Abstract
G protein-coupled receptors (GPCRs) are the most abundant receptor family encoded by the human genome and are the targets of a high percentage of drugs currently in use or in clinical trials for the treatment of diseases such as diabetes and its associated complications. Thus orphan GPCRs, for which the ligand is unknown, represent an important untapped source of therapeutic potential for the treatment of many diseases. We have identified the previously orphan GPCR, GPR146, as the putative receptor of proinsulin C-peptide, which may prove to be an effective treatment for diabetes-associated complications. For example, we have found a potential role of C-peptide and GPR146 in regulating the function of the retinal pigment epithelium, a monolayer of cells in the retina that serves as part of the blood–retinal barrier and is disrupted in diabetic macular edema. However, C-peptide signaling in this cell type appears to depend at least in part on extracellular glucose concentration and its interaction with insulin. In this review, we discuss the therapeutic potential of orphan GPCRs with a special focus on C-peptide and GPR146, including past and current strategies used to ‘deorphanize’ this diverse family of receptors, past successes, and the inherent difficulties of this process.
Keywords: C-peptide, diabetes, diabetes-associated complications, GPCR, GPR146, orphan GPCR
Orphan G protein-coupled receptors: an untapped source of therapeutic potential
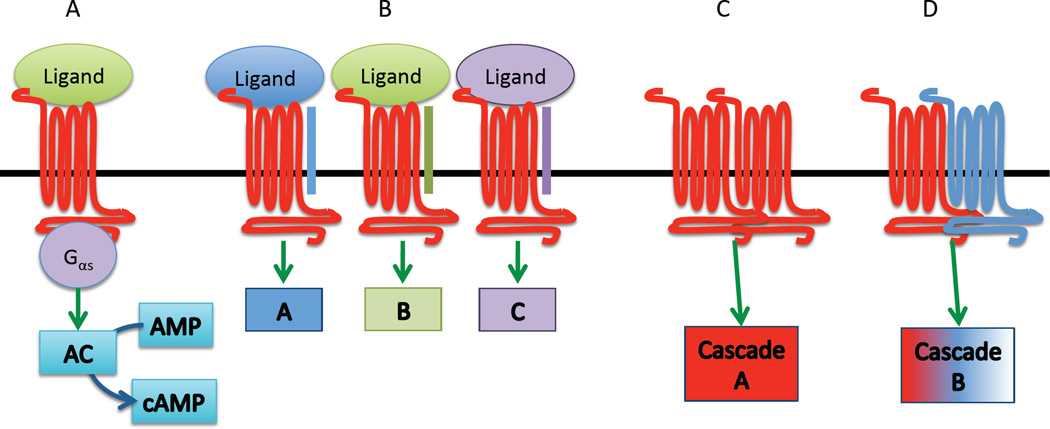
G protein-coupled receptors (GPCRs) represent the largest receptor family encoded by the human genome. Although all GPCRs share core structural features, including seven membrane-spanning domains and conserved residues in the C and N termini, this receptor family is remarkably diverse, particularly with respect to binding partners and functions [1, 2]. Known ligands of GPCRs include peptides, fatty acids, amino acids, steroids, nucleotides, and light, and activation of GPCRs by these ligands leads to a plethora of downstream signaling events initiated by stimulation of the associated G protein(s) subunits. Canonical GPCR signaling includes substitution of a guanosine diphosphate (GDP) for a guanosine triphosphate (GTP) by the alpha G protein subunit, resulting in the dissociation of the alpha subunit from the beta and gamma subunits and from the GPCR. This dissociation allows the alpha subunit to activate secondary signaling messengers, such as adenylyl cyclase, as is the case with Gαs (‘stimulatory’ G alpha protein) (Fig. 1). G alpha proteins possess intrinsic GTPase activity, and hydrolysis of the associated GTP back to GDP inactivates the G protein, wherein it can then re-associate with the beta and gamma subunits and the GPCR.
Fig. 1.
The complexity of G protein-coupled receptor (GPCR) signaling. (A) Canonical GPCR signaling via a stimulatory G alpha protein. (B) Association with receptor activity-modifying proteins (RAMPs) (blue, green, and purple bars) changes the ligand specificity of some GPCRs. Downstream signaling cascades may be altered as well (represented by colored boxes). Some GPCRs form homodimers (C) or heterodimers with other GPCRs (D), which can change ligand and/or downstream signaling molecule specificity.
These classical GPCR signaling mechanisms have been well studied and established for many years. However, it has been increasingly clear that GPCR signaling biology is remarkably more complex than originally thought. For instance, in addition to signaling through the G alpha subunit, the beta and gamma subunits are capable of activating independent signaling cascades [3, 4]. Additionally, beta arrestin, which was traditionally thought of as a scaffolding protein involved in desensitization of GPCRs, is now known to initiate signaling through the MAP kinase pathway [2, 5–7]. It also appears that GPCRs can be quite promiscuous in terms of their binding partners. While it was once thought that one receptor (or family of receptors) responded to only one ligand (or ligand family) (i.e. the so-called ‘one ligand, one receptor’ hypothesis), there have now been several reports of GPCRs that bind to multiple, unrelated ligands as a result of the association between the receptor and an adapter protein, such as a receptor activity-modifying protein (RAMP) [8]. Probably the most studied GPCR that interacts with RAMPs is the calcitonin receptor-like receptor (CLR) [9–13]. The CLR can be activated by several ligands, including calcitonin, alpha- and beta-calcitonin gene-related product (CGRP), amylin, adrenomedullin, and intermedin, depending upon its interaction with RAMPs 1, 2, or 3. GPCRs have been shown to interact with other types of membrane proteins, including integrins and other types of receptors [14]. In particular, GPCRs have been shown to transactivate tyrosine kinase receptors such as the epidermal growth factor receptor (EGFR) by activation of multiple GPCRs, including thrombin receptors, via ligand-dependent (involving matrix metalloproteinases) and ligand-independent mechanisms [15]. Furthermore, GPCRs can form homodimers or heterodimers with other GPCRs, which can have functional implications for receptor specificity and signal transduction [16]. In addition to ligand membrane-binding partner promiscuity, GPCRs are also known to be promiscuous in terms of signal transduction pathways. GPCRs can interact with multiple G protein alpha subunits (e.g. Gαs, Gαi, or Gαq) depending on the external and internal cellular milieu, and exhibit ‘biased agonism’ (i.e. functional selectivity) by which the GPCR ligand directs selectivity toward a specific signaling pathway [17–21]. While these complexities confer remarkable flexibility on GPCR-based signaling systems, they also present difficulties for studying this diverse family of receptors, particularly when the endogenous ligand is unknown.
GPCRs are categorized into five classes based on homology, although it has been suggested that these receptors are more appropriately classified based on ligand type [22]. Regardless of classification methodology, all groups of GPCRs include a subgroup referred to as ‘orphans,’ which are GPCRs that have been identified using bioinformatics or molecular cloning but as yet do not have a known endogenous ligand. Although there is some contention over the exact number of orphan GPCRs, according to the International Union of Basic and Clinical Pharmacology (IUPHAR) there are currently over 70 orphan GPCRs with unknown function or ligand [22]. Considering that 30–50% of drugs currently on the market in the USA target GPCRs [23], orphan GPCRs represent a significant untapped source of therapeutic potential for the treatment of diseases such as diabetes and its associated complications. As a testament to this assertion, several novel therapeutics have been developed in recent years that target orphan GPCRs or recently ‘deorphanized’ GPCRs to treat diabetes and its associated complications.
GPCRs, orphan GPCRs, and diabetes (Table 1)
Table 1.
| GPCR | Targeting compound | Trade name (if applicable) |
Action | Approval status | Reference |
|---|---|---|---|---|---|
| GLP1R | Exenatide | Byetta, Bydureon | Incretin mimetic |
Approved | [114, 115] |
| Liraglutide | Victoza, Saxenda | Incretin mimetic |
Approved | [116] | |
| Albiglutide | Tanzeum | Incretin mimetic |
Approved | [117] | |
| Dulaglutide | Trulicity | Incretin mimetic |
Approved | [118, 119] | |
| Semaglutide | Incretin mimetic |
Phase III | [120, 121] | ||
| Lixisenatide | Lyxumia | Incretin mimetic |
Phase III completed |
[12–124] | |
| Taspoglutide | Incretin mimetic |
Phase III terminated |
[125] | ||
| Insulin degludec and liraglutide | iDeg-Lira | Combination | Phase III | [126] | |
| Insulin glargine and lixisenatide | LixiLan | Combination | Phase III completed |
[123] | |
| Alogliptin | Nesina | DPP4 inhibitor | Approved | [127, 128] | |
| Saxagliptin | Onglyza | DPP4 inhibitor | Approved | [129] | |
| Sitagliptin | Januvia | DPP4 inhibitor | Approved | [130] | |
| Vildagliptin | Galvus | DPP4 inhibitor | Phase III | [131–133] | |
| Linagliptin | Tradjenta | DPP4 inhibitor | Approved | [134] | |
| Gemigliptin | DPP4 inhibitor | Phase III | [135–137] | ||
| GPR40 | Fasiglifam (Tak-875) | GPR 40 agonist | Phase III Terminated |
Reviewed in [138] |
|
| D2R | Bromocriptine | Cycloset | Reduces HbA1C |
[63, 70] | |
| CpepR | C-peptide | Ersatta | CpepR agonist | Phase IIb | [139] |
GPCR, G protein-coupled receptor.
Traditionally, patients with diabetes have been treated with injectable insulin or oral agents. Although insulin therapy has been the mainstay treatment of type 1 diabetes as it lowers blood glucose levels, it must be carefully administered in order to approximate physiological insulin delivery. Though many formulations exist (regular, intermediate-, and long-acting insulin, human and animal origins, etc.), the use of these products requires substantial empirical adjustment. Significant side effects limiting their therapeutic effectiveness can include hypoglycemia, allergic reactions, and antibody-mediated insulin resistance. Oral agents are typically the first-line therapy in patients with type 2 diabetes. These act by either stimulating insulin secretion from the beta cells of the pancreas (sulfonylureas such as glipizide or gliburide), or increasing insulin sensitivity, enhancing glucose uptake, and decreasing gluconeogenesis in peripheral tissues (biguanides). These approaches are not without their complications, however, and can include side effects such as hypoglycemia, abdominal pain, nausea, or diarrhea stemming from their effects on either the endocrine or peripheral tissues.
Targeting GPCRs for the treatment of diabetes and its associated complications poses some definite advantages. In many cases, therapies targeting GPCRs require only once or twice daily dosage regimens, providing a significant advantage for patient compliance. Monotherapy with these agents may have some utility but GPCR-based therapeutics may have a more substantial role as combination therapies with traditional oral agents. Current GPCR-targeting therapies include glucagon-like peptide 1 (GLP-1)-based agonists, the related DPP4 antagonists (both reviewed in [24]), bromocriptine, and serotonin agonists. More recently GPCR40 agonists and selective endothelin-A receptor antagonists have received interest.
The GLP-1 agonists and DPP4 inhibitors are the most well developed of the therapies for diabetes based on GPCRs. GLP-1 is an incretin hormone that is released from the gut after a meal and interacts through a GPCR, GLP1R. GLP-1 stimulates insulin secretion, islet proliferation, and cytoprotection while inhibiting glucagon secretion [25, 26]. It was suggested as a therapeutic target in type 2 diabetes in 1992 [27] and the first pharmaceutical agent based on GLP-1 came to market in 2005. GLP-1 has a short half-life of usually 1–2 min that is the result of inactivation by the DPP4 enzyme [28]. Several pharmaceutical strategies have aimed to remove this limitation. A GLP-1-mimetic from the Gila monster called exendin-4 has close to 50% homology and binds the receptor with the same affinity as human GLP-1 [29]. It is resistant to DPP4 and has a half-life of 30 min or 2–3 h depending on the route of administration (intravenous or subcutaneous, respectively). Synthetic analogs (exenatide) have now been developed, and in combination with metformin and sulphonylureas have been shown to lower body weight and HbA1c [30–32]. Alternatively the half-life of the native GLP-1 has been extended through structural modifications, such as amino acid substitutions and C16 fatty acid attachments, to promote binding to serum albumin (e.g., liraglutide) and to produce resistance to DPP4 activity and decreased clearance [33].
Inhibition of DPP4 serves as a therapeutic target, as it can also prolong endogenous GLP-1 concentrations that are typically observed after a meal, and increase fasting levels of GLP-1. Several DPP4 inhibitors have been approved by the FDA including algoliptin (xanthine-based compound), saxagliptin (substrate-like inhibitor), and sitagliptin (non-substrate-like inhibitor) [34–36]. However, both DPP4- and GLP-1-based therapies have been at the center of an academic debate [37–40] over the risk of pancreatitis and pancreatic cancer associated with incretin-based therapies. In 2013, the American Diabetes Association and The Endocrine Society called for efforts to investigate these potential complications [41, 42], though currently European and US federal agencies have not altered recommendations on the use of these agents.
Another potentially promising previously orphan GPCR that is being pursued as a therapeutic target is GPR40. GPR40 is expressed in pancreatic beta cells and is activated by medium- or long-chained free fatty acids to stimulate insulin secretion in a glucose-dependent manner (for reviews see [24, 43]). The secretion of insulin in response to high glucose occurs through two phases: (i) exocytosis of a pool of insulin granules already at the plasma membrane; and (ii) mobilization of intracellular granules to the plasma membrane. This second phase is potentiated by free fatty acids [44]. Half of the activity of free fatty acids can be attributed to GPR40 with the rest being the result of intracellular metabolism to long-chain coenzyme A esters [45, 46]. There is some debate regarding whether GPR40 has any impact on intracellular fuel metabolism [47, 48], probably due to differences in experimental models; nevertheless, it appears to act via a mechanism that is at least partially independent from intracellular fatty acid metabolism. In addition to its role in insulin secretion and hypoglycemic events, GPR40 has also been linked to incretin responses to free fatty acids [49]. A loss of function mutation in the GPR40 gene is found in 0.75% of healthy subjects and is associated with obesity and impaired insulin secretion [50]. A large number of GPR40 agonists are currently being developed. It has become clear in recent years that biased agonism among several GPCRs represents an important design consideration in the development of agonists. Different agonists may bias a receptor to certain activation states that result in the activation of specific signaling pathways and therefore potentially different biological outcomes [20]. This is particularly pertinent to GPR40 which has been coupled to Gαs and Gαq signaling pathways [51] and has been proposed to also activate Gαi in some circumstances [52]. Furthermore in the case of GPR40, as well as other GPCRs, alternative (allosteric) ligand-binding sites, even for the same agonist, exist and can have distinct or modulating effects on the receptor [53]. This has been demonstrated in the case of the most well-developed GPR40-based clinical candidate to date, fasiglifam (TAK-875; Takeda Pharmaceutical Company) [54]. Fasiglifam is an oral agent that enhances glucose-dependent insulin secretion in rat models and improves hyperglycemia [55, 56]. Importantly, there is a low risk of hypoglycemia in these models and no evidence of beta cell toxicity. In a human Phase II clinical trial, monotherapy reduced HbA1c significantly [57]. The Phase III clinical trial of Tak-875 was voluntarily terminated however due to concerns related to liver safety [56]. Considering that GPR40 has not been identified in the liver, it is likely not a direct GPR40 effect. Other agonists are being developed that target different allosteric binding sites on GPR40 in order to take advantage of the incretin-stimulating effects of GPR40 [58, 59].
GPR40 antagonists may eventually also play a role in long-term diabetes management. GPR40 null mice have some degree of protection of beta cells during high-fat diet-induced insulin resistance [60]. Pharmaceutical agents designed to exert antagonistic effects have been reported to reduce beta cell loss but do not impact hyperglycemia [61, 62]. Overall however this type of strategy may help preserve beta cell function in subjects with type 2 diabetes in the long term or serve as a protective strategy in pre-diabetic individuals.
The D2 dopamine receptor is also a GPCR and bromocriptine, a sympatholytic that targets this receptor, has recently been approved for the treatment of type 2 diabetes (reviewed in [63]). It is thought that low hypothalamic dopamine levels and excessive sympathetic tone in the CNS alter the metabolic state resembling changes in mammalian species that undergo hibernation. Such animals switch to a state of insulin resistance in muscle and liver along with accelerated hepatic glucose production and gluconeogenesis, hyperglycemia, adipocyte insulin resistance, increased lipolysis, enhanced fat oxidation with increased plasma free fatty acids and triglycerides as well as obesity [64–67]. Phase II and III clinical trials have shown improvement in levels of HbA1c, plasma triglycerides, and free fatty acids when given either as monotherapy or combination therapies with oral hypoglycemic agents [68–70]. There is also evidence from cardiovascular safety trials that bromocriptine (Cycloset) may improve major adverse cardiovascular outcomes (myocardial infarction, stroke, and death) in patients with type 2 diabetes [70].
Despite these advances in treating diabetes and its complications by targeting GPCRs and orphan GPCRs, diabetes-associated microvascular dysfunction remains an immense clinical challenge. More studies are needed to evaluate the therapeutic potential of additional GPCRs and orphan GPCRs to treat this devastating group of complications. However, the development of novel therapeutic agents based on orphan GPCRs depends heavily on the ability to identify their endogenous ligands, or to deorphanize them.
Deorphanization strategies: past, present, and future
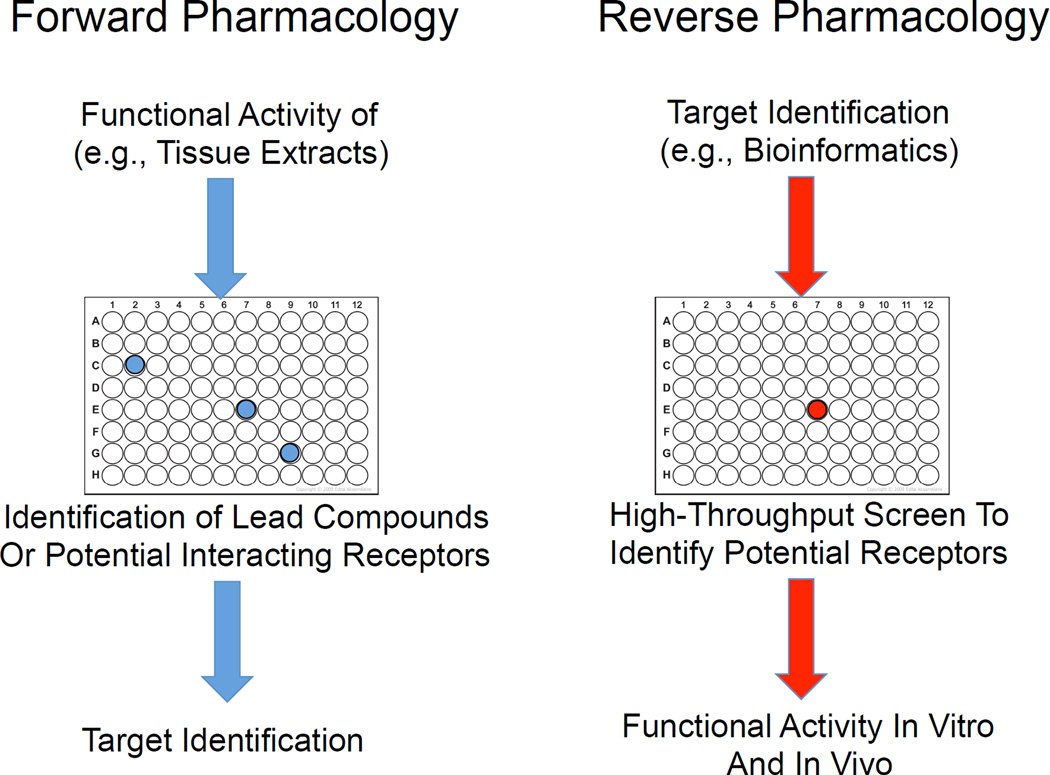
Classically, identification of ligand–receptor pairs depended upon purification of biologically active compounds from large quantities of tissue extracts, which were used to assess activity of cloned receptors overexpressed in null cell lines. With the advent of the Human Genome Project and the formation of readily accessible databases containing the genome sequences of humans and many other species, targeted drug development, or ‘reverse pharmacology’ became possible (Fig. 2) [71, 72]. Using this strategy, a potential drug target is identified based on sequence homology or gene expression, and synthetic ligands replace purified tissue extracts used in traditional or ‘forward pharmacology’. In recent years, reverse pharmacology has been used by the pharmaceutical industry to develop synthetic substances based on natural molecules to treat a variety of conditions. Most recently, companies have turned to computer modeling systems to predict the molecular activity of a drug at a particular receptor, which has allowed more focused in vitro experimentation.
Fig. 2.
Drug target discovery and receptor deorphanization strategies.
Both forward and reverse pharmacology depend upon high-throughput assays, which have been a mainstay of receptor deorphanization for many years. Robotic screens, in which hundreds of receptors are overexpressed with reporter molecules (e.g. SRE-luciferase reporter proteins) in separate cell populations are exposed to potential ligands or drugs, are in use by several pharmaceutical companies. Although undirected, this strategy has had some success in identifying ligand–receptor pairs, particularly when used in combination with reverse pharmacology and PCR-based homology screening technologies. For example, the orexins were identified based on the ability of orexin-containing tissue extracts to stimulate calcium release when applied to separate cell cultures expressing over 50 different GPCRs [71, 72]. The orexins and their receptors are now known to play important roles in sleep and arousal, as well as in metabolism, and orexin receptor-based drugs are currently in development for the treatment of narcolepsy [73–75]. Other examples include ghrelin, which is produced in the gut and stimulates appetite and was found to bind the growth hormone secretogue receptor (GHSR) [76, 77], and the adipokine apelin, which binds the previously orphan receptor APJ and plays important roles in glucose homeostasis as well as exerting cardiac and vascular effects [78].
In spite of these successes, many orphan GPCRs remain. Their deorphanization may be hindered by multiple factors, including technical difficulties inherent in high-throughput assays. For example, the downstream signaling machinery for most orphan receptors is unknown, and thus overexpression in non-native cell lines with engineered reporter proteins could prove detrimental to the activation of these receptors; their use in high-throughput assays could produce habitual false-negative results. Furthermore, some GPCRs require the presence of chaperone proteins for proper folding and trafficking to the cell membrane. Such chaperone proteins could be missing from high-throughput screening cell systems. Lastly, and most importantly, as discussed above, GPCR biology is remarkably more complex than originally realized, and thus any cell system lacking adapter proteins (RAMPs, integrins, etc), accessary receptors (e.g. additional GPCRs with which a receptor forms heterodimers), or intracellular scaffolding proteins necessary for proper signaling would render the affected orphan GPCR inactive in such a system. For these reasons, we have developed a directed ligand–receptor deorphanization strategy that is not dependent upon overexpression of the receptor in a non-native cell system but rather focuses on the pharmacological activity of the receptor in cells in which it is endogenously expressed. Thus far we have utilized this strategy to identify candidate receptors for three ligands [79–81], including a peptide hormone with tremendous potential for the treatment of diabetes-associated complications, proinsulin connecting peptide (C-peptide).
C-peptide and the deductive ligand –receptor matching strategy
Over the course of the last two decades, proinsulin C-peptide has gained recognition as a potential therapeutic target for the treatment of diabetes-associated microvascular dysfunction [82–85]. Although originally thought to be merely a byproduct of insulin prohormone processing, C-peptide is now known to possess biological activities, particularly in the microvessels, and has been shown to exert anti-inflammatory, anti-oxidant, and metabolic actions [83–86]. Additionally, C-peptide may act synergistically with insulin to maintain the vascular endothelium, although the mechanisms underlying this potential interaction have not been fully elucidated [87, 88]. However, in order to fully understand the role of C-peptide in normal physiology and, importantly, in order to develop C-peptide-based therapies, it is essential to identify the C-peptide receptor(s).
Identification of the C-peptide receptor has proven to be a difficult task. Several attempts to purify a membrane-bound receptor, with the use of affinity purification assays and molecular cloning, were unsuccessful. This difficulty could be due to the unique binding dynamics of C-peptide, as radioligand binding experiments revealed that C-peptide binds to human cell membranes likely through an interaction with a receptor complex or signalosome [89–91], which could include a GPCR coupled to an adapter protein, an integrin, a RAMP, or another type of receptor. The absence of these potential interacting proteins in non-native expression systems (i.e. C-peptide receptor ‘null’ cells) could complicate the isolation of the C-peptide receptor(s). Likewise, because the actions of C-peptide appear to be intimately linked with those of insulin [84, 88], C-peptide may exhibit cooperative binding with insulin, and thus the efficient binding and purification of a C-peptide receptor would require the presence of insulin. Therefore, isolation of the C-peptide receptor based on its biochemical and binding properties may not be feasible.
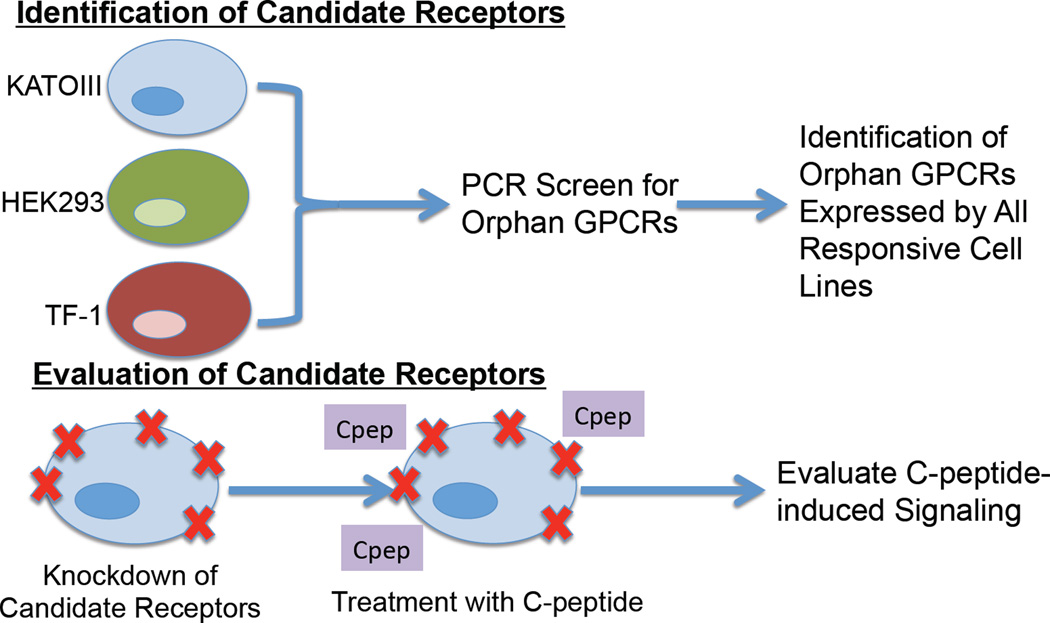
In order to identify a C-peptide receptor, we utilized a deductive ligand–receptor matching strategy developed in our laboratory to determine the receptor for the peptide hormone neuronostatin (Fig. 3) [80, 81]. Instead of depending upon the physical interaction between C-peptide and its receptor or receptor complex, this approach takes advantage of the known pharmacological effects of a ligand in vitro to identify receptor candidates. Briefly, cell lines or tissues known to respond to the peptide of interest (e.g. with changes in cFos mRNA expression or activation of protein kinase A) are screened for the expression of all known orphan GPCRs, as catalogued by IUPHAR [22]. Only orphan GPCRs that are expressed by all responsive cells and/or tissues, of which there are ideally three or more, are considered for further analysis. Next these receptors are subjected to bioinformatics analyses to identify homology with receptors with known ligands and potential expression profiles, which are compared to expected function and distribution based on known ligand actions. These steps are used to generate a short list of candidate receptors that are then knocked down individually using siRNA in a responsive cell line. If knockdown of a candidate receptor disrupts the normal signaling initiated by the ligand of interest, then that receptor is considered the best candidate receptor for the ligand, and is further analyzed using standard biochemical tests, including fluorescence co-localization, co-immunoprecipitation, and radioligand binding analyses.
Fig. 3.
Utilization of the deductive ligand–receptor matching strategy for the identification of C-peptide receptor candidates. C-peptide-responsive cell lines were screened for the expression of orphan G protein-coupled receptors (GPCRs). Orphan GPCRs expressed by all responsive cell lines were then knocked down individually using siRNA in KATOIII cells. Cells were treated with C-peptide, and C-peptide signaling was assessed. Knockdown of GPR146 resulted in loss of C-peptide-induced signaling, and thus was considered the best candidate for the C-peptide receptor.
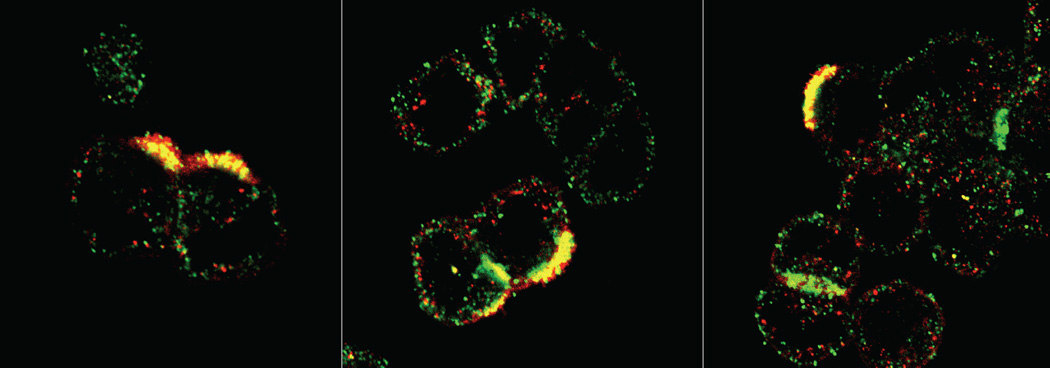
Using this method, we identified GPR146 as the best candidate receptor for proinsulin C-peptide, as knockdown of GPR146 in the human gastric tumor cell line KATOIII resulted in a loss of C-peptide-induced cFos mRNA expression [80]. We further demonstrated that C-peptide and GPR146 co-localized on KATOIII cell membranes (Fig. 4) [80], and that pre-incubation with a neutralizing antibody against GPR146 prevented C-peptide-induced inhibition of ATP release from human erythrocytes [88]. These data strongly suggest that GPR146 is a member of the C-peptide signalosome, perhaps acting as a C-peptide receptor. However, there are multiple limitations to this deorphanization strategy. First, using this strategy assumes that the receptor is a GPCR. While multiple lines of evidence suggest C-peptide interacts with a GPCR to exert its cellular effects [84, 85], other types of membrane receptors, particularly integrins, which have been shown to activate G proteins in some instances [92], could mimic the effects of GPCRs. Secondly, our system excludes GPCRs with known ligands and focuses only on orphan GPCRs as potential candidates. Given that GPCRs can exhibit ligand promiscuity, any potential interaction with a characterized GPCR could have been overlooked. Lastly, our deorphanization strategy does not provide evidence for a physical interaction between ligand and candidate receptor. Future experiments must therefore involve biochemical analyses of the potential physical association between C-peptide and GPR146, including radioligand binding experiments, fluorescence resonance energy transfer (FRET) or bioluminescence resonance energy transfer (BRET) assays, co-immunoprecipitation or pull down experiments, and re-evaluation of fluorescence co-localization using super resolution microscopy. The application of these tools has proven difficult for the study of the interaction of C-peptide with cell surface binding partners, because, in our hands, tagged forms of C-peptide (e.g. with fluorescent labels or biotin), which are necessary for most of the aforementioned techniques, do not exhibit biological activity in that they do not initiate signaling cascades normally observed in our cell systems. Furthermore, C-peptide appears to signal via an interaction with a receptor complex rather than with a signal receptor alone, and these protein–protein interactions appear to be transient or weak. In addition, we have found that some of the effects of C-peptide are dependent upon the glucose concentration of the culture medium and the relative concentration of insulin. These limitations could have important implications for understanding the role of C-peptide in normal physiology and in pathophysiological conditions, such as in diabetes-associated eye diseases.
Fig. 4.
Co-localization of C-peptide and GPR146 on KATOIII cell membranes. KATOIIl cells were co-incubated with 1 nM C-peptide and 1 nM insulin and stained using antibodies against C-peptide (red) and GPR146 (green). Co-localized particles appear yellow. Representative images from three visual fields.
C-peptide and the retinal pigment epithelium: effect of glucose and insulin on C-peptide signaling
Diabetic eye disease remains one of the major causes of visual impairment in the developed world. The etiology of diabetic eye disease is multifactorial and involves multiple pathological processes, including neovascularization and macular edema. Central to the development of diabetic macular edema is the retinal pigment epithelium (RPE), a monolayer of epithelial cells that forms the blood–retinal barrier and has essential metabolic roles in the retina [93]. In diabetes, the tight junctions between RPE cells are compromised, leading to blood–retinal barrier breakdown, leakage of excess fluid into the retina, and macular edema [94]. Although the mechanisms underlying RPE cell dysfunction have not been fully elucidated, over-secretion of vascular endothelial growth factor (VEGF) is thought to be involved, as high levels of retinal VEGF have been associated with the disruption of RPE tight junctions [95].
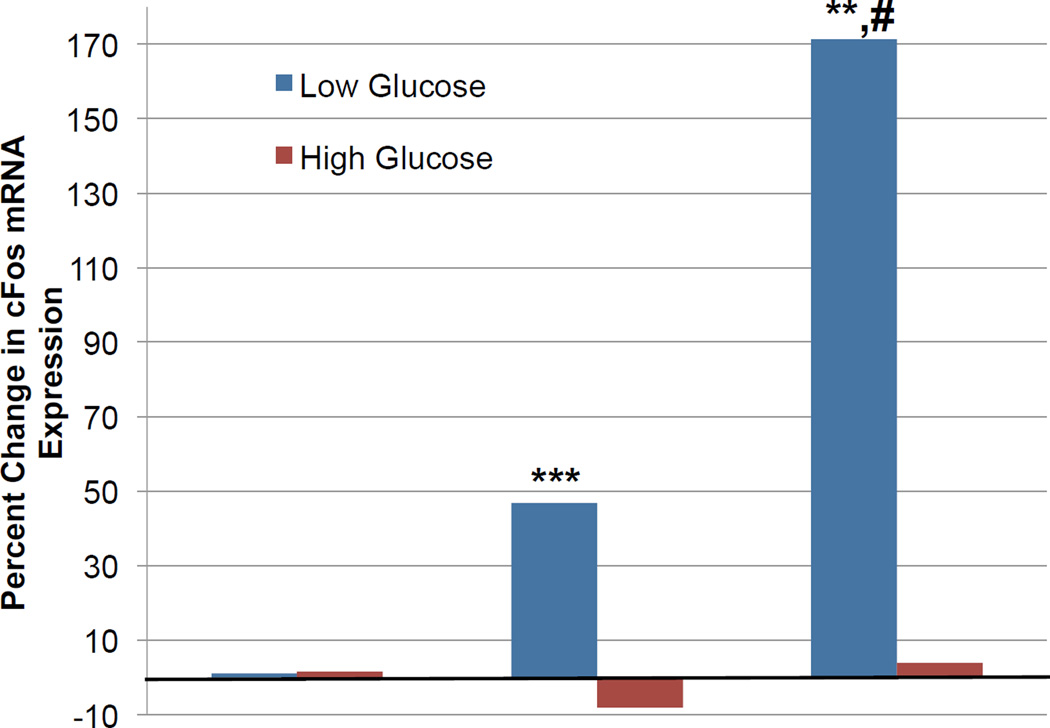
C-peptide has been shown to reverse blood flow abnormalities and protect against VEGF-mediated damage in the retina in the setting of experimentally induced diabetes [96, 97]. These data suggest that C-peptide could be used as an effective agent for the treatment of diabetic eye disease. Indeed, plasma levels of C-peptide were inversely correlated with the incidence of diabetic retinopathy in patients with type 1 diabetes [98–100]. However, in order to specifically evaluate the utility of C-peptide in the treatment of diabetic macular edema, we evaluated its ability to influence RPE cell biology. We found that GPR146 is expressed in the RPE and that C-peptide increases cFos mRNA expression in the human RPE cell line ARPE-19 (Fig. 5), as measured by quantitative PCR (qPCR). Importantly, the effect of C-peptide on cFos mRNA expression appeared to be dependent upon extracellular glucose concentration, as incubation with high glucose (25 mM, equivalent to a blood glucose concentration of approximately 450 mg/dL) for 24 h prior to exposure of ARPE-19 cells to C-peptide inhibited C-peptide-induced cFos mRNA expression. C-peptide has been shown to activate 5' adenosine monophosphate-activated protein kinase (AMPK) in human umbilical vein endothelial cells (HUVECs), and C-peptide-induced inhibition of reactive oxygen species (ROS) was mediated by this activation [101]. We therefore propose that at least some of the effects of C-peptide are dependent upon extracellular glucose concentration due to the interaction between C-peptide-initiated signaling cascades and AMPK activation, as AMPK functions as a metabolic sensor by detecting and reacting to changes in the AMP:ATP ratio within cells [102]. Given that the release of C-peptide from beta cells is tightly regulated by blood glucose levels, we also predict that the dependency of its effects on glucose concentration is not limited to ARPE-19 cells and HUVECs, but rather is a phenomenon common to many if not most C-peptide-responsive cell types.
Fig. 5.
C-peptide-induced cFos mRNA expression in the human retinal pigment epithelial cell line ARPE-19. ARPE-19 cells were incubated in either low (5.5 mM) or high (25 mM) glucose for 24 h prior to exposure to vehicle, 1 nM C-peptide, or 1 nM C-peptide with 1 nM insulin for 1 h. Cells were lysed, RNA isolated, and changes in cFos mRNA expression evaluated using quantitative PCR. **P < 0.01, ***P < 0.001 vs. low glucose, vehicle-treated cells; #P < 0.01 vs. low glucose, C-peptide-treated cells.
Because C-peptide is co-released with insulin in equimolar amounts, and C-peptide and insulin have been shown to initiate similar signaling cascades, including insulin receptor substrate 1 (IRS-1)- and Akt-mediated signaling events [103], it is possible that C-peptide and insulin interact with respect to their cellular activities and functions. Co-exposure of C-peptide and insulin in physiologically relevant ratios appeared to be critical for low oxygen tension-induced ATP release from human erythrocytes [87, 88]. Exposure of erythrocytes to either C-peptide or insulin alone was insufficient to restore ATP release and actually proved detrimental with regard to this function. We therefore tested the potential interaction between C-peptide and insulin in RPE cells. We found that co-treatment with C-peptide and insulin dramatically enhanced cFos mRNA expression in ARPE-19 cells compared to cells incubated with C-peptide alone (Fig. 5) and, importantly, this effect was reversed in the presence of a high glucose concentration (25 mM). Thus, C-peptide and insulin may interact in RPE cells, and this interaction may be important for the functions of both peptides.
However, these observations do not explain how C-peptide and insulin are interacting. Several possibilities exist. C-peptide and insulin could be interacting at the peptide level, perhaps acting as allosteric modulators of each other’s receptors. Alternatively, C-peptide- and insulin-initiated signaling cascades could be interacting intracellularly at the levels of secondary signaling molecules (e.g. Akt). We propose that the putative C-peptide receptor, GPR146, engages in G protein switching, depending in part on the concentration of insulin relative to C-peptide (hypothesis outlined in [84]). In this way, C-peptide, acting through its receptor, could either enhance or dampen insulin signaling through the activation or inhibition of appropriate downstream signaling molecules. This hypothesis could also explain discrepancies regarding the breadth of C-peptide-initiated signaling cascades, which range from the activation of protein kinase C (usually associated with Gαq) to pertussis toxin-sensitive events (indicating involvement of Gαi/o). Regardless, the potential interaction between C-peptide and insulin should be carefully considered when designing experiments to evaluate the biology of either peptide, because this interaction could have important implications for the function of these peptides in the RPE and other cell types as well.
C-peptide and RPE cell transdifferentiation
In addition to its metabolic roles in the retina, the RPE possesses the unique ability to transdifferentiate into other retinal cell types, including photoreceptors and ganglion cells [104–107]. The physiological significance of RPE transdifferentiation has not been fully elucidated, but this ability could be involved in retinal repair, with transdifferentiating RPE cells serving as a ‘stopgap’ when other retinal cell types have been damaged [108]. For example, injury to Bruch’s membrane or the endothelium of the choriocapillaris might in part drive the morphogenesis of RPE cells as a protective mechanism. In diabetes, aberrations in this process, including diminished activity of the Na+/K+ ATPase [109], lead to the transdifferentiation of RPE cells into fibroblasts and myofibroblasts that can form epiretinal membranes that contribute to retinal detachment [110]. The secretion of growth and angiogenic factors from these fibroblasts, including VEGF, could induce further damage to the retina, thus initiating a vicious cycle that spirals toward the progression of diabetic eye disease.
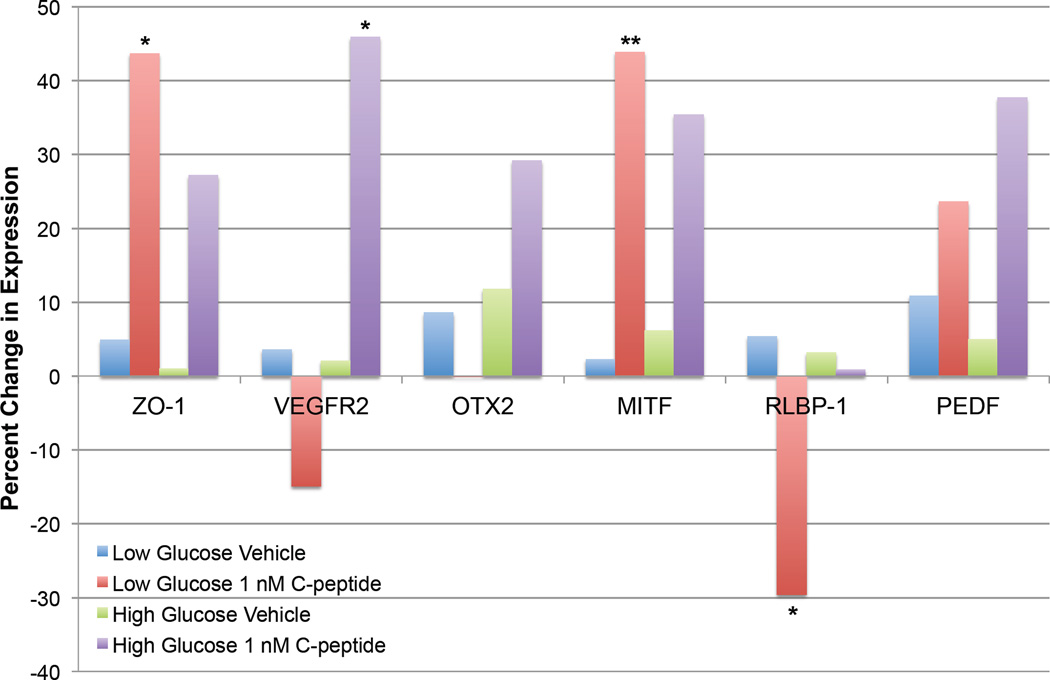
C-peptide has been shown to normalize Na+/K+ ATPase activity in multiple cell types [83, 111], therefore we hypothesized that exposure to C-peptide would prevent high glucose-induced transdifferentiation of ARPE-19 cells to a mesenchymal-like phenotype. To test this hypothesis, ARPE-19 cells were incubated with normal (5.5 mM) or high (25 mM) glucose for 30 days with or without 1 nM C-peptide, with weekly medium replenishment. Changes in expression of RPE-specific genes were evaluated using qPCR (Fig. 6). These experiments yielded two important findings concerning the role of C-peptide in RPE cell biology. First, C-peptide appeared to enhance expression of genes necessary for RPE cell function (Fig. 6), including ZO-1 (zona occludins 1), which encodes a tight junction protein that is essential for the function of the RPE as part of the blood–retinal barrier [112], and MITF (microphthalmia-associated transcription factor), which is a transcription factor that regulates the expression of genes essential for RPE cell identity [113]. Thus, C-peptide may protect against diabetic macular edema at least in part by maintaining RPE cell identity and protecting against RPE cell transdifferentiation. Secondly, in agreement with our previous data showing that C-peptide-induced changes in cFos mRNA expression were dependent upon extracellular glucose concentration (Fig. 6), the effect of C-peptide on gene expression changes were also modulated by the concentration of glucose in the culture medium. For example, while C-peptide significantly enhanced ZO-1 and MITF mRNA expression and inhibited RLBP-1 mRNA expression under normal glucose (5.5 mM) conditions, this effect was lost in cells cultured in high glucose (25 mM). Notably, although C-peptide had no effect on VEGF receptor 2 (VEGFR2) mRNA expression in low glucose-exposed cells, cells cultured in high glucose exhibited a significant increase in VEGFR2 mRNA expression, which could have important implications for the functionality of VEGF on the RPE. Thus, the modulatory action of C-peptide on gene expression appears to be dependent upon extracellular glucose concentration, perhaps reflecting an interaction with AMPK-mediated signaling cascades. However, further experiments are required to evaluate the signaling mechanisms underlying this important action of C-peptide.
Fig. 6.
C-peptide and retinal pigment epithelium (RPE) cell gene expression changes in a transdifferentiation assay. ARPE-19 cells were incubated in either low (5.5 mM) or high (25 mM) glucose for 30 days with or without 1 nM C-peptide. Medium +/− C-peptide was replenished weekly. To evaluate C-peptide-induced changes in gene expression, cells were lysed, RNA isolated, and changes in gene expression evaluated by quantitative PCR using primers specific against genes produced by RPE cells, including the tight junction protein zona occludens 1 (ZO-1), the VEGF receptor 2 (VEGFR2), the transcription factor orthodenticle homeobox 2 (OTX2), the transcription factor microphthalmia-associated transcription factor (MITF), which is involved in regulating RPE cell identity, retinaldehyde-binding protein 1 (RLBP-1), and pigment epithelium-derived factor (PEDF). Under low glucose conditions, C-peptide significantly altered the mRNA expression of several genes, including ZO-1, MITF, and RLBP-1. However, this effect was lost in cells cultured in high glucose, suggesting that C-peptide-induced changes in gene expression are dependent upon glucose concentration, perhaps reflecting an interaction with AMPK-mediated signaling cascades. *P ≤ 0.05, **P ≤ 0.01 versus vehicle-treated control.
These data suggest that experiments to evaluate the cellular effects of C-peptide must be carefully planned to control for glucose concentration and relative insulin levels. Likewise, these findings could have important implications for the development of C-peptide-based therapeutics, particularly with respect to plasma insulin and glucose levels at the time of C-peptide administration. Regardless of these difficulties, C-peptide remains a promising target for the treatment of diabetes-associated complications, including diabetic retinopathy, nephropathy, and neuropathy. However, until the full identity of the C-peptide signalosome is elucidated, the role of C-peptide in normal physiology cannot be fully understood. Evaluation of the physiological relevance of C-peptide and its receptor or receptor complex would not only enhance our understanding of the role of endocrine function in the maintenance of the microvasculature, but would likely lead to the development of novel therapeutic options for both type 1 and type 2 diabetic patients suffering from microvascular dysfunction.
Conclusions
GPCRs are the target of numerous drugs, including agents currently being developed as adjuvant therapy in diabetes. Over 70 GPCRs have been identified that as yet have an unidentified ligand. These orphan GPCRs represent a significant untapped source of therapeutic potential for diabetes and its complications, but first their endogenous ligands must be identified. Current deorphanization strategies have had major successes, including the use of the deductive ligand–receptor matching strategy to identify GPR146 as a candidate receptor for proinsulin C-peptide. However, further work is necessary to elucidate the full signalosome of C-peptide, which should facilitate the development of future C-peptide-based therapeutics. Future deorphanization of additional orphan GPCRs could yield important novel options for the treatment of diabetes and its complications.
Acknowledgments
The authors would like to thank Dr. John Wahren for many useful discussions and comments during the preparation of this manuscript. GLCY is supported by a grant from the National Institutes of Health (# HL121456). GRK is supported by the Edward Doisy Research Fund and the President’s Research Fund.
Footnotes
Conflict of interest statement
The authors do not have any conflicts of interest to declare.
References
- 1.Collins S, Lohse MJ, O'Dowd B, Caron MG, Lefkowitz RJ. Structure and regulation of G protein-coupled receptors: the beta 2-adrenergic receptor as a model. Vitamins and hormones. 1991;46:1–39. doi: 10.1016/s0083-6729(08)60681-0. PubMed PMID: 1660639. [DOI] [PubMed] [Google Scholar]
- 2.Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 2007;190(1):9–19. doi: 10.1111/j.1365-201X.2007.01693.x. PubMed PMID: 17428228. [DOI] [PubMed] [Google Scholar]
- 3.Daaka Y, Pitcher JA, Richardson M, Stoffel RH, Robishaw JD, Lefkowitz RJ. Receptor and G betagamma isoform-specific interactions with G protein-coupled receptor kinases. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(6):2180–2185. doi: 10.1073/pnas.94.6.2180. Epub 1997/03/18. PubMed PMID: 9122168; PubMed Central PMCID: PMCPmc20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang Y, Xie X, Zhang Y, Luo X, Wang X, Fan F, et al. Regulation of G-protein signaling by RKTG via sequestration of the G betagamma subunit to the Golgi apparatus. Molecular and cellular biology. 2010;30(1):78–90. doi: 10.1128/MCB.01038-09. Epub 2009/11/04. PubMed PMID: 19884349; PubMed Central PMCID: PMCPmc2798304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busnelli M, Sauliere A, Manning M, Bouvier M, Gales C, Chini B. Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. The Journal of biological chemistry. 2012;287(6):3617–3629. doi: 10.1074/jbc.M111.277178. Epub 2011/11/10. PubMed PMID: 22069312; PubMed Central PMCID: PMCPmc3281696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. Journal of cell science. 2002;115(Pt 3):455–465. doi: 10.1242/jcs.115.3.455. Epub 2002/02/28. PubMed PMID: 11861753. [DOI] [PubMed] [Google Scholar]
- 7.McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, et al. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290(5496):1574–1577. doi: 10.1126/science.290.5496.1574. Epub 2000/11/25. PubMed PMID: 11090355. [DOI] [PubMed] [Google Scholar]
- 8.Klein KR, Matson BC, Caron KM. The expanding repertoire of receptor activity modifying protein (RAMP) function. Crit Rev Biochem Mol Biol. 2016:1–7. doi: 10.3109/10409238.2015.1128875. PubMed PMID: 26740457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buhlmann N, Leuthauser K, Muff R, Fischer JA, Born W. A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology. 1999;140(6):2883–2890. doi: 10.1210/endo.140.6.6783. PubMed PMID: 10342881. [DOI] [PubMed] [Google Scholar]
- 10.Dackor RT, Fritz-Six K, Dunworth WP, Gibbons CL, Smithies O, Caron KM. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Molecular and cellular biology. 2006;26(7):2511–2518. doi: 10.1128/MCB.26.7.2511-2518.2006. PubMed PMID: 16537897; PubMed Central PMCID: PMCPMC1430335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flahaut M, Rossier BC, Firsov D. Respective roles of calcitonin receptor-like receptor (CRLR) and receptor activity-modifying proteins (RAMP) in cell surface expression of CRLR/RAMP heterodimeric receptors. The Journal of biological chemistry. 2002;277(17):14731–14737. doi: 10.1074/jbc.M112084200. PubMed PMID: 11854283. [DOI] [PubMed] [Google Scholar]
- 12.Moad HE, Pioszak AA. Selective CGRP and adrenomedullin peptide binding by tethered RAMP-calcitonin receptor-like receptor extracellular domain fusion proteins. Protein Sci. 2013;22(12):1775–1785. doi: 10.1002/pro.2377. PubMed PMID: 24115156; PubMed Central PMCID: PMCPMC3843631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. The Journal of pharmacology and experimental therapeutics. 2000;294(1):61–72. PubMed PMID: 10871296. [PubMed] [Google Scholar]
- 14.Teoh CM, Tam JK, Tran T. Integrin and GPCR Crosstalk in the Regulation of ASM Contraction Signaling in Asthma. Journal of allergy. 2012;2012:341282. doi: 10.1155/2012/341282. Epub 2012/10/12. doi: 10.1155/2012/341282. PubMed PMID: 23056062; PubMed Central PMCID: PMCPmc3465959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z. Transactivation of Epidermal Growth Factor Receptor by G Protein-Coupled Receptors: Recent Progress, Challenges and Future Research. International journal of molecular sciences. 2015;17(1) doi: 10.3390/ijms17010095. Epub 2016/01/16. PubMed PMID: 26771606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohse MJ. Dimerization in GPCR mobility and signaling. Current opinion in pharmacology. 2010;10(1):53–58. doi: 10.1016/j.coph.2009.10.007. Epub 2009/11/17. PubMed PMID: 19910252. [DOI] [PubMed] [Google Scholar]
- 17.Godin CM, Ferguson SS. Biased agonism of the angiotensin II type 1 receptor. Mini reviews in medicinal chemistry. 2012;12(9):812–816. doi: 10.2174/138955712800959134. Epub 2012/06/12. PubMed PMID: 22681254. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopal S, Bassoni DL, Campbell JJ, Gerard NP, Gerard C, Wehrman TS. Biased agonism as a mechanism for differential signaling by chemokine receptors. The Journal of biological chemistry. 2013;288(49):35039–35048. doi: 10.1074/jbc.M113.479113. Epub 2013/10/23. PubMed PMID: 24145037; PubMed Central PMCID: PMCPmc3853256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annual review of pharmacology and toxicology. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. Epub 2011/09/29. PubMed PMID: 21942629; PubMed Central PMCID: PMCPmc3628752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ. Recent developments in biased agonism. Current opinion in cell biology. 2014;27:18–24. doi: 10.1016/j.ceb.2013.10.008. Epub 2014/04/01. PubMed PMID: 24680426; PubMed Central PMCID: PMCPmc3971386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. British journal of pharmacology. 2008;153(Suppl 1):S379–S388. doi: 10.1038/sj.bjp.0707604. PubMed PMID: 18059321; PubMed Central PMCID: PMCPMC2268061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic acids research. 2014;42(Database issue):D1098–D106. doi: 10.1093/nar/gkt1143. Epub 2013/11/16. PubMed PMID: 24234439; PubMed Central PMCID: PMC3965070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams C, Hill SJ. GPCR signaling: understanding the pathway to successful drug discovery. Methods in molecular biology. 2009;552:39–50. doi: 10.1007/978-1-60327-317-6_3. PubMed PMID: 19513640. [DOI] [PubMed] [Google Scholar]
- 24.Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8(5):369–385. doi: 10.1038/nrd2782. PubMed PMID: 19365392. [DOI] [PubMed] [Google Scholar]
- 25.Ahren B. Glucagon-like peptide-1 (GLP-1): a gut hormone of potential interest in the treatment of diabetes. Bioessays. 1998;20(8):642–651. doi: 10.1002/(SICI)1521-1878(199808)20:8<642::AID-BIES7>3.0.CO;2-K. PubMed PMID: 9780839. [DOI] [PubMed] [Google Scholar]
- 26.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. PubMed PMID: 16517403. [DOI] [PubMed] [Google Scholar]
- 27.Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326(20):1316–1322. doi: 10.1056/NEJM199205143262003. PubMed PMID: 1348845. [DOI] [PubMed] [Google Scholar]
- 28.Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998;47(11):1663–1670. doi: 10.2337/diabetes.47.11.1663. PubMed PMID: 9792533. [DOI] [PubMed] [Google Scholar]
- 29.Ahren B. GLP-1-based therapy of type 2 diabetes: GLP-1 mimetics and DPP-IV inhibitors. Curr Diab Rep. 2007;7(5):340–347. doi: 10.1007/s11892-007-0056-9. PubMed PMID: 18173966. [DOI] [PubMed] [Google Scholar]
- 30.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628–2635. doi: 10.2337/diacare.27.11.2628. PubMed PMID: 15504997. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–1100. doi: 10.2337/diacare.28.5.1092. PubMed PMID: 15855572. [DOI] [PubMed] [Google Scholar]
- 32.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083–1091. doi: 10.2337/diacare.28.5.1083. PubMed PMID: 15855571. [DOI] [PubMed] [Google Scholar]
- 33.Madsen K, Knudsen LB, Agersoe H, Nielsen PF, Thogersen H, Wilken M, et al. Structure-activity and protraction relationship of long-acting glucagon-like peptide-1 derivatives: importance of fatty acid length, polarity, and bulkiness. J Med Chem. 2007;50(24):6126–6132. doi: 10.1021/jm070861j. PubMed PMID: 17975905. [DOI] [PubMed] [Google Scholar]
- 34.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9(5):733–745. doi: 10.1111/j.1463-1326.2007.00744.x. PubMed PMID: 17593236. [DOI] [PubMed] [Google Scholar]
- 35.Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10(5):376–386. doi: 10.1111/j.1463-1326.2008.00876.x. PubMed PMID: 18355324. [DOI] [PubMed] [Google Scholar]
- 36.Deacon CF. Alogliptin, a potent and selective dipeptidyl peptidase-IV inhibitor for the treatment of type 2 diabetes. Curr Opin Investig Drugs. 2008;9(4):402–413. PubMed PMID: 18393107. [PubMed] [Google Scholar]
- 37.Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: Are the GLP-1 therapies safe? Diabetes Care. 2013;36(7):2118–2125. doi: 10.2337/dc12-2713. PubMed PMID: 23645885; PubMed Central PMCID: PMCPMC3687282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nauck MA. A critical analysis of the clinical use of incretin-based therapies: The benefits by far outweigh the potential risks. Diabetes Care. 2013;36(7):2126–2132. doi: 10.2337/dc12-2504. PubMed PMID: 23645884; PubMed Central PMCID: PMCPMC3687264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen D. Has pancreatic damage from glucagon suppressing diabetes drugs been underplayed? BMJ. 2013;346:f3680. doi: 10.1136/bmj.f3680. PubMed PMID: 23748128. [DOI] [PubMed] [Google Scholar]
- 40.Moses A. Novo Nordisk replies to BMJ investigation on incretins and pancreatic damage. BMJ. 2013;347:f4386. doi: 10.1136/bmj.f4386. PubMed PMID: 23842435. [DOI] [PubMed] [Google Scholar]
- 41.American Diabetes Association; 2013. ADA/EASD/IDF Statement Concerning the Use of Incretin Therapy and Pancreatic Disease [Internet] Available from: http://www.diabetes.org/newsroom/press-releases/2013/recommendations-for.html. [Google Scholar]
- 42.The Endocrine Society; 2013. Society Joins Call for Review of Incretin-based Therapy for Diabetes Mellitus [Internet] Available from: https://www.endocrineorg/~/media/endosociety/files/advocacy-and-outreach/position-statements/2013/incretin-statement-18-jun-13.pdf?la=en. [Google Scholar]
- 43.Gromada J. The free fatty acid receptor GPR40 generates excitement in pancreatic beta-cells. Endocrinology. 2006;147(2):672–673. doi: 10.1210/en.2005-1388. PubMed PMID: 16418431. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci. 2009;122(Pt 7):893–903. doi: 10.1242/jcs.034355. PubMed PMID: 19295123; PubMed Central PMCID: PMCPMC2720925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferdaoussi M, Bergeron V, Zarrouki B, Kolic J, Cantley J, Fielitz J, et al. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia. 2012;55(10):2682–2692. doi: 10.1007/s00125-012-2650-x. PubMed PMID: 22820510; PubMed Central PMCID: PMCPMC3543464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56(4):1087–1094. doi: 10.2337/db06-1532. PubMed PMID: 17395749; PubMed Central PMCID: PMCPMC1853382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alquier T, Peyot ML, Latour MG, Kebede M, Sorensen CM, Gesta S, et al. Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes. 2009;58(11):2607–2615. doi: 10.2337/db09-0362. PubMed PMID: 19720802; PubMed Central PMCID: PMCPMC2768167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Azzouny M, Evans CR, Treutelaar MK, Kennedy RT, Burant CF. Increased glucose metabolism and glycerolipid formation by fatty acids and GPR40 receptor signaling underlies the fatty acid potentiation of insulin secretion. J Biol Chem. 2014;289(19):13575–13588. doi: 10.1074/jbc.M113.531970. PubMed PMID: 24675078; PubMed Central PMCID: PMCPMC4036363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57(9):2280–2287. doi: 10.2337/db08-0307. PubMed PMID: 18519800; PubMed Central PMCID: PMCPMC2518478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vettor R, Granzotto M, De Stefani D, Trevellin E, Rossato M, Farina MG, et al. Loss-of-function mutation of the GPR40 gene associates with abnormal stimulated insulin secretion by acting on intracellular calcium mobilization. J Clin Endocrinol Metab. 2008;93(9):3541–3550. doi: 10.1210/jc.2007-2680. PubMed PMID: 18583466. [DOI] [PubMed] [Google Scholar]
- 51.Hauge M, Vestmar MA, Husted AS, Ekberg JP, Wright MJ, Di Salvo J, et al. GPR40 (FFAR1) - Combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol Metab. 2015;4(1):3–14. doi: 10.1016/j.molmet.2014.10.002. PubMed PMID: 25685685; PubMed Central PMCID: PMCPMC4314522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroder R, Schmidt J, Blattermann S, Peters L, Janssen N, Grundmann M, et al. Applying label-free dynamic mass redistribution technology to frame signaling of G protein-coupled receptors noninvasively in living cells. Nat Protoc. 2011;6(11):1748–1760. doi: 10.1038/nprot.2011.386. PubMed PMID: 22015845. [DOI] [PubMed] [Google Scholar]
- 53.Sum CS, Tikhonova IG, Neumann S, Engel S, Raaka BM, Costanzi S, et al. Identification of residues important for agonist recognition and activation in GPR40. J Biol Chem. 2007;282(40):29248–29255. doi: 10.1074/jbc.M705077200. PubMed PMID: 17699519. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava A, Yano J, Hirozane Y, Kefala G, Gruswitz F, Snell G, et al. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature. 2014;513(7516):124–127. doi: 10.1038/nature13494. PubMed PMID: 25043059. [DOI] [PubMed] [Google Scholar]
- 55.Tsujihata Y, Ito R, Suzuki M, Harada A, Negoro N, Yasuma T, et al. TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J Pharmacol Exp Ther. 2011;339(1):228–237. doi: 10.1124/jpet.111.183772. PubMed PMID: 21752941. [DOI] [PubMed] [Google Scholar]
- 56.Kaku K, Enya K, Nakaya R, Ohira T, Matsuno R. Efficacy and safety of fasiglifam (TAK-875), a G protein-coupled receptor 40 agonist, in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise: a randomized, double-blind, placebo-controlled, phase III trial. Diabetes Obes Metab. 2015;17(7):675–681. doi: 10.1111/dom.12467. PubMed PMID: 25787200; PubMed Central PMCID: PMCPMC4676912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burant CF, Viswanathan P, Marcinak J, Cao C, Vakilynejad M, Xie B, et al. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2012;379(9824):1403–1411. doi: 10.1016/S0140-6736(11)61879-5. PubMed PMID: 22374408. [DOI] [PubMed] [Google Scholar]
- 58.Guo DY, Li DW, Ning MM, Dang XY, Zhang LN, Zeng LM, et al. Yhhu4488, a novel GPR40 agonist, promotes GLP-1 secretion and exerts anti-diabetic effect in rodent models. Biochem Biophys Res Commun. 2015;466(4):740–747. doi: 10.1016/j.bbrc.2015.09.130. PubMed PMID: 26417688. [DOI] [PubMed] [Google Scholar]
- 59.Luo J, Swaminath G, Brown SP, Zhang J, Guo Q, Chen M, et al. A potent class of GPR40 full agonists engages the enteroinsular axis to promote glucose control in rodents. PLoS One. 2012;7(10):e46300. doi: 10.1371/journal.pone.0046300. PubMed PMID: 23056280; PubMed Central PMCID: PMCPMC3467217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005;1(4):245–258. doi: 10.1016/j.cmet.2005.03.007. PubMed PMID: 16054069. [DOI] [PubMed] [Google Scholar]
- 61.Sun P, Wang T, Zhou Y, Liu H, Jiang H, Zhu W, et al. DC260126: a small-molecule antagonist of GPR40 that protects against pancreatic beta-Cells dysfunction in db/db mice. PLoS One. 2013;8(6):e66744. doi: 10.1371/journal.pone.0066744. PubMed PMID: 23776696; PubMed Central PMCID: PMCPMC3679087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X, Yan G, Li Y, Zhu W, Wang H. DC260126, a small-molecule antagonist of GPR40, improves insulin tolerance but not glucose tolerance in obese Zucker rats. Biomed Pharmacother. 2010;64(9):647–651. doi: 10.1016/j.biopha.2010.06.008. PubMed PMID: 20888730. [DOI] [PubMed] [Google Scholar]
- 63.Defronzo RA. Bromocriptine: a sympatholytic, d2-dopamine agonist for the treatment of type 2 diabetes. Diabetes Care. 2011;34(4):789–794. doi: 10.2337/dc11-0064. PubMed PMID: 21447659; PubMed Central PMCID: PMCPMC3064029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.AH C. Hypothalamic role in the insulin resistance syndrome. In: H B, S E, editors. Insulin Resistance Syndrome. London: Taylor and Francis; 2002. pp. 271–312. [Google Scholar]
- 65.Neel JV. Diabetes mellitus: a "thrifty" genotype rendered detrimental by "progress"? Am J Hum Genet. 1962;14:353–362. PubMed PMID: 13937884; PubMed Central PMCID: PMCPMC1932342. [PMC free article] [PubMed] [Google Scholar]
- 66.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. doi: 10.2337/diacare.14.3.173. PubMed PMID: 2044434. [DOI] [PubMed] [Google Scholar]
- 67.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. PubMed PMID: 3056758. [DOI] [PubMed] [Google Scholar]
- 68.Cincotta AH, Meier AH, Cincotta M., Jr Bromocriptine improves glycaemic control and serum lipid profile in obese Type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs. 1999;8(10):1683–1707. doi: 10.1517/13543784.8.10.1683. PubMed PMID: 11139820. [DOI] [PubMed] [Google Scholar]
- 69.Pijl H, Ohashi S, Matsuda M, Miyazaki Y, Mahankali A, Kumar V, et al. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care. 2000;23(8):1154–1161. doi: 10.2337/diacare.23.8.1154. PubMed PMID: 10937514. [DOI] [PubMed] [Google Scholar]
- 70.Gaziano JM, Cincotta AH, O'Connor CM, Ezrokhi M, Rutty D, Ma ZJ, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care. 2010;33(7):1503–1508. doi: 10.2337/dc09-2009. PubMed PMID: 20332352; PubMed Central PMCID: PMCPMC2890350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung S, Funakoshi T, Civelli O. Orphan GPCR research. British journal of pharmacology. 2008;153(Suppl 1):S339–S346. doi: 10.1038/sj.bjp.0707606. PubMed PMID: 18071299; PubMed Central PMCID: PMCPMC2268047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Civelli O, Reinscheid RK, Zhang Y, Wang Z, Fredriksson R, Schioth HB. G protein-coupled receptor deorphanizations. Annual review of pharmacology and toxicology. 2013;53:127–146. doi: 10.1146/annurev-pharmtox-010611-134548. PubMed PMID: 23020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Otlivanchik O, Sanders NM, Dunn-Meynell A, Levin BE. Orexin signaling is necessary for hypoglycemia-induced prevention of conditioned place preference. American journal of physiology Regulatory, integrative and comparative physiology. 2016;310(1):R66–R73. doi: 10.1152/ajpregu.00066.2015. Epub 2015/10/30. PubMed PMID: 26511522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samson WK, Resch ZT. The hypocretin/orexin story. Trends in endocrinology and metabolism: TEM. 2000;11(7):257–262. doi: 10.1016/s1043-2760(00)00273-3. Epub 2000/08/02. PubMed PMID: 10920381. [DOI] [PubMed] [Google Scholar]
- 75.Xu TR, Yang Y, Ward R, Gao L, Liu Y. Orexin receptors: multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cellular signalling. 2013;25(12):2413–2423. doi: 10.1016/j.cellsig.2013.07.025. Epub 2013/08/07. PubMed PMID: 23917208. [DOI] [PubMed] [Google Scholar]
- 76.Arnes L, Hill JT, Gross S, Magnuson MA, Sussel L. Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population. PloS one. 2012;7(12):e52026. doi: 10.1371/journal.pone.0052026. Epub 2012/12/20. PubMed PMID: 23251675; PubMed Central PMCID: PMC3520898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanger GJ, Furness JB. Ghrelin and motilin receptors as drug targets for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2016;13(1):38–48. doi: 10.1038/nrgastro.2015.163. PubMed PMID: 26392067. [DOI] [PubMed] [Google Scholar]
- 78.Chapman NA, Dupre DJ, Rainey JK. The apelin receptor: physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A GPCR. Biochem Cell Biol. 2014;92(6):431–440. doi: 10.1139/bcb-2014-0072. PubMed PMID: 25275559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stein LM, Yosten GL, Samson WK. Adropin Acts in Brain to Inhibit Water Drinking: Potential Interaction with the Orphan G Protein-Coupled Receptor, GPR19. American journal of physiology Regulatory, integrative and comparative physiology. 2016 doi: 10.1152/ajpregu.00511.2015. ajpregu 00511 2015. PubMed PMID: 26739651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yosten GL, Kolar GR, Redlinger LJ, Samson WK. Evidence for an interaction between proinsulin C-peptide and GPR146. The Journal of endocrinology. 2013;218(2):B1–B8. doi: 10.1530/JOE-13-0203. Epub 2013/08/28. PubMed PMID: 23980258. [DOI] [PubMed] [Google Scholar]
- 81.Yosten GL, Redlinger LJ, Samson WK. Evidence for an interaction of neuronostatin with the orphan G protein-coupled receptor, GPR107. American journal of physiology Regulatory, integrative and comparative physiology. 2012;303(9):R941–R949. doi: 10.1152/ajpregu.00336.2012. Epub 2012/08/31. PubMed PMID: 22933024; PubMed Central PMCID: PMC3517703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wahren J, Kallas A, Sima AA. The clinical potential of C-peptide replacement in type 1 diabetes. Diabetes. 2012;61(4):761–772. doi: 10.2337/db11-1423. Epub 2012/03/24. PubMed PMID: 22442295; PubMed Central PMCID: PMCPmc3314360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wahren J, Larsson C. C-peptide: New findings and therapeutic possibilities. Diabetes research and clinical practice. 2015;107(3):309–319. doi: 10.1016/j.diabres.2015.01.016. Epub 2015/02/05. PubMed PMID: 25648391. [DOI] [PubMed] [Google Scholar]
- 84.Yosten GL, Kolar GR. The Physiology of Proinsulin C-Peptide: Unanswered Questions and a Proposed Model. Physiology. 2015;30(4):327–332. doi: 10.1152/physiol.00008.2015. PubMed PMID: 26136546. [DOI] [PubMed] [Google Scholar]
- 85.Yosten GL, Maric-Bilkan C, Luppi P, Wahren J. Physiological effects and therapeutic potential of proinsulin C-peptide. American journal of physiology Endocrinology and metabolism. 2014;307(11):E955–E968. doi: 10.1152/ajpendo.00130.2014. Epub 2014/09/25. PubMed PMID: 25249503. PubMed Central PMCID: PMC4254984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luppi P, Cifarelli V, Wahren J. C-peptide and long-term complications of diabetes. Pediatric diabetes. 2011;12(3 Pt 2):276–292. doi: 10.1111/j.1399-5448.2010.00729.x. Epub 2010/12/07. PubMed PMID: 21129141. [DOI] [PubMed] [Google Scholar]
- 87.Richards JP, Stephenson AH, Ellsworth ML, Sprague RS. Synergistic effects of C-peptide and insulin on low O2-induced ATP release from human erythrocytes. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305(11):R1331–R1336. doi: 10.1152/ajpregu.00341.2013. Epub 2013/10/04. PubMed PMID: 24089376. PubMed Central PMCID: PMCPmc3882563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Richards JP, Yosten GL, Kolar GR, Jones CW, Stephenson AH, Ellsworth ML, et al. Low O2-induced ATP release from erythrocytes of humans with type 2 diabetes is restored by physiological ratios of C-peptide and insulin. American journal of physiology Regulatory, integrative and comparative physiology. 2014;307(7):R862–R868. doi: 10.1152/ajpregu.00206.2014. Epub 2014/09/25 2014/08/01. 10.1152/ajpregu.00206.2014. PubMed PMID: 25080497. [DOI] [PubMed] [Google Scholar]
- 89.Flatt PR, Swanston-Flatt SK, Hampton SM, Bailey CJ, Marks V. Specific binding of the C-peptide of proinsulin to cultured B-cells from a transplantable rat islet cell tumor. Bioscience reports. 1986;6(2):193–199. doi: 10.1007/BF01115006. Epub 1986/02/01. PubMed PMID: 3013335. [DOI] [PubMed] [Google Scholar]
- 90.Henriksson M, Pramanik A, Shafqat J, Zhong Z, Tally M, Ekberg K, et al. Specific binding of proinsulin C-peptide to intact and to detergent-solubilized human skin fibroblasts. Biochemical and biophysical research communications. 2001;280(2):423–427. doi: 10.1006/bbrc.2000.4135. Epub 2001/02/13. PubMed PMID: 11162533. [DOI] [PubMed] [Google Scholar]
- 91.Rigler R, Pramanik A, Jonasson P, Kratz G, Jansson OT, Nygren P, et al. Specific binding of proinsulin C-peptide to human cell membranes. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(23):13318–13323. doi: 10.1073/pnas.96.23.13318. Epub 1999/11/11. PubMed PMID: 10557318. PubMed Central PMCID: PMCPmc23945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen B, Delaney MK, Du X. Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Current opinion in cell biology. 2012;24(5):600–606. doi: 10.1016/j.ceb.2012.08.011. PubMed PMID: 22980731; PubMed Central PMCID: PMCPMC3479359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strauss O. The retinal pigment epithelium in visual function. Physiological reviews. 2005;85(3):845–881. doi: 10.1152/physrev.00021.2004. Epub 2005/07/01. PubMed PMID: 15987797. [DOI] [PubMed] [Google Scholar]
- 94.Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Seminars in ophthalmology. 1999;14(4):223–232. doi: 10.3109/08820539909069541. Epub 2000/04/12. PubMed PMID: 10758223. [DOI] [PubMed] [Google Scholar]
- 95.Ablonczy Z, Crosson CE. VEGF modulation of retinal pigment epithelium resistance. Experimental eye research. 2007;85(6):762–771. doi: 10.1016/j.exer.2007.08.010. Epub 2007/10/05. PubMed PMID: 17915218. PubMed Central PMCID: PMCPmc2199266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ido Y, Vindigni A, Chang K, Stramm L, Chance R, Heath WF, et al. Prevention of vascular and neural dysfunction in diabetic rats by C-peptide. Science. 1997;277(5325):563–566. doi: 10.1126/science.277.5325.563. Epub 1997/07/25. PubMed PMID: 9228006. [DOI] [PubMed] [Google Scholar]
- 97.Lim YC, Bhatt MP, Kwon MH, Park D, Lee S, Choe J, et al. Prevention of VEGF-mediated microvascular permeability by C-peptide in diabetic mice. Cardiovascular research. 2014;101(1):155–164. doi: 10.1093/cvr/cvt238. Epub 2013/10/22. PubMed PMID: 24142430. [DOI] [PubMed] [Google Scholar]
- 98.Bo S, Gentile L, Castiglione A, Prandi V, Canil S, Ghigo E, et al. C-peptide and the risk for incident complications and mortality in type 2 diabetic patients: a retrospective cohort study after a 14-year follow-up. European journal of endocrinology / European Federation of Endocrine Societies. 2012;167(2):173–180. doi: 10.1530/EJE-12-0085. Epub 2012/05/12. PubMed PMID: 22577110. [DOI] [PubMed] [Google Scholar]
- 99.Chung JO, Cho DH, Chung DJ, Chung MY. Relationship between serum C-peptide level and diabetic retinopathy according to estimated glomerular filtration rate in patients with type 2 diabetes. Journal of diabetes and its complications. 2015;29(3):350–355. doi: 10.1016/j.jdiacomp.2014.12.013. Epub 2015/01/28. PubMed PMID: 25623633. [DOI] [PubMed] [Google Scholar]
- 100.Lachin JM, McGee P, Palmer JP. Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes. 2014;63(2):739–748. doi: 10.2337/db13-0881. Epub 2013/10/04. PubMed PMID: 24089509. PubMed Central PMCID: PMCPmc3900540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhatt MP, Lim YC, Kim YM, Ha KS. C-peptide activates AMPKalpha and prevents ROS-mediated mitochondrial fission and endothelial apoptosis in diabetes. Diabetes. 2013;62(11):3851–3862. doi: 10.2337/db13-0039. Epub 2013/07/26. PubMed PMID: 23884890; PubMed Central PMCID: PMCPmc3806599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiological reviews. 2009;89(3):1025–1078. doi: 10.1152/physrev.00011.2008. Epub 2009/07/09. PubMed PMID: 19584320. [DOI] [PubMed] [Google Scholar]
- 103.Grunberger G, Qiang X, Li Z, Mathews ST, Sbrissa D, Shisheva A, et al. Molecular basis for the insulinomimetic effects of C-peptide. Diabetologia. 2001;44(10):1247–1257. doi: 10.1007/s001250100632. Epub 2001/11/03. PubMed PMID: 11692173. [DOI] [PubMed] [Google Scholar]
- 104.Azuma N, Tadokoro K, Asaka A, Yamada M, Yamaguchi Y, Handa H, et al. Transdifferentiation of the retinal pigment epithelia to the neural retina by transfer of the Pax6 transcriptional factor. Human molecular genetics. 2005;14(8):1059–1068. doi: 10.1093/hmg/ddi098. Epub 2005/03/11. PubMed PMID: 15757974. [DOI] [PubMed] [Google Scholar]
- 105.Dutt K, Douglas P, Cao Y. RPE-secreted factors: influence differentiation in human retinal cell line in dose- and density-dependent manner. Journal of ocular biology, diseases, and informatics. 2010;3(4):144–160. doi: 10.1007/s12177-011-9076-4. Epub 2010/12/01. PubMed PMID: 23316262; PubMed Central PMCID: PMCPmc3289158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghaderi S, Soheili ZS, Ahmadieh H, Davari M, Jahromi FS, Samie S, et al. Human amniotic fluid promotes retinal pigmented epithelial cells' trans-differentiation into rod photoreceptors and retinal ganglion cells. Stem Cells Dev. 2011;20(9):1615–1625. doi: 10.1089/scd.2010.0390. PubMed PMID: 21142973. [DOI] [PubMed] [Google Scholar]
- 107.Tamiya S, Liu L, Kaplan HJ. Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Investigative ophthalmology & visual science. 2010;51(5):2755–2763. doi: 10.1167/iovs.09-4725. PubMed PMID: 20042656. [DOI] [PubMed] [Google Scholar]
- 108.Chiba C. The retinal pigment epithelium: an important player of retinal disorders and regeneration. Experimental eye research. 2014;123:107–114. doi: 10.1016/j.exer.2013.07.009. Epub 2013/07/25. PubMed PMID: 23880527. [DOI] [PubMed] [Google Scholar]
- 109.Mony S, Lee SJ, Harper JF, Barwe SP, Langhans SA. Regulation of Na,K-ATPase beta1-subunit in TGF-beta2-mediated epithelial-to-mesenchymal transition in human retinal pigmented epithelial cells. Experimental eye research. 2013;115:113–122. doi: 10.1016/j.exer.2013.06.007. Epub 2013/07/03. PubMed PMID23810808; PubMed Central PMCID: PMCPmc3796007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuznetsova AV, Kurinov AM, Aleksandrova MA. Cell models to study regulation of cell transformation in pathologies of retinal pigment epithelium. Journal of ophthalmology. 2014;2014:801787. doi: 10.1155/2014/801787. Epub 2014/09/02. PubMed PMID: 25177495. PubMed Central PMCID: PMCPmc4142280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsimaratos M, Roger F, Chabardes D, Mordasini D, Hasler U, Doucet A, et al. C-peptide stimulates Na+,K+-ATPase activity via PKC alpha in rat medullary thick ascending limb. Diabetologia. 2003;46(1):124–131. doi: 10.1007/s00125-002-0996-1. Epub 2003/03/15. PubMed PMID: 12637991. [DOI] [PubMed] [Google Scholar]
- 112.Georgiadis A, Tschernutter M, Bainbridge JW, Balaggan KS, Mowat F, West EL, et al. The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PloS one. 2010;5(12):e15730. doi: 10.1371/journal.pone.0015730. Epub 2011/01/07. PubMed PMID: 21209887. PubMed Central PMCID: PMCPmc3012699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Capowski EE, Simonett JM, Clark EM, Wright LS, Howden SE, Wallace KA, et al. Loss of MITF expression during human embryonic stem cell differentiation disrupts retinal pigment epithelium development and optic vesicle cell proliferation. Human molecular genetics. 2014;23(23):6332–6344. doi: 10.1093/hmg/ddu351. Epub 2014/07/11. PubMed PMID: 25008112; PubMed Central PMCID: PMCPmc4222367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wysham CH, MacConell LA, Maggs DG, Zhou M, Griffin PS, Trautmann ME. Five-year efficacy and safety data of exenatide once weekly: long-term results from the DURATION-1 randomized clinical trial. Mayo Clin Proc. 2015;90(3):356–365. doi: 10.1016/j.mayocp.2015.01.008. PubMed PMID: 25744115. [DOI] [PubMed] [Google Scholar]
- 115.Elrick MM, Samson WK, Corbett JA, Salvatori AS, Stein LM, Kolar GR, et al. Neuronostatin acts via GPR107 to increase cAMP-independent PKA phosphorylation and proglucagon mRNA accumulation in pancreatic alpha-cells. American journal of physiology Regulatory, integrative and comparative physiology. 2016;310(2):R143–R155. doi: 10.1152/ajpregu.00369.2014. PubMed PMID: 26561648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuhadiya ND, Malik R, Bellini NJ, Patterson JL, Traina A, Makdissi A, et al. Liraglutide as additional treatment to insulin in obese patients with type 1 diabetes mellitus. Endocr Pract. 2013;19(6):963–967. doi: 10.4158/EP13065.OR. PubMed PMID: 23807520. [DOI] [PubMed] [Google Scholar]
- 117.Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2(4):289–297. doi: 10.1016/S2213-8587(13)70214-6. PubMed PMID: 24703047. [DOI] [PubMed] [Google Scholar]
- 118.Odawara M, Miyagawa J, Iwamoto N, Takita Y, Imaoka T, Takamura T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide significantly decreases glycated haemoglobin compared with once-daily liraglutide in Japanese patients with type 2 diabetes: 52 weeks of treatment in a randomized phase III study. Diabetes Obes Metab. 2016;18(3):249–257. doi: 10.1111/dom.12602. PubMed PMID: 26661514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu M, Van Brunt K, Varnado OJ, Boye KS. Patient-reported outcome results in patients with type 2 diabetes treated with once weekly dulaglutide: data from the AWARD Phase 3 clinical trial programme. Diabetes Obes Metab. 2015 doi: 10.1111/dom.12624. PubMed PMID: 26691396. [DOI] [PubMed] [Google Scholar]
- 120.Lau J, Bloch P, Schaffer L, Pettersson I, Spetzler J, Kofoed J, et al. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J Med Chem. 2015;58(18):7370–7380. doi: 10.1021/acs.jmedchem.5b00726. PubMed PMID: 26308095. [DOI] [PubMed] [Google Scholar]
- 121.Nauck MA, Petrie JR, Sesti G, Mannucci E, Courreges JP, Lindegaard ML, et al. A Phase 2, Randomized, Dose-Finding Study of the Novel Once-Weekly Human GLP-1 Analog, Semaglutide, Compared With Placebo and Open-Label Liraglutide in Patients With Type 2 Diabetes. Diabetes Care. 2016;39(2):231–241. doi: 10.2337/dc15-0165. PubMed PMID: 26358288. [DOI] [PubMed] [Google Scholar]
- 122.Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P) Diabetes Obes Metab. 2013;15(11):1000–1007. doi: 10.1111/dom.12121. PubMed PMID: 23627775. [DOI] [PubMed] [Google Scholar]
- 123.Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) Diabetes Care. 2013;36(9):2497–2503. doi: 10.2337/dc12-2462. PubMed PMID: 23564915; PubMed Central PMCID: PMCPMC3747901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosenstock J, Hanefeld M, Shamanna P, Min KW, Boka G, Miossec P, et al. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S) J Diabetes Complications. 2014;28(3):386–392. doi: 10.1016/j.jdiacomp.2014.01.012. PubMed PMID: 24650952. [DOI] [PubMed] [Google Scholar]
- 125.Raz I, Fonseca V, Kipnes M, Durrwell L, Hoekstra J, Boldrin M, et al. Efficacy and safety of taspoglutide monotherapy in drug-naive type 2 diabetic patients after 24 weeks of treatment: results of a randomized, double-blind, placebo-controlled phase 3 study (T-emerge 1) Diabetes Care. 2012;35(3):485–487. doi: 10.2337/dc11-1942. PubMed PMID: 22301126; PubMed Central PMCID: PMCPMC3322710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mathieu C, Rodbard HW, Cariou B, Handelsman Y, Philis-Tsimikas A, Ocampo Francisco AM, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON) Diabetes Obes Metab. 2014;16(7):636–644. doi: 10.1111/dom.12262. PubMed PMID: 24443830. [DOI] [PubMed] [Google Scholar]
- 127.Inagaki N, Onouchi H, Maezawa H, Kuroda S, Kaku K. Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: a randomised, double-blind, phase 3, non-inferiority study. Lancet Diabetes Endocrinol. 2015;3(3):191–197. doi: 10.1016/S2213-8587(14)70251-7. PubMed PMID: 25609193. [DOI] [PubMed] [Google Scholar]
- 128.Pratley RE, Fleck P, Wilson C. Efficacy and safety of initial combination therapy with alogliptin plus metformin versus either as monotherapy in drug-naive patients with type 2 diabetes: a randomized, double-blind, 6-month study. Diabetes Obes Metab. 2014;16(7):613–621. doi: 10.1111/dom.12258. PubMed PMID: 24400655. [DOI] [PubMed] [Google Scholar]
- 129.Davidson JA. Tolerability of saxagliptin in patients with inadequately controlled type 2 diabetes: results from 6 phase III studies. J Manag Care Pharm. 2014;20(2):120–129. doi: 10.18553/jmcp.2014.20.2.120. PubMed PMID: 24456313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roden M, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, et al. Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug-naive patients with type 2 diabetes: a double-blind extension of a Phase III randomized controlled trial. Cardiovasc Diabetol. 2015;14(1):154. doi: 10.1186/s12933-015-0314-0. PubMed PMID: 26701110; PubMed Central PMCID: PMCPMC4690334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Derosa G, Ragonesi PD, Carbone A, Fogari E, Bianchi L, Bonaventura A, et al. Vildagliptin added to metformin on beta-cell function after a euglycemic hyperinsulinemic and hyperglycemic clamp in type 2 diabetes patients. Diabetes Technol Ther. 2012;14(6):475–484. doi: 10.1089/dia.2011.0278. PubMed PMID: 22512264. [DOI] [PubMed] [Google Scholar]
- 132.Haidinger M, Werzowa J, Hecking M, Antlanger M, Stemer G, Pleiner J, et al. Efficacy and safety of vildagliptin in new-onset diabetes after kidney transplantation--a randomized, double-blind, placebo-controlled trial. Am J Transplant. 2014;14(1):115–123. doi: 10.1111/ajt.12518. PubMed PMID: 24279801. [DOI] [PubMed] [Google Scholar]
- 133.Kudo-Fujimaki K, Hirose T, Yoshihara T, Sato F, Someya Y, Ohmura C, et al. Efficacy and safety of nateglinide plus vildagliptin combination therapy compared with switching to vildagliptin in type 2 diabetes patients inadequately controlled with nateglinide. J Diabetes Investig. 2014;5(4):400–409. doi: 10.1111/jdi.12160. PubMed PMID: 25411599; PubMed Central PMCID: PMCPMC4210064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lajara R, Aguilar R, Hehnke U, Woerle HJ, von Eynatten M. Efficacy and safety of linagliptin in subjects with long-standing type 2 diabetes mellitus (>10 years): evidence from pooled data of randomized, double-blind, placebo-controlled, phase III trials. Clin Ther. 2014;36(11):1595–1605. doi: 10.1016/j.clinthera.2014.07.020. PubMed PMID: 25236917. [DOI] [PubMed] [Google Scholar]
- 135.Yang SJ, Min KW, Gupta SK, Park JY, Shivane VK, Pitale SU, et al. A multicentre, multinational, randomized, placebo-controlled, double-blind, phase 3 trial to evaluate the efficacy and safety of gemigliptin (LC15-0444) in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(5):410–416. doi: 10.1111/dom.12042. PubMed PMID: 23170990. [DOI] [PubMed] [Google Scholar]
- 136.Lim KS, Kim JR, Choi YJ, Shin KH, Kim KP, Hong JH, et al. Pharmacokinetics, pharmacodynamics, and tolerability of the dipeptidyl peptidase IV inhibitor LC15-0444 in healthy Korean men: a dose-block-randomized, double-blind, placebo-controlled, ascending single-dose, Phase I study. Clin Ther. 2008;30(10):1817–1830. doi: 10.1016/j.clinthera.2008.10.013. PubMed PMID: 19014837. [DOI] [PubMed] [Google Scholar]
- 137.Rhee EJ, Lee WY, Yoon KH, Yoo SJ, Lee IK, Baik SH, et al. A multicenter, randomized, placebo-controlled, double-blind phase II trial evaluating the optimal dose, efficacy and safety of LC 15-0444 in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(12):1113–1119. doi: 10.1111/j.1463-1326.2010.01303.x. PubMed PMID: 20977584. [DOI] [PubMed] [Google Scholar]
- 138.Mancini AD, Poitout V. GPR40 agonists for the treatment of type 2 diabetes: life after 'TAKing' a hit. Diabetes Obes Metab. 2015;17(7):622–629. doi: 10.1111/dom.12442. PubMed PMID: 25604916. [DOI] [PubMed] [Google Scholar]
- 139.Naas D, Morris T, Kousba A, Mazzoni M. A 9-Month Toxicity and Toxicokinetic Assessment of Subcutaneous Pegylated Human C-peptide (CBX129801) in Cynomolgus Monkeys. Int J Toxicol. 2015;34(4):318–324. doi: 10.1177/1091581815585854. PubMed PMID: 26111539. [DOI] [PubMed] [Google Scholar]