Abstract
It is from the discovery of leptin and the central nervous system as a regulator of bone remodeling that the presence of autonomic nerves within the skeleton transitioned from a mere histological observation to the mechanism whereby neurons of the central nervous system communicate with cells of the bone microenvironment and regulate bone homeostasis. This shift in paradigm sparked new preclinical and clinical investigations aimed at defining the contribution of sympathetic, parasympathetic, and sensory nerves to the process of bone development, bone mass accrual, bone remodeling, and cancer metastasis. The aim of this article is to review the data that led to the current understanding of the interactions between the autonomic and skeletal systems and to present a critical appraisal of the literature, bringing forth a schema that can put into physiological and clinical context the main genetic and pharmacological observations pointing to the existence of an autonomic control of skeletal homeostasis. The different types of nerves found in the skeleton, their functional interactions with bone cells, their impact on bone development, bone mass accrual and remodeling, and the possible clinical or pathophysiological relevance of these findings are discussed.
I. INTRODUCTION
The skeleton is a vital organ of mammals. It allows body posture and movement, protects fragile internal organs like the brain, and is the site of hematopoiesis and mineral storage. More recent findings support its role in endocrine regulations and general body homeostasis as well (48, 146, 312). Because of these important functions, the integrity and function of the skeleton must be maintained throughout life to adapt to the multiple physiological and environmental changes our body is exposed to. This is achieved by the process of bone remodeling, which consists of the continuous removal of small portions of bone, throughout the skeleton and life, and the subsequent replacement of this excavated bone by a new mineralized bone matrix rich in type 1 collagen. Osteoclasts are the cells doing the resorption work. These cells of hematopoietic origin can dissolve bone minerals and enzymatically degrade the bone extracellular matrix following differentiation and fusion into multinucleated cells (227). This osteoclastic activity and differentiated osteoclasts themselves release factors that recruit bone-forming osteoblasts, cells of mesenchymal origin able to secrete high amounts of bone matrix and mineralize it (145). In this review, the term osteoblast is used in a general manner to depict cells committed to this lineage, but several populations exist, which differ between their tissue localization (periosteal, endosteal, trabecular) or stage of differentiation (stem cells, osteochondroprogenitors, committed osteoblasts, differentiated osteoblasts). Postmitotic osteoblasts can embed themselves in the matrix they secrete, then become osteocytes. These cells buried within the cancellous and cortical bone have long extensions that connect them between each other and to cells of the bone marrow environment, including osteoblasts. This is in line with more of an instructive role in the bone remodeling process, acting as mechanosensor, endocrine cells and likely initiating the bone remodeling process at sites in need of repair (29, 37, 269).
For bone mass to be kept stable during adulthood, the new bone laid down by osteoblasts must exactly replace the amount of bone excavated by osteoclasts. This is achieved by the coupling between bone resorption and bone formation, which is driven by the action of multiple growth factors released from the bone matrix, both soluble and membrane-bound factors secreted by osteoclasts and topographical changes generated by osteoclasts at the bone surface (120, 283). An imbalance of this coupling is at the root of multiple bone diseases, one of which being osteoporosis, a progressive, silent, multicomponent and prevalent condition in our aging population, characterized by skeletal fragility and microarchitectural deterioration (35).
The fact that the bone remodeling process is regulated by endocrine, paracrine, and mechanical factors and the link between these regulatory mechanisms and pathologies impacting the skeleton are well-established. However, a series of observations in mutant mouse models of obesity and provocative new concepts derived from these observations initiated in the early 2000s a whole new field of investigations focused on the contribution of neuronal signals to the regulation of bone remodeling and maintenance of bone mass. The tenet of a central (i.e., mediated by the central nervous system) regulation of bone mass stems from a number of in vivo observations, initially from Dr. Karsenty and colleagues (73). Those include the high vertebral trabecular bone mass of mice deficient for leptin (the ob/ob mice), an adipose-derived hormone known to inhibit food intake and stimulate energy expenditure (73). These mice are morbidly obese and hypogonadal and have high corticosterone levels, which should lead to bone loss. Therefore, the high bone mass observed in these mice suggested the existence of a dominant mechanism maintaining bone formation at a high level in absence of leptin, despite high osteoclast activity. The fact that this hormone impacts energy homeostasis via action in hypothalamic centers and the rescue of the high bone mass phenotype of the ob/ob mice following infusion of minute amount of leptin within the third hypothalamic ventricle supported a central mode of action of this hormone on bone remodeling. The high bone mass of mice characterized by ablation of leptin-responsive ventromedial hypothalamic neurons further supported this model (73, 301). However, additional evidence of a direct osteogenic action of leptin in bone cells was also reported (62, 97, 119, 310), suggesting that leptin was acting both centrally and peripherally to control bone mass. Which of the two mechanism is more relevant to normal physiology remains a matter of debate, but the normal bone mass of mice lacking the leptin receptor ObRb in mature osteoblasts and the high bone mass of mice lacking this receptor specifically in neurons are strong evidence that leptin and ObRb-positive neurons in the hypothalmus have a dominant anti-osteogenic effect on the skeleton (282).
The logical next question was then to determine the mode of communication between hypothalamic neurons and bone cells. This question was first addressed through parabiosis experiments, used originally in the 1970s to identify leptin and its effect on body weight (61). In these experiments, the blood circulation of pairs of ob/ob mice was linked so that hormones from one mouse could reach target tissues in the contralateral mouse. With the use of this approach coupled with the delivery of leptin in the third cerebral ventricle (ICV) of one of the parabiosed mice, it was shown that only mice receiving leptin ICV lost bone, whereas the contralateral parabiosed mice did not. This observation suggested that the mediator of the anti-osteogenic central action of leptin was not humoral, but rather of neuronal nature. It was also in line with the histological observation of nerves located in areas of high osteogenic activity. The evidence of an association between bone remodeling and autonomic outflow, observed from dominant sympathetic activity during day hours, when bone resorption activity reaches its peak, and parasympathetic activity dominance in the night, when bone formation is more active, further supported this model (279).
II. INNERVATION OF THE SKELETON
A. Anatomy of the Peripheral Nervous System
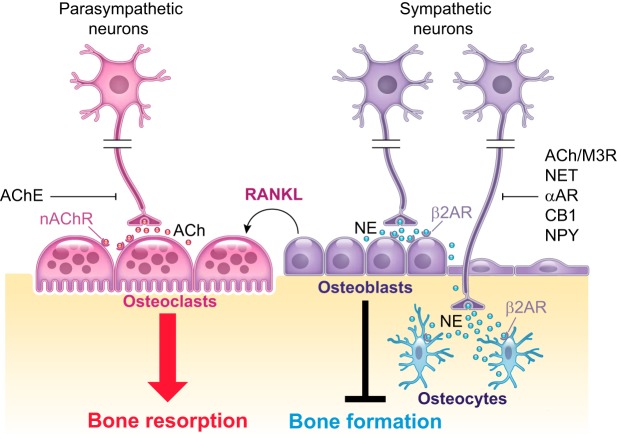
The central nervous system (CNS) collects information from the environment and from the interior milieu and sends commands to the rest of the body via efferent autonomic and somatic motor pathways. The autonomic nervous system (ANS) is structurally and functionally positioned to interface between the external and internal milieux and coordinates bodily functions to ensure homeostasis and adaptive response to various stresses. It is itself divided anatomically into two antagonistic arms: the sympathetic nervous system (SNS) and the parasympathetic nervous system (PSNS) (FIGURE 1). The sympathetic branch functions through the neurotransmitter norepinephrine (NE), which activates α- and β-adrenergic receptors in presynaptic and postsynaptic cells, respectively. The parasympathetic branch functions through the neurotransmitter acetylcholine (ACh), which activates muscarinic and nicotinic cholinergic receptors.
FIGURE 1.
Functional anatomy of the nervous system.
1. Sympathetic nerves
a) sympathetic neurotransmitters.
Norepinephrine is a major neurotransmitter of the SNS. Its availability for sympathetic transmission involves a highly coordinated process of NE synthesis, vesicular packaging, vesicular release upon action potential, and also reuptake into presynaptic nerve terminals by the NE transporter (NET). NE is synthesized from the amino acid tyrosine by action of tyrosine hydroxylase (TH), which is the rate-limiting enzyme of catecholamine biosynthesis. It converts tyrosine to l-DOPA, which is then converted to dopamine (DA) by l-aromatic amino acid decarboxylase (LAAD or DOPA decarboxylase). DA is then taken up into intracellular synaptic vesicles and converted to NE by dopamine β-hydroxylase (DBH) (FIGURE 2). NE is often colocalized and coreleased with neuropeptide Y (NPY) and ATP, with NPY being a potent inhibitor of NE and ATP release at the prejunctional level. NE can be metabolized by the enzymes monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT), or reuptaken into presynaptic neurons by NET.
FIGURE 2.
Norepinephrine (NE) synthesis, reuptake, and catabolism. NE is synthesized from tyrosine by action of tyrosine hydroxylase (TH). It converts tyrosine to l-DOPA (dihydroxyphenylalanine), which is then converted to dopamine (DA) by l-aromatic amino acid decarboxylase (LAAD or DOPA decarboxylase). DA is then taken up into intracellular synaptic vesicles and converted to NE by dopamine β-hydroxylase (DBH). Action potentials lead to vesicular exostosis and release of NE in the extracellular space where it can stimulate pre- and postsynaptic adrenergic receptors. 90% of NE is reuptaken by presynaptic nerves. The remaining NE is metabolized by the enzymes monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT).
b) sympathetic innervation of the skeleton.
The peripheral SNS is stimulated in a coordinated fashion by premotor neurons in the brain stem and hypothalamus which integrate internal and external signals from stressors, such as exercise, cold, and stress, to maintain homeostasis. Preganglionic sympathetic neurons are located in the intermediolateral nucleus in the spinal cord, extending from the first thoracic spinal segment to the second lumbar segment. These pre-ganglionic sympathetic neurons synapse with subpopulations of postganglionic neurons at the paravertebral ganglia of the sympathetic chain. Generally, preganglionic neurons are cholinergic, myelinated, and short, whereas postganglionic neurons are noradrenergic, unmyelinated, long, and of small diameter (<5 μm). Sympathetic postganglionic neurons project to most tissues of the body, including smooth and cardiac muscles, skin, digestive, urinary, and reproductive organs, as well as the skeleton (FIGURE 3). They commonly reach these tissues along with major nerves containing predominantly sensory and somatic motor nerve fibers. In humans, the femoral and sciatic nerves are for instance constituted of motor fibers, sensory fibers, and some autonomic fibers. However, most sympathetic nerve fibers appear to run along the main arteries feeding long bones through the nutrient foramina (34, 117, 299).
FIGURE 3.
Anatomy of the autonomic nervous system. Simplified diagram of the sympathetic and parasympathetic divisions of the peripheral nervous system. Sympathetic neuronal cell bodies (green) in the thoracolumbar segments of the spinal cord send short efferent myelinated preganglionic fiber projections to postganglionic neurons in different sets of the ganglia, including the paravertebral ganglia that are paired structures located bilaterally along the vertebral column. Postsynaptic sympathetic neurons then send long unmyelinated axons of small diameter to peripheral effector organs. Because the axons of the preganglionic neurons may branch and travel up and down the sympathetic chain, a single preganglionic neuron can synapse with many postganglionic neurons up and down this chain, leading to widespread stimulation. Postganglionic sympathetic neurons from the ganglia travel to specific organs, leading to a more localized effect. Parasympathetic preganglionic neurons (purple) originate in the brain stem and sacral spinal cord. They are long and synapse with postganglionic neurons in ganglia near or in the wall of effector organs.
The distribution of sympathetic nerves within the mammalian skeleton is still poorly described, despite the fact that original observations of bone innervation by histological techniques have now been refined by immunohistochemical and immunofluorescence approaches to visualize specific transmitters and thereby nerves. Both the periosteum and the bone marrow receive noradrenergic fibers (often associated with the vasculature) and nonadrenergic vesicular ACh transporter (VAChT)- and vasoactive intestinal polypeptide (VIP)-immunoreactive fibers (often associated with the parenchyma) (90, 117, 118, 200). Sympathetic periosteal fibers branch within the bone marrow and compact bone, as shown by immunoreactivity for TH, DBH, or NPY, which are markers of sympathetic nerves (5, 13, 33, 81, 117, 118, 123, 129, 200). Small branches of periosteal nerve fibers going through the cortical bone are associated with blood vessels in Volkmann and Haversian canals. Some can go through the bone marrow parenchyma and terminate on sinusoid walls, vascular elements, perivascular stromal cells, and bone surfaces (46, 193). TH-immunoreactive fibers generally have a spiral-like morphology and wrap around blood vessels with which they are associated (193, 200). In general, the areas of mineralized bone that receive the greatest mechanical stress and load have the highest metabolic rate and bone turnover. They are also the most vascularized and display the highest density of sympathetic and sensory fibers (see below).
c) interactions of sympathetic nerves with target cells.
Bone-embedded osteocytes respond to mechanical loading and microcracks and initiate the skeletal response to these stimuli (29, 156, 162, 170, 258, 269). NPY- and TH-immunoreactive fibers have been described in Volkman canals and close to osteocytes (33), but whether sympathetic signaling is part of the response of osteocytes to mechanical loading and microfractures has not been addressed directly. However, one study suggested that contribution of the SNS to the response to mechanical load appears minimal (65), while another one suggested sympathetic signaling contributes to unloading-induced bone loss (165). In fact, only a limited number of bone cells appear to be in direct contact with sympathetic nerve terminals (66, 301), and the sympathetic neuroeffector junction between skeletal nerve fibers and bone cells is poorly defined and seems to lack pre- and postjunctional specializations that are observed, for instance, in skeletal muscle end plates. Because of the lack or low density of recognizable synapses between skeletal neurons and bone cells, a diffusion mechanism could be at play to activate multiple target cells away from the nerve terminal, thus expanding the overall action of SNS activation and released NE on a higher number of bone cells than in the case of a direct synaptic contact. Communication via intercellular junctions following an effect on a limited number of target cells expressing the receptors for NE could be involved too, but such a putative mechanism is obviously challenging to address in vivo in hard tissues like bone.
It is also unknown whether distinct skeletal elements differ in terms of the density or activity of sympathetic nerves that innervate them, or to their response to signals from these nerves. In that regard, it is interesting to note that the ob/ob mice have a low sympathetic outflow associated with a high trabecular bone mass and a low cortical bone thickness in the vertebral axis (73, 105, 301). In long bones however, different phenotypes were reported. Ducy et al. (73) reported a high trabecular bone mass in the femurs of 6-mo-old ob/ob females, whereas Hamrick (104) reported a low trabecular and cortical bone mass in the femurs of 6-mo-old ob/ob males, in line with results from other groups in younger ob/ob mice (292). The Hamrick study also reported an increase in femoral adiposity in ob/ob mice, but not in the vertebrae, and a reduction in hindlimb muscle mass, both of which could contribute to the observed long bone phenotype. In addition, vertebrae were larger and long bones shorter in ob/ob mice (104). The effects of altered leptin signaling on bone might thus vary between different skeletal regions and between cortical and trabecular envelops. The mechanism(s) leading to these differential effects are unknown, and could involve an imbalance of stromal cell differentiation toward the adipocyte lineage and subsequent effect on femoral bone remodeling, or a different nerve supply, nerve outflow, and/or response to neuronal signals between vertebral and hindlimb bone elements. In the same vein, bilateral destruction of the inner ear vestibular structures, which was shown to stimulate sympathetic outflow, induced bone loss in long bones but not in the vertebral axis of mice and rats (327, 328). A better anatomical mapping of the innervation of the skeleton is thus necessary to better understand the role of sympathetic nerves on bone remodeling and bone pathologies, and is a first step required for the design of future investigations of “functional” mapping of skeletal innervation. Because of the existence of selective control of sympathetic outflow to individual tissues and thus expression of patterned autonomic responses (213, 284), the measurement of sympathetic autonomic outflow in specific bone elements also needs to be done as it would provide more interpretable and convincing results to implicate an effect of endogenous sympathetic activity and NE in the skeleton and in each specific bone element.
2. Parasympathetic nerves
a) parasympathetic neurotransmitters.
In addition to its major role in the activation of muscles at the neuromuscular junction, ACh is a neurotransmitter of preganglionic sympathetic nerves and of parasympathetic neurons. ACh is synthesized from choline and acetyl coenzyme A by the action of the enzyme choline acetyltransferase (ChAT) (FIGURE 4). Upon synthesis, ACh is loaded into synaptic vesicles by the vesicular ACh transporter (VAChT), whose expression increases progressively with proximity to nerve terminal (thus making this molecule a good marker of PSNS nerve fibers). Following release into the synaptic cleft and binding to its postsynaptic receptors, ACh is inactivated by action of the enzyme acetylcholine esterase (AChE), which hydrolyzes ACh into choline and acetate. Choline is then recycled into presynaptic terminals via choline transporter (CHT)-mediated uptake (the rate-limiting step of ACh synthesis). ACh and VIP are often coreleased from parasympathetic nerve terminals.
FIGURE 4.
Acetylcholine synthesis and catabolism. ACh is synthesized from choline and acetyl coenzyme A by the action of the enzyme choline acetyltransferase (ChAT). Upon synthesis, ACh is loaded into synaptic vesicles by the vesicular ACh transporter (VAChT). Following release into the synaptic cleft and binding to its postsynaptic receptors, ACh is inactivated by the action of the enzyme acetylcholine esterase (AChE) and butyrylcholinesterases (BChE). Choline is then recycled into presynaptic terminals via choline transporter (CHT)-mediated uptake.
b) parasympathetic innervation of the skeleton.
Parasympathetic cranial nerves originating from the brain stem innervate the rostral part of the body, whereas sacral preganglionic neurons innervate the caudal part of the body (2) (FIGURE 3). In contrast to SNS postsynaptic neurons, PSNS postsynaptic neurons are relatively short. They mainly supply cholinergic terminals to structures involved in excretory and reproductive functions; however, they have been detected within the bone microenvironment, via immunoreactivity against VAChT and ChAT (11, 16). The exact pattern and density of cholinergic innervation within bone and across bone elements is unknown.
3. Sensory nerves
a) sensory neurotransmitters.
Sensory nerves have substance P (SP) and calcitonin gene-related peptide (CGRP) as their main neurotransmitter. They also release glutamate and pituitary adenylase cyclase activating peptides (PACAP) 27 and 38. These nerves express neurofilament 200 (NF200) and TrkA, the high-affinity receptor for nerve growth factor (NGF). Of note is that calvarial differentiated osteoblasts as well as osteoclasts express VIP receptor type 1 and 2 (VPAC1 and VPAC2), which are receptors that bind PACAPs and VIP (187, 216, 247, 312).
b) sensory innervation of the skeleton.
Sensory fibers, emanating from dorsal root ganglia of the spinal cord (where the neuronal cell body is located), innervate tissues throughout the body. They can detect multiple environmental factors, including thermal and mechanical stimuli, as well as inflammatory cytokines, process these factors and relay signals to higher brain centers for further processing and integration (26, 246).
The periosteum and trabecular bone compartments are richly innervated with a dense network of sensory fibers that are sensitive to mechanical stimulation (193, 302). This sensitivity is at the origin of the pain sensation associated with trauma or fracture, or with bone malignancies and possibly genetic diseases like osteogenesis imperfecta. These sensory fibers are long and linear in appearance and include thickly and thinly myelinated A-fibers, as well as the peptide-rich C-fibers (50, 137). During embryonic development, the perichondrium of the femur is innervated by TrkA-positive axons originating from nerves of the lumbar plexus (L1–L6) as early as embryonic day 14.5. By birth, the entire bone collar is covered by sensory nerves, but no axons are detected within the cartilaginous ends (315). CGRP-immunoreactive sensory nerve fibers arising from collaterals that originate from nerve fibers that enter in large bundles via the nutrient foramen are found in close apposition to blood vessels in the bone marrow (193). Although the functional relevance of this observation is not yet addressed, both sensory and sympathetic nerve fibers in the limbs are able to sprout in response to inflammation (94, 136).
B. Neuronal Circuitry Between the CNS and the Skeleton
Multisynaptic tract-tracing methods have been critical to uncovering the circuitry linking neurons innervating the skeleton to CNS centers (66). These methods are based on the use of the fluorescently labeled neurotropic pseudorabies virus (PRV), which can be taken up by axon terminals. Upon delivery within long bones and following uptake by skeletal nerve terminals, the viral nucleocapsids of the PRV travel in a retrograde fashion to the neuronal cell body and replicate, and the new virions infect functionally connected neurons across synapses, thereby lighting up the succession of neurons connecting with the bone element where the virus was injected. These studies have shown that lumbar and thoracic sympathetic ganglia, as well as the spinal cord (T8-L1), were labeled within 4–5 days following injection of the PRV into the rat femur. Nerve transection in PRV-infected rats indicated that the major branches of sciatic, femoral, and obturator nerves have significant contribution to the innervation of the femoral bone marrow. Brain stem, pons, midbrain, and hypothalamic neurons were found positive within 5–6 days. Labeled structures included noradrenergic neurons within the locus coerelus and subcoeruleus area known to regulate the activity of the SNS (66, 221). Rostral ventrolateral medulla C1 adrenergic cells, known to play an important role in the reflex adjustments of sympathetic activity to internal and external stimuli (214, 273), were also labeled. In the hypothalamus, the paraventricular nucleus and the lateral hypothalamic area were identified as sources of afferent inputs to the spinal preganglionic neurons innervating the bone marrow. Some neurons in the arcuate nucleus were also labeled. Using a similar method of PRV injection in the femur, Bajayo et al. (16) confirmed labeling of neurons in the intermediolateral column at the thoracic level. Parasympathetic neurons in the central autonomic nucleus of the sacral spinal cord segment of mice and rats, which are anatomical sites with parasympathetic preganglionic cell bodies, were also detected in these two independent studies (16, 66). Together, these results revealed a hierarchically organized circuit that links several autonomic spinal and brain areas to femoral nerve fibers via sympathetic and parasympathetic preganglionic neurons. There are not yet more extensive studies that compare the innervation of several bone elements (long bones vs. vertebral elements or hindlimbs vs. forelimbs for instance) and the respective ganglionic and CNS structures they are connected to. In addition, because the bone environment contains blood vessels that have their own innervation, there is still some uncertainty as to whether the labeling observed originates from the bone marrow parenchyma or supplying vasculature, or both.
III. SYMPATHETIC REGULATION OF BONE REMODELING
A. Subtypes, Expression, and Signaling of Adrenergic Receptors in Bone
NE released by sympathetic nerve terminals signals through α- and β-adrenergic receptors (ARs), which belong to the large family of seven transmembrane spanning α-helical domain G protein-coupled receptors.
1. α-Adrenergic receptors
Three α1ARs have been cloned (A, B, and D). These receptors belong to the Gq protein-coupled receptor superfamily. Upon activation, Gq activates phospholipase C (PLC), which cleaves phosphatidylinositol 4,5-bisphosphate (PIP2), leading to an increase in inositol trisphosphate (IP3) and diacylglycerol (DAG), and eventually a rise in intracellular calcium content. Agonist potency order for α1ARs is NE > epinephrine (Epi) > isoproterenol (ISO). The α1AR is involved in the contraction of smooth muscles, hence its role in the control of blood pressure. It also regulates heart, salivary gland, and prostate function. There are also three α2ARs (A, B, C), which couple to Gi/o protein. These receptors are presynaptic and generally control neurotransmitter release. They have roles in the brain and peripheral nerves and in target tissues such as vascular smooth muscle cells, gastrointestinal tract, and pancreas. Agonist potency order for α2ARs is Epi > NE > ISO.
α1A, α1B, and α1DAR mRNA can be detected in both osteoblast (106, 121, 225, 307) and osteoclast (9, 166) lineages (TABLE 1). The α2AAR and, to a lesser extent, α2B and α2CAR transcripts can also be detected at low levels in bone tissues, mature osteoblasts, osteocytes, chondrocytes, and osteoclasts (87, 211). However, the weak expression level of these receptors in bone cells and the lack of strong in vivo evidence supporting the contribution of these receptors in bone cells to bone remodeling suggest that the βAR subtype may be the main AR receptor to mediate the action of sympathetic nerves on bone remodeling.
Table 1.
Main adrenergic receptor and enzyme mRNAs expressed in bone cells
| Cell Lineage | Cells Investigated | Receptor/Enzyme | Reference Nos. |
|---|---|---|---|
| Osteoclasts | Differentiated bone marrow macrophages and Raw 264.7 cells | β2AR | 9, 166 |
| β2AR | 6 | ||
| Chondrocytes | Mouse growth plate chondrocytes (IHC) | α2AAR and α2CAR | 87 |
| Immature osteoblasts (undifferentiated) | Rat bone marrow mesenchymal cells | α1AR, α1BAR, α1DAR | 106 |
| Mouse sarcoma C3H10T1/2 cells | α1AAR | 307 | |
| Mouse MC3T3 cells | α1AAR and α1DAR | 225 | |
| Mouse MC3T3 cells | β2AR, α2AAR | 87, 265 | |
| Human fetal long bone-derived osteoblasts | α1BAR and β2AR | 87, 121 | |
| Human osteosarcoma MG63 cells | β2AR | 121 | |
| Human osteosarcoma SaOS2 cells | β2AR, β1AR | 154 | |
| Human osteosarcoma TE-85 cells | β2AR, β1AR | 154 | |
| Human osteosarcoma OSH-4 cells | β1AR | 154 | |
| Rat ROS 17/2.8 cells | β2AR | 212 | |
| Human periosteum-derived osteoblastic SaM-1 cells | β2AR | 312 | |
| Human osteosarcoma HOS cells | β2AR | 312 | |
| Mouse calvarial osteoblasts | β2AR | 78, 189, 212, 301 | |
| Mouse bone marrow stromal cells | α1DAR | 307 | |
| Differentiated osteoblasts | Mouse bone marrow stromal cells | α1AAR and α1DAR | 307 |
| Osteocytes | Mouse IHC | β2AR | 12 |
2. β-Adrenergic receptors
βARs are postsynaptic receptors subdivided into three groups (β1, β2, and β3) and are classically detected in cardiac muscle, airway smooth muscle, and adipose tissue, respectively. They couple to Gs and their stimulation increases cAMP intracellular levels and protein kinase A (PKA) activity. Agonist potency order for β1/2ARs is ISO > Epi > NE and ISO > NE > Epi for the β3AR. Stimulation of the β1AR increases heart rate and contractibility. Stimulation of the β2AR leads to bronchodilation and vasodilatation. The β3AR is known for its effect on lipolysis and thermogenesis in adipose tissues.
Osteoblastic cell lines and primary osteoblast cultures mainly express the β2AR subtype (78, 189, 212), with weak to nondetectable expression of the β1AR and β3AR (6, 154, 212, 301, 312). The β2AR can be detected in osteoclasts too; however, the biological relevance of a direct effect of β2AR stimulation in osteoclasts needs to be further investigated; although a direct stimulatory effect of βAR stimulation on osteoclast differentiation was reported (6), the assays in this study were done with bone marrow cells, which may contain β2AR-expressing osteoblasts known to produce the osteogenic cytokine RANKL in response to NE (see below). In another study, a moderate pro-osteoclastogenic effect of ISO was reported in RAW264.7 cells in the presence of RANKL (166).
Stimulation of differentiating calvarial osteoblasts and SaOS-2 osteoblastic cells by the β1/β2AR nonselective agonist ISO stimulates cAMP accumulation, indicating that these receptors are functional in committed mature osteoblasts (301). Importantly, these cells can respond to βAR agonists, but they cannot generate catecholamines as they do not express DBH, the enzyme generating Epi and NE. However, both catabolic enzymes MAOα and MAOβ are expressed at both mRNA and protein levels in osteoblast precursor cells and in fully differentiated osteoblasts (6, 188), indicating that this cell lineage can catabolize NE. Differentiated osteoblasts also express the NET, which allows them to uptake NE (see below) (188). Therefore, bone cells have the machinery to modulate signals from sympathetic nerves.
It is worth noting that immune cells, including T cells, express the β2AR; hence, the immune cell compartment in bone represents an additional putative target of sympathetic nerves. This might have relevance to the control of bone remodeling, as T cells were shown to impact bone remodeling in mice (234, 332). The role of sympathetic nerves in the mobilization of hematopoietic stem cells, which reside in bone, is also to be noted (148, 206, 207).
B. βARs as Effector of SNS Action in Bone Cells
The in vivo biological relevance of postsynaptic βAR signaling has been addressed pharmacologically and genetically. Daily βAR stimulation by means of the β1/β2AR nonselective agonist ISO or by β2AR-selective pharmacological agonists triggers a bone catabolic response measured by increased osteoclast formation, reduced osteoblast function, and bone loss (39, 56, 181, 278, 301). The opposite manipulation, i.e., βAR blockade by the nonselective β1/β2AR antagonist propranolol, is anti-catabolic, and this action on the skeleton is best seen in conditions where bone remodeling is high. Young mice treated with propranolol, for instance, have a higher vertebral trabecular bone mass than vehicle-treated mice, and the bone loss induced by estrogen deficiency is attenuated in propranolol-treated mice (38, 241, 301). A low-dose propranolol (but not high dose) can prevent estrogen-induced bone loss in rats too, without significant effects on heart hemodynamic parameters (38, 40).
The specific condition of bone unloading provided data inconsistent with an anti-catabolic action of β1/β2AR blockade on bone. The β1AR agonist dobutamine indeed did not worsen but instead mitigated the reduction in cancellous bone mass induced by hindlimb unloading, and attenuated the associated increase in osteocyte apoptosis (296, 297). It had no effect in ambulatory rats. Although the data suggest opposing roles of the β1 and β2AR in the osteoblast lineage, it must be kept in mind that the condition of hindlimb suspension could impact sympathetic signaling or the response of target organs to sympathetic signaling, and that stimulation of the β1AR has an effect on insulin-like growth factor I (IGF1) expression, which could contribute to the reported effect of dobutamine on bone mass.
Genetic data have been valuable to further address the role of sympathetic signaling in bone cells and to provide further specificity in terms of which βAR is important in bone cells. β2AR-deficient mice, like β1AR-deficient mice, do not show any patterning or growth abnormalities, thus excluding a role of these receptors in bone development. However, β2AR-deficient mice display a high trabecular bone mass phenotype reminiscent of mice treated with β-blockers, associated with low bone resorption and high bone formation (79, 301). The inactivation of the β2AR specifically in osteoblasts induces a similar phenotype, which indicates that the osteoblast is the main target of sympathetic nerves for their effect on bone mass. Mice lacking Dbh, which is required for NE production in catecholaminergic neurons, displayed a high trabecular bone mass phenotype as well (301), further suggesting that the release of NE by sympathetic nerve fibers is required for normal bone accrual and remodeling.
Because other βAR subtypes than the β2AR have been detected in bone cell cultures, mice lacking all three βAR types have been analyzed for their bone phenotype too. The global absence of all three βARs resulted in a high trabecular bone mass with lower osteoclast surfaces in young mice (6 wk old) (43). However, these mice became obese and lost bone upon ovariectomy and aging, an observation in contrast to β2AR-deficient mice which had a normal body weight, were resistant to ovx-induced bone loss, and gained bone mass upon aging (79). The interpretation of these results is difficult because of the global nature of βAR ablation in this model and the development of obesity, which can affect bone mass in response to skeletal loading or to the indirect action of leptin or IGF1.
Consistent with the pharmacological data cited above, β1AR deficiency in mice caused a low bone mass phenotype in femurs and prevented the bone anabolic response to axial compression loading. This phenotype appeared to be dominant over the one induced by β2AR deficiency, as double β1/2AR mice also had a low bone mass phenotype associated with a reduction in bone formation rate and absence of response to compression loading (240). These results suggest that β1AR and β2AR signaling have opposite effects on bone, with β1AR signaling exerting a predominant anabolic stimulus, whereas β2AR signaling is catabolic. It must be noted here again that the effect of global β1AR deletion on bone mass was accompanied by a significant reduction in serum IGF1 and IGF-binding protein 3 during growth, which may confound interpretation of the results.
The molecular mechanisms whereby ISO or NE impact bone remodeling was shown to involve the stimulation of osteoclastogenesis and bone resorption via the action of ISO or NE in osteoblasts which induces an increase in the expression of the pro-osteoclastogenic cytokine RANKL. This effect was triggered by activation of the ATF4 transcription factor following phosphorylation of serine 254 by PKA (79). Interestingly, β2ARs and parathyroid hormone (PTH) signaling both promote Rankl expression and use the same second messengers (cAMP/PKA pathway) in the osteoblast lineage, but can elicit different transcriptional events. Obri et al. (228) provided evidence implicating a Smurf2-mediated ubiquitination of the class II histone deacetylase HDAC4 as a mechanism for PTH to favor RANKL expression in differentiated mouse calviaria osteoblasts, whereas ISO appears to favor the accumulation of HDAC4 in the nucleus of these cells and its association with ATF4. Despite using a common signaling pathway, it must be kept in mind that ISO and PTH may act at different stages of differentiation in the osteoblast lineage, thereby eliciting distinct genomic events and cellular effects. PTHR1 expression increases upon differentiation, with maximal expression in fully differentiated osteoblasts (84, 202), whereas the expression of the βAR is relatively constant along this process (189).
Although RANKL was the first β2AR target gene identified in the osteoblast lineage, more recent studies have shown that osteocyte-derived RANKL plays a predominant role in bone remodeling (337). It is thus possible that in vivo, sympathetic nerves or NE released by these nerves regulate bone remodeling through action on osteocytes, which express the β2AR (12). This hypothesis is further supported by the action of ISO on fibroblast growth factor (FGF)23 expression (151), which is made by mature osteoblasts/osteocytes. Although sympathetic nerves can be detected in Volkmann’s canals, it remains yet to be determined if NE is released at the vicinity of the osteocytic network, and if mice lacking the β2AR in osteocytes have the high bone mass phenotype expected from the current working model.
βAR stimulation in osteoblasts promotes osteoclastogenesis, but also restrains bone formation by inhibiting osteoblast proliferation via a cyclin D1 and Clock-dependent mechanism, thus uncoupling bone formation and bone resorption in vivo (91, 142, 198, 312). An in vitro study with the MC3T3-E1 osteoblastic cell line also provided evidence that β1/2AR stimulation suppresses BMP-induced alkaline phosphatase expression (339).
It is important to note that the results summarized above and their interpretation are mostly based on experiments in which postsynaptic βAR signaling was manipulated. These data may thus relate more to the action of cardiovascular drugs like sympathomimetics or β-blockers on bone remodeling than to the mechanism by which endogenous sympathetic outflow regulates bone remodeling. Experimental alteration of the number or function of skeletal sympathetic nerves per se, and in adults, will be required to truly assess the impact of sympathetic nerves and endogenous NE on bone remodeling. One study tackled this challenge through the generation of mice lacking FoxO1 in Dbh-positive catecholaminergic neurons. Results suggested that FoxO1 favors Dbh expression and thereby NE synthesis in sympathetic neurons, and showed that mice lacking FoxO1 in Dbh-neurons were characterized by low sympathetic outflow at 9 mo of age (in males) and a high bone mass phenotype similar to the one of the β2AR−/− mice, with high bone formation and reduced bone resorption (143).
C. Regulation of SNS Outflow
Sympathetic nerves activated by an action potential release NE at their synaptic terminals to stimulate postsynaptic target cells. The intensity and duration of this signal are controlled by a set of several feedback mechanisms, involving presynaptic αARs, the norepinephrine transporter, the cannabinoid system, and the NPYergic system.
1. Presynpatic autoinhibitory α-adrenergic receptors
The α2AAR and α2CAR are presynaptic receptors expressed in brain stem adrenergic neurons. Their activation by NE leads to a reduction in sympathetic tone, which can be functionally measured by a resultant decrease in heart rate and blood pressure. Accordingly, genetic ablation of the α2AAR leads to increased sympathetic outflow (8). On the basis of the current model of sympathetic regulation of bone mass (FIGURE 5), the increase in SNS outflow caused by α2AR blockade (no autoinhibitory activity) should theoretically lead to a low bone mass phenotype. However, genetic global deletion of α2A/α2CAR caused a generalized high bone mass phenotype in female mice, accompanied by low bone resorption and high bone formation. These mice had elevated levels of plasma NE, confirming the existence of a high sympathetic tone. Their bone phenotype became more evident as the mice aged, but was already detectable in young mice. It was also gender specific (not detected in males) (87). Consistent with the findings in α2A/α2CAR-deficient mice, a single oral dose of the α2AR agonist clonidine increased serum CTX (a marker of bone resorption) in healthy human volunteers (180). The picture becomes even murkier upon analysis of mice lacking the α2CAR only. These mice exhibit the expected low bone mass phenotype in long bones, in line with their elevated sympathetic activity, but a high bone mass phenotype in young female’s vertebrae (63). These observations indicate that the mechanisms whereby endogenous sympathetic tone regulates skeletal homeostasis are more complicated than previously thought, and also suggest a possible link between α2AR and estrogen signaling in the CNS. Elucidation of the role of the αAR in bone remodeling awaits the generation and analysis of mice with an inducible ablation of these receptor subtypes in neurons or bone cells, and in adult mice to circumvent any possible developmental phenotypes.
FIGURE 5.
Mechanism by which sympathetic nerves may inhibit bone formation and promote bone resorption. Noradrenergic nerve terminal in bone release NE at the vicinity of osteoblasts and osteocytes and stimulate the β2AR. This leads to inhibition of bone formation and to an increase in RANKL expression, leading to osteoclast formation and increased bone resorption. This brake on bone formation and stimulation of bone resorption by adrenergic agonists thus induces bone loss.
2. The norepinephrine transporter
The amount of endogenous NE released by sympathetic neurons is also controlled presynaptically by the NET and its reuptake of NE into presynaptic neurons, which clears 80–90% of NE released by central and peripheral sympathetic neurons. NE reuptake by NET provides a negative-feedback mechanism to limit the duration of SNS neurotransmission and allows NE recycling and repackaging into vesicles. Therefore, pharmacological NET blockade leads to acute NE spillover, but long-term NET inhibition, genetically or pharmacologically, leads to intracellular NE storage depletion and thus to a low sympathetic tone. Such characteristic was expected to cause a high bone mass phenotype; however, analysis of Net-deficient mice revealed a generalized low bone mass in male mice (188). Furthermore, chronic immobilization stress (CIS), which is an experimental means to increase endogenous sympathetic outflow, did not provoke bone loss as expected, but it did if combined with pharmacological NE reuptake blockade by reboxetine (188). These findings thus suggested that the control of NE reuptake by NET is an integral part of the homeostatic system by which bone remodeling is regulated.
In these studies, it was observed that differentiated osteoblasts and possibly osteocytes, like sympathetic neurons, express NET at both RNA and protein levels, and most importantly can uptake and catabolize NE ex vivo (188). This observation led to the hypothesis that NE uptake via NET in mature osteoblasts and osteocytes may contribute to NE clearance within the bone microenvironment and act as a catabolic sink for NE to buffer the catabolic action of sympathetic nerves on the skeleton. Of note is that osteoblasts and osteocytes compose 90% of resident bone cells in adults, making this putative osteocytic catabolic sink mechanism a potentially important one in the regulation of bone remodeling. In addition, one needs to note ISO cannot be uptaken by NET, hence ISO-treated mice can lose bone without the need of blocking NET uptake, unlike mice subjected to CIS. The fact that osteoblasts express the enzymes to degrade NE further supports this model. Like in the case of α-AR−/− mice, cell-specific inactivation of Net will be necessary to further understand if and how NET in bone cells modulate the response of the skeleton to endogenous sympathetic tone.
3. The cannabinoid receptor
A third mechanism involved in the modulation of sympathetic outflow relies on activation of the CB1 cannabinoid receptor, which induces presynaptic inhibition of NE release (132). CB1 and its paralog CB2 receptors are seven-transmembrane domain receptors that are coupled to the Gi/o subclass of G proteins. Their natural ligands are fatty acid derivatives of the cannabinoid family, which includes 2-arachidonoylglycerol (2-AG) (253). CB1 is mainly expressed in presynaptic neurons, centrally and peripherally (116, 132, 298), whereas CB2 is mostly present in peripheral tissues, including immune cells and bone cells (218).
There is considerably less evidence available to understand whether and how CB1 is involved in the regulation of bone remodeling, and the different bone phenotypes observed between various Cnr1-deficient mice have been difficult to reconcile. Mice lacking most of the protein-coding sequence of Cnr1 or only the NH2-terminal sequence of this gene encoding CB1 on the C57BL/6J background (males and females, 9–12 wk old) have a low bone mass with reduced bone formation rate and increased bone resorption (303), which is consistent with an inhibitory function of CB1 on NE release by presynaptic neurons (132) and the current model of sympathetic regulation of bone mass (Cnr1 deficiency releases presynaptic inhibition of NE secretion and is thus expected to increase SNS outflow, leading to bone loss, see FIGURE 5). However, the latter mutant mice on the CD1 background exhibited a high bone mass phenotype in 3-mo-old male mice (attributed to a reduction in bone resorption), and it is only in aging mice (12 mo old) that Idris et al. (126) also found a low bone mass in this CD1 mutant line, observed in both genders, in association with an increase in bone adiposity and low bone turnover. In vitro culture of MSCs prepared from these mutant mice had increased capacity to differentiate into adipocytes and reduced osteoblast differentiation potential, suggesting a bone-cell autonomous role of CB1, although this receptor is known to be mainly expressed in the CNS. Interestingly, CB1 expression in bone marrow cells was shown to increase with age, which may have something to do with the fact that these mice display their low bone mass as they age (126).
A consistent observation between these CB1-deficient mutant mice, regardless of their distinct basal bone phenotypes and genetic background, is their lack of increase in bone mass and 2-AG following traumatic brain injury (TBI), a condition known to cause heterotopic ossification and accelerated fracture healing (36, 203, 304). The model presented by these authors is that 2-AG is generated by osteoblasts (known to express the 2-AG synthesizing enzymes diacylglycerol lipase α and β) in response to TBI and stimulates CB1 on bone presynaptic neurons, thereby shutting down NE release, leading to an increase in bone formation (304). It is unclear how TBI leads to changes in 2-AG synthesis in osteoblasts, and how this may lead to the local lesions typical of heterotopic ossification (HO). In addition, deletion of Cnr1 in peripheral sympathetic neurons or 2-AG generating enzymes in osteoblasts specifically will be necessary to further support this model.
Data on the role of CB2 in bone remodeling are more consistent. All components of the endocannabinoid system, including this receptor and the main endocannabinoid biosynthetic and catabolic enzymes, are expressed in bone (260, 304). Osteoblasts, osteocytes, and osteoclasts express CB2, and mice lacking Cnr2 exhibit a late-onset, low bone mass phenotype associated with a high bone turnover (230). In osteoprogenitors, activation of CB2 signaling with CB2-specific agonists has a mitogenic effect that expands the pool of preosteoblasts (229, 230, 277) and promotes ALP activity and mineralization in more mature osteoblasts (230). In osteoclast precursors, CB2 signaling was associated with an inhibitory effect on cell proliferation, and this anti-osteoclastogenic effect might be reinforced by an inhibitory effect of CB2 signaling on RANKL expression in osteoblasts (230). Consistent with the anti-osteoclastogenic and pro-anabolic effect of CB2 signaling, the CB2 agonist HU-308 administered to ovariectomized mice inhibited their bone loss by reducing the number of osteoclasts and by stimulating endocortical bone formation (230). The bone anabolic properties of CB2 signaling and the clinical relevance of these findings are further supported by the significant association of polymorphisms in CNR2 and postmenopausal osteoporosis (144). CB2 thus appears to have a clear peripheral, bone cell-autonomous function in bone remodeling.
4. NPY receptors
NPY is an anxiolytic neurotransmitter and cotransmitter released by sympathetic nerves in conditions of sustained stress, with effects in the central and peripheral nervous systems (115, 350). This neuropeptide signals through five receptors (Y1R, Y2R, Y4R, Y5R, and Y6R), with Y1R and Y2R reported to be involved in bone homeostasis. The Baldock group provided the majority of the data supporting the role of NPY in the regulation of bone remodeling and showed that NPY in normal conditions works both centrally and peripherally to inhibit bone formation. In the CNS, increased level of NPY in the hypothalamus reduces bone mass and osteoblast activity (19, 21), whereas inhibition of central NPY signaling induces bone gain (22, 173). However, as an anxiolytic agent, NPY acts to dampen the behavioral and neuroendocrine effects of severe stress, which can induce bone loss. So, how can the same neuropeptide induce bone loss and dampen the bone loss effect of severe stress? This contradiction led these authors to assess the role of NPY in the setting of chronic stress and to show that in response to this condition, NPY expression is increased in the hypothalamus, and that NPY−/− mice display exaggerated bone loss compared with wild-type mice, thus supporting a protective action of NPY on bone formation in response to stress (20). Mice lacking the Y2 NPY receptor globally or specifically in hypothalamic arcuate neurons were also characterized by an exaggerated bone loss compared with wild-type mice, indicating that the Y2 receptor in the arcuate nuclei is of critical importance for NPY's action to protect against stress-induced bone loss (20). These data support the model by which NPY induction in hypothalamic noradrenergic neurons in response to stress has a protective function to limit the bone loss induced by increased NE release. The fact that germline and hypothalamic-specific deletion of Y2R protects mice from the bone loss induced by ovariectomy also supports a role of NPY signaling in response to gonadal failure-induced bone loss (7). Finally, there is also evidence of NPY and NPY receptor Y1 expression in calvarial-derived osteoblast/osteocytes and of an inhibitory effect of exogenous NPY on osteoblast differentiation, suggestive of a local role of NPY signaling in bone (127, 201, 288).
IV. PARASYMPATHETIC REGULATION OF BONE REMODELING
A. Subtypes, Expression, and Signaling of ACh Receptors in Bone Cells
ACh serves as the main neurotransmitter mediating synaptic neurotransmission at the preganglionic junction by both the SNS and PSNS. The PSNS uses ACh as its primary postganglionic neurotransmitter to modulate involuntary processes like heart rate, gastrointestinal mobility, smooth muscle tone, and exocrine gland secretion. ACh targets cholinergic receptors that are divided into two groups based on their responsiveness to the agonists nicotine and muscarine: the nicotinic (nAChRs) and muscarinic (mAChRs) ACh receptors. At the preganglionic junction, both SNS and PSNS use ACh to activate nAChRs on postganglionic neurons, which function primarily as ion channels. Postganglionic cholinergic neurons stimulate postsynaptic mAChRs expressed in target organs. The expression of nAChRs and mAChRs in bone cells is summarized in TABLE 2.
Table 2.
Main ACh receptor and enzyme mRNAs expressed in bone cells
| Cells | Cells Investigated | Receptor/Enzyme | Reference Nos. |
|---|---|---|---|
| Monocytes | Mouse bone marrow-derived monocytes | γ, δ, ε, α2, α10, and β2 nAChR β1 mAChR | 16 |
| Osteoclasts | Differentiated mouse bone marrow-derived osteoclasts | γ, δ, ε, α2, β2, and β4 nAChR and β1 mAChR | 16 |
| Differentiated mouse bone marrow-derived osteoclasts | α2–7, α9–10, β2–3 nAChR | 195 | |
| Differentiated RAW264.7 cells | α1–5, 7, 9, and 10 nAChR | 306 | |
| Immature osteoblasts | Mouse calvarial osteoblasts | β2, β4, α4, α7 nAChR; α1, β1, γ, δ, M1R, M2R, M4R mAChR AChE, VAChT, ChAT, and CHT1 | 16 |
| Human primary osteoblasts and MG63 osteosarcoma cells | α4 nAChR | 330 | |
| Human osteosarcoma SaOS2 cells | Carnitine acetyltransferase M3 and M5R mAChR α3, α5, α9, α10, β2 nAChR BChE | 80 | |
| Mouse MC3T3 cells | CarChT, VAChT, ChAT, (α1), α2, α3, α5, β2 and β4 nAChR M1R, M4R mAChR AChE and BChE | 80, 265 | |
| Differentiated osteoblasts | Mouse MC3T3 cells | CarChT, VAChT (α1), α6, α7, δ, ε, β2, β3, and β4 nAChR M1R, M2R, M4R mAChR AChE and BChE | 80, 265 |
| Mouse calvarial osteoblasts | AChE, VAChT α1, α6, α7, β1, β4, δ, and ε-nAChRs M1R, M2R, M4R mAChR | 236, 265 |
1. Nicotinic ACh receptors
Nicotinic receptors are composed of multiple units (α1, α2, α3, α4, α5, α6, α7, α9, α10, β1, β2, β3, β4, γ, δ, and ε) and are found in autonomic ganglia at the neuromuscular junction of skeletal muscles and in the CNS. RNA transcripts for neuronal and muscular type nAChR subunits can be detected in osteoclasts and osteoblasts, with α2nAChR being the most abundant subunit in osteoclasts (16, 80). The β2nAChR subunit is highly expressed in both osteoclasts and osteoblasts (16).
Differential expression of nicotinic subunits has been reported along the differentiation process of osteoblasts: undifferentiated osteoblasts express mainly the muscular type α1-, β1-, and γ-subunits, but also the neuronal subunits α4, α7, β2, and β4 (16, 236, 330), whereas the α1, α6, α7, β1, δ, and ε nAChRs are mainly expressed in differentiated osteoblasts (265).
2. Muscarinic ACh receptors
Five mAChRs have been identified (M1-5). Each of them has unique tissue distribution and pharmacological characteristics. Like α and βARs, mAChRs are seven transmembrane spanning proteins coupled to G proteins. The M1R, M3R, and M5R couple to G proteins of the Gq family, while the M2R and M4R preferentially activate Gi/Go type of G proteins (51, 52). Within the CNS, M2R and M4R are major autoreceptors for the control of ACh release. M3R controls exocrine gland secretion and smooth muscle function. M1R is mainly central and mediates cognitive functions. The expression of M1, M2, and M4 mAChR was detected by conventional RT-PCR in immature and differentiated osteoblasts (16, 80, 182, 265, 281).
3. Component of the ACh synthesis machinery
Osteoblasts express the cellular enzymes and transporters required to synthesize ACh, including the vesicular acetylcholine transporter enzyme VAChT, the choline transporter, and the ACh-synthesizing enzyme carnitine acetyltransferase (CarAT) (80, 265). The functional and biological relevance of ACh synthesis by osteoblasts remains unknown.
4. ACh catabolic enzymes
ACh is rapidly degraded into choline and acetate by AChE and butyrylcholinesterases (BChE). The expression of both enzymes has been detected at the RNA level in osteoblasts (80, 265), suggesting that cholinergic signaling may play a role in this lineage and that bone cells may be able to modulate or buffer the effect of PSNS outflow on the bone microenvironment. It must be noted that in the majority of these reports, the expression of genes encoding AChRs and ACh synthesis or degradation enzymes in bone cells was detected at the mRNA level. Evidence for protein and in vivo expression in bone tissues is lacking, and the most solid evidence for the contribution of these genes to bone remodeling comes from loss-of-function and functional studies (see below).
B. Role of ACh Signaling in Bone Remodeling
There are multiple observations to support a possible action of cholinergic signaling on bone remodeling. Those include the expression of cholinergic signaling molecules in bone cells, the presence of immunoreactivity for cholinergic nerve fibers within the bone microenvironment, the likely interplay between sympathetic and parasympathetic nervous systems for their action on bone remodeling, as well as preclinical and clinical observations.
Osteoclasts appear to be the main bone cell lineage targeted by cholinergic signaling, and effect of the latter is overall inhibitory in this cell lineage. nAChR agonists indeed upregulate osteoclast apoptosis ex vivo, and the peripherally acting AChE inhibitor (AChEi) pyridostigmine inhibits bone resorption in vivo (16). Nicotine can also diminish the ratio of proinflammatory to anti-inflammatory bone marrow monocytes (290) and the terminal differentiation and resorbing activity of osteoclasts (195, 306, 348). In line with these findings, α2nAChR−/− mice display a low bone mass phenotype associated with high number of osteoclasts, which are resistant to apoptosis (16, 17). However, bone mass was increased in α7nAChR−/− male mice, in association with reduced osteoclastogenesis and normal osteoblast parameters, whereas no bone phenotype was detected in females (195). The α7-specific agonist PNU282987 caused an increase in osteoclast numbers at low doses and a decrease in osteoclast numbers at high doses. These conflicting data may have to do with receptor desensitization, which is a key property of nAChRs (251), or effect of high doses of agonist on other receptors or ion channels. No bone phenotype was detected in β2nAChR−/− adult mice (195).
There is also experimental evidence of a direct action of cholinergic agonists on osteoblast proliferation and differentiation, but those are limited to in vitro data. Nicotine for instance induced the proliferation of MC3T3-E1 preosteoblasts in association with p53 downregulation and cyclin D1 upregulation, and reduced the differentiation of these cells (266). This stimulatory effect of cholinergic signaling on osteoblast proliferation was also observed by Bajayo et al. (16) with a low concentration of nicotine or carbamylcholine (an AChE-resistant cholinergic agonist); however, these authors did not detect any effect of treatment on osteoblast differentiation and function. UMR 106-01 rat osteoblastic osteosarcoma cells on the other hand responded to nicotine by a decrease in proliferation and higher alkaline phosphatase activity (i.e., increased osteoblast differentiation) (82), whereas MG63 human osteosarcoma cells responded in a biphasic manner, including toxic and antiproliferative effects at high concentration and stimulatory effects at low concentration (261). In conclusion, the direct effects of cholinergic signaling in both osteoclasts and osteoblasts and the detection of VAChT-positive neuronal fibers in bone medullary intertrabecular spaces support a local (skeletal) and anabolic action of ACh on bone mass, but the physiological relevance of this effect remains uncertain.
The local impact of cholinergic signaling on bone is certainly not the sole mechanism whereby ACh affects bone remodeling, as strong genetic and pharmacological evidence support a role of central ACh signaling in the regulation of bone homeostasis. Eimar et al. (75) for instance reported that healthy young female mice treated with the AChEi donepezil had increased bone mass accrual, accompanied by a decrease in osteoclast number and concomitant with a reduced locomotor activity and higher body weight and fat mass than saline-treated mice. Although these results could be explained by the action of increased levels of ACh in bone following AChE inhibition, these mice also had a lower level of urinary NE, suggesting that ACh may favor bone accrual via an inhibitory action on sympathetic outflow. This was further supported by the observation that cotreatment of these mice with donepezil and ISO dampened (although not significantly) bone volume compared with donepezil-treated mice. The low vertebral and appendicular bone mass phenotype of mice lacking M3R also supported a CNS-mediated action of ACh signaling on bone, as M3R is expressed in multiple brain areas, including the brain stem, that contain serotonergic and noradrenergic neurons shown to affect bone mass accrual (163, 301, 338). The bone phenotype of these mice, and the one of mutant mice lacking the M3R in nestin-positive neurons specifically, is essentially a phenocopy of mice treated with adrenergic agonists, i.e., low bone mass associated with high bone resorption and low bone formation (281). This observation and the fact that removing a copy of the β2AR in M3R-deficient mice rescued their low bone mass support the model of ACh signaling favoring bone mass accrual via a central and SNS-dependent mechanism. In conclusion, the PSNS may favor bone mass accrual via a central action and a M3R-dependent suppression of sympathetic signaling.
C. Regulation of PSNS Outflow
Data in transgenic mice suggest that inhibition of central interleukin-1 (IL-1) signaling dampens PSNS outflow to the heart and skeleton, as revealed by reduced skeletal VAChT expression and ACh levels (16). These mice with central inhibition of IL-1R signaling (hIL-1raAst) exhibit a low bone mass phenotype in both femurs and vertebrae, associated with increased bone resorption (17). This phenotype was not reversed by increasing peripheral (bone) ACh levels using inhibition of AChEi with pyridostigmine (16). These results thus suggest the existence of a central IL-1-driven regulation of bone remodeling that uses the PSNS and cholinergic nerves to inhibit osteoclast apoptosis. Whether there is a link between M3R and IL-1R signaling in the CNS remains to be addressed.
V. SENSORY CONTROL OF EMBRYONIC BONE DEVELOPMENT AND ADAPTATION TO MECHANICAL LOADING
A. Impact of Sensory Denervation on Embryonic Bone Development
Current evidence points to the role of sympathetic and parasympathetic neurons in the regulation of bone accrual and remodeling at adult stages. Sensory nerves, however, appear to have a predominant role during the formation of long bones and in their response to mechanical loading.
During formation of long bones, the process of endochondral bone formation proceeds through the formation of a cartilaginous anlagen that will define the position and shape of the future bones. This process is based on the action of intrinsic but also extrinsic inductive signals from multiple cell types to control skeletal patterning and the differentiation of skeletal progenitors (232). The secretion of VEGF by hypertrophic chondrocytes for instance is known to be critical to the invasion of blood vessel in the cartilaginous anlagen and to provide a conduit for progenitor cells and nutrients to reach the sites of primary ossification and generate calcified bone (93, 167). Using mice expressing LacZ under the control of the TrkA promoter to drive reporter expression in sensory nerves, Tomlinson et al. (315) described LacZ-positive neuronal projections innervating the hindlimb via the lumbar plexus by nerves from L1 to L6. These projections were observed extending into the limb and terminating near the femoral perichondrial region as early as embryonic day 14.5, a time of incipient ossification, and became progressively larger thereafter (315). A genetic approach to block TrkA signaling or NGF production by osteochondral progenitors during early development was then used by this group to interrogate the functional significance of this innervation. Their results support the model whereby perichondrial cells of the embryonic femur drive sensory innervation of this bone element via secretion of NGF to promote bone vascular invasion, osteoprogenitor lineage progression, and eventually the formation of the primary ossification centers (315). In an independent study, the deletion of Sema3A in sensory neurons also led to decreased sensory innervation of the skeleton and reduced bone mass, possibly via a similar mechanism to TrkA deletion (92). These genetic studies complement early ones based on the chemical destruction of capsaicin-sensitive sensory neurons at a neonatal stage in rats, which resulted in a mild reduction in trabecular bone mass accrual (114, 231). These results concur to support a predominant role of sensory skeletal innervation in bone development, which can impact peak bone mass acquisition. One study, however, showed that in adult rats too, destruction of capsaicin-sensitive sensory neurons reduced femoral and tibial bone mineral density (BMD), thus supporting the hypothesis that sensory neurons contribute to the maintenance of trabecular bone integrity as well (70). This latter interpretation will need to be further investigated to exclude possible indirect effects of the approach on bone homeostasis.
B. Adaptation to Mechanical Loading
In adults, bone mass and shape can adapt to functional demand by an increase in bone formation at the site of peak bone strain and an increase in bone resorption at sites that are underused. Osteocytes have been implicated in this response of the skeleton to transduce mechanical signals into molecular signals that drive bone turnover (37), but in general, the peripheral somatosensory system is the one responsible for sensing and responding to mechanical stimuli. The large density of sensory nerves within cortical bone prompted another study to investigate the putative role of these nerves in skeletal adaptation to mechanical load. The authors showed that inhibition of TrkA signaling in mice reduced the anabolic effect of forelimb axial compression and Wnt/β-catenin activity in osteocytes in the loaded bone, and that mechanical loading acutely stimulated NGF expression by osteoblasts (314). This increase in NGF expression in bone promoted nerve sprouting, and exogenous NGF administration before axial compression increased the anabolic response of the skeleton. Therefore, these data suggest that TrkA sensory nerves are important for bone development during late embryogenesis but also function in postnatal bone to potentiate the anabolic response to mechanical stimuli and achieve maximal load-induced bone formation [beside their role in osseous pain (197)]. This is in contrast to sympathetic nerves whose contribution to the response to mechanical load appears minimal (65). It will be of great interest to assess the impact of pathological conditions such as aging or diabetes on sensory nerves and their effect on response to mechanical load.
VI. CLINICAL RELEVANCE
The ANS is emerging as a key mediator in the pathophysiology of an increasing number of complex disorders, including anxiety, chronic fatigue, or regional pain syndrome, some of which are associated with bone abnormalities. The preclinical data accumulated over the past 10 yr suggest that some of these associations may reflect a significant influence of the ANS on bone homeostasis. In the following paragraphs, pathological conditions characterized by the coexistence of alterations in autonomic function and bone mass will be presented. Although these associations are only conjectural and lack strong evidence of causality, they bring forth potential clinical relevance to the preclinical observations cited above and areas of future investigations to address the existence of an autonomic control of skeletal homeostasis in humans.
A. Osteoporosis, Sympathetic Dominance During Aging, and Use of β-Blockers
In young women, the main regulator of the cardiovascular system is of parasympathetic nature. In contrast, the sympathetic tone dominates in postmenopausal women (109, 138, 147, 153). This transition from a parasympathetic to a sympathetic control of heart function may contribute to the increased cardiovascular morbidity associated with aging (172), and a number of observations suggest it may also contribute to the steady and estrogen-independent bone loss associated with aging. First, some reports described an inverse relationship between bone mineral content and blood pressure (49, 319). Others reported a correlation between high resting heart rate and increased risk of osteoporotic fractures (140). With the assumption that these cardiovascular parameters reflect alterations of autonomic outflow, an association or connection between the regulation of blood pressure, bone remodeling, and the ANS becomes evident. Importantly, an increased sympathetic activity and reduced parasympathetic activity was measured in postmenopausal women with osteoporosis compared with postmenopausal women without osteoporosis, using heart rate variability parameters and sympathetic skin responses, further supporting the clinical association between autonomic activity and bone loss (317). A more direct association was brought by another study, in which increased sympathetic activity was recorded in postmenopausal compared with premenopausal women, using microneurography at the peroneal nerve (a main nerve in the leg that provides sensation and motor function to the lower leg). In the two groups combined, after age adjustment, sympathetic activity was inversely correlated with trabecular bone volume fraction, thickness, and compressive bone strength, assessed by high-resolution pQCT and microfinite element analysis (83). Lastly, the increase in catecholamine levels in patients with pheochromocytoma (a catecholamine-producing neuroendocrine tumor) was found to be associated with an increase in bone resorption markers, which was normalized by adrenalectomy (324). These clinical observations all support a link between autonomic tone and bone mass regulation, with a more specific relevance to age-related bone loss. However, polymorphisms in ADRB2, known to impact the function of the β2AR, was not associated with BMD or fracture risk in the Utrecht Cardiovascular Pharmacogenetics and the Rotterdam Study cohorts (325). Some studies also reported that β1AR-selective agents were significantly more effective than nonselective β-blockers in reducing the risk of any fracture (287, 318).
The link between autonomic tone and bone mass regulation is further supported by the beneficial effect of β-blockers on BMD and fracture risk (41, 99, 248, 249, 252, 271, 287, 318, 335, 341, 342), although some studies showed conflicting results (175, 210, 250). Because these retrospective studies vary to a great extent in terms of patient number, age of patients, adrenergic receptor drug exposure time, doses, selectivity, concurrent drug treatment, menopausal status, and methodologies to assess bone parameters, prospective investigations will be necessary to further evaluate the effect of β-blockers on bone homeostasis. Veldhuis-Vlug et al. (326) performed an intervention study in a small group of postmenopausal women (32 healthy subjects) and concluded that selective β2AR agonists and nonselective βAR antagonists do not affect bone turnover. There is thus still much to learn from preclinical studies to obtain a better understanding of how endogenous ANS signaling impacts bone homeostasis in normal and pathological conditions, and to best design interventions and outcome measurements in related clinical studies.
B. Neuropathies
There is circumstantial evidence that sensory nerves are involved in the development of the human skeleton. For example, the hereditary sensory and autonomic neuropathies (HSANs) represent a group of neurodevelopmental disorders that give rise to sensory deficits, progressive autonomic dysfunction (14), and osteopenia (133). Mutations in TRKA cause congenital insensitivity to pain with anhidrosis (CIPA) (131), a syndrome of the HSAN family associated with short stature and delayed fracture healing (316). A reduced bone mass accrual was also reported in children with perinatal brachial plexus palsy (PBPP), which present with a flaccid paralysis of the arm at birth (125). These observations are consistent with the mouse data that support the requirement of functional sensory nerves for normal ossification and formation of the primary and secondary ossification centers during development of the skeleton (315).
Loss of peripheral nerve function can be the result of genetic mutations, but it also declines as a function of aging in the general population, and loss of peripheral nerve function is also a well-recognized complication of type 1 and type 2 diabetes (42, 100), all of which are associated with low BMD and increased risk of fracture (88, 89, 152, 161, 222, 254, 274). Whether and to what extent peripheral neuropathies contribute to the deleterious skeletal effects associated with these conditions is, however, unclear. It is a difficult question to address because of the multiple mechanisms likely involved in the bone fragility associated with diabetes (220), which include a negative impact on the nervous system and bone vasculature, and poor peripheral nerve function that may also indirectly increase risk of fracture following loss of balance and muscle mass, and thus greater risk of fall.
C. Dysautonomias
The term dysautonomia regroups a number of conditions affecting the autonomic nervous system. One of them is familial dysautonomia (FD), an autosomal recessive autonomic neuropathy most common in young Ashkenazi jews, caused by point mutation in the IKBKAP gene (192). Patients with FD present with poor development and degeneration of the autonomic nervous system, associated with impaired motor and sensory function. Skeletal manifestations contribute to the reduced quality of life of these patients and include spinal deformities (111) and high incidence of multiple fractures (171, 190), which is exacerbated by their insensitivity to bone pain and recurrent falls. The cause of these multiple fractures is likely a consequence of reduced bone density, which can be secondary to multiple factors, including reduced physical activity, low BMI, reduced caloric intake, consumption of antacids, delayed puberty, and perhaps uncontrolled blood pressure and bone perfusion. Therefore, even though the current model of sympathetic control of bone mass would predict a resistance to bone loss upon destruction of autonomic nerves, the impact of these multiple conditions is likely to be predominant and to cause bone loss despite a putative low sympathetic outflow to bone. Interestingly, these patients have reduced CGRP plasma levels (191) and C-fibers (237). Because CGRP inhibits bone resorption in vitro (349), increases mineral formation (23), and is involved in thermal, mechanical, and pain perception, a decrease in local CGRP levels in FD patients might explain their lack of bone pain and adversely affect bone turnover.
DBH deficiency is characterized by virtual absence of catecholamines and accumulation of dopamine in plasma, cerebrospinal fluid, and urine (158, 257). The lack of catecholamines is responsible for the severe hypotension and the common syncopes observed in these patients, which are predicted to increase fracture incidence. Pure autonomic failure (PAF), or Bradbury-Eggleston syndrome, is another degenerative disorder of the autonomic nervous system presenting in middle to late life (150). It is also characterized by very low levels of catecholamines and orthostatic hypotension. Despite their repetitive falls and reduced locomotor activity, which derive from their hypotension (98, 311), there is no report of increased fracture incidence in adult patients with DBH deficiency or PAF, thus suggesting, in line with the current model of autonomic control of bone mass and the high bone mass of the Dbh-deficient mice, that life-long deficiency in catecholamines in these patients protects them from bone loss.
Lastly, pathological activation of sympathetic nerves, as in the case of patients with postural tachycardia syndrome (186, 256), is expected according to this model to lead to bone loss. Unfortunately, the rarity of this condition makes it difficult to assess an association with BMD or fracture incidence, and there is thus here again no study to confirm this hypothesis.
D. Fracture Repair
Bone healing following fracture is a coordinated process that takes several weeks to complete. It can be divided into several phases, each of which is necessary for successful regeneration of the fractured bone and its functional recovery. Bone fracture first initiates the formation of a hematoma and an inflammatory response that provides an initial template for callus formation and stimuli for the recruitment of both hematopoietic and mesenchymal cells to the site of repair. The cartilaginous mold is then vascularized, resorbed, and replaced by a calcified bone matrix through the recruitment, proliferation, and differentiation of mesenchymal stem cells to regenerate bone and fully restore a normal bony structure.
Bone fracture negatively impacts preexisting nerve fibers in both cortical and bone marrow compartments, but reinnervation of the fractured bone was reported in mice (124, 177, 178, 183). The functional requirement of this reinnervation for successful bone healing or speed of healing remains unclear. The observation that peripheral nerve fibers grow into the callus before vascularization suggests that a restored nerve supply could be required for normal fracture healing (177). This would be in line with the data suggesting that sensory nerves provide osteogenic cues during formation of the primary ossification center during development, or play a permissive role for the invading vasculature to progress during this process or during bone regeneration (315). The observation that inflammatory cells in the initial phase of the repair process release immune mediators and nerve growth factors that can stimulate sensory nerve fibers further supports this hypothesis (124, 174, 272).
Studies based on limb denervation of ipsilateral peripheral nerve fibers have been performed to address whether callus reinnervation is required for successful bone healing. Although nerve resection did not provide a complete denervation of the healing fracture, it led to weaker tibial mechanical strength and bigger calluses in rats subjected to femoral and sciatic nerves resection (194, 226). Prefracture sympathectomy based on the use of 6-hydroxydopamine in adult mice led to low callus bone volume and weaker callus mechanical properties, and this was associated with an increased proportion of mesenchymal tissue and less cartilaginous tissues, suggesting that the absence of sympathetic nerves delays differentiation of mesenchymal callus tissue toward a cartilaginous matrix (223). These findings are not in agreement with what is known about the impact of βAR modulation on bone remodeling in nonpathological/fracture conditions. These former studies indeed supported a model whereby βAR blockade in the context of normal bone remodeling promotes bone formation and inhibits bone resorption, whereas in the context of bone repair, the lack of sympathetic nerves seems to inhibit cartilage and bone formation (194, 223, 226). One study in rats also reported no detectable effect of the β-blocker propranolol on femoral bone healing (286). It should be noted that the pharmacological drugs involved in denervation studies may have side effects on bone cells or on the bone microenvironment, which were not accounted for in the interpretation of the results. Mechanical loading or absence thereof following denervation may also interfere with the interpretation of results, even though some authors have attempted to compensate for this by immobilizing the leg of sham-operated mice with casts.
Interestingly, one study reported profuse ectopic sprouting of both sensory and sympathetic nerve fibers in the bone marrow space and periosteum in a mouse model of bone non-union. This ectopic sprouting remained for months following fracture and was associated with long-lasting pain (55). Although these observations suggest that the persistence of sensory nerves in the load-bearing callus of the nonhealed bone may contribute to increased pain sensitivity, the role of sympathetic nerves in this condition remains unknown.
In conclusion, the literature available to analyze the putative role of sympathetic nerves in bone repair is relatively scarce, and there is even fewer data available to evaluate the potential contribution of cholinergic nerves in this process. One study reported that cannabidiol, the major nonpsychoactive cannabis constituent, improves the biomechanical properties of femoral calluses in rats, possibly via an effect on collagen cross-linking enzymes (164).
E. Vestibular Aging
The vestibular system is a small bilateral sensory structure located in the inner ear, known as the organ of balance and spatial orientation. It senses head orientation and motion, as well as body motion in the three dimensions of our environment. It is also involved in the postural control of blood pressure, via anatomical nerve projections from vestibular nuclei to brain stem autonomic centers (343). Vestibular dysfunction is the cause of vertigo and significantly increases the likelihood of falls, which is further exacerbated in older individuals with weaker muscle mass. Vestibular function and bone mineral density both decline with age (3, 24, 239, 333), and as mentioned above, these changes are associated with an increase in sympathetic activity. Although the association between aging, vestibular dysfunction, increased sympathetic activity, and bone loss is conjectural, a model of induced vestibular dysfunction in young rats and mice led to bone loss, which was limited to hindlimbs and inhibited by the β-blocker propranolol or genetic ablation of the Adrß2 (67, 176, 327, 328). These observations led to the hypothesis that vestibular aging contributes to age-related bone loss, via an increase in sympathetic signaling in bone. The lower BMD recently measured in weight-bearing hip and lower extremity bones of individuals with reduced vestibular physiological function further supports this hypothesis (31, 208). Whether the bone loss detected in these studies is related to skeletal sympathetic overactivity associated with decreasing expression/activity of NET during aging, or reduced physical activity and bone unloading remains uncertain (110). Interestingly, a parallel can be made with the bone loss detected in astronauts, as saccular inputs are temporarily altered in microgravity. Experimental manipulations of vestibular inputs on hearth, such as in long-term head-down bedrest, are also associated with increases in sympathetic outflow and a reduction in bone mass (10, 285, 308). Like in aging individuals, drawing conclusions about the effect of vestibular dysfunction on bone from space medicine data is difficult due to the numerous potential confounders inherent to space flights.
F. Depression
Depression is a debilitating disease associated with mood, cognitive, and physical symptoms (245). This common condition, more prevalent in women than men (157), is associated with a high prevalence of osteoporosis (15, 59, 96, 128, 141, 209, 275, 276, 344, 345). The underlying mechanisms for this association can include alterations in the HPA axis and in glucocorticoid and mineralocorticoid receptor signaling (122) as well as elevated levels of proinflammatory cytokines such as IL‐1, IL‐6, tumor necrosis factor (TNF)‐α, C-reactive protein (CRP), and monocyte chemoattractant protein-1 (MCP‐1) (321, 347). Depression is also associated with high alcohol and nicotine intake (60, 71), unhealthy nutrition, and decreased physical activity (160), which all have a negative impact on bone. However, patients with depressive disorders are known to present with autonomic dysfunction as well (44, 155, 340). Although the contribution of altered autonomic function to the bone loss induced by depression in humans is speculative, a study in mice supports such a mechanism. In a model of depression in rodents, a low trabecular bone mass phenotype in long bones and spine was observed (346). This phenotype was associated with high bone NE content and was attenuated by βAR blockade using propranolol. These data thus suggest that severe and short-term experimental depression in mice induces bone loss via sympathetic activation. Of note is that antidepressant therapy with imipramine, a tricyclic antidepressant (TCAs) drug, prevented the behavioral responses to chronic mild stress (CMS) and attenuated the CMS-induced bone loss in this model. In patients, however, TCAs or selective serotonin reuptake inhibitors (SSRIs) have a negative impact on bone health and increase fracture risk (69, 107, 255, 280, 336). Related to these later clinical observations, a recent study in mice by Ducy and colleagues (233) provided preclinical evidence showing that long-term treatment with the SSRI fluoxetine triggers bone loss via a centrally mediated increase in sympathetic outflow.
G. Attention-Deficit/Hyperactivity Disorder
Children with attention-deficit/hyperactivity disorder (ADHD) have a higher risk of experiencing bone fracture than children without ADHD (58), and ADHD medication offers protection against fracture (64, 134, 238), possibly by lowering hyperactivity and risk taking behavior. The effect of these drugs on BMD and growth is controversial (168, 242, 243). Synthesized amphetamine analogs, or stimulant medications such as methylphenidate hydrochloride, are the first-line pharmacotherapies for pediatric ADHD (309). Amphetamines are classified as indirect sympathomimetic agents that inhibit presynaptic catecholamine reuptake and activate peripheral β-adrenergic receptors. Although it reduces fracture risk in young patients diagnosed with ADHD, the use of stimulant medication in the adult population is associated with lower BMD (149, 159, 215). From these observations and the fact that stimulants preferentially affect trabecular bone, like βAR agonists, it can be speculated that amphetamine use in adults augments the level NE in bone in a way that is not efficiently buffered by any of the homeostatic mechanisms reducing NE levels or activity, whereas in children, drug effect on behavior is dominant over effect on the growing skeleton to protect from fracture.
H. Breast Cancer Metastasis
Improved detection programs and better drugs to eradicate breast tumors have increased survival in women with breast cancer. However, patients subjected to psychosocial stress do not seem to benefit from these new treatments and present with shorter survival (45, 57, 95, 113, 259, 289, 320). More than 70% of women dying from breast cancer have bone metastases. Along with mutations in cancer cells (“the seeds”), many cell types of the host (“the soil”) as well as bone-derived cytokines are likely involved in the process of bone metastasis (235). Among these cytokines, RANKL has been implicated as a critical osteotropic factor, released by bone cells, for metastatic cancer cells from breast and prostate carcinomas (68, 139, 300). Because chronic stress and severe depression trigger activation of SNS outflow, and βAR stimulation upregulates RANKL expression in bone, it was hypothesized that psychosocial stress in patients with breast cancer, through activation of the SNS and RANKL expression in bone cells, promotes bone metastasis. This hypothesis was tested in mouse models of breast cancer bone metastasis. These studies showed that βAR stimulation by ISO, or endogenous SNS activation by chronic restraint stress, promotes the establishment of human MDA-MD231 breast cancer cells in bone, which could be inhibited by the β-blocker propranolol (47). Evidence also confirmed that RANKL was a crucial factor promoting breast cancer cell migration toward the osteoblastic niche in response to the βAR agonist ISO, independently of bone tumor load, turnover, or direct effect on cancer cells (47). An increase in VEGF expression and bone blood vessel density may also contribute to the mechanism whereby disseminated cancer cells engraft in the skeleton in response to β2AR stimulation in osteoblasts (217). These observations in mice suggested that 1) the colonization of the skeleton by metastatic breast cancer cells in response to chronic SNS activation, RANKL, and VEGF may explain, at least in part, the reduced survival of clinically depressed breast cancer patients and 2) β-blockers or RANKL blockade may be beneficial when used as adjuvant therapy to reduce the skeletal establishment of metastatic breast cancer cells and to favor their exposure to cytotoxic drugs. Studies reporting the beneficial effects of β-blockers, when taken early on at the time of cancer diagnosis, on survival in this population of patients, support this hypothesis (25, 204, 244).
Stress-related behavioral factors are also known to accelerate the progression of hematopoietic cancers such as acute lymphoblastic leukemia (ALL). As in the breast cancer studies cited above, chronic restraint stress significantly enhanced ALL tumor burden and dissemination, and this effect was blocked by the β-blocker propranolol (169). In this study too, the effect was shown to be mediated via cells of the host stroma rather than by a direct effect on cancer cells.
I. Complex Regional Pain Syndrome
Complex regional pain syndrome (CRPS) is a chronic pain syndrome that most frequently develops after limb injuries (28). It was initially called “reflex sympathetic dystrophy” based on the alleged therapeutic efficacy of surgical and pharmacological sympathectomy, which suggested local hyperactivity of the SNS as the main etiology of this condition. However, the ineffectiveness of sympathetic blockade and measurement of reduced sympathetic activity in patients with CRPS led to a change in the denomination of this condition and to the term of “Complex Regional Pain Syndrome” type I or II, relating to the absence or presence of neurological lesions, respectively (53, 291, 294). It was proposed that a local release of proinflammatory neuropeptides (CGRP, SP) and cytokines (TNF-α, IL-2, IL-1β, IL-6) contributes to the initial phase of the condition (associated with sweating, increased temperature, edema, eritrosis, pain, bone loss), followed by impaired capillary permeability that leads to edema, hypoxia, reduced temperature, and local acidosis. It was also hypothesized that an adrenergic supersensitivity and sympathetically mediated pain could be mediated by sympathetic sprouting and upregulation of αARs (32, 72, 108, 130, 264).
Models of CRPS have been generated in rats and mice using distal fracture of the tibia and cast immobilization. This approach leads to symptoms similar to what is observed in humans, including bone loss. Experimental strategies blocking the inflammatory mediators released in CRPS partially inhibited the nociceptive and vascular changes that develop after fracture, but had no effect on trabecular bone loss in the injured or contralateral limbs (103, 179, 262). Only bisphosphonate therapy was shown to inhibit pain, osteoclast activation, trabecular bone loss, and cutaneous inflammation in the rat fracture model of CRPS (331) and in clinical trials (54, 196, 322, 323). Therefore, CRPS might involve components of SNS signaling for pain and inflammation, but the SNS contribution to the associated local bone loss remains unclear.
J. Alzheimer's Disease and Use of Acetylcholine Esterase Inhibitors
Acetylcholine esterase inhibitors (AChEIs) belong to a group of drugs that increase acetylcholine levels by inhibiting the action of acetylcholine esterase in the CNS, and/or butyrylcholinesterase in peripheral tissues. AChEIs acting in the CNS are used for the treatment of Alzheimer's disease (AD), whereas peripheral acting AChEIs are used to treat myasthenia gravis and glaucoma. Progressive CNS atrophy and memory decline are well-known and visible traits of patients with AD, but these patients also present with low BMD and increased fracture risk (18, 205, 268, 329, 334). Importantly, this bone loss occurs before plaque development, reduced locomotor activity, and skeletal unloading typical of late-stage AD. AD is also associated with low parasympathetic and high sympathetic outflow (4, 313). Such evidence of cholinergic dysfunction at early stages of AD, progressing to loss of cholinergic innervation in later stages, led to the use of AChEIs to manage this condition (270). Interestingly, the use of AChEIs is associated with a reduced risk of fractures in patients with AD (305, 334) and improved bone healing in aged patients (76). These clinical observations suggest that reduced parasympathetic activity may contribute to the bone loss observed in patients with AD in addition to, and possibly before, disuse and vitamin D deficiency that occur at late stages of the disease. They also suggest that AChEI may counteract this bone catabolic effect, which is supported by the preclinical data cited above.
K. Multiple Sclerosis
Multiple sclerosis (MS) is a progressive autoimmune demyelinating disease of the CNS, in which chronic inflammation causes axonal injury and loss, resulting in deficits of motor and cognitive functions (295). It affects young adults, and bone loss is a common clinical presentation associated with this condition (112, 199). This bone loss is thought to be secondary to reduced physical mobility, thus reduced mechanical loading, and to the cumulative use of glucocorticoids known for their catabolic effect on bone. However, the correlation between BMD and MS disease severity (224, 293) and cognitive impairment (27) suggests a relationship between BMD and the MS neurodegenerative process. Alterations in both the sympathetic and parasympathetic responses have been reported in patients with MS, correlating with disease activity and progression (1, 85, 86, 102, 263). One can thus speculate that ANS dysfunction associated with the MS neurodegenerative process might contribute to some extent to the bone loss associated with this condition. This needs to be further explored.
VII. CONCLUSIONS
Despite the major progress made in this area of bone physiology over the last 10–15 yr, the current model of autonomic influence on bone homeostasis is most likely still quite inaccurate. A first reason for this opinion is that the current model is based on the interpretation of results that are often based on the use of mice with global and embryonic inactivation of neuropeptides and related receptors or enzymes. It is certainly possible that such genetic event creates developmental consequences on neuronal circuitry, activity, and/or homeostatic responses that could mislead interpretation of phenotypes observed in adults. This needs to be recognized and addressed experimentally. A second reason is that most studies have drawn conclusions from approaches based on alteration of postsynaptic neuropeptide signaling. The existence of multiple presynaptic mechanisms regulating autonomic outflow, their changes with aging or pathological conditions, and their impact on bone remodeling has not been very much considered yet. When this was done, observations did not support the current model. Moving forward, our current understanding of these pathways should thus be constantly questioned and revisited, and will gain in being based on the analysis of adult mice with conditional and inducible models, coupled to pharmacological approaches in wild-type mice. This will provide a more solid and biologically relevant description of the physiology involved in adults. In addition, altering presynaptic and postsynaptic neuronal genes and mechanisms is likely to provide a more complete and accurate picture of how the ANS impacts bone remodeling. We also must recognize the complex nature of this system and the current reductionist view we have of it, and integrate additional cellular components, such as immune cells, which are, like osteoblasts, responsive to autonomic signals (30, 185).
The impact of autonomic nerves on bone homeostasis and the related underlying mechanisms came into light from studies in mutant mouse models, but the pathophysiological and clinical relevance of these findings still remains rather speculative. Still, there are a number of intriguing clinical evidences supporting the possible contribution of the ANS to a number of bone loss conditions, which remain to be explored through intervention studies. Preliminary observations also provide many opportunities to link the current knowledge in neuroskeletal biology to additional clinical conditions such as osteoarthritis (74, 77, 94, 184, 219) and dental pathologies (101, 135, 267) for instance. Current models of how the ANS impacts bone homeostasis need to be refined, and putative species differences need to be investigated for transitioning this new knowledge from mouse data to the development of clinical strategies and treatment of specific bone health conditions. This being said, discoveries in lower organisms brought, and will continue to bring, an exceptionally rich set of data that allow scientists to generate new hypotheses and to better understand the physiology and modes of interactions between genes, cells, and tissues, including the unexpected interaction between the ANS and the skeleton.
GRANTS
The work from the author related to this review is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK082471, National Institute on Aging Grant R01 AG055394, National Cancer Institute Grant R01 CA168717-03, and National Aeronautics and Space Administration Grant NNX12AL35G.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
ACKNOWLEDGMENTS
I thank Gerard Portela for his editorial assistance.
Address for reprint requests and other correspondence: F. Elefteriou, One Baylor Plaza, ABBR-R716, Mail Stop BCM617, Houston, TX 77030 (e-mail: Florent.elefteriou@bcm.edu).
REFERENCES
- 1.Acevedo AR, Nava C, Arriada N, Violante A, Corona T. Cardiovascular dysfunction in multiple sclerosis. Acta Neurol Scand 101: 85–88, 2000. doi: 10.1034/j.1600-0404.2000.101002085.x. [DOI] [PubMed] [Google Scholar]
- 2.Agostoni E, Chinnock JE, De Daly MB, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol 135: 182–205, 1957. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch Intern Med 169: 938–944, 2009. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- 4.Aharon-Peretz J, Harel T, Revach M, Ben-Haim SA. Increased sympathetic and decreased parasympathetic cardiac innervation in patients with Alzheimer’s disease. Arch Neurol 49: 919–922, 1992. doi: 10.1001/archneur.1992.00530330041013. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed M, Bjurholm A, Kreicbergs A, Schultzberg M. Neuropeptide Y, tyrosine hydroxylase and vasoactive intestinal polypeptide-immunoreactive nerve fibers in the vertebral bodies, discs, dura mater, and spinal ligaments of the rat lumbar spine. Spine 18: 268–273, 1993. doi: 10.1097/00007632-199302000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Aitken SJ, Landao-Bassonga E, Ralston SH, Idris AI. Beta2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch Biochem Biophys 482: 96–103, 2009. doi: 10.1016/j.abb.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Allison SJ, Baldock P, Sainsbury A, Enriquez R, Lee NJ, Lin EJ, Klugmann M, During M, Eisman JA, Li M, Pan LC, Herzog H, Gardiner EM. Conditional deletion of hypothalamic Y2 receptors reverts gonadectomy-induced bone loss in adult mice. J Biol Chem 281: 23436–23444, 2006. doi: 10.1074/jbc.M604839200. [DOI] [PubMed] [Google Scholar]
- 8.Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, Kobilka BK, Hein L. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol Pharmacol 56: 154–161, 1999. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- 9.Arai M, Nagasawa T, Koshihara Y, Yamamoto S, Togari A. Effects of beta-adrenergic agonists on bone-resorbing activity in human osteoclast-like cells. Biochim Biophys Acta 1640: 137–142, 2003. doi: 10.1016/S0167-4889(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 10.Armbrecht G, Belavý DL, Backström M, Beller G, Alexandre C, Rizzoli R, Felsenberg D. Trabecular and cortical bone density and architecture in women after 60 days of bed rest using high-resolution pQCT: WISE 2005. J Bone Miner Res 26: 2399–2410, 2011. doi: 10.1002/jbmr.482. [DOI] [PubMed] [Google Scholar]
- 11.Artico M, Bosco S, Cavallotti C, Agostinelli E, Giuliani-Piccari G, Sciorio S, Cocco L, Vitale M. Noradrenergic and cholinergic innervation of the bone marrow. Int J Mol Med 10: 77–80, 2002. [PubMed] [Google Scholar]
- 12.Asada N, Katayama Y, Sato M, Minagawa K, Wakahashi K, Kawano H, Kawano Y, Sada A, Ikeda K, Matsui T, Tanimoto M. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell 12: 737–747, 2013. doi: 10.1016/j.stem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Asmus SE, Parsons S, Landis SC. Developmental changes in the transmitter properties of sympathetic neurons that innervate the periosteum. J Neurosci 20: 1495–1504, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axelrod FB, Gold-von Simson G. Hereditary sensory and autonomic neuropathies: types II, III, and IV. Orphanet J Rare Dis 2: 39, 2007. doi: 10.1186/1750-1172-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bab IA, Yirmiya R. Depression and bone mass. Ann N Y Acad Sci 1192: 170–175, 2010. doi: 10.1111/j.1749-6632.2009.05218.x. [DOI] [PubMed] [Google Scholar]
- 16.Bajayo A, Bar A, Denes A, Bachar M, Kram V, Attar-Namdar M, Zallone A, Kovács KJ, Yirmiya R, Bab I. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc Natl Acad Sci USA 109: 15455–15460, 2012. doi: 10.1073/pnas.1206061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajayo A, Goshen I, Feldman S, Csernus V, Iverfeldt K, Shohami E, Yirmiya R, Bab I. Central IL-1 receptor signaling regulates bone growth and mass. Proc Natl Acad Sci USA 102: 12956–12961, 2005. doi: 10.1073/pnas.0502562102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker NL, Cook MN, Arrighi HM, Bullock R. Hip fracture risk and subsequent mortality among Alzheimer’s disease patients in the United Kingdom, 1988-2007. Age Ageing 40: 49–54, 2011. doi: 10.1093/ageing/afq146. [DOI] [PubMed] [Google Scholar]
- 19.Baldock PA, Lee NJ, Driessler F, Lin S, Allison S, Stehrer B, Lin EJ, Zhang L, Enriquez RF, Wong IP, McDonald MM, During M, Pierroz DD, Slack K, Shi YC, Yulyaningsih E, Aljanova A, Little DG, Ferrari SL, Sainsbury A, Eisman JA, Herzog H. Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS One 4: e8415, 2009. doi: 10.1371/journal.pone.0008415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldock PA, Lin S, Zhang L, Karl T, Shi Y, Driessler F, Zengin A, Hörmer B, Lee NJ, Wong IP, Lin EJ, Enriquez RF, Stehrer B, During MJ, Yulyaningsih E, Zolotukhin S, Ruohonen ST, Savontaus E, Sainsbury A, Herzog H. Neuropeptide Y attenuates stress-induced bone loss through suppression of noradrenaline circuits. J Bone Miner Res 29: 2238–2249, 2014. doi: 10.1002/jbmr.2205. [DOI] [PubMed] [Google Scholar]
- 21.Baldock PA, Sainsbury A, Allison S, Lin EJ, Couzens M, Boey D, Enriquez R, During M, Herzog H, Gardiner EM. Hypothalamic control of bone formation: distinct actions of leptin and y2 receptor pathways. J Bone Miner Res 20: 1851–1857, 2005. doi: 10.1359/JBMR.050523. [DOI] [PubMed] [Google Scholar]
- 22.Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest 109: 915–921, 2002. doi: 10.1172/JCI0214588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballica R, Valentijn K, Khachatryan A, Guerder S, Kapadia S, Gundberg C, Gilligan J, Flavell RA, Vignery A. Targeted expression of calcitonin gene-related peptide to osteoblasts increases bone density in mice. J Bone Miner Res 14: 1067–1074, 1999. doi: 10.1359/jbmr.1999.14.7.1067. [DOI] [PubMed] [Google Scholar]
- 24.Baloh RW, Enrietto J, Jacobson KM, Lin A. Age-related changes in vestibular function: a longitudinal study. Ann N Y Acad Sci 942: 210–219, 2001. doi: 10.1111/j.1749-6632.2001.tb03747.x. [DOI] [PubMed] [Google Scholar]
- 25.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol 29: 2635–2644, 2011. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 26.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 139: 267–284, 2009. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batista S, Teter B, Sequeira K, Josyula S, Hoogs M, Ramanathan M, Benedict RH, Weinstock-Guttman B. Cognitive impairment is associated with reduced bone mass in multiple sclerosis. Mult Scler 18: 1459–1465, 2012. doi: 10.1177/1352458512440206. [DOI] [PubMed] [Google Scholar]
- 28.Beerthuizen A, Stronks DL, Van’t Spijker A, Yaksh A, Hanraets BM, Klein J, Huygen FJ. Demographic and medical parameters in the development of complex regional pain syndrome type 1 (CRPS1): prospective study on 596 patients with a fracture. Pain 153: 1187–1192, 2012. doi: 10.1016/j.pain.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int 94: 25–34, 2014. doi: 10.1007/s00223-013-9774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellinger DL, Lorton D. Autonomic regulation of cellular immune function. Auton Neurosci 182: 15–41, 2014. doi: 10.1016/j.autneu.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Bigelow RT, Semenov YR, Anson E, du Lac S, Ferrucci L, Agrawal Y. Impaired Vestibular Function and Low Bone Mineral Density: Data from the Baltimore Longitudinal Study of Aging. J Assoc Res Otolaryngol 17: 433–440, 2016. doi: 10.1007/s10162-016-0577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birder LA, Perl ER. Expression of alpha2-adrenergic receptors in rat primary afferent neurones after peripheral nerve injury or inflammation. J Physiol 515: 533–542, 1999. doi: 10.1111/j.1469-7793.1999.533ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjurholm A. Neuroendocrine peptides in bone. Int Orthop 15: 325–329, 1991. doi: 10.1007/BF00186871. [DOI] [PubMed] [Google Scholar]
- 34.Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M. Substance P- and CGRP-immunoreactive nerves in bone. Peptides 9: 165–171, 1988. doi: 10.1016/0196-9781(88)90023-X. [DOI] [PubMed] [Google Scholar]
- 35.Black DM, Rosen CJ. Clinical Practice. Postmenopausal Osteoporosis. N Engl J Med 374: 254–262, 2016. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 36.Boes M, Kain M, Kakar S, Nicholls F, Cullinane D, Gerstenfeld L, Einhorn TA, Tornetta P III. Osteogenic effects of traumatic brain injury on experimental fracture-healing. J Bone Joint Surg Am 88: 738–743, 2006. doi: 10.2106/JBJS.D.02648. [DOI] [PubMed] [Google Scholar]
- 37.Bonewald LF. The amazing osteocyte. J Bone Miner Res 26: 229–238, 2011. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnet N, Beaupied H, Vico L, Dolleans E, Laroche N, Courteix D, Benhamou CL. Combined effects of exercise and propranolol on bone tissue in ovariectomized rats. J Bone Miner Res 22: 578–588, 2007. doi: 10.1359/jbmr.070117. [DOI] [PubMed] [Google Scholar]
- 39.Bonnet N, Benhamou CL, Brunet-Imbault B, Arlettaz A, Horcajada MN, Richard O, Vico L, Collomp K, Courteix D. Severe bone alterations under beta2 agonist treatments: bone mass, microarchitecture and strength analyses in female rats. Bone 37: 622–633, 2005. doi: 10.1016/j.bone.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Bonnet N, Benhamou CL, Malaval L, Goncalves C, Vico L, Eder V, Pichon C, Courteix D. Low dose beta-blocker prevents ovariectomy-induced bone loss in rats without affecting heart functions. J Cell Physiol 217: 819–827, 2008. doi: 10.1002/jcp.21564. [DOI] [PubMed] [Google Scholar]
- 41.Bonnet N, Gadois C, McCloskey E, Lemineur G, Lespessailles E, Courteix D, Benhamou CL. Protective effect of beta blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone 40: 1209–1216, 2007. doi: 10.1016/j.bone.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care 27: 1458–1486, 2004. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 43.Bouxsein ML, Devlin MJ, Glatt V, Dhillon H, Pierroz DD, Ferrari SL. Mice lacking beta-adrenergic receptors have increased bone mass but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology 150: 144–152, 2009. doi: 10.1210/en.2008-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunoni AR, Kemp AH, Dantas EM, Goulart AC, Nunes MA, Boggio PS, Mill JG, Lotufo PA, Fregni F, Benseñor IM. Heart rate variability is a trait marker of major depressive disorder: evidence from the sertraline vs. electric current therapy to treat depression clinical study. Int J Neuropsychopharmacol 16: 1937–1949, 2013. doi: 10.1017/S1461145713000497. [DOI] [PubMed] [Google Scholar]
- 45.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ 330: 702, 2005. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calvo W. The innervation of the bone marrow in laboratory animals. Am J Anat 123: 315–328, 1968. doi: 10.1002/aja.1001230206. [DOI] [PubMed] [Google Scholar]
- 47.Campbell JP, Karolak MR, Ma Y, Perrien DS, Masood-Campbell SK, Penner NL, Munoz SA, Zijlstra A, Yang X, Sterling JA, Elefteriou F. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol 10: e1001363, 2012. doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cappariello A, Ponzetti M, Rucci N. The “soft” side of the bone: unveiling its endocrine functions. Horm Mol Biol Clin Investig 28: 5–20, 2016. doi: 10.1515/hmbci-2016-0009. [DOI] [PubMed] [Google Scholar]
- 49.Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA; Study of Osteoporotic Fractures Research Group . High blood pressure and bone-mineral loss in elderly white women: a prospective study. Lancet 354: 971–975, 1999. doi: 10.1016/S0140-6736(99)01437-3. [DOI] [PubMed] [Google Scholar]
- 50.Castañeda-Corral G, Jimenez-Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, Ghilardi JR, Mantyh PW. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience 178: 196–207, 2011. doi: 10.1016/j.neuroscience.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caulfield MP. Muscarinic receptors–characterization, coupling and function. Pharmacol Ther 58: 319–379, 1993. doi: 10.1016/0163-7258(93)90027-B. [DOI] [PubMed] [Google Scholar]
- 52.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50: 279–290, 1998. [PubMed] [Google Scholar]
- 53.Cepeda MS, Carr DB, Lau J. Local anesthetic sympathetic blockade for complex regional pain syndrome. Cochrane Database Syst Rev (4): CD004598, 2005. doi: 10.1002/14651858.CD004598.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Chapurlat RD, Duboeuf FP, Liens D, Meunier PJ. Dual energy X-ray absorptiometry in patients with lower limb reflex sympathetic dystrophy syndrome. J Rheumatol 23: 1557–1559, 1996. [PubMed] [Google Scholar]
- 55.Chartier SR, Thompson ML, Longo G, Fealk MN, Majuta LA, Mantyh PW. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and maintenance of chronic skeletal pain. Pain 155: 2323–2336, 2014. doi: 10.1016/j.pain.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chase LR, Aurbach GD. The effect of parathyroid hormone on the concentration of adenosine 3′,5′-monophosphate in skeletal tissue in vitro. J Biol Chem 245: 1520–1526, 1970. [PubMed] [Google Scholar]
- 57.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol 5: 466–475, 2008. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 58.Chou IC, Lin CC, Sung FC, Kao CH. Attention-deficit-hyperactivity disorder increases risk of bone fracture: a population-based cohort study. Dev Med Child Neurol 56: 1111–1116, 2014. doi: 10.1111/dmcn.12501. [DOI] [PubMed] [Google Scholar]
- 59.Cizza G, Ravn P, Chrousos GP, Gold PW. Depression: a major, unrecognized risk factor for osteoporosis? Trends Endocrinol Metab 12: 198–203, 2001. doi: 10.1016/S1043-2760(01)00407-6. [DOI] [PubMed] [Google Scholar]
- 60.Cohn AM, Cobb C, Hagman BT, Cameron A, Ehlke S, Mitchell JN. Implicit alcohol cognitions in risky drinking nicotine users with and without co-morbid major depressive disorder. Addict Behav 39: 797–802, 2014. doi: 10.1016/j.addbeh.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia 9: 294–298, 1973. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- 62.Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol 175: 405–415, 2002. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 63.Cruz Grecco Teixeira MB, Martins GM, Miranda-Rodrigues M, De Araújo IF, Oliveira R, Brum PC, Azevedo Gouveia CH. Lack of α2C-Adrenoceptor Results in Contrasting Phenotypes of Long Bones and Vertebra and Prevents the Thyrotoxicosis-Induced Osteopenia. PLoS One 11: e0146795, 2016. doi: 10.1371/journal.pone.0146795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dalsgaard S, Leckman JF, Mortensen PB, Nielsen HS, Simonsen M. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry 2: 702–709, 2015. doi: 10.1016/S2215-0366(15)00271-0. [DOI] [PubMed] [Google Scholar]
- 65.De Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, Chenu C. Sympathetic nervous system does not mediate the load-induced cortical new bone formation. J Bone Miner Res 20: 2159–2168, 2005. doi: 10.1359/JBMR.050812. [DOI] [PubMed] [Google Scholar]
- 66.Dénes A, Boldogkoi Z, Uhereczky G, Hornyák A, Rusvai M, Palkovits M, Kovács KJ. Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience 134: 947–963, 2005. doi: 10.1016/j.neuroscience.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 67.Denise P, Besnard S, Vignaux G, Sabatier JP, Edy E, Hitier M, Levasseur R. Sympathetic B antagonist prevents bone mineral density decrease induced by labyrinthectomy. Aviakosm Ekolog Med 43: 36–38, 2009. [PubMed] [Google Scholar]
- 68.Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi M, Yamamoto N. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother 60: 273–276, 2006. doi: 10.1016/j.biopha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 69.Diem SJ, Ruppert K, Cauley JA, Lian Y, Bromberger JT, Finkelstein JS, Greendale GA, Solomon DH. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab 98: 4355–4363, 2013. doi: 10.1210/jc.2013-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding Y, Arai M, Kondo H, Togari A. Effects of capsaicin-induced sensory denervation on bone metabolism in adult rats. Bone 46: 1591–1596, 2010. doi: 10.1016/j.bone.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 71.Dorn LD, Pabst S, Sontag LM, Kalkwarf HJ, Hillman JB, Susman EJ. Bone mass, depressive, and anxiety symptoms in adolescent girls: variation by smoking and alcohol use. J Adolesc Health 49: 498–504, 2011. doi: 10.1016/j.jadohealth.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drummond PD, Finch PM, Smythe GA. Reflex sympathetic dystrophy: the significance of differing plasma catecholamine concentrations in affected and unaffected limbs. Brain 114: 2025–2036, 1991. doi: 10.1093/brain/114.5.2025. [DOI] [PubMed] [Google Scholar]
- 73.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100: 197–207, 2000. doi: 10.1016/S0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 74.Dunn SL, Wilkinson JM, Crawford A, Le Maitre CL, Bunning RA. Cannabinoid WIN-55,212-2 mesylate inhibits interleukin-1β induced matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase expression in human chondrocytes. Osteoarthritis Cartilage 22: 133–144, 2014. doi: 10.1016/j.joca.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 75.Eimar H, Alebrahim S, Manickam G, Al-Subaie A, Abu-Nada L, Murshed M, Tamimi F. Donepezil regulates energy metabolism and favors bone mass accrual. Bone 84: 131–138, 2016. doi: 10.1016/j.bone.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Eimar H, Perez Lara A, Tamimi I, Márquez Sánchez P, Gormaz Talavera I, Rojas Tomba F, García de la Oliva T, Tamimi F. Acetylcholinesterase inhibitors and healing of hip fracture in Alzheimer’s disease patients: a retrospective cohort study. J Musculoskelet Neuronal Interact 13: 454–463, 2013. [PubMed] [Google Scholar]
- 77.Eitner A, Pester J, Nietzsche S, Hofmann GO, Schaible HG. The innervation of synovium of human osteoarthritic joints in comparison with normal rat and sheep synovium. Osteoarthritis Cartilage 21: 1383–1391, 2013. doi: 10.1016/j.joca.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 78.Elefteriou F. Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys 473: 231–236, 2008. doi: 10.1016/j.abb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434: 514–520, 2005. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 80.En-Nosse M, Hartmann S, Trinkaus K, Alt V, Stigler B, Heiss C, Kilian O, Schnettler R, Lips KS. Expression of non-neuronal cholinergic system in osteoblast-like cells and its involvement in osteogenesis. Cell Tissue Res 338: 203–215, 2009. doi: 10.1007/s00441-009-0871-1. [DOI] [PubMed] [Google Scholar]
- 81.Fan W, Bouwense SA, Crawford R, Xiao Y. Structural and cellular features in metaphyseal and diaphyseal periosteum of osteoporotic rats. J Mol Histol 41: 51–60, 2010. doi: 10.1007/s10735-010-9261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fang MA, Frost PJ, Iida-Klein A, Hahn TJ. Effects of nicotine on cellular function in UMR 106-01 osteoblast-like cells. Bone 12: 283–286, 1991. doi: 10.1016/8756-3282(91)90077-V. [DOI] [PubMed] [Google Scholar]
- 83.Farr JN, Charkoudian N, Barnes JN, Monroe DG, McCready LK, Atkinson EJ, Amin S, Melton LJ III, Joyner MJ, Khosla S. Relationship of sympathetic activity to bone microstructure, turnover, and plasma osteopontin levels in women. J Clin Endocrinol Metab 97: 4219–4227, 2012. doi: 10.1210/jc.2012-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fermor B, Skerry TM. PTH/PTHrP receptor expression on osteoblasts and osteocytes but not resorbing bone surfaces in growing rats. J Bone Miner Res 10: 1935–1943, 1995. doi: 10.1002/jbmr.5650101213. [DOI] [PubMed] [Google Scholar]
- 85.Flachenecker P, Reiners K, Krauser M, Wolf A, Toyka KV. Autonomic dysfunction in multiple sclerosis is related to disease activity and progression of disability. Mult Scler 7: 327–334, 2001. doi: 10.1177/135245850100700509. [DOI] [PubMed] [Google Scholar]
- 86.Flachenecker P, Wolf A, Krauser M, Hartung HP, Reiners K. Cardiovascular autonomic dysfunction in multiple sclerosis: correlation with orthostatic intolerance. J Neurol 246: 578–586, 1999. doi: 10.1007/s004150050407. [DOI] [PubMed] [Google Scholar]
- 87.Fonseca TL, Jorgetti V, Costa CC, Capelo LP, Covarrubias AE, Moulatlet AC, Teixeira MB, Hesse E, Morethson P, Beber EH, Freitas FR, Wang CC, Nonaka KO, Oliveira R, Casarini DE, Zorn TM, Brum PC, Gouveia CH. Double disruption of α2A- and α2C-adrenoceptors results in sympathetic hyperactivity and high-bone-mass phenotype. J Bone Miner Res 26: 591–603, 2011. doi: 10.1002/jbmr.243. [DOI] [PubMed] [Google Scholar]
- 88.Forsén L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trøndelag Health Survey. Diabetologia 42: 920–925, 1999. doi: 10.1007/s001250051248. [DOI] [PubMed] [Google Scholar]
- 89.Forst T, Beyer J, Pfützner A, Kann P, Schehler B, Lobmann R, Schäfer H, Andreas J, Bockisch A. Peripheral osteopenia in adult patients with insulin-dependent diabetes mellitus. Diabet Med 12: 874–879, 1995. doi: 10.1111/j.1464-5491.1995.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 90.Francis NJ, Asmus SE, Landis SC. CNTF and LIF are not required for the target-directed acquisition of cholinergic and peptidergic properties by sympathetic neurons in vivo. Dev Biol 182: 76–87, 1997. doi: 10.1006/dbio.1996.8464. [DOI] [PubMed] [Google Scholar]
- 91.Fu L, Patel MS, Karsenty G. The circadian modulation of leptin-controlled bone formation. Prog Brain Res 153: 177–188, 2006. doi: 10.1016/S0079-6123(06)53010-9. [DOI] [PubMed] [Google Scholar]
- 92.Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S, Sato T, Shibata S, Yoshida Y, Gu Z, Kimura A, Ma C, Xu C, Bando W, Fujita K, Shinomiya K, Hirai T, Asou Y, Enomoto M, Okano H, Okawa A, Itoh H. Sema3A regulates bone-mass accrual through sensory innervations. Nature 497: 490–493, 2013. doi: 10.1038/nature12115. [DOI] [PubMed] [Google Scholar]
- 93.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 5: 623–628, 1999. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 94.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Coughlin KA, Kaczmarska MJ, Castaneda-Corral G, Bloom AP, Kuskowski MA, Mantyh PW. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis Rheum 64: 2223–2232, 2012. doi: 10.1002/art.34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giese-Davis J, Wilhelm FH, Conrad A, Abercrombie HC, Sephton S, Yutsis M, Neri E, Taylor CB, Kraemer HC, Spiegel D. Depression and stress reactivity in metastatic breast cancer. Psychosom Med 68: 675–683, 2006. doi: 10.1097/01.psy.0000238216.88515.e5. [DOI] [PubMed] [Google Scholar]
- 96.Gold DT, Solimeo S. Osteoporosis and depression: a historical perspective. Curr Osteoporos Rep 4: 134–139, 2006. doi: 10.1007/s11914-996-0021-6. [DOI] [PubMed] [Google Scholar]
- 97.Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem 85: 825–836, 2002. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- 98.Graafmans WC, Ooms ME, Hofstee HM, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol 143: 1129–1136, 1996. doi: 10.1093/oxfordjournals.aje.a008690. [DOI] [PubMed] [Google Scholar]
- 99.Graham S, Hammond-Jones D, Gamie Z, Polyzois I, Tsiridis E, Tsiridis E. The effect of beta-blockers on bone metabolism as potential drugs under investigation for osteoporosis and fracture healing. Expert Opin Investig Drugs 17: 1281–1299, 2008. doi: 10.1517/13543784.17.9.1281. [DOI] [PubMed] [Google Scholar]
- 100.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L; 1999-2000 national health and nutrition examination survey . Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care 27: 1591–1597, 2004. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 101.Gu J, Ikeda H, Suda H. Sympathetic Regulation of Tertiary Dentinogenesis via Beta-2 Adrenergic Receptor on Rat Odontoblasts. J Endod 41: 1056–1060, 2015. doi: 10.1016/j.joen.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 102.Gunal DI, Afsar N, Tanridag T, Aktan S. Autonomic dysfunction in multiple sclerosis: correlation with disease-related parameters. Eur Neurol 48: 1–5, 2002. doi: 10.1159/000064949. [DOI] [PubMed] [Google Scholar]
- 103.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain 108: 95–107, 2004. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 104.Hamrick MW. Leptin, bone mass, and the thrifty phenotype. J Bone Miner Res 19: 1607–1611, 2004. doi: 10.1359/JBMR.040712. [DOI] [PubMed] [Google Scholar]
- 105.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34: 376–383, 2004. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 106.Han J, Zou Z, Zhu C, Deng J, Wang J, Ran X, Shi C, Ai G, Li R, Cheng T, Su Y. DNA synthesis of rat bone marrow mesenchymal stem cells through alpha1-adrenergic receptors. Arch Biochem Biophys 490: 96–102, 2009. doi: 10.1016/j.abb.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 107.Haney EM, Chan BK, Diem SJ, Ensrud KE, Cauley JA, Barrett-Connor E, Orwoll E, Bliziotes MM; Osteoporotic Fractures in Men Study Group . Association of low bone mineral density with selective serotonin reuptake inhibitor use by older men. Arch Intern Med 167: 1246–1251, 2007. doi: 10.1001/archinte.167.12.1246. [DOI] [PubMed] [Google Scholar]
- 108.Harden RN, Duc TA, Williams TR, Coley D, Cate JC, Gracely RH. Norepinephrine and epinephrine levels in affected versus unaffected limbs in sympathetically maintained pain. Clin J Pain 10: 324–330, 1994. doi: 10.1097/00002508-199412000-00014. [DOI] [PubMed] [Google Scholar]
- 109.Hart EC, Charkoudian N. Sympathetic neural regulation of blood pressure: influences of sex and aging. Physiology (Bethesda) 29: 8–15, 2014. doi: 10.1152/physiol.00031.2013. [DOI] [PubMed] [Google Scholar]
- 110.Harun A, Li C, Bridges JF, Agrawal Y. Understanding the Experience of Age-Related Vestibular Loss in Older Individuals: A Qualitative Study. Patient 9: 303–309, 2016. doi: 10.1007/s40271-015-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hayek S, Laplaza FJ, Axelrod FB, Burke SW. Spinal deformity in familial dysautonomia. Prevalence, and results of bracing. J Bone Joint Surg Am 82: 1558–1562, 2000. doi: 10.2106/00004623-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 112.Hearn AP, Silber E. Osteoporosis in multiple sclerosis. Mult Scler 16: 1031–1043, 2010. doi: 10.1177/1352458510368985. [DOI] [PubMed] [Google Scholar]
- 113.Heffner KL, Loving TJ, Robles TF, Kiecolt-Glaser JK. Examining psychosocial factors related to cancer incidence and progression: in search of the silver lining. Brain Behav Immun 17, Suppl 1: 109–111, 2003. doi: 10.1016/S0889-1591(02)00076-4. [DOI] [PubMed] [Google Scholar]
- 114.Heffner MA, Anderson MJ, Yeh GC, Genetos DC, Christiansen BA. Altered bone development in a mouse model of peripheral sensory nerve inactivation. J Musculoskelet Neuronal Interact 14: 1–9, 2014. [PMC free article] [PubMed] [Google Scholar]
- 115.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides 38: 213–224, 2004. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 116.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87: 1932–1936, 1990. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hill EL, Elde R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res 264: 469–480, 1991. doi: 10.1007/BF00319037. [DOI] [PubMed] [Google Scholar]
- 118.Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science 232: 868–871, 1986. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- 119.Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC. Leptin inhibits osteoclast generation. J Bone Miner Res 17: 200–209, 2002. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 120.Howard GA, Bottemiller BL, Turner RT, Rader JI, Baylink DJ. Parathyroid hormone stimulates bone formation and resorption in organ culture: evidence for a coupling mechanism. Proc Natl Acad Sci USA 78: 3204–3208, 1981. doi: 10.1073/pnas.78.5.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang HH, Brennan TC, Muir MM, Mason RS. Functional alpha1- and beta2-adrenergic receptors in human osteoblasts. J Cell Physiol 220: 267–275, 2009. doi: 10.1002/jcp.21761. [DOI] [PubMed] [Google Scholar]
- 122.Huang TL, Lin CC. Advances in biomarkers of major depressive disorder. Adv Clin Chem 68: 177–204, 2015. doi: 10.1016/bs.acc.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 123.Hukkanen M, Konttinen YT, Rees RG, Santavirta S, Terenghi G, Polak JM. Distribution of nerve endings and sensory neuropeptides in rat synovium, meniscus and bone. Int J Tissue React 14: 1–10, 1992. [PubMed] [Google Scholar]
- 124.Hukkanen M, Konttinen YT, Santavirta S, Paavolainen P, Gu XH, Terenghi G, Polak JM. Rapid proliferation of calcitonin gene-related peptide-immunoreactive nerves during healing of rat tibial fracture suggests neural involvement in bone growth and remodelling. Neuroscience 54: 969–979, 1993. doi: 10.1016/0306-4522(93)90588-7. [DOI] [PubMed] [Google Scholar]
- 125.Ibrahim AI, Hawamdeh ZM, Alsharif AA. Evaluation of bone mineral density in children with perinatal brachial plexus palsy: effectiveness of weight bearing and traditional exercises. Bone 49: 499–505, 2011. doi: 10.1016/j.bone.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 126.Idris AI, Sophocleous A, Landao-Bassonga E, Canals M, Milligan G, Baker D, van’t Hof RJ, Ralston SH. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab 10: 139–147, 2009. doi: 10.1016/j.cmet.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 127.Igwe JC, Jiang X, Paic F, Ma L, Adams DJ, Baldock PA, Pilbeam CC, Kalajzic I. Neuropeptide Y is expressed by osteocytes and can inhibit osteoblastic activity. J Cell Biochem 108: 621–630, 2009. doi: 10.1002/jcb.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ilias I, Alesci S, Gold PW, Chrousos GP. Depression and osteoporosis in men: association or casual link? Hormones (Athens) 5: 9–16, 2006. doi: 10.14310/horm.2002.11164. [DOI] [PubMed] [Google Scholar]
- 129.Imai S, Tokunaga Y, Maeda T, Kikkawa M, Hukuda S. Calcitonin gene-related peptide, substance P, and tyrosine hydroxylase-immunoreactive innervation of rat bone marrows: an immunohistochemical and ultrastructural investigation on possible efferent and afferent mechanisms. J Orthop Res 15: 133–140, 1997. doi: 10.1002/jor.1100150120. [DOI] [PubMed] [Google Scholar]
- 130.Inchiosa MA Jr, Kizelshteyn G. Treatment of complex regional pain syndrome type I with oral phenoxybenzamine: rationale and case reports. Pain Pract 8: 125–132, 2008. doi: 10.1111/j.1533-2500.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- 131.Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, Mitsubuchi H, Tonoki H, Awaya Y, Matsuda I. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet 13: 485–488, 1996. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- 132.Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol 118: 2023–2028, 1996. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jackson MZ, Gruner KA, Qin C, Tourtellotte WG. A neuron autonomous role for the familial dysautonomia gene ELP1 in sympathetic and sensory target tissue innervation. Development 141: 2452–2461, 2014. doi: 10.1242/dev.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jacob L, Kostev K. Impact of attention deficit hyperactivity disorder therapy on fracture risk in children treated in German pediatric practices. Osteoporos Int 28: 1265–1269, 2017. doi: 10.1007/s00198-016-3842-x. [DOI] [PubMed] [Google Scholar]
- 135.Jiao K, Niu LN, Li QH, Ren GT, Zhao CM, Liu YD, Tay FR, Wang MQ. β2-Adrenergic signal transduction plays a detrimental role in subchondral bone loss of temporomandibular joint in osteoarthritis. Sci Rep 5: 12593, 2015. doi: 10.1038/srep12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther 14: R101, 2012. doi: 10.1186/ar3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, Vanderah TW, Mantyh PW. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone 46: 306–313, 2010. doi: 10.1016/j.bone.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jindal RD, Vasko RC Jr, Jennings JR, Fasiczka AL, Thase ME, Reynolds CF III. Heart rate variability in depressed elderly. Am J Geriatr Psychiatry 16: 861–866, 2008. doi: 10.1097/JGP.0b013e318180053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, Komnenovic V, Kong YY, Schreiber M, Dixon SJ, Sims SM, Khokha R, Wada T, Penninger JM. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440: 692–696, 2006. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 140.Kado DM, Lui LY, Cummings SR; The Study Of Osteoporotic Fractures Research Group . Rapid resting heart rate: a simple and powerful predictor of osteoporotic fractures and mortality in older women. J Am Geriatr Soc 50: 455–460, 2002. doi: 10.1046/j.1532-5415.2002.50110.x. [DOI] [PubMed] [Google Scholar]
- 141.Kahl KG, Rudolf S, Stoeckelhuber BM, Dibbelt L, Gehl HB, Markhof K, Hohagen F, Schweiger U. Bone mineral density, markers of bone turnover, and cytokines in young women with borderline personality disorder with and without comorbid major depressive disorder. Am J Psychiatry 162: 168–174, 2005. doi: 10.1176/appi.ajp.162.1.168. [DOI] [PubMed] [Google Scholar]
- 142.Kajimura D, Hinoi E, Ferron M, Kode A, Riley KJ, Zhou B, Guo XE, Karsenty G. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med 208: 841–851, 2011. doi: 10.1084/jem.20102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kajimura D, Paone R, Mann JJ, Karsenty G. Foxo1 regulates Dbh expression and the activity of the sympathetic nervous system in vivo. Mol Metab 3: 770–777, 2014. doi: 10.1016/j.molmet.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Karsak M, Cohen-Solal M, Freudenberg J, Ostertag A, Morieux C, Kornak U, Essig J, Erxlebe E, Bab I, Kubisch C, de Vernejoul MC, Zimmer A. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet 14: 3389–3396, 2005. doi: 10.1093/hmg/ddi370. [DOI] [PubMed] [Google Scholar]
- 145.Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol 25: 629–648, 2009. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- 146.Karsenty G, Olson EN. Bone and Muscle Endocrine Functions: Unexpected Paradigms of Inter-organ Communication. Cell 164: 1248–1256, 2016. doi: 10.1016/j.cell.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Katayama PL, Dias DP, Silva LE, Virtuoso-Junior JS, Marocolo M. Cardiac autonomic modulation in non-frail, pre-frail and frail elderly women: a pilot study. Aging Clin Exp Res 27: 621–629, 2015. doi: 10.1007/s40520-015-0320-9. [DOI] [PubMed] [Google Scholar]
- 148.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124: 407–421, 2006. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 149.Katsuragawa Y. Effect of methamphetamine abuse on the bone quality of the calcaneus. Forensic Sci Int 101: 43–48, 1999. doi: 10.1016/S0379-0738(99)00010-9. [DOI] [PubMed] [Google Scholar]
- 150.Kaufmann H, Schatz IJ. Pure autonomic failure. In: Primer on the Autonomic Nervous System. San Diego, CA: Academic, 2004, p. 309. doi: 10.1016/B978-012589762-4/50084-0. [DOI] [Google Scholar]
- 151.Kawai M, Kinoshita S, Shimba S, Ozono K, Michigami T. Sympathetic activation induces skeletal Fgf23 expression in a circadian rhythm-dependent manner. J Biol Chem 289: 1457–1466, 2014. doi: 10.1074/jbc.M113.500850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kayath MJ, Dib SA, Vieira JG. Prevalence and magnitude of osteopenia associated with insulin-dependent diabetes mellitus. J Diabetes Complications 8: 97–104, 1994. doi: 10.1016/1056-8727(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 153.Kaye DM, Esler MD. Autonomic control of the aging heart. Neuromolecular Med 10: 179–186, 2008. doi: 10.1007/s12017-008-8034-1. [DOI] [PubMed] [Google Scholar]
- 154.Kellenberger S, Muller K, Richener H, Bilbe G. Formoterol and isoproterenol induce c-fos gene expression in osteoblast-like cells by activating beta2-adrenergic receptors. Bone 22: 471–478, 1998. doi: 10.1016/S8756-3282(98)00026-X. [DOI] [PubMed] [Google Scholar]
- 155.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry 67: 1067–1074, 2010. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 156.Kennedy OD, Lendhey M, Mauer P, Philip A, Basta-Pljakic J, Schaffler MB. Microdamage induced by in vivo Reference Point Indentation in mice is repaired by osteocyte-apoptosis mediated remodeling. Bone 95: 192–198, 2017. doi: 10.1016/j.bone.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health 34: 119–138, 2013. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim CH, Zabetian CP, Cubells JF, Cho S, Biaggioni I, Cohen BM, Robertson D, Kim KS. Mutations in the dopamine beta-hydroxylase gene are associated with human norepinephrine deficiency. Am J Med Genet 108: 140–147, 2002. doi: 10.1002/ajmg.10196. [DOI] [PubMed] [Google Scholar]
- 159.Kim EY, Kwon DH, Lee BD, Kim YT, Ahn YB, Yoon KY, Sa SJ, Cho W, Cho SN. Frequency of osteoporosis in 46 men with methamphetamine abuse hospitalized in a National Hospital. Forensic Sci Int 188: 75–80, 2009. doi: 10.1016/j.forsciint.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 160.Kim J, Nakamura T, Kikuchi H, Yoshiuchi K, Sasaki T, Yamamoto Y. Covariation of depressive mood and spontaneous physical activity in major depressive disorder: toward continuous monitoring of depressive mood. IEEE J Biomed Health Inform 19: 1347–1355, 2015. doi: 10.1109/JBHI.2015.2440764. [DOI] [PubMed] [Google Scholar]
- 161.Kim JH, Jung MH, Lee JM, Son HS, Cha BY, Chang SA. Diabetic peripheral neuropathy is highly associated with nontraumatic fractures in Korean patients with type 2 diabetes mellitus. Clin Endocrinol (Oxf) 77: 51–55, 2012. doi: 10.1111/j.1365-2265.2011.04222.x. [DOI] [PubMed] [Google Scholar]
- 162.Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J 9: 441–445, 1995. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 163.Kliemann K, Kneffel M, Bergen I, Kampschulte M, Langheinrich AC, Dürselen L, Ignatius A, Kilian O, Schnettler R, Lips KS. Quantitative analyses of bone composition in acetylcholine receptor M3R and alpha7 knockout mice. Life Sci 91: 997–1002, 2012. doi: 10.1016/j.lfs.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 164.Kogan NM, Melamed E, Wasserman E, Raphael B, Breuer A, Stok KS, Sondergaard R, Escudero AV, Baraghithy S, Attar-Namdar M, Friedlander-Barenboim S, Mathavan N, Isaksson H, Mechoulam R, Müller R, Bajayo A, Gabet Y, Bab I. Cannabidiol, a Major Non-Psychotropic Cannabis Constituent Enhances Fracture Healing and Stimulates Lysyl Hydroxylase Activity in Osteoblasts. J Bone Miner Res 30: 1905–1913, 2015. doi: 10.1002/jbmr.2513. [DOI] [PubMed] [Google Scholar]
- 165.Kondo H, Nifuji A, Takeda S, Ezura Y, Rittling SR, Denhardt DT, Nakashima K, Karsenty G, Noda M. Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J Biol Chem 280: 30192–30200, 2005. doi: 10.1074/jbc.M504179200. [DOI] [PubMed] [Google Scholar]
- 166.Kondo H, Takeuchi S, Togari A. β-Adrenergic signaling stimulates osteoclastogenesis via reactive oxygen species. Am J Physiol Endocrinol Metab 304: E507–E515, 2013. doi: 10.1152/ajpendo.00191.2012. [DOI] [PubMed] [Google Scholar]
- 167.Kronenberg HM. Developmental regulation of the growth plate. Nature 423: 332–336, 2003. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 168.Lahat E, Weiss M, Ben-Shlomo A, Evans S, Bistritzer T. Bone mineral density and turnover in children with attention-deficit hyperactivity disorder receiving methylphenidate. J Child Neurol 15: 436–439, 2000. doi: 10.1177/088307380001500702. [DOI] [PubMed] [Google Scholar]
- 169.Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, Ma JC, Arevalo JM, Morizono K, Cole SW. Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav Immun 26: 635–641, 2012. doi: 10.1016/j.bbi.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lanyon LE. Control of bone architecture by functional load bearing. J Bone Miner Res 7, Suppl 2: S369–S375, 1992. doi: 10.1002/jbmr.5650071403. [DOI] [PubMed] [Google Scholar]
- 171.Laplaza FJ, Turajane T, Axelrod FB, Burke SW. Nonspinal orthopaedic problems in familial dysautonomia (Riley-Day syndrome). J Pediatr Orthop 21: 229–232, 2001. doi: 10.1097/01241398-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 172.Lavi S, Nevo O, Thaler I, Rosenfeld R, Dayan L, Hirshoren N, Gepstein L, Jacob G. Effect of aging on the cardiovascular regulatory systems in healthy women. Am J Physiol Regul Integr Comp Physiol 292: R788–R793, 2007. doi: 10.1152/ajpregu.00352.2006. [DOI] [PubMed] [Google Scholar]
- 173.Lee NJ, Nguyen AD, Enriquez RF, Doyle KL, Sainsbury A, Baldock PA, Herzog H. Osteoblast specific Y1 receptor deletion enhances bone mass. Bone 48: 461–467, 2011. doi: 10.1016/j.bone.2010.10.174. [DOI] [PubMed] [Google Scholar]
- 174.Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci USA 91: 3739–3743, 1994. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Levasseur R, Dargent-Molina P, Sabatier JP, Marcelli C, Bréart G. Beta-blocker use, bone mineral density, and fracture risk in older women: results from the Epidemiologie de l’Osteoporose prospective study. J Am Geriatr Soc 53: 550–552, 2005. doi: 10.1111/j.1532-5415.2005.53178_7.x. [DOI] [PubMed] [Google Scholar]
- 176.Levasseur R, Sabatier JP, Etard O, Denise P, Reber A. Labyrinthectomy decreases bone mineral density in the femoral metaphysis in rats. J Vestib Res 14: 361–365, 2004. [PubMed] [Google Scholar]
- 177.Li J, Ahmad T, Spetea M, Ahmed M, Kreicbergs A. Bone reinnervation after fracture: a study in the rat. J Bone Miner Res 16: 1505–1510, 2001. doi: 10.1359/jbmr.2001.16.8.1505. [DOI] [PubMed] [Google Scholar]
- 178.Li J, Kreicbergs A, Bergström J, Stark A, Ahmed M. Site-specific CGRP innervation coincides with bone formation during fracture healing and modeling: a study in rat angulated tibia. J Orthop Res 25: 1204–1212, 2007. doi: 10.1002/jor.20406. [DOI] [PubMed] [Google Scholar]
- 179.Li W, Shi X, Wang L, Guo T, Wei T, Cheng K, Rice KC, Kingery WS, Clark JD. Epidermal adrenergic signaling contributes to inflammation and pain sensitization in a rat model of complex regional pain syndrome. Pain 154: 1224–1236, 2013. doi: 10.1016/j.pain.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Limonard EJ, Schoenmaker T, de Vries TJ, Tanck MW, Heijboer AC, Endert E, Fliers E, Everts V, Bisschop PH. Clonidine increases bone resorption in humans. Osteoporos Int 27: 1063–1071, 2016. doi: 10.1007/s00198-015-3312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lipski S. Effects of beta-adrenergic stimulation on bone-marrow function in normal and sublethally irradiated mice. I. The effect of isoproterenol on cAMP content in bone-marrow cells in vivo and in vitro. Int J Radiat Biol Relat Stud Phys Chem Med 29: 359–366, 1976. doi: 10.1080/09553007614550411. [DOI] [PubMed] [Google Scholar]
- 182.Liu PS, Chen YY, Feng CK, Lin YH, Yu TC. Muscarinic acetylcholine receptors present in human osteoblast and bone tissue. Eur J Pharmacol 650: 34–40, 2011. doi: 10.1016/j.ejphar.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 183.Long H, Ahmed M, Ackermann P, Stark A, Li J. Neuropeptide Y innervation during fracture healing and remodeling. A study of angulated tibial fractures in the rat. Acta Orthop 81: 639–646, 2010. doi: 10.3109/17453674.2010.504609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lorenz J, Schäfer N, Bauer R, Jenei-Lanzl Z, Springorum RH, Grässel S. Norepinephrine modulates osteoarthritic chondrocyte metabolism and inflammatory responses. Osteoarthritis Cartilage 24: 325–334, 2016. doi: 10.1016/j.joca.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 185.Lorton D, Bellinger DL. Molecular mechanisms underlying β-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int J Mol Sci 16: 5635–5665, 2015. doi: 10.3390/ijms16035635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS). Neurology 45, Suppl 5: S19–S25, 1995. [PubMed] [Google Scholar]
- 187.Lundberg P, Lundgren I, Mukohyama H, Lehenkari PP, Horton MA, Lerner UH. Vasoactive intestinal peptide (VIP)/pituitary adenylate cyclase-activating peptide receptor subtypes in mouse calvarial osteoblasts: presence of VIP-2 receptors and differentiation-induced expression of VIP-1 receptors. Endocrinology 142: 339–347, 2001. doi: 10.1210/endo.142.1.7912. [DOI] [PubMed] [Google Scholar]
- 188.Ma Y, Krueger JJ, Redmon SN, Uppuganti S, Nyman JS, Hahn MK, Elefteriou F. Extracellular norepinephrine clearance by NET is required for skeletal homeostasis. J Biol Chem 288: 30105–30113, 2013. doi: 10.1074/jbc.M113.481309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ma Y, Nyman JS, Tao H, Moss HH, Yang X, Elefteriou F. β2-Adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology 152: 1412–1422, 2011. doi: 10.1210/en.2010-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Maayan C, Bar-On E, Foldes AJ, Gesundheit B, Pollak RD. Bone mineral density and metabolism in familial dysautonomia. Osteoporos Int 13: 429–433, 2002. doi: 10.1007/s001980200050. [DOI] [PubMed] [Google Scholar]
- 191.Maayan C, Becker Y, Gesundheit B, Girgis SI. Calcitonin gene related peptide in familial dysautonomia. Neuropeptides 35: 189–195, 2001. doi: 10.1054/npep.2001.0863. [DOI] [PubMed] [Google Scholar]
- 192.Maayan C, Kaplan E, Shachar S, Peleg O, Godfrey S. Incidence of familial dysautonomia in Israel 1977-1981. Clin Genet 32: 106–108, 1987. doi: 10.1111/j.1399-0004.1987.tb03334.x. [DOI] [PubMed] [Google Scholar]
- 193.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O’Leary P, Mantyh PW. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 113: 155–166, 2002. doi: 10.1016/S0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 194.Madsen JE, Hukkanen M, Aune AK, Basran I, Møller JF, Polak JM, Nordsletten L. Fracture healing and callus innervation after peripheral nerve resection in rats. Clin Orthop Relat Res (351): 230–240, 1998. [PubMed] [Google Scholar]
- 195.Mandl P, Hayer S, Karonitsch T, Scholze P, Győri D, Sykoutri D, Blüml S, Mócsai A, Poór G, Huck S, Smolen JS, Redlich K. Nicotinic acetylcholine receptors modulate osteoclastogenesis. Arthritis Res Ther 18: 63, 2016. doi: 10.1186/s13075-016-0961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Manicourt DH, Brasseur JP, Boutsen Y, Depreseux G, Devogelaer JP. Role of alendronate in therapy for posttraumatic complex regional pain syndrome type I of the lower extremity. Arthritis Rheum 50: 3690–3697, 2004. doi: 10.1002/art.20591. [DOI] [PubMed] [Google Scholar]
- 197.Mantyh PW. The neurobiology of skeletal pain. Eur J Neurosci 39: 508–519, 2014. doi: 10.1111/ejn.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Maronde E, Schilling AF, Seitz S, Schinke T, Schmutz I, van der Horst G, Amling M, Albrecht U. The clock genes Period 2 and Cryptochrome 2 differentially balance bone formation. PLoS One 5: e11527, 2010. doi: 10.1371/journal.pone.0011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Marrie RA, Cutter G, Tyry T, Vollmer T. A cross-sectional study of bone health in multiple sclerosis. Neurology 73: 1394–1398, 2009. doi: 10.1212/WNL.0b013e3181beece8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Martin CD, Jimenez-Andrade JM, Ghilardi JR, Mantyh PW. Organization of a unique net-like meshwork of CGRP+ sensory fibers in the mouse periosteum: implications for the generation and maintenance of bone fracture pain. Neurosci Lett 427: 148–152, 2007. doi: 10.1016/j.neulet.2007.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Matic I, Matthews BG, Kizivat T, Igwe JC, Marijanovic I, Ruohonen ST, Savontaus E, Adams DJ, Kalajzic I. Bone-specific overexpression of NPY modulates osteogenesis. J Musculoskelet Neuronal Interact 12: 209–218, 2012. [PMC free article] [PubMed] [Google Scholar]
- 202.McCauley LK, Koh AJ, Beecher CA, Cui Y, Rosol TJ, Franceschi RT. PTH/PTHrP receptor is temporally regulated during osteoblast differentiation and is associated with collagen synthesis. J Cell Biochem 61: 638–647, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 203.Melamed E, Robinson D, Halperin N, Wallach N, Keren O, Groswasser Z. Brain injury-related heterotopic bone formation: treatment strategy and results. Am J Phys Med Rehabil 81: 670–674, 2002. doi: 10.1097/00002060-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 204.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, Gonzalez-Angulo AM. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 29: 2645–2652, 2011. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Melton LJ III, Beard CM, Kokmen E, Atkinson EJ, O’Fallon WM. Fracture risk in patients with Alzheimer’s disease. J Am Geriatr Soc 42: 614–619, 1994. doi: 10.1111/j.1532-5415.1994.tb06859.x. [DOI] [PubMed] [Google Scholar]
- 206.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452: 442–447, 2008. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 207.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466: 829–834, 2010. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mendy A, Vieira ER, Albatineh AN, Nnadi AK, Lowry D, Gasana J. Low bone mineral density is associated with balance and hearing impairments. Ann Epidemiol 24: 58–62, 2014. doi: 10.1016/j.annepidem.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 209.Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, Gold P. Bone mineral density in women with depression. N Engl J Med 335: 1176–1181, 1996. doi: 10.1056/NEJM199610173351602. [DOI] [PubMed] [Google Scholar]
- 210.Miyasaka N, Akiyoshi M, Kubota T. Relationship between autonomic nervous system activity and bone mineral density in non-medicated perimenopausal women. J Bone Miner Metab 32: 588–592, 2014. doi: 10.1007/s00774-013-0534-x. [DOI] [PubMed] [Google Scholar]
- 211.Mlakar V, Jurkovic Mlakar S, Zupan J, Komadina R, Prezelj J, Marc J. ADRA2A is involved in neuro-endocrine regulation of bone resorption. J Cell Mol Med 19: 1520–1529, 2015. doi: 10.1111/jcmm.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Moore RE, Smith CK II, Bailey CS, Voelkel EF, Tashjian AH Jr. Characterization of beta-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner 23: 301–315, 1993. doi: 10.1016/S0169-6009(08)80105-5. [DOI] [PubMed] [Google Scholar]
- 213.Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol 281: R683–R698, 2001. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- 214.Morrison SF, Milner TA, Reis DJ. Reticulospinal vasomotor neurons of the rat rostral ventrolateral medulla: relationship to sympathetic nerve activity and the C1 adrenergic cell group. J Neurosci 8: 1286–1301, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mosti MP, Flemmen G, Hoff J, Stunes AK, Syversen U, Wang E. Impaired skeletal health and neuromuscular function among amphetamine users in clinical treatment. Osteoporos Int 27: 1003–1010, 2016. doi: 10.1007/s00198-015-3371-z. [DOI] [PubMed] [Google Scholar]
- 216.Mukohyama H, Ransjö M, Taniguchi H, Ohyama T, Lerner UH. The inhibitory effects of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide on osteoclast formation are associated with upregulation of osteoprotegerin and downregulation of RANKL and RANK. Biochem Biophys Res Commun 271: 158–163, 2000. doi: 10.1006/bbrc.2000.2599. [DOI] [PubMed] [Google Scholar]
- 217.Mulcrone PL, Campbell JP, Clément-Demange L, Anbinder AL, Merkel AR, Brekken RA, Sterling JA, Elefteriou F. Skeletal Colonization by Breast Cancer Cells Is Stimulated by an Osteoblast and β2AR-Dependent Neo-Angiogenic Switch. J Bone Miner Res 32: 1442–1454, 2017. doi: 10.1002/jbmr.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365: 61–65, 1993. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 219.Muschter D, Schäfer N, Stangl H, Straub RH, Grässel S. Sympathetic Neurotransmitters Modulate Osteoclastogenesis and Osteoclast Activity in the Context of Collagen-Induced Arthritis. PLoS One 10: e0139726, 2015. doi: 10.1371/journal.pone.0139726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL; IOF Bone and Diabetes Working Group . Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol 13: 208–219, 2017. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 221.Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biol Psychiatry 46: 1131–1139, 1999. doi: 10.1016/S0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- 222.Nicodemus KK, Folsom AR; Iowa Women’s Health Study . Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care 24: 1192–1197, 2001. doi: 10.2337/diacare.24.7.1192. [DOI] [PubMed] [Google Scholar]
- 223.Niedermair T, Kuhn V, Doranehgard F, Stange R, Wieskötter B, Beckmann J, Salmen P, Springorum HR, Straub RH, Zimmer A, Grifka J, Grässel S. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification. Matrix Biol 38: 22–35, 2014. doi: 10.1016/j.matbio.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 224.Nieves J, Cosman F, Herbert J, Shen V, Lindsay R. High prevalence of vitamin D deficiency and reduced bone mass in multiple sclerosis. Neurology 44: 1687–1692, 1994. doi: 10.1212/WNL.44.9.1687. [DOI] [PubMed] [Google Scholar]
- 225.Nishiura T, Abe K. Alpha1-adrenergic receptor stimulation induces the expression of receptor activator of nuclear factor kappaB ligand gene via protein kinase C and extracellular signal-regulated kinase pathways in MC3T3-E1 osteoblast-like cells. Arch Oral Biol 52: 778–785, 2007. doi: 10.1016/j.archoralbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 226.Nordsletten L, Madsen JE, Almaas R, Rootwelt T, Halse J, Konttinen YT, Hukkanen M, Santavirta S. The neuronal regulation of fracture healing. Effects of sciatic nerve resection in rat tibia. Acta Orthop Scand 65: 299–304, 1994. doi: 10.3109/17453679408995457. [DOI] [PubMed] [Google Scholar]
- 227.Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annu Rev Pathol 3: 457–484, 2008. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- 228.Obri A, Makinistoglu MP, Zhang H, Karsenty G. HDAC4 integrates PTH and sympathetic signaling in osteoblasts. J Cell Biol 205: 771–780, 2014. doi: 10.1083/jcb.201403138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ofek O, Attar-Namdar M, Kram V, Dvir-Ginzberg M, Mechoulam R, Zimmer A, Frenkel B, Shohami E, Bab I. CB2 cannabinoid receptor targets mitogenic Gi protein-cyclin D1 axis in osteoblasts. J Bone Miner Res 26: 308–316, 2011. doi: 10.1002/jbmr.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, Mechoulam R, Zimmer A, Bab I. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA 103: 696–701, 2006. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Offley SC, Guo TZ, Wei T, Clark JD, Vogel H, Lindsey DP, Jacobs CR, Yao W, Lane NE, Kingery WS. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J Bone Miner Res 20: 257–267, 2005. doi: 10.1359/JBMR.041108. [DOI] [PubMed] [Google Scholar]
- 232.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol 16: 191–220, 2000. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 233.Ortuño MJ, Robinson ST, Subramanyam P, Paone R, Huang YY, Guo XE, Colecraft HM, Mann JJ, Ducy P. Serotonin-reuptake inhibitors act centrally to cause bone loss in mice by counteracting a local anti-resorptive effect. Nat Med 22: 1170–1179, 2016. doi: 10.1038/nm.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pacifici R. T cells, osteoblasts, and osteocytes: interacting lineages key for the bone anabolic and catabolic activities of parathyroid hormone. Ann N Y Acad Sci 1364: 11–24, 2016. doi: 10.1111/nyas.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8: 98–101, 1989. [PubMed] [Google Scholar]
- 236.Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, Kuo L, Shin DG, Rowe DW, Harris SE, Kalajzic I. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone 45: 682–692, 2009. doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pearson J, Dancis J, Axelrod F, Grover N. The sural nerve in familial dysautonomia. J Neuropathol Exp Neurol 34: 413–424, 1975. doi: 10.1097/00005072-197509000-00004. [DOI] [PubMed] [Google Scholar]
- 238.Perry BA, Archer KR, Song Y, Ma Y, Green JK, Elefteriou F, Dahir KM. Medication therapy for attention deficit/hyperactivity disorder is associated with lower risk of fracture: a retrospective cohort study. Osteoporos Int 27: 2223–2227, 2016. doi: 10.1007/s00198-016-3547-1. [DOI] [PubMed] [Google Scholar]
- 239.Peterka RJ, Black FO, Schoenhoff MB. Age-related changes in human vestibulo-ocular and optokinetic reflexes: pseudorandom rotation tests. J Vestib Res 1: 61–71, 1990-1991. [PubMed] [Google Scholar]
- 240.Pierroz DD, Bonnet N, Bianchi EN, Bouxsein ML, Baldock PA, Rizzoli R, Ferrari SL. Deletion of β-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res 27: 1252–1262, 2012. doi: 10.1002/jbmr.1594. [DOI] [PubMed] [Google Scholar]
- 241.Pierroz DD, Bouxsein ML, Rizzoli R, Ferrari SL. Combined treatment with a beta-blocker and intermittent PTH improves bone mass and microarchitecture in ovariectomized mice. Bone 39: 260–267, 2006. doi: 10.1016/j.bone.2006.01.145. [DOI] [PubMed] [Google Scholar]
- 242.Poulton A, Briody J, McCorquodale T, Melzer E, Herrmann M, Baur LA, Duque G. Weight loss on stimulant medication: how does it affect body composition and bone metabolism? - A prospective longitudinal study. Int J Pediatr Endocrinol 2012: 30, 2012. doi: 10.1186/1687-9856-2012-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Poulton AS, Bui Q, Melzer E, Evans R. Stimulant medication effects on growth and bone age in children with attention-deficit/hyperactivity disorder: a prospective cohort study. Int Clin Psychopharmacol 31: 93–99, 2016. doi: 10.1097/YIC.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 1: 628–638, 2010. doi: 10.18632/oncotarget.101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pratt LA, Brody DJ. Depression in the U.S. household population, 2009-2012. NCHS Data Brief 172: 1–8, 2014. [PubMed] [Google Scholar]
- 246.Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697, 2012. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- 247.Ransjö M, Lie A, Mukohyama H, Lundberg P, Lerner UH. Microisolated mouse osteoclasts express VIP-1 and PACAP receptors. Biochem Biophys Res Commun 274: 400–404, 2000. doi: 10.1006/bbrc.2000.3151. [DOI] [PubMed] [Google Scholar]
- 248.Reid IR. Effects of beta-blockers on fracture risk. J Musculoskelet Neuronal Interact 8: 105–110, 2008. [PubMed] [Google Scholar]
- 249.Reid IR, Gamble GD, Grey AB, Black DM, Ensrud KE, Browner WS, Bauer DC. beta-Blocker use, BMD, and fractures in the study of osteoporotic fractures. J Bone Miner Res 20: 613–618, 2005. doi: 10.1359/JBMR.041202. [DOI] [PubMed] [Google Scholar]
- 250.Reid IR, Lucas J, Wattie D, Horne A, Bolland M, Gamble GD, Davidson JS, Grey AB. Effects of a beta-blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab 90: 5212–5216, 2005. doi: 10.1210/jc.2005-0573. [DOI] [PubMed] [Google Scholar]
- 251.Reitstetter R, Lukas RJ, Gruener R. Dependence of nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J Pharmacol Exp Ther 289: 656–660, 1999. [PubMed] [Google Scholar]
- 252.Rejnmark L, Vestergaard P, Kassem M, Christoffersen BR, Kolthoff N, Brixen K, Mosekilde L. Fracture risk in perimenopausal women treated with beta-blockers. Calcif Tissue Int 75: 365–372, 2004. doi: 10.1007/s00223-004-0222-x. [DOI] [PubMed] [Google Scholar]
- 253.Rhee MH, Vogel Z, Barg J, Bayewitch M, Levy R, Hanus L, Breuer A, Mechoulam R. Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase. J Med Chem 40: 3228–3233, 1997. doi: 10.1021/jm970126f. [DOI] [PubMed] [Google Scholar]
- 254.Rix M, Andreassen H, Eskildsen P. Impact of peripheral neuropathy on bone density in patients with type 1 diabetes. Diabetes Care 22: 827–831, 1999. doi: 10.2337/diacare.22.5.827. [DOI] [PubMed] [Google Scholar]
- 255.Rizzoli R, Cooper C, Reginster JY, Abrahamsen B, Adachi JD, Brandi ML, Bruyère O, Compston J, Ducy P, Ferrari S, Harvey NC, Kanis JA, Karsenty G, Laslop A, Rabenda V, Vestergaard P. Antidepressant medications and osteoporosis. Bone 51: 606–613, 2012. doi: 10.1016/j.bone.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 256.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci 317: 75–77, 1999. doi: 10.1016/S0002-9629(15)40480-X. [DOI] [PubMed] [Google Scholar]
- 257.Robertson D, Haile V, Perry SE, Robertson RM, Phillips JA III, Biaggioni I. Dopamine beta-hydroxylase deficiency. A genetic disorder of cardiovascular regulation. Hypertension 18: 1–8, 1991. doi: 10.1161/01.HYP.18.1.1. [DOI] [PubMed] [Google Scholar]
- 258.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283: 5866–5875, 2008. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 259.Ross K. Mapping pathways from stress to cancer progression. J Natl Cancer Inst 100: 914–917, 2008. doi: 10.1093/jnci/djn229. [DOI] [PubMed] [Google Scholar]
- 260.Rossi F, Siniscalco D, Luongo L, De Petrocellis L, Bellini G, Petrosino S, Torella M, Santoro C, Nobili B, Perrotta S, Di Marzo V, Maione S. The endovanilloid/endocannabinoid system in human osteoclasts: possible involvement in bone formation and resorption. Bone 44: 476–484, 2009. doi: 10.1016/j.bone.2008.10.056. [DOI] [PubMed] [Google Scholar]
- 261.Rothem DE, Rothem L, Soudry M, Dahan A, Eliakim R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J Bone Miner Metab 27: 555–561, 2009. doi: 10.1007/s00774-009-0075-5. [DOI] [PubMed] [Google Scholar]
- 262.Sabsovich I, Guo TZ, Wei T, Zhao R, Li X, Clark DJ, Geis C, Sommer C, Kingery WS. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain 137: 507–519, 2008. doi: 10.1016/j.pain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sanya EO, Tutaj M, Brown CM, Goel N, Neundörfer B, Hilz MJ. Abnormal heart rate and blood pressure responses to baroreflex stimulation in multiple sclerosis patients. Clin Auton Res 15: 213–218, 2005. doi: 10.1007/s10286-005-0274-7. [DOI] [PubMed] [Google Scholar]
- 264.Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science 251: 1608–1610, 1991. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- 265.Sato T, Abe T, Chida D, Nakamoto N, Hori N, Kokabu S, Sakata Y, Tomaru Y, Iwata T, Usui M, Aiko K, Yoda T. Functional role of acetylcholine and the expression of cholinergic receptors and components in osteoblasts. FEBS Lett 584: 817–824, 2010. doi: 10.1016/j.febslet.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 266.Sato T, Abe T, Nakamoto N, Tomaru Y, Koshikiya N, Nojima J, Kokabu S, Sakata Y, Kobayashi A, Yoda T. Nicotine induces cell proliferation in association with cyclin D1 up-regulation and inhibits cell differentiation in association with p53 regulation in a murine pre-osteoblastic cell line. Biochem Biophys Res Commun 377: 126–130, 2008. doi: 10.1016/j.bbrc.2008.09.114. [DOI] [PubMed] [Google Scholar]
- 267.Sato T, Miyazawa K, Suzuki Y, Mizutani Y, Uchibori S, Asaoka R, Arai M, Togari A, Goto S. Selective β2-adrenergic Antagonist Butoxamine Reduces Orthodontic Tooth Movement. J Dent Res 93: 807–812, 2014. doi: 10.1177/0022034514536730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sato Y, Kanoko T, Satoh K, Iwamoto J. Risk factors for hip fracture among elderly patients with Alzheimer’s disease. J Neurol Sci 223: 107–112, 2004. doi: 10.1016/j.jns.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 269.Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int 94: 5–24, 2014. doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res 221: 555–563, 2011. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 271.Schlienger RG, Kraenzlin ME, Jick SS, Meier CR. Use of beta-blockers and risk of fractures. JAMA 292: 1326–1332, 2004. doi: 10.1001/jama.292.11.1326. [DOI] [PubMed] [Google Scholar]
- 272.Schmidt-Bleek K, Schell H, Schulz N, Hoff P, Perka C, Buttgereit F, Volk HD, Lienau J, Duda GN. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res 347: 567–573, 2012. doi: 10.1007/s00441-011-1205-7. [DOI] [PubMed] [Google Scholar]
- 273.Schreihofer AM, Stornetta RL, Guyenet PG. Regulation of sympathetic tone and arterial pressure by rostral ventrolateral medulla after depletion of C1 cells in rat. J Physiol 529: 221–236, 2000. doi: 10.1111/j.1469-7793.2000.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR; Study of Osteoporotic Features Research Group . Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 86: 32–38, 2001. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 275.Schweiger JU, Schweiger U, Hüppe M, Kahl KG, Greggersen W, Fassbinder E. Bone density and depressive disorder: a meta-analysis. Brain Behav 6: e00489, 2016. doi: 10.1002/brb3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Schweiger U, Deuschle M, Körner A, Lammers CH, Schmider J, Gotthardt U, Holsboer F, Heuser I. Low lumbar bone mineral density in patients with major depression. Am J Psychiatry 151: 1691–1693, 1994. doi: 10.1176/ajp.151.11.1691. [DOI] [PubMed] [Google Scholar]
- 277.Scutt A, Williamson EM. Cannabinoids stimulate fibroblastic colony formation by bone marrow cells indirectly via CB2 receptors. Calcif Tissue Int 80: 50–59, 2007. doi: 10.1007/s00223-006-0171-7. [DOI] [PubMed] [Google Scholar]
- 278.Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 25: 623–629, 1999. doi: 10.1016/S8756-3282(99)00215-X. [DOI] [PubMed] [Google Scholar]
- 279.Shao P, Ohtsuka-Isoya M, Shinoda H. Circadian rhythms in serum bone markers and their relation to the effect of etidronate in rats. Chronobiol Int 20: 325–336, 2003. doi: 10.1081/CBI-120019343. [DOI] [PubMed] [Google Scholar]
- 280.Sheu YH, Lanteigne A, Stürmer T, Pate V, Azrael D, Miller M. SSRI use and risk of fractures among perimenopausal women without mental disorders. Inj Prev 21: 397–403, 2015. doi: 10.1136/injuryprev-2014-041483. [DOI] [PubMed] [Google Scholar]
- 281.Shi Y, Oury F, Yadav VK, Wess J, Liu XS, Guo XE, Murshed M, Karsenty G. Signaling through the M(3) muscarinic receptor favors bone mass accrual by decreasing sympathetic activity. Cell Metab 11: 231–238, 2010. doi: 10.1016/j.cmet.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers MG Jr, Karsenty G. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci USA 105: 20529–20533, 2008. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Sims NA, Martin TJ. Coupling Signals between the Osteoclast and Osteoblast: How Are Messages Transmitted between These Temporary Visitors to the Bone Surface? Front Endocrinol (Lausanne) 6: 41, 2015. doi: 10.3389/fendo.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sipe LM, Yang C, Ephrem J, Garren E, Hirsh J, Deppmann CD. Differential sympathetic outflow to adipose depots is required for visceral fat loss in response to calorie restriction. Nutr Diabetes 7: e260, 2017. doi: 10.1038/nutd.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Smith SM, Zwart SR, Heer M, Hudson EK, Shackelford L, Morgan JL. Men and women in space: bone loss and kidney stone risk after long-duration spaceflight. J Bone Miner Res 29: 1639–1645, 2014. doi: 10.1002/jbmr.2185. [DOI] [PubMed] [Google Scholar]
- 286.Smitham P, Crossfield L, Hughes G, Goodship A, Blunn G, Chenu C. Low dose of propranolol does not affect rat osteotomy healing and callus strength. J Orthop Res 32: 887–893, 2014. doi: 10.1002/jor.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Song HJ, Lee J, Kim YJ, Jung SY, Kim HJ, Choi NK, Park BJ. β1 selectivity of β-blockers and reduced risk of fractures in elderly hypertension patients. Bone 51: 1008–1015, 2012. doi: 10.1016/j.bone.2012.08.126. [DOI] [PubMed] [Google Scholar]
- 288.Sousa DM, Baldock PA, Enriquez RF, Zhang L, Sainsbury A, Lamghari M, Herzog H. Neuropeptide Y Y1 receptor antagonism increases bone mass in mice. Bone 51: 8–16, 2012. doi: 10.1016/j.bone.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 289.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry 54: 269–282, 2003. doi: 10.1016/S0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 290.St-Pierre S, Jiang W, Roy P, Champigny C, LeBlanc É, Morley BJ, Hao J, Simard AR. Nicotinic Acetylcholine Receptors Modulate Bone Marrow-Derived Pro-Inflammatory Monocyte Production and Survival. PLoS One 11: e0150230, 2016. doi: 10.1371/journal.pone.0150230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Stanton-Hicks M, Jänig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain 63: 127–133, 1995. doi: 10.1016/0304-3959(95)00110-E. [DOI] [PubMed] [Google Scholar]
- 292.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept 92: 73–78, 2000. doi: 10.1016/S0167-0115(00)00152-X. [DOI] [PubMed] [Google Scholar]
- 293.Sternberg Z, Leung C, Sternberg D, Li F, Karmon Y, Chadha K, Levy E. The prevalence of the classical and non-classical cardiovascular risk factors in multiple sclerosis patients. CNS Neurol Disord Drug Targets 12: 104–111, 2013. doi: 10.2174/1871527311312010016. [DOI] [PubMed] [Google Scholar]
- 294.Straube S, Derry S, Moore RA, McQuay HJ. Cervico-thoracic or lumbar sympathectomy for neuropathic pain and complex regional pain syndrome. Cochrane Database Syst Rev (7): CD002918, 2010. doi: 10.1002/14651858.CD002918.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sturm D, Gurevitz SL, Turner A. Multiple sclerosis: a review of the disease and treatment options. Consult Pharm 29: 469–479, 2014. doi: 10.4140/TCP.n.2014.469. [DOI] [PubMed] [Google Scholar]
- 296.Swift JM, Hogan HA, Bloomfield SA. β-1 adrenergic agonist mitigates unloading-induced bone loss by maintaining formation. Med Sci Sports Exerc 45: 1665–1673, 2013. doi: 10.1249/MSS.0b013e31828d39bc. [DOI] [PubMed] [Google Scholar]
- 297.Swift JM, Swift SN, Allen MR, Bloomfield SA. Beta-1 adrenergic agonist treatment mitigates negative changes in cancellous bone microarchitecture and inhibits osteocyte apoptosis during disuse. PLoS One 9: e106904, 2014. doi: 10.1371/journal.pone.0106904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Szabo B, Nordheim U, Niederhoffer N. Effects of cannabinoids on sympathetic and parasympathetic neuroeffector transmission in the rabbit heart. J Pharmacol Exp Ther 297: 819–826, 2001. [PubMed] [Google Scholar]
- 299.Tabarowski Z, Gibson-Berry K, Felten SY. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem 98: 453–457, 1996. doi: 10.1016/S0065-1281(96)80013-4. [DOI] [PubMed] [Google Scholar]
- 300.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 62: 1832–1837, 2002. [PubMed] [Google Scholar]
- 301.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell 111: 305–317, 2002. doi: 10.1016/S0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 302.Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K, Ishii M, Terai K, Moriya M, Nakatsuji Y, Sakoda S, Sato S, Akira S, Takeda K, Inui M, Takai T, Ikawa M, Okabe M, Kumanogoh A, Kikutani H. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol 8: 615–622, 2006. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- 303.Tam J, Ofek O, Fride E, Ledent C, Gabet Y, Müller R, Zimmer A, Mackie K, Mechoulam R, Shohami E, Bab I. Involvement of neuronal cannabinoid receptor CB1 in regulation of bone mass and bone remodeling. Mol Pharmacol 70: 786–792, 2006. doi: 10.1124/mol.106.026435. [DOI] [PubMed] [Google Scholar]
- 304.Tam J, Trembovler V, Di Marzo V, Petrosino S, Leo G, Alexandrovich A, Regev E, Casap N, Shteyer A, Ledent C, Karsak M, Zimmer A, Mechoulam R, Yirmiya R, Shohami E, Bab I. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J 22: 285–294, 2008. doi: 10.1096/fj.06-7957com. [DOI] [PubMed] [Google Scholar]
- 305.Tamimi I, Ojea T, Sanchez-Siles JM, Rojas F, Martin I, Gormaz I, Perez A, Dawid-Milner MS, Mendez L, Tamimi F. Acetylcholinesterase inhibitors and the risk of hip fracture in Alzheimer’s disease patients: a case-control study. J Bone Miner Res 27: 1518–1527, 2012. doi: 10.1002/jbmr.1616. [DOI] [PubMed] [Google Scholar]
- 306.Tanaka H, Tanabe N, Kawato T, Nakai K, Kariya T, Matsumoto S, Zhao N, Motohashi M, Maeno M. Nicotine affects bone resorption and suppresses the expression of cathepsin K, MMP-9 and vacuolar-type H(+)-ATPase d2 and actin organization in osteoclasts. PLoS One 8: e59402, 2013. doi: 10.1371/journal.pone.0059402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tanaka K, Hirai T, Kodama D, Kondo H, Hamamura K, Togari A. α1B-Adrenoceptor signalling regulates bone formation through the up-regulation of CCAAT/enhancer-binding protein δ expression in osteoblasts. Br J Pharmacol 173: 1058–1069, 2016. doi: 10.1111/bph.13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Tanaka K, Nishimura N, Sato M, Kanikowska D, Shimizu Y, Inukai Y, Abe C, Iwata C, Morita H, Iwase S, Sugenoya J. Arterial pressure oscillation and muscle sympathetic nerve activity after 20 days of head-down bed rest. Auton Neurosci 177: 266–270, 2013. doi: 10.1016/j.autneu.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 309.Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet 387: 1240–1250, 2016. doi: 10.1016/S0140-6736(15)00238-X. [DOI] [PubMed] [Google Scholar]
- 310.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140: 1630–1638, 1999. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 311.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 319: 1701–1707, 1988. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 312.Togari A, Arai M, Mizutani S, Mizutani S, Koshihara Y, Nagatsu T. Expression of mRNAs for neuropeptide receptors and beta-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci Lett 233: 125–128, 1997. doi: 10.1016/S0304-3940(97)00649-6. [DOI] [PubMed] [Google Scholar]
- 313.Toledo MA, Junqueira LF Jr. Cardiac autonomic modulation and cognitive status in Alzheimer’s disease. Clin Auton Res 20: 11–17, 2010. doi: 10.1007/s10286-009-0035-0. [DOI] [PubMed] [Google Scholar]
- 314.Tomlinson RE, Li Z, Li Z, Minichiello L, Riddle RC, Venkatesan A, Clemens TL. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc Natl Acad Sci USA 114: E3632–E3641, 2017. doi: 10.1073/pnas.1701054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Tomlinson RE, Li Z, Zhang Q, Goh BC, Li Z, Thorek DLJ, Rajbhandari L, Brushart TM, Minichiello L, Zhou F, Venkatesan A, Clemens TL. NGF-TrkA Signaling by Sensory Nerves Coordinates the Vascularization and Ossification of Developing Endochondral Bone. Cell Reports 16: 2723–2735, 2016. doi: 10.1016/j.celrep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Toscano E, della Casa R, Mardy S, Gaetaniello L, Sadile F, Indo Y, Pignata C, Andria G. Multisystem involvement in congenital insensitivity to pain with anhidrosis (CIPA), a nerve growth factor receptor(Trk A)-related disorder. Neuropediatrics 31: 39–41, 2000. doi: 10.1055/s-2000-15296. [DOI] [PubMed] [Google Scholar]
- 317.Tosun A, Doğru MT, Aydn G, Keleş I, Arslan A, Güneri M, Orkun S, Ebinç H. Does autonomic dysfunction exist in postmenopausal osteoporosis? Am J Phys Med Rehabil 90: 1012–1019, 2011. doi: 10.1097/PHM.0b013e31822dea1a. [DOI] [PubMed] [Google Scholar]
- 318.Toulis KA, Hemming K, Stergianos S, Nirantharakumar K, Bilezikian JP. β-Adrenergic receptor antagonists and fracture risk: a meta-analysis of selectivity, gender, and site-specific effects. Osteoporos Int 25: 121–129, 2014. doi: 10.1007/s00198-013-2498-z. [DOI] [PubMed] [Google Scholar]
- 319.Tsuda K, Nishio I, Masuyama Y. Bone mineral density in women with essential hypertension. Am J Hypertens 14: 704–707, 2001. doi: 10.1016/S0895-7061(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 320.Vahdaninia M, Omidvari S, Montazeri A. What do predict anxiety and depression in breast cancer patients? A follow-up study. Soc Psychiatry Psychiatr Epidemiol 45: 355–361, 2010. doi: 10.1007/s00127-009-0068-7. [DOI] [PubMed] [Google Scholar]
- 321.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 150: 736–744, 2013. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 322.Varenna M, Adami S, Rossini M, Gatti D, Idolazzi L, Zucchi F, Malavolta N, Sinigaglia L. Treatment of complex regional pain syndrome type I with neridronate: a randomized, double-blind, placebo-controlled study. Rheumatology (Oxford) 52: 534–542, 2013. doi: 10.1093/rheumatology/kes312. [DOI] [PubMed] [Google Scholar]
- 323.Varenna M, Zucchi F, Ghiringhelli D, Binelli L, Bevilacqua M, Bettica P, Sinigaglia L. Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol 27: 1477–1483, 2000. [PubMed] [Google Scholar]
- 324.Veldhuis-Vlug AG, El Mahdiui M, Endert E, Heijboer AC, Fliers E, Bisschop PH. Bone resorption is increased in pheochromocytoma patients and normalizes following adrenalectomy. J Clin Endocrinol Metab 97: E2093–E2097, 2012. doi: 10.1210/jc.2012-2823. [DOI] [PubMed] [Google Scholar]
- 325.Veldhuis-Vlug AG, Oei L, Souverein PC, Tanck MW, Rivadeneira F, Zillikens MC, Kamphuisen PW, Maitland-van der Zee AH, de Groot MC, Hofman A, Uitterlinden AG, Fliers E, de Boer A, Bisschop PH. Association of polymorphisms in the beta-2 adrenergic receptor gene with fracture risk and bone mineral density. Osteoporos Int 26: 2019–2027, 2015. doi: 10.1007/s00198-015-3087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Veldhuis-Vlug AG, Tanck MW, Limonard EJ, Endert E, Heijboer AC, Lips P, Fliers E, Bisschop PH. The effects of beta-2 adrenergic agonist and antagonist on human bone metabolism: a randomized controlled trial. Bone 71: 196–200, 2015. doi: 10.1016/j.bone.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 327.Vignaux G, Besnard S, Ndong J, Philoxène B, Denise P, Elefteriou F. Bone remodeling is regulated by inner ear vestibular signals. J Bone Miner Res 28: 2136–2144, 2013. doi: 10.1002/jbmr.1940. [DOI] [PubMed] [Google Scholar]
- 328.Vignaux G, Ndong JD, Perrien DS, Elefteriou F. Inner Ear Vestibular Signals Regulate Bone Remodeling via the Sympathetic Nervous System. J Bone Miner Res 30: 1103–1111, 2015. doi: 10.1002/jbmr.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Visser H. Gait and balance in senile dementia of Alzheimer’s type. Age Ageing 12: 296–301, 1983. doi: 10.1093/ageing/12.4.296. [DOI] [PubMed] [Google Scholar]
- 330.Walker LM, Preston MR, Magnay JL, Thomas PB, El Haj AJ. Nicotinic regulation of c-fos and osteopontin expression in human-derived osteoblast-like cells and human trabecular bone organ culture. Bone 28: 603–608, 2001. doi: 10.1016/S8756-3282(01)00427-6. [DOI] [PubMed] [Google Scholar]
- 331.Wang L, Guo TZ, Wei T, Li WW, Shi X, Clark JD, Kingery WS. Bisphosphonates Inhibit Pain, Bone Loss, and Inflammation in a Rat Tibia Fracture Model of Complex Regional Pain Syndrome. Anesth Analg 123: 1033–1045, 2016. doi: 10.1213/ANE.0000000000001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Weitzmann MN, Pacifici R. T cells: unexpected players in the bone loss induced by estrogen deficiency and in basal bone homeostasis. Ann N Y Acad Sci 1116: 360–375, 2007. doi: 10.1196/annals.1402.068. [DOI] [PubMed] [Google Scholar]
- 333.Welgampola MS, Colebatch JG. Vestibulocollic reflexes: normal values and the effect of age. Clin Neurophysiol 112: 1971–1979, 2001. doi: 10.1016/S1388-2457(01)00645-9. [DOI] [PubMed] [Google Scholar]
- 334.Weller I, Schatzker J. Hip fractures and Alzheimer’s disease in elderly institutionalized Canadians. Ann Epidemiol 14: 319–324, 2004. doi: 10.1016/j.annepidem.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 335.Wiens M, Etminan M, Gill SS, Takkouche B. Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. J Intern Med 260: 350–362, 2006. doi: 10.1111/j.1365-2796.2006.01695.x. [DOI] [PubMed] [Google Scholar]
- 336.Wu Q, Liu J, Gallegos-Orozco JF, Hentz JG. Depression, fracture risk, and bone loss: a meta-analysis of cohort studies. Osteoporos Int 21: 1627–1635, 2010. doi: 10.1007/s00198-010-1181-x. [DOI] [PubMed] [Google Scholar]
- 337.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med 17: 1235–1241, 2011. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138: 976–989, 2009. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Yamada T, Ezura Y, Hayata T, Moriya S, Shirakawa J, Notomi T, Arayal S, Kawasaki M, Izu Y, Harada K, Noda M. β2 adrenergic receptor activation suppresses bone morphogenetic protein (BMP)-induced alkaline phosphatase expression in osteoblast-like MC3T3E1 cells. J Cell Biochem 116: 1144–1152, 2015. doi: 10.1002/jcb.25071. [DOI] [PubMed] [Google Scholar]
- 340.Yang AC, Tsai SJ, Yang CH, Kuo CH, Chen TJ, Hong CJ. Reduced physiologic complexity is associated with poor sleep in patients with major depression and primary insomnia. J Affect Disord 131: 179–185, 2011. doi: 10.1016/j.jad.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 341.Yang S, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Association between beta-blocker use and fracture risk: the Dubbo Osteoporosis Epidemiology Study. Bone 48: 451–455, 2011. doi: 10.1016/j.bone.2010.10.170. [DOI] [PubMed] [Google Scholar]
- 342.Yang S, Nguyen ND, Eisman JA, Nguyen TV. Association between beta-blockers and fracture risk: a Bayesian meta-analysis. Bone 51: 969–974, 2012. doi: 10.1016/j.bone.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 343.Yates BJ, Siniaia MS, Miller AD. Descending pathways necessary for vestibular influences on sympathetic and inspiratory outflow. Am J Physiol Regul Integr Comp Physiol 268: R1381–R1385, 1995. [DOI] [PubMed] [Google Scholar]
- 344.Yazici KM, Akinci A, Sütçü A, Ozçakar L. Bone mineral density in premenopausal women with major depressive disorder. Psychiatry Res 117: 271–275, 2003. doi: 10.1016/S0165-1781(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 345.Yirmiya R, Bab I. Major depression is a risk factor for low bone mineral density: a meta-analysis. Biol Psychiatry 66: 423–432, 2009. doi: 10.1016/j.biopsych.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 346.Yirmiya R, Goshen I, Bajayo A, Kreisel T, Feldman S, Tam J, Trembovler V, Csernus V, Shohami E, Bab I. Depression induces bone loss through stimulation of the sympathetic nervous system. Proc Natl Acad Sci USA 103: 16876–16881, 2006. doi: 10.1073/pnas.0604234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord 169: 15–20, 2014. doi: 10.1016/j.jad.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 348.Yuhara S, Kasagi S, Inoue A, Otsuka E, Hirose S, Hagiwara H. Effects of nicotine on cultured cells suggest that it can influence the formation and resorption of bone. Eur J Pharmacol 383: 387–393, 1999. doi: 10.1016/S0014-2999(99)00551-8. [DOI] [PubMed] [Google Scholar]
- 349.Zaidi M, Fuller K, Bevis PJ, GainesDas RE, Chambers TJ, MacIntyre I. Calcitonin gene-related peptide inhibits osteoclastic bone resorption: a comparative study. Calcif Tissue Int 40: 149–154, 1987. doi: 10.1007/BF02555699. [DOI] [PubMed] [Google Scholar]
- 350.Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature 452: 997–1001, 2008. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]