Abstract
Evidence from animal studies indicates that hyperinsulinemia, without changes in glucose, increases ventilation via a carotid body-mediated mechanism. However, whether insulin elevates ventilation in humans independently of changes in glucose remains unclear. Therefore, we tested the hypothesis that insulin increases ventilation in humans during a hyperinsulinemic-euglycemic clamp in which insulin was elevated to postprandial concentrations while glucose was maintained at fasting concentrations. First, in 16 healthy young men (protocol 1), we retrospectively analyzed respiration rate and estimated tidal volume from a pneumobelt to calculate minute ventilation during a hyperinsulinemic-euglycemic clamp. In addition, for a direct assessment of minute ventilation during a hyperinsulinemic-euglycemic clamp, we retrospectively analyzed breath-by-breath respiration rate and tidal volume from inspired/expired gasses in an additional 23 healthy young subjects (protocol 2). Clamp infusion elevated minute ventilation from baseline in both protocols (protocol 1: +11.9 ± 4.6% baseline, P = 0.001; protocol 2: +9.5 ± 3.8% baseline, P = 0.020). In protocol 1, peak changes in both respiration rate (+13.9 ± 3.0% baseline, P < 0.001) and estimated tidal volume (+16.9 ± 4.1% baseline, P = 0.001) were higher than baseline during the clamp. In protocol 2, tidal volume primarily increased during the clamp (+9.7 ± 3.7% baseline, P = 0.016), as respiration rate did not change significantly (+0.2 ± 1.8% baseline, P = 0.889). Collectively, we demonstrate for the first time in humans that elevated plasma insulin increases minute ventilation independent of changes in glucose.
Keywords: carotid body, chemoreceptors, respiration rate, tidal volume
INTRODUCTION
Carotid bodies contain chemoreceptors in the type I glomus cells that detect chemical stimuli from arterial blood in the carotid sinuses. Hypoxia and hypercapnia are primary signals that stimulate carotid bodies, triggering the release of several neurotransmitters (22), and sending afferent signals to the central nervous system, typically resulting in a reflex elevation in minute ventilation (9, 10, 18) The carotid bodies can also act as sensors for glucose. Indeed, several studies have reported that low glucose concentration in in vitro preparations augmented the release of catecholamines from carotid body cells (24, 25) and increased carotid sinus nerve activity (24). However, it should be noted that this is not a universal finding (5, 6, 8). Also, a direct effect of insulin on carotid chemoreceptors requires consideration. In this regard, low blood glucose concentration (hypoglycemia) evoked by insulin infusion (hyperinsulinemia) resulted in greater minute ventilation in rats (5), and greater respiration rate in humans (16, 33). Although these results have been interpreted to further suggest that glucose plays a role in the regulation of ventilation, potential effects of elevated insulin cannot be distinguished from reduced blood glucose. This is important since chemoreceptors sensitive to insulin have also been identified in the carotid bodies.
Several studies have indicated that insulin can stimulate carotid bodies. Indeed, incubation of carotid body cells in a solution with high insulin concentration resulted in phosphorylation of chemoreceptors and subsequent release of dopamine and ATP (28). Moreover, bolus injections of insulin into the carotid artery of rats caused a dose-dependent elevation in minute ventilation, which was abolished after carotid sinus denervation (28). Importantly, blood glucose in these animals was maintained at fasting values (euglycemia) via glucose infusion, indicating that insulin elicited the carotid body-mediated increase in ventilation (28). In humans, Ward et al. (33) reported an elevation in ventilation both during a hyperinsulinemic-hypoglycemic clamp infusion and during a glucose infusion that led to higher blood glucose (hyperglycemia) and endogenous hyperinsulinemia. Thus, despite largely disparate glycemic values, insulin and ventilation were both elevated, and it is reasonable to speculate that the greater ventilation might have been evoked by insulin per se; however, this hypothesis has not been directly tested in humans.
Therefore, we sought to isolate the effect of insulin on ventilation in humans. First, we calculated minute ventilation based on the respiration rate and estimated tidal volume from a pneumobelt trace during a hyperinsulinemic-euglycemic clamp in which insulin was elevated to postprandial concentrations while glucose was clamped at fasting concentrations (protocol 1). Additionally, for direct measurements of minute ventilation, we examined breath-by-breath respiration rate and tidal volume from inspired/expired gasses collected with a metabolic cart from a separate data set during a hyperinsulinemic-euglycemic clamp (protocol 2). We hypothesized that insulin increases ventilation during a hyperinsulinemic-euglycemic clamp.
METHODS
Study Population
Retrospective analyses were performed on subjects from two experimental protocols [protocol 1: 16 healthy men (age 27 ± 1 yr; height 179 ± 1 cm; weight 83 ± 2 kg); protocol 2: 23 healthy subjects (13 men, 10 women; age 28 ± 1 yr; height 176 ± 3 cm; weight 76 ± 3 kg)]. The majority of these data were from previously published work (13, 15, 21, 34); however, the analyses and results included were specific to the unique hypothesis raised. All data were analyzed at baseline and during hyperinsulinemic-euglycemic clamp conditions before any other intervention. Participants had no significant medical history, were nonsmokers, and were not using any prescribed or over-the-counter medications. All women had negative pregnancy test results and were studied in the early follicular phase of the menstrual cycle (days 1–5) or in the low hormone phase of oral contraceptives, based on self-report. Participants abstained from alcohol and physical activity for 24 h, and also fasted and abstained from caffeine for 12 h before experimental sessions. Subjects received written and verbal explanation of the experimental measurements and procedures after which they gave their written consent for participation. All experimental procedures conformed to the Declaration of Helsinki and were approved by University of Missouri Health Sciences Institutional Review Board (protocol 1) and Institutional Review Board at the Mayo Clinic (protocol 2).
Protocol 1
Procedures.
Subjects were studied in supine position at a constant ambient room temperature of 22–23°C. Intravenous catheters were placed in an antecubital vein and hand vein for the infusion of insulin/glucose and blood sampling, respectively. The hand for blood draws was placed in a heated box (50°C) to obtain arterialized venous blood samples (17). Insulin (Humulin, Eli Lilly, Indianapolis, IN) was diluted in 0.9% saline with 5 ml of the subject’s blood, and a 10-min priming infusion was followed by a constant infusion of 30 mU·m−2·min−1 (n = 7) or 40 mU·m−2·min−1 (n = 9) (average infusion rate relative to body weight: 0.88 ± 0.03 mU kg−1 min−1) for a total of 120 min. The goal was to raise and maintain plasma insulin concentrations at postprandial values. Whole blood glucose was determined every 5 min using a blood glucose meter (Accu-chek Compact Plus, Roche Diagnostics, Indianapolis, IN; StatStrip Xpress, Nova Biomedical, Waltham, MA) and maintained at euglycemic concentrations matching their morning fasting glucose via a variable 20% dextrose infusion.
Measurements.
Respiratory movements were continuously monitored using a strain-gauge pneumobelt placed in a stable position around the abdomen (1132 Pneumotrace II, UFI, Morro Bay, CA), sampled at 1,000 Hz and stored for off-line analyses (Powerlab, ADInstruments, Bella Vista, NSW, Australia). Respiration rate was the number of inspiratory peaks in the respiratory tracing, expressed as breaths per minute, calculated over a 5-min period at baseline (BL), 30, 60, 90, and 120 min of the hyperinsulinemic-euglycemic clamp. During the same periods, the amplitude of the respiratory movements was calculated in arbitrary units for each respiratory cycle as the range from the inspiratory peak to the next expiratory nadir of the tracing (7, 30). The mean range obtained in each period during the clamp was expressed as percent change from BL and was considered an estimate of change in tidal volume. Subsequently, percent changes in minute ventilation (i.e., the product of respiration rate and tidal volume) were estimated based on the percent changes in respiration rate and tidal volume. Percent changes in tidal volume and minute ventilation could not be estimated in 2 of the 16 subjects due to repositioning of the pneumobelt during the protocol. Beat-to-beat heart rate was continuously monitored using a lead II electrocardiogram (Q710; Quinton, Bothell, WA). Systolic and diastolic arterial blood pressure were measured with an automated sphygmomanometer (Welch Allyn, Skaneatles Falls, NY).
Blood samples were collected every 30 min during infusion and stored at –80°C for later analyses of insulin and glucose. Insulin was determined using enzyme-linked immunosorbent assays (Immulite 1000 Analyzer, Siemens Healthcare Diagnostics, Tarrytown, NY) and glucose was determined using the glucose oxidase method (ThermoFisher, Waltham, MA; Beckman Instruments, Brea, CA).
Protocol 2
Procedures.
Subjects were studied in supine position at a constant ambient room temperature of 22°C. An intravenous catheter was placed in the dominant arm for insulin and glucose infusion. Blood samples were obtained from an arterial catheter (20 gauge, 5 cm) placed in the nondominant arm using aseptic technique under local anesthesia (2% lidocaine). Insulin (Novolin, Novo Nordisk, Plainsboro, NJ) was infused at a constant rate of 1.0 mU·kgfat-free−1·min−1 (average infusion rate relative to body weight: 0.74 ± 0.01 mU·kg−1·min−1) for 60 min. Blood glucose was measured every 5–10 min at bedside using the glucose oxidase method (GM9 Glucose Analyzer, Analox Instruments, Stourbridge, UK; 2900D Biochemistry Analyzer, Yellow Springs Instruments, Yellow Springs, OH) and maintained at euglycemic concentrations matching their morning fasting glucose via a variable 50% dextrose infusion.
Measurements.
Breath-by-breath tidal volume (Universal Ventilation Meter, VacuMed, Ventura, CA), respiratory rate, and inspired/expired gasses (GE Datex-Ohmeda Cardiocap/5, GE Healthcare, Chicago, IL) were monitored and used to calculate minute ventilation and end-tidal CO2 expired fraction. Subjects breathed through a mouthpiece connected to a two-way nonrebreathing valve with a nose clip closing the nasal airways. The measures were averaged over a 5-min period at BL and 60 min of the hyperinsulinemic-euglycemic clamp. Beat-to-beat heart rate and blood pressure were continuously monitored using a lead II electrocardiogram (Cardiocap/5, Datex-Ohmeda, Louisville, CO) and brachial arterial catheter (TruWave Pressure Transducer; Edwards Lifescience, Irvine, CA), respectively.
Blood samples were collected 60 min after the start of infusion and stored at −80°C for later analyses of insulin and glucose. Insulin was assessed using a two-site immunoenzymatic assay (Beckman Instruments), and glucose was determined using a standard assay (Cobas c311, Roche Diagnostics).
Statistical Analysis
In protocol 1, one-way repeated-measures ANOVA was used to compare respiratory and metabolic measures before and over the course of the hyperinsulinemic-euglycemic clamp (i.e., BL, 30, 60, 90, and 120 min). Data are presented as absolute values and percent changes from baseline; however, all statistical comparisons were performed with absolute values. When a significant main effect was found, Fisher’s LSD post hoc tests were used for pairwise comparisons. Additionally, individual peak responses were averaged and compared with BL using Student’s paired t-tests. In protocol 2, respiratory and metabolic measures were compared between BL and 60 min using Student’s paired t-tests. Because of potential sex differences in the control of breathing (2, 11, 20), additional analyses were performed to compare the responses between men and women using Student’s t-tests for independent samples. Comparisons between protocols 1 and 2 (60-min time point) used Student’s t-tests for independent samples. All tests were performed with the software Statistica 8.0 (StatSoft, Tulsa, OK), and significance was set at P value < 0.05. Data are shown as means ± SE.
RESULTS
Protocol 1
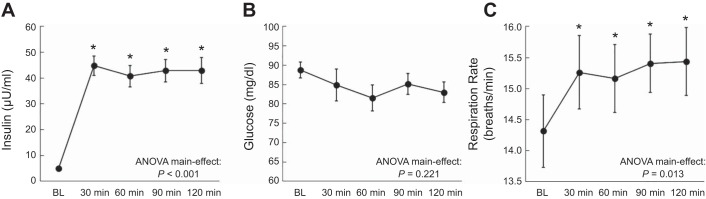
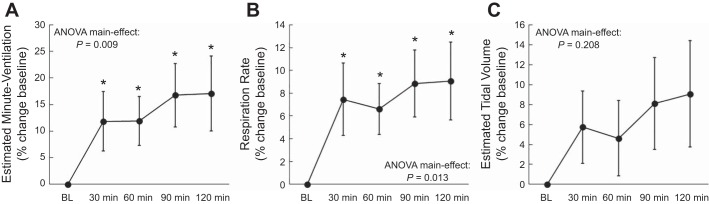
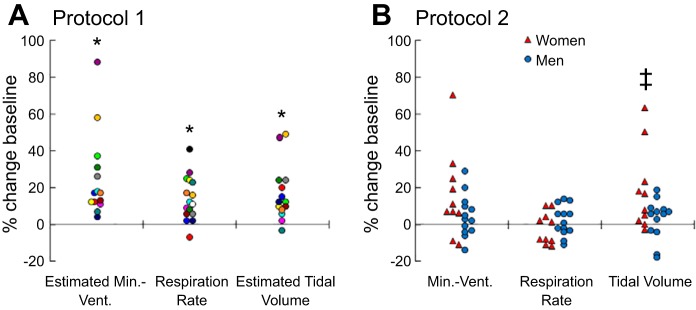
As expected, serum insulin was elevated during the clamp and maintained throughout the infusion period, while serum glucose concentration was maintained at fasting values (Fig. 1, A and B). Estimated minute ventilation 30 min after starting the clamp was higher than BL and remained elevated throughout the clamp (Figs. 2A and 3A) with a peak increase of 25.2 ± 6.1% (P = 0.001). Similarly, respiration rate was augmented throughout the hyperinsulinemic-euglycemic clamp (Fig. 1C) from a BL of 14.3 ± 0.6 breaths/min to a peak of 16.1 ± 0.5 breaths/min (P < 0.001). The elevation in respiration rate was also significant when expressed as a percent change from BL (Figs. 2B and 3A), with a peak of 13.9 ± 3.0% (P < 0.001). Furthermore, estimated tidal volume tended to be higher than BL throughout the clamp (Figs. 2C and 3A). When individual peak responses were compared with BL, there was a significant peak increase (16.9 ± 4.1%; P = 0.001) in tidal volume. Interestingly, 10 individuals elevated their minute ventilation via both respiration rate and tidal volume, 2 individuals increased minute ventilation solely via respiration rate, and 2 individuals increased minute ventilation solely via tidal volume (Fig. 4A). Heart rate increased slightly but significantly during the hyperinsulinemic-euglycemic clamp (e.g., BL: 58 ± 2 beats/min; 120 min: 60 ± 2 beats/min; ANOVA P = 0.015). Systolic blood pressure remained unchanged (e.g., BL: 118 ± 2 mmHg; 120 min: 118 ± 3 mmHg; ANOVA P = 0.595), whereas diastolic blood pressure tended to decrease throughout the hyperinsulinemic-euglycemic clamp (e.g., BL: 66 ± 2 mmHg; 120 min: 64 ± 2 mmHg; ANOVA P = 0.051).
Fig. 1.
Blood insulin concentration (A), blood glucose concentration (B), and respiration rate (C) throughout the hyperinsulinemic-euglycemic clamp in protocol 1. *P < 0.05 vs. baseline (BL).
Fig. 2.
Percent changes from baseline in estimated minute ventilation (A), respiration rate (B), and estimated tidal volume (C) throughout the hyperinsulinemic-euglycemic clamp in protocol 1. Estimated minute ventilation and tidal volume included 14 subjects. Respiration rate included 16 subjects. *P < 0.05 vs. baseline (BL) (using absolute values).
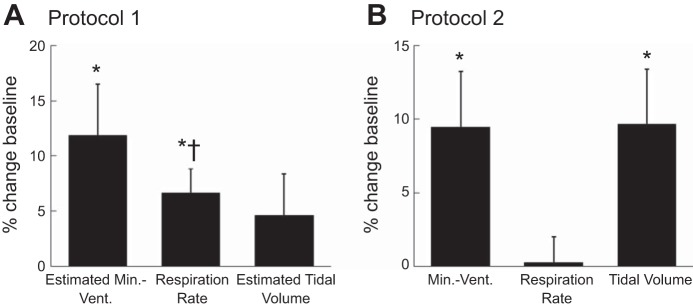
Fig. 3.
Percent changes from baseline in minute ventilation, respiration rate, and tidal volume at 60 min of the hyperinsulinemic-euglycemic clamp in protocol 1 (A) and protocol 2 (B). *P < 0.05 vs. baseline (BL); †P < 0.05 vs. protocol 2.
Fig. 4.
A: individual peak percent changes in estimated minute ventilation, respiration rate, and estimated tidal volume in protocol 1, with each subject identified by a specific color. B: individual percent changes in minute ventilation, respiration rate, and tidal volume for women (red triangles) and men (blue circles) in protocol 2. *P < 0.05 vs. baseline (BL); ‡P < 0.05 for sex difference.
Protocol 2
As expected, serum insulin concentration was elevated during the clamp (BL: 7.1 ± 0.6 µU/ml; Clamp: 50.5 ± 2.0 µU/ml; P < 0.001) while serum glucose concentration was maintained at fasting values (BL: 95 ± 1 mg/dl; Clamp: 96 ± 1 mg/dl; P = 0.221). Minute ventilation 60 min after starting the clamp was greater than BL (BL: 6.8 ± 0.3 l/min; Clamp: 7.3 ± 0.3 l/min; Fig. 3B) and was accompanied by a significant reduction in the end-tidal CO2 (BL: 5.7 ± 0.1%; Clamp: 5.5 ± 0.1%; P < 0.001). Similarly, tidal volume increased during the clamp (BL: 0.52 ± 0.04 liters; Clamp: 0.55 ± 0.03 liters; Fig. 3B). In contrast, respiration rate during the clamp did not change significantly from BL (BL: 13.8 ± 0.6 breaths/min; Clamp: 13.9 ± 0.7 breaths/min; Fig. 3B). The elevation in minute ventilation manifested in 16 of the 23 subjects, with 9 individuals increasing minute ventilation via both respiration rate and tidal volume, 6 individuals increasing minute ventilation solely via tidal volume, and 1 increasing minute ventilation solely via respiration rate. Heart rate increased during the clamp (BL: 64 ± 2 beats/min; Clamp: 67 ± 2 beats/min; P = 0.008), whereas systolic (BL: 137 ± 2 mmHg; Clamp: 136 ± 2 mmHg; P = 0.316) and diastolic blood pressure (BL: 77 ± 1 mmHg; Clamp: 77 ± 1 mmHg; P = 0.707) remained unchanged.
Figure 4B shows individual data for men and women in protocol 2. In terms of potential sex differences, women had a significantly greater increase in tidal volume during the clamp compared with men (women: +18.3 ± 6.9% baseline; men: +3.1 ± 3.0% baseline; P = 0.040), while changes in respiration rate were not significantly different between groups (women: −2.1 ± 2.7% baseline; men: +2.1 ± 2.3% baseline; P = 0.244). There was also no significant sex difference in the minute ventilation response during clamp infusion (women: +15.8 ± 7.4% baseline; men: +4.6 ± 3.2% baseline; P = 0.147).
Comparison Between Protocol 1 and Protocol 2
The average insulin infusion rate was slightly higher in protocol 1 (protocol 1: 0.88 ± 0.03 mU·kg−1·min−1; protocol 2: 0.74 ± 0.01 mU·kg−1·min−1; P < 0.001). Similarly, the average glucose infusion rate was greater in protocol 1 (protocol 1: 6.05 ± 0.63 mg·kg−1·min−1; protocol 2: 3.35 ± 0.18 mg·kg−1·min−1; P < 0.001). The elevation in minute ventilation and tidal volume during the hyperinsulinemic-euglycemic clamp conditions was not significantly different between protocols, whereas the elevation in respiration rate was higher in protocol 1 than in protocol 2 (Fig. 3). Importantly, the variables at the 60-min time point of protocol 1 were representative of the entire clamp period, i.e., there was no difference between the 60 min and the other time points.
DISCUSSION
Our major novel finding is that elevated insulin can increase ventilation independent of glucose in healthy young humans. This finding extends previous studies in humans in which hyperinsulinemia was used to cause hypoglycemia (16, 33), so that despite having reported an increase in ventilation, the effect of lowering glucose levels could not be ruled out. Here, for the first time in humans, we isolated the effect of insulin on ventilation via a hyperinsulinemic-euglycemic clamp, elevating plasma insulin to postprandial concentrations while preventing insulin-induced hypoglycemia.
Consistent with our findings, increased minute ventilation has been demonstrated in rats following bolus injections of insulin with simultaneous infusion of glucose to maintain euglycemia, an effect that was abolished after carotid sinus denervation (28). Moreover, in vitro studies have indicated that insulin can directly stimulate carotid body cells to release neurotransmitters (28). Indeed, it has been suggested that the carotid sinus nerve responds similarly to several chemical stimuli sensed by the carotid bodies, typically resulting in hyperventilation (5, 14). Thus, on the basis of these animal findings, it is possible that direct stimulation of carotid bodies via insulin receptors is a mechanism for the ventilatory responses obtained in the present study. Nevertheless, insulin effects cannot be restricted to direct actions on the carotid bodies, and other mechanisms require consideration. For example, previous studies demonstrated an elevated muscle sympathetic nerve activity during hyperinsulinemic-euglycemic clamp conditions (34). Thus it is possible that insulin-induced sympathoexcitation elicited hyperventilation due to norepinephrine effects on carotid bodies (1, 3, 12), or due to relative tissue hypoxia by reduction of carotid body microvascular blood flow (23, 27). Furthermore, greater glucose oxidation evoked by insulin, as previously demonstrated by an elevated respiratory quotient (19, 31), would result in high arterial CO2 content that could contribute to hyperventilation. However, it should be noted that end-tidal CO2 fraction was decreased during insulin infusion in protocol 2 as a result of hyperventilation, suggesting that CO2 production did not play a role in increasing ventilation. Moreover, because insulin crosses the blood-brain barrier and insulin receptors can be found in the central nervous system (4), a direct effect of insulin on central respiratory areas cannot be ruled out. Future studies are warranted.
Although both experimental protocols in the present study had an increase in minute ventilation and tidal volume, only protocol 1 had an increase in respiration rate, whereas no significant change in respiration rate was observed in protocol 2. One factor that may have led to different increases in respiration rate between protocols is the intensity of stimulus elicited by insulin. It is postulated that greater ventilation in response to metabolic and chemical stimuli is initially driven by tidal volume and accompanied by respiration rate when the stimulus is sufficiently high (32). Interestingly, subjects in protocol 1 received a greater insulin infusion rate. In addition, glucose infusion rate was higher in protocol 1 at 60 min of clamp, which suggests that this group had a higher insulin sensitivity. Thus it is possible that the combination of higher insulin infusion rate and higher insulin sensitivity might have represented a stronger chemical stimulus for the subjects in protocol 1 than in protocol 2, leading to increased respiration rate. The techniques used to measure ventilation may have also led to different increases in respiration rate between protocols. The pneumobelt (protocol 1) can be used to calculate respiration rate and characterize breathing patterns with an indirect measure of tidal volume, without obstructing the airways. When compared with the pneumobelt, the mouthpiece and nose clip connected to a metabolic cart (protocol 2) have the advantage of allowing a direct measurement of ventilation and tidal volume, but at the same time this technique forces a change from nasal to oral breathing, which is known to influence breathing patterns (11, 26). Possibly, this altered breathing pattern might have attenuated the elevation in respiration rate in protocol 2. Nevertheless, irrespective of the variables measured and possible differences in insulin sensitivity, both protocols independently resulted in greater minute ventilation during the hyperinsulinemic-euglycemic clamp. Interestingly, the results of protocol 2 also suggest potential sex differences in the ventilatory responses to hyperinsulinemia in which women may have greater tidal volume responses to insulin compared with men. Additional studies specifically designed to compare men and women are needed to substantiate these findings.
Perspectives and Significance
Although the functional significance for the insulin-induced increase in ventilation is not completely understood, some possibilities can be discussed. For example, from a clinical perspective, spontaneous respiration rate is elevated in animal models with chronic hyperinsulinemia (28). In this regard, cardiovascular and autonomic nervous systems are modulated by breathing patterns, in which slow breathing is generally associated with higher vagal activity and heart rate variability (29), both of which are associated with decreased cardiovascular risk. Therefore, chronic hyperinsulinemia in diseases such as Type 2 diabetes may expose these patients to increased respiration rate, contributing to impaired autonomic modulation of the cardiovascular system. Additionally, after a meal, plasma insulin and glucose levels are increased. Greater ventilation in this period would contribute to remove the excessive arterial CO2 from the elevated glucose oxidation (19). Nonetheless, ventilatory responses to insulin identified in the present study were of relatively low magnitude, so a contribution of insulin to normal breathing and maintenance of CO2 levels requires further investigation.
In summary, we demonstrate for the first time in humans that elevated plasma insulin increases minute ventilation independent of changes in glucose.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-093167 (P. J. Fadel), HL-127071 (P. J. Fadel), HL-130339 (J. K. Limberg), and HL-083947 (M. J. Joyner), American Heart Association Grants 20160072 (P. J. Fadel) and 15SDG25080095 (J. K. Limberg), and the Mayo Clinic Department of Anesthesiology and Perioperative Medicine (T. B. Curry, M. J. Joyner, J. K. Limberg).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.C.B., M.J.J., J.K.L., and P.J.F. conceived and designed research; T.C.B. and J.K.L. analyzed data; T.C.B., J.K., J.K.L., and P.J.F. interpreted results of experiments; T.C.B. prepared figures; T.C.B., J.K., and P.J.F. drafted manuscript; T.C.B., J.K., S.W.H., C.N.Y., T.B.C., J.P.T., M.J.J., J.K.L., and P.J.F. edited and revised manuscript; T.C.B., J.K., S.W.H., C.N.Y., T.B.C., J.P.T., M.J.J., J.K.L., and P.J.F. approved final version of manuscript; S.W.H., C.N.Y., T.B.C., J.P.T., M.J.J., J.K.L., and P.J.F. performed experiments.
ACKNOWLEDGMENTS
Experiments were performed at the Dept. of Medical Pharmacology and Physiology of Univ. of Missouri, Columbia, MO, and the Clinical Research Unit at Mayo Clinic, Rochester, MN. The time and effort expended by all the volunteer subjects are greatly appreciated.
REFERENCES
- 1.Acker H, O’Regan RG. The effects of stimulation of autonomic nerves on carotid body blood flow in the cat. J Physiol 315: 99–110, 1981. doi: 10.1113/jphysiol.1981.sp013735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aitken ML, Franklin JL, Pierson DJ, Schoene RB. Influence of body size and gender on control of ventilation. J Appl Physiol (1985) 60: 1894–1899, 1986. doi: 10.1152/jappl.1986.60.6.1894. [DOI] [PubMed] [Google Scholar]
- 3.Askanazi J, Forse RA, Weissman C, Hyman AI, Kinney JM. Ventilatory effects of the stress hormones in normal man. Crit Care Med 14: 602–605, 1986. doi: 10.1097/00003246-198607000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA. The source of cerebral insulin. Eur J Pharmacol 490: 5–12, 2004. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 5.Bin-Jaliah I, Maskell PD, Kumar P. Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat. J Physiol 556: 255–266, 2004. doi: 10.1113/jphysiol.2003.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conde SV, Obeso A, Gonzalez C. Low glucose effects on rat carotid body chemoreceptor cells’ secretory responses and action potential frequency in the carotid sinus nerve. J Physiol 585: 721–730, 2007. doi: 10.1113/jphysiol.2007.144261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadel PJ, Barman SM, Phillips SW, Gebber GL. Fractal fluctuations in human respiration. J Appl Physiol (1985) 97: 2056–2064, 2004. doi: 10.1152/japplphysiol.00657.2004. [DOI] [PubMed] [Google Scholar]
- 8.Gallego-Martin T, Fernandez-Martinez S, Rigual R, Obeso A, Gonzalez C. Effects of low glucose on carotid body chemoreceptor cell activity studied in cultures of intact organs and in dissociated cells. Am J Physiol Cell Physiol 302: C1128–C1140, 2012. doi: 10.1152/ajpcell.00196.2011. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 10.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han JN, Stegen K, Cauberghs M, Van de Woestijne KP. Influence of awareness of the recording of breathing on respiratory pattern in healthy humans. Eur Respir J 10: 161–166, 1997. doi: 10.1183/09031936.97.10010161. [DOI] [PubMed] [Google Scholar]
- 12.Heistad DD, Wheeler RC, Mark AL, Schmid PG, Abboud FM. Effects of adrenergic stimulation on ventilation in man. J Clin Invest 51: 1469–1475, 1972. doi: 10.1172/JCI106943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holwerda SW, Reynolds LJ, Restaino RM, Credeur DP, Leidy HJ, Thyfault JP, Fadel PJ. The influence of reduced insulin sensitivity via short-term reductions in physical activity on cardiac baroreflex sensitivity during acute hyperglycemia. J Appl Physiol (1985) 119: 1383–1392, 2015. doi: 10.1152/japplphysiol.00584.2015. [DOI] [PubMed] [Google Scholar]
- 14.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2: 141–219, 2012. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limberg JK, Johnson BD, Holbein WW, Ranadive SM, Mozer MT, Joyner MJ. Interindividual variability in the dose-specific effect of dopamine on carotid chemoreceptor sensitivity to hypoxia. J Appl Physiol (1985) 120: 138–147, 2016. doi: 10.1152/japplphysiol.00723.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limberg JK, Taylor JL, Dube S, Basu R, Basu A, Joyner MJ, Wehrwein EA. Role of the carotid body chemoreceptors in baroreflex control of blood pressure during hypoglycaemia in humans. Exp Physiol 99: 640–650, 2014. doi: 10.1113/expphysiol.2013.076869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D, Moberg E, Kollind M, Lins PE, Adamson U, Macdonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia 35: 287–290, 1992. doi: 10.1007/BF00400932. [DOI] [PubMed] [Google Scholar]
- 18.Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev 74: 543–594, 1994. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- 19.Menéndez JA, Atrens DM. Insulin increases energy expenditure and respiratory quotient in the rat. Pharmacol Biochem Behav 34: 765–768, 1989. doi: 10.1016/0091-3057(89)90272-4. [DOI] [PubMed] [Google Scholar]
- 20.Morelli C, Badr MS, Mateika JH. Ventilatory responses to carbon dioxide at low and high levels of oxygen are elevated after episodic hypoxia in men compared with women. J Appl Physiol (1985) 97: 1673–1680, 2004. doi: 10.1152/japplphysiol.00541.2004. [DOI] [PubMed] [Google Scholar]
- 21.Mozer MT, Holbein WW, Joyner MJ, Curry TB, Limberg JK. Reductions in carotid chemoreceptor activity with low-dose dopamine improves baroreflex control of heart rate during hypoxia in humans. Physiol Rep 4: e12859, 2016. doi: 10.14814/phy2.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci 120: 1–9, 2005. doi: 10.1016/j.autneu.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 23.O’Regan RG. Responses of carotid body chemosensory activity and blood flow to stimulation of sympathetic nerves in the cat. J Physiol 315: 81–98, 1981. doi: 10.1113/jphysiol.1981.sp013734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obeso A, Almaraz L, Gonzalez C. Effects of 2-deoxy-d-glucose on in vitro cat carotid body. Brain Res 371: 25–36, 1986. doi: 10.1016/0006-8993(86)90806-1. [DOI] [PubMed] [Google Scholar]
- 25.Pardal R, López-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci 5: 197–198, 2002. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- 26.Perez W, Tobin MJ. Separation of factors responsible for change in breathing pattern induced by instrumentation. J Appl Physiol (1985) 59: 1515–1520, 1985. doi: 10.1152/jappl.1985.59.5.1515. [DOI] [PubMed] [Google Scholar]
- 27.Purves MJ. The role of the cervical sympathetic nerve in the regulation of oxygen consumption of the carotid body of the cat. J Physiol 209: 417–431, 1970. doi: 10.1113/jphysiol.1970.sp009172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 62: 2905–2916, 2013. doi: 10.2337/db12-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo MA, Santarelli DM, O’Rourke D. The physiological effects of slow breathing in the healthy human. Breathe (Sheff) 13: 298–309, 2017. doi: 10.1183/20734735.009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shantsila A, McIntyre DB, Lip GY, Fadel PJ, Paton JF, Pickering AE, Fisher JP. Influence of age on respiratory modulation of muscle sympathetic nerve activity, blood pressure and baroreflex function in humans. Exp Physiol 100: 1039–1051, 2015. doi: 10.1113/EP085071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swaminathan R, Anderson E, Dean H, Wales JK. Metabolic response to insulin induced hypoglycaemia in lean and obese subjects. Horm Metab Res 18: 45–48, 1986. doi: 10.1055/s-2007-1012222. [DOI] [PubMed] [Google Scholar]
- 32.Tipton MJ, Harper A, Paton JFR, Costello JT. The human ventilatory response to stress: rate or depth? J Physiol 595: 5729–5752, 2017. doi: 10.1113/JP274596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward DS, Voter WA, Karan S. The effects of hypo- and hyperglycaemia on the hypoxic ventilatory response in humans. J Physiol 582: 859–869, 2007. doi: 10.1113/jphysiol.2007.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol 588: 3593–3603, 2010. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]