Abstract
Oxidative stress (OS) has been implicated in the causation of environmentally-induced diseases. However, the role of toxicants in the pathophysiology of disorders and diseases affecting the reproductive system are less understood. This review focuses on some of the mechanisms that underlie OS-induced reproductive toxicity at the cellular- and organ levels (germ cell damage and perturbed organ responses to endocrine stimuli). While most of the reproductive and developmental studies conducted in adult animals and transgenerational adult animals point to the involvement of genotoxicity, the part played by epigenetic alterations is accorded a recent recognition, thus warranting more studies in this area. Additionally, metabolomic, proteomic and transcriptomic approaches need to be employed to advance our understanding of key metabolites formed and the expression of anti-OS genes at the molecular level that are necessary for combating reactive oxygen species formation. The resulting data could be analyzed using bioinformatics tools to identify the pathways linked to disease causation and as a consequence, the adoption of therapeutic strategies, including but not limited to administering phytochemicals (many of which possess antioxidant properties) to improve disease outcomes.
Keywords: Free radicals, toxic chemicals, benzo(a)pyrene, hydroxyurea, endocrine disruption, epigenetics
1. Introduction
Procreation is a naturally endowed right of every couple. Regretfully, infertility among women of reproductive age in the USA was estimated as 4.5- 4.9 million annually between 1982 and 1988. By 1995 this estimate increased to 6.2 million and is predicted to increase to about 7.7 million by 2025 [1–2]. Diagnosis of infertility in women is complicated by the fact that only about 400 of the -2 million oocytes in primordial follicles at birth are ovulated during their reproductive life [3]. Incidentally, infertility does not affect females only. Roughly 50% of infertility cases in USA is of male partner origin when deviations from World Health Organization (W.H.O.) standards for normal semen are present in at least one of two semen analyses (SA; [4]). This problem is further compounded when no identifiable reason can be found for abnormal SA hence the diagnosis of idiopathic infertility. As a consequence, affected couples seek medical advice and interventions for improving their chances for successfully effecting fertilization and pregnancy. Currently, oxidative stress (OS) is believed to be an important and plausible cause for idiopathic male and female infertility [5–6].
OS is a condition that reflects an imbalance between the systemic generation of reactive oxygen species (ROS) and the ability of the body to readily detoxify (antioxidant defenses) the reactive intermediates or to repair the resulting damage. OS has become an area of great concern for clinicians and scientists due to the fact that this pathway of programmed health deterioration has also resulted in poor fertilization, poor embryonic development, pregnancy loss, birth defects (including autism), and childhood cancer (for review see Agarawal [5].
In this review, we will provide a comprehensive overview of the latest evidence regarding the mechanism of ROS production, the physiological roles of ROS, the pathophysiology of ROS, as well as the impact of OS on reproductive function. Furthermore, we will elaborate on different treatment strategies for reducing OS levels in the vicinity of gametes and embryos in infertile patients, with the ultimate intention of increasing the probability for normal natural fertilization and conception.
2. Production of free radicals in the body
Free radicals and other ROS are derived either from normal essential metabolic processes in the body or from external sources such as exposures to X-rays, ozone, cigarette smoking, air pollutants, and industrial chemicals. Free radical formation occurs continuously in the cells as a consequence of both enzymatic and non-enzymatic reactions. Enzymatic reactions, which serve as sources of free radicals, include those involved in the respiratory chain, phagocytosis, prostaglandin synthesis and the cytochrome P-450 system (for review, see Lobo et al. [7]). Free radicals can also be formed in non-enzymatic reactions of oxygen with organic compounds as well as those initiated by ionizing reactions.
Some internally generated sources of free radicals are [7]:
Mitochondria
Xanthine oxidase
Peroxisomes
Inflammation
Phagocytosis
Arachidonate pathways
Exercise
Ischemia/reperfusion injury
Some externally generated sources of free radicals are:
Cigarette smoke
Environmental pollutants
Radiation
Certain drugs, pesticides
Industrial solvents
Ozone
3. Need for Antioxidants
Antioxidants are low molecular weight molecules that are stable enough to each donate an electron to a rampaging free radical and neutralize it, thus reducing its capacity to cause damage. These antioxidants delay or inhibit cellular damage mainly through their free radical scavenging property [8]. Some of these antioxidants, including glutathione, ubiquinol, and uric acid, are produced during normal metabolism in the body [9]. Other lighter antioxidants are found in the diet. Although there are several enzymes system within the body that scavenge free radicals, the principal micronutrient (vitamins) antioxidants are vitamin E (α-tocopherol), vitamin C (ascorbic acid), and B-carotene [10]. The body cannot manufacture these micronutrients, so they must be supplied in the diet.
4. Sources of Antioxidants
Various antioxidants are supplied to the human body through vegetarian as well as non-vegetarian diets. Vitamins C and E, β-carotene and coenzyme Q are the most famous antioxidants of diet sources (for review see Akbarirad et al. [11]. Plants (fruits, vegetables, medicinal herbs) may contain a wide variety of free radical scavenging molecules such as phenolic compounds (Phenolic acids, flavonoids, quinones, coumarins, lignans, stilbenes, tannins etc.), nitrogen compounds (alkaloids, amines, betalains etc.), vitamins, terpenoids (including carotenoids) and some other endogenous metabolites which are rich in antioxidant activity [11].
5. Mechanism of action of antioxidants
Two principle mechanisms of action have been proposed for antioxidants [12]. The first is a chain-breaking mechanism by which the primary antioxidant donates an electron to the free radical present in the systems. The second mechanism involves removal of ROS/reactive nitrogen species initiators (secondary antioxidants) by quenching chain-initiating catalyst. Antioxidants may exert their effect on biological systems by different mechanisms including electron donation, metal ion chelation, co-antioxidants, or by gene expression regulation [13].
6. Potential Agent of Oxidative Stress to Reproductive Function
Any oxidizing radical is a potential agent of oxidative stress. Some are highly reactive with short half-lives, such as hydroxyl radicals, whereas others are less reactive but with longer half-lives, such as hydrogen peroxide (though ROS but not a free radical). A consequence of a longer half-life is the potential for a greater diffusion distance, which can allow the reactive species to do damage more remotely from its source. Oxidative damage can occur in many classes of molecules, including lipids, proteins, nucleic acids, and sugars, implying that cell, nuclear, and mitochondrial membranes, structural and cytoplasmic proteins, complex carbohydrates, RNA, and DNA are all susceptible to OS [14]. In gonads (testis and ovaries) with their high rates of metabolism and cell replication, OS can be very damaging, thus making the antioxidant capacity of gonadal tissues very important.
Excessive nitrosylated oxygen radicals (NO), play a role in amplifying testicular/ovarian injury. NO can form another potent oxidizing agent (peroxynitrite; ONOO2) by interacting with superoxide radicals [14–15]. Furthermore, NO can also react with CO2 to form nitrogen dioxide, a radical of less activity than peroxynitrite but of longer diffusion distance [14]. Peroxynitrite can modify proteins with thiol groups to generate nitrosothiols, which can trigger the generation of other metal-derived free radicals by disrupting metal-protein interactions [14–15].
NO is synthesized by nitric oxide synthase (NOS), which exists in the following 3 known forms: endothelial NOS (eNOS), inducible NOS (iNOS) and neuronal NOS. The latter appears to exist only in a truncated form in the gonads. NOS and/or NO have been found to be up-regulated in a number of experimental conditions known to induce gonadal OS, such as cryptorchidism, testicular torsion, obstructive azoospermia, and varicocele and during ischemia-reperfusion injury sustained during the treatment for ovarian torsion (for review see Turner and Lysiak [16]).
6.1. Conditions That Induce Testicular Oxidative Stress
Testicular OS is implicated in a number of conditions that are detrimental to male fertility. This review cannot cover all toxicants, however, specific examples will be given. Exposure of male rats to hexachlorocyclohexane, an organochlorine chemical used both as agricultural insecticide and pharmaceutical treatment for lice and scabies, exhibit a significant testicular OS and accompanying increases in germ cells apoptosis [17]. Other industrial pollutants such as 1,3-dinitrobenzene or nonylphenol have similar effects as hexachlorocyclohexane while methoxyethanol, (glycol ether used in paints and brake fluids) cause increased OS-induced testicular atrophy [16]. Other industrial/environmental toxicants such as benzo(a)pyrene, a prototypical polycyclic aromatic hydrocarbon (PAH) produced from burning coal and other fossil fuels also have a pro-oxidant effect in the testis [18]. The testis has two functional compartments: 1) the Leydig cell or steroidogenic compartment for the synthesis and release of the male hormone (testosterone) under luteinizing hormone (LH) stimulation; 2) the seminiferous tubule or spermatogenic compartment that produces spermatozoa under testosterone stimulation post follicle stimulating hormone (FSH) initiation of spermatogenic process. Toxicant-induced testicular failure is usually accompanied by shrinkage in the two testicular compartments albeit more so in the spermatogenic compartment as exemplified by the reduction in the testicular morphometric indices of BaP-exposed versus unexposed adult rats (Table 1; [18]). The shrinkage in the steroidogenic compartment was accompanied by a reduction in testosterone secretion (Figure 1) and ultimately a reduction in testosterone-regulated daily sperm production (Figure 2 [19]). Another endocrine disrupting AhR agonist that pollutes the environment is tetrachloro dibenzo-p-dioxin (TCDD). Exposure of C57BL/6 mice to TCDD (0.1–50 μg TCDD/kg) for 24 hr resulted in decreases in sperm mitochondrial membrane potential compared with control mice. The aforementioned effect was not evident in AhR-knockout mice, thus, demonstrating the need for AhR activation [20]. A toxicant such as 2,5-hexanedione that induces germ cell apoptosis may produce its toxic effect via the induction of OS even though other avenues to germ cell apoptosis exist. Furthermore, exposures to elevated levels of certain metals can induce OS. Case in point, high iron doses increase oxidative damage and deplete antioxidants in the testes of rats. Similarly, cadmium enhances OS in the testis and exposures to high levels of lead decrease spermatogenesis, increase and decrease ROS generation and sperm motility, respectively in stored sperm of exposed rats [16]. It is important to mention that excessive lifestyle indulgence such as alcohol consumption and cigarette smoking, contribute to male infertility via the generation of free-radical-induced OS [review in 16]. Furthermore, therapies such as the use of cancer chemotherapy agents are toxic to male gonads. The gonadotoxic effect(s) of chancer chemotherapeutic agents may result from factors ranging from endocrinopathy [20] to generalized cell-stress responses mediated by heat shock proteins. However, it is widely recognized that many chemotherapy agents like doxyrubicin, cyclophosphamide, cisplatin and hydroxyurea induce oxidative stress in a variety of tissues and cell types including spermatogenic and steroidogenic tissue [16 & 21]. Case in point, exposure of men with sickle cell disease and adult male mice to hydroxyurea disrupts spermatogenesis and testicular steroidogenesis via the induction of OS [21]. The adverse effect of hydroxyurea on the testis of exposed mouse is illustrated in Figure 3.
Table 1.
Morphometric analysis of testicular parameters in rats inhalationally exposed to 75μg/m3 benzo(a)pyrene (BaP).
| Parameter | UNC | BaP-exposed |
|---|---|---|
| Testis weight (gm/paired testis) | 3.0 ± 0.16 | 2.0 ± 0.11*1 |
| Tubule diameter (μm) | 250 ± 8.5 | 230 ± 8.2*2 |
| Tubules with elongated spermatids (%) | 99 ± 1.0 | 99 ± 1.0 |
| Tubular volume (X 109 μm3) | 2.0 ± 0.7 | 1.6 ± 0.004*3 |
| Total weight of tubules (gm) | 2.20 ± 0.7 | 1.6 ± 0.004 |
| Total tubular length (μm) | 55 ± 1.0 | 33 ± 1.0*5 |
| Total volume of interstitium per paired testis (μm3) | 0.43 ± 0.01 | 0.38 ± 0.01 |
| Total weight of interstitium per paired testis (gm) | 0.45 ± 0.01 | 0.37 ± 0.01*4 |
Figure 1.
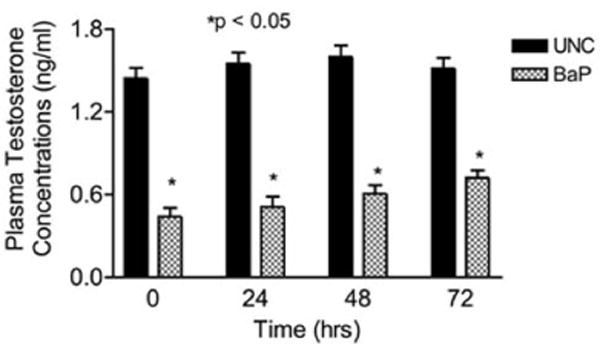
Effect of inhaled benzo(a)pyrene on plasma testosterone concentrations in F-344 male rats exposed to sub-chronic exposure concentrations of 75 μg BaP/m3; n = 10 per treatment or control group. Results are expressed as mean ± SE. UNC-unexposed control; CB-carbon black control; BaP-exposed rats. Asterisks indicate a significant difference from controls (P < 0.05). Data from [18].
Figure 2.
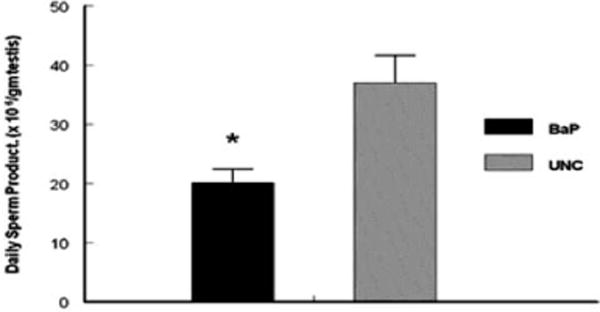
Effect of inhaled BaP on daily sperm production per gram of testis in F-344 male rats exposed to 75 μg BaP/m3 for 60 days; n = 10 per treatment or control group. Results are expressed as mean ± SE (UNC = unexposed control; BaP = BaP-inhaled rats. Asterisks indicate a significant difference from controls (P < 0.05). Data from [19].
Figure 3.
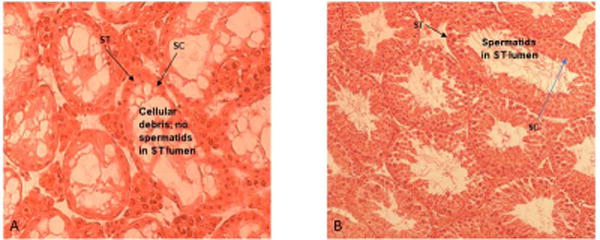
Photomicrographs of testis histologies from: A) adult male mice treated with 25 mg HU/kg; B) control adult male mice. Mice treated with HU have degenerative/atrophic seminiferous tubules (ST) with only Sertoli cells (SC) and cellular debris remaining in the tubules compared with controls. Image adapted from [21].
6.2. Conditions That Induce Ovarian Oxidative Stress
Among 75% of working reproductive age women in the USA, about 17% are exposed to environmental toxic chemicals that may result in infertility and early menopause via the destruction of follicles and oocytes. [3, 22–24]. The female reproductive system is regulated by pituitary gonadotropins and ovarian steroids and any environmental chemical insult on the pituitary/gonadal axis during the reproductive age of females may disrupt folliculogenesis, which can ultimately result in infertility. Because of the finite number of oocytes contained in the ovary, the destruction of follicles by toxic chemicals contributes to early menopause. Severe OS has been linked to rapid and effective impairment of ovarian function [25]. Exposure to pesticide residues is associated with menstrual cycle disturbances, reduced fertility, prolonged time-to-pregnancy, spontaneous abortion, stillbirths, and developmental defects. Because pesticides comprise a large number of distinct substances with dissimilar structures and diverse toxicity, it is most likely that their endocrine disrupting action and/or OS inducing properties are involved in pathophysiological pathways that trigger adverse reproductive outcomes [for review see 26]. AhR activating environmental/industrial pollutants such as PAHs, dioxins and polychlorinated biphenyls (PCBs) perturb the reproductive process via OS and endocrine disruption. Women who are exposed to the prototypical PAH (BaP) are more likely at a higher risk for infertility as exemplified by lower success rates in BaP-exposed women when using assisted reproductive technologies to attempt pregnancies [27]. In animal studies, exposure of adult F-344 adult female rats to inhaled BaP significantly reduced plasma concentrations of estradiol-17β (E2), and luteinizing hormone (LH) on proestrus, progesterone (P4) on diestrus I, ovulation rate and when mated, sustained a significant reduction in fetal survival compared to their unexposed counterparts [28]. The presence of dioxin and dioxin-like compounds in abdominal fat have for many years been believed to be associated with endometriosis-linked infertility albeit the literature is conflicting [29]. A small study that analyzed adipose tissue at the time of laparoscopic surgery demonstrated an association between adipose concentrations of dioxin and PCBs and the presence of endometriosis [30]. In utero and lactational (IUL) exposures of female pups’ hypothalamic-pituitary axis plus adult exposure of the whole body to TCDD significantly delayed puberty in female rats. Data analysis also revealed an accelerated onset of acyclicity by 5 months in all groups involving IUL exposure of the developing ovary to TCDD. Furthermore, E2 was significantly decreased in animals receiving TCDD during IUL exposure of the hypothalamic-pituitary axis; revealing a particular sensitivity of the developing ovary to TCDD leading to early loss of reproductive function with age [31]. It is generally accepted that some metals are essential for living organisms in small quantities, but toxic in higher concentrations or in other speciation forms, e.g. copper (Cu), chromium (Cr), manganese (Mn) and zinc (Zn). However, other metals that are widely distributed in the atmosphere, soil, and groundwater are not considered to have any specific metabolic role and are generally classified as obligate toxic metals, e.g. cadmium (Cd), mercury (Hg) and lead (Pb). The potential health disorders caused by chronic or acute exposures to the latter metals include the induction of estrogen-dependent diseases such as breast cancer, endometrial cancer, endometriosis and spontaneous abortions, as well as pre-term deliveries and stillbirths via OS induction (for review see Rzymski et al. [32]. Many female cancer survivors may have difficulty conceiving after treatment, but the infertility risks for different age groups and cancer treatments are variable. The risk of immediate and long-term amenorrhea after cancer treatment is impacted by both the specific chemotherapeutic agent/regimen used and the age at which the women are exposed. In addition, women may experience transient chemotherapy-induced amenorrhea, with menstruation resuming after cessation of treatment. The type of chemotherapy, such as the inclusion or absence of alkylating agents, has a significant effect on the resumption of menstrual cycle regularity [33].
7. Role of Epigenetic Mechanisms in Reproductive Toxicity
Exposures to environmental toxicants in the prenatal, postnatal periods and adult life will have long lasting consequences on sexual behavior and the reproductive physiology of animals [34]. While it is known that some of these changes are caused by altered expression of genes as a result of toxicant interaction with DNA sequence that could be transmitted through subsequent generations, the developmental reprogramming caused by toxicants is far from being understood (reviewed in [34]). Because heritable changes could occur through altered gene expression without any change in the DNA sequence, the altered traits are collectively termed as epigenetics [35]. Oxidative stress-induced epigenetic changes in germ cells carry the risk of transmitting epigenetic disorders to the offspring [36]. Several toxicants including heavy metals, trichloroethylene, particulate pollutants, bisphenol, dioxins, PCBs, and PAHs have been shown to induce epigenetic alterations [37]. The effect of exposure toxicants and the free radicals on the epigenome during cellular and tissue differentiation is one mechanism that deserves consideration [38].
As regards the male reproductive system, epigenetic modifications affect gametogenesis and infertility. Prominent among these modifications are DNA methylation, histone modifications and small non-coding RNAs; the former two impacting the proper maturation of gametes. Any changes in the process of spermatogenesis will lead to genome reprogramming during embryo development. The various epigenetic changes that occur during spermatogenesis depicted in Fig. 4 [39], could be targeted by toxicants with subsequent adverse impact on male fertility.
Figure 4.
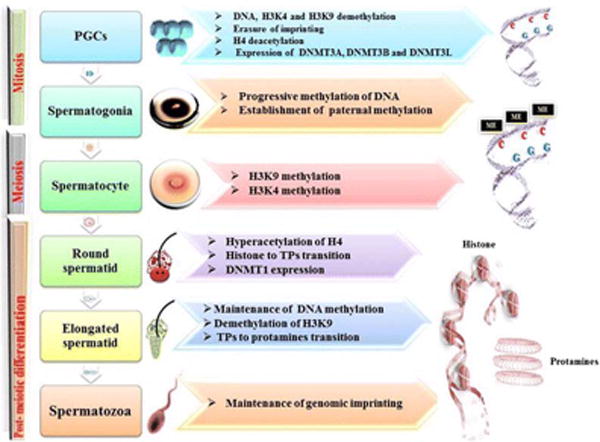
Epigenetic modifications during spermatogenesis (39). During the different steps of spermatogenesis, several epigenetic modifications involving DNA methylations and histone modifications occur. (1) Primordial germ cells (PGCs) undergo a process of demethylation involving DNA (with erasure of genomic imprinting) and histones (namely, K4 and K9 residues of histone H3, the testis-specific H3 variant in mammals). Also, a process of H4 deacetylation is present. DNA methyltransferases (DNMT3A, DNMT3B, and DNMT3L) are expressed at this time. (2) In spermatogonia, a progressive DNA methylation occurs, with establishment of paternal methylation. (3) In spermatocytes, histone H3 lysine 9;(H3K9) and histone H3 lysine 4 (H3K4) methylation are observed. (4) In round spermatids, H4 becomes hyperacetylated, DNMT1 is expressed, and the transition from histones to transition proteins (TPs) occurs. (5) Elongated spermatids show a maintenance of DNA methylation, together with H3K9 demethylation. The transition from TPs to protamines occurs at this step. (6) In spermatozoa, the genomic imprinting is maintained. Image taken from [39]. Use of this image is under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the authors give appropriate credit to the original author(s) and the source (BioMed Central-the Open Access Publisher).
The above-mentioned epigenetic changes also occur in females. Hypermethylation in ovaries [40], histone deacetylation in myometrium [41] are caused by endocrine disruptors. Other disorders associated with epigenetic changes are irregular ovulation, polycystic ovary syndrome and endometriosis [42].
8. Conclusions and Future Directions
The aforementioned account details disorders and diseases associated with reproductive system. Clearly, there are several areas that are amenable to extended research one of which is dietary manipulation. As paternal diet may have a bearing on gametogenesis, modified nutritional regimens (incorporation of some phytochemicals that possess antioxidant properties) could counter the effect of OS-induced epigenetic modifications of the germ line. From a therapeutic standpoint, focusing on anti-oxidative stress (AOS) proteins, which are downstream targets of transcription factors will aid in the identification of promising drug candidates. Additionally, screening of blood for key metabolites (after normalizing the data for covariants) will be instrumental in drawing a correlation among OS-induced weight gain, obesity and infertility. Future studies must leverage the strides made in bioinformatics to treat OS-induced idiopathic infertility.
Acknowledgments
This work was supported by the National Institutes of Health grants 5R25GM059994-11, G12MD007586-29, 5U54MD007593-09, and U54HD044315.
Footnotes
Conflict of interest
The authors declare no conflicts of interest
References
**Annotated for their contribution to the subject area
- 1.Abma JC, Chandra A, Mosher WD, Peterson LJ, Piccinino LJ. Fertility, family planning, and women’s health: estimates from the 1995 National Survey of Family Growth. Vital Health Stat. 1997;23:19. [PubMed] [Google Scholar]
- 2.Stephen EH, Chandra A. Updated projections of infertility in the United States: 1995-2025. Fertil Steril. 1998;70:30–34. doi: 10.1016/s0015-0282(98)00103-4. [DOI] [PubMed] [Google Scholar]
- 3.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 4.Jarow JP, Zirkin B. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann NY Sci. 2005;106:208–220. doi: 10.1196/annals.1336.023. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Virk G, Ong C, Plessis SS. Effect of Oxidative Stress on Male Reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalto DB, Roy M, Audet I, Palin M, Guay F, Lapointe J, Matte JJ. Interaction between vitamin B6 and source of selenium on the response of the selenium-dependent glutathione peroxidase system to oxidativestress induced by oestrus in pubertal pig. J Trace Elem Med Biol. 2015;32:21–29. doi: 10.1016/j.jtemb.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halliwell B. How to characterize an antioxidant- An update. Biochem Soc Symp. 1995;61:73–101. doi: 10.1042/bss0610073. [DOI] [PubMed] [Google Scholar]
- 9.Shi HL, Noguchi N, Niki N. Comparative study on dynamics of antioxidative action of α- tocopheryl hydroquinone, ubiquinol and α- tocopherol, against lipid peroxidation. Free Radic Biol Med. 1999;27:334–346. doi: 10.1016/s0891-5849(99)00053-2. [DOI] [PubMed] [Google Scholar]
- 10.Levine M, Ramsey SC, Daruwara R. Criteria and recommendation for Vitamin C intake. JAMA. 1991;281:1415–1423. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 11.Akbarirad H, Gohari AA, Kazemeini SM, Mousavi KA. An overview on some of important sources of natural antioxidants. Int Food Res J. 2016;23:928–933. [Google Scholar]
- 12.Rice-Evans CA, Diplock AT. Current status of antioxidant therapy. Free Radic Biol Med. 1993;15:77–96. doi: 10.1016/0891-5849(93)90127-g. [DOI] [PubMed] [Google Scholar]
- 13.Krinsky NI. Mechanism of action of biological antioxidants. Proc Soc Exp Biol Med. 1992;200:248–254. doi: 10.3181/00379727-200-43429. [DOI] [PubMed] [Google Scholar]
- 14.Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJA. Free radical biology and medicine: it’s a gas, man! Am J Physiol Regul Integr Comp Physiol. 2006;291:491–511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 15.Szabo C, Ischiropoulus H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev. 2007;6:662–679. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 16**.Turner TT, Lysiak JJ. Oxidative Stress: A Common Factor in Testicular Dysfunction. J Androl. 2008;29:488–498. doi: 10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- 17.Samanta L, Chainy GB. Comparison of hexachlorocyclohexaneinduced oxidative stress in the testis of immature and adult rats. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1997;118:319–327. doi: 10.1016/s0742-8413(97)00132-1. [DOI] [PubMed] [Google Scholar]
- 18.Ramesh A, Inyang F, Lunstra DD, Niaz MS, Kopsombut P, Jones KM, Hood DB, Hills ER, Archibong AE. Alteration of fertility endpoints in adult male F-344 rats by subchronic exposure to inhaled benzo(a)pyrene. Exp Toxic Pathol. 2008;60:269–280. doi: 10.1016/j.etp.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archibong AE, Ramesh A, Niaz MS, Brooks M, Roberson SI, Lunstra DD. Effects of benzo(a)pyrene on intra-testicular function in F-344 rats. Int J Environ Res Public Health. 2008;5:32–40. doi: 10.3390/ijerph5010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher MT, Nagarkatti M, Nagarkatti PS. Aryl hydrocarbon receptor-dependent induction of loss of mitochondrial membrane potential in epididydimal spermatozoa by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Lett. 2005;157:99–107. doi: 10.1016/j.toxlet.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 21**.Jones KM, Niaz MS, Brooks CM, Roberson SI, Aguinaga MP, Hills ER, Rice VM, Bourne P, Bruce D, Archibong AE. Adverse effects of a clinically relevant dose of hydroxyurea used for the treatment of sickle cell disease on male fertility endpoints. Int J Environ Res Public Health. 2009;6:1124–1144. doi: 10.3390/ijerph6031124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makuc D, Lalich N. Employment characteristics of mothers during pregnancy: Health United States and Prevention Profile 1983. National Center for Health Statistics. 1983;17:25–32. [Google Scholar]
- 23.Sharara FI, Seifer DB, Flaws JA. Environmental toxicants and female reproduction. Fertil Steril. 1998;70:613–622. doi: 10.1016/s0015-0282(98)00253-2. [DOI] [PubMed] [Google Scholar]
- 24.Mark-Kappeler CJ, Hoyer PB, Devine PJ. Xenobiotic effects on ovarian preantral follicles. Biol Reprod. 2011;85:871–883. doi: 10.1095/biolreprod.111.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu M, Che L, Yang Z, Zhang P, Shi J, Li J, Lin Y, Fang Z, Che L, Feng B, Wu D, Xu S. Effect of high fat dietary intake during maternal gestation on offspring ovarian health in a pig model. Nutrients. 2016;8:498. doi: 10.3390/nu8080498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bretveld RW, Thomas CMG, Scheepers PTJ, Gerhard A, Zielhuis GA, Roeleveld N. Pesticide exposure: the hormonal function of the female reproductive system disrupted? Reprod Biol Endocrinol. 2006;4:30. doi: 10.1186/1477-7827-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neal MS, Hughes EG, Holloway AC, Foster WG. Sidestream smoking is equally as damaging as mainstream smoking on IVF outcomes. Hum Reprod. 2005;20:2531–2535. doi: 10.1093/humrep/dei080. [DOI] [PubMed] [Google Scholar]
- 28.Archibong AE, Ramesh A, Inyang F, Niaz MS, Hood DB, Kopsombut P. Endocrine disruptive actions of inhaled benzo(a)pyrene on ovarian function and fetal survival in fisher F-344 adult rats. Reprod Toxicol. 2012;34:635–643. doi: 10.1016/j.reprotox.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soave I, Caserta D, Wenger JM, Dessole S, Perino A, Marci R. Environment and Endometriosis: a toxic relationship. Eur Rev Med Pharmacol Sci. 2015;19:1964–1972. [PubMed] [Google Scholar]
- 30.Martinez-Zamora MA, Mattioli L, Parera J, Abad E, Coloma JL, Van Babel B, Galceran MT, Balasch J, Carmona F. Increased levels of dioxin-like substances in adipose tissue in patients with deep infiltrating endometriosis. Hum Reprod. 2015;30:1059–1068. doi: 10.1093/humrep/dev026. [DOI] [PubMed] [Google Scholar]
- 31.Jablonska O, Zhanquan S, Valdez KE, Alison Y, Ting AY, Petroff BK. Temporal and anatomical sensitivities to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin leading to premature acyclicity with age in rats. Int J Androl. 2010;33:405–412. doi: 10.1111/j.1365-2605.2009.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rzymski P, Tomczyk K, Rzymski P, Poniedziałek B, Opala T, Wilczak M. Impact of heavy metals on the female reproductive system. Ann Agric Environ Med. 2015;22:259–264. doi: 10.5604/12321966.1152077. [DOI] [PubMed] [Google Scholar]
- 33.Waimey KE, Smith BM, Confino R, Jeruss JS, Pavone ME. Understanding Fertility in Young Female Cancer Patients. J Womens Health (Larchmt) 2015;24:812–818. doi: 10.1089/jwh.2015.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aluru N. Epigenetic effects of environmental chemicals: Insights from zebrafish. Current Opinion in Toxicology. 2017;6:26–33. doi: 10.1016/j.cotox.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science. 1999;286:481–486. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 36.Menezo YJ, Silvestris E, Dale B, Elder K. Oxidative stress and alterations in DNA methylation: two sides of the same coin in reproduction. Reprod Biomed Online. 2016;33:668–683. doi: 10.1016/j.rbmo.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 37**.Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21:243–51. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skinner MK. Endocrine disruptors in 2015: Epigenetic transgenerational inheritance. Nat Rev Endocrinol. 2016;12:68–70. doi: 10.1038/nrendo.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Stuppia L, Franzago M, Ballerini P, Gatta V, Antonucci I. Epigenetics and male reproduction: the consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin Epigenetics. 2015;7:120. doi: 10.1186/s13148-015-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Uzumcu M, Zama AM, Oruc E. Epigenetic mechanisms in the actions of endocrine-disrupting chemicals: gonadal effects and role in female reproduction. Reprod Domest Anim. 2012;47(Suppl 4):338–47. doi: 10.1111/j.1439-0531.2012.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laknaur A, Foster TL, Bobb LE, Ramesh A, Ladson GM, Hood DB, Al-Hendy A, Thota C. Altered expression of histone deacetylases, inflammatory cytokines and contractile-associated factors in uterine myometrium of Long Evans rats gestationally exposed to benzo[a]pyrene. J Appl Toxicol. 2016;36:827–35. doi: 10.1002/jat.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bunkar N, Pathak N, Lohiya NK, Mishra PK. Epigenetics: A key paradigm in reproductive health. Clin Exp Reprod Med. 2016;43:59–81. doi: 10.5653/cerm.2016.43.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]


