Abstract
Objective:
To provide consensus recommendations for health-care providers on the use of oral contraceptive pills (OCPs) in polycystic ovarian syndrome (PCOS) women in India.
Participants:
Extensive deliberations, discussions, and brainstorming were done with different fraternities (specialists) being involved. These included endocrinologists, gynecologists, reproductive endocrinologists, dermatologists, public health experts, researchers, and a project manager with a team to develop the guideline.
Evidence:
Published literature was retrieved through searches of Medline and The Cochrane Database from January 2003 to December 2017 using appropriate-controlled vocabulary (e.g., oral contraceptive pills, polycystic ovarian syndrome, long term outcomes, infertility). Clinical practice guideline collections, clinical trial registries, and national and international medical specialty societies' publications and data were also reviewed to suggest the recommendations.

Process:
The working group for guideline committee included members from the PCOS Society (India), Indian Society for Assisted Reproduction, The Mumbai Obstetric and Gynecological Society, The Endocrine Society of India, Indian Association of Dermatologists, Venereologists and Leprologists, Cosmetic Dermatology Society (India), Academicians from Medical Colleges, National Institute for Research in Reproductive Health, and a Research Associate. The core team included five reproductive endocrinologists, five gynecologists, five dermatologists, three endocrinologists, two public health experts and one research associate.
Conclusions:
This consensus statement provides the guidance/recommendations for Indian practitioners regarding the use of OCP in women with PCOS. PCOS is one of the common endocrinopathies encountered in gynecological/endocrine practice. The spectrum of this disorder may range from prepubertal girls with premature pubarche, young girls with hirsutism, acne and anovulatory cycles, married women with infertility, and elderly women. Although obesity is a common feature for most PCOS patients, 'lean PCOS' also exists. For several years, OCPs have played an important role in the symptom management of PCOS women. This is due to the fact that OCPs decrease the luteinizing hormone, reduce androgen production, and increase sex hormone-binding globulin, which binds androgens. Several new formulations of OCPs have been developed to decrease the side effects. This includes use of less androgenic progestins and lower doses of ethinyl estradiol. These consensus recommendations help the health provider to choose the right type of OCPs, which will alleviate the symptoms with least side effects. It also gives insight into the indications, contraindications, and concerns regarding its short, intermediate and long-term use.
KEYWORDS: Acne, anovulatory cycles, endometrial carcinoma, hirsutism, oral contraceptive pill, polycystic ovarian syndrome
BACKGROUND
Oral contraceptive pills (OCPs) have been the first-line therapy for concurrent treatment of menstrual irregularity, acne, and hirsutism in women with polycystic ovarian syndrome (PCOS), thus playing an important role in the symptom management of the PCOS women. The Combined OCP (COCP) also improve dysmenorrhea and menorrhagia, treat premenstrual syndrome, prevent menstrual migraines, treat pelvic pain related to endometriosis, and decrease the risk of endometrial and ovarian cancer.
The association of PCOS with metabolic syndrome brings up the debate regarding the risks versus benefits of OCP use in PCOS women. Moreover, the presence of confounding factors such as obesity, smoking, diabetes, and other cardiometabolic comorbidities is a significant limitation in comparing the different OCPs as well as the risks and benefits associated with their use. With this in mind, the PCOS Society (India) had a consensus meeting with a multidisciplinary faculty on providing recommendations for the use of OCPs in PCOS women in India. These recommendations were provided keeping in mind the phenotype of Indian women, who tend to have a higher risk of metabolic syndrome and insulin resistance.
PCOS is associated with several comorbidities such as obesity, diabetes, metabolic syndrome, and mood and anxiety disorders. Side effects of COCP such as weight gain, mood changes, and adverse effects on cardiometabolic risk factors may at times exacerbate the problems in PCOS women. Thus, before initiating treatment with OCPs, thorough counseling is essential and this should be backed up by stringent monitoring at every follow-up visit.
Prescribing information of OCPs in PCOS women should include the choice of cyclic versus continuous use and COCP with different types of progestins and pills with progestin only options.
Though weight reduction, exercise, and lifestyle modification are the first line of management with beneficial effects, especially in the obese PCOS women, COCP have been recommended to improve the clinical manifestations.
PHARMACOLOGY
There are two types of OCPs: combined pills (combined oral contraceptive [COCP]), containing an estrogen and a progestogen, and progestogen only pills. The COCP is a combination of two types of steroids; ethinyl estradiol (EE) as the main estrogenic component, with the dose varying from 20 to 50 μg per tablet (usually 30–35 micrograms), and progestins essentially derivatives of 19-nortestosterone (norethisterone, levonorgestrel, and more recently desogestrel, gestodene, norgestimate, and drospirenone). Reduction in the dose of estradiol was possible due to the availability of progestins with high antigonadotropic activity. The metabolism of EE is similar to that of endogenous estradiol, i.e., it undergoes oxidation at various carbon atoms.[1,2]
Contraceptive efficacy of COCP with 15 and 20 μg EE, measured by the Pearl index, is in the range of 0.07–0.88 and is therefore similar to that of COCP containing 30 μg EE (0.06–0.88).[3]
Estradiol undergoes an intensive first-pass effect in the cytochrome P450 (CYP) 3A system of the liver, leading to the formation of its metabolites: estrone, estrone sulfate, and estrone glucuronide.[4] Approximately 95% of the oral dose is metabolized before going into systemic circulation. The half-life of estradiol in plasma is ~2.5 h, whereas the terminal half-life is ~13–20 h and depends on enterohepatic circulation and on circulating levels of sulfate and glucuronide metabolites. On stopping treatment, estrogen concentrations return to basal levels within 2–3 days. Estradiol is mainly eliminated in the urine; ~10% is eliminated in feces.
MECHANISM OF ACTION OF ORAL CONTRACEPTIVE PILL
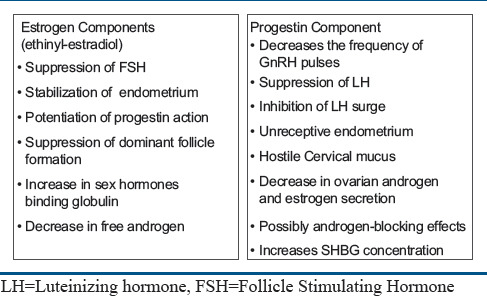
Mechanism for the action of OCPs is shown in Figure 1.
Figure 1.
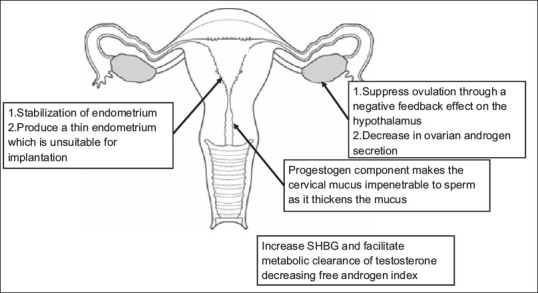
Mechanism of action of oral contraceptive pill
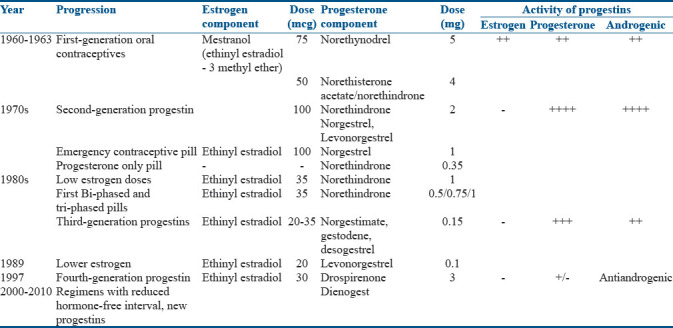
TYPES OF ORAL CONTRACEPTIVE PILL
OCPs contain potent, synthetic estrogen, EE, in combination with a progestin which may be derived from testosterone or those that are structurally unrelated to testosterone and function as androgen receptor antagonists (cyproterone acetate [CPA] and drospirenone). Low-dose OCPs contain EE (20–35 μg) along with low androgenic progestin, such as desogestrel or gestodene, or an antiandrogen, such as cyproterone acetate, chlormadinone acetate, or the spironolactone derivative drospirenone.
Originally, the manufactured combination OCP formulations were monophasic, with each active tablet containing a fixed dose of estrogen and progestin throughout the cycle. Multiphasic preparations (biphasic and triphasic) were developed in the 1980s to reduce the total dosage of progestin throughout the cycle without increasing the risk of breakthrough bleeding.
Different generations of OCPs[5] are highlighted in Table 1.
Table 1.
Different generation of oral contraceptive pills
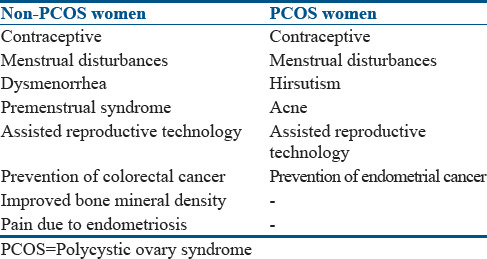
Indications and contraindications for the use of oral contraceptive pills
Indications for the use of OCPs are enumerated in Table 2.
Table 2.
Indications for the use of oral contraceptive pills in polycystic ovary syndrome and nonpolycystic ovary syndrome women
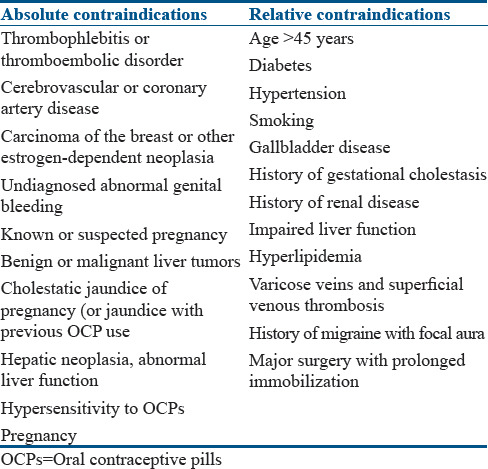
Contraindications for the use of OCPs are enumerated in Table 3.
Table 3.
Contraindications for the use of oral contraceptive pills
Summary of evidence
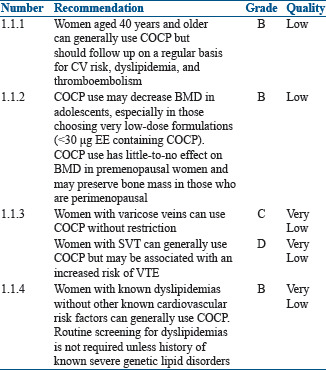
In women more than 40 years with PCOS, the use of OCPs outweighs the theoretical or proven risks. The use of OCPs results in protection against endometrial and ovarian cancers. One randomized controlled trial (RCT) (n = 83), one nonrandomized trial (n = 84), and 11 cohort studies (n = 3242) were evaluated by World Health Organization (WHO) in the 5th (2015) guidelines on 'Medical Eligibility Criteria for Contraceptive Use.' Only one study showed duration response effect and nine studies showed oral COCP use associated with less bone mineral density (BMD) gain (or greater loss) than nonuse.[6,7,8] One study suggested that among women using OCPs with varicose veins, the rate of venous thromboembolism (VTE) and superficial venous thrombosis (SVT) was higher as compared with nonusers but was not statistically significant and the number of events was small.[9] One study demonstrated that among women with SVT, the risk of VTE was higher in oral contraceptive (OC) users compared with nonusers.[10] One case–control study suggested an increased risk for myocardial infarction (MI)[11] and one retrospective cohort study suggested an increased risk for stroke and VTE[12] among COCP users with hypercholesterolemia compared to nonusers. One prospective cohort study suggested no worsening of lipid abnormalities among COCP users with dyslipidemia compared to nonusers with dyslipidemia.[13]
Recommendations
Drug interactions
Pharmacological interactions between OCPs and other compounds may be of two types: drugs may impair the effectiveness of the OCPs or OCPs may interfere with the metabolism of other compounds. Interactions of the first kind are due to interference with the absorption, metabolism, or excretion of estrogen, and interactions of the second kind are due to competition for metabolic pathway.
Summary of evidence
-
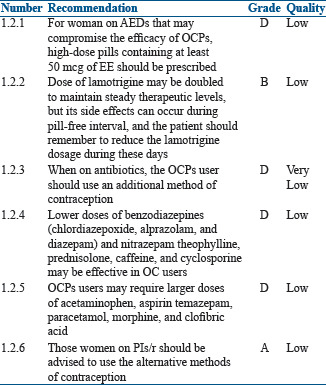
Drugs may impair the efficacy of OCPs: Drugs such as anticonvulsants (phenobarbital, phenytoin, and carbamazepine) and rifampicin induce a cytochrome P450, thereby increasing the clearance of the OCPs[14,15,16,17]
Other antibiotics such as ampicillin, metronidazole, quinolones, and tetracycline, which reduce the bacterial flora of the gastrointestinal tract and increase enterohepatic recirculation, affect OC efficacy as a result of low bioavailability of EE[18,19]
Ascorbic acid and paracetamol give rise to increased blood concentrations of EE due to competition for sulfation, which can increase the risk of its side effects[20]
-
OCPs may interfere with the metabolism of other drugs: OCPs reduce the clearance of benzodiazepines (chlordiazepoxide, alprazolam, diazepam) and nitrazepam.[21] The concentration of theophylline, prednisolone, caffeine, and cyclosporine is also reduced in OCP users.[22] Thus, lower doses of these drugs may be effective in OCPs users
The clearance of temazepam, salicylic acid, paracetamol, morphine, and clofibric acid apparently is increased.[23,24,25] OCPs users may require larger doses of these drugs. Serum concentration of some of the antiepileptic drug (AEDs) such as lamotrigine may vary during the cycle when OCPs are being used. Lamotrigine level decreases almost by 50% when on the pill resulting in poor seizure control. During 'pill-free' interval, the level may increase causing lamotrigine toxicities such as dizziness, double vision, and lack of coordination; therefore, the dose should be decreased during the pill-free period[26]
For women taking antiretroviral drugs that have significant pharmacokinetic interactions with hormonal contraceptives, an alternative or additional effective contraceptive method to prevent unintended pregnancy is recommended. Several ritonavir-boosted protease inhibitors (PIs/r), efavirenz (EFV), and elvitegravir/cobicistat (EVG/c) based regimens have drug interactions with COCP. These drugs decrease or increase the blood levels of EE, norethindrone, or norgestimate, which potentially decreases contraceptive efficacy or increases estrogen or progestin-related adverse effects (e.g., thromboembolism). EFV can decrease etonogestrel bioavailability and plasma progestin concentrations of COCP containing EE and norgestimate.[27] Several PI/r and EVG/c decrease OC estradiol levels.[28,29,30,31] Several pharmacokinetic studies have shown that etravirine, rilpivirine, and nevirapine use did not significantly affect estradiol or progestin levels in women with HIV using COCP.[32,33,34]
Recommendations
Side effects
Summary of evidence
Side effects of oral contraceptives vary depending on the dose and type of hormone used in the OCPs. Side effects, in general, include breast tenderness, bloating, nausea, weight gain, migraine, insulin resistance, increased blood sugars, dyslipidemia, VTE, cardiovascular disease (CVD), vaginal spotting, and abnormal bleeding. Mild increase in blood pressure might occur with some newer OCPs formulations.[35,36]
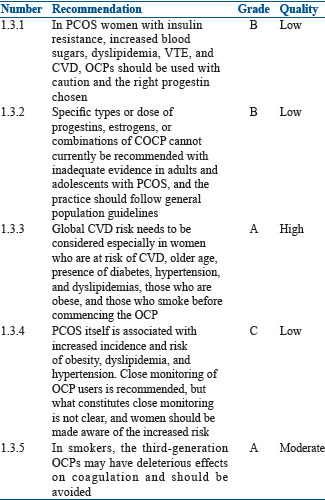
Specific types or dose of progestins, estrogens, or combinations of COCP cannot currently be recommended with inadequate evidence in adults and adolescents with PCOS, and the practice should follow general population guidelines.[37] In smokers, the third-generation OCPs may have deleterious effects[38] on and may increase the risk of VTE as compared to second-generation OCPs containing the androgenic progestin levonorgestrel.[39,40] Women who are at risk of CVD, older age, presence of diabetes, hypertension, and dyslipidemias, who are obese, and who smoke should be counseled about the increased risk of CVD. We also know that PCOS itself is associated with increased incidence and risk of obesity, hyperlipidemia, and hypertension.[37] As PCOS women are already at a higher risk of CVD, which can be exaggerated with the use of OCPs, close monitoring of OCP users though recommended but, what constitutes close monitoring is not clear. Pharmacovigilance is the key in OCP users.
Figure 2 describes the side effects related to the dose and drug in the OCP.
Figure 2.
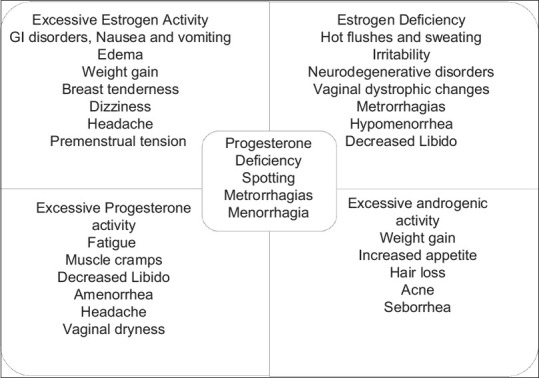
Side effects of oral contraceptive pills depending on dose and drug
Recommendations
COUNSELING
General counseling
Summary of evidence
To achieve maximum benefits and safety for each individual patient, for noncontraceptive use of COCP, detailed counseling regarding risks, benefits, and contraindications should be done. Counseling regarding the side effects such as weight gain, breakthrough bleeding, nausea, and breast tenderness is important. These women also need to be informed about the side effects such as decreased libido, melasma, and mood changes.[41]
It is also important to discuss the interactions of OCPs with anticonvulsants, antibiotics, benzodiazepines, salicylic acid, paracetamol, morphine, and clofibric acid. Women who want to start OCPs should also be counseled regarding the contraindications mentioned earlier.
Counseling is important when acne is treated with COCP as acne reduction may take a few months for the results to be evident, and therefore, combining COCP with other medications for early treatment of acne may be appropriate.
Recommendations

Investigations before initiation
Summary of evidence
Clinical examination and laboratory tests after proper history taking are essential before the initiation of OCPs.
-
Medical history
- Age
- Past and present medical conditions
- Any drug use
- Migraine
- CVD risk factors (smoking, obesity, hypertension, diabetes, dyslipidemia, and coronary artery disease)
- Thrombophilia (any known disorder of thrombophilia, personal or family history of previous VTE)
- History about usage of tobacco (oral, snuff, etc.)
- History of smoking
-
Physical examination[42]
- Blood pressure measurement
- Body mass index (BMI)
- Waist circumference
- Pelvic examination
- Breast examination
-
Laboratory tests in the presence of cardiometabolic risk[37]
- A fasting glucose level or in the presence of overweight or other diabetes risk factors a 75-g 2-h standard oral glucose tolerance test (OGTT)
- Lipid profile in overweight or obese PCOS.
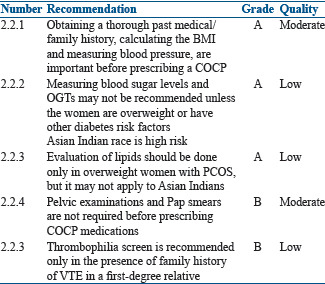
Contrary to previous practice, pelvic examinations and Pap smears are not required before prescribing COCP medications according to recommendations by the WHO, the American Congress of Obstetricians and Gynecologists, and Planned Parenthood. This makes it more feasible for dermatologists also to prescribe COCP medications without gynecology consultation.[43] Thrombophilia screen is not recommended routinely before prescribing an OCP unless there is a family history of VTE in a first-degree relative.
Recommendations
Starting and stopping of therapy with oral contraceptive pills
Summary of evidence
OCPs are ideally started on any day between day 1 and 5 of the cycle, given for 21 days and stopped. This will be followed by withdrawal bleed. A new packet of pills is started counting the 1st day of withdrawal bleed as day 1. The patient should be called for follow-up after 3 months for the assessment of blood pressure and enquired for the presence of any other problems. Thereafter, annual visit is required for assessing side effects, checking blood pressure, and evaluating glucose intolerance and lipids.
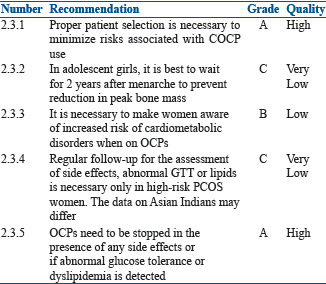
Since peak bone mass development occurs during adolescence and young adulthood, use of low-dose estrogen COCP early in the teen years may affect the bone mass,[44] although some studies refute this observation.[45,46] As definitive conclusions are not yet available, the use of COCP for acne should be avoided within 2 years of menarche or in patients who are <14 years of age unless it is clinically warranted. If the use of COCP is clinically warranted in the age group <14 years, it is preferable to use drospirenone and drospirenone/levomefolate, norgestimate, and norethindrone. During treatment, circulating androgen and sex hormone-binding globulin (SHBG) levels may be monitored to assess the adequacy of hormonal therapy, although clinical response will be the primary marker followed.
OCPs need to be stopped in the presence of any side effects or if abnormal glucose tolerance or dyslipidemia is detected.
Recommendations
Long-term concerns
Summary of evidence
Three issues that need to be addressed include cardiovascular risks, thrombosis with risk of embolism, and cancer risks.
Cardiovascular thrombosis risk
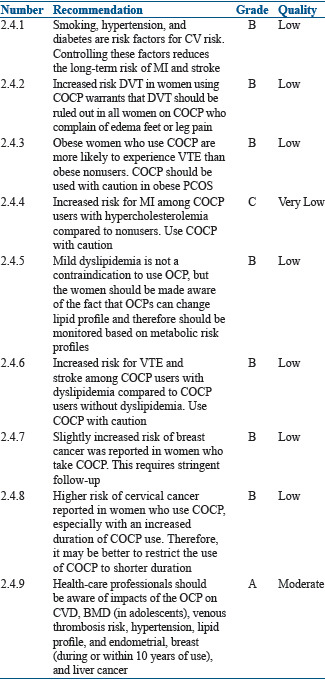
Smoking, hypertension, and diabetes are the risk factors for CVS risks. Controlling these factors reduces the long-term risk of MI and stroke. Thus, counseling regarding these modifiable factors is very important. There is also an increased risk of deep vein thrombosis (DVT) in women using COCP. This risk is 3.4/10,000 to 9–10/10,000 woman-years at 1 year of COCP use as compared to 1/10,000 woman-years in those who do not use COCP.[47] This risk increases in the presence of obesity, smoking, alcohol consumption, and positive family or personal history. Peragallo Urrutia et al. conducted a meta-analysis of 14 different studies, in which OCP users had three times increased odds for VTE compared with nonusers.
Initially, it was published that drospirenone-containing OCPs had an higher risk of DVT,[48,49] but a recent large prospective trial has shown that the risk of thrombosis is not higher with drospirenone containing OCPs as compared with other OCP formulations.[50] Obese women who use COCP are more likely to experience VTE than obese women who do not use COCP.[51,52] Among women with very high BMI using COCP, evidence for weight gain when on COCP is inconsistent.[53]
There is limited evidence and the results are inconsistent on the use of COCP among women with dyslipidemia and risk of cardiovascular outcomes. One study suggested an increased risk for MI among COCP users with hypercholesterolemia[11] compared to nonusers without hypercholesterolemia, one study suggested an increased risk for VTE and for stroke among COCP users with dyslipidemia[15] compared to COCP users without dyslipidemia, and another study suggested no worsening of lipid abnormalities among COCP users with dyslipidemia[13] compared to nonusers with dyslipidemia. The presence of dyslipidemia may not be a contraindication for the use of OCPs. Women should be made aware of the fact that OCPs can change their lipid profile and therefore should be monitored based on metabolic risk profiles. No evidence of risk for pancreatitis was identified in OCP users.
Effect on bone mineral density
The evidence on fracture risk in COCP users is still inconsistent,[54,55,56,57,58,59,60,61,62,63,64,65] although three recent studies show no effect.[45,66,67] COCP use may decrease BMD in adolescents, especially in those choosing very low-dose formulations (COCP containing <30 μg EE containing COCP).[67,68,69,70,71,72,73,74,75,76,77,78,79,80] It was also observed that COCP use has little-to-no effect on BMD in premenopausal women[8,66,68,69,70,71,72,73,74,75,76,77,81,82,83] and may preserve bone mass in those who are perimenopausal.[78,79,80,84,85,86,87,88,89,90] No impact of OCPs currently exists to caution their use with respect to bone health.
Visual disturbances
There is a cardiovascular background related to the expression of progesterone receptors in ocular tissues for visual disturbances in women receiving COCP. The incidence of ocular complications is estimated to be 1 in 230,000, including dry eye symptoms, corneal edema, lens opacities, and retinal neuro-ophthalmologic or vascular complications.[91]
Certain studies have reported an increased incidence of glaucoma in women over 40 years when OCPs were used for 3 years or more especially in those women who have additional risk factors for glaucoma.[92] Both the physicians prescribing OCPs and the patients receiving them need to be made aware that there may be an concurrent risk of open-angle glaucoma. At present, there is no high-quality evidence for the occurrence of glaucoma in peri and post-menopausal women. These women should therefore be counseled and reassured that the studies do not show any causative effects, but women who have used oral contraceptives for 3 years or more and who have additional risk factors for glaucoma should be checked annually for the disease during their ophthalmic examinations. Thus, ocular pharmacovigilance may be needed only for long-term users or elderly or high eye risk cases.
Cancer risk
Slightly increased risk of breast cancer was reported in women who take COCP and is not related to PCOS women as such.[93] The relative risk of breast cancer in the current COCP users was 1.24 (95% confidence interval [CI], 1.15–1.33), and this increased risk disappeared 10 years after discontinuation of COCP. There was also a higher risk of premature deaths due to breast cancer in the population who used COCP (P < 0.0001) for longer duration.[94]
Earlier 24 observational studies showed a higher risk of cervical cancer in women who use COCP, especially with an increased duration of COCP use. This increased risk, once again, was not related just to PCOS women but in general to OCP use. This risk reduced after discontinuation of COCP and disappeared after 10 years of non use.[95] Although a later published systematic review of nine pooled studies found no significant increase in the risk of cervical cancer among ever-users of COCP, it reported non significant increased risk of cervical cancer in women with >5 years of COCP use as compared to never users.[96]
The use of COCP is associated with reduced risk of occurrence of endometrial and ovarian cancer.[94,97] A 2008 meta-analysis of 45 different studies reported a significant correlation between duration of COCP use and reduction in risk of ovarian cancer (P < 0.0001).
Thus, healthcare professionals should be aware of impacts of the OCP on CVD, BMD (in adolescents), venous thrombosis risk, hypertension, lipid profile, and breast (during or within 10 years of use) and liver cancer. Pharmacovigilance is the key and awareness should not impede therapy in genuinely indicated cases, especially for short duration. Often detailed history taking is needed.
Recommendations
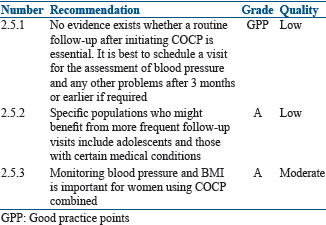
Follow-up on oral contraceptive pill therapy
Summary of evidence
No evidence exists whether a routine follow-up visit after initiating COCP improves correct or continued use and prevents side effects and/or long-term complications. After initiation of OCPs, it is best to review after 3 months. Monitoring blood pressure and BMI is important for women using COCP.
A systematic review identified five studies that examined the incidence of hypertension among women who began using a COCP versus those who started a non hormonal method of contraception or a placebo.[98] Few women developed hypertension after initiating COCP, and studies examining increase in blood pressure after COCP initiation found mixed results. At follow-up visit, it is important to ask for history of any side effects, breakthrough bleeding, and initiation of new medication.
Specific populations who might benefit from more frequent follow-up visits include adolescents and those with certain medical conditions.
Recommendations
CHOICE OF ORAL CONTRACEPTIVE PILL FOR NONCONTRACEPTIVE INDICATIONS
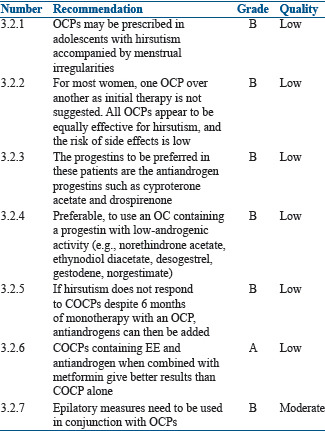
For acne
Summary of evidence
PCOS is associated with elevated androgen levels of ovarian origin. Use of COCP is a safe and effective option for women who suffer from acne. The antiandrogen effect of COCP is through the actions of estrogen, by stimulating the hepatic synthesis of SHBG, which binds androgens and decreases levels of free testosterone, with dehydroepiandrosterone sulfate. Estrogen also inhibits 5-alpha reductase, which prevents the conversion of testosterone to the more potent dihydrotestosterone. Estrogen through its negative feedback action on pituitary reduces luteinizing hormone (LH), consequently reducing the synthesis of ovarian and adrenal androgen.[99] Progestins may also contribute to the antiandrogenic properties of COCP by the reduction of gonadotropin-releasing hormone (GnRH) pulsatility and consequently LH production.[100]
COCP with third-generation progestins (e.g., norgestimate or desogestrel) or later generations (e.g., fourth or fifth-generation progestin-containing OCP formulations) are preferred because they have a lower androgenic activity as compared to first and second-generation COCP. Currently, four COCP are approved by the Food and Drug Administration (FDA) for the treatment of acne. They are EE/norgestimate, EE/norethindrone acetate/ferrous fumarate, EE/drospirenone, and EE/drospirenone/levomefolate.[31]
Several RCTs have assessed the efficacy of COCP in the management of acne.[101,102,103,104,105,106,107,108,109,110] A 2012 Cochrane meta-analysis assessed the effect of OCPs on acne in women and included 31 trials with a total of 12,579 women. Nine trials compared a COCP to placebo and the former reduced acne in almost all women. The progestins included in these nine trials were levonorgestrel, norethindrone acetate, norgestimate, drospirenone, dienogest, cyproterone acetate or chlormadinone acetate. Seventeen trials compared two different combinations of COCP but found no statistical difference between the two. Only one small study compared a COCP to an oral antibiotic, which found no significant difference.[100] A nonsystematic narrative review based on a literature search of the PubMed database showed high efficacy of CPA 2 mg/EE 35 μg in the treatment of severe acne and hirsutism.[111]
An Indian study by Bhattacharya et al. in the Indian population concluded that combination of EE and drospirenone (DRSP) is a good alternative to combination of EE and cyproterone acetate and has similar efficacy without affecting the insulin resistance.[112]
Recommendations

For hirsutism
Summary of evidence
Hirsutism is a clinical sign and is not a disease by itself. Its presence does not necessarily require treatment. However, as hirsutism can have great effect on the psychological well-being of women, treatment becomes necessary. Apart from plucking, shaving, waxing, electrolysis, and laser, OCPs are the first-line treatment for hirsutism, particularly in those women desiring contraception. Antiandrogens can be used for the treatment of hirsutism, but this can be associated with irregular uterine bleeding. When antiandrogens are combined with EE it not only regularizes the menstrual cycle but also provides endometrial protection and improves hirsutism and/or acne by reducing the production of ovarian androgens.[113,114]
Estrogen/progesterone combinations act by:
Reducing gonadotropin secretion and thereby reducing ovarian androgen production[115]
Increasing levels of SHBG resulting in lower levels of free testosterone[116]
Inhibiting adrenal androgen production.[116]
Cyproterone acetate (CPA) has strong progestogenic and antiandrogenic properties and decreases circulating testosterone and androstenedione levels through a reduction in circulating LH and has been used as an effective treatment for hirsutism.[117] CPA is available in combination with EE (2 mg CPA and 35 μg EE/tablet).
Medical treatment for hirsutism is never curative and therefore should be administered for long term. The PCOS adolescent and women must be counseled that the effect of medical treatment is evident only after several months and therefore requires monitoring by an expert while on treatment.[118,119] When OCPs and placebos were compared in two RCTs, OCPs showed a definite benefit with reduction in the hirsutism scores, although the evidence supporting this recommendation is of very low quality.[120,121] When COCP containing low-dose antiandrogen (CPA 2 mg) were compared with finasteride an antiandrogen, both had similar effects on the hirsutism score at the end of 9 months.[122]
Spironolactone 100 mg was more effective than COCP containing cyproterone acetate (2 mg/day) in combination with EE (35 μg/day). Moreover, cyproterone acetate may be associated with adrenal insufficiency and loss of libido.[117]
The use of OCPs containing recent generation low androgenicity progestins such as desogestrel and gestodene and androgen receptor antagonists such as CPA and drospirenone (DSP) may confer a 50%–100% increased risk of VTE compared with OCs containing the second-generation progestin levonorgestrel. It is also the best to use the lowest effective dose of EE (20 μg/day) to reduce the complications. However, one must remember that PCOS itself may represent an additional independent risk factor for VTE.[123,124]
Most general population guidelines do not recommend the use of OCPs with EE and cyproterone acetate (CPA) as the first-line treatment in adults and adolescents with PCOS. However, in the Indian context, OCPs containing an antiandrogen, such as cyproterone acetate, or the spironolactone derivative drospirenone rather than COCP containing other progestins may be used for the treatment of hirsutism.[125]
The use of OCPs results in a subjective improvement of symptoms in about 60%–100% of PCOS women.[126] An Indian study by Bobde et al. compared COCP containing EE 20 μg + drospirenone 3 mg with a combination of COCP with metformin and metformin alone. They found maximum improvement in hirsutism in the combination group followed by OCPs and least in the metformin group. Since the sample size was very small, the quality of evidence is of very low quality.[127] This was also demonstrated in two other publications.[128,129]
Recommendations
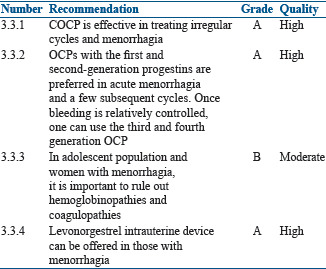
For menstrual irregularity and menorrhagia
Summary of evidence
PCOS is associated with menstrual irregularity and menorrhagia due to anovulation both in the adolescent girls and in women in their reproductive age. In the adolescent, a firm diagnosis of PCOS as a cause of menstrual irregularity and menorrhagia can be done only after 2 years of menarche.[130,131,132] OCPs with antiandrogenic properties can control menstrual irregularities and menorrhagia. OCPs with first and second generation progestins are preferred in acute menorrhagia for a few cycles. Once bleeding is relatively controlled, one can use the third and fourth generation OCPs.[133,134]
Rathod et al. reported menstrual problems in 84.88% of adolescent population who had either menstrual irregularity, menorrhagia, or dysmenorrhea.[135] As menorrhagia may be an important clinical manifestation in inherited bleeding disorders, it needs to be evaluated before embarking on the use of COCP.[136]
ESHRE/ASRM-sponsored PCOS consensus group recommendations[137] and The Endocrine Society's clinical practice guidelines[138] suggest OCPs as the first-line management for amelioration of clinical and biochemical androgen excess and menstrual irregularity. There is no difference in the efficacy with the use of various combination pills.[137,138]
Recommendations
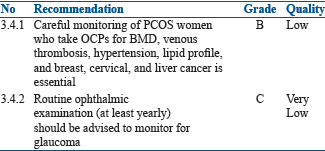
Oral contraceptive pills for long-term therapy in polycystic ovarian syndrome
Summary of evidence
The two main concerns when COCPs were used in PCOS women are decrease in bone density and fear of VTE. A positive linear relationship was also found between duration of OC use and risk of hypertension. The risk of hypertension increased by 13% for every 5-year increment in OC use.[139] The estrogen content also has a dose dependent effect on lipid profiles.[140] Moreover, OCPs use is also linked to cervical, breast, and liver cancers.[141] Long-term use of OCPs has also been associated with increased risk of glaucoma, especially when used for 3 years or more.[142,143]
Recommendations
During infertility management
Summary of evidence
Actions of COCP on reproductive function in PCOS women[144] are shown in Table 4.
Table 4.
Actions of combined oral contraceptives in PCOS
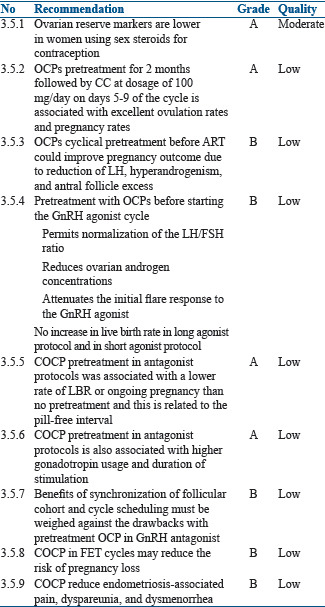
During treatment with hormonal contraceptives, serum concentrations of follicle-stimulating hormone (FSH), LH, and estradiol decrease.[145,146] Thus, COCP is associated with suppression of ovarian and endometrial function.[147,148,149,150] Markers of ovarian reserve are lower in women using sex steroids for contraception. When compared with non-users: anti-Müllerian hormone (AMH) 19% lower (95% CI 9.1%–29.3%); antral follicle count (AFC) 18% lower (95% CI 11.2%–24.8%); ovarian volume 50% lower (95% CI 45.1%–53.7%).[151,152,153] Thus, there is a risk of falsely identifying a low ovarian reserve in OCP users. Ovarian reserve, therefore, should be assessed with caution immediately after stopping the OCP. The AMH and AFC should ideally be tested only 3–6 months after stopping the pill.[153,154]
There is also a reduction of the endometrial thickness, and the pinopod expression is delayed in women using OCP as compared to normally cycling women or those using clomiphene citrate (CC).[155,156,157] When given for a minimum number of days, and if controlled ovarian stimulation (COS) is started after a washout period, OCP pretreatment might not have a negative effect on endometrial receptivity.[158]
The effect of OCP pretreatment on assisted reproductive technique (ART) outcome depends on the type and protocol of OCP used and washout period before initiation of ovarian stimulation.[159,160] No differences were found in ongoing pregnancy rate in GnRH agonist long protocol when pretreated with OCP.[161] Although initial publications did not show any negative impact on the ART outcome in GnRH antagonist cycles, recent publications and meta analyses suggest the contrary.[162] There were two studies which showed a beneficial effect of OCPs on the ART outcome in the GnRH antagonist cycle,[163,164] but later studies did not show any beneficial effect of pretreatment with OCPs in the ART cycles using GnRH antagonist.[165,166,167]
Outcome of ART after OCP pretreatment depends on pill-free interval and duration of pill administration. It is the pill-free interval that strongly influences gonadotropin recovery. After stopping OCPs, both pituitary and ovarian activity will recover. The LH levels remain low for a longer time (21–28 days),[168] as compared to FSH, which recovers in 5–7 days after stopping the OCP.[169,170,171] If the estrogen content of the OCP is <30 μg, there could be an earlier rise in FSH levels, which can have an effect on the COS. The progestin concentrations also become low 5–7 days after discontinuation of OCPs. Thus, a 5-day washout period after OCP is optimal before the initiation of COS.[170] Duration of pill administration may also have an impact on the reproductive hormones and endometrium.[169] More than 16 days of pill administration results in greater suppression of pituitary–ovarian axis, which is then associated with a longer duration and higher consumption of gonadotropins.[162,165] There was no difference in the ART outcome noted if the pills were administered only for 12–16 days and there was a washout period of 5 days.
OCP pretreatment before COS could also be successfully applied to schedule patients and for synchronization of follicular cohort, although the dose of gonadotropins required is higher and also associated with extra 1–2 days of stimulation.[172]
It was also reported that OCP pretreatment for 2 months followed by CC, at dosage of 100 mg/day on days 5–9 of the cycle, is associated with excellent ovulation rates and pregnancy rates.[173,176]
In PCOS women with OC induced menses in an antagonist protocol cycle resulted in a significantly lower clinical pregnancy rate and live birth rates as compared to spontaneous menses or progestin-induced menses. In an antagonist cycle where freeze-all policy was adopted and FET (Frozen Embryo Transfer) done where OCP was used to induce menstrual bleeding resulted in significantly higher rates of conception, clinical pregnancy, and live birth compared with fresh embryo transfer.[156]
The choice of COCP for the treatment of endometriosis as compared to other medical treatments such as progestogens (Level A), antiprogestogens (Level A), or GnRH agonists (Level A) depends on patient preferences, side effects, efficacy, costs, and availability. All these points should be taken into consideration when choosing hormonal treatment for endometriosis-associated pain.[174,175,176,177]
Despite limited evidence of effectiveness, COCP are widely used in women with endometriosis for the treatment of pain. COCP offer some practical advantages such as contraceptive protection, long-term safety, and control of menstrual cycle.[178]
Recommendations
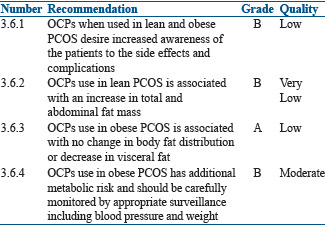
Use of oral contraceptive pills in lean and obese women
Summary of evidence
The prevalence of obesity is higher in PCOS women with increased visceral adiposity.[179,180] There is insufficient evidence to support a causal relationship between use of OCPs and weight gain.[181] In lean adolescents and adults, OCPs treatment is associated with an increase in total and abdominal fat mass,[182,183,184] despite significant decreases in serum androgens, suggesting other underlying mechanisms for potential increase in fat mass. On the contrary, no change in body fat distribution was observed in obese PCOS women after 12 months OCP use.[185]
In the OWL-PCOS study, treatment of overweight/obese PCOS women with OCPs for 4 months resulted in a significant decrease in visceral fat distribution.[186]
It is also reported that PCOS women showed 96% higher circulating C-reactive protein (CRP) levels as compared to controls.[187] In lean PCOS, slight increase or no change in CRP levels was seen after 6 months of OCP treatment.[188,189,190] No change was noted in obese PCOS.
The effects of OCPs on adiponectin levels (which has insulin-sensitizing, antiatherogenic, and anti-inflammatory properties) in women with PCOS is still unclear. Some studies report no change in adiponectin levels using a third-generation OCP for 6–12 months,[185,191] while others report an increase in serum adiponectin levels after 6 months of treatment in obese women.[187] Increase in inflammatory gene expression in subcutaneous adipose tissue biopsies was seen in adolescents, after treatment with OCPs.[188]
It is also important to consider the impact of OCPs on adipose tissue distribution and function in lean and obese PCOS women. The evidence is very low as all studies included small numbers of PCOS women. OCPs, when used in obese PCOS women, have increased negative metabolic effects on triglyceride and total cholesterol levels and also aggravate insulin resistance.[141] The use of OCPs in obese women especially with BMI of >30 kg/m2 will considerably increase the risk of CVD. Therefore, all obese women on OCPs should be carefully monitored. OCPs should not be used in obese women with BMI >30 kg/m2 associated with other risk factors for VTE.[192]
Recommendations
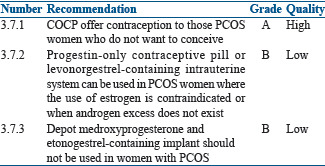
Oral contraceptive pills for added benefit of contraception
Summary of evidence
OCPs, apart from the other benefits also, offer contraception to those PCOS women who do not want to conceive. The data available on use of hormonal contraceptives other than OCPs in PCOS women are sparse; progestin only contraceptives pill or levonorgestrel containing intrauterine system can be used in PCOS women where use of estrogen is contraindicated or when androgen excess does not exist.
Depot medroxyprogesterone and etonogestrel containing implant should not be used in women with PCOS as they are associated with weight gain, insulin resistance, and worsening metabolic profile. The former is also associated with decreased BMD.[193]
Recommendations
For prevention of Cancer in Polycystic Ovarian Syndrome
Summary of evidence
Most PCOS women have anovulatory cycles with disruption of normal reproductive physiology, which increases the risk of the development of cancer of the endometrium, ovary, and/or breast, either directly or mediated by its associated reproductive metabolic alterations.[194,195,196,197] Women with PCOS have a 2.7-fold (95% CI, 1.0–7.3) increased risk for endometrial cancer, which is well differentiated and has a good prognosis. There are limited data on increased risk of ovarian and breast cancer in PCOS women. Cancer risk with PCOS is difficult to separate from other recognized risk factors such as nulliparity, infertility and its treatment, anovulation, and obesity.[134]
Use of OCPs in PCOS women showed a protective trend against endometrial cancer. In endometrial cancer, the estimated relative risk decrease is ∼50% with 4 years of use, ∼70% with 12 years of use, and decreasing with further use. After ceasing oral contraception, the risk begins to rise from its reduced levels, but it is still ∼50% even after >20 years after its last use.
Relative risk of ovarian cancer decreases by ∼20% for each 5 years of OCPs use. Risk is ∼50% for 15 years of use and decreasing with further use. The protective effect gained declines as time passes from its last use, but a significant effect remains for a long time after cessation of OCP use. OCPs do not protect from mucinous types of ovarian tumors.[198,199]
Linear coefficient exists for increased protective effects of OCPs, and duration of its use and is independent of type of formulation.
Recommendations

Use of other drugs with oral contraceptive pills in Polycystic Ovarian Syndrome
Summary of evidence
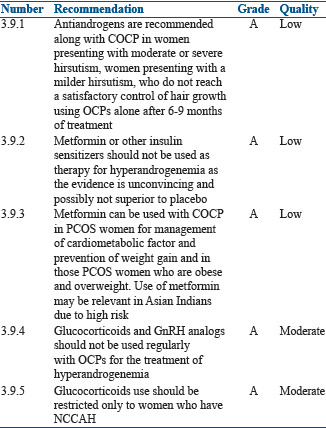
Anti-androgens
In PCOS women with moderate or severe hirsutism and acne not responding to COCP, one can add antiandrogens (androgen receptor blockers and 5 alpha-reductase inhibitors).[200]
Several RCTs have shown that flutamide 250 mg/day, finasteride 5 mg/day, or spironolactone 100 mg/day are effective in reducing the signs of hyperandrogenemia not responding to COCP of third and fourth generations.[201,202,203,204,205]
In three double-blind studies, combination of an OC containing 2 mg of cyproterone acetate and either spironolactone 100 mg or finasteride 5 mg had better results in the treatment of hirsutism than OC alone.[206,207,122]
At times, in PCOS women especially adolescent population, when antiandrogens such as cyproterone acetate and spironolactone alone are given, there may be menstrual disturbances or even amenorrhea because of their strong progestin effects.[207,122] This is the group which will benefit with combination of COCP and antiandrogens.[207,122] Antiandrogens can also be combined with COCP when the women need contraception.[208]
Insulin sensitizers
Insulin sensitizers improve insulin resistance and menstrual dysfunction and may also decrease serum androgen levels.[209] The main insulin sensitizer is metformin and others include pioglitazone and saroglitazar. However, data on these agents are still emerging but may be valuable adjuncts in individualized situations.
When metformin is used alone, it is not effective in reducing the signs and symptoms of hyperandrogenemia compared to COCP and antiandrogens.[36,210,211,212,213,214,215,216] While the literature on use of metformin plus OCP versus OCP alone is limited, metformin may lower risk and offset OCP exacerbated risk factors. Use of OCP plus flutamide and/or metformin reduced abnormal adipocytokine levels and adiposity, which are aggravated in women using OCPs alone increasing the risk of metabolic syndrome.[185] Hoeger et al. compared OCP plus placebo versus OCP plus metformin and found beneficial effect on waist circumference and higher high-density lipoprotein cholesterol when OCPs were used with metformin.[216] There was another study, which looked at the levels of adhesion molecules, vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, cyproterone containing OCPs with or without metformin and found significant reduction when metformin was used.[217] Metformin can be a valuable adjunct to OCPs in the treatment of Asian Indian PCOS women.
At present, there is either no evidence or it is sparse for the use of Myo-inositol or other inositols either alone or in combination with OCPs in women with PCOS. In the future, publications may become available on the use of Myo-inositol either alone or in combination with OCPs in PCOS women.
Glucocorticoids
There is no evidence for the use of glucocorticoids in women with adrenal[218] or unselected hyperandrogenism[219] and in women with idiopathic hirsutism[220] as compared to OCPs or antiandrogens. Glucocorticoids with OCPs are beneficial in women with nonclassical congenital adrenal hyperplasia (NCCAH) for prolonged remission.[221]
Use of glucocorticoids can be associated with side effects, such as adrenal atrophy, increased blood pressure, weight gain, Cushing's striae (particularly with dexamethasone), and decreased BMD. Therefore, the use of glucocorticoids along with OCPs should be restricted only to women who have NCCAH.[222]
Gonadotropin-releasing hormone analogs
Addition of a GnRH agonist to an OCP did not result in extra reduction in hirsutism scores when compared to OCP alone in two uncontrolled trials.[223,224] One randomized, placebo controlled trial concluded that there was greater reduction in chin hair diameter, but not in Ferriman–Gallwey score with combined GnRH agonist therapy and OCP therapy, as compared to OCP treatment alone.[225]
GnRH analogs can be used in selected patients with severe hyperandrogenism of ovarian origin who do not respond to other drugs as they reduce ovarian steroids including androgens. However, as GnRH analogs induce reversible menopause, it is usually used with OCPs to prevent menopausal symptoms.[226]
Recommendations
CONCLUSIONS ON USE OF ORAL CONTRACEPTIVE PILLS IN POLYCYSTIC OVARIAN SYNDROME
In PCOS women who do not desire fertility, OCPs are the first-line treatment to improve clinical symptoms of androgen excess such as acne and hirsutism and also help in regulating the menstrual cycles and protecting the endometrium against effects of unopposed estrogen action. Estrogen content in the OCP increases SHBG levels, thus decreasing the free circulating androgens. The progestin component of OCP suppresses the secretion of LH and decreases ovarian androgen production. It also competes for 5 alpha-reductase and the androgen receptor, with concomitant decrease in androgen action. OCPs may also reduce adrenal production of androgens. Menstrual irregularity gets corrected immediately, but acne and hirsutism may take a minimum of 6 months for its effects to be seen.
Low-dose COCP containing neutral or antiandrogenic progestins may be the OCPs of choice in the treatment of PCOS. Despite the potential adverse cardiovascular and metabolic effects of OCPs, the current evidence suggests that the benefits outweigh the risks for its use in most PCOS women. Use of OCPs in PCOS women should be individualized after risk stratification and not used if any contraindications exist.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge the guidance provided by the members of the working group in formulating the 'Consensus Statement on the Use of OCPs in PCOS'. We also acknowledge the immense personal assistance by Prof. Helena Teede, President of The Androgen Excess and Polycystic Ovary Syndrome Society, who guided us in the final preparation of this statement.
We thank Cipla Limited for their logistic support during the preparation of the Consensus Statement.
REFERENCES
- 1.De Leo V, Fruzzetti F, Musacchio MC, Scolaro V, Di Sabatino A, Morgante G, et al. Effect of a new oral contraceptive with estradiol valerate/dienogest on carbohydrate metabolism. Contraception. 2013;88:364–8. doi: 10.1016/j.contraception.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Christin-Maitre S. History of oral contraceptive drugs and their use worldwide. Best Pract Res Clin Endocrinol Metab. 2013;27:3–12. doi: 10.1016/j.beem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Poindexter A. The emerging use of the 20-μg oral contraceptive. Fertil Steril. 2001;75:457–65. doi: 10.1016/s0015-0282(00)01747-7. [DOI] [PubMed] [Google Scholar]
- 4.Hoy SM, Scott LJ. Estradiol valerate/dienogest: In oral contraception. Drugs. 2009;69:1635–46. doi: 10.2165/11202820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan B. Desogestrel, norgestimate, and gestodene: The newer progestins. Ann Pharmacother. 1995;29:736–42. doi: 10.1177/106002809502907-817. [DOI] [PubMed] [Google Scholar]
- 6.Cibula D, Skrenkova J, Hill M, Stepan JJ. Low-dose estrogen combined oral contraceptives may negatively influence physiological bone mineral density acquisition during adolescence. Eur J Endocrinol. 2012;166:1003–11. doi: 10.1530/EJE-11-1047. [DOI] [PubMed] [Google Scholar]
- 7.Gai L, Jia Y, Zhang M, Gai P, Wang S, Shi H, et al. Effect of two kinds of different combined oral contraceptives use on bone mineral density in adolescent women. Contraception. 2012;86:332–6. doi: 10.1016/j.contraception.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Sørdal T, Grob P, Verhoeven C. Effects on bone mineral density of a monophasic combined oral contraceptive containing nomegestrol acetate/17β-estradiol in comparison to levonorgestrel/ethinylestradiol. Acta Obstet Gynecol Scand. 2012;91:1279–85. doi: 10.1111/j.1600-0412.2012.01498.x. [DOI] [PubMed] [Google Scholar]
- 9.Oral contraceptives, venous thrombosis, and varicose veins. Royal College of General Practitioners' Oral Contraception Study. J R Coll Gen Pract. 1978;28:393–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Roach RE, Lijfering WM, van Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR, Cannegieter SC, et al. The risk of venous thrombosis in individuals with a history of superficial vein thrombosis and acquired venous thrombotic risk factors. Blood. 2013;122:4264–9. doi: 10.1182/blood-2013-07-518159. [DOI] [PubMed] [Google Scholar]
- 11.Tanis BC, van den Bosch MA, Kemmeren JM, Cats VM, Helmerhorst FM, Algra A, et al. Oral contraceptives and the risk of myocardial infarction. N Engl J Med. 2001;345:1787–93. doi: 10.1056/NEJMoa003216. [DOI] [PubMed] [Google Scholar]
- 12.Gronich N, Lavi I, Rennert G. Higher risk of venous thrombosis associated with drospirenone-containing oral contraceptives: A population-based cohort study. CMAJ. 2011;183:E1319–25. doi: 10.1503/cmaj.110463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Runnebaum B, Grunwald K, Rabe T. The efficacy and tolerability of norgestimate/ethinyl estradiol (250 micrograms of norgestimate/35 micrograms of ethinyl estradiol): Results of an open, multicenter study of 59,701 women. Am J Obstet Gynecol. 1992;166:1963–8. doi: 10.1016/0002-9378(92)91396-r. [DOI] [PubMed] [Google Scholar]
- 14.Back DJ, Bates M, Bowden A, Breckenridge AM, Hall MJ, Jones H, et al. The interaction of phenobarbital and other anticonvulsants with oral contraceptive steroid therapy. Contraception. 1980;22:495–503. doi: 10.1016/0010-7824(80)90102-x. [DOI] [PubMed] [Google Scholar]
- 15.Back DJ, Breckenridge AM, Crawford FE, Hall JM, MacIver M, Orme ML, et al. The effect of rifampicin on the pharmacokinetics of ethynylestradiol in women. Contraception. 1980;21:135–43. doi: 10.1016/0010-7824(80)90125-0. [DOI] [PubMed] [Google Scholar]
- 16.Hebbar S. Epilepsy and oral hormonal contraception-Indian perspective. Int J Pharm Pharm Sci. 2017;9:1–6. [Google Scholar]
- 17.Bhat M, Ramesha KN, Nirmala C, Sarma PS, Thomas SV. Knowledge and practice profile of obstetricians regarding epilepsy in women in Kerala state, India. Ann Indian Acad Neurol. 2011;14:169–71. doi: 10.4103/0972-2327.85877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Back DJ, Breckenbridge AM, Cross KJ, Orme ML, Thomas E. An antibiotic interaction with ethinyloestradiol in the rat and rabbit. J Steroid Biochem. 1982;16:407–13. doi: 10.1016/0022-4731(82)90053-x. [DOI] [PubMed] [Google Scholar]
- 19.Joshi JV, Joshi UM, Sankholi GM, Krishna U, Mandlekar A, Chowdhury V, et al. A study of interaction of low-dose combination oral contraceptive with ampicillin and metronidazole. Contraception. 1980;22:643–52. doi: 10.1016/0010-7824(80)90089-x. [DOI] [PubMed] [Google Scholar]
- 20.Back DJ, Breckenridge AM, MacIver M, Orme ML, Purba H, Rowe PH, et al. Interaction of ethinyloestradiol with ascorbic acid in man. Br Med J (Clin Res Ed) 1981;282:1516. doi: 10.1136/bmj.282.6275.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abernethy DR, Greenblatt DJ, Divoll M, Arendt R, Ochs HR, Shader RI, et al. Impairment of diazepam metabolism by low-dose estrogen-containing oral-contraceptive steroids. N Engl J Med. 1982;306:791–2. doi: 10.1056/NEJM198204013061307. [DOI] [PubMed] [Google Scholar]
- 22.Abernethy DR, Todd EL. Impairment of caffeine clearance by chronic use of low-dose oestrogen-containing oral contraceptives. Eur J Clin Pharmacol. 1985;28:425–8. doi: 10.1007/BF00544361. [DOI] [PubMed] [Google Scholar]
- 23.Hendrix-Treacy S, Wallace SM, Hindmarsh KW, Wyant GM, Danilkewich A. The effect of acetaminophen administration on its disposition and body stores of sulphate. Eur J Clin Pharmacol. 1986;30:273–8. doi: 10.1007/BF00541527. [DOI] [PubMed] [Google Scholar]
- 24.Miners JO, Grgurinovich N, Whitehead AG, Robson RA, Birkett DJ. Influence of gender and oral contraceptive steroids on the metabolism of salicylic acid and acetylsalicylic acid. Br J Clin Pharmacol. 1986;22:135–42. doi: 10.1111/j.1365-2125.1986.tb05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miners JO, Robson RA, Birkett DJ. Gender and oral contraceptive steroids as determinants of drug glucuronidation: Effects on clofibric acid elimination. Br J Clin Pharmacol. 1984;18:240–3. doi: 10.1111/j.1365-2125.1984.tb02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadam P, Dherai A, Naik P, Lokhande R, Udani V, Gursahani R, et al. Retrospective study on therapeutic drug monitoring of lamotrigine in Indian epileptic patients. Int J Pharm Pharm Sci. 2014;6:430–3. [Google Scholar]
- 27.Sevinsky H, Eley T, Persson A, Garner D, Yones C, Nettles R, et al. The effect of efavirenz on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and norgestimate in healthy HIV-negative women. Antivir Ther. 2011;16:149–56. doi: 10.3851/IMP1725. [DOI] [PubMed] [Google Scholar]
- 28.Vogler MA, Patterson K, Kamemoto L, Park JG, Watts H, Aweeka F, et al. Contraceptive efficacy of oral and transdermal hormones when co-administered with protease inhibitors in HIV-1-infected women: Pharmacokinetic results of ACTG trial A5188. J Acquir Immune Defic Syndr. 2010;55:473–82. doi: 10.1097/QAI.0b013e3181eb5ff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Chung E, Yones C, Persson A, Mahnke L, Eley T, et al. The effect of atazanavir/ritonavir on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and norgestimate in healthy women. Antivir Ther. 2011;16:157–64. doi: 10.3851/IMP1724. [DOI] [PubMed] [Google Scholar]
- 30.Food and Drug Administration. Stribild (package insert) 2016. [Last accessed on 2016 May 16]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203100s000lbl.pdf .
- 31.Food and Drug Administration. Genvoya (package insert). Gilead. [Last accessed on 2016 May 16]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207561s000lbl.pdf .
- 32.Schöller-Gyüre M, Kakuda TN, Woodfall B, Aharchi F, Peeters M, Vandermeulen K, et al. Effect of steady-state etravirine on the pharmacokinetics and pharmacodynamics of ethinylestradiol and norethindrone. Contraception. 2009;80:44–52. doi: 10.1016/j.contraception.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Crauwels HM, van Heeswijk RP, Buelens A, Stevens M, Hoetelmans RM. Lack of an effect of rilpivirine on the pharmacokinetics of ethinylestradiol and norethindrone in healthy volunteers. Int J Clin Pharmacol Ther. 2014;52:118–28. doi: 10.5414/CP201943. [DOI] [PubMed] [Google Scholar]
- 34.Stuart GS, Moses A, Corbett A, Phiri G, Kumwenda W, Mkandawire N, et al. Combined oral contraceptives and antiretroviral PK/PD in Malawian women: Pharmacokinetics and pharmacodynamics of a combined oral contraceptive and a generic combined formulation antiretroviral in Malawi. J Acquir Immune Defic Syndr. 2011;58:e40–3. doi: 10.1097/QAI.0b013e31822b8bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriplani A, Periyasamy AJ, Agarwal N, Kulshrestha V, Kumar A, Ammini AC, et al. Effect of oral contraceptive containing ethinyl estradiol combined with drospirenone vs. desogestrel on clinical and biochemical parameters in patients with polycystic ovary syndrome. Contraception. 2010;82:139–46. doi: 10.1016/j.contraception.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Luque-Ramírez M, Alvarez-Blasco F, Botella-Carretero JI, Martínez-Bermejo E, Lasunción MA, Escobar-Morreale HF, et al. Comparison of ethinyl-estradiol plus cyproterone acetate versus metformin effects on classic metabolic cardiovascular risk factors in women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2453–61. doi: 10.1210/jc.2007-0282. [DOI] [PubMed] [Google Scholar]
- 37.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018 doi: 10.1016/j.fertnstert.2018.05.004. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luque-Ramírez M, Mendieta-Azcona C, del Rey Sánchez JM, Matíes M, Escobar-Morreale HF. Effects of an antiandrogenic oral contraceptive pill compared with metformin on blood coagulation tests and endothelial function in women with the polycystic ovary syndrome: Influence of obesity and smoking. Eur J Endocrinol. 2009;160:469–80. doi: 10.1530/EJE-08-0725. [DOI] [PubMed] [Google Scholar]
- 39.Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: Case-control study using United States claims data. BMJ. 2011;342:d2151. doi: 10.1136/bmj.d2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parkin L, Sharples K, Hernandez RK, Jick SS. Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: Nested case-control study based on UK general practice research database. BMJ. 2011;342:d2139. doi: 10.1136/bmj.d2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Junkins-Hopkins JM. Hormone therapy for acne. J Am Acad Dermatol. 2010;62:486–8. doi: 10.1016/j.jaad.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Stewart FH, Harper CC, Ellertson CE, Grimes DA, Sawaya GF, Trussell J, et al. Clinical breast and pelvic examination requirements for hormonal contraception: Current practice vs. evidence. JAMA. 2001;285:2232–9. doi: 10.1001/jama.285.17.2232. [DOI] [PubMed] [Google Scholar]
- 43.Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: Update on women's health screening guidelines. J Am Acad Dermatol. 2008;58:781–6. doi: 10.1016/j.jaad.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 44.Lloyd T, Rollings N, Andon MB, Demers LM, Eggli DF, Kieselhorst K, et al. Determinants of bone density in young women. I. Relationships among pubertal development, total body bone mass, and total body bone density in premenarchal females. J Clin Endocrinol Metab. 1992;75:383–7. doi: 10.1210/jcem.75.2.1639940. [DOI] [PubMed] [Google Scholar]
- 45.Cromer BA, Bonny AE, Stager M, Lazebnik R, Rome E, Ziegler J, et al. Bone mineral density in adolescent females using injectable or oral contraceptives: A 24-month prospective study. Fertil Steril. 2008;90:2060–7. doi: 10.1016/j.fertnstert.2007.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lloyd T, Petit MA, Lin HM, Beck TJ. Lifestyle factors and the development of bone mass and bone strength in young women. J Pediatr. 2004;144:776–82. doi: 10.1016/j.jpeds.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 47.Peragallo Urrutia R, Coeytaux RR, McBroom AJ, Gierisch JM, Havrilesky LJ, Moorman PG, et al. Risk of acute thromboembolic events with oral contraceptive use: A systematic review and meta-analysis. Obstet Gynecol. 2013;122:380–9. doi: 10.1097/AOG.0b013e3182994c43. [DOI] [PubMed] [Google Scholar]
- 48.George R, Clarke S, Thiboutot D. Hormonal therapy for acne. Semin Cutan Med Surg. 2008;27:188–96. doi: 10.1016/j.sder.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 49.The American College of Obstetricians and Gynecologists. Committee opinion 540. Risk of Venous Thromboembolism among Users of Drospirenone-Containing Oral Contraceptive Pills. [Last accessed on 2018 May 10]. Available from: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Risk-of-Venous-Thromboembolism .
- 50.Dinger J, Möhner S, Heinemann K. Cardiovascular risks associated with the use of drospirenone-containing combined oral contraceptives. Contraception. 2016;93:378–85. doi: 10.1016/j.contraception.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Venous thromboembolic disease and combined oral contraceptives: Results of international multicentre case-control study. World Health Organization collaborative study of cardiovascular disease and steroid hormone contraception. Lancet. 1995;346:1575–82. [PubMed] [Google Scholar]
- 52.Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP, Jr, et al. Venous thromboembolic disease in users of low-estrogen combined estrogen-progestin oral contraceptives. Contraception. 2004;70:3–10. doi: 10.1016/j.contraception.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 53.McNicholas C, Zhao Q, Secura G, Allsworth JE, Madden T, Peipert JF, et al. Contraceptive failures in overweight and obese combined hormonal contraceptive users. Obstet Gynecol. 2013;121:585–92. doi: 10.1097/AOG.0b013e31828317cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in very young women using combined oral contraceptives. Contraception. 2008;78:358–64. doi: 10.1016/j.contraception.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Vestergaard P, Rejnmark L, Mosekilde L. Oral contraceptive use and risk of fractures. Contraception. 2006;73:571–6. doi: 10.1016/j.contraception.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Vessey M, Mant J, Painter R. Oral contraception and other factors in relation to hospital referral for fracture. Findings in a large cohort study. Contraception. 1998;57:231–5. doi: 10.1016/s0010-7824(98)00026-2. [DOI] [PubMed] [Google Scholar]
- 57.Michaëlsson K, Baron JA, Farahmand BY, Persson I, Ljunghall S. Oral-contraceptive use and risk of hip fracture: A case-control study. Lancet. 1999;353:1481–4. doi: 10.1016/S0140-6736(98)09044-8. [DOI] [PubMed] [Google Scholar]
- 58.Memon S, Iversen L, Hannaford PC. Is the oral contraceptive pill associated with fracture in later life? New evidence from the Royal College of General Practitioners Oral Contraception Study. Contraception. 2011;84:40–7. doi: 10.1016/j.contraception.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 59.Meier C, Brauchli YB, Jick SS, Kraenzlin ME, Meier CR. Use of depot medroxyprogesterone acetate and fracture risk. J Clin Endocrinol Metab. 2010;95:4909–16. doi: 10.1210/jc.2010-0032. [DOI] [PubMed] [Google Scholar]
- 60.La Vecchia C, Tavani A, Gallus S. Oral contraceptives and risk of hip fractures. Lancet. 1999;354:335–6. doi: 10.1016/S0140-6736(05)75239-9. [DOI] [PubMed] [Google Scholar]
- 61.Cooper C, Hannaford P, Croft P, Kay CR. Oral contraceptive pill use and fractures in women: A prospective study. Bone. 1993;14:41–5. doi: 10.1016/8756-3282(93)90254-8. [DOI] [PubMed] [Google Scholar]
- 62.Barad D, Kooperberg C, Wactawski-Wende J, Liu J, Hendrix SL, Watts NB, et al. Prior oral contraception and postmenopausal fracture: A women's health initiative observational cohort study. Fertil Steril. 2005;84:374–83. doi: 10.1016/j.fertnstert.2005.01.132. [DOI] [PubMed] [Google Scholar]
- 63.Michaëlsson K, Baron JA, Farahmand BY, Ljunghall S. Influence of parity and lactation on hip fracture risk. Am J Epidemiol. 2001;153:1166–72. doi: 10.1093/aje/153.12.1166. [DOI] [PubMed] [Google Scholar]
- 64.O'Neill TW, Marsden D, Adams JE, Silman AJ. Risk factors, falls, and fracture of the distal forearm in Manchester, UK. J Epidemiol Community Health. 1996;50:288–92. doi: 10.1136/jech.50.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mallmin H, Ljunghall S, Persson I, Bergström R. Risk factors for fractures of the distal forearm: A population-based case-control study. Osteoporos Int. 1994;4:298–304. doi: 10.1007/BF01622186. [DOI] [PubMed] [Google Scholar]
- 66.Berenson AB, Radecki CM, Grady JJ, Rickert VI, Thomas A. A prospective, controlled study of the effects of hormonal contraception on bone mineral density. Obstet Gynecol. 2001;98:576–82. doi: 10.1016/s0029-7844(01)01495-8. [DOI] [PubMed] [Google Scholar]
- 67.Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008;112:788–99. doi: 10.1097/AOG.0b013e3181875b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burr DB, Yoshikawa T, Teegarden D, Lyle R, McCabe G, McCabe LD, et al. Exercise and oral contraceptive use suppress the normal age-related increase in bone mass and strength of the femoral neck in women 18-31 years of age. Bone. 2000;27:855–63. doi: 10.1016/s8756-3282(00)00403-8. [DOI] [PubMed] [Google Scholar]
- 69.Cobb KL, Kelsey JL, Sidney S, Ettinger B, Lewis CE. Oral contraceptives and bone mineral density in white and black women in CARDIA. Coronary risk development in young adults. Osteoporos Int. 2002;13:893–900. doi: 10.1007/s001980200123. [DOI] [PubMed] [Google Scholar]
- 70.Elgán C, Dykes AK, Samsioe G. Bone mineral density changes in young women: A two year study. Gynecol Endocrinol. 2004;19:169–77. doi: 10.1080/09513590400012119. [DOI] [PubMed] [Google Scholar]
- 71.Elgán C, Samsioe G, Dykes AK. Influence of smoking and oral contraceptives on bone mineral density and bone remodeling in young women: A 2-year study. Contraception. 2003;67:439–47. doi: 10.1016/s0010-7824(03)00048-9. [DOI] [PubMed] [Google Scholar]
- 72.Endrikat J, Mih E, Düsterberg B, Land K, Gerlinger C, Schmidt W, et al. A 3-year double-blind, randomized, controlled study on the influence of two oral contraceptives containing either 20 microg or 30 microg ethinylestradiol in combination with levonorgestrel on bone mineral density. Contraception. 2004;69:179–87. doi: 10.1016/j.contraception.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Mazess RB, Barden HS. Bone density in premenopausal women: Effects of age, dietary intake, physical activity, smoking, and birth-control pills. Am J Clin Nutr. 1991;53:132–42. doi: 10.1093/ajcn/53.1.132. [DOI] [PubMed] [Google Scholar]
- 74.Nappi C, Di Spiezio Sardo A, Acunzo G, Bifulco G, Tommaselli GA, Guida M, et al. Effects of a low-dose and ultra-low-dose combined oral contraceptive use on bone turnover and bone mineral density in young fertile women: A prospective controlled randomized study. Contraception. 2003;67:355–9. doi: 10.1016/s0010-7824(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 75.Paoletti AM, Orrù M, Lello S, Floris S, Ranuzzi F, Etzi R, et al. Short-term variations in bone remodeling markers of an oral contraception formulation containing 3 mg of drospirenone plus 30 microg of ethinyl estradiol: Observational study in young postadolescent women. Contraception. 2004;70:293–8. doi: 10.1016/j.contraception.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 76.Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB, et al. Bone gain in young adult women. JAMA. 1992;268:2403–8. [PubMed] [Google Scholar]
- 77.Reed SD, Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM, et al. Longitudinal changes in bone density in relation to oral contraceptive use. Contraception. 2003;68:177–82. doi: 10.1016/s0010-7824(03)00147-1. [DOI] [PubMed] [Google Scholar]
- 78.Gambacciani M, Cappagli B, Ciaponi M, Benussi C, Genazzani AR. Hormone replacement therapy in perimenopause: Effect of a low dose oral contraceptive preparation on bone quantitative ultrasound characteristics. Menopause. 1999;6:43–8. [PubMed] [Google Scholar]
- 79.Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR, et al. Longitudinal evaluation of perimenopausal bone loss: Effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54:176–80. doi: 10.1016/j.maturitas.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 80.Gambacciani M, Ciaponi M, Cappagli B, Benussi C, Genazzani AR. Longitudinal evaluation of perimenopausal femoral bone loss: Effects of a low-dose oral contraceptive preparation on bone mineral density and metabolism. Osteoporos Int. 2000;11:544–8. doi: 10.1007/s001980070099. [DOI] [PubMed] [Google Scholar]
- 81.Nappi C, Di Spiezio Sardo A, Greco E, Tommaselli GA, Giordano E, Guida M, et al. Effects of an oral contraceptive containing drospirenone on bone turnover and bone mineral density. Obstet Gynecol. 2005;105:53–60. doi: 10.1097/01.AOG.0000148344.26475.fc. [DOI] [PubMed] [Google Scholar]
- 82.Gargano V, Massaro M, Morra I, Formisano C, Di Carlo C, Nappi C, et al. Effects of two low-dose combined oral contraceptives containing drospirenone on bone turnover and bone mineral density in young fertile women: A prospective controlled randomized study. Contraception. 2008;78:10–5. doi: 10.1016/j.contraception.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 83.Berenson AB, Breitkopf CR, Grady JJ, Rickert VI, Thomas A. Effects of hormonal contraception on bone mineral density after 24 months of use. Obstet Gynecol. 2004;103:899–906. doi: 10.1097/01.AOG.0000117082.49490.d5. [DOI] [PubMed] [Google Scholar]
- 84.Gambacciani M, Spinetti A, Cappagli B, Taponeco F, Maffei S, Piaggesi L, et al. Hormone replacement therapy in perimenopausal women with a low dose oral contraceptive preparation: Effects on bone mineral density and metabolism. Maturitas. 1994;19:125–31. doi: 10.1016/0378-5122(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 85.Gambacciani M, Spinetti A, Taponeco F, Cappagli B, Piaggesi L, Fioretti P, et al. Longitudinal evaluation of perimenopausal vertebral bone loss: Effects of a low-dose oral contraceptive preparation on bone mineral density and metabolism. Obstet Gynecol. 1994;83:392–6. [PubMed] [Google Scholar]
- 86.Hansen MA, Overgaard K, Riis BJ, Christiansen C. Potential risk factors for development of postmenopausal osteoporosis – Examined over a 12-year period. Osteoporos Int. 1991;1:95–102. doi: 10.1007/BF01880450. [DOI] [PubMed] [Google Scholar]
- 87.Shargil AA. Hormone replacement therapy in perimenopausal women with a triphasic contraceptive compound: A three-year prospective study. Int J Fertil. 1985;30:15, 18–28. [PubMed] [Google Scholar]
- 88.Taechakraichana N, Jaisamrarn U, Panyakhamlerd K, Chaikittisilpa S, Limpaphayom K. Difference in bone acquisition among hormonally treated postmenopausal women with normal and low bone mass. J Med Assoc Thai. 2001;84(Suppl 2):S586–92. [PubMed] [Google Scholar]
- 89.Taechakraichana N, Limpaphayom K, Ninlagarn T, Panyakhamlerd K, Chaikittisilpa S, Dusitsin N, et al. A randomized trial of oral contraceptive and hormone replacement therapy on bone mineral density and coronary heart disease risk factors in postmenopausal women. Obstet Gynecol. 2000;95:87–94. doi: 10.1016/s0029-7844(99)00493-7. [DOI] [PubMed] [Google Scholar]
- 90.Volpe A, Amram A, Cagnacci A, Battaglia C. Biochemical aspects of hormonal contraception: Effects on bone metabolism. Eur J Contracept Reprod Health Care. 1997;2:123–6. doi: 10.3109/13625189709167466. [DOI] [PubMed] [Google Scholar]
- 91.Leff SP. Side-effect of oral contraceptives: Occlusion of branch artery of the retina. Bull Sinai Hosp Detroit. 1976;24:227–9. [PubMed] [Google Scholar]
- 92.Pasquale LR, Kang JH. Female reproductive factors and primary open-angle glaucoma in the nurses' health study. Eye (Lond) 2011;25:633–41. doi: 10.1038/eye.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: Collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713–27. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 94.Charlton BM, Rich-Edwards JW, Colditz GA, Missmer SA, Rosner BA, Hankinson SE, et al. Oral contraceptive use and mortality after 36 years of follow-up in the nurses' health study: Prospective cohort study. BMJ. 2014;349:g6356. doi: 10.1136/bmj.g6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.International Collaboration of Epidemiological Studies of Cervical Cancer. Appleby P, Beral V, Berrington de González A, Colin D, Franceschi S, et al. Cervical cancer and hormonal contraceptives: Collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609–21. doi: 10.1016/S0140-6736(07)61684-5. [DOI] [PubMed] [Google Scholar]
- 96.Gierisch JM, Coeytaux RR, Urrutia RP, Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: A systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931–43. doi: 10.1158/1055-9965.EPI-13-0298. [DOI] [PubMed] [Google Scholar]
- 97.Collaborative Group on Epidemiological Studies of Ovarian Cancer. Beral V, Doll R, Hermon C, Peto R, Reeves G, et al. Ovarian cancer and oral contraceptives: Collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303–14. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 98.Steenland MW, Zapata LB, Brahmi D, Marchbanks PA, Curtis KM. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: A systematic review. Contraception. 2013;87:611–24. doi: 10.1016/j.contraception.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 99.Haider A, Shaw JC. Treatment of acne vulgaris. JAMA. 2004;292:726–35. doi: 10.1001/jama.292.6.726. [DOI] [PubMed] [Google Scholar]
- 100.Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi: 10.1002/14651858.CD004425.pub5. [DOI] [PubMed] [Google Scholar]
- 101.Lucky AW, Koltun W, Thiboutot D, Niknian M, Sampson-Landers C, Korner P, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: A randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143–50. [PubMed] [Google Scholar]
- 102.Maloney JM, Dietze P, JR, Watson D, Niknian M, Lee-Rugh S, Sampson-Landers C, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen: A randomized controlled trial. Obstet Gynecol. 2008;112:773–81. doi: 10.1097/AOG.0b013e318187e1c5. [DOI] [PubMed] [Google Scholar]
- 103.Maloney JM, Dietze P, Jr, Watson D, Niknian M, Lee-Rugh S, Sampson-Landers C, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 microg ethinylestradiol in the treatment of acne vulgaris: Lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837–44. [PubMed] [Google Scholar]
- 104.Plewig G, Cunliffe WJ, Binder N, Höschen K. Efficacy of an oral contraceptive containing EE 0.03 mg and CMA 2 mg (Belara) in moderate acne resolution: A randomized, double-blind, placebo-controlled phase III trial. Contraception. 2009;80:25–33. doi: 10.1016/j.contraception.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 105.Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-lidose) and the innovator isotretinoin formulation: A randomized, 4-treatment, crossover study. J Am Acad Dermatol. 2013;69:762–7. doi: 10.1016/j.jaad.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 106.Crockett SD, Porter CQ, Martin CF, Sandler RS, Kappelman MD. Isotretinoin use and the risk of inflammatory bowel disease: A case-control study. Am J Gastroenterol. 2010;105:1986–93. doi: 10.1038/ajg.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Etminan M, Bird ST, Delaney JA, Bressler B, Brophy JM. Isotretinoin and risk for inflammatory bowel disease: A nested case-control study and meta-analysis of published and unpublished data. JAMA Dermatol. 2013;149:216–20. doi: 10.1001/jamadermatol.2013.1344. [DOI] [PubMed] [Google Scholar]
- 108.Reddy D, Siegel CA, Sands BE, Kane S. Possible association between isotretinoin and inflammatory bowel disease. Am J Gastroenterol. 2006;101:1569–73. doi: 10.1111/j.1572-0241.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- 109.Lakshmi C. Hormone therapy in acne. Indian J Dermatol Venereol Leprol. 2013;79:322–37. doi: 10.4103/0378-6323.110765. [DOI] [PubMed] [Google Scholar]
- 110.Ghosh S, Chaudhuri S, Jain VK, Aggarwal K. Profiling and hormonal therapy for acne in women. Indian J Dermatol. 2014;59:107–15. doi: 10.4103/0019-5154.127667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bitzer J, Römer T, Lopes da Silva Filho A. The use of cyproterone acetate/ethinyl estradiol in hyperandrogenic skin symptoms - a review. Eur J Contracept Reprod Health Care. 2017;22:172–82. doi: 10.1080/13625187.2017.1317339. [DOI] [PubMed] [Google Scholar]
- 112.Bhattacharya SM, Ghosh M, Nandi N. Effects of drospirenone pill in Indian women with polycystic ovary syndrome. J Turk Ger Gynecol Assoc. 2011;12:144–7. doi: 10.5152/jtgga.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moghetti P. Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders. 2nd ed. Totowa, New Jersey: Humana Press Inc; 2006. Ovarian suppression and treatment of hirsuitism. [Google Scholar]
- 114.Batukan C, Muderris II, Ozcelik B, Ozturk A. Comparison of two oral contraceptives containing either drospirenone or cyproterone acetate in the treatment of hirsutism. Gynecol Endocrinol. 2007;23:38–44. doi: 10.1080/09637480601137066. [DOI] [PubMed] [Google Scholar]
- 115.Burkman RT., Jr The role of oral contraceptives in the treatment of hyperandrogenic disorders. Am J Med. 1995;98:130S–6S. doi: 10.1016/s0002-9343(99)80071-0. [DOI] [PubMed] [Google Scholar]
- 116.Shaw JC. Spironolactone in dermatologic therapy. J Am Acad Dermatol. 1991;24:236–43. doi: 10.1016/0190-9622(91)70034-y. [DOI] [PubMed] [Google Scholar]
- 117.Van der Spuy ZM, le Roux PA. Cyproterone acetate for hirsutism. Cochrane Database Syst Rev. 2003;4:CD001125. doi: 10.1002/14651858.CD001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39:85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 119.Fitzgerald C, Elstein M, Spona J. Effect of age on the response of the hypothalamo-pituitary-ovarian axis to a combined oral contraceptive. Fertil Steril. 1999;71:1079–84. doi: 10.1016/s0015-0282(99)00146-6. [DOI] [PubMed] [Google Scholar]
- 120.Saeed R, Akram J, Changezi HU, Saeed M. Treatment of hirsutism in polycystic ovarian syndrome with Diane, 50 mcg ethinyl estradiol and 2 mg cyproterone acetate. Specialist. 1993;9:109–12. [Google Scholar]
- 121.Porcile A, Gallardo E. Long-term treatment of hirsutism: Desogestrel compared with cyproterone acetate in oral contraceptives. Fertil Steril. 1991;55:877–81. doi: 10.1016/s0015-0282(16)54291-5. [DOI] [PubMed] [Google Scholar]
- 122.Sahin Y, Bayram F, Keleştimur F, Müderris I. Comparison of cyproterone acetate plus ethinyl estradiol and finasteride in the treatment of hirsutism. J Endocrinol Invest. 1998;21:348–52. doi: 10.1007/BF03350769. [DOI] [PubMed] [Google Scholar]
- 123.Stegeman BH, de Bastos M, Rosendaal FR, van Hylckama Vlieg A, Helmerhorst FM, Stijnen T, et al. Different combined oral contraceptives and the risk of venous thrombosis: Systematic review and network meta-analysis. BMJ. 2013;347:f5298. doi: 10.1136/bmj.f5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu CQ, Grandi SM, Filion KB, Abenhaim HA, Joseph L, Eisenberg MJ, et al. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: A systematic review. BJOG. 2013;120:801–10. doi: 10.1111/1471-0528.12210. [DOI] [PubMed] [Google Scholar]
- 125.Coenen CM, Hollanders JM, Rolland R, Spielmann D, Bulten J. The effects of a low-dose gestodene-containing oral contraceptive on endometrial histology in healthy women. Eur J Contracept Reprod Health Care. 1996;1:325–9. doi: 10.3109/13625189609150678. [DOI] [PubMed] [Google Scholar]
- 126.Guido M, Romualdi D, Giuliani M, Suriano R, Selvaggi L, Apa R, et al. Drospirenone for the treatment of hirsute women with polycystic ovary syndrome: A clinical, endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004;89:2817–23. doi: 10.1210/jc.2003-031158. [DOI] [PubMed] [Google Scholar]
- 127.Bobde JA, Bhosle D, Kadam R, Shelke S. Comparison of efficacy and safety of metformin, oral contraceptive combination of ethinyl estradiol and drospirenone alone or in combination in polycystic ovarian syndrome. J Obesity and Metabolic Research. 2014;1:112. [Google Scholar]
- 128.Cosma M, Swiglo BA, Flynn DN, Kurtz DM, Labella ML, Mullan RJ, et al. Clinical review: Insulin sensitizers for the treatment of hirsutism: A systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1135–42. doi: 10.1210/jc.2007-2429. [DOI] [PubMed] [Google Scholar]
- 129.Cibula D, Fanta M, Vrbikova J, Stanicka S, Dvorakova K, Hill M, et al. The effect of combination therapy with metformin and combined oral contraceptives (COC) versus COC alone on insulin sensitivity, hyperandrogenaemia, SHBG and lipids in PCOS patients. Hum Reprod. 2005;20:180–4. doi: 10.1093/humrep/deh588. [DOI] [PubMed] [Google Scholar]
- 130.Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, et al. An international consortium update: Pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm Res Paediatr. 2017;88:371–95. doi: 10.1159/000479371. [DOI] [PubMed] [Google Scholar]
- 131.Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibáñez L, et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015;83:376–89. doi: 10.1159/000375530. [DOI] [PubMed] [Google Scholar]
- 132.Metcalf MG, Skidmore DS, Lowry GF, Mackenzie JA. Incidence of ovulation in the years after the menarche. J Endocrinol. 1983;97:213–9. doi: 10.1677/joe.0.0970213. [DOI] [PubMed] [Google Scholar]
- 133.Apter D. Endocrine and metabolic abnormalities in adolescents with a PCOS-like condition: Consequences for adult reproduction. Trends Endocrinol Metab. 1998;9:58–61. doi: 10.1016/s1043-2760(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 134.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–92. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rathod AD, Chavan RP, Bhagat V, Pajai S, Padmawar A, Thool P, et al. Analysis of near-miss and maternal mortality at tertiary referral centre of rural India. J Obstet Gynaecol India. 2016;66:295–300. doi: 10.1007/s13224-016-0902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khosla AH, Devi L, Goel P, Saha PK. Puberty menorrhagia requiring inpatient admission. JNMA J Nepal Med Assoc. 2010;49:112–6. [PubMed] [Google Scholar]
- 137.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 138.Martin KA, Chang RJ, Ehrmann DA, Ibanez L, Lobo RA, Rosenfield RL, et al. Evaluation and treatment of hirsutism in premenopausal women: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1105–20. doi: 10.1210/jc.2007-2437. [DOI] [PubMed] [Google Scholar]
- 139.Liu H, Yao J, Wang W, Zhang D. Association between duration of oral contraceptive use and risk of hypertension: A meta-analysis. J Clin Hypertens (Greenwich) 2017;19:1032–41. doi: 10.1111/jch.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sitruk-Ware R, Nath A. Metabolic effects of contraceptive steroids. Rev Endocr Metab Disord. 2011;12:63–75. doi: 10.1007/s11154-011-9182-4. [DOI] [PubMed] [Google Scholar]
- 141.Cibula D, Gompel A, Mueck AO, La Vecchia C, Hannaford PC, Skouby SO, et al. Hormonal contraception and risk of cancer. Hum Reprod Update. 2010;16:631–50. doi: 10.1093/humupd/dmq022. [DOI] [PubMed] [Google Scholar]
- 142.Bayard F, Louvet JP, Moatti JP, Smilovici W, Duguet L, Boulard C, et al. Plasma concentrations of LH and of sex steroids during the normal menstrual cycle and during contraceptive treatment. J Gynecol Obstet Biol Reprod (Paris) 1975;4:915–26. [PubMed] [Google Scholar]
- 143.Bhanwra S, Ahluwalia K. The association of oral contraceptive pills with increase in intraocular pressure: Time for pharmacovigilance to step in. J Pharmacol Pharmacother. 2015;6:51–2. [Google Scholar]
- 144.Nader S, Diamanti-Kandarakis E. Polycystic ovary syndrome, oral contraceptives and metabolic issues: New perspectives and a unifying hypothesis. Hum Reprod. 2007;22:317–22. doi: 10.1093/humrep/del407. [DOI] [PubMed] [Google Scholar]
- 145.Deb S, Campbell BK, Pincott-Allen C, Clewes JS, Cumberpatch G, Raine-Fenning NJ, et al. Quantifying effect of combined oral contraceptive pill on functional ovarian reserve as measured by serum anti-Müllerian hormone and small antral follicle count using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2012;39:574–80. doi: 10.1002/uog.10114. [DOI] [PubMed] [Google Scholar]
- 146.Fitzgerald C, Feichtinger W, Spona J, Elstein M, Lüdicke F, Müller U, et al. A comparison of the effects of two monophasic low dose oral contraceptives on the inhibition of ovulation. Adv Contracept. 1994;10:5–18. doi: 10.1007/BF01986524. [DOI] [PubMed] [Google Scholar]
- 147.Archer DF, Kovalevsky G, Ballagh SA, Grubb GS. Ovarian activity and safety of a novel levonorgestrel/ethinyl estradiol continuous oral contraceptive regimen. Contraception. 2009;80:245–53. doi: 10.1016/j.contraception.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 148.Birtch RL, Olatunbosun OA, Pierson RA. Ovarian follicular dynamics during conventional vs. continuous oral contraceptive use. Contraception. 2006;73:235–43. doi: 10.1016/j.contraception.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 149.Legro RS, Pauli JG, Kunselman AR, Meadows JW, Kesner JS, Zaino RJ, et al. Effects of continuous versus cyclical oral contraception: A randomized controlled trial. J Clin Endocrinol Metab. 2008;93:420–9. doi: 10.1210/jc.2007-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kroll R, Seidman L, Ricciotti N, Howard B, Weiss H. A phase 1, multicentre, open-label study to evaluate ovarian follicular activity and hormone levels with an extended-regimen combined oral contraceptive with low-dose ethinyl estradiol supplementation. Eur J Contracept Reprod Health Care. 2015;20:249–58. doi: 10.3109/13625187.2014.979282. [DOI] [PubMed] [Google Scholar]
- 151.Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98:2106–15. doi: 10.1210/jc.2012-3995. [DOI] [PubMed] [Google Scholar]
- 152.Lauritsen MP, Bentzen JG, Pinborg A, Loft A, Forman JL, Thuesen LL, et al. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mullerian hormone. Hum Reprod. 2014;29:791–801. doi: 10.1093/humrep/det469. [DOI] [PubMed] [Google Scholar]
- 153.Birch Petersen K, Hvidman HW, Forman JL, Pinborg A, Larsen EC, Macklon KT, et al. Ovarian reserve assessment in users of oral contraception seeking fertility advice on their reproductive lifespan. Hum Reprod. 2015;30:2364–75. doi: 10.1093/humrep/dev197. [DOI] [PubMed] [Google Scholar]
- 154.D'Arpe S, Di Feliciantonio M, Candelieri M, Franceschetti S, Piccioni MG, Bastianelli C, et al. Ovarian function during hormonal contraception assessed by endocrine and sonographic markers: A systematic review. Reprod Biomed Online. 2016;33:436–48. doi: 10.1016/j.rbmo.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 155.Spona J, Binder N, Höschen K, Feichtinger W. Suppression of ovarian function by a combined oral contraceptive containing 0.02 mg ethinyl estradiol and 2 mg chlormadinone acetate given in a 24/4-day intake regimen over three cycles. Fertil Steril. 2010;94:1195–201. doi: 10.1016/j.fertnstert.2009.06.057. [DOI] [PubMed] [Google Scholar]
- 156.Wei D, Shi Y, Li J, Wang Z, Zhang L, Sun Y, et al. Effect of pretreatment with oral contraceptives and progestins on IVF outcomes in women with polycystic ovary syndrome. Hum Reprod. 2017;32:354–61. doi: 10.1093/humrep/dew325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sallam HN, Mohamed M, Aboul-Einein W, Safwat SH, Saleh F, Sallam A. Pinopod expression in women using oral contraceptives. Fertil Steril. 2005;84:S26. [Google Scholar]
- 158.Garcia-Velasco JA, Fatemi HM. To pill or not to pill in GnRH antagonist cycles: That is the question! Reprod Biomed Online. 2015;30:39–42. doi: 10.1016/j.rbmo.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 159.Griesinger G, Kolibianakis EM, Venetis C, Diedrich K, Tarlatzis B. Oral contraceptive pretreatment significantly reduces ongoing pregnancy likelihood in gonadotropin-releasing hormone antagonist cycles: An updated meta-analysis. Fertil Steril. 2010;94:2382–4. doi: 10.1016/j.fertnstert.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 160.Smulders B, van Oirschot SM, Farquhar C, Rombauts L, Kremer JA. Oral contraceptive pill, progestogen or estrogen pre-treatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques. Cochrane Database Syst Rev. 2010:CD006109. doi: 10.1002/14651858.CD006109.pub2. [DOI] [PubMed] [Google Scholar]
- 161.Barmat LI, Chantilis SJ, Hurst BS, Dickey RP. A randomized prospective trial comparing gonadotropin-releasing hormone (GnRH) antagonist/recombinant follicle-stimulating hormone (rFSH) versus GnRH-agonist/rFSH in women pretreated with oral contraceptives before in vitro fertilization. Fertil Steril. 2005;83:321–30. doi: 10.1016/j.fertnstert.2004.06.076. [DOI] [PubMed] [Google Scholar]
- 162.Bellver J, Albert C, Labarta E, Pellicer A. Early pregnancy loss in women stimulated with gonadotropin-releasing hormone antagonist protocols according to oral contraceptive pill pretreatment. Fertil Steril. 2007;87:1098–101. doi: 10.1016/j.fertnstert.2006.08.098. [DOI] [PubMed] [Google Scholar]
- 163.Biljan MM, Mahutte NG, Dean N, Hemmings R, Bissonnette F, Tan SL, et al. Effects of pretreatment with an oral contraceptive on the time required to achieve pituitary suppression with gonadotropin-releasing hormone analogues and on subsequent implantation and pregnancy rates. Fertil Steril. 1998;70:1063–9. doi: 10.1016/s0015-0282(98)00333-1. [DOI] [PubMed] [Google Scholar]
- 164.Fukuda M, Fukuda K, Yding Andersen C, Byskov AG. Does anovulation induced by oral contraceptives favor pregnancy during the following two menstrual cycles? Fertil Steril. 2000;73:742–7. doi: 10.1016/s0015-0282(99)00631-7. [DOI] [PubMed] [Google Scholar]
- 165.Kolibianakis EM, Papanikolaou EG, Camus M, Tournaye H, Van Steirteghem AC, Devroey P, et al. Effect of oral contraceptive pill pretreatment on ongoing pregnancy rates in patients stimulated with GnRH antagonists and recombinant FSH for IVF. A randomized controlled trial. Hum Reprod. 2006;21:352–7. doi: 10.1093/humrep/dei348. [DOI] [PubMed] [Google Scholar]
- 166.Meldrum DR, Scott RT, Jr, Levy MJ, Alper MM, Noyes N. Oral contraceptive pretreatment in women undergoing controlled ovarian stimulation in ganirelix acetate cycles may, for a subset of patients, be associated with low serum luteinizing hormone levels, reduced ovarian response to gonadotropins, and early pregnancy loss. Fertil Steril. 2009;91:1963–5. doi: 10.1016/j.fertnstert.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 167.Pinkas H, Sapir O, Avrech OM, Ben-Haroush A, Ashkenzi J, Fisch B, et al. The effect of oral contraceptive pill for cycle scheduling prior to GnRH-antagonist protocol on IVF cycle parameters and pregnancy outcome. J Assist Reprod Genet. 2008;25:29–33. doi: 10.1007/s10815-007-9189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Klein TA, Mishell DR. Gonadotropin, prolactin, and steroid hormone levels after discontinuation of oral contraceptives. Am J Obstet Gynecol. 1977;127:585–9. doi: 10.1016/0002-9378(77)90353-2. [DOI] [PubMed] [Google Scholar]
- 169.van Heusden AM, Fauser BC. Residual ovarian activity during oral steroid contraception. Hum Reprod Update. 2002;8:345–58. doi: 10.1093/humupd/8.4.345. [DOI] [PubMed] [Google Scholar]
- 170.Cédrin-Durnerin I, Bständig B, Parneix I, Bied-Damon V, Avril C, Decanter C, et al. Effects of oral contraceptive, synthetic progestogen or natural estrogen pre-treatments on the hormonal profile and the antral follicle cohort before GnRH antagonist protocol. Hum Reprod. 2007;22:109–16. doi: 10.1093/humrep/del340. [DOI] [PubMed] [Google Scholar]
- 171.van Heusden AM, Fauser BC. Activity of the pituitary-ovarian axis in the pill-free interval during use of low-dose combined oral contraceptives. Contraception. 1999;59:237–43. doi: 10.1016/s0010-7824(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 172.Rombauts L, Healy D, Norman RJ. Comparative ran- domized trial to assess the impact of oral contracep- tive pretreatment on follicular growth and hormone profiles in GnRH antagonist-treated patients. Hum Reprod. 2006;13:235–45. doi: 10.1093/humrep/dei302. [DOI] [PubMed] [Google Scholar]
- 173.Palomba S, Falbo A, Del Negro S, Russo T, Zullo F. Use of oral contraceptives in infertile patients: A descriptive review. Gynecol Endocrinol. 2006;22:537–46. doi: 10.1080/09513590601005672. [DOI] [PubMed] [Google Scholar]
- 174.Orief YI, Darwish EA, Elsamra MA, Ragab DH. Gestagen versus oral contraceptive pills to induce withdrawal bleeding before induction of ovulation by clomiphene citrate in polycystic ovary syndrome. Middle East Fertility Society J. 2014;19:115–123. [Google Scholar]
- 175.Gupta A, Goenka D, Goenka ML. Progesterone hypersensitivity: A challenge for luteal support. J Hum Reprod Sci. 2018;11:79–81. doi: 10.4103/jhrs.JHRS_116_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sangam K, Mitra N. Oral contraceptive pills in the management of clomiphene resistant anovulation. Int J Med Res Rev. 2015;3:1182–7. [Google Scholar]
- 177.Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2007;2:CD001019. doi: 10.1002/14651858.CD001019.pub2. [DOI] [PubMed] [Google Scholar]
- 178.Vercellini P, Frontino G, De Giorgi O, Pietropaolo G, Pasin R, Crosignani PG, et al. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril. 2003;80:560–3. doi: 10.1016/s0015-0282(03)00794-5. [DOI] [PubMed] [Google Scholar]
- 179.Vercellini P, Barbara G, Somigliana E, Bianchi S, Abbiati A, Fedele L, et al. Comparison of contraceptive ring and patch for the treatment of symptomatic endometriosis. Fertil Steril. 2010;93:2150–61. doi: 10.1016/j.fertnstert.2009.01.071. [DOI] [PubMed] [Google Scholar]
- 180.Vercellini P, Somigliana E, Viganò P, De Matteis S, Barbara G, Fedele L, et al. Post-operative endometriosis recurrence: A plea for prevention based on pathogenetic, epidemiological and clinical evidence. Reprod Biomed Online. 2010;21:259–65. doi: 10.1016/j.rbmo.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 181.Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, De Bie B, et al. ESHRE guideline: Management of women with endometriosis. Hum Reprod. 2014;29:400–12. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 182.Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, et al. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab. 2007;92:2500–5. doi: 10.1210/jc.2006-2725. [DOI] [PubMed] [Google Scholar]
- 183.Puder JJ, Varga S, Kraenzlin M, De Geyter C, Keller U, Müller B, et al. Central fat excess in polycystic ovary syndrome: Relation to low-grade inflammation and insulin resistance. J Clin Endocrinol Metab. 2005;90:6014–21. doi: 10.1210/jc.2005-1002. [DOI] [PubMed] [Google Scholar]
- 184.Gallo MF, Lopez LM, Grimes DA, Carayon F, Schulz KF, Helmerhorst FM. Combination contraceptives: Effects on weight. The Cochrane Database of Systematic Reviews. 2014;1:CD003987. doi: 10.1002/14651858.CD003987.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ibáñez L, de Zegher F. Ethinylestradiol-drospirenone, flutamide-metformin, or both for adolescents and women with hyperinsulinemic hyperandrogenism: Opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab. 2004;89:1592–7. doi: 10.1210/jc.2003-031281. [DOI] [PubMed] [Google Scholar]
- 186.Aydin K, Cinar N, Aksoy DY, Bozdag G, Yildiz BO. Body composition in lean women with polycystic ovary syndrome: Effect of ethinyl estradiol and drospirenone combination. Contraception. 2013;87:358–62. doi: 10.1016/j.contraception.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 187.Ibáñez L, Díaz M, Sebastiani G, Marcos MV, López-Bermejo A, de Zegher F, et al. Oral contraception vs. insulin sensitization for 18 months in nonobese adolescents with androgen excess: Posttreatment differences in C-reactive protein, intima-media thickness, visceral adiposity, insulin sensitivity, and menstrual regularity. J Clin Endocrinol Metab. 2013;98:E902–7. doi: 10.1210/jc.2013-1041. [DOI] [PubMed] [Google Scholar]
- 188.Glintborg D, Mumm H, Altinok ML, Richelsen B, Bruun JM, Andersen M, et al. Adiponectin, interleukin-6, monocyte chemoattractant protein-1, and regional fat mass during 12-month randomized treatment with metformin and/or oral contraceptives in polycystic ovary syndrome. J Endocrinol Invest. 2014;37:757–64. doi: 10.1007/s40618-014-0103-8. [DOI] [PubMed] [Google Scholar]
- 189.Legro RS, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI, et al. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100:4048–58. doi: 10.1210/jc.2015-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Escobar-Morreale HF, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: A systematic review and metaanalysis. Fertil Steril. 2011;95:1048–580. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Díaz M, Chacón MR, López-Bermejo A, Maymó-Masip E, Salvador C, Vendrell J, et al. Ethinyl estradiol-cyproterone acetate versus low-dose pioglitazone-flutamide-metformin for adolescent girls with androgen excess: Divergent effects on CD163, TWEAK receptor, ANGPTL4, and LEPTIN expression in subcutaneous adipose tissue. J Clin Endocrinol Metab. 2012;97:3630–8. doi: 10.1210/jc.2012-1754. [DOI] [PubMed] [Google Scholar]
- 192.Christakou C, Kollias A, Piperi C, Katsikis I, Panidis D, Diamanti-Kandarakis E, et al. The benefit-to-risk ratio of common treatments in PCOS: Effect of oral contraceptives versus metformin on atherogenic markers. Hormones (Athens) 2014;13:488–97. doi: 10.14310/horm.2002.1553. [DOI] [PubMed] [Google Scholar]
- 193.Orio F, Muscogiuri G, Giallauria F, Savastano S, Bottiglieri P, Tafuri D, et al. Oral contraceptives versus physical exercise on cardiovascular and metabolic risk factors in women with polycystic ovary syndrome: A randomized controlled trial. Clin Endocrinol (Oxf) 2016;85:764–71. doi: 10.1111/cen.13112. [DOI] [PubMed] [Google Scholar]
- 194.Moran LJ, Meyer C, Hutchison SK, Zoungas S, Teede HJ. Novel inflammatory markers in overweight women with and without polycystic ovary syndrome and following pharmacological intervention. J Endocrinol Invest. 2010;33:258–65. doi: 10.1007/BF03345790. [DOI] [PubMed] [Google Scholar]
- 195.World Health Organisation. Medical Eligibility Criteria for Contraceptive Use. 5th ed. Geneva: World Health Organisation; 2015. [PubMed] [Google Scholar]
- 196.Verhaeghe J. Hormonal contraception in women with the metabolic syndrome: A narrative review. Eur J Contracept Reprod Health Care. 2010;15:305–13. doi: 10.3109/13625187.2010.502583. [DOI] [PubMed] [Google Scholar]
- 197.Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol. 1998;51:581–6. doi: 10.1016/s0895-4356(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 198.Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: Results of a 31 year follow-up study. Hum Fertil (Camb) 2000;3:101–5. doi: 10.1080/1464727002000198781. [DOI] [PubMed] [Google Scholar]
- 199.Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol. 1996;88:554–9. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]
- 200.Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: A systematic review. Reprod Biomed Online. 2009;19:398–405. doi: 10.1016/s1472-6483(10)60175-7. [DOI] [PubMed] [Google Scholar]
- 201.Hannaford PC, Selvaraj S, Elliott AM, Angus V, Iversen L, Lee AJ, et al. Cancer risk among users of oral contraceptives: Cohort data from the Royal College of General Practitioner's Oral Contraception Study. BMJ. 2007;335:651. doi: 10.1136/bmj.39289.649410.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Balen A. Polycystic ovary syndrome and cancer. Hum Reprod Update. 2001;7:522–5. doi: 10.1093/humupd/7.6.522. [DOI] [PubMed] [Google Scholar]
- 203.Swiglo BA, Cosma M, Flynn DN, Kurtz DM, Labella ML, Mullan RJ, et al. Clinical review: Antiandrogens for the treatment of hirsutism: A systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008b;93:1153–60. doi: 10.1210/jc.2007-2430. [DOI] [PubMed] [Google Scholar]
- 204.Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: A randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000b;85:139–46. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 205.Calaf J, López E, Millet A, Alcañiz J, Fortuny A, Vidal O, et al. Long-term efficacy and tolerability of flutamide combined with oral contraception in moderate to severe hirsutism: A 12-month, double-blind, parallel clinical trial. J Clin Endocrinol Metab. 2007;92:3446–52. doi: 10.1210/jc.2006-2798. [DOI] [PubMed] [Google Scholar]
- 206.Belisle S, Love EJ. Clinical efficacy and safety of cyproterone acetate in severe hirsutism: Results of a multicentered Canadian study. Fertil Steril. 1986;46:1015–20. [PubMed] [Google Scholar]
- 207.Keleştimur F, Sahin Y. Comparison of Diane 35 and Diane 35 plus spironolactone in the treatment of hirsutism. Fertil Steril. 1998;69:66–9. doi: 10.1016/s0015-0282(97)00427-5. [DOI] [PubMed] [Google Scholar]
- 208.Kuttenn F, Rigaud C, Wright F, Mauvais-Jarvis P. Treatment of hirsutism by oral cyproterone acetate and percutaneous estradiol. J Clin Endocrinol Metab. 1980;51:1107–11. doi: 10.1210/jcem-51-5-1107. [DOI] [PubMed] [Google Scholar]
- 209.Lord JM, Flight IH, Norman RJ. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. 2003:CD003053–CD003053. doi: 10.1002/14651858.CD003053. [DOI] [PubMed] [Google Scholar]
- 210.Harborne L, Fleming R, Lyall H, Sattar N, Norman J. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4116–23. doi: 10.1210/jc.2003-030424. [DOI] [PubMed] [Google Scholar]
- 211.Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Martikainen HK, Tapanainen JS, et al. Endocrine and metabolic effects of metformin versus ethinyl estradiol-cyproterone acetate in obese women with polycystic ovary syndrome: A randomized study. J Clin Endocrinol Metab. 2000;85:3161–8. doi: 10.1210/jcem.85.9.6792. [DOI] [PubMed] [Google Scholar]
- 212.Morin-Papunen L, Vauhkonen I, Koivunen R, Ruokonen A, Martikainen H, Tapanainen JS, et al. Metformin versus ethinyl estradiol-cyproterone acetate in the treatment of nonobese women with polycystic ovary syndrome: A randomized study. J Clin Endocrinol Metab. 2003;88:148–56. doi: 10.1210/jc.2002-020997. [DOI] [PubMed] [Google Scholar]
- 213.Allen HF, Mazzoni C, Heptulla RA, Murray MA, Miller N, Koenigs L, et al. Randomized controlled trial evaluating response to metformin versus standard therapy in the treatment of adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2005;18:761–8. doi: 10.1515/jpem.2005.18.8.761. [DOI] [PubMed] [Google Scholar]
- 214.Lemay A, Dodin S, Turcot L, Déchêne F, Forest JC. Rosiglitazone and ethinyl estradiol/cyproterone acetate as single and combined treatment of overweight women with polycystic ovary syndrome and insulin resistance. Hum Reprod. 2006;21:121–8. doi: 10.1093/humrep/dei312. [DOI] [PubMed] [Google Scholar]
- 215.Meyer C, McGrath BP, Teede HJ. Effects of medical therapy on insulin resistance and the cardiovascular system in polycystic ovary syndrome. Diabetes Care. 2007;30:471–8. doi: 10.2337/dc06-0618. [DOI] [PubMed] [Google Scholar]
- 216.Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS, et al. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93:4299–306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bilgir O, Kebapcilar L, Taner C, Bilgir F, Kebapcilar A, Bozkaya G, et al. The effect of ethinylestradiol (EE)/cyproterone acetate (CA) and EE/CA plus metformin treatment on adhesion molecules in cases with polycystic ovary syndrome (PCOS) Intern Med. 2009;48:1193–9. doi: 10.2169/internalmedicine.48.2177. [DOI] [PubMed] [Google Scholar]
- 218.Frank-Raue K, Junga G, Raue F, Vecsei P, Ziegler R. Therapy of hirsutism in females with adrenal enzyme defects of steroid hormone biosynthesis: Comparison of dexamethasone with cyproterone acetate. Klin Wochenschr. 1990;68:597–601. doi: 10.1007/BF01660957. [DOI] [PubMed] [Google Scholar]
- 219.Carmina E, Lobo RA. The addition of dexamethasone to antiandrogen therapy for hirsutism prolongs the duration of remission. Fertil Steril. 1998;69:1075–9. doi: 10.1016/s0015-0282(98)00061-2. [DOI] [PubMed] [Google Scholar]
- 220.Devoto E, Aravena L, Ríos R. Treatment of hirsutism with spironolactone and with spironolactone plus dexamethasone. Rev Med Chil. 2000;128:868–75. [PubMed] [Google Scholar]
- 221.Prezelj J, Kocijancic A, Andolsek L. Dexamethasone and spironolactone in the treatment of non-tumorous hyperandrogenism. Gynecol Endocrinol. 1989;3:281–8. doi: 10.3109/09513598909152467. [DOI] [PubMed] [Google Scholar]
- 222.Spritzer P, Billaud L, Thalabard JC, Birman P, Mowszowicz I, Raux-Demay MC, et al. Cyproterone acetate versus hydrocortisone treatment in late-onset adrenal hyperplasia. J Clin Endocrinol Metab. 1990;70:642–6. doi: 10.1210/jcem-70-3-642. [DOI] [PubMed] [Google Scholar]
- 223.Carr BR, Breslau NA, Givens C, Byrd W, Barnett-Hamm C, Marshburn PB, et al. Oral contraceptive pills, gonadotropin-releasing hormone agonists, or use in combination for treatment of hirsutism: A clinical research center study. J Clin Endocrinol Metab. 1995;80:1169–78. doi: 10.1210/jcem.80.4.7714086. [DOI] [PubMed] [Google Scholar]
- 224.Heiner JS, Greendale GA, Kawakami AK, Lapolt PS, Fisher M, Young D, et al. Comparison of a gonadotropin-releasing hormone agonist and a low dose oral contraceptive given alone or together in the treatment of hirsutism. J Clin Endocrinol Metab. 1995;80:3412–8. doi: 10.1210/jcem.80.12.8530574. [DOI] [PubMed] [Google Scholar]
- 225.Vegetti W, Testa G, Maggioni P, Motta T, Falsetti L, Crosignani PG, et al. An open randomized comparative study of an oral contraceptive containing ethinyl estradiol and cyproterone acetate with and without the GnRH analogue goserelin in the long-term treatment of hirsutism. Gynecol Obstet Invest. 1996;41:260–8. doi: 10.1159/000292281. [DOI] [PubMed] [Google Scholar]
- 226.van der Spuy ZM, Tregoning S. Gonadotrophin-releasing hormone analogues for hirsutism (Protocol) Cochrane Database Syst Rev. 2008:CD001126. [Google Scholar]


