Abstract
Objective
An observational cross-sectional study was conducted in a national facioscapulohumeral muscular dystrophy (FSHD) expertise center to estimate the penetrance of FSHD1 and to evaluate phenotype–genotype correlations.
Methods
Ten FSHD1 probands carrying 4–9 D4Z4 unit alleles and 140 relatives were examined. All 150 participants were genetically characterized, including D4Z4 methylation levels in the mutation carriers. Mutation carriers were classified as (1) symptomatic: with symptoms of muscle weakness on history and muscle FSHD signs on examination; (2) asymptomatic: without symptoms of muscle weakness but with muscle FSHD signs on examination; and (3) nonpenetrant: without symptoms of muscle weakness on history and without muscle FSHD signs on examination. We assessed the relationship between age-corrected clinical severity score and repeat size, sex, and D4Z4 methylation levels.
Results
The maximum likelihood estimates of symptomatic and those of symptomatic plus asymptomatic FSHD showed that penetrance depends on repeat size and increases until late adulthood. We observed many asymptomatic carriers with subtle facial weakness with or without mild shoulder girdle weakness (25% [17/69]). Nonpenetrance was observed less frequently than in recent population studies (17% [12/69]), and most asymptomatic patients reported some shoulder pain. D4Z4 methylation tended to be lower in moderately to severely affected mutation carriers with 7 or 9 repeats.
Discussion
This family-based study detected a lower overall nonpenetrance than previously observed, probably due to many asymptomatic mutation carriers identified by careful examination of facial and shoulder muscles. The recognition of asymptomatic mutation carriers is essential for selection of participants for future trials, and the likelihood estimates are helpful in counseling.
Facioscapulohumeral muscular dystrophy (FSHD) type 1 (MIM 158,900) is one of the most common inherited adult muscular dystrophies characterized by progressive, often asymmetric muscle weakness.1,2 FSHD is in most cases associated with a contraction (1–10 units) of the polymorphic D4Z4 macrosatellite repeat in the chromosome 4q subtelomere. This leads to the production of the transcription factor DUX4 with numerous downstream myotoxic effects leading to muscular dystrophy.3,4 In control individuals, the D4Z4 array ranges between 11 and 100 units, a size range in which the D4Z4 embedded DUX4 gene is repressed by CpG methylation and other epigenetic modifications. DUX4 derepression in FSHD is caused either by a contraction to 1–10 D4Z4 units on a 4qA-specific FSHD-permissive haplotype (FSHD1) or by mutations in D4Z4 chromatin repressors SMCHD1 or DNMT3B in combination with a moderately sized D4Z4 repeat (11–20 units) on a 4qA allele (FSHD2).5–7 FSHD-permissive 4qA haplotypes are defined by the presence of a polymorphic DUX4 polyadenylation signal, which is necessary for DUX4 RNA stabilization in somatic tissue. Clinically, FSHD1 and FSHD2 are largely indistinguishable.8
In the premolecular era, estimation of penetrance of FSHD was based on clinical diagnostic criteria and examination of large pedigrees, which, in retrospect, were FSHD1 families. Disease presentation was considered age-dependent with almost complete (>95%) penetrance by the age of 20.1,9 These studies showed an approximately equal distribution of affected and nonaffected sibs in completely examined large sibships. With autosomal dominant inheritance and a 50% chance of carrying the mutation, this left little room for nonpenetrance. However, selected families were fairly large, with many sibs with clinically evident FSHD, as the authors were interested in linkage studies. Furthermore, follow-up genetic studies showed that patients in these studies carried mutations with moderate D4Z4 contractions (4–8 units). This selection bias is likely to have contributed to the assumed high penetrance by the age of 20.
More recent genetic studies have shown penetrance to be incomplete even at older age, with nonpenetrance of 32%–53% dependent on repeat size and degree of kinship.10–14 In addition, a recent study in the general population has shown a 1%–2% prevalence of a contracted D4Z4 repeat on a permissive 4qA haplotype without an FSHD family history.5,15
Together, these studies point to a role for additional causative (epigenetic) mechanisms contributing to developing FSHD1. This is supported by the observation that very large pedigrees of this apparently nonlethal disease are less common than would be expected based on the high mutation frequency and the high prevalence of the permissive haplotype.16 One of these epigenetic mechanisms includes the degree of D4Z4 methylation, which was not assessed in the population studies discussed above.10–14 Our recent studies have shown that D4Z4 methylation significantly correlates with repeat size, but is also likely to be modified by variants of known and unknown chromatin modifiers, especially affecting the severity in individuals with repeats of 8–10 units.3,5,7,17
We performed a cross-sectional, observational single-center study in 10 families with at least 2 affected family members, encompassing 10 probands and 140 first-, second-, and third-degree relatives. We aimed to estimate penetrance for FSHD1 for different repeat lengths and ages. Different from other population studies, we distinguished between (1) mutation carriers with muscle symptoms on history and muscle FSHD signs on examination (symptomatic carriers); (2) mutation carriers without muscle symptoms but with muscle signs of FSHD on examination (asymptomatic carriers); and (3) mutation carriers without muscle symptoms on history and without muscle FSHD signs on examination (nonpenetrant carriers). In addition, we assessed the relationship between age-corrected clinical severity score (CSS) and repeat size, sex, and D4Z4 methylation levels.
Methods
Standard protocol approvals, registrations, and patient consents
The study protocol was approved by the local medical ethics committee and informed consent was obtained from all participants (CMO 2001/031).
Outcome measures
The primary outcome measures were the maximum likelihood estimates of symptomatic and symptomatic plus asymptomatic FSHD. The secondary outcome measures were the relationships between age-corrected CSS and repeat size, sex, and D4Z4 methylation levels.
Patients
We recruited 11 genetically confirmed FSHD1 probands from families with at least 2 adult affected members. This excluded probands with D4Z4 repeats <4 units as they are frequently unique cases. One family with compound heterozygous contracted D4Z4 allele was excluded, resulting in 10 probands. We aimed to examine all available adult first-, second-, and third-degree family members irrespective of their clinical condition by home visits. Obligate mutation carriers not included in this study were defined as family members with genetically confirmed FSHD (in a diagnostic setting) or family members with offspring with genetically confirmed FSHD, both of whom were not able or willing to participate.
Clinical evaluation
All participants filled out a questionnaire on symptoms and signs of FSHD, including age at onset, initial and current weakness of facial, shoulder, and pelvic girdle, and foot extensor muscles. They were also questioned about other symptoms and their medical history and medication use. All participants were examined by the same well-trained observer (M.W.). Mutation carriers were classified as “symptomatic,” “asymptomatic,” and “nonpenetrant” as defined above. Muscle FSHD signs included both weakness and atrophy and related signs (incomplete forceful eye closure “signe de cils,” scapular winging, poly hill sign, horizontal axial folds, and Beevor sign).2,18 Facial weakness was scored as 0–2: 0 = no weakness; 1 = moderate weakness; 2 = severe weakness of the orbicularis oculi or oris muscles or the zygomaticus major muscles. Shoulder and pelvic girdle and foot extensor muscles were tested using manual muscle testing. In addition, subtle shoulder girdle weakness was specifically tested by repetition of slowly lowering the arms forwards and sideward (after maximal shoulder anteflexion and abduction). Clinical severity was also scored using the CSS and the age-corrected CSS.19 When a discrepancy was found between clinical observation and the genetic test, participants were evaluated by a second observer (G.W.P.) who was blinded for these results.
Genetic testing
All participants were analyzed for D4Z4 repeat size, haplotype, and D4Z4 methylation. Genomic DNA was isolated from peripheral blood mononuclear cells and detailed analysis of the D4Z4 repeat sizes and haplotype on chromosomes 4 and 10 was done by pulsed field gel electrophoresis (PFGE) and PCR as described.20 Further haplotype analysis was done by Southern blot hybridization with probes A and B and by analysis of simple sequence length polymorphism according to previously described protocols.20
Repeat array sizes ranging from 20 to 60 kb (4–16 units) were accurately determined using a modified PFGE program specific for fragment sizes between 5 and 75 kb and by adding a 5-kb molecular weight marker to each lane of the genomic DNA samples (Bio-Rad, Hercules, CA, 170–3,624).17 D4Z4 methylation was established at the FseI restriction site in the most proximal unit of the 4 D4Z4 repeat arrays as described previously.6 We used the delta1-methylation value, which is the difference between the predicted D4Z4 methylation level based on the repeat size and the observed methylation level.17 Control individuals and mutation carriers with an FSHD allele shorter than 7 units generally have delta1-methylation values between −10 and +10%. For mutation carriers with 7–10 unit FSHD alleles, the delta1-methylation value is more variable, which has been suggested to contribute to the variation in clinical severity.17,21 For FSHD2 patients, delta1-methylation values typically range between −21% and −45%.
Estimation of penetrance and genotype–phenotype correlations
The demographic, clinical, and genetic data were recorded as variables in a SPSS database (SPSS version 22, SPSS, Chicago, IL). Symptomatic mutation carriers had a self-reported age at onset preceding the age at examination. The age at onset of asymptomatic FSHD was unknown; by definition, it precedes the age at examination (age at onset is interval censored, type I). Nonpenetrant mutation carriers have an unknown age at onset that is always later than the age at examination (right-censored).
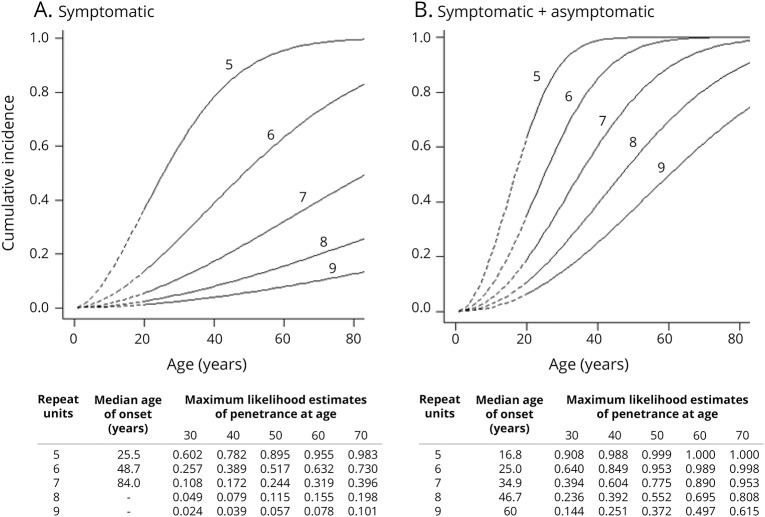
We determined the maximum likelihood estimates of the penetrance of symptomatic and symptomatic plus asymptomatic FSHD for 5, 6, 7, 8, and 9 D4Z4 units (from up to down) for age (figure 1). This represents the likelihood of reported symptoms by a patient at a certain age (symptomatic mutation carriership) and of reported symptoms by patients or observed signs by the neurologist at a certain age (symptomatic and asymptomatic mutation carriership). Both penetrances were modeled as Cox regression models with a Weibull baseline distribution and the logarithm of the number of D4Z4 units as a covariate. We estimated the unknown measures in the model by maximizing the conditional likelihood (by means of a grid search); the likelihood is conditional on the ascertainment event to correct for the selection of families (at least 2 known symptomatic mutation carriers).
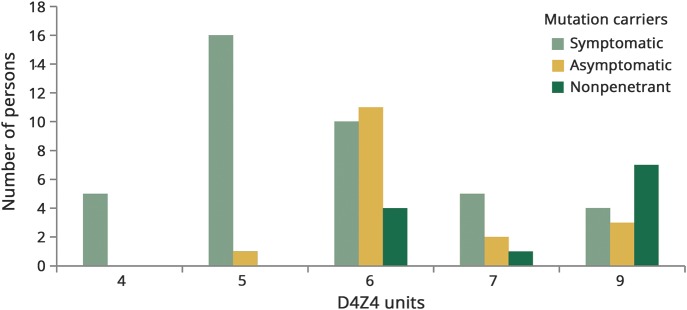
Figure 1. Penetrance for each contraction size.
Total number of facioscapulohumeral muscular dystrophy type 1 allele carriers grouped by repeat size in units and sorted by clinical status (symptomatic, asymptomatic and nonpenetrant mutation carriers).
The analysis thus (1) corrected for the selection of families with at least 2 known symptomatic mutation carriers; (2) distinguished for different repeat lengths with a presumed correlation to disease severity; and (3) took into account the unknown age at onset of asymptomatic mutation carriers. No correction for other factors that may influence disease severity (environmental, methylation, and epigenetic factors) was included. The R-code and mathematical calculations can be obtained on request by the corresponding author (Nicol Voermans, unpublished data, December 22, 2017).
Correlation between repeat length and age-corrected CSS was calculated with the Spearman correlation coefficient. We assessed the relationship between age-corrected CSS and repeat size, sex, and delta1-methylation value by linear regression analysis (without correction for other environmental and epigenetic factors that may influence CSS). The delta1-methylation value of mildly and severely affected patients (age-corrected CSS <50 and age-corrected CSS ≥50) was visualized in a graph.
Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Participants
We studied 10 families and invited 197 family members (95 male). Data are available from Dryad Pedigrees, doi.org/10.5061/dryad.cj4c2s1. Forty-seven family-members (29 male) were not able to participate for various reasons (living abroad, having lost contact with their family, or not willing to participate). Three of them were obligate mutation carriers. Hence, 10 probands (5 male) and 140 relatives were included (66 male), resulting in an inclusion rate of 76%. The median age was 44.5 years (range 18–85 years) (table 1: features of FSHD mutation carriers; table 2: distribution of mutation carriers and noncarriers by D4Z4 allele size in units).
Table 1.
Features of facioscapulohumeral muscular dystrophy (FSHD) type 1 mutation carriers (symptomatic, asymptomatic, and nonpenetrant)

Table 2.
Distribution of mutation carriers and noncarriers by D4Z4 allele size in units
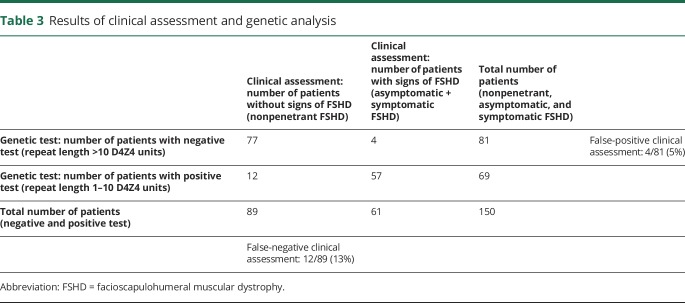
Genetic analysis identified 69 FSHD1 mutation carriers (28 male), of which 40 were symptomatic (10 probands [5 male] and 30 relatives [11 male]), 17 were asymptomatic (8 male), and 12 were nonpenetrant (4 male). This corresponded with a false-negative rate of the clinical assessment of 13% (12/89). In 4 nonmutation carriers, we observed mild asymmetrical weakness of the facial (n = 2) or shoulder muscles with shoulder pain (n = 2). These 4 participants were incorrectly scored by both examiners as asymptomatic with a CSS of 1 or 2 (CSS 1: facial weakness only; CSS 2: mild scapular weakness; no limitations of abduction or elevation; often asymptomatic). One of them was diagnosed before with idiopathic adhesive capsulitis of the shoulder, and one with rheumatoid arthritis. The other 2 showed very subtle facial weakness. Hence, the false-positive rate of clinical assessment for detection of the carrier status was 5% (4/81) (table 3: results of clinical assessment and genetic analysis).
Table 3.
Results of clinical assessment and genetic analysis
The pedigrees of these 10 families are deposited in Dryad (Data Dryad Digital Repository, doi:10.5061/dryad.cj4c2s1).
Clinical evaluation
The variation in estimated age at onset based on the recollection was large (median 20.0; range 10–60 years). Shoulder girdle weakness was the most frequent initial and current symptom (85%; 34 of the 40 symptomatic mutation carriers) (table 4: characteristics of individual patients). Pain, both chronic and episodic, was reported by 33 of the 40 symptomatic carriers, predominantly in shoulder, neck, and lower back region. Strikingly, 10 of the 17 asymptomatic carriers spontaneously reported shoulder girdle pain. Eight of these 10 had mild weakness of the shoulder girdle muscles (asymmetrical movement of the scapula); the other 2 young female patients only had mild facial weakness.
Table 4.
Characteristics of individual patients
The questionnaires on concomitant diseases and medication use showed no malignancies, and no remarkable other comorbidity or medication use. Four female mutation carriers reported worsening of the weakness after pregnancies or after menopause, and 2 mutation carriers reported a positive effect on muscle weakness when taking oral contraceptives. However, the number of female mutation carriers was too small to analyze correlation with hormonal effects.
Results of clinical examination are shown in table 2; overall, 40 mutation carriers were symptomatic (58%), 17 asymptomatic (25%; 26% in 4–6 units; 23% in 7–9 units), and 12 had neither symptoms nor signs of muscle weakness (nonpenetrant FSHD) (17%; 9% in 4–6 units; 36% in 7–9 units). The median CSS was 3 (range 0–9); the median age-corrected CSS was 94 (range 0–273).
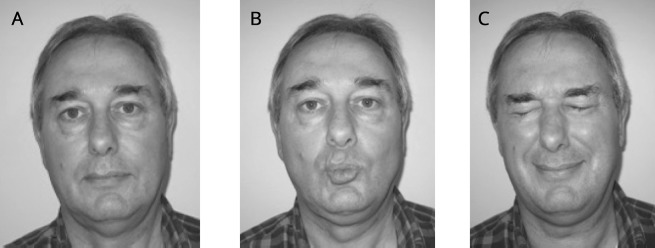
Relatives with symptomatic muscle weakness who attributed this to other disorders or aging were considered symptomatic. For example, we observed a carrier with unilateral foot drop and a carrier with a camptocormia, both with mild facial and shoulder girdle weakness. The asymptomatic mutation carriers were generally mildly affected (CSS 1–2) and all had at least mild facial weakness (figure 2). We did not observe any mutation carriers with shoulder girdle weakness without facial weakness.
Figure 2. Mild facioscapulohumeral muscular dystrophy features on examination in an asymptomatic patient.
(A) At rest. (B) Pursing of lips shows asymmetry of the orbicularis oris muscle. (C) Forceful closing of eyes: the eyelashes do not disappear: “signe de cils.”
Genetic testing
The disease allele size varied from 4 to 9 D4Z4 units (20–38 kb). Seven of the 12 nonpenetrant carriers were from 2 families carrying a 9-unit repeat. Interestingly, 6 asymptomatic carriers and 4 nonpenetrant carriers were found in family Rf208 with a 5 units repeat length. Mean age of these nonpenetrant carriers was 24.8 years (range 19–30 years), whereas the mean age of the asymptomatic carriers in this family was 43.0 (range 21–56 years). D4Z4 methylation levels were assessed in 66 mutation carriers and were variable with a delta1-methylation value range of −29.9 to 11.3.
Estimated penetrance and genotype–phenotype correlations
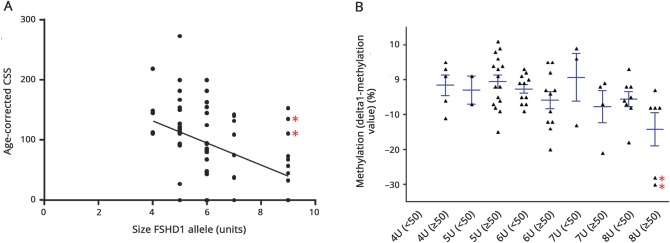
The maximum likelihood estimates of the penetrance for symptomatic and symptomatic plus asymptomatic mutation carriers showed that penetrance depends on age and repeat size (figure 3, A and B). We observed a moderate correlation between the age-corrected CSS and the size of the FSHD allele (R = −0.48 [p < 0.01]; figure 4A). We detected large differences between individual delta1-methylation values. Reduced methylation tended to be associated with a more severe phenotype (age-corrected CSS >50) in the carriers of the large size FSHD alleles (9 units) (figure 4B). Linear regression analysis confirmed this trend for methylation; in contrast, sex did not contribute to age-corrected CSS (β coefficients: −0.55 [p < 0.001], −0.224 [p = 0.06], −0.11 [p = 0.92] for repeat size, methylation level, and sex, respectively).
Figure 3. Maximum likelihood curves of the penetrance.
Maximum likelihood estimates of the penetrance of symptomatic (A) and symptomatic plus asymptomatic facioscapulohumeral muscular dystrophy (FSHD) (B) for 5, 6, 7, 8, and 9 D4Z4 units (from up to down) for age. This represents the likelihood of reported symptoms by the patient at a certain age (A: symptomatic mutation carriership) and of reported symptoms by the patients or observed signs by the neurologist at a certain age (B: symptomatic and asymptomatic mutation carriership). Both penetrances were modeled as Cox regression models with a Weibull baseline distribution and the logarithm of the number of D4Z4 units as a covariate. (A) Carriers with 8 repeats have a 20% (0.198) chance of being symptomatic at age 70. (B) Carriers of repeat size of 8 units have only a 24% (0.236) chance of being detected by clinical examination at age 30, which is in agreement with our clinical experience. These likelihood estimates are helpful in counseling; however, the number of patients on which the estimates are based calls for cautiousness. (A) Maximum likelihood estimates of penetrance of symptomatic FSHD (both symptoms + signs). (B) Maximum likelihood estimates of penetrance of symptomatic plus asymptomatic FSHD (only signs and both symptoms and signs).
Figure 4. Genotype–phenotype correlations.
(A) Correlation between the size of the facioscapulohumeral muscular dystrophy allele in units and the age-corrected clinical severity score (CSS). R = −0.48 (p < 0.01) (Spearman rho). (B) D4Z4 methylation (delta1-methylation value) and clinical severity (dichotomized age-corrected CSS) for each repeat length. For each repeat array size, we binned the mutation carriers based on their age-corrected CSS (<50: mild disease severity; ≥50: moderate–severe disease severity). For carriers of a 9-unit repeat, more severe disease tended to be associated with a lower delta1-methylation value (p = 0.06). *Mutation carriers with DNMT3B variant.
Family 210 is a family with a 9-unit D4Z4 allele. Three of the 6 mutation carriers in this family were clinically affected, 2 of whom had strongly reduced delta1-methylation values pointing to severe D4Z4 hypomethylation (patient 201: −28.4 and patient 212: –29.9). This suggested the presence of D4Z4 methylation modifiers. Indeed, genetic studies identified a pathogenic DNMT3B variant causing D4Z4 hypomethylation in this family.7
Discussion
This family-based study on penetrance in 10 probands and 140 relatives showed that prevalence of both symptomatic and asymptomatic FSHD1 increases into late adulthood (age >60 years) (figure 3, A and B). These maximum likelihood estimates are helpful in counseling; however, the number of patients on which the estimates are based calls for caution. We found a high percentage of asymptomatic mutation carriers (25% [17/69]; 26% in 4–6 units; 23% in 7–9 units). Nonpenetrance of FSHD1 was lower than in recent population studies with similar age ranges (17% [9% in 4–6 units; 36% in 7–9 units] vs 32%–52%), most likely since nonpenetrant FSHD1 and asymptomatic carriership were not distinguished in these studies.10–14,22 Furthermore, our approach of home visits allowed more family members to participate, resulting in inclusion of 76% of all existing first-, second-, and third-degree family members.
All asymptomatic mutation carriers showed at least mild, mostly asymmetric, facial weakness with or without mild shoulder girdle weakness. This observation challenges the concept of facial-sparing FSHD.23–27 Mild facial muscle weakness is likely to remain unnoticed both by the patient and the physician.2 Subtle weakness of the orbicularis oculi muscles leads to a “signe de cils”—an inability to bury the eyelashes completely when attempting to close the eyes tightly. Asymmetrical weakness of the orbicularis oris muscles causes asymmetrical pursing of the lips (figure 2). This differs from the normal facial asymmetry at rest observed in almost all healthy individuals. Hence, facial function should be actively tested, not only assessed by inspection during history. Likewise, subtle shoulder girdle weakness can be missed when not specifically tested by repetition of slowly lowering the arms forwards and sideward (after maximal shoulder anteflexion and abduction).2
We observed a moderate but significant (p < 0.01) inverse correlation between the size of the FSHD allele and the age-corrected CSS (figure 4A), confirming previous studies.17,28 In addition, we observed a great variability of D4Z4 methylation, both within and between families. Some moderately to severely affected carriers (age-corrected CSS >50) of larger FSHD alleles had very low delta1-methylation values (figure 4B). Both observations are in accordance with previous findings.17,29–32 In patients with repeat size of 1–5 units, the disease severity was mainly determined by the size of the FSHD allele with a minimal role for additional epigenetic factors.17 In contrast, in patients with 7–10 units, epigenetic regulation of the repeat, reflected in the delta1-methylation value, seems to co-determine the severity of FSHD.17 Furthermore, in family 210, a DNMT3B variant cosegregating with the D4Z4 contraction contributed to the severe D4Z4 hypomethylation and to clinical severity. Finally, in this family-based study we did not find significant sex differences in penetrance or clinical severity.17
This study also resulted in some other clinical observations. First, we confirmed a high prevalence of pain, especially shoulder pain, even in asymptomatic patients (75% of symptomatic and asymptomatic patients).33–36 Second, the observation of obviously affected mutation carriers who reported no symptoms (family member with foot drop) or had a very recent onset (family member with camptocormia) illustrates the fact that retrospective estimation of age at onset by patients is not reliable in all cases. We therefore prefer use of the age-corrected CCS.37 Indirectly, this study confirms that carrier status cannot be determined reliably by clinical examination alone (5% false-positive clinical assessment; 13% false-negative clinical assessment) and that genetic testing is required. Furthermore, because of the gray zone of 8–10 repeats, genetic testing in families with isolated cases should be counseled carefully as it is uncertain whether mutation carriers represent control or nonpenetrant population.
The main limitation of this study is its cross-sectional design. Furthermore, among the asymptomatic carriers were patients under the age of 30, who may still be presymptomatic. This underscores the need for longitudinal studies in FSHD.
In short, this study showed that penetrance of FSHD continues to increase in adulthood, and that nonpenetrance of FSHD is lower than shown in recent population studies. Penetrance and severity are related to both the number of residual D4Z4 units and, most likely in patients with longer repeats, to D4Z4 methylation. Assessment of family members at risk should include questions on shoulder girdle pain and examination of forceful eye closure, lip pouting, and inspection of the shoulder muscles after repeatedly and slowly lowering maximally abducted and anteflexed arms.
Glossary
- CSS
clinical severity score
- FSHD
facioscapulohumeral muscular dystrophy type 1
- PFGE
pulsed field gel electrophoresis
Author contributions
M. Wohlgemuth: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. R.J. Lemmers: analysis and interpretation, critical revision of the manuscript for important intellectual content. M. Jonker: analysis and interpretation, critical revision of the manuscript for important intellectual content. E. van der Kooi: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. C.G. Horlings: analysis and interpretation, critical revision of the manuscript for important intellectual content. B.G. van Engelen: analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. S.M. van der Maarel: analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. G.W. Padberg: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content, study supervision. N.C. Voermans: analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
Study funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Padberg GWAM. Facioscapulohumeral Disease Leiden. Leiden: Leiden University; 1982. [Google Scholar]
- 2.Mul K, Lassche S, Voermans NC, Padberg GW, Horlings CG, van Engelen BG. What's in a name? The clinical features of facioscapulohumeral muscular dystrophy. Pract Neurol 2016;16:201–207. [DOI] [PubMed] [Google Scholar]
- 3.Lemmers RJ, van der Vliet PJ, Klooster R, et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science 2010;329:1650–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snider L, Geng LN, Lemmers RJ, et al. Facioscapulohumeral dystrophy: incomplete suppression of a retrotransposed gene. PLoS Genet 2010;6:e1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Greef JC, Lemmers RJ, van Engelen BG, et al. Common epigenetic changes of D4Z4 in contraction-dependent and contraction-independent FSHD. Hum Mutat 2009;30:1449–1459. [DOI] [PubMed] [Google Scholar]
- 6.Lemmers RJ, Tawil R, Petek LM, et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat Genet 2012;44:1370–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Boogaard ML, Lemmers R, Balog J, et al. Mutations in DNMT3B modify epigenetic repression of the D4Z4 repeat and the penetrance of facioscapulohumeral dystrophy. Am J Hum Genet 2016;98:1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Greef JC, Lemmers RJ, Camano P, et al. Clinical features of facioscapulohumeral muscular dystrophy 2. Neurology 2010;75:1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunt PW, Compston DA, Harper PS. Estimation of age dependent penetrance in facioscapulohumeral muscular dystrophy by minimising ascertainment bias. J Med Genet 1989;26:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto K, Nishino I, Hayashi Y. Very low penetrance in 85 Japanese families with facioscapulohumeral muscular dystrophy 1A. J Med Genet 2004;41:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonini MM, Passos-Bueno MR, Cerqueira A, Matioli SR, Pavanello R, Zatz M. Asymptomatic carriers and gender differences in facioscapulohumeral muscular dystrophy (FSHD). Neuromuscul Disord 2004;14:33–38. [DOI] [PubMed] [Google Scholar]
- 12.Sakellariou P, Kekou K, Fryssira H, et al. Mutation spectrum and phenotypic manifestation in FSHD Greek patients. Neuromuscul Disord 2012;22:339–349. [DOI] [PubMed] [Google Scholar]
- 13.Ricci G, Scionti I, Sera F, et al. Large scale genotype-phenotype analyses indicate that novel prognostic tools are required for families with facioscapulohumeral muscular dystrophy. Brain 2013;136:3408–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salort-Campana E, Nguyen K, Bernard R, et al. Low penetrance in facioscapulohumeral muscular dystrophy type 1 with large pathological D4Z4 alleles: a cross-sectional multicenter study. Orphanet J Rare Dis 2015;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scionti I, Fabbri G, Fiorillo C, et al. Facioscapulohumeral muscular dystrophy: new insights from compound heterozygotes and implication for prenatal genetic counselling. J Med Genet 2012;49:171–178. [DOI] [PubMed] [Google Scholar]
- 16.Padberg GW, Frants RR, Brouwer OF, Wijmenga C, Bakker E, Sandkuijl LA. Facioscapulohumeral muscular dystrophy in the Dutch population. Muscle Nerve Suppl 1995:S81–S84. [PubMed] [Google Scholar]
- 17.Lemmers RJ, Goeman JJ, van der Vliet PJ, et al. Inter-individual differences in CpG methylation at D4Z4 correlate with clinical variability in FSHD1 and FSHD2. Hum Mol Genet 2015;24:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padberg GW, Lunt PW, Koch M, Fardeau M. Diagnostic criteria for facioscapulohumeral muscular dystrophy. Neuromuscul Disord 1991;1:231–234. [DOI] [PubMed] [Google Scholar]
- 19.Lemmers RJ, Van Overveld PG, Sandkuijl LA, et al. Mechanism and timing of mitotic rearrangements in the subtelomeric D4Z4 repeat involved in facioscapulohumeral muscular dystrophy. Am J Hum Genet 2004;75:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemmers RJ, van der Vliet PJ, van der Gaag KJ, et al. Worldwide population analysis of the 4q and 10q subtelomeres identifies only four discrete interchromosomal sequence transfers in human evolution. Am J Hum Genet 2010;86:364–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statland JM, Donlin-Smith CM, Tapscott SJ, Lemmers RJ, van der Maarel SM, Tawil R. Milder phenotype in facioscapulohumeral dystrophy with 7-10 residual D4Z4 repeats. Neurology 2015;85:2147–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zatz M, Marie SK, Cerqueira A, Vainzof M, Pavanello RC, Passos-Bueno MR. The facioscapulohumeral muscular dystrophy (FSHD1) gene affects males more severely and more frequently than females. Am J Med Genet 1998;77:155–161. [PubMed] [Google Scholar]
- 23.Krasnianski M, Eger K, Neudecker S, Jakubiczka S, Zierz S. Atypical phenotypes in patients with facioscapulohumeral muscular dystrophy 4q35 deletion. Arch Neurol 2003;60:1421–1425. [DOI] [PubMed] [Google Scholar]
- 24.Felice KJ, North WA, Moore SA, Mathews KD. FSH dystrophy 4q35 deletion in patients presenting with facial-sparing scapular myopathy. Neurology 2000;54:1927–1931. [DOI] [PubMed] [Google Scholar]
- 25.Butz M, Koch MC, Muller-Felber W, Lemmers RJ, van der Maarel SM, Schreiber H. Facioscapulohumeral muscular dystrophy: phenotype-genotype correlation in patients with borderline D4Z4 repeat numbers. J Neurol 2003;250:932–937. [DOI] [PubMed] [Google Scholar]
- 26.Pastorello E, Cao M, Trevisan CP. Atypical onset in a series of 122 cases with facioscapulohumeral muscular dystrophy. Clin Neurol Neurosurg 2012;114:230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He JJ, Lin XD, Lin F, et al. Clinical and genetic features of patients with facial-sparing Facioscapulohumeral Muscular Dystrophy. Eur J Neurol 2018;25:356–364. [DOI] [PubMed] [Google Scholar]
- 28.van den Boogaard ML, Lemmers RJ, Camano P, et al. Double SMCHD1 variants in FSHD2: the synergistic effect of two SMCHD1 variants on D4Z4 hypomethylation and disease penetrance in FSHD2. Eur J Hum Genet 2016;24:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lunt PW, Jardine PE, Koch M, et al. Phenotypic-genotypic correlation will assist genetic counseling in 4q35-facioscapulohumeral muscular dystrophy. Muscle Nerve Suppl 1995;S103–S109. [PubMed] [Google Scholar]
- 30.Tawil R, Forrester J, Griggs RC, et al. Evidence for anticipation and association of deletion size with severity in facioscapulohumeral muscular dystrophy. The FSH-DY Group. Ann Neurol 1996;39:744–748. [DOI] [PubMed] [Google Scholar]
- 31.Jones TI, King OD, Himeda CL, et al. Individual epigenetic status of the pathogenic D4Z4 macrosatellite correlates with disease in facioscapulohumeral muscular dystrophy. Clin Epigenetics 2015;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaillard MC, Roche S, Dion C, et al. Differential DNA methylation of the D4Z4 repeat in patients with FSHD and asymptomatic carriers. Neurology 2014;83:733–742. [DOI] [PubMed] [Google Scholar]
- 33.Bushby KM, Pollitt C, Johnson MA, Rogers MT, Chinnery PF. Muscle pain as a prominent feature of facioscapulohumeral muscular dystrophy (FSHD): four illustrative case reports. Neuromuscul Disord 1998;8:574–579. [DOI] [PubMed] [Google Scholar]
- 34.van der Kooi EL, Kalkman JS, Lindeman E, et al. Effects of training and albuterol on pain and fatigue in facioscapulohumeral muscular dystrophy. J Neurol 2007;254:931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miro J, Gertz KJ, Carter GT, Jensen MP. Pain location and intensity impacts function in persons with myotonic dystrophy type 1 and facioscapulohumeral dystrophy with chronic pain. Muscle Nerve 2014;49:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergsma A, Cup EH, Janssen MM, Geurts AC, de Groot IJ. Upper limb function and activity in people with facioscapulohumeral muscular dystrophy: a web-based survey. Disabil Rehabil 2017;39:236–243. [DOI] [PubMed] [Google Scholar]
- 37.van Overveld PG, Enthoven L, Ricci E, et al. Variable hypomethylation of D4Z4 in facioscapulohumeral muscular dystrophy. Ann Neurol 2005;58:569–576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.