; SP, sucrose preference; TLR2, toll-like receptor 2; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor-α; TST, tail suspension test
Inescapable shocks
Foot- or tail-shock is one of the most common aversive stressors used in rodent fear models, typically to examine acute stress responses, fear learning or depression-like effects under chronic exposure (e.g. learned helplessness model) (11). Although it is not considered ethologically valid, shock is highly feasible and doesn’t cause injury (Figure 1). A single exposure to foot shocks induce enduring (up to 56 days) PTSD-like phenotypes: hyperarousal, generalized avoidance, sleep disturbances, hippocampal-dependent memory deficits and thermal hyperalgesia (see Table S1 for details and references). The generalized avoidance and depression-like effects are sensitive to chronic administration of SSRIs (11–15). Parameters vary substantially however (e.g. 1-20 shocks, 0.3-1.5 mA, 0.5-10s) across laboratories, species, and strains. Susceptible and resilient animals have been defined by success or failure to escape subsequent shock exposures (16). Interestingly, REM immediately before (24 hrs) foot-shock exposure predicts long-term emergence of hyperarousal after the foot-shock protocol (17), suggesting that this paradigm may be useful in examining treatments. This paradigm also induces enhanced neuronal activity in the PFC and amygdala and decreased volume in the hippocampus (13; 18–23) (Table 2). Some of these behavioral and circuit effects manifest weeks after the trauma (Tables 2 and S1), which can be exploited to examine different preventative vs. treatment strategies for early vs. late effects of trauma.
Figure 1. Schema illustrating the several animal models of PTSD.
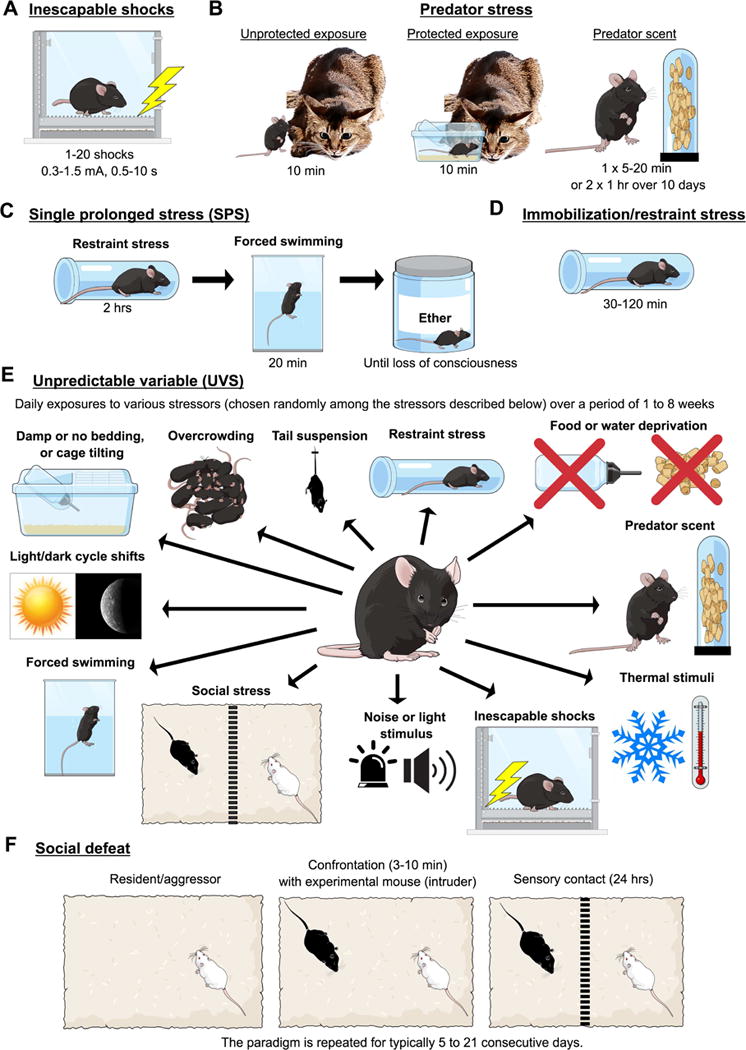
Inescapable shocks (A), predator stress (unprotected and protected exposure, and predator scent) (B), single prolonged stress (SPS) (C), immobilization or restraint stress (D), unpredictable variable stress (UVS) (E), and social defeat (F) models are described. Figure created on the Mind the Graph platform www.mindthegraph.com.
Table 2.
PTSD-related biological phenotypes in animal models of PTSD – Effects in males
| Model | Fear circuit dysfunctions (PFC-Amygdala) | HPA axis function | Peripheral vs. Central inflammation | Hippocampal structure/morphology | Sleep disturbances | Reversed by SSRIs |
|---|---|---|---|---|---|---|
| Inescapable foot shocks | ↑ cFos-positive cells (PFC, Amy) (90 min-6 wks) (13; 18) |
Plasma: ↑ CORT/ACTH (92; 93); ↑ feedback-DST (19); ↑ CORT in response to the situational reminder (2 wks) (21) Brain: N/A |
Plasma: N/A Brain: ↑ microglial activation (9 wks); ↑ pro-inflammatory cytokines (TNF-α, IL-6, IL-12); ↑ TLR4 levels; ↑ NLRP3 levels (23) | ↓ HP volume (4 wks) (22); ↓ number of cells and ↑ apoptosis rate (1 wk) (19) | ↑ awakening (3 wks); ↓ or ↑REm (3 wks) (18); ↑ total wakefulness and NREM time (1-8 wks) (94) | ↑ anxiety (EPM) (4 wks) (13); ↑ fear response in situational reminder (4 wks) (12–15); ↑ immobility (FST) (11) |
| Predator scent/stress | ↓ mPFC-Amy functional connectivity and ↑ cFos-positive cells in (PFC, Amy) (31); ↑ dendritic length and number (Amy) (8 days) (30) | Plasma: ↑ CORT/ACTH (9 days) (21; 33; 95); ↑ feedback-DST (25) Brain: ↑ GR (9 days) (96); ↑ CRFR1 levels (4 wks) and ↓ CRFR2 levels following situational reminder (4 wks) (96); ↑ or ↓ CRF (9 days) (97) |
Plasma: N/A Brain: ↑ pro-inflammatory cytokines (IL-1β, IL-18, TNF-α) and NLRP3 levels; ↑ TLR4 levels; ↓ anti-inflammatory cytokine IL-10 (4 wks) (27; 98; 99) |
↓ dendritic length and spine density (8 days) (29; 30) | N/A | ↑ anxiety (EPM) (1-4 wks) (28);↑ context and cued auditory freezing (4 wks) (28); ↑ startle (4 wks) (28); ↑ inflammation in brain (1 wk) (27) |
| Single prolonged stress | ↓ LTP (Amy) (1 wk) (100); ↓ glutamate (PFC) (1wk) (44; 101); ↑ apoptosis (Amy) (1-2 wks) (102) |
Plasma: ↑ feedback-DST (1 wk) (40; 100; 101); ↑ basal CORT and ACTH (100; 103–107) Brain: ↑ GR (1 wk) (41; 42; 101; 103; 106; 107); ↑ CRF and FKBP5 (1-3 wks) (100; 103–107) |
Plasma: N/A Brain:↑microglia,↑pro-inflammatory cytokines(TNF-α, IL-1β) (1-2wk) (104; 108) |
↑ apoptosis and ↓ volume (1 wk) (109; 110); ↓ LTP/LTD (1 wk) (100; 111) | Altered REM-NREM and EEG bands (1 wk) (112; 113) | ↑ fear learning (2 wks) (114); ↑ apoptosis (1 wk) (109); ↑ anxiety (2 wks) (104; 115); ↑ anhedonia (2 wks) (115); ↑ CORT (2 wks) (104); ↑ inflammation (104) |
| IMO/Restraint stress | ↓ HP-Amy coherence (11 days) (116); ↑ dendrites (Amy) (10 days) (117; 118); ↑ excitability (Amy) (10 days) (119) |
Plasma: ↓ and ↑Acth/cort for homo- and heterotypic stressors, respectively (1-4 wks) (120–122); ↑ feedback (18 days) (121; 122) Brain: N/A |
Plasma: N/A Brain: N/A |
↓ neurogenesis (5 wks) (123) | ↑ REM (1 day) (116; 124) | ↓ neurogenesis, ↑ immobility (FST), and ↑ anhedonia (SP) (5 wks) (123) |
| UVS | ↓ MD and ↑ neurite density (Amy) (52); ↑ cFos-positive cells (Amy) and ↑ damaged neurons (PFC) (53) | Plasma: ↑ basal CORT/ACTH(125; 126); ↓ CORT/ACTH hetero-response (3 wks) (127); ↑ feedback-DST (128) Brain: ↑ GR- and MR-positive neurons (2 days) (129) ; ↑ CRH levels (130) | Plasma: ↑ pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) (126); ↓ anti-inflammatory cytokine IL-10 (131)Brain: ↑ pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) (126; 132); ↑ TLR2 and NLRP3 levels (133; 134); ↑ microglial activation (134); ↓ IL-10 (135) | ↓ HP volume (136; 137); ↓ dendritic spine length/number (3 wks) (125; 138); ↑ apoptotic cells (139); ↓ cFos-positive cells (128) | ↑ wake state and ↓ REM sleep (140); “shift” of activity rhythms (more and less active during light and dark phases, respectively) (141) | ↑ anhedonia (SP) (2-4 wks) (48; 134); ↑ anxiety (novelty-suppressed feeding test) (2 wks) (128); ↑ immobility (FST, TST) (2 wks) (142); ↓ neurogenesis (4 wks) (143); ↑ CORT and inflammation (2 wks) (134; 135) |
| Social defeat | ↓ PFC control of Amy (10 days) (67); ↑ Amy activity during social defeat (1 day) (144; 145) | Plasma: ↑ basal CORT (12 days) (73); ↑ basal ACTH (68); ↓ feedback-DST (69) Brain: ↑↓ CRF; ↑CRFR2 (146) |
Plasma: ↑ pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) (6 days) (68) Brain: ↑ pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and microglial activation (6 days) (68) |
↑ HP to NAc activity (10 days) (147); ↓ HP volume (10 days) (148) | ↓ REM; ↑ NREM (1-28 days) (71; 72) | ↑ anhedonia (2 wks) (149); ↑ social avoidance (30 days) (150); ↑ fear memory (4 days) (61); ↑ CORT and inflammation (8 days) (58) |
ACTH, adrenocorticotropic hormone; Amy, amygdala; CORT, corticosterone; CRF, corticotropin-releasing factor; CRFR1, corticotropin-releasing factor receptor type 1; CRFR2, corticotropin-releasing factor receptor type 2; DST, dexamethasone suppression test; EEG, electroencephalography; GR, glucocorticoid receptor; HP, hippocampus; HPA, hypothalamic-pituitary-adrenal; LTP/LTD, long-term potentiation/depression; MD, mean diffusivity; MR, mineralocorticoid receptor; NAc, nucleus accumbens; NLRP3, NACTH, LRR and PYD domains-containing protein 3; NREM, non-rapid eye movement; PFC, prefrontal cortex; REM, rapid eye movement; SSRIs, selective serotonin reuptake inhibitors; TLR2, toll-like receptor 2; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor-α; UVS, unpredictable variable stress. In bold: most robust and reproduced findings. N.B.: The studies describing several PTSD-like phenotypes are not exclusive to this table, most comprehensive and robust findings as well as recency were used to prioritize addition of citation to the table.
Overall, the major strengths of shock exposure models are: (1) in some cases, enduring (up to 8 weeks) avoidance, hyperarousal, spatial memory deficits and fear-response to trauma cues (e.g. shock context) in both rats and mice; (2) sensitivity to sleep disturbances and induction of fear circuit pathology; and (3) the tight control over the stimulus parameters. Limitations include: (1) little data in females (Tables S2-S3); (2) the relatively non-ethological stressor; and (3) varied shock protocols across laboratories.
Predator stress model
Predator stress paradigms consist of a single-stress exposure, either unprotected exposure to a predator, exposure with a physical barrier, or exposure to a predator scent, that is inescapable, unpredictable and ethological (Figure 1) (24). These manipulations evoke enduring behavioral and physiological abnormalities up to 3 months after exposure, including general avoidance, exaggerated fear response, hyperarousal, and hyperalgesia (see Table S1 for details and references). In direct predator exposure paradigm, avoidance of trauma-related cues is assessed via subsequent exposure to predator odors in different contexts (25; 26) (Table S1). It is also sensitive to chronic administration of clinically effective SSRIs, mainly sertraline and amitriptyline (27; 28).
Predator exposure recapitulates some biological phenotypes in PTSD. Dendritic spines are reduced in hippocampus, while amygdala activity (cFos and dendritic spines number/length) is increased (29–31) (Table 2). It produces enhanced negative feedback of the HPA axis (25; 32), and an inverse correlation between post exposure levels of adrenocorticotropic hormone (ACTH) and corticosterone (CORT) and avoidance behaviors (33), suggesting that reduced HPA response to stress may predict long-term anxiety-like effects in this model (34). Predator stress can induce long-term inflammation in brain and is sensitive to anti-inflammatory treatments, however peripheral inflammation effects are not well described (for review see (10)).
Cohen and colleagues established a “cut-off behavioral criteria”, to classify susceptible vs. resilient animals based on a composite of extreme reductions in exploratory behavior and increased arousal (24) 7-90 days post-stress. This approach has been standardized (24) and meets the discriminant criteria to address the variance in individual response (35). Other groups identify resilient and susceptible individuals based on response to a “trauma reminder” (e.g. predator odor-paired chamber (36; 37)). Individual differences are not explained by differences in learning and memory per se, i.e. “resilient” animals are not simply bad at remembering past events (36).
Strengths of the predator stress model include (1) etiological validity with exposure to a single intense stressor; (2) robust behavioral and biological phenotypes (3) sensitivity to chronic SSRIs; and (4) established approach distinguishing susceptible vs. resilient animals. Limitations are the lack of data on sleep and depression/anhedonia measures (Table 2, Table S1). There are less data in females (Tables S2-S3), although females may be more susceptible to this paradigm than males (38). There are a number of variants of predator stress, from relatively severe (physical contact) to relatively mild (scent exposure only), thus moving across physical to purely “emotional” trauma models. Some paradigms require secondary stressors to increase efficacy (e.g. social instability post-stress (39)). Many of these parametric differences are laboratory specific, thus reliability across laboratories of specific protocols are difficult to assess.
Single prolonged stress (SPS)
SPS was developed by Liberzon et al. as a “single traumatic event”-like procedure to induce increased negative HPA feedback, a consistent neuroendocrinological characteristic of PTSD (40; 41). SPS applies three severe stressors in succession: a 2 h restraint-immobilization stress, followed by forced swimming for 20 min, and finally exposure to diethyl ether until loss of consciousness (Figure 1). It was proposed that SPS recapitulates the psychological, physiological and direct endocrine stress challenges that contribute to PTSD pathogenesis, although it is not clear how all 3 factors contribute in an additive/synergistic way to induce full symptomatology (42). Exposure to ether is required compared to other dissociative anesthetics (42), perhaps due to its unique effects on membrane permeability and/or neurotoxic effects. Ether may act via anoxia, as anoxia via underwater stress also produces some enduring PTSD-like phenotypes (43).
SPS reliably induces many PTSD-related behavioral (Table S1) and neurobiological phenotypes (Table 2). It produces elevated GR expression in the hippocampus and PFC, major negative feedback circuits for the HPA axis. Basal HPA axis activity and reactivity however (ACTH/CORT) are elevated post-trauma unlike in PTSD patients (Table 2) (i.e., low or normal cortisol) (6). SPS is also sensitive to chronic treatment with SSRIs (Table 2). Major strengths of SPS are (1) broad characterization of PTSD-relevant behavioral phenotypes and underlying neurobiological mechanisms (Tables 2 and S1), which (2) are fairly consistent across studies, and (3) a standardized procedure reproduced by multiple laboratories. Several phenotypes require a sensitization or incubation-like period (1 week) post-trauma to develop (41; 44), offering a well-defined time window for mechanistic studies targeting PTSD pathogenesis. Limitations include lack of data about (1) sex differences, (2) avoidance of trauma-related cues, and (3) less use of resilient vs. susceptible groupings (but see (45)).
Immobilization/restraint (IMO/RES) stress
In single IMO or RES stress, subjects are attached to a board or placed in a plastic restraint device for 30-120 min (Figure 1). Restraint is a strong, reliable psychogenic stressor inducing marked activation of the sympathetic nervous system and HPA axis. Restraint is more commonly used to probe mechanisms of acute stress responses and recovery or responses after chronic exposure, but enduring outcomes of single restraint is less well studied (Tables 2 and S1). Ressler and colleagues have developed an IMO variant, which uses a single 2 hrs board placement as the stressor. This stressor reliably induces long-term (>6 days after stress) generalized avoidance, increased startle, depression-like phenotype, and deficits in memory and fear extinction (see Table S1 for details and references). Extinction deficits are a consistent and relatively specific phenotype of PTSD and this is a construct with proven translation across animal and human studies (46).
The strengths of IMO are (1) extensive data on HPA axis-related changes and time course, (2) detailed and differential analysis of structural and functional changes in the prefrontal-hippocampal-amygdalar network, (3) available data on both sexes (although females are resilient) (Tables S2-S3), and (4) very reliable effects on fear-specific processes. In contrast, limitations are (1) limited data on other PTSD-relevant behavioral or biological outcomes, (2) few available studies using single exposure in combination with long-term measurements, and (3) limited use of vulnerable-resilient subgrouping approaches.
Unpredictable variable stress (UVS)
Although the UVS model is commonly considered a model of depression (47), this paradigm has face and predictive validities for at least some forms of PTSD, since it produces PTSD-relevant behavioral phenotypes and is responsive to chronic SSRIs (48) and fast-acting antidepressant ketamine (49–51). In this paradigm, behavioral abnormalities are induced following daily exposure to various stressors over a period of 1 to 8 weeks (Figure 1).
It is argued that exposing rodents to unpredictable stressors for several consecutive weeks mimics the prolonged and unpredictable stress experienced by soldiers during deployment (3). The UVS protocol induces most PTSD-relevant behaviors except for avoidance of trauma-specific cues (Table S1). Fear circuit abnormalities are also reported (higher c-fos in amygdala and its subregions) (52; 53), and susceptible and resilient groups differ in hippocampal and cortical functional activity (54). The UVS paradigm also induces enhanced negative feedback of the HPA axis in response to a subsequent stress or to dexamethasone (6) (Table 2). Behavioral markers of “pre-trauma risk” include pre-stress novelty-seeking and escape behavior predicting higher responses post-stress (53; 55).
Overall, UVS has (1) potential etiological validity for repeated, uncontrollable and unpredictable traumatic events such as deployment stress; (2) face validity for inducing long-lasting behavioral and physiological alterations similar to those observed in PTSD patients; and (3) approaches to identify susceptible and resilient groups, which allows researchers to identify potential biomarkers associated with higher vulnerability to stress. This is a problem for reproducibility, as it is not clear which variations are reliable in inducing long-term changes and if common mechanisms are shared across variations. Additionally, the strength of trauma-related avoidance cannot be determined due to the use of several stressors. There is also limited information in females (Tables S2-S3).
Social defeat stress (SDS)
SDS has been used extensively to assess behaviors and neurobiological mechanisms related to repeated stress exposure. SDS is typically performed in males using a resident-intruder procedure involving submission of the experimental animal (intruder) to an aggressive conspecific (resident) within the resident’s territory (see (56) for a review) (Figure 1). This procedure in rodents has been argued to model assault and social stress (57), however it can produce significant injury (58).
A consistent outcome of SDS is increased social avoidance (i.e., less time spent near a conspecific) (Table S1). Susceptible and resilient subgroups are also identified by social avoidance (e.g., susceptible animals spend <half of time near conspecific) (see Table S1 for details and references). Other consistent behavioral outcomes is hyperarousal, anhedonia (decreased sucrose preference, higher reward thresholds in intracranial self-stimulation), and several other impairments in reward and motivated behavior and in reward circuits (see Table S1 for details and references).
SDS has produced mixed effects on other PTSD-relevant phenotypes, such as hypoalgesia to thermal stimuli (59), and hyperalgesia to mechanical stimuli (60) (Table S1). Effects on fear learning are inconsistent, with susceptible mice typically showing increased contextual and cued fear learning (61) but there are also reports of decreased fear learning (62), increased fear learning in resilient animals only (63), or no effect of SDS in either group (64). Preliminary data indicate a slight impairment of extinction learning in socially defeated mice compared to non-stressed controls (65). Conversely, SDS in adolescent rats facilitates extinction learning in adulthood (66). There is some, but not completely consistent, evidence for impaired hippocampal-dependent cognition (see Table S1 for details and references).
In terms of PTSD-relevant biological phenotypes (Table 2), SDS increases amygdala activity, an effect thought to be mediated by decreased PFC control (67). SDS also robustly induces enduring peripheral and central inflammation (68). However, unlike PTSD and the other paradigms reviewed here, SDS suppresses negative feedback of HPA axis activity in a dexamethasone suppression test 1-3 weeks after stress (69). GR suppression may be a feature of chronic predictable stress, as although duration and intensity of daily defeat is unpredictable, the stressor type is not (70). Finally, SDS impairs sleep, resulting in decreased REM and increased non-rapid eye movement (NREM) slow-wave sleep (71; 72).
Overall strengths of the social defeat model is that it (1) reliably induces avoidance and reward alterations, fear circuit abnormalities and inflammation phenotypes; (2) is sensitive to anti-depressants (58; 64); and (3) offers a standardized protocol for the stressor to identify susceptibility and resilient groups. Limitations include that repeated exposure (10 days and 3-5 weeks for mice and rats, respectively) to a predictable stressor (defeat) is typically used to produce the phenomena, and it does not produce the relatively PTSD-specific phenotypes of extinction-deficits or increased HPA feedback. Finally, although modifications have been made in the procedure to test females (73), designing methodology that requires aggression toward female and adolescent rodents is challenging.
General discussion
Overall, the paradigms described above meet the requirement of inducing enduring behavioral and physiological abnormalities, typically from 7-90 days after stress termination. All paradigms produced enduring effects on general anxiety- and/or depression measures, although specific constructs mostly robustly affected varied across paradigms (Tables 2–3 and S1). There were some gaps in how each paradigm affected certain constructs. In terms of modeling behavioral phenotypes, avoidance of trauma-related cues is a critical feature of PTSD, but only half of the paradigms directly test this construct (SDS, inescapable foot shocks and variations of predator stress). At this point, only IMO and SPS produce robust deficits in fear extinction, another relatively specific phenotype to PTSD. In terms of biological phenotypes, all paradigms induced increased amygdala function, and all models except SDS produced GR hypersensitivity, a relatively specific phenotype for PTSD compared to anxiety and depression (Table 2). Other than SDS (74), little data exist on the peripheral inflammatory state evoked by these manipulations, which is a significant gap given that most of what is known about inflammation in PTSD is garnered from peripheral (e.g., plasma) studies. Sleep changes are also relatively understudied with only limited data in inescapable footshock, SDS and UVS. Although sleep and inflammation are relatively non-specific phenotypes, they are consistently changed in PTSD and enable homologous “biomarker” measures longitudinally across rodents and there is increasing evidence that they contribute directly to the etiology of PTSD risk and symptom maintenance (75; 76). For experimental design considerations, Tables 2 and S1 indicate the longest time post-stress in which PTSD phenotypes are observed, with most behavioral and biological changes tested <30 days post-stress. When feasible, expansion of assessments to longer periods post-trauma (2-3 months) to assess the trajectory of behavioral and circuit changes post-stress will support targeting of early vs. late interventions and better mimic the timeframe of treatment seeking in PTSD-like patients. All models except for SDS used unpredictable stressors, since repeated exposures to the same stressor may lead to adaptation/habituation, and HPA axis desensitization as seen in SDS (70). Unpredictability of the stressor can be controlled by exposing rats to different conspecifics each day in the SDS model, altering the stressor type (e.g. SPS), its duration (number of defeats, time to defeat in SDS or restraint duration) and also by adjusting its intensity (foot shock).
Table 3.
Reliability of PTSD-like behavioral and biological phenotypes produced in rodent stress paradigms
| Paradigm | Inescapable foot shocks | Predator scent/stress | Single prolonged stress | IMO/Restraint stress | UVS | Social defeat | |
|---|---|---|---|---|---|---|---|
| Behavioral phenotypes | ↑ Pain perception | ||||||
| General avoidance | |||||||
| Avoidance of trauma-related cues | |||||||
| ↑ Fear learning | |||||||
| ↓ Fear extinction | * | ||||||
| ↑ Arousal | |||||||
| Depression/Anhedonia | |||||||
| Memory deficits | |||||||
| Biological phenotypes | Circuits dysfunctions (PFC/Amygdala) | ||||||
| ↑ HPA negative feedback/GR sensitivity | * | ||||||
| Inflammation - Plasma | |||||||
| Inflammation - CNS | |||||||
| Hippocampal morphology/structure | |||||||
| Sensitive to chronic SSRIs | |||||||
| Sleep disturbances | |||||||
CNS, central nervous system; PFC, prefrontal cortex; UVS, unpredictable variable stress. Blank: phenotype not reported as yet; Light-blue: phenotype reported but reliability across laboratories unclear; Blue: robust phenotype reproducibly reported.
Opposite effects reported
Other gaps in the literature were how these models perform in female rodents (Tables S2-S3), across the lifespan (e.g., in adolescent or adult animals) (77) and if they are sensitive to early-life stress (i.e. “double-hit”). Females have higher prevalence of PTSD, potentially differing heritability for risk as well as treatment responses, thus the dearth of PTSD models using females is a significant problem for PTSD research. In terms of potential age effects, cortical circuitry matures during adolescence and early adulthood (78), suggesting that the adolescent brain may respond differently to trauma compared to the adult brain. This may be particularly important when attempting to model PTSD in military populations, in which combat trauma exposure is typically in late adolescence/early adulthood. There are also limited studies investigating the effects of early-life stressors or stress hormones on PTSD-related phenotypes (e.g. shift group to higher prevalence of “susceptible” animals) (38; 79; 80), despite the clear evidence that early-life trauma is one of the highest risk factors for PTSD (81).
Many paradigms have established approaches to take advantage of individual variance in responses to detect mechanisms of risk or resilience to enduring effects of stress. This approach has been increasingly favored in PTSD models to recapitulate the relatively low prevalence of PTSD even in highly traumatized populations (e.g. up to 20% in combat veterans), thus putatively providing greater face and etiological validity. The pros of this strategy include an inherent reliability test involving multiple measures in an individual, with one measurement to identify response group and then secondary tests to establish response to the planned experimental manipulation (e.g. treatment). It is not clear however how reliably cutoff methods are able to detect a true change in behavioral/biological response to the trauma vs. capturing random high trait-anxiety in the population of “susceptible” animals. Prevalence of resilience vs. susceptibility may also vary across species and strains (82–85). A future consideration is that these approaches currently rely on behavioral cutoffs to identify the “PTSD” group, and could incorporate biological changes to identify resilient and susceptible animals. With the advent of small animal imaging, telemetry and development of high throughput peripheral biomarkers, relatively non-invasive longitudinal assessments are possible. Indeed preliminary studies using non-invasive imaging such as MRI after stress are now emerging (86; 87). Movement towards these biological phenotypes can identify translational biomarkers of drug efficacy in proof-of-concept trials. Finally, there is little data on what translatable biological factors may predict which animals will be resilient or susceptible to trauma. There is some early evidence that individual differences in immune response (74) predict trauma response in the SDS model. Furthermore, another study demonstrated that low hippocampal levels of N-acetylaspartate assessed by proton magnetic resonance spectroscopy and differences in pre-trauma REM predicted persistent susceptibility to inescapable shocks in mice (17; 88).
For the future, as we gain better understanding of specific circuit and cell pathology in PTSD, techniques such as optogenetic and chemogenetic approaches (Table S4) can provide new models of targeted pathology within specific cell-types and circuits. For example, optogenetic methods targeting specific neuronal populations (e.g. glutamatergic neurons in PFC that project to the amygdala) could be used to mimic PFC-amygdala circuit abnormalities in PTSD, offering a highly specific drug target (e.g. drugs that enhance the activation of this circuit), as well as enable isolatation of potential neural circuits contributing to susceptibility to stress and treatment response (89; 90). Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) have promise to mimic long-term pathology via chronic clozapine-N-oxide treatment to modify second messenger cascades in a cell and circuit-specific manner. DREADD is particularly useful to manipulate large regions/circuits that cannot be manipulated via optogenetics due to spatial limitations (e.g. bed nucleus stria terminalis, microglia in PFC) (91). Overall, these tools should be used further in combination with stress models to enhance our understanding of the several circuits involved in the heterogeneity of PTSD symptoms.
Concluding remarks: In this review, we refrained from judgment of the “best” model of PTSD. PTSD is a heterogeneous disorder and the field is shifting towards targeting specific constructs/symptoms vs. overgeneralized diagnostic categories. In this context it is healthy to have multiple paradigms to enable appropriate hypothesis testing based on what is known about a given mechanism or drug target and its potential application to specific PTSD constructs. A long-term goal will be to identify the most robust underlying mechanisms for each model, to apply them to more targeted drug discovery and testing efforts. One approach to increase predictability of animal models is to incorporate homologous biological measures of risk and treatment response. Identifying reliable individual risk and treatment response predictors (e.g. high inflammatory response pre-trauma) in these models will be critical in informing appropriate clinical trial design on these models. Use of relatively non-invasive tools that allow longitudinal assessment (e.g. peripheral inflammation, sleep and non-invasive imaging) will complement behavioral outcomes upon which most paradigms are currently based (see Box S1). The hope is that treatments may be more readily translated to clinical trials enriched for patient groups endorsing the behavioral and biological phenotypes predicted by treatment efficacy in these models. Hence, achieving enhanced translational will require use of these homologous objective measures not only in animals, but across clinical trials designs as well.
Supplementary Material
Acknowledgments
Jessica Deslauriers, Ph.D. is recipient of a CIHR (Canadian Institutes of Health Research) postdoctoral fellowship. This work was also supported by a VA Merit Award and VA Center of Excellence for Stress and Mental Health support to VBR, R01 AA026560 for VBR and AD, UH2 MH109334 for AD and VBR, and National Research, Development and Innovation Office grant (No.116589) and Bolyai Janos Research Fellowship to MT. We thank members of the Cohen Veterans Biosciences Foundation PTSD Pre-clinical Workshop for inspiration for this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Koenen KC, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ, et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol Med. 2017:1–15. doi: 10.1017/S0033291717000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD) Int J Neuropsychopharmacol. 2012;15:825–840. doi: 10.1017/S1461145711001209. [DOI] [PubMed] [Google Scholar]
- 3.Goswami S, Rodríguez-Sierra O, Cascardi M, Paré D. Animal models of post-traumatic stress disorder: face validity. Front Neurosci. 2013;7:89. doi: 10.3389/fnins.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther. 2015;149:150–190. doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. doi: 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- 7.Siegmund A, Wotjak CT. Toward an animal model of posttraumatic stress disorder. Ann N Y Acad Sci. 2006;1071:324–334. doi: 10.1196/annals.1364.025. [DOI] [PubMed] [Google Scholar]
- 8.Bremner JD. Effects of traumatic stress on brain structure and function: relevance to early responses to trauma. J Trauma Dissociation. 2005;6:51–68. doi: 10.1300/J229v06n02_06. [DOI] [PubMed] [Google Scholar]
- 9.Acheson DT, Gresack JE, Risbrough VB. Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology. 2012;62:674–685. doi: 10.1016/j.neuropharm.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deslauriers J, Powell SB, Risbrough VB. Immune signaling mechanisms of PTSD risk and symptom development: insights from animal models. Current Opinion in Behavioral Sciences. 2017;14:123–132. doi: 10.1016/j.cobeha.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pryce CR, Azzinnari D, Spinelli S, Seifritz E, Tegethoff M, Meinlschmidt G. Helplessness: a systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacol Ther. 2011;132:242–267. doi: 10.1016/j.pharmthera.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Kao CY, Stalla G, Stalla J, Wotjak CT, Anderzhanova E. Norepinephrine and corticosterone in the medial prefrontal cortex and hippocampus predict PTSD-like symptoms in mice. Eur J Neurosci. (4) 2015;41:1139–1148. doi: 10.1111/ejn.12860. [DOI] [PubMed] [Google Scholar]
- 13.Bentefour Y, Rakibi Y, Bennis M, Ba-M’hamed S, Garcia R. Paroxetine treatment, following behavioral suppression of PTSD-like symptoms in mice, prevents relapse by activating the infralimbic cortex. Eur Neuropsychopharmacol. 2016;26:195–207. doi: 10.1016/j.euroneuro.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann L, Ionescu IA, Henes K, Golub Y, Wang NXR, Buell DR, et al. Long-lasting hippocampal synaptic protein loss in a mouse model of posttraumatic stress disorder. PLoS ONE. 2012;7:e42603. doi: 10.1371/journal.pone.0042603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Yang SH, Kwon J, Lee HW, Kim H. Mice subjected to uncontrollable electric shocks show depression-like behaviors irrespective of their state of helplessness. Behav Brain Res. 2017;322:138–144. doi: 10.1016/j.bbr.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Polta SA, Fenzl T, Jakubcakova V, Kimura M, Yassouridis A, Wotjak CT. Prognostic and symptomatic aspects of rapid eye movement sleep in a mouse model of posttraumatic stress disorder. Front Behav Neurosci. 2013;31:7–60. doi: 10.3389/fnbeh.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bin Yu, Cui S-Y, Zhang X-Q, Cui X-Y, Li S-J, Sheng Z-F, et al. Different neural circuitry is involved in physiological and psychological stress-induced PTSD-like “nightmares” in rats. Sci Rep. 2015:1–14. doi: 10.1038/srep15976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C. Glutamate and GABA imbalance promotes neuronal apoptosis in hippocampus after stress. Med Sci Monit. 2014;20:499–512. doi: 10.12659/MSM.890589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao C-Y, He Z, Zannas AS, Hahn O, Kühne C, Reichel JM, et al. Fluoxetine treatment prevents the inflammatory response in a mouse model of posttraumatic stress disorder. J Psychiatr Res. 2016;76:74–83. doi: 10.1016/j.jpsychires.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Bakshi VP, Alsene KM, Roseboom PH, Connors EE. Enduring sensorimotor gating abnormalities following predator exposure or corticotropin-releasing factor in rats: a model for PTSD-like information-processing deficits? Neuropharmacology. 2012;62:737–748. doi: 10.1016/j.neuropharm.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golub Y, Kaltwasser SF, Mauch CP, Herrmann L, Schmidt U, Holsboer F, et al. Reduced hippocampus volume in the mouse model of Posttraumatic Stress Disorder. J Psychiatr Res. 2011;45:650–659. doi: 10.1016/j.jpsychires.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y, Pardo M, de Armini RS, Martinez A, Mouhsine H, Zagury J-F, et al. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun. 2016;53:207–222. doi: 10.1016/j.bbi.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen H, Matar MA, Richter-Levin G, Zohar J. The contribution of an animal model toward uncovering biological risk factors for PTSD. Ann N Y Acad Sci. 2006;1071:335–350. doi: 10.1196/annals.1364.026. [DOI] [PubMed] [Google Scholar]
- 25.Zoladz PR, Park CR, Fleshner M, Diamond DM. Psychosocial predator-based animal model of PTSD produces physiological and behavioral sequelae and a traumatic memory four months following stress onset. Physiology & behavior. 2015;147:183–192. doi: 10.1016/j.physbeh.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Deslauriers J, van Wijngaarde M, Geyer MA, Powell S, Risbrough VB. Effects of LPS-induced immune activation prior to trauma exposure on PTSD-like symptoms in mice. Behav Brain Res. 2017;323:117–123. doi: 10.1016/j.bbr.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Wilson CB, McLaughlin LD, Ebenezer PJ, Nair AR, Dange R, Harre JG, et al. Differential effects of sertraline in a predator exposure animal model of post-traumatic stress disorder. Front Behav Neurosci. 2014;8:256. doi: 10.3389/fnbeh.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoladz PR, Fleshner M, Diamond DM. Differential effectiveness of tianeptine, clonidine and amitriptyline in blocking traumatic memory expression, anxiety and hypertension in an animal model of PTSD. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:1–16. doi: 10.1016/j.pnpbp.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman JR, Cohen H, Ostfeld I, Kaplan Z, Zohar J, Cohen H. Exercise Maintains Dendritic Complexity in an Animal Model of Posttraumatic Stress Disorder. Med Sci Sports Exerc. 2016;48:2487–2494. doi: 10.1249/MSS.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 30.Cohen H, Kozlovsky N, Matar MA, Zohar J, Kaplan Z. Distinctive hippocampal and amygdalar cytoarchitectural changes underlie specific patterns of behavioral disruption following stress exposure in an animal model of PTSD. Eur Neuropsychopharmacol. 2014;24:1925–1944. doi: 10.1016/j.euroneuro.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Janitzky K, D’Hanis W, Kröber A, Schwegler H. TMT predator odor activated neural circuit in C57BL/6J mice indicates TMT-stress as a suitable model for uncontrollable intense stress. Brain Res. 2015;1599:1–8. doi: 10.1016/j.brainres.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 32.Yehuda R. Neuroendocrine aspects of PTSD. Handb Exp Pharmacol. 2005:371–403. doi: 10.1007/3-540-28082-0_13. [DOI] [PubMed] [Google Scholar]
- 33.Whitaker AM, Gilpin NW. Blunted hypothalamo-pituitary adrenal axis response to predator odor predicts high stress reactivity. Physiology & behavior. 2015;147:16–22. doi: 10.1016/j.physbeh.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouthaan J, Sijbrandij M, Luitse JSK, Goslings JC, Gersons BPR, Olff M. The role of acute cortisol and DHEAS in predicting acute and chronic PTSD symptoms. Psychoneuroendocrinology. 2014;45:179–186. doi: 10.1016/j.psyneuen.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Belzung C, Lemoine M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol Mood Anxiety Disord. 2011;1:9. doi: 10.1186/2045-5380-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry. 2013;3:e296. doi: 10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreiber AL, Lu Y-L, Baynes BB, Richardson HN, Gilpin NW. Corticotropin-releasing factor in ventromedial prefrontal cortex mediates avoidance of a traumatic stress-paired context. Neuropharmacology. 2017;113:323–330. doi: 10.1016/j.neuropharm.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toth M, Flandreau EI, Deslauriers J, Geyer MA, Mansuy I, Merlo-Pich E, Risbrough V. Overexpression of Forebrain CRH During Early Life Increases Trauma Susceptibility in Adulthood. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zoladz PR, Diamond DM. Predator-based psychosocial stress animal model of PTSD: Preclinical assessment of traumatic stress at cognitive, hormonal, pharmacological, cardiovascular and epigenetic levels of analysis. Experimental Neurology. 2016;284:211–219. doi: 10.1016/j.expneurol.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–453. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 41.Liberzon I, López JF, Flagel SB, Vázquez DM, Young EA. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol. 1999;11:11–17. doi: 10.1046/j.1365-2826.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- 42.Knox D, Nault T, Henderson C, Liberzon I. Glucocorticoid receptors and extinction retention deficits in the single prolonged stress model. Neuroscience. 2012;223:163–173. doi: 10.1016/j.neuroscience.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 43.Ardi Z, Albrecht A, Richter-Levin A, Saha R, Richter-Levin G. Behavioral profiling as a translational approach in an animal model of posttraumatic stress disorder. Neurobiol Dis. 2016;88:139–147. doi: 10.1016/j.nbd.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Knox D, Perrine SA, George SA, Galloway MP, Liberzon I. Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci Lett. 2010;480:16–20. doi: 10.1016/j.neulet.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Dorze C, Gisquet-Verrier P. Sensitivity to trauma-associated cues is restricted to vulnerable traumatized rats and reinstated after extinction by yohimbine. Behav Brain Res. 2016;313:120–134. doi: 10.1016/j.bbr.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Risbrough VB, Glenn DE, Baker DG. On the Road to Translation for PTSD Treatment: Theoretical and Practical Considerations of the Use of Human Models of Conditioned Fear for Drug Development. Curr Top Behav Neurosci. 2016;28:173–196. doi: 10.1007/7854_2015_5010. [DOI] [PubMed] [Google Scholar]
- 47.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 48.Yin X, Guven N, Dietis N. Stress-based animal models of depression: Do we actually know what we are doing? Brain Res. 2016;1652:30–42. doi: 10.1016/j.brainres.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 49.Melo A, Kokras N, Dalla C, Ferreira C, Ventura-Silva AP, Sousa N, Pêgo JM. The positive effect on ketamine as a priming adjuvant in antidepressant treatment. Transl Psychiatry. 2015;5:e573. doi: 10.1038/tp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma X-C, Dang Y-H, Jia M, Ma R, Wang F, Wu J, et al. Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS ONE. 2013;8:e56053. doi: 10.1371/journal.pone.0056053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia LSB, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Progress in Neuropsychopharmacology & Biological Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Khan AR, Chuhutin A, Wiborg O, Kroenke CD, Nyengaard JR, Hansen B, Jespersen SN. Biophysical modeling of high field diffusion MRI demonstrates micro-structural aberration in chronic mild stress rat brain. Neuroimage. 2016;142:421–430. doi: 10.1016/j.neuroimage.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawley DF, Bardi M, Everette AM, Higgins TJ, Tu KM, Kinsley CH, Lambert KG. Neurobiological constituents of active, passive, and variable coping strategies in rats: integration of regional brain neuropeptide Y levels and cardiovascular responses. Stress. 2010;13:172–183. doi: 10.3109/10253890903144621. [DOI] [PubMed] [Google Scholar]
- 54.Harro J, Kanarik M, Kaart T, Matrov D, Kõiv K, Mällo T, et al. Revealing the cerebral regions and networks mediating vulnerability to depression: oxidative metabolism mapping of rat brain. Behav Brain Res. 2014;267:83–94. doi: 10.1016/j.bbr.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 55.Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiology & behavior. 2011;103:210–216. doi: 10.1016/j.physbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hammels C, Pishva E, De Vry J, van den Hove DLA, Prickaerts J, Van Winkel R, et al. Defeat stress in rodents: From behavior to molecules. Neurosci Biobehav Rev. 2015;59:111–140. doi: 10.1016/j.neubiorev.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Björkqvist K. Social defeat as a stressor in humans. Physiology & behavior. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- 58.Ramirez K, Sheridan JF. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive-like behaviors. Brain Behav Immun. 2016;57:293–303. doi: 10.1016/j.bbi.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Amato FR, Pavone F. Modulation of nociception by social factors in rodents: contribution of the opioid system. Psychopharmacology. 2012;224:189–200. doi: 10.1007/s00213-012-2863-1. [DOI] [PubMed] [Google Scholar]
- 60.Li C, Yang Y, Liu S, Fang H, Zhang Y, Furmanski O, et al. Stress induces pain transition by potentiation of AMPA receptor phosphorylation. Journal of Neuroscience. 2014;34:13737–13746. doi: 10.1523/JNEUROSCI.2130-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fuertig R, Azzinnari D, Bergamini G, Cathomas F, Sigrist H, Seifritz E, et al. Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: both effects are reversed by inhibition of indoleamine 23-dioxygenase. Brain Behav Immun. 2015:1–14. doi: 10.1016/j.bbi.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 62.Brzózka MM, Havemann-Reinecke U, Wichert SP, Falkai P, Rossner MJ. Molecular Signatures of Psychosocial Stress and Cognition Are Modulated by Chronic Lithium Treatment. Schizophrenia Bulletin. 2015 doi: 10.1093/schbul/sbv194. sbv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dulka BN, Lynch JF, Latsko MS, Mulvany JL, Jasnow AM. Phenotypic responses to social defeat are associated with differences in cued and contextual fear discrimination. Behav Processes. 2015;118:115–122. doi: 10.1016/j.beproc.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, et al. Ketamine as a Prophylactic Against Stress-Induced Depressive-like Behavior. Biol Psychiatry. 2016;79:776–786. doi: 10.1016/j.biopsych.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Narayanan V, Heiming RS, Jansen F, Lesting J, Sachser N, Pape H-C, Seidenbecher T. Social defeat: impact on fear extinction and amygdala-prefrontal cortical theta synchrony in 5-HTT deficient mice. PLoS ONE. 2011;6:e22600. doi: 10.1371/journal.pone.0022600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Novick AM, Mears M, Forster GL, Lei Y, Tejani-Butt SM, Watt MJ. Adolescent social defeat alters N-methyl-D-aspartic acid receptor expression and impairs fear learning in adulthood. Behav Brain Res. 2016;304:51–59. doi: 10.1016/j.bbr.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hultman R, Mague SD, Li Q, Katz BM, Michel N, Lin L, et al. Dysregulation of Prefrontal Cortex-Mediated Slow-Evolving Limbic Dynamics Drives Stress-Induced Emotional Pathology. Neuron. 2016;91:439–452. doi: 10.1016/j.neuron.2016.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience. 2015;289:429–442. doi: 10.1016/j.neuroscience.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buwalda B, de Boer SF, Schmidt ED, Felszeghy K, Nyakas C, Sgoifo A, et al. Long-lasting deficient dexamethasone suppression of hypothalamic-pituitary-adrenocortical activation following peripheral CRF challenge in socially defeated rats. J Neuroendocrinol. 1999;11:513–520. doi: 10.1046/j.1365-2826.1999.00350.x. [DOI] [PubMed] [Google Scholar]
- 70.Jaggi AS, Bhatia N, Kumar N, Singh N, Anand P, Dhawan R. A review on animal models for screening potential anti-stress agents. Neurol Sci. 2011;32:993–1005. doi: 10.1007/s10072-011-0770-6. [DOI] [PubMed] [Google Scholar]
- 71.Kamphuis J, Lancel M, Koolhaas JM, Meerlo P. Deep sleep after social stress: NREM sleep slow-wave activity is enhanced in both winners and losers of a conflict. Brain Behav Immun. 2015;47:149–154. doi: 10.1016/j.bbi.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 72.Meerlo P, Pragt BJ, Daan S. Social stress induces high intensity sleep in rats. Neurosci Lett. 1997;225:41–44. doi: 10.1016/s0304-3940(97)00180-8. [DOI] [PubMed] [Google Scholar]
- 73.Page GG, Opp MR, Kozachik SL. Sex differences in sleep, anhedonia, and HPA axis activity in a rat model of chronic social defeat. Neurobiology of Stress. 2016;3:105–113. doi: 10.1016/j.ynstr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baker DG, Nievergelt CM, O’Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology. 2012;62:663–673. doi: 10.1016/j.neuropharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 76.Kobayashi I, Cowdin N, Mellman TA. One’s sex, sleep, and posttraumatic stress disorder. Biol Sex Differ. 2012;3:29. doi: 10.1186/2042-6410-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hales S, Zimmermann C, Rodin G. The quality of dying and death. Arch Intern Med. 2008;168:912–918. doi: 10.1001/archinte.168.9.912. [DOI] [PubMed] [Google Scholar]
- 78.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 79.Siegmund A, Dahlhoff M, Habersetzer U, Mederer A, Wolf E, Holsboer F, Wotjak CT. Maternal inexperience as a risk factor of innate fear and PTSD-like symptoms in mice. J Psychiatr Res. 2009;43:1156–1165. doi: 10.1016/j.jpsychires.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Tsoory M, Guterman A, Richter-Levin G. Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: potential relevance for mood and anxiety disorders. Neuropsychopharmacology. 2008;33:378–393. doi: 10.1038/sj.npp.1301397. [DOI] [PubMed] [Google Scholar]
- 81.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 82.Dahlhoff M, Siegmund A, Golub Y, Wolf E, Holsboer F, Wotjak CT. AKT/GSK-3 beta/beta-catenin signalling within hippocampus and amygdala reflects genetically determined differences in posttraumatic stress disorder like symptoms. Neuroscience. 2010;169:1216–1226. doi: 10.1016/j.neuroscience.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 83.Cohen H, Geva AB, Matar MA, Zohar J, Kaplan Z. Post-traumatic stress behavioural responses in inbred mouse strains: can genetic predisposition explain phenotypic vulnerability? Int J Neuropsychopharmacol. 2008;11:331–349. doi: 10.1017/S1461145707007912. [DOI] [PubMed] [Google Scholar]
- 84.Ibarguen-Vargas Y, Surget A, Touma C, Palme R, Belzung C. Multifaceted strain-specific effects in a mouse model of depression and of antidepressant reversal. Psychoneuroendocrinology. 2008;33:1357–1368. doi: 10.1016/j.psyneuen.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 85.Razzoli M, Carboni L, Andreoli M, Michielin F, Ballottari A, Arban R. Strain-specific outcomes of repeated social defeat and chronic fluoxetine treatment in the mouse. Pharmacol Biochem Behav. 2011;97:566–576. doi: 10.1016/j.pbb.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 86.Montoya ER, van Honk J, Bos PA, Terburg D. Dissociated neural effects of cortisol depending on threat escapability. Hum Brain Mapp. 2015;36:4304–4316. doi: 10.1002/hbm.22918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang Z, King J, Zhang N. Neuroplasticity to a single-episode traumatic stress revealed by resting-state fMRI in awake rats. Neuroimage. 2014;103:485–491. doi: 10.1016/j.neuroimage.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siegmund A, Kaltwasser SF, Holsboer F, Czisch M, Wotjak CT. Hippocampal N-acetylaspartate levels before trauma predict the development of long-lasting posttraumatic stress disorder-like symptoms in mice. Biol Psychiatry. 2009;65:258–262. doi: 10.1016/j.biopsych.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 89.Daskalakis NP, Yehuda R. Principles for developing animal models of military PTSD. Eur J Psychotraumatol. 2014;5 doi: 10.3402/ejpt.v5.23825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sparta DR, Jennings JH, Ung RL, Stuber GD. Optogenetic strategies to investigate neural circuitry engaged by stress. Behav Brain Res. 2013;255:19–25. doi: 10.1016/j.bbr.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andero R, Dias BG, Ressler KJ. A role for Tac2, NkB, and Nk3 receptor in normal and dysregulated fear memory consolidation. Neuron. 2014;83:444–454. doi: 10.1016/j.neuron.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jin ZL, Liu JX, Liu X, Zhang LM, Ran YH, Zheng YY, et al. Anxiolytic effects of GLYX-13 in animal models of posttraumatic stress disorder-like behavior. J Psychopharmacol (Oxford) 2016;30:913–921. doi: 10.1177/0269881116645298. [DOI] [PubMed] [Google Scholar]
- 93.Philbert J, Pichat P, Palme R, Belzung C, Griebel G. The CRF1 receptor antagonist SSR125543 attenuates long-term cognitive deficit induced by acute inescapable stress in mice, independently from the hypothalamic pituitary adrenal axis. Pharmacol Biochem Behav. 2012;102:415–422. doi: 10.1016/j.pbb.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 94.Chen X, Li Y, Li S, Kirouac GJ. Early fear as a predictor of avoidance in a rat model of post-traumatic stress disorder. Behav Brain Res. 2012;226:112–117. doi: 10.1016/j.bbr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 95.Cohen H, Kaplan Z, Kozlovsky N, Gidron Y, Matar MA, Zohar J. Hippocampal microinfusion of oxytocin attenuates the behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. J Neuroendocrinol. 2010;22:889–904. doi: 10.1111/j.1365-2826.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- 96.Elharrar E, Warhaftig G, Issler O, Sztainberg Y, Dikshtein Y, Zahut R, et al. Overexpression of corticotropin-releasing factor receptor type 2 in the bed nucleus of stria terminalis improves posttraumatic stress disorder-like symptoms in a model of incubation of fear. Biol Psychiatry. 2013;74:827–836. doi: 10.1016/j.biopsych.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 97.Janitzky K, Peine A, Kröber A, Yanagawa Y, Schwegler H, Roskoden T. Increased CRF mRNA expression in the sexually dimorphic BNST of male but not female GAD67 mice and TMT predator odor stress effects upon spatial memory retrieval. Behav Brain Res. 2014;272:141–149. doi: 10.1016/j.bbr.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 98.Wilson CB, McLaughlin LD, Ebenezer PJ, Nair AR, Francis J. Valproic acid effects in the hippocampus and prefrontal cortex in an animal model of post-traumatic stress disorder. Behav Brain Res. 2014;268:72–80. doi: 10.1016/j.bbr.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 99.Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. In: Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J, editors. PLoS ONE. Vol. 8. 2013. p. e76146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kohda K, Harada K, Kato K, Hoshino A, Motohashi J, Yamaji T, et al. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: a putative post-traumatic stress disorder model. Neuroscience. 2007;148:22–33. doi: 10.1016/j.neuroscience.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 101.Perrine SA, Eagle AL, George SA, Mulo K, Kohler RJ, Gerard J, et al. Severe, multimodal stress exposure induces PTSD-like characteristics in a mouse model of single prolonged stress. Behav Brain Res. 2016;303:228–237. doi: 10.1016/j.bbr.2016.01.056. [DOI] [PubMed] [Google Scholar]
- 102.Ding J, Han F, Shi Y. Single-prolonged stress induces apoptosis in the amygdala in a rat model of post-traumatic stress disorder. J Psychiatr Res. 2010;44:48–55. doi: 10.1016/j.jpsychires.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 103.Laukova M, Alaluf LG, Serova LI, Arango V, Sabban EL. Early intervention with intranasal NPY prevents single prolonged stress-triggered impairments in hypothalamus and ventral hippocampus in male rats. Endocrinology. 2014;155:3920–3933. doi: 10.1210/en.2014-1192. [DOI] [PubMed] [Google Scholar]
- 104.Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm D-H. Effects of systemic administration of ibuprofen on stress response in a rat model of post-traumatic stress disorder. Korean J Physiol Pharmacol. 2016;20:357–366. doi: 10.4196/kjpp.2016.20.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Patki G, Li L, Allam F, Solanki N, Dao AT, Alkadhi K, Salim S. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiology & behavior. 2014;130:47–53. doi: 10.1016/j.physbeh.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 107.Wang H-T, Han F, Shi Y-X. Activity of the 5 HT1A receptor is involved in the alteration of glucocorticoid receptor in hippocampus and corticotropin-releasing factor in hypothalamus in SPS rats. Int J Mol Med. 2009;24:227–231. doi: 10.3892/ijmm_00000225. [DOI] [PubMed] [Google Scholar]
- 108.Sun R, Zhang Z, Lei Y, Liu Y, Lu C, Rong H, et al. Hippocampal activation of microglia may underlie the shared neurobiology of comorbid posttraumatic stress disorder and chronic pain. Mol Pain. 2016;12 doi: 10.1177/1744806916679166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang H, Zuo D, He B, Qiao F, Zhao M, Wu Y. Conditioned fear stress combined with single-prolonged stress: a new PTSD mouse model. Neurosci Res. 2012;73:142–152. doi: 10.1016/j.neures.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 110.Li XM, Han F, Liu DJ, Shi Y-X. Single-prolonged stress induced mitochondrial-dependent apoptosis in hippocampus in the rat model of post-traumatic stress disorder. J Chem Neuroanat. 2010;40:248–255. doi: 10.1016/j.jchemneu.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 111.Zer-Aviv TM, Akirav I. Sex differences in hippocampal response to endocannabinoids after exposure to severe stress. Hippocampus. 2016;26:947–957. doi: 10.1002/hipo.22577. [DOI] [PubMed] [Google Scholar]
- 112.Nedelcovych MT, Gould RW, Zhan X, Bubser M, Gong X, Grannan M, et al. A rodent model of traumatic stress induces lasting sleep and quantitative electroencephalographic disturbances. ACS Chem Neurosci. 2015;6:485–493. doi: 10.1021/cn500342u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vanderheyden WM, George SA, Urpa L, Kehoe M, Liberzon I, Poe GR. Sleep alterations following exposure to stress predict fear-associated memory impairments in a rodent model of PTSD. Exp Brain Res. 2015;233:2335–2346. doi: 10.1007/s00221-015-4302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takahashi T, Morinobu S, Iwamoto Y, Yamawaki S. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology. 2006;189:165–173. doi: 10.1007/s00213-006-0545-6. [DOI] [PubMed] [Google Scholar]
- 115.Lin C-C, Tung C-S, Liu Y-P. Escitalopram reversed the traumatic stress-induced depressed and anxiety-like symptoms but not the deficits of fear memory. Psychopharmacology. 2016;233:1135–1146. doi: 10.1007/s00213-015-4194-5. [DOI] [PubMed] [Google Scholar]
- 116.Hegde P, Singh K, Chaplot S, Shankaranarayana Rao BS, Chattarji S, Kutty BM, Laxmi TR. Stress-induced changes in sleep and associated neuronal activity in rat hippocampus and amygdala. Neuroscience. 2008;153:20–30. doi: 10.1016/j.neuroscience.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 117.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chauveau F, Lange MD, Jüngling K, Lesting J, Seidenbecher T, Pape H-C. Prevention of stress-impaired fear extinction through neuropeptide s action in the lateral amygdala. Neuropsychopharmacology. 2012;37:1588–1599. doi: 10.1038/npp.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Belda X, Fuentes S, Nadal R, Armario A. A single exposure to immobilization causes long-lasting pituitary-adrenal and behavioral sensitization to mild stressors. Hormones and behavior. 2008;54:654–661. doi: 10.1016/j.yhbeh.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 121.Belda X, Márquez C, Armario A. Long-term effects of a single exposure to stress in adult rats on behavior and hypothalamic-pituitary-adrenal responsiveness: comparison of two outbred rat strains. Behav Brain Res. 2004;154:399–408. doi: 10.1016/j.bbr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 122.Martí O, García A, Vallès A, Harbuz MS, Armario A, Vellès A. Evidence that a single exposure to aversive stimuli triggers long-lasting effects in the hypothalamus-pituitary-adrenal axis that consolidate with time. Eur J Neurosci. 2001;13:129–136. [PubMed] [Google Scholar]
- 123.Chu X, Zhou Y, Hu Z, Lou J, Song W, Li J, et al. 24-hour-restraint stress induces long-term depressive-like phenotypes in mice. Sci Rep. 2016;6:32935. doi: 10.1038/srep32935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meerlo P, Easton A, Bergmann BM, Turek FW. Restraint increases prolactin and REM sleep in C57BL/6J mice but not in BALB/cJ mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R846–54. doi: 10.1152/ajpregu.2001.281.3.R846. [DOI] [PubMed] [Google Scholar]
- 125.Zhang L-M, Wang Y-L, Liu Y-Q, Xue R, Zhang Y-Z, Yang R-F, Li Y-F. Antidepressant-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) in chronically stressed rats. Neuropharmacology. 2017;113:567–575. doi: 10.1016/j.neuropharm.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 126.Tao W, Dong Y, Su Q, Wang H, Chen Y, Xue W, et al. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav Brain Res. 2016;308:177–186. doi: 10.1016/j.bbr.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 127.Cordner ZA, Tamashiro KLK. Effects of chronic variable stress on cognition and Bace1 expression among wild-type mice. Transl Psychiatry. 2016;6:e854. doi: 10.1038/tp.2016.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Law J, Ibarguen-Vargas Y, Belzung C, Surget A. Decline of hippocampal stress reactivity and neuronal ensemble coherence in a mouse model of depression. Psychoneuroendocrinology. 2016;67:113–123. doi: 10.1016/j.psyneuen.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 129.Cotella EM, Durando PE, Suárez MM. A double-hit model of stress dysregulation in rats: implications for limbic corticosteroid receptors and anxious behavior under amitriptyline treatment. Stress. 2014;17:235–246. doi: 10.3109/10253890.2014.910649. [DOI] [PubMed] [Google Scholar]
- 130.Franco AJ, Chen C, Scullen T, Zsombok A, Salahudeen AA, Di S, et al. Sensitization of the Hypothalamic-Pituitary-Adrenal Axis in a Male Rat Chronic Stress Model. Endocrinology. 2016;157:2346–2355. doi: 10.1210/en.2015-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhuang F, Zhou X, Gao X, Lou D, Bi X, Qin S, et al. Cytokines and glucocorticoid receptors are associated with the antidepressant-like effect of alarin. Peptides. 2016;76:115–129. doi: 10.1016/j.peptides.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 132.Tagliari B, Tagliari AP, Schmitz F, da Cunha AA, Dalmaz C, Wyse ATS. Chronic variable stress alters inflammatory and cholinergic parameters in hippocampus of rats. Neurochem Res. (1st) 2010;36:487–493. doi: 10.1007/s11064-010-0367-0. [DOI] [PubMed] [Google Scholar]
- 133.Su W-J, Zhang Y, Chen Y, Gong H, Lian Y-J, Peng W, et al. NLRP3 gene knockout blocks NF-kB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav Brain Res. 2017;322:1–8. doi: 10.1016/j.bbr.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 134.Pan Y, Chen X-Y, Zhang Q-Y, Kong L-D. Microglial NLRP3 inflammasome activation mediates IL-1b-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014;41:90–100. doi: 10.1016/j.bbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 135.Xie H, Di Jin, Kang Y, Shi X, Liu H, Shen H, et al. The effect of Piper laetispicum extract (EAE-P) during chronic unpredictable mild stress based on interrelationship of inflammatory cytokines, apoptosis cytokines and neurotrophin in the hippocampus. BMC Complementary and Alternative Medicine. 2015:1–11. doi: 10.1186/s12906-015-0747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luo Y, Cao Z, Wang D, Wu L, Li Y, Sun W, Zhu Y. Dynamic study of the hippocampal volume by structural MRI in a rat model of depression. Neurol Sci. 2014;35:1777–1783. doi: 10.1007/s10072-014-1837-y. [DOI] [PubMed] [Google Scholar]
- 137.Li Y, Zhu X, Ju S, Yan J, Wang D, Zhu Y, Zang F. Detection of volume alterations in hippocampal subfields of rats under chronic unpredictable mild stress using 7T MRI: A follow-up study. J Magn Reson Imaging. 2017;30:111. doi: 10.1002/jmri.25667. [DOI] [PubMed] [Google Scholar]
- 138.Aslani S, Harb MR, Costa PS, Almeida OFX, Sousa N, Palha JA. Day and night: diurnal phase influences the response to chronic mild stress. Front Behav Neurosci. 2014;8:82. doi: 10.3389/fnbeh.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li W, Zhu Y, Saud SM, Guo Q, Xi S, Jia B, et al. Electroacupuncture relieves depression-like symptoms in rats exposed to chronic unpredictable mild stress by activating ERK signaling pathway. Neurosci Lett. 2017;642:43–50. doi: 10.1016/j.neulet.2017.01.060. [DOI] [PubMed] [Google Scholar]
- 140.Strekalova T, Evans M, Chernopiatko A, Couch Y, Costa-Nunes J, Cespuglio R, et al. Deuterium content of water increases depression susceptibility: the potential role of a serotonin-related mechanism. Behav Brain Res. 2015;277:237–244. doi: 10.1016/j.bbr.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 141.Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu X, McClung CA. Chronic Stress Induces Brain Region-Specific Alterations of Molecular Rhythms that Correlate with Depression-like Behavior in Mice. Biol Psychiatry. 2015;78:249–258. doi: 10.1016/j.biopsych.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pesarico AP, Sartori G, Brüning CA, Mantovani AC, Duarte T, Zeni G, Nogueira CW. A novel isoquinoline compound abolishes chronic unpredictable mild stress-induced depressive-like behavior in mice. Behav Brain Res. 2016;307:73–83. doi: 10.1016/j.bbr.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 143.Ayuob NN, Ali SS, Suliaman M, Wahab MGA, Ahmed SM. The antidepressant effect of musk in an animal model of depression: a histopathological study. Cell Tissue Res. 2016:1–14. doi: 10.1007/s00441-016-2468-9. [DOI] [PubMed] [Google Scholar]
- 144.Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learning & Memory. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP. Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience. 2009;162:5–13. doi: 10.1016/j.neuroscience.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Backström T, Winberg S. Central corticotropin releasing factor and social stress. Front Neurosci. 2013;7:117. doi: 10.3389/fnins.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bagot RC, Parise EM, Peña CJ, Zhang H-X, Maze I, Chaudhury D, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tse YC, Montoya I, Wong AS, Mathieu A, Lissemore J, Lagace DC, Wong TP. A longitudinal study of stress-induced hippocampal volume changes in mice that are susceptible or resilient to chronic social defeat. Hippocampus. 2014;24:1120–1128. doi: 10.1002/hipo.22296. [DOI] [PubMed] [Google Scholar]
- 149.Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry. 2014;76:542–549. doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Warren BL, Vialou VF, Iñiguez SD, Alcantara LF, Wright KN, Feng J, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


