Abstract
Nitrogen is fundamental to all of life and to many industrial processes. Nitrogen in its various oxidation states comprises the global nitrogen cycle, with the change between forms being redox reactions involving electrons and protons. The interchange of nitrogen oxidation states constitutes some of the most important industrial processes, with the energy for these processes being provided largely by fossil fuel. A key goal of research in the field of nitrogen chemistry is to minimize the use of fossil fuels by developing more efficient heterogeneous, homogeneous, or biological catalysts, or by inventing new energy-efficient processes that rely on catalysts. These approaches, as well as the challenges involved, are discussed in this review.
One-Sentence Summary
This review article reports on the current state of the field of nitrogen activation chemistry and discusses future directions.
Reduced forms of nitrogen, particularly ammonia (NH3), are vital to life. Prior to the early 1900’s, all reduced N came from biological nitrogen fixation occurring in microbes. This landscape changed in the early 1900’s with the invention of an industrial process to reduce N2 to NH3 (N2 + 3H2 ⇌ 2NH3) by Haber and Bosch (H-B). The H-B process, in turn, has led to fundamental changes in the way food is produced, and its impact is underscored by the fact that about 50% of the N atoms in humans today originate from the H-B process (1). For these reasons and others, the H-B process has been called the most impactful invention of the 20th century (2). The H-B process is, however, energy intensive, consuming 1–2% of the world’s annual energy output (3). The main energy requirements arise from the high reaction temperatures (~700 K) and pressures (~100 atm), and, most importantly, from the need for large quantities of H2. Nevertheless, at scale H-B is surprisingly energy efficient. In addition to the high energy demand, however, there is a second problem with H-B that is potentially more serious. Specifically, the source of H2 is usually natural gas, (4) and consequently H-B generates about 1.9 metric tons of CO2 per metric ton of NH3 produced (3 CO2 per 8 NH3) (5).
Nitrogen in its more oxidized forms is also industrially, biologically, and environmentally important. Oxidation of N2 to nitric oxide (NO) and nitrogen dioxide (NO2), which are collectively referred to as NOx (the representation of neutral forms of oxidized nitrogen), occurs naturally in lightning and also during combustion of fuels in air (6). In fact, plasma-driven oxidation of N2 to NO (the Birkeland-Eyde, or B-E, process) preceded H-B as the first commercial nitrogen fixation process (7). However, B-E is not economically competitive with the H-B process, and as a result the demand for oxidized nitrogen, primarily in the form of nitric acid (HNO3), is satisfied by catalytic oxidation of H-B-generated NH3 at elevated temperatures via the Ostwald process (8).
Nitrogen oxides (NOy, which represents both neutral and anionic forms of oxidized nitrogen) are also produced on a large scale by bacteria. These processes include oxidation of NH3 to nitrate (NO3-) by nitrifying bacteria and reduction of NO3- to nitrite (NO2-), NO, nitrous oxide (N2O), and ultimately to N2 by denitrifying bacteria. The conversion of NH3 and NO3- to N2 by the action of these bacteria is used in waste-water treatment and is the primary pathway for loss of applied fertilizers in agriculture. Nitrogen oxides are common environmental pollutants, and thus catalysts to reduce these oxides to N2 are also of considerable practical importance. For example, NOx generated during combustion is a primary component of photochemical smog (9). The development of active catalysts for reducing NOx to N2 (the TWC, or three-way catalytic converter) led to remarkable improvements in air quality in the late 1970’s (10), and there are now TWCs operating on hundreds of millions of vehicles worldwide. However, increasingly stringent emissions regulations coupled with the low exhaust temperatures from high efficiency engines present challenges for future combustion emissions control (11). The soluble forms of the nitrogen oxides, primarily NO2- and NO3- 2 3 , are similarly hazardous to human health and contribute to the eutrophication of waterways (12). Sustainable approaches for catalytically converting these pollutants to N2 remain an unmet challenge (13).
In the present article, we report on the results of a U. S. Department of Energy workshop held in October, 2016 that focused on the challenges and opportunities associated with fundamental aspects of nitrogen chemistry. We begin by discussing the thermochemistry of nitrogen transformations, and then focus on the reduced forms of nitrogen, primarily NH3, followed by the oxidized forms.
Thermochemistry of nitrogen fixation
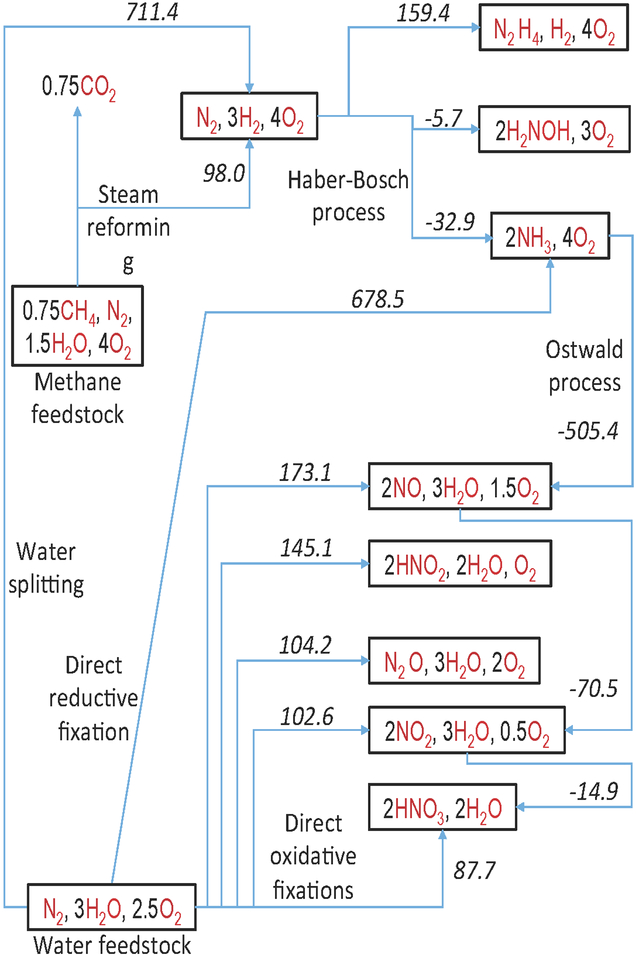
The energy expenditures for various reductive and oxidative N2 fixation pathways are compared in Figure 1 (14–16). Hydrocarbons, including biomass and fossil fuels (particularly methane), and water are the only two sources of hydrogen atoms that can sustain long-term, large-scale NH3 production. The use of water as the hydrogen source for NH3 production requires substantially more energy than methane (by 613.4 kJ/mol N2), but it is also more environmentally benign, does not contribute to the accumulation of greenhouse gases, and does not compete for valuable and limited hydrocarbon resources. However, these advantages can be fully realized only if the energy comes from a clean, renewable source such as the sun or wind.
Figure 1.
Atom and energy economy of nitrogen fixation. The numerical values represent standard (for gaseous reactants and products and liquid H2O, as defined in ref. 14) Gibbs free energies in kJ/mol of fixed N2 in the direction of the arrows. All thermochemical data are from ref. 15, except for H2NOH (ref. 16).
When water is used as the feedstock, there is only a small (32.9 kJ/mol N2) thermodynamic advantage for the direct synthesis of NH3 compared to a two-stage synthesis involving water splitting followed by H-B. The overall energy saving may, however, become more significant if the H-B process is avoided altogether and direct reductive N2 fixation is conducted under milder conditions.
The two most important N-containing commodity chemicals are NH3 and HNO3. In 2017, their worldwide production was estimated to be 150 (17) and 50 (18) million metric tons , respectively. Presently, nearly all HNO3 is manufactured using a three-stage process: steam reforming of methane, H-B, and then the Ostwald process (Figure 1), but this approach is energetically wasteful compared to direct oxidative N2 fixation.
An attractive alternative route to HNO3, which avoids the NH3 intermediate, is direct N2 oxidation to aqueous HNO3 (eq 1).
| (1) |
The equilibrium constant for this reaction is ~2.7×10−3 M4/bar3.5 (ΔG0 = 14.6 kJ/mol N2) (14–16, 19), which is large enough to drive spontaneous formation of ~0.1 M HNO3 in any pool of water on the surface of the earth. Moreover, if equilibrium were to be achieved between the earth’s atmosphere and the world’s oceans, the oceans would contain ~0.02 M HNO3 and ~90% of atmospheric O2 would be consumed. Fortunately for life on Earth, under ordinary conditions and in the absence of a catalyst, eq 1 is immeasurably slow in both directions.
Reduction of N2 to NH3
Overview.
As discussed in the previous two sections, the most important N2 reduction reaction is NH3 synthesis, specifically N2 + 3H2 ⇌ 2NH3. Given that this reaction is slightly thermodynamically favorable under ambient conditions, major effort has been devoted to developing alternative and environmentally friendly processes that would allow NH3 to be produced at distributed sources under more benign conditions rather than the large-scale centralized H-B process. This goal is particularly important in developing countries where the population, and hence the need for food, is increasing rapidly. In these countries, access to fertilizer is hampered by poor transportation infrastructure and insufficient capital to build large chemical plants. For example, there are no large-scale NH3 production facilities in all of East Africa. To address this need, there has been substantial effort expended to understand N2 reduction using a variety of catalysts; including: heterogeneous, enzymes, and homogeneous catalysts, and electro- and photocatalysts.
Heterogeneous catalysis.
The commercial H-B process is carried out using a heterogeneous catalyst based on iron and promoted with Al2O3 and potassium. Typically, 2500 tons of NH3 per day are produced in a single plant where natural gas and water are available in large quantities. It is now well accepted that the relative activity of metallic catalysts can be correlated to their binding energies with N-containing species in terms of a volcano-shaped relationship. Metals that bind nitrogen too strongly or too weakly are on either side of the volcano (20). For those having low binding energies, N2 dissociation is rate-limiting. For those metals exhibiting high binding energies, N2 dissociation occurs but desorption of the resulting atomic N (and other N-containing intermediates) is slow, limiting the number of available binding sites and thereby slowing the catalytic rate (21).
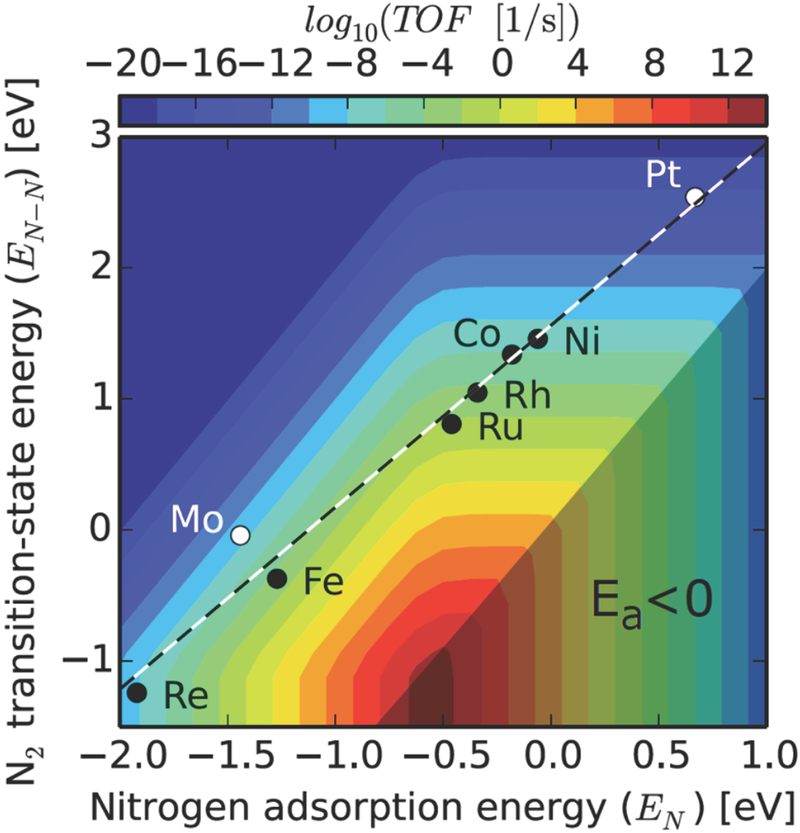
Recent calculations suggest that the energies of all intermediates and transition states involved in a conventional NH3 synthesis reaction network scale with the N binding energy. As shown in Figure 2, these correlations limit the rate of NH3 synthesis on transition metals to much lower values than would be possible on a material with both a low N2 dissociation barrier and more moderate intermediate binding energies (22). Clearly, an important challenge is to break this type of scaling relationship, which should lead to the development of catalysts yielding reaction rates that are potentially orders of magnitude higher than the current state of the art.
Figure 2.
Linear scaling between nitrogen binding energy and activation barrier for N2 dissociation. TOF = turnover frequency (from ref. 22).
Advances in the synthesis, characterization, and modeling of non-traditional heterogeneous catalysts, such as intermetallics, alloys containing a single heteroelement, and shape-controlled materials, whose discovery might be driven by computational models, offer the promise for more active catalysts. These combined efforts should lead to catalysts that could potentially operate at lower temperatures and thus reduce the operating pressures of the H-B process. Another opportunity to reduce the CO2 footprint of the H-B process is to replace the methane reformer with a water-electrolyzer to provide CO2-free H2, but this would require cost-effective, large-scale electrolyzers.
Enzyme catalysis.
A range of microbes (bacteria and archaea) living in all major ecosystems are able to reduce N2 to NH3 using the enzyme nitrogenase. Nitrogen fixation by these microbes contributes to global supply of NH3, but it is insufficient to support modern intensive agriculture food production. Exciting frontier areas for the use of microbial N2 fixation include engineering the nitrogen fixation genes into eukaryotes (23), such as plants, and using nitrogen-fixing microbes in microbial electrocatalysis cells that permit sustained N2 reduction to NH3 (24). Such systems offer the promise of providing localized solutions to N2 reduction, but are not likely to displace the need for large-scale N2 reduction in the near term.
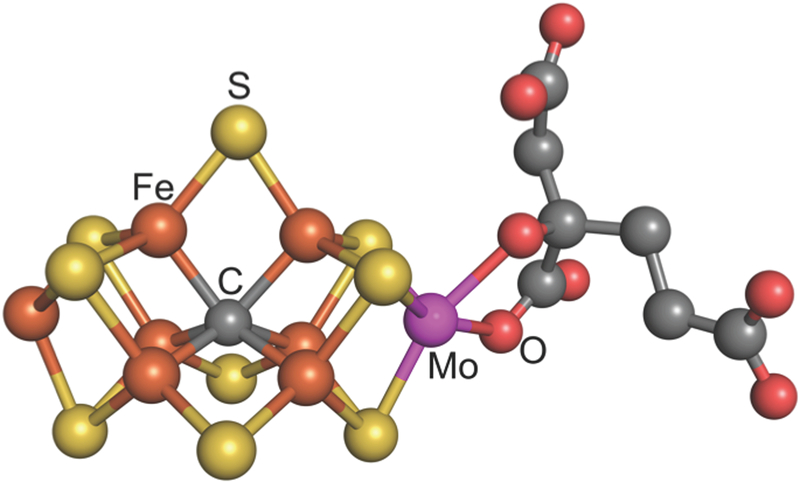
One important lesson that can be learned from biological nitrogen fixation is understanding how the enzyme nitrogenase accomplishes reduction of N2 to NH3 at low temperatures and pressures and without H2. The first nitrogenase to be purified (and the most widely distributed) is Mo-dependent and, as shown in Figure 3, has a metal-cluster active site containing Mo, Fe, S, C, and homocitrate (FeMo-cofactor).
Figure 3.
FeMo-cofactor with Fe in rust, S in yellow, C in gray, Mo in magenta, and O in red. Made from PDB: 1M1N
An important step toward mechanistic understanding of nitrogenase emerged from trapping an intermediate with four H+ and four e- accumulated immediately prior to binding N2. Characterization of this intermediate (termed the E4 state) has revealed the presence of bridging hydrides (Fe-H-Fe). The E4 state undergoes reductive elimination of the two hydrides with release of H2, with concomitant activation of the metal core by two e- for binding and reduction of N2 to a metal-bound diazenido species (HN=N-Fe). Although these studies have provided insights into the central catalytic step, which is the cleavage of the N2 triple bond, many challenges remain before the entire mechanism can be elucidated. These include a molecular level understanding of both the early and late stages of the N2 reduction pathways, an understanding of the roles of ATP hydrolysis in driving electron transfer, and then reconciliation of the resulting empirical mechanism with computational studies.
These foregoing challenges primarily involve substrate moieties bound as catalytic intermediates. In parallel, important questions remain about the FeMo-cofactor active site of Mo-dependent nitrogenase. For example, what changes in redox and spin states of the seven Fe centers and the Mo center accompany the stepwise transformations of substrates? Additionally, the FeMo-cofactor contains the first example of a carbide (C4–) in biology. The significance and function of this carbide with respect to mechanism are unknown. Understanding the structure of FeMo-cofactor intermediates and synthetic analogues could provide important clues to guide the design of new homogeneous and heterogeneous catalysts for N2 reduction (25). Biomimetic approaches could take advantage of not only the coordination chemistry in the FeMo-cofactor, but also the cooperative interactions with nearby acidic amino acid residues in the nitrogenase protein.
Electrochemical techniques offer a powerful alternative method of delivering electrons to nitrogenase without a requirement for ATP hydrolysis, and they also offer new avenues for addressing aspects of the nitrogenase mechanism. Electron transfer from an electrode, previously demonstrated for a number of redox enzymes, has been applied to nitrogenase but only via mediators (i.e., no direct electron transfer) (26–28). As illustrated in a recent study, this approach, in combination with computations (29), provides insights into catalytic nitrogen transformations.
A related frontier area is using nanomaterials to deliver electrons to nitrogenase. For example, it has been recently demonstrated that a CdS nanorod-nitrogenase MoFe protein hybrid achieves light-dependent N2 reduction to NH3 using a sacrificial electron donor (30). Such systems offer a means to use light as the exclusive energy source to drive N2 reduction.
Homogeneous catalysis.
Synthetic homogeneous catalysts designed for N2 reduction provide well-defined molecular pre-catalysts and intermediates, which can be thoroughly characterized by diverse spectroscopic techniques and thereby provide excellent opportunities for determining mechanistic information about N2 reduction to NH3. The reactivity of synthetic inorganic complexes provides insights into molecular reactivity and individual bond-making processes that occur in nitrogenases. The discovery of nitrogenase, along with a transition metal compound that contains N2 bound to ruthenium (31), initiated a race to prepare NH3 catalytically under mild conditions via a transition metal complex. Indeed, entire institutes were created in England and Russia in the late 1960s for this purpose. The stoichiometric N2-to-NH3 conversion using H2SO4 was demonstrated using low-valent Mo-N2 and W-N2 complexes in the 1970s (32); typically the electrons required came from the metal complex. In 1985, the synthesis of NH3 was accomplished by Pickett and Talarmin using an electrochemical route starting from the tungsten complex [W(N2)2(PMe2Ph)4], with the electrons supplied by the electrode and protons from an added acid (33). The catalytic reduction of N2 to NH3 by H+/e- was reported in subsequent studies, using, for example, a well-defined Mo-containing catalyst (34). Up to eight equivalents of NH3 per Mo were formed, along with H2. Both experiments and calculations support a mechanism involving the addition of 6H+ and 6e- to the N2 initially bound to a Mo(III) center. Altogether, eight of these proposed intermediates were prepared and characterized.
Additional Mo pre-catalysts for reduction of N2 using chemical reductants and an acid, a dimolybdenum–dinitrogen complex (35) and a molybdenum nitride (36), were subsequently reported in 2011 and 2015, respectively. In 2013, Fe complexes were reported that catalyze reduction of N2 to NH3 using a strong reducing agent (KC8) and a strong acid at 200 K (37); improvements to the Fe system recently produced as many as 84 equivalents of NH3 per Fe (38). These Fe complexes were designed to reflect the trigonal symmetry of the Fe in the catalytic face of FeMo-cofactor (Figure 3) and to allow variation of an axial ligand, so as to explore tuning of N2 binding/reduction by the unusual carbide at the center of the FeMo-cofactor.
In all these cases it appears that catalytic N2 reduction is a 6-electron process, involving many intermediate chemical species [M-N xHyn+], but unlike nitrogenase without a mechanistic requirement for production of H2. Nonetheless, in all cases N2 reduction is accompanied by production of H2, and at least with the Fe complexes, this has been shown to involve a catalytic process, with H+ reduction competing with N2 reduction.
Much work remains to move this field forward. For example, rational design of potential catalysts will be guided by accurate determination of the bond dissociation free energies (BDFEs) of N-H bonds, including likely intermediates such as M-N=NH, M=N-NH2, and M=N-NH3. Initial experimental and theoretical estimates have shown that N-H BDFEs of coordinated NH3 can vary significantly and demonstrate the importance of metal identity and its oxidation state as well as the possibility of ligand control to tune the stability of potential intermediates. In addition, determination of the energies of these bonds will allow evaluation of energetic requirements of the individual chemical steps that ultimately lead to NH3. It is also important to identify features that tune the reduction potentials of relevant complexes to ensure that the multiple reductions required for catalysis are energetically feasible. Thus, research should focus on exploring complexes designed to simultaneously promote N2 binding and multiple reductions at the metal site.
Electrocatalysis and photocatalysis.
The potential-pH predominance (Pourbaix) diagram for the N2-H2O system shown in Figure 4 reveals that reduction of N2 to NH4+/NH3 (eq 2 and the N2 reduction line in Figure 4) is thermodynamically possible under moderately reducing conditions.
| (2) |
Moreover, with a sufficiently active electrocatalyst, the N2 reduction reaction can, in principle, proceed in a narrow range of negative potentials without interference from water reduction (line a in Figure 4) over the entire pH range. However, at potentials below line a, an extremely selective electrocatalyst would be required to suppress parasitic water reduction. Note that the nitrogen reduction reaction could be paired with a water oxidation half-reaction to supply the H+ and e- required for a complete electrolysis cell. Such a cell would essentially combine water splitting with NH3 formation as depicted by the direct reductive fixation route in Figure 1. Despite the inviting prospect of a scalable NH3 electrolysis cell running on renewable electricity at or near room temperature, the difficulty of selectively catalyzing the nitrogen reduction reaction has prevented development of an efficient electrochemical system.
Figure 4.
Partial Pourbaix diagram for the N2-H2O system showing lines corresponding to N2 reduction to NH4+/NH3 and N2 oxidation to NO3− . Lines a and b straddle the region of water stability (reduction to H2 and oxidation to O2, respectively). See ref. 14 for details and refs. 15 and 19 for primary data.
At present, only a few examples of pairing the nitrogen reduction reaction with water oxidation at ambient temperature and pressure have been reported, and in all cases, due to competing H2 evolution, the faradaic efficiencies for NH3 are so low that even its reliable detection is challenging (39, 40). For example, an efficiency of 0.83% and a production rate of 9.4 × 10−10 mol-NH3 cm−2 s−1 were measured at 353 K at an applied cell voltage of 1.2 V using Pt electrodes and a Nafion electrolyte (41). Another electrochemical approach involved an electrochemical cycling process for producing NH3 (42). The cycling included separate steps of LiOH electrolysis, Li nitridation, and Li3N hydrolysis, thereby circumventing the parasitic H2 evolution reaction and leading to higher faradaic efficiencies for NH3.
Proton-conducting ceramic oxides have been explored as electrodes for nitrogen reduction, and when they operate at higher temperatures both the rate and faradaic efficiency increase (43). For example, efficiencies in excess of 50% and rates in the range of 10−9-10−8 mol-NH3 cm−2 s−1 have been attained above 673 K using oxide or molten chloride electrolytes and a variety of metal or metal oxide cathodes (43, 44). Note, however, that higher temperatures introduce an additional energy burden and hence compromise the advantage of this approach compared to H-B.
Photochemical reduction of N2 to NH3 has also been reported, and there may be guidance in these studies relevant to electrochemistry (45, 46). Photocatalytic routes to NH3 have been summarized recently (47). One of the biggest challenges for photocatalytic NH3 synthesis is the discovery of active and stable photoelectrode materials responsive to visible light. Synthetic nitrogenase mimics in the form of chalcogels, composed of Mo and Fe-containing biomimetic clusters, can potentially accomplish photocatalytic N2 fixation and conversion to NH3 at ambient temperature and pressure. Both Fe–S and Mo-Fe–S chalcogels have displayed promising activity toward N2 reduction (48).
Plasma-driven transformations.
The feasibility of using plasmas consisting of ions, electrons, and excited molecular species to drive chemical reactions that are thermally inaccessible has been known for many years (7). For example, NH3 was synthesized in laboratory-scale, non-thermal N2 + H2 plasmas at temperatures and pressures lower than those used in H-B as early as the 1970s, and more recently the synthesis has been demonstrated under much milder conditions (1 atm and 140°C) (49). The plasma synthesis of NH3 has been carried out both homogeneously and in combination with heterogeneous catalysts (7, 49, 50).
Several mechanisms have been proposed to account for enhancements in catalytic activity in the presence of non-thermal plasmas (51–53). However, the fundamental science and practical engineering of plasma-driven NH3 syntheses lag behind thermal and electrochemical routes and are therefore ripe for additional research. Further progress in this field will draw on the insights gained from traditional heterogeneous and electrochemical reductions.
Chemical looping.
Chemical looping is perhaps most familiar from combustion where two connected fluidized beds are used to cycle solid particles between an oxidized and a reduced state to combust a carbon-containing fuel with oxygen (54). Chemical looping could also be performed with two fixed beds instead of fluidized beds. Nitrogen can be used instead of oxygen for a similar looping approach in NH3 synthesis (55). First, N2 is contacted with a suitable solid-state transition metal to yield a nitride (activation). In the second step, NH3 is harvested by contacting the nitride with steam or H2 (56). Recently, lithium has been employed as the parent metal for the nitride, but this approach requires liquid-phase electrolysis to recycle lithium and this step is usually not present in chemical looping (42).
Potential advantages of chemical looping include the ability to independently control process conditions for N2 activation and product harvest. In this sense chemical looping enables breaking the scaling relationship alluded to in Figure 2. Operation at atmospheric pressure is another major advantage of chemical looping for nitrogen activation (57). Based on results from a pilot-scale plant, chemical looping for combustion, including CO2 capture, has been compared to conventional combustion and appears to be economically attractive (58). This may be an indication of the possible economic feasibility of chemical looping for nitrogen activation.
Oxidation of N2
Overview.
The dominance of the H-B process in commercial N2 fixation is arguably a direct consequence of the ready availability of H2 from fossil hydrocarbons. As shown in Figure 1, oxidative fixation of N2 can, in principle, be achieved with a lower energy input than reductive fixation if N2, H2O, and O2 are used as reactants. Other nitrogen oxides are accessible at even lower free energies, especially when coupled with water to form the corresponding oxoacids.
Although H-B and natural processes have motivated substantial research into N2 reductive fixation, direct N2 oxidation remains largely unexplored despite its great practical value as a replacement for the Ostwald process. The fact that living organisms have not evolved to consume N2 and O2 and produce aqueous HNO3 suggests fundamental chemical challenges that merit investigation. As a result, there is very little literature on the homogeneous oxidative chemistry of N2.
Direct oxidation of N2 with O2.
NOx is unavoidably generated at the high temperatures that prevail during combustion in air. NO is the primary combustion-generated component of NOx, and its formation can be viewed in the context of its equilibrium with N2 and O2 (eq 3).
| (3) |
The standard enthalpy of this reaction is 90.3 kJ/mol NO and the entropy is 12.4 J/(mol NO K) (14, 15), indicating that equilibrium is unfavorable for NO production under ambient conditions. Due to the positive entropy, however, the equilibrium NO concentration rises rapidly with temperature, exceeding 100 ppm above 2000 K (59).
The direct bimolecular reaction of N2 and O2 to produce NO is symmetry-forbidden and has a negligible rate even at combustion temperatures. Rather, NO is produced during combustion via eqs 4 and 5, where O (and similarly OH) radicals attack N2, as originally proposed by Zeldovich (60).
| (4) |
| (5) |
Analogous reactions are at play in high-temperature thermal plasmas, as in the original B-E arc process. Thermal plasmas are unlikely to be energy-competitive with the H-B process, even if the latter uses a renewable H2 source (61), because the overall energy efficiencies of N2 activation are low and rapid thermal quenching is required to suppress NO decomposition back to N2 and O2.
The aforementioned fundamental limitations can be mitigated using low-temperature, non-thermal plasmas generated by either a dielectric barrier discharge or microwave absorption at or below atmospheric pressure, potentially coupled with appropriate catalysts (7). According to current estimates, theoretical energy consumption for N2 fixation via eq 3 in a non-thermal plasma (~400 kJ/mol N2) is more than 2.5 times lower than that for the H-B process with methane-derived H2 (61–63), and the energy efficiencies already attained in the laboratory (600–1200 kJ/mol N2, assuming 100% efficient plasma generation (62, 63)) are better than the H-B process using H2 from water electrolysis (~3000 kJ/mol N2 (61)), 40% of which would go to electrolysis (64). Experiments and kinetic models show that the primary effect of the nonthermal plasma is to accelerate Zeldovich-like reactions involving athermal, vibrationally excited N2. Practical implementation of non-thermal plasmas is hampered by their relatively low power per unit reactant mass and thus low throughput.
A thermal, heterogeneous N2 oxidation catalyst would have to activate N2 and O2 and be stable at the extreme temperatures at which NO production becomes thermodynamically favored. Because of these stringent demands, non-thermal electro-, photo-, or plasma-assisted oxidation routes likely offer the greatest opportunities for progress. The development of catalytic systems that can effectively couple with these external energy sources will be key to practical advances. Preliminary results with non-thermal plasmas, especially coupled to catalysts, are promising. There is a pressing need to uncover the fundamental mechanisms that couple plasma chemistry, surface chemistry, catalysts, and theory to guide optimization of plasma and rational development of appropriate catalytic materials.
Electrochemical oxidation of N2 to HNO3.
The oxidation line in Figure 4 shows that N2 is electrochemically unstable toward NO3- under moderately oxidizing conditions (eq 6).
| (6) |
At pH > 1.3, this ten-electron reaction is more thermodynamically favorable than the parasitic four-electron water oxidation to O2. Thus, it is possible for NO3- to be the only product of an anodic process, particularly in neutral and alkaline solutions, if a sufficiently active and selective electrocatalyst for eq 6 can be discovered. The corresponding cell-completing cathodic reaction can be either water reduction to H2 or O2 reduction to water. No progress has yet been made in developing such catalysts, but there is no reason to believe that they cannot eventually be discovered given the necessary resources.
By coupling cathodic N2 reduction (eq 2) with anodic N2 oxidation (eq 6), an electrochemical cell consuming only atmospheric N2 and water as feedstock to yield aqueous NH4NO3 (eq 7) can be envisioned.
| (7) |
The additional H2 production is necessary for matching cathodic and anodic currents and maintaining the electrolyte pH constant. Such a cell would require a minimum of 1.08 V to operate in neutral or alkaline electrolyte, which is slightly lower than the minimum water electrolysis voltage of 1.23 V. Advances relating to electrochemical N2 oxidation will require development of catalysts that are sufficiently active (capable of good current densities at low overpotentials), selective (with respect to water oxidation), and stable (toward deactivation under harsh redox and pH operating conditions).
NOy Reduction and NH3 Oxidation
Overview.
The redox chemistry of forms of nitrogen other than N2 is immense and rich, and it is extensively incorporated into biological systems. Both NOy and NH3 are environmentally hazardous pollutants generated by industrial and transportation activity, and thus extensive research has gone into developing and applying NOy reduction and NH3 oxidation catalysts. As is often the case, the understanding of these catalysts lags their applications
Ammonia oxidation and NOy reduction are critical to environmental protection and are much less well understood or optimized than the synthesis of NH3. There are appealing opportunities to apply the experimental and computational tools of modern heterogeneous catalysis to these reactions, both to develop a fundamental understanding of catalytic processes and to identify superior materials and catalytic processes; for example, catalysts that can selectively reduce NOy to N2 using hydrocarbon reductants. Environmental applications generally demand high longevity, durability, and tolerance to poisons. Key gaps in understanding include how activity and selectivity can be achieved in relatively cool gas streams, how catalyst structure evolves over long periods of time, how sulfur and other common poisons interfere with reactions, and especially how these effects can be mitigated.
NOy reduction.
NO decomposition, the reverse of eq 3, is thermodynamically downhill but difficult to catalyze under the oxygen-rich conditions of interest for environmental protection (65). Nevertheless, NO decomposition has been demonstrated using both homogeneous and heterogeneous catalysts. Decomposition appears to involve a two-electron reduction of two NO molecules to a hyponitrite (eq 8).
| (8a) |
| (8b) |
| (8c) |
Coordination complexes of hyponitrites are not uncommon, and a tri-Cu hyponitrite coordination complex has been isolated as an intermediate in the catalytic decomposition of NO to N2 (66). A Cu dimer is similarly implicated as the active site in a Cu-exchanged zeolite catalyst for NO decomposition (67).
NOx can be catalytically reduced to N2 via several routes. Precious metals are active for NOx reduction by CO and hydrocarbons, but only under conditions in which O2 concentrations are low (10). Vanadia/titania and metal-exchanged zeolites catalyze selective reduction of NOx by NH3 (68). Nitrosamine appears to be the key N2-forming intermediate in the catalytic pathway (eq 9).
| (9a) |
| (9b) |
This N2-forming reaction is exothermic, implying significant, but perhaps not insurmountable, kinetic challenges to reversing the chemistry.
NOx can also be catalytically reduced with H2 over Pd and Pt catalysts (13), and N2 is typically the desired product (65). By suitable selection of promoters and control of the NO/H2 ratio, however, NH3 can be produced over Pt catalysts (69). Likewise, NH3 competes with N2 as the product of the catalytic reduction of NO2- and NO3- by H2 over Pd catalysts (13).
Bacteria analogously reduce NO3- and NO2- through a series of gaseous intermediates leading ultimately to N2 in a process called denitrification (eq 10). In these bacteria, NOy acts as a terminal electron acceptor in an anaerobic respiration process. Electrons flowing through membrane-bound proteins produces a proton gradient that in turn is used to make ATP. Each step in the denitrification process is catalyzed by well-studied metalloenzymes that use some rather unusual metal clusters to achieve their chemistry (70, 71). From a global nitrogen perspective, denitrification by these bacteria functions to convert fixed forms of N (NOy) into N2. In some cases, this process is beneficial, such as during waste-water treatment to remove nitrates. In other cases, such as in agriculture, denitrification results in loss of N that was applied to crops as fertilizer.
| (10) |
NH3 oxidation.
Heterogeneous NH3 oxidation with O2 is at the heart of the Ostwald process and is practiced in a variety of environmental protection applications. Precious metals remain the workhorse catalysts, and therefore opportunities exist to develop lower-cost materials having equivalent or better activity and selectivity. The preferred NH3 oxidation products shift from N2 at low temperatures to NO at higher temperatures, with N2O being a minor but highly undesirable product. At present, a full mechanistic understanding of this process and of the kinetic origins of selectivity is not available.
NH3 and/or NO2– are fuel for bacteria and archaea that employ multi-electron oxidative reactions to generate reducing equivalents for cellular respiration and to establish a proton gradient for ATP synthesis (72, 73). Ammonia-oxidizing bacteria (AOB) and archaea (AOA) stoichiometrically convert NH3 to NO2–. AOA and AOB first carry out the selective conversion of NH3 to hydroxylamine (NH2OH) using an integral membrane Cu monooxygenase called ammonia monooxygenase (AMO) (eq 11). Active AMO has never been purified, and thus this challenging transformation awaits mechanistic elucidation.
| (11) |
The NH2OH generated by AMO is oxidized to provide a net electron flow for cellular respiration. How AOA processes NH2OH is entirely unknown (74). In AOB, this process is carried out by hydroxylamine oxidoreductase (HAO), which contains a rare, covalently modified c-heme, heme P460, to which NH2OH binds at Fe and undergoes proton-coupled oxidation (72). Recent experiments strongly support NO as the enzymatic product of HAO reactivity, contrary to decades of dogma asserting NO2– as the enzymatic product (75). Assuming a 2-electron turnover of AMO, the 3-electron oxidation of NH2OH by HAO furnishes one net electron for cellular respiration. Stoichiometric production of NO2– from NH3 by AOB likely involves an additional, but as-yet unidentified, third enzyme in the pathway. Cytotoxicity of both NO and NH2OH may be managed by cytochrome P460, a monoheme enzyme that selectively produces N2O from the reaction of NH2OH with Fe-bound NO (76). NO2– is itself cellular fuel for nitrite-oxidizing bacteria (NOB) that effect the 2-electron oxidation of NO2– to NO3– and for recently discovered “complete ammonia oxidation” (COMAMMOX) bacteria that effect the 8-electron oxidation of (77).
Opportunity abounds to extract broadly applicable insights through the study of the oxidative enzymes operative in nitrification. Establishing the mechanism of NH3 oxidation by AMO remains a grand challenge whose difficulty is exacerbated by the integral membrane nature of the enzyme and the sluggish growth of its host organisms. The end goal is tantalizing technology: selective hydroxylation of relatively inert bonds generalizable beyond N-species, especially considering that enzymes related to AMO, such as particulate methane monooxygenase, effect similar transformations of unactivated alkanes (78).
Mechanistic understanding of NH2OH and NO2- oxidation in turn offers insights into proton-coupled, multi-electron transformations that are crucial to redox catalysis. NH2OH is an energetic, cytotoxic metabolite that AOB and AOA harness as a source of reducing equivalents. The absence of requisite c-heme biosynthesis pathways in AOA necessitates substitution of HAO for either a non-heme or Cu-based enzyme for NH2OH oxidation (79). Thus, opportunities exist to fill a “missing link” in the biogeochemical nitrogen cycle while expanding understanding of how transition metals can transduce energy from redox-active small molecules.
Concluding outlook
Since the beginning of the 20th century, both the ability to produce and the need to remediate nitrogen-containing compounds have been coupled to fossil fuels. Demands for greater energy efficiency, smaller and more flexible distributed processes, and environmental protection provide growing impetus to expand the scope of practically viable oxidative and reductive transformations of nitrogen that are not driven by fossil fuels. Opportunities exist to achieve radically improved, new, and different pathways, but progress in this regard will require a molecular-level understanding of nitrogen transformation reactions, as well as the translation of these core insights to the discovery of new catalytic systems and alternative means of delivering the energy needed to drive those reactions. These understandings will emerge through the collective knowledge and insights to be gained from fundamental research that integrates experiments and theory in hetero- and homogeneous catalysis science, in photon- and electron-driven processes, and in biochemistry and biology.
Acknowledgments
Funding: This article evolved from presentations and discussion at the workshop “Frontiers, Opportunities, and Challenges in Biochemical and Chemical N2 Activation” held in October 2016, in Gaithersburg, Maryland. The workshop was sponsored by the Council on Chemical Sciences, Geosciences and Biosciences of the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences. The authors thank the members of the Council for their encouragement and assistance in developing this workshop. The authors have no competing interests relevant to this publication.
Contributor Information
Jingguang G. Chen, Department of Chemical Engineering, Columbia University, New York, NY 10027, USA; Chemistry Department, Brookhaven National Laboratory, Upton, NY 11973, USA.
Richard M. Crooks, Department of Chemistry, The University of Texas at Austin, Austin, TX 78712, USA.
Lance C. Seefeldt, Department of Chemistry and Biochemistry, Utah State University, Logan, UT 84332, USA.
Kara L. Bren, Department of Chemistry, University of Rochester, Rochester, NY 14627, USA
R. Morris Bullock, Pacific Northwest National Laboratory, Richland, WA 99352, USA.
Marcetta Y. Darensbourg, Department of Chemistry, Texas A&M University, College Station, TX 77843, USA
Patrick L. Holland, Department of Chemistry, Yale University, New Haven, CT 06511, USA
Brian Hoffman, Department of Chemistry, Northwestern University, Evanston, IL 60208, USA.
Michael J. Janik, Department of Chemical Engineering, Pennsylvania State University, University Park, PA 16802, USA
Anne K. Jones, School of Molecular Sciences, Arizona State University, Tempe, AZ 85282, USA
Mercouri G. Kanatzidis, Department of Chemistry, Northwestern University, Evanston, IL 60208, USA
Paul King, National Renewable Energy Laboratory, Golden, CO 80401, USA.
Kyle M. Lancaster, Department of Chemistry and Chemical Biology, Cornell University, Baker Laboratory, Ithaca, NY 14853, USA
Sergei V. Lymar, Chemistry Department, Brookhaven National Laboratory, Upton, NY 11973, USA
Peter Pfromm, Voiland School of Chemical Engineering and Bioengineering, Washington State University, Pullman, WA 99164-6515, USA.
William F. Schneider, Department of Chemical and Biomolecular Engineering, University of Notre Dame, Notre Dame, IN 46556, USA
Richard R. Schrock, Department of Chemistry, MIT, 6-331, Cambridge MA 02139, USA.
References and Notes
- 1.Erisman JW, Sutton MA, Galloway J, Klimont Z, Winiwarter W, How a Century of Ammonia Synthesis Changed the World. Nat. Geosci 1, 636–639 (2008). [Google Scholar]
- 2.Smil V, Detonator of the population explosion. Nature 400, 415 (1999). [Google Scholar]
- 3.“Ammonia Production: Moving Towards Maximum Efficiency and Lower Ghg Emissions. ,” In Fertilizer Facts, International Fertilizer Industry Association http://www.fertilizer.org/ (2014).
- 4.Booth G, Zollinger H, McLaren K, Sharples W, Westwall AE, in Ullmanns encyclopedia of industrial chemistry, Elvers B, Hawkins S, Schulz G, Eds. (2002), vol. 17. [Google Scholar]
- 5.Rafiqul I, Weber C, Lehmann B, Voss A, Energy efficiency improvements in ammonia production - Perspectives and uncertainties. Energy 30, 2487–2504 (2005). [Google Scholar]
- 6.Miller JA, Bowman CT, Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Comb. Sci 15, 287–338 (1989). [Google Scholar]
- 7.Patil BS, Wang Q, Hessel V, Lang J, Plasma N2-fixation: 1900–2014. Catal. Today 256, 49–66 (2015). [Google Scholar]
- 8.Bartholomew CH, Farrauto RJ, Fundamentals of Industrial Catalytic Processes (Wiley, Hoboken, NJ, 2006). [Google Scholar]
- 9.Leighton PA, Photochemistry of Air Pollution (Academic Press, 1961). [Google Scholar]
- 10.Shelef M, McCabe RW, Twenty-five years after introduction of automotive catalysts: what next? Catalysis Today 62, 35–50 (2000). [Google Scholar]
- 11.Zammit M et al. , “Future automotive aftertreatment solutions: The 150C challenge workshop report,” Tech. Rep., U.S. Drive Report PNNL-22815,” (Southfield, Michigan, 2013). [Google Scholar]
- 12.National Research Council Clean Coastal Waters: Understanding and Reducing the Effects of Nutrient Pollution (Natl. Acad. Press, Washington DC, 2000). [Google Scholar]
- 13.Chaplin BP et al. , Critical Review of Pd-Based Catalytic Treatment of Priority Contaminants in Water. Environ. Sci. Technol 46, 3655–3670 (2012). [DOI] [PubMed] [Google Scholar]
- 14.The following standard states at 298 K and 1 bar are adopted throughout this paper: pure liquid for water, 1 mol/L for aqueous solutes, gaseous state for all other compounds; these states are designated by subscripts (liq), (aq), and (g), respectively. Aqueous nitric acid is considered to be fully ionized.
- 15.Wagman DD et al. , The NBS tables of chemical thermodynamic properties. J. Phys. Chem Ref. Data 11, Suppl. No. 2, (1982). [Google Scholar]
- 16.Gurvich LV, Veyts IV, Alcock CB, Thermochemical Properties of Individual Substances (Hemisphere Publishing Corp., New York, NY, 1989), vol. 1, part two. [Google Scholar]
- 17.U.S. Geological Survey, Mineral Commodity Summaries, 117 (2018). [Google Scholar]
- 18.The Essential Chemical Industry – Online: http://essentialchemicalindustry.org/chemicals/nitric-acid.html (accessed Feb. 22, 2018).
- 19.Armstrong DA et al. , Standard electrode potentials involving radicals in aqueous solution: inorganic radicals (IUPAC Technical Report). Pure Appl. Chem 87, 1139–1150 (2015). [Google Scholar]
- 20.Logadottir A et al. , The Brønsted–Evans–Polanyi Relation and the Volcano Plot for Ammonia Synthesis over Transition Metal Catalysts. J. Catal 197, 229–231 (2001). [Google Scholar]
- 21.Jacobsen CJH et al. , Catalyst Design by Interpolation in the Periodic Table: Bimetallic Ammonia Synthesis Catalysts. J. Am. Chem. Soc 123, 8404–8405 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Medford AJ et al. , From the Sabatier Principle to a Predictive Theory of Transition-Metal Heterogeneous Catalysis. J. Catal 328, 36 (2015). [Google Scholar]
- 23.Vicente EJ, Dean DR, Keeping The Nitrogen-Fixation Dream Alive. Proc. Natl. Acad. Sci. U.S.A 114, 3009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milton RD et al. , The In Vivo Potential-Regulated Protective Protein of Nitrogenase in Azotobacter vinelandii Supports Aerobic Bioelectrochemical Dinitrogen Reduction In Vitro. Journal of the American Chemical Society 139, 9044–9052 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Čorić I, Holland PL, Insight into the Iron–Molybdenum Cofactor of Nitrogenase from Synthetic Iron Complexes with Sulfur, Carbon, and Hydride Ligands. J. Am. Chem. Soc 138, 7200–7211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paengnakorn P et al. , Infrared spectroscopy of the nitrogenase MoFe protein under electrochemical control: Potential-triggered CO binding. Chem. Sci 8, 1500–1505 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milton RD et al. , Bioelectrochemical Haber-Bosch Process: An Ammonia-Producing H-2/N-2 Fuel Cell. Angew. Chem. Int. Ed 56, 2680–2683 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Danyal K et al. , Uncoupling nitrogenase: Catalytic reduction of hydrazine to ammonia by a MoFe protein in the absence of Fe protein-ATP. J. Am. Chem. Soc 132, 13197–13199 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khadka N et al. , Mechanism of Nitrogenase H2 Formation by Metal-Hydride Protonation Probed by Mediated Electrocatalysis and H/D Isotope Effects. J. Am. Chem. Soc 139, 13518–13524 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown KA et al. , Light-driven dinitrogen reduction catalyzed by a CdS:nitrogenase MoFe protein biohybrid. Science 352, 448–450 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Allen AD, Senoff CV, Nitrogenopentammineruthenium(II) complexes. Chem. Commun, 621–622 (1965). [Google Scholar]
- 32.Chatt J, Dilworth JR, Richards RL, Recent Advances in the Chemistry of Nitrogen Fixation. Chem. Rev 78, 589–625 (1978). [Google Scholar]
- 33.Pickett CJ, Talarmin J, Electrosynthesis of ammonia. Nature 317, 652–653 (1985). [Google Scholar]
- 34.Yandulov DV, Schrock RR, Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 301, 76–78 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Arashiba K, Miyake Y, Nishibayashi Y, A molybdenum complex bearing PNP-type pincer ligands leads to the catalytic reduction of dinitrogen into ammonia. Nat. Chem 3, 120–125 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Arashiba K et al. , Catalytic reduction of dinitrogen to ammonia by use of molybdenum–nitride complexes bearing a tridentate triphosphine as catalysts. J. Am. Chem. Soc 137, 666–5669 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Anderson JS, Rittle J, Peters JC, Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature 501, 84–87 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chalkley MJ, Del Castillo TJ, Matson BD, Roddy JP, Peters JC, Catalytic N2-to-NH3 conversion by Fe at lower driving force: A proposed role for metallocene-mediated PCET. ACS Cent. Sci 3, 217–223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosca V, Duca M, de Groot MT, Koper MTM, Nitrogen Cycle Electrocatalysis. Chem. Rev 109, 2209–2244 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Zhou F et al. , Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids. Energy Environ. Sci 10, 2516–2520 (2017). [Google Scholar]
- 41.Lan R, Tao S., Electrochemical synthesis of ammonia directly from air and water using a Li+/H+/NH4+ mixed conducting electrolyte. RSC Adv, 18016–18021 (2013). [Google Scholar]
- 42.McEnaney JM et al. , Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy & Environmental Science 7, 1621–1630 (2017). [Google Scholar]
- 43.Licht S et al. , Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science 345, 637–640 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Kyriakou V, Garagounis I, Vasileiou E, Vourros A, Stoukides M, Progress in the electrochemical synthesis of ammonia. Catalysis Today 286, 2–13 (2017). [Google Scholar]
- 45.Hamers RJ, Bandy JA, Zhu D, Zhang L, Photoemission from diamond films and substrates into water: dynamics of solvated electrons and implications for diamond photoelectrochemistry. Faraday Discuss 172, 397–411 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Guo C, Ran J, Vasileff A, Qiao S-Z, Rational design of electrocatalysts and photo(electro)catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energy Environ. Sci 11, 45–56 (2018). [Google Scholar]
- 47.Medford AJ, Hatzell MC, Photon-Driven Nitrogen Fixation: Current Progress, Thermodynamic Considerations, and Future Outlook. ACS Catal 7, 2624 (2017). [Google Scholar]
- 48.Liu J et al. , Nitrogenase-Mimic Iron-Containing Chalcogels for Photochemical Reduction of Dinitrogen to Ammonia. Proc. Natl. Acad. Sci. U.S.A 113, 5530–5535 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Helden JH et al. , Detailed study of the plasma-activated catalytic generation of ammonia in N2-H2 plasmas. J. Appl. Phys 101, 043305 (2007). [Google Scholar]
- 50.Hong J, Prawer S, Murphy AB, Plasma Catalysis as an Alternative Route for Ammonia Production: Status, Mechanisms, and Prospects for Progress. ACS Sustainable Chemistry & Engineering 6, 15–31 (2018). [Google Scholar]
- 51.Neyts EC, Ostrikov K, Sunkara MK, Bogaerts A, Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev 115, 13408–13446 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Whitehead JC, Plasma–catalysis: the known knowns, the known unknowns and the unknown unknowns. J. Phys. D: Appl. Phys 49, 243001 (2016). [Google Scholar]
- 53.Mehta P et al. , Overcoming Ammonia Synthesis Scaling Relations with Plasma-enabled Catalysis. Nature Catal In Press, (2018). [Google Scholar]
- 54.Adanez J, Abad A, Garcia-Labiano F, Gayan P, Diego L. F. d., Progress in Chemical-Looping Combustion and Reforming Technologies. Prog. Energy Combust. Sci 38, 215–282 (2012). [Google Scholar]
- 55.Michalsky R, Avram AM, Peterson BA, Pfromm PH, Peterson AA, Chemical looping of metal nitride catalysts: low-pressure ammonia synthesis for energy storage. Chem. Sci 6, 3965–3974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gálvez ME, Halmann M, Steinfeld A, Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 1. thermodynamic, environmental, and economic analyses. Ind. & Eng. Chem. Res 46, 2042–2046 (2007). [Google Scholar]
- 57.Michalsky R, Pfromm P, An Ionicity rationale to design solid phase metal nitride reactants for solar ammonia production. J. Phys. Chem C 116, 23243–22325 (2012). [Google Scholar]
- 58.Anders L, Leckner B, A 1000 MWth boiler for chemical-looping combustion of solid fuels - Discussion of design and cost. Appl. Energy 157, 475–487 (2015). [Google Scholar]
- 59.Schneider WF, in Environmental Catalysis, Grassian VH, Ed. (Wiley-VCH, 2004), pp. 233–268. [Google Scholar]
- 60.Dean AM, Bozzelli JW, in Gas-Phase Combustion Chemistry, Gardiner WC, Ed. (Springer New York, New York, NY, 2000), pp. 125–341. [Google Scholar]
- 61.Cherkasov N, Ibhadon AO, Fitzpatrick P, A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process. Process Intensif 90, 24–33 (2015). [Google Scholar]
- 62.Fridman A, Plasma Chemistry (Cambridge University Press, Cambridge, 2008). [Google Scholar]
- 63.Rusanov VD, Fridman AA, Sholin GV, The physics of a chemically active plasma with nonequilibrium vibrational excitation of molecules. Soviet Physics Uspekhi 24, 447–474 (1981). [Google Scholar]
- 64.Carmo M, Fritz D, Mergel J, Stolten D, A comprehensive review on PEM electrolysis (2013), vol. 38, pp. 4901–4934. [Google Scholar]
- 65.Pârvulescu VI, Grange P, Delmon B, Catalytic removal of NO. Catal. Today 46, 233–316 (1998). [Google Scholar]
- 66.Lionetti D, de Ruiter G, Agapie T, A trans-Hyponitrite Intermediate in the Reductive Coupling and Deoxygenation of Nitric Oxide by a Tricopper–Lewis Acid Complex. J. Am. Chem. Soc 138, 5008–5011 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Groothaert MH, van Bokhoven JA, Battiston AA, Weckhuysen BM, Schoonheydt RA, Bis(μ-oxo)dicopper in Cu-ZSM-5 and Its Role in the Decomposition of NO: A Combined in Situ XAFS, UV−Vis−Near-IR, and Kinetic Study. J. Am. Chem. Soc 125, 7629–7640 (2003). [DOI] [PubMed] [Google Scholar]
- 68.Paolucci C et al. , Dynamic Multinuclear Sites Formed By Mobilized Copper Ions In Nox Selective Catalytic Reduction. Science 357, 898 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Mergler YJ, Nieuwenhuys BE, NO reduction by H2 over promoted Pt catalysts. Appl. Catal., B 12, 95–110 (1997). [Google Scholar]
- 70.Martínez-Espinosa RM, Cole JA, Richardson DJ, Watmough NJ, Enzymology and ecology of the nitrogen cycle. Biochem. Soc. Trans 39, 175–178 (2011). [DOI] [PubMed] [Google Scholar]
- 71.Tavares P, Pereira AS, Moura JJG, Moura I, Metalloenzymes of the denitrification pathway. J. Inorg. Biochem 100, 2087–2100 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Hooper AB, Arciero D, Bergmann D, Hendrich MP, in Respiration in Archaea and Bacteria: Diversity of Prokaryotic Respiratory Systems. Advances in Photosynthesis and Respiration, Zannoni D, Ed. (Springer, Dordrecht, 2004), vol. 16, pp. 121–147. [Google Scholar]
- 73.Konneke M et al. , Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437, 543–546 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Vajrala N et al. , Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc. Natl. Acad. Sci. U.S.A 110, 1006–1011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caranto JD, Lancaster KM, Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase. Proc. Natl. Acad. Sci. U.S.A 114, 8217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caranto JD, Vilbert AC, Lancaster KM, Nitrosomonas europaea cytochrome P460 is a direct link between nitrification and nitrous oxide emission. Proc. Natl. Acad. Sci. U.S.A 113, 14704–14709 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daims H et al. , Complete nitrification by Nitrospira bacteria. Nature 528, 504–509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawton TJ, Ham J, Sun T, Rosenzweig AC, Structural conservation of the B subunit in the ammonia monooxygenase/particulate methane monooxygenase superfamily. Proteins Struct. Funct. Bioinf 82, 2263–2267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walker CB et al. , Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. U.S.A 107, 8818–8823 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]