Abstract
No-reflow phenomenon is defined as the reduced blood flow after myocardial ischemia. If prolonged it leads to profound damages in the myocardium. The lack of a detailed knowledge about the cells mediating no-reflow restricts the design of effective therapies. Recently, O'Farrell et al. (2017) by using state-of-the-art technologies, including high-resolution confocal imaging in combination with myocardial ischemia/reperfusion mouse model, reveal that pericytes contribute to the no-reflow phenomenon post-ischemia in the heart. Strikingly, intravenous adenosine increased vascular diameter at pericyte site after cardiac ischemia. This study provides a novel therapeutic target to inhibit no-reflow phenomenon after myocardial ischemia.
Keywords: Pericytes, Heart, Vasoconstriction, Ischemia
Cardiovascular diseases are among the major public health concerns in the world [1]. Myocardial ischemic disease leads to disability, and is considered the main cause of mortality worldwide [2]. Early coronary reperfusion of the infracted heart is the major therapeutic strategy to treat this heart disease [3,4]. After re-opening of the coronary artery post-temporary ischemia, portions of the microvasculature may fail to completely reperfuse, leading to the no-reflow phenomenon [5]. This phenomenon happens in approximately 30% of patients who receive coronary intervention after acute myocardial infarct. No-reflow leads to worse patients' prognosis, and is correlated with infarct expansion, more congestive heart failure, and increased death [6–8]. Thus, no-reflow may persist for several weeks, and predicts adverse clinical outcome [8]. Therefore, approaches to improve microvascular blood flow to treat myocardial ischemia are needed.
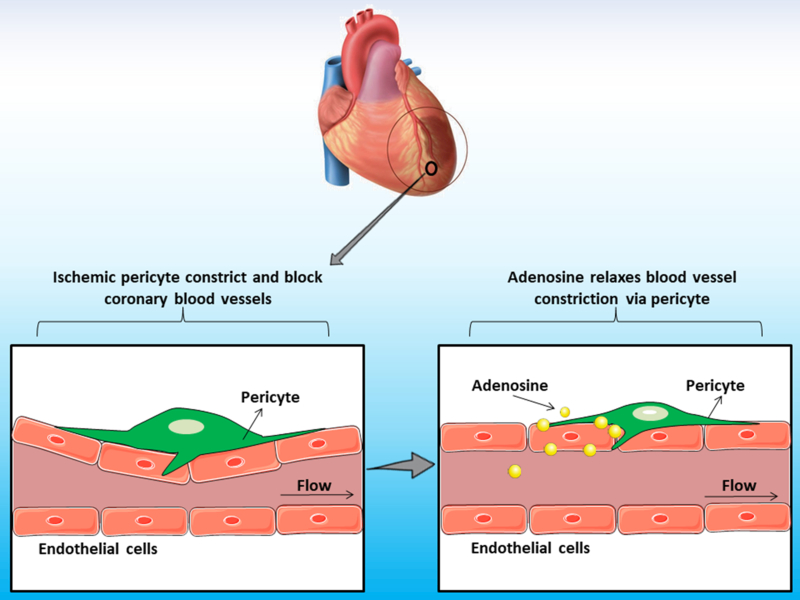
The pathophysiological mechanisms involved in no-reflow are poorly understood. Which are the cells and the underlying molecular mechanisms involved in this process that directly contribute to myocardial ischemic disease remains unknown. The lack of a detailed knowledge about the cellular contributors mediating no-reflow restricts the design of effective treatments. Elucidating the causes of the no-reflow phenomenon should be beneficial for patients with myocardial ischemia. Now, in a recent article in eLife, O'Farrell and colleagues reveal that pericytes participate in the no-reflow phenomenon post-ischemia in the heart [9]. The authors investigated the role of pericytes after transient cardiac ischemia by using state-of-the-art techniques, including high-resolution confocal imaging in combination with myocardial ischemia/reperfusion mouse model. O'Farrell and colleagues imaged cardiac pericytes in NG2-DsRed mice after induction of left anterior descending (LAD) coronary artery occlusion. These experiments unveiled that microvascular blockage localized predominantly in close proximity to pericytes, when compared with random distribution of hypothetical blockages throughout the heart vasculature [9]. Importantly, the diameter of the vascular lumen measured at pericyte's somata was reduced by more than one-third, indicating that pericytes' processes constrict cardiac blood vessels post-ischemia [9]. Strikingly, intravenous adenosine increased vascular diameter at pericyte somata after cardiac ischemia, suggesting that adenosine reduces no-reflow by reversing the constriction caused by pericytes evoked by ischemia [9]. This study provides a new role for pericytes in the cardiac microenvironment, and offers a novel therapeutic target to inhibit no-reflow phenomenon after myocardial ischemia (Fig. 1).
Fig. 1.
Cardiac pericytes induce vasoconstriction after myocardial ischemia.
Pericytes are present surrounding the vasculature of the heart. The study of O'Farrell and colleagues now suggests a novel role for pericytes in the vasculature of infarcted hearts [9]. Cardiac pericytes induce blood vessel constriction post-ischemia, which is reversed by adenosine delivery. Future studies will reveal in detail the cellular and molecular mechanisms involved in this process in the tissue microenvironment in the heart.
Here, we discuss the findings from this work, and evaluate recent advances in our understanding of the roles of pericytes in the heart.
1. Perspectives/future directions
The main findings from this study are based on the data obtained from NG2-DsRed mice [9]. Nevertheless, NG2 proteoglycan is not specific to pericytes, as other cells may express this protein [10]. Interestingly, pericytes that do not express NG2 proteoglycan were also reported [11]. Currently, there is no single molecular marker yet that can be used to unequivocally label exclusively the whole population of cardiac pericytes. Interestingly, not all perivascular cells are necessarily pericytes. In addition to pericytes, other cells have been described in this position surrounding the blood vessels, including macrophages [12], fibroblasts [13], adventitial cells [14], and vascular smooth muscle cells [15]. Altogether this brings the possibility that some of the observations by O'Farrell et al. (2017) are in a different, non-pericytic, cell population. Presently, the state-of-the-art identification of pericytes in tissue preparations relies on a combination of anatomical localization (covering endothelial cells and located below the basal lamina), morphology, and the co-expression of at least two pericytic molecular markers. The discovery of a single molecular marker specific to all cardiac pericytes will facilitate the study of the behavior of these cells in the heart.
Pericytes are heterogeneous regarding their distribution, phenotype, marker expression, origin, and function [16,31–47], several subtypes have been characterized in various organs [11,17–20], including the heart [21]. Cardiac pericytes subpopulations, type-1 (NG2 +/Nestin-GFP−) and type-2 (NG2 +/Nestin-GFP +) pericytes, were identified in the perivascular space of cardiac blood vessels using bitransgenic Nestin-GFP/NG2-DsRed mice [21]. Interestingly, after LAD coronary artery ligation, type-1 pericytes multiply, and are recruited to the infarcted area after myocardial infarction [21]. Whether both pericyte subpopulations contribute to the no-felow phenomenon after cardiac ischemia remains to be examined. Are present during brain embryogenesis remains unknown. Or, more intriguingly, is a specific pericyte subset responsible for vasoconstriction after ischemia in the heart?
Although O'Farrell and colleagues suggest that post-ischemic vasoconstriction depends on pericytes in the heart [9], they do not explore the molecular and cellular mechanisms involved in this process. Which signaling molecules are necessary for pericytes to reduce the vascular tone after cardiac ischemia? Recently, it has been shown, after spinal cord injury, that expression of the enzyme aromatic L-amino acid decarboxylase (AADC) is essential for pericyte-induced vasoconstriction [22]. Is this enzyme also important in cardiac pericytes post-ischemia? In addition to studies in genetic mouse models, transcriptomic and single cell analysis of cardial pericytes after infarct will allow us to understand better the molecular mechanisms involved in the no-reflow phenomenon in the cardiac microenvironment. Furthermore, it remains not understood whether other cells are involved and cross-talk with pericytes during the no-reflow phenomenon. Are other cells inducing pericytes to contract and promote vasoconstriction? Additionally, as discussed here, there are several other perivascular cells in the cardiac vasculature. How other perivascular cells participate in this process remains to be elucidated. It well known that pericytes produce several signals well as respond to various molecules, communicating with other cells, such as endothelial cells. In contrast, very little is known about the cross-talk within the population of pericytes. Future studies will need to explore how pericytes communicate with their peers in the heart.
The use of transgenic mouse models in which specific cell types can be genetically ablated has proved valuable for understanding the role of specific cell types in physiological and pathological states [48–54]. O'Farrell and colleagues place pericytes as central responsibles in cardiac vasoconstriction [9]. Nevertheless, pericytes have not yet been deleted from the heart after myocardial ischemia. Thus, the role of pericytes in the no-reflow phenomenon remains uncertain. Does the vasoconstriction still happen or alleviate in the absence of cardiac pericytes?
Adenosine is a purine nucleoside with several physiopathological roles [23]. This molecule is found endogenously in the extracellular space of multiple tissues [24]. O'Farrell and colleagues suggest that administered endogenously adenosine reduces pericyte-derived vasoconstriction after ischemia [9]. It remains to be explored whether the endogenous adenosine is essential for the role of pericytes in the normal cardiac vascular tone. Adenosine acts via several adenosine receptors which are all members of the G-protein-coupled receptor family [25]. Pericytes have been shown to express adenosine A2 receptors [26]. However, it remains unexplored which adenosine receptors are present on cardiac pericytes, and which of those are important for pericytes to constrict the underlying endothelial bed. Moreover, other cell populations may express adenosine receptors, including several cardiac vascular cells, such as endothelial cells [27]. Thus, it remains unclear whether the observed vasodilation after adenosine administration is exclusively due to pericytes, as other cells may be contributing as well. The modern technologies which delete single genes in specific cells in adult mice has allowed us to answer important questions regarding the roles of distinct cell types in the regulation of several physiologic and pathologic events. Adenosine receptors have not been conditionally deleted from pericytes in the heart, so there is no direct evidence that pericytes are the only/main functionally important cell responding to the adenosine treatment. This issue may be addressed by analyzing the effect of genetic ablation of adenosine receptors in cardiac pericytes on the vascular tone in the heart. The generation of A2R floxed mice to be crossed with pericyte-specific inducible CreER drivers [17], will allow us to specifically delete A2 adenosine receptor in pericytes. In addition to studies genetic mouse models, transcriptomic and single pericyte analysis represent fundamental tools that will help us understand the roles of pericytes within the heart.
Although pericytes are involved in the no-reflow phenomenon [9], it remains to be examined the molecular and cellular mechanisms involved in this process. Which endogenous signaling molecules are needed to activate pericytes-dependent vasoconstriction? A recent study, in the spinal cord, has shown that expression of the enzyme aromatic L-amino acid decarboxylase (AADC) is important for pericyte-induced vasoconstriction after spinal cord injury [22,55]. Is this enzyme also important in the cardiac pericyte after myocardial infarct? Interestingly, recently it has been shown that cerebral pericytes are plastic, and are able to extend processes and replace adjacent pericytes, exerting their function [28]. Pericytes' plasticity in the heart should be explored in future studies.
Interestingly, signals from the sympathetic nervous system regulate pericytes' function in the bone marrow microenvironment [29,30]. O'Farrell and colleagues showed that half of cardiac pericytes are located close to sympathetic neurons varicosities, implicating a possible role of noradrenergic regulation in pericytes' behavior [9]. Future experiments will reveal whether this regulation in fact occurs. Analysis of pericytes in the heart after genetic sympathectomy or achieved by neurotoxin 6-hydroxydopamine treatment will reveal the role of sympathetic axons in pericytes control of vascular tone. Additionally, deletion of adrenergic receptors in pericytes will allow to elucidate the role of noradrenaline on cardiac pericytes.
In conclusion, the study by O'Farrell and colleagues reveal a novel important role of pericytes in the ischemic heart. Nevertheless, our understanding of cross-talk between different cell types present in the cardiac vascular microenvironment remains limited, and the complexity of these interactions in distinct physiologic and pathologic conditions should be elucidated in future studies. A big challenge faced now is how to translate animal research into humans. Improving the availability of human tissue samples may help to reach this goal.
Acknowledgments
Alexander Birbrair is supported by a grant from Instituto Serrapilheira/Serra-1708-15285, and a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); Alexander Birbrair and Ricardo Gorçalves are supported by a grant from FAPEMIG [Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC, RED-00570-16)], and a grant from FAPEMIG [Rede De Pesquisa Em Doerças Infecciosas Humanas E Animais Do Estado De Minas Gerais (RED-00313-16)]; Akiva Mintz is supported by the National Institute of Health (1R01CA179072-01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13-121-01-CDD).
Footnotes
Disclosures
The authors indicate no potential conflicts of interest.
References
- [1].Hausenloy DJ, Yellon DM, Myocardial ischemia-reperfusion injury: a neglected therapeutic target, J. Clin. Invest 123 (1) (2013) 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fauconnier J, Meli AC, Thireau J, Roberge S, Shan J, Sassi Y, Reiken SR, Rauzier JM, Marchand A, Chauvier D, Cassan C, Crozier C, Bideaux P, Lompre AM, Jacotot E, Marks AR, Lacampagne A, Ryanodine receptor leak mediated by caspase-8 activation leads to left ventricular injury after myocardial ischemia-reperfusion, Proc. Natl. Acad. Sci. U. S. A 108 (32) (2011) 13258–13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American C Heart Association Statistics, S. Stroke Statistics, Heart disease and stroke statistics—2014 update: a report from the American Heart Association, Circulation 129 (3) (2014) e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zaman MJ, Stirling S, Shepstone L, Ryding A, Flather M, Bachmann M, Myint PK, The association between older age and receipt of care and outcomes in patients with acute coronary syndromes: a cohort study of the Myocardial Ischaemia National Audit Project (MINAP), Eur. Heart J 35 (23) (2014) 1551–1558. [DOI] [PubMed] [Google Scholar]
- [5].Reffelmann T, Kloner RA, The no-reflow phenomenon: a basic mechanism of myocardial ischemia and reperfusion, Basic Res. Cardiol 101 (5) (2006) 359–372. [DOI] [PubMed] [Google Scholar]
- [6].Bolognese L, Falsini G, Liistro F, Angioli P, Ducci K, Epicardial and microvascular reperfusion with primary percutaneous coronary intervention, Ital. Heart J 6 (6) (2005) 447–452. [PubMed] [Google Scholar]
- [7].Brosh D, Assali AR, Mager A, Porter A, Hasdai D, Teplitsky I, Rechavia E, Fuchs S, Battler A, Kornowski R, Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality, Am. J. Cardiol 99 (4) (2007) 442–445. [DOI] [PubMed] [Google Scholar]
- [8].Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T, Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction, J. Am. Coll. Cardiol 36 (4) (2000) 1202–1209. [DOI] [PubMed] [Google Scholar]
- [9].O'Farrell FM, Mastitskaya S, Hammond-Haley M, Freitas F, Wah WR, Attwell D, Capillary pericytes mediate coronary no-reflow after myocardial ischaemia, elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wohl SG, Schmeer CW, Friese T, Witte OW, Isenmann S, In situ dividing and phagocytosing retinal microglia express nestin, vimentin, and NG2 in vivo, PLoS One 6 (8) (2011) e22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S, Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct' them with pattern-recognition and motility programs, Nat. Immunol 14 (1) (2013) 41–51. [DOI] [PubMed] [Google Scholar]
- [12].Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R, Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages, Eur. J. Neurosci 14 (10) (2001) 1651–1658. [DOI] [PubMed] [Google Scholar]
- [13].Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, Lee JK, Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury, J. Neurosci 33 (34) (2013) 13882–13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Crisan M, Corselli M, Chen WC, Peault B, Perivascular cells for regenerative medicine, J. Cell. Mol. Med 16 (12) (2012) 2851–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wanjare M, Kusuma S, Gerecht S, Perivascular cells in blood vessel regeneration, Biotechnol. J 8 (4) (2013) 434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dias Moura PH, Sena Prazeres IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro Dos GS, Mintz Santos A, Delbono O, Birbrair A, Pericytes are heterogeneous in their origin within the same tissue, Dev. Biol 427 (1) (2017) 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma'ayan A, Frenette PS, Differential cytokine contributions of perivascular haematopoietic stem cell niches, Nat. Cell Biol 19 (3) (2017) 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, Pinho S, Ciero P, Nakahara F, Ma'ayan A, Bergman A, Merad M, Frenette PS, Fetal liver hematopoietic stem cell niches associate with portal vessels, Science 351 (6269) (2016) 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O, Skeletal muscle pericyte subtypes differ in their differentiation potential, Stem Cell Res 10 (1) (2013) 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J, A pericyte origin of spinal cord scar tissue, Science 333 (6039) (2011) 238–242. [DOI] [PubMed] [Google Scholar]
- [21].Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O, Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner, Stem Cell Res Ther 5 (6) (2014) 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li Y, Lucas-Osma AM, Black S, Bandet MV, Stephens MJ, Vavrek R, Sanelli L, Fenrich KK, Di Narzo AF, Dracheva S, Winship IR, Fouad K, Bennett DJ, Pericytes impair capillary blood flow and motor function after chronic spinal cord injury, Nat. Med 23 (6) (2017) 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fredholm BB, Adenosine, an endogenous distress signal, modulates tissue damage and repair, Cell Death Differ 14 (7) (2007) 1315–1323. [DOI] [PubMed] [Google Scholar]
- [24].Latini S, Pedata F, Adenosine in the central nervous system: release mechanisms and extracellular concentrations, J. Neurochem 79 (3) (2001) 463–484. [DOI] [PubMed] [Google Scholar]
- [25].Gessi S, Merighi S, Borea PA, Targeting adenosine receptors to prevent inflammatory skin diseases, Exp. Dermatol 23 (8) (2014) 553–554. [DOI] [PubMed] [Google Scholar]
- [26].Jackson JA, Carlson EC, Inhibition of bovine retinal microvascular pericyte proliferation in vitro by adenosine, Am. J. Phys 263 (2 Pt 2) (1992) H634–40. [DOI] [PubMed] [Google Scholar]
- [27].Fredholm BB, Irenius E, Kull B, Schulte G, Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem. Pharmacol 61 (4) (2001) 443–448. [DOI] [PubMed] [Google Scholar]
- [28].Berthiaume AA, Grant RI, McDowell KP, Underly RG, Hartmann DA, Levy M, Bhat NR, Shih AY, Dynamic remodeling of pericytes in vivo maintains capillary coverage in the adult mouse brain, Cell Rep 22 (1) (2018) 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hanoun M, Maryanovich M, Arnal-Estape A, Frenette PS, Neural regulation of hematopoiesis, inflammation, and cancer, Neuron 86 (2) (2015) 360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Birbrair A, Frenette PS, Niche heterogeneity in the bone marrow, Ann. N. Y. Acad. Sci 1370 (1) (2016) 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Azevedo PO, Sena IFG, Andreotti JP, Carvalho-Tavares J, Alves-Filho JC, Cunha TM, Cunha FQ, Mintz A, Birbrair A, Pericytes modulate myelination in the central nervous system, J. Cell. Physiol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, Mintz A, Delbono O, How plastic are pericytes? Stem Cells Dev 26 (2017) 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Birbrair A, Delbono O, Pericytes are essential for skeletal muscle formation, Stem Cell Rev 11 (2015) 547–548. [DOI] [PubMed] [Google Scholar]
- [34].Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista I, Nguyen VT, Messi ML, Solingapuram Sai KK, Marini FC, Delbono O, et al. , Novel peripherally derived neural-like stem cells as therapeutic carriers for treating glioblastomas, Stem Cells Transl. Med 6 (2017) 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O, Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle, PLoS One 6 (2011) e16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O, Role of pericytes in skeletal muscle regeneration and fat accumulation, Stem Cells Dev 22 (2013) 2298–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O, Skeletal muscle neural progenitor cells exhibit properties of NG2-glia, Exp. Cell Res 319 (2013) 45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O, Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle, Am. J. Physiol. Cell Physiol 305 (2013) C1098–C1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O, Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle, Front. Aging Neurosci 6 (2014) 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O, Pericytes at the intersection between tissue regeneration and pathology, Clin. Sci 128 (2015) 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O, Type-2 pericytes participate in normal and tumoral angiogenesis, Am. J. Physiol. Cell Physiol 307 (2014) C25–C38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Coatti GC, Frangini M, Valadares MC, Gomes JP, Lima NO, Cavacana N, Assoni AF, Pelatti MV, Birbrair A, de Lima ACP, et al. , Pericytes extend survival of ALS SOD1 mice and induce the expression of antioxidant enzymes in the murine model and in IPSCs derived neuronal cells from an ALS patient, Stem Cell Rev (2017). [DOI] [PubMed] [Google Scholar]
- [43].Prazeres P, Almeida VM, Lousado L, Andreotti JP, Paiva AE, Santos GSP, Azevedo PO, Souto L, Almeida GG, Filev R, et al. , Macrophages generate pericytes in the developing brain, Cell. Mol. Neurobiol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Santos GSP, Prazeres P, Mintz A, Birbrair A, Role of pericytes in the retina, Eye (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sena IFG, Borges IT, Lousado L, Azevedo PO, Andreotti JP, Almeida VM, Paiva AE, Santos GSP, Guerra DAP, Prazeres P, et al. , LepR + cells dispute hegemony with Glil + cells in bone marrow fibrosis, Cell Cycle (2017) 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sena IFG, Prazeres P, Santos GSP, Borges IT, Azevedo PO, Andreotti JP, Almeida VM, Paiva AE, Guerra DAP, Lousado L, et al. , Identity of Glil + cells in the bone marrow, Exp. Hematol 54 (2017) 12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sena IFG, Paiva AE, Prazeres PHDM, Azevedo PO, Lousado L, Bhutia SK, Salmina AB, Mintz A, Birbrair A, Glioblastoma-activated pericytes support tumor growth via immunosuppression, (2018) Cancer Medicine [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Andreotti JP, Lousado L, Magno LAV, Birbrair A, Hypothalamic neurons take center stage in the neural stem cell niche, Cell Stem Cell 21 (2017) 293–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Azevedo PO, Lousado L, Paiva AE, Andreotti JP, Santos GSP, Sena IFG, Prazeres P, Filev R, Mintz A, Birbrair A, Endothelial cells maintain neural stem cells quiescent in their niche, Neuroscience 363 (2017) 62–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Birbrair A, Stem cell microenvironments and beyond, Adv. Exp. Med. Biol 1041 (2017) 1–3. [DOI] [PubMed] [Google Scholar]
- [51].Borges I, Sena I, Azevedo P, Andreotti J, Almeida V, Paiva A, Santos G, Guerra D, Prazeres P, Mesquita LL, et al. , Lung as a niche for hematopoietic progenitors, Stem Cell Rev 13 (2017) 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Batista ML Jr., Birbrair A, Adipocytes role in the bone marrow niche, Cytom. A J. Int. Soc. Anal. Cytol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lousado L, Prazeres P, Andreotti JP, Paiva AE, Azevedo PO, Santos GSP, Filev R, Mintz A, Birbrair A, Schwann cell precursors as a source for adrenal gland chromaffin cells, Cell Death Dis 8 (2017) e3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Paiva AE, Lousado L, Almeida VM, Andreotti JP, Santos GSP, Azevedo PO, Sena IFG, Prazeres P, Borges IT, Azevedo V, et al. , Endothelial cells as precursors for osteoblasts in the metastatic prostate cancer bone, Neoplasia 19 (2017) 928–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Almeida VM, Paiva AE, Sena IFG, Mintz A, Magno LAV, Birbrair A, Pericytes make spinal cord breathless after injury, Neuroscientist (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]