Summary
Background
A third of deaths in the UK from ruptured abdominal aortic aneurysm (AAA) are in women. In men, national screening programmes reduce deaths from AAA and are cost-effective. The benefits, harms, and cost-effectiveness in offering a similar programme to women have not been formally assessed, and this was the aim of this study.
Methods
We developed a decision model to assess predefined outcomes of death caused by AAA, life years, quality-adjusted life years, costs, and the incremental cost-effectiveness ratio for a population of women invited to AAA screening versus a population who were not invited to screening. A discrete event simulation model was set up for AAA screening, surveillance, and intervention. Relevant women-specific parameters were obtained from sources including systematic literature reviews, national registry or administrative databases, major AAA surgery trials, and UK National Health Service reference costs.
Findings
AAA screening for women, as currently offered to UK men (at age 65 years, with an AAA diagnosis at an aortic diameter of ≥3·0 cm, and elective repair considered at ≥5·5cm) gave, over 30 years, an estimated incremental cost-effectiveness ratio of £30 000 (95% CI 12 000–87 000) per quality-adjusted life year gained, with 3900 invitations to screening required to prevent one AAA-related death and an overdiagnosis rate of 33%. A modified option for women (screening at age 70 years, diagnosis at 2·5 cm and repair at 5·0 cm) was estimated to have an incremental cost-effectiveness ratio of £23 000 (9500–71 000) per quality-adjusted life year and 1800 invitations to screening required to prevent one AAA-death, but an overdiagnosis rate of 55%. There was considerable uncertainty in the cost-effectiveness ratio, largely driven by uncertainty about AAA prevalence, the distribution of aortic sizes for women at different ages, and the effect of screening on quality of life.
Interpretation
By UK standards, an AAA screening programme for women, designed to be similar to that used to screen men, is unlikely to be cost-effective. Further research on the aortic diameter distribution in women and potential quality of life decrements associated with screening are needed to assess the full benefits and harms of modified options.
Funding
UK National Institute for Health Research Health Technology Assessment programme.
Introduction
Abdominal aortic aneurysm (AAA) traditionally has been considered a disease of men, strongly associated with smoking. However, a third of deaths caused by AAA rupture are in women.1, 2 In men, synthesis of four randomised trials has shown a benefit of population-based screening in reducing AAA-related mortality by up to 40% although any reduction in all-cause mortality is small.3, 4 Several countries including Sweden and the UK have introduced cost-effective population screening programmes for AAA in men aged at least 65 years, and screening for older men is available in the USA, and regionally in Italy and other countries.5, 6, 7, 8 The only randomised trial of AAA screening in women, which was done in the 1990s, was underpowered.9 In 2014, the US Preventive Services Task Force recommended against screening in women.7 The reasons for this recommendation include the reportedly lower prevalence of AAA in women, on the basis of the maximum aortic diameter threshold of at least 3 cm, and paucity of evidence about the management of AAA in women.10 However, with long-term health-economic modelling in men suggesting that population-based screening would be cost-effective with an AAA prevalence as low as 0·35–0·5%, smoking now almost as common in women as in men, and with the association between smoking and AAA almost twice as strong for women compared with men, the case for AAA screening in women needs to be formally assessed.11, 12, 13
There would be no quick answers from doing a randomised trial of AAA screening in women, because of the large sample size and long-term follow-up that would be required. The alternative is long-term modelling. This requires contemporary and reliable estimates of parameters that can influence the clinical and cost-effectiveness of screening in women. The aim of this project was to obtain this information and then apply discrete event simulation modelling to explore the hypothesis that a variation of the current AAA screening programmes for men might prove clinically beneficial and cost-effective in reducing deaths from ruptured AAA in women.
Research in context.
Evidence before this study
Historically, abdominal aortic aneurysm (AAA) has been considered to be a disease in older male smokers, with prevalence being 4–5 times higher in men than in women. We searched MEDLINE, Embase, and CENTRAL using the terms “abdominal aortic aneurysm”, “aneurysm”, “women” OR “gender” OR “sex” OR “women's health” OR “sex difference”, “prevalence” OR “incidence” OR “occurrence” OR “frequency”, “screening”, and “population” OR “population-based”. The search was restricted to major European languages and the final search date was Jan 15, 2017. Four randomised trials of population screening in older men have shown that screening can reduce AAA-related deaths by up to half, meta-analysis of these trials indicate a small decrease in all-cause mortality, and associated studies have shown that screening is cost-effective. Therefore, in many countries or regions, there are programmes for ultrasonographic AAA screening for men. In women, AAA screening is not recommended as there has been only a single underpowered randomised trial to date. However, the rupture rate of small AAAs is four times higher in women than in men, and a third of the deaths from AAA rupture are in women. Moreover, women with incidentally detected AAA are disadvantaged with respect to availability of elective repair, the types of treatment available, and the higher elective operative mortality and complication rates compared with men. Although the effect of AAA screening on quality of life has been assessed in men, the instruments used might not be sensitive to detect either small changes or changes in specific health domains such as depression and emotional status and, to our knowledge, there have been no studies in women to date.
Added value of this study
To our knowledge, this is the first study to formally assess the long-term benefits, costs, and harms of screening women aged 65 years and older for AAA. Contemporary systematic reviews, original data, clinical trials, and registries were used to obtain woman-specific parameters to feed into a discrete event simulation model for AAA screening, surveillance and intervention. The model estimated the numbers of key screening and clinical events over time for 10 million women enrolled in a screening programme with a UK costs perspective. The flexibility of the model permitted assessment of the clinical and cost-effectiveness of screening women using the same protocol used for the nationwide UK AAA screening programme in men and a range of different scenarios that might be more suitable for women (eg, changing the age at screening, lowering the diagnosis threshold, or reducing the intervention threshold).
Implications of all the available evidence
Our findings suggest that a screening programme of women aged 65 years—using the same screening, surveillance, and intervention protocol as defined for men in the UK—would need 3900 women to be invited to screening to save one death from AAA; a third of the screening-detected AAAs would be overdiagnosed and such a programme would not be cost-effective in the UK. The best alternative screening scenario for women would be screening at age 70 years, diagnosis of AAA when the maximum aortic diameter reaches 2·5 cm, and with intervention considered when the AAA diameter reaches 5·0 cm. In this scenario 1800 women would need to be invited to screening to save one death from AAA, but overdiagnosis would occur in more than half of screen-detected AAAs. By contrast, for AAA screening in men aged 65 years, recent estimates have shown that fewer than 700 men need to be screened to avoid one AAA-related death. There is considerable uncertainty as to whether this best alternative scenario in women would be cost-effective because of uncertainty in the key input parameter of AAA prevalence at different ages and a lack of information about the quality of life decrements associated with screening. Therefore, urgent research on the population-based aortic diameter distribution in older women, and on the quality of life decrements associated with screening, is necessary before closing the door on the possibility that in some health-care systems, population screening for AAA in women might be cost-effective.
Methods
Study design and participants
A decision model was developed to assess the differences in life-years, quality-adjusted life-years (QALYs) and costs for a population of women invited to AAA screening versus a population who were not invited to screening (the status quo). The West of Scotland Research Ethics Committee (number 5) provided a favourable ethical opinion for the project (reference 15/WS/0136). A public focus group was convened to provide input to the project (appendix).
The reference case assessed the long-term cost-effectiveness of an invitation to a single ultrasound screen for all women in the UK at age 65 years, based on the UK National Abdominal Aortic Aneurysm Screening Programme (NAAASP) for men; namely a diagnosis of AAA when aortic diameter was at least 3·0 cm, annual surveillance for individuals with the smallest AAA (3·0–4·4 cm) at their most recent scan, a surveillance scan every 3 months for those with a medium AAA (4·5–5·4 cm), and referral to a vascular surgeon for a large AAA (5·5 cm).6 Those with no AAA detected would not be rescreened. We took a UK National Health Service (NHS) perspective for costs including invitation, screening, consultations, elective surgery, and emergency surgery for ruptured AAAs.14
A total of 12 different screening options were assessed, including changing the age at screening from 65 to 70 years, lowering the diagnosis threshold from 3·0 cm to 2·5 cm (on the basis of available data on aortic diameter distribution in older women15), and reducing the intervention threshold from 5·5 cm to 5·0 cm or 4·5 cm. The most cost-effective option was selected as the best alternative screening strategy.
Modelling
A discrete event simulation model was designed and implemented, as described in detail elsewhere.16 Briefly, a previous multi-state Markov model of AAA screening in men11 was redeveloped and programmed as a more flexible discrete event simulation model to allow rapid assessment of different screening options, and was validated for men against data from the randomised Multicentre Aneurysm Screening Study.16 The model simulated a sequence of key screening and clinical events for 10 million women (with results presented per 1 million women invited) from the time of invitation to screening up to their date of death or age 95 years (the time horizon). Each woman had a counterpart who shared some key characteristics (age, aortic diameter at baseline, rate of aortic growth, or potential time of non-AAA-related death), except that the counterpart was not invited to screening. The structure of the model (appendix) allowed women to drop out of the surveillance programme or for an AAA to be incidentally detected. Women who were referred for a consultation could either be returned to surveillance if their diameter, as confirmed by a CT scan, was less than the intervention threshold; placed on a waiting list for elective surgery; or not offered repair because of the high surgical risk associated with their comorbidities.17 Overdiagnosis and over-treatment rates were calculated by comparing those with screen-detected AAA with their unscreened counterpart. All analyses used R software for statistical computing (version 3.2.4).
Outcomes
Predefined outcomes from the model were death caused by AAA, life-years, QALYs, costs, and the incremental cost-effectiveness ratio (ICER). Both costs and life-years were discounted at 3·5% per annum (appendix). Secondary outcomes included the number of women who were overdiagnosed (screen-detected AAAs in which the disease would without screening have remained without symptoms or incidental detection), and the number of women who were over-treated (repairs of screen-detected AAA that would without screening not have resulted in AAA death or surgery).
Data sources
Input parameters for women were obtained from a combination of literature reviews, clinical trial data, bespoke hospital datasets, and analysis of routine and registry data sources. Parameter values used in the reference case are described briefly here (for full details see appendix).
Prevalence of AAA at different ages, based on the standard definition of an aortic diameter of at least 3 cm was obtained from a systematic review of the literature,10 and estimated as 0·43% (95% CI 0·23–0·80) and 0·70% (0·37–1·34) for those aged 65 and 70 years respectively (the prevalence at age 70 being an interpolation between estimates at 65 and 75 years). The baseline aortic diameter distribution for women was obtained either from the screening of 70-year-old women in Sweden15 or using data from NAAASP6 re-weighted to give the appropriate prevalence for women (appendix). On the basis of the definition of the aortic diameter in AAAs being 50% larger than a normal aortic diameter,18 the former data suggested that 2·5 cm might be an appropriate alternative AAA threshold for women (appendix).
AAA growth rates were estimated from a previous study using linear mixed effects models,19 with 1743 women providing 4800 person-years of observation for analysis (appendix). Rupture rates from the same study19 were modelled as a function of AAA diameter using joint longitudinal and time-to-event models, with rupture rates increasing with larger diameters (appendix).
Data on operative mortality for both endovascular (EVAR) and open aneurysm repairs, and elective and emergency operations were extracted from the UK National Vascular Registry20 and Hospital Episode Statistics (HES).21 The overall estimated 30-day mortality rates were 2·4% for elective EVAR, 8·1% for elective open repair, 35·9% for emergency EVAR and 44·2% for emergency open repair (appendix).
Attendance rates were obtained from the randomised AAA screening trial in women,9 72·7% at age 65 years, decreasing to 67·6% at age 70 years. More recent audit data from Leicester and London, UK, showed little evidence of differential loss to surveillance between women and men, or between self-referred and screen-detected individuals. Therefore, the loss to AAA surveillance was assumed constant at 5·5 per 100 person-years (based on unpublished NAAASP data for men [Jo Jacomelli, Public Health England, personal communication]). The incidental detection rate was calculated as 2·93 per 100 person-years derived from data reported from New Zealand (similar to unpublished UK data given in the appendix).22
Randomised trials of AAA screening have shown little evidence that an AAA screened-positive population has lower long-term health-related quality of life (HRQoL) than a population screened-negative or a population not invited to screening.23, 24 For the reference case, QALYs for all women, screened and unscreened, were therefore calculated using age and sex-specific UK population EuroQoL-5D utility survey data25 and any transient reduction in HRQoL following elective AAA repair was not considered. The HRQoL weights used were 0·78 for ages 65–74 years, and 0·71 for ages of 75 years or older. In a sensitivity analysis, we investigated how possible HRQoL decrements resulting from diagnosis and surveillance, surgery (elective and emergency), and non-intervention for elective repair could affect mean QALYs and the cost-effectiveness ratio.
Age-specific non-AAA mortality rates for women were estimated using two datasets: overall life tables from the UK population 2012–14 and rates of death by age and cause.26, 27 Non-AAA mortality was calculated by subtracting AAA-specific death rates from overall mortality.
Costs were obtained from the NAAASP, 2014–15 NHS reference costs4 (pre-surgical consultation, post-surgical monitoring),14 and previous microeconomic modelling of the EVAR-1 (elective)28 and IMPROVE (emergency)29 surgery trials. Key components of the trial-based elective and emergency repair costs (vascular ward and critical care hospital stay) were from contemporaneous women-specific data from HES and updated 2014–2015 NHS reference costs. Other components were inflated to 2014–15 prices to reflect general NHS inflation, by use of published indices.30 The costs of re-interventions were taken from published trial data,28 which were inflated by use of published indices.30
Parameter uncertainty
We did probabilistic sensitivity analyses to investigate the effect of parameter uncertainty on the cost-effectiveness results (for distributions of the input parameters see appendix). We also did a series of deterministic sensitivity analyses to investigate robustness of results to changes in individual or groups of parameters; key sensitivity analyses investigated varying the prevalence and using a re-weighted aortic diameter distribution from the Swedish screening study in women.15 Changes to the aortic diameter distribution used the same prevalence as in the reference case but included a higher proportion of women with more than 4·5 cm diameter AAA (17% of detected AAAs at screening compared with 0·8% in the reference case).
Role of the funding source
The National Institute of Health Research (NIHR) had no role in study design, data collection, data analysis, data interpretation, in the writing of the report or in the decision to submit the article for publication. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Health Technology Assessment (HTA) programme, NIHR, UK NHS, or Department of Health. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
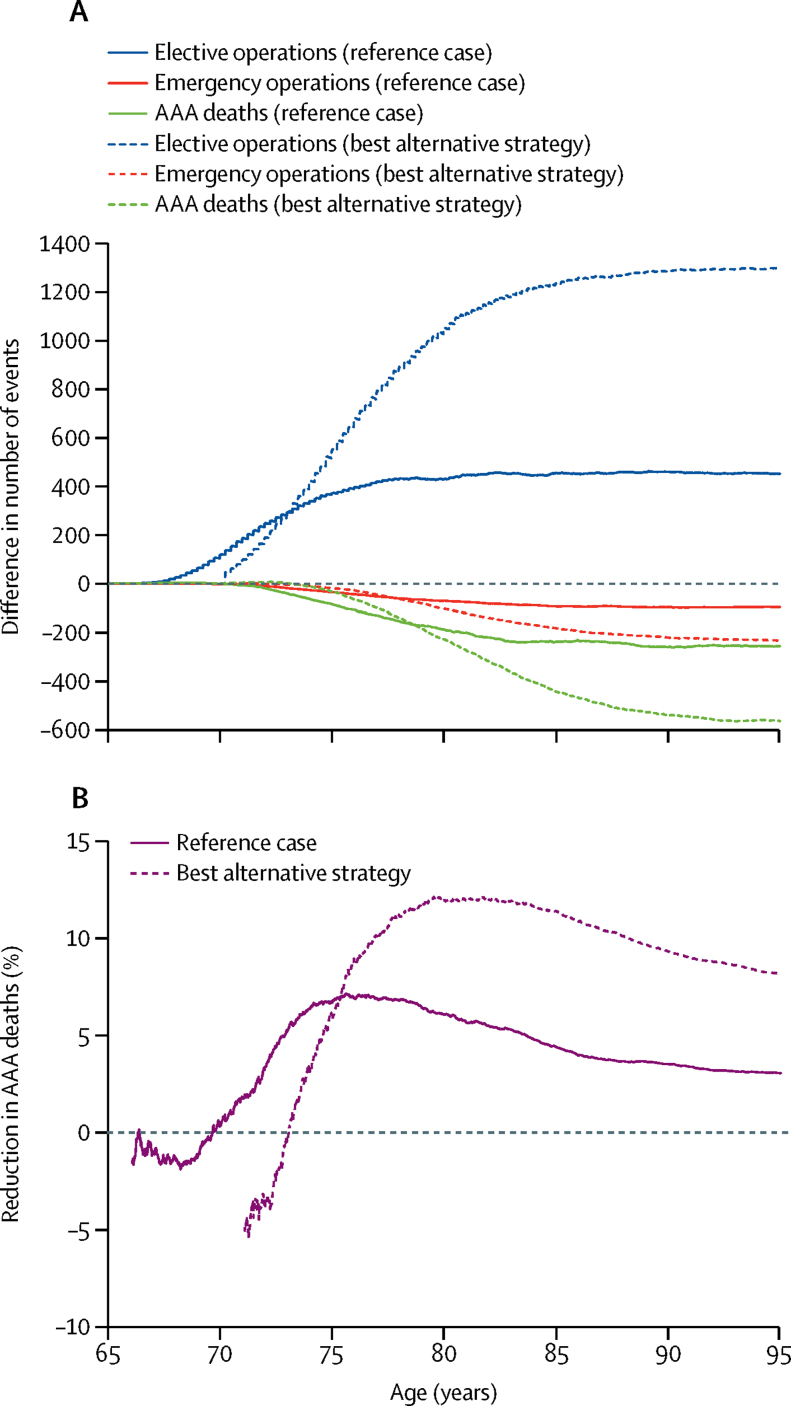
Using the same screening protocol as for 65-year-old men in NAAASP, screening detected AAA in 0·31% of the population resulting in 23% more AAAs detected in total from age 65 to 95 years, leading to an additional 452 elective operations per 1 million women invited to screening, 21% more than predicted to occur under no screening (table 1). Most of these additional operations occurred between the ages of 67 and 77 years (figure 1A, appendix). By contrast, there were 97 (4%) of 2336 fewer emergency operations (appendix), and 396 (4%) of 9235 fewer AAA ruptures, predominantly occurring after age 70 years (figure 1A). Approximately half of the AAA ruptures in the group invited to screening occurred in women with aortic diameter of less than 3 cm at initial screening (appendix). There were 85 (7%) of 1269 fewer AAA-related deaths in the first 10 years after screening and 257 (3%) of 8388 fewer deaths overall from age 65 to 95 years. For every four women who avoided an AAA-death because of successful screening, one woman died due to additional elective repair (342 avoiding AAA-related deaths because of screening vs 85 deaths caused by elective surgery after screening; table 1). There was an early negative effect of screening on deaths due to AAA because of the high operative mortality for elective repair, particularly since less than half of the repairs in women aged younger than 75 years used EVAR (figure 1B).20 Overall, 3900 women would need to be invited to screening to prevent one AAA-related death.
Table 1.
Clinical benefits and harms of AAA screening in 1 million women from screening age until age 95 years
|
Reference case* |
Best alternative strategy† |
||||||
|---|---|---|---|---|---|---|---|
| Not invited to screening | Invited to screening | Difference (% of that in non-invited group) | Not invited to screening | Invited to screening | Difference (% of that in non-invited group) | ||
| Diagnosis and treatment | |||||||
| AAA detected | 9529 | 11 697 | 2168 (23%) | 13 835 | 22 924 | 9089 (66%) | |
| Screen detected | 0 | 3101 | .. | 0 | 12 309 | .. | |
| Incidentally detected | 9529 | 8596 | .. | 13 835 | 10 615 | .. | |
| Elective AAA repair | 2165 | 2618 | 452 (21%) | 2375 | 3676 | 1301 (55%) | |
| Elective AAA repair contraindicated | 1173 | 1398 | 225 (19%) | 1261 | 1956 | 695 (55%) | |
| AAA rupture | 9235 | 8839 | −396 (−4%) | 7465 | 6555 | −910 (−12%) | |
| Emergency AAA repair | 2336 | 2239 | −97 (−4%) | 1869 | 1636 | −233 (−13%) | |
| AAA-related deaths | 8388 | 8131 | −257 (−3%) | 6886 | 6321 | −566 (−8%) | |
| Elective surgery or long-term complications of elective repair | 308 | 393 | 85 (28%) | 324 | 547 | 223 (69%) | |
| Rupture or long-term complications of emergency repair | 8080 | 7738 | −342 (−4%) | 6562 | 5774 | −789 (−12%) | |
| Non AAA-related deaths | 855 079 | 855 285 | 186 (<1%) | 849 789 | 850 220 | 431 (<1%) | |
| Re-intervention after elective repair | 505 | 619 | 114 (23%) | 543 | 913 | 370 (68%) | |
| Re-intervention after emergency repair | 322 | 302 | −20 (−6%) | 234 | 193 | −41 (−18%) | |
| Surveillance measurements | 13 773 | 16 367 | 2594 (19%) | 17 995 | 26 648 | 8653 (48%) | |
| Overdiagnosis and overtreatment | |||||||
| Overdiagnosis of AAA‡ | .. | 1036/3101 (33%) | .. | .. | 6732/12 308 (55%) | .. | |
| Overtreatment of AAA§ | .. | 94/752 (13%) | .. | .. | 494/2077 (24%) | .. | |
AAA=abdominal aortic aneurysm.
Invitation to screening at age 65 years, diagnosis threshold 3·0 cm, intervention threshold 5·5 cm.
Invitation to screening at age 70 years, diagnosis threshold 2·5 cm, intervention threshold 5·0 cm.
Screen-detected AAAs in which the disease would not have otherwise become evident (incidentally detected) or caused any problems (AAA rupture) within the woman's lifetime.
Elective AAA repair arising from screen-detection of AAA that in the absence of screening would not have resulted in AAA death or surgery.
Figure 1.
Aneurysm deaths and aneurysm repairs for reference case and best alternative strategy
Data for reference case and best alternative strategy are shown. Reference case: invitation to screening at age 65 years, diagnosis threshold 3·0 cm; intervention threshold 5·5 cm. Best alternative strategy: invitation to screening at age 70 years, diagnosis threshold 2·5 cm, intervention threshold 5·0 cm. Differences in elective operations, emergency operations and AAA deaths in 1 million women (invited to screening minus not invited to screening group; A). Percentage reduction in number of AAA deaths from screening by age (B). Because of small number volatility, the percent reduction in AAA deaths is not shown when the number of AAA deaths is less than 50 (approximately the first year after invitation to screening). AAA=abdominal aortic aneurysm.
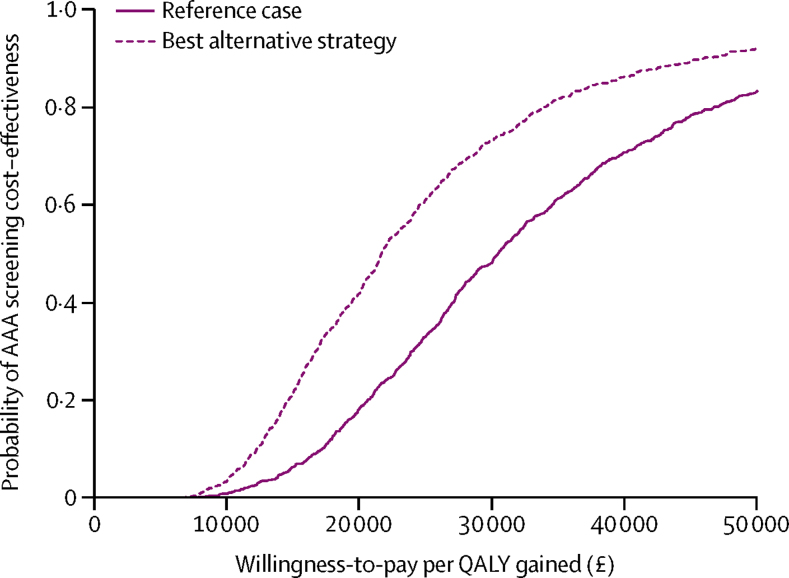
For those invited to screening, the increase in mean QALYs was 0·0011 (SD 0·0008) and costs, which were discounted at 3·5% per year, increased by a mean of £34 (4·7), which gave an ICER of £30 000 (95% CI 12 000–87 000) per QALY gained (table 2). The ICER fell considerably as the model time horizon increased because benefits from screening continued to accrue over a 30-year period (appendix). The wide confidence interval for the ICER was mainly due to uncertainty in the incremental QALYs (appendix). The probability that the reference case was cost-effective for different willingness-to-pay thresholds is shown in figure 2. Willingness-to-pay is the amount that a particular health provider is prepared to pay for each additional QALY of benefit, which for the National Institute of Health and Care Excellence is usually considered in the range of £20 000–30 000.
Table 2.
Mean life-years and costs for reference case and best alternative strategy from screening age until age 95 years
|
Reference case* |
Best alternative strategy† |
|||||
|---|---|---|---|---|---|---|
| Not invited to screening | Invited to screening | Difference | Not invited to screening | Invited to screening | Difference | |
| Life-years | ||||||
| Undiscounted | 20·5451 | 20·5480 | 0·0029 | 16·4305 | 16·4353 | 0·0048 |
| Discounted | 13·9351 | 13·9367 | 0·0016 | 11·8599 | 11·8627 | 0·0028 |
| Discounted, QA | 10·4484 | 10·4495 | 0·0011 | 8·7257 | 8·7277 | 0·0020 |
| Costs (£) | ||||||
| Undiscounted | 90·33 | 126·23 | 35·90 | 84·53 | 134·93 | 50·40 |
| Discounted | 50·55 | 84·36 | 33·81 | 52·76 | 97·83 | 45·07 |
| ICER (£ per life-year or QALY gained) | ||||||
| Discounted, life-years | .. | .. | 21 620 (95% CI 8862–61 794) | .. | .. | 16 016 (95% CI 6800–50 039) |
| Discounted, QA | .. | .. | 30 170 (95% CI 12 238–87 002) | .. | .. | 22 540 (95% CI 9522–70 638) |
Selective sampling of individuals above the diagnosis threshold was used to calculate accurate incremental estimates whereas mean life-years and costs within groups were obtained from full population sampling. For consistency, estimates in the Invited group are therefore obtained by adding the incremental estimates to the estimates from the Not Invited group. QA=quality-adjusted. ICER=incremental cost-effectiveness ratio. QALY=quality-adjusted life-year.
Invitation to screening at age 65 years, diagnosis threshold 3·0 cm, intervention threshold 5·5 cm.
Invitation to screening at age 70 years, diagnosis threshold 2·5cm, intervention threshold 5·0cm.
Figure 2.
Cost-effectiveness acceptability curves of invitation to AAA screening from the probabilistic sensitivity analyses.
Willingness-to-pay is the amount that a particular health provider is prepared to pay for each additional QALY of benefit, which for the National Institute of Health and Care Excellence is usually considered in the range of £20 000–30 000. AAA=abdominal aortic aneurysm. QALY=quality-adjusted life year. The probability of cost-effectiveness at £20 000 per QALY is 0·18 for the reference case and 0·42 for the best alternative strategy.
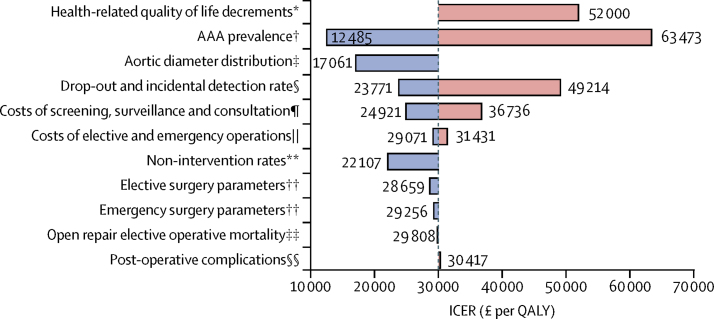
Doubling the AAA prevalence or changes to the aortic diameter distribution in the model both resulted in the ICER dropping below £20 000 per QALY gained (figure 3). Conversely, a halving of AAA prevalence or a doubling of the incidental detection and drop out rates from surveillance both resulted in a much larger ICER, whereas changes to other parameters were less influential. Possible reductions in HRQoL associated with an AAA diagnosis resulted in large changes in the ICER (a decrease in utility of 0·01, about 1·3% of the baseline value, during surveillance resulted in the ICER increasing to £43 000; appendix). Transient reductions in HRQoL following either elective or emergency surgery (affecting one in 200 invited women) did not affect the ICER but sizeable lifetime reductions in HRQoL following non-intervention for repair (affecting one in 700 invited women) would have a larger effect. A combination of these effects resulted in the ICER increasing to £52 000 (figure 3).
Figure 3.
Tornado plot showing ICER estimates for sensitivity analyses.
Blue bars show a decrease in the ICER from the reference case (grey vertical line; £30 170), red bars show an increasing ICER from the reference case. Details of changes to all parameter values are given in the appendix. ICER=incremental cost-effectiveness ratio. AAA=abdominal aortic aneurysm. NAAASP=National Abdominal Aortic Aneurysm Screening Programme. NVR/HES=National Vascular Registry/Hospital Episode Statistics. QALY=quality-adjusted life year. *Health-related quality of life decrements for diagnosis, surgery, and non-intervention for elective surgery (appendix). †Used the NAAASP-based distribution but doubled and halved the AAA prevalence. ‡NAAASP-based AAA distribution was replaced with one based on 5140 women aged 70 years screened in Sweden, while keeping the prevalence of AAA constant.15 §Halved and doubled the drop-out from surveillance and incidental detection rates simultaneously. ¶Reduced (by 20%) and increased (by 25%) the screening, surveillance, and consultation costs. ||Reduced (by 20%) elective surgery costs while increasing (by 25%) emergency surgery costs, and vice-versa. **Allowed non-intervention rate to depend on age. ††Sensitivity of operative parameters investigated by using systematic review data (rather than NVR/HES) to inform elective and emergency operative parameters.17, 25 ‡‡Reduced the open repair operative mortality from 8·1% estimated from NVR/HES to 5%. §§Increased re-intervention rate after elective open repair and AAA mortality after emergency repair.
A best alternative strategy was considered: offering screening at age 70 years, together with lowering the threshold for AAA diagnosis to 2·5 cm and lowering the threshold for considering surgery to 5·0 cm (table 1). This resulted in a substantially larger number of screen-detected AAAs (1·2% of the population) and more elective repairs were done because of the screening programme (1301 more elective repairs in the best alternative strategy vs 452 more elective repairs in the reference case per 1 million invited women; figure 1A, appendix), attributable to the higher age and lowered intervention threshold. There was a greater overall reduction in emergency operations (appendix) and AAA-related deaths (figure 1A), with 229 (12%) of 1914 fewer AAA-related deaths from age 70 to 80 years and 566 (8%) of 6886 fewer deaths overall from age 70 to 95 years, per 1 million women (figure 1B). However, there was also an increase in the number of AAA-deaths after elective repair: for every seven women who avoided an AAA-related death because of successful screening, two women died due to elective repair of screen-detected AAA (789 avoiding AAA-related deaths because of screening vs 223 deaths caused by elective surgery after screening; table 1). About a quarter of AAA ruptures in the group of women invited to screening occurred in those who initially had aortic diameter of less than 2·5 cm (appendix). Overall, 1800 women would need to be invited to screening to prevent one AAA-related death.
The best alternative strategy gave an overall ICER of £23 000 (95% CI 9500–71 000; table 2). Across the probability sensitivity analyses there was a wide spread in the estimated incremental QALYs highlighting uncertainty in parameters that affect differences in average life-expectancy between populations invited and not invited to screening, with narrower spread for incremental costs (appendix). The cost-effectiveness acceptability curve for this strategy was more favourable than the reference case (figure 2).
In the reference case, 33% of screen-detected AAAs would not otherwise have been identified during the woman's lifetime (table 1). In the best alternative strategy, the rate of overdiagnosis increased to 55%. Of the 752 screen-detected AAA elective repairs in the reference case and 2077 in the best alternative strategy per 1 million women, 94 (13%) and 494 (24%) were overtreated, respectively.
Discussion
Comprehensive modelling has shown that offering women screening for AAA, using the same screening protocol as for men in the UK, would reduce deaths from AAA in the UK by 7% in women aged from 65 to 75 years and by 3% in women aged from 65 to 95 years, would require 3900 screening invitations to avoid one AAA-death, and would be unlikely to be cost-effective. The best alternative screening strategy was based on screening at age 70 years, giving a reduction of 12% in AAA-related deaths at age 70–80 years and by 8% at age 70–95 years, reducing both the number of screening invitations needed to prevent one AAA-death to 1800 and the ICER to £23 000 but with an overdiagnosis rate of more than 50%. This is in stark contrast to AAA screening in men, for which less than 700 men need to be invited to screening to avoid one AAA-death,5 and for which contemporary modelling, on the basis of current AAA prevalence in the UK, estimates screening to reduce AAA-related deaths by 18% from age 65 to 75 years and by 6% from age 65 to 95 years with a corresponding ICER of £7400.11
Addressing sex-specific clinical issues might reduce the harms from screening and improve the future clinical benefit and cost-effectiveness estimates for women; these include expanding the use of EVAR in women (to reduce both the non-intervention rate and mortality from elective repair). This should be achievable given that the UK-wide quality improvement programme reduced overall elective in-hospital mortality for AAA repair from 5·4% in 2006 to 2·9% in 2016–1731, 32 and that mortality is even lower for elective repair of screen-detected aneurysms (in men).6
The best alternative strategy at age 70 years that used woman-specific definitions of AAA (maximum aortic diameter ≥2·5 cm) is likely to identify many more aneurysms; however, in over half of these women the AAA would have remained asymptomatic without incidental detection. The concern of overdiagnosis must therefore be recognised. The previous definition of AAA for men (a maximum aortic diameter of ≥3 cm) was used in most published studies of AAA prevalence in women,10 so that prevalence appears to be much lower in women than in men. This is a major driver of the lower cost-effectiveness in women compared with men. In women, the average aortic diameter is smaller than in men,33 providing reasonable justification for sex-specific diagnosis thresholds, since an aneurysm could be defined by a more than 50% focal increase in arterial diameter.
Nevertheless, screening women at age 70 years, as in the best alternative strategy, means that many of these women would not require intervention during their lifetime. Still, after the intervention threshold is reached, elective repair is recommended for most women as was shown by the relatively low proportion of over-treatment in both the reference case (threshold 5·5 cm) and the best alternative strategy (threshold 5·0 cm). This adds to the debate about whether the threshold for intervention of 5·5 cm, derived from randomised trials in which women were under-represented, could be lowered in women. Women have a four times increased risk of rupture in AAA of less than 5·5 cm diameter for a given AAA diameter compared with men.19 This risk, together with inspection of the available data for the population distribution of aortic diameters in women, indicates that it would be reasonable to consider lower intervention thresholds in women. A lowered threshold would have the effect of potentially offering elective repair at a younger age, reducing the non-intervention rates and operative mortality.
The possible deleterious effects of a positive AAA diagnosis and subsequent surveillance on quality of life could have a sizeable effect on the cost-effectiveness of screening, as shown in our sensitivity analysis. Small and temporary changes in utility associated with screening might be important, given that there are concerns that EuroQoL EQ-5D is not sufficiently sensitive to identify such effects. Furthermore, psychosocial consequences of AAA screening, for which the available quantitative studies have been deemed insufficient,34, 35 could affect health-care costs,36 and complications following elective repair, which could be more common in women than in men,37 could further reduce quality of life. Although the magnitude of any decrements needs clarification, their effect is only likely to reduce cost-effectiveness of AAA screening.
Our model also did not consider the probably higher cardiovascular risk in women with AAA nor the potential benefits of cardiovascular risk management, given that women often are undertreated.38 A higher risk of other cardiovascular deaths in women with AAA would lower the cost-effectiveness of AAA screening, whereas a screening programme that incorporated risk management could reduce operative mortality and cardiovascular risk, and thus improve cost-effectiveness. Opinion in our convened patient and public focus group initially favoured universal screening and did not favour the screening of only high-risk subgroups. Given the strength of the association between smoking and AAA in women, there might be merit and public support in formally assessing the effectiveness of a screening programme for women who have ever smoked. Alternatively, for women it might be more appropriate to consider a combined cardiovascular disease screening programme.23
This project has been underpinned by the development and implementation of a bespoke discrete event simulation model to assess AAA screening.16 This model, which builds on a previously developed Markov model, gives more flexibility to assess different screening options, allows heterogeneity in AAA growth rates between individuals, and permits parameters to depend on patient characteristics, such as elective operative mortality increasing with age and AAA diameter. Other strengths of this research stem from the systematic reviews of the recent literature to obtain best estimates for women-specific parameters, with individual patient clinical trial and registry data providing accurate information on post-operative outcomes for both elective and emergency repair.
A limitation of this research is that there were key quantities for which information was limited or lacking, especially the prevalence of AAA in women (based on woman-specific definition of AAA) and the effect of both screening and elective repair on quality of life. Prevalence estimates were obtained from a systematic review of studies from between 2000 and 2012, with over half of women recruited from self-purchase of screening, which could lead to underestimation of general population prevalence. A population prevalence at age 65 years of 0·8% (the upper limit of our sensitivity analysis) would result in a more favourable cost-effectiveness ratio. Conversely, it is possible that prevalence has decreased since the studies were done, reflecting trends in both AAA prevalence seen in men and smoking in women, which could lower the cost-effectiveness of screening. Furthermore, since costs were all UK-based, it is not clear how relevant our cost-effectiveness results are to other health-care providers; the results on clinical benefit and harm are likely to be more generalisable.
In conclusion, based on current evidence and definitions of AAA in men, population AAA screening of women would yield little benefit. Other screening options have more favourable cost-effectiveness but could lead to more overdiagnosis and overtreatment. Further research on the population-based aortic size distribution in older women is needed, to provide a female-specific definition of AAA, together with better quantitative studies of the effect of screening on quality of life.
Acknowledgments
Acknowledgments
We are grateful to a number of people who kindly provided input, data, or analyses to help us with this project, including: Prof Jonothan Earnshaw, Jo Jacomelli, and Lisa Summers for data on screening, surveillance, and referral to surgery for men in NAAASP; Dr Sverker Svensjö (Uppsala, Sweden) for individual data on women screened for AAA in Sweden; Dr David Epstein (Granada, Spain) for analyses of resource use and costs in the EVAR-1 trial; Dr Manuel Gomes (London, UK) for analyses of resource use and costs in the IMPROVE trial; Prof Simon Griffin (Cambridge, UK) for additional discussion on possible quality of life decrements. Additional support for this project for work done at the University of Cambridge came from the UK Medical Research Council (MR/L003120/1), the British Heart Foundation (RG/13/13/30194), and the UK National Institute for Health Research (Cambridge Biomedical Research Centre). Patient and public involvement was supported by the UK National Institute for Health Research (Leicester Biomedical Research Centre).
Contributors
MJS, KLM, and JTP co-wrote the first draft of the paper; all other authors contributed substantial amendments and critical review. MJS led the statistical and computational components of the project. KLM ran the discrete event simulation models and produced tables and figures. EJ constructed and programmed the discrete event simulation model, undertook model validation, and produced tables and figures. PU undertook systematic reviews. MJG modelled costings and provided health economics input. JAM analysed Hospital Episode Statistics data. MJB provided clinical input, developed the project website and set-up, and oversaw the public and patient involvement group. JTP provided clinical input, sourced woman-specific data, and led the systematic reviews. SGT was the principal investigator and directed all aspects of the project.
Declaration of interests
All authors declare support from the UK National Institute for Health Research Health Technology Assessment programme for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the past 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Supplementary Material
References
- 1.Office for National Statistics Deaths registered in England and Wales (Series DR) 2016. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsregisteredinenglandandwalesseriesdrreferencetables
- 2.Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg. 2012;43:161–166. doi: 10.1016/j.ejvs.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007;2 doi: 10.1002/14651858.CD002945.pub2. CD002945. [DOI] [PubMed] [Google Scholar]
- 4.Lederle FA. The last (randomized) word on screening for abdominal aortic aneurysms. JAMA Intern Med. 2016;176:1767–1768. doi: 10.1001/jamainternmed.2016.6663. [DOI] [PubMed] [Google Scholar]
- 5.Wanhainen A, Hultgren R, Linne A. Outcome of the Swedish nationwide abdominal aortic aneurysm screening program. Circulation. 2016;134:1141–1148. doi: 10.1161/CIRCULATIONAHA.116.022305. [DOI] [PubMed] [Google Scholar]
- 6.Jacomelli J, Summers L, Stevenson A, Lees T, Earnshaw JJ. Impact of the first 5 years of a national abdominal aortic aneurysm screening programme. Br J Surg. 2016;103:1125–1131. doi: 10.1002/bjs.10173. [DOI] [PubMed] [Google Scholar]
- 7.Guirguis-Blake JM, Beil TL, Sun X, Senger CA, Whitlock EP. US Agency for Healthcare Research and Quality; Rockville, MD: 2014. Primary care screening for abdominal aortic aneurysm: a systematic evidence review for the US Preventive Services Task Force. [PubMed] [Google Scholar]
- 8.Giardina S, Pane B, Spinella G. An economic evaluation of an abdominal aortic aneurysm screening program in Italy. J Vasc Surg. 2011;54:938–946. doi: 10.1016/j.jvs.2011.03.264. [DOI] [PubMed] [Google Scholar]
- 9.Scott R, Bridgewater S, Ashton H. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg. 2002;89:283–285. doi: 10.1046/j.0007-1323.2001.02014.x. [DOI] [PubMed] [Google Scholar]
- 10.Ulug P, Powell J, Sweeting M, Bown M, Thompson S. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097–1104. doi: 10.1002/bjs.10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glover M, Kim L, Sweeting M, Thompson S, Buxton M. Cost-effectiveness of the National Health Service abdominal aortic aneurysm screening programme in England. Br J Surg. 2014;101:976–982. doi: 10.1002/bjs.9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svensjö S, Mani K, Bjorck M, Lundkvist J, Wanhainen A. Screening for abdominal aortic aneurysm in 65-year-old men remains cost-effective with contemporary epidemiology and management. Eur J Vasc Endovasc Surg. 2014;47:357–365. doi: 10.1016/j.ejvs.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Stackelberg O, Bjorck M, Larsson SC, Orsini N, Wolk A. Sex differences in the association between smoking and abdominal aortic aneurysm. Br J Surg. 2014;101:1230–1237. doi: 10.1002/bjs.9526. [DOI] [PubMed] [Google Scholar]
- 14.Department of Health . Department of Health and Social Care; London: 2015. NHS reference costs 2014 to 2015. [Google Scholar]
- 15.Svensjö S, Björck M, Wanhainen A. Current prevalence of abdominal aortic aneurysm in 70-year-old women. Br J Surg. 2013;100:367–372. doi: 10.1002/bjs.8984. [DOI] [PubMed] [Google Scholar]
- 16.Glover MJ, Jones E, Masconi KL, Sweeting MJ, Thompson SG, SWAN Collaborators Discrete event simulation for decision making in health care: lessons from abdominal aortic aneurysm screening. Med Decis Making. 2018;38:439–451. doi: 10.1177/0272989X17753380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulug P, Sweeting MJ, von Allmen RS, Thompson SG, Powell JT, SWAN Collaborators Morphological suitability for endovascular repair, non-intervention rates, and operative mortality in women and men assessed for intact abdominal aortic aneurysm repair: systematic reviews with meta-analysis. Lancet. 2017;389:2482–2491. doi: 10.1016/S0140-6736(17)30639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101–2108. doi: 10.1056/NEJMcp1401430. [DOI] [PubMed] [Google Scholar]
- 19.Thompson SG, Brown LC, Sweeting MJ. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: implications for surveillance intervals and their cost-effectiveness. Health Technol Assess. 2013;17:1–118. doi: 10.3310/hta17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sidloff DA, Saratzis A, Sweeting MJ. Sex differences in mortality after abdominal aortic aneurysm repair in the UK. Br J Surg. 2017;104:1656–1664. doi: 10.1002/bjs.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NHS Digital Hospital Episode Statistics. 2016. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics
- 22.Khashram M, Jones G, Roake J. Prevalence of abdominal aortic aneurysm (AAA) in a population undergoing computed tomography colonography in Canterbury, New Zealand. Eur J Vasc Endovasc Surg. 2015;50:199–205. doi: 10.1016/j.ejvs.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Lindholt JS, Sogaard R. Population screening and intervention for vascular disease in Danish men (VIVA): a randomised controlled trial. Lancet. 2017;390:2256–2265. doi: 10.1016/S0140-6736(17)32250-X. [DOI] [PubMed] [Google Scholar]
- 24.Ashton H, Buxton M, Day N. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 25.Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316:736–741. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Office for National Statistics National Life Tables, United Kingdom: 2012–2014. 2015. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandandwalesreferencetables
- 27.Office for National Statistics Deaths registered in England and Wales (Series DR) 2015. https://www.ons.gov.uk/releases/deathsregisteredinenglandandwalesseriesdr2013
- 28.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM, for the EVAR trial investigators Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366–2374. doi: 10.1016/S0140-6736(16)31135-7. [DOI] [PubMed] [Google Scholar]
- 29.IMPROVE Trial Investigators Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ. 2017;359:j4859. doi: 10.1136/bmj.j4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis L, Burns A. University of Kent; Canterbury: 2015. Unit costs of health and social care 2015. [Google Scholar]
- 31.Mani K, Lees T, Beiles B. Treatment of abdominal aortic aneurysm in nine countries 2005–2009: a vascunet report. Eur J Vasc Endovasc Surg. 2011;42:598–607. doi: 10.1016/j.ejvs.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 32.Waton S, Johal A, Heikkila K, Cromwell D, Boyle J, Loftus I. The Royal College of Surgeons of England; London: 2017. National Vascular Registry: 2017 annual report. [Google Scholar]
- 33.Rogers IS, Massaro JM, Truong QA. Distribution, determinants, and normal reference values of thoracic and abdominal aortic diameters by computed tomography (from the Framingham Heart Study) Am J Cardiol. 2013;111:1510–1516. doi: 10.1016/j.amjcard.2013.01.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson M, Jorgensen KJ, Brodersen J. Harms of screening for abdominal aortic aneurysm: is there more to life than a 0·46% disease-specific mortality reduction? Lancet. 2016;387:308–310. doi: 10.1016/S0140-6736(15)00472-9. [DOI] [PubMed] [Google Scholar]
- 35.Cotter AR, Vuong K, Mustelin L. Do psychological harms result from being labelled with an unexpected diagnosis of abdominal aortic aneurysm or prostate cancer through screening? A systematic review. BMJ Open. 2017;7:e017565. doi: 10.1136/bmjopen-2017-017565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasmussen JF, Siersma V, Pedersen JH, Heleno B, Saghir Z, Brodersen J. Healthcare costs in the Danish randomised controlled lung cancer CT-screening trial: a registry study. Lung Cancer. 2014;83:347–355. doi: 10.1016/j.lungcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Deery SE, Soden PA, Zettervall SL. Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J Vasc Surg. 2017;65:1006–1013. doi: 10.1016/j.jvs.2016.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alabas OA, Gale CP, Hall M. Sex differences in treatments, relative survival, and excess mortality following acute myocardial infarction: national cohort study using the SWEDEHEART registry. J Am Heart Assoc. 2017;6:e007123. doi: 10.1161/JAHA.117.007123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.