Abstract
Background
Obesity alters adipose tissue immunology, and these changes may be reflected in circulating soluble inflammatory biomarker and T cell subset profiles measured in HIV research studies.
Methods
We recruited 70 adults with HIV (50% obese) on efavirenz, tenofovir, and emtricitabine, virologic suppression for >2 years, and no rheumatologic or other known inflammatory conditions. We measured fasting plasma levels of several markers of innate immunity and major CD4+ and CD8+ T cell subsets. We assessed relationships between measurements of total adiposity (body mass index [BMI], DEXA fat mass index [FMI], and plasma leptin) and the immunologic parameters using covariate-adjusted Spearman’s rank correlations.
Results
The cohort was 43% female, 54% non-white, and median age was 45 years. Higher BMI, FMI and plasma leptin were consistently associated with higher C-reactive protein, serum amyloid A, and interleukin (IL)-6 (p<0.01 for all), but lower IL-10 (p≤0.02 for all). BMI and FMI were positively associated with soluble tumor necrosis factor-α receptor 1 levels (p<0.02 for both), and a positive correlation approached significance for all three body composition measurements with soluble CD163 (p≤0.09 for all). Higher BMI and FMI were associated with lower CD38 expression on CD4+ T cells (p≤0.04 for both), but higher CD69 expression (p≤0.01 for BMI and FMI, p=0.07 for leptin).
Conclusions
Greater adiposity is associated with alterations in a limited set of circulating immune markers, potentially reflecting changes known to occur in adipose tissue with treated HIV infection. Measuring total fat mass radiographically did not yield substantively different results compared to BMI.
Introduction
Relationships between circulating soluble immune mediators or T cell subsets and health outcomes among people living with HIV (PLWH) are reported in many studies.1-8 As examples, higher circulating levels of C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are associated with increased risk of cardiovascular events, insulin resistance, and all-cause mortality among PLWH.1,2,9-13 Similarly, a greater proportion of activated CD8+ T cells is associated with poor CD4+ T cell reconstitution on antiretroviral therapy (ART), subclinical carotid artery disease, and impaired arterial relaxation.14-16 Ideally, these studies illuminate either the causal contribution of a given innate or adaptive immune actor in a pathophysiologic process, reflect an immune perturbation arising from a clinical condition, or both.
A concern in studies of biomarkers and health outcomes is confounding by participant characteristics that affect both the biomarker and outcome of interest, thus contributing to a spurious statistical relationship or a false-negative finding. In HIV-negative persons, serum levels of CRP and IL-6 increase with adiposity,17-19 and it is estimated that adipose tissue-derived IL-6 constitutes up to 35% of circulating levels in obese individuals.20 Furthermore, HIV-negative overweight and obese women have significantly higher CD4+ and total lymphocyte counts compared to normal weight women.21 At present there are few similar data for PLWH.
The increasing prevalence of obesity among PLWH in the US22 raises the importance of understanding how immunologic biomarkers are affected by body composition. In this study, we characterize relationships between body fat and circulating levels of over 20 plasma markers of innate immunity and major CD4+ and CD8+ T cell subsets in PLWH on ART, with the goal of identifying the immune parameters most affected by adiposity as estimated by body mass index (BMI), dual energy X-ray absorptiometry (DEXA)-quantified fat mass index (FMI), and plasma leptin (an adipokine produced in proportion to fat mass).
Methods
We enrolled 70 adults with HIV on ART from the Vanderbilt Comprehensive Care Clinic, distributed approximately equally between four BMI categories (<25.0, 25.0-29.9, 30.0-34.9, and ≥35.0 kg/m2). Within each BMI strata, similar numbers of males and females, and whites and non-whites, were enrolled. All participants were on a single-tablet regimen of co-formulated efavirenz, tenofovir, and emtricitabine for at least 6 months, and had persistent HIV-1 RNA <50 copies/mL on ART for at least the previous 2 years. Additional inclusion criteria were CD4+ T cell count >350 cells/μl at enrollment, no use of any anti-diabetic agent or statin (i.e., HMG CoA reductase inhibitor), no self-reported heavy alcohol or cocaine/amphetamine use, no active infectious condition aside from HIV, and no previously diagnosed diabetes, cardiovascular disease (CVD), rheumatologic disease, or other inflammatory condition.
Venous blood was drawn in the morning between 8 and 11am after a minimum 8 hour fast. Samples were collected in an EDTA-containing vacutainer, centrifuged for 10 minutes at 4°C, and the plasma removed and immediately frozen at −80°C. High-sensitivity CRP (hs-CRP) was measured by nephelometry in the Vanderbilt Clinical Chemistry Laboratory. Plasma levels of soluble CD14 (sCD14) and CD163 (sCD163), two surface markers released into circulation by activated macrophages, were measured using ELISA (R&D Systems, Minneapolis, MN). Other plasma cytokines including interleukins, serum amyloid A, interferon-γ, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 α and β (MIP-1α/β), TNF-α, and soluble TNF-α receptors 1 and 2 (sTNFRI and sTNFRII) were measured in duplicate using a standard multiple immunoassay panel (MesoScale, Rockville, MD).
Peripheral blood mononuclear cells (PBMCs) were obtained from fasting whole blood samples collected in EDTA, separated by Ficoll-Paque Plus density gradient, and cryopreserved in FBS with 10% DMSO. After study enrollment was completed, PBMC aliquots were thawed, stained, and run on a Fortessa (Becton Dickson Biosciences, San Jose, CA) flow cytometer. We used three different fluorochrome panels incorporating CD8-APC-A750 (Life Technologies, Carlsbad, CA); CD4-PcP-Cy5.5, CD3-BV711, CD14-V500, CD19-V500, CD57-FITC, PD-1-PE, CD69-APC, CD38-PE-Cy7, HLA-DR-V450A, CD25-PE, CD27-PE-Cy7, CD28-APC, CCR7-BV421, (Becton Dickson Biosciences); and CD45RO-PETxR, CD127-PE-Cy5.5 (Beckman Coulter, Pasadena, CA). We measured the proportion of CD4+ and CD8+ T cells expressing activation (CD38, HLA-DR, and CD69), senescence (CD57), and exhaustion (PD-1) markers. We used memory (CCR7, CD45RO, CD27) surface markers to markers identify naïve (CD45RO−, CCR7+, CD27+), central memory (Tcm; CD45RO+, CCR7+, CD27+), transitional memory (CD45RO+, CCR7−, CD27+), effector memory (Tem; CD45RO+, CCR7−, CD27−), and effector memory RA+ (TemRA; CD45RO−, CCR7−, CD27−) T cell phenotypes. Lastly, in the CD4+ population, we also measured the percentage of regulatory (CD25high, CD127−) T cells.
Height and weight were measured in duplicate to calculate BMI. A full body DEXA (GE Lunar Prodigy, GE Healthcare, Little Chalfont, United Kingdom) measured total fat mass to calculate FMI (total fat in kilograms divided by height in meters, squared). FMI is a variant of BMI that accounts for individual variability in the ratio of fat to lean mass.23 Lastly, plasma leptin was measured in duplicate using an immunoassay (MesoScale, Rockville, MD).
Statistical analyses
Demographic, clinical, and body composition characteristics were compared between BMI categories using Kruskal-Wallis rank sum or chi-square tests.
Due to the large number of biomarkers with heterogeneous distributions characterized by high skewness for some and assay detection limits for others, we assessed the relationships between adiposity measurements (BMI, FMI, and leptin) and the immunologic parameters using covariate-adjusted Spearman’s rank correlations robust for these types of data.24 Covariates were pre-specified and included age, sex, race (white versus non-white), entry CD4+ T cell count (square root transformed), ART duration, and smoking status. This method first fits separate cumulative probability models (with logit link functions) to each adiposity and immunological measure as a function of covariates.25 Probability-scale residuals (PSRs) are then calculated, and Spearman’s correlations computed as the correlation between PSRs.
Secondary analyses included nadir CD4+ T cell count as a covariate. We also calculated adjusted Spearman’s rank correlations conditional on sex to assess whether relationships between adiposity measures and immune parameters differed by sex. No adjustments were made for multiple comparisons for this exploratory study.26 Analyses were conducted using SPSS 22.0.0 (IBM) and R Statistical Software, Version 3.4.2 (http://www.R-project.org).
Results
Seventy PLWH were enrolled. The cohort was 43% female and 54% non-white (Supplementary Table 1). Median age was 45 years, BMI 30.3 kg/m2, CD4+ T cell count 701 cells/μl, and ART duration 6.2 years. Age, race, sex, smoking status, entry CD4+ count, ART initiation, duration of ART treatment, and hepatitis C prevalence were similar across the BMI categories (p>0.05 for all comparisons).
Adjusted rank correlations between BMI, FMI, or plasma leptin and each of the immunologic parameters are shown in the heat map (Figure); correlations with a P-value <0.05 and <0.10 are indicated. Higher BMI, FMI and plasma leptin were consistently associated with higher hs-CRP, serum amyloid A, and IL-6 (P<0.01 for all), but lower IL-10 (p≤0.02 for all; significant associations are shown in the Table). BMI and FMI were positively associated with sTNFRI levels (p<0.02 for both), and the correlation with plasma leptin approached significance (p=0.07). A positive correlation between sCD163 and adiposity approached significance for all three body composition measurements (p≤0.09 for all). Adjusted correlations for all measured biomarkers are shown in Supplementary Table 2.
Figure. Heat map showing adjusted correlation of body compositon measurements with immune parameters.
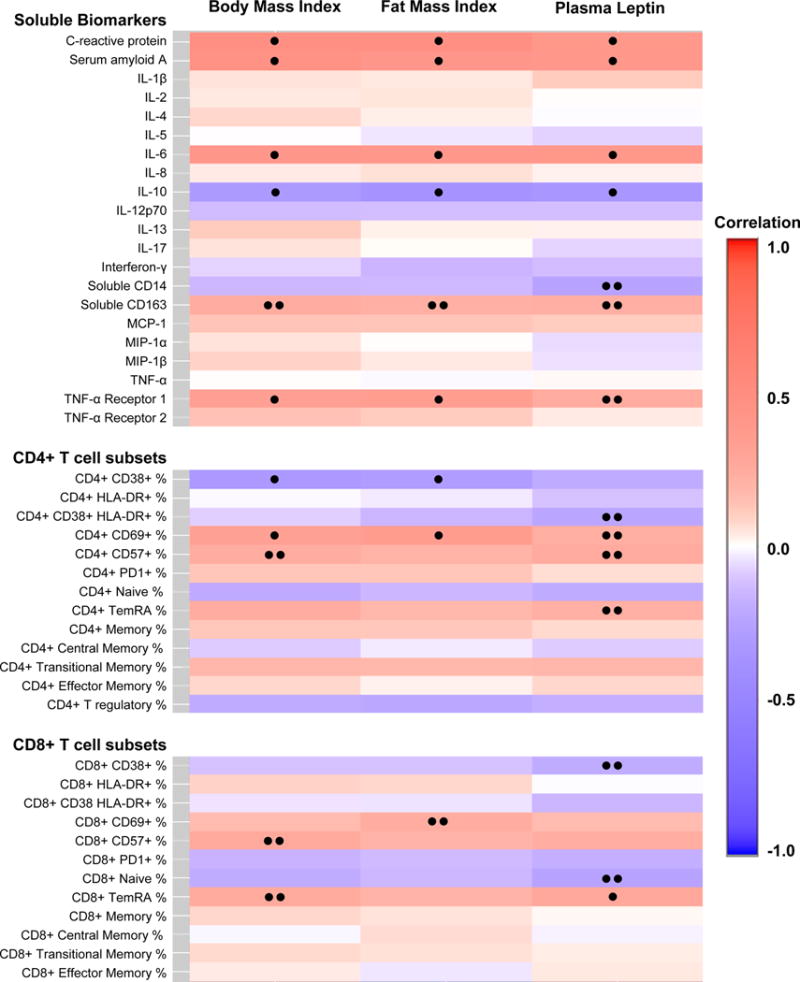
Relationships between adiposity measurements (BMI, FMI, and plasma leptin) and the immunologic parameters assessed using covariate-adjusted Spearman’s rank correlations. Covariates included age, sex, race, CD4 count, duration of ART, and smoking status.
• p<0.05, •• p<0.10
Table.
Adjusted correlation of selected soluble innate immune markers and T cell subsets with measures of total body adiposity
| Body mass index | Fat mass index | Plasma leptin | ||||
|---|---|---|---|---|---|---|
| Soluble Biomarkers | Correlation (95% CI) |
P-value | Correlation (95% CI) |
P-value | Correlation (95% CI) |
P-value |
| C-reactive protein |
0.48 (0.23, 0.67) |
< 0.001 |
0.48 (0.22, 0.67) |
< 0.001 |
0.40 (0.14, 0.6) |
0.003 |
| Serum amyloid A |
0.45 (0.22, 0.64) |
< 0.001 |
0.41 (0.17, 0.6) |
0.001 |
0.40 (0.14, 0.6) |
0.003 |
| IL-6 |
0.41 (0.15, 0.61) |
0.002 |
0.39 (0.14, 0.6) |
0.003 |
0.4 (0.18, 0.58) |
< 0.001 |
| IL-10 |
−0.30 (−0.52, −0.05) |
0.02 |
−0.36 (−0.57, −0.11) |
0.006 |
−0.33 (−0.55, −0.07) |
0.01 |
| Soluble CD14 |
−0.14 (−0.38, 0.11) |
0.27 |
−0.14 (−0.38, 0.11) |
0.28 |
−0.24 (−0.47, 0.02) |
0.07 |
| Soluble CD163 |
0.23 (−0.03, 0.47) |
0.08 |
0.22 (−0.03, 0.45) |
0.09 |
0.22 (−0.03, 0.45) |
0.09 |
| Soluble TNF-α receptor 1 |
0.33 (0.07, 0.55) |
0.01 |
0.34 (0.07, 0.57) |
0.02 |
0.25 (−0.02, 0.48) |
0.07 |
| CD4+ T cell subsets | ||||||
| CD4+ CD38+ % |
−0.32 (−0.55, −0.05) |
0.02 |
−0.29 (−0.52, −0.01) |
0.04 |
−0.2 (−0.44, 0.07) |
0.15 |
| CD4+ CD38+ HLA-DR+ % |
−0.08 (−0.35, 0.2) |
0.57 |
−0.15 (−0.4, 0.11) |
0.26 |
−0.23 (−0.46, 0.03) |
0.08 |
| CD4+ CD69+ % |
0.32 (0.08, 0.53) |
0.01 |
0.35 (0.12, 0.55) |
0.004 |
0.23 (−0.02, 0.44) |
0.07 |
| CD4+ CD57+ % |
0.24 (−0.03, 0.47) |
0.09 | 0.21 (−0.08, 0.46) |
0.15 |
0.25 (−0.03, 0.49) |
0.07 |
| CD4+ TemRA % | 0.23 (−0.05, 0.48) |
0.10 | 0.18 (−0.09, 0.42) |
0.18 |
0.22 (−0.03, 0.45) |
0.09 |
| CD8+ T cell subsets | ||||||
| CD8+ CD38+ % |
−0.11 (−0.36, 0.15) |
0.41 |
−0.11 (−0.35, 0.14) |
0.39 |
−0.19 (−0.4, 0.04) |
0.10 |
| CD8+ CD69+ % | 0.16 (−0.1, 0.41) |
0.23 |
0.23 (−0.04, 0.48) |
0.09 | 0.16 (−0.1, 0.4) |
0.23 |
| CD8+ CD57+ % |
0.26 (−0.02, 0.5) |
0.07 | 0.19 (−0.11, 0.47) |
0.21 | 0.23 (−0.07, 0.49) |
0.13 |
| CD8+ Naive % |
−0.19 (−0.43, 0.07) |
0.15 |
−0.15 (−0.39, 0.11) |
0.27 |
−0.24 (−0.46, 0.01) |
0.06 |
| CD8+ TemRA % |
0.25 (−0.01, 0.49) |
0.06 | 0.2 (−0.07, 0.44) |
0.14 |
0.27 (0.01, 0.49) |
0.04 |
Table shows only biomarkers or T cell subsets with at least one correlation with p<0.10.
Bold indicates p-values <0.10, bold italics indicates p-values <0.05.
Abbreviations: IL, interleukin; TemRA, T effector memory RA+ cells; TNF-α, tumor necrosis factor-α.
We observed more heterogeneity between adiposity and the CD4+ and CD8+ T cell subsets than the soluble markers. Higher BMI and FMI were associated with lower CD38 expression on CD4+ T cells (p≤0.04 for both), but higher CD69 expression (p≤0.01 for BMI and FMI, p=0.07 for leptin). Greater BMI and leptin levels were accompanied by higher expression of CD57 on CD4+ T cells, and this relationship approached significance (p≤0.09 for both). In contrast, the only significant association for CD8+ T cell subsets was a positive correlation between leptin and CD8+ TemRA cells.
Results were not substantively different when the models were further adjusted for nadir CD4+ T cell count. When the 6 participants with BMI <20 kg/m2 were excluded, results were similar with the exception that CD38 expression on CD4+ T cells was no longer significantly associated with BMI or FMI. Lastly, we did not find that the adjusted correlations conditioned on sex indicated a significant difference for in any of the adiposity and immune parameter relationships for males vs. females (p>0.05 for all)
Discussion
In a cohort of PLWH on long-term ART and without known rheumatologic or other inflammatory conditions, higher levels of adiposity were associated with greater plasma levels of several markers of innate immune activation, and variable changes in CD4+ and CD8+ T cell subsets. Notably, while hs-CRP, serum amyloid A, IL-6, sCD163, and sTNFRI levels demonstrated similar proportional increases for BMI, FMI, and plasma leptin, the changes in circulating CD4+ and CD8+ T cell subsets were markedly less consistent. Notably, several of the biomarkers that changed with adiposity in our cohort are known to increase in adipose tissue in the setting of obesity and exposure to HIV,27 while lower levels of IL-10, a cytokine with pleiotropic anti-inflammatory effects on T cells and macrophages, are linked to poor metabolic health.28,29 We interpret these findings to indicate that levels of circulating markers of innate immune function in PLWH may be determined, in part, by changes occurring within adipose tissue in response to progressive weight gain. Of note, measuring total fat mass with DEXA to adjust for participant adiposity did not substantively alter results compared to BMI.
The stromal vascular fraction of adipose tissue contains a diverse mix of cells from the innate and adaptive arms of the immune system that form a complex paracrine signaling milieu, modulating local inflammation and adipocyte function. These include monocyte-derived tissue macrophages and several T cell subsets, which may infiltrate adipose tissue from the bloodstream or lymphatic system, or be tissue-resident immune cells. HIV infection intervenes on this environment at many points, including changes in adipocyte metabolic characteristics and signaling, changes in circulating monocyte and T cell populations, and potential latent infection of adipose tissue CD4+ T cells.27
With obesity, adipose tissue depots primarily expand through adipocyte hypertrophy rather than hyperplasia, the former of which is accompanied by a disproportionate rise in IL-6 and TNF-α.30-32 Adipocyte hypertrophy in obesity is also accompanied by increased MCP-1 and MIP-1α expression, which promote macrophage infiltration, and increased IL-8, which promotes neutrophil chemotaxis.33-35 Adipose tissue biopsies from obese human and animals contain higher absolute numbers of macrophages, which demonstrate greater polarization towards a pro-inflammatory M1 cytokine phenotype (characterized by high IL-6, TNF-α and inducible nitric oxide synthase production).36-38
In our cohort, greater adiposity, as measured by both BMI and FMI, was most strongly associated with lower CD38 expression and higher CD69 expression on CD4+ T cells. A link between adiposity and cellular immunity is supported by studies from the pre-ART era demonstrating that a higher BMI was associated with slower HIV disease progression.39-41 In the combination ART era, a higher BMI is associated with more robust CD4+ T cell recovery.42 Notably, this may not reflect an HIV-related phenomenon, as HIV-negative overweight and obese women have significantly higher CD4+ and total lymphocyte counts compared to normal weight women.21
Strengths of this study include a wide distribution of BMI values, restriction of participants to a single ART regimen, and long-term (≥2 years) virologic suppression, which allowed for the inflammatory effects of plasma viremia to fade. Limitations included the use of DEXA as opposed to CT or MRI for adipose tissue quantification, and lack of adjustment for diet and exercise. The absence of an HIV-negative control group precluded the assessment of potential differences in relationships between biomarkers and body composition by HIV status. Lastly, since all participants were on efavirenz, tenofovir, and emtricitabine, our results may not be generalizable to persons on a protease inhibitors or integrase strand transfer inhibitors.
As two-thirds the US HIV population is overweight or obese, there is an acute need to understand the health outcomes of this population and, ultimately, optimize care for PLWH. Clinical studies should consider the potential effects of body composition on immunologic parameters.
Supplementary Material
Acknowledgments
The authors thank the participants in the Adiposity and Immune Activation Cohort study.
Funding support:
This work was supported by NIAID grants K23 AI100700 and K23 AI110532, NIDDK grant R01 DK112262, the NIH-funded Vanderbilt Clinical and Translational Science award from NCRR/NIH grant UL1 RR024975, and the NIH-funded Tennessee Center for AIDS Research grant P30 AI110527. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- IL
interleukin
- MIP-1α/β
macrophage inflammatory protein-1α/β
- MCP-1
monocyte chemoattractant protein-1
- TemRA
T effector memory RA+ cells
- TNF-α
tumor necrosis factor-α
Footnotes
Disclosures: No authors report a conflict of interest.
References
- 1.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33(10):2244–2249. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51(3):268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordell AD, McKenna M, Borges AH, et al. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014;3(3):e000844. doi: 10.1161/JAHA.114.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krastinova E, Lecuroux C, Leroy C, et al. High Soluble CD14 Levels at Primary HIV-1 Infection Predict More Rapid Disease Progression. J Infect Dis. 2015 doi: 10.1093/infdis/jiv145. Epub March 6, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Sandler NG, Wand H, Roque A, et al. Plasma Levels of Soluble CD14 Independently Predict Mortality in HIV Infection. J Infect Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25(17):2123–2131. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 10.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry K, Kitch D, Dube M, et al. C-Reactive protein levels over time and cardiovascular risk in HIV-infected individuals suppressed on an indinavir-based regimen: AIDS Clinical Trials Group 5056s. AIDS. 2004;18(18):2434–2437. [PubMed] [Google Scholar]
- 12.Shikuma CM, Ribaudo HJ, Zheng Y, et al. Change in High-Sensitivity C-Reactive Protein Levels Following Initiation of Efavirenz-Based Antiretroviral Regimens in HIV-Infected Individuals. AIDS Res Hum Retroviruses. 2011;27(5):461–468. doi: 10.1089/aid.2010.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford ES, Greenwald JH, Richterman AG, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24(10):1509–1517. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187(10):1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203(4):452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grome HN, Barnett L, Hagar CC, Harrison DG, Kalams SA, Koethe JR. Association of T Cell and Macrophage Activation with Arterial Vascular Health in HIV. AIDS Res Hum Retroviruses. 2017;33(2):181–186. doi: 10.1089/aid.2016.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 18.Bo M, Raspo S, Morra F, et al. Body fat and C-reactive protein levels in healthy non-obese men. Nutr Metab Cardiovasc Dis. 2004;14(2):66–72. doi: 10.1016/s0939-4753(04)80012-7. [DOI] [PubMed] [Google Scholar]
- 19.Greenfield JR, Samaras K, Jenkins AB, et al. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation. 2004;109(24):3022–3028. doi: 10.1161/01.CIR.0000130640.77501.79. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82(12):4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 21.Womack J, Tien PC, Feldman J, et al. Obesity and immune cell counts in women. Metabolism. 2007;56(7):998–1004. doi: 10.1016/j.metabol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koethe JR, Jenkins CA, Lau B, et al. Rising Obesity Prevalence and Weight Gain Among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32(1):50–58. doi: 10.1089/aid.2015.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52(6):953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Li C, Wanga V, Shepherd BE. Covariate-adjusted Spearman’s rank correlation with probability-scale residuals. Biometrics. 2017 doi: 10.1111/biom.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Shepherd BE, Li C, Harrell FE., Jr Modeling continuous response variables using ordinal regression. Stat Med. 2017;36(27):4316–4335. doi: 10.1002/sim.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. Am J Epidemiol. 1995;142(9):904–908. doi: 10.1093/oxfordjournals.aje.a117737. [DOI] [PubMed] [Google Scholar]
- 27.Koethe JR. Adipose Tissue in HIV Infection. Compr Physiol. 2017;7(4):1339–1357. doi: 10.1002/cphy.c160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esposito K, Pontillo A, Giugliano F, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab. 2003;88(3):1055–1058. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- 29.Choi KM, Ryu OH, Lee KW, et al. Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res Clin Pract. 2007;75(2):235–240. doi: 10.1016/j.diabres.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Bastard JP, Lagathu C, Caron M, Capeau J. Point-counterpoint: Interleukin-6 does/does not have a beneficial role in insulin sensitivity and glucose homeostasis. J Appl Physiol. 2007;102(2):821–822. doi: 10.1152/japplphysiol.01353.2006. author reply 825. [DOI] [PubMed] [Google Scholar]
- 31.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92(3):1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 32.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83(8):2907–2910. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 33.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90(4):2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 34.Lee YH, Nair S, Rousseau E, et al. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia. 2005;48(9):1776–1783. doi: 10.1007/s00125-005-1867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jernas M, Palming J, Sjoholm K, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20(9):1540–1542. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 36.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 39.Shor-Posner G, Campa A, Zhang G, et al. When obesity is desirable: a longitudinal study of the Miami HIV-1-infected drug abusers (MIDAS) cohort. J Acquir Immune Defic Syndr. 2000;23(1):81–88. doi: 10.1097/00126334-200001010-00011. [DOI] [PubMed] [Google Scholar]
- 40.Jones CY, Hogan JW, Snyder B, et al. Overweight and human immunodeficiency virus (HIV) progression in women: associations HIV disease progression and changes in body mass index in women in the HIV epidemiology research study cohort. Clin Infect Dis. 2003;37(Suppl 2):S69–80. doi: 10.1086/375889. [DOI] [PubMed] [Google Scholar]
- 41.Shuter J, Chang CJ, Klein RS. Prevalence and predictive value of overweight in an urban HIV care clinic. J Acquir Immune Defic Syndr. 2001;26(3):291–297. doi: 10.1097/00042560-200103010-00013. [DOI] [PubMed] [Google Scholar]
- 42.Koethe JR, Jenkins CA, Lau B, et al. Higher Time-Updated Body Mass Index: Association With Improved CD4+ Cell Recovery on HIV Treatment. J Acquir Immune Defic Syndr. 2016;73(2):197–204. doi: 10.1097/QAI.0000000000001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


