Abstract
When individual neurons in a circuit contain multiple neuropeptides, these peptides can target different sets of follower neurons. This endows the circuit with a certain degree of flexibility. Here we identified a novel family of peptides, the Aplysia SPTR-Gene Family-Derived peptides (apSPTR-GF-DPs). We demonstrated apSPTR-GF-DPs, particularly apSPTR-GF-DP2, are expressed in the Aplysia CNS using immunohistochemistry and MALDI-TOF MS. apSPTR-GF-DP2 is present in single projection neurons, e.g., in the cerebral-buccal interneuron-12 (CBI-12). Previous studies have demonstrated that CBI-12 contains two other peptides, FCAP/CP2. In addition, CBI-12 and CP2 promote shortening of the protraction phase of motor programs. Here, we demonstrate that FCAP shortens protraction. Moreover, we show that apSPTR-GF-DP2 also shortens protraction. Surprisingly, apSPTR-GF-DP2 does not increase the excitability of retraction interneuron B64. B64 terminates protraction and is modulated by FCAP/CP2 and CBI-12. Instead, we show that apSPTR-GF-DP2 and CBI-12 increase B20 excitability and B20 activity can shorten protraction. Taken together, these data indicate that different CBI-12 peptides target different sets of pattern-generating interneurons to exert similar modulatory actions. These findings provide the first definitive evidence for SPTR-GF’s role in modulation of feeding, and a form of molecular degeneracy by multiple peptide cotransmitters in single identified neurons.
Keywords: Aplysia, neuropeptides, SPTR-Gene Family-Derived peptides, neuromodulation, projection interneuron, feeding
Table of Contents
INTRODUCTION
Many, if not all, neural circuits are multifunctional, due, in part, to actions of neuromodulators 1–4, such as neuropeptides. Neuropeptides act on G protein coupled receptors to exert slow and persistent actions in the circuit and alter intrinsic properties and/or synaptic connections of circuit elements so as to produce multiple outputs. In peptidergic neurons, neuropeptides are often colocalized with small molecular transmitters so that single neurons exert both fast and slow actions on their postsynaptic targets 3–7. An additional, prevalent level of complexity in peptide neuromodulation is that two or more peptide transmitters can be localized to one neuron 6–13, making it challenging to decipher the unique function of one peptide cotransmitter as opposed to another. For example, one important question that arises is, do colocalized peptides have similar functions? And if so, do they target the same or different circuit elements? We address this issue by studying peptidergic projection interneurons in the feeding circuit of an experimentally advantageous model system, the mollusc Aplysia californica. Earlier work suggested that different peptides in single neurons may target the same set of neurons to produce similar functional effects 12–15. Here we provide evidence suggesting that different peptides in single neurons may target different sets of neurons but still fulfill a similar function.
These experiments focus on the SPTR-Gene Family peptides. The SPTR precursor was first discovered in Lymnaea 16, which like Aplysia, is a gastropod mollusc. More recent work has identified SPTR gene family (SPTR-GF) precursors in other molluscs and in several annelids 17–24. For example, in Aplysia, the SPTR precursor, referred to as Whitnin, has been deposited into the NCBI database 25. Nevertheless, there is little definitive information regarding the possible functions of SPTR-GF-derived peptides (SPTR-GF-DPs). In Lymnaea, SPTR-GF-DPs were examined for their potential role in axonal growth, but no effect was found 16. Two other studies suggested that SPTR-GF-DPs might be differentially expressed during the life cycle of a mollusc 18, 19. This might suggest a role in development, but to date none has been demonstrated. More relevant to the present work, there has been no demonstration of a behavioral or circuit effect of the SPTR-GF-DPs in any species.
Since many peptides are present in the Aplysia feeding system 26–38, we sought to determine whether Aplysia SPTR-GF-DPs (apSPTR-GF-DPs) play a role in modulating the feeding central pattern generator (CPG). We demonstrated the presence of apSPTR-GF-DPs in the Aplysia CNS using MALDI-TOF MS, and mapped the distribution of apSPTR-GF-DP-like reactivity in neurons using immunohistochemistry. We found that the apSPTR-GF-DPs are present in single neurons of the cerebral and buccal ganglia that are involved in the control of feeding. In particular, they are present in a projection interneuron, the cerebral-buccal interneuron-12 (CBI-12) which also contains two other peptides, feeding circuit activating peptide (FCAP) 30, 39 and cerebral peptide 2 (CP2) 40, 41. Previous work has demonstrated that stimulation of CBI-12 can shorten the duration of the protraction phase of motor programs 42. Presumably this effect is at least partially mediated by effects of FCAP and CP2, which increase the excitability of the interneuron B64 12. B64 terminates protraction and initiates retraction when it is activated 43, 44. We found that one of the apSPTR-GF-DPs, i.e., apSPTR-GF-DP2, also shortens protraction duration. Surprisingly, however, apSPTR-GF-DP2 did not enhance B64 excitability. Instead, apSPTR-GF-DP2 enhanced the excitability of a different interneuron, B20, which we now show can also shorten protraction. Overall, the data suggest that different peptides in CBI-12, i.e., FCAP/CP2 and apSPTR-GF-DP2, may target different sets of CPG interneurons to produce the same modulatory effect. This may constitute a form of molecular degeneracy.
RESULTS
Bioinformatics of SPTR-Gene Family and apSPTR Precursor
The apSPTR Precursor was identified using an Expressed Sequence Tag (EST) approach, and the gene sequence, named Whitnin, was deposited into the NCBI database (GenBank™ accession number AAV84472.1) 25. Given its 86% identity to the SPTR neuropeptide precursor identified in Lymnaea 16, and first use in Aplysia under this name45,we continue with the apSPTR precursor nomenclature for the prohormone.
The apSPTR precursor consists of 116 amino acids and has a structure characteristic of a neuropeptide prohormone, having a signal peptide and encoding several putative peptides. Its signal peptide was predicted by the SignalP tool46 with the most likely signal peptide cleavage site between Ser23 and Leu24. Our MS analysis described later indicated that the peptide detected closest to the N terminus, named apSPTR-GF-DP1, was Leu24-Asn42 (see Figure 4), which matches with the signal peptide prediction.
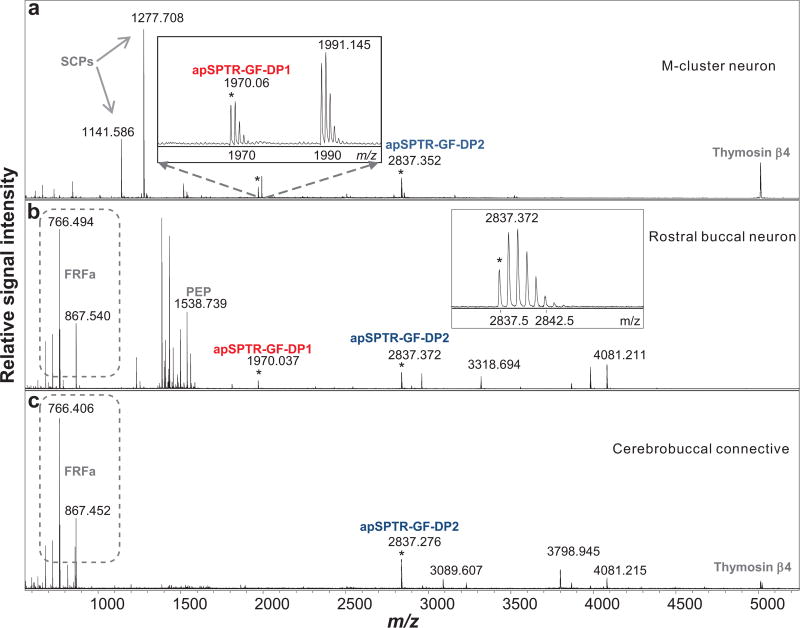
Figure 4.
Processing of the Aplysia SPTR prohormone as detected by MALDI-TOF MS in individual cerebral and buccal neurons, and cerebrobuccal connective. (a), a cerebral neuron in the M cluster; (b), a buccal neuron on the rostral surface; (c), cerebrobuccal connective. Detected peptides, apSPTR-GF-DP1 and apSPTR-GF-DP2 are labeled. Average mass accuracy of assignment calculated from six measurements (4 neurons and 2 samples of CBC) is 8 ppm and 4 ppm, respectively. Additional known detected peptides: SCPs, FRF, PEP, and thymosin β4 are also labeled.
We used NeuroPred47 to predict posttranslational processing of the apSPTR precursor. Five potential neuropeptides were predicted. However, the cleavage site at Lys101 is not utilized due to the formation of a disulfide bridge on peptide Met92-Ser114 as determined by MALDI-TOF MS, thus three putative neuropeptides are listed in Figure 1a. The peptide amidation was also verified by MALDI-TOF MS, and the peptide Met92-Ser114 has been named apSPTR-GF-DP2. The localization of precursor processing sites is similar to those described in Lymnaea 16, but actual processing cannot be compared due to lack of peptide structure confirmation in Lymnaea. Koert et al 16 suggested processing of the prohormone C terminus region into two peptides, ERYM-amide and pQRYMGICMRKQYNNFVPVPCLRS-amide based on MALDI MS data from the fractionated ganglia extracts, but the suggestion remained to be verified.
Figure 1.
Aplysia SPTR precursor and alignment of apSPTR-GF-DP2 with related sequences in other species. (a), Cleavage and processing of the Aplysia SPTR precursor. The sequence in green represents the signal peptide. Confirmed peptide sequences are underlined. The sequence in red represents apSPTR-GF-DP1. The sequence in orange represents the linker peptide. The sequence in blue represents apSPTR-GF-DP2. The confirmed amidation site is in bold, and an intramolecular disulfide bridge is confirmed as shown. The amino acids highlighted in yellow represent the cleavage sites of endopeptidase for the precursor. (b), Alignment of apSPTR-GF-DP2 with 16 C-terminal neuropeptides from SPTR-gene family precursors from 11 molluscs and 5 annelids using Bioedit. Note that all of the C-terminal neuropeptides have two cysteines, which may form a disulfide bridge. Not all of the C-terminal neuropeptides are amidated. *, represents amidated C termini which have been verified by MALDI; #, represents potential amidated C termini; peptides not labeled represent potential nonamidated C termini.
In addition to mollusca, precursors from five annelid species were found to have some similarity with the SPTR precursor. Finally, a study on the mollusc Lottia gigantea 17 suggested that the PKYMDT region of the molluscan SPTR may be homologous to an arthropod peptide proctolin (RYLPT). A similar idea was also suggested by other authors who analyzed both evolutionary relationships between neuropeptide precursors and evolutionary relationships between peptide receptors 20, 48. The arthropod proctolin precursors have been aligned with two molluscan and two annelid SPTR-Gene Family precursors (Supplemental sld.pdf in Mirabeau and Joly 2013)20. In order to determine how similar these precursors are, we compared 18 SPTR related precursors from 13 molluscs and 5 annelids, and proctolin precursors from 12 arthropods (Table S1). We used Bioedit to align them (Figure S1). The alignment suggested that proctolin precursors from Danaus, Chilo, Operophtera and Bombyx have little similarity with other precursors in this group, partly because their proctolin sequences are different from RYLPT. We therefore did another alignment without these four species (Figure S2).
This latter alignment showed that the similarity between species was largely related to the similarity between the sequence PKYMXT in mollusca, AXWLXT in annelida, and RYLPT in arthropoda, e.g., all of these sequences have a T at the end. However, despite the conservation for these three short sequences within each phylum, the similarity between phyla is poor. Further, the predicated peptides that contain the PKYMDT were not found to be expressed in any of the three molluscan species examined with MALDI-TOF MS (Table S1), including Lymnaea 16, Haliotis 18, and Aplysia (this work). This contrasts with what was observed in arthropods in which proctolin is clearly expressed 49.
We studied one peptide from Aplysia (apSPTR-GF-DP2) located toward the C-terminus of the precursor (in the region marked with arrows, Figure S1–3). We compared the sequence of this peptide with the other 17 related sequences from different species, and found that all contain two cysteines except Charonia, which may simply reflect the fact that its precursor sequence is incomplete. Thus, we aligned apSPTR-GF-DP2 with 16 other sequences without that of Charonia (Figure 1b). The alignment showed similarities between molluscan and annelidan sequences. In addition to the shared two cysteines, more than half (i.e., 9 out of 17, Figure 1b) of the molluscan and annelidan precursors terminate with a Gly, which suggests that some other peptides are like apSPTR-GF-DP2 in that they are amidated. Interestingly, in this part of the arthropod precursors, no peptides are present.
Overall, our bioinformatics data suggested that arthropod proctolin precursors are possibly not closely related to the SPTR precursor. On the other hand, genes in molluca and annelida are definitively related (aligned in Figure S3), and belong to SPTR-Gene Family. Thus, we generated a phylogenic tree with 18 SPTR-Gene Family precursors from 18 species (Figure S4). Overall, the phylogenetic tree suggested that there are three clusters of closely related SPTR-Gene Family precursors: 1) Gastropod mollusca, 2) Bivalvia and cephalopoda molluscs, 3) Annelida (with one exception: Ruditapes, a bivalvia).
Distribution of apSPTR-GF-DPs Immunoreactivity in the Aplysia CNS and Immunopositive CBI-12
To map apSPTR-GF-DPs positive neurons and their processes in the CNS of Aplysia, we performed immunohistochemistry experiments on whole mounts (n = 3 for each ganglion). To accomplish this, we raised a polyclonal antibody against apSPTR-GF-DP2, which is presumably amidated with a disulfide bridge (see the next section). The specificity of the antibody was verified in preabsorption experiments (Figure S5).
Importantly, apSPTR-GF-DP2 positive cell bodies were distributed in the buccal and cerebral ganglia (Figure 2a, 2b), which are involved in the control of feeding behavior in Aplysia 43, 50–54. The buccal ganglion contains motoneurons and CPG interneurons that form a pattern generator to drive radula rhythmic protraction-retraction movements. The cerebral ganglion contains somata of cerebral-buccal interneurons that send their axons to the buccal ganglion to drive or modulate the CPG there. The cerebral ganglion contains the most apSPTR-GF-DP2 positive cell bodies, which are scattered on both the dorsal and ventral surfaces (Figure 2b1, 2b2). Most notably, the “M” cluster and adjacent areas on the ventral surface, where some CBIs are located contain several positive somata. In the buccal ganglion, there were two or three apSPTR-GF-DP2 positive neurons on the rostral surface (Figure 2a1), whereas no positive neurons were found on the caudal surface (Figure 2a2).
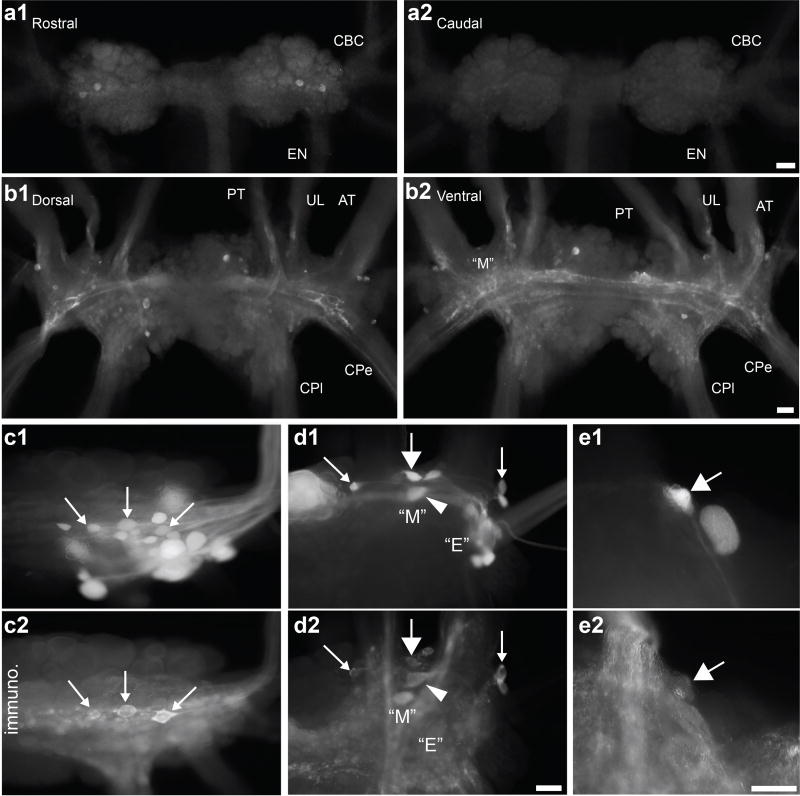
Figure 2.
Distribution of apSPTR-GF-DP2 immunopositive neurons in the buccal and cerebral ganglia (a, b) and some BCIs and CBIs (including CBI-12) are apSPTR-GF-DP2 immunoreactive (c, d, e). (a), Immunostaining in the buccal ganglion. There are 2–3 apSPTR-GF-DP2 positive somata on the rostral surface (a1), whereas there is no staining on the caudal surface (a2). (b), Immunostaining in the cerebral ganglion. There are several positive neurons distributed over the dorsal (b1) and ventral (b2) surface. Extensive fibers and neuropile in the cerebral ganglion are also stained. Scale Bars: 200 µm (scale bar in a2 is for a1 and a2, scale bar in b2 is for b1 and b2). Nerve abbreviations: EN, esophageal nerve; CBC, cerebrobuccal connective; UL, upper labial nerve; AT, anterior tentacular nerve; CPe, cerebropedal connective; CPl, cerebropleural connective. (c, d), CBCs were backfilled with biocytin (c1, d1) and immunostained with an antibody to apSPTR-GF-DP2 (c2, d2). Right buccal hemiganglion is shown in (c) and Right cerebral ganglion ventral M and E clusters are shown in (d). Immunostaining CBIs and BCIs are shown with arrows or an arrowhead. For panel d, possible CBIs from left to right: CBI-11, CBI-12, CBI-3 (arrowhead), CBI-8/9. (e), Carboxyfluorescein injected CBI-12 (large arrow) and C12 cell (e1). CBI-12 immunostains for apSPTR-GF-DP2 (e2, large arrow), but C12 does not. Scale Bars: 100 µm (scale bar in d2 is for c1, c2, d1, d2; scale bar in e2 is for e1 and e2).
To determine whether the apSPTR-GF-DP2 positive neurons may be previously identified neurons in the feeding circuit, we performed immunohistochemistry in conjunction with backfills of cerebrobuccal connectives (CBCs) (Figure 2c, d). When the buccal end of the CBC was backfilled, a number of neurons on the rostral surface of the buccal ganglion were stained (Figure 2c1). Three of these neurons were also immunopositive (Figure 2c2), indicating that these three apSPTR-GF-DP2 positive neurons are buccal-cerebral interneurons which send axons to the cerebral ganglion. When the cerebral end of the CBC was backfilled, several neurons in and around the “M” cluster on the ventral surface of the cerebral ganglion were stained (Figure 2d1). Four of these neurons were also immunoreactive (Figure 2d2), suggesting that these apSPTR-GF-DP2 positive neurons are CBIs. Based on its location, one of the CBIs (Figure 2d, large arrow in the middle) appeared to be CBI-12. To verify this, we injected CBI-12 (Figure 2e1, large arrow) and the adjacent C12 with carboxyfluorescein dye, and found that CBI-12 was immunostained (Figure 2e2, large arrow) whereas C12 was not.
We also performed immunohistochemistry on two other major central ganglia: pleural-pedal and abdominal ganglia (Figure 3). We observed several apSPTR-GF-DP2 positive neurons on the ventral side of the pleural ganglion (Figure 3a2, a4, arrows). A cluster of smaller neurons were located near the root of cerebropedal connective on the ventral side of the pedal ganglion (Figure 3a2, a4, arrowheads). Several apSPTR-GF-DP2 positive neurons were also observed on the ventral side (Figure 3b, arrows), but not on the dorsal side of the abdominal ganglia. In summary, there are relatively few apSPTR-GF-DP2 positive somata in all ganglia, with the cerebral ganglion having the largest number of positive somata. On the other hand, apSPTR-GF-DP2 positive neuropile and fibers are extensively distributed in the cerebral ganglion and the ventral side of the pedal ganglia.
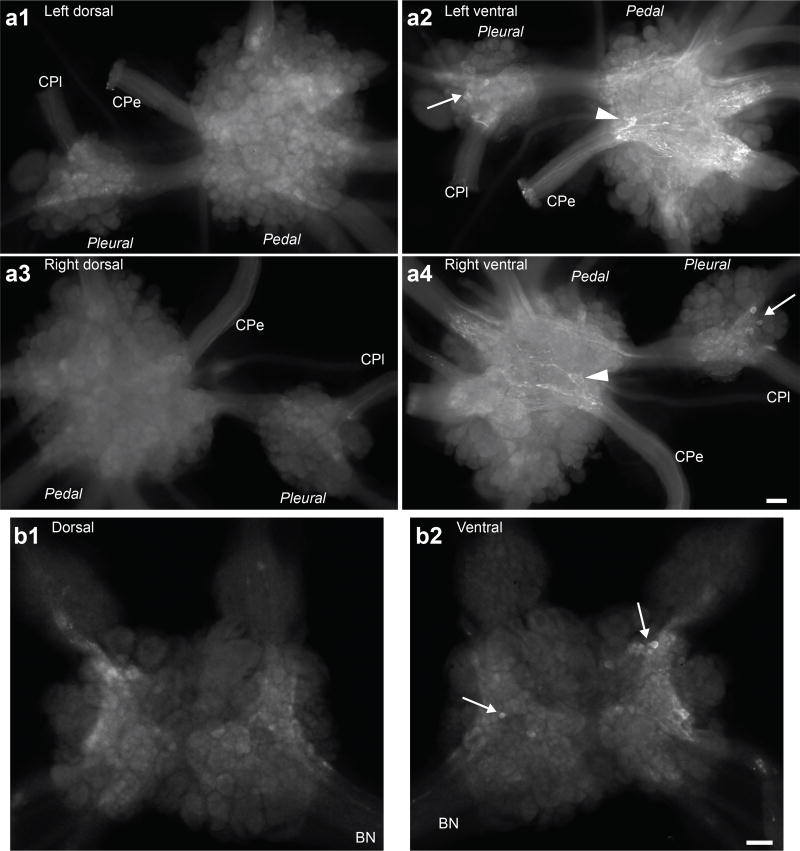
Figure 3.
Distribution of apSPTR-GF-DP2 positive neurons and fibers in the pleural-pedal ganglia and the abdominal ganglion (whole mounts). (a), pleural-pedal ganglion. (a1, a2), left ganglion, dorsal (a1) and ventral (a2) surfaces. (a3, a4), right ganglion, dorsal (a3) and ventral (a4) surfaces. There is no obvious staining on the dorsal surfaces (a1, a3), but there are several somata and processes on the ventral surfaces (a2, a4) in the pleural and pedal ganglia. (b), abdominal ganglion. There is no obvious staining on the dorsal surface (b1), but there are several somata on the ventral surfaces (b2) in the abdominal ganglia. Extensive fibers and neuropile in the pedal ganglion are also stained. Scale Bars: 200 µm (scale bar in a4 is for a1, a2, a3 and a4; scale bar in b2 is for b1 and b2). Nerve abbreviations: CPe, cerebropedal connective; CPl, cerebropleural connective; BN, branchial nerve.
Mass Spectrometric Analysis of Prohormone Processing and Posttranslational Modifications (PTMs) in Individual Neurons
To determine processing and PTMs of the apSPTR precursor, we performed MALDI-TOF MS on single neurons in the cerebral and buccal ganglia, an approach well suited for characterizing prohormone processing 55–57. Based on the above immunohistochemistry experiments (Figure 2) showing that some cells in the cerebral “M” cluster and rostral buccal surface are immunopositive, we isolated two single neurons from the cerebral region and two cells from rostral surface in the buccal ganglion. We observed mass peaks matching protonated ions of two predicted peptides, i.e., apSPTR-GF-DP1 (M+H 1970.019) and apSPTR-GF-DP2 (M+H 2837.37) (Figure 4a, b). We also observed a mass peak matching protonated ions of apSPTR-GF-DP2 from a sample derived from the CBC (Figure 4c). Importantly, the data verified that apSPTR-GF-DP2 is present in an amidated form with a disulfide bridge. The linker peptide containing PKYMDT (M+H 5235.94) was not detected. These data also supported the idea that the antibody we used is specific. Because the PTMs such as amidation 26, 27, 29, 30, 33–35, 38 and the formation of a disulfide bridge35 often suggest bioactivity, we focused the rest of the study on apSPTR-GF-DP2.
Modulatory Actions of apSPTR-GF-DP2 in the Feeding Circuit
To determine the functional role of apSPTR-GF-DP2 in the Aplysia feeding circuit, we first sought to determine whether apSPTR-GF-DP2 is bioactive. Specifically, we tested the effect of apSPTR-GF-DP2 on motor programs elicited by the command neuron CBI-2 50–53. We elicited a single cycle of a motor program by stimulating CBI-2 at 8–10 Hz. The inter-stimulation interval was 1.5 min. A representative example is showed in Figure 5a. Under control conditions (Figure 5a1), CBI-2 stimulation (9 Hz) elicited an ingestive motor program (i.e. B8 was predominantly active during retraction). When the preparation was superfused with 10−6 M apSPTR-GF-DP2 (Figure 5a2) and 10−5 M apSPTR-GF-DP2 (Figure 5a3), there was a concentration-dependent shortening of protraction (Figure 5a5, F (3, 21) = 33.47, p < 0.001, n = 8). Other phases of the motor program were not significantly altered (i.e. there was no significant effect on retraction duration (Figure 5a6, F (3, 21) = 0.7241, p > 0.05, n = 8), or on B8 activity during protraction (Figure 5a7, F (3, 21) = 0.5283, p > 0.05, n = 8) or retraction (Figure 5a8, F (3, 21) = 0.9161, p > 0.05, n = 8).
Figure 5.
apSPTR-GF-DP2 modulates feeding motor programs elicited by CBI-2 through its actions on the interneuron B20, but not B64. (a), apSPTR-GF-DP2 shortens protraction duration in a concentration-dependent manner. (a1–4), a representative example; a single cycle of a motor program was elicited by stimulating CBI-2 at 9 Hz until the end of the protraction phase, detected by the onset of the sharp synaptic inhibition of CPG neuron B31 and the abrupt ending of I2 nerve activity. Protraction phase (open bar) is defined by the activity in the I2 nerve. Retraction phase (filled bar) is defined by a period of hyperpolarization of B31 after the protraction phase is terminated and also by a period of high-frequency activity of the radula closing motoneuron B8. Upon wash, protraction duration returns to its control value. (a5), group data on protraction duration. In contrast, apSPTR-GF-DP2 has no significant effect on the duration of retraction (a6) or B8 activity during either protraction (a7) or retraction (a8). (b), apSPTR-GF-DP2 has no effect on B64 excitability. (b1), a representative example, (b2), group data. (c), apSPTR-GF-DP2 increases B20 excitability and shortens B20 spike latency. (c1), a representative example, (c2, c3), group data. Recordings in (a) were made in ASW, whereas recordings in (b) and (c) were made in high divalent saline. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (Bonferroni post hoc tests). Error bars, S.E.
Circuit Targets of apSPTR-GF-DP2
Similar to most rhythmic behaviors, feeding in Aplysia is controlled by a CPG. Therefore, we sought to determine whether apSPTR-GF-DP2 shortens the duration of protraction via modulation of the CPG. Specifically, we examined the effects of apSPTR-GF-DP2 on the excitability of the CPG element, interneuron B64. Previous studies have shown that B64 is active during retraction and acts as a terminator of protraction 43, 44, 58. However, we found that apSPTR-GF-DP2 had no significant effect on B64 excitability (Figure 5b, F (3, 12) = 1.691, p > 0.05, n = 5). These data suggest that apSPTR-GF-DP2 does not shorten protraction by acting on B64.
In the next set of experiments, we sought to find other interneurons that may be targeted by apSPTR-GF-DP2. We found that apSPTR-GF-DP2 increased the excitability of B20 in a concentration-dependent manner (Figure 5c1, c2, F (3, 15) = 11.98, p < 0.001, n = 6). It also decreased the latency to spike (Figure 5c1, c3, F (3, 15) = 5.35, p < 0.05, n = 6).
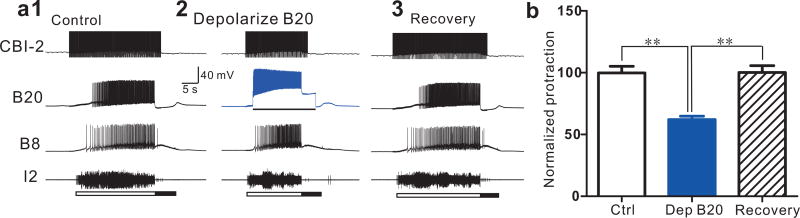
We hypothesized that B20 may promote protraction shortening. To test this, we elicited motor programs by stimulating CBI-2 and depolarized B20 with a moderate amount of current (i.e. 3 nA), which increased B20 firing frequency (Control: 5.12 ± 1.21 Hz, B20 depolarization: 12.31 ± 0.76 Hz, Recovery: 5.85 ± 1.36 Hz). Protraction duration did decrease (Figure 6, F (2, 8) = 15.14, p < 0.01, n = 5). Thus, apSPTR-GF-DP2 may shorten protraction, at least partly, by acting on B20.
Figure 6.
Depolarizing interneuron B20 shortens the protraction duration of CBI-2-elicited motor programs. (a1–3), A representative example; a single cycle of a motor program was elicited by stimulating CBI-2 at 9 Hz until the end of the protraction phase. Bar in (a2) denotes current injections in B20. (b), Group data. Recordings were made in ASW. **, p < 0.01 (Bonferroni post hoc tests). Error bars, S.E.
Neuronal Source of apSPTR-GF-DP2: Roles of CBI-12
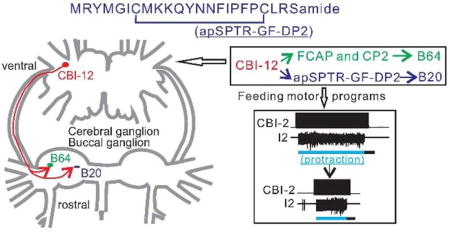
CBI-12 is apSPTR-GF-DP2 immunoreactive (Figure 2d, e). This is intriguing because previous work has demonstrated that CBI-12 stimulation also shortens the duration of the protraction phase of CBI-2-elicted programs 42, like apSPTR-GF-DP2. We therefore hypothesized that CBI-12 might shorten protraction duration, partly through apSPTR-GF-DP2. If this hypothesis were correct, CBI-12 should increase the B20 excitability (as does apSPTR-GF-DP2). To test this, we stimulated CBI-12 at different frequencies (i.e. 5 Hz, 10 Hz, and 15 Hz) and measured the B20 excitability. Excitability was increased in a frequency-dependent manner (Figure 7a, b) (5 Hz, F (2, 8) = 53.39, p < 0.001, n = 5; 10 Hz, F (2, 8) = 74.45, p < 0.001, n = 5; 15 Hz, F (2, 8) = 114.3, p < 0.001, n = 5). Thus, this provided at least a partial explanation for why CBI-12 shortens protraction.
Figure 7.
CBI-12 increases excitability of B20 (a, b) and B64 (c, d) in a frequency-dependent manner. (a, c), representative examples showing the effect of CBI-12 stimulation at 15 Hz. (b, d), Group data. Recordings were made in high divalent saline. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (Bonferroni post hoc tests). Error bars, S.E.
Additional Targets of CBI-12
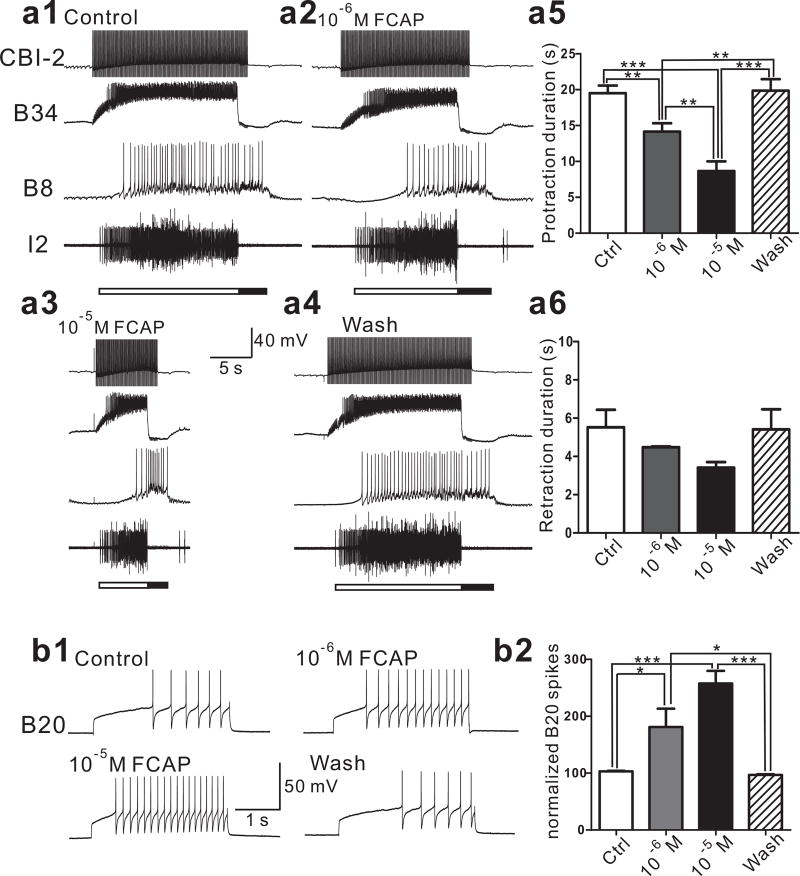
Although the above data provide a possible mechanism underlying the ability of CBI-12 to shorten protraction, these findings do not exclude the possibility that CBI-12 may target additional neuronal elements to shorten protraction. In fact, previous work has shown that CBI-12 also contains two other peptides, FCAP 39 and CP2 40, 41. In addition, CP2 has been shown to shorten protraction 41. Here, we demonstrated that FCAP could also shorten protraction (F (3, 9) = 38.89, p < 0.001, n = 4), and had no significant effect on retraction (F (3, 9) = 2.481, p > 0.05, n = 4) (Figure 8a). This protraction shortening effect of FCAP/CP2 may be mediated, at least partly, through B64 because both peptides enhance the excitability of B64 12. Thus, we hypothesized that CBI-12 might also increase B64 excitability. To test this, we stimulated CBI-12 at 10, 15 and 20 Hz. B64 excitability was increased in a frequency-dependent manner (Figure 7c, d) (10 Hz, F (2, 6) = 9.52, p < 0. 05, n = 4; 15 Hz, F (2, 6) =19.52, p < 0. 01, n = 4; 20 Hz, F (2, 6) =34.58, p < 0.001, n = 4). Although apSPTR-GF-DP2 does not target B64, the data suggest that CBI-12 may use its other peptide co-transmitters, FCAP/CP2, to target B64. Finally, we also showed that FCAP increased B20 excitability (F (3, 9) = 21.28, p < 0.001, n = 4) (Figure 8b). Thus, the protraction shortening effect of FCAP may also be partly mediated by its actions on B20, in addition to B64 12. Overall, CBI-12 might shorten protraction duration by acting via at least two parallel peptidergic modulatory pathways (Figure 9).
Figure 8.
FCAP shortens the protraction duration of CBI-2-elicited motor programs in a concentration-dependent manner partly through its actions on interneuron B20. (a1–4), FCAP shortens protraction duration of CBI-2-elicited programs, representative examples; a single cycle of a motor program was elicited by stimulating CBI-2 at 10 Hz until the end of the protraction phase, detected by the onset of the sharp synaptic inhibition of CPG neuron B34 and the abrupt ending of I2 nerve activity. Protraction phase (open bar) is defined by the activity in the I2 nerve. Retraction phase (filled bar) is defined by a period of hyperpolarization of B34 after the protraction phase is terminated. Upon wash, protraction duration returns to its control value. (a5), group data on protraction duration. In contrast, FCAP has no significant effect on the duration of retraction (a6). (b), FCAP increases B20 excitability in a concentration-dependent manner. (b1), a representative example, (b2), group data. Recordings in (a) were made in ASW, whereas recordings in (b) were made in high divalent saline. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (Bonferroni post hoc tests). Error bars, S.E.
Figure 9.
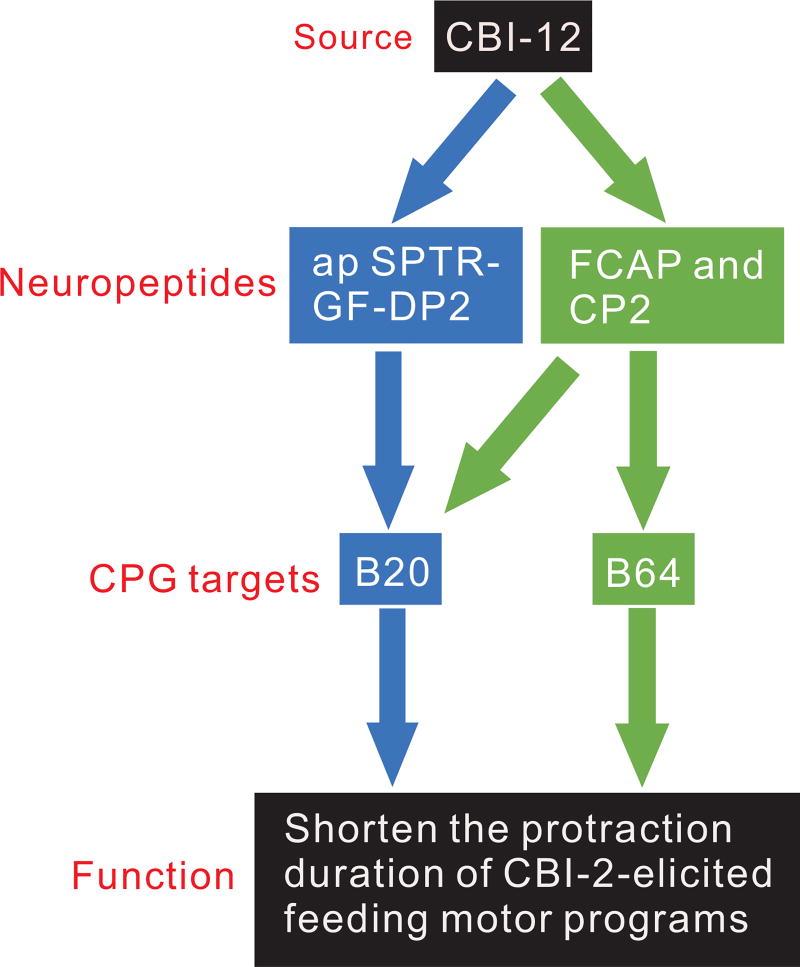
A schematic diagram of two parallel peptidergic pathways through which CBI-12 may modulate the protraction duration of feeding motor programs. CBI-12 peptides: apSPTR-GF-DP2 acts on B20, whereas FCAP/CP2 act on B64 to shorten protraction. Note that FCAP also acts on B20, whereas the effect of CP2 on B20 was unknown.
DISCUSSION
In this work, we studied SPTR-Gene Family-Derived peptides in Aplysia, and provided the first definite evidence for a function of SPTR-GF-DPs: i.e., a modulatory role in the feeding motor network. Perhaps more importantly, this study enabled us to identify novel example of molecular degeneracy, i.e., we demonstrated that a single effect (protraction shortening) could be mediated by multiple peptide cotransmitters contained in a single projection neuron.
The Precursors for SPTR-Gene Family-Derived Peptides and Their Functional Roles: Modulatory Mechanisms of Neuropeptides
Since the initial discovery of the SPTR precursor in Lymnaea in 2001 16, a number of precursors from SPTR-Gene Family have been identified in Mollusca and Annelida, i.e., lophotrochozoa 17–24. Although there have been suggestions that the PKYMDT sequence in the SPTR gene family in molluscs may be homologous to the arthropod proctolin sequence RYLPT 17, 20, 48, our sequence alignments (Figure S1–3) suggest that this may not be the case for the following three reasons: 1) the similarity is poor even for this region (For Annelids, the sequence is AXWLXT). 2) current MALDI-TOF MS data (Table S1) provide evidence that PKYMDT or larger peptides that may contain the PKYMDT region is not processed in the three molluscs, whereas proctolin is expressed in arthropods 49; 3) the sequences that show more similarity between molluscs and annelids actually correspond to the amidated apSPTR-GF-DP2 with a disulfide bridge, whereas no such similar sequence is present in any of the arthropod proctolin precursors. If this is the case, it would suggest that the SPTR gene family is present in molluscs and annelids, but not in arthropods (but see 20, 48). However, it is also possible that arthropods contain other, as yet unidentified peptide precursors that are related to SPTR gene family. This is an important issue to resolve in the future to determine whether the SPTR gene family is restricted to lophotrochozoa or is more broadly distributed.
Prior to our work, little was known about the functions of SPTR-GF-DPs. Although there is a possibility that they may play a role in development 18, 19, this has not been established. In contrast, our study demonstrates that the SPTR-GF-DPs, particularly apSPTR-GF-DP2, modulate feeding in Aplysia. We demonstrated that apSPTR-GF-DPs are detected in single neurons of the central ganglia using immunohistochemistry and MALDI-TOF MS. We then took advantage of the well-defined feeding circuit, and showed that apSPTR-GF-DP2 modifies a specific parameter of motor programs elicited by command neuron CBI-2, i.e., it shortens protraction duration. Interestingly, a recent report demonstrated that another family of peptides, ALKs, have a similar effect, i.e., they shorten protraction without affecting other parameters of feeding motor programs 38. However, the two types of peptides apparently target different CPG elements. Specifically, apSPTR-GF-DP2 acts on B20, whereas the ALKs act on B64. B64, a retraction interneuron, shortens protraction directly by inhibiting protraction neurons 43, 44. In contrast, B20, a protraction interneuron 51, 59, likely affects protraction duration indirectly. Importantly, the neuronal sources of the two peptides are also different. We have identified CBI-12 as one major source of apSPTR-GF-DP2, but the neurons that might release the ALKs have not been identified. Although a number of ALK positive neurons have been mapped, CBI-12 is not one of them, i.e., there are no ALK positive neurons in the “M” cluster of the cerebral ganglion 38.
Role of Multiple Peptide Cotransmitters in Single Neurons: Molecular Degeneracy and Divergent vs. Convergent Neuromodulation
Perhaps more significantly, the localization of apSPTR-GF-DP2 to the peptidergic interneuron CBI-12 allowed us to compare the functional roles of different peptide cotransmitters in a single neuron (Figure 9). Specifically, our previous work showed that stimulation of CBI-12 shortens the protraction duration of motor programs elicited by CBI-2 42. This might be mediated in part by CBI-12 releasing FCAP and CP2. CP2 has been shown previously to shorten protraction 41, whereas we have now shown that FCAP also shortens protraction (Figure. 8b), Furthermore, both FCAP and CP2 increase the excitability of B64 12. In support of this idea, we show here that CBI-12 mimicks the effects of FCAP/CP2 by enhancing B64 excitability. In addition, we show that apSPTR-GF-DP2 also shortens protraction, but increases the excitability of B20 rather than B64. Importantly this effect is also mimicked by CBI-12 stimulation. Interestingly, FCAP may promote short protraction by also acting on B20. Overall, the data suggest that CBI-12 exerts its effects via two parallel peptidergic pathways (Figure 9).
In neural networks, work in Aplysia indicates that different configurations of circuit elements may produce a similar form of motor output, perhaps enabling the same output to be generated under different network states 60. This network degeneracy is an active area of research and growing number of examples are forthcoming 61. In the present work, the type of two parallel modulatory pathways (Figure 9) may be a form of molecular degeneracy whereby a single neuron uses two molecules (i.e., modulatory peptides) to target distinct CPG elements so as to fulfill a similar function. Demonstration of molecular degeneracy provides supports for a prevalent role of degeneracy in systems neurosciences.
In addition, the use of two peptidergic pathways in single neurons to target different sets of CPG elements presumably provides the circuit with a divergent modulatory mode, therefore offering more flexibility in neuromodulation. Although both apSPTR-GF-DP2 and FCAP/CP2 are co-localized in CBI-12, other neurons may contain only one type, e.g., CBI-2 only has FCAP/CP2 30, 39, 41 but not apSPTR-GF-DP2, allowing differential modulation from different neurons. Concordant with this, under resting conditions, CBI-2 tends to promote programs with a longer protraction duration, whereas CBI-12 tends to promote programs with a shorter protraction 42. In other studies in Aplysia, data suggest that the effects of FCAP and CP2, which result from two different peptide genes 30, 62, colocalized in CBI-2, are largely convergent. Specifically, CBI-2 appears to use both peptides to enhance the excitability of similar sets of target neurons and contribute to synaptic facilitation and priming 12–15. Similarly, in the crustacean stomatogastric nervous systems, various peptides, many of which originate from projection neurons, have been shown to have both convergent 63 and divergent 64–66 actions on the central pattern generating circuit elements.
In summary, we have uncovered the modulatory functions of novel peptides, apSPTR-GF-DP2 from SPTR gene family in molluscs and annelids, in the feeding circuit of Aplysia. We confirmed its in vivo translation using immunohistochemistry and MALDI-TOF MS, and mapped apSPTR-GF-DP2 positive neurons in the central ganglia involved in the feeding. We found it modulates a specific parameter of feeding motor programs, which mimicked the effects of CBI-12 that also contains the peptide. We further demonstrated that CBI-12 may use two peptidergic pathways to modulate distinct CPG circuit elements to fulfill a specific function, suggesting a form of molecular degeneracy. The study provides the first definitive evidence for a functional role of SPTR gene family in any animals, and further proof for Aplysia as an advantageous model system for the study of peptidergic neuromodulation.
METHODS
Animals
Experiments were performed on Aplysia californica (10–300 g), which are purchased from Marinus Scientific (Long Beach, CA) and the Aplysia Research Facility (Miami, FL). Aplysia are hermaphroditic (i.e., each animal has reproductive organs normally associated with both male and female sexes). Animals were kept in an aquarium containing aerated and filtered artificial seawater (Instant Ocean, Aquarium Systems Inc., Mentor, OH) at 14–16 °C until use. The animal room was equipped with a 24 h light-dark cycle with light period from 6:00 am to 6:00 pm. Prior to dissection, animals were anesthetized by injection of isotonic 333 mM MgCl2 (about 50% of body weight) into the body cavity.
Reagents and Peptides
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. apSPTR-GF-DP2 was synthesized by Synpep, Inc and ChinaPeptides Co., Ltd. FCAP precursor 30 contains 8 peptides that are very similar, all end with HGWamide, and were named FCAPa-h. We used the one with the most copies in the precursor, FCAPg, which was obtained from SynPep, Dublin, CA. The peptide standards for mass spectrometry (MS) calibration were purchased from Bruker Daltonics (Billerica, MA).
Sequence Comparison
We compared 18 SPTR-Gene Family and 12 arthropod proctolin precursors (Table S1, Figure S1) and also a subset of these sequences (Figure S2 –3). For precursor comparison in Figure 1b and Figure S1–3, all precursor sequences were downloaded from NCBI protein database (https://www.ncbi.nlm.nih.gov/protein). We generated the sequence alignment (Figure 1b and Figure S1–3) using BioEdit software. We used MEGA7 software (http://mega.co.nz) to generate a phylogenetic tree. First, the sequences were aligned by ClustalW using default parameters. Second, the alignment result was used to perform phylogenetic analysis (Figure S4) with Construct/Test Neighbor-Joining Tree, of which, a phylogeny was chosen using Bootstrap method. The other parameters were set as default.
Antibodies
Antibodies were generated to apSPTR-GF-DP2. The peptide antigens were prepared as described in detail previously 29, 30, 67. Briefly, the antigen was made by coupling apSPTR-GF-DP2 to BSA (Sigma, catalog #A0281) using 1-ethyl-3-(dimethylaminopropyl) carbodiimide (EDC) (Sigma, catalog #E7750), and then purified. For each antigen, two male Sprague Dawley rats (250–300 g; Taconic Farms) were immunized by intraperitoneal injections. At days 21 and 42, the rats were boosted by intraperitoneal injections. Animals were killed by decapitation at 49 d, and the blood was harvested and processed for serum. Sera were aliquoted, frozen, and lyophilized or stored at 4°C with EDTA (25 mM final concentration) and thimerosal (0.1% final concentration) added as stabilizers. For antibodies that produced immunostaining, specificity was confirmed by pre-incubation overnight of the primary antibodies with the corresponding synthetic peptide, i.e., apSPTR-GF-DP2 (100 µM), which abolished the staining (Figure S5).
Immunohistochemistry
Immunohistochemistry in wholemounts was performed as described previously 29, 32. The tissue was fixed in a buffer (4% paraformaldehyde, 0.2% picric acid, 25% sucrose, and 0.1 M NaH2PO4, pH 7.6), for either 3h at room temperature or overnight at 4°C. All subsequent incubations were done at room temperature. The tissue was washed with PBS, and was permeabilized and blocked by overnight incubation in blocking buffer (10% normal goat serum, 2% Triton X-100, 1% BSA, 154 mM NaCl, 50 mM EDTA, 0.01% thimerosal, and 10 mM Na2HPO4, pH 7.4). The primary antibody was diluted 1:250 in blocking buffer and incubated with the tissue for 4–7 d. The tissue was then washed twice per day for 2–3 d with washing buffer (2% Triton X-100, 1% BSA, 154 mM NaCl, 50 mM EDTA, 0.01% thimerosal, and 10 mM Na2HPO4, pH 7.4). After washing, the tissue was incubated with a 1:500 dilution of secondary antibody (lissamine rhodamine goat anti-rat; Jackson ImmunoResearch) for 2–3 d and then washed again two times with washing buffer for 1 d and four times with storage buffer (1% BSA, 154 mM NaCl, 50 mM EDTA, 0.01% thimerosal, and 10 mM Na2HPO4, pH 7.4) for 1 d. We observed and photographed the tissue under fluorescence microscope.
For double labeling of physiologically identified CBI-12 cell with apSPTR-GF-DP2 immunohistochemistry, neurons were identified based on morphology and electrophysiological characters and injected with carboxyfluorescein 53. For double labeling with backfills, CBCs of live buccal or cerebral ganglia were cut and the buccal ends or the cerebral ends of the CBCs were incubated overnight in a Vaseline well containing biocytin. This allowed biocytin transport to the somata of neurons with axons in the CBC. Ganglia were then fixed, and processed with avidin-fluorescein so that somata were green. Tissues were also processed for immuohistochemistry as described above.
Mass Spectrometric Analysis of Peptides
To characterize apSPTR-GF-DPs in the Aplysia CNS, we used matrix-assisted laser desorption/ionization (MALDI) time-of flight (TOF) MS on individual apSPTR-GF-DP2-immunoreactive neurons from the cerebral and buccal ganglia. The ganglia were treated with 1% protease IX in ASW with antibiotics (100 units/mL penicillin G, 100µg/mL streptomycin, and 100 µg/mL gentamicin) for 45 min at 34°C and then desheathed. Individual cerebral neurons were manually isolated according to the apSPTR-GF-DP2 immunohistochemistry staining map using electrolytically sharpened tungsten needles. Isolated neurons were transferred one by one onto a stainless steel MALDI sample plate (Bruker Daltonics, Billerica, MA) using homemade plastic micropipettes filled with deionized water. Excess liquid was aspirated from the sample plate and 0.3 µl of saturated DHB matrix (DHB: 2, 5-dihydroxybenzoic acid, 20 mg/ml deionized water) was applied onto the sample spots. Peptide profiles were measured using ultrafleXtreme MALDI-TOF/TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with a Smartbeam II frequency tripled Nd:YAG solid state laser. Mass spectra were manually acquired in positive reflectron mode and calibrated externally using Bruler Peptide Mix II standards. Signals from 500–4000 laser shots fired at 1000 Hz frequency at multiple locations within each sample spot were summed into representative cell spectrum. Obtained mass spectra were processed using flexAnalysis 3.4 software (Bruker Daltonics, Billerica, MA).
To aid interpretation of the MS results, putative peptides encoded by the apSPTR-GF-DP2 prohormone were predicted using the Neuropred prediction tool (http://stagbeetle.animal.uiuc.edu/cgi-bin/neuropred.py) 47, 68 and assignment of detected peptides was performed by peptide mass fingerprint approach. Location of the putative signal peptide cleavage site was inferred using SignalP (http://www.cbs.dtu.dk/services/SignalP/) model 46.
Electrophysiology
Intracellular and extracellular recording techniques were utilized as described previously 36, 37, 58, 69, 70. Briefly, ganglia were desheathed, transferred to a recording chamber containing 1.5 mL of artificial seawater (ASW, 460mM NaCl, 10 mM KCl, 11 mM CaCl2, 55 mM MgCl2, and 10 mM HEPES, pH 7.6), continuously perfused at 0.3mL/min, and maintained at 14–17°C. The peptide, apSPTR-GF-DP2, was dissolved in ASW immediately before each physiological test, and the peptide/ASW solution was perfused into the recording chamber. Some experiments were also performed in a high divalent (HiDi) saline (368 mM NaCl, 8 mM KCl, 13.8 mM CaCl2, 115 mM MgCl2, and 10 mM HEPES, pH 7.6), which increases the spiking threshold of neurons and therefore curtails polysynaptic influences. Intracellular recordings were obtained using 5–10 MΩ sharp microelectrodes filled with an electrolyte (0.6 M K2SO4 plus 60 mM KCl). Extracellular recordings were acquired from polyethylene suction electrodes. Grass S88 and WPI Pulsemaster A300 stimulators were used to provide timing signals for intracellular stimulation.
To test the effects of apSPTR-GF-DP2 and FCAP on the feeding circuit, we performed experiments with the cerebral and buccal ganglia. The buccal ganglion innervates the feeding organ (radula). Feeding motor programs were elicited by stimulation of command-like interneuron CBI-2 at 8–10 Hz, and were monitored by cyclic bursts in the I2 nerve of the buccal ganglion 51, 71. The interval of CBI-2 stimulation was 1.5 min. Electrophysiological recordings were digitized on line using AxoScope software (version 10.7, Molecular Devices, LLC, Sunnyvale, CA), and were plotted by CorelDraw (version X7, Corel Corporation, Ottawa, ON, Canada). Bar graphs are plotted with Prism (version 5, GraphPad Software, La Jolla, CA). Data are expressed as mean ± S.E. All statistical tests (e.g., repeated measures one-way analysis of variance) were performed using Prism. When the data showed significant effects in analysis of variance, further individual comparisons were performed with a Bonferroni’s correction.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Natural Science Foundation of China (Grants 31671097, 31371104 (J.J.), J1103512, and J1210026 (School of Life Sciences, Nanjing University)); Award Nos. RO1 NS066587, RO1 NS070583 (K.R.W/E.C.C), and RO1 NS031609 (J.V.S.) from the National Institute of Neurological Disorders and Stroke; RO1 NS031609 (E.C.C.) from the National Institute of Mental Health, the National Institutes of Health, Award No. P30 DA018310 from the National Institute on Drug Abuse, and Award No. CHE-16-067915 from the National Science Foundation (J.V.S.).
ABREVIATIONS
- apSPTR-GF-DPs
Aplysia SPTR-Gene Family-Derived peptides
- apSPTR-GF-DP1
Aplysia SPTR-Gene Family-Derived peptide1
- apSPTR-GF-DP2
Aplysia SPTR-Gene Family-Derived peptide2
- CBI-12
cerebral-buccal interneuron-12
- CNS
central nervous system
- apSPTR Precursor
Aplysia SPTR Precursor
- EST
expressed sequence tag
- MALDI-TOF MS
matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry
- CBC
cerebrobuccal connective
- FCAP
feeding circuit activating peptide
- CP2
cerebral peptide 2
- CPG
central pattern generator
- PTMs
posttranslational modifications
- ASW
artificial seawater
- HiDi
high divalent
Footnotes
ASSOCIATED CONTENT
There are a total of one supplemental table and 5 supplemental figures (Figure S1–5).
Author Contributions
Conceived and designed the experiments: GZ, FSV, EVR, ECC, JVS, KRW, JJ. Performed the experiments and analyzed the data: GZ, WY (Wang-ding Yuan), FSV, EVR, KY, SY (Si-yuan Yin), ZL (Zi-wei Le), YX (Ying-yu Xue), TC (Ting-ting Chen), GC (Guo-kai Chen), SC (Song-an Chen), ECC, JVS, KRW, JJ. Wrote the paper: GZ, FSV, EVR, ECC, JVS, KRW, JJ.
The authors declare no competing financial interest.
References
- 1.Briggman KL, Kristan WB. Multifunctional pattern-generating circuits. Annu. Rev. Neurosci. 2008;31:271–294. doi: 10.1146/annurev.neuro.31.060407.125552. [DOI] [PubMed] [Google Scholar]
- 2.Jing J, Gillette R, Weiss KR. Evolving concepts of arousal: insights from simple model systems. Rev. Neurosci. 2009;20:405–427. doi: 10.1515/revneuro.2009.20.5-6.405. [DOI] [PubMed] [Google Scholar]
- 3.Nusbaum MP, Blitz DM. Neuropeptide modulation of microcircuits. Curr. Opin. Neurobiol. 2012;22:592–601. doi: 10.1016/j.conb.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kupfermann I. Modulatory actions of neurotransmitters. Annu. Rev. Neurosci. 1979;2:447–465. doi: 10.1146/annurev.ne.02.030179.002311. [DOI] [PubMed] [Google Scholar]
- 6.Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- 7.Nusbaum MP, Blitz DM, Marder E. Functional consequences of neuropeptide and small-molecule co-transmission. Nat. Rev. Neurosci. 2017;18:389–403. doi: 10.1038/nrn.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cropper EC, Lloyd PE, Reed W, Tenenbaum R, Kupfermann I, Weiss KR. Multiple neuropeptides in cholinergic motor neurons of Aplysia: evidence for modulation intrinsic to the motor circuit. Proc. Natl. Acad. Sci. U S A. 1987;84:3486–3490. doi: 10.1073/pnas.84.10.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sossin WS, Sweet-Cordero A, Scheller RH. Dale's hypothesis revisited: different neuropeptides derived from a common prohormone are targeted to different processes. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4845–4848. doi: 10.1073/pnas.87.12.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brezina V, Orekhova IV, Weiss KR. Functional uncoupling of linked neurotransmitter effects by combinatorial convergence. Science. 1996;273:806–810. doi: 10.1126/science.273.5276.806. [DOI] [PubMed] [Google Scholar]
- 11.Vilim FS, Cropper EC, Price DA, Kupfermann I, Weiss KR. Peptide cotransmitter release from motorneuron B16 in Aplysia californica: costorage, corelease, and functional implications. J. Neurosci. 2000;20:2036–2042. doi: 10.1523/JNEUROSCI.20-05-02036.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh HY, Weiss KR. Activity-dependent peptidergic modulation of the plateau-generating neuron B64 in the feeding network of Aplysia. J. Neurophysiol. 2007;97:1862–1867. doi: 10.1152/jn.01230.2006. [DOI] [PubMed] [Google Scholar]
- 13.Friedman AK, Weiss KR. Repetition priming of motoneuronal activity in a small motor network: intercellular and intracellular signaling. J. Neurosci. 2010;30:8906–8919. doi: 10.1523/JNEUROSCI.1287-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh HY, Weiss KR. Peptidergic contribution to posttetanic potentiation at a central synapse of Aplysia. J. Neurophysiol. 2005;94:1281–1286. doi: 10.1152/jn.00073.2005. [DOI] [PubMed] [Google Scholar]
- 15.Dacks AM, Weiss KR. Latent modulation: a basis for non-disruptive promotion of two incompatible behaviors by a single network state. J. Neurosci. 2013;33:3786–3798. doi: 10.1523/JNEUROSCI.5371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koert CE, Spencer GE, van Minnen J, Li KW, Geraerts WP, Syed NI, Smit AB, van Kesteren RE. Functional implications of neurotransmitter expression during axonal regeneration: serotonin, but not peptides, auto-regulate axon growth of an identified central neuron. J. Neurosci. 2001;21:5597–5606. doi: 10.1523/JNEUROSCI.21-15-05597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veenstra JA. Neurohormones and neuropeptides encoded by the genome of Lottia gigantea, with reference to other mollusks and insects. Gen. Comp. Endocrinol. 2010;167:86–103. doi: 10.1016/j.ygcen.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 18.York PS, Cummins SF, Degnan SM, Woodcroft BJ, Degnan BM. Marked changes in neuropeptide expression accompany broadcast spawnings in the gastropod Haliotis asinina. Frontiers in Zoology. 2012;9 doi: 10.1186/1742-9994-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conzelmann M, Williams EA, Krug K, Franz-Wachtel M, Macek B, Jekely G. The neuropeptide complement of the marine annelid Platynereis dumerilii. Bmc Genomics. 2013;14:906. doi: 10.1186/1471-2164-14-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirabeau O, Joly JS. Molecular evolution of peptidergic signaling systems in bilaterians. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E2028–2037. doi: 10.1073/pnas.1219956110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simakov O, Marletaz F, Cho SJ, Edsinger-Gonzales E, Havlak P, Hellsten U, Kuo DH, Larsson T, Lv J, Arendt D, Savage R, Osoegawa K, de Jong P, Grimwood J, Chapman JA, Shapiro H, Aerts A, Otillar RP, Terry AY, Boore JL, Grigoriev IV, Lindberg DR, Seaver EC, Weisblat DA, Putnam NH, Rokhsar DS. Insights into bilaterian evolution from three spiralian genomes. Nature. 2013;493:526–531. doi: 10.1038/nature11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn SJ, Martin R, Rao S, Choi MY. Neuropeptides predicted from the transcriptome analysis of the gray garden slug Deroceras reticulatum. Peptides. 2017;93:51–65. doi: 10.1016/j.peptides.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Bose U, Suwansa-Ard S, Maikaeo L, Motti CA, Hall MR, Cummins SF. Neuropeptides encoded within a neural transcriptome of the giant triton snail Charonia tritonis, a Crown-of-Thorns Starfish predator. Peptides. 2017;98:3–14. doi: 10.1016/j.peptides.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Wang T, Zhao M, Liang D, Bose U, Kaur S, McManus DP, Cummins SF. Changes in the neuropeptide content of Biomphalaria ganglia nervous system following Schistosoma infection. Parasit Vectors. 2017;10:275. doi: 10.1186/s13071-017-2218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L, Jezzini S, Lovell P, Iannucculli W, Chen M, Nguyen T, Sheng H, Shaw R, Kalachikov S, Panchin YV, Farmerie W, Russo JJ, Ju J, Kandel ER. Neuronal transcriptome of Aplysia: neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cropper EC, Tenenbaum R, Kolks MA, Kupfermann I, Weiss KR. Myomodulin: a bioactive neuropeptide present in an identified cholinergic buccal motor neuron of Aplysia. Proc. Natl. Acad. Sci. U S A. 1987;84:5483–5486. doi: 10.1073/pnas.84.15.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cropper EC, Miller MW, Vilim FS, Tenenbaum R, Kupfermann I, Weiss KR. Buccalin is present in the cholinergic motor neuron B16 of Aplysia and it depresses accessory radula closer muscle contractions evoked by stimulation of B16. Brain Res. 1990;512:175–179. doi: 10.1016/0006-8993(90)91189-n. [DOI] [PubMed] [Google Scholar]
- 28.Church PJ, Lloyd PE. Expression of diverse neuropeptide cotransmitters by identified motor neurons in Aplysia. J. Neurosci. 1991;11:618–625. doi: 10.1523/JNEUROSCI.11-03-00618.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furukawa Y, Nakamaru K, Wakayama H, Fujisawa Y, Minakata H, Ohta S, Morishita F, Matsushima O, Li L, Romanova E, Sweedler JV, Park JH, Romero A, Cropper EC, Dembrow NC, Jing J, Weiss KR, Vilim FS. The enterins: a novel family of neuropeptides isolated from the enteric nervous system and CNS of Aplysia. J. Neurosci. 2001;21:8247–8261. doi: 10.1523/JNEUROSCI.21-20-08247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweedler JV, Li L, Rubakhin SS, Alexeeva V, Dembrow NC, Dowling O, Jing J, Weiss KR, Vilim FS. Identification and characterization of the feeding circuit-activating peptides, a novel neuropeptide family of Aplysia. J. Neurosci. 2002;22:7797–7808. doi: 10.1523/JNEUROSCI.22-17-07797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dembrow NC, Jing J, Proekt A, Romero A, Vilim FS, Cropper EC, Weiss KR. A newly identified buccal interneuron initiates and modulates feeding motor programs in Aplysia. J. Neurophysiol. 2003;90:2190–2204. doi: 10.1152/jn.00173.2003. [DOI] [PubMed] [Google Scholar]
- 32.Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, Yashina I, Kupfermann I, Sweedler JV, Weiss KR. From hunger to satiety: reconfiguration of a feeding network by Aplysia neuropeptide Y. J. Neurosci. 2007;27:3490–3502. doi: 10.1523/JNEUROSCI.0334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jing J, Sweedler JV, Cropper EC, Alexeeva V, Park JH, Romanova EV, Xie F, Dembrow NC, Ludwar BC, Weiss KR, Vilim FS. Feedforward compensation mediated by the central and peripheral actions of a single neuropeptide discovered using representational difference analysis. J. Neurosci. 2010;30:16545–16558. doi: 10.1523/JNEUROSCI.4264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vilim FS, Sasaki K, Rybak IA, Alexeeva V, Cropper EC, Jing J, Orekhova IV, Brezina V, Price D, Romanova EV, Rubakhin SS, Hatcher NG, Sweedler JV, Weiss KR. Distinct mechanisms produce functionally complementary actions of neuropeptides that are structurally related but derived from different precursors. J. Neurosci. 2010;30:131–147. doi: 10.1523/JNEUROSCI.3282-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romanova EV, Sasaki K, Alexeeva V, Vilim FS, Jing J, Richmond TA, Weiss KR, Sweedler JV. Urotensin II in invertebrates: from structure to function in Aplysia californica. PLoS One. 2012;7:e48764. doi: 10.1371/journal.pone.0048764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai L, Livnat I, Romanova EV, Alexeeva V, Yau PM, Vilim FS, Weiss KR, Jing J, Sweedler JV. Characterization of GdFFD, a D-amino acid-containing neuropeptide that functions as an extrinsic modulator of the Aplysia feeding circuit. J. Biol. Chem. 2013;288:32837–32851. doi: 10.1074/jbc.M113.486670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livnat I, Tai HC, Jansson ET, Bai L, Romanova EV, Chen TT, Yu K, Chen SA, Zhang Y, Wang ZY, Liu DD, Weiss KR, Jing J, Sweedler JV. A D-amino acid-containing neuropeptide discovery funnel. Anal. Chem. 2016;88:11868–11876. doi: 10.1021/acs.analchem.6b03658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang G, Vilim FS, Liu DD, Romanova EV, Yu K, Yuan WD, Xiao H, Hummon AB, Chen TT, Alexeeva V, Yin SY, Chen SA, Cropper EC, Sweedler JV, Weiss KR, Jing J. Discovery of leucokinin-like neuropeptides that modulate a specific parameter of feeding motor programs in the molluscan model, Aplysia. J. Biol. Chem. 2017;292:18775–18789. doi: 10.1074/jbc.M117.795450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh HY, Vilim FS, Jing J, Weiss KR. Two neuropeptides colocalized in a command-like neuron use distinct mechanisms to enhance its fast synaptic connection. J. Neurophysiol. 2003;90:2074–2079. doi: 10.1152/jn.00358.2003. [DOI] [PubMed] [Google Scholar]
- 40.Phares GA, Walent JH, Niece RL, Kumar SB, Ericsson LH, Kowalak JA, Lloyd PE. Primary structure of a new neuropeptide, cerebral peptide 2, purified from cerebral ganglia of Aplysia. Biochemistry (Mosc) 1996;35:5921–5927. doi: 10.1021/bi953081y. [DOI] [PubMed] [Google Scholar]
- 41.Morgan PT, Perrins R, Lloyd PE, Weiss KR. Intrinsic and extrinsic modulation of a single central pattern generating circuit. J. Neurophysiol. 2000;84:1186–1193. doi: 10.1152/jn.2000.84.3.1186. [DOI] [PubMed] [Google Scholar]
- 42.Jing J, Weiss KR. Generation of variants of a motor act in a modular and hierarchical motor network. Curr. Biol. 2005;15:1712–1721. doi: 10.1016/j.cub.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 43.Hurwitz I, Susswein AJ. B64, a newly identified central pattern generator element producing a phase switch from protraction to retraction in buccal motor programs of Aplysia californica. J. Neurophysiol. 1996;75:1327–1344. doi: 10.1152/jn.1996.75.4.1327. [DOI] [PubMed] [Google Scholar]
- 44.Wu JS, Due MR, Sasaki K, Proekt A, Jing J, Weiss KR. State dependence of spike timing and neuronal function in a motor pattern generating network. J. Neurosci. 2007;27:10818–10831. doi: 10.1523/JNEUROSCI.1806-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vilim FS, Jing J, Wu JS, Alexeeva V, Moroz LL, Weiss KR. Identification and characterization of the Aplysia SPTR prohormone related peptides (ASPRP) Society for Neuroscience Annual Meeting 2003 2003 [Google Scholar]
- 46.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 47.Southey BR, Amare A, Zimmerman TA, Rodriguez-Zas SL, Sweedler JV. NeuroPred: a tool to predict cleavage sites in neuropeptide precursors and provide the masses of the resulting peptides. Nucleic Acids Res. 2006;34:W267–272. doi: 10.1093/nar/gkl161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jekely G. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc. Natl. Acad. Sci. U. S. A. 2013;110:8702–8707. doi: 10.1073/pnas.1221833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starratt AN, Brown BE. Structure of the pentapeptide proctolin, a proposed neurotransmitter in insects. Life Sci. 1975;17:1253–1256. doi: 10.1016/0024-3205(75)90134-4. [DOI] [PubMed] [Google Scholar]
- 50.Hurwitz I, Kupfermann I, Susswein AJ. Different roles of neurons B63 and B34 that are active during the protraction phase of buccal motor programs in Aplysia californica. J. Neurophysiol. 1997;78:1305–1319. doi: 10.1152/jn.1997.78.3.1305. [DOI] [PubMed] [Google Scholar]
- 51.Jing J, Weiss KR. Neural mechanisms of motor program switching in Aplysia. J. Neurosci. 2001;21:7349–7362. doi: 10.1523/JNEUROSCI.21-18-07349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jing J, Weiss KR. Interneuronal basis of the generation of related but distinct motor programs in Aplysia: implications for current neuronal models of vertebrate intralimb coordination. J. Neurosci. 2002;22:6228–6238. doi: 10.1523/JNEUROSCI.22-14-06228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jing J, Vilim FS, Wu JS, Park JH, Weiss KR. Concerted GABAergic actions of Aplysia feeding interneurons in motor program specification. J. Neurosci. 2003;23:5283–5294. doi: 10.1523/JNEUROSCI.23-12-05283.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasaki K, Cropper EC, Weiss KR, Jing J. Functional differentiation of a population of electrically coupled heterogeneous elements in a microcircuit. J. Neurosci. 2013;33:93–105. doi: 10.1523/JNEUROSCI.3841-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubakhin SS, Romanova EV, Nemes P, Sweedler JV. Profiling metabolites and peptides in single cells. Nat Methods. 2011;8:S20–29. doi: 10.1038/nmeth.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romanova EV, Aerts JT, Croushore CA, Sweedler JV. Small-volume analysis of cell-cell signaling molecules in the brain. Neuropsychopharmacology. 2014;39:50–64. doi: 10.1038/npp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buchberger A, Yu Q, Li L. Advances in mass spectrometric tools for probing neuropeptides. Annu Rev Anal Chem (Palo Alto Calif) 2015;8:485–509. doi: 10.1146/annurev-anchem-071114-040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jing J, Alexeeva V, Chen SA, Yu K, Due MR, Tan LN, Chen TT, Liu DD, Cropper EC, Vilim FS, Weiss KR. Functional characterization of a vesicular glutamate transporter in an interneuron that makes excitatory and inhibitory synaptic connections in a molluscan neural circuit. J. Neurosci. 2015;35:9137–9149. doi: 10.1523/JNEUROSCI.0180-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teyke T, Rosen SC, Weiss KR, Kupfermann I. Dopaminergic neuron-B20 generates rhythmic neuronal activity in the feeding motor circuitry of Aplysia. Brain Res. 1993;630:226–237. doi: 10.1016/0006-8993(93)90661-6. [DOI] [PubMed] [Google Scholar]
- 60.Cropper EC, Dacks AM, Weiss KR. Consequences of degeneracy in network function. Curr. Opin. Neurobiol. 2016;41:62–67. doi: 10.1016/j.conb.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marder E, Gutierrez GJ, Nusbaum MP. Complicating connectomes: Electrical coupling creates parallel pathways and degenerate circuit mechanisms. Dev Neurobiol. 2017;77:597–609. doi: 10.1002/dneu.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vilim FS, Alexeeva V, Moroz LL, Li L, Moroz TP, Sweedler JV, Weiss KR. Cloning, expression and processing of the CP2 neuropeptide precursor of Aplysia. Peptides. 2001;22:2027–2038. doi: 10.1016/s0196-9781(01)00561-7. [DOI] [PubMed] [Google Scholar]
- 63.Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J. Neurosci. 2000;20:6752–6759. doi: 10.1523/JNEUROSCI.20-18-06752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wood DE, Stein W, Nusbaum MP. Projection neurons with shared cotransmitters elicit different motor patterns from the same neural circuit. J. Neurosci. 2000;20:8943–8953. doi: 10.1523/JNEUROSCI.20-23-08943.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thirumalai V, Marder E. Colocalized neuropeptides activate a central pattern generator by acting on different circuit targets. J. Neurosci. 2002;22:1874–1882. doi: 10.1523/JNEUROSCI.22-05-01874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein W, DeLong ND, Wood DE, Nusbaum MP. Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur. J. Neurosci. 2007;26:1148–1165. doi: 10.1111/j.1460-9568.2007.05744.x. [DOI] [PubMed] [Google Scholar]
- 67.Fujisawa Y, Furukawa Y, Ohta S, Ellis TA, Dembrow NC, Li L, Floyd PD, Sweedler JV, Minakata H, Nakamaru K, Morishita F, Matsushima O, Weiss KR, Vilim FS. The Aplysia mytilus inhibitory peptide-related peptides: identification, cloning, processing, distribution, and action. J. Neurosci. 1999;19:9618–9634. doi: 10.1523/JNEUROSCI.19-21-09618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Southey BR, Sweedler JV, Rodriguez-Zas SL. A python analytical pipeline to identify prohormone precursors and predict prohormone cleavage sites. Front Neuroinform. 2008;2:7. doi: 10.3389/neuro.11.007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu JS, Wang N, Siniscalchi MJ, Perkins MH, Zheng YT, Yu W, Chen SA, Jia RN, Gu JW, Qian YQ, Ye Y, Vilim FS, Cropper EC, Weiss KR, Jing J. Complementary interactions between command-like interneurons that function to activate and specify motor programs. J. Neurosci. 2014;34:6510–6521. doi: 10.1523/JNEUROSCI.5094-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang CY, Yu K, Wang Y, Chen SA, Liu DD, Wang ZY, Su YN, Yang SZ, Chen TT, Livnat I, Vilim FS, Cropper EC, Weiss KR, Sweedler JV, Jing J. Aplysia locomotion: Network and behavioral actions of GdFFD, a D-amino acid-containing neuropeptide. PLoS One. 2016;11:e0147335. doi: 10.1371/journal.pone.0147335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hurwitz I, Neustadter D, Morton DW, Chiel HJ, Susswein AJ. Activity patterns of the B31/B32 pattern initiators innervating the I2 muscle of the buccal mass during normal feeding movements in Aplysia californica. J. Neurophysiol. 1996;75:1309–1326. doi: 10.1152/jn.1996.75.4.1309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.