Abstract
Statement of the Problem:
Extraction of the impacted third molar is often associated with severe postoperative pains, management of which are a big challenge. Lamotrigine is a new antiepileptic drug with pre-emptive analgesic properties, which is hypothesized to alleviate postoperative pain.
Purpose:
This study aimed to evaluate the efficacy of pre-operative administration of single oral 200 mg lamotrigine in reducing the postoperative pain of impacted third molar surgery.
Materials and Method:
In this randomized controlled trial, 100 adult patients were divided into two groups (n= 50) to receive either 200 mg oral lamotrigine or placebo 1 hour before the removal of impacted third molar. The patients were monitored for 4 hours in the recovery room and pain intensity was measured through visual analogue scale (VAS) for the next 12 hours at 30-minute intervals. The time and number of rescue analgesics used in 12 hours was also recorded.
Results:
Two groups were not statistically significantly different regarding the severity of postoperative pain. (p= 0.512)
Conclusion:
Accordingly, pre-emptive administration of lamotrigine was not effective in diminishing the postoperative acute pain of impacted third molar extraction.
Keywords: Lamotrigine , Third Molar , Pain Management
Introduction
One of the most frequent procedures in oral and maxillofacial surgery offices is the extraction of impacted third molars. It often involves soft tissue flaps and removal of bone tissue; thus, the postoperative inflammation is sometimes accompanied by severe pain, edema, and limited mouth opening. The wide range of complications goes from small normal consequences of postoperative pain and swelling, to persistent nerve damage, mandibular fractures, and severe infections. Minor complications are those whose recovery does not take any particular treatment. Molar extraction causes moderate to severe pain, which are challenges in pain management.[1-2]
Pain is a complex phenomenon, which concerns both nerve mechanisms and psychological perceptions. It is generally classified as acute pain in which normal pain pathways are triggered and chronic pain in which persists for longer than three months. Chronic pain might also have caused by nerve damage, which is known as neuropathic pain, or by an underlying disease such as cancer.3 Acute pain is often associated with physical signs originating from the sympathetic branch of the autonomic nervous system, which manifest as tachycardia, hypertension, sweating, mydriasis, and pallor.[1] Therefore, it seems that the symptoms resulting from surgical trauma after removal of the third molars are an excellent clinical model for studying acute pain.[2,4]
Among the most important parts of postsurgical care is finding an efficient way for pain management. Pre-emptive analgesia is described as a treatment that is introduced before the surgical procedure to prevent neurophysiological and biochemical consequences of an injury to CNS, which is triggered by a procedure like surgery.[5-6] The notion of pre-emptive analgesia is to prevent CNS alterations, which consequently lead to pain amplification after initial pain experience.[6-7] Regarding this protective effect on the nociceptive pathways, pre-emptive analgesia might hypothetically be more helpful than a similar analgesic treatment started after surgery and analgesia prescribed before an initial injurious stimulus may be more effective than the same dose given later.[8-10]
Accordingly, this might reduce the immediate post-surgical pain and preclude the development of subsequent chronic pain.[6,11]
Antiepileptic drugs have been administered for the treatment of neuropathic pain since lamotrigine was initially introduced for treatment trigeminal neuralgia in the 1960s.[12] Other antiepileptic medications have been surveyed, with acceptable efficacy for gabapentin[13] and pregabalin,[14] and these two are now commonly administered. Being used to treat different types of epilepsy, lamotrigine has also been proposed for treatment of chronic neuropathic pain and fibromyalgia in adults in many studies.[12]
Lamotrigine is a medication from phenyltriazine group and is chemically dissimilar to other antiepileptic medications. The drug is presented as standard oral tablets (25 to 200 mg) and chewable, dispersible tablets (2 to 25mg), and a new extended release tablet is available in some parts of the world.[12] Lamotrigine is a new generation antiepileptic medication employing its anticonvulsant effect by influencing the sodium channels. It is reported that the medications, which block sodium channels, could be helpful in the treatment of neuropathic pain.[15-16] An animal model study has reported the efficacy of lamotrigine use in neuropathic pain and showed its effect in experimental pain models such as cold induced pain in humans.[15,17-18] The role of lamotrigine as a pre-emptive analgesic to reduce postsurgical pain has also been reported.[16,19]
Observations indicate that increased activity of sodium channels is seemingly the basis for hyperalgesia, like the positive effect of sodium channel inhibitors (e.g. lidocaine) on increasing the pain threshold.[8,16,20] Lamotrigine inhibits the function of neuronal sodium channels in a concentration and voltage-dependent manner, decreasing the release of excitatory neurotransmitters, especially glutamate and aspartate.[12,18,21-23] However, up to now, the exact principles responsible for the lamotrigine-induced anticonvulsive effect remain obscure, and probably other targets might control and regulate such effect.[18]
The frequent plausible side effects of lamotrigine are dizziness, tremors, sleepiness, loss of coordination, headache, double vision, blurred vision, nausea, vomiting, stomach pain, dry mouth, changes in menstrual periods, back pain, sore throat, runny nose, or sleeping problems (insomnia), however, the reported incidence is low and clinically insignificant.[12,16,18,24]
According to the findings of uncontrolled clinical trials with small sample sizes, lamotrigine positively helped pain management in healthy volunteers or those suffering from different neuropathic syndromes.[20] On the other side, there are investigations reporting either a lack of benefit or an inconsistent benefit. [12,25-27] Trying to find out about the efficacy of lamotrigine in neuropathy, a systematic review of the available placebo-controlled studies declared that, so far, there are no evidences to confirm that lamotrigine can be effective in treatment of chronic pain.[12] However, in this systematic review, no clinical trials on acute pain have been considered and they reported there was little or no use or intended use of lamotrigine and similar medications in acute pain, or other forms of chronic pain.[12]
It is believed that surgical procedures, even skin incisions, may result in initial sensitization .On the other hand, the observations on the genesis and perception of pain brought about the notion that analgesia administered before an initial noxious stimulus may be more effective than the same dose given later.[9-10] Moreover, the literature review shows that the effect of lamotrigine on acute post-surgical pain of third molar extraction has not been studied, hence, the current study decided to assess the effect of pre-emptive administration of this medication after this surgical procedure because of the mild to moderate postoperative pain experienced in recovery, which necessitates analgesics.
Therefore, the current placebo-controlled study was designed to evaluate the efficacy and safety of a single dose of 200 mg oral lamotrigine as preoperative analgesia agent in patients undergoing impacted third molar surgery.
Materials and Method
This study was a prospective double-blind placebo-controlled randomized clinical trial, designed in concordance with the CONSORT guidelines (consolidated standards of reporting trials).[28] Before beginning the study, approval was obtained from the Committee for the Protection of Human Subjects in the related hospital. The study was also approved by the Local Ethical Committee of Shiraz University of Medical Sciences (IRCT201510111674N12).
At the beginning, 111 patients were selected from those who referred for the removal of impacted third molars to the Department of Oral and Maxillofacial Surgery in School of Dentistry, Shiraz University of Medical Sciences. The details of the study, the possible complications of the surgery, and the drug were fully explained to the patients, and informed consent was obtained. Eleven patients were excluded from the study for not meeting the inclusion criteria or not signing the consent form.
All the enrolled participants met the inclusion criteria determined by good medical, clinical and systemic health status and being in the age range of 14-55 years. They all had indications for extraction of impacted third molars according to clinical and radiographic evaluation.[29]
The exclusion criteria were asymmetric bilateral anatomic position of the mandibular-third molars, local contraindications such as periodontitis, odontogenic cysts or tumors (associated or not associated with the third molar), trauma to the region, any symptom of infection, and also general health issues such as systemic diseases, history of drug consumption and drug allergic reactions, history of using opioid, alcohol or analgesics 3 days before the study, and history of using anticonvulsants, use of any medication within the 30 preceding days, hepatic and renal dysfunction, patients with a previous history of using steroid and nonsteroidal anti-inflammatory drugs, intolerance to other materials used in the research, menstruation, pregnancy, or lactation at the time of surgery.[2]
The sample size was determined based on a power test (available on amostragem/amostra.html) proposing a sample size of nine patients per group to achieve a power of 0.8. [2] (Figure 1)
Figure1.
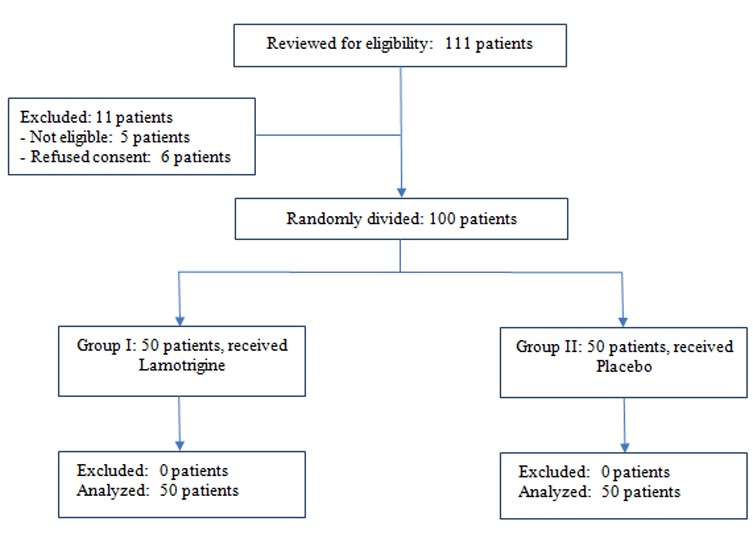
CONSORT flow diagram of the study.
The remaining 100 patients were divided into two groups (n=50). The experimental group received lamotrigine, and the control group received placebo in the preoperative setting one hour before the surgery. The side to undergo extraction first and the drug to be used were randomly chosen by using the relevant website (www.randomization.com) regarding the CONSORT guidelines and flow diagram.[2,28] Figure 1
The patient underwent extraction of the right or left lower third molar, randomly chosen by the research assistant. Then, the patient was orally administered with 200 mg lamotrigine 1 hour before the extraction of the other lower third molar. The control group received 20 cc of water as placebo 1 hour before the surgery. Neither the surgeon nor the patient was aware which drug was administered for which side.
An assistant collected the data regarding the operation sides and drug treatments. The surgical procedures were all performed by the same operator. Intraoral antisepsis was done through vigorous rinsing with aqueous 0.12% chlorhexidine gluconate for 1 minute. The extraoral antisepsis was done with an alcoholic solution of 0.5% chlorhexidine.
The operations underwent 2% lidocaine with epinephrine (1:100,000) local anesthesia. Then, bone removal and tooth section were completed. After impacted tooth extraction, profuse irrigation was done with sterile 0.9% saline solution.[2]
The wounds were closed with interrupted No. 3-0 absorbable surgical sutures. The patients with any postoperative complications such as bleeding, dry, or purulent alveolitis were treated and excluded from the study. Throughout the surgery, the heart rate, blood pressure, and blood oxygen saturation were recorded at specified intervals. By the end of the operation, the analgesics usage according to the patients' self-assessment of pain in the VAS was also registered.
After the surgery, the patients were monitored for up to 4 hours in the recovery room. A blind operator assessed the severity of the postoperative pain by using visual analogue scale (VAS, 100-mm) at 30-minute intervals at rest. VAS is an accepted method for assessment of post-operative pain.[31-32] It is considered as the most valid scale of subjective self-reported pain and probably the most frequently used self-rating instrument for the measurement of pain in clinical and research settings. It can be easily administered by any health professional.[31-33]
The patients were instructed to record the time and number of rescue analgesics (Acetaminophen 500 mg) used within the next 12 postoperative hours. The record of analgesic ingestions could reveal whether any of the treatments provided sufficient analgesia. Moreover, the postoperative complications were asked through questionnaires.
The obtained data were statistically analyzed by using SPSS software. Chi-square, Fisher’s exact test, and Mann-Whitney U test were employed as appropriated. Significance level was set atp < 0.05.
Results
This study was first concerned with the pain intensity over the first 12 postoperative hours, and second with the efficacy, measures included analgesic consumption as well as the safety and tolerability of lamotrigine. One hundred eligible patients completed the study in form of two random intervention groups. Figure 1 illustrates the flowchart of the trail.
The mean age of the studied patients was 25.9±7.5 years, the oldest being 55, and the youngest 14. Regarding the sex, 46% of patients were male and 54% were female, although the study was not looking for sex-based findings. Table 1 shows the demographic characteristics of patients in the two studied groups. Other clinical characteristics including type of impacted tooth, length of operation, duration of anesthesia, surgery duration, and analgesic use are displayed in Table 2.
Table 1.
Demographic characteristics of the patients in lamotrigine group
| Features | Group | p Value | |
|---|---|---|---|
| Lamotrigine N=50 | Placebo N=50 | ||
| Sex | |||
| Male | 24 (48) | 22 (44) | 0.688† |
| Female | 26 (52) | 28 (56) | |
| Age (years) | 24.3±6.1 | 27.5±8.4 | 0.033* |
| Weight (kg) | 66.1±15.7 | 65.8±12.4 | 0.922* |
| Height (cm) | 167.1±9.5 | 167.7±9.3 | 0.767* |
| BMI (kg/m2) | 23.4±4.2 | 23.2±3.0 | 0.784* |
Data are mean ± SD and number (%).
p Values calculated by using †Chi-square test or *Independent sample t-test
Table 2.
Comparison of clinical characteristics and postoperative outcome after impacted third molar surgery
| Features | Group | p Value | |
|---|---|---|---|
| Lamotrigine N=50 | Placebo N=50 | ||
| Type of impacted tooth | |||
| Mesioangular | 30 (60) | 23 (46) | 0.197† |
| Vertical | 8 (16) | 11 (22) | |
| Distoangular | 2 (4) | 0 | |
| Horizontal | 10 (20) | 16 (32) | |
| Length of operation (min) | 30 [15.0-41.25] | 30 [30.0-41.25] | 0.349* |
| Duration of local anesthesia (hr) | 2 [1.5-2.81] | 2 [1.5-2.81] | 0.975* |
| Surgery duration (hr) | 2 [1.0-2.5] | 2.25 [1.5-3.0] | 0.092* |
| Pain (VAS) | 0.512† | ||
| Mild | 13 (26) | 17 (34) | |
| Moderate | 3 (6) | 5 (10) | |
| Severe | 34 (68) | 28 (56) | |
| Analgesic use | 40 (80) | 50 (100) | 0.001 |
Data are mean ± SD and number (%).
p Values calculated by using †Chi square or Fisher exact test and *Mann-Whitney U test.
The outcome of VAS was subjected to Chi-square test to find out the difference between the groups. The results indicated that the two groups were not statistically significantly different in terms of the severity of postoperative pain. (p= 0.512)
Discussion
Acute post-surgical pain is a complicated physiological reaction to the tissue damage. This pain is concerned since it may intensify patient’s discomfort and moreover, it may transform into chronic pain by triggering the peripheral and central pain pathways.[9-35] Pre-emptive analgesia is an antinociceptive therapy that begins before surgery and precludes establishment of transformed processing of afferent input after incisional and inflammatory injuries, which amplifies postoperative pain.[5]
Previous studies have reported the significance of the pre-emptive effect of some medications such as opioids, local anaesthetics, intravenous anaesthetics, and cyclooxygenase inhibitors in postsurgical pain management.[10,35-36] Surgical interventions, even skin incisions, may lead to this initial sensitization. These studies scrutinized the idea that analgesia given before an initial injury may be more effective than the same dose administered after intervention.[9-10,35]
There has also been discussion on the efficacy of lamotrigine as a pre-emptive analgesic to reduce postsurgical pain.[19] Moreover, the intensity of acute postoperative pain was reported to be associated with the development of chronic pain. More recently, it has been shown that neuronal alpha-4-beta2-nicotinic acetylcholine receptors may be a target for lamotrigine, which may regulate its antiepileptic effects.[12,16,18,24]
McCleane et al.[16] reported that treatment of neuropathic pains could benefit from agents that block sodium channels. Likewise, Bonicalzi et al.[19] discussed the effect of lamotrigine as a preemptive analgesic on decreasing the postoperative pain. Systematic reviews performed by Wiffen et al.[12,16,24] reported that Bonicalzi et al. introduced the use of lamotrigine for acute pain, however, in their study all patients were given buprenorphine, a potent analgesic.[19] Seven other research studied the central post stroke pain, diabetic neuropathy, HIV related neuropathy, intractable neuropathic pain,[26] spinal cord injury related pain and trigeminal neuralgia.[37]
In the study of McCleane,[26] use of lamotrigine 200 mg in patients with symptoms of shooting/lancinating pain, burning, numbness, allodynia and paraesthesia/dysesthesia were examined but no useful analgesic benefit could be achieved. Although a reduction in the overall pain score of 1 mm was reported, but it was not statistically significant.
In a placebo-controlled trial, Zakrzewska et al.[37] noted that lamotrigine helped the treatment of refractory trigeminal neuralgia.Fourteen participants recruited in their crossover study comparing lamotrigine with placebo in two two-week phases with a three-day long washout. Lamotrigine showed to be slightly more effective than placebo in this study with limited cases and statistical analysis achieved a hardly significant power.[12]
As mentioned earlier, lamotrigine has been studied in the past for management of painful neuropathic conditions with only few establishing their efficacy in trigeminal neuralgia. In addition, this drug has negligible effects on hematological and biochemical indices;[38] does not induce hepatic enzyme, has very slight drug interactions and protein binding which makes them proper for use in older patients.[38] Likewise, in comparison to other drugs, this medication requires only twice daily prescription, which consequently increases the patient’s compliance. The adverse-effect reported this medication has also been recognized to be acceptable.[38]
The systematic reviews show that there are not consistently regarding the adverse effects of lamotrigine.[12,16] They stated that the incidence of mild and severe adverse effects could not be possibly determined. Lamotrigine can produce rash with the incidence of approximately 7%. Severe possible life-threatening rashes such as Stevens Johnson Syndrome are appraised to occur at an incidence of one in 1000.[12]
Other significant symptoms included drowsiness, headache, and insomnia, which were not more than the control group.[12]
The present investigation was focused on the efficacy of lamotrigine monotherapy in postoperative pain management after extraction of the mandibular third molar since there is little or no study on the use of lamotrigine and similar drugs as pre-emptive agents in acute pain, or other forms of chronic pain particularly after oral and dental surgeries. The enrolled systematic reviews did not recruit any studies regarding the efficacy of this drug in management of pains after orodental surgeries. There have not yet been any studies evaluating the clinical pain control and postoperative benefits of lamotrigine when used as pre-emptive analgesia after third molar surgical extraction. The literature seems to lack results from studies on acute dental pains whilst this pain is recognized as one of the most annoying post-surgical conditions in general and third molar extraction in particular, for the control of which, various medicines are used.[1,21] Management of surgical patients is challenging in several aspects, one of which is postoperative pain control. The pain experienced within the first 24 hours after the surgery is called the immediate postoperative pain; it demands help from the physicians. Meanwhile, the attempts to provide satisfying postoperative analgesia might fail due to several reasons including inadequate knowledge, poor pain evaluation, limited staffs, and worrying about the complications of analgesic agents.[2,21] According to Leach et al.[23] by decreasing the severity of postoperative pain, lamotrigine might also decrease the upcoming adverse events.
Although some uncontrolled clinical trials with small sample sizes claimed lamotrigine to be helpful in treating pain in normal individuals or those with neuropathic syndromes, there are reports on lack of benefit or inconsistent benefit to this agent.[26-27,39]
Aiming to investigate the advantages of lamotrigine in neuropathic pains, consequent systematic reviews of all the placebo-controlled found no evidence to confirm the efficacy of lamotrigine as a therapy for pain syndromes up to date of their study.[12,16,24]
The results of the current study indicated that the difference between study and control group was not statistically significant considering the severity of acute postoperative pain which confirms and adds to the results yielded by Wiffen et al.[12] that lamotrigine does not have a significant place in therapy based on available evidence.
Nevertheless, more studies with larger samples of study and more sophisticated pain assessments need to be conducted to oppose or strengthen the findings of our study.
Conclusion
Within the limitations of a small sample size and the subjective nature of the assessments, the current results revealed that lamotrigine was not effective more than placebo in controlling the postoperative pain of third molar surgery.
Acknowledgement
This study was financially supported by the Research Vice-chancellor of Shiraz University of Medical Sciences, Shiraz, Iran (Grant#94-01-03-10165). Appreciations are also expressed to the maxillofacial nursing staff in the School of Dentistry for their cooperation.
Conflict of Interest:None declared.
References
- 1.Bui CH, Seldin EB, Dodson TB. Types, frequencies, and risk factors for complications after third molar extraction. J Oral Maxillofac Surg. 2003; 61: 1379–1389. doi: 10.1016/j.joms.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Silva de Oliveira JC, Grossi de Oliveira GA, Bassi AP. Comparative Assessment of the Effect of Ibuprofen and Etodolac on Edema, Trismus, and Pain in Lower Third Molar Surgery: A Randomized Clinical Trial. J Oral Maxillofac Surg. 2016; 74: 1524–1530. doi: 10.1016/j.joms.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006; 10: 287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell B, Max RKP, Laska EM. The design of analgesic clinical trials (Advances in pain research and therapy) 1th ed. Lippincott Williams & Wilkins: New York: Raven Press; 1991. p. 18. [Google Scholar]
- 5.Kissin I. Preemptive analgesia. Anesthesiology. 2000; 93: 1138–1143. doi: 10.1097/00000542-200010000-00040. [DOI] [PubMed] [Google Scholar]
- 6.Shah P, Bhosale UA, Gupta A, Yegnanarayan R, Sardesai S. A Randomized Double-Blind Placebo-Controlled Study to Compare PreemptiveAnalgesic Efficacy of Novel Antiepileptic Agent Lamotrigine in Patients Undergoing Major Surgeries. N Am J Med Sci. 2016; 8: 93–99. doi: 10.4103/1947-2714.177315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werner MU, Søholm L, Rotbøll-Nielsen P, Kehlet H. Does an acute pain service improve postoperative outcome? Anesth Analg. 2002; 95: 1361–1372. doi: 10.1097/00000539-200211000-00049. [DOI] [PubMed] [Google Scholar]
- 8.Shah P, Bhosale UA, Gupta A, Yegnanarayan R, Sardesai S. A Randomized Double-Blind Placebo-Controlled Study to Compare PreemptiveAnalgesic Efficacy of Novel Antiepileptic Agent Lamotrigine in Patients Undergoing Major Surgeries. N Am J Med Sci. 2016; 8: 93–99. doi: 10.4103/1947-2714.177315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl JB, Møiniche S. Pre-emptive analgesia. Br Med Bull. 2004; 71: 13–27. doi: 10.1093/bmb/ldh030. [DOI] [PubMed] [Google Scholar]
- 10.Kashefi P, Honarmand A, Safavi M. Effects of preemptive analgesia with celecoxib or acetaminophen on postoperative pain relief following lower extremity orthopedic surgery. Adv Biomed Res. 2012;1:66. doi: 10.4103/2277-9175.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woolf CJ, Chong MS. Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993; 77: 362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 12.Wiffen PJ, Derry S, Moore RA. Lamotrigine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2013; 12: CD006044. doi: 10.1002/14651858.CD006044.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011; 3: CD007938. doi: 10.1002/14651858.CD007938.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009; 3: CD007076. doi: 10.1002/14651858.CD007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCleane GJ. Lamotrigine in the management of neuropathic pain: a review of the literature. Clin J Pain. 2000; 16: 321–326. doi: 10.1097/00002508-200012000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Wiffen PJ, Rees J. Lamotrigine for acute and chronic pain. Cochrane Database Syst Rev. 2007; 2: CD006044. doi: 10.1002/14651858.CD006044.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Lees G, Leach MJ. Studies on the mechanism of action of the novel anticonvulsant lamotrigine (Lamictal) using primary neurological cultures from rat cortex. Brain Res. 1993; 612: 190–199. doi: 10.1016/0006-8993(93)91660-k. [DOI] [PubMed] [Google Scholar]
- 18.Zheng C, Yang K, Liu Q, Wang MY, Shen J, Vallés AS, et al. The anticonvulsive drug lamotrigine blocks neuronal {alpha}4{beta}2 nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2010; 335: 401–408. doi: 10.1124/jpet.110.171108. [DOI] [PubMed] [Google Scholar]
- 19.Bonicalzi V, Canavero S, Cerutti F, Piazza M, Clemente M, Chió A. Lamotrigine reduces total postoperative analgesic requirement: a randomized double-blind, placebo-controlled pilot study. Surgery. 1997; 122: 567–570. doi: 10.1016/s0039-6060(97)90129-x. [DOI] [PubMed] [Google Scholar]
- 20.Webb J, Kamali F. Analgesic effects of lamotrigine and phenytoin on cold-induced pain: a crossover placebo-controlled study in healthy volunteers. Pain. 1998; 76: 357–363. doi: 10.1016/S0304-3959(98)00068-2. [DOI] [PubMed] [Google Scholar]
- 21.Comfort MB, Tse AS, Tsang AC, McGrath C. A study of the comparative efficacy of three common analgesics in the control of painafter third molar surgery under local anaesthesia. Aust Dent J. 2002; 47: 327–330. doi: 10.1111/j.1834-7819.2002.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheung H, Kamp D, Harris E. An in vitro investigation of the action of lamotrigine on neuronal voltage-activatedsodium channels. Epilepsy Res. 1992; 13: 107–112. doi: 10.1016/0920-1211(92)90065-2. [DOI] [PubMed] [Google Scholar]
- 23.Leach MJ, Marden CM, Miller AA. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action. Epilepsia 1986; 27: 490–497. doi: 10.1111/j.1528-1157.1986.tb03573.x. [DOI] [PubMed] [Google Scholar]
- 24.Wiffen PJ, Derry S, Moore RA. Lamotrigine for acute and chronic pain. Cochrane Database Syst Rev. 2011; 2: CD006044. doi: 10.1002/14651858.CD006044.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devulder JE. Lamotrigine in refractory cancer pain. A case report. J Clin Anesth 2000; 12: 574–575. doi: 10.1016/s0952-8180(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 26.McCleane G. 200 mg daily of lamotrigine has no analgesic effect in neuropathic pain: a randomised, double-blind, placebo controlled trial. Pain. 1999; 83: 105–107. doi: 10.1016/s0304-3959(99)00095-0. [DOI] [PubMed] [Google Scholar]
- 27.Klamt JG, Posner J. Effects of lamotrigine on pain-induced chemo-somatosensory evoked potentials. Anaesthesia. 1999; 54: 774–777. doi: 10.1046/j.1365-2044.1999.00959.x. [DOI] [PubMed] [Google Scholar]
- 28.Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010; 340: c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santosh P. Impacted Mandibular Third Molars: Review of Literature and a Proposal of a Combined Clinical and Radiological Classification. Ann Med Health Sci Res. 2015; 5: 229–234. doi: 10.4103/2141-9248.160177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myles PS, Troedel S, Boquest M, Reeves M. The pain visual analog scale: is it linear or nonlinear? Anesth Analg. 1999; 89: 1517–1520. doi: 10.1097/00000539-199912000-00038. [DOI] [PubMed] [Google Scholar]
- 31.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986; 27: 117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 32.Morone NE, Weiner DK. Pain as the fifth vital sign: exposing the vital need for pain education. Clin Ther. 2013; 35: 1728–1732. doi: 10.1016/j.clinthera.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell JN. APS 1995 Presidential address. Pain Forum. 1996; 5:85–88. [Google Scholar]
- 34.Taleska G, Trajkovska T, Kokareva A, Popovska A, Naumoska M, Gavrilovska A, et al. Preemptive Epidural Analgesia with Bupivacaine and Sufentanyl and the Effects of Epiduraly Added Epinephrine for Thoracic Surgery. Maced J Med Sci. 2010; 3: 46–53. [Google Scholar]
- 35.Gupta A, Bhosale UA, Shah P, Yegnanarayan R, Sardesai S. Comparative Pre-Emptive Analgesic Efficacy Study of Novel Antiepileptic AgentsLamotrigine and Topiramate in Patients Undergoing Major Surgeries at a Tertiary CareHospital: A Randomized Double Blind Clinical Trial. Ann Neurosci. 2016; 23: 162–170. doi: 10.1159/000449182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canbay O, Karakas O, Celebi N, Peker L, Coskun F, Aypar U. The preemptive use of diclofenac sodium in combination with ketamine and remifentanil does not enhance postoperative analgesia after laparoscopic gynecological procedures. Saudi Med J. 2006;27: 642–645. [PubMed] [Google Scholar]
- 37.Zakrzewska JM, Chaudhry Z, Nurmikko TJ, Patton DW, Mullens EL. Lamotrigine (lamictal) in refractory trigeminal neuralgia: results from a double-blindplacebo controlled crossover trial. Pain. 1997;73: 223–230. doi: 10.1016/S0304-3959(97)00104-8. [DOI] [PubMed] [Google Scholar]
- 38.Rustagi A, Roychoudhury A, Bhutia O, Trikha A, Srivastava MV. Lamotrigine Versus Pregabalin in the Management of Refractory Trigeminal Neuralgia: A Randomized Open Label Crossover Trial. J Maxillofac Oral Surg. 2014;13: 409–418. doi: 10.1007/s12663-013-0513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vinik Al, Tuchman M, Safirstein B, Corder c, Kirby L, Wilks K, et al. Lamotrigine for treatment of pain associated with diabetic neuropathy: results of two randomized, double-blind, placebo-controlled studies. Pain. 2007;128: 169–179. doi: 10.1016/j.pain.2006.09.040. [DOI] [PubMed] [Google Scholar]


