Abstract
The human autoimmune disease-associated HLA alleles HLA-DR2b (DRB1*1501) and HLA-DR4 (DRB1*0401) are strongly linked to increased susceptibility for multiple sclerosis (MS) and rheumatoid arthritis (RA), respectively. The underlying mechanisms are not fully understood, but these MHC alleles may shape the repertoire of pathogenic T cells via central tolerance. The transcription factor autoimmune regulator (AIRE) promotes central T cell tolerance via ectopic expression of tissue-specific antigens (TSAs). Aire deficiency in humans causes autoimmune polyendocrinopathy syndrome type 1 (APS1), and Aire knockout mice (Aire−/−) develop spontaneous autoimmune pathology characterized by multi-organ lymphocytic infiltrates.
Here, we asked whether impaired TSAs gene expression in the absence of Aire promoted spontaneous MS- or RA-like autoimmune pathology in the context of human HLA alleles in HLA-DR2b or HLA-DR4 transgenic (tg) mice.
The results show that reduced TSAs gene expression in the thymus of Aire-deficient HLA-DR2b or HLA-DR4 tg mice corresponded to mild spontaneous inflammatory infiltrates in salivary glands, liver, and pancreas. Moreover, Aire-deficiency modestly enhanced experimental autoimmune encephalomyelitis (EAE) in HLA-DR tg mice, but the animals did not show signs of spontaneous neuroinflammation or arthritis. No significant changes were observed in CD4+ T cell numbers, T cell receptor (TCR) distribution, regulatory T cells (Treg), or antigen-induced cytokine production. Abrogating Treg function by treatment with anti-CTLA-4 or anti-CD25 mAb in Aire-deficient HLA-DR tg mice did not trigger EAE or other autoimmune pathology. Our results suggest a redundant role for Aire in maintaining immune tolerance in the context of autoimmune disease-associated human HLA alleles.
Keywords: EAE, T cells, Aire
Introduction
While the exact cause for most human autoimmune diseases is unknown, it is believed that autoreactive T cells are key mediators of pathology in many conditions, including multiple sclerosis (MS) and rheumatoid arthritis (RA) [1–3]. Central tolerance is a key mechanism involved in eliminating autoreactive T cells in the thymus during the process of negative selection of thymocytes via presentation of self-antigens in the thymic medulla [4, 5]. In the thymus, autoimmune regulator (AIRE in humans, Aire in mice) promotes central tolerance by inducing ectopic transcription of tissue specific antigens (TSAs) normally expressed in peripheral sites [6, 7]. AIRE is primarily expressed in the thymus, but it can also be expressed in secondary lymphatic tissues. Within the thymus, it is expressed mostly by thymic medullary epithelial cells (mTECs), but also by dendritic cells (DCs), although at much lower levels [6–8]. In secondary lymphoid tissues, Aire is expressed in extrathymic Aire-expressing cells (eTACs), which may help to enforce peripheral T cell tolerance [9]. The limited overlap between Aire-dependent gene expression in eTACs and mTECs suggests that peripherally expressed Aire may regulate the expression of a unique set of self-antigens.
In humans, it has been suggested that failure in T-cell tolerance caused by mutations in AIRE gene may result in the autoimmune condition observed in autoimmune polyendocrine syndrome type 1 (APS1) [10, 11]. It is also known as autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) and is characterized by spontaneous multi-organ failure and chronic mucocutaneous candidiasis due to immune cell destruction and dysfunction [12]. Aire-deficient mice show loss of T-cell tolerance, mimicking the phenotype of human APS1. Spontaneous autoimmune disease characterized by lymphocytic infiltrates in multiple organs and tissues has also been observed in these mice [13–15]. Moreover, human gene association studies have reported that SNPs in the AIRE gene are associated with susceptibility to RA [16–18].
Major histocompatibility complex (MHC) class II molecules (human leukocyte antigen (HLA) in humans) are expressed by antigen-presenting cells (APCs), where they function in antigen processing and presentation to CD4+ T helper (Th) cells. HLA-DR2b (DRB1*1501) and HLA-DR4 (DRB1*0401) alleles are associated with MS and RA, respectively [15, 19–21]. HLA-DR2b and HLA-DR4 tg mice are useful to test the role of these molecules in processing and presentation of autoantigens, shaping the T cell repertoire in the thymus and the periphery, and to interrogate their function in the pathogenesis of human autoimmune diseases [22–24]. Since these animals are devoid of endogenous murine MHC class II molecules (Ia), antigen presentation is solely the property of the human HLA-DR2b and HLA-DR4 molecules [15, 20, 25]. We showed previously that T cell tolerance was maintained in these mice and depended on the expression of self-antigen [26]. To determine if Aire was critical in maintaining T cell tolerance in the context of HLA-DR2b and HLA-DR4 molecules, we developed HLA-DR tg Aire−/− mice on the experimental autoimmune encephalomyelitis (EAE)-susceptible C57BL/6 background [27, 28]. Specifically, we asked if the absence of Aire promoted the development of spontaneous organ-specific autoimmune pathology akin to MS or RA in HLA-DR tg mice. Of note, Aire deficiency on the conventional C57BL/6 background displays only a mild autoimmune phenotype in mice [29, 30].
Our studies revealed a decrease in gene expression of TSAs in thymus, which was paralleled by mild, spontaneous inflammatory infiltrates in a small number of Aire−/− HLA-DR2b or HLA-DR4 tg mice in salivary glands, liver, and pancreas, as well as serum autoantibodies against gastric tissue, in particular in older mice. Similarly, we noted a mild decrease in thymic gene expression of neuroantigens, which corresponded to modestly enhanced active EAE, but no spontaneous neuroinflammatory disease or autoimmune arthritis was observed. No significant changes were observed in the numbers and distribution of CD4+ T cells, TCR distribution, percentages of regulatory T cells, and antigen-induced cytokine production. Furthermore, abrogation of T regulatory (Treg) function by treatment with anti-CTLA-4 and anti-CD25 mAb did not trigger EAE or autoimmune arthritis in these mice. Our results support a role for the transcription factor Aire in modulating autoimmune pathology, but argue against its essential role in regulating autoimmunity in the context of the human HLA-DR2b and -DR4 alleles associated with MS and RA, respectively.
Materials and Methods
Mice
Aire−/− C57BL/6 mice [6] were originally purchased from Jackson lab (stock no. 004743) and were then maintained on the C57BL/6 background. For the experiments described here, Aire+/− mice were crossed to Ia−/− C57BL/6 mice (Jackson lab; stock no. 003584) and the progeny were then interbred to obtain Aire+/−Ia−/− animals devoid of all endogenous murine MHC II. The HLA- DR2b and HLA-DR4 tg mice have been described previously [23, 26, 31, 32]. Aire+/−Ia−/− mice were crossed to HLA-DR2b or HLA-DR4 tg mice to obtain Aire+/−Ia−/− HLA-DR2b or Aire+/− Ia−/− HLA-DR4 tg mice. F1 offspring of Aire+/−Ia−/− HLA-DR2b or Aire+/−Ia−/− HLA-DR4 tg mice were then interbred to obtain Aire+/−Ia−/−, Aire+/+ Ia−/−, and Aire−/− Ia−/− HLA-DR2b or HLA-DR4 tg mice, respectively. All mice were bred at The University of Texas at San Antonio (UTSA) under specific pathogen-free conditions. All studies were performed per UTSA Institutional Animal Care and Use Committee guidelines and approved protocols.
Genotyping
Genomic DNA from tail snips was extracted using DNeasy Blood and Tissue kit (Qiagen). PCR primers were custom-ordered from Invitrogen. Standard PCR parameters were employed using Go Taq Green polymerase mix (Promega) on Thermal Cycler 2720 (Applied Biosystems). Primers used in the study are as following: forward primer for Aire gene, 5′-GTC ATG TTG ACG GAT CCA GGG TAG AAA GT- 3′; reverse primer for Aire gene, 5′-AGA CTA GGT GTT CCC TCC CAA CCT CAG- 3′; forward primer for mouse MHC II (I-A) gene, 5′-GGG GTG GAA TTT GAC CTC TT- 3′; reverse primer for mouse MHC II (I-A) gene, 5′-TGG AGA CAT TGG CCA GTA CA- 3′; forward primer for HLA-DR2b gene, 5′-GTT TCC TGT GGC AGC CTA AGA GG- 3′; reverse primer for HLA-DR2b gene, 5′-TCC ACC GCG GCC CGC GC- 3′; forward primer for HLA-DR4 gene, 5′-CGT TTC TTG GAG CAG GTT AAA CA- 3′; reverse primer for HLA-DR4 gene, 5′-AAG CGC ACG TAC TCC TCT TGG TG- 3′.
Real-Time PCR
Total RNA was extracted from the thymus of Aire−/− and Aire+/+ HLA-DR2b or HLA-DR4 tg mice using RNeasy fibrous tissue mini kit (Qiagen). The RNA was reverse transcribed to cDNA using the cDNA reverse transcription kit (Life technologies). Real-time PCR was performed with the CFX96 Touch Deep Well Real-Time PCR Detection System (BioRad) using RT2 SYBR Green master mix (Qiagen) per manufacturer’s instructions. The amplification program included an initial denaturation step at 95 °C for 10 min, followed by denaturation at 95 °C for 15s and annealing and extension at 60 °C for 1 min for 40 cycles. SYBR Green fluorescence was measured after each extension step, and the specificity of amplification was evaluated by melting curve analysis. RT2 qPCR primer assay for mouse Tff3 (NM_011575), Ins2 (NM_001185083), Spt1(NM_009267), Rbp3 (NM_015745), Muc6 (NM_181729), Csn1s1 (NM_007784), Igf2 (NM_001122736), Krt8 (NM_031170), Mog (NM_010814), Plp1 (NM_011123) and Mbp (NM_001025245) from Qiagen were used. Gene expression was normalized by Keratin 8 expression, which is specifically expressed in the thymic epithelial cell fraction and is not influenced by Aire [6, 33].
Treg depletion
Aire−/− and Aire+/+ HLA-DR2b and HLA-DR4 tg mice (3 to 9 months old) were divided into groups and injected with mAb as indicated. Aire−/− and Aire+/+ HLA-DR4 tg mice were injected i.p. with 800 μg of anti-CTLA-4 mAb (Bio X cell; UC10-4F10) and Aire−/− and Aire+/+ HLA-DR2b tg mice were given i.p a combination of 100 μg of anti-CTLA-4 mAb and 1 mg of anti-CD25 mAb (Bio X cell; PC-61.5.3) once a week for three continuous weeks.
Immunofluorescence (IF) staining
Mouse organs (liver, pancreas, lung, stomach, and intestine) from the studies were immersed in OCT (Fisher Scientific) and frozen in −80 ºC, then cryosectioned (10 μm) onto slides. Slides were fixed and stained with Alexa Fluor 488-conjugated anti-mouse CD4 mAb (eBioscience; GK1.5), APC-conjugated anti-mouse CD8 mAb (eBioscience; 53-6.7), followed by microscopic examination and imaging of positive staining cells (Olympus microscope with DP72 camera, CellSens Standard 1.5 software). The scoring system is described in Table 1. For indirect immunofluorescence staining to detect IgG autoantibodies, sera from Aire−/− and Aire+/+ HLA-DR2b and HLA-DR4 tg mice were incubated with tissue sections of organs from C57BL/6 SCID mice, followed by staining with FITC goat anti-mouse IgG Ab (Jackson Immunoresearch).
Table 1.
Scoring table for tissue sections obtained from Aire−/− and Aire+/+ HLA-DR2b and HLA-DR4 tg mice.
| Score | Average no. of lesions/section (2–3 sections per slide) |
|---|---|
| 0 | No lesions at all |
| 1 | 1–4 lesions |
| 2 | 5–15 lesions |
| 3 | 15–25 lesions |
The tissue sections were obtained from the Aire−/− and Aire+/+ HLA-DR2b and HLA-DR4 tg with 2 slides per mouse (staggered sections ensuring spanning across the organ). The scoring was performed based on the average number of lesions per section with 2–3 sections per slide.
mice were incubated with tissue sections of organs from C57BL/6 SCID mice, followed by staining with FITC goat anti-mouse IgG Ab (Jackson Immunoresearch).
Histology
Tissues (adrenal glands, salivary glands, testis and ovaries) from mice were harvested and fixed by immersion in 10% buffered formalin overnight. They were then rinsed in PBS, dehydrated through increasing concentrations of ethanol, cleared and embedded in paraffin. Each tissue block was cut into 3 sections, with each section 20 μm apart and mounted on slides. The sections were stained with hematoxylin and eosin (H&E) followed by microscopic examination for cellular infiltrates (Olympus microscope with DP72 camera, CellSens Standard 1.5 software).
Cytokine ELISPOT
ELISPOT plates (Millipore; Multiscreen IP) were coated with mouse IFN-γ-specific (eBioscience; AN-18), IL-17-specific (Bio X Cell; 17F3) or GM-CSF-specific (MP1-22E9) capture mAbs diluted in PBS. The plates were blocked with 1% BSA in PBS for 1 h at room temperature and then washed four times with PBS. Splenocytes (1 × 106 cells/well) were collected and incubated with or without Ag for 24 h at 37 °C. The plates were washed three times with PBS and four times with PBS-Tween (0.05%) and incubated with mouse IFN-γ-specific biotinylated detection mAb (eBioscience; R4-6A2), IL-17-specific biotinylated detection mAb (TC11-81-14) or GM-CSF-specific biotinylated detection mAb (MP1-31G6) at 4 °C overnight. The plates were washed four times with PBS-Tween (0.05%) and incubated with streptavidin-alkaline phosphatase (Invitrogen) for 2 h at room temperature, followed by four washes with PBS. Cytokine spots were visualized by 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium phosphatase substrate (Kirkegaard & Perry Laboratories). Image analysis of ELISPOT assays was performed on a Series 2 ImmunoSpot analyzer and software (Cellular Technology).
EAE induction and disease evaluation
Active immunization EAE was induced in HLA-DR2b and HLA-DR4 tg mice (6–10 weeks of age) by s.c. injection of 300 μg of myelin oligodendrocyte glycoprotein (MOG)35–55 peptide and 200 μg of MOG97–108 peptide respectively, in 50 μl of emulsion with complete Freund’s adjuvant (CFA). CFA was prepared by adding 5 mg/ml of Mycobacterium tuberculosis H37RA (Difco laboratories) in incomplete Freund’s adjuvant (IFA). Mice also received i.p injections of 400 ng of pertussis toxin (PTX) on day 0 and day 1 post immunization.
Mice were monitored and graded daily for clinical signs of EAE using the following scoring system [34]: 0, no abnormality; 1, limp tail; 2, moderate and hind limb weakness; 3, complete hind limb paralysis; 4, quadriplegia or premoribund state; 5, death.
Flow Cytometry Analysis- Cell Surface Staining
Murine splenocytes (1×106 cells/sample) and thymocytes (1×106 cells/sample) were collected and stained for various cell surface markers after washing and blocking with anti-mouse CD16/32 to block binding through the Fc-receptor (eBioscience). The samples were then analyzed by Becton Dickinson (BD) FACS-Aria II using BD FACS Diva Software. All antibodies to cell surface markers were purchased from eBioscience or BD: PE-Cy7 anti-CD4 mAb (GK1.5), APC anti-CD8 mAb (53-6.7), PE anti-CD11c mAb (N418), Alexa Fluor 488 anti-CD11b mAb (M1/70), Alexa Fluor 488 anti-CD25 mAb (eBio7D4), FITC anti-HLA-DR mAb (LN3), PE anti-Ia mAb (AF6-120.1, BD) and PE-Cy7 anti-CD45 mAb (GK1.5). The FITC labeled mAbs for 14 Vβ and 3 Vα families were purchased from BD.
Flow Cytometry Analysis- Intracellular Staining
Murine splenocytes were collected, fixed and permeabilized in 96-well round bottom plates (1 ×106 cells/well), and stained for the intracellular marker using PerCP-Cy5.5 anti-Foxp3 mAb (eBioscience; FJK-16s). The samples were then analyzed by BD FACS-Aria II using BD FACS Diva Software.
Statistical analysis
For comparisons using two experimental groups, the t test was used. Analysis of variance (ANOVA) was used for statistical analysis involving multiple groups followed by Bonferroni posttest. Comparisons of all analyses were performed using Sigma Plot 12.5. A difference was considered statistically significant when p ≤ 0.05.
Results
Downregulation of Aire-dependent TSAs gene expression in thymus of Aire-deficient HLA-DR2b and HLA–DR4 tg mice
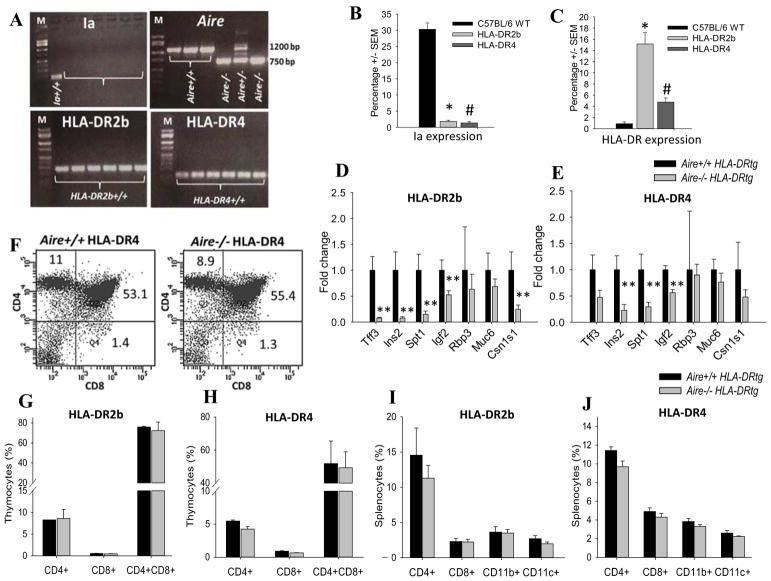
To test the effect of Aire-deficiency in the context of the human HLA-DR2b and HLA-DR4 alleles we generated Aire-deficient (Aire−/−) Ia−/− HLA-DR2b and HLA-DR4 tg mice as described in Materials and Methods. The deletion of Aire and Ia and the presence of HLA-DR2b and HLA-DR4 were confirmed by PCR (Fig. 1A). The expression of HLA-DR2b, HLA-DR4 and absence of Ia was corroborated by flow cytometry (Fig. 1B, C).
FIGURE 1.
Downregulation of Aire-dependent genes and immune cell distribution in Aire-deficient HLA-DR2b and HLA–DR4 tg mice. Genomic DNA extracted from off-springs of Aire+/− HLA-DR2b or Aire+/− HLA-DR4 breeders were genotyped by PCR with primers for Aire, Ia, HLA-DR2b and HLA-DR4 genes as described in Materials and Methods. (A) Shown is the representative PCR result run on 1.8% agarose gel. Arrows indicate Aire knockout band of 750 bp and Aire Wt band of 1200 bp respectively. All the mice are either positive for HLA-DR2b or HLA-DR4 and show absence of Ia. M, marker (100 bp low scale DNA ladder); −/−, Aire knockout; +/+, Aire Wt; +/−, Aire heterozygous. (B and C) Flow cytometry analysis of splenocytes from C57BL/6 Wt, HLA-DR2b, and HLA-DR4 tg mice using fluorochrome conjugated anti-CD45, anti-Ia and anti-HLA-DR mAb. (D) Aire+/+ and Aire−/− HLA-DR2b tg or (E) Aire+/+ and Aire−/− HLA-DR4 tg mice were euthanized, their thymi were harvested and RNA was extracted and used for performing qPCR against a panel of Aire-dependent genes as described in Materials and Methods. (F – J) Thymus and spleens of HLA-DR2b or HLA–DR4 mice were procured and stained with fluorochrome conjugated anti-CD4, CD8, CD11c, CD11b, CD25, Foxp3 mAbs followed by flow cytometry analysis as described in Materials and Methods. (F) Shown are representative flow plots of CD4+, CD8+ and double positive thymocytes in (F) Aire+/+ HLA-DR4 vs Aire−/− HLA-DR4 mice. (G, H) Quantification for percentages of CD4+, CD8+ and double positive thymocytes in (G) Aire−/− HLA-DR2b and (H) Aire−/− HLA-DR4 mice in comparison with their Aire+/+ HLA-DR littermates. (I, J) Distribution of CD4+, CD8+, CD11c+, CD11b+ cells in the spleens of Aire−/− HLA-DR2b (I) and Aire−/− HLA-DR4 mice (J) in comparison with their respective Aire+/+ HLA-DR littermates. Data are representative for two independent experiments (n= 3 – 4 mice/groups), (Mean ± SEM) * indicates significant difference between HLA-DR2b group and C57BL/6 group, # indicates significant difference between HLA-DR4 group and C57BL/6 group; * indicates significant difference between Aire−/− group and Aire+/+ group.
Quantitative RT-PCR (qRT-PCR) analysis of thymic tissue was performed to confirm the effect of Aire deletion on the expression of known Aire-dependent TSA genes in HLA-DR tg mice (Fig. 1D, E), including trefoil factor-3 (Tff3), insulin-2 (Ins2), mouse salivary protein-1 (Spt1), interphotoreceptor retinoid-binding protein (Rbp3), mucin 6 (Muc6), casein alpha (Csn1s1) and insulin like growth factor II (Igf2) as described previously [6, 30, 35, 36]. Specifically, mRNA expression for Ins2, Spt1, Igf2 was substantially decreased in Aire−/− HLA-DR tg mice as compared with Aire+/+ HLA-DR tg littermates (Fig. 1D, E). Our findings agree with previous reports showing a similar reduction of these genes in Aire−/− mice on other genetic backgrounds, i.e. BALB/c [6, 36, 37].
Aire-deficiency does not affect immune cell distribution in HLA-DR tg mice
To begin to investigate the effect of Aire-deficiency on the immune system in the HLA-DR tg mice, particularly in the T cell compartment, we determined the distribution and numbers of T cells, DCs, and monocytes/macrophages in thymus and spleen of 6 – 9-week-old Aire−/− and Aire+/+ HLA-DR tg mice by flow cytometry.
As shown in Fig. 1F, G & H, the percentages of single positive (SP) CD4+ T cells, SP CD8+ T cells, or double positive (DP) CD4+CD8+ T cells were comparable in thymus of Aire+/+ versus Aire−/− HLA-DR2b or HLA-DR4 tg mice. No significant gender differences were observed (not shown). Furthermore, the overall percentage and the absolute numbers of CD4+ T cells, CD8+ T cells, DCs (CD11c+), and macrophages (CD11b+) were similar in the spleens of Aire−/− mice as compared to Aire+/+ mice, irrespective of whether the mice were on the HLA-DR2b or HLA-DR4 background (Fig. 1I, J). We also investigated the thymus architecture in our mice by H&E staining and did not note abnormalities on histology (Supplemental Fig. 1A–D). Thus, the results showed that Aire did not have a notable effect on the numbers and distribution of T cells, monocytes, and DCs in naïve HLA-DR tg mice.
Mild spontaneous inflammatory tissue infiltrates in HLA-DR tg mice
Aire-deficient mice have been reported to exhibit spontaneous lymphocytic tissue infiltrates [14, 15]. Therefore, we investigated the HLA-DR tg mice for clinical or histopathological signs of spontaneous autoimmune pathology in other tissues.
Tissue sections from representative organs from Aire−/− HLA-DR2b and HLA-DR4 tg mice up to 15 months of age were procured and investigated by immunofluorescence microscopy for the presence of CD4+ and CD8+ T cells, and CD11c+ cells. Of note, the results showed a small percentage (~20%) of Aire−/− HLA-DR2b and HLA-DR4 tg mice 12 months and older with inflammatory infiltrates, mostly confined to liver and pancreas, with an average immune cell infiltration score of 1 – 2 (Fig. 2, Table 1 & 2). The inflammatory infiltrates in Aire−/− HLA-DR tg mice consisted predominantly of CD4+ T cells (Fig. 2B, D), with very few CD8+ T cells present (data not shown). CD4+ T cell infiltrates were not observed in stomach, intestine and lungs (data not shown). A mild gender bias was observed, with older females showing more CD4+ T cell infiltrates as compared with older males (Table 2). This observation is in line with the literature, where immunopathology was more pronounced in older female Aire−/− mice as compared with male Aire−/− mice [38]. However, in contrast, we did not observe splenomegaly in our Aire−/− HLA-DR tg mice and the number of splenocytes was comparable between the Aire−/− HLA-DR tg and Aire+/+ HLA-DR tg mice (data not shown), conceivably due to the younger average age of the mice in our studies. Finally, no immune cell infiltration was noted in organs of Aire+/+ HLA-DR tg littermates.
FIGURE 2.
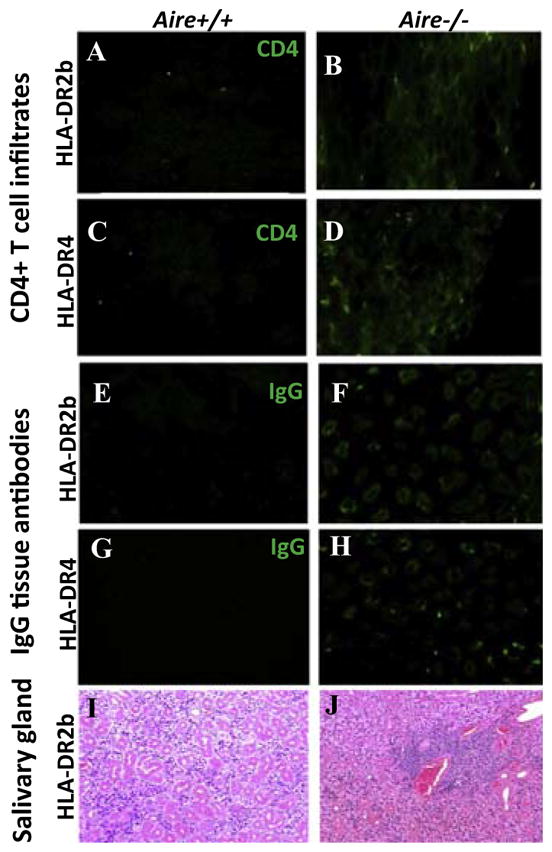
Mild spontaneous immune pathology in Aire-deficient HLA-DR2b and HLA-DR4 tg mice. (A, B) Shown are representative IF microscopy images for CD4+ T cells in the liver of 13-months-old Aire+/+ HLA-DR2b tg mice (A) versus Aire−/− HLA-DR2b tg mice (B). (C, D) Representative IF microscopy images for CD4+ T cells in the liver of 16-months old Aire+/+ HLA-DR4 mice (C) vs (D) Aire−/− HLA-DR4 mice. Magnification 200x. (E, F) Detection of autoantibodies against gastric tissues by IF microscopy images of gastric tissue sections from SCID mice in sera from 12-month-old Aire+/+ HLA-DR2b tg mice (E) vs Aire −/− littermates (F), and 12-month-old Aire+/+ HLA-DR4 mice (G) vs Aire−/− littermates (H). Magnification 200x. Representative H&E staining images from salivary glands of Aire+/+ HLA-DR2b tg mice (I) vs Aire−/− HLA-DR2b tg mice (J). Magnification 100x.
Table 2.
Incidence of lymphocytic infiltrates in the organs of Aire−/− and Aire+/+ HLA-DR2b and HLA-DR4 tg mice.
| CD4+ T cells | CD8+ T cells | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Organ | Liver | Pancreas | Liver | Pancreas | ||||
|
|
|
|
|
|||||
| Genotype | Male | Female | Male | Female | Male | Female | Male | Female |
| Aire+/+ HLA-DR2b mice | 0/5 | 0/3 | 0/5 | 0/3 | 0/5 | 0/3 | 0/5 | 0/3 |
| Aire−/− HLA- DR2b mice | 1/11 | 3/7 | 1/11 | 0/7 | 0/11 | 1/7 | 0/11 | 0/7 |
| Aire+/+ HLA-DR4 mice | 0/7 | 0/4 | 0/7 | 0/4 | 0/7 | 0/4 | 0/7 | 0/4 |
| Aire−/− HLA- DR4 mice | 0/8 | 3/7 | 0/8 | 1/7 | 0/8 | 1/7 | 0/8 | 1/7 |
Immune infiltration was examined in Aire−/−, Aire+/+ HLA-DR2b and HLA-DR4 tg mice by I.F. staining on tissue sections from different organs (liver, lung, pancreas, stomach and intestine) with anti-CD4 and anti-CD8 mAbs as described in Materials and Methods. Infiltrates were mostly observed in liver and pancreas of a few aged Aire−/− HLA-DR tg mice. Shown are the incidences of mice that have immune infiltrates found in indicated organs (n = 3–11).
The mild gender bias noted in our studies prompted us to examine reproductive organs from these mice. However, no inflammatory infiltrates were noted in ovaries or testis procured from Aire−/− HLA-DR tg mice or Aire+/+ HLA-DR tg littermates (data not shown).
In APS1 the endocrine manifestations include Addison’s disease [39], which lead us to examine the adrenal glands of the animals in our studies. However, in agreement with previous studies, we did not detect inflammation of the adrenal glands in Aire−/− HLA-DR tg mice or littermate controls (data not shown) [13, 38].
Aire-deficient mice have been reported to exhibit lymphocytic infiltration in salivary glands [6, 40]. Similarly, we observed inflammatory infiltrates in salivary glands in 3 out of 4 Aire−/− HLA-DR2 tg mice, but not in Aire+/+ HLA-DR2 tg mice by H&E examination (Fig. 2I, J). Of note, our qRT-PCR results showed a significantly reduced expression of Spt1 in Aire−/− HLA-DR2 and – DR4 tg mice as compared with Aire+/+ littermates (Fig. 1D, E), which would be consistent with increased induction of autoimmunity directed towards salivary gland tissue.
The occurrence of autoantibodies in the sera of APS1 patients often precedes the onset of clinical disease and signals ongoing autoimmune responses [41]. Therefore, to examine the presence of autoantibodies, sera were obtained from Aire−/− and Aire+/+ HLA-DR2b and HLA-DR4 tg mice (1 to 16 months of age) and tissue sections from SCID mice were used to test for the presence of IgG autoantibodies. Of note, IgG autoantibodies were present in sera of 22% of Aire−/− HLA-DR tg mice 10 months and older, which were directed against gastric tissue, but not against any other tissues (Fig. 2F, H; Table 3); this corresponded to the reduced expression of stomach specific gene Muc6 in the thymus. Minimal IgG autoantibodies were detected in sera of Aire+/+ HLA-DR tg littermates (Fig. 2E, G).
Table 3.
Incidence of IgG autoantibodies in the sera of Aire−/− and Aire+/+ HLA-DR2b and HLA-DR4 tg mice.
| Genotype | HLA-DR2b | HLA-DR4 | ||
|---|---|---|---|---|
|
|
|
|||
| Gender | Male | Female | Male | Female |
| Aire+/+ mice | 1/3 | 0/2 | 0/7 | 1/3 |
| Aire−/− mice | 2/10 | 2/9 | 2/10 | 2/8 |
IgG autoantibodies detected in the sera of Aire+/+ and Aire−/− HLA-DR2b or HLA-DR4 tg mice which was stained against a panel of organs (stomach, lung, liver, bowel, pancreas and kidney) from SCID mice, IgG binding was observed in the gastric tissue. Shown are the incidences of mice that have IgG auto-antibodies (n = 3–10).
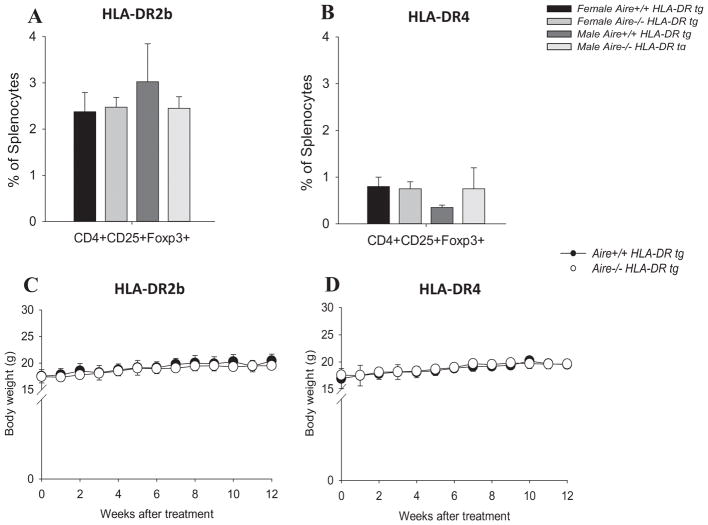
Despite the presence of autoantibodies, Aire−/− HLA-DR2b and HLA-DR4 tg mice did not show obvious clinical signs of pathology and maintained similar body weights as compared with age and gender matched Aire+/+ HLA-DR2b or HLA-DR4 tg littermates (Supplemental Fig. 2A–D). Moreover, no signs of spontaneous neuroinflammatory disease (e.g. EAE) were noted in Aire−/− HLA-DR2b tg mice or their Aire+/+ HLA-DR2b tg littermates (data not shown). Similarly, Aire−/− HLA-DR4 tg mice or their Aire+/+ HLA-DR4 tg littermates did not show joint swelling or increased articular inflammation suggestive of autoimmune arthritis (data not shown).
Collectively, the results showed that a small percentage of older Aire−/− mice expressing the human autoimmune disease-associated MHC class II alleles HLA-DR2b and HLA-DR4 exhibited mild spontaneous inflammatory infiltrates dominated by CD4+ T cells, predominantly in liver and pancreas. Moreover, a small percentage of older Aire−/− HLA-DR tg mice showed IgG autoantibodies directed against gastric tissue. However, no evidence of spontaneous CNS-demyelinating disease or autoimmune arthritis was observed.
Earlier disease onset and mild increase in EAE severity in Aire-deficient HLA-DR tg mice
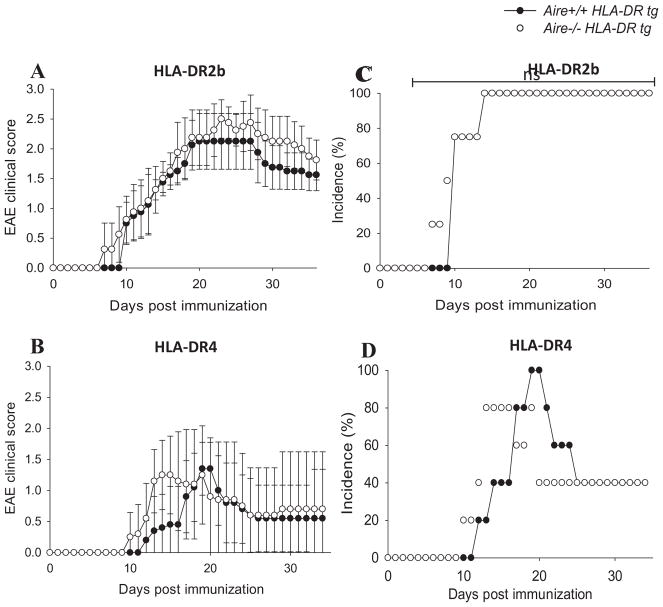
HLA-DR2b and –DR4 tg mice did not show evidence of spontaneous neuroinflammatory disease or arthritis. However, Ko et. al. reported previously that Aire−/− mice showed earlier EAE onset [42]. Therefore, we asked whether Aire-deficiency in mice transgenic for the MS susceptibility allele HLA-DR2b would lead to more severe disease in Aire−/− mice compared with Aire+/+ mice. To evaluate the effect of Aire-deficiency on autoimmune pathology in HLA-DR2b and HLA-DR4 tg mice, EAE was induced by active immunization with MOG35–55 or MOG97–108 peptide, respectively, and mice were observed for clinical signs of disease for up to 4 weeks as previously described [31, 32].
Shown in Fig. 3, disease onset occurred slightly earlier in Aire−/− HLA-DR2b and HLA-DR4 tg mice (day 7 and day 10, respectively) as compared with Aire+/+ HLA-DR2b and HLA-DR4 littermates (day 10 and day 12, respectively; Fig. 3A, B), but the results did not reach statistical significance. Disease severity (Fig. 3A, B) and incidence (Fig. 3C, D) of Aire−/− HLA-DR tg versus Aire+/+ HLA-DR tg mice were comparable and no statistically significant differences were noted. Taken together, the results suggested that Aire-deficiency had a minor effect on the induction of EAE in mice expressing autoimmune disease-associated HLA-DR alleles and promoted a slightly earlier onset of disease.
FIGURE 3.
Earlier onset and mild increase in EAE severity in Aire-deficient HLA-DR tg mice. Aire+/+ and Aire−/− HLA-DR2b tg mice (A and C) or HLA-DR4 tg mice (B and D) were immunized for EAE and scored for clinical signs of disease as described in Materials and Methods. Data are the representative of 3–4 independent experiments (n= 3 – 4 mice/group; Mean ± SEM).
Aire-deficiency alters Th17 cell responses in HLA-DR tg mice but does not skew the T cell receptor repertoire
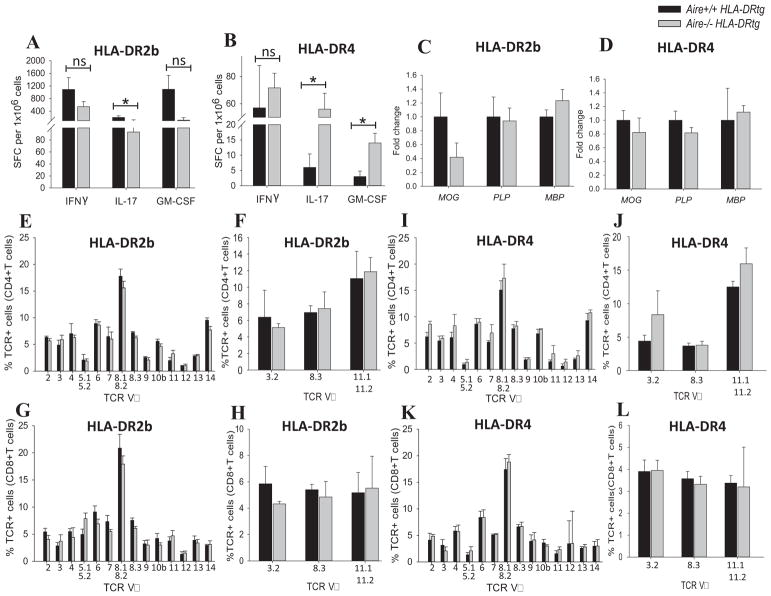
To determine whether Aire-deficiency modulated antigen-specific T cell responses in HLA-DR tg mice, IFN-γ, IL-17 and GM-CSF production was measured by cytokine ELISPOT assay from splenocytes at EAE remission. As shown in Fig. 4A, the frequencies of T cells producing IFN-γ, IL-17, or GM-CSF were mildly decreased in Aire−/− mice expressing the HLA-DR2b allele, as compared with the control mice, with only IL-17 reaching statistical significance (p < 0.05). In contrast, frequencies of IL-17 and GM-CSF producing T cells were significantly increased in Aire−/− HLA-DR4 tg mice (p < 0.05), whereas IFN-γ was not significantly increased (Fig. 4B). Conceivably, higher frequencies of T cells in Aire−/− HLA-DR4 tg mice as compared with Aire+/+ HLA-DR4 tg could have contributed to the slightly more severe EAE phenotype noted in these mice. However, differences in cytokine production could not sufficiently explain the earlier disease onset and slight increase in disease severity noted in HLA-DR2b tg mice.
FIGURE 4.
Antigen-specific cytokine production, myelin-specific gene expression, and T cell receptor repertoire unaltered in Aire-deficient HLA-DR tg mice. Mice immunized for EAE were euthanized during disease remission (day 34 – 36), splenocytes of Aire−/− and Aire+/+ HLA-DR2b tg (A) or HLA-DR4 tg mice (B) were harvested and recalled with peptides MOG35–55 (HLA-DR2b tg) or MOG97–108 (HLA-DR4 tg) and tested by cytokine ELISPOT assay to quantify IFN-γ, IL-17 and GM-CSF producing cells as described in Materials and Methods. Shown is mean ± SEM of the number of cytokine forming cells. Data are the representative of 3–4 independent experiments (n= 3 – 4 mice/group; * indicates significant difference between Aire−/− group and Aire+/+ group). C) naïve Aire+/+ and Aire−/− HLA-DR2b or (D) naïve Aire+/+ and Aire−/− HLA-DR4 mice were euthanized, their thymus was harvested, and RNA was extracted and used for performing qPCR against myelin-specific genes (MOG, PLP, MBP) as described in Materials and Methods. Data are representative for two independent experiments (n= 3 – 4 mice/groups), (mean ± SEM of mRNA expression). (E–H) Shown is the flow cytometry analysis of TCR Vβ and Vα distribution of splenic CD4+ (E, F) and CD8+ T cells (G, H) of Aire−/− vs Aire+/+ HLA-DR2b tg mice. (I–L) Flow cytometry analysis of TCR Vβ and Vα distribution of splenic CD4+ (I, J) and CD8+ T cells (K, L) of Aire−/− vs Aire+/+ HLA-DR4 tg mice. Flow cytometry analysis was performed using panels of mAbs for TCR families as described in Materials and Methods. Shown are pooled data of two independent experiments (n = 2 – 4 mice/group, Mean ± SEM).
Ko et. al. observed a reduction in MOG gene expression in their Aire−/− mouse line [42], but not in the expression of MBP or PLP. To address this question in our model we analyzed the expression of myelin specific genes MOG, PLP, and MBP by qRT-PCR. We noted decreased MOG gene expression in Aire−/− mice, particularly in mice expressing HLA-DR2b (Fig. 4C), whereas the expression of MBP or PLP was essentially not altered (Fig. 4C, D). Thus, decreased MOG expression may have contributed to the slightly more severe EAE observed in our model. Conceivably, altered expression of TSAs in Aire−/− HLA-DR tg mice could have affected the positive and/or negative selection of T cells expressing autoreactive TCRs [4, 5]. Therefore, we tested whether Aire-deficiency globally affected TCR selection in HLA-DR2b and HLA-DR4 tg mice and resulted in a skewed TCR repertoire. To address this question, we examined the repertoire of TCRVβ and Vα families in the thymus and spleen by flow cytometry using a panel of anti-TCR monoclonal antibodies.
Shown in Fig. 4E–L, the TCR repertoire was comparable between Aire−/− HLA-DR tg and Aire+/+ HLA-DR tg mice and no significant alterations were observed in the representation of TCR Vα or Vβ families. Thus, our results suggest that Aire expression did not significantly affect the overall TCR repertoire distribution in the context of human HLA-DR2b and HLA-DR4 molecules in the transgenic mice.
Anti-CD25/CTLA-4 mAb treatment does not trigger overt autoimmune pathology in Aire-deficient HLA-DR tg mice
Natural Tregs (nTreg; CD4+ CD25+ Foxp3+) develop in the thymus and are a population of lymphocytes implicated in the regulation of autoimmune T cell responses, whereas inducible Tregs (iTreg) develop in the immune periphery and similarly keep aberrant T cell responses in check [43, 44]. The role of Aire in positive selection of Tregs has been controversial. However, growing evidence suggests that Aire may influence the thymic development of Treg cells [45–49]. Therefore, we asked whether the percentage of Tregs was altered in this model. However, as shown in Fig. 5A & B, the percentage of CD4+CD25+Foxp3+ Tregs in the spleen was comparable between Aire−/− HLA-DR tg mice and Aire+/+ HLA-DR tg mice.
FIGURE 5.
No significant increase in autoimmune pathology in Aire−/− and Aire+/+ HLA-DR2b or HLA-DR4 mice after CLTA-4 and/or CD25 blockade. Spleens of naïve HLA-DR2b or HLA–DR4 mice were procured and stained with fluorochrome conjugated anti-CD4, CD25, Foxp3 mAbs followed by flow cytometry analysis as described in Materials and Methods. (A, B) Distribution of CD4+CD25+Foxp3+ cells in the spleens of Aire−/− HLA-DR2b (A) and Aire−/− HLA-DR4 mice (B) in comparison with their respective Aire+/+ HLA-DR littermates. (C) Aire−/− and Aire+/+ HLA-DR2b mice treated with anti-CTLA-4 and anti-CD25 mAbs, or (D) Aire−/− and Aire+/+ HLA-DR4 mice treated with anti-CTLA-4 mAb were weighed and no significant differences were found between the body weights of Aire−/− and Aire+/+ HLA-DR tg mice (t-test). Data are representative of two independent experiments (n = 3 – 4 mice/group, Mean ± SEM).
Nevertheless, while the data showed that the overall percentage of nTregs was not affected by Aire-deficiency in naïve HLA-DR2b or HLA-DR4 tg mice, conceivably, their function could still be altered in the absence of Aire. Along these lines, CTLA-4 is an inhibitory receptor expressed by regulatory and conventional T cells, which is necessary for maintaining T cell homeostasis and self-tolerance. In conventional T cells, CTLA-4 cell surface expression is induced after TCR signaling [50]. In contrast, CTLA-4 is constitutively expressed on Tregs, where it serves to control self-reactive T cells [51]. In vivo blockade of CTLA-4 with anti-CTLA-4 mAb can promote organ-specific autoimmune pathology [51]. Moreover, the administration of anti-CD25 mAb results in functional inactivation of Tregs [52, 53].
To test whether inhibition of CTLA-4 and/or CD25 triggered autoimmune disease in Aire−/− and Aire+/+ HLA-DR2b or HLA-DR4 tg mice, 3–9-month-old animals were treated with a combination of anti-CTLA-4 and anti-CD25 mAbs (for HLA-DR2b tg mice), or with anti-CTLA-4 mAb alone (for HLA-DR4 tg mice) and observed for evidence of autoimmune pathology for up to 12 weeks. Of note, no clinical signs of overt autoimmune pathology, including neuroinflammatory disease or arthritis were noted in Aire−/− or Aire+/+ HLA-DR2b or HLA-DR4 tg mice (data not shown). Moreover, body weight remained comparable between Aire−/− and Aire+/+ mice (Fig. 5C, D). To corroborate the lack of clinical disease, histopathology of liver, lungs, pancreas, stomach, and small and large intestine was performed by IF staining as described in Materials and Methods. Summarized in Table 4, Aire−/− and Aire+/+ HLA-DR2b tg mice injected with a combination of anti-CD25/CTLA-4 mAb did not show inflammatory infiltrates in any of the tissues examined. However, approximately half of the Aire−/− HLA-DR4 tg mice, and 1/3 of the Aire+/+ HLA-DR4 tg mice injected with anti-CTLA-4 mAb showed inflammatory infiltrates, which were mostly noted in liver and pancreas, and which consisted predominantly of CD4+ T cells.
Table 4.
Incidence of immune cell infiltrates in Aire−/− and Aire+/+ HLA-DR2b and HLA-DR4 tg mice injected with anti-CTLA-4 + anti-CD25 and anti-CTLA-4 respectively.
| Genotype | HLA-DR2b (anti-CTLA-4 + anti-CD25) | HLA-DR4 (anti-CTLA-4) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Organ | Liver | Pancreas | Liver | Pancreas | ||||
|
|
|
|
|
|||||
| CD4+ | CD8+ | CD4+ | CD8+ | CD4+ | CD8+ | CD4+ | CD8+ | |
| Aire+/+ mice | 0/4 | 0/4 | 0/4 | 0/4 | 1/3 | 0/3 | 1/3 | 0/3 |
| Aire−/− mice | 0/5 | 0/5 | 0/5 | 0/5 | 3/7 | 1/7 | 1/7 | 0/7 |
Staining was performed using AF488 anti-CD4 mAb and APC anti-CD8 mAb against a panel of organs (liver, lungs, pancreas, stomach and intestine). Shown are the incidences of mice (injected with anti-CTLA-4 and CD-25 mAb) that have immune infiltrates found in indicated organs (n = 3–7).
Taken together, the results showed that treatment with anti-CTLA-4 and anti-CD25 antibodies accelerated the development of lymphocytic infiltrates in younger mice, whereas infiltrates in the absence of antibody treatment were only observed in older mice. However, the effect of antibody treatment was independent of the presence or absence of Aire.
Discussion
In this study, we show that deficiency of Aire in mice expressing human MS and RA-associated HLA-DR2b or HLA-DR4 alleles did not lead to spontaneous neuroinflammatory disease or arthritis. However, Aire-deficiency resulted in mild spontaneous autoimmune phenomena in the HLA-DR tg mice characterized by mild inflammatory infiltrates and increased autoantibodies, predominantly in mice 12 months or older. Inhibition of regulatory mechanisms by treatment with anti-CTLA-4 and anti-CD25 mAb did not trigger neuroinflammation or arthritis in the HLA-DR2b or HLA-DR4 tg mice, irrespective of the presence or absence of Aire. Moreover, induction of active EAE in Aire-deficient HLA-DR2b or HLA-DR4 tg mice resulted in earlier disease onset, but comparable disease severity. Thus, overall, the results suggest a mild and non-essential effect of Aire on modulating autoimmune disease pathology in the context of the human MS and arthritis-associated HLA-DR2b and HLA-DR4 alleles.
Central tolerance requires presentation of self-antigens by MHC molecules in the thymus to promote negative selection of potentially autoreactive T cells. Ectopic expression of self-antigens promoted by the transcription factor Aire in mTECs is important for tolerance and elimination of autoreactive T cells [54]. Aire may also promote the selection of Tregs [8]. Deficiency of Aire can lead to breakdown of central tolerance and result in multi-organ autoimmune disease [8]. Certain MHC Class II alleles, including HLA-DR2b and HLA-DR4, are associated with human autoimmune conditions, such as MS and RA, respectively [21, 55]. However, it has remained unresolved why certain HLA alleles are associated with specific autoimmune diseases. Thus, we asked whether Aire plays a role in preventing or promoting autoimmune pathology in the context of the human HLA-DR2b or HLA-DR4 alleles in HLA-DR tg mice. Specifically, we asked whether spontaneous neuroinflammation or arthritis was observed in Aire−/− mice expressing these autoimmune disease-associated HLA alleles.
Of note, our studies confirmed that absence of Aire resulted in downregulation of Aire-dependent ectopic tissue antigens in thymus. Consistent with impaired central T cell tolerance, and reduced expression of TSAs, specifically Spt1, Ins2 and Muc6 in the thymus, we observed mild tissue pathology in salivary glands, liver, and pancreas of some HLA-DR2b or HLA-DR4 tg mice, and, in addition, some of the animals developed increased autoantibodies directed against gastric tissue. The pathological consequences of these autoimmune phenomena appeared to be limited, because Aire−/− HLA-DR2b or HLA-DR4 tg mice did not show notable loss of bodyweight or other clinical signs of autoimmune disease. Most of the mice showing inflammatory infiltrates or autoantibodies were older females, suggesting an age-related effect and gender bias.
Previously it was shown that the HLA-DR transgenes in HLA-DR tg mice behave similar to endogenous murine MHC Class II genes and instruct normal thymic development and maintain normal lymphocyte development and homeostasis in the thymus and periphery [22]. Evaluation of the immune compartment of these mice for proportion and numbers of DCs, macrophages, T helper cells, cytotoxic T cells, and nTregs did not reveal dramatic changes between Aire−/− and Aire+/+ mice. Moreover, we did not observe alterations in thymus architecture. These observations are consistent with previous reports in the literature that Aire may have a minor effect on overall thymocyte composition and development [56]. The limited effect of Aire-deficiency on global CD4+ T cell development in the context of human HLA-DR alleles was further supported by lack of significant changes in the distribution of major TCR families between the Aire−/− HLA-DR tg mice and their Aire+/+ HLA-DR tg littermates. We cannot exclude that more sensitive techniques, such as gene melting spectral pattern (GMSP assay) analysis might uncover minor differences in TCR selection that were not apparent in our flow cytometry analyses due to the limited coverage imposed by the available mAbs for these studies [57]. However, based on our results we would predict minor effects on the TCR repertoire.
It is conceivable that more than one mechanisms accounted for the absence of more striking autoimmune pathology in our model. Along these lines, it is conceivable that the C57BL/6 genetic background of the HLA-DR tg mice is less conducive to development of spontaneous autoimmune phenomena associated with Aire-deficiency, which is supported by literature showing that Aire−/− mice on the C57BL/6 background consistently show a very mild autoimmune disease phenotype as compared with other backgrounds, e.g. BALB/c, NOD [7, 29]. Jiang et. al. showed that Aire−/− NOD mice crossed to Aire−/− B6 displayed a mild autoimmune phenotype which, was observed only in a minority of animals [29]. Thus, the lack of Aire may be most notable in the context of a particular set of disease susceptibility background genes. However, these disease susceptibility genes do not include HLA alleles known to promote autoimmune pathology. Thus, the lack of spontaneous autoimmune pathology in our Aire-deficient HLA-DR tg mice is consistent with reports showing that HLA alleles do not strongly influence autoantibody formation in APS1 patients [58]. Similarly, Ahonen et al. and others reported that HLA-DR alleles were not associated with APS1 related pathology [58–60].
The similar numbers of Tregs in our Aire−/− HLA-DR tg mice compared with their Aire+/+ littermates suggested that Treg development was not impaired. To further investigate this question, we asked whether an autoimmune phenotype was elicited by interfering with other regulatory mechanisms, for example via blockade of the regulatory CTLA-4 molecule. This was inspired by reports who observed spontaneous development of chronic organ-specific autoimmune disease after in vivo blockade of CTLA-4 mAb [51], and by studies showing functional inactivation of Tregs upon treatment with anti-CD25 mAb [52, 53]. Also, it has been shown that CTLA-4 has dual function in Tregs and conventional T cells to prevent multi-organ autoimmunity [61]. However, in our studies, treatment of Aire−/− HLA-DR2b tg mice with anti-CTLA-4/CD25 mAb did not result in significantly increased immune-mediated pathology, and treatment of Aire−/− HLA-DR4 tg mice with anti-CTLA-4 mAb only induced mild inflammatory infiltrates in less than half of the animals. Nevertheless, the mAb treatment accelerated development of lymphocytic infiltrates in younger mice, whereas without antibody treatment we only observed these infiltrates in a small subset of older mice. Of note, we found that the percentages of Tregs in our HLA-DR tg mice were generally lower as compared with the percentages observed in C57BL/6 Wt mice (1–4% versus 10–15%). Thus, it is conceivable that the antibody treatment may not have been as effective in further reducing the Treg compartment in the HLA-DR tg mice as compared with Wt mice.
Last, we asked whether autoimmune disease pathology was increased in Aire-deficient HLA-DR tg mice using a well-established EAE model [62, 63]. However, the effect of Aire-deficiency on EAE incidence and severity in HLA-DR2b or HLA-DR4 tg mice was also mild, and mostly characterized by earlier disease onset and slight disease increase. The results of these studies are noteworthy for somewhat divergent effects of Aire-deficiency on cytokine production by autoreactive T cells in HLA-DR2b versus HLA-DR4 tg mice. While the effect of Aire on cytokine producing T cells was marginal in HLA-DR2b tg mice, Aire−/− HLA-DR4 tg mice showed an increase in the frequencies of T cells producing proinflammatory cytokines, in particular for IL-17 and GM-CSF. Thus, it is conceivable that the increase in T cells producing these cytokines in the HLA-DR4 tg mice was, at least partially, compensated by regulatory mechanisms, such as Treg cells.
The interpretation of our results should take under consideration that TSA expression is not completely abolished by the absence of Aire [33]. Takaba et al. demonstrated the existence of another transcriptional regulator, FEZF2, which is specific for and highly expressed in mTECs in humans, and, similarly, numerous TRA transcripts are downregulated in Fezf2-deficient mTECs in mice. Thus, Fezf2 has an Aire-independent and non-redundant role in promoting ectopic expression of TSAs, and this mechanism may have played a compensatory role in our model [64].
In summary, our studies showed that mice deficient for Aire and tg for human autoimmune disease-associated HLA alleles developed limited spontaneous autoimmune pathology, mostly restricted to salivary glands, liver, and pancreas, and predominantly in older animals. The animals showed no major changes in immune cell subsets, particularly CD4+ T cells, and increased systemic autoimmune pathology was not triggered upon treatment with anti-CTLA-4 or CD25 mAb. Active induction of EAE resulted in earlier disease onset, but with similar disease severity in Aire-deficient mice as compared with their Aire+/+ HLA-DR tg littermates. However, the animals did not develop spontaneous neuroimmune disease or arthritis despite the presence of the human MS- and RA-associated HLA-DR2b and -DR4 alleles.
Our results support that the transcription factor Aire plays a general role in modulating autoimmune pathology, but indicate that it does not have an essential role in regulating autoimmunity in the context of the human HLA-DR2b and -DR4 alleles associated with MS and RA, respectively.
Supplementary Material
Highlights.
HLA-DR2b and HLA-DR4 transgenic (tg) Aire−/− mice were generated on the experimental autoimmune encephalomyelitis (EAE)-susceptible C57BL/6 background.
These mice were used to investigate the role of Aire for autoimmune pathology in the context of the multiple sclerosis and rheumatoid arthritis-associated human HLA-DR2b and -DR4 molecules.
The mice showed a decrease in thymic expression of gene expression of tissue-specific antigens in parallel with mild spontaneous inflammatory infiltrates in salivary glands, liver, and pancreas and gastric autoantibodies.
The mice showed a modes increase in the severity of actively induced experimental autoimmune encephalomyelitis.
However, the animals did not develop spontaneous neuroinflammatory disease or autoimmune arthritis.
No significant effect of the Aire-deletion was noted on the immune compartment in these mice.
The results support a role for the transcription factor Aire in modulating autoimmune pathology, but argue against its essential role in regulating autoimmunity in the context of the human HLA-DR2b and -DR4 alleles associated with MS and RA, respectively.
Acknowledgments
We would like to thank Francisco Gomez-Rivera for his help with flow cytometry. We thank the RCMI Biophotonics core for assistance with imaging. This work was supported by grants NS42809, NS84201, and G12MD007591 from the National Institute of Health, and grants RG3701, RG5501, and RG1602 from the National Multiple Sclerosis Society (T.G.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359(9313):1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 2.Fournier C. Where do T cells stand in rheumatoid arthritis? Joint, bone, spine: revue du rhumatisme. 2005;72(6):527–532. doi: 10.1016/j.jbspin.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Smith DA, Germolec DR. Introduction to immunology and autoimmunity. Environ Health Perspect. 1999;107(Suppl 5):661–665. doi: 10.1289/ehp.99107s5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20(5):509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- 5.Pender MP. Activation-induced apoptosis of autoreactive and alloreactive T lymphocytes in the target organ as a major mechanism of tolerance. Immunol Cell Biol. 1999;77(3):216–223. doi: 10.1046/j.1440-1711.1999.00818.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 7.Venanzi ES, Melamed R, Mathis D, Benoist C. The variable immunological self: genetic variation and nongenetic noise in Aire-regulated transcription. Proc Natl Acad Sci U S A. 2008;105(41):15860–15865. doi: 10.1073/pnas.0808070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2(4):301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 9.Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321(5890):843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson P, Org T, Rebane A. Transcriptional regulation by AIRE: molecular mechanisms of central tolerance. Nat Rev Immunol. 2008;8(12):948–957. doi: 10.1038/nri2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91(8):2843–2850. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 12.Ahonen P, Myllarniemi S, Sipila I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322(26):1829–1836. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- 13.Hubert FX, Kinkel SA, Crewther PE, Cannon PZ, Webster KE, Link M, Uibo R, O’Bryan MK, Meager A, Forehan SP, et al. Aire-deficient C57BL/6 mice mimicking the common human 13-base pair deletion mutation present with only a mild autoimmune phenotype. J Immunol. 2009;182(6):3902–3918. doi: 10.4049/jimmunol.0802124. [DOI] [PubMed] [Google Scholar]
- 14.Kurisaki H, Nagao Y, Nagafuchi S, Mitsuyama M. Autoimmune gastro-pancreatitis with anti-protein disulfide isomerase-associated 2 autoantibody in Aire-deficient BALB/cAnN mice. PLoS One. 2013;8(8):e73862. doi: 10.1371/journal.pone.0073862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, Eskelin P, Pelto-Huikko M, Peltonen L. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11(4):397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Lozano JR, Torres-Agrela B, Montes-Cano MA, Ortiz-Fernandez L, Conde-Jaldon M, Teruel M, Garcia A, Nunez-Roldan A, Martin J, Gonzalez-Escribano MF. Association of the AIRE gene with susceptibility to rheumatoid arthritis in a European population: a case control study. Arthritis Res Ther. 2013;15(1):R11. doi: 10.1186/ar4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng ZJ, Zhang SL, Wen HF, Liang Y. Association of rs2075876 polymorphism of AIRE gene with rheumatoid arthritis risk. Hum Immunol. 2015;76(4):281–285. doi: 10.1016/j.humimm.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Berczi B, Gerencser G, Farkas N, Hegyi P, Veres G, Bajor J, Czopf L, Alizadeh H, Rakonczay Z, Vigh E, et al. Association between AIRE gene polymorphism and rheumatoid arthritis: a systematic review and meta-analysis of case-control studies. Sci Rep. 2017;7(1):14096. doi: 10.1038/s41598-017-14375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapitany A, Zilahi E, Szanto S, Szucs G, Szabo Z, Vegvari A, Rass P, Sipka S, Szegedi G, Szekanecz Z. Association of rheumatoid arthritis with HLA-DR1 and HLA-DR4 in Hungary. Ann N Y Acad Sci. 2005;1051:263–270. doi: 10.1196/annals.1361.067. [DOI] [PubMed] [Google Scholar]
- 20.McDevitt HO. The role of MHC class II molecules in susceptibility and resistance to autoimmunity. Curr Opin Immunol. 1998;10(6):677–681. doi: 10.1016/s0952-7915(98)80088-5. [DOI] [PubMed] [Google Scholar]
- 21.Olerup O, Hillert J. HLA class II-associated genetic susceptibility in multiple sclerosis: a critical evaluation. Tissue Antigens. 1991;38(1):1–15. doi: 10.1111/j.1399-0039.1991.tb02029.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheng S, Smart M, Hanson J, David CS. Characterization of HLA DR2 and DQ8 transgenic mouse with a new engineered mouse class II deletion, which lacks all endogenous class II genes. J Autoimmun. 2003;21(3):195–199. doi: 10.1016/s0896-8411(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, Bian HJ, Molina M, Han J, Magram J, Saar E, Belunis C, Bolin DR, Arceo R, Campbell R, et al. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J Exp Med. 1996;183(6):2635–2644. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A. 1999;96(18):10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lincoln MR, Montpetit A, Cader MZ, Saarela J, Dyment DA, Tiislar M, Ferretti V, Tienari PJ, Sadovnick AD, Peltonen L, et al. A predominant role for the HLA class II region in the association of the MHC region with multiple sclerosis. Nat Genet. 2005;37(10):1108–1112. doi: 10.1038/ng1647. [DOI] [PubMed] [Google Scholar]
- 26.Kawamura K, McLaughlin KA, Weissert R, Forsthuber TG. Myelin-reactive type B T cells and T cells specific for low-affinity MHC-binding myelin peptides escape tolerance in HLA-DR transgenic mice. Journal of immunology. 2008;181(5):3202–3211. doi: 10.4049/jimmunol.181.5.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuerten S, Kostova-Bales DA, Frenzel LP, Tigno JT, Tary-Lehmann M, Angelov DN, Lehmann PV. MP4- and MOG:35–55-induced EAE in C57BL/6 mice differentially targets brain, spinal cord and cerebellum. Journal of neuroimmunology. 2007;189(1–2):31–40. doi: 10.1016/j.jneuroim.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol. 1995;25(7):1951–1959. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- 29.Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202(6):805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi RR, Fong L, Anderson MS. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203(12):2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forsthuber TG, Shive CL, Wienhold W, de Graaf K, Spack EG, Sublett R, Melms A, Kort J, Racke MK, Weissert R. T cell epitopes of human myelin oligodendrocyte glycoprotein identified in HLA-DR4 (DRB1*0401) transgenic mice are encephalitogenic and are presented by human B cells. J Immunol. 2001;167(12):7119–7125. doi: 10.4049/jimmunol.167.12.7119. [DOI] [PubMed] [Google Scholar]
- 32.Rich C, Link JM, Zamora A, Jacobsen H, Meza-Romero R, Offner H, Jones R, Burrows GG, Fugger L, Vandenbark AA. Myelin oligodendrocyte glycoprotein-35–55 peptide induces severe chronic experimental autoimmune encephalomyelitis in HLA-DR2-transgenic mice. Eur J Immunol. 2004;34(5):1251–1261. doi: 10.1002/eji.200324354. [DOI] [PubMed] [Google Scholar]
- 33.Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202(1):33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofstetter HH, Shive CL, Forsthuber TG. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund’s adjuvant: induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. Journal of immunology. 2002;169(1):117–125. doi: 10.4049/jimmunol.169.1.117. [DOI] [PubMed] [Google Scholar]
- 35.Gavanescu I, Kessler B, Ploegh H, Benoist C, Mathis D. Loss of Aire-dependent thymic expression of a peripheral tissue antigen renders it a target of autoimmunity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(11):4583–4587. doi: 10.1073/pnas.0700259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kont V, Laan M, Kisand K, Merits A, Scott HS, Peterson P. Modulation of Aire regulates the expression of tissue-restricted antigens. Mol Immunol. 2008;45(1):25–33. doi: 10.1016/j.molimm.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eldershaw SA, Sansom DM, Narendran P. Expression and function of the autoimmune regulator (Aire) gene in non-thymic tissue. Clin Exp Immunol. 2011;163(3):296–308. doi: 10.1111/j.1365-2249.2010.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassler S, Ramsey C, Karlsson MC, Larsson D, Herrmann B, Rozell B, Backheden M, Peltonen L, Kampe O, Winqvist O. Aire-deficient mice develop hematopoetic irregularities and marginal zone B-cell lymphoma. Blood. 2006;108(6):1941–1948. doi: 10.1182/blood-2006-04-019679. [DOI] [PubMed] [Google Scholar]
- 39.Buzi F, Badolato R, Mazza C, Giliani S, Notarangelo LD, Radetti G, Plebani A, Notarangelo LD. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome: time to review diagnostic criteria? J Clin Endocrinol Metab. 2003;88(7):3146–3148. doi: 10.1210/jc.2002-021495. [DOI] [PubMed] [Google Scholar]
- 40.Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y, Bando Y, Izumi K, Takahashi T, Nomura T, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174(4):1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 41.Soderbergh A, Myhre AG, Ekwall O, Gebre-Medhin G, Hedstrand H, Landgren E, Miettinen A, Eskelin P, Halonen M, Tuomi T, et al. Prevalence and clinical associations of 10 defined autoantibodies in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 2004;89(2):557–562. doi: 10.1210/jc.2003-030279. [DOI] [PubMed] [Google Scholar]
- 42.Ko HJ, Kinkel SA, Hubert FX, Nasa Z, Chan J, Siatskas C, Hirubalan P, Toh BH, Scott HS, Alderuccio F. Transplantation of autoimmune regulator-encoding bone marrow cells delays the onset of experimental autoimmune encephalomyelitis. Eur J Immunol. 2010;40(12):3499–3509. doi: 10.1002/eji.201040679. [DOI] [PubMed] [Google Scholar]
- 43.Papatriantafyllou M. Tolerance: both natural and induced TReg talents needed. Nat Rev Immunol. 2011;11(8):500. doi: 10.1038/nri3030. [DOI] [PubMed] [Google Scholar]
- 44.Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8(4):351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 46.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bosl MR, Hollander GA, Hayashi Y, de Malefyt RW, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208(2):383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339(6124):1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perry JS, Lio CW, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, Hsieh CS. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41(3):414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348(6234):589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perkins D, Wang Z, Donovan C, He H, Mark D, Guan G, Wang Y, Walunas T, Bluestone J, Listman J, et al. Regulation of CTLA-4 expression during T cell activation. J Immunol. 1996;156(11):4154–4159. [PubMed] [Google Scholar]
- 51.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176(6):3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 53.Stephens LA, Gray D, Anderton SM. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proc Natl Acad Sci U S A. 2005;102(48):17418–17423. doi: 10.1073/pnas.0507454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 55.Fugger L, Svejgaard A. Association of MHC and rheumatoid arthritis. HLA-DR4 and rheumatoid arthritis: studies in mice and men. Arthritis Res. 2000;2(3):208–211. doi: 10.1186/ar89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gillard GO, Dooley J, Erickson M, Peltonen L, Farr AG. Aire-dependent alterations in medullary thymic epithelium indicate a role for Aire in thymic epithelial differentiation. J Immunol. 2007;178(5):3007–3015. doi: 10.4049/jimmunol.178.5.3007. [DOI] [PubMed] [Google Scholar]
- 57.Yang JZ, Li MW, Wang JG, Lu HF, Yao XS, He JQ, Li LJ. Rapid detection of clonal expansion of T-cell receptor-beta gene in patients with HBV using the real-time PCR with DNA melting curve analysis. Hepatol Res. 2010;40(4):407–414. doi: 10.1111/j.1872-034X.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- 58.Halonen M, Eskelin P, Myhre AG, Perheentupa J, Husebye ES, Kampe O, Rorsman F, Peltonen L, Ulmanen I, Partanen J. AIRE mutations and human leukocyte antigen genotypes as determinants of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy phenotype. J Clin Endocrinol Metab. 2002;87(6):2568–2574. doi: 10.1210/jcem.87.6.8564. [DOI] [PubMed] [Google Scholar]
- 59.Ahonen P, Koskimies S, Lokki ML, Tiilikainen A, Perheentupa J. The expression of autoimmune polyglandular disease type I appears associated with several HLA-A antigens but not with HLA-DR. J Clin Endocrinol Metab. 1988;66(6):1152–1157. doi: 10.1210/jcem-66-6-1152. [DOI] [PubMed] [Google Scholar]
- 60.Huang W, Connor E, Rosa TD, Muir A, Schatz D, Silverstein J, Crockett S, She JX, Maclaren NK. Although DR3-DQB1*0201 may be associated with multiple component diseases of the autoimmune polyglandular syndromes, the human leukocyte antigen DR4-DQB1*0302 haplotype is implicated only in beta-cell autoimmunity. J Clin Endocrinol Metab. 1996;81(7):2559–2563. doi: 10.1210/jcem.81.7.8675578. [DOI] [PubMed] [Google Scholar]
- 61.Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci U S A. 2010;107(4):1524–1528. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klehmet J, Shive C, Guardia-Wolff R, Petersen I, Spack EG, Boehm BO, Weissert R, Forsthuber TG. T cell epitope spreading to myelin oligodendrocyte glycoprotein in HLA-DR4 transgenic mice during experimental autoimmune encephalomyelitis. Clinical immunology. 2004;111(1):53–60. doi: 10.1016/j.clim.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 63.Ji N, Somanaboeina A, Dixit A, Kawamura K, Hayward NJ, Self C, Olson GL, Forsthuber TG. Small molecule inhibitor of antigen binding and presentation by HLA-DR2b as a therapeutic strategy for the treatment of multiple sclerosis. Journal of immunology. 2013;191(10):5074–5084. doi: 10.4049/jimmunol.1300407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takaba H, Morishita Y, Tomofuji Y, Danks L, Nitta T, Komatsu N, Kodama T, Takayanagi H. Fezf2 Orchestrates a Thymic Program of Self-Antigen Expression for Immune Tolerance. Cell. 2015;163(4):975–987. doi: 10.1016/j.cell.2015.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.