Short abstract
Objective
To evaluate the antioxidant and apoptotic inductive effects of Withania somnifera (Ashwagandha) leaf extract against a hepatocellular carcinoma cell line.
Methods
After treating HepG2cells with Ashwagandha water extract (ASH-WX; 6.25 mg/ml–100 mg/ml), cell proliferation was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Antioxidant activities (total antioxidant, glutathione S-transferase and glutathione reductase), Fas-ligand level, tumour necrosis factor-α (TNF-α) level and caspase-3, -8, and -9 activities were measured. Molecular modelling assessed the binding-free energies of Ashwagandha in the cyclin D1 receptor.
Results
The MTT assay demonstrated increased cytotoxicity following treatment of HepG2 cells with ASH-WX compared with control untreated cells and theIC50was 5% (approximately 5.0 mg/ml). Antioxidant activities, Fas-ligand levels and caspase-3, -8 and -9 activities significantly increased, while TNF-α level significantly decreased following ASH-WX treatment compared with control untreated cells. Molecular docking analysis revealed a good prediction of binding between cyclin D1 and Ashwagandha. There was significant accumulation of ASH-WX-treated HepG2cells in the G0/G1 and G2/M phases compared with the control untreated cells.
Conclusion
Ashwagandha could be a powerful antioxidant and a promising anticancer agent against HCC.
Keywords: Ashwagandha, HepG2, cytotoxicity, antioxidants, apoptosis
Introduction
Cancer is a group of heterogeneous hereditary disorders that share common alterations in different cellular signalling pathways. 1 Apoptosis is one of the main alterations that dictate malignant growth. 2 Moreover, other characteristics include self-sufficiency in growth signalling, alteration of cellular bioenergetics, evasion of immune detection and tissue invasion and metastasis have been described.3,4 Genome instability and mutations are essential for tumour progression and facilitate acquisition of these hallmarks. 5 Coordinated processes such as cell proliferation, differentiation, and apoptosis are modified, producing altered cellular phenotypes with these specific characteristics. 6
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer in the world and the second largest contributor to cancer mortality. 7 More than 80% of HCC cases around the world occur in sub-Saharan Africa and in Eastern Asia, with typical incidence rates of more than 20 per 100 000 individuals. 8 HCC represents approximately 11.75% of all the gastrointestinal cancers, and about 1.68% of the total malignancies in Egypt. 9 In Egypt, HCC arises mainly as complications of cirrhosis, which results from hepatitis C virus. 10 According to the Egyptian National Cancer Institute, HCC is the third most common cancer after lymphoma in both genders (8.1%), first in males (12.1%) and fifth in females (4.0%). 11
There are various treatment options for HCC such as curative resection, liver transplantation, radiofrequency ablation, transarterial chemoembolization, radioembolization and systemic targeted agents such as sorafenib. 12 Although the short-term survival of patients with HCC has improved, these treatments have many side-effects, which are toxic and harmful for patients, such as pain, fatigue, emotional distress and anaemia, in addition to their high cost. 13
There is now a trend toward the use of complementary medicines for treating and reducing cancer symptoms and pain. 14 Since ancient times, natural products had been utilized as conventional drugs in various parts of the world including Egypt, China, Greece and India. 15 These botanical products had been used as prophylactic agents for the treatment of many diseases including cancer, as they have an anticancer effects against different types of cancer. 16 These natural products have different mechanisms of action including the inhibition of cell progression, alteration of cell differentiation and induction of apoptosis. 17 Ashwagandha (Withania somnifera) is a natural herb that has been investigated in a wide range of conditions including muscle strain, 18 fatigue, 19 aches, skin infections, rheumatoid joint inflammation 20 and as an anticancer agent.21,22 Recent studies demonstrated that Ashwagandha water extract (ASH-WX) is a powerful antioxidant and it can inhibit cancer cell growth, thus it might be a good example of a natural and economic anticancer medicine.23,24
The aim of this study was to investigate the effects of ASH-WX as an antioxidant and an anticancer agent against HCC.
Materials and methods
Ashwagandha water extract preparation
Egyptian Ashwagandha leaves were collected from Rafah, El-Arish, Egypt in September 2015, as fresh wet leaves, which were then sun-dried, ground and filtered by sieving to get a fine dry powder as previously described. 25 ASH-WX was prepared by suspending 100 g of dry powder in 1 l of double-distilled water with stirring overnight at 45 ± 5°C. Then the mixture was filtered under sterile conditions. The sterile filtrate was treated as 100% ASH-WX as previously described and stored at –20°C for future use. 26
HepG2 cell culture and treatment with ASH-WX
The HCC cell line HepG2 was obtained from the National Cancer Institute, Cairo, Egypt. The cells were cultured in RPMI 1640 medium (Sigma-Aldrich, St Louis, MO, USA) as described previously. 27 The cells (1 × 106 cells/ml) were then treated with a range of concentrations of ASH-WX (6.25 mg/ml–100 mg/ml) as described below.
Cytotoxicity analysis by MTT assay and examination of cell viability
The effect of ASH-WX on cell viability was estimated using a3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously. 28 Briefly, after maintaining the cells in RPMI 1640 medium for 24 h, the medium of each well was removed, the cells were washed twice using 1.0 M phosphate-buffered saline (PBS; pH7.4) at 25°C, and the medium was replaced with 100 μl of ASH-WX at a range of concentrations (6.25 mg/ml–100 mg/ml). The cells were incubated at 37°C in a 5% CO2 incubator for 24 h. Then, 10 μl MTT solution (5 mg/1 ml of 1.0 M PBS pH 7.4) was added and the cells incubated for 4 h at 37°C in a 5% CO2 incubator. The medium was discarded and each well was supplemented with 100 μl of dimethyl sulphoxide, mixed thoroughly using a pipette, and incubated in a dark room for 2 h. The absorbance of each well was read at 570 nm with a plate reader (Sunrise™; Tecan Group, Männedorf, Switzerland). GraphPad Prism 7 software (GraphPad Software, La Jolla, CA, USA) was used to calculate the percentage viability and the half-maximal inhibitory concentration (IC50) of ASH-WX.
The viability of the cells was determined using an inverted microscope (Zeiss Axio Vert.A1; Zeiss, Gottingen, Germany) at 40 × magnification after incubation of HepG2 cells with ASH- WX at a range of concentrations (6.25 mg/ml–100 mg/ml) at 37 °C in a 5% CO2 incubator for 24 h.
Assessment of antioxidant activity
Antioxidants are synthesized or natural compounds that may prevent or delay some types of cell damage. 29 Total antioxidant, glutathione S-transferase and glutathione reductase levels were measured in HepG2cells treated with ASH-WX at three concentrations (2.5, 5.0, and 10.0 mg/ml) after incubation for 48 h at 37 °C in a 5% CO2 incubator using colorimetric assay kits (Biodiagnostic, Giza, Egypt) following the manufacturer’s instructions.
Assessment of concentrations of FAS-ligand and TNF-α
The Fas/Fas-ligand pathway is one of the most important pathways that induce apoptosis inside cells. After incubation of HepG2 cells with ASH-WX at the IC50 concentration (5.0 mg/ml) for 48 h at 37 °C in a 5% CO2 incubator, the Fas-ligand concentration in HepG2 cells was measured using a colorimetric human factor-related apoptosis ligand (FAS-L) enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer’s instructions (Wkea Med Supplies, Changchun, China).
Tumour necrosis factor-α (TNF-α) is one on the most important cell signalling proteins (cytokines), which promotes necrosis of some types of tumours and stimulates the growth of other types of tumours. 30 After incubation of HepG2 cells with ASH-WX at the IC50concentration (5.0mg/ml) for 48 h at 37 °C in a 5% CO2 incubator, human TNF-α levels were measured using an ELISA Kit (K0331131P; KomaBiotech, Seoul, South Korea) following the manufacturer’s instructions.
Assessment of caspase-3, caspase-8 and caspase-9 activity
Apoptosis is mediated by proteolytic enzymes, called caspases, which trigger cell death. Caspase-3 is related to the promotion and enhancement of the ‘death cascade’ and is therefore considered an important factor involved in the cell’s entrance into the apoptotic signalling pathway. 31 Caspase-8 is involved in apoptosis and cytokine processing. 32 Caspase-9 is one of the most common caspases, which is responsible for the activation of other caspases that result in apoptosis. 33 After incubation of HepG2 cells with ASH-WX at the IC50concentration (5.0 mg/ml) for 48 h at 37 °C in a 5% CO2 incubator, the caspase-3, -8 and -9 activities were measured using colorimetric assay kits (K106-25, K113-25and K119-25 for caspase-3, -8 and -9, respectively; BioVision, Milpitas, CA, USA) following the manufacturer’s instructions.
Molecular modelling and docking study
Cyclin D1 is one of the most important regulators of cell cycle progression inside the cell. Cyclin D1 over expression has been associated with cancer progression.34 As the inhibition of cyclin D1 in cancer is considered to be one of the most important targeted therapeutic pathways, calculations of molecular modelling and local docking were estimated using Molecular Operating Environment (MOE) software (Chemical Computing Group, Montreal, Canada) to evaluate the binding-free energies of Ashwagandha in the cyclin D1 receptor. For the docking calculations, the structure of the target protein cyclin D1 was obtained from the Protein Data Bank (Protein Data Bank ID: 2w96). Based on PyMOL software calculations, the binding affinity between cyclin D1 and Ashwagandha was calculated using the binding-free energies (S-score, kcal/mol), hydrogen bonds, and root-mean-square deviation (RMSD) values . The RMSD value is usually used to validate the docking protocol, which provides a method of considering the crystallographic complex protein with a ligand docked within it; the optimized complex protein has the lowest RMSD values.
Cell cycle analysis
The HepG2 cells were treated with ASH-WX at the IC50 concentration (5.0 mg/ml) for 24 h at 37 °C in a 5% CO2 incubator and stained with the Cytell™ Cell Cycle Kit (GE Healthcare Japan, Tokyo, Japan) and then analysed using a Cytell™ cell imaging system (GE Healthcare Japan).
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 15.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Data are presented as mean ± SD or median (range). Comparison of numerical variables between the study groups was undertaken using Mann–Whitney U-test for independent samples. Two-tailed P-values < 0.05 were considered statistically significant.
Results
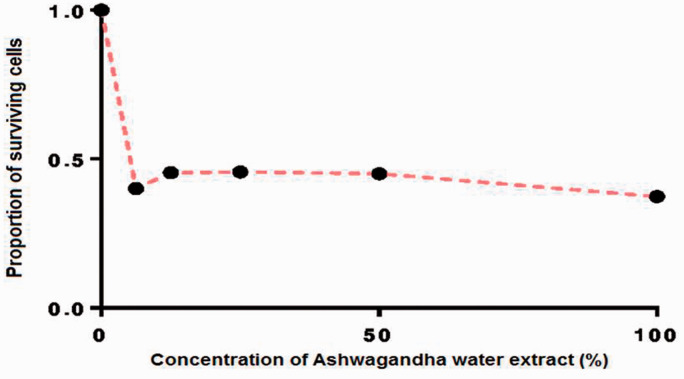
Figure 1 shows the growth response curve for HepG2 cells following incubation for 24 h with a range of concentrations of ASH-WX (6.25 mg/ml–100 mg/ml) as determined using the MTT assay and presented as the survival fraction of treated cells compared with control untreated cells. The IC50 value of ASH-WX in HepG2 cells was equivalent to approximately 5.0 mg/ml.
Figure 1.
Growth response curve for HepG2 cells treated with a range of concentrations of Ashwagandha water extract (6.25mg/ml–100 mg/ml) at 37 °C in a 5% CO2 incubator for 24 h calculated using GraphPad Prism 7 software. The IC50 value was 5.0 mg/ml.
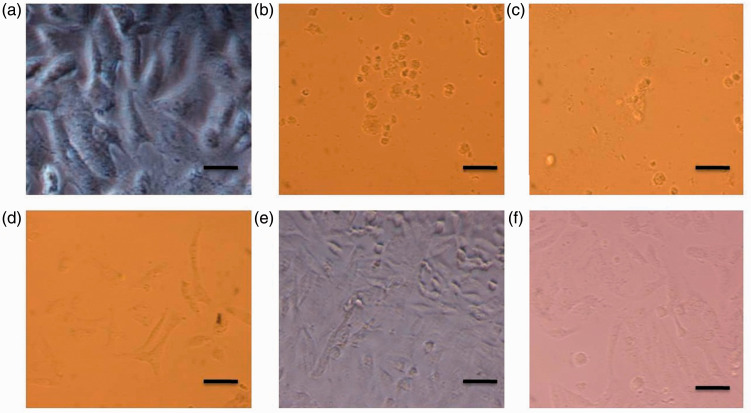
At all concentrations of ASH-WX (6.25 mg/ml–100 mg/ml), treated cells showed marked signs of shrinkage and accumulation of dead cells after 24 h of incubation (Figure 2). In addition, cells appeared to undergo apoptosis compared with the control untreated cells.
Figure 2.
Representative photomicrographs showing the viability of HepG2 cells treated with a range of concentrations of Ashwagandha water extract (ASH-WX) at 37 °C in a 5% CO2 incubator for 24 h. (a) Control untreated cells; (b) cells treated with 100 mg/ml ASH-WX; (c) cells treated with 50 mg/ml ASH-WX; (d) cells treated with 25 mg/ml ASH-WX; (e) cells treated with 12.5 mg/ml ASH-WX; and (f) cells treated with 6.25 mg/ml ASH-WX. HepG2 cells incubated in all concentrations showed signs of marked shrinkage and accumulation of dead cells compared with the control untreated cells. The higher the concentration of ASH-WX, the higher the percentage of dead cells and shrinkage of cells. Scale bar 20 µm. The colour version of this figure is available at: http://imr.sagepub.com.
HepG2 cells treated with different concentrations of ASH-WX (2.5, 5.0, and 10.0 mg/ml) for 48 h demonstrated a significant increase in antioxidant activities compared with the control untreated cells (P < 0.05 for all comparisons) (Table 1). The highest activity of total antioxidant (1.8 ± 0.1 µM/ml) was observed when HepG2 cells were treated with 5.0 mg/ml ASH-WX, while the highest activity of glutathione reductase (80.0 ± 6.0 mg/dl) was observed when HepG2cells were treated with 10.0 mg/ml of ASH-WX. Glutathione S-transferase showed the highest activity (1706.7 ± 7.3 µM/ml) following treatment of HepG2 cells with 5.0 mg/ml ASH-WX.
Table 1.
Total antioxidant, glutathione S-transferase and glutathione reductase activities in HepG2 cells treated with different concentrations of Ashwagandha water extract at 37°C in a 5% CO2 incubator for 48 h.
| Antioxidant | Control untreated cells |
Cells treated with Ashwagandha water extract |
Statistical signficancea | ||
|---|---|---|---|---|---|
| 2.5 mg/ml | 5.0 mg/ml | 10.0 mg/ml | |||
| Total antioxidant, µM/ml | 0.6 ± 0.0 | 0.8 ± 0.0 | 1.8 ± 0.1 | 1.5 ± 0.1 | P < 0.05 |
| Glutathione S-transferase, µM/ml | 382.1 ± 61.0 | 610.4 ± 6.0 | 1706.7 ± 7.3 | 1375.0 ± 5.3 | P < 0.05 |
| Glutathione reductase, mg/dl | 3.9 ± 0.5 | 13.3 ± 0.3 | 22.8 ± 0.5 | 80.0 ± 6.0 | P < 0.05 |
Data presented as mean ± SD.
aComparison between all three test groups and the control untreated group; Mann–Whitney U-test.
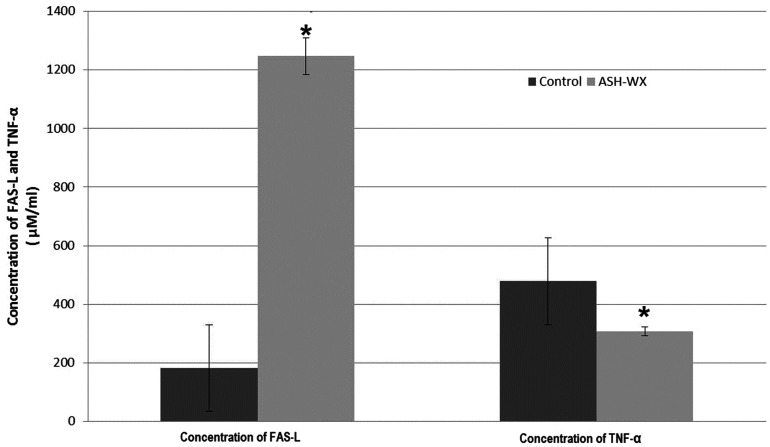
The results of the ELISA analyses showed a significant increase in the FAS-L concentration (P < 0.05) and a significant decrease in the TNF-α concentration (P < 0.05) of HepG2 cells treated with ASH-WX at the IC50 concentration (5.0 mg/ml) for 48 h compared with the control untreated cells (Figure 3).
Figure 3.
The concentration of Fas-ligand (FAS-L) and tumour necrosis factor-α (TNF-α) in HepG2 cells treated with the IC50 concentration (5.0 mg/ml) of Ashwagandha water extract (ASH-WX) at 37 °C in a 5% CO2 incubator for 48 h. Data presented as mean ± SD. *P < 0.05 compared with the control untreated cells; Mann–Whitney U-test.
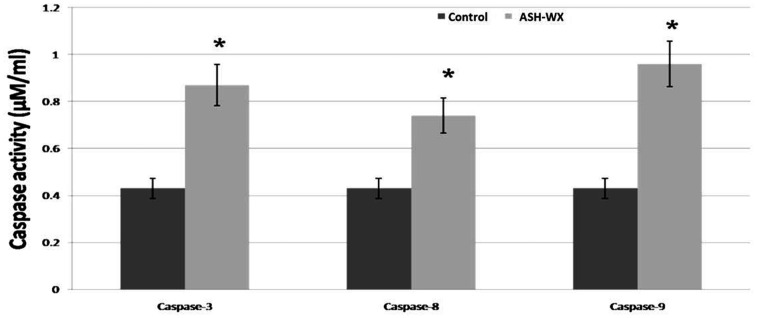
The results of the caspase colorimetric assays showed significant increases in the activity of the three caspases in HepG2 cells treated with ASH-WX at the IC50 concentration (5.0 mg/ml) for 48 h compared with the control untreated cells (P < 0.05 for all comparisons) (Figure 4).
Figure 4.
The activity of caspase-3, caspase-8 and caspase-9 in HepG2 cells treated with the IC50 concentration (5.0 mg/ml) of Ashwagandha water extract (ASH-WX) at 37 °C in a 5% CO2 incubator for 48 h. Data presented as mean ± SD. *P < 0.05 compared with the control untreated cells; Mann–Whitney U-test.
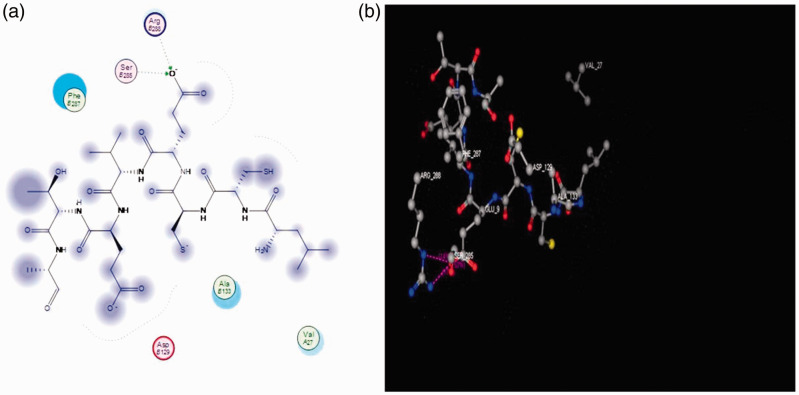
Molecular docking studies suggested good interactions between the three-dimensional structure of the drug targets (CCND1; Protein Data Bank ID: 2w96) and ASH-WX. Molecular docking revealed that ASH-WX was able to bind in the ligand-binding domain of cyclin D1 and inhibit its activity. The best docking scores depend on the binding-free energy and by measuring the hydrogen bond length, which should not exceed 3 A and RMSD from the native legend. The current results showed a score energy of –24.151, hydrogen bond lengths of 2.61A and2.34A; and the RMSD was less than 1.5A (0.98A) (Figure 5).
Figure 5.
Ligand interaction and binding model analysis of Ashwagandha with the cyclin D 1 receptor showing the pharmacophore model (a) and docking drug model (b). Ashwagandha exhibited two hydrogen bonds with the amino acids in cyclin D1: arginine β 256 and serine β 258 (hydrogen bonds shown in green). Arg B256, arginine β 256; Ser B258, serine β 258; Phe B287, phenylalanine β 287; Ala B1, alanine β1; Val A27, valine α 27; Arg B129, arginine β 129. The colour version of this figure is available at: http://imr.sagepub.com.
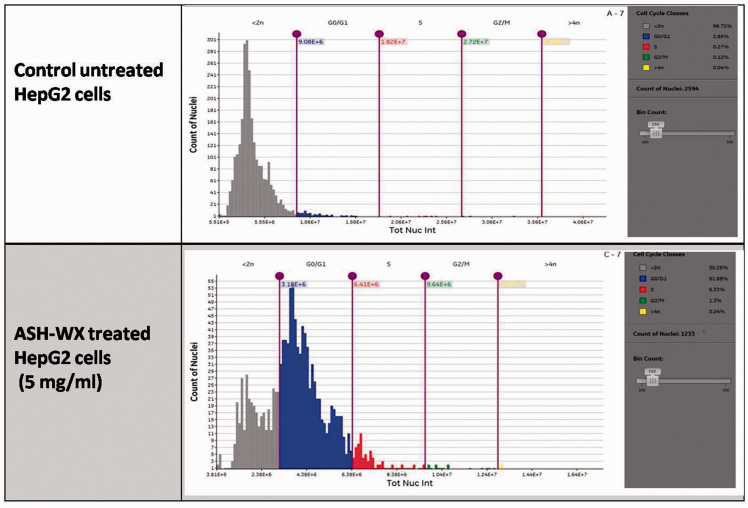
Cell cycle analysis demonstrated the presence of a marked accumulation of ASH-WX-treated HepG2 cells in the G0/G1 (61.9%) and G2/M (1.3%) phases; and a marked decrease of HepG2 cells in the <2n phase (30.3%) compared with the control untreated cells (<2n phase: 96.7%; G0/G1 phase: 2.9%; G2/M phase: 0.1%) (Figure 6).
Figure 6.
Cell cycle analysis of HepG2 cells treated with the IC50 concentration (5.0 mg/ml) of Ashwagandha water extract (ADH-WX) at 37 °C in a 5% CO2 incubator for 24 h analysed using a Cytell™ cell imaging system. The colour version of this figure is available at: http://imr.sagepub.com.
Discussion
Hepatocellular carcinoma receives a great deal of attention from scientists,35,36 due to its late presentation, aggressiveness and poor sensitivity to traditional therapies for cancer, 37 which, in turn, limits the success of many cancer therapies that are also associated with many side-effects. 38 Some natural products have been used as traditional medicines against various diseases, where they have fewer side-effects and have been shown to act as anticarcinogenic agents. 39
This present study investigated the anticancer effects of Egyptian Ashwagandha leaves, a well-known herbal medicine that is full of antioxidants, against a HCC cell line HepG2. Previous studies of Ashwagandha leaves found that their water extract was effective against many types of cancer. For example, one study found that Ashwagandha leaves activated tumour suppressor proteins (p53 and pRB) and increased levels of cyclin B1 in cancer cells. 25 Another study showed an efficient cytotoxic effect of Ashwagandha leaves on MCF-7 breast cancer cells, which was stronger than that exerted on PA-1 and A-459 cancer cell lines. 40 The phytochemical analysis of Egyptian Ashwagandha leaves suggests that it belongs to chemotype III, which is different to the Indian Ashwagandha regarding the antioxidant activity.41,42 The results of the current study showed that ASH-WX had a strong cytotoxic effect on HepG2 cells. ASH-WX showed a marked effect on the cells causing shrinkage and accumulation of dead HepG2 cells when compared with control untreated cells. This current finding agreed with previous research that reported a decrease in the viability as well as inhibition of growth of HepG2 cells when treated of ASH-WX. 43 The current study of the effect of ASH-WX on total antioxidant, glutathione S-transferase and glutathione reductase activities demonstrated a significant increase in the activities of these antioxidants when the HepG2 cells were treated with different concentrations of ASH-WX. The highest activity of total antioxidant (1.8 ± 0.1 µM/ml) was observed when HepG2 cells were treated with 5.0 mg/ml ASH-WX, while the highest activity of glutathione reductase (80.0 ± 6.0 mg/dl) was observed when HepG2 cells were treated with 10.0 mg/ml of ASH-WX. Glutathione S-transferase showed the highest activity (1706.7 ± 7.3 µM/ml) following treatment of HepG2 cells with 5.0 mg/ml ASH-WX. These current findings were in agreement with a previous study that demonstrated that Ashwagandha was a rich source of powerful antioxidants. 44 That previous study found an increase in the levels of three natural antioxidants (superoxide dismutase, catalase, and glutathione peroxidase) when they tested the antioxidant compounds extracted from ASH-WX on rat brains. 44 Another study confirmed the antioxidant effect of ASH-WX in aging spinal cords of laboratory mice, where it stopped the lipid peroxidation that can lead to heart disease in humans. 45 This present study also showed that ASH-WX significantly increased apoptotic markers compared with control treatment and these results agreed with a study that reported an induction of apoptosis, accompanied by an up-regulation of Bim, t-Bid, caspase-8, caused by withaferin A, the main active constituent of ASH-WX. 46 Also, the current study showed a significant decrease in the concentration of TNF-α in cells treated with ASH-WX compared with control untreated cells. This current findings agreed with a report that withaferin A inhibited TNF-α and induced NF-kB activation in endothelial cells treated with the IC50 (0.5 μM) dose. 47 ASH-WX was shown to decrease the proteins or cytokines that are involved in systemic inflammation and it also inhibited tumour cell growth. 47 The current molecular docking analysis that investigated the interaction between Ashwagandha and cyclin D1, one of the most important regulators of cell cycle progression and its over expression is considered to be a hallmark in various types of cancer, 48 revealed a good and promising interaction between Ashwagandha and cyclin D1;with a score energy of –24.151, hydrogen bond lengths of 2.61A and 2.34A, and the RMSD was less than 1.5A (0.98A). The current molecular docking results were confirmed by the results obtained from cell cycle analysis, which revealed an accumulation of HepG2 cells in the G0/G1 and G2/M phases and a corresponding decrease of HepG2 cells in the <2n phase following treatment with ASH-WX compared with control untreated cells. These findings agreed with those of a previous study that reported a progressive accumulation of cells in the G0/G1 phase that correlated with a decrease in the proportion of cells in the S phase and G2/M phase in HepG2 cells treated with Ashwagandha extract. 49
In conclusion, Ashwagandha (Withania somnifera) water extract is a powerful antioxidant and has anticancer properties in HepG2 cells. It might have potential as a promising anticancer agent against HCC and these results should be confirmed in animal studies.
Acknowledgements
The authors would like to thank the members of staff of the Department of Cancer Biology, National Cancer Institute, Cairo University, Cairo, Egypt for providing all of the necessary facilities to conduct this study and Dr Omar Dahruog for his efforts in the molecular docking work.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Sever R andBrugge JS.. Signal transduction in cancer. Cold Spring Harb Perspect Med 2015; 5. Epub ahead of print 1 April 2015. DOI: 10.1101/cshperspect.a006098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D andWeinberg RA.. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 3.Fouad YA andAanei C.. Revisiting the hallmarks of cancer. Am J Cancer Res 2017; 7: 1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 4.Adjiri A. Identifying and targeting the cause of cancer is needed to cure cancer. Oncol Ther 2016; 4: 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charames GS andBapat B.. Genomic instability and cancer. Curr Mol Med 2003; 3: 589–596. [DOI] [PubMed] [Google Scholar]
- 6.Martin TA, Ye L, Sanders AJ, et al. Cancer invasion and metastasis: molecular and cellular perspective. https://www.ncbi.nlm.nih.gov/books/NBK164700/ (2013, accessed 8 October 2017).
- 7.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spach DH andKim HN. Module 5. Treatment of Chronic Hepatitis C Infection. Lesson 4. Treatment of HCV Genotype 4. Hepatitis C Online. https://www.hepatitisc.uw.edu/go/treatment-infection/treatment-genotype-4/core-concept/all (2017, accessed 8 October 2017).
- 10.Zhu RX, Seto WK, Lai CL, et al. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver 2016; 10: 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim AS, Khaled HM, Mikhail NN, et al. Cancer incidence in Egypt: results of the national population-based Cancer Registry Program. J Cancer Epidemiol 2014; 2014: 437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Best J, Schotten C, Theysohn JM, et al. Novel implications in the treatment of hepatocellular carcinoma. Ann Gastroenterol 2017; 30: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karaman B, Battal B, Sari S, et al. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J Gastroenterol 2014; 20: 18059–18060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Njamen D, Zingue S, Mvondo MA, et al. The efficacy of some comestible natural products in treatment of cancer. Altern Integr Med 2014; 3:158. doi:10.4172/2327-5162.1000158. [Google Scholar]
- 15.Soladoye MO, Amusa NA, Raji-Esan SO, et al. Ethnobotanical survey of anti-cancer plants in Ogun State, Nigeria. Ann Biol Res 2010; 1: 261–273. [Google Scholar]
- 16.Cragg GM andNewman DJ.. Plants as a source of anti-cancer agents. J Ethnopharmacol 2005; 100: 72–79. [DOI] [PubMed] [Google Scholar]
- 17.Chahar MK, Sharma N, Dobhal MP, et al. Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev 2011; 5: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wankhede S, Langade D, Joshi K, et al. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial. J Int Soc Sports Nutr 2015; 12: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswal BM, Sulaiman SA, Ismail HC, et al. Effect of Withania somnifera (Ashwagandha) on the development of chemotherapy-induced fatigue and quality of life in breast cancer patients. Integr Cancer Ther 2013; 12: 312.–. [DOI] [PubMed] [Google Scholar]
- 20.Khan MA, Subramaneyaan M, Arora VK, et al. Effect of Withania somnifera (Ashwagandha) root extract on amelioration of oxidative stress and autoantibodies production in collagen-induced arthritic rats. J Complement Integr Med 2015; 12: 117–125. [DOI] [PubMed] [Google Scholar]
- 21.Sandhu JS, Shah B, Shenoy S, et al. Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int J Ayurveda Res 2010; 1: 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prakash J, Gupta SK, and Dinda AK. Withania somnifera root extract prevents DMBA-induced squamous cell carcinoma of skin in Swiss albino mice. Nutr Cancer 2002; 42: 91.–. [DOI] [PubMed] [Google Scholar]
- 23.Jayaprakasam B, Zhang Y, Seeram NP, et al. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci 2003; 74: 125–132. [DOI] [PubMed] [Google Scholar]
- 24.Panjamurthy K, Manoharan S, Menon VP, et al. Protective role of withaferin-A on 7,12– dimethylbenz(a)anthracene-induced genotoxicity in bone marrow of Syrian golden hamsters. J Biochem Mol Toxicol 2008; 22: 251–258. [DOI] [PubMed] [Google Scholar]
- 25.Wadhwa R, Singh R, Gao R, et al. Water extract of Ashwagandha leaves has anticancer activity: identification of an active component and its mechanism of action. PloS One 2013; 8: e77189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar P, Singh R, Nazmi A, et al. Glioprotective effects of Ashwagandha leaf extract against lead induced toxicity. Biomed Res Int 2014; 2014: 182029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mokhtar MM, Shaban HM, Hegazy MEF, et al. Evaluating the potential cancer chemopreventive efficacy of two different solvent extracts of Seriphidiumherba-alba in vitro. Bulletin of Faculty of Pharmacy, Cairo University 2017; 55: 195–201. http://www.sciencedirect.com/science/article/pii/S111009311730011X (2017, accessed 9 October 2017). [Google Scholar]
- 28.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 29.Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 2010; 4: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parameswaran N andPatial S.. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 2010; 20: 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoch KM Madathil SK andSaatman KE.. Genetic manipulation of cell death and neuroplasticity pathways in traumatic brain injury. Neurotherapeutics 2012; 9: 323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang TB, Ben-Moshe T, Varfolomeev EE, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol 2004; 173: 2976–2984. [DOI] [PubMed] [Google Scholar]
- 33.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007; 35: 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer 2007; 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai ZJ, Wang XJ, Li ZF, et al. Scutellaria barbate extract induces apoptosis of hepatoma H22 cells via the mitochondrial pathway involving caspase-3. World J Gastroenterol 2008; 14: 7321–7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafik N, Elshimy R, Rahouma M, et al. Circulating MiR-150 and miR-130b as promising novel biomarkers for hepatocellular carcinoma. Cancer Biol 2017; 7; 1–8. [Google Scholar]
- 37. Tejeda-Maldonado J, García-Juárez I, Aguirre-Valadez J, et al . Diagnosis and treatment of hepatocellular carcinoma: An update. . ; : – . World J Hepatol. 2015;7:362. doi: 10.4254/wjh.v7.i3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samlowski WE, Yim CY, McGregor JR, et al. Effectiveness and toxicity of protracted nitric oxide synthesis inhibition during IL-2 treatment of mice. J Immunother Emphasis Tumor Immunol 1995; 18: 166–178. [DOI] [PubMed] [Google Scholar]
- 39. Jain S, Dwivedi J, Jain PK, et al . Medicinal plants for treatment of cancer: A brief review. . ; : – . Pharmacognosy Journal. 2016;8:87. [Google Scholar]
- 40.Nema R, Khare S, Jain P, et al. Anticancer activity of Withania somnifera (leaves) flavonoids compound. Int J Pharm Sci Rev Res 2013; 19: 103–106. [Google Scholar]
- 41.Mahrous RSR, Ghareeb DA, Fathy HM, et al. The protective effect of Egyptian Withania somnifera against Alzeheimer’s. Med Aromat Plants 2017; 6: 285. doi:10.4172/2167-0412.1000285. [Google Scholar]
- 42.Scartezzini P, Antognoni F, Conte L, et al. Genetic and phytochemical difference between some Indian and Italian plants of Withania somnifera (L.) Dunal. Nat Prod Res 2007; 21: 923–932. [DOI] [PubMed] [Google Scholar]
- 43.Pretorius E Oberholzer H andBecker P.. Comparing the cytotoxic potential of Withania somnifera water and methanol extracts. African Journal of Traditional, Complementary and Alternative Medicines 2009; 6: 275–280. https://www.ajol.info/index.php/ajtcam/article/view/57173 (2009, accessed 9 October 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattacharya SK, Bhattacharya A, Sairam K, et al. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: an experimental study. Phytomedicine 2000; 7: 463–469. [DOI] [PubMed] [Google Scholar]
- 45.Andallu B andRadhika B.. Hypoglycemic, diuretic and hypocholesterolemic effect of winter cherry (Withania somnifera, Dunal) root. Indian J Exp Biol 2000; 38: 607–609. [PubMed] [Google Scholar]
- 46.Morishima N, Nakanishi K, Takenouchi H, et al. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 2002; 277: 34287–34294. [DOI] [PubMed] [Google Scholar]
- 47.Mohan R, Hammers HJ, Bargagna-Mohan P, et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 2004; 7: 115–122. [DOI] [PubMed] [Google Scholar]
- 48.Casimiro MC, Crosariol M, Loro E, et al. Cyclins and cell cycle control in cancer and disease. Genes Cancer 2012; 3: 649.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed WA, Mohamed A, Nasser EA, et al. Potential toxicity of Egyptian Ashwagandha: significance for their therapeutic bioactivity and anticancer properties. International Journal of Science and Research 2015; 4: 2170.–. [Google Scholar]