Summary
Due to its lack of both innate and acquired immune responses to human cells, the NODSCIDIl2rγ−/− (NSG) mouse model has become an important tool for human stem cell research. When compared with the mouse, the rat is physiologically more similar to humans and offers advantages in preclinical efficacy studies on human stem cells, particularly in evaluating neural, hepatic, and cardiac functions. Therefore, we generated a human SIRPα+Prdkc−/−Il2rγ−/− rat model, denoted NSG-like (NSGL) rat, which expresses human SIRPα and is abolished in the development of B, T, and natural killer cells. When compared with Prdkc−/−Il2rγ−/− (SG) rats, NSGL rats allow more efficient engraftment of human cancer cells and human pluripotent stem cells. In addition, only NSGL rats, but not SG rats, can be engrafted with human hematopoietic stem cells to reconstitute the human immune system. Therefore, NSGL rats represent an improved xenotransplantation model for efficacy studies of human stem cells.
Keywords: immunodeficient rat models, innate immunity, acquired immunity, human stem cells, hematopoietic stem cells
Highlights
-
•
Generation of human SIRPα+Prkdc−/−Il2rγ−/− NSG-like (NSGL) rat model
-
•
NSGL rats lack B, T, and NK cells but express human SIRPα
-
•
NSGL rats can be efficiently engrafted with human stem cells
-
•
NSGL rats can be reconstituted by human HSCs to generate a human immune system
Due to the significant difference in physiology between mouse and human, mouse models are not appropriate for preclinical efficacy studies of some human stem cell-based therapies, especially in the context of neural and cardiac functions. To mitigate this challenge, Xu and colleagues established NSG-like rat model that can be efficiently engrafted with human stem cells.
Introduction
Immunodeficient animal models play critical roles in biomedical research, such as in the evaluation of stem cell functions in vivo. Severe combined immunodeficient (SCID) mice lack an adaptive immune response due to deficiencies in genes such as Prdkc (Rongvaux et al., 2014). However, innate immune cells such as natural killer (NK) cells and macrophages can also reject human cells transplanted into mice, and thus significantly reduce the engraftment efficiency of human cells in SCID mice (Strowig et al., 2011, Takenaka et al., 2007, Yamauchi et al., 2013). These bottlenecks are resolved by the findings that NK cells in mice do not develop after the Il2rγ gene is disrupted (DiSanto et al., 1995). In addition, the inhibitory receptor signal regulatory protein alpha (SIRPα), which is primarily expressed on the surface of monocytes and macrophages, is responsible for the macrophage-mediated rejection of xenografts by sensing through the interaction between SIRPα and CD47 (Jaiswal et al., 2009, Oldenborg et al., 2000). Non-obese diabetic (NOD) mice harbor a polymorphism in the Sirpα gene that enhances the binding of mouse SIRPα to human CD47, preventing the macrophage-mediated rejection of human cells in NOD mice (Takenaka et al., 2007). The transgenic expression of human SIRPα gene in SCID mice also improves the engraftment of human stem cells (Strowig et al., 2011, Takenaka et al., 2007, Yamauchi et al., 2013). Therefore, NODSCIDIl2rγ−/− (NSG) mice represent an optimized transplantation model for human stem cell research, especially for human hematopoietic stem cells (HSCs). In this context, NSG mice can be efficiently engrafted with human HSCs to reconstitute the human immune system, providing an important in vivo model to study human immune responses (Koboziev et al., 2015, Zhang et al., 2009). For example, these immune system-humanized mice have been successfully used to study human immune responses to cells derived from human pluripotent stem cells (He et al., 2017, Rong et al., 2014, Zhao et al., 2015).
Compared with the mouse, the rat is metabolically and physiologically more similar to humans and is the preferred species for modeling metabolic diseases and carrying out physiological, pharmacological (including pharmacokinetics and pharmacodynamics), and toxicological studies to provide preclinical efficacy and safety data (Floresco et al., 2005, Gibbs et al., 2004, Martignoni et al., 2006) (Blais et al., 2017, Goutianos et al., 2015). The rat is also the preferred species to evaluate the behavioral, psychological, and cognitive functions in response to drug treatment and stem cell therapy of neurological diseases (Ellenbroek and Youn, 2016, Gibbs et al., 2004, Robbins, 2017). Because of the enormous difference between the heart rates of mice and humans, the mouse model is inappropriate to evaluate the functions of human stem cell-based therapy of heart diseases. Larger animal models with slower heart rates, such as the rat, are more suitable for this purpose (Terrovitis et al., 2010). In support of this notion, rat models have been extensively used to evaluate the efficacy of human stem cell therapy of heart diseases, such as myocardial infarction and heart failure (Terrovitis et al., 2010). Additionally, compared with mice, the body size of the rat is significantly larger, and thus allows more sophisticated surgical procedures for stem cell transplantation and provides more blood and sample volume to evaluate the efficacy of stem cell-based therapy. Therefore, the NSG rat can serve as a better alternative to the NSG mouse for preclinical evaluation of the efficacy of human stem cell-based therapy.
While the genetic manipulation of the mouse has become routine during the past 40 years, the genetic manipulation of rats has been technically challenging, and NSG rats have not yet been reported. Two recent publications described the generation of Prkdc−/− (SCID) and Il2rγ−/− rats, which exhibit immunodeficiency similar to that of their mouse counterparts (Mashimo et al., 2010, Mashimo et al., 2012). However, while the Prkdc−/−Il2rγ−/− (SG) rats lack acquired immunity (B and T cells) and NK cells, they cannot be engrafted with human HSCs due to the macrophage-mediated rejection of human xenografts, as expected from mouse studies (Mashimo et al., 2012). Therefore, it is necessary to generate NSG rats for human stem cell research.
The breakthrough technology of CRISPR (clustered regularly interspaced short palindromic repeat) and Cas (CRISPR-associated) protein (CRISPR/Cas9 system) has enabled the efficient genetic modification of various animal species, including rats. Taking advantage of the CRISPR/Cas9 technology, we disrupted the Prkdc and Il2rγ genes in rats. We further established human SIRPα (hSIRPα) transgenic rats via zygote injection and intercrossed these genetically modified rats to generate hSIRPα+Prkdc−/−Il2rγ−/− rats, denoted NSG-like (NSGL) rats. Compared with SG rats, NSGL rats can be more efficiently engrafted with human cancer cells and human embryonic stem cells (hESCs), but only NSGL rats can be engrafted with human HSCs. Therefore, the NSGL rats will have broad applications in human stem cell research.
Results
Generation and Characterization of SG and NSGL Rats
To generate hSIRPα+ transgenic rats, a 200-kb bacterial artificial chromosome (BAC; RP11-993C19) harboring the entire coding region of human SIRPα (hSIRPα) was linearized and injected into rat zygotes, which were implanted into pseudo-pregnant female rats to generate hSIRPα+ transgenic rats. Flow cytometric analysis demonstrated the co-expression of rat Sirpα (rSirpα) and hSIRPα on the surface of the leukocytes of the hSIRPα+ transgenic mice, confirming the successful generation of hSIRPα+ transgenic mice (Figure S1).
To generate the SCID rats, we employed CRISPR/Cas9 technology to disrupt the rat Prkdc gene. Two guide RNAs (gRNAs) were designed to achieve the disruption of the gene (Figure S2A). The sequencing of F1 rats indicated a deletion of 95 bases in the Prkdc gene (Figure S2B). Compared with wild-type (WT) rats, the percentage of B and T cells was reduced in the Prkdc+/− rats (Figure S2C). The development of B cells was abolished in the Prkdc−/− rats, indicating the successful disruption of the Prkdc gene (Figure S2C). However, there remained a small fraction of CD4+ cells in the Prkdc−/− rats, suggesting a leaky mutation that retained a low level of DNA-dependent protein kinase activity similar to that found in SCID mice (Figures S2C). The percentage of NK cells was increased in Prkdc−/− rats due to the large reduction of B and T cells (Figure S2C).
We used a similar approach to disrupt the Il2rγ gene in the rat, leading to a 662-bp deletion of the Il2rγ gene (Figures S3A and S3B). The disruption of the Il2rγ gene abolished the development of NK and B cells in rats and significantly reduced the number of T cells in the rats (Figure S3C). Therefore, the Il2rγ chain is required for the development of the lymphoid lineages in rats.
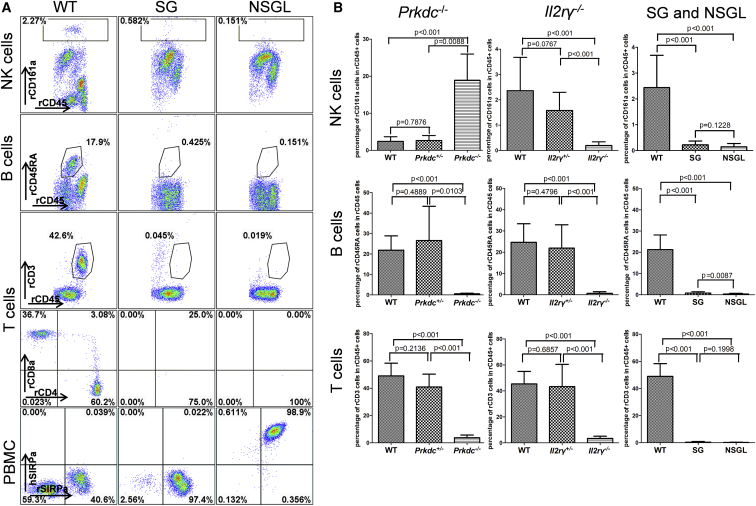
To generate rats that are lacking in acquired immunity and NK cells, we intercrossed Prkdc+/− Il2rγ+/− rats to generate SG rats. While a small fraction of T cells remained in Prkdc−/− and Il2rγ−/− rats, T cells were abolished in SG rats (Figures 1A and 1B). We also introduced the hSIRPα transgene into the SG background through multiple generations of intercrossing to generate NSGL rats, which express the hSIRPα in leukocytes but do not have B, T, and NK cells (Figures 1A and 1B).
Figure 1.
Defective Development of B, T, and NK Cells in Various Genetically Mutant Rats
(A) NK cells, B cells, and T cells are abolished in SG (Prdkc−/−Il2rγ−/−) and NSGL (hSIRPα+Prdkc−/−Il2rγ−/−) rats. In addition, NSGL rats co-expressed human SIRPα and rat SIRPα on the peripheral blood leukocytes. The genotypes are indicated on the top and cell types on the left. The percentage of the gated cells within the CD45+ leukocytes is indicated. PBMC, peripheral blood mononuclear cells.
(B) Statistical analysis of the percentage of NK cells, B cells, and T cells in the CD45+ leukocytes in the peripheral blood of WT (N = 10), SG (N = 14), and NSGL (N = 18) rats. Mean value ± SD are presented. p values are indicated.
NSGL Rats Can Be More Efficiently Engrafted with Human Cancer Cells and hESCs than SG Rats
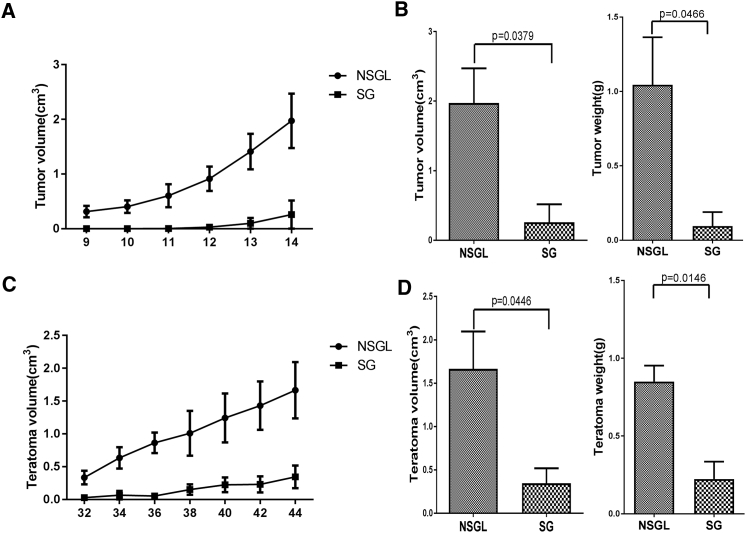
Based on the roles of SIRPα in the macrophage-mediated rejection of human cells, we predicted that NSGL rats could be more efficiently engrafted with human cells. We first compared the engraftment efficiency of human cancer cells (H460) subcutaneously transplanted in SG and NSGL rats. While the implanted NSGL rats rapidly developed tumors after transplantation, the implanted SG rats developed smaller tumors with slower kinetics (Figures 2A and 2B). Therefore, NSGL rats can be more efficiently engrafted with human cancer cells than SG rats.
Figure 2.
NSGL Rats Were More Efficiently Engrafted with Human Cancer Cells and hESCs than SG Rats
Growth curve of tumors formed by human lung cancer cells H460 (A) and teratomas formed by H9 hESCs (C) after subcutaneous injection into the SG and NSGL rats. The tumor volume was plotted against the days after transplantation. N = 3. Mean value ± SD are presented. Statistical analysis of the volume and weight of the tumors formed by H460 cells 2 weeks after transplantation (B) or those of the teratomas formed by hESCs at 6 weeks after transplantation (D) in SG and NSGL rats. N = 3. p values are indicated.
hESCs can spontaneously form teratomas after subcutaneous transplantation in NSG mice (Rong et al., 2012, Rong et al., 2014). Therefore, we used a teratoma formation assay to compare the engraftment efficiency of hESCs in SG and NSGL rats. After subcutaneous transplantation, all implanted NSGL rats developed teratomas (Figures 2C and 2D). However, the implanted SG rats either failed to develop teratomas or developed teratomas at a much slower rate (Figures 2C and 2D). Therefore, NSGL rats can be more efficiently engrafted with hESCs than SG rats.
Engraftment of Human HSCs in SG and NSGL Rats
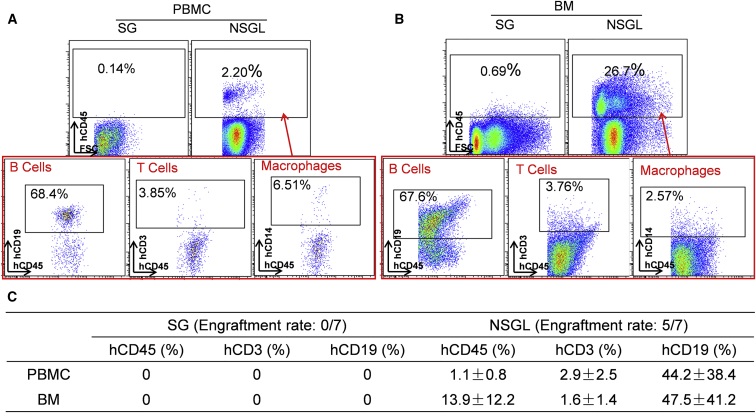
Previous studies have shown that SG mice or rats cannot be engrafted with human HSCs (hHSCs) to reconstitute the human immune system (Mashimo et al., 2012). Therefore, we tested the possibility of engrafting NSGL rats with hHSCs by transfusing CD34+ cells derived from human fetal liver into SG and NSGL rats pre-conditioned with ionizing radiation. Consistent with previous findings (Mashimo et al., 2012), none of the seven analyzed SG rats transplanted with CD34+ hHSCs developed human immune cells up to 10 weeks after implantation (Figure 3C). In contrast, five of the seven NSGL rats that survived the transfusion developed human CD45+ cells in the peripheral blood 5 weeks after transfusion (Figure 3A). There was an efficient reconstitution of CD19+ human B cells in the NSGL rats transfused with human fetal liver CD34+ cells (Figures 3A and 3B). However, there was a lack of human T cells in these rats that was likely due to the absence of human thymus required for efficient human T cell development.
Figure 3.
NSGL Rats Can Be Engrafted with Human Hematopoietic Stem Cells to Develop Multi-lineage hCD45+ Human Leukocytes
(A and B) Human B cells and macrophages, but not human T cells, were readily detected within the PBMCs (A) and bone marrow (BM) (B) of NSGL rats 5 weeks after being transfused with CD34+ human fetal liver cells. The percentage of the hCD45+ cells within the leukocyte population is indicated in the top panel. The percentage of human B cells, T cells, and macrophages within the hCD45+ human leukocyte population is indicated in the bottom panel. PBMC and BM of the WT rats were used as negative controls for immunostaining.
(C) Statistical analysis of the percentage of the hCD45+ cells within all leukocytes as well as the percentage of B cells and T cells within the gated hCD45+ cell population in SG and NSGL rats transplanted with hHSCs. The engraftment rate (the number of rats with human cell engraftment versus the total rats transplanted) is indicated. Mean values ± SD are presented.
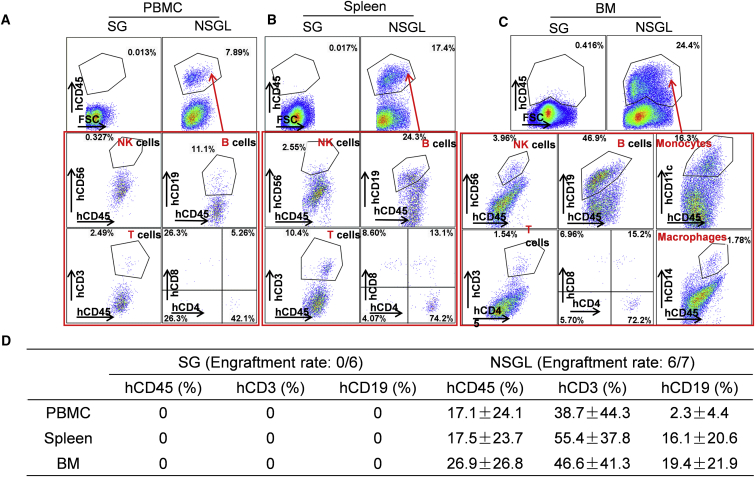
To promote T cell development in NSGL rats engrafted with hHSCs, we transplanted a small piece of autologous human fetal thymus under the renal capsule of pre-conditioned SG and NSGL rats that were simultaneously transfused with hHSCs, as we previously described to establish the mice with a humanized immune system (He et al., 2017, Rong et al., 2014, Zhao et al., 2015). While we could not detect any human CD45+ cells in six SG rats 5 weeks after being transplanted with CD34+ fetal liver cells and fetal thymus, we detected human CD45+ cells in six of the seven surviving NSGL rats 5 weeks after transplantation (Figure 4A). In addition to the reconstitution of human B cells, human T cells were also efficiently reconstituted in the NSGL rats 5 weeks after transplantation (Figures 4A–4D and S4). Human NK cells and macrophages were easily detectable in the bone marrow of the reconstituted NSGL rats (Figure 4C). In summary, in contrast to SG rats, NSGL rats can be engrafted with hHSCs to develop a human immune system.
Figure 4.
The reconstitution of Human Immune System in NSGL Rats Engrafted with CD34+ Fetal Liver Cells and Autologous Fetal Thymus
(A–C) Human B cells, T cells, and NK cells were readily detected in the PBMC (A), spleen (B), and BM (C) of NSGL rats 5 weeks after transplantation. No reconstitution of the immune cells could be detected in SG rats up to 10 weeks after transplantation. The percentage of hCD45+ cells within all leukocytes is indicated in the top panels. The percentage of NK cells, macrophages, B cells, and T cells within the gated hCD45+ human leukocyte population is indicated in the bottom panels.
(D) Statistical analysis of the percentage of the hCD45+ cells within all leukocytes as well as the percentage of B cells and T cells within the gated hCD45+ cell population in SG and NSGL rats engrafted with CD34+ fetal liver cells and autologous fetal thymus. The engraftment rate (the number of rats with human cell engraftment versus the total rats transplanted) is indicated. Mean values ± SD are presented.
Discussion
Immunodeficient animal models have become indispensable transplantation models in stem cell research. Among various experimental animal species, the mouse has become the most extensively used animal species in biomedical research and drug development due to the long-established feasibility of genetically modifying the mouse genome. In this context, mouse ESCs were successfully established more than 40 years ago to allow efficient genetic modification of the mouse genome in vitro (Evans and Kaufman, 1981, Martin, 1981), and the development of chimeric mouse technology has enabled the transmission of the genetic mutations of mouse ESCs into the mouse germline (Wood et al., 1993). Various spontaneous and genetically modified immunodeficient mouse models, including nude mice, SCID mice, NSG mice, and RAG1/2-deficient mice, have been widely used for biomedical research (Rongvaux et al., 2013). These technical advances have been delayed for other animal species, such as the rat and pig, which are evolutionarily and physiologically closer to humans than the mouse (Aitman et al., 2008, Gibbs et al., 2004).
With the accumulating knowledge of the immune responses to human xenografts in mice, it has been well established that both acquired and innate immunity are involved in rejecting human cells transplanted in mice (Strowig et al., 2011, Takenaka et al., 2007, Yamauchi et al., 2013). Several lines of immunodeficient mice (Nude, SCID or Prkdc−/−, RAG1/2 deficient) are defective in acquired immunity (Rongvaux et al., 2013). The innate immunity mediated by NK cells and macrophages is also required for the immune rejection of human cells, especially human stem cells (Ishikawa et al., 2005, Ito et al., 2002, Ito et al., 2012). These problems are mitigated by the abolishment of NK cells in Il2Rγ−/− mice and a mutation in the Sirpα gene of NOD mice that prevents macrophage-mediated rejection of human cells by promoting the interaction between mouse SIRPα and human CD47 (Strowig et al., 2011, Takenaka et al., 2007, Yamauchi et al., 2013). These discoveries led to the generation of NSG mice that are lacking in both innate and acquired immunity. Therefore, NSG mice have become an important transplantation model for human stem cell research (Rongvaux et al., 2013). Considering the significant physiological differences between the mouse and human immune systems, NSG mice reconstituted with a functional human immune system have broad applications in studying human immunity, from infection to cancer and human stem cells (Liu et al., 2017, Rongvaux et al., 2013).
Despite the importance of NSG mice in biomedical research, their rat counterparts represent a better xenotransplantation model to evaluate the neural, cardiac, and hepatic functions of stem cell therapy (Aitman et al., 2008, Pijacka et al., 2016). Therefore, the establishment of the NSGL rat represents a major advance in preclinical efficacy studies of human stem cell-based therapy. In addition, in contrast to SG rats, only NSGL rats can be engrafted with human HSCs to reconstitute a human immune system. Therefore, once the conditions to reconstitute the human immune system in NSGL rats are optimized, we expect that the NSGL rats and immune system-humanized rats will become important new tools for human stem cell research and therapeutic development.
Experimental Procedures
Genetic Manipulation of Rats
We disrupted the Prkdc and Il2rγ genes in Sprague-Dawley (SD) rats by injecting a pair of gRNAs and CAS9 mRNA into rat zygotes, which were then implanted into pseudo-pregnant female SD rats. We generated human SIRPα transgenic rats via zygote injection of BAC DNA. The rat genomic DNA used for genotyping was isolated from a small piece of ear. All animal work was approved by the Institutional Animal Care and Use Committee.
Cell Culture
The H9 hESCs were cultured on a CF1 mouse embryonic fibroblast feeder layer in DMEM/F12 supplemented with 20% knockout serum replacement, 0.1 mM nonessential amino acids, 2 mM Glutamax, 1% penicillin/streptomycin, 10 ng/mL basic fibroblast growth factor, and 110 μM β-mercaptoethanol. H9 hESCs were dissociated with collagenase type IV (1 mg/mL) and passaged on a feeder layer with a 1:4–1:6 dilution. H460 lung cancer cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM Glutamax, 1% penicillin/streptomycin, and 55μM β-mercaptoethanol. All cell culture reagents were purchased from Invitrogen unless indicated otherwise.
Xenotransplantation Studies
Five-week-old male rats were subcutaneously injected with human cells. The length (a) and width (b) of the tumors or teratoma were measured with calipers, using the formula V = ab2/2 to calculate the tumor or teratoma volume (V). Female rats 6–10 weeks old were used for the reconstitution of the immune system by transplanting human HSCs and/or fetal thymus cells. After pre-conditioning with sublethal (250 cGy or 200 cGy, 100 cGy/min) total body ionizing irradiation, SG and NSGL rats were intravenously transfused with 1 × 106 human CD34+ fetal liver cells with or without implantation of human fetal thymic tissue (1 mm3) under the kidney capsule. Human fetal tissues were obtained from Advanced Bioscience Resources (ABR) under informed consent, and their use in this study was approved by ABR as well as University of California, San Diego and Southern Medical University. Human CD34+ cells were isolated by a magnetic-activated cell sorter separation system using anti-CD34 microbeads (Miltenyi Biotec) as we previously described (He et al., 2017, Zhao et al., 2015).
Flow Cytometry Analysis
The single cell suspension of rat tissues was prepared as previously described (He et al., 2017). After the red blood cells were lysed with ammonium-chloride-potassium buffer, the cells were stained with human or rat leukocyte-specific antibodies and analyzed with a BD LSRFortessa cytometer using FlowJo software. The antibodies used were the following: hCD45 (BD Bioscience, 561864), hCD19 (BD Bioscience, 560994), hCD3 (BD Bioscience, 563423), hCD4 (BD Bioscience, 555347), hCD8 (BD Bioscience, 555634), hCD56 (Biolegend, 318332), hCD14 (BD Bioscience, 557831), rCD45 (Biolegend, 202216), rCD45RA (Biolegend, 202318), rCD3 (Biolegend, 201403), rCD4 (Biolegend, 201516), rCD8a (Biolegend, 200610), rCD161a (BD Bioscience, 555009), hSIRPα (Biolegend, 323810), and rSIRPα (Biolegend, 204706).
Statistical Analysis
All statistical analyses were performed using a two-tailed Student's t test with GraphPad Prism 5 software. The mean ± SD and p value are shown. p < 0.05 indicates statistical significance.
Author Contributions
Y.X. designed the research. X.Y., J.H., and J.Z. performed the majority of experiments with the help of J.L., Y.L., H.W., T.J., and Q.Z. X.Y., J.H., X.F., and Y.X. interpreted the data. X.F. and Y.X. provided the administrative support. X.Y. and Y.X. were responsible for the initial draft of the manuscript, whereas other authors contributed to the final version.
Acknowledgments
We thank Dr. Zhili Rong for technical advice. This study was supported by the National Natural Science Foundation of China (nos. 815300045, 81373166, 81430032, and U1601222), a grant from the National High-tech R&D Program (863 Program no. 2015AA020310), the leading talents of Guangdong Province Program (no. 00201516), the Guangdong Provincial Key Laboratory of Tumor Immunotherapy, the Guangzhou Key Laboratory of Tumor Immunology Research, the South Wisdom Valley Innovative Research Team Program (2014) no. 365, the Guangdong Province Key Special Science and Technology Project (2015B020225004), and a major basic research developmental project of the Natural Science Foundation of Guangdong Province.
Published: July 5, 2018
Footnotes
Supplemental Information includes four figures and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.06.004.
Contributor Information
Xuemei Fu, Email: fxmzj2004@163.com.
Yang Xu, Email: yangxu@ucsd.edu.
Supplemental Information
References
- Aitman T.J., Critser J.K., Cuppen E., Dominiczak A., Fernandez-Suarez X.M., Flint J., Gauguier D., Geurts A.M., Gould M., Harris P.C. Progress and prospects in rat genetics: a community view. Nat. Genet. 2008;40:516–522. doi: 10.1038/ng.147. [DOI] [PubMed] [Google Scholar]
- Blais E.M., Rawls K.D., Dougherty B.V., Li Z.I., Kolling G.L., Ye P., Wallqvist A., Papin J.A. Reconciled rat and human metabolic networks for comparative toxicogenomics and biomarker predictions. Nat. Commun. 2017;8:14250. doi: 10.1038/ncomms14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSanto J.P., Muller W., Guy-Grand D., Fischer A., Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek B., Youn J. Rodent models in neuroscience research: is it a rat race? Dis. Model. Mech. 2016;9:1079. doi: 10.1242/dmm.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Floresco S.B., Geyer M.A., Gold L.H., Grace A.A. Developing predictive animal models and establishing a preclinical trials network for assessing treatment effects on cognition in schizophrenia. Schizophr. Bull. 2005;31:888–894. doi: 10.1093/schbul/sbi041. [DOI] [PubMed] [Google Scholar]
- Gibbs R.A., Weinstock G.M., Metzker M.L., Muzny D.M., Sodergren E.J., Scherer S., Scott G., Steffen D., Worley K.C., Burch P.E. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- Goutianos G., Tzioura A., Kyparos A., Paschalis V., Margaritelis N.V., Veskoukis A.S., Zafeiridis A., Dipla K., Nikolaidis M.G., Vrabas I.S. The rat adequately reflects human responses to exercise in blood biochemical profile: a comparative study. Physiol. Rep. 2015;3 doi: 10.14814/phy2.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Rong Z., Fu X., Xu Y. A safety checkpoint to eliminate cancer risk of the immune evasive cells derived from human embryonic stem cells. Stem Cells. 2017;35:1154–1161. doi: 10.1002/stem.2568. [DOI] [PubMed] [Google Scholar]
- Ishikawa F., Yasukawa M., Lyons B., Yoshida S., Miyamoto T., Yoshimoto G., Watanabe T., Akashi K., Shultz L.D., Harada M. Development of functional human blood and immune systems in NOD/SCID/Il2 receptor gamma chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K., Ueyama Y., Koyanagi Y., Sugamura K., Tsuji K. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- Ito R., Takahashi T., Katano I., Ito M. Current advances in humanized mouse models. Cell. Mol. Immunol. 2012;9:208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Jamieson C.H.M., Pang W.W., Park C.Y., Chao M.P., Majeti R., Traver D., van Rooijen N., Weissman I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koboziev I., Jones-Hall Y., Valentine J.F., Reinoso Webb C., Furr K.L., Grisham M.B. Use of humanized mice to study the pathogenesis of autoimmune and inflammatory diseases. Inflamm. Bowel Dis. 2015;21:1652–1673. doi: 10.1097/MIB.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Li W., Fu X., Xu Y. The immunogenicity and immune tolerance of pluripotent stem cell derivatives. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni M., Groothuis G.M.M., de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T., Takizawa A., Kobayashi J., Kunihiro Y., Yoshimi K., Ishida S., Tanabe K., Yanagi A., Tachibana A., Hirose J. Generation and characterization of severe combined immunodeficiency rats. Cell Rep. 2012;2:685–694. doi: 10.1016/j.celrep.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Mashimo T., Takizawa A., Voigt B., Yoshimi K., Hiai H., Kuramoto T., Serikawa T. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PLoS One. 2010;5:e8870. doi: 10.1371/journal.pone.0008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg P.A., Zheleznyak A., Fang Y.F., Lagenaur C.F., Gresham H.D., Lindberg F.P. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- Pijacka W., McBryde F.D., Marvar P.J., Lincevicius G.S., Abdala A.P.L., Woodward L., Li D., Paterson D.J., Paton J.F.R. Carotid sinus denervation ameliorates renovascular hypertension in adult Wistar rats. J. Physiol. 2016;594:6255–6266. doi: 10.1113/JP272708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T.W. Cross-species studies of cognition relevant to drug discovery: a translational approach. Br. J. Pharmacol. 2017;174:3191–3199. doi: 10.1111/bph.13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Z.L., Fu X.M., Wang M.Y., Xu Y. A scalable approach to prevent teratoma formation of human embryonic stem cells. J. Biol. Chem. 2012;287:32338–32345. doi: 10.1074/jbc.M112.383810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Z.L., Wang M.Y., Hu Z., Stradner M., Zhu S.Y., Kong H.J., Yi H.F., Goldrath A., Yang Y.G., Xu Y. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell. 2014;14:121–130. doi: 10.1016/j.stem.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A., Takizawa H., Strowig T., Willinger T., Eynon E.E., Flavell R.A., Manz M.G. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu. Rev. Immunol. 2013;31:635–674. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A., Willinger T., Martinek J., Strowig T., Gearty S.V., Teichmann L.L., Saito Y., Marches F., Halene S., Palucka A.K. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T., Rongvaux A., Rathinam C., Takizawa H., Borsotti C., Philbrick W., Eynon E.E., Manz M.G., Flavell R.A. Transgenic expression of human signal regulatory protein alpha in Rag2(-/-)gamma(-/-)(c) mice improves engraftment of human hematopoietic cells in humanized mice. Proc. Natl. Acad. Sci. USA. 2011;108:13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K., Prasolava T.K., Wang J.C., Mortin-Toth S.M., Khalouei S., Gan O.I., Dick J.E., Danska J.S. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- Terrovitis J.V., Smith R.R., Marbán E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ. Res. 2010;106:479. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.A., Allen N.D., Rossant J., Auerbach A., Nagy A. Non-injection methods for the production of embryonic stem cell-embryo chimaeras. Nature. 1993;365:87–89. doi: 10.1038/365087a0. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Takenaka K., Urata S., Shima T., Kikushige Y., Iwamoto C., Miyamoto T., Akashi K. Polymorphic SIRPA is the genetic determinant for nod-based mouse lines to achieve efficient human cell engraftment. Exp. Hematol. 2013;41:S34. doi: 10.1182/blood-2012-06-440354. [DOI] [PubMed] [Google Scholar]
- Zhang B.J., Duan Z.Y., Zhao Y. Mouse models with human immunity and their application in biomedical research. J. Cell. Mol. Med. 2009;13:1043–1058. doi: 10.1111/j.1582-4934.2008.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Zhang Z.N., Westenskow P.D., Todorova D., Hu Z., Lin T., Rong Z., Kim J., He J., Wang M. Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell. 2015;17:353–359. doi: 10.1016/j.stem.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.