Abstract
Although neurotoxicity and hepatotoxicity have long been associated with exposure to polychlorinated biphenyls (PCBs), less is known about the selective toxicity of those hydroxylated PCBs (OH-PCBs) and PCB sulfates that are metabolites derived from exposure to PCBs found in indoor air. We have examined the toxicity of OH-PCBs and PCB sulfates derived from PCBs 3, 8, 11, and 52 in two neural cell lines (N27 and SH-SY5Y) and an hepatic cell line (HepG2). With the exception of a similar toxicity seen for N27 cells exposed to either OH-PCB 52 or PCB 52 sulfate, these OH-PCBs were more toxic to all three cell-types than their corresponding PCB or PCB sulfate congeners. Differences in the distribution of individual OH-PCB and PCB sulfate congeners between the cells and media, and the ability of cells to interconvert PCB sulfates and OH-PCBs, were important components of cellular sensitivity to these toxicants.
Keywords: PCB, hydroxy PCB, OH-PCB, PCB sulfate, neurotoxicity, hepatotoxicity
1. Introduction
The man-made environmental contaminants known as polychlorinated biphenyls (PCBs) continue to persist in the environment and cause or contribute to various harmful health effects including neurotoxicity (Schantz et al. 2003; Tilson and Kodavanti 1997, 1998) and hepatotoxicity (Cave et al. 2010). Although global levels of PCBs have decreased since their production and use have been limited worldwide (Nyberg et al. 2014), the lower-chlorinated PCBs (i.e., ≤4 chlorine atoms per congener) are found in environmental samples (Basu et al. 2009; Hu et al. 2011; Rodenburg et al. 2010) including indoor and outdoor air from both urban and rural areas (Egsmose et al. 2016; Pedersen et al. 2016) and as unintended byproducts from current production of consumer products such as pigments and dyes (Hu and Hornbuckle 2010; Shang et al. 2014; Vorkamp 2016). Exposure to these semi-volatile compounds has been proposed to occur through multiple pathways that include inhalation (Hu et al. 2014) as well as the consumption of contaminated food or water (Ampleman et al. 2015; Chen et al. 2017).
Lower-chlorinated PCBs are highly susceptible to metabolic transformation and have often been considered transient species in the body. This metabolic vulnerability, however, also carries with it the potential for production of reactive and toxic compounds (Grimm et al. 2015b; Hansen 2001; Sethi et al. 2017). Hydroxylated PCB metabolites (OH-PCBs) have been detected in human blood samples and in biological samples from various species (Gutleb et al. 2010; Koh et al. 2016; Marek et al. 2014; Schafer et al. 2009). The oxidation of PCBs to hydroxylated metabolites allows for further metabolism, of which sulfation represents a potentially important route (Grimm et al., 2015b). Although sulfation of a phenolic compound is traditionally considered a mode for its removal from the body due to increased polarity, water solubility, and excretion of the sulfated product, the potential for biological retention may also exist. A study in Sprague-Dawley rats has indicated that hydroxylation followed by sulfation accounts for more than half of the metabolic fate after exposure to the monochlorinated PCB 3 (Dhakal et al. 2012). Additional studies in rats following administration of 4-PCB 11 sulfate indicated that some PCB sulfates, however, may be retained in vivo (Grimm et a1. 2015a). Furthermore, the presence of 4-PCB 11 sulfate in human serum samples has recently been reported (Grimm et al. 2017). In vitro studies have shown that, while OH-PCBs can inhibit the sulfation of endogenous molecules including dehydroepiandrosterone (DHEA) and estradiol, many OH-PCBs also serve as substrates for sulfate conjugation (Ekuase et al. 2011; Kester et al. 2002; Liu et al. 2006; Parker et al. 2018). The resulting PCB sulfates bind to the thyroid hormone carrying protein transthyretin, where, in some cases, they bind with similar affinity to that observed with thyroxine (Grimm et al. 2013). Moreover, PCB sulfates have been shown to bind with high affinity to the major drug binding sites of human serum albumin (HSA), the most abundant protein in human plasma (Rodriguez et al. 2016). In the case of both transthyretin and HSA, protein binding was influenced by the degree of chlorination and the substitution patterns of the PCB congeners, and PCB sulfates generally bound with a higher or equal affinity than the corresponding PCB or OH-PCB, thereby potentially increasing their retention and distribution in the body. These studies suggest that, contrary to the general assumption that sulfation of a xenobiotic is simply a mode for its excretion, the sulfates derived from lower-chlorinated OH-PCBs may be retained, transported, and have distinct biological and/or toxicological activities.
While little is known about the in vivo toxic effects of PCB metabolites, the neurotoxic and hepatotoxic effects of various PCB mixtures and individual congeners have been well documented in the scientific literature. Exposure to PCBs has been associated with non-alcoholic fatty liver (Cave et al. 2010), and PCBs have been identified as promoting and initiating agents in hepatic carcinogenesis (Ludewig et al., 2008). Epidemiological studies on the neurotoxic effects of PCB exposure indicate correlations with neurodevelopmental dysfunction and with incidences of neurodegenerative diseases (Hatcher-Martin et al. 2012; Steenland et al. 2006). Environmental PCB exposure-related effects on mood, depression, social and reproductive behaviors, cognition and motor function have also been reported (Berghuis et al. 2015; Berghuis et al. 2013; Jurewicz et al. 2013; Polanska et al. 2013). In vitro studies using cultured neuronal cells have often focused on the cytotoxic effects of higher-chlorinated PCB congeners and Aroclor mixtures (Tilson and Kodavanti 1997; Tilson et al. 1998). Lower-chlorinated PCBs are, however, of increasing interest, as seen in the recent study of the effect of PCB 11 and its hydroxylated and sulfated metabolites on axonal and dendritic growth in cultured primary rat neuronal cells (Sethi et al. 2017).
We hypothesized that OH-PCB and corresponding PCB sulfate metabolites of lower-chlorinated PCBs exhibit toxicity in cultured cells that is influenced by PCB congener, metabolite, and target cell type. The cell lines used in this study were of neural (rat midbrain N27 and human neuroblastoma SH-SY5Y) and hepatic (human hepatic HepG2) origins. Cellular toxicity was measured using two orthogonal cell viability assays (i.e., the reduction of MTT and the release of lactate dehydrogenase (LDH)). The PCBs and PCB metabolites included in this study (Figure 1) were chosen to represent some of the most frequently detected PCB congeners in air samples and encompass varying degrees of chlorination and substitution patterns (Grimm et al. 2015b). Moreover, since the presence of ortho-substituted chlorines among PCB congeners can dictate their three dimensional structure by influencing the dihedral angle between the phenyl rings, with significant influences on their biological effects (Van den Berg et al. 2006), we have examined both ortho-substituted and non ortho-substituted congeners. The fate of these molecules in vitro was monitored by HPLC to determine their distribution between cells and extracellular medium. Finally, to determine the effects of albumin-binding on cytotoxicity, studies were performed with HSA supplementation in the incubation media.
Fig. 1.
Chemical structures of the PCBs, OH-PCBs, and PCB sulfates used in these studies.
The studies presented here probe the roles that metabolism of lower-chlorinated PCBs, particularly hydroxylation and subsequent sulfation, may play in the toxic effects of certain PCB congeners. These changes impart complex differences regarding toxicity profiles, distribution of the metabolites into cells from different tissues, as well as their potential for further metabolic reactions within those cells that influence toxic outcomes.
2. Materials and Methods
Cell culture media and media components that were obtained from Gibco (Life Technologies, Madison, WI, USA) included: Roswell Park Memorial Institute (RPMI) medium, Dulbecco’s Modified Eagle’s Medium (DMEM), Opti-MEM, Dulbecco’s phosphate buffered saline (DPBS), Trypsin -EDTA (0.25%), penicillin/streptomycin, sodium pyruvate (100mM), fetal bovine serum (FBS), horse serum (HS), and MEM non-essential amino acids (MEM NEAA). Corning Falcon tissue culture 100 mm2 petri dishes, Corning Costar 96-well plates, and dimethyl sulfoxide (DMSO, ≥99.9%) were purchased from Fisher Scientific (Radnor, PA, USA). Collagen Type I, rat tail, was purchased from BD Biosciences (San Jose, CA, USA). Human serum albumin (HSA, fatty acid and globulin free, ≥99%), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 98%), and NADH (≥99% by HPLC) were purchased from Sigma Aldrich (St. Louis, MO, USA). Authenticity of the human cell lines was confirmed by analysis of genomic DNA conducted by the University of Arizona Genetics Core (Arizona Research Laboratories, Tucson, AZ). Cell culture and incubations were performed under the standard conditions of 37°C in a 5% CO2 atmosphere. All PCBs and their corresponding hydroxylated and sulfated metabolites were prepared and characterized as previously described (Lehmler et al. 2013; Lehmler and Robertson 2001; Li et al. 2010; Rodriguez et al. 2016). Spectroscopic analyses were performed using a Spectramax M5 fluorimeter (Molecular Devices, Sunnyvale, CA, USA), and statistical analyses and sigmoidal dose response cytotoxicity analyses were obtained using SigmaPlot v. 11.0, Systat Software (Chicago, IL, USA).
N27 cells
Rat midbrain-derived immortalized N27 cells were a generous gift from Dr. Jau-Shyong Hong, Neuropharmacology Group, National Institute of Environmental Health Sciences. N27 cells were seeded at a density of 1 × 106 cells in collagen-coated 100 mm2 tissue culture dishes and maintained in RPMI 1640 medium supplemented with 10% heat-inactivated HS, penicillin (100 I.U./mL) and streptomycin (100 μg/mL), Medium was changed every other day until the cells were near confluence (approximately four days). The N27 cells used in this study were between passages 17 through 30.
SH-SY5Y cells
The human neuroblastoma-derived SH-SY5Y cells were grown in collagen-coated 100 mm2 tissue culture dishes and maintained in Opti-Mem medium supplemented with 10% heat-inactivated FBS, non-essential amino acids, sodium pyruvate (500 μM), penicillin (100 I.U./mL), and streptomycin (100 μg/mL). Medium was changed every other day until the cells were near confluence (approximately seven days). The SH-SY5Y cells used in this study were between passages 15 through 30.
HepG2 cells
The immortalized human liver-derived HepG2 cells were provided by Ms. Susanne Flor of the University of Iowa Superfund Research Center. HepG2 cells were grown in 100 mm2 tissue culture dishes and maintained in DMEM supplemented with 10% heat-inactivated FBS, penicillin (100 I.U./mL), and streptomycin (100 μg/mL). Medium was changed every other day until the cells were near confluence (approximately 4 days). The HepG2 cells used in this study were between passages 21 through 32.
2.1. Effects of PCBs and PCB Metabolites on Cell Viability
Following culture in 100 mm2 tissue culture dishes, cells were seeded at 25 × 103 cells/well in 96-well plates (plates were collagen-coated for the N27 and SH-SY5Y cells) and allowed to grow for 48 h with a change to fresh medium after 24 h. The cells were then washed twice with 100 μL DPBS, and treated with 100 μL phenol red-free, serum-free medium (prepared respective to cell line-specific medium composition), containing the desired PCB, OH-PCB, or PCB sulfate concentration. The experiments in this study were performed under serum-free conditions because serum proteins bind with high affinity to PCBs, OH-PCBs, and PCB sulfates (Grimm et al. 2013; Rodriguez et al. 2016), which might alter their effective concentration in situ. The solutions of PCBs, OH-PCBs, and PCB sulfates were obtained using serial dilutions of a stock solution (i.e., 100 μM in serum-free medium). The concentrated stock solutions of PCBs and metabolites were made in DMSO at a concentration of 200 mM, and the resulting concentration of DMSO in the media stock solutions following dilution was 0.1% (v/v). The untreated controls in these experiments contained the respective DMSO amounts (i.e., ≤ 0.1% v/v), although no change in cell viability was seen at these DMSO concentrations. Each plate contained four sets of three control wells. The cells were incubated for 24 h in the presence of compound. Toxicity was assessed by measuring both the cellular reduction of MTT and the release of LDH.
For the HSA supplementation and toxicity studies, these same conditions were used, however, stock solutions of 50 μM compound were made in serum free media, and the HSA stock solutions were made in the same medium at 100 μM based on a molecular weight of 66.5 kDa. Untreated serum free medium was used to make the final dilution to the desired concentration.
Cellular release of LDH was measured according to a previously described procedure (Vassault 1974). After 24 h of exposure to each concentration of PCB, OH-PCB, or PCB sulfate, 10 μL of a Triton-X 100 solution (20% v/v in serum free medium) was added to the high-control wells (2% v/v final concentration of Triton-X 100 in each well) in the plate, and the plate was incubated for 15 min. Lysis of untreated cells and subsequent complete release of cellular LDH provided the high control for this assay. A 50 μL volume of medium from each well was then transferred to a new 96-well plate. This step was performed carefully so as to not disrupt the cells that were to be assessed using the MTT-reduction assay (see method below). To each 50 μL-containing well in the new 96-well plate was added 50 μL of a DPBS solution containing 200 μM NADH (100 μM final concentration) and 3.2 mM sodium pyruvate (1.6 mM final concentration). The oxidation of NADH was determined by monitoring absorbance at 340 nm, and results were reported as a percent of control catalytic activity of NADH oxidation (i.e., pyruvate reduction catalyzed by total LDH from lysed cells).
The reduction of MTT as a measure of cell viability was determined according to the procedure described by (van Meerloo et al. 2011). After the 24 h exposure of cells to PCBs or PCB metabolites, 50 μL of the medium was carefully removed and placed in a separate 96-well plate for analysis of LDH release (see method above). To the remaining cells in each well, 50 μL of a 1 mg/mL MTT solution in DPBS was added, and they were incubated for 1 h. The plate was removed, 100 μL of acetonitrile was added to each well, and the water-insoluble formazan crystals were dissolved with a multi-pipettor. Visible absorption of each well was then determined at 570 nm and 650 nm. The absorbance at 650 nm was subtracted from that at 570 nm, and the resulting absorbance values were then plotted as a percent of control versus the ligand concentration.
2.2. Graphical and statistical analyses
The graphs of the MTT-reduction and LDH-release experiments are shown in the Supplementary Material in Figures S1 through S3. Results were plotted as the percentage of vehicle-treated control values (for either MTT reduction or LDH release) vs. the log of the concentration of PCB or PCB metabolite. Plotting the data in a logarithmic form allowed for analysis by fitting to a sigmoidal dose response curve from which an effective concentration that afforded half of the total effect (EC50) was calculated. For those cases where a sigmoidal dose response model was appropriate, curve-fitting and statistical analyses were performed using SigmaPlot v. 11.0 (Systat Software, Chicago, IL). Statistical significance of differences in EC50 values was determined by one way ANOVA with Bonferoni post hoc analysis.
2.3. Analysis of OH-PCBs and PCB sulfates in cellular and extracellular compartments
Cells were grown, seeded, and treated with solutions containing 25 μM of the desired test compound as described in the previous sections. After 24 h, the 96-well plate was centrifuged at 2300 × g for 5 min. The media from two identically treated wells were carefully removed and pooled in microcentrifuge tubes (0.5-mL high-clarity polypropylene microcentrifuge tubes). The cells were gently washed with 100 μL DPBS, centrifuged at 2300 × g for 5 min, and the supernatant medium was removed. This washing procedure was repeated once. HPLC analyses indicated that these washes showed no detectable amounts of OH-PCBs or PCB sulfates. One hundred μL of trypsin solution was added to the cells remaining in each well and the plate was incubated for 30 min at room temperature. The plate was subjected to water bath-sonication for 10 min, cells were dislodged from each well using a multi-pipettor, and quantitative removal of the cells was verified using a light microscope. The contents of two identically treated wells were pooled in a microcentrifuge tube and subjected to sonication (Fisher Scientific Model 100 hand-held sonicator). To each of the extracellular and cellular pooled extracts was added 200 μL acetonitrile and 15 mg NaCl. Each mixture was then thoroughly vortexed and centrifuged at 4400 × g for 5 minutes. The organic acetonitrile (MeCN) layer was collected and analyzed by HPLC using a Shimadzu Model LC-20-AT liquid chromatograph equipped with an SPD-20-AT UV/VIS detector and a C18 AQ 5μm (4.6 × 250 mm) column (Grace, Deerfield, IL). Elution of the HPLC column was accomplished with a mobile phase containing 0.04% (v/v) triethylammonium acetate (pH 7.4) and the indicated concentration of MeCN: from 0–1 min, 15% MeCN; from 1–10 min, a linear gradient from 15% to 100% MeCN; from 10–14 min, 100% MeCN; and from 14–15 min, a linear gradient from 100% to 15% MeCN. Analysis of the eluate was carried out by absorbance at 254 nm. Concentrations were determined by relating the peak area to standard curves, ranging from 0.8 μM to 50 μM, obtained using synthetic standards. Standard curves exhibited acceptable linearity with r2 values ranging between 0.97 and 0.99. The limit of detection (LOD) and limit of quantitation (LOQ) for the compounds were calculated using the relationships 3:1 and 10:1 signal to noise (S/N) ratio, respectively. The S/N ratio was calculated from the standard curve by the relationship:, where Sy is the y-intercept standard deviation and K is the slope of the best fit line. The LOD and LOQ for the compounds in this study were: 4’-OH-PCB 3, 4-PCB 52 sulfate (0.02 nmol/100 μL and 0. 07 nmol/100 μL); 4’-PCB 3 sulfate, 4-PCB 8 sulfate, 4-PCB 11 sulfate (0.03 nmol/100 μL and 0. 10 nmol/100 μL); 4-OH-PCB 8 (0.05 nmol/100 μL and 0. 17 nmol/100 μL); 4-OH-PCB 11 (0.06 nmol/100 μL and 0.20 nmol/100 xL) and 4-OH-PCB 52 (0.01 nmol/100 μL and 0. 04 nmol/100 μL), respectively.
3. Results
3.1. Cytotoxicity as assessed by MTT reduction
As shown in Table 1 and Supplemental Material Figures S1 through S3 (panels A), the parent PCBs in this study exhibited little toxicity, within the range of concentrations tested, to the three cell lines as assessed by the reduction of MTT. The di-ortho-substituted congener PCB 52, however, showed moderate toxicity to the neural N27 cell line. In fact, in the N27 cells, all PCBs indicated some toxicity within the range of concentrations (i.e., exposures of up to 100 μM PCB), however to a lesser extent than PCB 52.
Table 1.
Summary of EC50 values for viability of N27, SH-SY5Y, and HepG2 cells as determined by the MTT assay following 24 h exposure to PCBs or their metabolites
| PCB or Metabolite |
N27 EC50 (μM) |
SH-SY5Y EC50 (μM) |
HepG2 EC50 (μM) |
|---|---|---|---|
| PCB 3 | >50 | >50 | >50 |
| PCB 8 | >50 | >50 | >50 |
| PCB 11 | >50 | >50 | >50 |
| PCB 52 | 28.5 ± 2.4 | >50 | >50 |
| 4’-OH-PCB 3 | 31.4 ± 4.2 | >50 | >50 |
| 4-OH-PCB 8 | 13.8 ± 2.1b | 16.2 ± 0.1 | 27.0 ± 0.7b,c |
| 4-OH-PCB 11 | 15.2 ± 0.2a | 20.7 ± 0.1a | >50 |
| 4-OH-PCB 52 | 7.9 ± 0.1 a,b | 6.7 ± 0.2a,c | 13.0 ± 0.3b,c |
| 4’-PCB 3 sulfate | >50 | >50 | >50 |
| 4-PCB 8 sulfate | >50 | >50 | >50 |
| 4-PCB 11 sulfate | >50 | >50 | >50 |
| 4-PCB 52 sulfate | 14.3 ± 0.6a,b | 32.7 ± 2.2a | 28.8 ± 3.9 b |
Significant difference between N27 and SH-SY5Y (p<0.05)
Significant difference between N27 and HepG2 (p<0.05)
Significant difference between SH-SY5Y and HepG2 (p<0.05)
Exposure to OH-PCBs elicited greater toxic responses than the corresponding PCBs in all three cell lines, however to a lesser extent in the hepatic cells. The mono-chlorinated 4’-OH-PCB 3 showed the least potent toxicity throughout, and the ortho-chlorinated congeners (4-OH-PCB 8 and 4-OH-PCB 52) presented the highest. The dichlorinated non-ortho-substituted 4-OH-PCB 11 exhibited selective toxicity to the non-hepatic cells with significantly higher potency in the N27 cells. Interestingly, the tetra-chlorinated di-ortho-substituted 4-OH-PCB 52 showed the highest toxicity in all three cell lines, with the greatest effect in the SH-SY5Y cells. With the exception of the effects seen with 4-OH-PCB 52, the rat N27 cells generally exhibited the highest susceptibility to cytotoxicity following exposure to the OH-PCBs examined in this study.
Similar to the effects observed with PCBs, most of the PCB sulfates showed limited toxicity as determined by the MTT assay. Interestingly, exposure to 4-PCB 52 sulfate elicited a toxic response in all cell lines within the concentration range tested. Furthermore, the N27 cell line exhibited a significantly lower EC50 value (i.e., higher potency) for 4-PCB 52 sulfate than either the SH-SY5Y or HepG2 cells. Of further note is that, in the N27 cells, 4-PCB 52 sulfate exhibited a toxic response similar to that of some OH-PCBs.
3.2. Cytotoxicity as assessed by LDH release
As seen in Table 2 and Supplementary Material Figures S1 through S3 (panels B), incubation with PCBs resulted in a minimal release of LDH. It was noted, however, that although exposure of N27 cells to PCB 52 at the highest concentration (100 μM) indicated a complete loss of cell viability by a lack of mitochondrial reduction of MTT, a complete release of cellular LDH was not observed at this concentration. None of the PCBs exhibited inhibition of LDH catalytic activity (data not shown), indicating that some cell membrane integrity remained, even when the cells were not metabolically active. Therefore, in many cases, minimal changes in LDH release upon exposure to these PCBs precluded the quantitative assessment of toxicity using this method within the range of doses examined.
Table 2.
Summary of EC50 values for viability of N27, SH-SY5Y, and HepG2 cells as determined by LDH release following 24 h exposure to PCBs or their metabolites
| PCB or Metabolite |
N27 EC50 (μM) |
SH-SY5Y EC50 (μM) |
HepG2 EC50 (μM) |
|---|---|---|---|
| PCB 3 | >50 | >50 | >50 |
| PCB 8 | >50 | >50 | >50 |
| PCB 11 | >50 | >50 | >50 |
| PCB 52 | >50 | >50 | >50 |
| 4’-OH-PCB 3 | >50 | >50 | >50 |
| 4-OH-PCB 8 | 18.5 ± 0.4a,b | 14.0 ± 0.5a,c | 25.1 ± 2.6 b,c |
| 4-OH-PCB 11 | 22.2 ± 0.9b | 16.8 ± l.lc | 39.8 ± 4.1b,c |
| 4-OH-PCB 52 | 8.3 ± 0.6a,b | 10.2 ± 0.5a,c | 15.3 ± 0.5b,c |
| 4’-PCB 3 sulfate | >50 | >50 | >50 |
| 4-PCB 8 sulfate | >50 | >50 | >50 |
| 4-PCB 11 sulfate | >50 | >50 | >50 |
| 4-PCB 52 sulfate | >50 | 33.3 ± 2.5 | 29.2 ± 1.3 |
Significant difference between N27 and SH-SY5Y (p<0.05)
Significant difference between N27 and HepG2 (p<0.05)
Significant difference between SH-SY5Y and HepG2 (p<0.05)
A comparison of the results of cell viability assays measuring LDH release and MTT reduction following incubation with OH-PCBs yielded similar trends between the two methods. By both methods, 4’-OH-PCB 3 exhibited the lowest toxicity to all cells tested. The assays for LDH release indicated that the di- and tetra-chlorinated OH-PCBs displayed a selective toxicity to the neural cells versus the hepatic cells, with the ortho-substituted congeners (i.e., 4-OH PCB 8 and 4-OH PCB 52) exhibiting a greater potency. For example, the di-ortho-substituted 4-OH-PCB 52 showed a significantly lower EC50 value for LDH release in the N27 cells when compared with either the SH-SY5Y or HepG2 cells. The relative EC50 values for LDH release following exposure to 4-OH-PCB 52 in the two neural cell lines were reversed from those seen with the MTT assay, however, in both assay methods, selective toxicity was observed for the non-hepatic cells.
With the exception of N27 cells treated with 4-PCB 52 sulfate, cells exposed to PCB sulfates exhibited similar results between the two cell viability methods. For the experiments in which LDH release-EC50 values could be calculated (i.e., in the SH-SY5Y and HepG2 cells), the data corresponded well with the experimental values observed for MTT reduction.
3.3. Supplementation of N27 cells with serum proteins during exposure to PCB metabolites
Since previous studies have shown that OH-PCBs and PCB sulfates bind with high affinity to human serum albumin (HSA), we examined the effect of addition of either HSA or horse serum (HS; a common constituent of growth medium for N27 cells) to the cell culture medium during exposure of N27 cells to OH-PCBs and PCB sulfates. Results of these experiments are shown in Supplementary Material Figures S4 through S7. Incubation of the cells with 25 μM 4-OH-PCB 52 or its corresponding sulfate (Figure S4) resulted in a significant decrease in cell viability that correlated with the results seen in Table 2. This concentration of 25 μM for PCB metabolites was chosen to facilitate comparison across all compounds in the N27 cells (i.e., it includes both toxic and non-toxic responses). The concentrations of HSA were chosen to represent a 1:2, 1:1, 2:1 molar ratio of metabolite:HSA, and the HS supplementation was chosen to be in a range to mimic the media composition of serum (i.e., 2%−10%) in previously published PCB-exposure studies (Angus and Contreras 1996; Costa et al. 2007; De et al. 2010; Lee and Opanashuk 2004; Shimokawa et al. 2006). These results, as well as those for the remaining PCB metabolites included in this study (Figures S5 through S6), indicate that even at a 2:l-metabolite:HSA ratio, the protective effects of albumin-binding were sufficient to decrease toxicity substantially to a level that was statistically indistinguishable from untreated controls.
3.4. The disposition of OH-PCB and PCB sulfates between media and cells
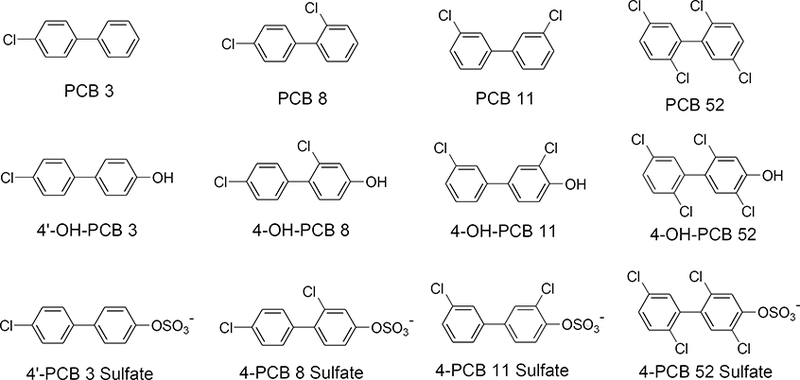
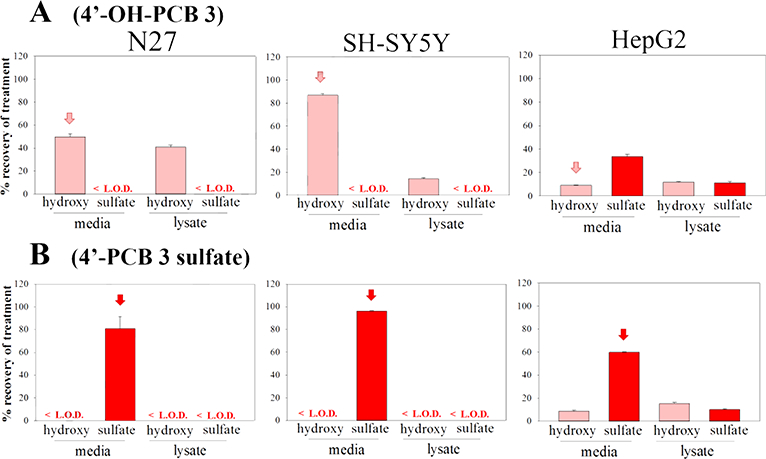
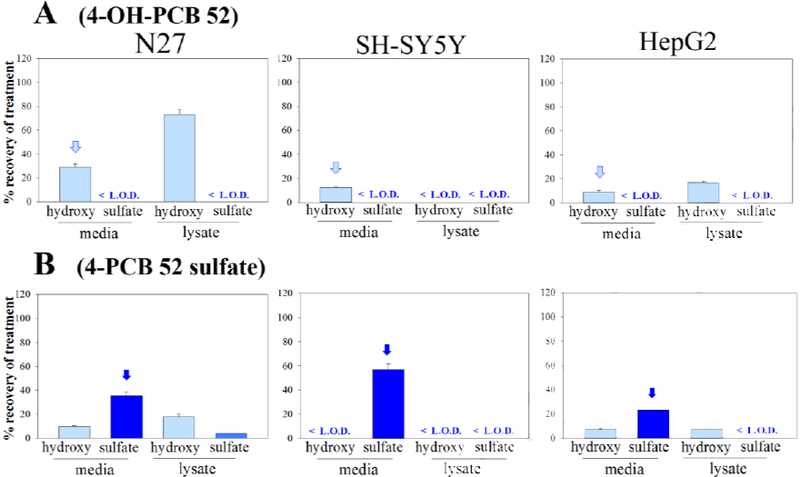
In the analysis of our results on the toxicity of OH-PCB and PCB sulfate metabolites, the question arose as to the extent to which these metabolites are taken up into the cells and/or further metabolized within those cells. To address this question, each cell-type was treated for 24 h with 25 μM (2.5 nmol in 100 μL of serum-free medium) of the OH-PCB or PCB sulfate. The cellular and extracellular compartments were analyzed by HPLC, and the distributions of these metabolites between the cells and the extracellular media are presented in Figures 2 through 5, with the treatment compound denoted with an arrow.
Fig. 2.
The distribution of: A) 4’-OH-PCB 3 and B) 4’-PCB 3 sulfate, in N27, SH-SY5Y, and HepG2 cells as determined by HPLC analysis. Cells were treated with 25 μM (2.5nmol) compound for 24h, then extracellular media and intracellular contents were collected and analyzed. The treatment compound is annotated with an arrow. The values shown are the means ± SE, n=3.
Fig. 5.
The distribution of: A) 4-OH-PCB 52 and B) 4-PCB 52 sulfate, in N27, SH-SY5Y, and HepG2 cells as determined by HPLC analysis. Cells were treated with 25μM (2.5nmol) compound for 24h, and subjected to analysis of the extracellular media and intracellular contents. The treatment compound is annotated with an arrow. The values shown are the means ± SE, n=3.
The results for 4’-OH-PCB 3 and 4-OH-PCB 11 (i.e., the hydroxylated metabolites without ortho-chlorine substitutions) are shown in Figures 2 and 3, respectively. A comparison across cell lines indicates that, after 24 h, there was a distribution of 4’-OH-PCB 3 into all three cell-types, however, the cell lines differed in the amount of OH-PCB found within the cells and the total amount recovered in the two compartments. For N27 and SH-SY5Y cells, 4’-OH-PCB 3 and 4-OH-PCB 8 were found in the cell lysate, although less of these two OH-PCBs were detected in the SH-SY5Y cell lysates. While exposure of the HepG2 cells to either 4’-OH-PCB 3 or 4-OH-PCB 11 resulted in the sulfation of these hydroxylated congeners and further redistribution of the PCB sulfate into the medium, there was no evidence of sulfation of these two OH-PCBs in either neural cell line. Exposure of N27 and SH-SY5Y cells to either 4’-PCB 3 sulfate or 4-PCB 11 sulfate indicated that these sulfated congeners did not enter the neural cells, given their near quantitative recovery in the media. However, with the HepG2 cells, exposure to 4’-PCB 3 sulfate or 4-PCB 11 sulfate resulted in its cellular uptake and distribution between the cellular and extracellular compartments. Moreover, the corresponding hydroxylated derivatives were also detected in these cells. These OH-PCBs are most likely present due to hydrolysis of the sulfates catalyzed by sulfatases within the HepG2 cells, since control experiments showed no spontaneous sulfate-hydrolysis in the medium under the experimental conditions. In those cases where there was a lack of quantitative recovery of the OH-PCB and PCB sulfate, it is likely that further metabolism may account for this discrepancy. Although binding of the OH-PCB and PCB sulfate to the plastic surfaces of the culture plates was considered, this was unlikely due to the near quantitative recovery of several OH-PCB and PCB sulfate congeners from some cell-types under identical extraction conditions.
Fig. 3.
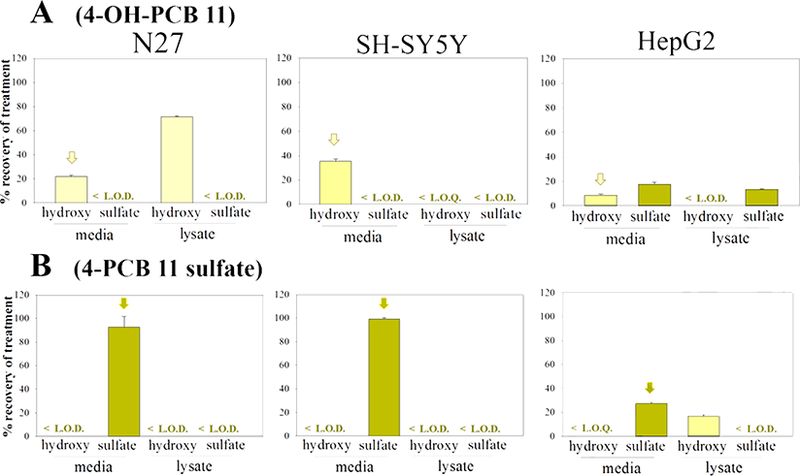
The distribution of: A) 4-OH-PCB 11 and B) 4-PCB 11 sulfate, in N27, SH-SY5Y, and HepG2 cells as determined by HPLC analysis. Cells were treated with 25μM (2.5nmol) compound for 24h, and subjected to analysis of the extracellular media and intracellular contents. The treatment compound is annotated with an arrow. The values shown are the means ± SE, n=3.
Exposure of the three cell-types to the metabolites of PCBs containing ortho-chlorine substitutions (4-OH-PCB 8, 4-OH-PCB 52, 4-PCB 8 sulfate, and 4-PCB 52 sulfate) exhibited qualitative and quantitative differences from the results obtained with the metabolites of PCB 3 and PCB 11. As seen in Figures 4 and 5, 4-OH-PCB 8 distributed well into the N27 cells while relatively lower concentrations were found in the lysate of SH-SY5Y cells exposed to this metabolite. Furthermore, overall recovery of the 4-OH-PCB 8 after incubation was lower in the SH-SY5Y cells. As observed for the sulfated metabolites of PCB 3 and PCB 11, 4-PCB 8 sulfate was not found in the lysate of either N27 or SH-SY5Y cells. The 4-PCB 52 sulfate, however, was taken up by the N27 cells and the presence of 4-OH-PCB 52 in both the medium and cell lysates indicated metabolic hydrolysis of the sulfate and redistribution between the extracellular and intracellular compartments. While both 4-OH-PCB 8 and 4-OH-PCB 52 were taken up by HepG2 cells, only 4-OH-PCB 8 was detected as its corresponding sulfate conjugate. Incubation of HepG2 cells with either 4-PCB 8 sulfate or 4-PCB 52 sulfate resulted in substantial amounts of the corresponding 4-OH-PCB metabolites. This indicated that hydrolysis of the PCB sulfate occurred in these cells.
Fig. 4.
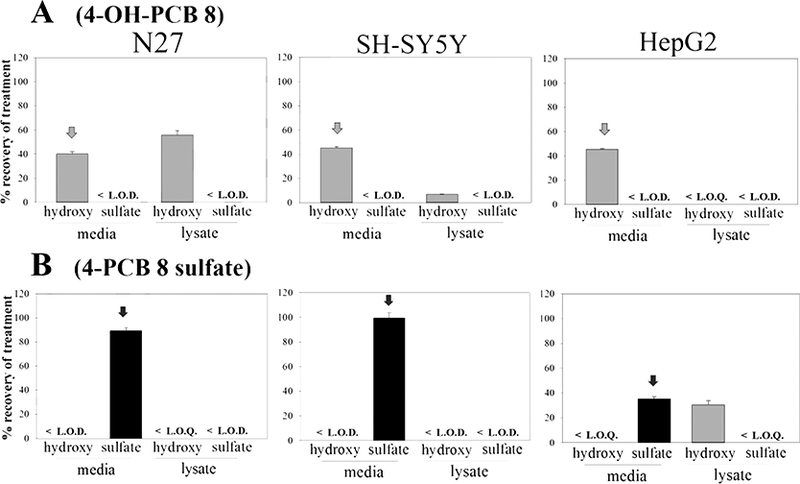
The distribution of: A) 4-OH-PCB 8 and B) 4-PCB 8 sulfate, in N27, SH-SY5Y, and HepG2 cells as determined by HPLC analysis. Cells were treated with 25μM (2.5nmol) compound for 24h, and subjected to analysis of the extracellular media and intracellular contents. The treatment compound is annotated with an arrow. The values shown are the means ± SE, n=3.
4. Discussion
4.1. Selectivity in the toxicities of lower-chlorinated PCBs and their metabolites
Our results on cytotoxicity indicated that the lower-chlorinated OH-PCBs examined were more toxic than their corresponding PCBs or PCB sulfates. Moreover, in most cases, rat and human cells of neural origin exhibited increased toxicity when compared with the hepatic HepG2 cells, and the ortho-substituted OH-PCBs elicited the most potent toxic responses. Although the present experiments were not designed to distinguish among specific mechanisms of toxicity, it is noteworthy that OH-PCBs have been shown to be potent inducers of reactive oxygen species (ROS) in both neural and hepatic cells (Dreiem et al. 2009; Spencer et al. 2009; Xu et al. 2015), and this may represent a potential starting point for future studies on the mechanisms of these toxic responses.
Most of the PCBs and PCB sulfates examined here did not exhibit a significant toxic response to any cell line, as determined by either viability assay. The exception in both cases was the di-ortho-substituted PCB 52 and its sulfate conjugate, where the latter exhibited a toxic response in N27 cells that was comparable to that observed for most of the OH-PCBs in all cell lines. The toxicity of PCBs with ortho-chlorine substituents has been reported (Lilienthal et al. 2014; Tan et al. 2004), and toxic responses to the di-ortho-substituted PCB 52 have been proposed to proceed through multiple pathways, such as cell membrane dysfunction, disruption of cellular calcium homeostasis, and covalent binding to proteins and DNA (Lin et al. 2000; Pessah et al. 2010; Tan et al. 2003). A comparison of PCB 52- and PCB 52 sulfate-mediated cytotoxicity in N27 cells (Figure S1) indicates that, under the current experimental conditions, mitochondrial dysfunction may have been more substantial than alteration in cell membrane integrity when considering cellular insult. While this interpretation was supported by the lack of quantitative LDH release in the medium at concentrations where the MTT-reduction capability was essentially eliminated, the exact cause of this difference between the two responses remains to be elucidated.
4.2. Serum supplementation and PCB metabolite toxicities
Upon supplementing N27 cells with either human serum albumin (HSA) or horse serum (HS; commonly used in culture of N27 cells) the PCB-mediated toxic responses were mitigated. Even at molar concentrations of protein that were half those of the PCB derivatives, the toxic response was eliminated. Serum proteins such as HSA bind lower-chlorinated PCBs, OH-PCBs, and PCB sulfates with high affinity (Rodriguez et al. 2016), and their presence would likely decrease the concentration of the free form of each toxicant in the cell culture media (vs. protein-bound which is not available for diffusion or transport across the cell membrane). Many published studies on PCB-related assessments of cellular viability, calcium homeostasis, ROS formation, and other endpoints use serum supplemented media (ranging from 2–10% horse or fetal calf serum, or some mix thereof) throughout the duration of PCB exposure. Although it is unknown the extent to which binding to serum proteins might protect either neural or hepatic cells from exposure to these PCB metabolites in vivo, previous studies indicate that this binding may serve as a mode for their retention and distribution (Cutler et al. 1967; Kragh-Hansen et al. 2002). Moreover, our results highlight the observation that in vitro studies on PCBs and PCB metabolites may be affected by serum proteins in media components. These results also have implications in vivo since, variations in the concentrations of albumin may impart organ-specific susceptibility to PCB-mediated toxicites. For example, the steady state concentration of albumin in cerebrospinal fluid is only approximately 0.5% of that found in plasma (Cutler et al., 1967).
4.3. Selectivity in cellular distribution of PCB metabolites
The further metabolism and distribution of the lower-chlorinated PCB metabolites between the cells and the surrounding medium may contribute to the observed selective toxicity in the neural versus the hepatic cell lines. Using a mass balance approach, our experiments gave some insight into the movement of these PCB metabolites into the cells, as well as their potential for further metabolism, degradation, or other modifications (e.g., covalently bound to protein). It is important to note that our approach did not allow accounting for all possible metabolites in all cells.
The OH-PCBs that showed the highest toxicity to all three cell lines generally exhibited an appreciable distribution into the cells. The extent of distribution, however, could not be correlated with toxic potency. These experiments were not designed to discern between a lack of uptake into and an efficient efflux out of the cells. Likewise, metabolic reactions other than sulfation or de-sulfation could not be distinguished by this method. Previous results from in vitro and in vivo studies indicate that oxidized metabolites of lower chlorinated PCBs may become covalently adducted to proteins or DNA (Flor and Ludewig 2010; Ludewig et al. 2008; Zhao et al. 2004). Moreover, the formation of such covalent adducts in the livers and brains of rats treated with PCB 52 have been reported (Lin et al., 2000). Protein/DNA-binding, or other sequestration of metabolites, may explain the lack of quantitative recovery for many of the experiments that, nonetheless, resulted in highly potent toxic responses (e.g., 4-OH-PCB 52 in the SH-SY5Y and HepG2 cells). However, this explanation cannot account for all trends in toxicity such as that seen with exposure to 4-OH-PCB 8, where the N27 cells exhibited higher quantitative recovery, better distribution into the cells, and higher potency when compared to the HepG2 cells.
HepG2 cells have been reported to display multiple drug-metabolizing enzymes and transporters (Guo et al. 2011), and our studies indicated substantial metabolic capacity in the sulfation of OH-PCBs and hydrolysis of PCB sulfates. This capability was seen for all compounds tested, and it may contribute to the reduced toxicity of these OH-PCBs to the HepG2 cells (i.e., due to sulfation or other conjugation reactions, thereby decreasing the intracellular concentration of the OH-PCB). The ability of the HepG2 cells to catalyze hydrolysis of the sulfate conjugates, however, is likely responsible for the toxic response to 4-PCB 52 sulfate associated with the intracellular formation of 4-OH-PCB 52. The N27 cells displayed similar metabolic potential with the 4-PCB 52 sulfate, and this may also explain the toxic effects of this metabolite to these cells, where the relatively high potency of 4-PCB 52 sulfate (as judged by the MTT assay) may be due to the presence of 4-OH-PCB 52, as evidenced by the HPLC analysis.
5. Conclusions
The number of studies concerning the cytotoxic potential of individual PCB congeners with lower numbers of chlorine atoms is small in comparison to those studies involving the higher-chlorinated PCBs and Aroclor mixtures. This is certainly true for investigations on PCB metabolites, where previous experiments have primarily focused on hydroxylated PCBs. The results of our present studies suggest that metabolism of lower-chlorinated PCBs by hydroxylation and subsequent sulfation may mediate or influence PCB-related health effects. This was particularly evident in the differential effects on neural cells, indicating a need for further investigation of potential effects on neurological function. The use of cell lines derived from different origins (i.e., midbrain vs. neuroblastoma vs. liver) and from different species (i.e., rat and human) in the present experiments indicate that hydroxylated and sulfated metabolites derived from PCBs exhibit distinct toxicological profiles that differ by congener and cell-type.
The conclusions from our study of the distribution of OH-PCBs and PCB sulfates in these cells are summarized in Scheme 1. Generally, with all three cell lines, OH-PCBs were able to enter the cells and elicit variable toxic responses. However, only in the hepatic cell line was there evidence of subsequent sulfation, presumably through sulfotransferase-mediated metabolism, which may explain the reduced toxicity in these cells when compared to those of neural origin. The PCB sulfates were also able to enter the HepG2 cells, where, in some cases, their hydrolysis resulted in detectable OH-PCBs. The neural cells were not permeant to all PCB sulfates, but exhibited sulfatase activity for those congeners that were able to enter the cells. Sulfatase-catalyzed formation of the OH-PCBs may explain the toxicity detected upon exposure of N27 cells to 4-PCB 52 sulfate. Thus, our results indicate the need for more information on the transport of PCB sulfates and OH-PCBs into and out of different cell types as well as the congener selectivity in relation to intracellular sulfatases and sulfotransferases.
Scheme 1.
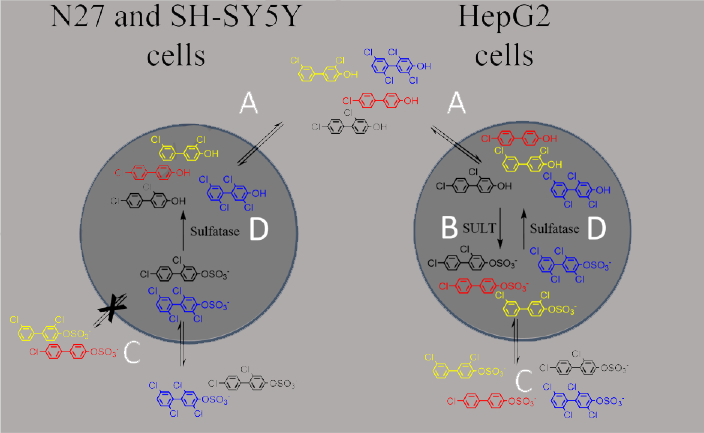
A summary of the observed cell-type specific differences in PCB metabolite permeability and potential for further metabolic transformations. Colors indicate OH-PCB and PCB sulfate metabolites of PCB 3 (red), PCB 8 (black), PCB 11 (yellow), and PCB 52 (blue). A) OH-PCBs were able to enter all three cell lines tested, where B) some congeners were susceptible to sulfation in the hepatic HepG2 cells. n=3. C) All PCB sulfates were able to enter HepG2 cells, while uptake in the neural cell lines was more selective. D) Both the HepG2 and N27 cell lines exhibited sulfatase activity for some congeners, thereby resulting in in situ production of OH-PCBs.
Although this study does not provide details on the exact mechanism(s) of toxicity for these compounds, it does highlight the need to consider that metabolism may play a key role in the toxic effects associated with PCB-exposure to cells of neural origin. Previous studies using rat hippocampal slices and primary cultured hippocampal neurons have indicated no primary metabolism of parent PCBs, however no such studies with lower-chlorinated PCBs were reported (Wu et al. 2013; Yang et al. 2014). Furthermore, while OH-PCBs have not been identified in human brain samples, they have been reported in brain samples collected from East Greenland polar bears (Gebbink et al. 2008). The CNS may not be a site for the initial oxidative metabolism of lower chlorinated PCBs, however, our studies indicate that it may be a site for further metabolism, and the production of potentially toxic species. In addition to the direct toxic insult of these PCB metabolites, the sulfation of hormones and neurotransmitters is a crucial component of cellular maintenance and regulation in the CNS, and disruption of this component of the neuroendocrine system may have damaging and chronic effects (Coughtrie 2002).
An additional consideration is the fact that the EC50 values reported in this study are generally higher than PCB concentrations commonly reported in an exposed population. A chronic exposure to these compounds may, however, allow for their accumulation, resulting in high localized concentrations that might exhibit similar toxic outcomes. A recent study on the elimination kinetics of PCB 28 metabolites in a PCB-exposed human population indicated half-lives on the time-scale of years, with some of the OH-PCB metabolites showing a longer half-life than the parent PCB (Quinete et al. 2017). Thus, our results on the distinct toxicity profiles of OH-PCBs and PCB sulfates, and the growing evidence that these metabolites may play roles in the dysregulation of hormonal, neurotoxicological, and neurodevelopmental events, indicate the need for more extensive studies on the mechanism(s) of these toxic effects and the dependence upon congener structure and metabolic fate.
Supplementary Material
Highlights.
Phenolic and sulfated metabolites of PCBs 3, 8, 11, and 52 were studied in vitro.
The OH-PCBs displayed toxicity to N27, SHSY-5Y, and HepG2 cells.
OH-PCBs were generally more toxic than the corresponding PCBs or PCB sulfates.
4-PCB 52 sulfate, however, showed significant toxicity to neural and hepatic cells.
Mechanisms for 4-PCB 52 sulfate toxicity may require its metabolism to 4-OH-PCB 52.
Acknowledgements
The authors thank Dr. Xueshu Li of the Synthesis Core of the Iowa Superfund Research Program for the synthesis and characterization of the various PCB derivatives.
Funding Sources
This work was supported by NIH grant P42 ES013661 from the National Institute of Environmental Health Sciences and the University of Iowa Environmental Health Sciences Research Center (NIEHS/NIH P30 ES05605).
Abbreviations
- DMEM
Dulbecco’s modified eagle’s medium
- DPBS
Dulbecco’s phosphate buffered saline
- HS
horse serum
- HSA
human serum albumin
- LDH
lactate dehydrogenase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OH-PCB
hydroxylated polychlorinated biphenyl
- 4’-OH PCB 3
4-chloro-4’-hydroxybiphenyl
- 4-OH-PCB 8
2,4’-dichloro-4-hydroxybiphenyl
- 4-OH-PCB 11
3,3’-dichloro-4-hydroxybiphenyl
- 4-OH PCB 52
2,2’,5, 5’-tetrachloro-4-hydroxybiphenyl
- PCB
polychlorinated biphenyl
- PCB 3
4-chlorobiphenyl
- PCB 8
2,4’-dichlorobiphenyl
- PCB 11
3,3’-dichlorobiphenyl
- PCB 28
2,4,4’-trichlorobiphenyl
- PCB 52
2,2’,5,5’-tetrachlorobiphenyl
- 4’-PCB 3 sulfate
4-chloro-4’-biphenylsulfate
- 4-PCB 8 sulfate
2,4’-dichloro-4-biphenylsulfate
- 4-PCB 11 sulfate
3,3’-dichloro-4-biphenylsulfate
- 4-PCB 52 sulfate
2,2’,5, 5’-tetrachloro-4-biphenylsulfate
- RPMI medium
Roswell Park Memorial Institute medium.
Footnotes
Conflict of Interest
The authors state that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ampleman MD, Martinez A, DeWall J, Rawn DF, Hornbuckle KC and Thorne PS 2015. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ Sci Technol 49, 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus WG and Contreras ML 1996. Effects of polychlorinated biphenyls on dopamine release from PC12 cells. Toxicol Lett 89, 191–199. [DOI] [PubMed] [Google Scholar]
- Basu I, Arnold KA, Venier M and Hites RA 2009. Partial pressures of PCB-11 in air from several Great Lakes sites. Environ Sci Technol 43, 6488–6492. [DOI] [PubMed] [Google Scholar]
- Berghuis SA, Bos AF, Sauer PJ and Roze E 2015. Developmental neurotoxicity of persistent organic pollutants: an update on childhood outcome. Arch Toxicol 89, 687–709. [DOI] [PubMed] [Google Scholar]
- Berghuis SA, Soechitram SD, Hitzert MM, Sauer PJ and Bos AF 2013. Prenatal exposure to polychlorinated biphenyls and their hydroxylated metabolites is associated with motor development of three-month-old infants. Neurotoxicology 38, 124–130. [DOI] [PubMed] [Google Scholar]
- Cave M, Appana S, Patel M, Falkner KC, McClain CJ and Brock G 2010. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect 118, 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lin Y, Dang K and Puschner B 2017. Quantification of Polychlorinated Biphenyls and Polybrominated Diphenyl Ethers in Commercial Cows’ Milk from California by Gas Chromatography-Triple Quadruple Mass Spectrometry. PLoS One 12, e0170129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Fattori V, Giordano G and Vitalone A 2007. An in vitro approach to assess the toxicity of certain food contaminants: methylmercury and polychlorinated biphenyls. Toxicology 237, 65–76. [DOI] [PubMed] [Google Scholar]
- Coughtrie MW 2002. Sulfation through the looking glass--recent advances in sulfotransferase research for the curious. Pharmacogenomics J 2, 297–308. [DOI] [PubMed] [Google Scholar]
- Cutler RW, Deuel RK and Barlow CF 1967. Albumin exchange between plasma and cerebrospinal fluid. Arch Neurol 17, 261–270. [DOI] [PubMed] [Google Scholar]
- De S, Ghosh S, Chatterjee R, Chen YQ, Moses L, Kesari A, Hoffman EP and Dutta SK 2010. PCB congener specific oxidative stress response by microarray analysis using human liver cell line. Environ Int 36, 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal K, He X, Lehmler HJ, Teesch LM, Duffel MW and Robertson LW 2012. Identification of sulfated metabolites of 4-chlorobiphenyl (PCB3) in the serum and urine of male rats. Chem Res Toxicol 25, 2796–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiem A, Rykken S, Lehmler HJ, Robertson LW and Fonnum F 2009. Hydroxylated polychlorinated biphenyls increase reactive oxygen species formation and induce cell death in cultured cerebellar granule cells. Toxicol Appl Pharmacol 240, 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egsmose EL, Brauner EV, Frederiksen M, Morck TA, Siersma VD, Hansen PW, Nielsen F, Grandjean P and Knudsen LE 2016. Associations between plasma concentrations of PCB 28 and possible indoor exposure sources in Danish school children and mothers. Environ Int 87, 13–19. [DOI] [PubMed] [Google Scholar]
- Ekuase EJ, Liu Y, Lehmler HJ, Robertson LW and Duffel MW 2011. Structure-activity relationships for hydroxylated polychlorinated biphenyls as inhibitors of the sulfation of dehydroepiandrosterone catalyzed by human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol 24, 1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor S and Ludewig G 2010. Polyploidy-induction by dihydroxylated monochlorobiphenyls: structure-activity-relationships. Environ Int 36, 962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink WA, Sonne C, Dietz R, Kirkegaard M, Riget FF, Born EW, Muir DC and Letcher RJ 2008. Tissue-specific congener composition of organohalogen and metabolite contaminants in East Greenland polar bears (Ursus maritimus). Environ. Pollut 152, 621–629. [DOI] [PubMed] [Google Scholar]
- Grimm FA, He X, Teesch LM, Lehmler HJ, Robertson LW and Duffel MW 2015a. Tissue Distribution, Metabolism, and Excretion of 3,3’-Dichloro-4’-sulfooxy-biphenyl in the Rat. Environ Sci Technol 49, 8087–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A and Robertson LW 2015b. Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol 45, 245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Lehmler HJ, He X, Robertson LW and Duffel MW 2013. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ Health Perspect 121, 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Lehmler HJ, Koh WX, DeWall J, Teesch LM, Hornbuckle KC, Thorne PS, Robertson LW and Duffel MW 2017. Identification of a sulfate metabolite of PCB 11 in human serum. Environ Int 98, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Dial S, Shi L, Branham W, Liu J, Fang JL, Green B, Deng H, Kaput J and Ning B 2011. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab Dispos 39, 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutleb AC, Cenijn P, Velzen M, Lie E, Ropstad E, Skaare JU, Malmberg T, Bergman A, Gabrielsen GW and Legler J 2010. In vitro assay shows that PCB metabolites completely saturate thyroid hormone transport capacity in blood of wild polar bears (Ursus maritimus). Environ Sci Technol 44, 3149–3154. [DOI] [PubMed] [Google Scholar]
- Hansen LG (Ed) 2001. Identification of steady state and episodic PCB congeners from multiple pathway exposures, The University Press of Kentucky, Lexington, Kentucky, US, pp. 47–56. [Google Scholar]
- Hatcher-Martin JM, Gearing M, Steenland K, Levey AI, Miller GW and Pennell KD 2012. Association between polychlorinated biphenyls and Parkinson’s disease neuropathology. Neurotoxicology 33, 1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D and Hornbuckle KC 2010. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ Sci Technol 44, 2822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Martinez A and Hornbuckle KC 2011. Sedimentary Records of Non-Aroclor and Aroclor PCB mixtures in the Great Lakes. J Great Lakes Res 37, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Adamcakova-Dodd A and Thorne PS 2014. The fate of inhaled (14)C-labeled PCB11 and its metabolites in vivo. Environ Int 63, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J, Polanska K and Hanke W 2013. Chemical exposure early in life and the neurodevelopment of children--an overview of current epidemiological evidence. Ann Agric Environ Med 20, 465–486. [PubMed] [Google Scholar]
- Kester MH, Bulduk S, van Toor H, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Schuur AG, Brouwer A and Visser TJ 2002. Potent inhibition of estrogen sulfotransferase by hydroxylated metabolites of polyhalogenated aromatic hydrocarbons reveals alternative mechanism for estrogenic activity of endocrine disrupters. J Clin Endocrinol Metab 87, 1142–1150. [DOI] [PubMed] [Google Scholar]
- Koh WX, Hornbuckle KC, Marek RF, Wang K and Thorne PS 2016. Hydroxylated polychlorinated biphenyls in human sera from adolescents and their mothers living in two U.S. Midwestern communities. Chemosphere 147, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh-Hansen U, Chuang VT and Otagiri M 2002. Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol Pharm Bull 25, 695–704. [DOI] [PubMed] [Google Scholar]
- Lee DW and Opanashuk LA 2004. Polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress plays a role in dopaminergic cell injury. Neurotoxicology 25, 925–939. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, He X, Li X, Duffel MW and Parkin S 2013. Effective synthesis of sulfate metabolites of chlorinated phenols. Chemosphere 93, 1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler HJ and Robertson LW 2001. Synthesis of hydroxylated PCB metabolites with the Suzuki-coupling. Chemosphere 45, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Li X, Parkin S, Duffel MW, Robertson LW and Lehmler HJ 2010. An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ Int 36, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienthal H, Heikkinen P, Andersson PL, van der Ven LT and Viluksela M 2014. Dopamine-dependent behavior in adult rats after perinatal exposure to purity-controlled polychlorinated biphenyl congeners (PCB52 and PCB180). Toxicol Lett 224, 32–39. [PubMed] [Google Scholar]
- Lin PH, Sangaiah R, Ranasinghe A, Upton PB, La DK, Gold A and Swenberg JA 2000. Formation of quinonoid-derived protein adducts in the liver and brain of Sprague-Dawley rats treated with 2,2’,5, 5’-tetrachlorobiphenyl. Chem Res Toxicol 13, 710–718. [DOI] [PubMed] [Google Scholar]
- Liu Y, Apak TI, Lehmler HJ, Robertson LW and Duffel MW 2006. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol 19, 1420–1425. [DOI] [PubMed] [Google Scholar]
- Ludewig G, Lehmann L, Esch H and Robertson LW 2008. Metabolic Activation of PCBs to Carcinogens in Vivo - A Review. Environ Toxicol Pharmacol 25, 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek RF, Thorne PS, DeWall J and Hornbuckle KC 2014. Variability in PCB and OH-PCB serum levels in children and their mothers in urban and rural U.S. communities. Environ Sci Technol 48, 13459–13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg E, Danielsson S, Eriksson U, Faxneld S, Miller A and Bignert A 2014. Spatio-temporal trends of PCBs in the Swedish freshwater environment 1981–2012. Ambio 43 Suppl 1, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker VS, Squirewell EJ, Lehmler HJ, Robertson LW and Duffel MW 2018. Hydroxylated and sulfated metabolites of commonly occurring airborne polychlorinated biphenyls inhibit human steroid sulfotransferases SULT1E1 and SULT2A1. Environ Toxicol Pharmacol 58, 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen EB, Ebbehoj NE, Goen T, Meyer HW and Jacobsen P 2016. Exposure to 27 polychlorinated biphenyls in the indoor environment of a workplace: a controlled bio-monitoring study. Int Arch Occup Environ Health 89, 43–47. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Cherednichenko G and Lein PJ 2010. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther 125, 260–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska K, Jurewicz J and Hanke W 2013. Review of current evidence on the impact of pesticides, polychlorinated biphenyls and selected metals on attention deficit / hyperactivity disorder in children. Int J Occup Med Environ Health 26, 16–38. [DOI] [PubMed] [Google Scholar]
- Quinete N, Esser A, Kraus T and Schettgen T 2017. PCB 28 metabolites elimination kinetics in human plasma on a real case scenario: Study of hydroxylated polychlorinated biphenyl (OH-PCB) metabolites of PCB 28 in a highly exposed German Cohort. Toxicol Lett 276, 100–107. [DOI] [PubMed] [Google Scholar]
- Rodenburg LA, Guo J, Du S and Cavallo GJ 2010. Evidence for unique and ubiquitous environmental sources of 3,3’-dichlorobiphenyl (PCB 11). Environ Sci Technol 44, 2816–2821. [DOI] [PubMed] [Google Scholar]
- Rodriguez EA, Li X, Lehmler HJ, Robertson LW and Duffel MW 2016. Sulfation of Lower Chlorinated Polychlorinated Biphenyls Increases Their Affinity for the Major Drug Binding Sites of Human Serum Albumin. Environ Sci Technol 50, 5320–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer P, Muller M, Kruger A, Steinberg CE and Menzel R 2009. Cytochrome P450-dependent metabolism of PCB52 in the nematode Caenorhabditis elegans. Arch Biochem Biophys 488, 60–68. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ and Rice DC 2003. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect 111, 357–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Keil KP, Chen H, Hayakawa K, Li X, Lin Y, Lehmler HJ, Puschner B and Lein PJ 2017. Detection of 3,3’-Dichlorobiphenyl in Human Maternal Plasma and Its Effects on Axonal and Dendritic Growth in Primary Rat Neurons. Toxicol Sci 158, 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang H, Li Y, Wang T, Wang P, Zhang H, Zhang Q and Jiang G 2014. The presence of polychlorinated biphenyls in yellow pigment products in China with emphasis on 3,3’-dichlorobiphenyl (PCB 11). Chemosphere 98, 44–50. [DOI] [PubMed] [Google Scholar]
- Shimokawa N, Miyazaki W, Iwasaki T and Koibuchi N 2006. Low dose hydroxylated PCB induces c-Jun expression in PC12 cells. Neurotoxicology 27, 176–183. [DOI] [PubMed] [Google Scholar]
- Spencer WA, Lehmler HJ, Robertson LW and Gupta RC 2009. Oxidative DNA adducts after Cu(2+)-mediated activation of dihydroxy PCBs: role of reactive oxygen species. Free Radic Biol Med 46, 1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Hein MJ, Cassinelli RT 2nd Prince MM, Nilsen NB, Whelan EA, Waters MA, Ruder AM and Schnorr TM 2006. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology 17, 8–13. [DOI] [PubMed] [Google Scholar]
- Tan Y, Li D, Song R, Lawrence D and Carpenter DO 2003. Ortho-substituted PCBs kill thymocytes. Toxicol Sci 76, 328–337. [DOI] [PubMed] [Google Scholar]
- Tan Y, Song R, Lawrence D and Carpenter DO 2004. Ortho-substituted but not coplanar PCBs rapidly kill cerebellar granule cells. Toxicol Sci 79, 147–156. [DOI] [PubMed] [Google Scholar]
- Tilson HA and Kodavanti PR 1997. Neurochemical effects of polychlorinated biphenyls: an overview and identification of research needs. Neurotoxicology 18, 727–743. [PubMed] [Google Scholar]
- Tilson HA and Kodavanti PR 1998. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology 19, 517–525. [PubMed] [Google Scholar]
- Tilson HA, Kodavanti PR, Mundy WR and Bushnell PJ 1998. Neurotoxicity of environmental chemicals and their mechanism of action. Toxicol Lett 102-103, 631–635. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N and Peterson RE 2006. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 93, 223–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meerloo J, Kaspers GJ and Cloos J 2011. Cell sensitivity assays: the MTT assay. Methods Mol Biol 731, 237–245. [DOI] [PubMed] [Google Scholar]
- Vassault A 1974. Lactate dehydrogenase In: H.U.B. Bergmeyer J; Grassl M (Ed), Methods of enzymatic analysis, Verlag Chemie; Academic Press, Weinheim, New York, pp. 118–126. [Google Scholar]
- Vorkamp K 2016. An overlooked environmental issue? A review of the inadvertent formation of PCB-11 and other PCB congeners and their occurrence in consumer products and in the environment. Sci Total Environ 541, 1463–1476. [DOI] [PubMed] [Google Scholar]
- Wu X, Kania-Korwel I, Chen H, Stamou M, Dammanahalli KJ, Duffel M, Lein PJ and Lehmler HJ 2013. Metabolism of 2,2’,3,3’,6,6’-hexachlorobiphenyl (PCB 136) atropisomers in tissue slices from phenobarbital or dexamethasone-induced rats is sex-dependent. Xenobiotica 43, 933–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Li L, Liu L, Dong H, Deng Q, Yang X, Song E and Song Y 2015. Polychlorinated biphenyl quinone induces mitochondrial-mediated and caspase-dependent apoptosis in HepG2 cells. Environ. Toxicol 30, 1063–1072. [DOI] [PubMed] [Google Scholar]
- Yang D, Kania-Korwel I, Ghogha A, Chen H, Stamou M, Bose DD, Pessah IN, Lehmler HJ and Lein PJ 2014. PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol Sci 138, 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Narang A, Ding X and Eadon G 2004. Characterization and quantitative analysis of DNA adducts formed from lower chlorinated PCB-derived quinones. Chem Res Toxicol 17, 502–511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


