Abstract
Background and objectives
The prevalence of ESKD is increasing worldwide. Treating ESKD is disproportionately costly in comparison with its prevalence, mostly due to the direct cost of dialysis therapy. Here, we aim to provide a contemporary cost description of dialysis modalities, including facility-based hemodialysis, peritoneal dialysis, and home hemodialysis, provided with conventional dialysis machines and the NxStage System One.
Design, setting, participants, & measurements
We constructed a cost-minimization model from the perspective of the Canadian single-payer health care system including all costs related to dialysis care. The labor component of costs consisted of a breakdown of activity-based per patient direct labor requirements. Other costs were taken from statements of operations for the kidney program at Seven Oaks General Hospital (Winnipeg, Canada). All costs are reported in Canadian dollars.
Results
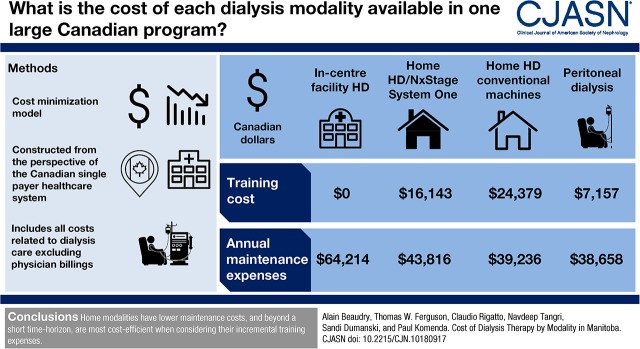
Annual maintenance expenses were estimated as $64,214 for in-center facility hemodialysis, $43,816 for home hemodialysis with the NxStage System One, $39,236 for home hemodialysis with conventional dialysis machines, and $38,658 for peritoneal dialysis. Training costs for in-center facility hemodialysis, home hemodialysis with the NxStage System One, home hemodialysis with conventional dialysis machines, and peritoneal dialysis are estimated as $0, $16,143, $24,379, and $7157, respectively. The threshold point to achieve cost neutrality was determined to be 9.7 months from in-center hemodialysis to home hemodialysis with the NxStage System One, 12.6 months from in-center hemodialysis to home hemodialysis with conventional dialysis machines, and 3.2 months from in-center hemodialysis to peritoneal dialysis.
Conclusions
Home modalities have lower maintenance costs, and beyond a short time horizon, they are most cost efficient when considering their incremental training expenses.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2018_07_18_CJASNPodcast_18_8_F.mp3
Keywords: chronic dialysis; chronic hemodialysis; clinical epidemiology; dialysis; Economic Analysis; Economic Impact; Epidemiology and outcomes; hemodialysis; renal dialysis; Prevalence; Hospitals, General; Kidney Failure, Chronic; peritoneal dialysis; Kidneys, Artificial; Cost of Illness
Introduction
The prevalence of ESKD is expected to double between 2010 and 2030 (1,2). This increase seems to be driven by an aging population combined with increasing rates of diabetes mellitus and hypertension (3–5). Although kidney transplantation is the preferred treatment for ESKD (6–8), the current supply of donor organs is insufficient. Furthermore, because of comorbid conditions and frailty, the aging end stage kidney failure population may be less suitable for kidney transplantation and derive less overall benefit (9,10). For these reasons, the majority of patients with end stage kidney failure are treated with dialysis therapy.
The cost of providing dialysis treatment for patients with end stage kidney failure is substantial, totaling over $1.8 billion each year in Canada (6) and accounting for 7.2% of total Medicare claims in the United States (totaling $32.8 billion) (11), and it makes up 2% of national health care budgets across Europe (12). Most patients requiring dialysis therapy are treated with thrice weekly in-center hemodialysis (HD) (11,13–15). Emerging literature, however, suggests that the priorities of patients with end stage kidney failure are primarily related to quality of life, which has contributed to home dialysis modalities becoming increasingly appealing options for patients with end stage kidney failure (16–22). The increasing prevalence of end stage kidney failure and dialysis alongside the financial burden of dialysis care necessitates a thorough, contemporary examination of the cost of dialysis modalities, including costs of home HD programs, which have been increasing in size in recent years (13).
This study aims to provide a description of the costs of each dialysis modality available to patients with kidney failure in one large Canadian program that provided dialysis care for over 290 patients on in-center HD, over 90 patients on peritoneal dialysis (PD), and over 45 patients on home HD (with both conventional dialysis machines and the NxStage System One) in 2016. A cost-minimization approach is used, and it can be used to estimate costs of other programs and inform the selection of modality and the time to payoff for investments in patient training.
Materials and Methods
The presented cost-minimization model is constructed from the perspective of the Canadian single-payer health care system and includes all costs related to dialysis care and management (direct labor, supplies, equipment, utilities, drugs related to dialysis, overhead, training, and capital costs), excluding physician billings due to heterogeneity of billing models (e.g., capitation versus salary versus fee for service). Results were presented as real 2016 Canadian dollars (CAD) and on a per patient, per year basis. An overview of the demographics of patients on dialysis in Manitoba is available in a previously published report (23). Because only aggregate cost estimates, publicly available information, or previously published figures were used in this analysis, we did not seek study approval from a research ethics board.
The presented model comprises three summed components for each modality: a labor component, a consumables component (including dialysis-related drugs), and a capital costs component. Each is expressed in a per capita format and may, therefore, be scaled appropriately. The model presented here provides a blended annual maintenance cost that accounts for a mix of dosing regimens for patients on HD (frequency of dialysis distributed as three times per week, 3%; four times per week, 35%; five times per week, 54%; and six times per week, 8%) and the divide between continuous ambulatory PD and continuous cycling PD (22% continuous ambulatory PD and 78% continuous cycling PD), and as such, it will represent the average expected cost of a patient in a given modality and allows for movement between dosing regimens during annual maintenance.
The labor component of the model consists of a granular breakdown of estimated per patient direct labor requirements. Specific activities analyzed include pharmacy, dietician services, clerking, nursing, social work, and equipment maintenance by a staff technician. Estimates of direct time expenditures by activity were generated using quantitative and qualitative analyses of a previously validated costing model by the British Columbia Renal Agency (24), a program with a home dialysis program similar in size to Manitoba. Time requirements for these resources are recognized as variable when a patient is training for a given modality compared with during their maintenance period, and therefore, training time is estimated independently. Time spent on indirect care of patients (e.g., administrative and organizational tasks) is estimated as a linear function of direct care—relative ratios are extrapolated as they relate to home HD and generalized to other modalities, which yield estimates of indirect labor requirements per specialty (24) (Supplemental Table 1). Summed direct and indirect labor requirements for each specialty are then multiplied by region-specific wage data from the Manitoba Renal Program to arrive at a total expenditure per patient in our center. Benefits, relief hours, sick time, and vacation were assessed as 41.6% of total direct human resources expenditures on the basis of what is observed at the Manitoba Renal Program. Labor estimates generated by the activity-based costing estimates used in the model were validated against actual human resources expenditures in statements of operations from the Manitoba Renal Program.
Cost estimates for consumables, equipment, sundry, and drugs used in the model were taken directly from historical statements of operations for the Renal Program at Seven Oaks General Hospital in Winnipeg, Canada (under direct management of the Manitoba Renal Program) (25). Seven Oaks General Hospital provides dialysis care for over 290 patients on in-center HD, over 90 patients on PD, and over 45 patients on home HD as of the end of 2016. Primary costs of NxStage System One supplies were sourced from consultation with suppliers. Where utilities are concerned, unit costs are drawn from current (2016–2017) rates applicable to Winnipeg, Manitoba, Canada (26,27), and estimates of utility usage for large community hospitals were assumed (28). Note that some centers choose to use home monitoring techniques for their clients—these are not typically used in the jurisdiction described here, and therefore, applicable costs have not been included. Capital expenditures are derived directly from capital cost estimates of units constructed within the Winnipeg Regional Health Authority, and they are taken to represent costs related to depreciation of existing capital for dialysis units assuming a 30-year useful life. Lastly, we assumed that patients on home modalities would have an average of 11 in-center dialysis runs per year in accordance with a previous analysis (29).
Labor costs were modeled separately for both maintenance therapy and training a patient to perform a home modality. In the case of home HD with the NxStage System One, because of a simpler and shorter training regimen, patients were assumed to require 61% of the nursing hours needed to train a patient with conventional dialysis machines on the basis of training data sourced from the home HD unit at Seven Oaks General Hospital.
To establish model validity, labor cost assumptions derived using activity-based costing estimates were compared with actual outputs by the Manitoba Renal Program. Human resources expenditures observed in statements of operations for in-center facility HD and PD were within 5% of estimates produced by the activity-based labor component of the model.
Cost minimization for HD is achieved by overlaying the three HD modality cost functions and selecting the lowest-cost alternative given a certain probable time on dialysis therapy. In this sense, the intercept of the post-training and installation linear relationship of cost over time is represented by the upfront costs of establishing a patient on a home modality, and the post-training slope is determined by the expected monthly costs of each respective HD modality. Sensitivity analyses on threshold points for cost savings were performed by varying the cost of facility HD by ±25% in the baseline scenario.
Results
Model outputs included training costs, maintenance therapy costs, and points of efficient modality change. Annual maintenance expenses are estimated as $64,214 CAD for in-center facility HD, $43,816 CAD for home HD with the NxStage System One, $39,236 CAD for home HD with conventional dialysis machines, and $38,658 CAD for PD (Table 1). Primary cost drivers shift from human resources in facility HD (68% of total costs for facility HD versus 12%–16% at home) to supplies in home modalities (45%–72% of total costs at home versus 13% for facility HD). Training costs for in-center facility HD, home HD with the NxStage System One, home HD with conventional dialysis machines, and PD are estimated as $0, $16,143 CAD, $24,379 CAD, and $7157 CAD, respectively (Table 2).
Table 1.
Annual per patient cost of dialysis maintenance therapy by modality in Manitoba, Canada (2016 Canadian dollars)
| Cost | Facility Hemodialysis, $ | Peritoneal Dialysis, $ | Home Hemodialysis Conventional, $ | Home Hemodialysis NxStage, $ |
|---|---|---|---|---|
| Human resources (direct) | ||||
| Registered nurse | 18,499 | 1825 | 1048 | 1048 |
| Unit clerk | 1099 | 279 | 254 | 254 |
| Licensed practical nurse | 9072 | — | — | — |
| Dietician | 647 | 446 | 535 | 535 |
| Dialysis technician | 609 | — | 1834 | 1834 |
| Clinical pharmacist | 405 | 310 | 822 | 822 |
| Social worker | 443 | 295 | 369 | 369 |
| Total human resources | 30,773 | 3155 | 4862 | 4862 |
| Benefits | 6770.13 | 694.19 | 1069.65 | 1069.65 |
| Vacation and relief | 6037.73 | 619.09 | 953.94 | 953.94 |
| Supplies—medical, surgical, and laboratory | 7406 | 27,551 | 14,727 | 20,083 |
| Supplies—other (e.g., housekeeping, maintenance) | 791 | 315 | 2745 | 2745 |
| Drug expenses | 5669 | 3056 | 3007 | 3007 |
| Equipment expenses | 548 | — | 4488 | 3689 |
| Departmental sundry/miscellaneous | 136 | 161 | 135 | 158 |
| Hospital utilities/overhead (electricity/heat) | 204 | 73 | 73 | 73 |
| Water | 404 | — | — | — |
| Capital cost | 5475 | 3033 | 3033 | 3033 |
| In-center runs | — | — | 4142 | 4142 |
| Total | 64,214 | 38,658 | 39,236 | 43,816 |
—, not applicable.
Table 2.
Training cost overview by modality in Manitoba, Canada (2016 Canadian dollars)
| Facility Hemodialysis, $ | Peritoneal Dialysis, $ | Home Hemodialysis Conventional, $ | Home Hemodialysis NxStage, $ | |
|---|---|---|---|---|
| Human resources (direct) | ||||
| Registered nurse | — | 4538 | 14,375 | 8769 |
| Clerk | — | 237 | 42 | 42 |
| Licensed practical nurse | — | — | — | — |
| Dietician | — | 211 | 169 | 169 |
| Dialysis technician | — | — | 2265 | 2055 |
| Clinical pharmacist | — | 68 | 68 | 68 |
| Social worker | — | — | 296 | 296 |
| Total human resources | — | 5054 | 17,215 | 11,399 |
| Benefits | — | 1111.80 | 3787.19 | 2553.82 |
| Relief hours and vacation | — | 991.52 | 3377.49 | 2277.54 |
| Total | — | 7157 | 24,379 | 16,143 |
—, not applicable.
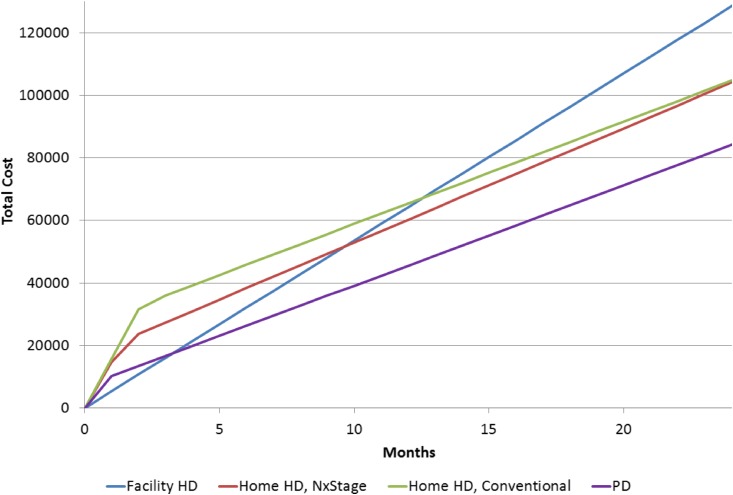
Threshold points to achieve cost savings for one modality versus another were calculated as 9.7 months from in-center HD to home HD with the NxStage System One, 12.6 months from in-center HD to home HD with conventional dialysis machines, and 3.2 months from in-center HD to PD. Sensitivity analysis suggests that the transition between in-center HD and home HD with the NxStage System One is of moderate elasticity. If we assumed a 25% lower cost of facility HD, the maximum threshold between in-center HD and home HD with NxStage System One is 45.3 months to reach the breakeven threshold; it is 35.2 months for conventional home HD and 8.7 months for PD (Figure 1, Table 3).
Figure 1.
Home hemodialysis provided with the NxStage machine results in cost savings versus home hemodialysis provided with a conventional machine if treatment duration is <25.6 months. HD, hemodialysis; PD, peritoneal dialysis.
Table 3.
Months on therapy required to achieve cost savings
| Evaluated Therapy | Comparator Therapy | Months Required for Cost Savings | Sensitivity Analysis (25% Lower Cost of Facility HD) | Sensitivity Analysis (25% Higher Cost of Facility HD) |
|---|---|---|---|---|
| Home HD (NxStage) | Facility HD | 9.7 | 45.3 | 5.4 |
| Home HD (conventional) | Facility HD | 12.6 | 35.2 | 7.7 |
| PD | Facility HD | 3.2 | 8.7 | 2.0 |
| Home HD (conventional) | Home HD (NxStage) | 25.6 | N/A | N/A |
HD, hemodialysis; PD, peritoneal dialysis; N/A, not applicable.
The transition between NxStage System One and conventional home HD is sensitive to variation in relative training costs. This is due primarily to the similar annual maintenance costs between conventional and NxStage System One modalities. From a strictly financial perspective, these modalities are similar, favoring home HD with NxStage System One if dialysis treatment duration is expected to be <25.6 months.
Discussion
This cost-minimization analysis provides a comprehensive examination of the training and annual maintenance costs of each dialysis modality available to patients with end stage kidney failure in Canada and a tool with which they might be compared. In keeping with previous cost analyses (30–32), this study affirms a considerable cost saving related to home dialysis modalities over in-center HD provided that a sufficiently long period of dialysis is expected. Building on these previous analyses, we compare the costs of the NxStage System One with other available dialysis modalities.
The model also provides a specific minimum period over which the initial cost of training a patient may be recovered, and this period may be determined using specific regional characteristics. Lastly, a contemporary reanalysis of the required costs of dialysis therapy can help provide accurate assessments of costs to the health care payer in the current marketplace, accounting for temporal changes in prices for dialysis-related consumables (e.g., PD supplies and erythropoietin) as well as the expanding size of many home HD programs.
Selecting a modality on the basis of a patient’s estimated time on dialysis allows for treatment costs to be minimized. Regarding HD modalities, patients with the shortest expected duration of treatment should be considered for in-center HD, whereas placing those with the longest expected duration of treatment on conventional home HD will minimize costs. An intermediate expected duration should be optimally addressed using the NxStage System One, which although costing 36% more for consumables, has a 39% lower cost associated with training.
There are multiple different modalities for home dialysis, and in each modality, there are differing platforms. Each of these is characterized by a different spectrum of advantages and disadvantages. For example, patients with space constraints may prefer a modality that minimizes or avoids modifications to the home, such as home water filtration systems or space requirements for storing dialysis-related supplies. In addition, there may be financial, electrical, water supply, plumbing, or waste disposal concerns that influence modality choice (33). Further consideration should also be given to the technical knowledge required for using and maintaining the dialysis machine, wherein a tradeoff of cost for ease of use could be considered reasonable if it can contribute to patient uptake or retention (34). Relative ease of shipping supplies for patients may also be important, especially with respect to remote locations with expensive logistics requirements (35). Ultimately, these factors and others should help guide the decision to recommend patients for home therapy and the specific choice of modality.
Consideration must also be given to the clinical and cost implications of switching patients from one dialysis modality to another. Unfortunately, because of the difficulty of conducting a randomized, controlled trial comparing self-care home dialysis with full-care in-center dialysis, much of the evidence for comparative effectiveness relies on observational studies that have a high risk of selection bias. With the inability to completely rule out residual confounding, these findings should be interpreted cautiously, and per capita costs associated with shifting a part of the patient population from one modality to another may indeed differ from the existing cohort given varying baseline characteristics. Notwithstanding, after adjustment for patient characteristics, many studies have found no higher risk of mortality among patients receiving home dialysis (PD or HD) in comparison with in-center HD (36–38). In addition, evidence suggests that there is no additional risk of all-cause hospitalization in the home dialysis group (39). The risk of technique failure is also important, because it is associated with adverse outcomes and increased costs, and it can result in a cost-ineffective treatment choice if a patient does not remain on a home therapy long enough to justify the cost of training and setup (40).
Program size may also be an important factor to consider in evaluating the costs of home HD programs. The prevalence of home HD in Canada totaled 4.8% in 2014, having almost doubled since 2005 (13). Because many home dialysis programs are still in their early stages and may not have achieved optimal operational efficiency, consideration of how fixed costs adapt to changing program sizes for home programs would be prudent. Moreover, in larger dialysis programs, it may seem more favorable to introduce home HD programs, because economies of scale could be achieved quicker and the startup costs of a program would represent a smaller fraction of overall capital costs (41). Data provided by this model should be interpreted with this in mind, and the reader should recognize that newer and/or smaller programs may experience some per capita cost differences compared with those presented herein.
Many patients are not suitable to perform full self-care dialysis in the home, and as such, consideration of introducing fully or partially assisted home PD and home HD programs may be a cost-saving strategy, particularly with an aging dialysis population. However, the preferred method of administering these programs is unclear; some care models may prefer the use of registered nurses for more complex patients, whereas other models could be considered for patients with simpler cases, providing opportunity for improved resource use (42). It may also be worth evaluating the potential synergies of supporting assisted dialysis resources in other areas of the health system that interact with a relatively high number of patients on dialysis, such as long-term care or personal care home services (43). Several studies have considered the potential benefits of these assisted dialysis programs (43,44), but a more thorough analysis of the costs of various assisted home dialysis staffing models is warranted.
Our model has many strengths. First, the activity-based human resources model is on the basis of figures generated from actual program expenditures, and the estimated expenditures are validated against data from a different region and time period. This strongly supports the model’s generalizability to other publically funded jurisdictions, especially in Canada and likely in other publicly administered systems, such as Australia and the United Kingdom. Second, all equipment and supply costs represent actual expenditures provided by statements of operations from the Manitoba Renal Program and accurately reflect the differences in supply requirements between dialysis departments.
The most important limitation of the model is a product of its perspective. In considering costs applicable to the public payer, those costs borne by the patients and their caregivers are not factored into this model. The estimates provided account for public health care costs related specifically to dialysis therapy directly and do not account for the total cost of caring for these patients; they exclude transportation, caregiver costs or assistance with activities of daily living, access costs, patient opportunity costs, and other items. Access costs maybe higher with more frequent dialysis, regardless of whether it is delivered in center or at home, and daily dialysis prescriptions could increase these costs preferentially for home modalities (45). Heterogeneity should also be recognized between programs: data used to estimate consumables costs are generated from the Manitoba Renal Program, and the reader is reminded that the unit costs and quantities may vary between institutions on the basis of local supplier arrangements and patient mix. As well, differences may exist between modalities for items not accounted for in the model, such as vascular and PD access costs. In addition, although capital costs in the presented model are easily adjustable to local costing using the provided tools, this accommodates for differences in construction costs for comparable units and does not accommodate for units with differing forms or standards. Lastly, validation of costs determined with the activity-based model against the home HD program was difficult to interpret due to a recent trend of transferring patients between conventional and NxStage System One machines, incurring atypical human resource expenses for training and education.
In summary, all forms of dialysis are associated with substantial use of health care resources. Home modalities (home HD and PD) have lower overall maintenance costs, and beyond a short time horizon, they are more cost efficient when including incremental training expenses.
Disclosures
P.K. is a member of the scientific advisory board for NxStage Medical Inc. Financial support for this study was provided by NxStage Medical Inc. Analysis was performed independently. NxStage Medical Inc. did not have any influence over analysis or conclusions drawn in this manuscript.
Supplementary Material
Acknowledgments
Financial support for this study was provided by NxStage Medical Inc.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10180917/-/DCSupplemental.
References
- 1.Eggers PW: Has the incidence of end-stage renal disease in the USA and other countries stabilized? Curr Opin Nephrol Hypertens 20: 241–245, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V: Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 385: 1975–1982, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Lutz W, Sanderson W, Scherbov S: The coming acceleration of global population ageing. Nature 451: 716–719, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J: Global burden of hypertension: Analysis of worldwide data. Lancet 365: 217–223, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Klarenbach SW, Tonelli M, Chui B, Manns BJ: Economic evaluation of dialysis therapies. Nat Rev Nephrol 10: 644–652, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N: A study of the quality of life and cost-utility of renal transplantation. Kidney Int 50: 235–242, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Concepcion BP, Forbes RC, Schaefer HM: Older candidates for kidney transplantation: Who to refer and what to expect? World J Transplant 6: 650–657, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wynn JJ, Alexander CE: Increasing organ donation and transplantation: The U.S. experience over the past decade. Transpl Int 24: 324–332, 2011 [DOI] [PubMed] [Google Scholar]
- 11.United States Renal Data System: 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, 2016. Available at: https://www.usrds.org/adr.aspx. Accessed September 7, 2017
- 12.European Kidney Health Alliance: Policy Options for Kidney Health in Europe, 2017. Available at: http://ekha.eu/. Accessed September 7, 2017
- 13.Canadian Institute for Health Information: Canadian Organ Replacement Register Annual Report, 2017. Available at: https://www.cihi.ca/en/corr-annual-statistics-2007-to-2016. Accessed January 5, 2018
- 14.ANZDATA Registry: Prevalence of End Stage Kidney Disease ANZDATA Registry, 2017. Available at: http://www.anzdata.org.au/v1/report_2016.html. Accessed September 7, 2017 [Google Scholar]
- 15.Caskey F, Cullen R: UK renal registry 18th annual report: Introduction. Nephron 132: 1–8, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Walker RC, Howard K, Morton RL: Home hemodialysis: A comprehensive review of patient-centered and economic considerations. Clinicoecon Outcomes Res 9: 149–161, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker RC, Howard K, Morton RL, Palmer SC, Marshall MR, Tong A: Patient and caregiver values, beliefs and experiences when considering home dialysis as a treatment option: A semi-structured interview study. Nephrol Dial Transplant 31: 133–141, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Tong A, Crowe S, Chando S, Cass A, Chadban SJ, Chapman JR, Gallagher M, Hawley CM, Hill S, Howard K, Johnson DW, Kerr PG, McKenzie A, Parker D, Perkovic V, Polkinghorne KR, Pollock C, Strippoli GF, Tugwell P, Walker RG, Webster AC, Wong G, Craig JC: Research priorities in CKD: Report of a national workshop conducted in Australia. Am J Kidney Dis 66: 212–222, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Tonelli M, Molzahn AE, Wiebe N, Davison SN, Gill JS, Hemmelgarn BR, Manns BJ, Pannu N, Pelletier R, Thompson S, Klarenbach SW; Alberta Kidney Disease Network : Relocation of remote dwellers living with hemodialysis: A time trade-off survey. Nephrol Dial Transplant 30: 1767–1773, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Morton RL, Devitt J, Howard K, Anderson K, Snelling P, Cass A: Patient views about treatment of stage 5 CKD: A qualitative analysis of semistructured interviews. Am J Kidney Dis 55: 431–440, 2010 [DOI] [PubMed] [Google Scholar]
- 21.François K, Bargman JM: Evaluating the benefits of home-based peritoneal dialysis. Int J Nephrol Renovasc Dis 7: 447–455, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker RC, Hanson CS, Palmer SC, Howard K, Morton RL, Marshall MR, Tong A: Patient and caregiver perspectives on home hemodialysis: A systematic review. Am J Kidney Dis 65: 451–463, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Chartier M, Dart A, Tangri N, Komenda P, Walld R, Bogdanovic B, Burchill C, Koseva I, McGowan K-L, Rajotte L: Care of Manitobans Living with Chronic Kidney Disease, 2015. Available at: http://mchp-appserv.cpe.umanitoba.ca/reference/ckd_final.pdf. Accessed September 7, 2017
- 24.Komenda P, Copland M, Makwana J, Djurdjev O, Sood MM, Levin A: The cost of starting and maintaining a large home hemodialysis program. Kidney Int 77: 1039–1045, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Program MR: Kidneyhealth.ca, 2017. Available at: http://www.kidneyhealth.ca/wp/. Accessed September 7, 2017
- 26.City of Winnipeg: Water and Waste Development, 2017. Available at: http://www.winnipeg.ca/waterandwaste/. Accessed September 7, 2017
- 27.Hydro M: Current Electricity Rates, 2017. Available at: https://www.hydro.mb.ca/regulatory_affairs/energy_rates/electricity/current_rates.shtml. Accessed September 7, 2017
- 28.Sure Solutions Inc.: Energy Efficiency Opportunities in Ontario Hospitals, 2006. Available at: http://studylib.net/doc/18873604/energy-efficiency-opportunities-in-ontario-hospitals. Accessed September 7, 2017
- 29.Komenda P, Gavaghan MB, Garfield SS, Poret AW, Sood MM: An economic assessment model for in-center, conventional home, and more frequent home hemodialysis. Kidney Int 81: 307–313, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, Donaldson C: Cost analysis of ongoing care of patients with end-stage renal disease: The impact of dialysis modality and dialysis access. Am J Kidney Dis 40: 611–622, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Howard K, Salkeld G, White S, McDonald S, Chadban S, Craig JC, Cass A: The cost-effectiveness of increasing kidney transplantation and home-based dialysis. Nephrology (Carlton) 14: 123–132, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Karopadi AN, Mason G, Rettore E, Ronco C: Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant 28: 2553–2569, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Agar JW, Perkins A, Heaf JG: Home hemodialysis: Infrastructure, water, and machines in the home. Hemodial Int 19[Suppl 1]: S93–S111, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Clark WR, Turk JE Jr .: The NxStage system one. Semin Dial 17: 167–170, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Ferguson TW, Zacharias J, Walker SR, Collister D, Rigatto C, Tangri N, Komenda P: An economic assessment model of rural and remote satellite hemodialysis units. PLoS One 10: e0135587, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehrotra R, Chiu Y-W, Kalantar-Zadeh K, Bargman J, Vonesh E: Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171: 110–118, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ: Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 21: 499–506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeates K, Zhu N, Vonesh E, Trpeski L, Blake P, Fenton S: Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant 27: 3568–3575, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Ishani A, Slinin Y, Greer N, MacDonald R, Messana J, Rutks I, Wilt TJ: Comparative Effectiveness of Home-Based Kidney Dialysis Versus In-Center or Other Outpatient Kidney Dialysis Locations—A Systematic Review, Washington, DC, Department of Veterans Affairs Health Services Research & Develoipment Service, 2015, pp 1–153 [PubMed] [Google Scholar]
- 40.Chui BK, Manns B, Pannu N, Dong J, Wiebe N, Jindal K, Klarenbach SW: Health care costs of peritoneal dialysis technique failure and dialysis modality switching. Am J Kidney Dis 61: 104–111, 2013 [DOI] [PubMed] [Google Scholar]
- 41.McFarlane P, Komenda P: Economic considerations in frequent home hemodialysis. Semin Dial 24: 678–683, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Quinn RR, Oliver MJ: Is assisted peritoneal dialysis an alternative to in-center hemodialysis? Perit Dial Int 26: 650–653, 2006 [PubMed] [Google Scholar]
- 43.Oliver MJ, Quinn RR, Richardson EP, Kiss AJ, Lamping DL, Manns BJ: Home care assistance and the utilization of peritoneal dialysis. Kidney Int 71: 673–678, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Couillerot-Peyrondet AL, Sambuc C, Sainsaulieu Y, Couchoud C, Bongiovanni-Delarozière I: A comprehensive approach to assess the costs of renal replacement therapy for end-stage renal disease in France: The importance of age, diabetes status, and clinical events. Eur J Health Econ 18: 459–469, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Suri RS, Larive B, Sherer S, Eggers P, Gassman J, James SH, Lindsay RM, Lockridge RS, Ornt DB, Rocco MV, Ting GO, Kliger AS; Frequent Hemodialysis Network Trial Group : Risk of vascular access complications with frequent hemodialysis. J Am Soc Nephrol 24: 498–505, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.