Supplemental Digital Content is Available in the Text.
Ranibizumab was effective in treating choroidal neovascularization of various etiologies, with a treatment effect of +9.9 letters versus sham at Month 2 and a mean gain of 11.0 letters from baseline to Month 12. The beneficial effects of ranibizumab were observed across all etiology subgroups.
Key words: anti–vascular endothelial growth factor, choroidal neovascularization, choroidal neovascularization etiologies, angioid streak, uveitis, central serous chorioretinopathy, idiopathic, phase III, ranibizumab, randomized
Abstract
Purpose:
To evaluate the efficacy and safety of ranibizumab 0.5 mg in adult patients with choroidal neovascularization because of an uncommon cause enrolled in the 12-month MINERVA study.
Methods:
In this Phase III, double-masked study, adult (≥18 years) patients (N = 178) were randomized 2:1 to receive either ranibizumab (n = 119) or sham (n = 59) at baseline and, if needed, at Month 1 and open-label individualized ranibizumab from Month 2. Best-corrected visual acuity change from baseline to Month 2 (primary endpoint) and Month 12, treatment exposure, and safety over 12 months were reported. Subgroup analysis was conducted on five predefined choroidal neovascularization etiologies (angioid streak, postinflammatory, central serous chorioretinopathy, idiopathic, and miscellaneous).
Results:
Ranibizumab showed superior efficacy versus sham from baseline to Month 2 (adjusted least-squares mean best-corrected visual acuity: +9.5 vs. −0.4 letters; P < 0.001). At Month 12, the mean best-corrected visual acuity change was +11.0 letters (ranibizumab) and +9.3 letters (sham). Across the 5 subgroups, the treatment effect ranged from +5.0 to +14.6 letters. The mean number of ranibizumab injections was 5.8 (ranibizumab arm) with no new ocular or nonocular adverse events.
Conclusion:
Ranibizumab 0.5 mg resulted in clinically significant treatment effect versus sham at Month 2. Overall, ranibizumab was effective in treating choroidal neovascularization of various etiologies with no new safety findings.
Choroidal neovascularization (CNV) is characterized by the growth of abnormal blood vessels originating from the choriocapillaris through Bruch membrane into the subretinal pigment epithelium or subretinal space.1–5 Choroidal neovascularization in adults is most commonly associated with neovascular age-related macular degeneration (nAMD) and pathologic myopia.2 It can also be associated with other conditions such as uveitis, central serous chorioretinopathy (CSC), angioid streaks, trauma, tumor, and retina or macular dystrophies. Choroidal neovascularization can also be idiopathic, which is usually defined as “CNV with no apparent cause or no clinical evidence of a predisposing abnormality.”2,5–7 Choroidal neovascularization because of causes other than nAMD and myopic CNV is rare and usually occur in younger, working-age adults (<50 years).4
Vascular endothelial growth factor (VEGF) plays an important role in the pathogenesis of CNV and the use of anti-VEGF agents such as ranibizumab (Lucentis; Novartis Pharma AG, Basel, Switzerland, and Genentech Inc, South San Francisco, CA), specifically designed for ocular use, has emerged as an effective approved therapy in the treatment of various eye diseases, including CNV due to nAMD and CNV secondary to pathologic myopia (myopic CNV).8–18 Other anti-VEGF agents such as aflibercept (Eylea; Regeneron Pharmaceuticals, Inc, Tarrytown, NY) are also approved for these conditions, whereas only off-label use of bevacizumab (Avastin; Genentech, Inc) has been reported.19–28
Previous reports of case series and clinical studies in patients with CNV associated with diseases other than nAMD and myopic CNV treated with off-label bevacizumab and ranibizumab showed promising results in improving functional and anatomical parameters irrespective of the underlying etiology.2,29–44 There was an unmet need in the management of patients with CNV associated with diseases other than nAMD and myopic CNV because of the lack of an approved therapy or standard of care that can improve vision. If left untreated, patients with CNV may develop irreversible and rapid vision loss, which may further deteriorate with progression of the CNV lesion. The efficacy and safety profile of ranibizumab in treating CNV associated with nAMD and myopic CNV is well established, and it, therefore, was reasonable to expect that patients with CNV lesions due to different causes may benefit in a similar manner.8–10,12–15,17 In the European Union, ranibizumab has been recently approved in November 2016 for the treatment of visual impairment due to CNV in adult patients.45
Here, we report the 12-month results of adult patients from the MINERVA study (group members of this study are cited in Supplemental Digital Content 1, http://links.lww.com/IAE/A677). The study was specifically designed to evaluate the efficacy and safety of ranibizumab 0.5 mg versus sham, using an individualized pro re nata (PRN) regimen based on disease activity, in adult and adolescent patients with visual impairment due to CNV associated with any cause other than nAMD and myopic CNV.
Methods
Study Design
The MINERVA study was a 12-month, Phase III, prospective, double-masked, sham-controlled, multicenter, randomized clinical study with an open-label setting from Month 2. The study, initiated in September 2013 and completed in November 2015, was conducted across 20 countries worldwide (see Supplemental Digital Content 2, http://links.lww.com/IAE/A678). The study was conducted in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by an Independent Ethics Committee or Institutional Review Board at each contributing center. Patients provided written informed consent before entering the study. The study is registered with www.clinicaltrialsregister.eu (EudraCT no. 2012-005417-38).
Population
The study population consisted of patients aged ≥18 years, with a confirmed diagnosis of CNV lesion in the study eye with involvement of the central macular area (subfoveal, juxtafoveal with involvement of the central macular area, extrafoveal with involvement of the central macular area, or margin of the optic disk with involvement of the central macular area). The other key inclusion criteria included best-corrected visual acuity (BCVA) between ≥24 and ≤83 Early Treatment Diabetic Retinopathy Study (ETDRS) letters.
The key exclusion criteria were as follows: CNV associated with nAMD or myopic CNV; polypoidal choroidal vasculopathy, or retinal angiomatous proliferation lesions (in patients aged ≥50 years); any type of systemic advanced, severe, or unstable disease or its treatment that could interfere with primary and/or secondary outcome evaluations; uncontrolled systemic inflammation or infection, related directly to the underlying causal disease of CNV; active diabetic retinopathy, and active ocular or periocular infectious disease or active severe intraocular inflammation; history of laser photocoagulation with involvement of the macular area, verteporfin photodynamic therapy (vPDT), and vitreoretinal surgery and intravitreal implants at any time; and use of anti-VEGF agents and intravitreal steroids, within 6 months of the baseline visit. In addition, pregnant women were excluded (inclusion and exclusion criteria are listed in detail in Supplemental Digital Content 3, http://links.lww.com/IAE/A679).
Randomization and Treatment
At baseline, all eligible patients were randomized in a 2:1 ratio to receive either ranibizumab 0.5 mg or sham. The randomization list was generated using an interactive response technology that automated the random assignment of patients to either of the treatment arms in the specified ratio. The randomization was stratified by type of underlying categories of ocular pathophysiologic mechanisms (angioid streaks vs. others). Although this was an open-label study from Month 2, the examiner who assessed the BCVA outcomes was masked and was not allowed to perform any other study tasks that would have unmasked him or her to the patients' treatment received throughout the study.
In the ranibizumab group, patients received ranibizumab 0.5 mg intravitreal injections at baseline and PRN from Month 1 based on disease activity (visual acuity [VA] impairment, presence of intraretinal/subretinal fluid, hemorrhage, or leakage) as judged by the masked investigator at each visit (Figure 1). In the sham group, patients received sham injection at baseline and PRN from Month 1 based on disease activity as judged by the masked investigator at each visit. From Month 2, patients in both the groups were treated with open-label PRN ranibizumab based on disease activity.
Fig. 1.
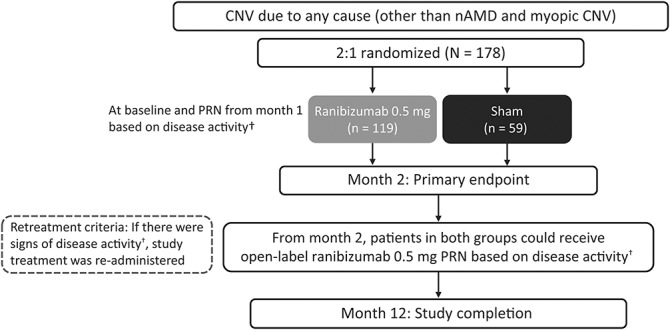
Study design (randomized set*). *Consisted of all patients who were randomized. †VA impairment, intraretinal/subretinal fluid presence, hemorrhage, or leakage.
Maintenance and Retreatment
All patients were monitored and evaluated by the masked investigator monthly for disease activity for consideration of retreatment. Retreatment can be performed based on any of the following: BCVA impairment of 1 or more ETDRS letter; presence of any intraretinal or subretinal fluid on optical coherence tomography; any new hemorrhage or persistent hemorrhage; or fluorescein angiography leakage, which can be performed at any visit if required by the investigator.
In both arms, the last possible treatment was administered at Month 11, and the last assessment was performed at Month 12. If both eyes were eligible for treatment at screening and baseline, the study eye was selected by the investigator based on the most appropriate active CNV lesion characteristics in addition to visual impairment. The fellow eye was allowed to receive ranibizumab treatment if it presented with or developed CNV due to the same underlying disease as in the study eye during the course of the study. Treatment of the fellow eye with anti-VEGF agents other than ranibizumab was not allowed in the study.
Rescue Medication
Use of rescue medication, either thermal laser photocoagulation or vPDT, as per the standard clinical practice was allowed at Month 1 in the study at the investigator's discretion and if the patient had a VA loss of >5 letters due to disease activity from baseline to Month 1.
Objectives
The primary objective of the study was to demonstrate the superior efficacy of individualized ranibizumab 0.5 mg treatment compared with sham in adult patients with CNV because of any cause other than nAMD and myopic CNV, assessed by change in BCVA from baseline to Month 2 (primary endpoint).
Preplanned subgroup analyses were conducted on the primary endpoint for the following predefined subgroups: 1) baseline BCVA (≤60 and >60 letters); 2) baseline CNV etiology (CNV-idiopathic chorioretinopathy [CNV-idiopathic], CNV-angioid streaks, CNV-postinflammatory retinochoroidopathy, CNV-CSC, and CNV-miscellaneous [etiologies that did not fit into the other CNV etiology subgroups and were insufficiently frequent to form a separate subgroup]); and 3) baseline age (≤60 and >60 years).
The secondary objectives were as follows: 1) to evaluate the efficacy of ranibizumab treatment compared with sham by assessing a) change in BCVA from baseline by visit to Month 2, b) changes in central subfield thickness (CSFT) and central subfield volume (CSFV) from baseline to Month 2, c) presence of intraretinal/subretinal fluid at Month 2, d) presence of active leakage at Month 2, and e) requirement of rescue treatment at Month 1; 2) to evaluate the efficacy of ranibizumab treatment by original treatment assignment by assessing a) change in BCVA from baseline to Month 12, b) mean average change in BCVA from baseline to Month 1 through Month 6 and to Month 1 through Month 12, c) changes in CSFT and CSFV from baseline to Month 12, d) presence of intraretinal/subretinal fluid at Month 12, e) presence of leakage at Month 12, and f) proportion of patients gaining ≥5, ≥10, and ≥15 letters or reaching 84 letters from baseline and losing >5, >10, and >15 letters at Months 2 and 12; 3) to assess the treatment exposure to ranibizumab before Month 12; and 4) to evaluate the safety of ranibizumab treatment up to Months 2 and 12.
Efficacy and Safety Assessments
Study assessments were performed at screening, baseline (Day 1), and at all monthly visits up to the last visit.
Best-corrected visual acuity
Best-corrected visual acuity was assessed by certified VA examiners at every study visit using ETDRS VA testing charts at an initial testing distance of 4 m, reduced to 1 m if necessary.
Optical coherence tomography
Optical coherence tomography was performed by certified site personnel at every study visit using either time domain or spectral domain optical coherence tomography (e.g., Cirrus, Spectralis, TopCon, Nidek, Optovue, and Opko). Because the same equipment was used for assessment throughout the study, the change in thickness was the same across different equipment; however, the baseline value differed. The investigator or designated study staff evaluated the images according to the standard clinical practice and recorded both the quantitative and qualitative parameters in the clinical database. The raw images were forwarded to a central reading center (CRC; Bern Photographic Reading Center, Bern, Switzerland) to ensure a standardized evaluation of the quantitative (e.g., CSFT and CSFV) and qualitative (e.g., macular edema, cysts, and intraretinal and subretinal fluid) anatomical parameters and their change over time as endpoints. The CSFT was defined as the average retinal thickness of the circular area with 1-mm diameter around the foveal center, and the CSFV (macular volume) was defined as the average volume of the 3-mm field centered around the fovea.
Fluorescein angiography and color fundus photography
Fluorescein angiography was performed in conjunction with 7-field color fundus photography on study eyes at screening, Month 2, Month 6, and the last study visit. These fluorescein angiography and color fundus photography images were evaluated by the investigator for the presence or absence of active leakage and were forwarded to the CRC for standardized evaluation.
Treatment exposure
Data were collected on the number of ranibizumab treatments and retreatments before Month 12.
Safety assessments
Safety assessments included type, frequency, and severity of adverse events (AEs) and serious AEs (SAEs) up to Months 2 and 12.
Statistical Analysis
Assuming a SD of 15 ETDRS letters for the change in BCVA at Month 2 compared with baseline, based on a randomization ratio of 2:1, a sample size of 112 and 56 patients in the ranibizumab and sham arms, respectively, was considered. With this sample size, the resulting power for analysis of covariance was 90.5% to detect a mean treatment difference of 8 ETDRS letters at a one-sided α level of 0.025. Conservative sample size calculations were performed using the two-sample t test. The primary and secondary efficacy outcomes were analyzed in a full analysis set using observed data and the randomized treatment. The full analysis set included all randomized patients to whom treatment regimen was assigned. Hypothesis tests were evaluated at a one-sided significance level of 2.5%, and 2-sided asymptotic 95% confidence intervals (CIs) were reported. The primary efficacy outcome, defined as the change in BCVA from baseline to Month 2, was analyzed using a Mixed-Effect Repeated Measure Model. Frequencies and percentages, with corresponding Clopper–Pearson exact 2-sided 95% CIs, were provided for selected binary efficacy variables. The least-squares (LS) mean and 95% CI were used to support the conclusions of statistical inferences. Descriptive statistics included number of observations (n), mean, SD, SE (as required), median and ranges for continuous variables, and frequencies and percentages for categorical values; and where appropriate, estimates of treatment group differences, CIs, and P values were presented. All safety analyses were descriptive and were performed using the safety set that included all patients who received ≥1 administration of the study treatment and had ≥1 postbaseline safety assessment. Statistical analyses were performed using SAS (version 9.4).
Results
Patient Disposition
Overall, 178 patients were randomized to receive either ranibizumab (n = 119) or sham (n = 59), of whom 167 (93.8%) completed the 12-month study (ranibizumab group, n = 112; sham group, n = 55). The most common reasons for study discontinuation were consent withdrawal (n = 3), physician's decision (n = 3), and AEs (n = 2) (Figure 2). All patients in the full analysis set received the randomized treatment in the study eye. The safety set included 119 patients receiving ranibizumab and 59 patients receiving sham from baseline to Month 2. Of the latter, 52 patients received at least one ranibizumab injection in the study eye in the open-label period. For the safety analyses, these patients were designated as the “sham with ranibizumab” group. The seven patients who did not receive any ranibizumab injection in the study eye during the study were designated as the “sham without ranibizumab” group.
Fig. 2.
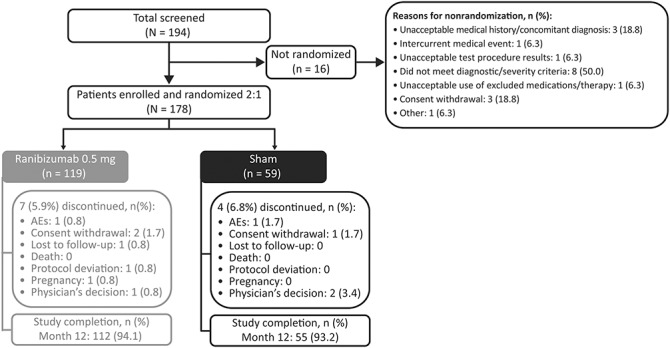
Patient disposition (randomized set*). *Consisted of all patients who were randomized.
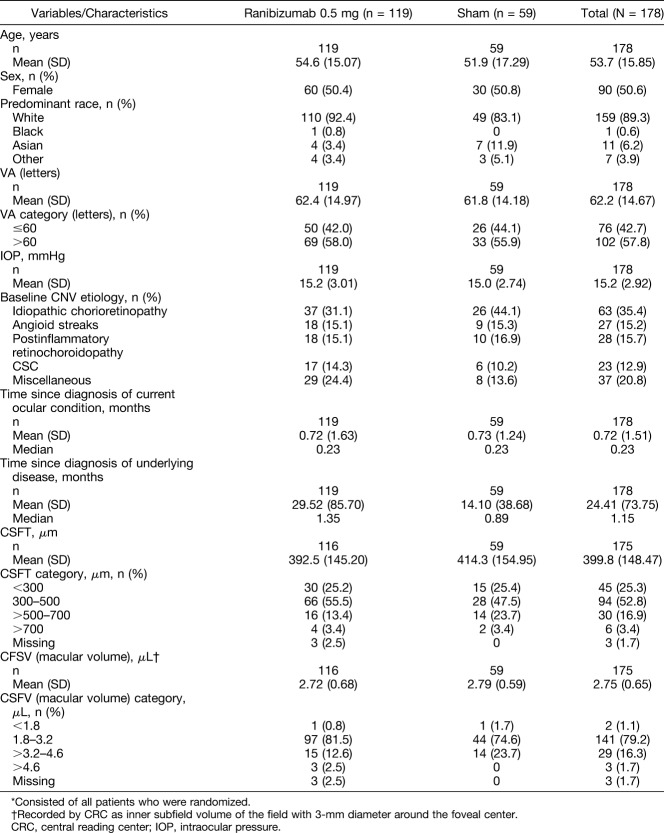
Patient demographics and baseline characteristics were well balanced between both treatment arms (Table 1). Overall, the mean (±SD) age of the patients was 53.7 (±15.9) years, the proportion of male to female patients was approximately 1:1 (49.4% vs. 50.6%, respectively), and 89.3% of the patients were white (Table 1). At baseline, the mean (±SD) BCVA was 62.2 (±14.7) letters (Table 1). At baseline, the majority of the patients (52.8%) had a CSFT in the range of 300 to 500 μm (Table 1).
Table 1.
Baseline Demographic, Ocular, and Disease Characteristics (Randomized Set*)
Efficacy
Best-corrected visual acuity
Ranibizumab treatment showed superior efficacy compared with sham from baseline to Month 2 (adjusted LS means [95% CI]: +9.5 [7.6–11.4] letters vs. −0.4 [−2.8 to 1.9] letters; one-sided P < 0.001); hence, the primary endpoint was met (Figure 3). Subgroup analyses showed that patients with lower baseline BCVA (≤60 letters) had a numerically higher treatment effect to Month 2 compared with those with a higher baseline BCVA (>60 letters) (adjusted LS means [95% CI]: 12.2 [7.0–17.4] letters vs. 8.1 [4.6–11.7] letters; P = 0.27). In the CNV etiology subgroups, the treatment effect (adjusted LS means [95% CI]) with ranibizumab ranged from 5.0 (−3.1 to 13.2) letters to 14.6 (6.1–23.0) letters (P = 0.65), demonstrating that the etiology of the CNV was not contributory to the overall positive result. A treatment effect (adjusted LS means) of >10 letters was observed with ranibizumab compared with sham in the CNV-angioid streaks, CNV-idiopathic, and CNV-miscellaneous groups (list of diagnosis listed in Supplemental Digital Content 4, http://links.lww.com/IAE/A680). From baseline to Month 2, patients aged ≤60 years had a significantly higher treatment effect with ranibizumab compared with patients aged >60 years (adjusted LS means [95% CI]: 13.2 [9.2–17.2] letters vs. 5.0 [0.8–9.3] letters; P < 0.001) (Figure 4).
Fig. 3.
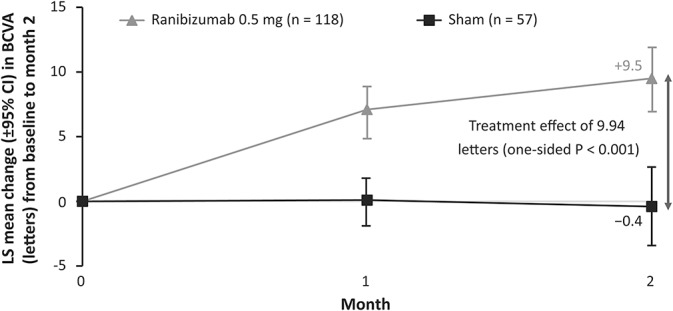
Change in BCVA from baseline to Month 2 (full analysis set* [observed]). *Consisted of all randomized patients to whom treatment regimen has been assigned.
Fig. 4.
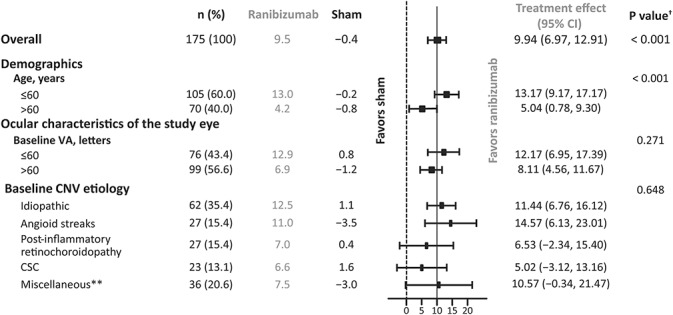
Change in BCVA from baseline to Month 2 in each of the specified subgroups (full analysis set* [observed]). *Consisted of all randomized patients to whom treatment regimen has been assigned. †P value is the interaction between the subgroup and treatment; P values > 0.05 are consistent with an equal treatment effect across the subgroup categories. **Etiologies that did not fit into the other CNV etiology subgroups and were insufficiently frequent to form a separate subgroup.
The mean (95% CI) change in BCVA at Month 2 was higher with ranibizumab treatment compared with sham (+9.4 [7.4–11.5] letters vs. −0.3 [−2.6 to 2.0] letters) (Figure 5). At Month 12, the mean (95% CI) change in BCVA was +11.0 (8.5–13.6) letters and +9.3 (5.7–12.9) letters in patients treated with ranibizumab and sham, respectively (Figure 5). The mean average (95% CI) change in BCVA from baseline to Month 1 through Month 6 was 9.5 (7.6–11.4) letters with ranibizumab and 4.7 (2.4–7.0) letters with sham and from baseline to Month 1 through Month 12 was 10.0 (7.9–12.1) letters and 6.6 (3.8–9.4) letters with ranibizumab and sham, respectively.
Fig. 5.
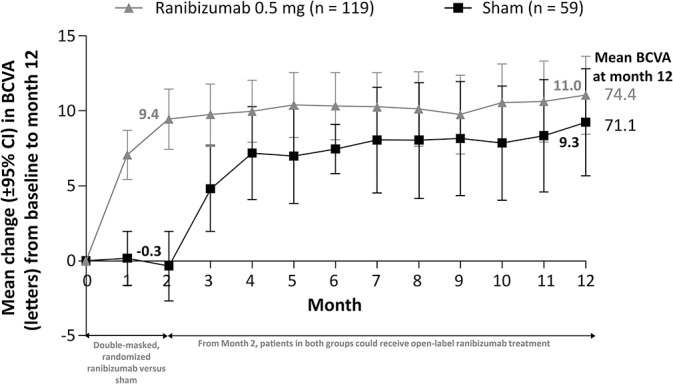
Change in BCVA from baseline to Month 12 (full analysis set* [observed]). *Consisted of all randomized patients to whom treatment regimen has been assigned.
At Months 2 and 12, a higher proportion of patients gained ≥5, ≥10, and ≥15 letters or reached 84 letters from baseline with ranibizumab treatment compared with patients originally randomized to sham (see Supplemental Digital Content 5, http://links.lww.com/IAE/A681). The proportion of patients who lost >5, >10, and >15 letters at Months 2 and 12 are shown in Supplemental Digital Content 6 (http://links.lww.com/IAE/A682).
Anatomical outcomes
From baseline to Month 2, among patients treated with ranibizumab compared with sham, there was a significantly greater reduction in CRC-assessed CSFT (adjusted LS means [95% CI]: −77.0 [−94.5 to −59.5] μm vs. +9.8 [−25.6 to 45.2] μm; P < 0.001; Figure 6) and CSFV (adjusted LS means [95% CI]: −0.4 [−0.5 to −0.3] μL vs. 0.0 [−0.1 to 0.2] μL; P < 0.001). At Month 12, the reductions in CRC-assessed mean CSFT (Figure 7) and mean CSFV (see Supplemental Digital Content 7, http://links.lww.com/IAE/A683) were similar in both treatment arms.
Fig. 6.
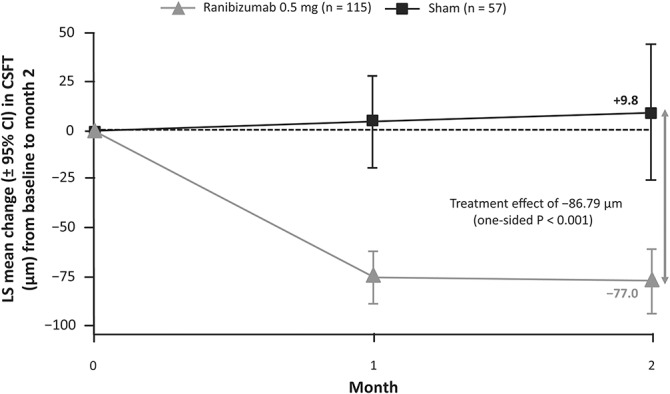
Change in CSFT from baseline to Month 2 (full analysis set* [observed]). *Consisted of all randomized patients to whom treatment regimen has been assigned.
Fig. 7.
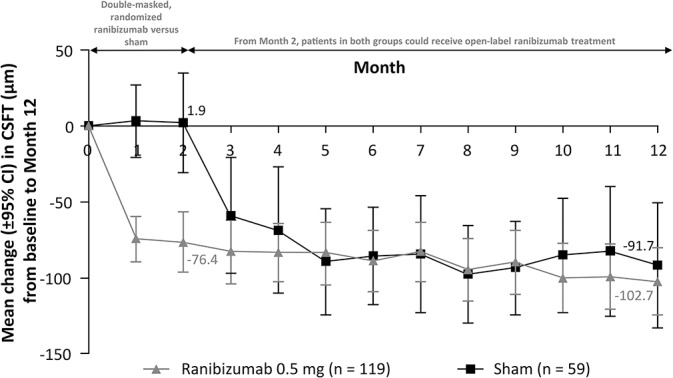
Change in CSFT from baseline to Month 12 (full analysis set* [observed]). *Consisted of all randomized patients to whom treatment regimen has been assigned.
At Month 2, ranibizumab treatment significantly lowered the odds for the presence of intraretinal fluid (odds ratio: 0.16; 95% CI: 0.067–0.369; one-sided P < 0.001) and subretinal fluid (odds ratio: 0.12; 95% CI: 0.048–0.28; one-sided P < 0.001) in the study eye compared with sham. From baseline to Month 2, intraretinal and subretinal fluid in the study eye was resolved in 67.4% and 56.0% of patients treated with ranibizumab compared with 29.2% and 14.0% of patients treated with sham, respectively (see Supplemental Digital Content 8, http://links.lww.com/IAE/A684). At Month 2, treatment with ranibizumab significantly lowered the odds of having active leakage compared with sham (odds ratio: 0.20; 95% CI: 0.058, 0.58; one-sided P < 0.001). From baseline to Month 2, active leakage was resolved in a greater proportion of patients treated with ranibizumab compared with sham, whereas resolution of active leakage was similar at Month 12 in patients treated with ranibizumab and sham (see Supplemental Digital Content 9, http://links.lww.com/IAE/A685).
The treatment effect on the functional and anatomical outcomes was in favor of ranibizumab compared with sham from baseline to Month 2.
Treatment Exposure
Ranibizumab injections
Before Month 2, the mean number of ranibizumab or sham injections received in the study eye was similar (1.7 vs. 1.8 injections, respectively). Approximately 75% of patients in each treatment group received 2.0 of a possible 2.0 injections. At Month 12, the mean number of ranibizumab injections was 5.8 of a possible 12 injections in the ranibizumab group and 5.4 of a possible 10 injections in the “sham with ranibizumab” group (Figure 8).
Fig. 8.
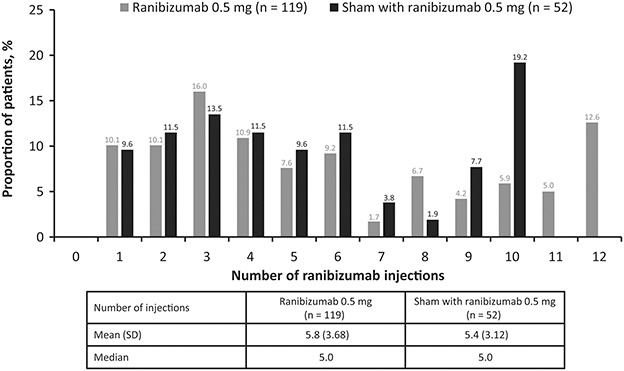
Number of injections in the study eye at Month 12 (safety set*). *Consisted of all adult patients who received at least one application of study treatment and had at least one postbaseline safety assessment.
Rescue treatment
At Month 1, one (1.7%) patient in the sham group received rescue treatment with vPDT as per protocol. None of the patients in the ranibizumab group required rescue treatment at Month 1.
Safety
Serious adverse events
No ocular SAEs were reported in the study eye up to Month 12 (see Supplemental Digital Content 10, http://links.lww.com/IAE/A686.) Up to Month 12, one ocular SAE (retinal detachment) was reported in the fellow untreated eye. Up to Month 12, nonocular SAEs were reported in 6.7% and 7.7% of patients in the ranibizumab and “sham with ranibizumab” arms, respectively (see Supplemental Digital Content 10, http://links.lww.com/IAE/A686). No deaths were reported during the study period. None of the ocular or nonocular SAEs were suspected by the investigator to be related to the study drug or ocular injection.
Adverse events
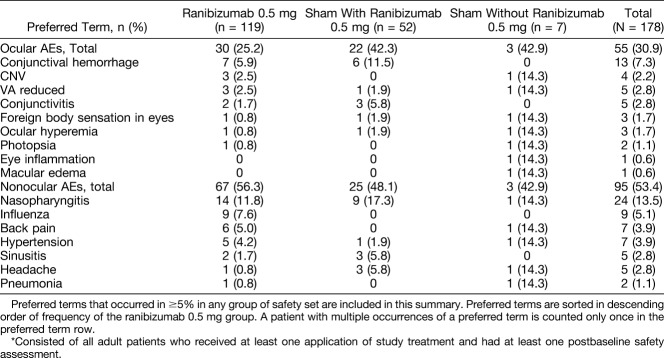
Up to Month 2, ocular AEs in the study eye were reported in 11.8% and 18.6% of patients in the ranibizumab and sham groups, respectively. Conjunctival hemorrhage (4.2%) and reduced VA (3.4%) were the most commonly reported ocular AEs among patients receiving ranibizumab and sham, respectively. Up to Month 12, ocular AEs were reported in 25.2%, 42.3%, and 42.9% of patients in the ranibizumab, “sham with ranibizumab”, and “sham without ranibizumab” groups, respectively (Table 2). Up to Month 12, the incidence of nonocular AEs was similar between the 2 groups (Table 2). None of the ocular AEs of the study eye or nonocular AEs up to Month 2 were suspected by the investigator to be related to the study drug. Ocular and nonocular AEs up to Month 12 suspected to be related to the study drug treatment and ocular injection are listed in Supplemental Digital Content 11, http://links.lww.com/IAE/A687, and Supplemental Digital Content 12, http://links.lww.com/IAE/A688, respectively. No ocular or nonocular AEs leading to study drug discontinuation were reported during the 12-month study.
Table 2.
Ocular (Study Eye) and Nonocular AEs Up to Month 12 Regardless of Study Drug Relationship (Safety Set*)
Discussion
The MINERVA study is the first Phase III randomized controlled clinical trial to evaluate the efficacy and safety of ranibizumab 0.5 mg in patients with CNV associated with causes other than nAMD and myopic CNV. Ranibizumab, which selectively binds to VEGF-A and inhibits all active forms of VEGF-A, plays an essential role in the pathogenesis of CNV. Furthermore, because ranibizumab is effective and approved for the treatment of CNV due to nAMD and myopic CNV worldwide including the US Food and Drug Administration, it is likely that it would have a beneficial effect in a more diverse population with CNV lesions due to other etiologies as well.6,11,42,43,46 Previously published case reports and case studies have shown the potential efficacy of anti-VEGF agents in treating CNV lesions (e.g., CNV-CSC, CNV-punctate inner choroidopathy, CNV-idiopathic, CNV-angioid streaks, and CNV-choroidal osteoma), thus supporting the rationale for the use of anti-VEGF agents.29–40 There was no approved or standard of care therapy for the management of patients with CNV because of causes other than nAMD and myopic CNV, thus creating an unmet medical need. If left untreated, CNV could lead to further deterioration and rapid loss of VA because of the sequelae of CNV lesions including fibrosis and atrophy with complications of secondary histoplasmosis. Previous treatments such as vPDT or thermal laser photocoagulation have been used in the past; however, these treatment modalities do not restore lost vision in patients with subfoveal, extrafoveal, or juxtafoveal CNV.47 The efficacy of ranibizumab in treating nAMD and myopic CNV is already well established based on data from several randomized clinical trials in patients with nAMD and myopic CNV.8–10,12–15,17 Based on the results from MINERVA, ranibizumab has been recently approved by the European Union for the treatment of visual impairment due to CNV other than nAMD and myopic CNV in adult patients.45 In the MINERVA study, no active comparators were used because there is no licensed standard of care for the treatment of CNV because of any cause other than nAMD and myopic CNV. Previous studies have shown that vPDT might affect the physiological choriocapillaris, indirectly affecting VEGF upregulation, which might cause further CNV growth.48–51 Laser photocoagulation was also not considered because the benefits are limited and might result in visual loss particularly in lesions with foveal involvement.52 Moreover, vPDT and laser photocoagulation are not standards of care in these uncommon conditions.
Results from previous small uncontrolled studies in patients with CNV lesions other than nAMD and myopic CNV have shown that anti-VEGF treatment results in a potential better gain in BCVA when compared with other treatment options such as vPDT.29–40 In MINERVA, patients treated with ranibizumab PRN from the beginning of the study had a mean (±SD) BCVA gain of 11.0 (±13.4) letters from baseline to Month 12, whereas those treated initially with sham up to Month 1 and switched to ranibizumab PRN from Month 2 had a mean (±SD) BCVA gain of +9.3 (±13.4) letters at Month 12. In the multicenter, 2-year, double-blind, sham-controlled, Phase III, Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration (MARINA) trial, ranibizumab treatment led to a mean BCVA gain of approximately 5.5 letters and 7.2 letters at Months 2 and 12, respectively, in patients with nAMD.14 In the first pivotal 12-month, randomized, double-masked, multicenter, active-controlled, Phase III, Ranibizumab And PDT (verteporfin) Evaluation In myopic Choroidal Neovascularization (RADIANCE) clinical trial, there was a mean BCVA gain of 10.9 letters and 14.4 letters at Months 2 and 12, respectively, in patients with visual impairment because of myopic CNV treated with ranibizumab 0.5 mg guided by disease activity.17 The mean BCVA gain observed in MINERVA is consistent with that observed in MARINA and RADIANCE, further confirming the efficacy of ranibizumab for the treatment of CNV. However, comparison with these studies should be interpreted with caution because of the differences in study design, study duration, patient population, and treatment regimen.
In MINERVA, early initiation of ranibizumab treatment after diagnosis in most of the patients resulted in a significant gain in mean BCVA at Month 2 compared with sham, and this gain in BCVA was sustained until Month 12. Patients in the sham group who were eligible to receive open-label ranibizumab from Month 2 also showed a consistent improvement in BCVA from Month 2 to Month 12; however, these gains were numerically lower compared with those in patients treated with ranibizumab from baseline. Therefore, a timely initiation of treatment should be followed, although in case of a “reasonable” delay, good visual outcomes might still be achieved. As in other conditions complicated by CNV, it is assumed that delaying anti-VEGF treatment might result in worse functional outcomes.
The subgroup analyses in our study showed that the individualized PRN ranibizumab treatment regimen based on disease activity criteria was effective in improving BCVA irrespective of the baseline BCVA and underlying CNV etiology. In pivotal clinical trials such as MARINA, the Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration (ANCHOR) (2-year, multicenter, double-blind study in patients with predominantly classic nAMD), and RADIANCE, ranibizumab was equally effective in the treatment of the underlying disease irrespective of the baseline BCVA, age, and CNV lesions.9,10,14,17 In this study, patients with lower baseline BCVA (≤60 letters) had a numerically higher treatment effect with ranibizumab at Month 2 compared with those with a higher baseline BCVA (>60 letters). Because of the heterogeneity of CNV etiologies, five predefined baseline CNV etiology subgroups were determined based on grouping same etiologies (e.g., CNV-angioid streaks) or similar pathologies (e.g., CNV-postinflammatory retinochoroidopathy). Across the baseline CNV etiology subgroups, ranibizumab showed a treatment effect of 5.0 letters to 14.6 letters. In MINERVA, patients with CNV due to CSC and postinflammatory retinochoroidopathy had a treatment effect of 5.0 letters and 6.5 letters, respectively, at Month 2 after ranibizumab treatment. It might be possible that the visual improvement for these CNV due to CSC or inflammation could enhance further using combination therapy of ranibizumab and vPDT and corticosteroid or immunomodulatory therapy, respectively.29,53–55 However, combination therapy could have several challenges, such as different therapies, doses, and timing, making it difficult to conduct large and definitive trials for determining the safest and most effective treatment regimen.55 It should be noted that MINERVA was not designed to evaluate ranibizumab in combination with other therapies for the treatment of CNV due to uncommon causes. At Month 2, the treatment effect was significantly higher in younger patients (≤60 years) treated with ranibizumab compared with patients aged >60 years. Clinical studies have demonstrated that ranibizumab treatment is effective in reducing CNV leakage in patients with myopic CNV.17,56 Likewise, in the MINERVA study, it was observed that at Month 2, individualized ranibizumab treatment was effective in reducing active leakage in fluorescein angiography compared with sham in patients with CNV due to any cause other than nAMD and myopic CNV.
In MINERVA, individualized PRN treatment with ranibizumab 0.5 mg based on disease activity resulted in a significant treatment effect of approximately 10 letters (P < 0.001) compared with sham from baseline to Month 2. The individualized dosing regimen of ranibizumab in MINERVA is in line with the current Summary of Product Characteristics57 posology of ranibizumab, which recommends that treatment be initiated with one injection per month until maximum VA is achieved, and/or there are no signs of disease activity. In patients with nAMD, diabetic macular edema and retinal vein occlusion, initially, three or more consecutive monthly injections may be needed. By contrast, in patients with myopic CNV, only one or two injections are often sufficient, although a few patients may need more frequent dosing based on the disease activity.57
There were no new safety concerns identified with ranibizumab treatment in the MINERVA study. No SAEs were suspected to be related to the study drug or ocular injection. There were no reports of deaths or endophthalmitis in the study. Overall, the safety findings were consistent with the well-established safety profile of ranibizumab reported previously.9,10,12,14,17,18
One limitation of this study might be the lack of compulsory indocyanine green angiography at baseline examination, as indocyanine green angiography was not available in some centers. The diagnoses of various etiologies were generally based on the investigators' clinical judgment. However, indocyanine green angiography could be performed at the investigators' discretion for a more accurate diagnosis before enrollment into the study.
In summary, an individualized PRN ranibizumab treatment regimen based on disease activity in the 12-month MINERVA study showed highly clinically relevant VA gains for the treatment of CNV irrespective of the underlying etiology and was well tolerated. The study included a diverse patient population with respect to the CNV etiology at baseline and a large sample size considering the rarity of the disease condition. Despite the heterogeneity and some associated diseases being extremely rare, this well-powered randomized study was able to demonstrate compelling clinically significant treatment effect in the overall population and predefined subgroups. The study findings support early initiation of treatment to achieve the best possible outcomes. Overall, ranibizumab was effective, with no new safety findings up to Month 12 in comparison with the previously well-established safety profile of ranibizumab.
Supplementary Material
Acknowledgments
The authors thank Sabyasachi Ghosh (Scientific Services Practice—Product Lifecycle Services, Novartis Healthcare Pvt. Ltd, Hyderabad, India) for medical writing and editorial assistance toward the development of this article. T. Y. Y. Lai, G. Staurenghi, and P. G. Hykin are the steering committee members of the MINERVA study and have contributed significantly toward the design of the study, interpretation of data, and development of this manuscript.
T. Y. Y. Lai: Consultant—Allergan, Bayer Healthcare, Novartis Pharmaceuticals, and Genentech; Grants/grants pending—Bayer Healthcare and Novartis Pharmaceuticals; Lecture fees—Allergan, Bausch & Lomb, Bayer Healthcare, and Novartis Pharmaceuticals. G. Staurenghi: Consultant—Novartis, Bayer HealthCare, Allergan, Genentech, Roche, Heidelberg Engineering, and Alcon; Travel support to meetings—Bayer HealthCare, Centervue, Heidelberg Engineering, and Novartis; Payment for lectures—Zeiss; Patent holder—in conjunction with Ocular Instruments, Inc.; Payment for development of educational presentations—Roche. P. Lanzetta: Consultant—Alcon, Alimera, Allergan, Bausch & Lomb, Bayer, Novartis, Roche, and Teva. F. G. Holz: Consultant—Acucela, Boehringer-Ingelheim, Genentech, and Merz; Consultant and has received grants—Alcon and Allergan; Consultant for and has received lecture and speaker fees—Bayer Healthcare and Pfizer; Consultant for and has received grants, lecture fees, and speaker's bureau fees—Heidelberg Engineering and Novartis; received grants—Optos, GlaxoSmithKline, and Carl Zeiss Meditec. S. H. Melissa Liew: Employee—Novartis Pharmaceuticals Corporation, USA. S. Desset-Brethes: Employee—Novartis Pharma AG, Basel, Switzerland. H. Staines: Employee—Novartis Pharma AG, Basel, Switzerland at the time of development of the manuscript. Currently, he is the director of Sigma Statistical Services, Balmullo KY16 0BJ, United Kingdom. P. G. Hykin: Grants—Bayer HealthCare, Allergan and Novartis; Consultant—Bayer HealthCare, Novartis and Allergan; Travel support to meetings for participation in review activities and provision of writing assistance, medicines, equipment, or administrative support—Bayer HealthCare; Payment for lectures and support for conference attendance—Allergan and Novartis.
Footnotes
This multicenter study was funded and managed by Novartis Pharma AG and is registered with www.clinicaltrialsregister.eu (EudraCT no. 2012-005417-38).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
References
- 1.Grossniklaus HE, Gass JD. Clincopathologic correlations of surgically excised type 1 and type 2 submacular choroidal neovsacular membranes. Am J Ophthalmol 1998;126:59–69. [DOI] [PubMed] [Google Scholar]
- 2.Carneiro AM, Silva RM, Veludo MJ, et al. Ranibizumab treatment for choroidal neovascularization from causes other than age-related macular degeneration and pathological myopia. Ophthalmologica 2011;225:81–88. [DOI] [PubMed] [Google Scholar]
- 3.Sivaprasad S, Moore AT. Choroidal neovascularisation in children. Br J Ophthalmol 2008;92:451–454. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SY, Laroche A, Leguen Y, et al. Etiology of choroidal neovascularization in young patients. Ophthalmology 1996;103:1241–1244. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol 2004;137:486–495. [DOI] [PubMed] [Google Scholar]
- 6.Spaide RF. Choroidal neovascularization in younger patients. Curr Opin Ophthalmol 1999;10:177–181. [DOI] [PubMed] [Google Scholar]
- 7.Derosa JT, Yannuzzi LA, Marmor M, et al. Risk factors for choroidal neovascularization in young patients: a case-control study. Doc Ophthalmol 1995;91:207–222. [DOI] [PubMed] [Google Scholar]
- 8.Antoszyk AN, Tuomi L, Chung CY, Singh A. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol 2008;145:862–874. [DOI] [PubMed] [Google Scholar]
- 9.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–1444. [DOI] [PubMed] [Google Scholar]
- 10.Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009;116:57–65.e5. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006;26:859–870. [DOI] [PubMed] [Google Scholar]
- 12.Heier JS, Boyer DS, Ciulla TA, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: year 1 results of the FOCUS Study. Arch Ophthalmol 2006;124:1532–1542. [DOI] [PubMed] [Google Scholar]
- 13.Holz FG, Amoaku W, Donate J, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology 2011;118:663–671. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–1431. [DOI] [PubMed] [Google Scholar]
- 15.Tufail A, Narendran N, Patel PJ, et al. Ranibizumab in myopic choroidal neovascularization: the 12-month results from the REPAIR study. Ophthalmology 2013;120:1944–1945.e1. [DOI] [PubMed] [Google Scholar]
- 16.Tufail A, Patel PJ, Sivaprasad S, et al. Ranibizumab for the treatment of choroidal neovascularisation secondary to pathological myopia: interim analysis of the REPAIR study. Eye (Lond) 2013;27:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology 2014;121:682–692.e2. [DOI] [PubMed] [Google Scholar]
- 18.Dhoot DS, Kaiser PK. Ranibizumab for age-related macular degeneration. Expert Opin Biol Ther 2012;12:371–381. [DOI] [PubMed] [Google Scholar]
- 19.Brue C, Pazzaglia A, Mariotti C, et al. Aflibercept as primary treatment for myopic choroidal neovascularisation: a retrospective study. Eye (Lond) 2016;30:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikuno Y, Ohno-Matsui K, Wong TY, et al. Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR study. Ophthalmology 2015;122:1220–1227. [DOI] [PubMed] [Google Scholar]
- 21.Pece A, Milani P. Intravitreal aflibercept for myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 2016;254:2327–2332. [DOI] [PubMed] [Google Scholar]
- 22.Szabo SM, Hedegaard M, Chan K, et al. Ranibizumab vs. aflibercept for wet age-related macular degeneration: network meta-analysis to understand the value of reduced frequency dosing. Curr Med Res Opin 2015;31:2031–2042. [DOI] [PubMed] [Google Scholar]
- 23.Gupta B, Elagouz M, Sivaprasad S. Intravitreal bevacizumab for choroidal neovascularisation secondary to causes other than age-related macular degeneration. Eye (Lond) 2010;24:203–213. [DOI] [PubMed] [Google Scholar]
- 24.Hashemi S, Faramarzi MA, Ghasemi Falavarjani K, Abdollahi M. Bevacizumab for choroidal neovascularization secondary to age-related macular degeneration and pathological myopia. Expert Opin Biol Ther 2014;14:1837–1848. [DOI] [PubMed] [Google Scholar]
- 25.Kasahara K, Moriyama M, Morohoshi K, et al. Six-year outcomes of intravitreal bevacizumab for choroidal neovascuarlzation in patients with pathologic myopia. Retina 2017;37:1055–1064. [DOI] [PubMed] [Google Scholar]
- 26.Maier M, Feucht N, Haas K, et al. Intravitreal injection of bevacizumab for exsudative AMD with occult or minimal classic choroidal neovascularisation (CNV) [in German]. Klin Monbl Augenheilkd 2008;225:818–824. [DOI] [PubMed] [Google Scholar]
- 27.Pece A, Milani P, Monteleone C, et al. A randomized trial of intravitreal bevacizumab vs. ranibizumab for myopic CNV. Graefes Arch Clin Exp Ophthalmol 2015;253:1867–1872. [DOI] [PubMed] [Google Scholar]
- 28.Tan CS, Cheong KX, Lim LW, Tan S. A randomized trial of intravitreal bevacizumab vs. ranibizumab for myopic CNV. Graefes Arch Clin Exp Ophthalmol 2016;254:1433–1434. [DOI] [PubMed] [Google Scholar]
- 29.Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (avastin) for choroidal neovascularization secondary to central serous chorioretinopathy, secondary to punctate inner choroidopathy, or of idiopathic origin. Am J Ophthalmol 2007;143:977–983. [DOI] [PubMed] [Google Scholar]
- 30.Chhablani J, Kozak I, Pichi F, et al. Outcomes of treatment of choroidal neovascularization associated with central serous chorioretinopathy with intravitreal antiangiogenic agents. Retina 2015;35:2489–2497. [DOI] [PubMed] [Google Scholar]
- 31.Chhablani J, Pichi F, Silva R, et al. Antiangiogenics in choroidal neovascularization associated with laser in central serous chorioretinopathy. Retina 2016;36:901–908. [DOI] [PubMed] [Google Scholar]
- 32.Finger RP, Charbel Issa P, Hendig D, et al. Monthly ranibizumab for choroidal neovascularizations secondary to angioid streaks in pseudoxanthoma elasticum: a one-year prospective study. Am J Ophthalmol 2011;152:695–703. [DOI] [PubMed] [Google Scholar]
- 33.Finger RP, Charbel Issa P, Schmitz-Valckenberg S, et al. Long-term effectiveness of intravitreal bevacizumab for choroidal neovascularization secondary to angioid streaks in pseudoxanthoma elasticum. Retina 2011;31:1268–1278. [DOI] [PubMed] [Google Scholar]
- 34.Khan MA, DeCroos FC, Storey PP, et al. Outcomes of anti-vascular endothelial growth factor therapy in the management of choroidal neovascularization associated with choroidal osteoma. Retina 2014;34:1750–1756. [DOI] [PubMed] [Google Scholar]
- 35.Mansour AM, Arevalo JF, Al Kahtani E, et al. Role of intravitreal antivascular endothelial growth factor injections for choroidal neovascularization due to choroidal osteoma. J Ophthalmol 2014;2014:210458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansour AM, Mackensen F, Arevalo JF, et al. Intravitreal bevacizumab in inflammatory ocular neovascularization. Am J Ophthalmol 2008;146:410–416. [DOI] [PubMed] [Google Scholar]
- 37.Mansour AM, Mackensen F, Mahendradas P, et al. Five-year visual results of intravitreal bevacizumab in refractory inflammatory ocular neovascularization. Clin Ophthalmol 2012;6:1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosina C, Romano M, Cigada M, et al. Intravitreal bevacizumab for choroidal neovascularization secondary to angioid streaks: a long-term follow-up study. Eur J Ophthalmol 2015;25:47–50. [DOI] [PubMed] [Google Scholar]
- 39.Rouvas A, Petrou P, Douvali M, et al. Intravitreal ranibizumab for the treatment of inflammatory choroidal neovascularization. Retina 2011;31:871–879. [DOI] [PubMed] [Google Scholar]
- 40.Sudhalkar A, Yogi R, Chhablani J. Anti-vascular endothelial growth factor therapy for naive idiopathic choroidal neovascularization: a comparative study. Retina 2015;35:1368–1374. [DOI] [PubMed] [Google Scholar]
- 41.Bares M, Rektor I. Basal ganglia involvement in sensory and cognitive processing. A depth electrode CNV study in human subjects. Clin Neurophysiol 2001;112:2022–2030. [DOI] [PubMed] [Google Scholar]
- 42.Heier JS, Brown D, Ciulla T, et al. Ranibizumab for choroidal neovascularization secondary to causes other than age-related macular degeneration: a phase I clinical trial. Ophthalmology 2011;118:111–118. [DOI] [PubMed] [Google Scholar]
- 43.Ishibashi T, Hata Y, Yoshikawa H, et al. Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 1997;235:159–167. [DOI] [PubMed] [Google Scholar]
- 44.Martin G, Schlunck G, Hansen LL, Agostini HT. Differential expression of angioregulatory factors in normal and CNV-derived human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol 2004;242:321–326. [DOI] [PubMed] [Google Scholar]
- 45.European Medicines Agency. Summary of Product Characteristics. Lucentis 10 mg/mL solution for injection. Novartis 2016. Available at: http://ec.europa.eu/health/documents/community-register/2016/20161114136324/anx_136324_en.pdf. Accessed December 13, 2016. [Google Scholar]
- 46.Cui JZ, Kimura H, Spee C, et al. Natural history of choroidal neovascularization induced by vascular endothelial growth factor in the primate. Graefes Arch Clin Exp Ophthalmol 2000;238:326–333. [DOI] [PubMed] [Google Scholar]
- 47.Diaz RI, Sigler EJ, Rafieetary MR, Calzada JI. Ocular histoplasmosis syndrome. Surv Ophthalmol 2015;60:279–295. [DOI] [PubMed] [Google Scholar]
- 48.Azab M, Boyer DS, Bressler NM, et al. Verteporfin therapy of subfoveal minimally classic choroidal neovascularization in age-related macular degeneration: 2-year results of a randomized clinical trial. Arch Ophthalmol 2005;123:448–457. [DOI] [PubMed] [Google Scholar]
- 49.Kaiser PK. Combination therapy with verteporfin and anti-VEGF agents in neovascular age-related macular degeneration: where do we stand? Br J Ophthalmol 2010;94:143–145. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt-Erfurth U, Michels S, Barbazetto I, Laqua H. Photodynamic effects on choroidal neovascularization and physiological choroid. Invest Ophthalmol Vis Sci 2002;43:830–841. [PubMed] [Google Scholar]
- 51.Schmidt-Erfurth U, Schlotzer-Schrehard U, Cursiefen C, et al. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sci 2003;44:4473–4480. [DOI] [PubMed] [Google Scholar]
- 52.Virgili G, Bini A. Laser photocoagulation for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2007;CD004763. [DOI] [PubMed] [Google Scholar]
- 53.Neri P, Lettieri M, Fortuna C, et al. Inflammatory choroidal neovascularization. Middle East Afr J Ophthalmol 2009;16:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res 2015;48:82–118. [DOI] [PubMed] [Google Scholar]
- 55.Augustin AJ, Offermann I. Combination therapy for choroidal neovascularisation. Drugs Aging 2007;24:979–990. [DOI] [PubMed] [Google Scholar]
- 56.Lai TY, Cheung CM. Myopic choroidal neovascularization: diagnosis and treatment. Retina 2016;36:1614–1621. [DOI] [PubMed] [Google Scholar]
- 57.European Medicines Agency. Summary of Product Characteristics. Lucentis 10 mg/mL solution for injection. Novartis 2015. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000715/WC500043546.pdf. Accessed May 18, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.