Abstract
Purpose
To study the regulation and functions of oviductal glycoprotein 1 (OVGP1) in endometrial epithelial cells.
Methods
Expression of OVGP1 in mouse endometrium during pregnancy and in the endometrial epithelial cell line (Ishikawa) was studied by immunofluorescence, Western blotting, and RT-PCR. Regulation of OVGP1 in response to ovarian steroids and human chorionic gonadotropin (hCG) was studied by real-time RT-PCR. OVGP1 expression was knockdown in Ishikawa cells by shRNA, and expression of receptivity associated genes was studied by real-time RT-PCR. Adhesion of trophoblast cell line (JAr) was studied by in vitro adhesion assays.
Results
OVGP1 was localized exclusively in the luminal epithelial cells of mouse endometrium at the time of embryo implantation. Along with estrogen and progesterone, hCG induced the expression of OVGP1 in Ishikawa cells. Knockdown of OVGP1 in Ishikawa cells reduced mRNA expression of ITGAV, ITGB3, ITGA5, HOXA10, LIF, and IL15; it increased the expression of HOXA11, MMP9, TIMP1, and TIMP3. Supernatants derived from OVGP1 knockdown Ishikawa cells reduced the adhesiveness of JAr cells in vitro. Expression of OVGP1 mRNA was found to be significantly lowered in the endometrium of women with recurrent implantation failure.
Conclusion
OVGP1 is specifically induced in the luminal epithelium at the time of embryo implantation where it regulates receptivity-related genes and aids in trophoblast adhesion.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1231-4) contains supplementary material, which is available to authorized users.
Keywords: Endometrium, Implantation, Receptivity, Oviductal glycoprotein 1, Glycoprotein, Trophoblast
Introduction
Since its first clinical report, assisted reproductive technologies have undergone several advances; however, the success rates remain low. The failure of implantation of morphologically, developmentally, and genetically competent embryos yet remains a major challenge in the success of assisted reproduction [1, 2]. A significant proportion of women also serially experience implantation failure, and the cause in most of them is unknown [1]. This is because there are gaps in our understanding of fundamental processes of embryo implantation.
Successful implantation involves a receptive endometrium, a developmentally competent embryo, and a synchronized dialogue between maternal and embryonic tissues [3–5]. Even though the blastocyst can implant in different tissues, in the endometrium, it can do so only during a narrow time frame termed as window of implantation or receptive phase [6, 7]. Achieved under the influence of ovarian steroids, the molecular signature of the receptive-stage endometrium is well defined [8, 9]. It is believed that failure to attain receptivity or inability to synchronize embryo transfer with receptive endometrium is a major cause of recurrent implantation failure in women undergoing assisted reproduction [10, 11].
According to the current notion, a receptive endometrium is a passive tissue which readily promotes embryo implantation. However, notwithstanding this notion, we and others have demonstrated an extensive remodeling of the endometrium by the embryo at the time of implantation [3, 12–15]. It has been shown [16, 17] that the endometrial remodeling at the time of implantation is mediated by embryo-derived soluble factors like chorionic gonadotropin (CG) and interleukin 1β (IL1β). Both in presence of embryo and in response to the soluble factors, the endometrial luminal epithelium undergoes hyperproliferation, stromal cell compaction, decidualization, and increased vascularization [15–17]. These morphological changes are also associated with extensive changes in expression of genes having roles in immune modulation, proteolysis, transcription regulation, and cell signaling [15]. Furthermore, the expression of these genes is altered in the endometria of infertile women or those experiencing recurrent implantation failure [8, 18]. This has led to the emergence of a new step in our understanding of embryo implantation, where the receptive endometrium is further modulated by a blastocyst-driven signature to promote implantation [19]. However, limited information is available regarding the molecular features of this embryo-endometrium crosstalk.
The luminal epithelium of the endometrium is highly enriched in glycoproteins and have diverse functions [20]. Among these, Glycodelin and MUC1 are the most extensively studied glycoproteins for their roles in embryo implantation [20, 21]. Glycodelin-A is found abundantly in the secretory endometrium, decidua, and amniotic fluid that plays important roles in fertilization, embryo implantation, and placentation [22]. Its levels are altered in the endometria of women with endometriosis and recurrent implantation failure [23, 24]. MUC1 is abundantly expressed in the receptive-stage endometrium; its lower levels in serum or uterine flushing are associated with recurrent implantation failure [23, 25]. The other glycoproteins in the endometrial epithelium are the selectin and galectins that play role in embryo apposition and implantation [20, 25, 26]. Oviductal glycoprotein 1 (OVGP1) is a high-molecular-weight glycoprotein thought to be exclusively secreted by the non-ciliated epithelial cells of the fallopian tube [27, 28]. Identified first as an estrogen-induced secretory protein in the oviduct, OVGP1 aids in sperm capacitation, fertilization, and early embryonic development [29, 30]. The endometrial epithelium is a continuum of the oviductal epithelium and shares the same embryonic origin, and it is not surprising that several genes including steroid receptors, transcription factors, and growth factors are expressed commonly by both the uterine and oviductal epithelium [31]. In this context, OVGP1 is considered as an exception as its transcripts and protein are not detected in the endometrium of most species, except the hamster [29, 32, 33]. In our attempts to identify embryo-regulated molecules, we serendipitously discovered the expression of OVGP1 in the mouse endometrial epithelium during embryo implantation.
In the present study, we report a detailed analysis of the expression, regulation, and requirement of OVGP1 in the endometrial epithelial cells during embryo implantation. The results reveal that in the endometrial epithelial cells, OVGP1 regulates the expression of receptivity-related genes and aids in trophoblast adhesion.
Materials and methods
Ethics approval
The study was approved by the Institutional Animal Ethics Committee (IAEC) of the National Institute for Research in Reproductive (NIRRH).
Animal/tissue collection
Ten-week-old C57BL/6 mice housed in an experimental animal facility of NIRRH were used. Female mice were mated with males of proven fertility, and the day of vaginal plug was defined as day 1. The mice were sacrificed on different days after detection of vaginal plug corresponding to various stages of embryo implantation [7]. The uteri were collected on day 4 morning (9:00 AM) when the embryo enters the endometrial lumen and it is in the receptive phase and day 4 evening (9:00 PM) when the embryo initiates apposition. The uteri were also collected on day 5 morning (9:00 AM) when the embryo attaches to the endometrial luminal epithelium and also day 5 evening (9:00 PM) when the embryo initiates epithelial breaching. The oviduct and uterus were also harvested from non-pregnant mice in both estrus and diestrus stages. Uteri from three mice were collected at each time points/stages as biological replicates point/stage. The tissues were fixed overnight in 4% paraformaldehyde (Sigma-Aldrich, MO, USA) at 4 °C and processed for paraffin embedding and sectioning.
Cell culture
Ishikawa cells are derived from well-differentiated adenocarcinoma of the endometrium. These cells express steroid receptors and mimic most features of the endometrial epithelium [34, 35]. Ishikawa cells were kindly provided to us by Dr. Geetanjali Sachdeva (NIRRH). The cells were cultured in DMEM/F12 with 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic (all from Gibco, Thermo Fisher Scientific, MA, USA). JAr cells are placental choriocarcinoma cell lines that were kindly provided to us by Dr. Geetanjali Sachdeva and were cultured in RPMI with 10% FBS and 1% antibiotic-antimycotic (all from Gibco). The cells were maintained in 5% CO2 at 37 °C.
Hormonal treatment
Ishikawa cells were cultured in DMEM/F12 with steroid-stripped 2% FBS for 24 h and then treated with 17-estradiol (10−8 M) and progesterone (10−6 M) (both from Sigma) alone or in combination as detailed previously [36]. Cells were treated with 1 IU of human chorionic gonadotropin (hCG) (Bharat Serum and Vaccines Ltd., Thane, Maharashtra, India) alone or in combination with estrogen and progesterone. Cells were harvested at 6, 12, 18, and 24 h of treatment. Cells without any treatment were harvested at each time point and were used as controls.
OVGP1 gene silencing
Gene silencing was performed using plasmid containing shRNA (pGIPZ vector) targeting OVGP1 gene (V3LHS_361729; Dharmacon, Colorado, USA). As controls, a plasmid containing scrambled shRNA (V3LHS_65890S; Dharmacon) was used. Endotoxin-free plasmid was transfected in Ishikawa cells using Xtreme Gene HP transfection reagent (Sigma) as per the manufacturer’s protocol. Post-72 h of transfection, the cells were treated 8 μg/ml of puromycin (Gibco) for 15 days. The cells were then serially subcultured and harvested at 70% confluency for further experiments. The knockdown efficiency of OVGP1 gene was examined by qPCR and immunofluorescence. All the experiments were performed between passages 30–35.
Total RNA isolation and real-time PCR
Chilled Trizol reagent (Invitrogen, Thermo Fisher Scientific) was directly added to the cells, and RNA was extracted as described previously [36]. RNA was treated with DNase I (New England Biolabs, MA, USA) and reverse-transcribed using high-capacity cDNA reverse transcriptase kit (Applied Biosystems, Thermo Fisher Scientific). Quantitative PCR was performed (in duplicates) in the CFX-96 thermal cycler (Bio-Rad, CA, USA) using iQ SYBR green chemistry (Bio-Rad). The amplification conditions for all genes (GAPDH, HOXA10, HOXA11, IL6, IL15, IL11, ITGAV, ITGA5, ITGA6, ITGB3, LIF, MMP2, MMP9, OVGP1, PAEP, TGFB1, TIMP1, TIMP2, TIMP3) were initial denaturation 95 °C for 30 s, specific annealing temperature (Supplementary Table 1) for 30 s, and extension at 72 °C for 30 s for 35 cycles. The fluorescence emitted at each cycle was collected for the entire period of 30 s during the extension step of each cycle. The homogeneity of the PCR amplicons was verified using the melt curve method. The sequences of the primers and annealing temperature used are given in Supplementary Table 1. Gene expression was normalized to GAPDH, and fold change was estimated using Livak’s method [36].
Immunofluorescence
Five-micrometer-thick paraffin embedded sections were prepared on poly-l-lysine (Sigma)-coated slides (HiMedia, Maharashtra, India), deparaffinized in xylene, and rehydrated in decreasing concentrations of alcohol. Immunofluorescence was performed as described previously [37]. In brief, the antigenic sites were unmasked by incubation of sections at 90 °C in Tris EDTA buffer (10 mM, pH 9) followed by incubation in 10 mM sodium borohydrate (Sigma) to reduce autofluorescence. The sections were blocked in 5% bovine serum albumin (BSA; MP Biomedicals, Maharashtra, India), followed by overnight incubation with the anti-OVGP1 (ab74544) antibody (Abcam, Cambridge, UK), at a dilution of 1:500 in phosphate-buffered saline (PBS). The sections were washed with PBS, and the bound antibodies were detected with Alexa Fluor 568 donkey anti-rabbit IgG (Molecular Probes, Thermo Fisher Scientific). The sections were counterstained in 0.1 μg/ml of DAPI (Sigma) and mounted in the antifade medium. Imaging was done on inverted fluorescence microscope equipped with sCMOS camera (Leica Microsystems DMi8, Mannheim, Germany). The images were analyzed, and the quantification was done using LAS X software (Leica Microsystems).
Ishikawa cells were grown on a coverslip and fixed in absolute methanol for 20 min, followed by blocking in 5% BSA, and OVGP1 immunofluorescence was carried out as mentioned above.
Western blotting
Total cell lysates were prepared in ice-cold lysis buffer (150 mM NaCl, 50 mM Hepes, 5 μg/ml digitonin, 0.1% Triton X-100) containing protease inhibitor (all from Sigma). Thirty-microgram protein was separated on 8% SDS gel, transferred overnight at 30 V onto a nitrocellulose membrane (GE Healthcare Life Sciences, IL, USA) in a transfer buffer (0.03% SDS and 10% methanol). The membrane was blocked in 5% non-fat dried milk (Nestle, Uttar Pradesh, India) and probed with anti-OVGP1 (1:500) antibody (Abcam). After extensive washings, the blots were probed with a secondary antibody labeled with horseradish peroxidase-conjugated anti-rabbit IgG (ABclonal, MA, USA). Detection was done using chemiluminescence kit (Pierce, Thermo Fisher Scientific).
Trophoblast adhesion assay
JAr cells were treated with conditioned media derived from OVGP1 knockdown cells and scrambled cells for 24 h. JAr cells were trypsinized and allowed to adhere to a 96-well plate. At the end of 6 h, the wells were washed three times with PBS, and cells were fixed and stained with 0.5% crystal violet (Sigma). After extensive washing, the cells were lysed, and the absorbance was measured at 590 nm. The experiment was done using two independent pools of media derived from scrambled and OVGP1 knockdown cells. Each experiment was carried out in triplicates.
Statistical analysis
The mean ± SD for all the experimental data was calculated, and statistical analysis was done using GraphPad Prism, version 5, either by Student’s t test or by one-way ANOVA using Tukey’s all column comparison test. p < 0.05 was accepted as statistically significant.
Results
OVGP1 is expressed in the mouse endometrium at the time of implantation
By immunofluorescence, the signals for OVGP1 were detected exclusively in the luminal epithelial cells of the endometrium from the pregnant mice (Fig. 1). Faint OVGP1 staining was observed in the luminal epithelium on the morning of day 4, the intensity increased on the evening of day 4 (Fig. 1a, b). This increase was not statistically significant (Fig. 1i). On the morning of day 5, OVGP1 expression increased further; and this increase was statistically significant (Fig. 1c, i). On the evening of day 5, the expression of OVGP1 appeared weaker as compared to day 5 morning; this reduction was statistically significant (Fig. 1d, i). In the oviduct (positive control), intense cytoplasmic staining for OVGP1 staining was detected; no staining was observed in the endometrium at both estrus and diestrus stages (Fig. 1e–g). No staining was observed in negative control without primary antibody (Fig. 1h).
Fig. 1.
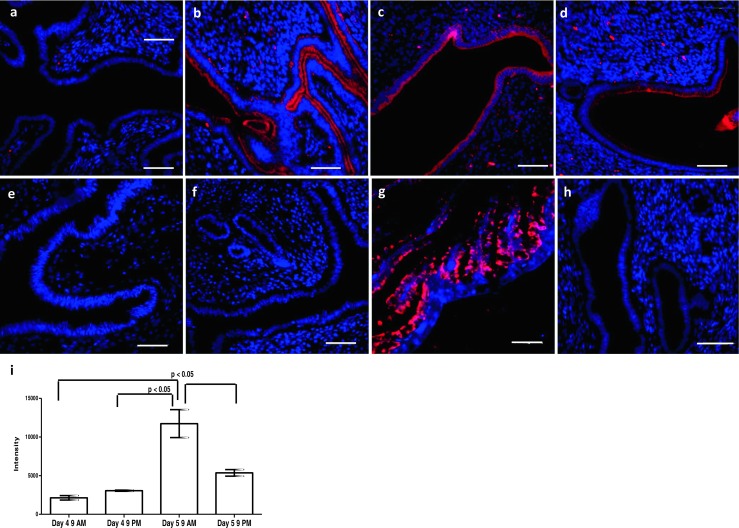
Expression of OVGP1 in mouse endometrium at time of embryo implantation. Paraffin sections were probed with OVGP1 antibody and detected with Alexa Fluor 568. a Day 4 morning. b Day 4 evening. c Day 5 morning. d Day 5 evening. e Estrus stage. f Diestrus stage. g Oviduct. h Negative control (without primary antibody). Red staining is for OVGP1, and blue is for the nuclei. Scale bar is 50 μm. i Intensity of OVGP1 staining during the course of embryo implantation. Y-axis is the intensity values for OVGP1 staining. Data is mean ± SD for the three biological replicates at each point. * indicates significant difference as compared to control (p < 0.05)
OVGP1 is expressed in Ishikawa cells
A single band of expected size (172 bp) for OVGP1 was detected by RT-PCR in cDNA of Ishikawa; no bands were observed in the reactions without reverse transcriptase. GAPDH is an internal control (Fig. 2a). The PCR products of OVGP1 showed 100% sequence similarity to human OVGP1 (data not shown). By immunofluorescence, intense cytoplasmic staining for OVGP1 was detected in Ishikawa cells; at higher magnification, the localization appears perinuclear (Fig. 2b). No OVGP1 staining was observed in the cell incubated without primary antibody. By Western blotting, three bands of approximately 110–130 kDa, 60–66 kDa, and 54 kDa were detected in Ishikawa cell lysate probed with anti-OVGP1 antibody. No bands were detected in negative control without primary antibody (Fig. 2c).
Fig. 2.
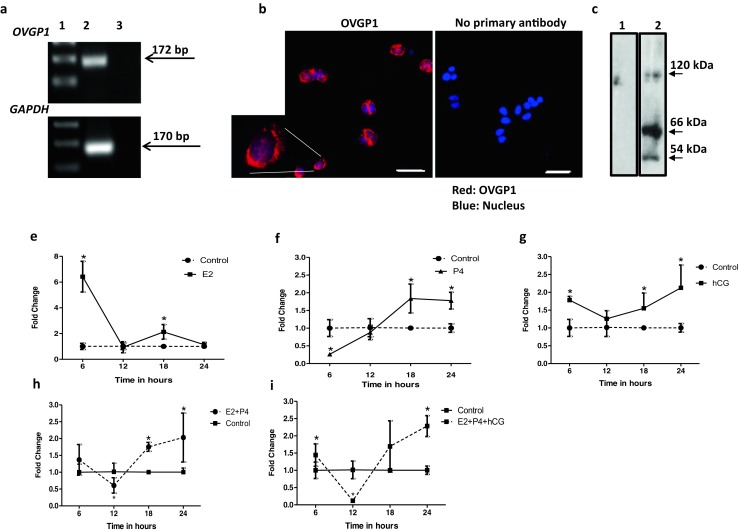
Effects of estrogen, progesterone, and human chorionic gonadotropin (hCG) on OVGP1 expression in human endometrial epithelial cells. a Expression of OVGP1 mRNA from Ishikawa cells. Lane 1 is 100 bp ladder, lane 2 cDNA from Ishikawa mRNA, and lane 3 no reverse transcriptase control. The bands for OVGP1 (172 bp) and GAPDH (170 bp) are shown by arrow marks. b Immunofluorescence for OVGP1 in Ishikawa cells. Red staining is for OVGP1, and blue is for the nuclei. Scale bar is 50 μm. A cell is digitally zoomed × 5 times and shown. c Western blotting for OVGP1 in protein isolated from Ishikawa cells. Lane 1 is negative control (incubated without primary antibody), and lane 2 is sample incubated with primary antibody. The approximate size of the bands is marked with an arrow. All the experiments were done at least three times. Ishikawa cells were treated with estrogen (E2), progesterone (P4), and hCG alone or in combinations (e–i) for varying time points, and level of OVGP1 mRNA was measured by real-time PCR. Y-axis is fold change as compared to untreated control at each time point (control value taken as 1). X-axes are time points in hours post-treatment. Values are mean ± SD for six replicates. * indicates mean value significant different (p < 0.05) as compared to respective control
Steroid hormones and human chorionic gonadotropin (hCG) regulate expression of OVGP1 in Ishikawa cells
As compared to controls, OVGP1 mRNA levels increased significantly (p < 0.05) at 6 and 18 h of estrogen treatment; the levels were not significantly different at 12 and 24 h (Fig. 2e). As compared to untreated controls, treatment with progesterone significantly reduced (p < 0.05) OVGP1 levels at 6 h of treatment, which further increases significantly (p < 0.05) at 18 h and remained high at 24 h post-treatment (Fig. 2f). The levels of OVGP1 were significantly (p < 0.05) high through the course of hCG treatment as compared to control, except for 12 h where the increase in expression was not significant (Fig. 2g).
As compared to controls, a combination of estrogen and progesterone significantly increased (p < 0.05) OVGP1 levels at 18 and 24 h of treatment (Fig. 2h). As compared to controls, the inclusion of hCG along with estrogen and progesterone increased OVGP1 mRNA levels at 6, 18, and 24 h. However, the increase was statistically significant (p < 0.05) only at 6 and 24 h (Fig. 2i). At 12 h, the levels of OVGP1 were significantly low (p < 0.05) as compared to controls.
Downregulation of OVGP1 alters the expression of endometrial receptivity-related genes in Ishikawa cells
As compared to scrambled shRNA-transfected Ishikawa cells (controls), there was 60% reduction in OVGP1 mRNA in cells transfected with shRNA against OVGP1 (Fig. 3a). The intensity of OVGP1 protein was also reduced. There was 50% reduction in levels of OVGP1 protein in knockdown cells as compared to control (Fig. 3b, c). In both cases, the reduction was statistically significant (p < 0.05).
Fig. 3.
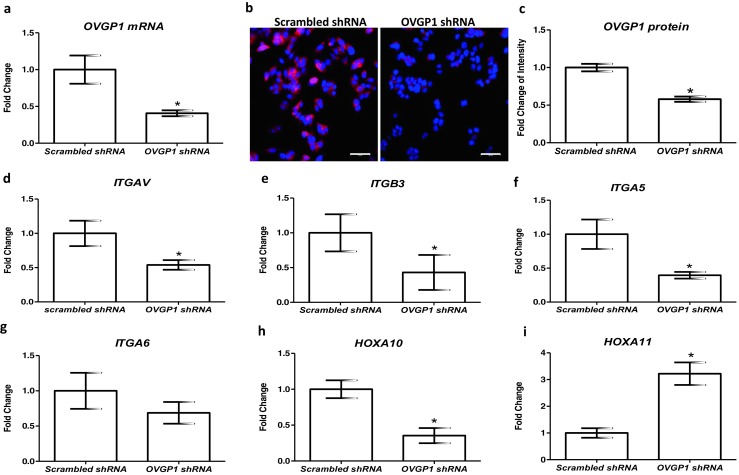
Effect of loss of OVGP1 on expression of receptivity markers in the endometrial epithelial cells. a mRNA levels of OVGP1 in scrambled and OVGP1 shRNA-transfected Ishikawa cells. Y-axis is fold change where values obtained from scrambled cells were taken as 1. b Immunofluorescence for OVGP1 in scrambled and knockdown Ishikawa cells. Red staining is for OVGP1, and blue is for the nuclei. Scale bar is 50 μm. c Intensity of OVGP1 staining in scrambled and OVGP1 shRNA-transfected Ishikawa cells. Y-axis is intensity values for OVGP1 staining. In both a and b, data is mean ± SD for the three independent replicates. * indicates significant difference as compared to control (p < 0.05). Effect of loss of OVGP1 on mRNA levels of ITGAV, ITGB3, ITGA5, ITGA6, HOXA10, and HOXA11 (d–i). Y-axis is fold change where values obtained from scrambled cells were taken as 1. Data is the mean ± SD for the three independent replicates. * indicates significant difference as compared to control (p < 0.05)
As compared to controls, the expression of ITGAV, ITGB3, and ITGA5 was significantly (p < 0.05) reduced in cells knockdown for OVGP1; ITGA6 mRNA levels were not significantly altered (Fig. 3d–g). The expression of HOXA10 mRNA was reduced significantly (p < 0.05), whereas HOXA11 mRNA levels were significantly (p < 0.05) increased in OVGP1 knockdown cells as compared to controls (Fig. 3h, i).
Downregulation of OVGP1 alters expression of cytokines, matrix metalloproteases, and tissue inhibitor of matrix metalloproteases in Ishikawa cells
As compared to controls, the mRNA levels of LIF and IL15 were significantly (p < 0.05) lowered in Ishikawa cells knockdown for OVGP1 (Fig. 4a, d). The expression of IL6, IL11, TGFB1, and PAEP was not significantly altered in OVGP1 knockdown cells as compared to controls (Fig. 4b–f). As compared to control, there was significant (p < 0.05) increase in the levels of MMP9, TIMP1, and TIMP3 in OVGP1 knockdown cells; the levels of MMP3 and TIMP2 were unaltered (Fig. 5).
Fig. 4.
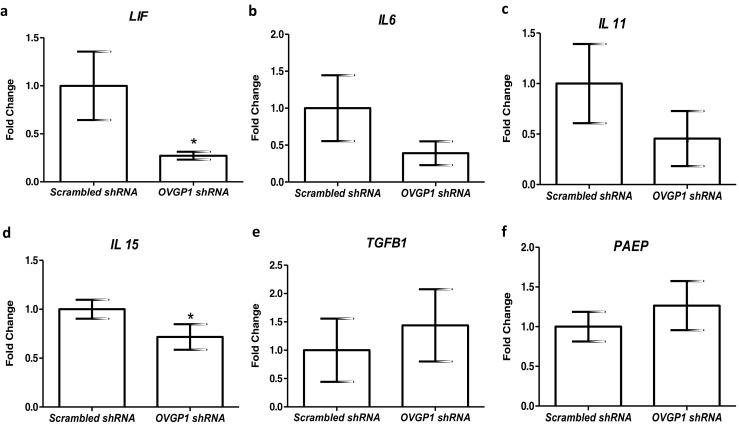
Effect of loss of OVGP1 on mRNA levels of LIF, IL6, IL11, IL15, TGFB1, and PAEP (a–f). Y-axis is fold change where values obtained from scrambled cells were taken as 1. Data is the mean ± SD for the three independent replicates. * indicates significant difference as compared to control (p < 0.05)
Fig. 5.
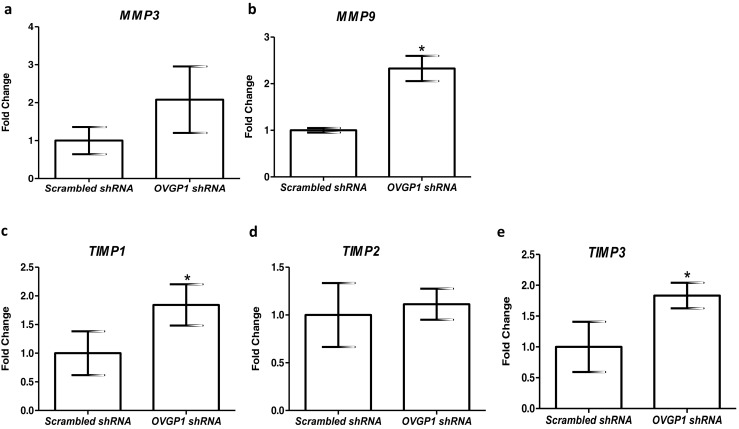
Effect of loss of OVGP1 on mRNA levels of MMP3, MMP9, TIMP1, TIMP2, and TIMP3 (a–d). Y-axis is fold change where values obtained from scrambled cells were taken as 1. Data is the mean ± SD for three independent replicates. * indicates significant difference as compared to control (p < 0.05)
Loss of OVGP1 in Ishikawa cells reduces the adhesion of trophoblast cells
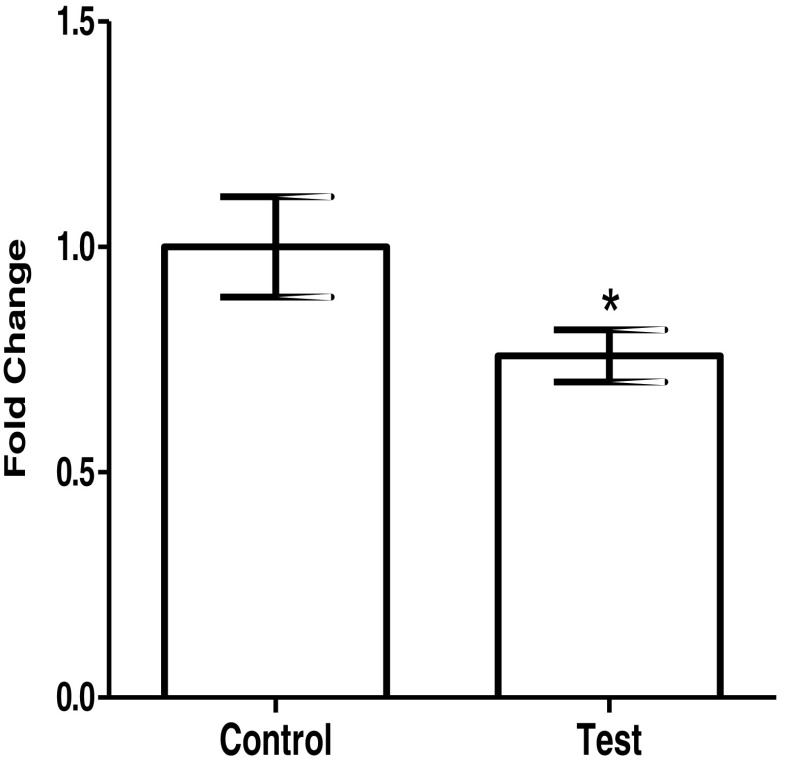
As compared to JAr cells incubated with conditioned media from scrambled shRNA-transfected Ishikawa cells, there was a decrease in adhesion of JAr cells treated with conditioned medium from OVGP1 knockdown cells (Fig. 6). There was 30% reduction in the adhesion, and this was statistically significant (p < 0.05).
Fig. 6.
Effect of loss of OVGP1 in the endometrial epithelial cells on adhesion of trophoblast cells. Control is conditioned media from Ishikawa cells transfected with scrambled shRNA. Test is conditioned media from Ishikawa cells transfected with OVGP1 shRNA. Trophoblast cells (JAr cell line) were treated with the conditioned media for 24 h, trypsinized, and allowed to adhere for 6 h. The number of adherent cells was measured as described in the “Materials and methods” section. Values on Y indicate fold change where values obtained from control were taken as 1. Data are the mean ± SD of absorbance from two independent experiments done in triplicates. * indicates significant difference as compared to control (p < 0.05)
Discussion
The results of the present study demonstrate that OVGP1 is expressed exclusively in the luminal epithelial cells of the mouse at the time of embryo apposition. Further, we demonstrate that loss of OVGP1 in human endometrial epithelial cells alters the expression of genes involved in endometrial receptivity. Finally, the results also reveal that culture medium derived from epithelial cells knockdown for OVGP1 reduces the in vitro adhesiveness of trophoblast cells.
Previous studies have demonstrated that OVGP1 is not expressed by the cycling endometrium of mice and monkeys [27, 29, 32]. However, microarray analysis of isolated cells from human endometrium reveals significant enrichment of OVGP1 mRNA in the epithelial cells (Supplementary Fig. 1). Interestingly, in the present study, we observed that in the presence of the embryo, OVGP1 protein is expressed by the luminal epithelial cells at the time of implantation. This gain of expression is due to the transcription of Ovgp1 by the endometrial cells as its mRNA is detected in the endometria of pregnant mice (Supplementary Fig. 2). A similar increase in OVGP1 expression is observed in the luminal epithelial cells at the time of embryo apposition in the hamster [33]. These results together suggest that OVGP1 is induced in the endometrial epithelium at the time of implantation.
Since OVGP1 is induced by the endometrial epithelial cells in the presence of the embryo, we next asked if embryonic signals induce OVGP1 expression in the endometrial epithelium. For this purpose, we screened several cell lines of human endometrium and observed that Ishikawa cells express significant amounts of OVGP1 mRNA and protein; three bands of OVGP1 corresponding to pre-processed (60–66 kDa), processed (54 kDa), and glycosylated (110–130 kDa) forms were detected. Furthermore, the protein was localized in the perinuclear region; OVGP1 was also detected on the surface of Ishikawa cells and conditioned medium (data not shown) suggesting that OVGP1 is transcribed, translated, and secreted by Ishikawa cells. Thus, Ishikawa cells were chosen to carry out further studies on regulation and roles of OVGP1. Embryo implantation involves coordinated signaling from the embryo and also the ovarian steroids estrogen and progesterone. It is known, that in the progesterone-primed endometrium, a peak in estrogen is essential to promote implantation, and this peak of estrogen brings about the coordinated expression of several genes essential for receptivity [3–7, 38]. Furthermore, this steroid-driven signature of the endometrium is modulated by hCG secreted from the embryo [15–17]. Thus, we studied the effects of estrogen, progesterone, and hCG alone or in combination to test the regulation of OVGP1 in the endometrial epithelium. Treatment of estrogen transiently increases the OVGP1 expression in Ishikawa cells. Interestingly, in the human endometrium, OVGP1 mRNA levels are highest in the proliferative phase which coincides with estrogen peak (Supplementary Fig. 3). Indeed, in the oviduct, OVGP1 is known to be induced by estrogen [29], and OVGP1 promoter contains ten half-palindromic estrogen responsive elements [28]. Interestingly, along with estrogen, hCG consistently and significantly increased the OVGP1 expression in Ishikawa cells. These results suggest that the increase in OVGP1 observed in the luminal epithelium at the time of embryo implantation is perhaps due to the combined effect of estrogen and hCG.
We next assessed the functional significance of OVGP1 in the endometrial epithelial cells in the context of embryo implantation. For this purpose, endogenous expression OVGP1 was silenced in Ishikawa cells using shRNA, and the expression of genes associated with receptivity was studied. Integrins are required for embryo implantation, and the aberrant expression of integrins is observed in the endometria of women with unexplained infertility [39, 40]. HOXA10 and HOXA11 are essential for uterine receptivity and mice null for both these are infertile due to the failure of the embryo to implant (reviewed in [41, 42]). In addition, women with unexplained infertility have reduced expression of HOXA10 in their uteri [42]. From our study, it appears that OVGP1 is required to maintain the expression of integrins and HOXA10 in the endometrial epithelium, as inhibition of OVGP1 expression reduced the mRNA levels of HOXA10, ITGAV, ITGA5, and ITGB3. Interestingly, at the same time, the levels of HOXA11 were elevated upon the reduction of OVGP1; the levels of PAEP and TGFB1 were unaltered. Since HOXA10 controls integrin expression and permits pinopode formation [43, 44], it is possible that OVGP1 aids this process by maintaining the levels of these molecules in the receptive endometrium. Together, these results suggest that OVGP1 is required to maintain the balance of receptivity-related molecules in the endometrial epithelium.
Cytokines play an integral role in embryo implantation and stromal cell decidualization. LIF, IL6, IL11, and IL15 are secreted by the uterine epithelium that are required for decidualization; they also promote trophoblast proliferation, adhesion, and invasion [45–50]. Thus, we tested if OVGP1 has any role in the regulation of cytokine expression. The results revealed that the levels of LIF and IL15 were reduced in cell knockdown for OVGP1; the levels of IL6 and IL11 were also low, albeit not significantly. These results indicate that endometrial OVGP1 might aid embryo implantation by controlling cytokine expression for decidualization and trophoblast invasion.
One of the requirements for embryo implantation is degradation of extracellular matrix proteins of the endometrium. This is achieved by increasing the production of proteases such as matrix metalloproteases (MMPs) and decreasing the activity of tissue inhibitors of matrix metalloproteases (TIMPs). To test if OVGP1 has any role in the regulation of protease activity at the time of implantation, we measured the mRNA levels of MMP3, MMP9, TIMP1, TIMP2, and TIMP3 in Ishikawa cells knockdown for OVGP1. The results revealed an increase in the expression of MMP9, TIMP1, and TIMP3 in response to the loss of OVGP1. MMP9 is a key enzyme required for degradation of collagenase, and an increase in its expression is associated with loss of tissue integrity [51]. Thus, an excess of MMP9 might be detrimental for embryo and endometrial cells. While TIMPs are considered as an antagonist for MMPs, mice transgenic for TIMP1 have smaller deciduas and higher resorption rates [52]. These observations suggest that high TIMP1 is also detrimental for implantation. Excess of TIMP3 is known to inhibit matrix degradation at the time of implantation and trophoblast invasion [46]. Thus, it is plausible that OVGP1 in the epithelium may be required to balance the production of MMPs and TIMPs and control ECM remodeling for implantation.
For successful implantation, the embryonic trophoblast must adhere to the endometrial epithelium. While the role of epithelial integrins and membrane-bound proteins in the process of trophoblast adhesion is undisputable [6, 21], the process is also governed by secretory growth factors, cytokines, chemokines, and other molecules [37, 47, 50]. From our results, it is evident that the levels of cytokines, MMPs, and TIMPs are altered in epithelial cells knockdown for OVGP1. Since these molecules are known to alter trophoblast physiology [46], we asked if secretions from OVGP1 knockdown cells have any effects on trophoblast adhesion. Indeed, trophoblast cells treated with conditioned medium of OVGP1 knockdown cells had lower adhesiveness suggesting that epithelial OVGP1 probably maintains a secretome that is conducive for embryonic attachment. However, it is intriguing to note that mouse knockout for Ovgp1 is fertile, but they have reduced litter size [53]. Also, OVGP1 transcripts are significantly reduced in the secretory phase endometria of women with recurrent implantation failure (Supplementary Fig. 4). Based on these results, we suggest that OVGP1 might have critical roles in embryo implantation.
To summarize, in the present study, for the first time, we demonstrate that OVGP1 is specifically induced in the luminal epithelium at the time of embryo implantation; this increase in OVGP1 is required to maintain the balanced expression of receptivity-related genes and aids in trophoblast adhesion. This study adds one more molecule to the growing list of embryo-induced factors in endometrium required for implantation. We believe that identification of such factors will expand our knowledge of the mechanisms involved in embryo implantation, and in long-term such studies might aid in improving pregnancy rates of assisted reproduction.
Electronic supplementary material
(DOCX 22 kb)
Distribution of OVGP1 mRNA in different cell types of human endometrium. Data was extracted from GEO dataset accession no. GDS4987. In that study, endometrial cell types were separated using fluorescence-assisted cell sorting from proliferative stage endometrial biopsies and subjected microarray analysis. The values of the Y-axis are mean ± SEM of fold change. Fold change for each sample was calculated using mean values of epithelial cells taken as 1. The number of samples analyzed for each cell type is indicated (n). Each dot represents levels of OVGP1 in an individual. (PNG 168 kb)
mRNA expression of Ovgp1 in mouse endometrium at time of embryo implantation. Panel A is the representative gel image for Ovgp1, and panel B is the representative gel image for housekeeping gene Gapdh. The bands for Ovgp1 (185 bp) and Gapdh (179 bp) are shown by arrows. In both panels, lane 1 is the 100 bp ladder, lane 2 is the oviduct, lane 3 is the uterus at diestrus stage, lane 4 is the uterus on day 4 morning, lane 5 is the uterus on day 4 evening, lane 6 is the uterus on day 5 morning, (day of vaginal plus as day 1), and lane 7 is the negative control without template. (PNG 228 kb)
Cyclic changes in OVGP1 mRNA in human endometrium. Data was extracted from GEO dataset GDS2052. In this study, RNA from endometrial tissue from women at different stages of menstrual cycle were collected and subjected to microarray. The values of the Y-axis are mean ± SEM of fold change. Fold change for each sample was calculated using mean values of proliferative stage taken as 1. The number of samples analyzed for each stage of the menstrual cycle is indicated (n). Each point represents the levels of OVGP1 in an individual. (PNG 173 kb)
Levels of OVGP1 mRNA in the endometrium of women with recurrent implantation failure. The data was retrieved from GEO dataset accession no. GSE58144. In that study, endometrial biopsies were collected at mid-luteal phase from women undergoing IVF/ICSI. The control group consisted of a woman who conceived within first three IVF/ICSI cycles, whereas women with more than three failed IVF/ICSI treatment or replacement ≥ 10 embryos were categorized as recurrent implantation failure. The RNA was extracted and subjected to microarray. The values of the Y-axis are mean ± SEM of fold change. Fold change for each sample was calculated using mean values of control was taken as 1. The number of samples in each group is indicated (n). Each dot represents the levels of OVGP1 in an individual. (PNG 205 kb)
Acknowledgements
We express our gratitude to Dr. Geetanjali Sachdeva for generously giving the Ishikawa and JAr cells. We are thankful to Mr. Abhishek Tiwari (intern at NIRRH) for the technical help. The present study (RA/597/01-2018) is funded by intra-mural grants from ICMR to DM and NMIMS (Deemed-to-be University) to PB.
Funding source
Indian Council of Medical Research (ICMR), Government of India; Department of Biotechnology, Government of India; and SVKM’s NMIMS (Deemed-to-be University).
Compliance with ethical standards
The study was approved by the Institutional Animal Ethics Committee (IAEC) of the National Institute for Research in Reproductive (NIRRH).
The authors declare that they have no conflict of interests.
References
- 1.Polanski LT, Baumgarten MN, Quenby S, Brosens J, Campbell BK, Raine-Fenning NJ. What exactly do we mean by ‘recurrent implantation failure’? A systematic review and opinion. Reprod BioMed Online. 2014;28(4):409–423. doi: 10.1016/j.rbmo.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 2.European IVF-monitoring Consortium Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Hum Reprod. 2017;32(10):1957–1973. doi: 10.1093/humrep/dex264. [DOI] [PubMed] [Google Scholar]
- 3.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modi DN, Bhartiya P. Physiology of embryo-endometrial cross talk. Biomed Res J. 2015;2:83–104. [Google Scholar]
- 5.Evans J, Salamonsen LA, Winship A, Menkhorst E, Nie G, Gargett CE, Dimitriadis E. Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol. 2016;12(11):654–667. doi: 10.1038/nrendo.2016.116. [DOI] [PubMed] [Google Scholar]
- 6.Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25(3):341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 8.Altmäe S, Koel M, Võsa U, Adler P, Suhorutšenko M, Laisk-Podar T, Kukushkina V, Saare M, Velthut-Meikas A, Krjutškov K, Aghajanova L. Meta-signature of human endometrial receptivity: a meta-analysis and validation study of transcriptomic biomarkers. Sci Rep. 2017;7(1):10077. doi: 10.1038/s41598-017-10098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Díaz-Gimeno P, Ruiz-Alonso M, Sebastian-Leon P, Pellicer A, Valbuena D, Simón C. Window of implantation transcriptomic stratification reveals different endometrial subsignatures associated with live birth and biochemical pregnancy. Fertil Steril. 2017;108(4):703–710. doi: 10.1016/j.fertnstert.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Teh WT, McBain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet. 2016;33(11):1419–1430. doi: 10.1007/s10815-016-0773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdes CT, Schutt A, Simon C. Implantation failure of endometrial origin: it is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil Steril. 2017;108(1):15–18. doi: 10.1016/j.fertnstert.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 12.Rosario GX, Modi DN, Sachdeva G, Manjramkar DD, Puri CP. Morphological events in the primate endometrium in the presence of a preimplantation embryo, detected by the serum preimplantation factor bioassay. Hum Reprod. 2005;20:61–71. doi: 10.1093/humrep/deh534. [DOI] [PubMed] [Google Scholar]
- 13.Godbole GB, Modi DN, Puri CP. Regulation of homeobox A10 expression in the primate endometrium by progesterone and embryonic stimuli. Reproduction. 2007;134(3):513–523. doi: 10.1530/REP-07-0234. [DOI] [PubMed] [Google Scholar]
- 14.Nimbkar-Joshi S, Katkam RR, Chaudhari UK, Jacob S, Manjramkar DD, Metkari SM, Hinduja I, Mangoli V, Desai S, Kholkute SD, Puri CP, Sachdeva G. Endometrial epithelial cell modifications in response to embryonic signals in bonnet monkeys (Macaca radiata) Histochem Cell Biol. 2012;138:289–304. doi: 10.1007/s00418-012-0951-2. [DOI] [PubMed] [Google Scholar]
- 15.Modi DN, Godbole G, Suman P, Gupta SK. Endometrial biology during trophoblast invasion. Front Biosci (Schol Ed) 2012;4:1151–1171. doi: 10.2741/s323. [DOI] [PubMed] [Google Scholar]
- 16.Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci U S A. 1999;96:2543–2548. doi: 10.1073/pnas.96.5.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, Brudney A, Knight O, Fazleabas AT. In vivo infusion of interleukin-1β and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinol. 2005;146:4097–4104. doi: 10.1210/en.2005-0380. [DOI] [PubMed] [Google Scholar]
- 18.Koot YE, Van Hooff SR, Boomsma CM, Van Leenen D, Koerkamp MJ, Goddijn M, Eijkemans MJ, Fauser BC, Holstege FC, Macklon NS. An endometrial gene expression signature accurately predicts recurrent implantation failure after IVF. Sci Rep. 2016;6:19411. doi: 10.1038/srep19411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashary N, Tiwari A, Modi D. Embryo implantation: war in times of love. Endocrin. 2018;159:1188–1198. doi: 10.1210/en.2017-03082. [DOI] [PubMed] [Google Scholar]
- 20.Clark GF. Functional glycosylation in the human and mammalian uterus. Fertil Res Pract. 2015;1:17. doi: 10.1186/s40738-015-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tu Z, Ran H, Zhang S, Xia G, Wang B, Wang H. Molecular determinants of uterine receptivity. Int J Dev Biol. 2014;58:147–154. doi: 10.1387/ijdb.130345wh. [DOI] [PubMed] [Google Scholar]
- 22.Lee CL, Lam KK, Vijayan M, Koistinen H, Seppala M, Ng EH, Yeung WS, Chiu PC. The pleiotropic effect of Glycodelin-A in early pregnancy. Am J Reprod Immunol. 2016;75:290–297. doi: 10.1111/aji.12471. [DOI] [PubMed] [Google Scholar]
- 23.Bastu E, Mutlu MF, Yasa C, Dural O, Aytan AN, Celik C, Buyru F, Yeh J. Role of Mucin 1 and Glycodelin A in recurrent implantation failure. Fertilit Steril. 2015;103:1059–1064. doi: 10.1016/j.fertnstert.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Focarelli R, Luddi A, De Leo V, Capaldo A, Stendardi A, Pavone V, Benincasa L, Belmonte G, Petraglia F, Piomboni P. Dysregulation of GdA expression in endometrium of women with endometriosis: implication for endometrial receptivity. Reprod Sci 2017;1933719117718276. [DOI] [PubMed]
- 25.Singh H, Aplin JD. Adhesion molecules in endometrial epithelium: tissue integrity and embryo implantation. J Anat. 2009;215(1):3–13. doi: 10.1111/j.1469-7580.2008.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Y, Ma X, Deng L, Yao B, Xiong Y, Wu Y, Wang L, Ma Q, Ma F. Role of selectins and their ligands in human implantation stage. Glycobiology. 2017;27(5):385–391. doi: 10.1093/glycob/cwx009. [DOI] [PubMed] [Google Scholar]
- 27.Natraj U, Bhatt P, Vanage G, Moodbidri SB. Overexpression of monkey oviductal protein: purification and characterization of recombinant protein and its antibodies. Biol Reprod. 2002;67:1897–1906. doi: 10.1095/biolreprod67.6.1897. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt P, Kadam K, Saxena A, Natraj U. Fertilization, embryonic development and oviductal environment: role of estrogen induced oviductal glycoprotein. Indian J Exp Biol. 2004;42:1043–1055. [PubMed] [Google Scholar]
- 29.Buhi WC. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction. 2002;123:355–362. doi: 10.1530/rep.0.1230355. [DOI] [PubMed] [Google Scholar]
- 30.Choudhary S, Kumaresan A, Kumar M, Chhillar S, Malik H, Kumar S, Kaushik JK, Datta TK, Mohanty AK. Effect of recombinant and native buffalo OVGP1 on sperm functions and in vitro embryo development: a comparative study. J Anim Sci Biotechnol. 2017;8:69. doi: 10.1186/s40104-017-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- 32.Laheri S, Modi D, Bhatt P. Extra-oviductal expression of oviductal glycoprotein 1 in mouse: detection in testis, epididymis and ovary. J Biosci. 2017;42:69–80. doi: 10.1007/s12038-016-9657-2. [DOI] [PubMed] [Google Scholar]
- 33.Roux E, Bleau G, Kan FW. Fate of hamster oviductin in the oviduct and uterus during early gestation. Molecular Reproduction and Development Mol Reprod Dev. 1997;46:306–317. doi: 10.1002/(SICI)1098-2795(199703)46:3<306::AID-MRD9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 34.Uchida H, Maruyama T, Nishikawa-Uchida S, Oda H, Miyazaki K, Yamasaki A, Yoshimura Y. Studies using an in vitro model show evidence of involvement of epithelial-mesenchymal transition of human endometrial epithelial cells in human embryo implantation. J Biol Chem. 2012;287:4441–4450. doi: 10.1074/jbc.M111.286138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruane PT, Berneau SC, Koeck R, Watts J, Kimber SJ, Brison DR, Westwood M, Aplin JD. Apposition to endometrial epithelial cells activates mouse blastocysts for implantation. Mol Hum Reprod. 2017;23:617–627. doi: 10.1093/molehr/gax043. [DOI] [PubMed] [Google Scholar]
- 36.Godbole G, Modi D. Regulation of decidualization, interleukin-11 and interleukin-15 by homeobox A 10 in endometrial stromal cells. J Reprod Immunol. 2010;85:130–139. doi: 10.1016/j.jri.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Godbole G, Suman P, Malik A, Galvankar M, Joshi N, Fazleabas A, Gupta SK, Modi D. Decrease in expression of HOXA10 in the decidua after embryo implantation promotes trophoblast invasion. Endocrin. 2017;158:2618–2633. doi: 10.1210/en.2017-00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhurke AS, Bagchi IC, Bagchi MK. Progesterone-regulated endometrial factors controlling implantation. Am J Reprod Immunol. 2016;75:237–245. doi: 10.1111/aji.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Germeyer A, Savaris RF, Jauckus J, Lessey B. Endometrial beta3 integrin profile reflects endometrial receptivity defects in women with unexplained recurrent pregnancy loss. Reprod Biol Endocrinol. 2014;12(1):53. doi: 10.1186/1477-7827-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elnaggar A, Farag AH, Gaber ME, Hafeez MA, Ali MS, Atef AM. AlphaVBeta3 integrin expression within uterine endometrium in unexplained infertility: a prospective cohort study. BMC Womens Health. 2017;17:90. doi: 10.1186/s12905-017-0438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modi D, Godbole G. HOXA10 signals on the highway through pregnancy. J Reprod Immunol. 2009;83:72–78. doi: 10.1016/j.jri.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Du H, Taylor HS. The role of Hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb Perspect Med. 2016;6(1):a023002. doi: 10.1101/cshperspect.a023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagot CN, Kliman HJ, Taylor HS. Maternal Hoxa10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev Dyn. 2001;222:538–544. doi: 10.1002/dvdy.1209. [DOI] [PubMed] [Google Scholar]
- 44.Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. Direct regulation of β3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol. 2002;16(3):571–579. doi: 10.1210/mend.16.3.0792. [DOI] [PubMed] [Google Scholar]
- 45.Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J Endocrinol. 2011;210(1):5–14. doi: 10.1530/JOE-10-0461. [DOI] [PubMed] [Google Scholar]
- 46.Sharma S, Godbole G, Modi D. Decidual control of trophoblast invasion. Am J Reprod Immunol. 2016;75:341–350. doi: 10.1111/aji.12466. [DOI] [PubMed] [Google Scholar]
- 47.Dimitriadis E, Menkhorst E, Salamonsen LA, Paiva PLIF. IL11 in trophoblast-endometrial interactions during the establishment of pregnancy. Placenta. 2010;31:99–104. doi: 10.1016/j.placenta.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 48.Shuya LL, Menkhorst EM, Yap J, Li P, Lane N, Dimitriadis E. Leukemia inhibitory factor enhances endometrial stromal cell decidualization in humans and mice. PLoS One. 2011;6:e25288. doi: 10.1371/journal.pone.0025288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35:851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 50.Gupta SK, Malhotra SS, Malik A, Verma S, Chaudhary P. Cell signaling pathways involved during invasion and syncytialization of trophoblast cells. Am J Reprod Immunol. 2016;75:361–371. doi: 10.1111/aji.12436. [DOI] [PubMed] [Google Scholar]
- 51.Salamonsen LA, Evans J, Nguyen HPT, Edgell TA. The microenvironment of human implantation: determinant of reproductive success. Am J Reprod Immunol. 2016;75:218–225. doi: 10.1111/aji.12450. [DOI] [PubMed] [Google Scholar]
- 52.Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, Werb Z. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122:1723–1736. doi: 10.1242/dev.122.6.1723. [DOI] [PubMed] [Google Scholar]
- 53.Araki Y, Nohara M, Yoshida-Komiya H, Kuramochi T, Mamoru IT, Hoshi H, Shinkai Y, Sendai Y. Effect of a null mutation of the oviduct-specific glycoprotein gene on mouse fertilization. Biochem J. 2003;374:551–557. doi: 10.1042/bj20030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 22 kb)
Distribution of OVGP1 mRNA in different cell types of human endometrium. Data was extracted from GEO dataset accession no. GDS4987. In that study, endometrial cell types were separated using fluorescence-assisted cell sorting from proliferative stage endometrial biopsies and subjected microarray analysis. The values of the Y-axis are mean ± SEM of fold change. Fold change for each sample was calculated using mean values of epithelial cells taken as 1. The number of samples analyzed for each cell type is indicated (n). Each dot represents levels of OVGP1 in an individual. (PNG 168 kb)
mRNA expression of Ovgp1 in mouse endometrium at time of embryo implantation. Panel A is the representative gel image for Ovgp1, and panel B is the representative gel image for housekeeping gene Gapdh. The bands for Ovgp1 (185 bp) and Gapdh (179 bp) are shown by arrows. In both panels, lane 1 is the 100 bp ladder, lane 2 is the oviduct, lane 3 is the uterus at diestrus stage, lane 4 is the uterus on day 4 morning, lane 5 is the uterus on day 4 evening, lane 6 is the uterus on day 5 morning, (day of vaginal plus as day 1), and lane 7 is the negative control without template. (PNG 228 kb)
Cyclic changes in OVGP1 mRNA in human endometrium. Data was extracted from GEO dataset GDS2052. In this study, RNA from endometrial tissue from women at different stages of menstrual cycle were collected and subjected to microarray. The values of the Y-axis are mean ± SEM of fold change. Fold change for each sample was calculated using mean values of proliferative stage taken as 1. The number of samples analyzed for each stage of the menstrual cycle is indicated (n). Each point represents the levels of OVGP1 in an individual. (PNG 173 kb)
Levels of OVGP1 mRNA in the endometrium of women with recurrent implantation failure. The data was retrieved from GEO dataset accession no. GSE58144. In that study, endometrial biopsies were collected at mid-luteal phase from women undergoing IVF/ICSI. The control group consisted of a woman who conceived within first three IVF/ICSI cycles, whereas women with more than three failed IVF/ICSI treatment or replacement ≥ 10 embryos were categorized as recurrent implantation failure. The RNA was extracted and subjected to microarray. The values of the Y-axis are mean ± SEM of fold change. Fold change for each sample was calculated using mean values of control was taken as 1. The number of samples in each group is indicated (n). Each dot represents the levels of OVGP1 in an individual. (PNG 205 kb)