Abstract
The successful targeting of oncogenic BRAF V600 represents one of the landmark breakthroughs in therapy for advanced melanoma. While the initial clinical benefit can be dramatic, resistance is common due to a number of mechanisms, including MAPK pathway reactivation. Recent data have revealed a novel role for copper (Cu) in BRAF signaling with potential clinical implications. The history, preclinical data and efficacy of Cu chelating agents in cancer, specifically tetrathiomolybdate, will be reviewed with a focus on the rationale for targeting the MAPK cascade in melanoma through novel combination strategies.
KEYWORDS : BRAF, cobimetinib, copper, dabrafenib, MAPK, MEK, melanoma, tetrathiomolybdate, trametinib, vemurafenib
Practice points.
Successfully targeting the MAPK pathway in BRAF V600 mutated melanoma represents a landmark breakthrough in the treatment of metastatic disease.
MAPK pathway reactivation plays an important role in the development of resistance to BRAF inhibitor monotherapy, less is known about the development of resistance in patients treated with dual BRAF + MEK inhibition.
There is marked heterogeneity in BRAF inhibitor resistance and multitargeted treatment approaches are likely needed.
Copper (Cu) plays an integral role in MAPK pathway signaling at the level of the MEK1/2 kinases to regulate signal transduction and is required for BRAF mutation-positive tumorigenesis.
Reducing Cu availability via Cu chelation may be a new strategy to target the MAPK pathway in melanoma.
Preclinical and early phase clinical trials confirm the feasibility and tolerability of the potent Cu chelator, tetrathiomolybdate.
Background
Copper (Cu) is an essential metal for an array of biological processes including cellular respiration, free radical detoxification and neuropeptide processing [1,2]. These functions are carried out by Cu-dependent enzymes that harness the redox-active nature of Cu as either a structural or catalytic cofactor. The importance of proper Cu homeostasis in human physiology is underscored by disease states that are caused by an over-abundance of Cu or by deficits in its acquisition or distribution [3]. Germline mutations in the gene encoding the Cu transporting ATPase ATP7B cause a single known phenotype, Wilson disease (WD). WD is an autosomal recessive disorder affecting cellular Cu transport leading to an accumulation of Cu in the liver, brain and cornea. This leads to liver dysfunction and an array of neuropsychiatric sequelae [4]. The treatment of WD is Cu chelation therapy for rapid removal of accumulated tissue Cu and prevention of Cu reaccumulation. The safety and feasibility of Cu chelation therapy has been role modeled for decades in the treatment of WD. Cu acquisition is achieved through the high affinity Cu+ transport function of the integral membrane protein CTR1 encoded by the gene SLC31A1 [5]. CTR1 is the major mode of cellular Cu+ uptake. Germline knockout of the Cu+ transporter Ctr1 in mice results in retardation of growth and embryonic lethality, further supporting the pivotal role of elemental Cu [6].
The risk of dying from cancer has been associated with higher levels of Cu [7]. Cancer may also increase serum Cu levels several years prior to its diagnosis [8]. Several studies have suggested Cu homeostasis plays a role in angiogenesis and the neovascularization of tumor tissue [9,10]. Specifically, Cu has been shown in a number of diverse models to promote angiogenesis [11,12]. In the setting of cancer, Cu-reduced diets or Cu chelators have been found to reduce angiogenesis and tumor growth in a number of xenograft models of tumorigenesis [13–15]. Tyrosinase is a copper-containing oxidase which is integral in mammalian melanogenesis. Copper chelating agents have been proposed as possible competitive tyrosinase inhibitors and have been studied in preclinical trials. The copper chelator D-penicillamine strongly inhibited melanin production and tyrosinase activity in human SKMEL-188 melanoma cells [16,17]. This work has made Cu chelation therapy an attractive therapeutic option in preclinical and early phase clinical trials in an array of solid tumor cancers. In particular, the concept of repurposing currently available drugs has real-world implications, given that globally the cost of new oncology drugs is often prohibitive [18].
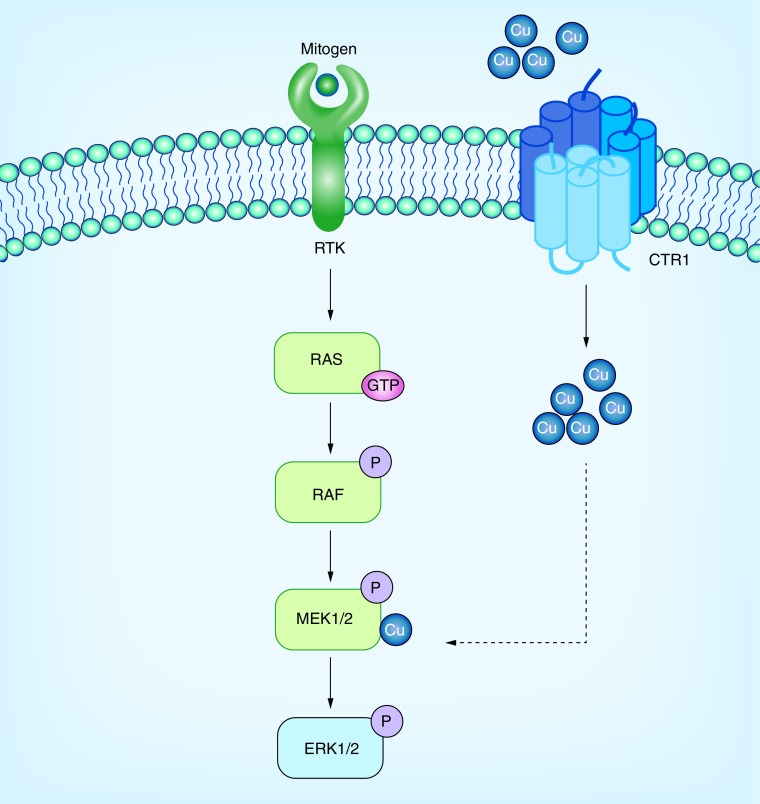
Novel preclinical data have emerged that Cu+ influences the strength of the RAF-MEK-ERK MAPK cascade in response to proliferative signals. Specifically, Cu+ directly binds MEK1/2 and allosterically potentiates the ability of MEK1/2 to phosphorylate ERK1/2 in a dose-dependent manner [19]. Leveraging the dependence of BRAF V600E mutation-positive melanomas on MEK1/2 for tumorigenesis, it was demonstrated that decreasing the levels of CTR1 and introducing surface accessible mutations in MEK1 that disrupt Cu+ binding reduces BRAFV600E-driven signaling and tumorigenesis [20]. Importantly, Cu chelators reduced tumor growth of both BRAF V600E transformed cells and cells resistant to BRAF inhibition [20]. Given the dependence of the oncogenic mutation BRAF v600E for MEK1/2 activity and the unexpected link between Cu+ influx and MAPK pathway activation, decreasing Cu+ availability may be an alternative strategy to limit MEK1/2 activity in BRAFV600E-driven MAPK signaling and tumorigenesis.
Melanoma & targeted therapies
There have been landmark breakthroughs in the treatment of advanced melanoma in the last decade with therapies targeting the MAPK pathway. Approximately 40–60% of patients with metastatic melanoma possess an activating mutation of the intracellular signaling kinase, BRAF [21,22]. BRAF is a serine/threonine protein kinase activating the MAP kinase/ERK-signaling pathway. In most cases, the mutation is due to a single point mutation at amino acid position 600 resulting in the substitution of a glutamic acid for valine, known as BRAF V600E [23]. The negatively charged glutamic acid in mutant BRAF V600E destabilizes the inhibitory interaction between the activation loop and the phosphate-binding loop in the BRAF kinase domain, resulting in a constitutively activated protein, which binds to MEK [24]. Targeted inhibition of BRAFV600E has demonstrated improved overall and progression-free survival in melanoma in several clinical trials using the selective BRAF inhibitors vemurafenib and dabrafenib [25,26]. Despite high initial response rates, half of patients treated with targeted monotherapies relapse within 6–7 months, highlighting the problem with BRAF inhibitor resistance.
Several studies have identified MAPK pathway reactivation via MEK signaling as a major pathway in the development of resistance to BRAF inhibitors [27–36]. However, the mechanism of resistance to BRAF inhibition is a heterogeneous process mediated through multiple effectors including PDGFR-β, IGF-1R, PI3K and EGFR. Acquired resistance mechanisms have been identified due to mutations in NRAS/KRAS, BRAF splice variants, BRAFV600 amplifications, MEK 1/2 mutations and non-MAPK pathway alterations with marked heterogeneity observed within tumors and patients, suggesting multimodality approaches are needed in the treatment of BRAFV600 mutated melanoma to target the MAPK pathway [37].
Co-targeting BRAF + MEK with selective inhibitors has resulted in increased response rates, improved progression free survival and improved overall survival compared with BRAF inhibitor monotherapy in patients with V600E or V600K mutated melanoma [38–40]. However, the combination of MEK and BRAF therapy is limited in that patients who have already developed resistance to BRAF inhibition do not appear to derive great benefit [41]. Also, despite initial response with combination therapy, responses are rarely durable [38]. Resistance to combined BRAF+ MEK inhibition is less well understood. Early data suggest that MAPK pathway reactivation is still a critical mechanism in the development of resistance to BRAF + MEK therapy [42]. Despite major breakthroughs in the combination of targeted therapies in the treatment of BRAF V600 mutated melanoma, novel approaches to delay or overcome resistance are needed.
Overview of Cu metabolism & chelation
Cu is an essential trace element that is abundant in the human diet. It is a transition metal existing as a tightly regulated metabolite in a reduced or oxidized state [43]. The ability of Cu to exchange between a reduced and oxidized state allows for its coordination to diverse ligands in specific proteins as either a structural or catalytic cofactor [44]. Cu is an essential transition metal for a diverse array of biological processes, such as cellular respiration, free radical detoxification, pigmentation and neuropeptide processing [1,2]. Excess Cu+ accumulation is toxic due to free Cu+ ions reacting with hydrogen peroxide to form hydroxyl radicals [4]. There are a number of inherent intracellular mechanisms such as metallochaperones that function to efficiently distribute Cu [45] and molecular buffers such as glutathione and metallothionein that serve to inhibit Cu accumulation in mammals.
Current US FDA approved Cu chelating agents include d-penicillamine, trientine hydrochloride and zinc acetate. Penicillamine removes Cu from less tightly bound sites on proteins, peptides, and membranes and promotes urinary excretion. Its side effect profile is limiting with nearly 30% of patients requiring discontinuation of drug due to toxicities. Common toxicities include sensitivity reactions, fever, pancytopenia and proteinuria [46]. Trientine has been used successfully in patients unable to tolerate penicillamine [13]. Trientine chelates Cu by forming a stable complex with its four constituent nitrogens removing Cu on less tightly bound proteins and peptides. Zinc blocks Cu absorption from the gastrointestinal tract and is reserved for maintenance therapy due to its slow acting effects. Penicillamine and trientine can worsen neurologic symptoms irreversibly in a subset of Wilson disease patients. In order to fill this therapeutic void, a potent Cu chelator tetrathiomolybdate was developed [47].
Cu & the MAPK pathway connection
A number of studies have linked Cu or the Cu+ transporter CTR1 to the MAPK pathway. While interrogating the function of Ctr1 in Xenopus laevis, an unexpected link between this transporter and MAPK signaling was uncovered [6]. Specifically, it was shown that Xenopus CTR1 is required for FGF-mediated MAPK pathway activation during gastrulation in Xenopus, as evidenced by a reduction in FGF-mediated ERK1/2 phosphorylation after CTR1 depletion. In searching for therapeutic targets, a genome RNAi screen undertaken in Drosophila S2 cells identified CTR1 as a potential target to reduce the phosphorylation of ERK1/2 [48]. Data confirm CTR1 and intracellular Cu+ play important roles in RAS/MAPK pathway activation. In both flies, mice, and in vivo, reduction of intracellular Cu+ imposed by loss of Ctr1 Cu+ transporter protein levels resulted in impaired activation of RAS/MAPK signaling, as evidenced by a reduction in ERK phosphorylation [19].
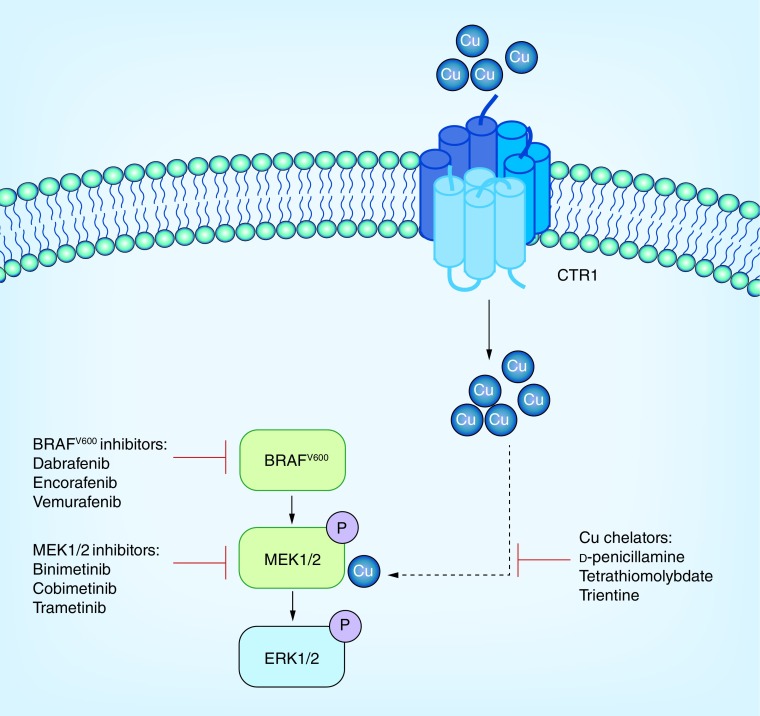
Recent data suggest Cu+ increases MEK1 phosphorylation of ERK2 [19]. Since BRAF inhibitor resistance is likely dependent on MAPK reactivation though MEK signaling, this data suggest a potential for Cu chelation therapy to be used in conjunction with other therapies to overcome resistance (Figure 1 & Figure 2). Further research has shown that genetic loss of the high affinity Cu+ transporter Ctr1 and mutations in MEK1 disrupting Cu+ binding reduced BRAFV600E-driven signaling and tumorigenesis [20].
Figure 1. . Effective Cu influx mediated by CTR1 modulates MEK1/2-mediated phosphorylation of ERK1/2 downstream of oncogenic signaling through a direct interaction between copper and the MEK1/2 kinases.
Oncogenic drivers RTKs, RAS, ARAF, BRAF, CRAF, MEK1, and MEK2 are shown in green.
Cu: Copper; P: Phosphorylated; RTK: Receptor tyrosine kinase.
Figure 2. . Leveraging MAPK inhbitors in combination with copper chelators for both naive and MAPKi resistant forms of BRAF mutation-positive melanoma.
Oncogenic drivers BRAF, MEK1, and MEK2 are shown in green.
Cu: Copper; P: Phosphorylated.
Tetrathiomolybdate: a potent Cu chelating agent
Tetrathiomolybate (TM) is a potent decoppering agent which has a dual mechanism of action. TM forms a stable tripartite complex with Cu and protein, blocking intestinal absorption of Cu when taken with meals and binding Cu from plasma when taken between meals [46]. It has been studied in the setting of both Wilson Disease and as an antineoplastic agent in patients with normal Cu metabolism. Though not commercially available, TM has been tested in clinical trials for Wilson disease and has shown promise in this setting as it does not appear to be associated with the neurological deterioration that can be seen with penicillamine [47]. Its dual mechanism of action and potent anti-Cu activity compared with other agents make it an attractive Cu chelator to consider as oncologic therapy. It has been largely studied as antiangiogenic therapy in preclinical and clinical models. In a series of early phase trials in solid tumors, the administration of TM either alone or in combination with other agents is feasible and well tolerated, summarized in Table 1 [49–54].
Table 1. . Summary of tetrathiomolybate use and toxicity in early phase clinical trials.
| Study (year) | Study design: patient population | Subjects (n) | Drug dose | Concurrent therapies | Adverse events | Efficacy | Ref. |
|---|---|---|---|---|---|---|---|
| Brewer et al. (2000) | Phase I open-label dose escalation: solid metastatic cancers | 18 | 90, 105 and 250 mg/daily divided into six doses† | Trastuzumab (one patient) IFN and radiotherapy (two patients) |
Anemia, sulfur eructation | SD in 5/6 patients | [53] |
| Redman et al. (2003) | Phase II open-label: advanced kidney cancer | 15 | 180 mg/daily divided into four doses† | N/A | Fatigue, sulfur eructation, nausea, diarrhea, dizziness, self-limiting macular rash | 31% SD for at least 6 months during copper depletion (median: 34.5 weeks) | [49] |
| Henry et al. (2006) | Phase II open-label: hormone-refractory prostate cancer | 19 | 180 mg/daily divided into four doses† | N/A | Hematuria, neutropenia, lymphopenia, musculoskeletal pain, unstable angina | 14 PD, 1 BR | [52] |
| Pass et al. (2008) | Phase II open-label: post-operative malignant mesothelioma | 30 | 180 mg/daily divided into four doses† Maintenance: 80 mg/daily† |
N/A | Dizziness, fatigue, neutropenia, anemia, thrombocytopenia, fatigue, granulocytopenia, anemia | TTP 20 months in TM-treated vs 10 months non-TM-treated (p = 0.046) | [54] |
| Gartner et al. (2009) | Phase II open-label: metastatic colorectal cancer | 24 | 180 mg/daily divided into four doses† Maintenance: 80 mg/daily† |
Irinotecan 125 mg/m2, 5-FU 500 mg/m2, and leucovorin 20 mg/m2 | Neutropenia, anemia, diarrhea, nausea, vomiting | ORR 25%, median TTP 5.6 months | [51] |
| Jain et al. (2013) | Phase II open-label: histologically confirmed stage 3 or 4 breast cancer with NED | 40 | 180 mg/daily in four divided doses† Maintenance: 100 mg/daily† |
N/A | Anemia, leukopenia, neutropenia, sulfur eructation, fatigue | 10-month RFS was 85.0% | [50] |
| Nackos et al. (2015) | Phase II open-label: breast cancer with high risk for recurrence | 75 | 180 mg/daily divided into four doses† Maintenance: 100 mg/daily† |
N/A | Neutropenia, anemia | PFS 81% | [55] |
†Dose was titrated to optimal ceruloplasmin levels.
BR: Biochemical recurrence; ORR: Overall response rate; PD: Progressive disease; PFS: Progression-free survival; RFS: Relapse-free survival; SD: Stable disease; TTP: Time to progression.
Initial studies using TM in Wilson disease reported that the drug can be used safely and can rapidly reduce serum Cu levels [47,56–57]. Formal pharmacokinetic (PK) analyses of TM were not performed in early phase clinical trials, presumably due to extensive data in veterinary studies. There is also general acceptance of serum Cu and ceruloplasmin levels as reliable pharmacodynamic markers of efficacy. Toxicity has been manageable, and consistent across numerous studies. Mild bone marrow suppression resulting in reversible leukopenia, anemia and thrombocytopenia has been reported. Elevations in liver transaminases and alkaline phosphatase were also seen. In subsequent larger clinical trials, these all appeared reversible with appropriate treatment breaks and/or dose reductions [58–60].
Preclinical models in Cu chelation & cancer
TM has demonstrated antitumor activity in a number of murine models of human cancer and has been well tolerated in these animal studies. UM-SCC-11B squamous cell head and neck cancer cell line injected into xenograft models treated with TM (0.7 mg/day per mouse and then titrated biweekly to maintain ceruloplasmin suppressed at 20 to 30% of baseline) showed decreased VEGF expression, 54% reduction in the tumor size and increased tumor necrosis [61]. Daily oral treatment of TM at the same dose was shown to roughly halve lung lesions detected in mice after injection of the human oral squamous carcinoma cancer cell line OSCC-3 Luc into the tail vein [62] and reduce the growth of SUM149 breast cancer xenograft tumors in mice [14].
TM has also shown synergy with concurrent chemotherapeutic agents. Daily oral TM (1.25 mg initial dose for 3 days followed by 0.7 mg daily) greatly enhanced the efficacy of 5 mg/kg/week doxorubicin in this model by increasing the sensitivity of tumors to undergo apoptosis (113% increase in apoptosis) [63]. In addition, daily oral TM (0.7–1.5 mg/day) also enhanced the antimetastatic effect of the invasion inhibitor peptide Ac-PHSCN-NH2 in a xenograft mouse model as well as a plasma fibronectin-induced rat model of invasion involving human DU145 prostate cancer cells [15]. Treatment with TM provided in the drinking water reduced tumor growth in a Lewis lung high metastatic carcinoma mouse model to a similar extent as single-dose 15 Gy radiotherapy (RT; 50–60% volume reduction) [15]. Finally, 0.7 mg/day TM administered in the drinking water for 70 days completely blocked xenograft tumor growth of the SCCVII/SF human squamous cell carcinoma cell line. This treatment effect was reversible, as mice taken off treatment rapidly developed tumors [64]. Together, these data suggest that TM has potential antitumor activity across a wide spectrum of cancer models.
Tetrathiomolybdate in oncology: clinical experience
Several early phase clinical trials have demonstrated the feasibility and safety of TM in an array of tumor types, as summarized in Table 1. The first in human trial of TM involved 18 patients with a variety of metastatic solid tumors. TM dosing was variable (90, 105 or 250 mg daily) with a goal of 20% ceruloplasmin (Cp) reduction. TM was tolerated well with only mild anemia. Disease stabilization was achieved in the majority of patients adequately Cu depleted [53]. TM (40 mg PO TID with meals/60 mg at bedtime) was used in a Phase II clinical trial enrolling 15 patients with advanced kidney cancer [49]. A target serum ceruloplasmin level of 5–15 mg/dl was defined as having achieved Cu depletion. All of the patients rapidly became Cu depleted at this dosing. Stable disease was achieved in 31% of patients for at least 6 months during Cu depletion (median, 34.5 weeks). TM was well tolerated with reversible granulocytopenia. A Phase II trial of mesothelioma patients treated with TM showed that time to progression for all stage I or II TM patients was 20 months versus 10 months in non-TM-treated patients (p = 0.046) [54].
Due to its promising anti-angiogenic properties, TM was combined with irinotecan, 5-fluorouracil, and leucovorin (IFL) in 24 patients with metastatic colon cancer. The combination with IFL was well tolerated and dose intensity of IFL was maintained during combination therapy with TM. The overall response rate (RR) was 25% and the median time to progression (TTP) was 5.6 months. VEGF levels were correlated with TTP [51]. This study showed that TM was well tolerated and had no apparent impact on toxicity patterns observed when administered with a standard chemotherapy backbone.
The tumor microenvironment has emerged a therapeutic target in solid tumors and is critical for tumor progression [65,66]. Among the many components of the tumor microenvironment are bone marrow-derived progenitor cells including VEGFR1+ hematopoietic progenitor cells and VEGFR2+ endothelial progenitor cells (EPCs) which have been demonstrated to play a pivotal role in breast cancer progression. A large Phase II study has explored the effect of TM induced Cu depletion on VEGFR2+ EPCs and other factors in the tumor microenvironment in patients at high risk for breast cancer recurrence [55]. In an initial report of 40 high risk breast cancer patients without evidence of disease, TM 100 mg orally was administered to maintain ceruloplasmin <17 mg/dl for 2 years or until relapse [50]. The primary end point was change in VEGFR2+ EPCs. Seventy-five percent of patients achieved the Cu depletion target by 1 month. In Cu-depleted patients only, there was a significant reduction in EPCs. TM was tolerated well with mild toxicity, consistent with that noted in previous studies. Because of encouraging results in the initial cohort, the trial was expanded to accrue 75 patients with enrichment of patients with triple negative breast cancer as a ‘signal’ as observed in the initial cohort [55]. TM was again shown to effectively deplete Cu, and showed promising clinical activity. With more than 5 years of follow-up, PFS was 81% for the entire study population, and was 94% for patients with triple negative disease – a particularly high-risk cohort. Correlative analyses revealed that VEGFR2+ EPCs and serum lysyl oxidase (a Cu dependent enzyme required for conditioning the microenvironment or premetastatic niche) were markedly reduced only in the Cu depleted patients. While these data are encouraging, a Phase III trial will be necessary to evaluate the effect of Cu depletion in a high risk for relapse breast cancer to assess whether it reduces the risk of relapse over standard care. To date, there have been no clinical trials investigating the potential role of TM in BRAF V600 mutated tumors specifically, though trials are being planned.
Conclusion
An increased understanding of the role MAPK signaling plays in melanomagenesis has led to the development of novel therapies that can effectively target this pathway. Additionally, recent preclinical data has uncovered a potential intersection between MAPK activation and Cu+ metabolism, demonstrating that Cu+ is a necessary cofactor required for MEK mediated activation of ERK 1/2. Furthermore, a decrease in levels of this critical element, either through silencing of the CTR1 protein or through chelation results in decreased growth in BRAF driven tumor models. This novel mechanism may provide a foundation for further exploration of compounds with synergistic potential.
Future perspective
Despite major breakthroughs in the combination of targeted therapies in the treatment of BRAFV600E mutated melanoma, novel approaches to delay or overcome BRAF inhibitor resistance are needed. Acquired resistance mechanisms have been identified due to mutations in NRAS, BRAF splice variants, BRAFV600E/K amplifications, MEK 1/2 mutations and non-MAPK pathway alterations with marked heterogeneity. Multiple mechanisms of resistance are often detected in the same patients [37]. Multitargeted approaches will be needed to overcome resistance in BRAF mutated melanoma in targeting the MAPK pathway. Recent preclinical data have emerged that MEK requires Cu+ for both kinase activity and BRAFV600E tumor growth. The Cu chelator, TM reduced tumor growth of both BRAFV600E transformed cells and cells resistant to BRAF inhibition. TM is safe and well tolerated in several early phase clinical trials. Cu chelation using TM may represent a component of a rationally designed strategy to limit MEK1/2 activity and delay resistance in BRAFV600 driven tumorigenesis. Additional preclinical data exploring synergy between dual BRAF/MEK inhibition and copper chelation are needed, with the potential to inform future combination strategies for further clinical investigation.
Footnotes
Financial & competing interests disclosure
AKS Salama receives financial support to conduct clinical trials from Bristol-Myers Squibb, Merck, Genentech, Reata, Celldex and Immunocore for research funding to conduct clinical trials that is paid to institution (no personal compensation). AKS Salama has also served on an advisory board for Bristol-Myers Squibb (personal compensation). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Prohaska JR. Impact of copper deficiency in humans. Ann. NY Acad. Sci. 2014;1314:1–5. doi: 10.1111/nyas.12354. [DOI] [PubMed] [Google Scholar]
- 2.Kaler SG. Translational research investigations on ATP7A: an important human copper ATPase. Ann. NY Acad. Sci. 2014;1314:64–68. doi: 10.1111/nyas.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Fontaine S, Mercer JF. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch. Biochem. Biophys. 2007;463(2):149–167. doi: 10.1016/j.abb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson's disease. Lancet. 2007;369(9559):397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 5.Dancis A, Yuan DS, Haile D, et al. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994;76(2):393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 6.Haremaki T, Fraser ST, Kuo YM, Baron MH, Weinstein DC. Vertebrate Ctr1 coordinates morphogenesis and progenitor cell fate and regulates embryonic stem cell differentiation. Proc. Natl Acad. Sci. USA. 2007;104(29):12029–12034. doi: 10.1073/pnas.0701413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu T, Sempos CT, Freudenheim JL, Muti P, Smit E. Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann. Epidemiol. 2004;14(3):195–201. doi: 10.1016/S1047-2797(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 8.Coates RJ, Weiss NS, Daling JR, Rettmer RL, Warnick GR. Cancer risk in relation to serum copper levels. Cancer Res. 1989;49(15):4353–4356. [PubMed] [Google Scholar]
- 9.Brewer GJ. Copper control as an antiangiogenic anticancer therapy: lessons from treating Wilson's disease. Exp. Biol. Med. (Maywood) 2001;226(7):665–673. doi: 10.1177/153537020222600712. [DOI] [PubMed] [Google Scholar]
- 10.Brewer GJ, Merajver SD. Cancer therapy with tetrathiomolybdate: antiangiogenesis by lowering body copper – a review. Integr. Cancer Ther. 2002;1(4):327–337. doi: 10.1177/1534735402238185. [DOI] [PubMed] [Google Scholar]
- 11.Raju KS, Alessandri G, Ziche M, Gullino PM. Ceruloplasmin, copper ions, and angiogenesis. J. Natl Cancer Inst. 1982;69(5):1183–1188. [PubMed] [Google Scholar]
- 12.Parke A, Bhattacherjee P, Palmer RM, Lazarus NR. Characterization and quantification of copper sulfate-induced vascularization of the rabbit cornea. Am. J. Pathol. 1988;130(1):173–178. [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshii J, Yoshiji H, Kuriyama S, et al. The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. Int. J. Cancer. 2001;94(6):768–773. doi: 10.1002/ijc.1537. [DOI] [PubMed] [Google Scholar]
- 14.Pan Q, Kleer CG, Van Golen KL, et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62(17):4854–4859. [PubMed] [Google Scholar]; • Among the initial preclinical studies to suggest tetrathiomolybate (TM) could have antitumor activity in addition to its effects as a potent copper lowering agent.
- 15.Khan MK, Miller MW, Taylor J, et al. Radiotherapy and antiangiogenic TM in lung cancer. Neoplasia. 2002;4(2):164–170. doi: 10.1038/sj.neo.7900218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brozyna AA, Vanmiddlesworth L, Slominski AT. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int. J. Cancer. 2008;123(6):1448–1456. doi: 10.1002/ijc.23664. [DOI] [PubMed] [Google Scholar]
- 17.Chang T-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009;10(6):2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertolini F, Sukhatme VP, Bouche G. Drug repurposing in oncology-patient and health systems opportunities. Nat. Rev. Clin. Oncol. 2015;12(12):732–742. doi: 10.1038/nrclinonc.2015.169. [DOI] [PubMed] [Google Scholar]
- 19.Turski ML, Brady DC, Kim HJ, et al. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol. Cell. Biol. 2012;32(7):1284–1295. doi: 10.1128/MCB.05722-11. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Preclinical data reporting the role of copper in potentiating MEK 1/2 signaling.
- 20.Brady DC, Crowe MS, Turski ML, et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature. 2014;509(7501):492–496. doi: 10.1038/nature13180. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The initial report on the role of copper in BRAF-mediated oncogenesis, highligting the potential antitumor activity of TM.
- 21.Wellbrock C, Hurlstone A. BRAF as therapeutic target in melanoma. Biochem. Pharmacol. 2010;80(5):561–567. doi: 10.1016/j.bcp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. 2011;29(10):1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 23.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]; •• Initial work characterizing the high incidence of BRAF mutations in melanoma.
- 24.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 25.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, Phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 27.Whittaker S, Kirk R, Hayward R, et al. Gatekeeper mutations mediate resistance to BRAF-targeted therapies. Sci. Transl. Med. 2010;2(35):35–41. doi: 10.1126/scitranslmed.3000758. [DOI] [PubMed] [Google Scholar]
- 28.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trunzer K, Pavlick AC, Schuchter L, et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J. Clin. Oncol. 2013;31(14):1767–1774. doi: 10.1200/JCO.2012.44.7888. [DOI] [PubMed] [Google Scholar]
- 30.Nathanson KL, Martin AM, Wubbenhorst B, et al. Tumor genetic analyses of patients with metastatic melanoma treated with the BRAF inhibitor dabrafenib (GSK2118436) Clin. Cancer Res. 2013;19(17):4868–4878. doi: 10.1158/1078-0432.CCR-13-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4(1):80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paraiso KH, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71(7):2750–2760. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18(6):683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508(7494):118–122. doi: 10.1038/nature13121. [DOI] [PubMed] [Google Scholar]
- 36.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, Phase 3 randomised controlled trial. Lancet. 2015;386(9992):444–451. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 37.Johnson DB. BRAF inhibitor acquired resistance: a multicenter meta-analysis of the spectrum and clinical implications of resistance mechanisms. J. Clin. Oncol. 2015;33(Suppl.) doi: 10.1016/j.ejca.2015.08.022. Abstract 9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2014;372(1):30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 40.Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 41.Johnson DB, Flaherty KT, Weber JS, et al. Combined BRAF (dabrafenib) and MEK inhibition (trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J. Clin. Oncol. 2014;32(33):3697–3704. doi: 10.1200/JCO.2014.57.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagle N, Allen EMV, Frederick DT, et al. Whole exome and whole transcriptome sequencing in melanoma patients to identify mechanisms of resistance to combined RAF/MEK inhibition. J. Clin. Oncol. 2013;31(Suppl.) Abstract 9015. [Google Scholar]
- 43.Thiele DJ, Gitlin JD. Assembling the pieces. Nat. Chem. Biol. 2008;4(3):145–147. doi: 10.1038/nchembio0308-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Festa RA, Thiele DJ. Copper: an essential metal in biology. Curr. Biol. 2011;21(21):R877–R883. doi: 10.1016/j.cub.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson NJ, Winge DR. Copper metallochaperones. Annu. Rev. Biochem. 2010;79:537–562. doi: 10.1146/annurev-biochem-030409-143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47(6):2089–2111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- 47.Brewer GJ, Dick RD, Yuzbasiyan-Gurkin V, Tankanow R, Young AB, Kluin KJ. Initial therapy of patients with Wilson's disease with tetrathiomolybdate. Arch. Neurol. 1991;48(1):42–47. doi: 10.1001/archneur.1991.00530130050019. [DOI] [PubMed] [Google Scholar]
- 48.Friedman A, Perrimon N. A functional RNAi screen for regulators of receptor tyrosine kinase and ERK signalling. Nature. 2006;444(7116):230–234. doi: 10.1038/nature05280. [DOI] [PubMed] [Google Scholar]
- 49.Redman BG, Esper P, Pan Q, et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin. Cancer Res. 2003;9(5):1666–1672. [PubMed] [Google Scholar]
- 50.Jain S, Cohen J, Ward MM, et al. Tetrathiomolybdate-associated copper depletion decreases circulating endothelial progenitor cells in women with breast cancer at high risk of relapse. Ann. Oncol. 2013;24(6):1491–1498. doi: 10.1093/annonc/mds654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gartner EM, Griffith KA, Pan Q, et al. A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil, and leucovorin for metastatic colorectal cancer. Invest. New Drugs. 2009;27(2):159–165. doi: 10.1007/s10637-008-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henry NL, Dunn R, Merjaver S, et al. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology. 2006;71(3–4):168–175. doi: 10.1159/000106066. [DOI] [PubMed] [Google Scholar]
- 53.Brewer GJ, Dick RD, Grover DK, et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin. Cancer Res. 2000;6(1):1–10. [PubMed] [Google Scholar]; •• The first study of TM as an anticancer therapy, demonstrating safety of the agent in this population.
- 54.Pass HI, Brewer GJ, Dick R, Carbone M, Merajver S. A Phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. Ann. Thorac. Surg. 2008;86(2):383–389. doi: 10.1016/j.athoracsur.2008.03.016. discussion 390. [DOI] [PubMed] [Google Scholar]
- 55.Nackos EN, Kornhauser N, Ward MM, et al. Altering the tumor microenvironment: a Phase II study of copper depletion using tetrathiomolybdate (TM) in patients (pts) with breast cancer (BC) at high risk for recurrence. J. Clin. Oncol. 2015;33(15):11008. [Google Scholar]; •• A more recent study of TM, demonstrating early promising results in patients with high-risk breast cancer.
- 56.Brewer GJ, Dick RD, Johnson V, et al. Treatment of Wilson's disease with ammonium tetrathiomolybdate. I. Initial therapy in 17 neurologically affected patients. Arch. Neurol. 1994;51(6):545–554. doi: 10.1001/archneur.1994.00540180023009. [DOI] [PubMed] [Google Scholar]
- 57.Brewer GJ, Johnson V, Dick RD, Kluin KJ, Fink JK, Brunberg JA. Treatment of Wilson disease with ammonium tetrathiomolybdate. II. Initial therapy in 33 neurologically affected patients and follow-up with zinc therapy. Arch. Neurol. 1996;53(10):1017–1025. doi: 10.1001/archneur.1996.00550100103019. [DOI] [PubMed] [Google Scholar]
- 58.Brewer GJ, Hedera P, Kluin KJ, et al. Treatment of Wilson disease with ammonium tetrathiomolybdate: III. Initial therapy in a total of 55 neurologically affected patients and follow-up with zinc therapy. Arch. Neurol. 2003;60(3):379–385. doi: 10.1001/archneur.60.3.379. [DOI] [PubMed] [Google Scholar]
- 59.Brewer GJ, Askari F, Lorincz MT, et al. Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease. Arch. Neurol. 2006;63(4):521–527. doi: 10.1001/archneur.63.4.521. [DOI] [PubMed] [Google Scholar]
- 60.Brewer GJ, Askari F, Dick RB, et al. Treatment of Wilson's disease with tetrathiomolybdate: V. Control of free copper by tetrathiomolybdate and a comparison with trientine. Transl. Res. 2009;154(2):70–77. doi: 10.1016/j.trsl.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Hassouneh B, Islam M, Nagel T, Pan Q, Merajver SD, Teknos TN. Tetrathiomolybdate promotes tumor necrosis and prevents distant metastases by suppressing angiogenesis in head and neck cancer. Mol. Cancer Ther. 2007;6(3):1039–1045. doi: 10.1158/1535-7163.MCT-06-0524. [DOI] [PubMed] [Google Scholar]
- 62.Kumar P, Yadav A, Patel SN, et al. Tetrathiomolybdate inhibits head and neck cancer metastasis by decreasing tumor cell motility, invasiveness and by promoting tumor cell anoikis. Mol. Cancer. 2010;9:206. doi: 10.1186/1476-4598-9-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan Q, Bao LW, Kleer CG, Brewer GJ, Merajver SD. Antiangiogenic tetrathiomolybdate enhances the efficacy of doxorubicin against breast carcinoma. Mol. Cancer Ther. 2003;2(7):617–622. [PubMed] [Google Scholar]
- 64.Cox C, Merajver SD, Yoo S, et al. Inhibition of the growth of squamous cell carcinoma by tetrathiomolybdate-induced copper suppression in a murine model. Arch. Otolaryngol. Head Neck Surg. 2003;129(7):781–785. doi: 10.1001/archotol.129.7.781. [DOI] [PubMed] [Google Scholar]
- 65.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer. 2009;9(4):285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]