Supplemental Digital Content is available in the text
Keywords: choline, glioma, meta-analysis, necrosis, pet, recurrence
Abstract
Objectives:
Distinguishing glioma recurrence from the necrosis after radiation therapy and/or chemotherapy is a crucial clinical issue, for the different diagnosis will lead to divergent treatments. The accurate judgment is barely achieved by conventional imaging methods. We therefore assume it is of need to exert a meta-analysis to evaluate the diagnostic accuracy of 11C-choline positron emission tomography (PET), to achieve this goal.
Material and methods:
We searched the PubMed, Embase, and Chinese Biomedical databases comprehensively to select eligible studies and assessed the quality of each article included (up to May 31, 2018). Fixed-effects models were used. Summary diagnostic accuracy of 11C-choline PET was obtained from pooled analysis.
Results:
Five articles comprising 6 studies with total 118 patients (134 scans) were enrolled for the meta-analysis. There was no heterogeneity or publication bias among the included studies. The pooled sensitivity and specificity were 0.87 (95% confidence interval [CI]: 0.78, 0.93) and 0.820 (95% CI: 0.69, 0.91), respectively. The pooled diagnostic odds ratio was 35.50 (95% CI: 11.70, 107.75). The area under the curve was 0.9170 (95% CI: 0.8504, 0.9836), with Q∗ index equaling to 0.8499. The diagnostic accuracy of each subgroup showed no statistical differences with that of the overall group.
Conclusions:
This meta-analysis indicated 11C-choline has high diagnostic accuracy for the identification of tumor relapse from radiation induced necrosis in gliomas.
1. Introduction
Glioma is the most common and aggressive primary malignant brain tumors with exceptionally poor prognosis in adults.[1] Glioma can be further classified into low-grade glioma (LGG, Grade 1–2) and high-grade glioma (HGG, Grade 3–4) according to its malignancy. LGG is a well differentiated tumor, which causes relatively good prognosis. Although HGG is a low differentiated malignant tumor and always leads to poor prognosis. The first-line therapeutic strategy for glioma is maximal tumor excision followed by radiation therapy (RT) with the concomitant or assisted chemotherapy.[2] RT plays a central part in brain tumor therapy, while radiation necrosis after RT is a sever complication. Owing to similar appearance on conventional imagines,[3] it is difficult to distinguish glioma relapse from radiation necrosis. Early discovery of tumor recurrence from radiation necrosis is crucial, for the following therapies are totally different. Given the limitations of conventional imaging methods, a few clinical studies using diffusion magnetic resonance imaging (MRI),[4,5] perfusion MRI,[3] and MR spectroscopy[6,7] have been undertaken to differentiate these 2 kinds of lesions, which showed improved diagnosis accuracy compared with the conventional MR or computed tomography (CT). Furthermore, many studies have been focusing on physiological and metabolic characteristics of tumors; 201thallium-SPECT[8] and 18F-fluorodeoxyglucosepositron emission tomography (18F-FDGPET)[9,10] were pointed out to be more effective for identifying glioma recurrence from radiation necrosis. However, it was reported that FDG-PET shows poor sensitivity (SEN) and/or specificity (SPE) in evaluation of some sorts of cancers.[11–13] Malignant tumors contain substantial phospholipid, especially phosphatidylcholine,[14] which makes it possible to use 11C-choline as a tracer. 11C-choline has become an alternative tracer, and it is supposed to be effective in brain tumor diagnosis in recent studies,[15,16] yet no meta-analysis has been made to evaluate the accuracy of 11C-choline PET in differentiating glioma recurrence from the radiation necrosis. The aim of this meta-analysis is doing so.
2. Materials and methods
2.1. Ethical review
This review was approved by the clinical ethics committee of the Second Affiliated Hospital of Zhejiang University School of Medicine.
2.2. Search strategy
The 3 electronic databases, Pubmed, Embase, and Chinese Biomedical (CBM) database, were included to retrieve eligible published articles (up to May 31, 2018). Keywords were listed as follows: “glioma” or “glioblastoma” or “astrocytoma” or “oligodendroglioma” or “brain neoplasm” or “brain tumor” or “brain tumour”; and “positron emission tomography” or “PET”; and “recurrence” or “recurrent” or “relapse” or “regrowth” or “necrosis” or “posttreatment”. Additionally, the references of all eligible articles were also checked for possible correlative articles, which may be included in this study. The detailed search strategy was displayed in Table 1.
Table 1.
Search terms and strategies of 11C-choline PET for the differential diagnosis of glioma recurrence from radiation necrosis in different databases.

2.3. Inclusion and exclusion criteria
Inclusion criteria are[1]: the patients were confirmed to have glioma according to surgical pathology[2]; all these patients received the resultant RT together with chemotherapy or not[3]; 11C-choline PET was applied to identify tumor relapse from the radiation necrosis[4]; the criterion standard was pathology and/or clinical follow-up[5]; every study contained at least 10 cases[6]; true positive (TP), false positive (FP), false negative (FN), and true negative (TN) could be gained or computed from the data[7]; no repeated data[8]; the language is Chinese and English. Exclusion criteria: the letters, the reviews, the case reports, the editorials, the conference papers, the abstracts, and the proceedings. Two authors (T.L. and J.Z.) independently evaluated the retrieved articles by screening the titles, and then another 2 reviewers (L.G. and W.X.) independently perused the abstracts of the underlying eligible articles after preliminary screening. Any disagreements were settled by the senior authors (G.C.).
2.4. Data extraction and quality assessment
We carried out the meta-analysis following the PRISMA guidelines (Supplemental Table 1).
Two authors (LG and TL) separately reviewed and extracted data with uniform criteria until an agreement being reached. The core data extracted from every study should contain the following information: study design, number of cases and scans, classifications of glioma, pathology, RT type, analysis method, parameters and cutoff value. The figures of TP, FP, FN, and TN were either listed in each publication or could be calculated. Other related factors, such as names of authors, year of publication, institute of publication, the sex and age of patients, and the tracer dosage that was used for treatment were extracted as well. The Quality Assessment Tool for Diagnostic Accuracy Studies version 2 (QUADAS-2) recommended by Cochrane was adopted to assess the document qualities.[17] Any disagreements were disposed by another reviewer (GC). The quality evaluation was operated and the bias risk map was plotted using Review Manager 5.3.[17]
2.5. Statistical analysis
The meta-analysis was carried out according to the recommendation for diagnostic accuracy meta-analysis.[18,19]
In step 1, the heterogeneity among studies ascribe to threshold effect was speculated by threshold analysis. Spearman correlation coefficient between the logit of SEN and the logit of (1−SPE) was applied to determine the threshold effect. A high relevance with P < .05 suggests a significant threshold effect.
In step 2, Cochran-Q test, χ2 test and the inconsistency index (I2) of the diagnostic odds ratio (DOR) were applied to estimate the extent of heterogeneity among studies ascribe to nonthreshold effect, and P < .05 or I2 > 50% indicates a notable heterogeneity. If the results of calculation showed a significant heterogeneity, random-effects coefficient binary regression model was then used to summarize the data; SEN analysis and meta-regression analysis were also applied to search for the possible source of heterogeneity. The fixed-effects coefficient binary regression model was applied if no notable heterogeneity was found.[17,19]
In step 3, random- or fixed-effects models were applied to calculate the pooled SEN, SPE, positive likelihood (LR+), negative likelihood (LR−), and DOR with 95% confidence intervals (CIs) on the basis of the above-mentioned analysis. In case any of the numbers of TP, FP, FN, and TN appears to be zero in the table, a value of 0.5 was added to prevent the SENs or SPEs being 100%.
In step 4, summary receiver-operating characteristic curve (SROC) was plotted, the area under curve (AUC) and Q∗ index were computed accordingly. Q∗ index is the value of SEN at the SROC curve where SEN equals SPE. The diagnostic accuracy was assessed as reported before:[9] the value of AUC from 51% to 70% denotes low accuracy, from 71% to 90% denotes moderate accuracy, and ≥90% denotes high accuracy. Subgroup analysis was carried out to analyze the heterogeneity according to the same principle elaborated above. Every subgroup should contain at least 3 studies with uniform characteristics according to the same parameter. Z test was used to compare the value of AUC and P < .05 denotes significant differences. The above-mentioned statistical analysis methods were operated using Meta-Disc statistical software version 1.4.[17,19]
At last, Deek funnel plot and linear regression method were applied to estimate the publication bias. The x-axis indicates DOR while the y-axis indicates the reciprocal of the root of the effective sample quantity.[15]P < .05 suggested obvious asymmetry that is evidence of publication bias.[13] The statistical analysis was operated using Stata statistical software 14.0 (StataCorp LP, College Station, TX).
3. Results
3.1. Study selection and its characteristics
Total 194, 1034 and 9 articles were retrieved from Pubmed, Embase, and CBM databases, respectively. Additional records identified through other sources were zero. Records remained 1103 articles after duplicates removed. There were 81 potentially eligible records left for further full-text assessment after titles and abstracts screening. After the reviews, the case reports, the articles whose data cannot be extracted, and all repeated data being removed, finally 5 articles comprising 6 studies with 118 patients satisfied all inclusion criteria and without situations should be excluded were included in the meta-analysis.[20–24]
The PRISMA flow diagram of the document selection procedure is shown in Figure 1. The characteristics of all 6 studies were summarized in Table 2. The final 5 articles contained 6 retrospective studies with 118 patients (134 scans) from the United States, Japan, and China. The sample size of each study ranged from 10 to 50. Eighty-seven patients received routine RT, whereas 31 patients received stereotactic RT (SRT). Semiquantitative analysis method was used in 4 studies; 1 study used visual analysis method. All studies adopted lesion/normal ratio (LNR) or tumor/normal ratio (TNR) as the parameters. The cutoff value ranged from 2 to 8.92. All the studies used both pathology and/or clinical follow-up as the gold standard. The methodological quality graph and methodological quality summary graph of every article is shown in Figure 2. Most articles were of low or unclear risk of bias, suggesting acceptable quality.
Figure 1.
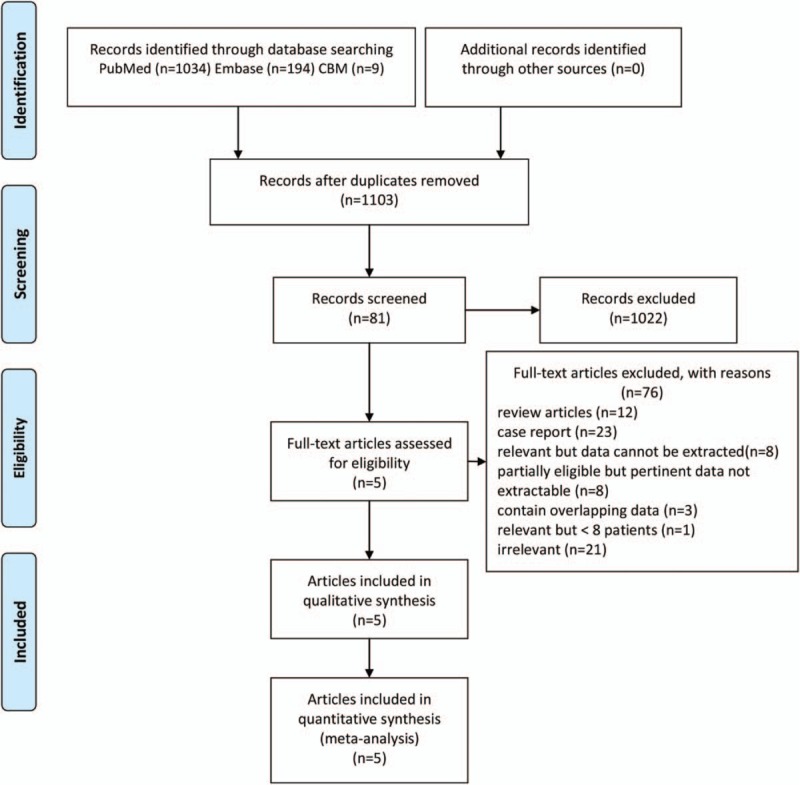
Flow diagram of the study selection process.
Table 2.
Characteristics of studies included in the meta-analysis of 11C-choline PET for the differential diagnosis of glioma recurrence from radiation necrosis.
Figure 2.
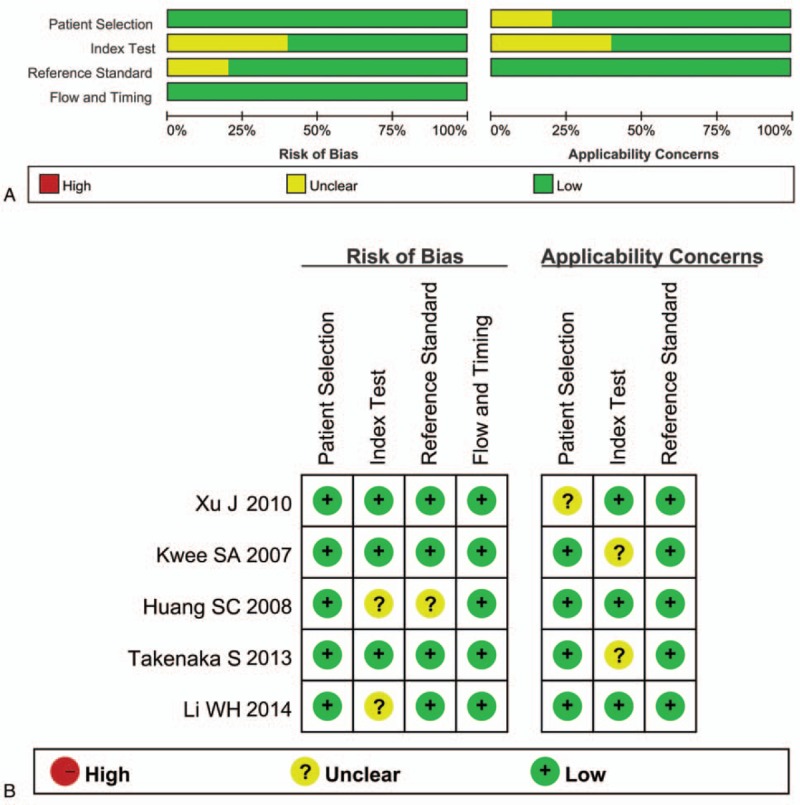
Methodological quality graph (A) and methodological quality summary graph (B) of each study.
3.2. Quantitative synthesis
3.2.1. Overall analysis
There were finally 5 articles including 6 studies with 118 patients (134 scans) of 11C-choline PET to identify glioma recurrence from radiation necrosis after primary surgery and RT and/or chemotherapy. The heterogeneity among studies ascribe to threshold effect was estimated with spearman correlation coefficient equaling to 0.266 (P = .610), which implied no obvious threshold effect. The Cochran-Q index and the inconsistency index (I2) of the DOR was 1.89 (P = .865) and 0.0%, respectively, indicating there was no significant heterogeneity observed among studies, thus the fixed-effects coefficient binary regression model was selected. The pooled SEN was 0.87, with 95% CIs between 0.78 and 0.93; the pooled SPE was 0.82, with 95% CIs between 0.69 and 0.91 (Fig. 3A); the pooled positive LR was 4.90, with 95% confidence intervals between 2.63 and 9.13; the pooled negative LR was 0.16, with 95% CIs between 0.09 and 0.29; the pooled DOR was 35.50, with 95% CIs between 11.70 and 107.75 (Fig. 4A). The AUC was 0.9170 (95% CI: 0.8504, 0.9836), with Q∗ index equaling to 0.8499 (Fig. 5A), indicating high diagnostic accuracy.
Figure 3.
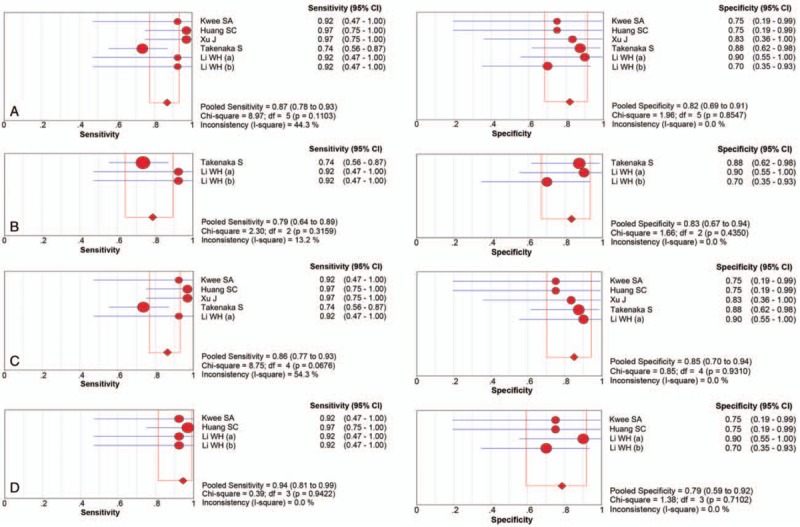
Forest plot of the sensitivity and specificity with the 95% confidence interval of the overall group (A) and each subgroup (B–D). CI = confidence interval, df = degrees of freedom, OR = odds ratio.
Figure 4.
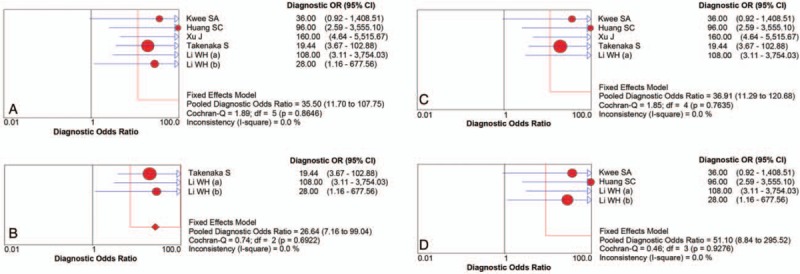
Forest plot of the DOR with the 95% confidence interval of the overall group (A) and each subgroup (B–D). df = degrees of freedom.
Figure 5.
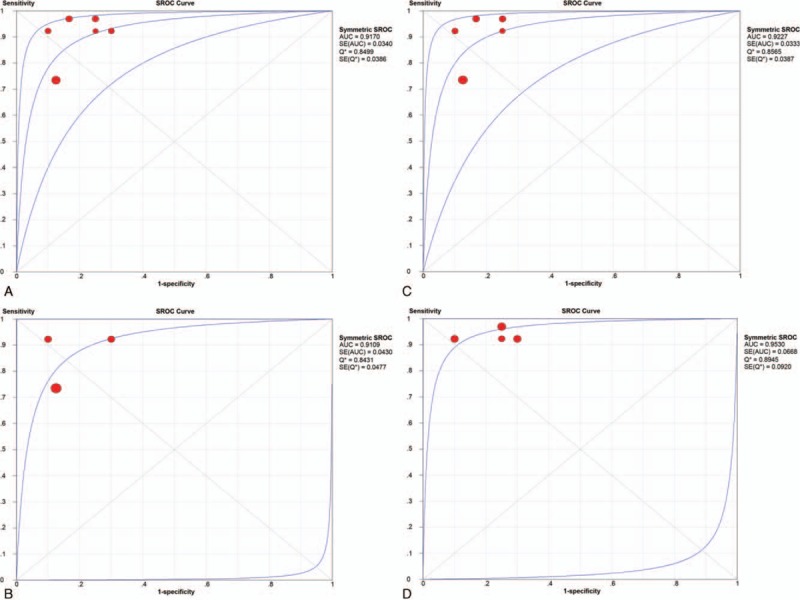
Summary receiver operating characteristic curve of the overall group (A) and each subgroup (B–D). AUC = area under the curve, SE = standard error.
3.2.2. Subgroup analysis
Although there was no heterogeneity, subgroup analysis with important clinical values was still conducted according to 3 parameters. Regarding to the HGG only, 3 studies containing 66 patients with 82 scans were included. The pooled SEN and SPE were: 0.79 (95% CI: 0.64, 0.89) and 0.83 (95% CI: 0.67, 0.94), respectively (Fig. 3B). The pooled LR+ and LR− were 5.20 (95% CI: 2.37, 11.44) and 0.22 (95% CI: 0.12, 0.42), respectively. The pooled DOR was 26.64 (95% CI: 7.17, 99.04, Fig. 4B) and the AUC was 0.9109 (95% CI: 0.8266, 0.9952, Fig. 5B).
Focusing on the semiquantitative analysis only, 5 studies containing 118 patients with 118 scans were included. The pooled SEN and SPE were: 0.86 (95% CI: 0.77, 0.93) and 0.85 (95% CI: 0.70, 0.94), respectively (Fig. 3C). The pooled LR+ and LR− were 5.45 (95% CI: 2.61, 11.38) and 0.17 (95% CI: 0.10, 0.31), respectively. The pooled DOR was 36.91 (95% CI: 11.29, 120.68, Fig. 4C) and the AUC was 0.9227 (95% CI: 0.8574, 0.9880, Fig. 5C).
Focusing on the patients only receiving RT, 4 studies containing 46 patients with 62 scans were included. The pooled SEN and SPE were: 0.94 (95% CI: 0.81, 0.99) and 0.79 (95% CI: 0.59, 0.92), respectively (Fig. 3D). The pooled LR+ and LR− were 4.23 (95% CI: 2.03, 8.81) and 0.09 (95% CI: 0.02, 0.34), respectively. The pooled DOR was 51.10 (95% CI: 8.84, 295.52, Fig. 4D) and the AUC was 0.9530 (95% CI: 0.8221, 1.0000, Fig. 5D). The results of the overall group and each subgroup were summarized in Table 3.
Table 3.
Subgroup analyses of 11C-choline PET for the differential diagnosis of glioma recurrence from radiation necrosis.
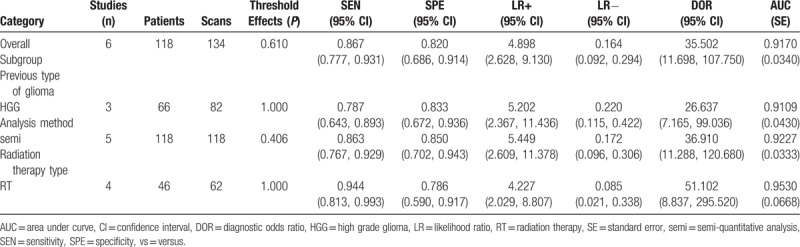
The diagnostic accuracy of each subgroup showed no statistical differences with that of the overall group. Subgroup analysis according to other parameters was ineligible because of insufficient numbers of studies despite of good clinical values.
3.3. Heterogeneity analysis
No heterogeneity was discovered in the summary analysis of the overall group and any of the subgroups with I2 of DOR all equaling to 0.0% (Fig. 4).
3.4. Publication bias
Deek funnel plot of the overall group and each subgroup showed no publication bias among enrolled studies (Fig. 6).
Figure 6.

Deek's funnel plot of publication bias of the overall group (A) and each subgroup (B–D), as determined by linear regression of the inverse root of effective sample sizes (ESS) on log diagnostic odds ratio.
4. Discussion
Glioma is the most common malignant brain tumor, occupying >60% of all primary brain tumors.[25] The first randomized trial in the 1970s proved that the postoperative 60 Gy whole-brain radiation therapies were beneficial for the survival.[26] RT in combination with synchronous and adjuvant chemotherapy with temozolomide after surgery has been proven to increase the survival rate of patients with glioma. Although it has become the first-line treatment for glioma,[2] the problem it brings about is radiation necrosis. Despite multifarious treatments for this disease, glioma still trends to recrudesce near or distally to the primary site, which may be visualized as newly enhanced lesions with surrounding edema on conventional MRI.[27]
Imaging plays a crucial part in diagnosing and following up patients with glioma. Differentiation between radiation necrosis and tumor relapse has been proved to be a particularly difficult diagnostic problem, which may affect the subsequent treatment plan. The patients with tumor recurrence may require reoperation, whereas the patients with radiation necrosis may only need symptomatic treatments. Both contrast-enhanced CT and MR show low performance in identifying radiation necrosis from tumor recurrence. The enhancement may appear from several days to a few months at the surgical position, or months delayed after RT, which makes the differentiation really difficult. 18F-FDG PET has been used to discriminate the relapse of glioma from radiation necrosis for >30 years.[10] However, 18F-FDG has inevitable limitations because of the high accumulation in normal gray matter[28]; Yang and Aghi et al[29] supposed that 18F-FDG PET has high FP or FN rate. Several PET tracers have been developed to diagnosis the glioma and other brain tumors other than 18F-FDG, which include 11C-methionine, 18F-FDOPA, 18F-fluoroethyltyrosine (FET), and 11C-tyrosine. These tracers all showed superior performance than 18F-FDG.[30–35] However, they also have some limitations. One of the most important problems is the uptake of these tracers in normal brain is relatively high, which causes low TNR and may obscure the uptake of tumor tissue.
Recently, 11C-choline has become a widely applied tracer for diagnosis of tumors, such as prostate cancer, hepatocellular cancer, head and neck, bone, soft tissue, and especially the brain tumors.[36] Tian et al[37] found 11C-choline PET shows high contrast between glioma and normal brain tissue, and the AUC was high, which indicated good diagnostic accuracy. Huang et al[38] reported 11C-choline PET has higher diagnostic accuracy than 18F-FDG PET and underlined 11C-choline PET was superior compared with 18F-FDG PET for the discovery of brain tumors. Tan et al[39] also reported that 11C-Choline PET had higher SEN and SPE and may be better in discriminating recurrent brain tumor from necrosis compared with F-18 FDG PET and MRI. An important application of 11C-choline PET is to distinguish between tumor recurrence and necrosis for the differential diagnosis of previously treated gliomas. The 11C-choline PET showed great value in discriminating glioma recurrence from radiation necrosis as reported in the included studies in our meta-analysis, possibly because of its metabolic characteristics.
Phosphatidylcholine is a chief phospholipid constituent of cell membranes in mammalian, which is synthesized from choline.[40] The activity of choline kinase in malignant tumor cells appears high, which leads to raised level of phosphorylcholine, an intermediate product involved in phospholipids synthesis.[41] The uptake of choline in the normal brain tissue is very low; however, the synthesis of cell membrane is obviously increased in glioma, which makes the uptake of choline become much higher in glioma than in normal and necrotic brain tissues. It is the possible reason why choline could be used as a measure to evaluate the cell proliferation. As reported by Hara et al[42] the standardized uptake values of 11C-choline in glioma ranged from 0.394 to 1.769 at 5 minutes after injection and from 0.444 to 1.942 at 20 minutes after injection, respectively; the standardized uptake values of 11C-choline in normal brain tissue ranged from 0.072 to 0.127 at 5 minutes after injection and from 0.074 to 0.151 at 20 minutes after injection, respectively. It has been pointed out that 11C-choline accumulates high in living glioma tissues, whereas low in natural brain tissues and necrotic glioma tissues; thus, it had a high TNR and could show the mass boundary distinctly.[39] Instead of other PET tracers, 11C-choline could give much higher TNR,[42] which could help to distinguish glioma recurrence more easily. Recurrent HGG or untreated primary HGG often has a clear display in 11C-choline PET and lack performance of reactive inflammatory.[43] Besides, the TNR of 11C-choline could evaluate the prognosis of patients with suspected glioma relapse. Patients with a lower TNR may enjoy a longer lifetime compared with those with a higher one. Similar conclusion was drawn by Santra et al in their prospective study.[44]
Up to now, there are few studies focusing on the accuracy of 11C-choline PET in differentiating glioma recurrence from the necrosis, and the synthetic research is still absent. Our meta-analysis included 5 articles with 6 studies containing 118 patients (134 scans), which summarized the diagnostic performance of 11C-choline PET in distinguishing glioma recurrence from radiation necrosis using pathology or clinical follow-up as criterion standard. The results of quantitative synthesis indicated that 11C-choline PET had a high diagnostic accuracy (AUC = 0.9170) independently of tumor grade, image analysis method, RT type, and other parameters. 11C-choline PET had preferable diagnostic SEN (0.87) and SPE (0.82), which implied low rate of missed diagnosis and misdiagnosis. DOR is another index reporting diagnostic accuracy that combines both SEN and SPE.[45] The pooled DOR for all the analysis was 35.50, which also showed the accuracy of 11C-choline PET in glioma differential diagnosis. No publication bias was observed according to Deek funnel plot, indicating statistical credibility of this study.
There were a small amount of meta-analyses considering the accuracy of different PET tracers for diagnosing primary/recurrent glioma and other brain tumors. Zhao et al reported that for the diagnosis of primary and recurrent brain tumors, the pooled SEN, pooled SPE, and AUC of 11C-methionine PET were 0.91, 0.86, and 0.94, respectively.[46] Nihashi et al[47] found that 11C-methionine PET had a summary SEN of 0.70 and SPE of 0.93 for distinguishing the recurrent HGG from necrosis. Considering the differential diagnosis between the recurrent glioma and the necrosis combined with pseudoprogression, our previous studies showed that the pooled SEN, SPE, and AUC of 11C-methionine PET were 0.88, 0.85, and 0.9352, respectively.[48] In addition, Yu et al[49] reported 18F-FDOPA and 18F-FET also showed good accuracy in diagnosing recurrent glioma from necrosis. Above all, these tracers all displayed excellent diagnostic accuracy. However, the inconsistencies in the inclusion criteria and research objectives, the few research numbers, the heterogeneity among studies, different types of tumor, and the incomplete data made the results of these studies of no comparability. The results of our study suggested that 11C-choline has high performance for the diagnosis of glioma recurrence from radiation necrosis, whereas it is hard to determine whether it is the best. Moreover, it should also be pointed out that one study included in our meta-analysis showed that 11C-methionine PET was superior to 11C-choline PET in distinguishing glioma recurrence from necrosis.[23] However, it is a retrospective study thus with low level of evidence and may partly contribute to the heterogeneity of our meta-analysis as well. As there is no meta-analysis comparing the diagnostic accuracy among 11C-choline and other PET tracers or the conventional diagnostic modalities, further research is needed.
In our study, although there was no heterogeneity in the overall group, subgroup analysis with important clinical values was still conducted according to 3 parameters. Most studies used 2 analysis methods to assess the diagnostic accuracy of 11C-choline PET in differentiating glioma recurrence, which included the semiquantitative analysis and the visual analysis. The semiquantitative analysis is based on an optimal cutoff value, which lets the diagnostic test has the best SEN and SPE as well as the biggest AUC, thus reducing the misdiagnosis and missed diagnosis. If the LNR or TNR is higher than the cutoff value, a diagnosis of glioma recurrence is made; otherwise, the necrosis is determined. The visual analysis depends more on the experience of the radiologist, whereas no cutoff value is set. Most studies thought the diagnostic accuracy of semiquantitative analysis was higher than that of the visual analysis in diagnosing glioma recurrence. Focusing on the grading of glioma, HGG shows obviously different biological characteristics compared with LGG. HGG is a type of highly malignant tumor and expands rapidly, which makes it always form necrosis in the center of the tumor spontaneously or after RT, whereas the tumor tissue alive is at the border of the tumor. On the contrary, LGG grows slowly and seldom forms necrosis very different from HGG. Two types of RT were most commonly applied to glioma post operation, which are RT and SRT. The shape of tumor is not fully consistent with the radiation field of RT, which causes much normal brain tissue to be exposed to radiation. SRT overcome this short come. It uses lots of radioactive sources distributed along the sphere, which focuses on the target area. It can provide bigger radiation dosage to the tumor, whereas lower impact on normal brain tissues around, which causes more necrosis in the tumor. SRT is a more effective kind of RT in treating glioma compared with RT. Because of limited number of studies, we only extracted the data of one subgroup according to each parameter; thus, the comparison among different subgroups referring to each parameter could not be conducted.
In addition, it should be pointed out that despite high diagnostic accuracy and effectiveness of 11C-choline PET in identifying glioma relapse from radiation necrosis, it could still cause FP and FN, which should be remembered when analyzing the results of 11C-choline PET.[37]
5. Limitations
There were also some limitations in our study. First, only 6 studies met our criterions and were included in our analysis, making our results lack powerful support from larger samples. Second, despite no publication bias in the overall analysis, only English and Chinese publications with full text were enrolled in this meta-analysis, which might lead to a few appropriate articles unpublished or published in other languages being missed. That indicates potential existence of publication bias.
6. Conclusions
This meta-analysis indicated 11C-choline PET has high diagnostic accuracy in the confirmation of glioma recurrence from radiation-induced necrosis in glioma independent of tumor grade, image analysis method, RT type, and other parameters. Additionally, more multicenter trials studies involving a larger number of patients concerning the application of 11C-choline PET in glioma diagnosis are needed to be performed.
Author contributions
This meta-analysis was designed by Liansheng Gao and Gao Chen, data collection and analysis were conducted by Weilin Xu, Tao Li and Jingwei Zheng, this article was checked by Gao Chen. This article was written by Liansheng Gao, Weilin Xu and Tao Li.
Conceptualization: Liansheng Gao, Weilin Xu, Gao Chen.
Data curation: Liansheng Gao, Tao Li.
Formal analysis: Weilin Xu, Jingwei Zheng.
Investigation: Jingwei Zheng.
Methodology: Liansheng Gao, Jingwei Zheng.
Resources: Tao Li.
Software: Weilin Xu.
Supervision: Gao Chen.
Writing – original draft: Liansheng Gao, Weilin Xu, Tao Li.
Writing – review & editing: Gao Chen.
Supplementary Material
Footnotes
Abbreviations: 18F-FDGPET = 18F-fluorodeoxyglucosepositron emission tomography, AUC = area under the curve, CI = confidence interval, CT = computed tomography, DOR = diagnostic odds ratio, ESS = effective sample sizes, FN = false negative, FP = false positive, HGG = high-grade glioma, LGG = low-grade glioma, LNR = lesion/normal ratio, LR− = negative likelihood, LR+ = positive likelihood, MRI = magnetic resonance imaging, PET = positron emission tomography, QUADAS-2 = Quality Assessment Tool for Diagnostic Accuracy Studies version 2, RT = radiation therapy, semi = semi-quantitative, SEN = sensitivity, SPE = specificity, SROC = summary receiver-operating characteristic curve, SRT = stereotactic radiation therapy, TN = and true negative, TNR = tumor/normal ratio, TP = true positive.
LG, WX and TL have equally contributed to this work as co-first authors.
This work was supported by the National Natural Science Foundation of China (No. 81400951, No. 81771246).
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;359:492–507. [DOI] [PubMed] [Google Scholar]
- [2].Skvortsova TY, Brodskaya ZL, Gurchin AF. [PET using 11C-methionine in recognition of pseudoprogression in cerebral glioma after combined treatment]. Zh Vopr Neirokhir Im N N Burdenko 2014;78:50–8. [PubMed] [Google Scholar]
- [3].Hoffman JM. New advances in brain tumor imaging. Curr Opin Oncol 2001;13:148–53. [DOI] [PubMed] [Google Scholar]
- [4].Hein PA, Eskey CJ, Dunn JF, et al. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 2004;25:201–9. [PMC free article] [PubMed] [Google Scholar]
- [5].Sundgren PC, Fan X, Weybright P, et al. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magnetic resonance imaging 2006;24:1131–42. [DOI] [PubMed] [Google Scholar]
- [6].Matsusue E, Fink JR, Rockhill JK, et al. Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology 2010;52:297–306. [DOI] [PubMed] [Google Scholar]
- [7].Rock JP, Scarpace L, Hearshen D, et al. Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery 2004;54:1111–7. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- [8].Sonoda Y, Kumabe T, Takahashi T, et al. Clinical usefulness of 11C-MET PET and 201T1 SPECT for differentiation of recurrent glioma from radiation necrosis. Neurol Med Chir 1998;38:342–7. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- [9].Ogawa T, Kanno I, Shishido F, et al. Clinical value of PET with 18F-fluorodeoxyglucose and L-methyl-11C-methionine for diagnosis of recurrent brain tumor and radiation injury. Acta Radiol 1991;32:197–202. [PubMed] [Google Scholar]
- [10].Patronas NJ, Di Chiro G, Brooks RA, et al. Work in progress: [18F] fluorodeoxyglucose and positron emission tomography in the evaluation of radiation necrosis of the brain. Radiology 1982;144:885–9. [DOI] [PubMed] [Google Scholar]
- [11].Singhal T, Narayanan TK, Jain V, et al. 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol Imaging Biol 2008;10:1–8. [DOI] [PubMed] [Google Scholar]
- [12].Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 2008;49:694–9. [DOI] [PubMed] [Google Scholar]
- [13].Tsuyuguchi N, Takami T, Sunada I, et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery—in malignant glioma. Ann Nucl Med 2004;18:291–6. [DOI] [PubMed] [Google Scholar]
- [14].Ridgway ND. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit Rev Biochem Mol biol 2013;48:20–38. [DOI] [PubMed] [Google Scholar]
- [15].Kato T, Shinoda J, Nakayama N, et al. Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose, and 11C-choline positron-emission tomography. AJNR Am J Neuroradiol 2008;29:1176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ohtani T, Kurihara H, Ishiuchi S, et al. Brain tumour imaging with carbon-11 choline: comparison with FDG PET and gadolinium-enhanced MR imaging. Eur J Nucl Med 2001;28:1664–70. [DOI] [PubMed] [Google Scholar]
- [17].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [18].Deville WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2002;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huang SC, Qin CJ, Ma JQ, et al. Diagnostic value of 11C-choline PET/CT in detection of the remnant or relapse tumor after operation in patients with cerebral gliomas. Guangxi Med J 2008;30:15–7. [Google Scholar]
- [21].Kwee SA, Ko JP, Jiang CS, et al. Solitary brain lesions enhancing at MR imaging: evaluation with fluorine 18 fluorocholine PET. Radiology 2007;244:557–65. [DOI] [PubMed] [Google Scholar]
- [22].Li W, Ma L, Wang X, et al. (11)C-choline PET/CT tumor recurrence detection and survival prediction in post-treatment patients with high-grade gliomas. Tumour Biol 2014;35:12353–60. [DOI] [PubMed] [Google Scholar]
- [23].Takenaka S, Asano Y, Shinoda J, et al. Comparison of (11)C-methionine, (11)C-choline, and (18)F-fluorodeoxyglucose-PET for distinguishing glioma recurrence from radiation necrosis. Neurol Med Chir 2014;54:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xu J, Liu ZF, Zhu YF, et al. The application of 11C-choline PET/CT in diagnosing glioma residual or recurrence after treatment. Shandong Med 2010;50:83–4. [Google Scholar]
- [25].Ullrich RT, Kracht L, Brunn A, et al. Methyl-L-11C-methionine PET as a diagnostic marker for malignant progression in patients with glioma. J Nucl Med 2009;50:1962–8. [DOI] [PubMed] [Google Scholar]
- [26].Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg 1978;49:333–43. [DOI] [PubMed] [Google Scholar]
- [27].Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000;217:377–84. [DOI] [PubMed] [Google Scholar]
- [28].Gulyas B, Halldin C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl Med Mol Imag 2012;56:173–90. [PubMed] [Google Scholar]
- [29].Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol 2009;6:648–57. [DOI] [PubMed] [Google Scholar]
- [30].Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 2006;47:904–11. [PubMed] [Google Scholar]
- [31].Karunanithi S, Sharma P, Kumar A, et al. 18F-FDOPA PET/CT for detection of recurrence in patients with glioma: prospective comparison with 18F-FDG PET/CT. Eur J Nucl Med Mol Imag 2013;40:1025–35. [DOI] [PubMed] [Google Scholar]
- [32].Minamimoto R, Saginoya T, Kondo C, et al. Differentiation of brain tumor recurrence from post-radiotherapy necrosis with 11C-methionine PET: visual assessment versus quantitative assessment. PLoS One 2015;10:e0132515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pruim J, Willemsen AT, Molenaar WM, et al. Brain tumors: L-[1-C-11]tyrosine PET for visualization and quantification of protein synthesis rate. Radiology 1995;197:221–6. [DOI] [PubMed] [Google Scholar]
- [34].Sharma R, D'Souza M, Jaimini A, et al. A comparison study of (11)C-methionine and (18)F-fluorodeoxyglucose positron emission tomography-computed tomography scans in evaluation of patients with recurrent brain tumors. Indian J Nucl Med 2016;31:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Weber WA, Wester HJ, Grosu AL, et al. O-(2-[18F]fluoroethyl)-L-tyrosine and L-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med 2000;27:542–9. [DOI] [PubMed] [Google Scholar]
- [36].Kirienko M, Sollini M, Lopci E, et al. Applications of PET imaging with radiolabelled choline (11C/18F-choline). Q J Nucl Med Mol Imag 2015;59:83–94. [PubMed] [Google Scholar]
- [37].Tian M, Zhang H, Oriuchi N, et al. Comparison of 11C-choline PET and FDG PET for the differential diagnosis of malignant tumors. Eur J Nucl Med Mol Imag 2004;31:1064–72. [DOI] [PubMed] [Google Scholar]
- [38].Huang Z, Zuo C, Guan Y, et al. Misdiagnoses of 11C-choline combined with 18F-FDG PET imaging in brain tumours. Nucl Med Commun 2008;29:354–8. [DOI] [PubMed] [Google Scholar]
- [39].Tan H, Chen L, Guan Y, et al. Comparison of MRI, F-18 FDG, and 11C-choline PET/CT for their potentials in differentiating brain tumor recurrence from brain tumor necrosis following radiotherapy. Clin Nucl Med 2011;36:978–81. [DOI] [PubMed] [Google Scholar]
- [40].Fagone P, Jackowski S. Phosphatidylcholine and the CDP-choline cycle. Biochim Biophys Acta 2013;1831:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ramirez de Molina A, Rodriguez-Gonzalez A, Gutierrez R, et al. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun 2002;296:580–3. [DOI] [PubMed] [Google Scholar]
- [42].Hara T, Kondo T, Hara T, et al. Use of 18F-choline and 11C-choline as contrast agents in positron emission tomography imaging-guided stereotactic biopsy sampling of gliomas. J Neurosurg 2003;99:474–9. [DOI] [PubMed] [Google Scholar]
- [43].Hara T. 11C-choline and 2-deoxy-2-[18F]fluoro-D-glucose in tumor imaging with positron emission tomography. Mol Imaging Biol 2002;4:267–73. [DOI] [PubMed] [Google Scholar]
- [44].Santra A, Kumar R, Sharma P, et al. F-18 FDG PET-CT for predicting survival in patients with recurrent glioma: a prospective study. Neuroradiology 2011;53:1017–24. [DOI] [PubMed] [Google Scholar]
- [45].Nakajima T, Kumabe T, Kanamori M, et al. Differential diagnosis between radiation necrosis and glioma progression using sequential proton magnetic resonance spectroscopy and methionine positron emission tomography. Neurol Med Chir 2009;49:394–401. [DOI] [PubMed] [Google Scholar]
- [46].Zhao C, Zhang Y, Wang J. A meta-analysis on the diagnostic performance of (18)F-FDG and (11)C-methionine PET for differentiating brain tumors. AJNR Am J Neuroradiol 2014;35:1058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nihashi T, Dahabreh IJ, Terasawa T. Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am J Neuroradiol 2013;34:944–50. S1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xu W, Gao L, Shao A, et al. The performance of 11C-Methionine PET in the differential diagnosis of glioma recurrence. Oncotarget 2017;8:91030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yu J, Zheng J, Xu W, et al. Accuracy of (18)F-FDOPA positron emission tomography and (18)F-FET positron emission tomography for differentiating radiation necrosis from brain tumor recurrence. World Neurosurg 2018;114:e1211–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


