Abstract
Background
A challenge facing clinical neuroscientists is how best to synthesize diverse and sometimes inconsistent evidence for neuropsychological deficits and brain system dysfunction found in psychiatric disorders into models that guide etiological and treatment research. Multiple pathway models suggest psychiatric symptoms might arise from pathophysiology in different neural systems. This study tested “dual pathway” model predictions for Attention Deficit Hyperactivity Disorder (ADHD) that reward and executive function cognitive deficits should be related to abnormalities in corresponding functionally-specialized neural systems.
Methods
Behavioral inhibition and preference for immediate rewards were assessed in N=251 adolescent boys and girls ages 12–18 diagnosed with DSM-IV Combined-subtype ADHD or non-ADHD controls. Following taxometric analyses of test performance, the resulting subgroups were compared on an fMRI Monetary Incentive Delay task probing reward anticipation and Go/NoGo task of motor response inhibition.
Results
Three ADHD subgroups were identified consistent with different proposed pathways – ADHD with executive function/motor inhibition deficits, ADHD with both executive and reward deficits, and ADHD with relatively normal test performance. Each cognitive domain mapped to different ADHD brain dysfunction features as expected. However, no brain abnormalities were found common to all ADHD subgroups despite the fact they had nearly identical ADHD-related clinical characteristics.
Conclusions
The results suggest Combined-subtype ADHD is a collection of discrete disorders for which a comparable behavioral endpoint arises through different neurobiological pathways. The findings raise caution about applying common cause, single-deficit conceptual models to individual ADHD patients and should prompt researchers to consider biologically-defined, multifactorial etiological models for other psychiatric diagnoses.
Keywords: ADHD, inhibition, reward, subgroup, fMRI, pathway
Introduction
Psychiatric researchers formulate neurocognitive models to understand relationships among diagnostic symptoms, associated cognitive abnormalities, and neurobiological dysfunction. While many models were initially advanced to understand how brain abnormalities could underlie one particular disorder-related cognitive feature, some have evolved into etiological models. However, such single deficit models typically do not attempt to explain the diverse and sometimes inconsistent evidence for brain function abnormalities in different functional contexts that has accumulated over the past several decades for many disorders. For instance in Attention-Deficit Hyperactivity Disorder (ADHD), there is considerable heterogeneity in both neuropsychological (1–3) and neural system function (4). Despite this, ADHD researchers almost exclusively use case-control designs to identify neurobiological dysfunction common to the broad behaviorally-defined diagnostic phenotype. But what if no single neurobiological abnormality is common to all patients who meet criteria for a specific diagnosis? This study tests this fundamental assumption to learn whether ADHD patients can manifest all, some, or none of well-replicated neuropsychological and neural abnormalities previously linked to the behaviorally-based diagnosis.
Multiple pathway neurocognitive models for ADHD eschew single-cognitive deficit explanations, instead proposing symptoms arise from abnormality in several distinct neural systems. The first “dual pathway” ADHD model proposed patients could have either a primary deficit in behavioral inhibition-related executive function, or in reward and motivation-related reinforcement processes that produce a characteristic aversion to delay (5, 6). This is mirrored by later models that recognize similar abnormalities (7–9). Although models disagree which cognitive test deficits are most representative of each pathway and how exactly they relate to ADHD etiology, there is strong neuropsychological evidence for distinct executive- and reward-related impairment in ADHD. Data reduction techniques separate the two domains in ADHD-diagnosed patients (10, 11). Reward and executive test performance independently predict ADHD symptoms (12). Scores on tests from the two domains can discriminate ADHD from non-ADHD with reasonably high accuracy (13). Perhaps most useful for experimentation, ADHD-diagnosed children, adolescents, and adults can be differentiated into subgroups with test deficits on only motor inhibition tests, reward tests, in both domains, or in neither (10, 11, 13–15).
It has been proposed these different causal pathways are associated with abnormalities in different brain systems. Impairments on tests assessing reward or executive control possibly arise from dysfunctional mesolimbic and mesocortical dopaminergic neural systems, respectively (5, 9). Neuroimaging ADHD studies have found abnormalities in ventral striatum and orbitofrontal cortex within the reward system, and lateral and medial prefrontal cortices related to executive control (4, 16). Strict dual pathway ADHD models would predict that different pathways could result in separate and distinct profiles of brain dysfunction, each mapping only to the corresponding functional domain. Alternatively, it is possible pathway-specific brain activity abnormalities exist, but manifest as variation around a core profile of ADHD neural dysfunction. Although these models have considerably different implications for understanding ADHD neurobiology and ultimately ADHD etiology, no neuroimaging studies have yet offered evidence to support or refute either possibility.
To describe how putative dual pathway causal mechanisms typically are expressed in ADHD-diagnosed adolescents across neurocognitive, clinical, and neural system levels of inquiry, this study sought to better characterize the nature of pathway-related neurocognitive deficits in ADHD. We first asked if ADHD patients fell somewhere along a normal continuum of impairment in executive or reward domains, or if pathways were expressed in a way that formed neurocognitively distinct ADHD subgroups. We used a combination of data reduction and taxometric classification analyses to examine ADHD and non-ADHD participants’ performance on a battery where several theoretically-relevant tests were given to assess each pathway. Then, we characterized brain dysfunction in the ADHD subgroups that resulted, using a subsample who performed fMRI reward (Monetary Incentive Delay; [MID]) and motor inhibition (Go/NoGo; [GNG]) tasks. We hypothesized ADHD-diagnosed adolescents who had only inhibition or only reward test deficits would show neural function abnormalities only in brain regions functionally specialized for inhibition or reward, while patients with both types of test deficits would show brain dysfunction in both neural systems.
Methods and Materials
Participants
DSM-IV Combined-Subtype ADHD (n=117) and non-ADHD (n=134) adolescent boys and girls ages 12–18 were recruited via community advertisements. Study exclusion criteria were major medical disorders, current DSM-IV substance dependence, mood or anxiety disorders, lifetime bipolar, OCD, PTSD, Tourette’s, psychotic, or Pervasive Developmental Disorder, abnormal brain structure on MRI, and Full Scale IQ estimate <80. Only un-medicated patients or patients who took short half-life psychostimulant medications were enrolled. ADHD participants who took medications not amenable to a 24 hour “washout” procedure prior to cognitive/MRI assessment were excluded.
Clinical assessment
Following informed consent/parental permission using procedures approved by Hartford Hospital’s Institutional Review Board, the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL) (17) assessed Axis-I disorders using standard guidelines with information synthesized from both parent and participant interviews. The cognitive battery (Table 1) included several tests measuring either preference for immediate or delayed rewards (“reward”), or a tendency for impulsive responding when executive control or motor inhibition is required (“executive”). Comparison of ADHD versus non-ADHD test performance found the ADHD adolescents were impaired on nearly every measure (Supplementary Table S1). A detailed description of the clinical and neurocognitive battery is in SI text.
Table 1.
Taxometric-derived ADHD subgroup characteristics compared to non-ADHD control participants by one-way Analysis of Variance (ANOVA) and post hoc pairwise group comparison results.
| Controls n=134 |
ADHD-EF n=40 |
ADHD- EF/REW n=31 |
ADHD- NONE n=46 |
p | Planned comparison | |
|---|---|---|---|---|---|---|
| Delay Discounting (AUC) | 0.386 (0.02) | 0.318 (0.04) | 0.274 (0.05) | 0.353 (0.04) | .128 | HC > ADHD-EF/REW |
| Experiential Discounting (AUC) | 0.638 (0.01) | 0.582 (0.03) | 0.656 (0.03) | 0.586 (0.02) | .059 | - |
| SKIP average IRT (sec) | 13.375 (1.67) | 6.943 (3.01) | 2.445 (3.43) | 11.682 (2.82) | .023 | HC > ADHD-EF/REW |
| Stop Signal RT (msec) | 278.8 (5.96) | 295.9 (10.72) | 347.4 (12.24) | 287.2 (10.05) | <.001 | HC > ADHD-EF/REW |
| IMT Impulsivity (ratio) | 0.506 (0.02) | 0.674 (0.03) | 0.806 (0.03) | 0.532 (0.03) | <.001 | HC > ADHD-EF/REW, ADHD-EF |
| DMT Impulsivity (ratio) | 0.495 (0.03) | 0.731 (0.05) | 1.092 (0.06) | 0.596 (0.05) | <.001 | HC > ADHD-EF/REW, ADHD-EF |
| MMF Errors (#) | 16.663 (1.15) | 33.771 (2.06) | 36.954 (2.36) | 23.537 (1.93) | <.001 | HC > all 3 ADHD |
| CPT-II Commissions (#) | 20.426 (0.57) | 23.536 (1.02) | 26.61 (1.17) | 18.529 (0.96) | <.001 | HC > ADHD-EF/REW, ADHD-EF |
SKIP – Single Key Impulsivity Paradigm; IMT/DMT – Immediate and Delayed Memory Test, MMF – Matching Familiar Figures Test, CPT-II – Conner’s Continuous Performance Test – II
AUC – Area under the curve; IRT – Inter-response interval, RT – Reaction Time
Taxometric classification
Principal component analysis (PCA) prepared test data for ADHD pathway characterization. The resulting ADHD three-factor structure was superficially similar to non-ADHD, but several tests had different loading profiles (see Supplementary Table S2 and SI text for details). In brief, executive and reward tests more often co-loaded on the same factors in ADHD, consistent with prior observations that the two pathways likely interact (18), and there were differences in the how EDT and DDQ tests of impulsive choice loaded onto each groups’ factors. Next, an iterative taxometric analysis examined pairwise combinations of ADHD PCA factors. The comparative curve fit index (CCFI) calculated on average MAMBAC curves (19) can determine whether a latent construct is categorical in nature (CCFI > .60) or dimensional (CCFI < .40) through comparison to simulated data. When applied to the ADHD PCA data, MAMBAC-derived CCFI = .638 and the estimated taxon base rate (P) was .398. Multiple methods validation (20) using another taxometric algorithm MAXSLOPE gave comparable results (CCFI = .621, P = .412). Average MAMBAC and MAXSLOPE curves are depicted in Fig. S3. Cases were assigned to the n=46 taxon and n=71 complement using the average P. A second taxometric analysis determined if either of these subgroups should be further split. Results for the n=71 complement also suggested categorical subgroups (MAMBAC CCFI = .580; MAXSLOPE CCFI = .682), so it was divided into n=31 and n=40 subgroups based on average P.
fMRI Methods
All participants were invited for MRI assessment, but over a third declined or were excluded because they had orthodontia or were left-handed. After quality control data inspection (see SI text), the final fMRI subsample included n=63 non-ADHD and n=62 ADHD GNG fMRI datasets and n=69 non-ADHD and n=62 ADHD MID fMRI datasets. The final fMRI subsamples’ mean age and sex proportion did not statistically differ from the full sample.
MRI data collection, data preparation, and fMRI activation modeling details are in SI text. The Go/No-Go (GNG) task (21) quantified brain activation to both correctly inhibited motor responses and errors of commission, designed to provide insight into both response inhibition and error processing aspects of cognitive control. The Monetary Incentive Delay (MID) task modified the original fMRI paradigm (22) to dissociate the prospect of reward (cue indicating possible win or loss of money) from the subsequent period where participants exert effort to obtain the reward.
A two-stage analysis plan was used. First, ADHD subgroup brain activation abnormalities were identified by contrasting each subgroup to non-ADHD controls in a series of two-sample t tests. These results were evaluated using a clusterwise inference framework (23). The cluster-determining threshold was the same for all analyses (p< .005) with the target whole brain-corrected p < .05. Anatomical localization was assisted using anatomical atlases (24). Second, we examined the specificity of subgroup deficits. ANOVA tested if any activation levels differed among the ADHD subgroups in brain regions-of-interest found in the primary analyses to be abnormal in at least one subgroup. Conjunction analyses (25) asked if any abnormality was found jointly in all three ADHD subgroups.
Results
ADHD Neurocognitive Subgroup Analysis and Results
CCFI results from the taxometric analyses used to test for a polytomous group structure indicated executive and reward-related cognitive abilities were not dimensionally arranged in ADHD as they were for non-ADHD (see Fig. S1). Instead, there were three discrete, categorically different ADHD subgroups with different test profiles: 1) An ADHD subgroup impaired only on executive tests (ADHD-EF), 2) a subgroup impaired relative to non-ADHD on tests of both reward and executive function (ADHD-EF/REW), and 3) a subgroup who was largely intact on dual pathway tests (ADHD-NONE), differing from non-ADHD in a slightly elevated number of MMFT non-reflective responses. The profile of cognitive impairments validated the putative ADHD dual pathways, and dictated the subsequent analysis strategy. Table 1 lists mean test scores and results for statistical comparison between non-ADHD and each subgroup.
As expected, all three ADHD subgroups significantly differed from non-ADHD controls on clinical measures of ADHD symptom severity, functional impairment, and common ADHD-associated clinical problems. In contrast, the ADHD subgroups’ demographic and clinical profiles were indistinguishable for proportion male (ADHD-EF 77.5%, ADHD-EF/REW 83.9%, ADHD-NONE 80.4%; X2=0.449, p=.799), mean age (ADHD-EF 14.8, ADHD-EF/REW 15.0, ADHD-NONE 15.1; F2,114=0.182, p=.834), and mean IQ (26) (ADHD-EF 102.5, ADHD-EF/REW 102.6, ADHD-NONE 102.0; F2,114=0.027, p=.973). There were no subgroup differences in Hyperactive or Inattentive symptom counts (27), ADHD-associated behaviors (28) or functional impairment (29), mood or anxiety symptoms, and common psychiatric comorbidities or substance misuse (Table 2). ADHD subgroups did not differ in the proportion treated with pharmacotherapy, though ADHD-NONE showed a trend towards more frequent lisdexamfetamine use (p=.061). Trend-level evidence suggested families of the ADHD-EF/REW and ADHD-EF subgroups had higher familial ADHD than ADHD-NONE (ANOVA p=.069).
Table 2.
Taxometric-derived ADHD subgroup demographic and clinical characteristics compared to non-ADHD control participants by one-way Analysis of Variance (ANOVA) and post hoc pairwise group comparison results.
| Controls n=134 |
ADHD- EF/REW n=31 |
ADHD-EF n=40 |
ADHD- NONE n=46 |
Sample p | ADHD Subgroup p | |
|---|---|---|---|---|---|---|
| ADHD Inattentive Symptoms | 0.007 (0.09) | 7.57 (0.19) | 7.189 (0.16) | 7.409 (0.15) | <.001 | ns |
| ADHD Hyperactive/Impulsive Symptoms | 0.003 (0.10) | 6.745 (0.21) | 6.624 (0.19) | 6.247 (0.17) | <.001 | ns |
| Brown ADD Scales Parent | 14.392 (2.11) | 63.859 (4.57) | 69.528 (4.29) | 70.435 (3.86) | <.001 | ns |
| Brown ADD Scales Self | 26.914 (1.98) | 58.65 (4.32) | 53.522 (3.84) | 60.26 (3.69) | <.001 | ns |
| % Currently Medicated | 0.8% | 77.4% | 67.5% | 66.7% * | <.001 | ns |
| ADHD Family History | 25.7% | 60.7% | 64.6% | 40.5% | <.001 | .069 |
| % Conduct Disorder Diagnosis | 0.8% | 3.2% | 2.5% | 13.0% | .002 | ns |
| BDI-II Total | 4.321 (0.63) | 8.956 (1.28) | 8.495 (1.15) | 9.64 (1.09) | <.001 | ns |
| MASC Total T | 43.846 (0.97) | 45.272 (1.98) | 48.9 (1.8) | 46.907 (1.69) | ns | ns |
| Alcohol/Drug use (AADIS) | 10.447 (1.47) | 4.261 (2.88) | 13.339 (2.67) | 11.139 (2.5) | ns | .085 |
| Fagerstrom | 2.4% | 3.2% | 11.1% | 12.2% | .039 | ns |
| CBCL Total T | 41.896 (0.86) | 59.73 (1.59) | 60.69 (1.49) | 61.258 (1.35) | <.001 | ns |
MASC – Multidimensional Anxiety Scale for Children
AADIS – Adolescent Alcohol and Drug Involvement Scale
CBCL – Child Behavior Checklist
ADHD Subgroup Brain Dysfunction Profiles
We examined a subsample of n=74 ADHD participants who agreed to fMRI with two tasks chosen for their relevance to the executive and reward pathways. Whole brain renderings of simple activation effects to all four of these conditions of interest are shown in Supplementary Fig. S2 (p<.01 uncorrected). The overall non-ADHD vs. ADHD activation differences depicted in Supplementary Fig. S3 (p<.01 uncorrected) resembled abnormalities seen prior ADHD fMRI studies (4).
We contrasted each ADHD taxometrically-derived subgroup to the non-ADHD sample to identify subgroup-specific neural dysfunction, using statistical corrections for searching the whole brain. Results for each task condition of interest are shown in Figs. 1–4. Localized functional abnormalities depicted in these figures are listed in Tables 3–5.
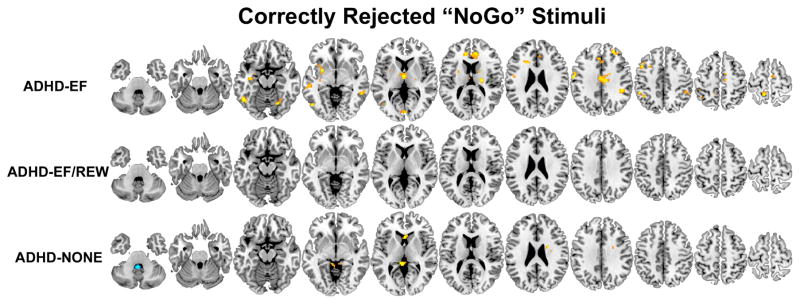
Figure 1.
Brain regions where participants classified by taxometric analysis into the three ADHD subgroups (ADHD-EF/REW, ADHD-EF, and ADHD-NONE) had less (orange-yellow) or more (blue-light blue) activation compared to non-ADHD controls during correctly-inhibited NoGo ‘K’ stimuli on the GNG fMRI task. Results are thresholded at p<.05 clusterwise corrected for searching the whole brain.
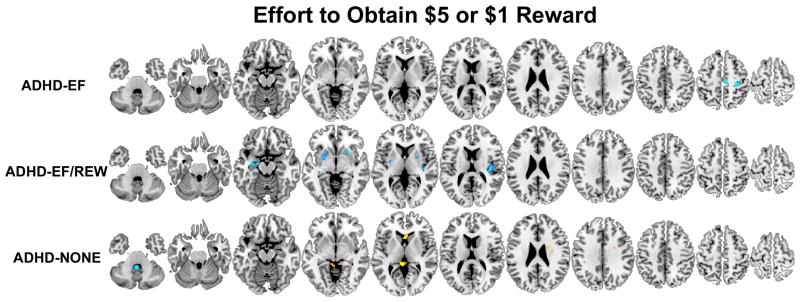
Figure 4.
Brain regions where participants classified by taxometric analysis into the three ADHD subgroups (ADHD-EF/REW, ADHD-EF, and ADHD-NONE) had less (orange-yellow) or more (blue-light blue) activation compared to non-ADHD controls measured during the exertion of effort to obtain monetary reward on the MID fMRI task. Results are thresholded at p<.05 clusterwise corrected for searching the whole brain.
Table 3.
Brain activation amplitude differences between the ADHD-EF taxometrically- determined subgroup and non-ADHD. The table lists Montreal Neurological Institute stereotactic space coordinates of peak study group difference for each cluster that was found to be significant in two-sample t test (p<.001 corrected for searching the whole brain). Results are reported for motor inhibition and error processing on the Go/NoGo task and reward prospect and reward anticipation for the Monetary Incentive Delay task. For each brain region, the Cohen’s d effect size is depicted using sparkline graph to facilitate comparison of ADHD subgroup differences with non-ADHD. The final columns report omnibus F significance p levels from a secondary ANOVA comparing the ADHD subgroups. Subgroup ANOVA-identified regions that survive False Discovery Rate corrections for multiple comparisons are denoted with†.
| Max Peak x, y, z |
t | Effect Size Pattern* | ADHD Subgroup ANOVA | ADHD Subgroup Activation Level Pairwise | |
|---|---|---|---|---|---|
| Motor Inhibition HC > ADHD-EF | |||||
| Right posterior medial-frontal gyrus | 9, −10, 62 | 3.94 |
|
.017 † | EF < REW/EF, NONE |
| Right middle frontal gyrus | 24, 41, 32 | 4.17 |
|
.012 † | EF < REW/EF, NONE |
| Left precentral gyrus (BA 44) | −57, −7, 35 | 3.65 |
|
.210 | - |
| Left superior medial frontal/anterior cingulate gyrus | −6, 29, 38 | 3.51 |
|
.031 | EF < NONE |
| Left anterior cingulate gyrus (BA 24) | −12, 38, 17 | 4.12 |
|
.073 | - |
| Right mid-cingulate gyrus (BA 33) | 9, −19, 35 | 3.71 |
|
.003 † | EF < NONE |
| Left precuneus | −12, −46, 65 | 4.10 |
|
.095 | - |
| Left inferior parietal lobule | −39, −52, 47 | 3.72 |
|
.020 | EF < REW/EF, NONE |
| Right inferior parietal lobule (hIP1/hIP3) | 42, −55, 50 | 3.19 |
|
.133 | - |
| Right supramarginal gyrus (hiP2/PF/PFm) | 42, −40, 38 | 4.32 |
|
.051 | - |
| Left middle temporal gyrus | −54, −28, −4 | 3.23 |
|
.020 | EF < REW/EF, NONE |
| Right inferior temporal gyrus | 54, −46, −4 | 3.44 |
|
< .001 † | EF < REW/EF, NONE |
| Left hippocampus | −36, −16, −13 | 3.53 |
|
.014 † | EF < REW/EF, NONE |
| Right lingual gyrus (hOc1/hOc2) | 3, −82, 2 | 4.01 |
|
.106 | - |
| Thalamus (temporal/prefrontal) | −3, −10, 5 | 3.64 |
|
.051 | - |
| Right thalamus (premotor/motor) | 21, −16, 14 | 4.30 |
|
.005 † | EF < REW/EF, NONE |
| Left putamen | −27, −1, −10 | 3.64 |
|
.012 † | EF < REW/EF, NONE |
| Right cerebellum (VI) | 24, −67, −16 | 3.97 |
|
.006 † | EF < REW/EF, NONE |
| Error Processing ADHD-EF > HC | |||||
| Right middle orbital gyrus (Fo3) | 24, 32, −13 | 5.26 |
|
.069 | - |
| Rectal gyrus (Fp2/Fo1) | 0, 56, −10 | 3.93 |
|
.187 | - |
| Left posterior cingulate/precuneus | 0, −55, 17 | 4.57 |
|
.040 | EF > NONE |
| Left nucleus accumbens | −15, 8, −7 | 3.77 |
|
.027 | EF > NONE |
| Reward Prospect HC > ADHD-EF | |||||
| Left middle occipital gyrus (hOc4lp/hOc3v/hOc4v) | −24, −91, 5 | 3.54 |
|
.110 | |
| Effort to Obtain Reward ADHD-EF > HC | |||||
| Right posterior medial frontal gyrus (4a) | 7, −22, 59 | 4.74 |
|
.185 | - |
For sparkline graphs, the initial grey datapoint represents the Cohen’s d effect size from a supplemental two-sample t test that compared non-ADHD to all of the ADHD participants. Afterwards, subgroups ADHD-EF, ADHD-EF/REW, and ADHD-NONE are depicted (left-to-right). All sparkline values on this table are scaled relative to the maximum Cohen’s d=1.16 found across all four fMRI task conditions for the ADHD-EF subgroup.
Table 5.
Brain activation amplitude differences between the ADHD-NONE taxometrically- determined subgroup and non-ADHD. The table lists Montreal Neurological Institute stereotactic space coordinates of peak study group difference for each cluster that was found to be significant in two-sample t test (p<.001 corrected for searching the whole brain). Results are reported for motor inhibition and error processing on the Go/NoGo task and reward prospect and reward anticipation for the Monetary Incentive Delay task. For each brain region, the Cohen’s d effect size is depicted using sparkline graph to facilitate comparison of ADHD subgroup differences with non-ADHD. The final columns report omnibus F significance p levels from a secondary ANOVA comparing the ADHD subgroups. Subgroup ANOVA-identified regions that survive False Discovery Rate corrections for multiple comparisons are denoted with †.
| Max Peak x, y, z |
t | Effect Size Pattern* | ADHD Subgroup ANOVA | ADHD Subgroup Activation Level Pairwise | |
|---|---|---|---|---|---|
| Motor Inhibition HC > ADHD-NONE | |||||
| Left caudate | −6, 17, 2 | 2.91 |
|
.010 † | EF, NONE < REW/EF |
| Motor Inhibition ADHD-NONE > HC | |||||
| Cerebellum (X) | −3, −43, -37 | 4.26 |
|
.101 | - |
For sparkline graphs, the initial grey datapoint represents the Cohen’s d effect size from a supplemental two-sample t test that compared non-ADHD to all of the ADHD participants. Afterwards, subgroups ADHD1, ADHD2, and ADHD3 are depicted. All sparkline values on this table are scaled relative to the maximum Cohen’s d=1.01 found across all four fMRI task conditions for the ADHD3 subgroup.
ADHD-EF
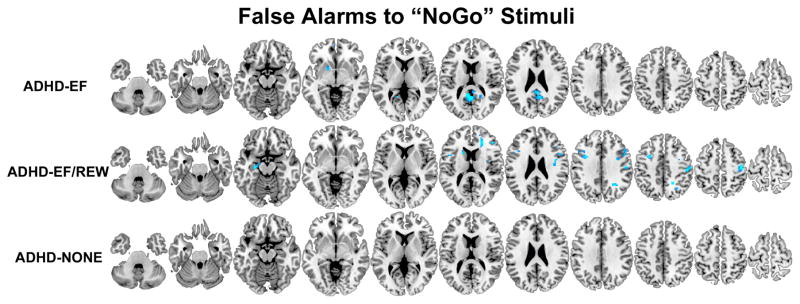
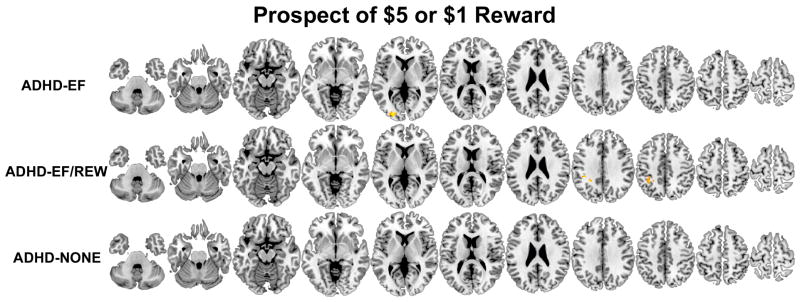
Brain function abnormalities in ADHD-EF indicate the executive pathway is related to failure to engage bilateral middle frontal gyri, right posterior medial frontal gyrus, left precentral gyrus, anterior and mid-cingulate gyri, superior medial frontal gyrus, left inferior parietal lobule and right supramarginal gyrus, several temporal and occipital lobe regions, thalamus, left putamen and right cerebellum during GNG response inhibition. During error processing, ADHD-EF failed to deactivate posterior cingulate/precuneus, gyrus rectus, and over-engaged left nucleus accumbens. ADHD-EF showed far fewer abnormalities on the MID task. When confronted with the prospect of reward, they under-activated left middle occipital gyrus. When exerting effort to obtain reward and awaited outcome, they over-engaged right posterior medial frontal gyrus.
ADHD-EF/REW
ADHD-diagnosed adolescents who had deficits on both executive and reward tasks did not show response inhibition-related hypofunction. Instead, they had prominent frontoparietal over-activation during error processing. They more greatly engaged left middle frontal gyrus, right superior frontal gyrus, bilateral inferior frontal gyri (bilateral pars opercularis and right pars triangularis), left hippocampus/amygdala and left caudate. The only abnormality these ADHD-diagnosed adolescent showed during the prospect of reward MID condition was left inferior parietal lobule hypoactivation. In contrast, when exerting effort to obtain reward, ADHD-EF/REW participants showed greater activation than non-ADHD in bilateral ventral striatum, right amygdala, left amygdala/hippocampus, and right posterior insula.
ADHD-NONE
Brain dysfunction in the final ADHD subgroup least resembled the other two subgroups. During GNG successful response inhibition, ADHD-NONE participants under-activated left caudate and over-engaged one region of the cerebellum (X). No other effects survived clusterwise Type I error control extent thresholds.
The seeming subgroup-specificity of these deficits was borne out in secondary analyses. First, conjunction analyses for each fMRI task condition failed to find any specific regional brain function abnormality that was shared by all three ADHD subgroups, even at the most liberal p < .05 uncorrected statistical thresholds. Secondary ANOVA found activation levels differed among ADHD subgroups in most regions found to be abnormal in at least one subgroup, with many differences surviving familywise Type I error rate corrections. When a subgroup abnormality was found, it alone appeared to typically drive any non-ADHD vs. overall ADHD sample effect.
Discussion
This study and nearly half a dozen others (10, 11, 13–15) have distinguished reward and executive pathways in ADHD at the level of neurocognitive test performance, despite different test batteries, participant ages, sample characteristics, or analytic methodology. At the risk of over-simplifying the precise localization of fMRI-measured ADHD subgroup differences, we found ADHD-diagnosed adolescents with only executive function/inhibition cognitive deficits primarily showed hypofunction within various frontal lobe, parietal lobe, subcortical, and cerebellar regions when inhibiting motor responses and abnormalities of posterior default mode and ventral striatum during error processing. In contrast, the ADHD subgroup with both reward and inhibition deficits had hyperactivation in mostly non-overlapping cortical and subcortical regions during error processing and over-engaged amygdala and ventral striatum regions involved in processing reward and salience information when exerting effort to obtain rewards. The third, largest ADHD subgroup had intact reward and mostly normal executive test performance, as well as the fewest brain function abnormalities compared to the other subgroups.
ADHD-EF deficits correspond to prior meta-analysis of ADHD response inhibition brain dysfunction that found ADHD hypofunction in pre-SMA, middle and precentral frontal gyri, insula, caudate and thalamus (30–32). Interestingly, these deficits were observed in only two of three brain regions best linked to motor inhibition in cognitive neuroscience studies of non-clinical populations – pre-SMA and right middle frontal gyrus, but not inferior frontal gyrus (33–35). Instead, other ADHD-EF hypofunction was seen in cingulate and parietal lobe regions specialized for attention and executive processing during GNG tasks. This suggests what gives rise to ADHD symptoms in this subgroup might be abnormal higher-order neural processing involved with motor inhibition, not inhibition itself. The error processing-related ADHD-EF/REW cortical and subcortical hyperfunction and abnormal ADHD-EF default mode disengagement differs from most prior ADHD error-processing neuroimaging studies (36–39). Those studies mostly used region-of-interest analyses or data reduction techniques to focus on hypothesized cingulo-opercular brain abnormalities. Supplementary contrast of all ADHD vs. non-ADHD confirmed our sample had similar cingulo-opercular network hypofunction (Supplementary Fig. S3B). But compared to the subgroup-specific abnormalities we observed, cingulate hypofunction appears to be a weaker, likely non-pathway-specific abnormality in ADHD. The hyperfunctional regions we found in ADHD-EF/REW are part of several extended error-processing networks shown engaged across many task contexts that operate at different timescales (40). Their over-engagement could be a direct cause of poor inhibition, represent neural adaptation to inhibition deficits (e.g., possibly manifesting as a deliberate, resource-heavy approach to performance monitoring), or perhaps relate to reward system-related hyperfunction also seen in ADHD-EF/REW. In contrast, ADHD-EF adolescents most prominently showed abnormality disengaging the default mode network during error processing, as some previously have theorized for ADHD (41).
ADHD-EF/REW MID-measured brain dysfunction matches regions implicated in delay aversion models (42). But when we examined discrete early MID task phases separately in this study, ADHD-EF/REW did not show abnormalities when initially confronted with the prospect of reward. Instead, ventral striatal, amygdala and posterior insular regions over-engaged when these participants made a speeded response to obtain the reward. This striatal over-engagement runs contrary to most prior ADHD MID studies that report ventral striatal under-signaling during MID-elicited reward anticipation (43). However, no prior ADHD fMRI study separated these contiguous reward prospect and response MID phases. If these two early MID task phases had been modeled together, the differences we observed in ADHD-ER/REW likely would have had a poor fit to any hemodynamic model that presumed a uniform response throughout both periods (i.e., resulting in the lower ADHD “activation” estimate seen in prior studies). This specificity in our results runs contrary to a generalized “reward deficit” model of the ADHD reward pathway assuming generally blunted mesolimbic dopaminergic region response (44) in reward contexts. Because this hyperfunction was not seen to reward-signaling cues, it is unlikely to reflect abnormal ADHD processing of expected value (45). Instead, it might reflect abnormal neural processing of anticipated or actual exerted effort, as seen in studies of non-ADHD samples where effort is experimentally manipulated (46, 47). Supporting this possibility, a recent fMRI study found greater ADHD ventral striatum activation compared to non-ADHD when ADHD-diagnosed patients were motivated by a highly salient contextual factor (i.e., to escape delays (48)).
Study findings support proposed ADHD multiple pathway theories not only because ADHD subgroups had distinct profiles of brain dysfunction, but also as previously hypothesized (5, 9) reward-related behavioral abnormality predicted reward brain system abnormality, while inhibition impairment was associated with dysfunction of frontoparietal systems involved in executive function and attention. Neither abnormality was seen in the ADHD subgroup without reward or EF test deficits. If one believes that brain dysfunction is closer to etiology than test behavior, this reflects three distinct pathways. However, these brain-behavior associations were not always the simple, one-to-one relationship between neurocognitive domain and neural system impairment one would expect from a strict dual pathway model. ADHD EF test deficits were associated with two different profiles of brain dysfunction – one primarily during response inhibition and another during error processing. This does not reflect a simple “additive” model where the difference between ADHD-EF and ADHD-EF/REW is merely the presence or absence of additional reward-related neural dysfunction. Unfortunately, our taxometric analysis did not identify a reward deficit-only ADHD subgroup which would have given us an opportunity to assess whether the same striatal hyperfunction seen during reward-related effort could be present without GNG error processing-related frontal lobe hyperfunction.
The neurobiological support for multiple ADHD pathways suggests a theoretical step forward. It is noteworthy that our study could not find a single brain function abnormality that was shared by all three neurocognitively-defined ADHD subgroups, even when assessed across four theoretically-relevant fMRI task conditions using the most liberal statistical thresholds. Yet, all three subgroups were demographically and intellectually similar, and expressed equivalent, typical ADHD symptom severity and associated clinical impairments. This is evidence for a model wherein the diverse behavioral symptoms of ADHD are equally likely to arise from brain dysfunction in discretely different neural systems, each suggesting unique etiologies. As researchers develop and refine ADHD neurocognitive models, they should test causal models that conceptualize the disorder as a constellation of different ADHD subgroups whose brains function abnormally in wholly different ways, rather than a single disorder with minor etiology-specific variations. We temper this interpretation by recognizing the obvious need for replication and examination of other cognitive deficits found in ADHD (1–3). The ADHD-NONE subgroup might reflect a wholly separate ADHD pathway – either related to a neurocognitive domain we did not assess (e.g., perhaps temporal information processing, working memory, or motor function) or one in which no specific test deficits identify it. Also, considerable supportive evidence will be needed to support such a radical shift to a conceptual model for ADHD arising from several different etiologies. The need for specialized neurocognitive batteries, theoretically-relevant fMRI probes, and large samples pose challenges for subsequent replication and new research into ADHD multi-etiological models. Research might begin by isolating cogent and reproducible fMRI-measured brain dysfunction profiles in large ADHD samples that are most informative about discrete neural subgroups, e.g., perhaps starting with resting state fMRI data from the ADHD-200 effort (49) and community detection algorithms that already have proven informative in ADHD (2). Then, attempts could be made using other datasets that possess rich behavioral phenotypic information to link those features to neurocognitive or clinical characteristics. Much effort would be needed, but consider the implications should it ultimately support a behavioral equifinality model of ADHD neurobiology. The long-recognized diversity of ADHD symptom, clinical comorbidity, neurocognitive profile, and long-term outcomes might be readily explainable as sampling of different predominant neurobiologically-grounded pathways and physiological mechanisms. Although there always will be a place for examining the broad ADHD diagnostic phenotype as a starting point to identify genes, molecular pathways, or physiological abnormalities, future research would need to more prominently consider how those variables relate to the likely handful of discrete ADHD neural subtypes that would be expected in any DSM 5-based clinical sample.
The executive and reward neurocognitive pathways are reminiscent of Research Domain Criteria (RDoC) constructs (50) and several brain function abnormalities found here align with RDoC predictions, e.g., hyperfunctional amygdala linked to RDoC approach motivation in ADHD-EF/REW and dysfunction in prefrontal and parietal cortex regions relevant to RDoC cognitive control in ADHD-EF. However, study results are not wholly consistent with RDoC expectations (51). Neurocognitive features fit dimensional expectations for non-ADHD, but not ADHD participants. This differs from a recent study comparing neuropsychological heterogeneity in ADHD and non-ADHD, but the differences can be traced back to different methodological approaches (2). In this study, the ADHD test factor structure differed from non-ADHD. Taxometric analyses also indicated the ADHD subgroups had discretely different test impairment profiles that did not fall along a simple dimension of strengths or weaknesses, as in psychosis (52). RDoC is not antithetical to the same EF abnormality arising from different neural dysfunction as found in ADHD-EF and ADHD-EF/REW, but such findings represent a challenge to RDoC conceptualizations of ADHD.
Study strengths include using both fMRI and neuropsychological test data to test the dual pathway model for the first time, moderate ADHD sample size, diverse test battery, proper use of fMRI clusterwise statistical thresholds (53), and the largely unambiguous fMRI findings suggesting multifactorial ADHD etiology. Among its limitations, we chose to sample only Combined-subtype ADHD adolescents because it seemed likely a priori that dual pathway-related brain dysfunction might be most relevant to impulsive symptomatology (54). While the results may not generalize to predominantly Inattentive ADHD DSM 5 presentations, we did not find a special relationship between the neurocognitive pathways and Hyperactive/Impulsive symptom expression. Just over half the ADHD sample contributed useable fMRI data after considering refusals, orthodontia, and quality control exclusions. Although this subsample was adequate for “whole brain” fMRI Type I error control, more participants would lend greater confidence in representativeness as well as possibly finding more subgroups, especially if a broader neurocognitive battery that assessed more than the dual pathway model test indicators were used. There also were insufficient girls for stable sex difference analyses and the study was not designed to assess the effect of pubertal status or age on relationships between subgroup membership, test performance, and brain dysfunction. The latter is important because the idea of an etiology-related “pathway” is not merely that there might be several neurobiological mechanisms underlying symptom expression, but that each likely reflects many influences that interact over time to ultimately produce a characteristic profile of brain (dys)function. It remains to be seen whether pathway-related neural features or neurocognitive subgroup membership are stable across development in ways that ADHD symptom presentation seems not to be (55).
In conclusion, this study takes an important step towards understanding how different pathways that are putatively related to etiology manifest in ADHD-diagnosed patients. It found neurocognitive markers of proposed executive and delay aversion pathways are linked to specific brain function differences. The fMRI-measured profiles described here should not be considered final biomarkers that underpin Sonuga-Barke’s proposed dual ADHD pathways (5, 6). More research is needed to discover how many neurocognitively-defined pathways exist, to make clearer predictions for what behavioral and neural features should be found in each pathway, and ultimately, to relate those observations to genetic factors, molecular mechanisms or even experiential factors that shape neural development throughout childhood to move us closer to full etiological understanding.
Supplementary Material
Figure 2.
Brain regions where participants classified by taxometric analysis into the three ADHD subgroups (ADHD-EF/REW, ADHD-EF, and ADHD-NONE) had less (orange-yellow) or more (blue-light blue) activation compared to non-ADHD controls during “false alarm” errors to ‘K’ stimuli on the GNG fMRI task. Results are thresholded at p<.05 clusterwise corrected for searching the whole brain.
Figure 3.
Brain regions where participants classified by taxometric analysis into the three ADHD subgroups (ADHD-EF/REW, ADHD-EF, and ADHD-NONE) had less (orange-yellow) or more (blue-light blue) activation compared to non-ADHD controls measured to cues signaling the availability of monetary reward on the MID fMRI task. Results are thresholded at p<.05 clusterwise corrected for searching the whole brain.
Table 4.
Brain activation amplitude differences between the ADHD-REW/EF taxometrically- determined subgroup and non-ADHD. The table lists Montreal Neurological Institute stereotactic space coordinates of peak study group difference for each cluster that was found to be significant in two-sample t test (p<.001 corrected for searching the whole brain). Results are reported for motor inhibition and error processing on the Go/NoGo task and reward prospect and reward anticipation for the Monetary Incentive Delay task. For each brain region, the Cohen’s d effect size is depicted using sparkline graph to facilitate comparison of ADHD subgroup differences with non-ADHD. The final columns report omnibus F significance p levels from a secondary ANOVA comparing the ADHD subgroups. Subgroup ANOVA-identified regions that survive False Discovery Rate corrections for multiple comparisons are denoted with †.
| Max Peak x, y, z |
t | Effect Size Pattern* | ADHD Subgroup ANOVA | ADHD Subgroup Activation Level Pairwise | |
|---|---|---|---|---|---|
| Error Processing ADHD-EF/REW > HC | |||||
| Right precentral gyrus (BA 3b/4p) | 45, −16, 56 | 3.93 |
|
.025 | REW/EF > EF |
| Left middle frontal gyrus | −33, 5, 38 | 4.61 |
|
.016 | REW/EF > EF, NONE |
| Right superior frontal gyrus (Fp1) | 24, 35, 14 | 4.22 |
|
.173 | - |
| Left inferior frontal gyrus (pars opercularis BA 44) | −54, 8, 17 | 3.97 |
|
.071 | - |
| Right inferior frontal gyrus (pars opercularis BA 44/45) | 51, 11, 23 | 3.64 |
|
.008 † | REW/EF > EF, NONE |
| Right Rolandic operculum (OP3/BA 44) | 39, −10, 23 | 4.07 |
|
.003 † | REW/EF > EF, NONE |
| Right inferior frontal gyrus (pars triangularis BA 45) | 45, 35, 11 | 3.66 |
|
.101 | - |
| Left hippocampus/amygdala | −27, −19, −13 | 3.65 |
|
.117 | - |
| Left caudate | −15, 11, 20 | 3.77 |
|
.003 † | REW/EF > EF, NONE |
| Reward Prospect HC > ADHD-EF/REW | |||||
| Left inferior parietal lobule | −39, −37, 35 | 3.38 |
|
.027 | REW/EF > NONE |
| Effort to Obtain Reward ADHD-EF/REW > HC | |||||
| Right insula (Ig2/Ig1/TE1/OP3) | 39, −22, 11 | 4.18 |
|
0.078 | - |
| Left amygdala/hippocampus | −21, −10, −16 | 3.93 |
|
0.074 | - |
| Right amgydala | 24, −1, −7 | 3.37 |
|
0.047 | REW/EF > EF, NONE |
| Right putamen | 24, 14, −4 | 3.59 |
|
0.008 † | REW/EF > EF, NONE |
For sparkline graphs, the initial grey datapoint represents the Cohen’s d effect size from a supplemental two-sample t test that compared non-ADHD to all of the ADHD participants. Afterwards, subgroups ADHD-EF, ADHD-EF/REW, and ADHD-NONE are depicted (left-to-right). All sparkline values on this table are scaled relative to the maximum Cohen’s d=1.05 found across all four fMRI task conditions for the ADHD-EF/REW subgroup.
Acknowledgments
This project was funded by NIH grant R01MH080956. Thanks go to the research staff on the project, including Danielle Francois, Nicole Pompay, Ethan Rosenfeld, and Christina Wong. Special thanks go to Dr. Ralitza Gueorguieva for her input on statistical modeling options.
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coghill DR, Seth S, Matthews K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychol Med. 2014;44:1989–2001. doi: 10.1017/S0033291713002547. [DOI] [PubMed] [Google Scholar]
- 2.Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A. 2012;109:6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57:1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. The American journal of psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Sonuga-Barke EJ, Sergeant JA, Nigg J, Willcutt E. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: nosologic and diagnostic implications. Child Adolesc Psychiatr Clin N Am. 2008;17:367–384. ix. doi: 10.1016/j.chc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Durston S, van Belle J, de Zeeuw P. Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:1178–1184. doi: 10.1016/j.biopsych.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- 9.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28:397–419. doi: 10.1017/S0140525X05000075. discussion 419–368. [DOI] [PubMed] [Google Scholar]
- 10.de Zeeuw P, Weusten J, van Dijk S, van Belle J, Durston S. Deficits in cognitive control, timing and reward sensitivity appear to be dissociable in ADHD. PLoS One. 2012;7:e51416. doi: 10.1371/journal.pone.0051416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Thorell LB. Do delay aversion and executive function deficits make distinct contributions to the functional impact of ADHD symptoms? A study of early academic skill deficits. J Child Psychol Psychiatry. 2007;48:1061–1070. doi: 10.1111/j.1469-7610.2007.01777.x. [DOI] [PubMed] [Google Scholar]
- 13.Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, et al. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- 14.Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Sonuga-Barke EJ, Dalen L, Remington B. Do executive deficits and delay aversion make independent contributions to preschool attention-deficit/hyperactivity disorder symptoms? J Am Acad Child Adolesc Psychiatry. 2003;42:1335–1342. doi: 10.1097/01.chi.0000087564.34977.21. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy H, Skokauskas N, Frodl T. Identifying a consistent pattern of neural function in attention deficit hyperactivity disorder: a meta-analysis. Psychol Med. 2014;44:869–880. doi: 10.1017/S0033291713001037. [DOI] [PubMed] [Google Scholar]
- 17.Ambrosini PJ. Historical development and present status of the schedule for affective disorders and schizophrenia for school-age children (K-SADS) J Am Acad Child Adolesc Psychiatry. 2000;39:49–58. doi: 10.1097/00004583-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Ma I, van Duijvenvoorde A, Scheres A. The interaction between reinforcement and inhibitory control in ADHD: A review and research guidelines. Clin Psychol Rev. 2016;44:94–111. doi: 10.1016/j.cpr.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Ruscio J, Ruscio AM, Carney LM. Performing Taxometric Analysis to Distinguish Categorical and Dimensional Variables. J Exp Psychopathol. 2011;2:170–196. doi: 10.5127/jep.010910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruscio J, Walters GD, Marcus DK, Kaczetow W. Comparing the relative fit of categorical and dimensional latent variable models using consistency tests. Psychol Assess. 2010;22:5–21. doi: 10.1037/a0018259. [DOI] [PubMed] [Google Scholar]
- 21.Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 24.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. Neuroimage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- 27.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Brown TE. Brown Attention-Deficit Disorder Scales for Adolescents and Adults. San Antonio, TX: Harcourt Assessment; 2001. [Google Scholar]
- 29.Altepeter TS, Breen MJ. The Home Situations Questionnaire (HSQ) and the School Situations Questionnaire (SSQ): Normative data an an evaluation of pscychometric properties. Journal of Psychoeducational Assessment. 1989;7:312–322. [Google Scholar]
- 30.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 31.Lei D, Du M, Wu M, Chen T, Huang X, Du X, et al. Functional MRI reveals different response inhibition between adults and children with ADHD. Neuropsychology. 2015;29:874–881. doi: 10.1037/neu0000200. [DOI] [PubMed] [Google Scholar]
- 32.Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, et al. Structural and Functional Brain Abnormalities in Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder: A Comparative Meta-analysis. JAMA psychiatry. 2016;73:815–825. doi: 10.1001/jamapsychiatry.2016.0700. [DOI] [PubMed] [Google Scholar]
- 33.Rae CL, Hughes LE, Weaver C, Anderson MC, Rowe JB. Selection and stopping in voluntary action: a meta-analysis and combined fMRI study. Neuroimage. 2014;86:381–391. doi: 10.1016/j.neuroimage.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugman S, Schuhmann T. A network approach to response inhibition: dissociating functional connectivity of neural components involved in action restraint and action cancellation. The European journal of neuroscience. 2014;39:821–831. doi: 10.1111/ejn.12425. [DOI] [PubMed] [Google Scholar]
- 35.Criaud M, Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci Biobehav Rev. 2013;37:11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Braet W, Johnson KA, Tobin CT, Acheson R, McDonnell C, Hawi Z, et al. fMRI activation during response inhibition and error processing: the role of the DAT1 gene in typically developing adolescents and those diagnosed with ADHD. Neuropsychologia. 2011;49:1641–1650. doi: 10.1016/j.neuropsychologia.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Plessen KJ, Allen EA, Eichele H, van Wageningen H, Hovik MF, Sorensen L, et al. Reduced error signalling in medication-naive children with ADHD: associations with behavioural variability and post-error adaptations. J Psychiatry Neurosci. 2016;41:77–87. doi: 10.1503/jpn.140353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. The American journal of psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 39.Vasic N, Plichta MM, Wolf RC, Fallgatter AJ, Sosic-Vasic Z, Gron G. Reduced neural error signaling in left inferior prefrontal cortex in young adults with ADHD. J Atten Disord. 2014;18:659–670. doi: 10.1177/1087054712446172. [DOI] [PubMed] [Google Scholar]
- 40.Neta M, Miezin FM, Nelson SM, Dubis JW, Dosenbach NU, Schlaggar BL, et al. Spatial and temporal characteristics of error-related activity in the human brain. J Neurosci. 2015;35:253–266. doi: 10.1523/JNEUROSCI.1313-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Sonuga-Barke EJ, Wiersema JR, van der Meere JJ, Roeyers H. Context-dependent dynamic processes in attention deficit/hyperactivity disorder: differentiating common and unique effects of state regulation deficits and delay aversion. Neuropsychol Rev. 2010;20:86–102. doi: 10.1007/s11065-009-9115-0. [DOI] [PubMed] [Google Scholar]
- 43.Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev. 2014;38:125–134. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blum K, Chen AL, Braverman ER, Comings DE, Chen TJ, Arcuri V, et al. Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatr Dis Treat. 2008;4:893–918. doi: 10.2147/ndt.s2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters J, Buchel C. Neural representations of subjective reward value. Behav Brain Res. 2010;213:135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 46.Kurniawan IT, Guitart-Masip M, Dayan P, Dolan RJ. Effort and valuation in the brain: the effects of anticipation and execution. J Neurosci. 2013;33:6160–6169. doi: 10.1523/JNEUROSCI.4777-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vassena E, Silvetti M, Boehler CN, Achten E, Fias W, Verguts T. Overlapping neural systems represent cognitive effort and reward anticipation. PLoS One. 2014;9:e91008. doi: 10.1371/journal.pone.0091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemiere J, Danckaerts M, Van Hecke W, Mehta MA, Peeters R, Sunaert S, et al. Brain activation to cues predicting inescapable delay in adolescent Attention Deficit/Hyperactivity Disorder: an fMRI pilot study. Brain Res. 2012;1450:57–66. doi: 10.1016/j.brainres.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 49.Bellec P, Chu C, Chouinard-Decorte F, Benhajali Y, Margulies DS, Craddock RC. The Neuro Bureau ADHD-200 Preprocessed repository. NeuroImage. 2017;144:275–286. doi: 10.1016/j.neuroimage.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 50.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuthbert BN. Research Domain Criteria: toward future psychiatric nosologies. Dialogues Clin Neurosci. 2015;17:89–97. doi: 10.31887/DCNS.2015.17.1/bcuthbert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. The American journal of psychiatry. 2016;173:373–384. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI clustering in AFNI: False positive rates redux. Brain Connectivitiy. doi: 10.1089/brain.2016.0475. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012;121:991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.