Abstract
Purpose
Primary ovarian insufficiency (POI) is a clinical condition observed in women younger than 40 years of age, characterized by amenorrhea, hypoestrogenism, high levels of follicle-stimulating hormone (FSH), and infertility. Mutations in some master regulators of the development, maturation, and maintenance of ovarian follicles such as BMP15, FSHR, FOXL2, and GDF9 have been suggested as etiological factors in the development of POI. The aim of this study, the first in the Mexican population, is to evaluate the presence of mutations or polymorphisms in these four candidate genes.
Methods
In a sample of 20 Mexican patients with idiopathic POI, we looked for and analyzed genetic variants in BMP15, FSHR, FOXL2, and GDF9 genes.
Results
We observed two polymorphisms: a coding change, c.919G>A (p.Ala307Thr), in the FSHR gene and a synonymous variant, c.447C>T (p.Thr149Thr), in the GDF9 gene. These two variants have been reported previously as polymorphisms (rs6165 and rs254286, respectively). We observed no significant difference associated with POI in the patients when compared with a healthy control group (p > 0.05). Also, no exonic variants were found for the genes BMP15 and FOXL2 in the individuals tested.
Conclusions
The lack of association of the evaluated genes in this sample of Mexican women is consistent with the complex genetic etiology of POI that is observed across cohorts studied thus far.
Keywords: Primary ovarian insufficiency, BMP15, FSHR, FOXL2, GDF9, Mexican population
Introduction
Primary ovarian insufficiency (POI) is a heterogeneous and multifactorial disorder characterized by absent menarche (primary amenorrhea) or cessation of menses for a period of at least 4–6 months (secondary amenorrhea), hypoestrogenism, infertility, and high FSH levels (> 40UI/L, measured in at least two occasions with a month apart), before the age of 40 [1–3]. This clinical condition affects 1 in 100 women younger than 40 years of age (1%), 1 in 1000 women below 30 years of age (0.1%), and 1 in 10,000 women younger than 20 years of age (0.01%) [4]. Approximately 10–28% of patients present primary amenorrhea and 4–18%, secondary amenorrhea [5].
Based on the presence or absence of follicles, POI is classified as follicular or afollicular. The former has ovarian follicles whose function has been impaired by other pathologies, whereas the latter is characterized by the complete depletion of ovarian follicles [4]. POI is further classified into sporadic (the majority) and familial cases [6]; the latter comprising 4–30% of patients (depending on the studied population), thus suggesting it is a hereditary disorder [7, 8].
POI etiology is not well understood [9], but the condition has been widely reported in association with (1) low initial number of follicles, (2) accelerated follicular atresia, (3) compromised maturation process, or (4) folliculogenesis blockage previous to antral stage and ovulation inhibition [10, 11]. Reports have suggested that such mechanisms can be activated by iatrogenic, metabolic, infectious, chromosomal, autoimmune, environmental, or genetic factors [12, 13]. Concerning the latter, and based on murine knockout models [14], several candidate genes have been considered for association with the recruitment, development, and maturation of follicles and their oocytes [15]. Some point mutations in autosomal or X-linked genes (INHA, FOXL2, ESR, FSHR, FMR1, GDF9, BMP15, and others) have been found in both syndromic and non-syndromic cases [2, 9, 16]. However, < 25% of POI cases show direct association with such mutations, and therefore, POI etiology still remains unexplained in most patients [6, 17]. Among the most studied and proposed candidate genes are BMP15, FSHR, FOXL2, and GDF9 [7, 9].
BMP15: Bone morphogenetic protein 15, found within the superfamily of transforming growth factors β (TGF-β), is located in Xp11.22 [18] and is expressed in the oocytes of humans and other mammalian species [19]. It stimulates the proliferation, development, and maturation of granulosa cells, follicles, and oocytes and also acts in the regulation of ovulation [20–22]. BMP15 mutations have been associated with both primary and secondary amenorrhea, with a prevalence of 1.5–15% [7].
FSHR: Follicle-stimulating hormone receptor is located in 2p21-p16 [23]. It participates in normal reproductive function regulating the development and maturation of follicles and oocytes, stimulating the production of estrogens, estradiol, and progesterone by granulosa and thecal cells [14, 16, 23].
FOXL2: Blepharophimosis-ptosis-epicanthus inversus syndrome (BPES) (OMIM 110100) is a hereditary autosomal dominant syndrome characterized by alterations in the eyelids and POI in type I, although POI is absent in type II [24]. BPES, in both types I and II, is caused by FOXL2 (Forkhead Box L2) mutations; this gene is located in 3q23 [25]. FOXL2 participates in ovarian and eyelid development, and in the maintenance of female gonads [26, 27]. Further, it intervenes in the differentiation and maintenance of granulosa cells and the development and maturation of oocytes [24, 28]. Mutations in this gene have also been found in cases without BPES and also associated with infertility due to folliculogenesis blockage [29, 30].
GDF9: Growth differentiation factor-9 is found within the TGF-β superfamily, located in 5q31.1, participates in the proliferation and differentiation of granulosa and theca cells, and regulates follicular development and ovulation [20, 23, 31].
The aim of this initial study in a Mexican patient sample is to evaluate the presence of mutations or polymorphisms in the BMP15, FSHR, FOXL2, and GDF9 genes.
Material and methods
Patients
We recruited 20 Mexican women under 40 years old in a period of 3 years, with idiopathic, sporadic or family IOP, normal karyotype (46,XX), primary or secondary amenorrhea, and elevated FSH (> 40UI/L) in two measurements. Pregnancy was not excluded in the patient group. All participants signed an informed consent letter previously approved by the local ethics committee and the study has been performed in accordance with the ethical standards as laid down in the Declaration of Helsinki. In addition, healthy women with an age range of 15–39 years and unrelated to the patients, participated as a control group (n = 50 for FSHR and n = 100 for GDF9). We conducted a descriptive, observational, and comparative study.
Molecular analysis
Genomic DNA was extracted from peripheral blood samples obtained from each patient using standard protocols (Miller et al. 1988) [32]. The exome of each gene (divided into fragments: BMP15 1, 2A, and 2B; FSHR 7–8 and 10; FOXL2 1, 2, and 3; and GDF9 1, 2A, and 2B) was amplified by polymerase chain reaction (PCR) (primers, exons, and conditions are presented in Table 1). The amplicons were analyzed by agarose gel electrophoresis and then sequenced by Sanger’s method in an automated sequencer (ABI 3130; Applied Biosystems, Foster City, CA). The genetic variants identified were confirmed in three different experiments.
Table 1.
BMP15, FSHR, FOXL2, and GDF9 primers
| Gene | Forward | Reverse | Fragment size | Exon | Conditions |
|---|---|---|---|---|---|
| BMP15 | AGTGACGTCCCTTGGGCTTG | CAAAGCCTGACAGTAAACCC | 477 bp | 1 | 95 °C for 4 min, followed by 28 cycles at 95 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min, and 72 °C for 7 min |
| GGGCTGATTATAGCTATCAGTC | GGAAGAGGCAGTAACCTCAGCTG | 617 bp | 2A | 95 °C for 4 min, followed by 28 cycles at 95 °C for 1 min, 65 °C for 1 min, 72 °C for 1 min, and 72 °C for 7 min | |
| GGGAATCTCTTCTCCGGAGAACC | CTAGCTCACAAGTGGGGGAAGAGAC | 555 bp | 2B | 95 °C for 4 min, followed by 29 cycles at 95 °C for 1 min, 64 °C for 1 min, 72 °C for 1 min, and 72 °C for 7 min | |
| FSHR | CCGTGTATTGTTTGCATCTG | GAAGCTTCTCCCCTAGCTGC | 395 bp | 7–8 | 94 °C for 2 min, followed by 33 cycles at 94 °C for 1 min, 60 °C for 30 s, 72 °C for 75 s, and 72 °C for 4 min |
| CCTGCACAAAGACAGTGATG | GCCCCAGTTTGCCAGTC | 540 bp | 10 | 94 °C for 2 min, followed by 33 cycles at 94 °C for 1 min, 60 °C for 30 s, 72 °C for 75 s, and 72 °C for 4 min | |
| FOXL2 | CAGCGCCTGGAGCGGAGAG | CTTGCCGGGCTGGAAGTGC | 546 bp | 1 | 95 °C for 10 min, followed by 30 cycles at 95 °C for 1 min, 66 °C for 1 min, 72 °C for 2 min, and 72 °C for 10 min |
| GACCCGGCCTGCGAAGACA | GGCCGCGTGCAGATGGTGT | 516 bp | 2 | 95 °C for 10 min, followed by 35 cycles at 95 °C for 1 min, 66 °C for 1 min, 72 °C for 2 min, and 72 °C for 10 min | |
| CGCGGCCGCTGTGGTCAAG | GCTGGCGGCGGCGTCGTC | 501 bp | 3 | 94 °C for 2 min, followed by 30 cycles at 95 °C for 30 s, 66 °C for 30 s, 72 °C for 2 min, and 72 °C for 10 min | |
| GDF9 | TTCCTCACTAGTTCTCCCAAGC | CATCTTCCCTCCACCCAGT | 461 bp | 1 | 95 °C for 4 min, followed by 28 cycles at 95 °C for 1 min, 58 °C for 1 min, 72 °C for 1 min, and 72 °C for 7 min |
| TTCAAGCACTACTGGTAG | AGCCTGAGCACTTGTGTCATT | 460 bp | 2A | 95 °C for 4 min, followed by 27 cycles at 95 °C for 1 min, 58 °C for 1 min, 72 °C for 1 min, and 72 °C for 7 min | |
| ATGAAAGACCAGCTGGAGCA | TTTGCCAAATAGGCTCAAGG | 686 bp | 2B | 95 °C for 4 min, followed by 29 cycles at 95 °C for 1 min, 64 °C for 1 min, 72 °C for 1 min, and 72 °C for 7 min |
Statistical analysis
Statistical analysis was performed using the software SPSS v.22.0 (SPSS Inc., Chicago, IL). Descriptive statistics were performed for categorical variables expressed as frequencies. Quantitative variables were expressed as mean ± standard deviations (SD). A chi-square test was used to compare allele and genotype frequencies between patients and a healthy control group. A p < 0.05 was considered significant.
Results
Twenty patients with idiopathic POI were included in the study. The average age for POI onset was of 24.8 ± 6.8 years; most cases started between the ages of 20 and 25 years (n = 7, 35%), 30% before 20 years old (n = 6), 30% between 26 and 35 years (n = 6), and only 5% after 35 years old (n = 1). The average age at diagnosis was of 30.5 ± 6.7 years; most women were diagnosed between 26 and 30 years old (n = 9, 45%), 35% after 30 years (n = 7), and 20% were diagnosed before 25 years old (n = 4). Concerning amenorrhea, most cases presented secondary amenorrhea (85%), with sporadic type POI (80%) and fertility problems (found in 80% of the studied cases) (Table 2).
Table 2.
Clinical characteristics of patients with POI
| Primary amenorrhea | Secondary amenorrhea | Sporadic type | Familiar type | Fertility | Infertility | |
|---|---|---|---|---|---|---|
| Number patients | 3 | 17 | 16 | 4 | 4 | 16 |
| Frequency (%) | 15% | 85% | 80% | 20% | 20% | 80% |
| FSH and LH average levels | ||||||
| FSH | 76.4 mUI/ml ± 23.2 (2.5–10.2 mUI/ml normal levels) | |||||
| LH | 47.2 mUI/ml ± 14.4 (1.9–12.5 mUI/ml normal levels) | |||||
Frequencies obtained with 20 patients. Quantitative variables are expressed as mean ± SD and categorical variables as frequencies (in percentage)
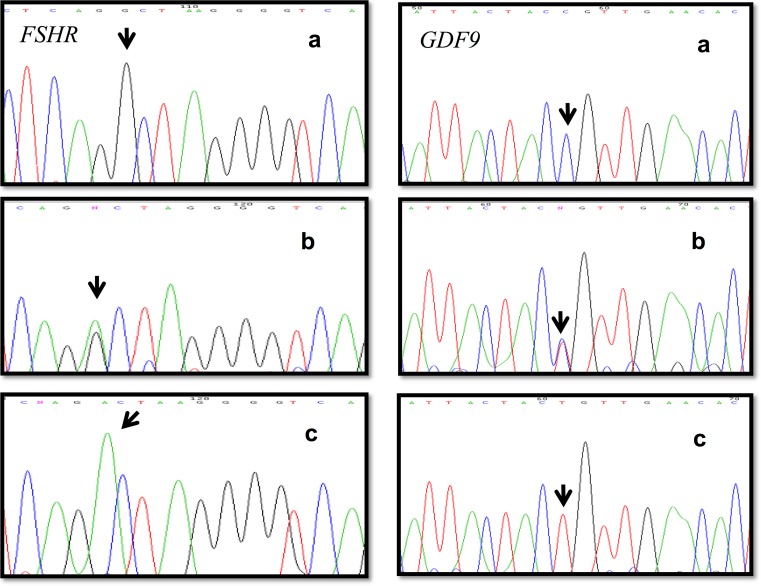
Molecular analysis revealed no variants in BMP15 and FOXL2, but found variants in exons of two genes, a transition c. 919G>A (p.Ala307Thr) located in exon 10 of the FSHR gene, and a transition c.447C>T (p.Thr149Thr) in exon 2A of the GDF9 gene. Both have been previously reported as polymorphisms (rs6165 and rs254286). These two genetic variants were observed as either wild type, heterozygous, or polymorphic homozygous (Fig. 1). The allele and genotype frequencies for both polymorphisms are shown in Table 3. The analysis found no significant difference between patients and controls (p > 0.05), and the distribution was similar in both patient and control groups (p = 0.691 and 0.729, respectively). The population was in Hardy–Weinberg equilibrium. The allele frequencies of the two polymorphisms observed in the Mexican population (rs6165 and rs254286) were compared with those reported in the NCBI-SNPs HapMap project, finding that the allele frequencies of the rs6165 polymorphism were similar to the Sub-Saharan African population (G = 25% and A = 76%) and the allele frequencies of the rs254286 polymorphism were similar to the Japanese population (C = 60% and T = 40%).
Fig. 1.
Electropherogram showing the polymorphisms c. 919G>A (p.Ala307Thr) in the FSHR gene and c.447C>T (p.Thr149Thr) in the GDF9 gene in the three evaluated conditions: (a) wild type, (b) heterozygous, and (c) polymorphic homozygous
Table 3.
Frequency of the polymorphisms c. 919G>A and c.447C>T
| Gene | Polymorphism | AA change | Exon | Genotype | Genotype frequency (%) (POI patients) | Genotype frequency (%) (control population) | p | Allele | Allele frequency (%) (POI patients) | Allele frequency (%) (control population) | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FSHR | c.919G>A | p.Ala307Thr | 10 | G/G | 2 (10%) | 7 (14%) | 0.46 | G | 14 (35%) | 31 (31%) | 0.691 |
| G/A | 10 (50%) | 17 (34%) | A | 26 (65%) | 69 (69%) | ||||||
| A/A | 8 (40%) | 26(52%) | |||||||||
| Total | 20 (100%) | 50 (100%) | 40 (100%) | 100 (100%) | |||||||
| GDF9 | c.447C>T | p.Thr149Thr | 2A | C/C | 6 (30%) | 26 (26%) | 0.88 | C | 23 (58%) | 107 (54%) | 0.729 |
| C/T | 11 (55%) | 55 (55%) | T | 17 (42%) | 93 (46%) | ||||||
| T/T | 3 (15%) | 19 (19%) | |||||||||
| Total | 20 (100%) | 100 (100%) | 40 (100%) | 200 (100%) |
p < 0.05 was considered significant
Discussion
POI candidate genes have been evaluated in diverse world populations [37]; mutations in these genes could modify the amino acid sequence affecting the function of the coded protein and resulting in amenorrhea and infertility. However, the genetic variants identified and associated with POI are somewhat disparate from one population to another, and it has been suggested that differences could be due to the heterogeneity in the design of the studies or of the analyzed populations [16, 29].
This is the first study conducted in the Mexican population concerning the genes BMP15, FSHR, FOXL2, and GDF9. All of which have been reported to be mutated in at least some cases of POI in other populations [7]. We found two genetic variants, c.919G>A (p.Ala307Thr) in exon 10 of the FSHR gene (extracellular domain) and c.447C>T (p.Thr149Thr) in exon 2 of the GDF9 gene (pro-region), but found no significant difference for either of them in cases vs. control (p > 0.05). Regarding the FSHR gene, past reports are varied, e.g., Sundblad (2004), Woad (2013), Ma (2015), and Ghezelayagh (2018) found similar results to ours, meaning that though they observed the polymorphism c.919G>A (p.Ala307Thr), they did not find any significant difference in 20 Argentinean women with POI, 80 from New Zealand, 63 from China, and 84 from Iran, respectively [38–41]. Contrary to these findings, the genetic variant was associated with POI in a Brazilian population (n = 100) [16], but in countries including Japan, Greece, India, Germany, Denmark, and Singapore, no changes were identified in this gene; and thus the genetic variants associated with POI are geographically restricted [42, 43].
As for GDF9 gene, like FSHR, the reported results differ depending upon the population studied. For example, whereas we found the synonymous variant c.447C>T (p.Thr149Thr), in either wild type, heterozygous, or polymorphic homozygous configurations in both patients and control subjects, Kumar (2017) observed no variation in 54 women from India, nor has it been found in women from New Zealand (n = 38) [44]. However, in USA, China, and Caucasian populations, this and other variants have been reported [29, 36], although mostly in heterozygous state but only in POI patients, with none seen in the controls [7, 15].
Despite the differences found in diverse populations of the world, we observed that the allele frequencies of the rs6165 and rs254286 polymorphisms identified in the Mexican population were similar to those reported in the Sub-Saharan African and Japanese populations respectively (NCBI-SNPs HapMap project), although this has been mainly attributed to a coincidence, given that the Mexican population has greater European ancestry, than Asian or African.
Mexican patients with POI showed no variations in the BMP15 or FOXL2 genes. Similarly, neither Takebayashi (2000) nor Ni (2010) found any BMP15 gene variants in 15 Japanese and 118 Chinese women, respectively [33, 45]. Nevertheless, Laissue (2009) showed evidence implicating FOXL2 gene variants in non-syndromic POI (without BPES) in a Tunisian population [46]. Further, Kumar (2017) recently identified diverse BMP15 gene variants in a sample of 53 women with idiopathic POI, although with no clear association [44]. Taking into account the previous data, the overall sample size that has been explored in various groups is sufficient to indicate that ethnic differences must be considered in the search of genetic variants linked to POI.
As for clinical characteristics, the average age for symptom onset in the patients from our population was 24.8 ± 6.8 years of age, but most started before 25 (n = 13, 65%); in 80% of these cases, POI was sporadic type (n = 16) and 80% could not achieve pregnancy (n = 16). This is of particular concern now that women are increasingly delaying the age for first pregnancy and in this initial study, we did not find variants in any of the four proposed genes in the Mexican population as a common cause for POI, although the results are consistent with the complex genetic etiology of POI that is observed across cohorts studied thus far. However, other candidate genes could be studied as a cause of POI in the Mexican population.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
References
- 1.Chaloutsou K, Aggelidis P, Pampanos A, Theochari E, Michala L. Premature ovarian insufficiency: an adolescent series. J Pediatr Adolesc Gynecol. 2017;30:615–619. doi: 10.1016/j.jpag.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Carlosama C, Elzaiat M, Patiño LC, Mateus HE, Veitia RA, Laissue P. A homozygous donor splice-site mutation in the meiotic gene MSH4 causes primary ovarian insufficiency. Hum Mol Genet. 2017;26:3161–3166. doi: 10.1093/hmg/ddx199. [DOI] [PubMed] [Google Scholar]
- 3.Desai S, Wood-Trageser M, Matic J, Chipkin J, Jiang H, Bachelot A, et al. MCM8 and MCM9 nucleotide variants in women with primary ovarian insufficiency. J Clin Endocrinol Metab. 2017;102:576–582. doi: 10.1210/jc.2016-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunsha N, Rojas J, Bermúdez V. Osteoporosis in a 30-yr old woman with premature ovarian insufficiency. Case report. Archivos Venezolanos de Farmacología y Terapéutica. 2015;34:31–35. [Google Scholar]
- 5.Košir Pogačnik R, Meden Vrtovec H, Vizjak A, Uršula Levičnik A, Slabe N, Ihan A. Possible role of autoimmunity in patients with premature ovarian insufficiency. Int J Fertil Steril. 2014;7:281–290. [PMC free article] [PubMed] [Google Scholar]
- 6.Guo T, Zhao S, Zhao S, Chen M, Li G, Jiao X, et al. Mutations in MSH5 in primary ovarian insufficiency. Hum Mol Genet. 2017;26:1452–1457. doi: 10.1093/hmg/ddx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossetti R, Ferrari I, Bonomi M, Persani L. Genetics of primary ovarian insufficiency. Clin Genet. 2017;91:183–198. doi: 10.1111/cge.12921. [DOI] [PubMed] [Google Scholar]
- 8.Bouilly J, Beau I, Barraud S, Bernard V, Azibi K, Fagart J. Identification of multiple gene mutations accounts for a new genetic architecture of primary ovarian insufficiency. J Clin Endocrinol Metab. 2016;101:4541–4550. doi: 10.1210/jc.2016-2152. [DOI] [PubMed] [Google Scholar]
- 9.Pu D, Xing Y, Gao Y, Gu L, Wu J. Gene variation and premature ovarian failure: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;182:226–237. doi: 10.1016/j.ejogrb.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 10.Mohamadhashem F, Rafati M, Hoseininasab F, Rostami S, Tabatabaie R, Rezai S, et al. Primary ovarian insufficiency with t(5;13): a case report and literature review on disrupted genes. Climacteric. 2017;20:498–502. doi: 10.1080/13697137.2017.1316255. [DOI] [PubMed] [Google Scholar]
- 11.Vabre P, Gatimel N, Moreau J, Gayrard V, Picard-Hagen N, Parinaud J, et al. Environmental pollutants, a possible etiology for premature ovarian insufficiency: a narrative review of animal and human data. Environ Health. 2017;16:37. doi: 10.1186/s12940-017-0242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J, Li XL, Song H, Li Q, Wang MY, Qiu XM, et al. A general description for Chinese medicine in treating premature ovarian failure. Chin J Integr Med. 2017;23:91–97. doi: 10.1007/s11655-016-2642-7. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen HH, Milat F, Vincent A. Premature ovarian insufficiency in general practice: meeting the needs of women. Aust Fam Physician. 2017;46:360–366. [PubMed] [Google Scholar]
- 14.Qin Y, Jiao X, Simpson JL, Chen ZJ. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21:787–808. doi: 10.1093/humupd/dmv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.França MM, Funari MFA, Nishi MY, Narcizo AM, Domenice S, Costa EMF, et al. Identification of the first homozygous 1-bp deletion in GDF9 gene leading to primary ovarian insufficiency by using targeted massively parallel sequencing. Clin Genet. 2018;93:408–411. doi: 10.1111/cge.13156. [DOI] [PubMed] [Google Scholar]
- 16.Cordts EB, Santos MC, Bianco B, Barbosa CP, Christofolini DM. Are FSHR polymorphisms risk factors to premature ovarian insufficiency? Gynecol Endocrinol. 2015;31:663–666. doi: 10.3109/09513590.2015.1032933. [DOI] [PubMed] [Google Scholar]
- 17.Brauner R, Pierrepont S, Bignon-Topalovic J, McElreavey K, Bashamboo A. Etiology of primary ovarian insufficiency in a series young girls presenting at a pediatricendocrinology center. Eur J Pediatr. 2015;174:767–773. doi: 10.1007/s00431-014-2457-5. [DOI] [PubMed] [Google Scholar]
- 18.Al-ajoury R, Kassem E, Al-halabi B, Moassess F, Al-achkar W. Investigation of some genetic variations in BMP15 accompanied with premature ovarian failure (POF) in Syrian women. Middle East Fertil Soc J. 2015;20:91–96. doi: 10.1016/j.mefs.2014.02.005. [DOI] [Google Scholar]
- 19.Fonseca DJ, Ortega-Recalde O, Esteban-Perez C, Moreno-Ortiz H, Patiño LC, Bermúdez OM, et al. BMP15 c.-9C>G promoter sequence variant may contribute to the cause of non-syndromic premature ovarian failure. Reprod BioMed Online. 2014;29:627–633. doi: 10.1016/j.rbmo.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Mayer A, Fouquet B, Pugeat M, Misrahi M. BMP15 “knockout-like” effect in familial premature ovarian insufficiency with persistent ovarian reserve. Clin Genet. 2017;92:208–212. doi: 10.1111/cge.12970. [DOI] [PubMed] [Google Scholar]
- 21.Patiño LC, Walton KL, Mueller TD, Johnson KE, Stocker W, Richani D, et al. BMP15 mutations associated with primary ovarian insufficiency reduce expression, activity, or Synergy With GDF9. J Clin Endocrinol Metab. 2017;102:1009–1019. doi: 10.1210/jc.2016-3503. [DOI] [PubMed] [Google Scholar]
- 22.Auclair S, Rossetti R, Meslin C, Monestier O, Di Pasquale E, Pascal G, et al. Positive selection in bone morphogenetic protein 15 targets a natural mutation associated with primary ovarian insufficiency in human. PLoS One. 2013;8:e78199. doi: 10.1371/journal.pone.0078199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barasoain M, Barrenetxea G, Huerta I, Télez M, Criado B, Arrieta I. Study of the genetic etiology of primary ovarian insufficiency: FMR1 Gene. Genes (Basel) 2016;7:E123. doi: 10.3390/genes7120123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue M, Zheng J, Zhou Q, Hejtmancik JF, Wang Y, Li S. Novel FOXL2 mutations in two Chinese families with blepharophimosis-ptosis-epicanthus inversus syndrome. BMC Med Genet. 2015;16:73. doi: 10.1186/s12881-015-0217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuovo S, Passeri M, Di Benedetto E, Calanchini M, Meldolesi I, Di Giacomo MC, et al. Characterization of endocrine features and genotype-phenotypes correlations in blepharophimosis-ptosis-epicanthus inversus syndrome type 1. J Endocrinol Investig. 2016;39:227–233. doi: 10.1007/s40618-015-0334-3. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Chai P, Fan J, Wang X, Lu W, Li J, et al. A novel FOXL2 mutation implying blepharophimosis-ptosis-epicanthus inversus syndrome type I. Cell Physiol Biochem. 2018;45:203–211. doi: 10.1159/000486358. [DOI] [PubMed] [Google Scholar]
- 27.Yang XW, He WB, Gong F, Li W, Li XR, Zhong CG, et al. Novel FOXL2 mutations cause blepharophimosisptosis-epicanthus inversus syndrome with premature ovarian insufficiency. Mol Genet Genomic Med. 2018; 6:261–7. [DOI] [PMC free article] [PubMed]
- 28.Elzaiat M, Todeschini AL, Caburet S, Veitia RA. The genetic make-up of ovarian development and function: the focus on the transcription factor FOXL2. Clin Genet. 2017;91:173–182. doi: 10.1111/cge.12862. [DOI] [PubMed] [Google Scholar]
- 29.Laven JS. Primary ovarian insufficiency. Semin Reprod Med. 2016;34:230–234. doi: 10.1055/s-0036-1585402. [DOI] [PubMed] [Google Scholar]
- 30.Settas N, Anapliotou M, Kanavakis E, Fryssira H, Sofocleous C, Dacou-Voutetakis C, et al. A novel FOXL2 gene mutation and BMP15 variants in a woman with primary ovarian insufficiency and blepharophimosis-ptosis-epicanthus inversus syndrome. Menopause. 2015;22:1264–1268. doi: 10.1097/GME.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 31.Patiño LC, Silgado D, Laissue P. A potential functional association between mutant BMPR2 and primary ovarian insufficiency. Syst Biol Reprod Med. 2017;63:145–149. doi: 10.1080/19396368.2017.1291767. [DOI] [PubMed] [Google Scholar]
- 32.Miller SA, Dikes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takebayashi K, Takakura K, Wang HQ, Kimura F, Kasahara K, Noda Y. Mutation analysis of the growth differentiation factor-9 and 9B genes in patients with premature ovarian failure and polycystic ovary syndrome. Fert Steril. 2000;74:976–979. doi: 10.1016/S0015-0282(00)01539-9. [DOI] [PubMed] [Google Scholar]
- 34.Doherty E, Pakarinen P, Tiitinen A, Kiilavuori A, Huhtaniemi I, Forrest S, et al. A novel mutation in the FSH receptor inhibiting signal transduction and causing primary ovarian failure. J Clin Endocrinol Metab. 2002;87:1151–1155. doi: 10.1210/jcem.87.3.8319. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Liu J, Zhang Q. FOXL2 mutations in Chinese patients with blepharophimosis-ptosis-epicanthus inversus syndrome. Mol Vis. 2007;13:108–113. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H, Qin Y, Kovanci E, Simpson JL, Chen ZJ, Rajkovic A. Analyses of GDF9 mutation in 100 Chinese women with premature ovarian failure. Fertil Steril. 2007;88:1474–1476. doi: 10.1016/j.fertnstert.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katari S, Wood-Trageser MA, Jiang H, Kalynchuk E, Muzumdar R, Yatsenko SA, et al. Novel inactivating mutation of the FSH receptor in two siblings of Indian origin with premature ovarian failure. J Clin Endocrinol Metab. 2015;100:2154–2157. doi: 10.1210/jc.2015-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sundblad V, Chiauzzi VA, Escobar ME, Dain L, Charreau EH. Screening of FSH receptor gene in Argentine women with premature ovarian failure (POF) Mol Cell Endocrinol. 2004;222:53–59. doi: 10.1016/j.mce.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Woad KJ, Prendergast D, Winship IM, Shelling AN. FSH receptor gene variants are rarely associated with premature ovarian failure. Reprod BioMed Online. 2013;26:396–399. doi: 10.1016/j.rbmo.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Ma L, Chen Y, Mei S, Liu C, Ma X, Li Y, et al. Single nucleotide polymorphisms in premature ovarian failure-associated genes in a Chinese Hui population. Mol Med Rep. 2015;12:2529–2538. doi: 10.3892/mmr.2015.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghezelayagh Z, Totonchi M, Zarei-Moradi S, Asadpour O, Maroufizadeh S, Eftekhari-Yazdi P, et al. The impact of genetic variation and gene expression level of the follicle-stimulating hormone receptor on ovarian reserve. Cell J. 2018;19:620–626. doi: 10.22074/cellj.2018.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prakash GJ, Kanth VV, Shelling AN, Rozati R, Sujatha M. Absence of 566C>T mutation in exon 7 of the FSHR gene in Indian women with premature ovarian failure. Int J Gynaecol Obstet. 2009;105:265–266. doi: 10.1016/j.ijgo.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 43.Tong Y, Liao WX, Roy AC, Ng SC. Absence of mutations in the coding regions of follicle-stimulating hormone receptor gene in Singapore Chinese women with premature ovarian failure and polycystic ovary syndrome. Horm Metab Res. 2001;33:221–226. doi: 10.1055/s-2001-14941. [DOI] [PubMed] [Google Scholar]
- 44.Kumar R, Alwani M, Kosta S, Kaur R, Agarwal S. BMP15 and GDF9 gene mutations in premature ovarian failure. J Reprod Infertil. 2017;18:185–189. [PMC free article] [PubMed] [Google Scholar]
- 45.Ni F, Wen Q, Wang B, Zhou S, Wang J, Mu Y, et al. Mutation analysis of FOXL2 gene in Chinese patients with premature ovarian failure. Gynecol Endocrinol. 2010;26:246–249. doi: 10.3109/09513590903225358. [DOI] [PubMed] [Google Scholar]
- 46.Laissue P, Lakhal B, Benayoun BA, Dipietromaria A, Braham R, Elghezal H, et al. Functional evidence implicating FOXL2 in non-syndromic premature ovarian failure and in the regulation of the transcription factor OSR2. J Med Genet. 2009;46:455–457. doi: 10.1136/jmg.2008.065086. [DOI] [PubMed] [Google Scholar]