Abstract
Renal obstruction is a common cause of renal failure in adults and children and is suspected when hydronephrosis is detected on imaging. Because not all cases of hydronephrosis are associated with renal damage, biomarkers are needed to guide intervention to relieve obstruction. We performed gene expression profiling on the kidneys from adult mice over a detailed time course after obstruction and compared these data with a neonatal model of bilateral high-grade obstruction induced by conditional deletion of the calcineurin β1 gene. Having identified a set of 143 transcripts modulated in both adult and neonatal obstruction, we tested their expression in a model of short-term obstruction (1 day), where renal damage is transient and reversible, and long-term obstruction (5 days), where significant renal damage is permanent. A significant number of transcripts increased early after obstruction, and later normalized, while 26 transcripts remained elevated 10 and 28 days after relief of 5 days of ureteral obstruction. With the use of qPCR, elevated levels of several of these candidate RNA biomarkers of renal damage were detected in urine from obstructed mice. In addition, several of these candidate RNA biomarkers of damage resulting from obstruction were detectable in catheterized urine samples from children undergoing surgery for ureteropelvic junction obstruction. Measurement of urinary transcripts modulated in response to renal obstruction could serve as biomarkers of renal damage with important clinical applications.
Keywords: gene expression, renal damage, renal obstruction, urine biomarker
INTRODUCTION
Obstructive nephropathy is a common cause of chronic kidney disease in adults and children (25). Hydronephrosis, a common radiographic sign of obstruction, is a poor predictor of subsequent loss of renal function since many cases are partial, and compensatory changes, including dilation of the ureter, decreased renal blood flow, and decreased production of urine, can relieve the back pressure on the nephron that leads to damage. In cases of congenital hydronephrosis, such as ureteropelvic junction obstruction (UPJO), spontaneous resolution of obstruction is common, although a subset of cases show loss of function and require surgical repair (32). Currently, the decision to intervene is based on worsening hydronephrosis, loss of renal function on functional imaging studies, or worsening serum chemistries. Based on these imprecise methods of documenting renal function loss, therapies are often delayed until irreversible renal damage has occurred. An ongoing challenge for clinicians is determining whether and when an intervention is necessary to relieve obstruction and protect renal function.
One approach to mitigate obstructive renal damage would be to use molecular biomarkers as indicators of renal tubular injury (15). Gene expression profiling has been used to identify candidate biomarkers in a variety of conditions, including ischemia-reperfusion (I/R) injury, diabetic nephropathy, hypertension-induced nephropathy, membranous nephropathy, renal obstruction, and in response to nephrotoxins (3, 8, 13, 21, 28, 38, 39). From this and other work, common pathways of renal damage response have been identified, and candidate biomarkers have been nominated. For example, LCN2 (NGAL), KIM-1, MCP-1, and TGF-β1 have been tested in several clinic contexts and have shown considerable promise as early markers of damage (2, 20, 30, 34).
For renal obstruction, several challenges have slowed development of biomarkers that can be used to identify renal damage early so that obstruction can be relieved in time to preserve renal function. We and others have described broad changes in gene expression after obstruction, but identification of which transcripts are associated with or mediate renal damage has proven difficult (4, 5, 35). In addition, it is unknown whether biomarkers identified in adult models will predict damage in congenital obstruction, particularly since changes in gene expression as a result of obstruction are overlaid on large-scale orchestrated changes in gene expression that occur as the kidney grows and develops (36). To identify transcripts modulated after obstruction and associated with renal damage in adult and neonatal kidneys, we measured gene expression profiles on a common platform in three separate model systems of renal obstruction.
METHODS
Induction of unilateral ureteral obstruction in mice.
Postnatal day 42 C57BL/6 mice kept under standard conditions were anesthetized with ketamine and subjected to unilateral ureteric obstruction for 0 h, 0.5 h, 1 h, 3 h, 5 h, 7 h, 12 h, 1 day, 3 days, 5 days, or 7 days (n = 5–6/time point). The left ureter was completely obstructed with a nontraumatic microvascular clip (5–15 g/mm2, 7-mm S&T Vascular Clamp; Fine Science Tools, Foster City, CA). For each time point, six additional mice underwent laparotomy and dissection of the left ureter without clipping to serve as sham controls. All animal experiments were approved by the Animal Care and Use Committee at Stanford University and were maintained and used in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council 2003).
Calcineurin β1 knockout mice.
Mice with conditional knockout of calcineurin β1 (Cnb KO mice) develop high-grade bilateral ureteral obstruction and were obtained as a gift from C. P. Chang, G. Crabtree, and J. Epstein from the University of Pennsylvania. Methods of breeding and genotyping have been described previously (7). Cnb1 KO mice were compared with littermate controls with at least one intact Cnb1 allele.
Temporary unilateral ureteral obstruction in mice.
We performed temporary obstruction of the ureter using a spring-loaded removable microvascular clip in two separate sets of mice. In the first experiment, postnatal day 42 C57BL/6 mice were subjected to left unilateral obstruction for 1, 4, or 6 days with a nontraumatic microvascular clip, and the animals were allowed to recover. The mice underwent a second laparotomy, and the microvascular clip was removed after 1, 4, or 6 days of obstruction. After the animals recovered for 7 days, the contralateral (nonobstructed) kidney was removed so that the previously obstructed kidney was responsible for all renal function. A blood sample was taken by retro-orbital puncture at the time the ureter was obstructed, when the clip was removed, at the time of contralateral nephrectomy, and at days 1, 3, 7, 14, 21, 28, and 35 after nephrectomy. Serum blood urea nitrogen (BUN) was measured on all blood samples using standard spectrophotometry in the clinical chemistry laboratory.
For the second experiment, the ureter was obstructed using the methods described above although the nonobstructed kidney was not removed. After obstruction for 1 or 5 days, the obstructed kidney was removed from one-third of the mice (time 0 h) for gene expression profiling. The remaining mice recovered for an additional 10 (1/3 of mice) or 28 (1/3 of mice) days after the obstructing clip had been removed (i.e., after either 1 or 5 days of complete obstruction), and the kidneys then were removed for gene expression profiling.
Collection of kidneys from mice and RNA isolation.
The kidneys were rapidly excised and stored at −80°C. Total RNA was purified from kidneys homogenized in TRIzol (Invitrogen, Carlsbad, CA) on ice using an RNeasy microarray minikit according to the manufacturer’s instructions (Qiagen, Valencia, CA). RNA concentration and integrity were determined using the NanoDrop 1000 (Thermo Scientific) and the Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA), and only high-quality RNA (28S/18S ≥1.8) was used for microarray and qPCR analysis.
Gene expression analysis.
Gene expression analysis was carried out using the Agilent 44K Mouse Whole Genome Oligonucleotide Microarray (G4140-90050 version 5.7; Agilent) following the manufacturer’s protocol. The array signal intensities for each channel were analyzed using Agilent Gene-Spring GX software (version 12.5; Agilent Technologies), and gene expression values of all datasets were normalized and log transformed after scanning. We used Significance Analysis of Microarrays to identify transcripts significantly modulated because of obstruction [Significance Analysis of Microarrays (SAM) 3.0; www-stat.stanford.edu] to compare all obstructed mice with all sham controls (31). In addition, we used GeneSpring to identify differentially expressed transcripts at each time point by comparing obstructed mice with sham controls using a threshold of ≥1.5-fold change and corrected P ≤ 0.05. Heatmaps were generated in R using gplots. All data are available through GEO (accession no.: GSE96571).
Quantitation of transcript levels in mouse urine.
Mouse urine was collected from either the renal pelvis or bladder, immediately placed on ice, and processed within 4 h. Urine was centrifuged at 4,000 revolutions/min at 4°C for 15 min, and the cell pellet was washed in PBS and resuspended in RNAlater (Qiagen). Urine RNA was purified using the RNeasy microkit (Qiagen) and stored at −80°C. Urine transcript levels were measured with a CFX384 Real-Time System (Bio-Rad, Hercules, CA). PCR primers for Sprr2f, Fos, Serpina3n, Atf3, Krt19, and Gadd45b were purchased from Life Technologies (Thermo Fisher Scientific). The expression level of each gene was represented as fold increase normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 2−ΔCt, where ΔCT = [CT(sample)] − [CT(gapdh)].
Urine transcript levels in patients with UPJO.
Urine was collected under an Institutional Review Board-approved protocol and signed consent from the parent. Bladder urine was collected from patients with UPJO and control subjects undergoing surgery for nonobstructive pathologies by catheterization after induction of anesthesia. The urine was placed on ice and transported to the laboratory within 4 h where it was processed as above. RNA was purified from the resuspended cell pellet using the MagMAX Viral RNA isolation kit (Invitrogen). Purified human RNA was converted into cDNA and preamplified using the QuantiTect Whole Transcriptome kit (Qiagen). Urine transcript levels were measured using SYBR with a CFX384 Real-Time System (Bio-Rad). PCR primer sequences for human Sprr2f, Bcl3, Krt20, Timp1, Lcn2, Tgfb1, Actb, Gapdh, and Ppia are provided in Table 1. The expression level of each gene was represented as fold increase normalized to the average of three housekeeping genes (Actb, Gapdh, and Ppia): 2−ΔCt, where ΔCT = [CT(sample)] − [CT(3 internal control genes)].
Table 1.
Primer sequences for detection of RNA from human urine
| Gene | Description | qPCR Primer Sequence |
|---|---|---|
| SPRR2F | Small proline-rich protein 2F | Forward 5′-ATCAGGAGCATGAAAGGATAAGG-3′ |
| Reverse 5′-GGAAAGAAGCTCCCTGTGTATC-3′ | ||
| BCL3 | B cell CLL/lymphoma 3 | Forward 5′-TCGACGCAGTGGACATTAA-3′ |
| Reverse 5′-ACATTTGCGCGTTCACG-3′ | ||
| KRT20 | Keratin 20 | Forward 5′-CTCCCAGAGCCTTGAGAT-3′ |
| Reverse 5′-GGCCTTGGTCTCCTCTAGA-3′ | ||
| TIMP1 | TIMP metallopeptidase inhibitor 1 | Forward 5′-TCCTGTTGTTGCTGTGGC-3′ |
| Reverse 5′-CTTGGCCCTGATGACGA-3′ | ||
| LCN2 | Lipocalin 2 | Forward 5′-GGAGCTGACTTCGGAACTAAAG-3′ |
| Reverse 5′-CGTCGATACACTGGTCGATTG-3′ | ||
| TGFB1 | Transforming growth factor-β1 | Forward 5′-GGCCTTTCCTGCTTCTCAT-3′ |
| Reverse 5′-CGTGGAGCTGAAGCAATAGT-3′ | ||
| ACTB | Actin, β | Forward 5′-GGACCTGACTGACTACCTCAT-3′ |
| Reverse 5′-CGTAGCACAGCTTCTCCTTAAT-3′ | ||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Forward 5′-CTTTGTCAAGCTCATTTCCTGG-3′ |
| Reverse 5′-TCTTCCTCTTGTGCTCTTGC-3′ | ||
| PPIA | Peptidylprolyl isomerase A (cyclophilin A) | Forward 5′-GGTCCCAAAGACAGCAGAAA-3′ |
| Reverse 5′-GTCACCACCCTGACACATAAA-3′ |
RESULTS
Gene expression time course in mice with unilateral ureteral obstruction.
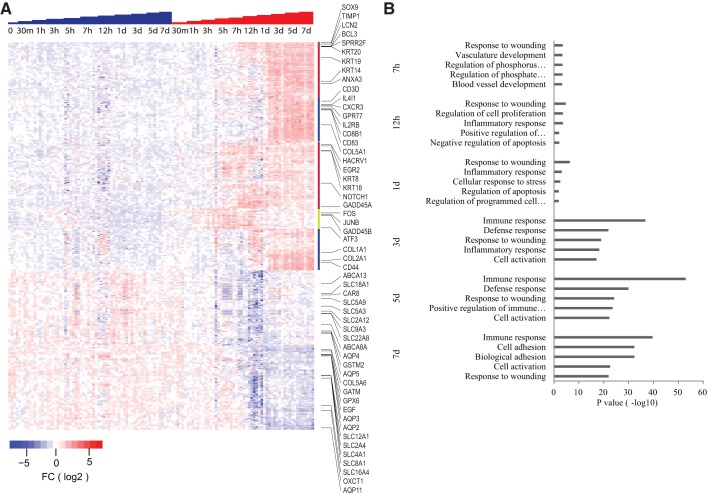
Gene expression profiling of whole kidneys at 0.5 h, 1 h, 3 h, 5 h, 12 h, 1 day, 3 days, 5 days, and 7 days after obstruction identified 1,084 transcripts modulated over the entire time course compared with controls (Fig. 1A and Supplemental Table S1). Gene expression changes could be observed as early as 30 min after obstruction and included many transcription factors and stress response genes such as Gadd45b, Plk3, Fos, and Jun. Expression of these genes increased over the first several hours and then became attenuated by 1 day after obstruction. A larger set of genes showed increased expression by 7 h to 1 day and continued with elevated expression levels through the time course. These transcripts encode for proteins of diverse functions, and many have been previously identified as responders to diverse nephrotoxic insults (3–5, 8, 11, 13, 28, 29, 35, 38, 39). Transcripts included in this group include Lcn2 (Ngal), Sox9, Timp1, Bcl3, Atf3, Egr1, Egr2, as well as several keratins.
Fig. 1.
A: heat map displaying transcripts at hours 0.5, 1, 3, 5, 7, and 12 and days 1, 3, 5, and 7 for sham (blue steps) and unilateral ureteral obstruction (UUO, red steps). Each column represents a single mouse. Transcripts significantly elevated were identified by Significance Analysis of Microarrays (SAM) comparing obstructed with sham controls. Each mouse kidney sample is represented in a column, and individual transcripts are displayed in rows. Red indicates relative increased expression level of transcripts relative to the median level across the samples, whereas blue represents relative decrease in expression levels; the degree of color saturation corresponds to the degree of change. The yellow bar at the edge of the heat map highlights transcripts elevated by 30 min–3 h after obstruction, the red bars by 7–24 h, and blue bars by 3 days. B: GOTERM pathway analysis of transcripts with increased expression from each time point of obstructed mouse kidneys compared with controls. The graph shows −log P values calculated using Benjamini-Hochberg-corrected 2-tailed t-test for the enrichment of a specific pathway. The top 5 pathways for each time point from 7 h to 7 days postobstruction were listed. FC, fold change.
Another large set of transcripts was up- and downregulated beginning at 3–7 days and were distinct from earlier time points. Induced transcripts at 3 days included members of the cytokine signaling pathway (Cxcr3, Il2rb, Gpr77, Il1, Il4, and others) and immune cell markers (Cd83, Cd8b1, Cd3d, H2-DM, and many others). Ingenuity Pathway Analysis (IPA) of all genes modulated after 3 days of obstruction showed significant enrichment for nephrotoxicity, including renal damage, inflammation, nephritis, and glomerular injury. In addition, there was clear enrichment of inflammation pathways and immune cell trafficking (Fig. 1B).
Gene expression in Cnb1 KO mice with bilateral high-grade renal obstruction.
To investigate whether transcripts modulated in response to obstruction showed overlap between the adult and neonatal kidney, we performed gene expression profiling on a mouse model with selective deletion of the calcineurin β1-subunit (Cnb1) in the mesenchyme of developing urinary tract that results in bilateral high-grade ureteral obstruction, renal failure, and death by postnatal day 20 (7). We confirmed the Pax3CreT/;Cnb1F/F or F/Δ genotype in the Cnb1 KO mice and the intact genotype in the littermate controls. Gene expression profiling was performed in whole kidneys at postnatal days 0, 1, 2, 3, 7, and 14. A SAM analysis [false discovery rate (FDR) <0.1] comparing Cnb1 KO mice kidney tissues with littermate controls identified 137 transcripts were significantly upregulated and 159 transcripts were downregulated (Fig. 2 and Supplemental Table S2). Most of these transcripts were found to be significantly altered immediately after birth in the Cnb1 KO kidneys, and the differential expression increased for most of them by 7–14 days after birth. IPA showed enrichment of pathways related to nephrotoxicity similar to those observed in the unilateral ureteral obstruction (UUO) model, although pathways associated with immune cell infiltration and inflammation were not enriched. Cnb1 KO mice also showed significant enrichment for pathways associated with renal hypoplasia, with downregulated genes associated with renal and urologic system development and function.
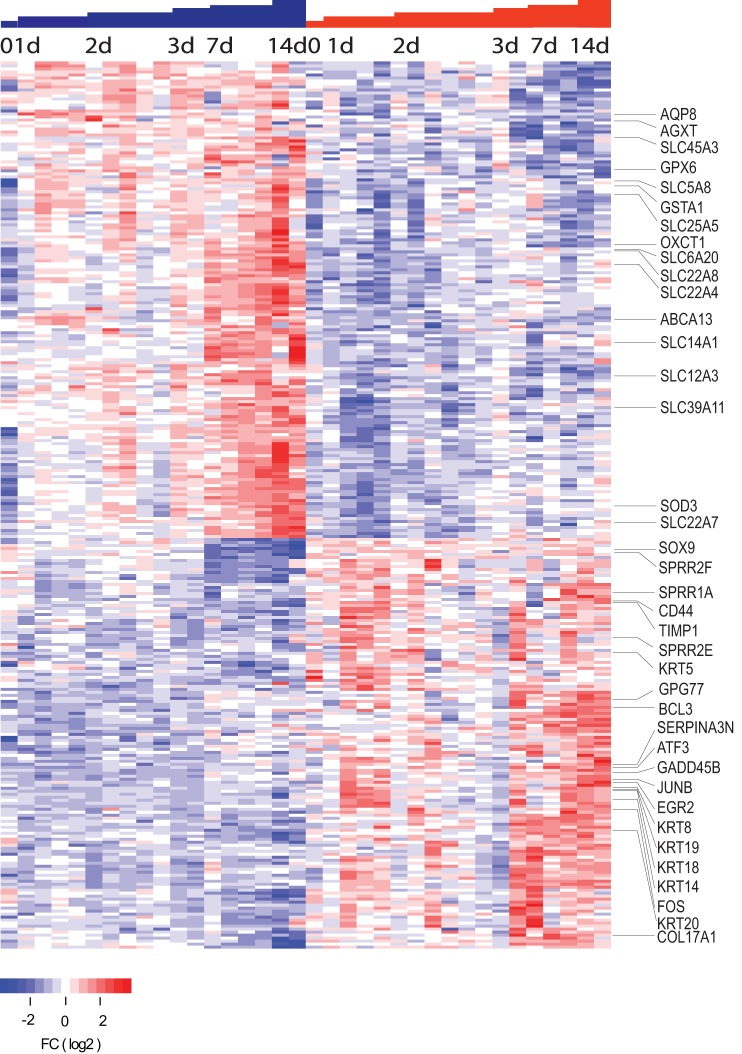
Fig. 2.
Heat map of gene expression for kidneys of calcineurin β1 knockout (Cnb1 KO) mice and littermates at postnatal days 0, 1, 2, 3, 7, and 14 as determined by SAM. Each column represents a single mouse. Gene expression differences showed progressively greater differences with increasing age.
There was significant overlap between the UUO model performed in adult kidneys and the transcripts identified in the Cnb1 KO mice. Of the 296 genes found to be altered by SAM in the Cnb1 knockout mice, 143 overlapped with those identified in the UUO model. Several transcripts observed in other models of renal damage were likewise observed in this dataset, including upregulation of Sox9, Cd44, Timp1, Bcl3, Atf3, Egr1, Egr2, Fos, and Junb. Once again, a significant number of keratin types were upregulated and included Krt5, Krt8, Krt14, Krt17, Krt18, Krt19, and Krt20, as were Sprr2f, Sprr1a, and Sprr2e transcripts. Both keratin family members and Sprr1a and Sprr2f transcripts have been observed to be elevated in a megacystis model of obstruction (5).
Gene expression in mice with reversible UUO.
Based on the detailed time course after UUO, it appeared that significant renal damage with immune infiltration and inflammation occurred after 3 days. This observation was consistent with previous work in C57BL/6 mice showing that obstruction for <3 days caused transient and reversible renal compromise, whereas obstruction for >3 days caused irreversible renal damage and renal scarring (27). Based on these findings, we decided to test whether a model of transient obstruction could be used to identify transcripts that are modulated early after obstruction and remained persistently elevated in kidneys with documented renal damage.
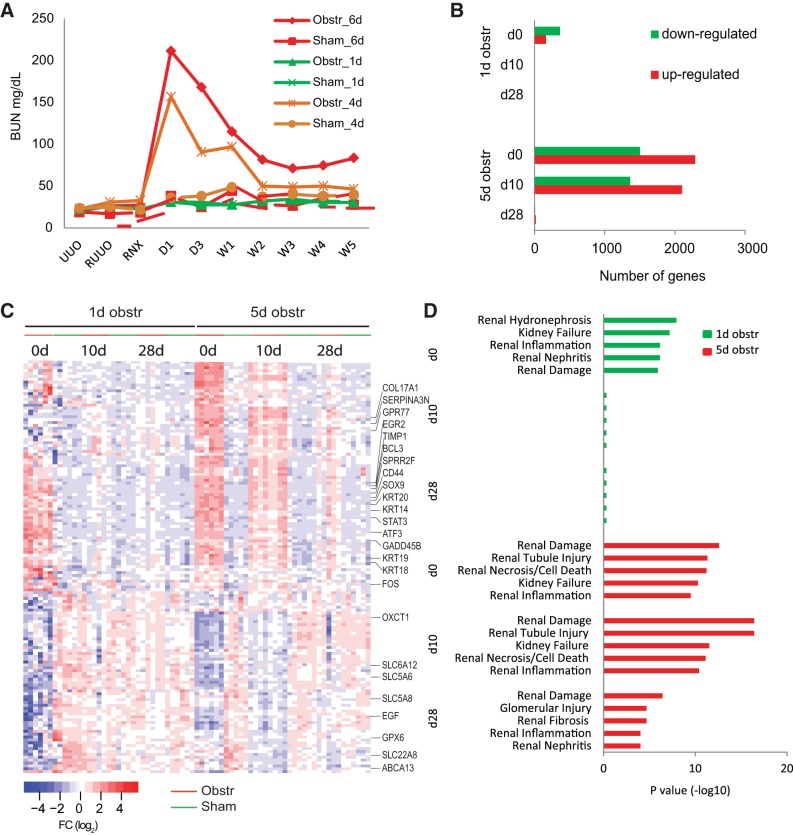
Using micro spring-loaded hemoclips, we obstructed the left kidney in adult mice for 1, 4, and 6 days (with parallel groups of sham-operated animals), allowed them to recover for 7 days, and then removed or obstructed the contralateral kidney so that renal function was wholly subsumed by the previously obstructed kidney. The animals who had been subjected to only 1 day of obstruction showed no rise in serum BUN levels after contralateral nephrectomy, identical to sham controls and implying that renal function had been preserved in these animals (Fig. 3A). However, persistent BUN elevations were observed 5 wk after relief of obstruction in the animals obstructed for 4 and 6 days, implying these kidneys had been damaged irreversibly.
Fig. 3.
A: serum blood urea nitrogen (BUN) concentration (mg/dl) in mice before and after obstruction of the ureter for 1, 4, and 6 days. BUN levels return to baseline after 1 day of obstruction but remain elevated after 4 or 6 days of temporary obstruction. B: no. of differentially upregulated and downregulated genes from 1- and 5-day obstructed mice at 0, 10, and 28 days after relief of obstruction. C: heat map of 143 genes modulated in both the UUO model and neonatal obstruction in the Cnb1 KO mouse. Each column represents the gene expression levels for these 143 transcripts in a single mouse in the temporary obstruction mouse sets [1 day (reversible damage) and 5 day (permanent damage)]. After 1 day of obstruction, all genes returned to expression levels comparable to sham controls. Five days of obstruction result in persisting changes in many genes 10 and 28 days after relief of obstruction. D: Ingenuity Pathway Analysis (IPA) of transcripts modulated in the kidneys from both 1- and 5-day obstructed mice. Top 5 nephrotoxicity functions from each time point were listed.
Based on those findings, we transiently obstructed kidneys for either 1 or 5 days and then harvested the kidneys at 0, 10, and 28 days after relief of obstruction and performed gene expression profiling. At 5 days without recovery, a SAM analysis showed a greater number of genes modulated compared with those obstructed only 1 day, as expected (Figs. 1A and 3B and Supplemental Table S3). For the kidneys obstructed 1 day, gene expression alterations normalized (i.e., were comparable to sham controls) by 10 and 28 days after relief of obstruction (Fig. 3, B and C). However, for kidneys obstructed for 5 days, 3,465 transcripts differed significantly from sham controls 10 days after relief of obstruction, and 26 transcripts remained significantly different 28 days after relief of obstruction (FDR <0.05). IPA analysis showed that these transcript signatures were most highly associated with renal damage. These changes were completely reversed 10 and 28 days after recovery from 1 day of obstruction but persisted at both time points after 5 days of obstruction (Fig. 3D).
Many, but not all, of the 143 transcripts modulated in both the UUO model and the Cnb1 KO mice showed expression changes after 1 day of nondamaging obstruction and then returned to normal after relief of obstruction. Interestingly, many of these transcripts remained elevated by 5 days after obstruction and were persistently elevated 10 and 28 days later, despite relief of obstruction (Fig. 3C). Because these transcripts were induced early and were associated with damage, they were selected as candidate early biomarkers of renal damage resulting from obstruction.
Detection of candidate transcripts in urine from both kidney and urine of UUO mice.
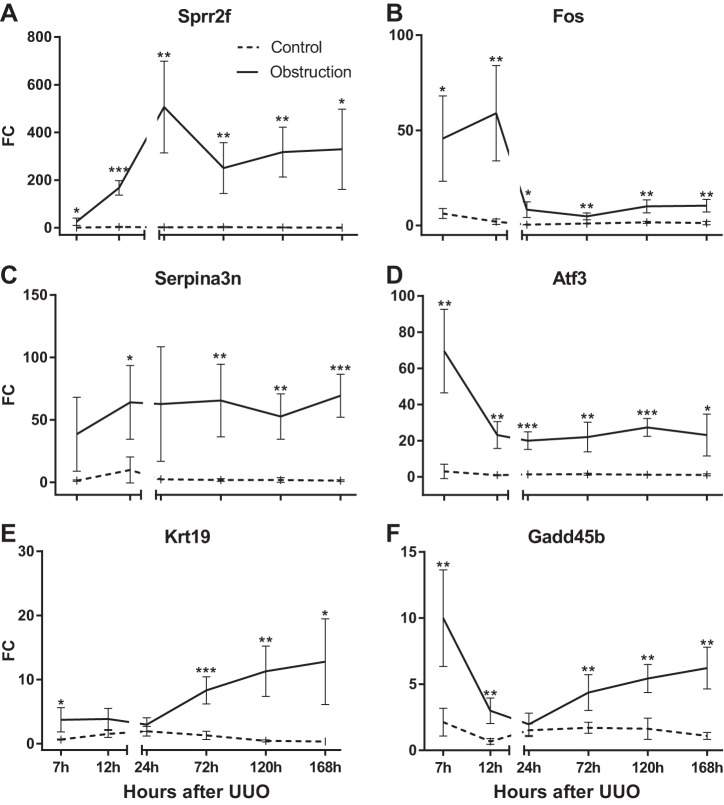
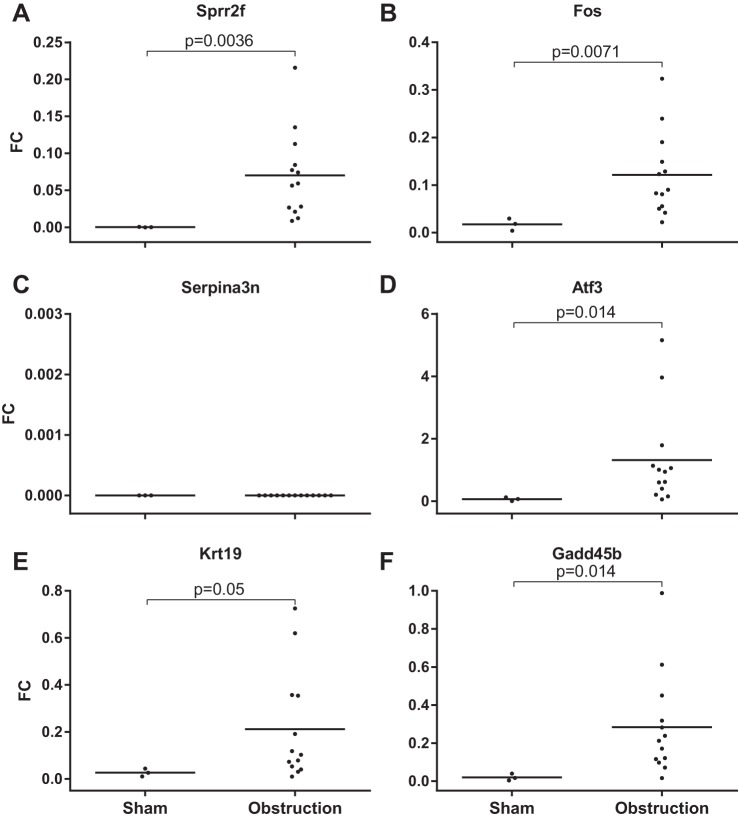
Because several transcripts showed significant increases in expression in renal tissues, we were curious whether they could be measured in the urine of obstructed animals. We developed a qPCR assay for six of the transcripts (Sprr2f, Fos, Serpina3n, Atf3, Krt19, and Gadd45b) and confirmed their expression using RNA extracted from kidney tissues (Fig. 4, A–F).
Fig. 4.
Expression of mRNA from kidney tissue of UUO mice compared with nonobstructed controls. Transcript levels relative to GAPDH were measured using qRT-PCR. *P < 0.05, **P < 0.01, and ***P < 0.001, 2-tailed Student's t-test.
Urine was collected from the renal pelvis of obstructed animals and either the renal pelvis or bladder of nonobstructed animals. The urine from the obstructed animals showed elevated levels compared with controls for five of the six transcripts, including Sprr2f, Fos, Atf3, Krt 19, and Gadd45b, but not Serpina3n (Fig. 5, A–F). There was some heterogeneity in expression of the transcripts in that low expression of one was not associated with low expression of the other transcripts in the same mouse. Therefore, most, but not all, transcripts appeared to be released in the urine after obstruction and could serve as candidate biomarkers of renal damage resulting from obstruction.
Fig. 5.
Expression of mRNA from the cell pellet from urine of UUO mice compared with nonobstructed controls. Transcript levels relative to GAPDH were measured using qRT-PCR. Horizontal bar indicates median fold increase.
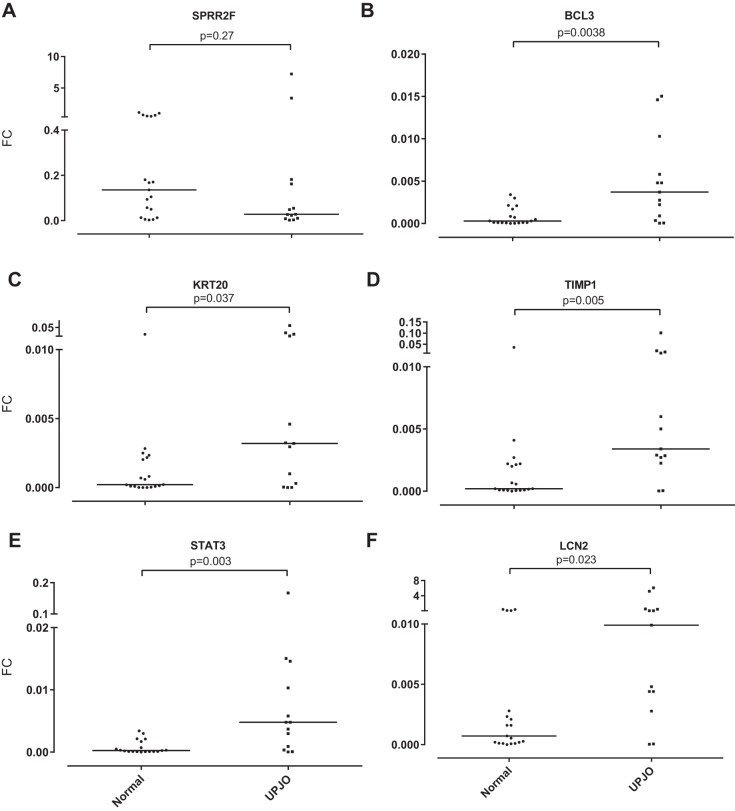
Detection of genes in urine from UPJO patients.
Based on promising findings in the mouse urine, we tested whether transcripts upregulated in response to renal obstruction could be found in the catheterized urine of children undergoing surgery for repair of congenital UPJO. In parallel, catheterized urine samples were collected from age- and sex-matched control subjects undergoing surgery for nonobstructive processes, primarily hypospadias repair. qPCR was used to assess gene expression for Sprr2f, Timp1, Krt20, and Bcl3 using human-specific sequences and preamplification. We also included Lcn2 (Ngal) and Tgfb1 since protein levels for these genes have been reported to be elevated in the urine after renal injury and obstruction (2, 9). Transcript levels for both Lcn2 and Tgfb1 were elevated in the urine of patients undergoing surgery for UPJO. Likewise, Timp1, Krt20, and Bcl3 transcript levels were significantly elevated in the urine of patients with UPJO compared with control subjects, although Sprr2f was not (Fig. 6, A–F). Again, heterogeneity in expression of these transcripts was observed, since expression levels of any one of the transcripts did not correlate with expression levels of other transcripts in the same individual.
Fig. 6.
mRNA expression levels normalized against 3 control transcripts measured by qRT-PCR from the cell pellet from the urine of patients with ureteropelvic junction obstruction (UPJO) compared with nonobstructed controls. Horizontal bar indicates median fold increase.
DISCUSSION
By using a common gene expression platform in three unique mouse models of obstruction, we have identified a set of genes that respond to renal obstruction in adults and neonates that are correlated with renal damage. Many of the transcripts and pathways identified in our obstruction models have been observed after a variety of nephrotoxic insults, using diverse gene expression platforms and over limited time courses (3, 4, 5, 8, 13, 21, 22, 28, 35, 38, 39). For example, relatively early induction of NGAL validates its role as an early marker of renal damage (1, 9, 20, 21, 34). In addition, our findings validate the recent demonstration of induction of a variety of keratins in response to renal damage (11) and nominates Krt20 as another potential damage marker. However, our detailed time course and model of transient obstruction document the relatively late induction of transcripts such as Gpr77, cytokines, immune cell markers, and several collagens as induced only after irreversible renal damage has occurred, making them less reliable as predictors of incipient damage (4).
Our work provides several novel insights into the renal response to obstruction and validates this approach to identifying candidate biomarkers of renal damage because of obstruction. As mentioned, previous profiling experiments have been limited to a few time points after renal insult. By performing the most detailed time course reported to date, we identified a cascade of transcriptional events that occur after renal damage. Several transcription factors such as Fos and Junb are induced almost immediately after obstruction, reflecting cellular stress. By 1 day the expression levels of these transcripts become attenuated, even before renal damage occurs, making them less optimal candidate biomarkers for renal damage. By 7 h to 1 day a second wave of transcripts is induced, and these include diverse genes and pathways, including Sprr2f, Krt20, and Bcl3. By 3 days the largest portion of transcripts is induced and is associated with inflammation, collagen deposition, and scar formation. Repressed transcripts from 3 to 7 days include many solute transport molecules, including aquaporins and small molecule transporters, as well as enzymes that metabolize a variety of toxins (17). Overall, the expression patterns after 3 days of obstruction remain remarkably consistent and reflect significant changes in the state of the kidney that encompass irreversible damage, scar formation, leukocyte infiltration, and nephron cell loss.
Using the Cnb1 KO mouse, we were able to show significant overlap with the UUO model, with nearly one-half of the genes shared between them. Transcripts modulated in the Cnb1 KO mouse also overlap with those observed in the obstructed kidneys of the megabladder (mgb−/−) mouse model in which a subset of mice develop high-grade obstruction with hydronephrosis (5). This overlap validates the expression changes found in the Cnb1 KO mouse and demonstrates that they are the product of obstruction and not simply the result of deletion of Cnb1 or Mgb. In addition, IPA analysis of the Cnb1 KO profiles shows enrichment for many of the same pathways and functions as seen in the UUO model, further validating that these transcripts are changing in direct response to obstruction. The Cnb1 KO also showed downregulation of pathways associated with renal development, and hypoplasia is a well-documented consequence of obstruction in the pediatric population (14). As observed in the UUO model, many keratin genes were upregulated in the neonatal model of obstruction as were small proline-rich region (SPRR) proteins. SPRR proteins have been extensively studied in the skin, where they are expressed at high levels and are critical to keratinization by cross-linking proteins (6, 10). In the epidermis, the SPRR proteins function to maintain epithelial integrity, and it is possible that they play a similar role in the obstructed kidney. SPRR2 proteins are also induced after bile duct obstruction, implying that they might have a broader role in maintaining epithelial integrity (23). This finding also suggests that their expression might be regulated by mechanical forces such as those caused by back pressure on the nephron and tubular dilation. SPRR proteins are also induced in response to ischemia in cardiomyocytes and in an ischemia reperfusion injury model in the kidney (26, 37). In the skin, SPRR proteins participate in wound healing and have been reported to quench reactive oxygen species (33).
The reversible model of obstruction provided unique insights into which transcripts could serve as biomarkers that correlate with renal damage. The transcripts that are induced early (7 h–1 day) and remain elevated in the damaged kidney are candidate early biomarkers associated with renal damage resulting from obstruction. Based on data from both mouse and human urine samples, these nominated transcripts showed promise as urinary biomarkers, although our evaluation using qPCR is very preliminary. The use of urine biomarkers has been proposed because of the relative ease in obtaining urine samples, particularly in the pediatric population (1, 8, 9, 12, 19). Several urine-based biomarkers have been identified and validated in discrete clinical contexts. Ngal transcript levels have been shown to be upregulated in renal ischemia, and NGAL protein levels are detectable in the urine (1, 20). However, for patients with obstruction, the performance of NGAL protein detection has been mixed. Several studies show significantly higher levels of NGAL protein levels in the urine of patients undergoing surgical repair for UPJO (9, 19), whereas others have not (12, 24). The inconsistency of results is possibly because of the limits of detection of proteins in the urine or because it is variably modulated by obstruction-induced damage, possibly depending on the degree or duration of obstruction. On the other hand, measurement of urinary transcript levels has a theoretical advantage of improved sensitivity, particularly as technologies for quantitatively detecting low levels of transcripts continue to improve. Our preliminary finding that several transcripts are elevated in obstructed patients might mean that a panel of transcripts will be useful in characterizing those with renal damage. Notably, the several transcripts we tested showed elevated levels in nonoverlapping patients. One of the challenges of any biomarker measurement in the urine, including transcripts, is correcting for urine concentration. However, this challenge should not be insurmountable since commercially available urine assays exist for RNA species for detection of prostate cancer, such as PCA3 and the TMPRSS2:ERG fusion transcript (16, 18).
Moving forward, we are actively screening urinary transcripts for their association with renal damage in an expanded set of patients with UPJO to narrow the list of candidate biomarkers. A significant future challenge for developing these (or any) biomarkers as clinically useful tools will be to evaluate prospectively whether they predict progression of obstructed (hydronephrotic) kidneys to renal damage before conventional imaging and functional studies such as MAG-3. One shortcoming of our and other profiling studies is our inability to localize the cells in the nephron where transcripts are modulated. Although immunohistochemistry and RNA in situ hybridization can be used for single markers, the vast scale of the transcript changes will make this process challenging. Recently, laser capture microdissection has been used to localize gene expression changes in the nephron induced by I/R injury and hypovolemia, and this technology can be readily adapted to any renal injury (37). In that study, strikingly different gene expression patterns were found between I/R and hypovolemia, despite similar physiological findings of elevated serum creatinine. This finding raises the possibility that specific renal insults might show distinct gene expression changes. Because obstruction and hydronephrosis are associated with mechanical distortion of the epithelial cells of the nephron because of dilation and stretch, it is possible there will be unique genes associated with obstructive nephropathy, such as the SPRR proteins. Future work will be necessary to understand whether there are unique gene regulation pathways induced by mechanical forces experienced by the nephron during obstruction.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.W. performed experiments; B.W., X.G., and J.D.B. analyzed data; B.W., X.G., W.A.K., and J.D.B. interpreted results of experiments; B.W., X.G., and J.D.B. prepared figures; J.D.B. conceived and designed research; J.D.B. drafted manuscript; J.D.B. edited and revised manuscript; J.D.B. approved final version of manuscript.
ACKNOWLEDGMENTS
This research was supported by the Keith and Jan Hurlbut Research Fund and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-101736 (to J. D. Brooks).
REFERENCES
- 1.Abassi Z, Shalabi A, Sohotnik R, Nativ O, Awad H, Bishara B, Frajewicki V, Sukhotnik I, Abbasi A, Nativ O. Urinary NGAL and KIM-1: biomarkers for assessment of acute ischemic kidney injury following nephron sparing surgery. J Urol 189: 1559–1566, 2013. doi: 10.1016/j.juro.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Almodhen F, Loutochin O, Capolicchio JP, Jednak R, El-Sherbiny M. The role of bladder urine transforming growth factor-beta1 concentrations in diagnosis and management of unilateral prenatal hydronephrosis. J Urol 182: 292–298, 2009. doi: 10.1016/j.juro.2009.02.132. [DOI] [PubMed] [Google Scholar]
- 3.Arbillaga L, Vettorazzi A, Gil AG, van Delft JH, García-Jalón JA, López de Cerain A. Gene expression changes induced by ochratoxin A in renal and hepatic tissues of male F344 rat after oral repeated administration. Toxicol Appl Pharmacol 230: 197–207, 2008. doi: 10.1016/j.taap.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Arvaniti E, Moulos P, Vakrakou A, Chatziantoniou C, Chadjichristos C, Kavvadas P, Charonis A, Politis PK. Whole-transcriptome analysis of UUO mouse model of renal fibrosis reveals new molecular players in kidney diseases. Sci Rep 6: 26235, 2016. doi: 10.1038/srep26235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becknell B, Carpenter AR, Allen JL, Wilhide ME, Ingraham SE, Hains DS, McHugh KM. Molecular basis of renal adaptation in a murine model of congenital obstructive nephropathy. PLoS One 8: e72762, 2013. doi: 10.1371/journal.pone.0072762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabral A, Voskamp P, Cleton-Jansen AM, South A, Nizetic D, Backendorf C. Structural organization and regulation of the small proline-rich family of cornified envelope precursors suggest a role in adaptive barrier function. J Biol Chem 276: 19231–19237, 2001. doi: 10.1074/jbc.M100336200. [DOI] [PubMed] [Google Scholar]
- 7.Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest 113: 1051–1058, 2004. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cianciolo R, Yoon L, Krull D, Stokes A, Rodriguez A, Jordan H, Cooper D, Falls JG, Cullen J, Kimbrough C, Berridge B. Gene expression analysis and urinary biomarker assays reveal activation of tubulointerstitial injury pathways in a rodent model of chronic proteinuria (Doxorubicin nephropathy). Nephron, Exp Nephrol 124: 1–10, 2013. doi: 10.1159/000355542. [DOI] [PubMed] [Google Scholar]
- 9.Cost NG, Noh PH, Devarajan P, Ivancic V, Reddy PP, Minevich E, Bennett M, Haffner C, Schulte M, DeFoor WR Jr. Urinary NGAL levels correlate with differential renal function in patients with ureteropelvic junction obstruction undergoing pyeloplasty. J Urol 190, Suppl: 1462–1467, 2013. doi: 10.1016/j.juro.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Koning HD, van den Bogaard EH, Bergboer JG, Kamsteeg M, van Vlijmen-Willems IM, Hitomi K, Henry J, Simon M, Takashita N, Ishida-Yamamoto A, Schalkwijk J, Zeeuwen PL. Expression profile of cornified envelope structural proteins and keratinocyte differentiation-regulating proteins during skin barrier repair. Br J Dermatol 166: 1245–1254, 2012. doi: 10.1111/j.1365-2133.2012.10885.x. [DOI] [PubMed] [Google Scholar]
- 11.Djudjaj S, Papasotiriou M, Bülow RD, Wagnerova A, Lindenmeyer MT, Cohen CD, Strnad P, Goumenos DS, Floege J, Boor P. Keratins are novel markers of renal epithelial cell injury. Kidney Int 89: 792–808, 2016. doi: 10.1016/j.kint.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Gerber C, Harel M, Lynch ML, Herbst KW, Ferrer FA, Shapiro LH. Proximal tubule proteins are significantly elevated in bladder urine of patients with ureteropelvic junction obstruction and may represent novel biomarkers: a pilot study. J Pediatr Urol 12: 120–127, 2016. doi: 10.1016/j.jpurol.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Hauser PV, Perco P, Mühlberger I, Pippin J, Blonski M, Mayer B, Alpers CE, Oberbauer R, Shankland SJ. Microarray and bioinformatics analysis of gene expression in experimental membranous nephropathy. Nephron, Exp Nephrol 112: e43–e58, 2009. doi: 10.1159/000213505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang WY, Peters CA, Zurakowski D, Borer JG, Diamond DA, Bauer SB, McLellan DL, Rosen S. Renal biopsy in congenital ureteropelvic junction obstruction: evidence for parenchymal maldevelopment. Kidney Int 69: 137–143, 2006. doi: 10.1038/sj.ki.5000004. [DOI] [PubMed] [Google Scholar]
- 15.Kiley SC, Chevalier RL. Urinary biomarkers: the future looks promising. Kidney Int 76: 133–134, 2009. doi: 10.1038/ki.2009.124. [DOI] [PubMed] [Google Scholar]
- 16.Leyten GH, Hessels D, Jannink SA, Smit FP, de Jong H, Cornel EB, de Reijke TM, Vergunst H, Kil P, Knipscheer BC, van Oort IM, Mulders PF, Hulsbergen-van de Kaa CA, Schalken JA. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol 65: 534–542, 2014. doi: 10.1016/j.eururo.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Li ZZ, Xing L, Zhao ZZ, Li JS, Xue R, Chandra A, Nørregaard R, Wen JG. Decrease of renal aquaporins 1-4 is associated with renal function impairment in pediatric congenital hydronephrosis. World J Pediatr 8: 335–341, 2012. doi: 10.1007/s12519-012-0378-9. [DOI] [PubMed] [Google Scholar]
- 18.Lin DW, Newcomb LF, Brown EC, Brooks JD, Carroll PR, Feng Z, Gleave ME, Lance RS, Sanda MG, Thompson IM, Wei JT, Nelson PS; Canary Prostate Active Surveillance Study Investigators . Urinary TMPRSS2:ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the Canary Prostate Active Surveillance Study. Clin Cancer Res 19: 2442–2450, 2013. doi: 10.1158/1078-0432.CCR-12-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen MG. Urinary biomarkers in hydronephrosis. Dan Med J 60: B4582, 2013. [PubMed] [Google Scholar]
- 20.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 21.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa S, Nishihara K, Miyata H, Shinke H, Tomita E, Kajiwara M, Matsubara T, Iehara N, Igarashi Y, Yamada H, Fukatsu A, Yanagita M, Matsubara K, Masuda S. Molecular markers of tubulointerstitial fibrosis and tubular cell damage in patients with chronic kidney disease. PLoS One 10: e0136994, 2015. doi: 10.1371/journal.pone.0136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nozaki I, Lunz JG III, Specht S, Stolz DB, Taguchi K, Subbotin VM, Murase N, Demetris AJ. Small proline-rich proteins 2 are noncoordinately upregulated by IL-6/STAT3 signaling after bile duct ligation. Lab Invest 85: 109–123, 2005. doi: 10.1038/labinvest.3700213. [DOI] [PubMed] [Google Scholar]
- 24.Olvera-Posada D, Dayarathna T, Dion M, Alenezi H, Sener A, Denstedt JD, Pautler SE, Razvi H. KIM-1 Is a Potential Urinary Biomarker of Obstruction: Results from a Prospective Cohort Study. J Endourol 31: 111–118, 2017. doi: 10.1089/end.2016.0215. [DOI] [PubMed] [Google Scholar]
- 25.Pain VM, Strandhoy JW, Assimis DG. Pathophysiology of urinary tract obstruction. In: Campbell-Walsh Urology (9th ed). Kavoussi LR, Novick AC, Partin AW, Peters CA, Wein AJ. Philadelphia, PA: Saunders Elsevier, 2007, p. 1227–1273. [Google Scholar]
- 26.Pradervand S, Yasukawa H, Muller OG, Kjekshus H, Nakamura T, St Amand TR, Yajima T, Matsumura K, Duplain H, Iwatate M, Woodard S, Pedrazzini T, Ross J, Firsov D, Rossier BC, Hoshijima M, Chien KR. Small proline-rich protein 1A is a gp130 pathway- and stress-inducible cardioprotective protein. EMBO J 23: 4517–4525, 2004. doi: 10.1038/sj.emboj.7600454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puri TS, Shakaib MI, Chang A, Mathew L, Olayinka O, Minto AW, Sarav M, Hack BK, Quigg RJ. Chronic kidney disease induced in mice by reversible unilateral ureteral obstruction is dependent on genetic background. Am J Physiol Renal Physiol 298: F1024–F1032, 2010. doi: 10.1152/ajprenal.00384.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skogstrand T, Leh S, McClure J, Dashti M, Iversen BM, Graham D, McBride MW, Hultström M. Identification of a common molecular pathway in hypertensive renal damage: comparison of rat and human gene expression profiles. J Hypertens 33: 584–596, 2015. doi: 10.1097/HJH.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 29.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int 63: 1714–1724, 2003. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 30.Taranta-Janusz K, Wasilewska A, Dębek W, Waszkiewicz-Stojda M. Urinary cytokine profiles in unilateral congenital hydronephrosis. Pediatr Nephrol 27: 2107–2113, 2012. doi: 10.1007/s00467-012-2230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulman I, Jayanthi VR, Koff SA. The long-term followup of newborns with severe unilateral hydronephrosis initially treated nonoperatively. J Urol 164: 1101–1105, 2000. doi: 10.1016/S0022-5347(05)67262-X. [DOI] [PubMed] [Google Scholar]
- 33.Vermeij WP, Backendorf C. Skin cornification proteins provide global link between ROS detoxification and cell migration during wound healing. PLoS One 5: e11957, 2010. doi: 10.1371/journal.pone.0011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wasilewska A, Taranta-Janusz K, Dębek W, Zoch-Zwierz W, Kuroczycka-Saniutycz E. KIM-1 and NGAL: new markers of obstructive nephropathy. Pediatr Nephrol 26: 579–586, 2011. doi: 10.1007/s00467-011-1773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu B, Brooks JD. Gene expression changes induced by unilateral ureteral obstruction in mice. J Urol 188: 1033–1041, 2012. doi: 10.1016/j.juro.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Wu B, Sahoo D, Brooks JD. Comprehensive gene expression changes associated with mouse postnatal kidney development. J Urol 189: 2385–2390, 2013. doi: 10.1016/j.juro.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Xu K, Rosenstiel P, Paragas N, Hinze C, Gao X, Huai Shen T, Werth M, Forster C, Deng R, Bruck E, Boles RW, Tornato A, Gopal T, Jones M, Konig J, Stauber J, D’Agati V, Erdjument-Bromage H, Saggi S, Wagener G, Schmidt-Ott KM, Tatonetti N, Tempst P, Oliver JA, Guarnieri P, Barasch J. Unique Transcriptional Programs Identify Subtypes of AKI. J Am Soc Nephrol 28: 1729–1740, 2017. doi: 10.1681/ASN.2016090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuen PS, Jo SK, Holly MK, Hu X, Star RA. Ischemic and nephrotoxic acute renal failure are distinguished by their broad transcriptomic responses. Physiol Genomics 25: 375–386, 2006. doi: 10.1152/physiolgenomics.00223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng S, Huang Y, Yang L, Chen T, Xu J, Epstein PN. Uninephrectomy of diabetic OVE26 mice greatly accelerates albuminuria, fibrosis, inflammatory cell infiltration and changes in gene expression. Nephron, Exp Nephrol 119: e21–e32, 2011. doi: 10.1159/000327586. [DOI] [PMC free article] [PubMed] [Google Scholar]