Abstract
Objective
Executive functioning deficits have been documented among congenital heart disease (CHD) survivors and may contribute to emotional distress. Little research has investigated the role of coping in this association. This study examined the role of coping in accounting for the association between self-reported executive function problems and internalizing symptoms among adolescents and emerging adults (AEAs), as well as young adults (YAs) with CHD.
Methods
Participants included 74 AEA (Mage = 19.32 3.47 years, range 15–25 years) and 98 YA CHD survivors (Mage = 32.00 3.69 years, range 26–39 years), recruited from pediatric and adult outpatient cardiology clinics. Participants completed self-report measures of executive function problems, coping (primary control, secondary control, and disengagement coping), and internalizing symptoms. Lesion severity classification and functional impairment due to symptoms of heart failure were determined from medical chart review.
Results
Significant problems in executive function were reported by 5% of AEA and 13% of YA. Coping was not associated with executive function problems or internalizing symptoms for AEA. However, among YA, less use of adaptive coping strategies and more maladaptive coping responses was associated with both more executive function problems and internalizing symptoms. An indirect effect of executive function problems on internalizing symptoms via secondary control coping emerged for YA.
Conclusions
Executive function problems may disrupt the ability to use important adaptive coping skills, such as cognitive reappraisal, positive thinking, and acceptance, thereby resulting in greater emotional distress among YA CHD survivors.
Keywords: congenital heart disease, coping, emotional distress, executive function
Introduction
Congenital heart disease (CHD) is a common birth defect that includes many different structural malformations of the heart. Recent estimates indicate that there are over 2.4 million individuals living with CHD in the United States, and this population continues to grow because of advancements in treatment (Gilboa et al., 2016). Among CHD survivors, health outcomes are diverse across age ranges, ranging from no functional impairment to significant disease burden (e.g., shortness of breath, fatigue, and palpitations during physical activity) and premature death (Kovacs, Sears, & Saidi, 2005), due in part to the type of cardiac lesion and surgical interventions required. Greater functional impairment is associated with poorer patient-reported emotional well-being (Apers et al., 2016; Jackson, Hassen, Gerardo, Vannatta, & Daniels, 2016). Furthermore, as CHD survivors transition from adolescence to adulthood, they assume more responsibility for managing their condition, which may coincide with greater disease-related stress that persists into young adulthood, resulting in poorer patient-reported outcomes (Jackson et al., 2016) and elevated risk for significant emotional distress (Westhoff-Bleck et al., 2016). Disease-related stressors identified among CHD survivors include uncertainty about future health, scarring from medical interventions, and having a device (i.e., pacemaker or implantable cardioverter defibrillator) (Jackson et al., 2016).
Deficits in executive function (e.g., planning and self-regulation) are also more likely to be experienced by survivors with moderate to complex CHD, and may contribute to elevated emotional distress (Calderon, 2016; Calderon & Bellinger, 2015; Neal et al., 2015). Evidence is mounting that cumulative risk factors for brain injury related to CHD and its interventions, such as inadequate oxygenation of the blood (cyanosis) because of the cardiac lesion and/or perioperative hypoxic ischemia from surgery, may impact executive function across the life span (Marelli, Miller, Marino, Jefferson, & Newburger, 2016). The effect of executive function on emotional functioning may be partly explained by disruption in one’s ability to cope adaptively. Indeed, across multiple patient populations, including traumatic brain injury (Krpan, Levine, Stuss, & Dawson, 2007), acute lymphocytic leukemia (Campbell et al., 2009), and multiple sclerosis (Grech et al., 2016), impaired executive function is associated with reductions in adaptive coping (e.g., acceptance, cognitive restructuring, generating alternative solutions) and elevations in maladaptive coping (e.g., avoidance, distancing, positive comparisons), leading to greater emotional distress. Prior research has not investigated the relationship between executive function and coping in this population or the effect this relationship may have on emotional functioning.
The current study examined whether less use of adaptive coping serves as a pathway through which problems in executive function may contribute to more emotional distress. We drew from a well-established framework (Connor-Smith, Compas, Wadsworth, Thomsen, & Saltzman, 2000) that distinguishes three forms of coping: primary control, secondary control, and disengagement. Primary control coping directly addresses the source of stress (e.g., problem-solving) or one’s emotional reactions to stress (e.g., emotional expression) and is proposed to be beneficial when individuals face controllable stressors (Band & Weisz, 1988; Compas, Jaser, Dunn, & Rodriguez, 2012). Secondary control coping is used to adapt to stress (e.g., cognitive reappraisal, positive thinking, acceptance) and may be most beneficial when faced with uncontrollable stressors (Band & Weisz, 1988; Compas et al., 2012). Finally, disengagement coping involves coping efforts oriented away from stress (e.g., avoidance, denial). There is evidence that primary and secondary control coping is adaptive and beneficial to those with serious health conditions, while disengagement coping is maladaptive and associated with more internalizing symptoms (Andreotti et al., 2013). Problems in executive function, which affect planning and emotional control, are theorized to affect emotional distress via coping strategies (Campbell et al., 2009), and have important implications for designing interventions to improve effective coping in populations at risk for executive function problems, such as CHD survivors.
The current study aimed to (1) examine differences in problems with executive function among adolescents and emerging adults (AEAs) and young adults (YAs) with CHD of varying cardiac lesion severities (simple, moderate, and complex) and functional status (no impairment, impairment), (2) determine associations between self-reported problems with executive function, coping responses (primary control, secondary control, and disengagement), and internalizing symptoms, and (3) test the indirect effects of self-reported problems with executive function on internalizing symptoms via coping. Individuals with more complex disease and poorer functional status were predicted to report more problems with executive function than those with less complex disease and no functional status impairment. Adaptive coping (primary control coping and secondary control coping) was expected to be negatively associated with internalizing symptoms, whereas maladaptive coping (disengagement coping) was expected to be positively associated with internalizing symptoms. Finally, problems with executive function were hypothesized to be positively associated with internalizing symptoms, and this link may be partially explained by less use of adaptive coping and more use of maladaptive coping.
Methods
Participants and Procedures
Participants were recruited from outpatient cardiology clinics at a pediatric and an adult hospital. Individuals were eligible if they (a) had structural CHD, (b) were 15–39 years old, and (c) were fluent in English. Individuals were excluded if they had been diagnosed with a genetic syndrome or had cognitive limitations that would prevent completion of self-report measures. Participants aged 15–25 years were considered AEAs, whereas those aged 26–39 years were considered YAs. Participants were grouped by these ages because potential developmental differences between AEA and YA were of interest, and age 25 years is a commonly accepted upper limit in age for emerging adulthood (Arnett, 2000). Verbal consent and assent were obtained via phone or in person during an outpatient clinic visit.
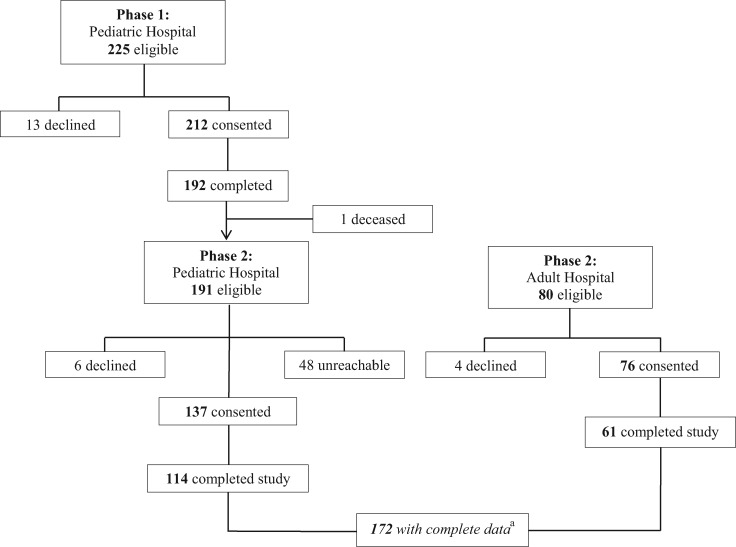
As shown in the study flow diagram (Figure 1), participants from pediatric and adult cardiology clinics at the pediatric hospital were initially recruited to examine disease knowledge and health-related quality of life (Phase 1). Self-report measures of internalizing symptoms and problems with executive function were added to the study protocol after data collection was initiated at the pediatric institution, requiring participants to be recontacted and reconsented in a Phase 2 of the study. As participants from the pediatric hospital were being recontacted, recruitment efforts began at the adult hospital. As a result, 172 participants completed all measures. For those patients who declined participation at either phase (n = 23), were unreachable at Phase 2 from the pediatric hospital (n = 48), did not complete the study at either phase (n = 58), or died before Phase 2 (n = 1), 14% were non-White in race. The number of non-White patients who did not participate in current sample did not statistically differ from those who did (χ2 = 0.55, p = .46). Participants completed measures online at home or in clinic, and demographic and medical variables were abstracted from hospital and clinic charts. The study protocol and informed consent/assent procedures were approved by the institutional review board.
Figure 1.
Study flow diagram.
aIncludes having data for the Behavior Rating Inventory of Executive Function, Responses to Stress Questionnaire, and the Youth Self-Report/Adult Self-Report.
Demographics and Medical Status
Participants self-reported sex, race/ethnicity, and years of education. Estimated family income was derived by the Federal Financial Institutions Examination Council (FFIEC) 2013 Census estimate, which is based on participants’ home address. The FFIEC uses loan application information, in conjunction with self-reported federal census data, to estimate family income within a specific geographic census tract.
Cardiac lesion severity was determined based on diagnosis and/or surgical procedure as outlined by the American College of Cardiology/American Heart Association (Warnes et al., 2008). New York Heart Association (NYHA) functional class was extracted from medical charts, categorizing participants into two groups based on the presence of shortness of breath, heart palpitations, or fatigue limiting physical activity: no impairment (NYHA = 1) and at least some impairment (NYHA > 1). CHD survivors with and without these functional limitations have reported significant differences in physical and emotional health-related quality of life (Apers et al., 2016; Jackson, Gerardo, Daniels, & Vannatta, 2017; Jackson et al., 2016).
Self-Report Measures
Executive Function
The Behavior Rating Inventory of Executive Function (BRIEF), Self-Report (ages 11–18 years) and Adult Self-Report (ASR; ages 18+ years) versions, assess problems with executive function, including difficulty initiating tasks, working memory, planning, completing tasks, and organization (Guy, Isquith, & Gioia, 2004; Roth, Isquith, & Gioia, 2005). The BRIEF has been used among children and adolescents with CHD (Cassidy, White, DeMaso, Newburger, & Bellinger, 2015). The 80 items on the Self-Report version and 75 on the Adult version are totaled to create a Global Executive Composite, which are converted into T-scores based on age with higher T-scores indicative of worse executive function. T-scores above 64 for adolescent males, 63 for adolescent females, and 65 for adults are above the 90th percentile. The BRIEF has demonstrated moderate to high internal consistency (Cronbach’s α = 0.96 [Self-Report]; 0.96 [Adult Self-Report]), as well as discriminant (i.e., depression and anxiety) validity (Roth, Isquith, & Gioia, 2005). For the current study, the internal consistency of the both versions was strong (α = 0.96 [Self-Report], 0.99 [Adult Self-Report]).
Coping
The Responses to Stress Questionnaire (RSQ) (Connor-Smith, Compas, Wadsworth, Thomsen, & Saltzman, 2000) has been adapted to assess coping in relation to a variety of contextual and disease-related stressors (Compas et al., 2017), including CHD (Jackson et al., 2016). Participants were asked to think about a list of CHD-related stressors and rate how often they engaged in various coping and involuntary responses to stress on a four-point Likert scale (“Not at all,” “A Little,” “Somewhat,” and “Very”). Coping responses included: primary control coping (9 items; e.g., problem-solving, emotional expression, emotion regulation), secondary control coping (12 items; e.g., cognitive restructuring, positive thinking, acceptance), and disengagement coping (9 items; e.g., behavioral avoidance, wishful thinking). In accordance with recommended scoring of the RSQ based on confirmatory factor analysis (Connor-Smith et al., 2000), proportion scores were computed by dividing totals of subscale items by the grand total of all items to adjust for differences in response tendencies, such as endorsing all items in the extremes, thereby emphasizing which coping strategies are used more or less by each respondent. Each coping variable was computed as the mean of the subscale items divided by the total score on the measure. The reliability and validity of the coping scales have been documented (Connor-Smith et al., 2000). For the current study, all coping scales demonstrated adequate to good internal consistency (Cronbach’s α = 0.76–0.84).
Internalizing Symptoms
The Youth Self-Report (YSR; ages 15–17 years) and ASR (ages 18+ years) were used to measure symptoms of depression and anxiety (Achenbach & Rescorla, 2001, 2003). The Internalizing subscale includes 31 items for the YSR and 39 items for the ASR on a three-point Likert scale (“Not True,” “Somewhat or Sometimes True,” and “Very True or Often True”). The YSR and ASR produce T-scores, which if >63 are in the clinical range and are above the 90th percentile. The reliability and validity of these measures have been well documented (Achenbach & Rescorla, 2001, 2003). Excellent internal consistency was demonstrated in the current study (α = 0.91 [YSR], 0.93 [ASR]).
Plan of Analysis
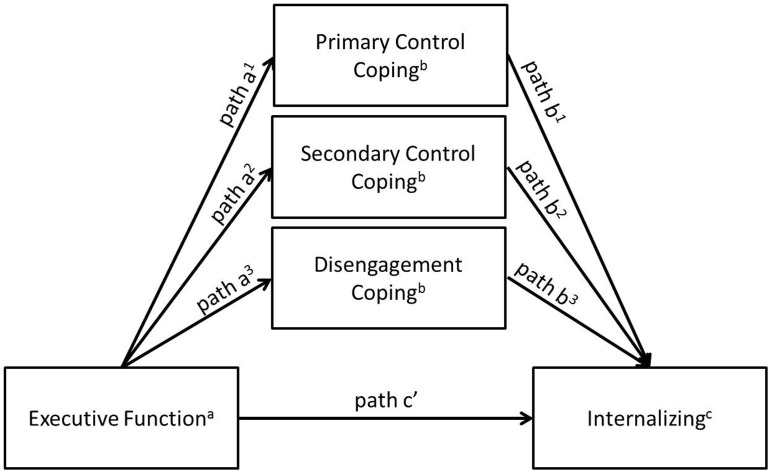
Separate analyses were conducted for AEA and YA. Analyses of variance and planned contrasts or t-tests were used to determine if problems with executive function varied between lesion severity categories and groups with and without functional impairment. Pearson correlations were computed to determine associations between executive function, coping, and internalizing symptoms. To test the hypothesized indirect effect of problems with executive function on internalizing symptoms via coping, an ordinary least squares path analytic framework was used via the PROCESS macro in SPSS (Hayes, 2013). As shown in Figure 2, the three types of coping were simultaneously entered into the model to examine whether problems with executive function had an indirect effect on internalizing symptoms via each coping dimension. This procedure yields unstandardized path coefficients (betas) for each individual path of the model. Unstandardized path coefficients are scaled according to the measurement of variables in each path of the model and are preferable over standardized coefficients in this type of modeling (Deegan, 1978). The significance of the indirect effects was determined using bootstrapped (n = 10,000) confidence intervals.
Figure 2.
Conceptual mediation model.
aBehavior Rating Inventory of Executive Function.
bResponses to Stress Questionnaire.
cYouth Self-Report (ages 15–17 years) and Adult Self-Report (ages 18+ years).
Results
Demographic information and means for study variables are reported in Table I. Approximately 10% of the sample (n = 17) reported clinically significant problems in global executive function (at or above the 90th percentile), which is similar to the general population. When broken down by age group, only 5% of AEA reported clinically significant T-scores, as compared with 13% of YA. Problems with executive function did not differ by lesion severity [AEA: F(2, 71) = 1.97, p = .147; η2 = 0.05; YA: F(2, 97) = 0.32, p = .727; η2 = 0.01] nor functional impairment status (AEA: t = −1.30, df = 72, p = .199, d = 0.35; YA: t = −1.74, df = 96, p = .084, d = 0.36) across the age groups.
Table I.
Demographics, Coping, Executive Functioning, and Internalizing Symptoms by Age Grouping
| Adolescents and emerging adults (n = 74) |
Young adults (n = 98) |
|||
|---|---|---|---|---|
| M (SD) or % | Range | M (SD) or % | Range | |
| Age | 19.32 (3.47) | 15–25 | 32.00 (3.69) | 26–39 |
| Sex (female) | 51 | 62 | ||
| Race/ethnicity (minority) | 10 | 12 | ||
| Living independently | 34 | 83 | ||
| Education | ||||
| Currently students | 72 | 8 | ||
| Not currently students | 28 | 92 | ||
| Completed high school or GED program | 46 | 78 | ||
| Completed collegea | 29 | 40 | ||
| Works full time | 16 | 55 | ||
| Estimated family incomeb ($) | 77,253 (36,519) | 12,718–167,509 | 65,483 (27,514) | 20,642–167,509 |
| Lesion severity | ||||
| Simple | 35 | 25 | ||
| Moderate | 41 | 41 | ||
| Complex | 24 | 34 | ||
| Functional limitations | ||||
| None | 78 | 63 | ||
| At least some | 22 | 37 | ||
| Global Executive Compositec | 46.93 (9.43) | 32–76 | 50.53 (12.37) | 34–100 |
| Copingd | ||||
| Primary control coping | 0.17 (0.03) | 0.10–0.26 | 0.17 (0.04) | 0.02–0.26 |
| Secondary control coping | 0.30 (0.06) | 0.10–0.44 | 0.30 (0.07) | 0.04–0.44 |
| Disengagement coping | 0.14 (0.03) | 0.09–0.21 | 0.14 (0.03) | 0.03–0.23 |
| Internalizinge | 50.19 (11.53) | 30–76 | 52.19 (14.18) | 30–98 |
Note. NYHA = New York Heart Association.
Completed at least 4 years of college.
Federal Financial Institutions Examination Council uses loan application information, in conjunction with self-reported federal census data, to derive estimate family income within a specific geographic census tract.
Behavior Rating Inventory of Executive Function (T-Score); higher scores indicate poorer self-report executive functioning.
Responses to Stress Questionnaire; higher scores indicate greater use of that coping response.
Youth Self-Report (ages 15–17 years) and Adult Self-Report (ages 18+ years) (T-Score); higher scores indicate greater internalizing symptoms.
Correlations among the study variables by age group are presented in Table II. For AEA, more internalizing symptoms were associated with more problems with executive function. Problems with executive function and internalizing symptoms were not associated with coping. Great disease complexity was associated with more internalizing symptoms and was therefore included as a covariate in the regression model. A different pattern emerged for YA, such that more problems with executive function were associated with less use of primary and secondary control coping, and greater use of disengagement coping. Similarly, all three dimensions of coping were associated in expected directions with internalizing symptoms.
Table II.
Pearson Correlations Among Measures of Executive Functioning, Coping, Functional Class, and Internalizing Symptoms by Age Grouping
| A. | B. | C. | D. | E. | F. | |
|---|---|---|---|---|---|---|
| Adolescents and emerging adults | ||||||
| A. Global executive functiona | – | |||||
| B. Primary control copingb | −0.09 | – | ||||
| C. Secondary control copingb | −0.06 | 0.24* | – | |||
| D. Disengagement copingb | 0.01 | −0.54* | −0.52* | – | ||
| E. Lesion severity | 0.21 | 0.05 | −0.00 | −0.00 | – | |
| F. Functional impairment | 0.15 | −0.10 | −0.14 | 0.07 | 0.38* | – |
| G. Internalizingc | 0.56* | 0.05 | −0.13 | −0.02 | 0.29* | 0.19 |
| Young adults | ||||||
| A. Global executive functiona | – | |||||
| B. Primary control copingb | −0.40* | – | ||||
| C. Secondary control copingb | −0.43* | 0.43* | – | |||
| D. Disengagement copingb | 0.29* | −0.45* | −0.28* | – | ||
| E. Lesion severity | 0.02 | −0.04 | −0.09 | −0.02 | – | |
| F. Functional impairment | 0.18 | 0.93 | −0.31* | 0.01 | 0.23* | – |
| G. Internalizingc | 0.81* | −0.37* | −0.48* | 0.34* | 0.10 | 0.12 |
Behavior Rating Inventory of Executive Function (T-Score); higher scores indicate poorer self-report executive functioning.
Responses to Stress Questionnaire; higher scores indicate greater use of that coping response.
Youth Self-Report (ages 15–17 years) and Adult Self-Report (ages 18+ years) (T-Score); higher scores indicate greater internalizing symptoms.
p ≤ .01; two-tailed tests.
The results of the ordinary least squares path analyses by age group are presented in Table III. When all three coping responses were included in the model for AEA (Model R2 = 0.37), problems with executive function (Path c′) and lesion severity continued explaining a significant portion of the variance in internalizing symptoms, but problems with executive functioning did not predict coping (Paths a1–3), and coping did not predict internalizing symptoms (Paths b1–3). Additionally, there were no indirect effects of executive functioning via coping on internalizing symptoms. In contrast for YA (Model R2 = 0.67), more problems with executive function were associated with less use of primary and secondary control coping (Paths a1–2) and greater use of disengagement coping (Path a3). Greater use of secondary control coping was associated with fewer internalizing symptoms (Path b2). Primary control and disengagement were no longer associated with internalizing symptoms (Paths b1 and b3). An indirect effect of problems with executive function on internalizing symptoms via secondary control coping emerged, such that those with more executive function problems used less secondary control coping strategies, which in turn was associated with increased emotional distress.
Table III.
Indirect Effect of Executive Function on Internalizing Symptoms via Coping by Age Grouping
| B (SE) | t | p | Model R2 | ||
|---|---|---|---|---|---|
| Adolescents and emerging adults | |||||
| Executive functionapredicting coping | |||||
| Model 1: Primary control copingb (Path a1) | −0.00 (0.00) | −0.85 | .3997 | 0.01 | |
| Model 2: Secondary control copingb (Path a2) | −0.00 (0.00) | −0.72 | .4757 | 0.02 | |
| Model 3: Disengagement copingb (Path a3) | 0.00 (0.00) | −0.03 | .6063 | 0.00 | |
| Predicting internalizing symptomsc | |||||
| Model 4: | 0.37 | ||||
| Primary control coping (Path b1) | 31.05 (41.11) | 0.76 | .4528 | ||
| Secondary control coping (Path b2) | −34.72 (20.40) | −1.70 | .0933 | ||
| Disengagement coping (Path b3) | −41.22 (56.63) | −0.73 | .4691 | ||
| Global executive function | 0.62 (0.12) | 5.13 | .0000 | ||
| Lesion severity | 3.04 (1.52) | 2.01 | .0483 | ||
|
B |
95% CI |
||||
| Indirect effects | |||||
| Primary control coping | −0.01 | −0.10 to 0.01 | |||
| Secondary control coping | 0.02 | −0.01 to 0.12 | |||
| Disengagement coping | 0.00 | −0.05 to 0.05 | |||
| Young adults | |||||
| Executive functionapredicting copingd | |||||
| Model 1: Primary control copingb (Path a1) | −0.00 (0.00) | −4.19 | .0000 | 0.16 | |
| Model 2: Secondary control copingb (Path a2) | −0.00 (0.00) | −4.56 | .0000 | 0.18 | |
| Model 3: Disengagement copingb (Path a3) | 0.00 (0.00) | 2.93 | .0043 | 0.08 | |
| Predicting internalizing symptomsc | |||||
| Model 4: | 0.67 | ||||
| Primary control coping (Path b1) | 8.48 (24.83) | 0.34 | .7335 | ||
| Secondary control coping (Path b2) | −30.15 (14.25) | −2.12 | .0371 | ||
| Disengagement coping (Path b3) | 36.19 (31.27) | 1.16 | .2502 | ||
| Global executive function | 0.83 (0.08) | 10.52 | .0000 | ||
|
B |
95% CI |
||||
| Indirect effects | |||||
| Primary control coping | −0.01 | −0.08 to 0.06 | |||
| Secondary control coping | 0.07 | 0.01–0.16e | |||
| Disengagement coping | 0.03 | −0.02 to 0.11 | |||
Note. CI = confidence interval; NYHA = New York Heart Association.
Behavior Rating Inventory of Executive Function (T-Score); higher scores indicate poorer self-report executive functioning.
Responses to Stress Questionnaire; higher scores indicate greater use of that coping response.
Youth Self-Report (ages 15–17 years) and Adult Self-Report (ages 18+ years) (T-Score); higher scores indicate greater internalizing symptoms.
Values listed are based on the inclusion of NYHA class as a covariate in the model.
When the CI does not include 0, it supports an indirect effect of executive function on internalizing symptoms via secondary control coping.
Discussion
CHD survivors are at risk for emotional distress and problems with executive function because of their cardiac malformations, surgical interventions, and functional limitations, as well as the stress generated by living with a chronic heart condition (Jackson et al., 2016; Kovacs et al., 2009; Marelli et al., 2016). However, little is known about how problems with executive function may interfere with coping and account for variations in emotional functioning in this growing, yet understudied, population of AEA and YA CHD survivors. The current study suggest that self-reported problems with executive function among YA, but not AEA, with CHD may disrupt adaptive coping and that interference in secondary control coping (e.g., cognitive reappraisal, acceptance) in particular may account for variations in internalizing symptoms.
Neither mean scores nor the proportion of participants in the clinical range suggested that CHD survivors in this study were experiencing problems with executive functioning that exceeded the general population. This was unanticipated given that survivors with complex lesions and those with a history open-heart surgery are at risk for executive function deficits (Calderon, 2016; Marino et al., 2012), and within the current sample, ∼70% of participants met those risk factors. Two indicators of disease complexity were examined in association with executive function problems, but neither lesion severity classification nor functional impairment was associated with executive function problems. Other indicators of disease severity may better capture executive function problems, such blood oxygenation and hypoxic ischemia (Marelli et al., 2016). Self-report of executive function may also lack of sensitivity to detect problems, although such measures have identified problems in pediatric illness survivors with white matter disruption (Ellenberg et al., 2009) similar to that which may occur in at-risk CHD survivors (Morton, Ishibashi, Jonas, & Gallo, 2015). Furthermore, the BRIEF may measure emotion dysregulation specifically, rather than other deficits of executive function that would be identified via performance-based measures (Rouel, Raman, Hay, & Smith, 2016). Adolescents in particular may also underreport executive function deficits (Steward, Tan, Delgaty, Gonzales, & Bunner, 2017), suggesting that adolescents’ self-report on the BRIEF should ideally be accompanied by multiple informant ratings, such as parents and/or teachers (Guy et al., 2004) and performance-based measures.
Problems with executive function were associated with internalizing symptoms for both age groups. While the link between executive function problems and depression and anxiety symptoms has not previously been examined among adult CHD survivors, it has among children and adolescents with CHD (Marelli et al., 2016; Neal et al., 2015), as well as in other medical populations (Ellenberg et al., 2009; Grech et al., 2016). Interestingly, the predicted associations between executive function and coping were found for YA, but not AEA. This is in contrast to a study by Evans and colleagues (Evans, Kouros, Samanez-Larkin, & Garber, 2016) who found that working memory and cognitive flexibility, components of executive function, are associated with less use of primary control and secondary control coping among healthy adolescents. CHD survivors are more likely to encounter changes in their health as they age, and age-related increases in CHD-related stress (Jackson et al., 2016) may suggest that there is less demand for coping with these stressors for adolescent than YA survivors.
The indirect effect of problems with executive function on internalizing symptoms as partially explained by less use of secondary control coping has been found among adults who have multiple sclerosis (Grech et al., 2016). Other studies have shown a mediating effect of problem-focused coping among individuals with acquired brain injury (Wolters Gregório et al., 2015), as well as primary and secondary control coping among healthy children and adolescents (Evans et al., 2016) when predicting emotional distress. These findings suggest that disruptions in executive function may make engaging in more complex cognitive processes difficult, such as those required for problem-solving (i.e., primary control coping) or reappraisal and acceptance (i.e., secondary control coping). In the current study, secondary control coping emerged as the only coping response to explain unique variance in the relationship between executive function and internalizing symptoms, suggesting this may be a particularly important skill set that is negatively affected by problems with executive function, resulting in higher levels of emotional distress. Many aspects of living with CHD are uncontrollable, including the need for future procedures, visible signs of intervention, and/or paying for health care, suggesting that effective use of secondary control coping is important for this population.
The current study has additional limitations that should be noted. First, the study was cross-sectional. Causal relationships between executive function, coping, and emotional distress cannot be ascertained and the direction of the associations between these variables may differ from the current model. Alternative models are viable and should be explored, such as interference with coping producing more stress, which in turn exacerbates or creates new stressors, thereby resulting in greater emotional distress. Additionally, other variables could be influential in these effects, such as individual differences in cognitive appraisal styles, stress reactivity, or posttraumatic stress symptoms. Second, self-report measures of internalizing and executive function problems were added at a later time for some participants. This may have introduced more measurement error and inhibited our ability to detect relationships between coping and problems with executive functioning. Third, the sample may be biased toward enrolling CHD survivors who have better executive function because of still being engaged in care and able to complete online surveys. CHD survivors are at greater risk for gaps in care, especially during the period of transition to adulthood (Mackie et al., 2009), and the differences between those who do and do not remain in care are not well understood.
In summary, the current study is the first to explore the relationship between problems with executive function, coping, and emotional distress among AEA and YA CHD survivors. Cognitive behavioral interventions, including cognitive restructuring, are being explored for adult survivors of CHD to address the unique disease-related stressors (Kovacs et al., 2015). Results of the current study suggest that YA CHD survivors with self-reported executive function problems may struggle more with using secondary control coping, such as cognitive restructuring and acceptance, which are commonly taught during cognitive behavioral therapy, and may need more assistance with bolstering these skill sets. Problem-solving therapy, an approach that has been included in cognitive behavioral therapy protocols or used as a stand-alone treatment in other populations at risk for executive function problems, including traumatic brain injury (Wade et al., 2015) and epilepsy (Caller et al., 2016), may also be helpful for these individuals given that it targets aspects of primary control coping. Although additional longitudinal research using performance-based measures of executive function is needed to determine the impact of executive function problems on coping over time, results from the current study suggest that executive function problems should be considered as one contributing factor for increased risk of emotional distress in this growing population of adult CHD survivors.
Acknowledgments
The authors would like to acknowledge the support of congenital heart disease survivors and their families by participating in this work.
Funding
This work was supported by National Institutes of Health (grant number T32HL-098039) to J.L.J., the Heart Center at Nationwide Children’s Hospital to K.V., and Clinical and Translational Science Award (grant number UL1TR001070) to the Ohio State University and Nationwide Children’s Hospital.
Conflicts of interest: None declared.
References
- Achenbach T. M., Rescorla L. A. (2001). Manual for the ASEBA school-age forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Achenbach T. M., Rescorla L. A. (2003). Manual for the ASEBA adult forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Andreotti C., Thigpen J. E., Dunn M. J., Watson K., Potts J., Reising M. M., Robinson K. E., Rodriguez E. M., Roubinov D., Luecken L., Compas B. E. (2013). Cognitive reappraisal and secondary control coping: Associations with working memory, positive and negative affect, and symptoms of anxiety/depression. Anxiety, Stress, and Coping, 26, 20–35. doi: 10.1080/10615806.2011.631526 [DOI] [PubMed] [Google Scholar]
- Apers S., Kovacs A. H., Luyckx K., Thomet C., Budts W., Enomoto J., Sluman M. A., Wang J. K., Jackson J. L., Khairy P., Cook S. C., Chidambarathanu S., Alday L., Eriksen K., Dellborg M., Berghammer M., Mattsson E., Mackie A. S., Menahem S., Caruana M., Veldtman G., Soufi A., Romfh A. W., White K., Callus E., Kutty S., Fieuws S., Moons P.; APPROACH-IS consortium and ISACHD. (2016). Quality of life of adults with congenital heart disease in 15 countries: Evaluating country-specific characteristics. Journal of the American College of Cardiology, 67, 2237–2245. doi: 10.1016/j.jacc.2016.03.477 [DOI] [PubMed] [Google Scholar]
- Arnett J. J. (2000). Emerging adulthood. A theory of development from the late teens through the twenties. American Psychologist, 55, 469–480. doi: 10.1037/0003-066X.55.5469 [DOI] [PubMed] [Google Scholar]
- Band E. B., Weisz J. R. (1988). How to feel better when it feels bad: Children’s perspectives on coping with everyday stress. Developmental Psychology, 24, 247–253. doi: 10.1037//0012-1649.24.2.247 [DOI] [Google Scholar]
- Calderon J. (2016). Executive function in patients with congenital heart disease: Only the tip of the iceberg? Journal of Pediatrics, 173, 7–9. doi: 10.1016/j.jpeds.2016.02.066 [DOI] [PubMed] [Google Scholar]
- Calderon J., Bellinger D. C. (2015). Executive function deficits in congenital heart disease: Why is intervention important? Cardiology in the Young, 25, 1238–1246. doi: 10.1017/S1047951115001134 [DOI] [PubMed] [Google Scholar]
- Caller T. A., Ferguson R. J., Roth R. M., Secore K. L., Alexandre F. P., Zhao W., Tosteson T. D., Henegan P. L., Birney K., Jobst B. C. (2016). A cognitive behavioral intervention (HOBSCOTCH) improves quality of life and attention in epilepsy. Epilepsy and Behavior, 57, 111–117. doi: 10.1016/j.yebeh.2016.01.024 [DOI] [PubMed] [Google Scholar]
- Campbell L. K., Scaduto M., Van Slyke D., Niarhos F., Whitlock J. A., Compas B. E. (2009). Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. Journal of Pediatric Psychology, 34, 317–327. doi: 10.1093/jpepsy/jsn080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy A. R., White M. T., DeMaso D. R., Newburger J. W., Bellinger D. C. (2015). Executive function in children and adolescents with critical cyanotic congenital heart disease. Journal of the International Neuropsychological Society, 21, 34–49. doi: 10.1017/S1355617714001027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas B. E., Jaser S. A., Bettis A. H., Watson K. H., Gruhn M., Dunbar J. P., Williams E., Thigpen J. C. (2017). Coping, emotion regulation and psychopathology in childhood and adolescence: A meta-analysis and narrative review. Psychological Bulletin, 143, 939–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas B. E., Jaser S. S., Dunn M. J., Rodriguez E. M. (2012). Coping with chronic illness in childhood and adolescence. Annual Review of Clinical Psychology, 8, 455–480. doi: 10.1146/annurev-clinpsy-032511-143108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor-Smith J. K., Compas B. E., Wadsworth M. E., Thomsen A. H., Saltzman H. (2000). Responses to stress in adolescence: Measurement of coping and involuntary stress responses. Journal of Consulting and Clinical Psychology, 68, 976–992. doi: 10.1037//0022-006X.68.6.976 [DOI] [PubMed] [Google Scholar]
- Deegan J. (1978). On the occurrence of standardized regression coefficients greater than one. Educational and Psychological Measurement, 38, 873–888. 10.1177/001316447803800404 [DOI] [Google Scholar]
- Ellenberg L., Liu Q., Gioia G., Yasui Y., Packer R. J., Mertens A., Donaldson S. S., Stovall M., Kadan-Lottick N., Armstrong G., Robison L. L., Zeltzer L. K. (2009). Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the Childhood Cancer Survivor Study. Neuropsychology, 23, 705–717. doi: 10.1037/a0016674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. D., Kouros C. D., Samanez-Larkin S., Garber J. (2016). Concurrent and short-term prospective relations among neurocognitive functioning, coping, and depressive symptoms in youth. Journal of Clinical Child and Adolescent Psychology, 45, 6–20. doi: 10.1080/15374416.2014.982282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa S. M., Devine O. J., Kucik J. E., Oster M. E., Riehle-Colarusso T., Nembhard W. N., Xu P., Correa A., Jenkins K., Marelli A. J. (2016). Congenital heart defects in the United States: Estimating the magnitude of the affected population in 2010. Circulation, 134, 101–109. doi: 10.1161/CIRCULATIONAHA.115.019307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grech L. B., Kiropoulos L. A., Kirby K. M., Butler E., Paine M., Hester R. (2016). Coping mediates and moderates the relationship between executive functions and psychological adjustment in multiple sclerosis. Neuropsychology, 30, 361–376. doi: 10.1037/neu0000256 [DOI] [PubMed] [Google Scholar]
- Guy S. C., Isquith P. K., Gioia G. A. (2004). Behavior rating inventory of executive function—self-report version. Lutz, FL: PAR. [Google Scholar]
- Hayes A. F. (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press. [Google Scholar]
- Jackson J. L., Gerardo G. M., Daniels C. J., Vannatta K. (2017). Perceptions of disease-related stress: A key to better understanding patient-reported outcomes among survivors of congenital heart disease. Journal of Cardiovascular Nursing, 32, 587–593. Advance online publication. doi: 10.1097/JCN.0000000000000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. L., Hassen L., Gerardo G. M., Vannatta K., Daniels C. J. (2016). Medical factors that predict quality of life for young adults with congenital heart disease: What matters most? International Journal of Cardiology, 202, 804–809. doi: 10.1016/j.ijcard.2015.09.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs A. H., Bandyopadhyay M., Grace S. L., Kentner A. C., Nolan R. P., Silversides C. K., Irvine M. J. (2015). Adult congenital heart disease-coping and REsilience (ACHD-CARE): Rationale and methodology of a pilot randomized controlled trial. Contemporary Clinical Trials, 45, 385–393. doi: 10.1016/j.cct.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Kovacs A. H., Saidi A. S., Kuhl E. A., Sears S. F., Silversides C., Harrison J. L., Ong L., Colman J., Oechslin E., Nolan R. P. (2009). Depression and anxiety in adult congenital heart disease: Predictors and prevalence. International Journal of Cardiology, 137, 158–164. doi: 10.1016/j.ijcard.2008.06.042 [DOI] [PubMed] [Google Scholar]
- Kovacs A. H., Sears S. F., Saidi A. S. (2005). Biopsychosocial experiences of adults with congenital heart disease: Review of the literature. American Heart Journal, 150, 193–201. doi: 10.1016/j.ahj.2004.08.025 [DOI] [PubMed] [Google Scholar]
- Krpan K. M., Levine B., Stuss D. T., Dawson D. R. (2007). Executive function and coping at one-year post traumatic brain injury. Journal of Clinical Experimental Neuropsychology, 29, 36–46. doi: 10.1080/13803390500376816 [DOI] [PubMed] [Google Scholar]
- Mackie A. S., Ionescu-Ittu R., Therrien J., Pilote L., Abrahamowicz M., Marelli A. J. (2009). Children and adults with congenital heart disease lost to follow-up: Who and when? Circulation, 120, 302–309. [DOI] [PubMed] [Google Scholar]
- Marelli A., Miller S. P., Marino B. S., Jefferson A. L., Newburger J. W. (2016). Brain in congenital heart disease across the lifespan: The cumulative burden of injury. Circulation, 133, 1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino B. S., Lipkin P. H., Newburger J. W., Peacock G., Gerdes M., Gaynor J. W., Mussatto K. A., Uzark K., Goldberg C. S., Johnson W. H. Jr., Li J., Smith S. E., Bellinger D. C., Mahle W. T.; American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council. (2012). Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the American Heart Association. Circulation, 126, 1143–1172. doi: 10.1161/CIR.0b013e318265ee8a [DOI] [PubMed] [Google Scholar]
- Morton P. D., Ishibashi N., Jonas R. A., Gallo V. (2015). Congenital cardiac anomalies and white matter injury. Trends in Neurosciences, 38, 353–363. doi: 10.1016/j.tins.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal A. E., Stopp C., Wypij D., Bellinger D. C., Dunbar-Masterson C., DeMaso D. R., Newburger J. W. (2015). Predictors of health-related quality of life in adolescents with tetralogy of Fallot. Journal of Pediatrics, 166, 132–138. doi: 10.1016/j.jpeds.2014.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. M., Isquith P. K., Gioia G. A. (2005). Behavior rating inventory of executive function—adult version. Lutz, FL: PAR. [Google Scholar]
- Rouel M., Raman J., Hay P., Smith E. (2016). Validation of the Behavior Rating Inventory of executive function – adult version (BRIEF-A) in the obese with and without binge eating disorder. Eating Behaviors, 23, 58–65. doi: 10.1016/j.eatbeh.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Steward K. A., Tan A., Delgaty L., Gonzales M. M., Bunner M. (2017). Self-awareness of executive functioning deficits in adolescents with ADHD. Journal of Attention Disorders, 21, 316–322. doi: 10.1177/1087054714530782. [DOI] [PubMed] [Google Scholar]
- Wade S. L., Kurowski B. G., Kirkwood M. W., Zhang N., Cassedy A., Brown T. M., Nielsen B., Stancin T., Taylor H. G. (2015). Online problem-solving therapy after traumatic brain injury: A randomized controlled trial. Pediatrics, 135, e487–e495. doi: 10.1542/peds.2014-1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes C. A., Williams R. G., Bashore T. M., Child J. S., Connolly H. M., Dearani, J. A., del Nido P., Fasules J. W., Graham T. P. Jr., Hijazi Z. M., Hunt S. A., King M. E., Landzberg M. J., Miner P. D., Radford M. J., Walsh E. P., Webb G. D., Smith S. C. Jr., Jacobs A. K., Adams C. D., Anderson J. L., Antman E. M., Buller C. E., Creager M. A., Ettinger S. M., Halperin J. L., Hunt S. A., Krumholz H. M., Kushner F. G., Lytle B. W., Nishimura R. A., Page R. L., Riegel B., Tarkington L. G., Yancy C. W.; American College of Cardiology, American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease), American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons. (2008). ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Journal of the American College of Cardiology, 52, e143–e263. doi: 10.1016/j.jacc.2008.10.001 [DOI] [PubMed] [Google Scholar]
- Westhoff-Bleck M., Briest J., Fraccarollo D., Hilfiker-Kleiner D., Winter L., Maske U., Busch M. A., Bleich S., Bauersachs J., Kahl K. G. (2016). Mental disorders in adults with congenital heart disease: Unmet needs and impact on quality of life. Journal of Affective Disorders, 204, 180–186. doi: 10.1016/j.jad.2016.06.047 [DOI] [PubMed] [Google Scholar]
- Wolters Gregório G., Ponds R. W., Smeets S. M., Jonker F., Pouwels C. G., Verhey F. R., van Heugten C. M. (2015). Associations between executive functioning, coping, and psychosocial functioning after acquired brain injury. British Journal of Clinical Psychology, 54, 291–306. doi: 10.1111/bjc.12074 [DOI] [PubMed] [Google Scholar]