Abstract
Introduction:
Non-fermenting Gram-negative bacilli are at the center of the antimicrobial resistance epidemic. Acinetobacter baumannii and Pseudomonas aeruginosa are both designated with a threat level to human health of “serious” by the Centers for Disease Control and Prevention. Two other major non-fermenting Gramnegative bacilli, Stenotrophomonas maltophilia and Burkholderia cepacia complex, while not as prevalent, have devastating effects on vulnerable populations, such as those with cystic fibrosis, as well as immunosuppressed or hospitalized patients.
Areas covered:
In this review, we summarize the clinical impact, presentations, and mechanisms of resistance of these four major groups of non-fermenting Gram-negative bacilli. We also describe available and promising novel therapeutic options and strategies, particularly combination antibiotic strategies, with a focus on multidrug resistant variants.
Expert commentary:
We finally advocate for a therapeutic approach that incorporates in vitro antibiotic susceptibility testing with molecular and genotypic characterization of mechanisms of resistance, as well as pharmacokinetics and pharmacodynamics (PK/PD) parameters. The goal is to begin to formulate a precision medicine approach to antimicrobial therapy: a clinical-decision making model that integrates bacterial phenotype, genotype and patient’s PK/PD to arrive at rationally-optimized combination antibiotic chemotherapy regimens tailored to individual clinical scenarios.
Keywords: Gram-negative bacteria; glucose-non-fermenting; antimicrobial resistance; nosocomial infections; mechanisms of resistance; combination therapy, precision medicine
1. Introduction
During the past decade, the scientific, public health, and political communities realized the alarming public health crisis that antimicrobial resistance (AMR) represents. Based on a landmark report from the Centers for Disease Control and Prevention (CDC) published in 2013, we now know that AMR affects more than 2 million people every year, resulting in a minimum estimate of 23,000 deaths per year in the United States (U.S.) and 25,000 deaths per year in the European Union [1,2]. These figures pale in comparison to the estimate put forth by the British Government in May 2016 where authorities predict that by 2050, approximately 10 million people would die every year globally as a result of AMR, more people than currently die from cancer [3]. The problem has therefore reached proportions of significant importance for both human health and the economy, enough to propel the issue of AMR to the forefront of national and global health concerns. The Infectious Disease Society of America (IDSA) and the World Health Organization (WHO) regard AMR as one of the three greatest threats to human health worldwide [4,5]. National and world health leaders describe antibiotic-resistant bacteria as “nightmare bacteria” that “pose a catastrophic threat” to people in every country in the world [1].
Among such bacterial pathogens, two lactose- and glucose non-fermenting Gram-negative bacteria (GNB) are described by the Centers for Disease Control and Prevention (CDC) report as microorganisms with a threat level of serious: multidrug resistant (MDR) Pseudomonas aeruginosa and Acinetobacter baumannii. Two other groups of non-fermenting GNB did not make the list, probably due to their relatively lower incidence of infection: Stenotrophomonas maltophilia and Burkholderia cepacia complex (Bcc). Despite their lower incidence, these two groups of organisms also represent growing concerns, particularly to vulnerable hospitalized and immunosuppressed patients. Of particular concern, non-fermenting GNB are intrinsically resistant to a wide array of antibiotics, diminishing available therapies to only a few active antibiotics. In recent years, acquired resistance to many of these active agents has reduced therapeutic options even further, straining our resources in combating their rise. Note that the CDC and the European CDC define MDR as acquired non-susceptibility to at least one agent in three or more antimicrobial categories, and extensive-drug resistance (XDR) as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e. bacterial isolates remain susceptible to only one or two categories) [6].
In this review, we offer a general summary of the clinical impact and mechanisms of resistance of the four major groups of non-fermenting GNB: P. aeruginosa, A. baumannii, S. maltophilia, and Bcc organisms. We also present available and promising novel therapeutic options and strategies, particularly combination antibiotic strategies, with a focus on multidrug resistant variants.
2. Clinical impact and mechanisms of resistance
2.1. Pseudomonas aeruginosa
2.1.1. Clinical impact
Since their discovery by Professor Migula of the Karlsruhe Institute in Germany at the end of the 19th century, Pseudomonas spp. have proved to be formidable pathogens, efficiently adapting to and colonizing the environments they inhabit, resulting in serious and devastating infections [7,8]. Owing to these qualities, in addition to its intrinsic resistance to many antibiotics, P. aeruginosa is successful as a nosocomial and opportunistic pathogen in vulnerable populations, namely the immunosuppressed, critically ill, and burn patients [9,10]. Infections caused by P. aeruginosa are also notorious in patients with severe structural lung disease and cystic fibrosis, in whom rates of multidrug resistance are even higher [8,11–13]. The clinical impact of P. aeruginosa is as such the most significant among non-fermenting bacteria in the Western world, with converging epidemiologic data from the U.S. and Europe [14,15].
In the U.S., among healthcare-associated infections reported to the National Healthcare Safety Network (NHSN) in its latest report between 2011 and 2014, P. aeruginosa represented 7% of pathogens, ranking sixth overall but second and third for ventilator-associated pneumonias (VAP) and catheter-associated urinary tract infections, respectively [16]. Carbapenem resistance in that report averaged 26% for catheter-associated bloodstream infections (2011–2014) and 28% for VAP (2011–2012) [16].
National and global trends of infections caused by carbapenem-resistant P. aeruginosa are alarming, knowing that such infections portend a significantly worse prognosis than infections caused by susceptible strains. A meta-analysis of 17 studies comprising 6660 patients with P. aeruginosa infections indicated that infections caused by carbapenem-resistant P. aeruginosa are associated with a significantly higher mortality than those caused by carbapenem-susceptible P. aeruginosa (adjusted OR=2.38; 95% Cl=1.53, 3.69) [17]. Similarly, in a prospective cohort of 709 consecutive episodes of bloodstream infections caused by P. aeruginosa, Morata et al. observed a 32% mortality rate for episodes caused by MDR P. aeruginosa vs. 17% for those caused by non-MDR isolates (p<0.0001) [18]. Overall mortality rate in a Korean cohort of 100 episodes of bacteremic pneumonias due to P. aeruginosa reached 51% at 28 days, a rate comparable to death from Ebola virus disease [19,20]. Finally, data from a 13-year prospective cohort of bloodstream infections from Duke University Medical Center (2002–2015), P. aeruginosa bloodstream infection was associated with the highest mortality relative to S. aureus or other GNB, even after adjustment for patient and treatment factors [21].
2.1.2. Mechanisms of resistance
In general, resistance to β-lactam antibiotics in P. aeruginosa is due to mutations that lead to hyperproduction of AmpC cephalosporinases (Pseudomonas-derived cephalosporinases, PDC). In the case of carbapenem-resistant strains, there usually is an interplay of mutations in the OprD porin (sufficient to exclude imipenem), up-regulation of drug-efflux pumps (usually needed to exclude meropenem), mutations in the penicillin-binding-proteins (PBP) and decrease in PBP expression [22–24]. These mutational mechanisms are involved in in vivo emergence of pan-ß-lactam resistance. Fortunately, many such strains retain susceptibility to the new antipseudomonal cephalosporin, ceftolozane, which is co-formulated with the β-lactamase inhibitor tazobactam [25]. Similarly, the combination of ceftazidime with avibactam, the novel β-lactamase inhibitor against ESBLs, class A, some class D β-lactamases, and AmpC class C enzymes, also has significant activity against these types of strains resistant to all other β-lactams. Of note, neither ceftazidime-avibactam nor ceftolozane-tazobactam are active against P. aeruginosa with acquired carbapenemases, such as metallo-β-lactamases (MBLs). Nevertheless, pre-existent resistance against ceftazidime-avibactam is not infrequent in P. aeruginosa, likely due to changes in membrane permeability and drug efflux [26]. Resistance to ceftolozane-tazobactam can occur prior to treatment, and is associated with therapeutic failure [27]. Emergence of resistance during treatment has been observed, and attributed to mutations resulting in AmpC or PDC overexpression [28]. MBLs are only detected sporadically in the U.S. [24,29]. In a recent survey of 290 meropenem non-susceptible P. aeruginosa from 34 US hospitals, an extensive genotypic characterization revealed the presence of VIM (an MBL) among only 4% of the subset of isolates non-susceptible to ceftazidime-avibactam, ceftolozane-tazobactam or both. MBL-producing P. aeruginosa have with VIM-7 and VIM-2 have been detected in cancer patients in Houston and VIM-2 producing P. aeruginosa have caused outbreaks in Chicago and Northeast Ohio [30,31]. In contrast, VIM-2 mediated carbapenem-resistance in P. aeruginosa has long been observed in the Mediterranean basin [32]. Indeed, among 529 isolates of P. aeruginosa collected from 2009–2011 from 14 European and Mediterranean countries, there was increasing prevalence of MBL producers, from 12% in 2010 to 31% in 2011 [33]. P. aeruginosa-harboring Klebsiella pneumoniae carbapenemase (KPC) has been detected in Puerto Rico and other Latin American locales [34]. Note that the major mechanisms of resistance for P. aeruginosa as well as the other organisms discussed are summarized in Table 1.
Table 1.
Major mechanisms of resistance
| β-lactamases | Others | ||||
|---|---|---|---|---|---|
| Class A | Class B | Class C | Class D | ||
|
Pseudomonas aeruginosa [22,24] |
PER-1, PER-2, VEB-1, VEB-2, VEB-3, GES-1, GES-2, GES-5, GES-8, GES-9, GES-13, GES-18, BEL-1, BEL-2, BEL-3, PME-1 Rarely identified: TEM-, SHV-, CTX-M-, KPC-, and NDM- types |
IMP-1, VIM-1, VIM-2, SPM-1, GIM-1, FIM-1 | AmpC overexpression |
OXA-50 (naturally occurring), OXA-1, OXA-2, OXA-10, OXA-18, OXA-24/40, OXA-45, OXA-56, OXA-198 subfamilies (acquired) |
OprD porin deficiency; Alterations of efflux systems (MexAB-OprM RND-type system, mexCD-oprJ and MexEF-oprN opérons); PBP mutations; Aminoglycoside-modifying enzymes (RmtA and RmtD enzymes); LPS alteration (cobstin resistance) |
|
Acinetobacter baumannii [22] |
PER-1, PER-2, PER-3, PER-7, VEB-1, VEB-3, GES-1, GES-11, GES-12, GES-14, GES-22 Rarely identified: TEM-, SHV-, CTX-M- and KPC- types |
IMP-1, IMP-2, IMP-4, IMP-5, IMP-8, IMP-11, IMP-14, IMP-19, VIM-1, VIM-2, VIM-4, VIM-6, VIM-11, SIM-1, NDM-1, NDM-2 | AmpC overexpression |
OX A-51 (naturally occurring), OXA-23, OXA- 24/40, OXA-58, OXA-143, OXA- 23 5 subfamilies (acquired) | Overexpression of efflux systems (adeABC, adeIJK opérons and AbeM efflux system); PBP mutations; Aminoglycoside-modifying enzymes (ArmA and RmtB enzymes); LPS alteration (cobstin resistance) |
| Stenotrophomon as maltophilia [51,52] | L2 cephalosporinase Rarely identified: TEM-2, CTX-M |
L1 carbapenemase Rarely identified: NDM-1 |
Overexpression of efflux systems (RND pumps SmeABC, SmeDEF, SmeOP-TolC, SmeYZ, SmeVWX,SmeIJK; ABC pumps MacABC, SmrA, FuaABC; MFS pumps FloR, MfsA, tetA, Ter A) FPS alteration; Aminoglycoside-modifying enzymes; Bypassing dihydropteroate synthase and dihydrofolate reductase; Presence of class I integron carrying sal and dhfr genes |
||
|
Burkholderia cepacia complex [72,77,79,85] |
PenA, PenB carbapenemases |
AmpC, AmpD overexpression |
Overexpression of efflux systems (RND-3, RND-4 and RND-9 systems); FPS alteration; Modification of dihydrofolate reductase; Mutation of the QRDR region of topoisomerase II; Presence of class I integron |
||
RND Resistance-Nodulation-Division; PBP Proptein Binding Proteins; LPS lipopolysaccharide
2.2. Acinetobacter baumannii
2.2.1. Clinical impact
The genus Acinetobacter contains more than 55 species, most of which are non-pathogenic and are recovered from soil, water, and even hydrocarbon sludge [35,36]. In the 1970s, several members of the species, collectively named Acinetobacter baumannii-calcoaceticus complex (ABC), emerged as serious pathogens, involved in numerous nosocomial outbreaks. The ABC complex includes A. baumannii, A. nosocomialis and A. calcoceticus [37].
Initially susceptible to several classes of antibiotics, A. baumanni isolates are now routinely XDR, including resistance to carbapenems [38]. A pooled analysis of patients with carbapenem-resistant A. baumanni revealed that they face a significantly higher risk of mortality than patients with carbapenem-susceptible isolates, with a crude OR of 2.22 (95% CI = 1.66, 2.98). The risk of mortality is perhaps increased because patients with carbapenem-resistant A. baumanni are more likely to have severe underlying illness and receive inappropriate antimicrobial treatment than patients with carbapenem-susceptible isolates. [39].
In the U.S., A. baumannii is a cause of less than 2% of all healthcare-associated infections reported to the NHSN. In the latest NHSN report spanning 2011–2014, it was no longer in the top 15 most prevalent species reported across all hospital-associated infections [16]. Whether there is indeed a “secular trend” towards lower rates of infections caused by A. baumannii in the US healthcare system requires confirmation. Nonetheless, A. baumannii displays the highest carbapenem-resistance rates relative to all the other reported pathogens: 52% and 64% for isolates causing catheter-associated bloodstream infections and catheter-associated urinary tract infections respectively (for 2011–2014), and 60% for isolates causing VAP (2011–2012) [16].
2.2.2. Mechanisms of resistance
A. baumannii demonstrates low permeability to antibiotics and constitutive efflux which allows it to inherently evade a broad array of antibiotics [38]. The former is the result of reduced outer membrane porin content compared to other Gram-negative organisms, and the latter is caused by constitutive low expression of one or more active efflux systems [38,40].
In addition to these inherent characteristics, acquired resistance mechanisms include numerous ß-lactamases (most commonly AmpC or Acinetobacter-derived cephalosporinases, ADC), OXA type-carbapenemases (intrinsic OXA-51, and the acquired OXA-type enzymes belonging to the OXA-23, OXA-24/40, OXA-58, OXA-143, and OXA-235 subfamilies), efflux pumps, as well as alterations of outer membrane proteins and alteration in expression of penicillin binding proteins (PBP). Reports of isolates containing KPC, and MBLs such as the New Delhi MBL (NDM-1) also exist [41].
A. baumannii acquires resistance genes from other organisms via horizontal gene transfer [42]. Globally, two clones labeled global clones 1 and 2 (GC1 and GC2) currently account for the majority of MDR isolates of A. baumannii and were first identified in the 1970s. Hall and colleagues recently dissected the evolutionary history of these clones, particularly GC1. The Australian team revealed a high mutation rate, coupled with extensive horizontal gene transfer and homologous recombinations that overtime have resulted in a diversity of MDR subclones [43]. Fournier et al. described a remarkably resistant strain of A. baumannii, the AYE epidemic strain. This strain had an 86 kilobase genomic island (AbaR1) containing 88 open reading frames (ORF) likely acquired from other Gram-negative organisms such as Salmonella, Escherichia coli and Pseudomonas spp. Of the 52 resistance genes found in this strain, as many as 45 were localized to this resistance island alone [44]. This and other examples illustrate the outstanding ability of the Species to diversify and lead to new resistant and versatile pathogens [42].
2.3. Stenotrophomonas maltophilia
2.3.1. Clinical impact
Stenotrophomonas maltophilia is an emerging pathogenic non-fermenting Gram-negative rod most commonly encountered among immunocompromised hosts in the hospital setting. Risk factors for infection in non-cystic fibrosis patients include malignancy (especially hematologic), treatment with corticosteroids or immunosuppressant medications, exposure to broad spectrum antibiotics, prolonged hospitalization, intensive care unit admission, mechanical ventilation, presence of an indwelling central catheter, and hemodialysis [45]. Recent surveillance studies estimate its prevalence among isolates from all sources at 1.3% to 1.7% [46]. S. maltophilia is an environmental pathogen found in water and soil, and has also been isolated in multiple medical devices and products. Most common infectious syndromes are pneumonia, including hemorrhagic pneumonia, bloodstream infection, including catheter-associated bloodstream infections, and urinary tract and wound infections. Other less common syndromes include post-neurosurgical meningitis, endocarditis, sinusitis, eye infections and septic arthritis [47]. Although difficult to ascertain, in a systematic review, Falagas et al. estimated attributable mortality of S. maltophilia infections at up to 38% [48]
Mortality is even higher in patients with malignancy, in whom it can reach more than 50% [49,50].
2.3.2. Mechanisms of resistance
S. maltophilia is intrinsically resistant to ß-lactams including carbapenems, as well as aminoglycosides. Like A. baumannii, it possesses a relatively impermeable membrane, and like P. aeruginosa, it expresses efflux pumps and acquires additional resistance determinants in class 1 integrons. Key mechanisms of resistance include the production of inducible ß-lactamases, and aminoglycosides-modifying enzymes, listed in Table 1 [45,51,52]. The S. maltophilia chromosome harbors two ß-lactamases: L1, an MBL with carbapenemase activity that does not hydrolyze aztreonam, and L2, a serine cephalosporinase that can be inhibited by clavulanic acid [51,53]. Trimethoprim-sulfamethoxazole is left as the main antibiotic option to reliably treat infections caused by S. maltophilia, although resistance has emerged, associated with the genes sul1 and sul2. These have been linked to the presence of class 1 integrons in plasmids in the main, but also in the chromosomal genome [51]. Fluoroquinolones, colistin, and especially tigecycline and minocycline may offer activity [54].
2.4. Burkholderia cepacia complex
2.4.1. Clinical impact
B. cepacia complex (Bcc) organisms are ubiquitous environmental pathogens found worldwide in water, soil and plants. Bcc were originally described by Burkholder in 1950 as the causative agents of the bacterial rot of onion bulbs. While agents from this group, particularly B. multivorans, are important pathogens in patients with cystic fibrosis and chronic granulomatous disease, they remain infrequent and their epidemiology poorly defined in other populations [55]. Information about clinical characteristics and outcome of patients with Bcc bloodstream infections is mostly derived from small cohorts in the context of outbreaks. These cohorts indicate that patients with Bcc bloodstream infections are critically ill older adults with serious underlying diseases, receive intensive care, and have undergone invasive procedures [56–58]. When sources of infection were identified, the respiratory tract and intravascular catheters were the most common portals of entry [56–60].
Bcc organisms are also remarkable in their ability to contaminate environmental surfaces and drug solutions and cause outbreaks in vulnerable populations including hospitalized and intensive care unit patients, cancer patients and transplant recipients [61–69]. A recent report describes a multi-state outbreak related to oral liquid docusate sodium, a common stool softener [70,71]. Owing to lifestyle-specific chlorhexidine tolerance mechanisms via efflux pump mutants, Bcc organisms can also contaminate disinfecting solutions such as the widely used chlorhexidine and can be a cause of outbreaks in that context [72,73].
In addition to their outbreak and contamination potential, these organisms have bioenvironmental and agricultural applications. While they are no longer used for these purposes, prior use has raised concerns for selective pressure and resistance promotion through human environment contamination, including water reservoirs, food supply and household plants [74–76]. This, in addition to tolerance to chlorhexidine, render Bcc an under-recognized potential public health threat.
2.4.2. Mechanisms of resistance
Bcc organims are intrinsically resistant to multiple antimicrobial classes, and acquired resistance is prevalent. Mechanisms of resistance are varied and selection of mutant clones is favored by the propensity of these organisms to cause long-standing and difficult-to-eradicate colonization and infections in patients exposed to frequent antibiotic courses, such as cystic fibrosis patients [77]. Antimicrobial treatment of infections caused by Bcc is more difficult due to their ability to form biofilms, particularly in patients with cystic fibrosis and those with intravascular catheters [78].
Resistance to ß-lactams in Bcc is attributable to the production of inducible or constitutively expressed chromosomal or plasmid-mediated ß-lactamases. One major ß-lactamase is PenA, a chromosomally-encoded inhibitor-resistant class-A-carbapenemase that structurally resembles Klebsiella pneumoniae carbapenemase (KPC) [77,79]. Others are also described in the literature (PenB, AmpC), as well as efflux pumps and mutations in the cell-wall recycling enzyme AmpD, but the role of these mechanisms with treatment failure in clinical practice is not well understood and their relative impact may be differentially represented in individual Bcc species [55,77,80–86].
The main mechanisms of fluoroquinolones resistance occur through efflux transporter systems and through the accumulation of mutations in the quinolone resistance-determining region of the topoisomerase genes. Efflux systems are also implicated in resistance to aminoglycosides and trimethoprim. A modification of the dihydrofolate reductase enzyme also contributes to trimethoprim resistance, and changes in outer membrane affecting the binding sites on the lipopolysaccharide layer is related to intrinsic resistance to the polymyxins and aminoglycosides [83,85,87,88].
3. Implications for present and future antibiotic therapy
3.1. Introduction of concepts of therapy: monotherapy vs. combination antibiotic chemotherapy
The rise of MDR Gram-negative organisms and the dwindling pipeline of new agents and novel therapies have led to renewed interest in combination antibiotic regimens for MDR and XDR GNB, particularly carbapenem-resistant, polymyxin-only-susceptible isolates [89]. The supporting data for such use is mixed and mostly based on retrospective or nonrandomized prospective cohorts of clinically heterogeneous populations using a large number of different combinations [89,90]. With few exceptions [91], these studies so far point to an advantage of combination schemes over monotherapy only for carbapenem-resistant Enterobacteriaceae (CRE) but not for other MDR GNB infections [89,92]. Combination therapy seems beneficial in two instances in particular: i) for isolates that show a meropenem or imipenem Minimum Inhibitory Concentration (MIC) of < 16 mg/ L [93]; ii) for patients with CRE bloodstream infections and a high mortality score as measured by the validated mortality score INCREMENT-CPE, in whom combination therapy was associated with improved survival [94].
Despite limited data and concerns that combination chemotherapy is more expensive and potentially more toxic, it remains appealing for many reasons. Combination chemotherapies have proven their utility for various viral, mycobacterial and parasitic infections (HIV, MTB, malaria, filariasis etc.). The rationale for its use in MDR and XDR Gram-negative infections is based on maximizing bacterial killing and minimizing the emergence of resistance through synergistic combination of antibiotics [92]. Monotherapy with agents like polymyxins and fosfomycin, in many cases the only agents carbapenem-resistant Gram-negative organisms remain susceptible to, has been shown to lead to the development of resistance during therapy [8,89,92]. Additionally, we know that multidrug resistance has been associated with patients being more likely to receive inappropriate antimicrobial therapy and Micek et al. found that appropriate initial antimicrobial therapy was more likely to be administered in patients receiving combination chemotherapy. Hence combination chemotherapy might increase the likelihood of administering initial appropriate empiric therapy [8,95]. Further research is still needed to confirm whether the microbiologic rationale for combination chemotherapy translates into clinical benefit, but in the setting of multidrug resistance limiting effective options, careful consideration is warranted. So far three published randomized controlled trial (RCT) studied combination antibiotic chemotherapy for MDR GNB infections, and the three addressed the treatment of MDR A. baumannii alone (Table 2).
Table 2.
List of major randomized, controlled trials of combination antibiotic therapy versus monotherapy for drug-resistant Gram-negative bacteria
| Name | Start date/completion date | Location | Organisms/Syndrome | Intervention* | Primary Outcome | Status or Results |
|---|---|---|---|---|---|---|
| For treatment of drug-resistant Acinetobacter baumannii alone | ||||||
| - Durante- Mangoni et al, 2013 [154] - Multi-center, open-label, parallel, phase 3 randomized controlled trial - n=209 |
November 2008 – July 2011 | Italy | - Colistin- susceptible XDR- Acinetobacter baumannii (resistant to carbapenems MIO 16 mg/L and all other antibiotics except colistin) - BSI, HAP, VAP, complicated intra-abdominal infections |
Colistin 2 Million units IV every 8 hours and and rifampicin 600 mg orally every 12 hours vs. colistin alone (same dose) for 10–21 days |
30-day all-cause mortality | - Primary outcome: 45/104 (43.3%) mortality in the combination arm vs. 45/105 (42.9%) in the colistin arm (NS) - Microbiologie eradication: 63/104 (60.6%) in the combination arm vs. 47/105 (44.8%) in the colistin arm (p=0.034) |
| - Sirijatuphat R. and Thamlikitkul V., 2014 [155] - Single-center, open-label, parallel, phase 3, randomized controlled trial - n=94 |
January 2010 – March 2011 | Thailand | - Carbapenem- resistant Acinetobacter baumannii - Pneumonia, BSI, UP, skin and soft tissue infections, intraabdominal infections |
Colistin base activity 5 mg/ Kg/ day and fosfomycin 4g IV every 12 hours vs. colistin alone (same dose) for 7–14 days |
- Favorable clinical outcome defined as “cure or improvement at 28 days” | - Primary outcome: 62.8% favorable outcome in the combination arm vs. 56.4% in the colistin arm (NS) Microbiologie eradication: 100% in the combination arm vs. 84.5% in the monotherapy arm (p=0.023) |
| - Aydemir H. et al. [153] - Single-center, open-label, parallel, phase 3, randomized controlled trial - n=43 |
March 2011 – March 2012 | Turkey | - Carbapenem- resistant Acinetobacter baumannii VAP alone £ |
Colistin base activity 300 mg daily and rifampicin 600 mg daily vs. colistin alone (same dose) |
Clinical response (normothermia, normal respiratory secretions, PaO2/FiO2>240 or nomechanical ventilation) |
- Primary outcome: 11 (52.4%) clinical response in the combination arm vs. 9 (40.9%) in the monotherapy arm (NS) - Time to microbiologie clearance: 3.1+/− 0. 5 days in the combination arm vs. 4.5+/−1.7 days in the monotherapy arm (p=0.029) |
| For treatment of other drug-resistant Gram-negative bacteria | ||||||
| -NCT01732250 (responsible party: Mical Paul, Rabin Medical Center, Israel) - Multi-center, open label, parallel, phase 4 trial, randomized controlled trial |
March 2013 – February 2017 | Greece, Israel, Italy |
- Carbapenem- resistant, colistin- susceptible Gram-negative bacilli - BSI, HAP, VAP, UH |
Colistin 9 Million units loading IV once then 4.5 Million units every 12 hours and meropenem 2g IV every 8 hours vs. colistin alone (same dose) for 10 days |
Clinical success at 14 days (composite of: being alive, not in septic shock or improved SOFA score, stable or improved Pa02/ Fi02 in the case of HAP/VAP, and negative blood cultures if still febrile). | Completed - pending publication of results |
| -NCT 01597973 (responsible party: Keith Kaye, University of Michigan) - Multi-center, triple-blind, parallel, phase 3, randomized controlled trial |
October 2012 – September 2021 | United States, Israel, Taiwan, Thailand | - XDR- Acinetobacter baumannii, XDR- Pseudomonas aeruginosa, and carbapenem- resistant Enterobactericea e -BSI or pneumonia |
Colistin and meropenem vs. cobstin and placebo | 30-day all-cause mortality |
Currently recruiting |
Doses renally-adjusted as needed
XDR Extensively drug-resistant; MIC Minimum Inhibitory Concentration; BSI Bloodstream Infection; HAP Hospital-Acquired Pneumonia; YAP Ventilator-Associated Pneumonia; UTI Urinary Tract Infection; SOFA Sequential Organ Failure Assessment score
Two RCTs are currently ongoing to compare combination chemotherapy to monotherapy. They compare colistin and meropenem vs. colistin alone; one in patients with XDR A. baumanii and P. aeruginosa, and CRE (NCT01597973), the other in carbapenem-resistant, colistin-susceptible GNB (NCT01732250). While the former is still recruiting, we are awaiting the results of the latter which the authors, led by Mical Paul from Israel, completed in February 2017 (Table 2).
The results of these trials will shed further light on the clinical benefit of combination chemotherapy or lack thereof for infections caused mostly by A. baumanii, but the question remains for other non-fermenters such as P. aeruginosa, as well as Bcc and S. maltophilia. Indeed, these last two organisms present extraordinary challenges. Bcc are intrinsically resistant to both carbapenems and polymyxins and S. maltophilia is intrinsically resistant to multiple antibiotics including carbapenems; however, they are not the target of current studies or drug development efforts. These unfortunate realities underscore the urgent need to study combinations of agents active against these two organisms.
3.2. P. aeruginosa
3.2.1. Monotherapy
Traditionally, the anti-pseudomonal antibiotics include ceftazidime, cefepime, piperacillin/ tazobactam, aztreonam, carbapenems (except ertapenem), fluoroquinolones (ciprofloxacin, levofloxacin), aminoglycosides, and polymyxins. These agents should always be administered at the appropriate higher dosages described in the literature [96]. Of note, in vitro data demonstrate a lower MICs for doripenem against P. aeruginosa, followed by those of meropenem and imipenem [97]. However, the superior clinical efficacy of doripenem is not borne out by clinical studies.
Since the introduction of ceftolozane-tazobactam, several case reports and case series of MDR P. aeruginosa infections treated with ceftolozane-tazobactam have demonstrated the clinical efficacy of this formulation, including its use to treat infections in critically ill patients, and those with cystic fibrosis [98,99]. In contrast, experience with ceftazidime-avibactam in the management of P. aeruginosa infections is more limited, although adding avibactam to ceftazidime is successful in lowering MICs of many XDR P. aeruginosa isolates [100,101]. When considering XDR P. aeruginosa, an important concept is that although ceftolozane-tazobactam is more likely to be active than ceftazidime-avibactam, there are some ceftolozane-tazobactam-resistant strains that can be susceptible to ceftazidime-avibactam [102]. Therefore, in vitro susceptibilities to ceftazidime-avibactam should be obtained.
MBL-producing P. aeruginosa strains demonstrate resistance against all anti-pseudomonal ß-lactams, including ceftazidime-avibactam and ceftolozane-tazobactam. The monobactam aztreonam is unique in demonstrating stability to hydrolysis by MBLs and MBL-producing P. aeruginosa may retain susceptibility to aztreonam [103,104]. Many strains of MBL-producing P. aeruginosa will also contain mechanisms of resistance against aztreonam, such as increased expression of PDCs. Nevertheless, aztreonam is an attractive component for rationally optimized combination with ceftazidime-avibactam for the treatment of infections caused by MBL-producing P. aeruginosa [105], as well as by MBL-producing S. maltophilia [53,106], and MBL-producing Enterobacteriaceae [107].
Analyses of PK/PD characteristics and clinical outcomes demonstrates that anti-pseudomonal ß-lactams ought to be administered as an extended infusion, as this dosing strategy likely results in lower mortality relative to intermittent infusions, particularly for P. aeruginosa strains with higher MICs [108]. This strategy is based on the possibility of attaining longer periods of free drug exposure above the isolates’ MICs [89]. In one study of patients with invasive P. aeruginosa infections admitted to the hospital between 2008 and 2010, mortality was significantly lower in the group that received extended cefepime infusion compared to those who were administered intermittent infusion (1/33 or 3% vs. 11/54 or 20%, p=0.03) [109]. The same group also observed a significant reduction in 14-day mortality in patients treated with extended infusions of piperacillin-tazobactam compared to intermittent infusions. Other studies have corroborated these findings [108], including large, double-blind placebo-controlled randomised controlled trials, as long as patients were not undergoing renal replacement therapy [110,111].
The anti-pseudomonal fluoroquinolones, ciprofloxacin and levofloxacin, when active, are important options against P. aeruginosa [112], especially because of their high oral bioavailability. Similarly, among aminoglycosides, tobramycin and amikacin offer reliable activity against P. aeruginosa, even MDR strains. Reservations about the efficacy and toxicity of monotherapy with aminoglycosides remain, but may be overcome with careful dosing guided by therapeutic drug monitoring, with special attention to peak and trough levels, and fluctuations in renal function [113].
The polymyxins, colistimethate-sodium and polymyxin B, have become a mainstay in the treatment of carbapenem-resistant and other MDR P. aeruginosa, as agents of “last resort”. Unfortunately, our confident use of polymyxins is hampered by evolving definitions of their different pharmacologic characteristics and nephrotoxic potential. Nonetheless, consensus is emerging regarding the superiority of colistin versus polymyxin B for treatment of urinary tract infections, but inferiority in all other infections, in addition to increased nephrotoxicity of colistin relative to that of polymyxin B [89]. Interestingly, case series and retrospective comparisons validate the efficacy of monotherapy with this class of agents against P. aeruginosa. Nevertheless, in vitro studies raise concerns about the reliability of polymyxins as monotherapy against P. aeruginosa, in terms of attaining requisite PK/PD targets and succumbing to resistance.
3.2.2. Combination chemotherapy
Both intrinsic and acquired resistance frequently result in delays before an appropriate antimicrobial regimen is initiated for P. aeruginosa. Such delays correlate with worse outcomes and higher mortality [114]. Therefore, there is a strong argument to support combination therapy for serious infections caused by P. aeruginosa, aiming to deliver effective empiric therapy. A multicenter retrospective study of 183 monomicrobial P. aeruginosa VAP cases in Spain showed that the administration of two empiric antipseudomonal antibiotics resulted in improved survival and fewer microbiological failures [115]. Conversely, a meta-analysis of 11 P. aeruginosa VAP clinical trials comprising 1,805 patients comparing monotherapy to combination chemotherapy, demonstrated no significant differences in either efficacy or treatment failure between both regimens, albeit the overall quality of the studies was low [116]. Similarly, a multicenter randomized trial conducted by Heyland and colleagues [117] among critically ill patients with late onset VAP found no statistically significant difference between combination chemotherapy and monotherapy. Although the trial revealed enhanced microbiologic clearance and shorter intensive care unit stays for patients receiving combination antimicrobials, the differences were not statistically significant. Neutropenic patients infections caused by P. aeruginosa may be more vulnerable to ineffective empiric therapy [118]. Nevertheless, it is firmly established that there is no additional benefit of aminoglycosides to ß-lactams monotherapy, and that they augment nephrotoxicity [119,120].
An additional theoretical argument for combination therapy is to achieve antimicrobial synergy, but this is difficult to demonstrate clinically [121]. The checkerboard technique has emerged as a means to examine various combinations and test for possible synergy in the management of MDR P. aeruginosa infections. Combination chemotherapy with piperacillin-tazobactam and tobramycin as well as ceftazidime and tobramycin displayed the highest synergy ratios [122]. The following combinations also displayed synergy: ceftazidime and colistin [123], clarithromycin and tobramycin [124], polymyxins and rifampin [125], meropenem and levofloxacin [126], and prolonged infusion fosfomycin (16 to 24 g) in combination with a carbapenem [127]. A concept that is invoked to support the use of combination therapy against P. aeruginosa is the potential suppression of the emergence of resistance. For example, the mechanistic analysis of a combined regimen of β-lactams and tobramycin demonstrated resistance suppression in P. aeruginosa, through reduction of AmpC β-lactamase expression due to inhibition of protein synthesis by tobramycin [128,129]. Furthermore, it is the antibiotic exposure achieved during the first dose that may be relevant for predicting the microbial response and prevention of resistance to a therapeutic intervention, and the likelihood of achieving this may be increased with combination therapy [130].
A special category is the use of polymyxins-containing combination “salvage regimens” for patients with serious infections caused by XDR P. aeruginosa. There is a considerable number of in vitro studies supporting the use of polymyxins in combination with carbapenems (i.e. doripenem) and other agents (e.g., fosfomycin). Unlike CRE, observational evidence demonstrating the value of combination therapy with polymyxin-based regimens (usually with carbapenems) against P. aeruginosa is scarce. In contrast with Acinetobacter, there are no published randomized controlled trials comparing polymyxin monotherapy vs. combination therapy. Nevertheless, clinicians treating patients with these challenging infections would be wise to carefully evaluate combinations on an individual basis. A suggested approach to interpretation of susceptibility testing and treatment of P. aeruginosa is presented in Figure 1.
Figure 1.
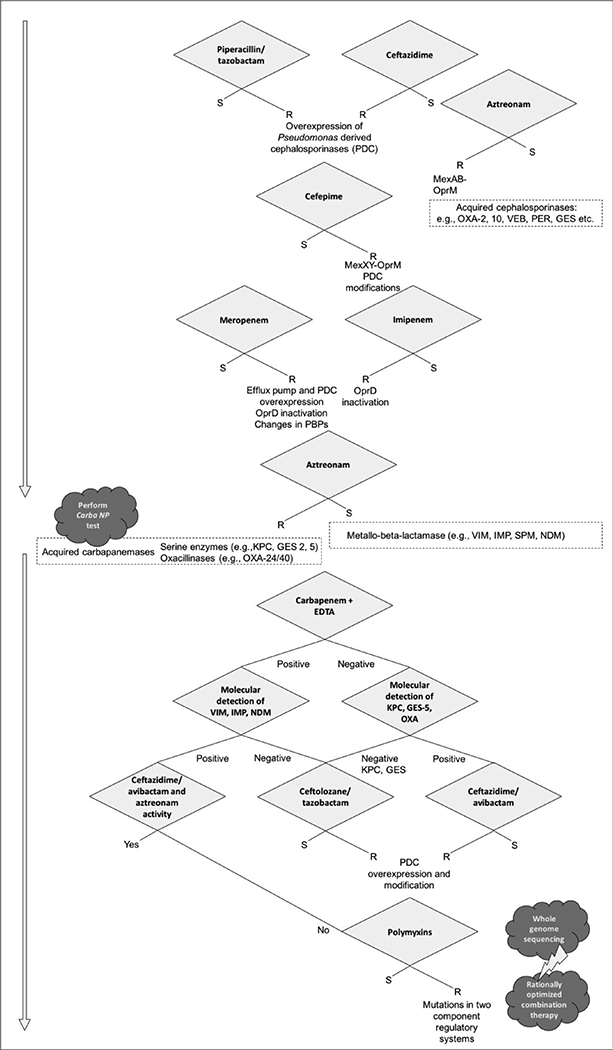
Proposed algorithm for the interpretation of antimicrobial susceptibility testing and treatment of Pseudomonas aeruginosa
3.2.3. Novel therapies
As discussed above, the recently introduced antibiotics ceftolozane-tazobactam and ceftazidime-avibactam offer activity against some carbapenem-resistant P. aeruginosa, but MBL producing isolates are categorically excluded. Although aztreonam-avibactam (currently in phase II studies) offers activity against MBL-producing Enterobacteriaceae, the MIC90 against a large collection of P. aeruginosa, which included MBL-producing isolates, was 32 μg/mL [131]. Meropenem-vaborbactam, also recently approved, is a combination with a ß-lactams with a novel boronic acid ß-lactamase inhibitor; unfortunately, its activity is similar to that of meropenem alone against P. aeruginosa [132]. In contrast, rates of susceptibility of P. aeruginosa to imipenem were improved from 70.3% to 94.2% with the addition of relebactam, a diazabicyclooctane similar to avibactam [133]. Imipenem-relebactam (MK-7655A) is currently in a phase III study to compare its efficacy against colistimethate-sodium in patients with infections caused by imipenem-resistant Gram-negative bacteria, including P. aeruginosa (NCT03293485).
Cefiderocol (S-649266), the novel siderophore cephalosporin able to penetrate the outer cell membrane through bacterial iron transporters, is stable against all ß- lactamases and very potent against P. aeruginosa. Among 82 carbapenem-resistant P. aeruginosa from Greek hospitals, the MIC90 of cefiderocol was 0.5 mg/L [134], similar to those from a global survey; however, isolates with higher MICs were also reported [135]. The recently announced results of a multicenter randomized controlled trial in patients with complicated urinary tract infections (APEKs-cUTI) demonstrated superiority against imipenem/cilastatin (clinical cure and microbiologic eradication of 72.6 % vs. 54.6 %) (NCT02321800). Additional trials vs. meropenem for hospital-acquired and ventilator-associated pneumonia (NCT03032380) and against “best available therapy” for resistant Gram-negative bacteria (NCT02714595) are underway.
There are anti-pseudomonal products that are not traditional antibiotics such as monoclonal antibodies [136] and bacterial phages. Aerubumab is an anti-P. aeruginosa IgG1 monoclonal antibody that binds to surface polysaccharide alginate to enhance immune response, with a prolonged half-life of 3–4 weeks. It is currently in phase-2 trials as adjunct treatment in addition to standard of care antibiotics for pneumonia caused by P. aeruginosa (NCT03027609). Similarly, MEDI-3902 is an anti-P. aeruginosa IgG monoclonal antibody that targets virulence factors Psl and PcrV and is in clinical trials for the prevention of ventilator-associated pneumonia in colonized patients (NCT02696902). The use of monoclonal antibodies beyond adjunctive of pre-emptive therapy, as an alternative to traditional antibiotics remains to be proven. A trial evaluating phage therapy for the treatment of wound infections, including those caused by P. aeruginosa, in burn patients (PHAGOBURN: NCT02116010) might offer more insight on use in humans although considerable work remains to be done before clinical development for systemic therapy is underway. Antimicrobial peptides [137] are also in development but remain in early stages.
3.3. A. baumannii
3.3.1. Monotherapy
Polymyxins (polymyxin B and polymyxin E or colistin, administered as colisthimetate sodium) are the backbone antibiotics for the treatment of carbapenem-resistant A. baumannii. However, high rates of renal injury, and, in the case of colistin, difficulty in achieving adequate and consistent plasma levels, as well as poor lung penetration, make their use challenging [138,139]. Resistance to polymyxins is also increasing, mostly mediated by preventing drug binding through modification of the lipid A moiety of lipopolysaccharide [140].
Sulbactam, in addition to being a ß-lactamase inhibitor, also acts as a ß-lactams by binding to PBP2, and is as such an active agent against A. baumannii. When susceptible, sulbactam-based therapies are a viable option to treat carbapenem-resistant A. baumannii, or even as a carbapenem-sparing option for carbapenem-susceptible strains [141]. Sulbactam is available only as ampicillin/sulbactam or cefoperazone/sulbactam in certain locations. A small meta-analysis of four studies suggest that sulbactam-based therapies are similarly efficacious to various comparator drugs (e.g., fluoroquinolones, tetracyclines, polymyxins, carbapenems) [141–143].
Minocycline and doxycycline are tetracycline antibiotics that are currently being investigated for the treatment of MDR A.baumannii infections. Most of the data is from small retrospective studies, where these agents were used in combination with other therapies. A systematic review by Falagas et al. summarized ten retrospective studies regarding doxycycline and minocycline for the treatment of 185 A. baumannii infection. In this review, tetracyclines in combination with other antibiotics (notably ampicillin-sulbactam, cefoperazone-sulbactam, aminoglycosides, carbapenems, tigecycline, and colistin) for the treatment of several infections caused by A. baumanii were successful in about 77% of patients with an observed mortality of 22% [144]. Tigecycline, a derivative of minocycline, is bacteriostatic and achieves low plasma concentrations. Levels in urine and different lung compartments might also not be sufficient to eliminate bacteria in patients with pneumonia [145,146]. Resistance has also emerged, in addition to breakthrough A. baumannii bacteremia during treatment with tigecycline. All these limitations, in addition to a black box warning about increased risk of death based on multiple systematic reviews [147], make tigecycline a last resort therapeutic option when no other alternative is available. High dose tigecycline-based regimens might overcome these limitations in MDR GNB including A. baumannii [148,149]. Fosfomycin does not have activity against A.baumannii. However, it demonstrated in vitro synergy with colistin and sulbactam [150]. Finally, use of rifampicin as monotherapy is limited by rapid development of resistance. However it has shown some promise as a part of combination chemotherapy.
3.3.2. Combination chemotherapy
Multiple studies have compared monotherapy to combination chemotherapy for the treatment of A. baumannii, but many are retrospective and propose heterogeneous combinations, precluding firm conclusions. A high-quality prospective observational study conducted in 28 Spanish hospitals did not find an association of combination therapy with improved mortality in infections caused by A. baumannii [151].
Polymyxin-carbapenem combinations are commonly used for the treatment of MDR A. baumannii, driven by evidence of in vitro synergy. In a systematic review and meta-analysis of in vitro synergy between the two classes comprising 1,054 bacterial isolates, Zusman et al. showed synergy rates as high as 77% in time-kill studies for combination chemotherapies for A. baumannii. This was the highest observed synergy rate when compared to other organisms such as P. aeruginosa (50%) or K. pneumoniae (44%). All had low antagonism rates [152].
Clinically, a number of prospective studies have examined polymyxin-based combination chemotherapies for the treatment of A. baumannii and share similar conclusions: i) a small, prospective randomized study of treatment of carbapenem-resistant A.baumannii VAP with colistin or colistin and rifampicin, showed improved time to microbiologic eradication in the combination treatment group (4.5 vs 3.1 days), but improvement in mortality was not evident [153]; ii) a RCT of colistin alone compared to colistin and rifampicin for the treatment of serious infections caused by A. baumannii showed improved microbiologic eradication with combination chemotherapy (60.6 vs 44.8) but again, significant differences in mortality were not seen [154]; iii) a study of colistin versus colistin plus fosfomycin for the treatment of carbapenem-resistant A. baumanii infections showed significant microbiologic eradication in favor of the combination (87.8% vs 65.7%), but no significant difference in clinical response [155]. Table 2 provides a summary of available and ongoing RCTs studying combination chemotherapies for A. baumannii infections.
In contrast to these studies, a prospective multicenter observational study showed increased mortality associated with a colistin-tigecycline combination (when the MIC of tigecycline exceeded 2 mg/L) compared to the colistin-carbapenem combination [156]. Another polymyxin-based combination that has shown synergistic killing in vitro as well as in animal models is colistin-glycopeptides but clinical data is inconclusive [157–159]. Lastly, Lenhard et al. recently demonstrated additivity or synergy against two clinical isolates of A. baumannii using triple therapy with polymyxin B, high-dose ampicillin-sulbactam, and meropenem in a 14-day hollow-fiber infection model. The isolates were resistant to all 3 antibiotics yet the combination resulted in rapid (96-hour) eradication of A. baumannii in 24-hour time-kill experiment [160].
Finally, none of the studies reviewed here show significant mortality benefit as a primary outcome with using combination chemotherapy, only improved microbiologic eradication with different combinations. These and others are presented in the figure 2. A suggested approach to interpretation of susceptibility testing and treatment of A. baumannii is also presented in Figure 3.
Figure 2.

Forest plot comparing mortality for combination therapy versus monotherapy in major cohort studies of Acinetobacter baumannii infections (both retrospective and prospective)[151,153–155,187–193]
Figure 3.
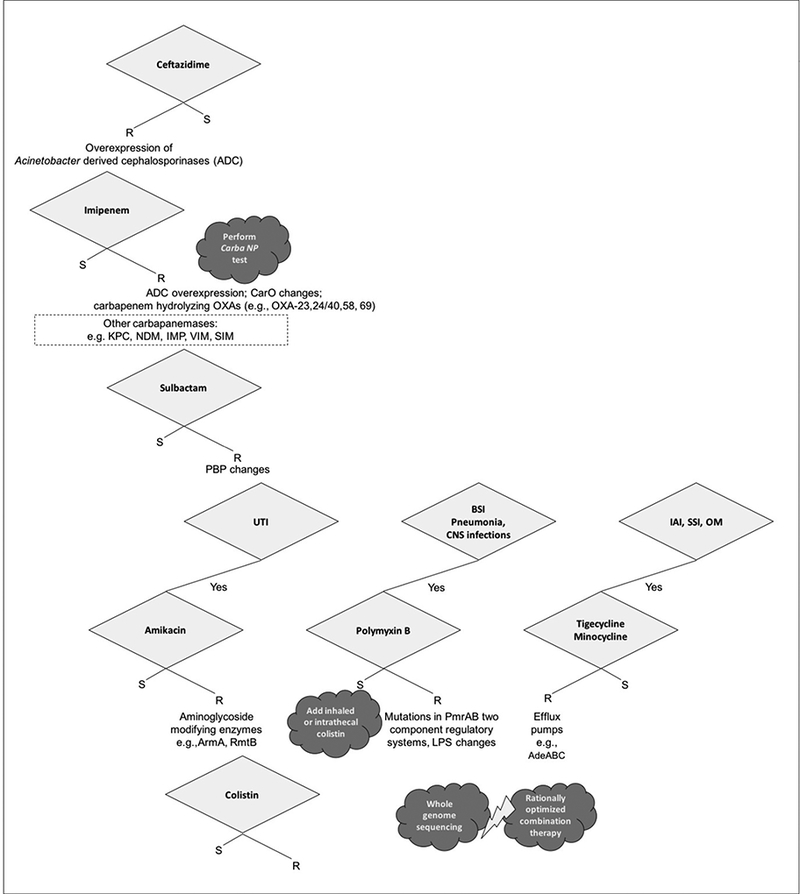
Proposed algorithm for the interpretation of antimicrobial susceptibility testing and treatment of Acinetobacter baumannii UTI Urinary Tract Infection; BSI Bloodstream Infection; CNS Central Nervous System; IAI Intraabdominal Infection; OM Osteomyelitis
3.3.3. Novel therapies
Since the approval of tigecycline in 2005, no new antibiotics with activity against A. baumannii spp. were approved for clinical use. Fortunately, several drugs are currently in Phase 2 and 3 trials that show some activity against A. baumannii. Eravacycline, a novel fluorocycline of a tetracycline class, is currently in phase 3 development. In in vitro experiments with collection of 158 A.baumannii isolates, eravacycline showed to be 4-fold more active than tigecycline by comparison of MIC90 values (1 mcg/mL vs 4 mcg/mL). However, the study also included isolates with MICs reaching 2 and 4 mcg/mL, attributable to increased expression of efflux pump AdeABC [161]. Similarly TP-6076, another synthetic tetracycline more potent than tigecycline against A. baumanii, has elevated MICs against isolates overexpressing the adeABC pump [162].
A next generation aminoglycoside, plazomicin has been the subject of in vitro studies, alone and in combination with other Gram-negative agents against 69 carbapenem-resistant A. baumannii obtained from clinical samples in Spain. MIC90 values were 16- and 32-fold lower than those of amikacin and gentamicin. Studies also looked at plazomicin’s performance in combination with imipenem or meropenem and showed improved synergistic killing compared to amikacin combinations [163]. Cefiderocol, a novel parenteral siderophore cephalosporin, which is currently in Phase 3 development, has shown in vitro activity against A. baumannii, including carbapenem-resistant strains. Cefiderocol was tested against two types of global isolate collections of A. baumanii. One included 104 randomly collected clinical isolates of A. baumannii and another included 99 ß-lactam-resistant strains, including carbapenem-resistant and multidrug resistant strains containing IMP-1, OXA-23, OXA-24/40, OXA-51/ISAba, and OXA-58 enzymes. For the clinical isolates MIC50 and MIC90 were 0.125 mcg/mL and 2 mcg/mL respectively. Among the 99 ß-lactam-resistant strains, only two had an MIC of >32 mg/L and seven strains with MIC of 8mg/L [164]. Though in vitro results look promising for these new agents, how they will perform in the clinical setting remains to be seen. The presence of clinical strains that are already non-susceptible to these new drugs is also discouraging.
Neither avibactam, vaborbactam or relebactam inhibit the class D or OXA-type β-lactamases that confer resistance to carbapenems in A. baumannii. Another diazabicyclooctane β-lactamase inhibitor, ETX2514 does inhibit such enzymes and promises activity against A. baumannii in combination with other agents, but is only in early stages of development [165].
In addition to these new agents, several antibiotic alternatives, adjuvant therapies and vaccines are currently in development. Schooley et al. recently achieved a successful clinical outcome with personalized bacteriophage-based therapy in a patient with necrotizing pancreatitis complicated by MDR A. baumannii infection [166]. As with phage therapy for P. aeruginosa, this case supports the merits of studying phage-based therapies further for MDR and XDR infections with limited therapeutic options.
Another potential adjuvant therapy is inhibition of LpxC, a zinc-dependent deacetylase that catalyzes the first committed step in the biosynthesis of lipid A. Garcia-Quintanilla and her team from the University of Sevilla were recently able to show that the LpxC inhibitor PF-5081090 significantly increased cell permeability and significantly potentiated the activity of existing antibiotics including rifampin, vancomycin, azithromycin, imipenem and amikacin against MDR A. baumannii [167].
3.4. S. maltophilia
3.4.1. Monotherapy
Antibiotics potentially active against S. maltophilia include trimethoprim-sulfamethoxazole, piperacillin-tazobactam, ticarcillin-clavulanate, fluoroquinolones, minocycline, tigecycline, colistin and chloramphenicol. High dose trimethoprim-sulfamethoxazole is the preferred treatment, supported by clinical experience [55]. Some cephalosporins have also been reportedly used successfully. Similar to carbapenems, treatment with the same cephalosporins (especially ceftazidime and cefepime) is also a risk factor for the emergence of S. maltophilia infections. Clinical experience with tetracycline derivatives is limited, but these agents have shown reliable in vitro activity and are an alternate option. The use of colistin is limited by the lack of experience and toxicity.
3.4.2. Combination chemotherapy
Combination chemotherapy is a necessary strategy when S. maltophilia is resistant to trimethoprim-sulfamethoxazole. Data supporting this approach are lacking but the high-level intrinsic resistance and the emergence of acquired resistance under antibiotic pressure makes combination chemotherapy the only available option, especially in severe invasive infections.
Proposed combinations include trimethoprim-sulfamethoxazole with any second-line agent, including ceftazidime, ticarcillin-clavulanate, aztreonam, or a fluoroquinolone, provided both agents used are active against the particular pathogenic strain on in vitro testing [47,55,168–170]. A noteworthy and recent case report describes a MDR S. maltophilia bloodstream infection in a young immunosuppressed renal transplant patient [53]. The organism was resistant to trimethoprim-sulfamethoxazole, ceftazidime, meropenem, and levofloxacin. The authors used PCR to confirm the presence of a L1 MBL and L2 cephalosporinase, both inducible β-lactamases. Disc diffusion susceptibility testing showed resistance to the novel agent ceftazidime-avibactam and aztreonam but the authors demonstrated in vitro synergy between these agents. The patient eventually responded to a “triple combination” of ceftazidime-avibactam and aztreonam with clearance of his bacteremia. This approach is promising, both for S. maltophilia infections as well as infections caused by other Gram-negative organisms with a MBL. However, more studies are needed to confirm the efficacy and safety of such approach. Finally, a suggested approach to interpretation of susceptibility testing and treatment of S. maltophilia is presented in Figure 4.
Figure 4.
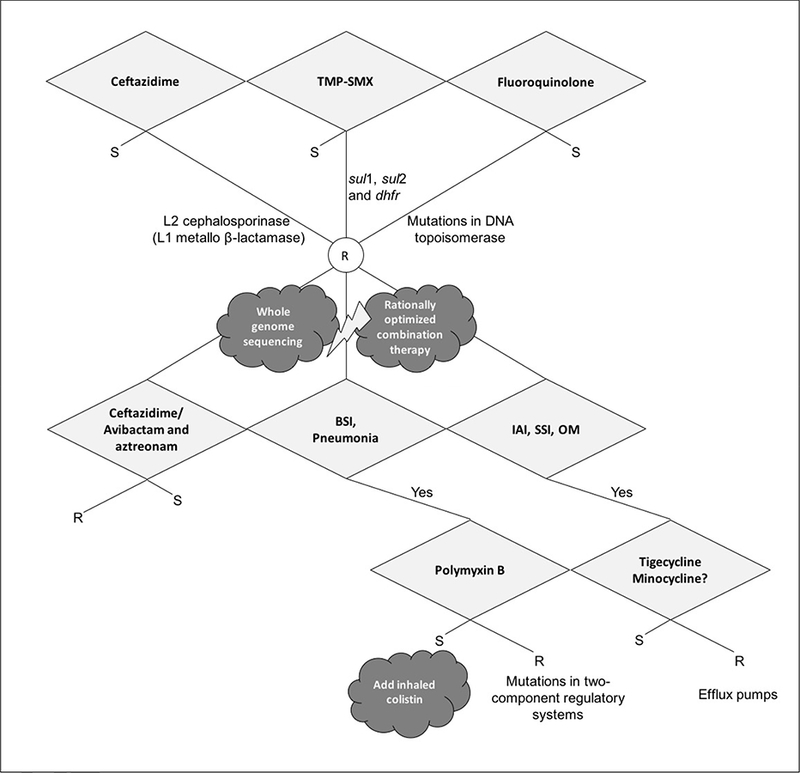
Proposed algorithm for the interpretation of antimicrobial susceptibility testing and treatment of Stenotrophomonas maltophilia TMP-SMX Trimethoprim Sulfamethoxazole; BSI Bloodstream Infection; IAI Intraabdominal Infection; SSI Skin and Soft Tissue Infection; OM Osteomyelitis
3.4.3. Novel therapies
Similar to other MDR organisms, there is a lack of promising agents in development that appear to be available in the near future for clinical use. Parenteral fosfomycin and polymyxins, alone or in combination, are considered possible last-resort agents in desperate situations. However, this is hindered by the variability and difficult interpretation of in vitro testing. Novel agents at different stages of development carry some hope, although it is difficult to predict the outcome of such efforts at this early stage [45,171]. The in vitro efficacy of aztreonam-avibactam against selected clinical strains of S. maltophilia due to the inhibition of the L2 β-lactamase by avibactam has been demonstrated [106]. Also, the novel siderophore cephalosporin cefiderocol is stable against both serine β-lactamases and MBLs harbored by S. maltophilia. The MIC90 of cefiderocol against S. maltophilia were 0.5 μg/ml for North American isolates (n = 152) and 0.25 μg/ml for European isolates (n = 276); none of the isolates had an MIC >4 μg/ml [135].
3.5. Burkholderia cepacia complex
3.5.1. Monotherapy
Bcc are remarkable for being intrinsically resistant to a wider range of antibiotics compared to other non-fermenting GNB. They are inherently resistant to penicillins, cephalosporins (with the exception of ceftazidime), monobactams, carbapenems (with the exception of meropenem), polymyxins, aminoglycosides (with the exception of B. vietnamiensis), and fosfomycin. Additionally, Bcc are resistant to all antimicrobial classes that are usually inactive against non-fermentative GNB. Antibiotics that may be active include: ticarcillin-clavulanate, piperacillin-tazobactam, ceftazidime, meropenem, minocycline, ciprofloxacin, levofloxacin, trimethoprim-sulfamethoxazole, and chloramphenicol. Clinical Laboratory Standards Institute (CLSI) provides interpretative criteria for susceptibility reporting for trimethoprim-sulfamethoxazole, ticarcillin- clavulanate, ceftazidime, minocycline, levofloxacin, and chloramphenicol [172].
Although trimethoprim-sulfamethoxazole is often recommended, high-quality evidence to guide treatment in cystic fibrosis patients and other settings is lacking [173]. The recommendation is nonetheless based on susceptibility profiles reported in retrospective clinical studies. These susceptibility profiles are most favorable for trimethoprim-sulfamethoxazole followed by ceftazidime and meropenem [56–58,174–176], albeit recent studies found elevated resistance to the latter (25%−35% non-susceptiblity to ceftazidime) [58,85]. Readers are referred to Avgeri et al. for a compilation of some of these papers [168]. The authors summarized more than fifty studies and concluded that ceftazidime, meropenem and piperacillin are appropriate choices in monotherapy or in combination with other antibiotics when the administration of trimethoprim-sulfamethoxazole is inappropriate or ineffective. In a cohort of 248 bacteremic cases, overall <20% were treated with trimethoprim-sulfamethoxazole, with no effect on clinical outcome [58]. This and other major studies of bloodstream infections caused by Bcc are summarized in Table 3 and a suggested approach to interpretation of susceptibility testing and treatment of B. cepacia complex is presented in Figure 5.
Table 3.
Summary of the major studies of Burkholderia cepacia complex bloodstream infections
| Characteristics | El Chakhtoura et al. [58] | Liao et al. [174] | Bressler et al. [175]; Woods et al. and [1761 |
Lu et al. [56] | Huang et al. [57] |
|---|---|---|---|---|---|
| Study Population | 1999-2015 | 2004-2007 | 1996-2002 | 1982-1995 | 1997-1999 |
| Time Frame | 1999-2015 | 2004-2007 | 1996-2002 | 1982-1995 | 1997-1999 |
| Number of cases | 248 | 95 | 53 | 70 | 42 |
| Age – mean (range) | 68 (22-94) | 69 (55-87) | 46 (24-82) | 34 (median, <1-83) | 70 (2-92) |
| Source | |||||
| Catheter-related infection | 155/248 (63) | 21/95 (22) | --- | 11/70(16) | 8/42 (19) |
| Respiratory tract infection | 49/248 (20) | 47/95 (49) | --- | 17/70 (24) | 20/42 (48) |
| Nosocomial | 225/248 (91) | All cases | All cases | 64/70 (91) | 40/42 (95) |
| Susceptibility pattern | |||||
| Trimethoprim-sulfamethoxazole | 193/205 (89) | 0/73 (0) | 4/53 (83) | --- | 36/42 (86) |
| Ceftazidime | 152/212 (72) | 71/73 (97) | 37/40 (92) | 41/43 (95) | 40/42 (95) |
| Meropenem | 22/32 (69) | 73/73 (100) | --- | --- | --- |
| Imipenem | 54/136 (40) | --- | 5/40 (13) | 23/38 (61) | 32/42 (76) |
| Recorded outcomes | |||||
| 14-day mortality | 40/248 (16) | 16/95 (17) | 25/53 (47) | 8/70(11) | --- |
| In-hospital mortality | --- | 51/95 (54) | --- | --- | 27/42 (64) |
Adapted from El Chakhtoura et al. [58]
Values in No. (%) unless otherwise specified
Figure 5.
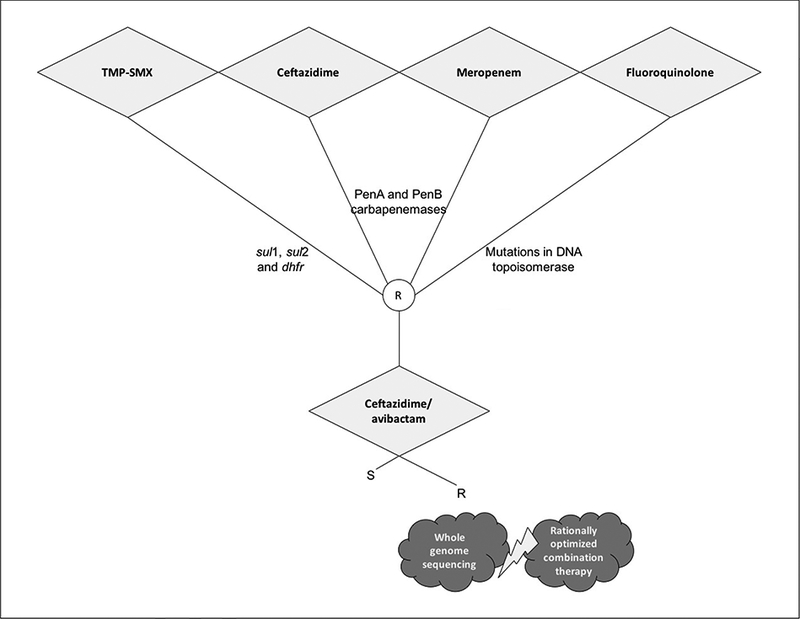
Proposed algorithm for the interpretation of antimicrobial susceptibility testing and treatment of Burkholderia cepacia complex
3.5.2. Combination chemotherapy
The above listed first and second-line agents may also be used in combination of two or more active antibiotics, particularly for salvage treatment. However, as of this writing, reliable data on combination chemotherapy for Bcc infections for either cystic fibrosis or non-cystic fibrosis patients are not yet available.
3.5.3. Novel therapies
The novel non-β-lactam β-lactamase inhibitor avibactam has potent activity against the PenA β-lactamase present in Bcc and as such, the administration of ceftazidime-avibactam may serve as an option in cases where first-line agents are not active. In vitro assays demonstrate that the addition of avibactam to ceftazidime resulted in a ≥4 fold reduction in MIC among 35 of 49 ceftazidime-resistant Bcc strains [177]. In another study, addition of avibactam to ceftazidime also restored susceptibility to ceftazidime in all ceftazidime-resistant B. multivorans isolates from cystic fibrosis patients [178]. Ceftolozane-tazobactam possesses in vitro activity against some organisms from this group. However, the MIC range was broad (≤0.5 to >64 μg/ml), there were differences between species, and clinical efficacy has not been evaluated [179]. Cefiderocol had MICs ≤4 μg/ml against all but one of 12 strains of Bcc included in a recent global survey [135]. Unfortunately, only few agents in development, of which information is currently available to the public, seem to target or be collaterally active against pathogens of the Bcc group.
4. Expert commentary
The challenges presented by non-fermenting GNB infections are remarkable. In addition to their intrinsically resistant nature, the rise in recent years of acquired resistance, particularly to carbapenems, has propelled A. baumannii and P. aeruginosa to the center of the epidemic of AMR. Our experience in treating these and other carbapenem-resistant bacterial infections, such as CRE, is limited to less than a decade and the evidence is scarce, mostly derived from observational cohort studies. Last, but not least, the progress in the development of new antibiotics remains slow.
As a result, infectious disease clinicians have resorted to strategies that utilize basic principles of microbiology and PK/PD against infections caused by MDR and XDR GNB. These include inhalation antibiotic therapy for respiratory infections, reviewed elsewhere [180], in addition to the use of combination antibiotic chemotherapy or the prolonged infusion of antibiotics. The study of such strategies, particularly combination antibiotic chemotherapy, is challenging owing to difficulties in recruitment but also in design. For example, combination chemotherapy has so far consisted of two agents but skeptics have raised the specter of possible resistance selection with only two agents [181–183]. Therefore, should study protocols for the treatment of MDR GNB include three or more agents such as is the case for the treatment of HIV or Mycobacterium tuberculosis? Another challenge concerns which backbone agent to use, should we include a carbapenem in combination chemotherapies for carbapenem-resistant organisms and if we should, what cutoff MIC would we use in such trials? What other agents should we use in combination?
A central notion in attempting to answer these questions is that there is no single set of combination of agents that is effective for individual MDR and XDR GNB, whether fermenters or non-fermenters. This is the result of the multitude of mechanisms of resistance that make up each observed resistance phenotype and the genetic heterogeneity that underlies it. Unfortunately, available therapeutic studies do not address the genetic makeup of the organisms they study and its impact on treatment. While the geographic diversity of these studies is remarkable, it is important to understand that the treatment of carbapenem-resistant A. baumannii or P. aeruginosa originating from the Mediterranean basin is not necessarily the same as for those from the U.S. or Southeast Asia. Molecular characterization of the organisms and the identification of mechanisms of resistance are essential to precisely formulate a choice of combination chemotherapy that would be effective against the epidemiologically predominant strains in a particular location, in what we call “molecularly-targeted” therapy. Similarly, Zavascki et al., have advanced the notion of “rationally-optimized combination regimens” [92]. These regimens ought to be designed based on thorough in vitro antibiotic susceptibility testing including combination testing, screening for resistance genes, including β-lactamases and others, in addition to PK/PD parameters. Such an approach is illustrated in the S. maltophilia case report of Mojica et al. described previously, where the clinicians and microbiologists went from “the bench to the bedside and back”, using genetic and in vitro testing of various combinations to arrive to an effective “rationally-optimized” and “molecularly-targeted” regimen for that individual patient [53].
Unfortunately, the systems currently in place do not, most of the time, allow us in practice to obtain such information in a timely fashion. Yiying Cai and his team have nonetheless proven that this bench-to-bedside approach is feasible [184]. In a pragmatic and effectively designed study, the Singaporean team put in place an “in vitro antibiotic combination testing service” with the catchy acronym “iACT”. The service provided clinicians with the information they needed, based on the parameters we detailed above, to select a rationally-optimized combination antibiotic regimen for the precise treatment of XDR GNB with a rapid turnover of 48 hours. While their study lacked a control group, their prospectively-determined outcomes compared very favorably to historical cohorts, with a low 30-day infection-related mortality of 15% and high clinical response and microbiologic eradication of approximately 80%.
While we await further much needed novel agents, this individualized methodical, rational scheme is the sound alternative to approaches to combination therapy that are otherwise based on generalizations that ignore the marked molecular heterogeneity in resistant bacteria. Despite our optimism about rational, molecularly targeted combinations, we are not naïve to the fact that the current body of clinical evidence does not support the use of combination therapy for non-fermenter GNB. The gap between the existing anecdotal experiences and high quality evidence needs to be filled with systematic studies that rely on the molecular characterization of isolates to determine therapy. We acknowledge that this is not feasible today, but advances in whole genome sequencing promise to rapidly deliver interpretable data to the bedside, allowing for the identification and selection of subjects to be enrolled in such studies. Such breakthroughs will be crucial to bridge the extraordinary obstacles that prevent the development and implementation of molecularly-targeted and rationally-optimized combination therapies.
5. Five-year view
There is an urgent need for further novel antibiotic agents to address the growing epidemic of AMR in general and MDR and XDR non-fermenting GNB in particular. In addition to traditional antibiotic development, other novel therapies are showing promise as adjuvants to antibiotic agents. These include biotherapeutics like monoclonal antibodies or small molecules that target certain bacterial virulence factors such as toxins, biofilms and adherence, and signaling pathways [185]. Other agents that could be used as adjuvants are target immune receptors, which allow for the modulation of host immunity and the enhancement of protective antimicrobial immunity [186].
As these new agents become available, we need to build evidence towards a systematic, standardized approach for the use of rationally-optimized and molecularly-targeted combination antibiotic chemotherapies for the treatment of MDR and XDR GNB. In order to achieve this in the near future, we need to not only develop our rapid diagnostic capabilities geared towards the detection of resistance mechanisms, but to incorporate such information into clinical decision making regarding therapy of infections caused by GNB. This approach will take into consideration the genotypic makeup of resistant isolates, together with their phenotype, in order to make the best therapeutic decisions. Beyond the logistical and technological aspects, this new clinical practice model represents a cultural shift in the way we approach these infections. In other areas such as cancer chemotherapy, this new paradigm has been referred to as “precision medicine”, a term that we think should be applied to antibiotic chemotherapy [89]. We will need this and other innovative approaches as we persevere on our perilous journey to fight infections caused by MDR and XDR GNB. This is particularly true for non-fermenters, which cause serious infection in some of the most vulnerable patients in our healthcare system, are tenaciously resilient in the nosocomial environment, and for which the therapeutic options are limited due to broad intrinsic resistance.
6. Key issues
Non-fermenting Gram-negative bacteria, especially P. aeruginosa and A. baumannii, are critically important in the epidemic of antimicrobial resistance due to broad intrinsic resistance to a wide array of antibiotics, in addition to growing acquired resistance patterns.
Although less prevalent, S. maltophilia and B. cepacia complex have even broader intrinsic resistance, encompasssing most β-lactams, including carbapenems, and polymyxins. They have a disproportionate impact on vulnerable populations, including immunosuppressed and hospitalized patients, and those with cystic fibrosis.
Cefiderocol is a promising agent in the antibiotic pipeline with activity against all four major groups of non-fermenters: P. aeruginosa, A. baumannii, S. maltophilia, and B. cepacia complex, but is still undergoing clinical development.
Ceftolozane-tazobactam is a new option to treat infections caused by carbapenem-resistant, XDR P. aeruginosa where resistance is not mediated by acquired carbapenemases or MBLs. However, the use of ceftolozane-tazobactam should be guided by susceptibility testing because it is not universally active and treatment failures occur with resistance.
Ceftazidime-avibactam has less activity against carbapenem-resistant, XDR P. aeruginosa than ceftolozane-tazobactam. Nevertheless, it may still be an option against some ceftolozane-tazobactam-resistant strains of P. aeruginosa.
No new agents targeting carbapenem and XDR A. baumannii have become available since the introduction of tigecycline in 2005. Given the limitations of tigecycline, polymyxin remains the backbone of antibiotic treatment of XDR A. baumannii.
Eravacycline has in vitro activity against A. baumannii, but it may be vulnerable to efflux pumps present in tigecycline resistant strains, and requires further clinical development.
Trimethoprim-sulfamethoxazole (TMP-SMX) remains the most reliable first-line agent for the treatment of infections caused by S. maltophilia and B. cepacia complex.
Ceftazidime-avibactam B. cepacia complex may serve as an option for strains resistant to TMP-SMX.
Aztreonam is an attractive component for rationally-optimized therapy in combination with ceftazidime-avibactam for infections caused by the MBL-producing non-fermenters P. aeruginosa and S. maltophilia. This is in part due to aztreonam’s stability to hydrolysis by MBLs, a unique feature among β-lactams.
Data from randomized controlled trials demonstrate that combination therapy of A. baumannii polymyxins with rifampin or fosfomycin is not associated with improved survival. There is insufficient clinical data on combination antibiotic therapy against P. aeruginosa, S. maltophilia or B. cepacia complex.
For serious infections caused by MDR and XDR non-fermenters where there are no clear therapeutic options, we advocate a “precision medicine” approach that integrates information on the bacterial phenotype and genotype, in vitro assessments of antibiotic combination, and PK/PD considerations to achieve “rationally-optimized” and “molecularly-targeted” antibiotic regimens.
Acknowledgements
The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Funding
This manuscript was funded by grants from the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program award number 1I01BX001974; the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R01AI063517 and R01AI10056; the Antibiotic Resistance Leadership Group under National Institutes of Health award number UM1AI104681 and the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health.
Footnotes
Declaration of interest
F Perez received grants from Pfizer, Merck; Consulting fee: Allergan, Achaogen. R A. Bonomo received grants from Merck, Allergan, Wockhart. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Reference annotations
* of interest
** of considerable interest
- 1.U.S. Department of Health and Human Services, CDC [Internet]. Atlanta (GA): CDC; c2013. Antibiotic Resistance Threats in the United States [cited 2017 Dec 26] Available from: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
- 2.The European Centre for Disease Control and Prevention (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2014. European Food Safety Authority Journal, 14(2), 4380 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.**. The Review on Antimicrobial Resistance [Internet]. London (UK): O’neill J; c2016. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations [cited 2017 Dec 26] Available from:https://amr-review.org This is a landmark report on the state of antimicrobial resistance worlwdwide. It was commissioned in 2014 U.K. Prime Minister David Cameron and the Wellcome Trust, and led by economist Lord Jim O’Neill. [Google Scholar]
- 4.Spellberg B, Blaser M, Guidos RJ et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis, 52 Suppl 5, S397–428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) [Internet]. Geneva (Switzerland): WHO; c2015. Draft global action plan on antimicrobial resistance [cited 2017 Dec 26] Available from: http://apps.who.int/iris/bitstream/10665/193736/1/9789241509763_eng.pdf?ua=1
- 6.Magiorakos AP, Srinivasan A, Carey RB et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect, 18(3), 268–281 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Palleroni NJ. The Pseudomonas story. Environ Microbiol, 12(6), 1377–1383 (2010). [DOI] [PubMed] [Google Scholar]
- 8.McCarthy K Pseudomonas aeruginosa: evolution of antimicrobial resistance and implications for therapy. Semin Respir Crit Care Med, 36(1), 44–55 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Azzopardi EA, Azzopardi E, Camilleri L et al. Gram negative wound infection in hospitalised adult burn patients--systematic review and metanalysis. PLoS One, 9(4), e95042 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lachiewicz AM, Hauck CG, Weber DJ et al. Bacterial Infections After Burn Injuries: Impact of Multidrug Resistance. Clin Infect Dis, 65(12), 2130–2136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez F, Adachi J, Bonomo RA. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin Infect Dis, 59 Suppl 5, S335–339 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurley MN, Camara M, Smyth AR. Novel approaches to the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. Eur Respir J, 40(4), 1014–1023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andremont A, Lucet J-C. Extensively resistant VIM-2-positive Pseudomonas aeruginosa. The Lancet Infectious Diseases, 13(10), 828–829 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Magill SS, Edwards JR, Bamberg W et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med, 370(13), 1198–1208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarb P, Coignard B, Griskeviciene J et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill, 17(46) (2012). [DOI] [PubMed] [Google Scholar]
- 16.Weiner LM, Webb AK, Limbago B et al. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol, 37(11), 1288–1301 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Li X, Li W et al. Influence of carbapenem resistance on mortality of patients with Pseudomonas aeruginosa infection: a meta-analysis. Sci Rep, 5, 11715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morata L, Cobos-Trigueros N, Martinez JA et al. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother, 56(9), 4833–4837 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SY, Park HJ, Moon SM et al. Impact of adequate empirical combination therapy on mortality from bacteremic Pseudomonas aeruginosa pneumonia. BMC Infect Dis, 12, 308 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) [Internet]. Geneva (Switzerland): WHO; c2016. Ebola Virus Disease [cited 2017 Dec 26] Available from: http://www.who.int/mediacentre/factsheets/fs103/en
- 21.**. Thaden JT, Park LP, Maskarinec SA et al. Results from a 13-Year Prospective Cohort Study Show Increased Mortality Associated with Bloodstream Infections Caused by Pseudomonas aeruginosa Compared to Other Bacteria. Antimicrob Agents Chemother, 61(6), e00954–17 (2017). This is a remarkable prospective study of all bloodstream infections at Duke University Medical Center over a period of 14 years. Authors used propensity-score models to examine species specific bacterial bloodstream infection mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and epidemiology. Int J Antimicrob Agents, 45(6), 568–585 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Papp-Wallace KM, Endimiani A, Taracila MA et al. Carbapenems: past, present, and future. Antimicrob Agents Chemother, 55(11), 4943–4960 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clinical microbiology reviews, 22(4), 582–610 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moya B, Beceiro A, Cabot G et al. Pan-beta-lactam resistance development in Pseudomonas aeruginosa clinical strains: molecular mechanisms, penicillin binding protein profiles, and binding affinities. Antimicrob Agents Chemother, 56(9), 4771–4778 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkler ML, Papp-Wallace KM, Hujer AM et al. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother, 59(2), 1020–1029 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munita JM, Aitken SL, Miller WR et al. Multicenter Evaluation of Ceftolozane/Tazobactam for Serious Infections Caused by Carbapenem-Resistant Pseudomonas aeruginosa. Clin Infect Dis, 65(1), 158–161 (2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.*. Haidar G, Philips NJ, Shields RK et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: Clinical Effectiveness and Evolution of Resistance. Clin Infect Dis, 65(1), 110–120 (2017). The authors have assembled here one of the largest cohorts of patients with MDR P. aeruginosa infections treated with ceftolozane-tazobactam. They also examined emergence of resistance and performed whole genome sequencing and molecular characterization of longitudinal isolates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez F, Hujer AM, Marshall SH et al. Extensively drug-resistant Pseudomonas aeruginosa isolates containing blaVIM-2 and elements of Salmonella genomic island 2: a new genetic resistance determinant in Northeast Ohio. Antimicrob Agents Chemother, 58(10), 5929–5935 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toleman MA, Rolston K, Jones RN et al. blaVIM-7, an evolutionarily distinct metallo-beta-lactamase gene in a Pseudomonas aeruginosa isolate from the United States. Antimicrobial agents and chemotherapy, 48(1), 329–332 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aboufaycal H, Sader HS, Rolston K et al. blaVIM-2 and blaVIM-7 carbapenemase-producing Pseudomonas aeruginosa isolates detected in a tertiary care medical center in the United States: report from the MYSTIC program. J Clin Microbiol, 45(2), 614–615 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornaglia G, Giamarellou H, Rossolini GM. Metallo-beta-lactamases: a last frontier for beta-lactams? The Lancet Infectious Diseases, 11(5), 381–393 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Castanheira M, Deshpande LM, Costello A et al. Epidemiology and carbapenem resistance mechanisms of carbapenem-non-susceptible Pseudomonas aeruginosa collected during 2009–11 in 14 European and Mediterranean countries. J Antimicrob Chemother, 69(7), 1804–1814 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Akpaka PE, Swanston WH, Ihemere HN et al. Emergence of KPC-producing Pseudomonas aeruginosa in Trinidad and Tobago. J Clin Microbiol, 47(8), 2670–2671 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visca P, Seifert H, Towner KJ. Acinetobacter infection--an emerging threat to human health. IUBMB life, 63(12), 1048–1054 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Nemec A Classification and nomenclature of the genus Acinetobacter. (2017). [Google Scholar]
- 37.Gerner-Smidt P, Tjernberg I, Ursing J. Reliability of phenotypic tests for identification of Acinetobacter species. J Clin Microbiol, 29(2), 277–282 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.**. Wong D, Nielsen TB, Bonomo RA et al. Clinical and Pathophysiological Overview of Acinetobacter Infections: a Century of Challenges. Clin Microbiol Rev, 30(1), 409–447 (2017). This is a remarkably comprehensive and very timely clinical and pathophysiological review of Acinetobacter infections in the typical and unique Clinical Microbiology Reviews style. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemos EV, de la Hoz FP, Einarson TR et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect, 20(5), 416–423 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Vila J, Marti S, Sanchez-Cespedes J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J Antimicrob Chemother, 59(6), 1210–1215 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Doi Y, Murray GL, Peleg AY. Acinetobacter baumannii: evolution of antimicrobial resistance-treatment options. Semin Respir Crit Care Med, 36(1), 85–98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Touchon M, Cury J, Yoon EJ et al. The genomic diversification of the whole Acinetobacter genus: origins, mechanisms, and consequences. Genome Biol Evol, 6(10), 2866–2882 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holt K, Kenyon JJ, Hamidian M et al. Five decades of genome evolution in the globally distributed, extensively antibiotic-resistant Acinetobacter baumannii global clone 1. Microb Genom, 2(2), e000052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fournier PE, Vallenet D, Barbe V et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genetics, 2(1), e7 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.*. Chang YT, Lin CY, Chen YH et al. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol, 6, 893 (2015). This well-written review provides an exhaustive survey of most reported S. maltophilia resistance mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sader HS, Farrell DJ, Flamm RK et al. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: results from the SENTRY Antimicrobial Surveillance Program, 2009–2012. Int J Antimicrob Agents, 43(4), 328–334 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Looney WJ, Narita M, Muhlemann K. Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect Dis, 9(5), 312–323 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Falagas ME, Kastoris AC, Vouloumanou EK et al. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol, 4(9), 1103–1109 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Tada K, Kurosawa S, Hiramoto N et al. Stenotrophomonas maltophilia infection in hematopoietic SCT recipients: high mortality due to pulmonary hemorrhage. Bone Marrow Transplant, 48(1), 74–79 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Cho SY, Lee DG, Choi SM et al. Stenotrophomonas maltophilia bloodstream infection in patients with hematologic malignancies: a retrospective study and in vitro activities of antimicrobial combinations. BMC Infect Dis, 15, 69 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanchez MB. Antibiotic resistance in the opportunistic pathogen Stenotrophomonas maltophilia. Front Microbiol, 6, 658 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X-Z, Li J. Antimicrobial Resistance in Stenotrophomonas maltophilia: Mechanisms and Clinical Implications In: Antimicrobial Drug Resistance: Clinical and Epidemiological Aspects, Volume 2 Mayers DL, Sobel JD, Ouellette M, Kaye KS, Marchaim D (Eds.) (Springer International Publishing, Cham, 2017) 937–958. [Google Scholar]
- 53.*. Mojica MF, Ouellette CP, Leber A et al. Successful Treatment of Bloodstream Infection Due to Metallo-beta-Lactamase-Producing Stenotrophomonas maltophilia in a Renal Transplant Patient. Antimicrob Agents Chemother, 60(9), 5130–5134 (2016). Elegant report describing the design of an effective “rationally-optimized” combination antibiotic chemotherapy regimen for the treatment of a MBL-producing S. maltophilia using phenotypic and genotypic data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clinical Microbiology Reviews, 25(1), 2–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abbott IJ, Peleg AY. Stenotrophomonas, Achromobacter, and nonmelioid Burkholderia species: antimicrobial resistance and therapeutic strategies. Semin Respir Crit Care Med, 36(1), 99–110 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Lu DC, Chang SC, Chen YC et al. Burkholderia cepacia bacteremia: a retrospective analysis of 70 episodes. J Formos Med Assoc, 96(12), 972–978 (1997). [PubMed] [Google Scholar]
- 57.Huang CH, Jang TN, Liu CY et al. Characteristics of patients with Burkholderia cepacia bacteremia. J Microbiol Immunol Infect, 34(3), 215–219 (2001). [PubMed] [Google Scholar]
- 58**.El Chakhtoura NG, Saade E, Wilson BM et al. A 17-Year Nationwide Study of Burkholderia cepacia Complex (Bcc) Blood Stream Infections (BSI) Among Patients in the Veterans Health Administration (VHA). Clinical Infectious Diseases, 65(8), 1327–1334 (2017). This retrospective cohort of bloodstream infections caused by B. cepacia complex organims represents one of the largest reported to date. It is a national cohort spanning a period of 17 years and provides insight into the clinical and therapeutic behavior of B. cepacia complex infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu WL, Wang DY, Lin CW et al. Endemic Burkholderia cepacia bacteraemia: clinical features and antimicrobial susceptibilities of isolates. Scand J Infect Dis, 31(3), 293–298 (1999). [DOI] [PubMed] [Google Scholar]
- 60.Righi E, Girardis M, Marchegiano P et al. Characteristics and outcome predictors of patients involved in an outbreak of Burkholderia cepacia complex. J Hosp Infect, 85(1), 73–75 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Nannini EC, Ponessa A, Muratori R et al. Polyclonal outbreak of bacteremia caused by Burkholderia cepacia complex and the presumptive role of ultrasound gel. Braz J Infect Dis, 19(5), 543–545 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Souza Dias MB, Cavassin LG, Stempliuk V et al. Multi-institutional outbreak of Burkholderia cepacia complex associated with contaminated mannitol solution prepared in compounding pharmacy. Am J Infect Control, 41(11), 1038–1042 (2013). [DOI] [PubMed] [Google Scholar]
- 63.De Smet B, Veng C, Kruy L et al. Outbreak of Burkholderia cepacia bloodstream infections traced to the use of Ringer lactate solution as multiple-dose vial for catheter flushing, Phnom Penh, Cambodia. Clin Microbiol Infect, 19(9), 832–837 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Martins IS, Pellegrino FL, Freitas A et al. Case-crossover study of Burkholderia cepacia complex bloodstream infection associated with contaminated intravenous bromopride. Infect Control Hosp Epidemiol, 31(5), 516–521 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Romero-Gomez MP, Quiles-Melero MI, Pena Garcia P et al. Outbreak of Burkholderia cepacia bacteremia caused by contaminated chlorhexidine in a hemodialysis unit. Infect Control Hosp Epidemiol, 29(4), 377–378 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Boszczowski I, do Prado GV, Dalben MF et al. Polyclonal outbreak of bloodstream infections caused by Burkholderia cepacia complex in hematology and bone marrow transplant outpatient units. Rev Inst Med Trop Sao Paulo, 56(1), 71–76 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lalitha P, Das M, Purva PS et al. Postoperative endophthalmitis due to Burkholderia cepacia complex from contaminated anaesthetic eye drops. Br J Ophthalmol, 98(11), 1498–1502 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Moehring RW, Lewis SS, Isaacs PJ et al. Outbreak of bacteremia due to Burkholderia contaminans linked to intravenous fentanyl from an institutional compounding pharmacy. JAMA Intern Med, 174(4), 606–612 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Wiener-Well Y, Segonds C, Mazuz B et al. Successful outbreak investigation of Burkholderia cepacia complex bacteremia in intensive care patients. Am J Infect Control, 42(5), 580–581 (2014). [DOI] [PubMed] [Google Scholar]
- 70.U.S. Department of Health and Human Services CDC [Internet]. Atlanta (GA): CDC; c2016. Multistate Outbreak of Burkholderia cepacia Infections [cited 2017 Dec 26] Available from: https://www.cdc.gov/hai/outbreaks/b-cepacia/index.html
- 71.Marquez L, Jones KN, Whaley EM et al. An Outbreak of Burkholderia cepacia Complex Infections Associated with Contaminated Liquid Docusate. Infect Control Hosp Epidemiol, 38(5), 567–573 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Coenye T, Van Acker H, Peeters E et al. Molecular mechanisms of chlorhexidine tolerance in Burkholderia cenocepacia biofilms. Antimicrob Agents Chemother, 55(5), 1912–1919 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ko S, An HS, Bang JH, Park SW. An outbreak of Burkholderia cepacia complex pseudobacteremia associated with intrinsically contaminated commercial 0.5% chlorhexidine solution. Am J Infect Control, 43(3), 266–268 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Mahenthiralingam E, Urban TA, Goldberg JB. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol, 3(2), 144–156 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Walsh F, Duffy B. The culturable soil antibiotic resistome: a community of multi-drug resistant bacteria. PLoS One, 8(6), e65567 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allen HK, Donato J, Wang HH et al. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol, 8(4), 251–259 (2010). [DOI] [PubMed] [Google Scholar]
- 77.Papp-Wallace KM, Taracila MA, Gatta JA et al. Insights into beta-lactamases from Burkholderia species, two phylogenetically related yet distinct resistance determinants. J Biol Chem, 288(26), 19090–19102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peeters E, Nelis HJ, Coenye T. In vitro activity of ceftazidime, ciprofloxacin, meropenem, minocycline, tobramycin and trimethoprim/sulfamethoxazole against planktonic and sessile Burkholderia cepacia complex bacteria. J Antimicrob Chemother, 64(4), 801–809 (2009). [DOI] [PubMed] [Google Scholar]
- 79.*. Papp-Wallace KM, Becka SA, Taracila MA et al. Exploring the role of the Omega-loop in the evolution of ceftazidime resistance in the PenA beta-lactamase from Burkholderia multivorans, an important Cystic Fibrosis pathogen. Antimicrob Agents Chemother, 61(2), e01941–16 (2016). This is one of few studies that provide a biochemical basis for ceftazidime resistance in B. cepacia complex, a growing problem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drevinek P, Mahenthiralingam E. Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin Microbiol Infect, 16(7), 821–830 (2010). [DOI] [PubMed] [Google Scholar]
- 81.Hwang J, Kim HS. Cell Wall Recycling-Linked Coregulation of AmpC and PenB beta-Lactamases through ampD Mutations in Burkholderia cenocepacia. Antimicrob Agents Chemother, 59(12), 7602–7610 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trepanier S, Prince A, Huletsky A. Characterization of the penA and penR genes of Burkholderia cepacia 249 which encode the chromosomal class A penicillinase and its LysR-type transcriptional regulator. Antimicrob Agents Chemother, 41(11), 2399–2405 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Podnecky NL, Rhodes KA, Schweizer HP. Efflux pump-mediated drug resistance in Burkholderia. Front Microbiol, 6, 305 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poirel L, Rodriguez-Martinez JM, Plesiat P, Nordmann P. Naturally occurring Class A ss-lactamases from the Burkholderia cepacia complex. Antimicrob Agents Chemother, 53(3), 876–882 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tseng SP, Tsai WC, Liang CY et al. The contribution of antibiotic resistance mechanisms in clinical Burkholderia cepacia complex isolates: an emphasis on efflux pump activity. PLoS One, 9(8), e104986 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nzula S, Vandamme P, Govan JR. Influence of taxonomic status on the in vitro antimicrobial susceptibility of the Burkholderia cepacia complex. J Antimicrob Chemother, 50(2), 265–269 (2002). [DOI] [PubMed] [Google Scholar]
- 87.Desai M, Buhler T, Weller PH et al. Increasing resistance of planktonic and biofilm cultures of Burkholderia cepacia to ciprofloxacin and ceftazidime during exponential growth. J Antimicrob Chemother, 42(2), 153–160 (1998). [DOI] [PubMed] [Google Scholar]
- 88.Pope CF, Gillespie SH, Pratten JR et al. Fluoroquinolone-resistant mutants of Burkholderia cepacia. Antimicrob Agents Chemother, 52(3), 1201–1203 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez F, El Chakhtoura NG, Papp-Wallace KM et al. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply “precision medicine” to antimicrobial chemotherapy? Expert Opin Pharmacother, 17(6), 761–781 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.**. Paul M, Carmeli Y, Durante-Mangoni E et al. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother, 69(9), 2305–2309 (2014). This is a valuable critical appraisal of the available literature looking at combination therapy for carbapenem-resistant Gram-negative bacteria. Written by leading experts in the field. [DOI] [PubMed] [Google Scholar]
- 91.Gomez-Simmonds A, Nelson B, Eiras DP et al. Combination Regimens for Treatment of Carbapenem-Resistant Klebsiella pneumoniae Bloodstream Infections. Antimicrob Agents Chemother, 60(6), 3601–3607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.*. Zavascki AP, Bulitta JB, Landersdorfer CB. Combination therapy for carbapenem-resistant Gram-negative bacteria. Expert Rev Anti Infect Ther, 11(12), 1333–1353 (2013). Comprehensive review of the concepts and rationales for the use of combination therapy for carbapenem-resistant Gram-negative bacteria. The authors are among the first to advocate for “rationally-optimized” combination antibiotic chemotherapy strategies. [DOI] [PubMed] [Google Scholar]
- 93.Machuca I, Gutierrez-Gutierrez B, Gracia-Ahufinger I et al. Mortality Associated with Bacteremia Due to Colistin-Resistant Klebsiella pneumoniae with High-Level Meropenem Resistance: Importance of Combination Therapy without Colistin and Carbapenems. Antimicrob Agents Chemother, 61(8), e00406–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.**. Gutierrez-Gutierrez B, Salamanca E, de Cueto M et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis, 17(7), 726–734 (2017). This is an important study that demonstrates that combination therapy for bloodstream infections caused by carbapenemase-producing Enterobacteriaceae was associated with improved survival only in patients with a high mortality score as measured by the now well-validated REMENT score. [DOI] [PubMed] [Google Scholar]
- 95.Micek ST, Lloyd AE, Ritchie DJ et al. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother, 49(4), 1306–1311 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin Proc, 86(3), 250–259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pillar CM, Torres MK, Brown NP et al. In vitro activity of doripenem, a carbapenem for the treatment of challenging infections caused by gram-negative bacteria, against recent clinical isolates from the United States. Antimicrob Agents Chemother, 52(12), 4388–4399 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuti JL, Pettit RS, Neu N et al. Microbiological activity of ceftolozane/tazobactam, ceftazidime, meropenem, and piperacillin/tazobactam against Pseudomonas aeruginosa isolated from children with cystic fibrosis. Diagn Microbiol Infect Dis, 83(1), 53–55 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Vickery S, McClain D, Wargo KA. Successful Use of Ceftolozane-Tazobactam to Treat a Pulmonary Exacerbation of Cystic Fibrosis Caused by Multidrug- Resistant Pseudomonas aeruginosa. Pharmacotherapy, 36(10), e154–e159 (2016). [DOI] [PubMed] [Google Scholar]
- 100.Chalhoub H, Tunney M, Elborn JS et al. Avibactam confers susceptibility to a large proportion of ceftazidime-resistant Pseudomonas aeruginosa isolates recovered from cystic fibrosis patients. J Antimicrob Chemother, 70(5), 1596–1598 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Flamm RK, Stone GG, Sader HS et al. Avibactam reverts the ceftazidime MIC90 of European Gram-negative bacterial clinical isolates to the epidemiological cut-off value. J Chemother, 26(6), 333–338 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Humphries RM, Hindler JA, Wong-Beringer A et al. Activity of ceftolozane-tazobactam and ceftazidime-avibactam against beta-lactam resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother, 61(12), e01858–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bellais S, Mimoz O, Leotard S et al. Efficacy of beta-lactams for treating experimentally induced pneumonia due to a carbapenem-hydrolyzing metallo-beta-lactamase-producing strain of Pseudomonas aeruginosa. Antimicrob Agents Chemother, 46(6), 2032–2034 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mushtaq S, Ge Y, Livermore DM. Doripenem versus Pseudomonas aeruginosa in vitro: activity against characterized isolates, mutants, and transconjugants and resistance selection potential. Antimicrob Agents Chemother, 48(8), 3086–3092 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davido B, Fellous L, Lawrence C et al. Ceftazidime-Avibactam and Aztreonam, an Interesting Strategy To Overcome beta-Lactam Resistance Conferred by Metallo-beta-Lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother, 61(9), e01008–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.**. Mojica MF, Papp-Wallace KM, Taracila MA et al. Avibactam Restores the Susceptibility of Clinical Isolates of Stenotrophomonas maltophilia to Aztreonam. Antimicrob Agents Chemother, 61(10), e00777–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.**. Marshall S, Hujer AM, Rojas LJ et al. Can Ceftazidime-Avibactam and Aztreonam Overcome beta-Lactam Resistance Conferred by Metallo-beta- Lactamases in Enterobacteriaceae? Antimicrob Agents Chemother, 61(4), e02243–16 (2017). These two publications shed light on the novel combination of avibactam and aztreonam and its role in the treatment of MBL-producing organisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lodise TP Jr.</au, Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis, 44(3), 357–363 (2007). [DOI] [PubMed] [Google Scholar]
- 109.Bauer KA, West JE, O’Brien JM, Goff DA. Extended-infusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob Agents Chemother, 57(7), 2907–2912 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dulhunty JM, Roberts JA, Davis JS et al. A Multicenter Randomized Trial of Continuous versus Intermittent beta-Lactam Infusion in Severe Sepsis. Am J Respir Crit Care Med, 192(11), 1298–1305 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med, 42(10), 1535–1545 (2016). [DOI] [PubMed] [Google Scholar]
- 112.Kaye KS, Kanafani ZA, Dodds AE, Engemann JJ, Weber SG, Carmeli Y. Differential effects of levofloxacin and ciprofloxacin on the risk for isolation of quinolone-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother, 50(6), 2192–2196 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Layeux B, Taccone FS, Fagnoul D et al. Amikacin monotherapy for sepsis caused by panresistant Pseudomonas aeruginosa. Antimicrob Agents Chemother, 54(11), 4939–4941 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tam VH, Rogers CA, Chang KT et al. Impact of multidrug-resistant Pseudomonas aeruginosa bacteremia on patient outcomes. Antimicrob Agents Chemother, 54(9), 3717–3722 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garnacho-Montero J, Sa-Borges M, Sole-Violan J et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med, 35(8), 1888–1895 (2007). [DOI] [PubMed] [Google Scholar]
- 116.Aarts MA, Hancock JN, Heyland D et al. Empiric antibiotic therapy for suspected ventilator-associated pneumonia: a systematic review and meta-analysis of randomized trials. Crit Care Med, 36(1), 108–117 (2008). [DOI] [PubMed] [Google Scholar]
- 117.Heyland DK, Dodek P, Muscedere J et al. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med, 36(3), 737–744 (2008). [DOI] [PubMed] [Google Scholar]
- 118.Lin MY, Weinstein RA, Hota B. Delay of active antimicrobial therapy and mortality among patients with bacteremia: impact of severe neutropenia. Antimicrob Agents Chemother, 52(9), 3188–3194 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paul M, Benuri-Silbiger I, Soares-Weiser K et al. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ, 328(7441), 668 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev, 25(3), 450–470 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klastersky J, Zinner SH. Synergistic combinations of antibiotics in gram-negative bacillary infections. Rev Infect Dis, 4(2), 294–301 (1982). [DOI] [PubMed] [Google Scholar]
- 122.Dundar D, Otkun M. In-vitro efficacy of synergistic antibiotic combinations in multidrug resistant Pseudomonas aeruginosa strains. Yonsei Med J, 51(1), 111–116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gunderson BW, Ibrahim KH, Hovde LB et al. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother, 47(3), 905–909 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saiman L, Chen Y, Gabriel PS et al. Synergistic activities of macrolide antibiotics against Pseudomonas aeruginosa, Burkholderia cepacia, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans isolated from patients with cystic fibrosis. Antimicrob Agents Chemother, 46(4), 1105–1107 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Timurkaynak F, Can F, Azap OK et al. In vitro activities of non-traditional antimicrobials alone or in combination against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii isolated from intensive care units. Int J Antimicrob Agents, 27(3), 224–228 (2006). [DOI] [PubMed] [Google Scholar]
- 126.Louie A, Liu W, VanGuilder M et al. Combination treatment with meropenem plus levofloxacin is synergistic against Pseudomonas aeruginosa infection in a murine model of pneumonia. J Infect Dis, 211(8), 1326–1333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Samonis G, Maraki S, Karageorgopoulos DE et al. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur J Clin Microbiol Infect Dis, 31(5), 695–701 (2012). [DOI] [PubMed] [Google Scholar]
- 128.Drusano GL, Bonomo RA, Bahniuk N et al. Resistance emergence mechanism and mechanism of resistance suppression by tobramycin for cefepime for Pseudomonas aeruginosa. Antimicrob Agents Chemother, 56(1), 231–242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Louie A, Liu W, Fikes S et al. Impact of meropenem in combination with tobramycin in a murine model of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother, 57(6), 2788–2792 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martinez MN, Papich MG, Drusano GL. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother, 56(6), 2795–2805 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karlowsky JA, Kazmierczak KM, de Jonge BLM et al. In Vitro Activity of Aztreonam-Avibactam against Enterobacteriaceae and Pseudomonas aeruginosa Isolated by Clinical Laboratories in 40 Countries from 2012 to 2015. Antimicrob Agents Chemother, 61(9), e00472–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Castanheira M, Huband MD, Mendes RE et al. Meropenem-Vaborbactam Tested against Contemporary Gram-Negative Isolates Collected Worldwide during 2014, Including Carbapenem-Resistant, KPC-Producing, Multidrug-Resistant, and Extensively Drug-Resistant Enterobacteriaceae. Antimicrob Agents Chemother, 61(9), e00472–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lob SH, Hackel MA, Kazmierczak KM et al. In Vitro Activity of Imipenem-Relebactam against Gram-Negative ESKAPE Pathogens Isolated by Clinical Laboratories in the United States in 2015 (Results from the SMART Global Surveillance Program). Antimicrob Agents Chemother, 61(6), e00472–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.*. Falagas ME, Skalidis T, Vardakas KZ et al. Activity of cefiderocol (S-649266) against carbapenem-resistant Gram-negative bacteria collected from inpatients in Greek hospitals. J Antimicrob Chemother, 72(6), 1704–1708 [DOI] [PubMed] [Google Scholar]
- 135.*. Hackel MA, Tsuji M, Yamano Y et al. In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against a Recent Collection of Clinically Relevant Gram-Negative Bacilli from North America and Europe, Including Carbapenem-Nonsusceptible Isolates (SIDERO-WT-2014 Study). Antimicrob Agents Chemother, 61(9), e00472–17 (2017). Two of the largest studies that demonstrate the effectiveness of the new agent Cefiderocol, which is active against all the major non-fermenter Gram-negative organism groups discussed in this review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.DiGiandomenico A, Sellman BR. Antibacterial monoclonal antibodies: the next generation? Curr Opin Microbiol, 27, 78–85 (2015). [DOI] [PubMed] [Google Scholar]
- 137.Papareddy P, Kasetty G, Kalle M et al. NLF20: an antimicrobial peptide with therapeutic potential against invasive Pseudomonas aeruginosa infection. J Antimicrob Chemother, 71(1), 170–180 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Ortwine JK, Kaye KS, Li J, Pogue JM. Colistin: Understanding and Applying Recent Pharmacokinetic Advances. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 35(1), 11–16 (2015). [DOI] [PubMed] [Google Scholar]
- 139.Boisson M, Jacobs M, Grégoire N et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother, 58(12), 7331–7339 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qureshi ZA, Hittle LE, O’Hara JA et al. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis, 60(9), 1295–1303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wood GC, Hanes SD, Croce MA et al. Comparison of ampicillin-sulbactam and imipenem-cilastatin for the treatment of Acinetobacter ventilator-associated pneumonia. Clin Infect Dis, 34(11), 1425–1430 (2002). [DOI] [PubMed] [Google Scholar]
- 142.Choi JY, Kim CO, Park YS et al. Comparison of efficacy of cefoperazone/sulbactam and imipenem/cilastatin for treatment of Acinetobacter bacteremia. Yonsei Med J, 47(1), 63–69 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chu H, Zhao L, Wang M, Liu Y et al. Sulbactam-based therapy for Acinetobacter baumannii infection: a systematic review and meta-analysis. Braz J Infect Dis, 17(4), 389–394 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Falagas ME, Vardakas KZ, Kapaskelis A et al. Tetracyclines for multidrug-resistant Acinetobacter baumannii infections. Int J Antimicrob Agents, 45(5), 455–460 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Burkhardt O, Rauch K, Kaever V et al. Tigecycline possibly underdosed for the treatment of pneumonia: a pharmacokinetic viewpoint. Int J Antimicrob Agents, 34(1), 101–102 (2009). [DOI] [PubMed] [Google Scholar]
- 146.Rose WE, Rybak MJ. Tigecycline: First of a New Class of Antimicrobial Agents. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 26(8), 1099–1110 (2006). [DOI] [PubMed] [Google Scholar]
- 147.U.S. Federal Drug Administration (FDA) [Internet]. Washington D.C.: FDA; c2016. Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new Boxed Warning [cited 2017 Dec 26] Available from: https://www.fda.gov/Drugs/DrugSafety/ucm369580.htm
- 148.Cunha BA, Baron J, Cunha CB. Once daily high dose tigecycline - pharmacokinetic/pharmacodynamic based dosing for optimal clinical effectiveness: dosing matters, revisited. Expert Rev Anti Infect Ther, 15(3), 257–267 (2017). [DOI] [PubMed] [Google Scholar]
- 149.De Pascale G, Montini L, Pennisi M et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care, 18(3), R90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Santimaleeworagun W, Wongpoowarak P, Chayakul P et al. In vitro activity of colistin or sulbactam in combination with fosfomycin or imipenem against clinical isolates of carbapenem-resistant Acinetobacter baumannii producing OXA-23 carbapenemases. The Southeast Asian Journal of Tropical Medicine and Public Health, 42(4), 890–900 (2011). [PubMed] [Google Scholar]
- 151.Lopez-Cortes LE, Cisneros JM, Fernandez-Cuenca F et al. Monotherapy versus combination therapy for sepsis due to multidrug-resistant Acinetobacter baumannii: analysis of a multicentre prospective cohort. J Antimicrob Chemother, 69(11), 3119–3126 (2014). [DOI] [PubMed] [Google Scholar]
- 152.Zusman O, Avni T, Leibovici L et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother, 57(10), 5104–5111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aydemir H, Akduman D, Piskin N et al. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiology And Infection, 141(6), 1214–1222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Durante-Mangoni E, Signoriello G, Andini R et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis, 57(3), 349–358 (2013). [DOI] [PubMed] [Google Scholar]
- 155.Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother, 58(9), 5598–5601 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cheng A, Chuang YC, Sun HY et al. Excess Mortality Associated With Colistin-Tigecycline Compared With Colistin-Carbapenem Combination Therapy for Extensively Drug-Resistant Acinetobacter baumannii Bacteremia: A Multicenter Prospective Observational Study. Crit Care Med, 43(6), 1194–1204 (2015). [DOI] [PubMed] [Google Scholar]
- 157.Hornsey M, Wareham DW. In vivo efficacy of glycopeptide-colistin combination therapies in a Galleria mellonella model of Acinetobacter baumannii infection. Antimicrob Agents Chemother, 55(7), 3534–3537 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Garnacho-Montero J, Amaya-Villar R, Gutierrez-Pizarraya A et al. Clinical efficacy and safety of the combination of colistin plus vancomycin for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Chemotherapy, 59(3), 225–231 (2013). [DOI] [PubMed] [Google Scholar]
- 159.Petrosillo N, Giannella M, Antonelli M et al. Clinical experience of colistin-glycopeptide combination in critically ill patients infected with Gram-negative bacteria. Antimicrob Agents Chemother, 58(2), 851–858 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.*. Lenhard JR, Smith NM, Bulman ZP et al. High-Dose Ampicillin-Sulbactam Combinations Combat Polymyxin-Resistant Acinetobacter baumannii in a Hollow-Fiber Infection Model. Antimicrob Agents Chemother, 61(3), e00472–17 (2017). Elegant report that shows synergy of the three-drug combination of high-dose ampicillin-sulbactam, meropenem and polymyxin B in two clinical A. aumannii isolates resistant to all three drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abdallah M, Olafisoye O, Cortes C et al. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob Agents Chemother, 59(3), 1802–1805 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu F, Myers AG. Development of a platform for the discovery and practical synthesis of new tetracycline antibiotics. Curr Opin Chem Biol, 32, 48–57 (2016). [DOI] [PubMed] [Google Scholar]
- 163.Garcia-Salguero C, Rodriguez-Avial I, Picazo JJ et al. Can Plazomicin Alone or in Combination Be a Therapeutic Option against Carbapenem-Resistant Acinetobacter baumannii? Antimicrob Agents Chemother, 59(10), 5959–5966 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ito A, Kohira N, Bouchillon SK et al. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J Antimicrob Chemother, 71(3), 670–677 (2016). [DOI] [PubMed] [Google Scholar]
- 165.Durand-Reville TF, Guler S, Comita-Prevoir J et al. ETX2514 is a broad-spectrum beta-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol, 2, 17104 (2017). [DOI] [PubMed] [Google Scholar]
- 166.*. Schooley RT, Biswas B, Gill JJ et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother, 61(10), e00954–17 (2017). The authors describe a method used to produce a personalized bacteriophage-based therapeutic treatment for a patient with disseminated infection sue to MDR A. baumannii. This is a rare clinical account of the successful use of bacteriophage therapeutics for MDR Gram-negative infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.*. Garcia-Quintanilla M, Caro-Vega JM, Pulido MR et al. Inhibition of LpxC Increases Antibiotic Susceptibility in Acinetobacter baumannii. Antimicrob Agents Chemother, 60(8), 5076–5079 (2016). Describes the activity of LpxC inhibitors as potential agents that could be used to potentiate existing antibiotics in an alternative treatment strategy for multidrug-resistant A. baumannii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Avgeri SG, Matthaiou DK, Dimopoulos G et al. Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: a systematic review of the clinical evidence. Int J Antimicrob Agents, 33(5), 394–404 (2009). [DOI] [PubMed] [Google Scholar]
- 169.Milne KE, Gould IM. Combination antimicrobial susceptibility testing of multidrug-resistant Stenotrophomonas maltophilia from cystic fibrosis patients. Antimicrob Agents Chemother, 56(8), 4071–4077 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Church D, Lloyd T, Peirano G et al. Antimicrobial susceptibility and combination testing of invasive Stenotrophomonas maltophilia isolates. Scand J Infect Dis, 45(4), 265–270 (2013). [DOI] [PubMed] [Google Scholar]
- 171.Personne Y, Curtis MA, Wareham DW et al. Activity of the type I signal peptidase inhibitor MD3 against multidrug-resistant Gram-negative bacteria alone and in combination with colistin. J Antimicrob Chemother, 69(12), 3236–3243 (2014). [DOI] [PubMed] [Google Scholar]
- 172.Patel JB, Cockerill FR, Alder J et al. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. CLSI standards for antimicrobial susceptibility testing, 34(1), 1–226 (2014). [Google Scholar]
- 173.Horsley A, Jones AM, Lord R. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Syst Rev, (1), CD009529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liao CH, Chang HT, Lai CC et al. Clinical characteristics and outcomes of patients with Burkholderia cepacia bacteremia in an intensive care unit. Diagn Microbiol Infect Dis, 70(2), 260–266 (2011). [DOI] [PubMed] [Google Scholar]
- 175.Bressler AM, Kaye KS, LiPuma JJ et al. Risk factors for Burkholderia cepacia complex bacteremia among intensive care unit patients without cystic fibrosis: a case-control study. Infect Control Hosp Epidemiol, 28(8), 951–958 (2007). [DOI] [PubMed] [Google Scholar]
- 176.Woods CW, Bressler AM, LiPuma JJ et al. Virulence associated with outbreak-related strains of Burkholderia cepacia complex among a cohort of patients with bacteremia. Clin Infect Dis, 38(9), 1243–1250 (2004). [DOI] [PubMed] [Google Scholar]
- 177.Mushtaq S, Warner M, Livermore DM. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother, 65(11), 2376–2381 (2010). [DOI] [PubMed] [Google Scholar]
- 178.**. Papp-Wallace KM, Becka SA, Zeiser ET et al. Overcoming an Extremely Drug Resistant (XDR) Pathogen: Avibactam Restores Susceptibility to Ceftazidime for Burkholderia cepacia Complex Isolates from Cystic Fibrosis Patients. ACS Infect Dis, 3(7), 502–511 (2017). This study presents compelling data on the efficacy of ceftazidime-avibactam for the treatment of B. cepacia complex infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mazer DM, Young C, Kalikin LM et al. In Vitro Activity of Ceftolozane-Tazobactam and Other Antimicrobial Agents against Burkholderia cepacia Complex and Burkholderia gladioli. Antimicrob Agents Chemother, 61(9), e0076–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.**. Wenzler E, Fraidenburg DR, Scardina T et al. Inhaled Antibiotics for Gram- Negative Respiratory Infections. Clin Microbiol Rev, 29(3), 581–632 (2016). Comprehensive review of inhaled antibiotics for Gram-negative respiratory infections in the typical style of Clinical Microbiology Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Michel JB, Yeh PJ, Chait R et al. Drug interactions modulate the potential for evolution of resistance. Proc Natl Acad Sci USA, 105(39), 14918–14923 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Munck C, Gumpert HK, Wallin AI et al. Prediction of resistance development against drug combinations by collateral responses to component drugs. Sci Transl Med, 6(262), 262ra156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rodriguez de Evgrafov M, Gumpert H, Munck C et al. Collateral Resistance and Sensitivity Modulate Evolution of High-Level Resistance to Drug Combination Treatment in Staphylococcus aureus. Mol Biol Evol, 32(5), 1175–1185 (2015). [DOI] [PubMed] [Google Scholar]
- 184.**. Cai Y, Chua NG, Lim TP et al. From Bench-Top to Bedside: A Prospective In Vitro Antibiotic Combination Testing (iACT) Service to Guide the Selection of Rationally Optimized Antimicrobial Combinations against Extensively Drug Resistant (XDR) Gram Negative Bacteria (GNB). PLoS One, 11(7), e0158740 (2016). Cai et al. present here an elegantly designed dissemination and implementation campaign aimed at bringing the use rationally-optimized antibiotic combinations into clinical practice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hauser AR, Mecsas J, Moir DT. Beyond Antibiotics: New Therapeutic Approaches for Bacterial Infections. Clin Infect Dis, 63(1), 89–95 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hancock RE, Nijnik A, Philpott DJ. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol, 10(4), 243–254 (2012). [DOI] [PubMed] [Google Scholar]
- 187.Shields RK, Clancy CJ, Gillis LM et al. Epidemiology, clinical characteristics and outcomes of extensively drug-resistant Acinetobacter baumannii infections among solid organ transplant recipients. PLoS One, 7(12), e52349 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hernandez-Torres A, Garcia-Vazquez E, Gomez J et al. Multidrug and carbapenem-resistant Acinetobacter baumannii infections: Factors associated with mortality. Med Clin (Barc), 138(15), 650–655 (2012). [DOI] [PubMed] [Google Scholar]
- 189.Kuo LC, Lai CC, Liao CH et al. Multidrug-resistant Acinetobacter baumannii bacteraemia: clinical features, antimicrobial therapy and outcome. Clin Microbiol Infect, 13(2), 196–198 (2007). [DOI] [PubMed] [Google Scholar]
- 190.Tasbakan MS, Pullukcu H, Sipahi OR et al. Is tigecyclin a good choice in the treatment of multidrug-resistant Acinetobacter baumannii pneumonia? J Chemother, 23(6), 345–349 (2011). [DOI] [PubMed] [Google Scholar]
- 191.Tseng YC, Wang JT, Wu FL et al. Prognosis of adult patients with bacteremia caused by extensively resistant Acinetobacter baumannii. Diagn Microbiol Infect Dis, 59(2), 181–190 (2007). [DOI] [PubMed] [Google Scholar]
- 192.Lim SK, Lee SO, Choi SH et al. The outcomes of using colistin for treating multidrug resistant Acinetobacter species bloodstream infections. J Korean Med Sci, 26(3), 325–331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ye JJ, Lin HS, Kuo AJ et al. The clinical implication and prognostic predictors of tigecycline treatment for pneumonia involving multidrug-resistant Acinetobacter baumannii. J Infect, 63(5), 351–361 (2011). [DOI] [PubMed] [Google Scholar]


